Abstract
Purpose of Review
In this review, we discuss the mechanism of action of gabapentinoids and the potential consequences of long-term treatment with these drugs on the musculoskeletal system.
Recent Findings
Gabapentinoids, such as gabapentin (GBP) and pregabalin (PGB) were designed as antiepileptic reagents and are now commonly used as first-line treatment for neuropathic pain and increasingly prescribed off-label for other pain disorders such as migraines and back pain. GBP and PGB exert their analgesic actions by selectively binding the α2δ1 auxiliary subunit of voltage-sensitive calcium channels, thereby inhibiting channel function. Numerous tissues express the α2δ1 subunit where GBP and PGB can alter calcium-mediated signaling events. In tissues such as bone, muscle, and cartilage, α2δ1 has important roles in skeletal formation, mechanosensation, and normal tissue function/repair that may be affected by chronic use of gabapentinoids.
Summary
Long-term use of gabapentinoids is associated with detrimental musculoskeletal outcomes, including increased fracture risk. Therefore, understanding potential complications is essential for clinicians to guide appropriate treatments.
Keywords: Gabapentin, Pregabalin, α2δ1, Bone, Muscle, Cartilage
Introduction
Neuropathic pain is caused by a lesion or disease of the somatosensory system that leads to altered transmission of sensory signals in the spinal cord and brain. Neuropathic pain is often experienced as burning and/or electric-like sensations, including pain from non-painful stimuli (allodynia), and low responsiveness to pain medications [1]. Neuropathic changes result from alterations in ion channels within affected nerves leading to increased neurotransmitter release, upregulated neuronal expression of the α2δ1 subunit of voltage-sensitive calcium channels (VSCCs) [2, 3], and enhanced excitatory synaptic transmission in the nociceptive circuit. Conditions associated with neuropathic pain include post-herpetic neuralgia, diabetic neuropathy, painful radiculopathy, HIV infection, leprosy, amputation, peripheral nerve injury, and stroke [4].
Gabapentinoids, such as gabapentin (GBP) and pregabalin (PGB), are synthetic compounds originally designed to treat epileptic seizures but are now used as first-line treatments for neuropathic pain. GBP (Neurontin®) and PGB (Lyrica®) are approved by the Federal Drug Administration (FDA) for the treatment of four neuropathic pain conditions, including post-herpetic neuralgia, fibromyalgia, neuropathic pain associated with diabetes, and spinal cord injuries [5]. However, in practice, these drugs are commonly prescribed, often as an alternative to opioid therapy, for essentially any type of chronic pain, including off-label conditions such as low back pain, osteoarthritis-related pain [5], and migraines [6]. In fact, in 2018, PGB was the 10th highest-selling drug globally [7] and the 15th most sold drug in the United States [8]. Furthermore, between 2012 and 2016, there was a 64% increase in the number of GBP prescriptions in the United States [5]. GBP and PGB are currently taken by millions of individuals each year, and forecasts indicate that both drugs will remain among the top-selling medications for the foreseeable future. In the ranking of the pharmaceutical products by global sales compiled from GlobalData’s pharmaceutical revenue figures, Lyrica (PGB) is projected to continue to be among the top 50 produced pharmaceuticals through 2023, the last year included in the analysis [9]. More recently, in a market research report, the global GBP market size in 2020 was valued at more than 1.5 billion USD and is expected to continue growing at a compound annual growth rate (CAGR) of 1.8% during 2021–2027 [10].
While GBP and PGB decrease neuronal transmission and are therefore effective treatments for epileptic seizures, uncontrolled pain, and allodynia [3], these drugs have unintended effects in other tissues because of their effects on VSCCs. In this narrative review, we detail the mechanisms by which GBP and PGB function. We delineate how the function of these drugs differs within various tissue types and emphasize their often-unappreciated effects on the musculoskeletal system, including bone, muscle, and cartilage. The implications to medicine and physical rehabilitation of musculoskeletal tissues also are discussed.
Gabapentin and Pregabalin Mechanisms of Action
GBP and PGB were designed originally as pharmacological mimetics of gamma-amino acid butyric acid (GABA) [11, 12], the primary inhibitory neurotransmitter within the cerebral cortex, which maintains the inhibitory tone necessary for counterbalancing neuronal excitation [13]. An imbalance within this feedback system increases seizure activity.
Despite their structural similarity to GABA, GBP [14, 15] and PGB [12] are essentially inactive against the canonical GABAA and GABAB receptors. Instead, GBP binds with nearly exclusive affinity to the extracellular α2δ1 subunit of VSCCs [16], whereas PGB has direct and selective interactions with the α2δ1 and α2δ2 subunits [12].
VSCCs are transmembrane ion channels that selectively enable calcium (Ca2+) influx across the electrochemical gradient of the plasma membrane. There are several classes and subclasses of these channels (for review, see [17•]). VSCCs are typically found in excitable tissues, such as neurons and skeletal muscle, and are essential for transducing changes in the electrical membrane potential into biochemical signals. The influx of Ca2+ through VSCCs regulates many physiological processes, including cardiac action potentials, neurotransmitter release, and muscle contraction. VSCCs typically are composed of several protein subunits forming a multimeric complex [18]. The α1 subunit comprises the pore of the VSCC complex enabling translocation of Ca2+ across the plasma membrane. While the α1 pore is the primary functional subunit of the VSCC complex, several auxiliary subunits associate with the pore, contributing to VSCC activity [19]. These auxiliary subunits include the intramembranous γ subunit, the intracellular β subunit, and the extracellular α2δ subunit, which is the proteolytically cleaved protein product of a single gene [20]. The α2δ subunit remains membrane-bound via a glycosylphosphatidylinositol (GPI) anchor on the C-terminal portion of the protein [21], and associates with the α1 pore via a von Willebrand Factor A domain (vWFA) within the α2 portion of the peptide [22]. Regulation of VSCCs by α2δ subunits is mediated in two ways. First, the association of α2δ increases trafficking of the α1 subunit to the plasma membrane, thereby increasing channel density, and second, α2δ subunits influence the biophysical properties of the calcium currents (for review, see [23]). There are four variants of α2δ, each encoded by a distinct gene (Cacna2d-1, Cacna2d-2, Cacna2d-3, or Cacna2d-4) [24, 25], and they all share a similar topology.
GBP binds to α2δ1 with high affinity but binds to α2δ2 with only 1/3 of the affinity to that of α2δ1 [16]. GBP reduces the cell surface accumulation of α2δ2 and affects VSCC trafficking [26]. GBP does not bind α2δ3 [27] nor α2δ4 [25]. Thus, α2δ1 is the predominant binding site for GBP and this interaction is mediated by an Arg-Arg-Arg (RRR) motif within the extracellular (α2) region of the subunit [28]. Mutation of a single amino acid in the RRR motif of α2δ1 or α2δ2 prevents GBP binding [26].
GBP does not disrupt the association of α2δ1 to the α1 pore-forming unit [29]; however, it does alter the properties of VSCCs. GBP impairs the trafficking of the α1 subunit to the plasma membrane, reducing the magnitude of Ca2+ currents [30, 31]. Deletion or disruption of α2δ1 renders similar effects to that of chronic GBP administration, where the absence of α2δ1 impairs Ca2+ influx and decreases pore opening times [19, 26] (Fig. 1).
Fig. 1.
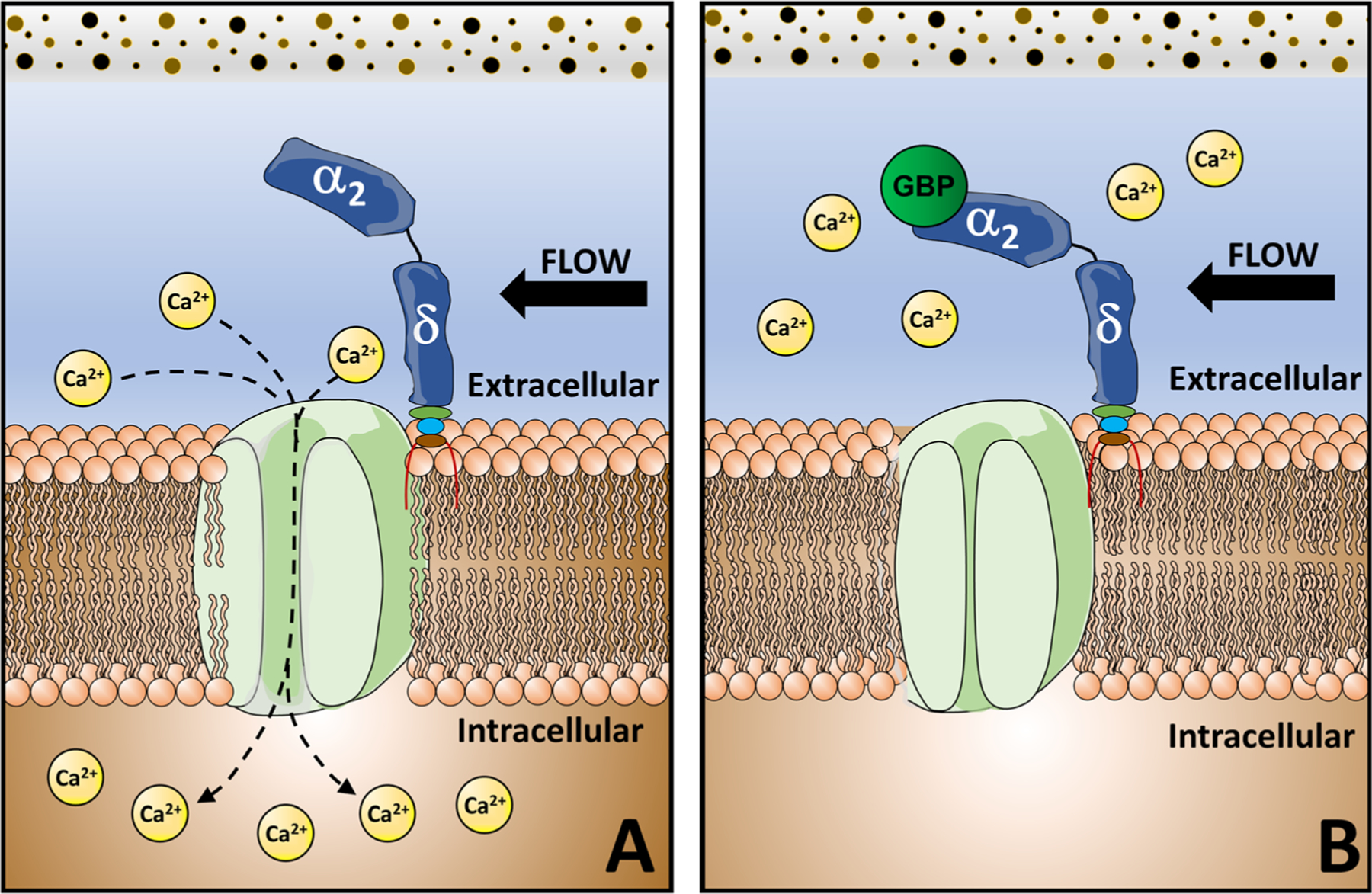
Gabapentinoids bind the α2δ1 subunit of VSCC impairing Ca2+ influx. A Opening of the voltage-sensitive Ca2+ channels (VSCC) is modulated by the α2δ1 auxiliary unit and activated by Ca2+ influx. B) The α2δ1 subunit is the predominant binding site for gabapentinoids, whereby these drugs alter VSCC function and impair Ca2+ currents
Altered expression of α2δ1 leads to current density changes in calcium channels, thereby affecting sensory information processing [2]. In neurons, elevated neuronal α2δ1 expression contributes to specific pain states through a mechanism partially mediated by enhanced VSCC activity in sensory neurons and hyperexcitability in dorsal horn neurons in response to peripheral stimulation. Thus, modulation of α2δ and VSCC activity by gabapentinoids likely underlies the analgesic actions of these drugs [2].
The α2δ subunit has been proposed to interact with other proteins to influence VSCC function, and, in some cases, α2δ exerts its function independent of VSCCs [23]. The binding of α2δ with the low-density lipoprotein receptor-related protein 1 (LRP1) influences N-type VSCC trafficking [32]. In neurons, α2δ1 binds matricellular proteins called thrombospondins (TSPs) [33] to promote synaptogenesis. The α2δ subunit also binds N-methyl-D-aspartate (NMDA) receptors to modulate their activity [34], and synaptic cell-adhesion molecules α-neurexins [35]. In many cases, gabapentinoids block the association of these proteins with α2δ1 and impair their respective actions in various tissues, not just neurons. Gabapentinoids modify specific NMDA-sensitive glutamate receptors and limit some neurexin-1α, and TSP actions [36•]. These effects could contribute to the analgesic actions of gabapentinoids.
Binding of GBP and PGB to α2δ is not tissue specific. Non-selective binding to α2δ subunits has been associated with adverse outcomes in patients treated long-term with these drugs [37, 38, 39••, 40••, 41–48]. VSCCs have critical physiological functions in tissues other than neurons, including bone, skeletal muscle, and smooth muscle [49]. In bone, VSCCs are critical regulators of skeletal formation and are necessary for the anabolic responses of bone to mechanical loading [50–52]. In muscle, α2δ1 regulates satellite cell commitment to the muscle lineage [53], muscle development, and repair [54]. The following sections will summarize the potential unintended adverse effects of GBP and PGB in musculoskeletal tissues resultant from the targeting of α2δ1 in patients treated with GBP or PGB for extended periods of time.
Effects of GBP and PGB in Bone
In vitro and in vivo studies demonstrate the function of VSCCs in skeletal development and maintenance. Treatment with the long-lasting activation (L-type) VSCC inhibitors, verapamil, and nifedipine, impairs skeletal structure and inhibits osteogenesis, resulting in vertebral defects, decreased mineral apposition rates, and impaired bone formation in animals [51, 55].
VSCCs are critical for the anabolic bone responses to mechanical stimuli. During cell mechanotransduction, the earliest event that takes place within 1 min of mechanical stimulation is an increase in the intracellular Ca2+ concentration [56]. This Ca2+ mobilization is triggered by the activation of VSCCs [57]. Our group showed that L-type channels are predominantly expressed in osteoblasts [58]. In contrast, osteocytes produce transiently activated (T-type) channels but lack L-type channels [59]. Treatment with L-type channel inhibitors partially suppressed the load-induced skeletal response in mice compared to vehicle-treated controls [52]. As osteocytes are the most abundant and mechanosensitive cells within bone [60], the presence of T-type channels in these cells likely maintains skeletal mechanosensitivity in the absence of L-type VSCC activity. T-type VSCCs are necessary for mechanically induced Ca2+ oscillations in osteocytes [61]. While auxiliary subunits do not bind T-type VSCCs in all tissues [62], we have shown that the α2δ1 subunit binds T-type (Cav3.2) VSCCs in osteocytes, where it influences channel activity and plays an essential function in mechanical activation in vitro [50].
Mice with α2δ1 global deletion were generated by targeting exon 2 of Cacna2d1 (the gene encoding α2δ1). These animals lack high-affinity GBP binding sites, resulting in altered Ca2+ currents but no changes in other VSCC subunits [63]. Our group used these mice to study the effects of α2δ1 in the bone at baseline and in response to mechanical loading. We assessed structure (DXA), microarchitecture (μCT), and morphological changes (dynamic histomorphometry) of bones from 18-week-old mice lacking α2δ1 and their respective controls (n= 8–6 mice/genotype) subjected to axial ulnar loading (1 bout of loading ever-other-day during a 10-day period). We found α2δ1 knockout mice exhibited osteopenia with impaired bone mass, density, trabecular structure, and blunted anabolic bone responses to mechanical loading compared with control mice (unpublished data). Impaired bone responses to mechanical stimuli were also observed in mice with pharmacological inhibition of the α2δ1 subunit by GBP treatment. In 16-week-old male C57BL/6J mice injected daily with either GBP (300mg/kg) or saline during a 4-week ulnar loading scheme, we found that in contrast to vehicle-injected mice, which had increased bone mineralizing surface (MS/BS) and bone formation rate (BFR/BS) with loading, the GBP treated mice did not [64]. Similarly, transcriptomic analyses identified Cacna2d1 as an important differentially expressed gene in response to mechanosensation in bone [65, 66•]. While Cacna2d1 mRNA expression increased following mechanical stimulation in vertebral osteocytes [65], Cacna2d1 mRNA levels decreased in the bones of mice subjected to hindlimb unloading [66•]. Taken together, these studies demonstrate not only that the α2δ1 subunit is necessary for skeletal responses to mechanical force, but that production of this auxiliary VSCC subunit is sensitive to applied loads.
Several studies in rodents [67, 68] and population-based analyses in humans [37–41] report adverse skeletal effects after chronic treatment with gabapentinoids. Oral administration of GBP in rats (equivalent dosing to the human regimen of 1200 mg/day) negatively affected bone repair after femoral fracture and biomechanical strength of the callus [67]. In another study, Sprague-Dawley rats treated with GBP (150 mg/kg, administrated orally) daily for 12 weeks demonstrated decreased bone formation and enhanced bone resorption compared to controls, ultimately decreasing bone mass and strength. This work also showed that GBP regulates both osteoblasts and osteoclasts by impairing the former (i.e., decreased mineralization surface and serum osteocalcin levels), while activating the latter (i.e., increased osteoclast number/surface, and TRAP serum levels) [68].
In a recent study, feeding with GBP- and PGB-enriched diets (30 mg/kg of PGB or GBP) for 12 weeks significantly increased serum RANKL levels (a marker for osteoclast activity) in orchiectomized rats, but this effect was not observed in gonadally-intact animals. Neither bone mineral density (BMD) nor biomechanical strength were affected with GBP or PGB [69]. These data suggest that the negative impact of these drugs on bone has a dose-response effect and is dependent on the hormonal status of the animals. In the same study, a PGB-containing diet increased sclerostin levels, a negative regulator of bone formation, in gonadally-intact animals compared with untreated controls [69]. Thus, although bone changes were not found in this study, the changes in biochemical markers observed could precede later changes in bone mass and strength, which were not apparent in these studies due to the length of the intervention.
In humans, long-term treatment with antiepileptic drugs (AEDs) is associated with detrimental bone health. In a large population-based sample of males and females, the use of anticonvulsants, including PGB and GBP, was associated with lower bone quantity (BMD) and quality (lower bone strength and higher risk of bone fragility), determined by DXA at the spine and hip, and quantitative heel ultrasound (QUS), respectively [39••]. A new cross-sectional study investigated the association between AEDs and decreased BMD in a large population of Danish patients with epilepsy. Even after accounting for several risk factors, this study showed that both enzyme-inducing antiepileptic drugs (EIAEDs) and polytherapy with AEDs were associated with osteoporosis risk [40••]. Consistent with these data, the use of AEDs (including GBP and PGB) significantly increased rates of bone loss at the hip in a cohort of older men (≥65 years old) [38], while most AEDs were associated with an increased risk of non-traumatic fractures in individuals aged 50 years or older [37]. Although age is an increasing risk factor for bone loss, similar results were shown in young males in which long-term treatment with AEDs correlated with significant bone loss at the hip in the absence of vitamin D deficiency [41].
A recent study investigated the cellular and molecular effects of different AEDs on human primary bone cells [70]. This work showed that GBP inhibits both osteoclastogenesis and osteoblastogenesis by significantly increasing osteoclast apoptosis and decreasing expression of osteoclast- (TRAP, CATK) and osteoblast-specific genes (COL1, ALP, and BMP2). Interestingly, we showed the α2δ1 subunit of VSCC is absent (protein levels) in primary mouse osteoclasts (unpublished data). Thus, GBP modulation of osteoclast behavior may involve a mechanism other than direct binding to α2δ1 in these cells. The impact of GBP on osteoblast and osteoclast functions observed in culture could account for some of the harmful bone effects of GBP in vivo. In contrast, a recent study evaluated the local cellular effects of PGB treatment on human primary osteoblasts (hOB), human mesenchymal stem cells (hMSC), and the human osteosarcoma cell line (MG63) [71]. This work showed short-term osteoanabolic effects on all cells tested after 14 days of PGB treatment (i.e., increased cell proliferation, and increased activity of the osteogenic markers ALP, von Kossa, and Alizarin red). Conflicting data reported in vivo could be attributed to the differences in administration (delivery), the systemic effects, and the duration of PGB treatment versus the in vitro short-term effects evaluated in this study.
Several reports describe skeletal anomalies associated with prenatal GBP exposure.
GBP treatment during pregnancy can disrupt bone development in utero, including those leading to delayed ossification, growth retardation, skull and vertebral column deformities in rodents [72, 73], as well as jaw and oral malformation in humans [74]. Collectively, these studies highlight the adverse skeletal consequences of GBP and PGB treatment and the need for regular monitoring of changes in bone density among patients prescribed gabapentinoids. Bone loss or other bone outcomes are not included in the list of PGB or GBP side effects. It is essential to acknowledge the potential disruption of bone metabolism in individuals taking increasing doses of gabapentinoids over long periods and those with an already compromised hormonal status (i.e., menopause, testosterone, or estradiol depletion). For these patients, osteoprotective strategies may need to be combined with gabapentinoids to reduce the potential side effects of these drugs on musculoskeletal tissues.
Effects of GBP and PGB in Skeletal Muscle
Cross-analysis of three human multi-omics data sets in different tissues (HPA, GTEx, and FANTOM5) showed that gene expression and mRNA levels of Cacna2d1 are 3-fold higher in skeletal muscle than in the brain. Protein profiles of α2δ1 in human normal tissues also showed that α2δ1 expression is higher in skeletal muscle compared to the hippocampus, cerebral cortex, and cerebellum [75].
Similar to its function in bone, α2δ1 regulates the density of L-type VSCCs and activation of Ca2+ current kinetics in skeletal muscle [76]. The α2δ1 subunit is also a component of the dihydropyridine receptor (DHPR), a slowly activating calcium channel that functions as the voltage sensor in excitation-contraction (E.C.) coupling and supports Ca2+ current dynamics [76, 77]. The DHPR α2δ1 subunit is the major α2δ isoform of skeletal muscle, and although α2δ1 is not necessary for E.C. coupling, it is a functional modulator of the VSCC function in muscle [77].
In addition to the direct function of α2δ1 in the regulation of VSCCs, α2δ1 influences extracellular signaling and interactions of muscle cells with their environment [54]. Knocking down α2δ1 in myotubes, using small interfering RNA (siRNA), disrupted the cells’ interactions with the extracellular matrix (ECM), resulting in reduced attachment, spreading, and myoblast migration. These changes were independent of calcium currents and were attributed to direct non-gating functions of α2δ1. Such findings suggest a role for α2δ1 in muscle development and repair, where cell migration is crucial for these processes [54]. Consistent with these observations, α2δ1 is expressed in isolated satellite cells [53], the skeletal muscle stem cells (MuSCs), involved in muscle maintenance and regeneration in response to injury (for review see [78]). α2δ1 protein was detected in MuSCs earlier and at higher levels than other VSCC subunits, and cells that expressed α2δ1 were more likely to commit to muscle lineage than those cells lacking this protein [53]. These data indicate α2δ1 serves an essential function in muscle cell differentiation in addition to direct modulation of gating of VSCCs.
Data demonstrating the effects of gabapentinoids on skeletal muscle are lacking. Modulation of calcium channels in skeletal myocytes by gabapentinoids through binding to α2δ1 has been hypothesized as the mechanism associated with various pathologies; however, the exact pathogenesis of the detrimental effects of gabapentinoids in skeletal muscle in patients is not yet fully understood. Because the α2δ1 subunit influences VSCC gating kinetics, E.C. coupling, and interactions with the extracellular matrix, GBP binding and disruption of these events can reprogram multiple aspects of skeletal muscle structure and/or function.
In a recent report, GBP treatment showed no effects on Ca2+ currents in cardiac myocytes; however, GBP did attenuate Ca2+ currents in skeletal myotubes. These alterations in Ca2+ currents led to uncoupling of DHPR function in GBP-treated myotubes, resulting in impaired ability of the DHRP to trigger Ca2+ release and function as a calcium channel [79]. In this study, mice were exposed to one-time (1, 2, or 5 g/kg) or daily (20, 40, or 80 mg/kg) intraperitoneal injections of PGB for 21 days to test acute and sub-acute toxicity, respectively. PGB administration, particularly at higher doses and for longer-term interventions, resulted in muscle atrophy, inflammatory cell infiltration, cell degeneration, and increased serum levels of muscle injury markers creatinine kinase (CK-MM) and troponin I (fsTn1) [80].
Muscles require innervation to function and thus maintain muscle mass and strength. In this context, neuromuscular junctions allow the motor neuron to transmit signals to the muscle fiber. The basic properties of synaptic transmission at the neuromuscular junction are similar to the process of neuron-to-neuron synaptic transmission, in which Ca2+ influx through VSCCs at nerve terminals enables action potentials and transmitter release [81]. Transmission at the neuromuscular junction begins when an action potential reaches the presynaptic terminal of a motor neuron activating VSCCs to allow Ca2+ to enter the neuron. Ca2+ then triggers neurotransmitter release, causing a cascade of events resulting in muscle contraction [81]. While there is no data directly linking GBP treatment with changes in neuromuscular signaling, compromised synaptic transmission between a motor neuron and a muscle cell could result in neuromuscular dysfunction. As such, calcium channel blockers [82] have been associated with exacerbating weakness in myasthenia gravis (a neuromuscular disorder characterized by muscle weakness). Similarly, although rarer, clinical studies caution about the use of GBP and PGB, raising concern about the development or worsening of muscle conditions, such as myasthenia gravis [45, 46] and myoclonus [47, 48]. Particularly, the latter condition has been noted to occur even with short, low dosage treatments and among patients with normal renal function. Thus, GBP and/or PGB could alter Ca2+ currents at the neuromuscular junction, resulting in decreased neuromuscular transmission, impaired skeletal muscle contraction, and ultimately muscle weakness.
While the mechanisms regulating GBP/PGB-induced muscle dysfunction remain unclear, several clinical studies indicate that these drugs are associated with muscular pathology. Case reports in humans found an association between GBP and PGB treatment with the occurrence of myopathy [42, 43], myositis, and rhabdomyolysis [44]. Additionally, muscle pain and weakness, muscle cramps, rhabdomyolysis, myalgia, and myasthenia are listed in the FDA’s approved labeling for the consumer as possible side effects associated with PGB/GBP use [83, 84].
Effects of GBP and PGB in Cartilage
Similar to bone and muscle cells, VSCCs are expressed in chondrocytes, where they regulate intracellular Ca2+ influx [85]. Several in vitro and in vivo studies using VSCC inhibitors demonstrate an essential function of these channels in chondrocyte biology at various developmental stages. VSCCs influence resting membrane potential in chondrocytes, extracellular matrix synthesis, chondrogenic differentiation and proliferation, chondrocyte metabolism during cartilage formation, endochondral ossification, and chondrocyte mechanosensation and mechanotransduction [86].
While blockage of VSCCs in healthy chondroblasts and chondrocytes is deleterious (i.e., impaired cell proliferation and primary and terminal differentiation), VSCCs have a contrasting function in the setting of inflammatory osteoarthritis (OA). In this context, the increase in VSCC activity and voltage-sensitive Ca2+ signaling in early-degenerated cartilage could be involved in the pathogenesis of OA [86, 87]. In response to the altered Ca2+ signaling, chondrocytes increase the expression of matrix metalloproteinases (MMPs) and inflammatory cytokines, potentially contributing to the onset/progression of OA [87]. Thus, selective modulation and targeting of VSCC function could be beneficial for developing new means to treat OA. While these studies suggest important functions of VSCCs in cartilage, less is known regarding how auxiliary subunits, such as α2δ, influence cartilage pathophysiology.
In one study, the effects of the gabapentin-like analog PD-0200347, an α2δ ligand of VSCCs that selectively blocks Ca2+ currents, were examined in an experimental canine model of OA. Treatment with PD-0200347 significantly reduced the progression of structural changes in cartilage by reducing the production of MMPs and nitric oxide synthase (iNOS) (catabolic factors involved in cartilage degradation) [88] and inhibiting ERK1/2 activation via a Ras-independent mechanism [89]. The pharmacological properties of PD-0200347 in these experiments were mainly associated with its binding to α2δ1 and the subsequent reduction in Ca2+ influx in chondrocytes. This work suggests that this gabapentin-like drug may prevent cartilage degradation. While the mechanisms underlying those effects remain unclear, unregulated Ca2+ influx leading to high intracellular calcium is cytotoxic [90]. Thus, one could speculate that the protective effects observed by this group reflect the reduction of Ca2+ influx by PD-0200347 treatment, reducing chondrocyte cell apoptosis. As is the case with the use of GBP and PGB to treat neuropathic pain, chronic use of α2δ1 ligands such as PD-0200347, although beneficial in certain pathologies, may have unintended, even deleterious, effects on healthy bone, cartilage, or muscle that should be considered when prescribing these drugs.
Other α2δ Ligands
While gabapentinoids can alleviate neuropathic pain, these drugs are not always effective and often are accompanied by severe side effects. Gabapentinoids are the first-line therapy for neuropathic pain; however, they are only 30% effective in 30% of patients, and based on the average number of people who must be treated to achieve 50% pain relief in one person, these drugs can be considered to be largely ineffective [91]. Moreover, many patients cannot continue their treatment because of common side effects including somnolence, dizziness, and edema [92]. In the past decade, efforts and resources have been directed to discover new drugs to treat neuropathic pain with increased efficacy and fewer or more tolerable side effects. Like PD-0200347, some of these new drugs also bind α2δ. In the following section, we will describe the use and potential of some of these alternative drugs.
Mirogabalin Besilate
Developed by Daiichi Sankyo Co., Ltd. (Tokyo, Japan), Mirogabalin (MGB) ([(1R,5S,6S)-6-(aminomethyl)-3-ethylbicyclo[3.2.0]hept3-en-6-yl] acetic acid) is a selective ligand for α2δ designed to treat pain associated with diabetic peripheral neuropathy and post-herpetic neuralgia [93]. Mirogabalin was approved to treat peripheral neuropathic pain in Japan in January 2019 under the market name Tarlige®, and in Korea and Taiwan in 2020. MGB is being evaluated as a therapy for diabetic neuropathy [94] and effectively provides pain relief in rat models of fibromyalgia [95••]. However, this drug still is awaiting approval for its use in other countries [96]. MGB has a selective and potent binding affinity for α2δ subunits in humans, which reduces Ca2+ influx. The binding affinity of MGB to α2δ1 is higher (KD = 13.5 nmol/L) [97] than that of GBP (KD = 59 nmol/L) [96] or PGB (KD = 62.5 nmol/L), but the association time for the α2δ1 (11.1 h ) and α2δ2 subunits (2.4 h) is longer for MGB than for PGB (1.4 h from both α2δ1 and α2δ2 subunits) [97]. The high affinity and slow dissociation kinetics from α2δ1 contribute to MGB’s greater analgesic efficacy. Thus, the equianalgesic daily dose for 30 mg of MGB is 600 mg of PGB and over 1200 mg of GBP [96]. In other words, MGB is equally effective at smaller doses, at least when considering its effects on pain relief. Additionally, recent evidence from several clinical trials and case reports found that, compared to PGB, MGB had fewer adverse side effects, less patient withdrawal, and lower abuse potential [96]. Although binding of MGB to α2δ1 is necessary for the pain-relieving effect of this drug, a new study reported that the mechanism of MGB’s analgesic effects may be due to the actions of this drug on the activity of sodium-gated channels in excitable cells [98].
Collectively, these data indicate that MGB may offer a superior and more efficient treatment for various neuropathic pain syndromes compared to GBP and PGB. However, the fact that MGB binds with both higher affinity and for a longer period to α2δ1 could lead to unintended effects on off-target tissues. Thus, the long-term effects of this drug on the musculoskeletal system remain unknown, thus warranting future research.
Phenibut
Phenibut (PHB), also known as Anvifen, Fenibut, or Noofen [99], is a clinically used anxiolytic and nootropic drug structurally related to gabapentin. R-phenibut ((3R)-phenyl-4-aminobutyric acid) mimics the structure of the neurotransmitter GABA, and unlike GBP and PGB, its activity correlates with its binding affinity to GABAB receptors [100]. However, subsequent binding experiments in rat brain membrane preparations revealed that the affinity of R-phenibut for α2δ is four times higher than its affinity for the GABAB receptors. Additionally, the anti-nociceptive effects of this drug, in an experimental pain model, were associated with its action on α2δ rather than on GABAB receptors [101]. Although PHB has been suggested as a novel candidate to treat neuropathic pain, there have been recent reports of acute toxicity/withdrawal [99] and physical dependence associated with its use [102]. PHB was synthesized at the Department of Organic Chemistry of the Herzen Pedagogic Institute in St. Petersburg [103] and is used for various medical conditions in Russia, Ukraine, Belarus, and Latvia. To date, the US Food and Drug Administration (FDA) has not approved the use of PHB as a prescription medication [104•], and has recently issued several warning letters for the use of PHB, classifying it as an unsafe product [105]. However, PHB has been marketed as a dietary supplement in the USA and can be obtained via the internet or over-the-counter, in higher doses (up to 450%) than the typical pharmaceutical tablet (250mg) [104•]. Considering that PHB could result in adverse musculoskeletal effects due to its binding to α2δ1, together with its potential toxic and addictive effects and its marketing as an uncontrolled substance, extra caution should be taken when this drug is used for therapeutic purposes.
Enacarbil
Gabapentin enacarbil (GEn) (also known as XP13512, GSK1838262, Horizant) is a prodrug to gabapentin, specifically designed (XenoPort, Inc., California) for increased oral bioavailability over gabapentin [106]. GBP absorption from the upper small intestine occurs by an active, saturable pathway, dependent on system L-amino acid transporters. The saturable absorption of GBP results in dose-dependent pharmacokinetics reflected as a decrease in GBP bioavailability as the dose of the drug increases [107]. XP13512 was engineered to be recognized as a substrate by two high-capacity nutrient transporters broadly distributed in the intestinal tract of humans: the monocarboxylate transporter type 1 (MCT-1) and the sodium-dependent multivitamin transporter (SMVT) [106]. This property allows absorption throughout the length of the intestine, including the colon, and sustained, dose-dependent exposure to GBP as the prodrug is metabolized. Pharmacokinetic comparisons in humans of the XP13512 immediate- and extended-release formulations with oral GBP showed that XP13512 had overall higher bioavailability and prolonged delivery to the circulation than GBP [108]. These results suggest treatment with GEn could reduce dosing frequency, improve compliance, and have prolonged effects in patients compared to those treated with GBP. In 2011, the FDA approved the use of the extended-release formulation of GEn (Horizant) to treat moderate-to-severe primary restless legs syndrome (RLS), for which this prodrug has shown efficacy in several clinical trials [109, 110], and in 2012, for the management of postherpetic neuralgia. As there is no therapeutically equivalent version of Horizant available in the USA (i.e., generic product), the costs of Horizant are significantly higher than GBP.
Long-Term Use of Gabapentinoids—Implications for Physical Rehabilitation
As gabapentinoids in general, and GBP in particular, are widely used for many different conditions that influence pain and thus physical mobility, the effects of these drugs on the musculoskeletal system should be considered when designing pharmacological and physical rehabilitation regimens. While GBP can enable restoration of function by alleviation of pain in some individuals, there may be additional long-term adverse consequences that affect long-term rehabilitation because of interactions in musculoskeletal tissues that are not always considered in treatment planning. Additionally, withdrawal effects of GBP/PGB include confusion, disorientation, and weakness that can last for up to 10 days after quitting the medication [111], which could increase the risk of falls in adults.
Long-term treatment with gabapentinoids could compromise bone and muscle quality and quantity, repair processes, cartilage formation, and the anabolic bone and cartilage in which VSCCs are critical for various functions (Fig. 2). As these effects are not well known in the treatment community, the detrimental outcomes in skeletal tissues resulting from the long-term pharmacological blockage of α2δ1 with GBP/PGB in patients treated with these drugs are not routinely considered in clinical practice. It is unclear if some of these adverse effects appear after the GBP/PGB therapy has ceased, as they have not been studied systematically.
Fig. 2.
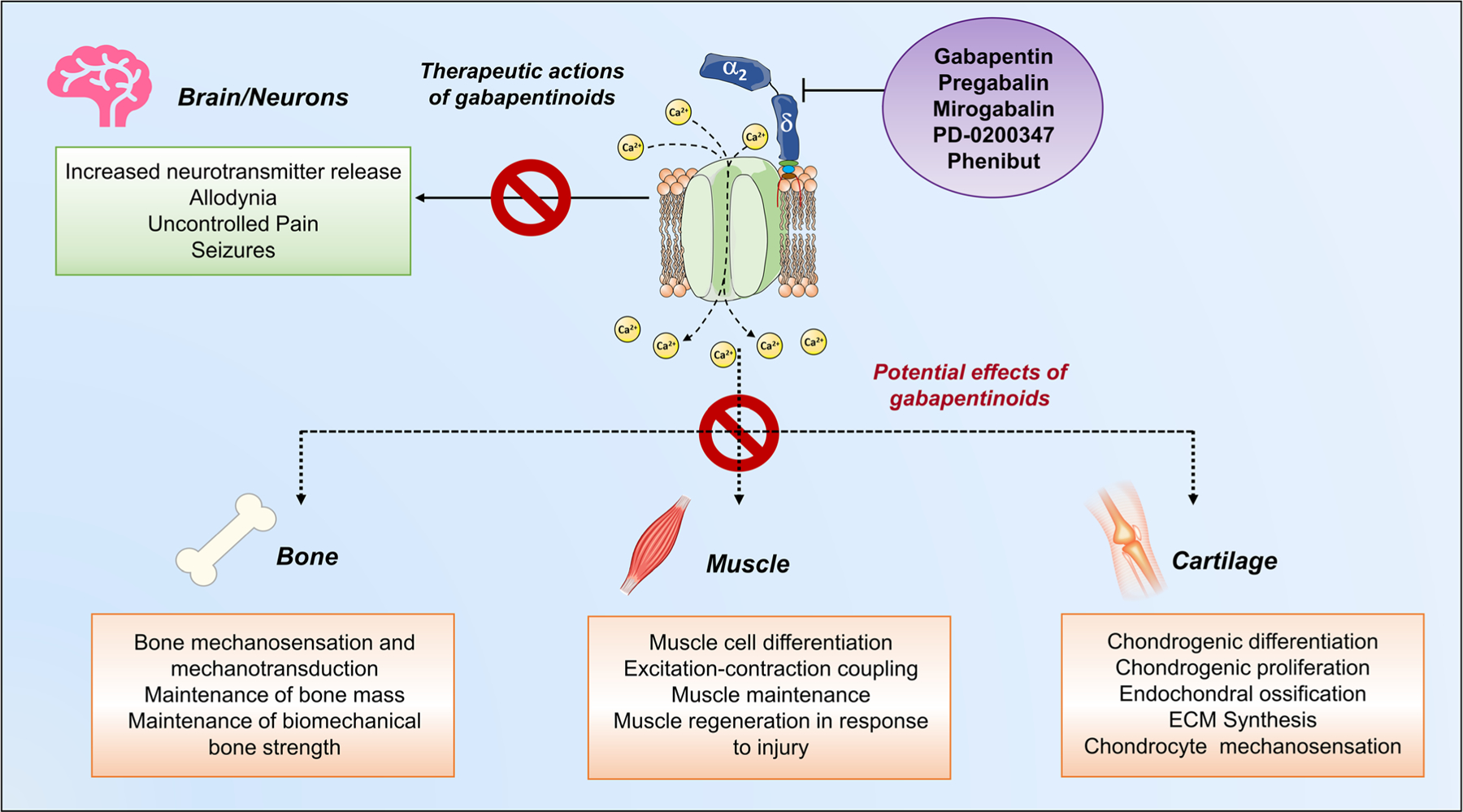
Potential effects of gabapentinoids in musculoskeletal tissues. Ca2+ influx through VSCCs regulates many physiological processes, including neurotransmitter release, bone, and chondrocyte mechanosensation, and muscle contraction. In neurons, elevated neuronal α2δ1 expression contributes to specific pain states through a mechanism partially mediated by enhanced VSCC activity (green square). Thus, gabapentinoids modulate VSCC activity by acting through α2δ1, which underlies the analgesic actions of these drugs (purple circle). However, the binding of gabapentinoids to α2δ subunits in bone, muscle, and cartilage potentially interferes with normal VSCC function (orange squares), resulting in adverse physiological impairments in patients treated long-term with these drugs
We identified a series of potential considerations for clinicians and rehabilitation specialists when working with patients taking GBP/PGB. These are summarized below:
Increased use of GPB and PGB. Both drugs are prescribed largely worldwide as an alternative to addictive opioids. Moreover, the US generic availability of GBP in 2014 and PGB in 2019 has lowered the costs, increased the availability, and amplified the use of already heavily prescribed medications.
Off-label uses of gabapentinoids. Gabapentinoids often are prescribed in the periand post-operative periods, and to treat generalized, multifactorial, non-specific pain, despite the lack of data showing efficacy for these uses.
Adverse effects and potential misuse of PGB and GBP. Besides the negative impact of GBP/PGB on bone and muscle, the use of gabapentinoids is associated with adverse reactions in the central nervous system, and there is growing evidence reporting withdrawal symptoms, and the abuse and misuse of these drugs.
Questionable effectiveness of GBP/PGB. Although some alternatives have been developed to improve the efficacy and selectivity of current medications to treat neuropathic pain, much work remains to be done. GBP and PGB often are ineffective for pain conditions and other pathologies for which their use is approved. Some of the reasons for ineffectiveness include poor drug bioavailability and a substantial inter-individual variation in drug absorption. The average bioavailability of a 600 mg oral dose of GBP is reported to be 49% in healthy individuals, and it is even lower with greater GBP doses [112]. Similarly, significant variation in GBP absorption is seen among individual subjects ranging from 5 to 74%, which could account for the large placebo responses and inadequate response to the drugs in some individuals as observed in multiple clinical trials [112].
Physical rehabilitation relies on mechanical stimuli. Physical therapy interventions use extrinsic and/or intrinsic mechanical forces (i.e., via therapist intervention or exercise therapy) to enhance tissue regeneration, healing, or remodeling [113]. Patients treated with gabapentinoids may exhibit desensitized responses to mechanical signals at the cellular level in musculoskeletal tissues. Thus, the efficacy of these interventions may be blunted among individuals taking these medications. GBP is absorbed slowly after oral administration, peak plasma concentrations occur within 3 to 4 h, both GBP and PGB exhibit short elimination half-life of approximately 6 h [114]. Thus, traditional GBP treatment regimens include doses of 600 mg, 3 times a day, for a maximum of 1800 mg/day. Pain relief may start after one week, reach a maximum effect after 4 weeks, and the drug is usually taken for long periods of times. Understanding how gabapentinoids influence mechanically induced anabolic responses in musculoskeletal tissues will provide physicians and rehabilitation specialists with critical information to inform the design of both pharmacological and non-pharmacological interventions for pain and musculoskeletal disorders.
Conclusions and Future Directions
Given the consequences associated with gabapentinoids, musculoskeletal rehabilitation in patients taking these drugs can be challenging. Hence, health professionals must exercise caution and appropriately consider the risks posed when recommending the use of gabapentinoids, particularly in vulnerable individuals. At risk populations include elderly patients presenting with age-associated bone loss, those experiencing sarcopenia and loss of physical function; postmenopausal women with increased fracture-risk; patients recovering from surgery for which these drugs are often prescribed long-term; and conditions in which bone healing/repair could be compromised, such as post-surgery after total knee replacement (TKR) or total hip arthroplasty (THA). As gabapentinoids are often co-administered with other medications, the potentially harmful effects of drug synergism must be considered. Finally, several questions remain unclear regarding gabapentinoid therapy, including the following: How long is the calcium channel function altered after treatment? Should physical rehabilitation be delayed until concentrations decline and mechanosensation has recovered? Can drug regimen be adjusted to account for musculoskeletal effects? Could an increase in physical activity and/or channel function offset the negative effects of the drugs on the musculoskeletal system?
There is a need to develop safer, more effective, and specific drugs acting on new pain targets. The evidence is growing indicating that gabapentinoids may have modest efficacy in many patients receiving them. Given the growth in prescriptions for off-label use, the potential effects on unintended tissues such as the musculoskeletal system are concerning. Future clinical trials should include better phenotypic patient profiling and long-term evaluations of the risks/benefits of gabapentinoids and related drugs. Additionally, genome-wide association studies could be helpful to identify appropriate therapeutic targets and biomarkers for responses to gabapentinoids, reduce adverse drug-associated outcomes, and improve drug efficacy in individual patients [115]. Educational efforts to reduce off-label prescriptions, increase monitoring of patients treated with gabapentinoids, and incorporate therapies to protect the musculoskeletal system by limiting long-term prescriptions in new patients may improve outcomes in patients receiving rehabilitative therapies.
Funding
This study was supported by 1F32AR074893-01 to CSW, 1R01AR074473-01 to WRT, UL1 TR001108 to WRT, and Faculty Research Development funds through Marian University to WRT.
Footnotes
Compliance with Ethical Standards
Conflict of Interest All authors declare no conflicts of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci 2009;32:1–32. 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li CY, Zhang XL, Matthews EA, Li KW, Kurwa A, Boroujerdi A, Gross J, Gold MS, Dickenson AH, Feng G, Luo DZ. Calcium channel alpha2delta1 subunit mediates spinal hyperexcitability in pain modulation. Pain 2006;125(1–2):20–34. 10.1016/j.pain.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo ZD, Chaplan SR, Higuera ES, Sorkin LS, Stauderman KA, Williams ME, et al. Upregulation of dorsal root ganglion α2δ calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats 2001;21(6):1868–75. 10.1523/JNEUROSCI.21-06-01868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, Freeman R, Truini A, Attal N, Finnerup NB, Eccleston C, Kalso E, Bennett DL, Dworkin RH, Raja SN. Neuropathic pain. Nat Rev Dis Primers 2017;3(1):17002. 10.1038/nrdp.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodman CW, Brett AS. Gabapentin and pregabalin for pain is increased prescribing a cause for concern? N Engl J Med 2017;377(5):411–4. 10.1056/NEJMp1704633. [DOI] [PubMed] [Google Scholar]
- 6.Urits I, Gress K, Charipova K, Zamarripa AM, Patel PM, Lassiter G, et al. Pharmacologic options for the treatment of chronic migraine pain 2020. 10.1016/j.bpa.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Urquhart L Market watch: Top drugs and companies by sales in 2017. Nat Rev Drug Discov 2018;17(4):232. 10.1038/nrd.2018.42. [DOI] [PubMed] [Google Scholar]
- 8.Philippidis AJGE, News B. Top 15 best-selling drugs of 2018: sales for most treatments grow year-over-year despite concerns over rising prices 2019;39(4):16–7. 10.1089/genedge.1.1.05. [DOI] [Google Scholar]
- 9.GlobalData. PMLive Top 50 pharmaceutical products by global sales https://www.pmlive.com/top_pharma_list/Top_50_pharmaceutical_products (2017). Accessed 10/14/21.
- 10.Press Release. At a 1.8% CAGR, Gabapentin Market 2021 is expected to register a significant growth by increasing demand from end-user industries including Epilepsy and Neuropathic Pain https://www.marketwatch.com/press-release/at-a-18-cagr-gabapentin-market-2021-is-expected-to-register-a-significant-growth-by-increasing-demand-from-end-user-industries-including-epilepsy-and-neuropathic-pain-2021-07-25 (2021). Accessed 10/14/21.
- 11.Goa KL, Sorkin EM. Gabapentin. A review of its pharmacological properties and clinical potential in epilepsy. Drugs 1993;46(3): 409–27. 10.2165/00003495-199346030-00007. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Taylor CP, Weber M, Piechan J, Prior F, Bian F, et al. Pregabalin is a potent and selective ligand for α2δ−1 and α2δ−2 calcium channel subunits 2011;667(1–3):80–90. 10.1016/j.ejphar.2011.05.054. [DOI] [PubMed] [Google Scholar]
- 13.Treiman DM. GABAergic mechanisms in epilepsy. Epilepsia 2001;42(Suppl 3):8–12. 10.1046/j.1528-1157.2001.042suppl.3008.x. [DOI] [PubMed] [Google Scholar]
- 14.Jensen AA, Mosbacher J, Elg S, Lingenhoehl K, Lohmann T, Johansen TN, Abrahamsen B, Mattsson JP, Lehmann A, Bettler B, Bräuner-Osborne H. The anticonvulsant gabapentin (neurontin) does not act through gamma-aminobutyric acid-B receptors. Mol Pharmacol 2002;61(6):1377–84. 10.1124/mol.61.6.1377. [DOI] [PubMed] [Google Scholar]
- 15.Lanneau C, Green A, Hirst WD, Wise A, Brown JT, Donnier E,J. Charles K, Wood M, Davies CH, Pangalos MN. Gabapentin is not a GABAB receptor agonist. Neuropharmacology 2001;41(8): 965–75. 10.1016/s0028-3908(01)00140-x. [DOI] [PubMed] [Google Scholar]
- 16.Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem 1996;271(10):5768–76. 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- 17.•.Wright CS, Robling AG, Farach-Carson MC, Thompson WR. Skeletal functions of voltage sensitive calcium channels. Current osteoporosis reports 2021;19(2):206–21. 10.1007/s11914-020-00647-7. A comprehensive review of the function of voltage-gated calcium channels in bone cells and their regulation of bone development, bone formation, and mechanotransduction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev 2005;57(4):411–25. 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- 19.Dolphin AC. Voltage-gated calcium channels and their auxiliary subunits: physiology and pathophysiology and pharmacology. J Physiol 2016;594(19):5369–90. 10.1113/JP272262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Jongh KS, Warner C, Catterall WA. Subunits of purified calcium channels. Alpha 2 and delta are encoded by the same gene. J Biol Chem 1990;265(25):14738–41. 10.1016/S0021-9258(18)77174-3. [DOI] [PubMed] [Google Scholar]
- 21.Davies A, Kadurin I, Alvarez-Laviada A, Douglas L, Nieto-Rostro M, Bauer CS, Pratt WS, Dolphin AC. The alpha2delta subunits of voltage-gated calcium channels form GPI-anchored proteins, a posttranslational modification essential for function. Proc Natl Acad Sci U S A 2010;107(4):1654–9. 10.1073/pnas.0908735107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canti C, Nieto-Rostro M, Foucault I, Heblich F, Wratten J, Richards MW, et al. The metal-ion-dependent adhesion site in the Von Willebrand factor-A domain of alpha2delta subunits is key to trafficking voltage-gated Ca2+ channels. Proc Natl Acad Sci U S A 2005;102(32):11230–5. 10.1073/pnas.0504183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dolphin AC. Voltage-gated calcium channel alpha 2delta subunits: an assessment of proposed novel roles. F1000Res 2018;7. 10.12688/f1000research.16104.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klugbauer N, Lacinova L, Marais E, Hobom M, Hofmann F. Molecular diversity of the calcium channel alpha2delta subunit. J Neurosci 1999;19(2):684–91. 10.1523/JNEUROSCI.19-02-00684.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin N, Yagel S, Momplaisir ML, Codd EE, D’Andrea MR. Molecular cloning and characterization of the human voltage-gated calcium channel alpha(2)delta-4 subunit. Mol Pharmacol 2002;62(3):485–96. 10.1124/mol.62.3.485. [DOI] [PubMed] [Google Scholar]
- 26.Hendrich J, Van Minh AT, Heblich F, Nieto-Rostro M, Watschinger K, Striessnig J, et al. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc Natl Acad Sci U S A 2008;105(9):3628–33. 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marais E, Klugbauer N, Hofmann F. Calcium channel alpha(2)delta subunits-structure and Gabapentin binding. Mol Pharmacol 2001;59(5):1243–8. 10.1124/mol.59.5.1243. [DOI] [PubMed] [Google Scholar]
- 28.Wang M, Offord J, Oxender DL, Su TZ. Structural requirement of the calcium-channel subunit alpha2delta for gabapentin binding. Biochem J 1999;342(Pt 2):313–20. 10.1042/bj3420313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cassidy JS, Ferron L, Kadurin I, Pratt WS, Dolphin AC. Functional exofacially tagged N-type calcium channels elucidate the interaction with auxiliary alpha2delta-1 subunits. Proc Natl Acad Sci U S A 2014;111(24):8979–84. 10.1073/pnas.1403731111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fink K, Meder W, Dooley DJ, Gothert M. Inhibition of neuronal Ca(2+) influx by gabapentin and subsequent reduction of neurotransmitter release from rat neocortical slices. Br J Pharmacol 2000;130(4):900–6. 10.1038/sj.bjp.0703380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Hooft JA, Dougherty JJ, Endeman D, Nichols RA, Wadman WJ. Gabapentin inhibits presynaptic Ca(2+) influx and synaptic transmission in rat hippocampus and neocortex. Eur J Pharmacol 2002;449(3):221–8. 10.1016/s0014-2999(02)02044-7. [DOI] [PubMed] [Google Scholar]
- 32.Kadurin I, Rothwell SW, Lana B, Nieto-Rostro M, Dolphin ACJSr. LRP1 influences trafficking of N-type calcium channels via interaction with the auxiliary α 2 δ−1 subunit 2017;7(1):1–17. 10.1038/srep43802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eroglu C, Allen NJ, Susman MW, O’Rourke NA, Park CY, Özkan E, et al. Gabapentin receptor α2δ−1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis 2009;139(2):380–92. 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, Li L, Chen S-R, Chen H, Xie J-D, Sirrieh RE, et al. The α2δ−1-NMDA receptor complex is critically involved in neuropathic pain development and gabapentin therapeutic actions 2018;22(9):2307–21. 10.1016/j.celrep.2018.02.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brockhaus J, Schreitmuller M, Repetto D, Klatt O, Reissner C, Elmslie K, et al. alpha-Neurexins together with alpha2delta-1 auxiliary subunits regulate Ca(2+) influx through Cav2.1 channels. J Neurosci 2018;38(38):8277–94. 10.1523/JNEUROSCI.0511-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.•.Taylor CP, Harris EW. Analgesia with gabapentin and pregabalin may involve N-methyl-d-aspartate receptors, neurexins, and thrombospondins. J Pharmacol Exp Ther 2020;374(1):161–74. 10.1124/jpet.120.266056. Interesting review discussing the interaction of a2d-1 with N-methyl-D-aspartate–sensitive glutamate receptors, neurexin1a, thrombospondins, and other presynaptic proteins, in addition to actions at calcium channels, and the importance of these findings for gabapentin and pregabalin therapeutic effects. [DOI] [PubMed] [Google Scholar]
- 37.Jette N, Lix LM, Metge CJ, Prior HJ, McChesney J, Leslie WD. Association of antiepileptic drugs with nontraumatic fractures: a population-based analysis. Arch Neurol 2011;68(1):107–12. 10.1001/archneurol.2010.341. [DOI] [PubMed] [Google Scholar]
- 38.Ensrud KE, Walczak TS, Blackwell TL, Ensrud ER, Barrett-Connor E, Orwoll ES, For the Osteoporotic Fractures in Men (MrOS) Study Research Group. Antiepileptic drug use and rates of hip bone loss in older men: a prospective study. Neurology 2008;71(10):723–30. 10.1212/01.wnl.0000324919.86696.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.••.Chandrasekaran V, Pasco JA, Stuart AL, Brennan-Olsen SL, Berk M, Hodge JM, et al. Anticonvulsant use and bone health in a population-based study of men and women: cross-sectional data from the Geelong Osteoporosis Study. Bmc Musculoskel Dis 2021;22(1):172. 10.1186/s12891-021-04042-w. This study evaluated the association between anticonvulsant use and bone health in a population-based sample of men and women. The main findings were that bone quantity and quality were lower for users of anticonvulsants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.••.Baddoo DR, Mills AA, Kullab RB, Al-Mashat H, Andersen NB, Jorgensen NR, et al. Metabolic bone disease in patients with epilepsy and the use of antiepileptic drugs-Insight from a Danish cross-sectional study. Seizure-Eur J Epilep 2021;86:29–34. 10.1016/j.seizure.2021.01.008. A recent cross-sectional study showing a clear association between the use of AEDs and decreased bone mineral density in a large population of Danish patients with epilepsy. [DOI] [PubMed] [Google Scholar]
- 41.Andress DL, Ozuna J, Tirschwell D, Grande L, Johnson M, Jacobson AF, Spain W. Antiepileptic drug-induced bone loss in young male patients who have seizures. Arch Neurol 2002;59(5): 781–6. 10.1001/archneur.59.5.781. [DOI] [PubMed] [Google Scholar]
- 42.Hegde V, Shekar N, Garrett F, Baz M, Anstead M. Pregabalin-induced myopathy in a double lung transplant recipient. Cureus 2020;12(12):e11935. 10.7759/cureus.11935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tuccori M, Lombardo G, Lapi F, Vannacci A, Blandizzi C, Del Tacca M. Gabapentin-induced severe myopathy. Ann Pharmacother 2007;41(7):1301–5. 10.1345/aph.1K077. [DOI] [PubMed] [Google Scholar]
- 44.Coupal TM, Chang DR, Pennycooke K, Ouellette HA, Munk PL. Radiologic findings in gabapentin-induced myositis. J Radiol Case Rep 2017;11(4):30–7. 10.3941/jrcr.v11i4.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheschonka A, Beuche W. Treatment of post-herpetic pain in myasthenia gravis: exacerbation of weakness due to gabapentin. Pain 2003;104(1–2):423–4. 10.1016/S0304-3959(03)00004-6. [DOI] [PubMed] [Google Scholar]
- 46.Chien JL, Baez V, Mody HR. Uncontrolled recurrent myasthenia gravis exacerbations secondary to chronic gabapentin use. J Community Hosp Int 2019;9(4):371–2. 10.1080/20009666.2019.1643220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim JB, Jung JM, Park MH, Lee EJ, Kwon DY. Negative myoclonus induced by gabapentin and pregabalin: a case series and systematic literature review. J Neurol Sci 2017;382:36–9. 10.1016/j.jns.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 48.Koide Y, Ikeda H, Inoue Y. Development or worsening of myoclonus associated with gabapentin therapy. Rinsho Shinkeigaku 2009;49(6):342–7. 10.5692/clinicalneurol.49.342. [DOI] [PubMed] [Google Scholar]
- 49.Zamponi GW, Striessnig J, Koschak A, Dolphin AC. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol Rev 2015;67(4):821–70. 10.1124/pr.114.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson WR, Majid AS, Czymmek KJ, Ruff AL, Garcia J, Duncan RL, et al. Association of the alpha(2)delta(1) subunit with Ca(v)3.2 enhances membrane expression and regulates mechanically induced ATP release in MLO-Y4 osteocytes. J Bone Miner Res 2011;26(9):2125–39. 10.1002/jbmr.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li J, Duncan RL, Burr DB, Gattone VH, Turner CH. Parathyroid hormone enhances mechanically induced bone formation, possibly involving L-type voltage-sensitive calcium channels. Endocrinology 2003;144(4):1226–33. 10.1210/en.2002-220821. [DOI] [PubMed] [Google Scholar]
- 52.Li J, Duncan RL, Burr DB, Turner CH. L-type calcium channels mediate mechanically induced bone formation in vivo. J Bone Miner Res 2002;17(10):1795–800. 10.1359/jbmr.2002.17.10.1795. [DOI] [PubMed] [Google Scholar]
- 53.Tamayo T, Grajales L, Garcia J. Commitment of satellite cells expressing the calcium channel alpha2delta1 subunit to the muscle lineage. J Signal Transduct 2012;2012:460842. 10.1155/2012/460842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garcia K, Nabhani T, Garcia J. The calcium channel alpha2/delta1 subunit is involved in extracellular signalling. J Physiol 2008;586(3):727–38. 10.1113/jphysiol.2007.147959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duriez J, Flautre B, Blary MC, Hardouin P. Effects of the calcium channel blocker nifedipine on epiphyseal growth plate and bone turnover: a study in rabbit. Calcif Tissue Int 1993;52(2):120–4. 10.1007/BF00308320. [DOI] [PubMed] [Google Scholar]
- 56.Robling AG, Turner CH. Mechanical signaling for bone modeling and remodeling. Crit Rev Eukaryot Gene Expr 2009;19(4):319–38. 10.1615/critreveukargeneexpr.v19.i4.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davidson RM, Tatakis DW, Auerbach AL. Multiple forms of mechanosensitive ion channels in osteoblast-like cells. Pflugers Arch 1990;416(6):646–51. 10.1007/BF00370609. [DOI] [PubMed] [Google Scholar]
- 58.Caffrey JM, Farach-Carson MC. Vitamin D3 metabolites modulate dihydropyridine-sensitive calcium currents in clonal rat osteosarcoma cells. J Biol Chem 1989;264(34):20265–74. 10.1016/S0021-9258(19)47057-9. [DOI] [PubMed] [Google Scholar]
- 59.Shao Y, Alicknavitch M, Farach-Carson MC. Expression of voltage sensitive calcium channel (VSCC) L-type Cav1.2 (alpha1C) and T-type Cav3.2 (alpha1H) subunits during mouse bone development. Dev Dyn 2005;234(1):54–62. 10.1002/dvdy.20517. [DOI] [PubMed] [Google Scholar]
- 60.Bonewald LF. The amazing osteocyte. J Bone Miner Res 2011;26(2):229–38. 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown GN, Leong PL, Guo XE. T-Type voltage-sensitive calcium channels mediate mechanically-induced intracellular calcium oscillations in osteocytes by regulating endoplasmic reticulum calcium dynamics. Bone 2016;88:56–63. 10.1016/j.bone.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perez-Reyes E Molecular characterization of T-type calcium channels. Cell Calcium 2006;40(2):89–96. 10.1016/j.ceca.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 63.Fuller-Bicer GA, Varadi G, Koch SE, Ishii M, Bodi I, Kadeer N, Muth JN, Mikala G, Petrashevskaya NN, Jordan MA, Zhang SP, Qin N, Flores CM, Isaacsohn I, Varadi M, Mori Y, Jones WK, Schwartz A. Targeted disruption of the voltage-dependent calcium channel alpha2/delta-1-subunit. Am J Physiol Heart Circ Physiol 2009;297(1):H117–24. 10.1152/ajpheart.00122.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reyes Fernandez PC, Wright CS, Masterson AN, Yi X, Tellman TV, Bonteanu A, et al. Gabapentin disrupts binding of perlecan to the α2δ1 voltage sensitive calcium channel subunit and impairs skeletal mechanosensation. bioRxiv 2022. 10.1101/2022.07.20.500827v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wasserman E, Webster D, Kuhn G, Attar-Namdar M, Muller R, Bab I. Differential load-regulated global gene expression in mouse trabecular osteocytes. Bone 2013;53(1):14–23. 10.1016/j.bone.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 66.•.Hong AR, Kim K, Lee JY, Yang JY, Kim JH, Shin CS, et al. Transformation of mature osteoblasts into bone lining cells and rna sequencing-based transcriptome profiling of mouse bone during mechanical unloading. Endocrinol Metab 2020;35(2):456–69. 10.3803/EnM.2020.35.2.456. An important study using RNA sequencing-based transcriptome profiling in bone cells to better understand the effect of mechanical unloading on bone loss and identify the genes involved in this process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sofu H, Kockara N, Aydin BK, Suleyman B, Tayfur M, Malkoc I. Should orthopedic surgeons consider the effects of gabapentin administration on bone healing while treating a long bone fracture: experimental study in a rat model. SICOT J 2016;2:36. 10.1051/sicotj/2016028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kanda J, Izumo N, Kobayashi Y, Onodera K, Shimakura T, Yamamoto N, Takahashi HE, Wakabayashi H. Effects of the antiepileptic drugs phenytoin, gabapentin, and levetiracetam on bone strength, bone mass, and bone turnover in rats. Biol Pharm Bull 2017;40(11):1934–40. 10.1248/bpb.b17-00482. [DOI] [PubMed] [Google Scholar]
- 69.Simko J, Karesova I, Kremlacek J, Eva Z, Horacek J, Fekete S, Malakova J, Zivna H, Palicka V. The effect of gabapentin and pregabalin on bone turnover and bone strength: a prospective study in Wistar rats. Pharmacol Rep 2019;71(6):1213–8. 10.1016/j.pharep.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 70.Rocha S, Ferraz R, Prudencio C, Fernandes MH, Costa-Rodrigues J. Differential effects of antiepileptic drugs on human bone cells. J Cell Physiol 2019;234(11):19691–701. 10.1002/jcp.28569. [DOI] [PubMed] [Google Scholar]
- 71.Wagener N, Di Fazio P, Boker KO, Matziolis G. Osteogenic effect of pregabalin in human primary mesenchymal stem cells, osteoblasts, and osteosarcoma cells. Life (Basel) 2022;12(4):496. 10.3390/life12040496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singh KP, Gupta K, Singh M. Effect of in utero exposure of gabapentin and valproic acid on skeletal anomalies in rat fetuses. Natl Acad Sci Lett 2014;37(2):117–21. 10.1007/s40009-013-0206-3. [DOI] [Google Scholar]
- 73.Badawy GM, Atallah MN, Sakr SA. Morphological and skeletal malformations induced by gabapentin in rat fetuses and their amelioration by ginger. Asian J Adv Basic Sci 2019;7(1):01–12. 10.33980/AJABS.2019.V07I01.001. [DOI] [Google Scholar]
- 74.Koo J, Zavras A. Antiepileptic drugs (AEDs) during pregnancy and risk of congenital jaw and oral malformation. Oral Dis 2013;19(7):712–20. 10.1111/odi.12061. [DOI] [PubMed] [Google Scholar]
- 75.The Human Protein Atlas Database. Search: calcium voltage-gated channel auxiliary subunit alpha2delta 1 gene, RNA consensus tissue gene data. Version: 20.1 https://www.proteinatlas.org/ENSG00000153956-CACNA2D1/tissue#top (2021). Accessed 10/12/2021.
- 76.Obermair GJ, Kugler G, Flucher BE. The role of the calcium channel alpha 2 delta-1 subunit in skeletal muscle. J Muscle Res Cell Motil 2004;25(3):239–40. 10.1023/b:jure.0000038361.47060.fe. [DOI] [PubMed] [Google Scholar]
- 77.Flucher BE, Obermair GJ, Tuluc P, Schredelseker J, Kern G, Grabner M. The role of auxiliary dihydropyridine receptor subunits in muscle. J Muscle Res Cell Motil 2005;26(1):1–6. 10.1007/s10974-005-9000-2. [DOI] [PubMed] [Google Scholar]
- 78.Relaix F, Bencze M, Borok MJ, Der Vartanian A, Gattazzo F, Mademtzoglou D, et al. Perspectives on skeletal muscle stem cells. Nat Commun 2021;12(1):692. 10.1038/s41467-020-20760-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alden K, Garcia J. Effect of gabapentin on excitation-contraction coupling in skeletal myotubes. Biophysical Journal: Biophysical Society 9650 Rockville Pike, Bethesda, MD 20814–3998 USA; 2002. p. 642A-A. [Google Scholar]
- 80.Moshiri M, Moallem SA, Attaranzadeh A, Saberi Z, Etemad L. Injury to skeletal muscle of mice following acute and sub-acute pregabalin exposure. Iran J Basic Med Sci 2017;20(3):256–9. 10.22038/ijbms.2017.8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rodríguez Cruz PM, Cossins J, Beeson D, Vincent A. The neuromuscular junction in health and disease: molecular mechanisms governing synaptic formation and homeostasis. Frontiers in molecular neuroscience 2020;13:610964. 10.3389/fnmol.2020.610964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sheikh S, Alvi U, Soliven B, Rezania K. Drugs that induce or cause deterioration of myasthenia gravis: an update. Journal of Clinical Medicine 2021;10(7):1537. ARTN 1537 10.3390/jcm10071537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lyrica. Highlights of prescribing information Reference ID: 4252171. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021446s035,022488s013lbl.pdf (2018). Accessed 10/14/2021.
- 84.Neurotin. Highlights of prescribing information Reference ID: 4168942. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020235s064_020882s047_021129s046lbl.pdf (2017). Accessed 10/14/2021.
- 85.Fodor J, Matta C, Olah T, Juhasz T, Takacs R, Toth A, et al. Store-operated calcium entry and calcium influx via voltage-operated calcium channels regulate intracellular calcium oscillations in chondrogenic cells. Cell Calcium 2013;54(1):1–16. 10.1016/j.ceca.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 86.Matta C, Zakany R, Mobasheri A. Voltage-dependent calcium channels in chondrocytes: roles in health and disease. Curr Rheumatol Rep 2015;17(7):43. 10.1007/s11926-015-0521-4. [DOI] [PubMed] [Google Scholar]
- 87.Gong X, Xie W, Wang B, Gu L, Wang F, Ren X, Chen C, Yang L. Altered spontaneous calcium signaling of in situ chondrocytes in human osteoarthritic cartilage. Sci Rep 2017;7(1):17093. 10.1038/s41598-017-17172-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boileau C, Martel-Pelletier J, Brunet J, Tardif G, Schrier D, Flory C, el-Kattan A, Boily M, Pelletier JP. Oral treatment with PD-0200347, an alpha2delta ligand, reduces the development of experimental osteoarthritis by inhibiting metalloproteinases and inducible nitric oxide synthase gene expression and synthesis in cartilage chondrocytes. Arthritis Rheum 2005;52(2):488–500. 10.1002/art.20809. [DOI] [PubMed] [Google Scholar]
- 89.Boileau C, Martel-Pelletier J, Brunet J, Schrier D, Flory C, Boily M, Pelletier JP. PD-0200347, an alpha2delta ligand of the voltage gated calcium channel, inhibits in vivo activation of the Erk1/2 pathway in osteoarthritic chondrocytes: a PKCalpha dependent effect. Ann Rheum Dis 2006;65(5):573–80. 10.1136/ard.2005.041855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kass GE, Orrenius S. Calcium signaling and cytotoxicity. Environ Health Perspect 1999;107 Suppl 1(suppl 1):25–35. 10.1289/ehp.99107s125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.King A Analgesia without opioids. Nature 2019;573(7773):S4-S. [Google Scholar]
- 92.Davari M, Amani B, Amani B, Khanijahani A, Akbarzadeh A, Shabestan R. Pregabalin and gabapentin in neuropathic pain management after spinal cord injury: a systematic review and meta-analysis. Korean J Pain 2020;33(1):3–12. 10.3344/kjp.2020.33.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zajaczkowska R, Mika J, Leppert W, Kocot-Kepska M, Malec-Milewska M, Wordliczek J. Mirogabalin-a novel selective ligand for the alpha2delta calcium channel subunit. Pharmaceuticals (Basel) 2021;14(2):112. 10.3390/ph14020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Javed S, Alam U, Malik RA. Mirogabalin and emerging therapies for diabetic neuropathy. J Pain Res 2018;11:1559–66. 10.2147/JPR.S145999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.••.Saeki K, Yasuda S-i, Kato M, Kano M, Domon Y, Arakawa N, et al. Analgesic effects of mirogabalin, a novel ligand for α2δ subunit of voltage-gated calcium channels, in experimental animal models of fibromyalgia 2019;392(6):723–8. 10.1007/s00210-019-01628-z. Study discussing mirogabalin as a novel ligand for the α2δ subunit of voltage-gated calcium channels and its potential analgesic effects in fibromyalgia. [DOI] [PubMed] [Google Scholar]
- 96.Kim JY, Abdi S, Huh B, Kim KH. Mirogabalin: could it be the next generation gabapentin or pregabalin? Korean J Pain 2021;34(1):4–18. 10.3344/kjp.2021.34.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Domon Y, Arakawa N, Inoue T, Matsuda F, Takahashi M, Yamamura N, Kai K, Kitano Y. Binding characteristics and analgesic effects of mirogabalin, a novel ligand for the alpha2delta subunit of voltage-gated calcium channels. J Pharmacol Exp Ther 2018;365(3):573–82. 10.1124/jpet.117.247551. [DOI] [PubMed] [Google Scholar]
- 98.Wu C-L, Chuang C-W, Cho H-Y, Chuang T-H, Wu S-N. The Evidence for Effective inhibition of I Na produced by mirogabalin ((1R, 5S, 6S)-6-(aminomethyl)-3-ethyl-bicyclo [3.2. 0] hept-3-ene-6-acetic acid), a known blocker of CaV channels. Int J Mol Sci 2022;23(7):3845. 10.3390/ijms23073845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hardman MI, Sprung J, Weingarten TN. Acute phenibut withdrawal: a comprehensive literature review and illustrative case report. Bosn J Basic Med Sci 2019;19(2):125–9. 10.17305/bjbms.2018.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dambrova M, Zvejniece L, Liepinsh E, Cirule H, Zharkova O, Veinberg G, Kalvinsh I. Comparative pharmacological activity of optical isomers of phenibut. Eur J Pharmacol 2008;583(1): 128–34. 10.1016/j.ejphar.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 101.Zvejniece L, Vavers E, Svalbe B, Veinberg G, Rizhanova K, Liepins V, Kalvinsh I, Dambrova M. R-phenibut binds to the alpha2-delta subunit of voltage-dependent calcium channels and exerts gabapentin-like anti-nociceptive effects. Pharmacol Biochem Behav 2015;137:23–9. 10.1016/j.pbb.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 102.Mash JE, Leo RJ. Phenibut: a novel nootropic with abuse potential. Prim Care Companion CNS Disord 2020;22(4):0-. 10.4088/PCC.19l02587. [DOI] [PubMed] [Google Scholar]
- 103.Lapin I. Phenibut (beta-phenyl-GABA): a tranquilizer and nootropic drug. CNS Drug Rev 2001;7(4):471–81. 10.1111/j.1527-3458.2001.tb00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.•.Cohen PA, Ellison RR, Travis JC, Gaufberg SV, Gerona R. Quantity of phenibut in dietary supplements before and after FDA warnings. Clin Toxicol 2022;60(4):486–8. 10.1080/15563650.2021.1973020. An analysis of over-the-counter supplements by liquid chromatography time-of-flight mass spectrometry warns us about the higher quantities of phenibut present in various of these supplements. [DOI] [PubMed] [Google Scholar]
- 105.CFSAN Constituent Updates, https://www.fda.gov/food/cfsan-constituent-updates/fda-acts-dietary-supplements-containing-dmha-and-phenibut. 2019. https://www.fda.gov/food/cfsan-constituent-updates/fda-acts-dietary-supplements-containing-dmha-and-phenibut. Accessed 03/14/2022.
- 106.Cundy KC, Branch R, Chernov-Rogan T, Dias T, Estrada T, Hold K, Koller K, Liu X, Mann A, Panuwat M, Raillard SP, Upadhyay S, Wu QQ, Xiang JN, Yan H, Zerangue N, Zhou CX, Barrett RW, Gallop MA. XP13512 [(±)-1-([(α-Isobutanoyloxyethoxy) carbonyl] aminomethyl)-1-cyclohexane acetic acid], a novel gabapentin prodrug: I. Design, synthesis, enzymatic conversion to gabapentin, and transport by intestinal solute transporters. J Pharmacol Exp Ther 2004;311(1):315–23. 10.1124/jpet.104.067934. [DOI] [PubMed] [Google Scholar]
- 107.Stewart BH, Kugler AR, Thompson PR, Bockbrader HN. A saturable transport mechanism in the intestinal absorption of gabapentin is the underlying cause of the lack of proportionality between increasing dose and drug levels in plasma. Pharmaceut Res 1993;10(2):276–81. 10.1023/a:1018951214146. [DOI] [PubMed] [Google Scholar]
- 108.Cundy KC, Sastry S, Luo W, Zou J, Moors TL, Canafax DM. Clinical pharmacokinetics of XP13512, a novel transported prodrug of gabapentin. J Clin Pharmacol 2008;48(12):1378–88. 10.1177/0091270008322909. [DOI] [PubMed] [Google Scholar]
- 109.Lal R, Ellenbogen A, Chen D, Zomorodi K, Atluri H, Luo W, Tovera J, Hurt J, Bonzo D, Lassauzet ML, Vu A, Cundy KC. A randomized, double-blind, placebo-controlled, dose-response study to assess the pharmacokinetics, efficacy, and safety of gabapentin enacarbil in subjects with restless legs syndrome. Clin Neuropharmacol 2012;35(4):165–73. 10.1097/WNF.0b013e318259eac8. [DOI] [PubMed] [Google Scholar]
- 110.Lee DO, Ziman RB, Perkins AT, Poceta JS, Walters AS, Barrett RW, XP053 Study Group. A randomized, double-blind, placebo-controlled study to assess the efficacy and tolerability of gabapentin enacarbil in subjects with restless legs syndrome. J Clin Sleep Med 2011;7(3):282–92. 10.5664/JCSM.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mersfelder TL, Nichols WH. Gabapentin: abuse, dependence, and withdrawal. Ann Pharmacother 2016;50(3):229–33. 10.1177/1060028015620800. [DOI] [PubMed] [Google Scholar]
- 112.Gidal BE, Radulovic LL, Kruger S, Rutecki P, Pitterle M, Bockbrader HN. Inter- and intra-subject variability in gabapentin absorption and absolute bioavailability. Epilepsy Res 2000;40(2–3):123–7. 10.1016/s0920-1211(00)00117-0. [DOI] [PubMed] [Google Scholar]
- 113.Thompson WR, Scott A, Loghmani MT, Ward SR, Warden SJ. Understanding mechanobiology: physical therapists as a force in mechanotherapy and musculoskeletal regenerative rehabilitation. Phys Ther 2016;96(4):560–9. 10.2522/ptj.20150224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bockbrader HN, Wesche D, Miller R, Chapel S, Janiczek N, Burger P. A comparison of the pharmacokinetics and pharmaco-dynamics of pregabalin and gabapentin. Clin Pharmacokinet 2010;49(10):661–9. 10.2165/11536200-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 115.McInnes G, Yee SW, Pershad Y, Altman RB. Genomewide association studies in pharmacogenomics. Clin Pharmacol Ther 2021;110(3):637–48. 10.1002/cpt.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]


