Abstract
Introduction:
Traditional Chinese medicine (TCM) injections, as a relatively safe and low-cost treatment, have been widely used in the prevention and treatment of anthracyclines-induced cardiotoxicity in China. However, the quality of the relevant systematic reviews and meta-analyses published in recent years is uneven, so that the effectiveness and safety of TCM injections in preventing and treating anthracyclines-induced cardiotoxicity remain to be discussed. A systematic overview is therefore needed to provide a more advanced evidentiary reference for clinical practice.
Methods:
Eight Chinese and English databases were searched by computer to screen the meta-analyses/systematic reviews on the efficacy of traditional Chinese medicine injections for the prevention and treatment of anthracyclines-induced cardiotoxicity from the database establishment to October 2022. The methodological quality and evidence quality of outcome indicators included in the study were evaluated by AMSTAR 2 tool, PRISMA statement and GRADE classification.
Results:
A total of 7 articles were included in the study. The quality evaluation of AMSTAR 2 showed that 7 studies were extremely low-level; PRISMA stated that the evaluation results showed that the reports of 7 studies were of intermediate quality; The GRADE rating indicated that most of the evidence was of low quality.
Conclusion:
The methodological quality and evidence quality of meta-analysis/system evaluation concerning the prevention and treatment of anthracyclines-induced cardiotoxicity by Chinese medicine are currently low, and the effectiveness of Chinese medicine in the treatment of anthracyclines-induced cardiotoxicity needs more high-quality evidence-based evidence.
Keywords: Chinese medicine injection, TCM, Anthracyclines, Cardiac toxicity, Overview
With the prolongation of survival time of tumor patients, complications related to anti-tumor treatment have received more and more attention. Cardiovascular disease, the second leading cause of death among tumor survivors after tumor recurrence, has received increasing attention in recent years.1-3 As the most common chemotherapeutic drugs causing cardiotoxicity, anthracyclines can cause heart injury when used for the first time, and the toxicity accumulates with the increase of dose and the prolongation of medication duration. The acute reactions are mainly manifested as arrhythmia and myocarditis. The chronic response is manifested as congestive heart failure, which greatly limits its application.4,5
In the prevention and treatment of anthracyclines-induced cardiotoxicity, apart from the only available dexrazoxane approved by the FDA (United States Food and Drug Administration), basic drugs for heart disease such as ACEIs/ARBs, β-receptor blockers, diuretics, and aldosterone antagonists can be used in combination. However, studies have shown that dexrazoxane may increase the risk of secondary malignant tumors in children with tumors, and there is wide controversy about its safety.6,7 The effectiveness of basic cardiac drugs such as ACEIs/ARBs lacks credibility due to the lack of large-scale randomized controlled trials.8,9 Therefore, brand-new treatments are urgently needed to solve the problem of anthracyclines causing cardiotoxicity to tumor patients. As an effective drug for the treatment of drug-induced injury and the prevention of cardiovascular disease,10,11 Chinese medicine has received extensive attention in China. Clinical studies on the use of Chinese medicine injections to prevent cardiotoxicity of anthracyclines have been increasing year by year. As of now, meta-analysis/systematic evaluation has concluded that TCM injections have certain advantages in preventing anthracycline-induced cardiotoxicity. However, as the application of TCM injections for anthracycline-induced cardiotoxicity is relatively short, and the methods and quality of relevant studies are uneven, their effectiveness and safety need to be further confirmed. Therefore, this study aims to re-evaluate the meta-analysis of the efficacy of Chinese herbal injections against anthracycline-induced cardiotoxicity by using the AMSTAR 2 tool, PRISMA statement and GRADE classification, to give a further developed proof based reason for important clinical choices.
Data and Methods
Study Data
Type of study
All systematic evaluations/meta-analyses related to the use of Chinese herbal injections for the prevention and treatment of anthracycline-induced cardiotoxicity.
Study population
Patients with a definite diagnosis of malignancy and on a chemotherapy regimen containing anthracyclines, irrespective of patient gender, age, race and tumor type.
Type of intervention
The control group was treated with an anthracycline-based chemotherapy regimen with or without cardioprotective agents such as dexrazoxane; the test group was treated with Chinese herbal injections on top of the control group.
Outcome indicators
(1) Primary outcome indicators included clinical efficiency, cardiotoxicity, abnormal ECG rate, and left ventricular ejection fraction (LVEF).
(2) Secondary outcome indicators: change in left ventricular end-diastolic internal diameter (LVIDD), change in left ventricular end-systolic internal diameter (LVISD), change in left ventricular short-axis shortening (FS), creatine kinase isoenzyme-MB (CK-MB), troponin I (cTnI), B-type natriuretic peptide (BNP).
Exclusion criteria
Study population
Patients who received radiation therapy or who were accompanied by chronic heart disease and who also developed clinical symptoms similar to cardiotoxicity caused by anthracyclines were excluded; Cancer patients whose intervention was an alternative chemotherapy regimen that did not contain anthracyclines; Study type: reticulated meta-analysis, systematically evaluated study protocols (protocols), traditional reviews, conference abstracts; Duplicate publications; Articles in languages other than Chinese and English.
Literature Search Strategy
The following databases were searched: China Biomedical Literature Database (CBM), China National Knowledge Infrastructure (CNKI), Wanfang Data Knowledge Service Platform (Wanfang Data), VIP Chinese sci-tech journal full-text database (VIP), the Cochrane Library, PubMed, Web of Science, Embase. The retrieval time was from the database establishment to October 2022. The Chinese search terms include: Chinese medicine, Chinese traditional medicine, injection, granule, tablet, Chinese medicine compound, Chinese medicine decoction, anthracycline, epirubicin, pyradoxorubicin, daunorubicin, aclacinomycin, doxorubicin, pirarubicin, cardiac toxicity, cardiac injury, meta-analysis, meta-analyses, systematic review, systematic evaluation. The retrieval adopts the method of combining subject words and free words, and is adjusted according to the specific database, and all retrieval strategies are determined after multiple pre-retrieval.
Literature Screening and Data Extraction
The literature screening was first conducted independently by 2 investigators: all potentially included literature was retrieved, imported into Endnote X9 to remove duplicates, then the titles and abstracts of the literature were read for initial screening, and finally the full text was read to determine whether final inclusion was achieved. And 2 researchers independently performed data extraction, which included: first author, year of publication, type of original study included, number of original studies included and sample size, interventions, outcome indicators, risk of bias evaluation tools, and main findings. The above process was cross-checked by the 2 researchers, and any disagreement was adjudicated by a third-party personnel. For studies with missing key information, the original authors were contacted for data whenever possible.
Evaluation of Methodological Quality and Reporting Quality of Included Studies
Two investigators independently used the AMSTAR 2 tool. 12 The methodological quality of the included studies was evaluated: entries 2, 4, 7, 9, 11, 13, and 15 of the 16 entries were considered critical; If 0 or 1 non-key item did not conform, the evaluation was deemed as high in credibility; If more than 1 non-critical item does not conform, it is assessed as within the credibility; If 1 key item did not conform with or without non-key items did not conform, the evaluation was deemed as low in reliability; If more than 1 key item did not conform, with or without non-key items did not conform, it was rated as extremely low confidence.
Use the PRISMA statements 13 to evaluate the quality of reporting of included studies: for reporting of each entry, a score of 1 was given for complete reporting, 0.5 for partial reporting, and 0 for not reporting. The total score ≥22 was considered relatively complete in reporting, while ≤15 was considered as serious missing in information reporting, and the intermediate score range indicated certain reporting defect.
Outcome indicators included in the study were graded for quality of evidence using the GRADE system. 14 The RCTs were first set as high-level evidence, and then the outcome measures were downgraded from 5 aspects, namely risk of bias, inconsistency, indirectness, imprecision and publication bias. If there is no downgrading in all 5 aspects, it is high-level evidence; 1 aspect of downgrading is moderate-level evidence; Demotion in 2 aspects was considered as low-level evidence; Downgrades in 3 or more areas are very low-level evidence.
Results
Literature Screening Process and Results
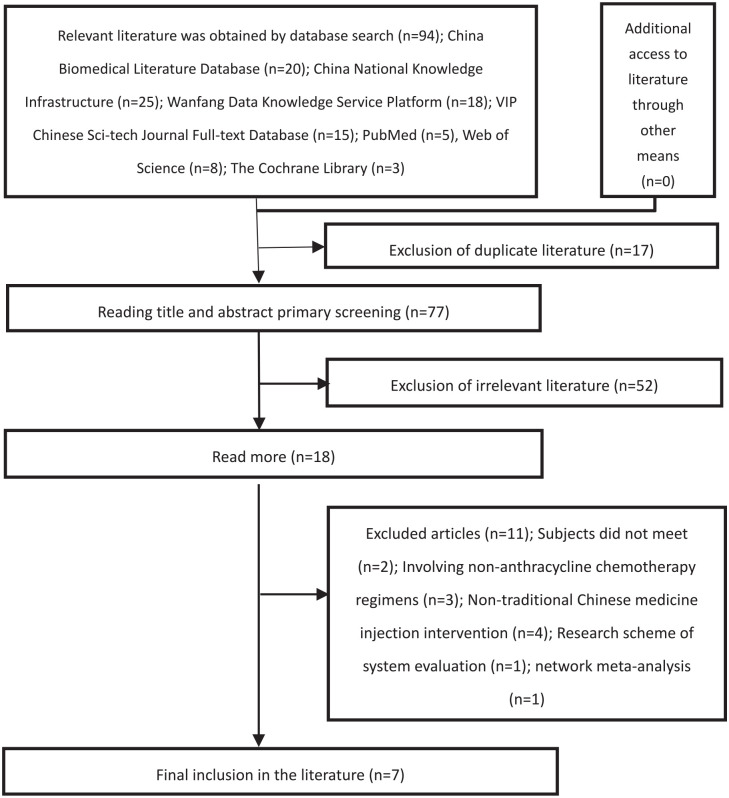
The initial search yielded 94 papers, and 11 papers were finally included after being screened by Endnote X9 with the inclusion and exclusion criteria. The screening process is shown in Figure 1.
Figure 1.
Literature screening process.
Characteristics of the Included Literature
A total of 7 systematic evaluations were included,15-21 all of which are in Chinese, 6 of which are journal papers.15-20 One is a degree thesis. 21 The original study types included in the 7 studies were randomized controlled trials (RCT). The number of included original literature was 6 to 24, including sample size of 486 to 1705. As for interventions, the control group was an anthracycline-containing chemotherapy regimen, or the addition of dexrazoxane, vitamin E and coenzyme Q, and the experimental group was treated with Chinese herbal injections on top of the control group, including Shenqi Fuzheng injection, Xinmailong injection, Shenmai injection, Shengmai injection; And in terms of methodological quality assessment of the included original literature, Cochrane risk of bias assessment tool was used in 6 studies,15-20 and the Jadad scale was used in 1 study. 21 The basic characteristics of the included studies are shown in Table 1.
Table 1.
Characteristic Table of Included Articles.
| Study | Type of research | Number of original studies (items) | Original study sample size (cases) | Interventions | Risk of bias assessment tool | Closing indicators | Main conclusions | |
|---|---|---|---|---|---|---|---|---|
| Control group | Test group | |||||||
| Yu et al 15 | RCT | 19 | 1705 | Anthracycline-containing chemotherapy regimens | Anthracycline chemotherapy regimen + herbal injection | Cochrane Risk of Bias Assessment Tool | ②③④⑧⑨ | Chinese herbal injection is effective against cardiotoxicity caused by anthracycline chemotherapy regimen. |
| Long et al 16 | RCT | 8 | 486 | Anthracycline-containing chemotherapy regimens | Anthracycline-based chemotherapy regimen + Ginseng-Qi Fuzheng injection | Cochrane Risk of Bias Assessment Tool | ③④⑧⑩ | Shenqi Fuzheng injection can improves cardiac function in patients on anthracycline chemotherapy regimens. |
| Ma et al 17 | RCT | 8 | 660 | Anthracycline-containing chemotherapy regimens | Anthracycline-based chemotherapy regimen + cardiac veinron injection | Cochrane Risk of Bias Assessment Tool | ③④⑤⑥⑨ | Cardioplegia injection is effective in reducing the rate of ECG abnormalities in patients treated with anthracycline chemotherapy regimens. |
| Zhang et al 18 | RCT | 12 | 775 | Anthracycline-containing chemotherapy regimens | Anthracycline chemotherapy regimen + Shenmai injection | Cochrane Risk of Bias Assessment Tool | ①② | Shenmai injection is effective in the treatment of acute leukemia and can alleviate the adverse effects caused by chemotherapy. |
| Ma et al 19 | RCT | 10 | 612 | Anthracycline-containing chemotherapy regimen + dexrazoxane | Anthracycline chemotherapy regimen + dexraxozane + Shenmai injection | Cochrane Risk of Bias Assessment Tool | ③④⑧⑩ | Combination of dexrazoxane with Shenmai injection is superior to dexrazoxane alone in preventing anthracycline chemotherapy-induced cardiotoxicity. |
| Yang et al 20 | RCT | 6 | 615 | Anthracycline-containing chemotherapy regimen + vitamin E and coenzyme Q, etc. | Anthracycline-containing chemotherapy regimens + Biovein injection | Cochrane Risk of Bias Assessment Tool | ③⑤⑥⑦ | Shengmai injection may prevent cardiotoxicity caused by anthracycline chemotherapy drugs. |
| Li 21 | RCT | 24 | 1702 | Anthracycline-containing chemotherapy regimens | Anthracycline chemotherapy regimen + herbal injection | Jadad Scale | ③④⑧⑨ | Chinese herbal injections are protective against cardiotoxicity caused by anthracycline-based chemotherapy regimens |
① Clinical effective rate, ② Cardiac toxicity, ③ Abnormal rate of ECG, ④ Left ventricular ejection fraction, LVEF, ⑤ Change of left ventricular end diastolic diameter, LVIDD, ⑥ Change of left ventricular end systolic diameter, LVISD, ⑦ Change of left ventricular short axis shortening rate, FS, ⑧ Creatine kinase isoenzyme-MB, CK-MB, ⑨ Cardiac troponin I, cTnI, ⑩ Type B natriuretic peptide, BNP.
Abbreviations: RCT, randomized controlled trial.
Methodological Quality Assessment of the Included Studies
The methodological quality of the included literature was assessed strictly according to the AMSTAR 2 tool, 12 and the results showed that all 7 publications had a very low methodological quality rating. The results of the specific scores are shown in Table 2.
Table 2.
AMSTAR 2 Scoring Results Included in the Literature.
| Study | Item | Credibility | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||
| Yu et al 15 | Y | N | N | PY | Y | N | N | Y | Y | N | Y | Y | Y | Y | Y | Y | Extremely low |
| Long et al 16 | Y | N | N | PY | Y | Y | N | NY | Y | N | Y | N | N | N | Y | N | Extremely low |
| Ma et al 17 | Y | N | N | PY | Y | Y | N | PY | Y | N | Y | Y | Y | Y | Y | N | Extremely low |
| Zhang et al 18 | Y | N | N | Y | Y | N | N | Y | Y | N | Y | Y | Y | N | Y | N | Extremely low |
| Ma et al 19 | Y | N | N | PY | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | N | Extremely low |
| Yang et al 20 | Y | N | N | PY | Y | Y | N | Y | Y | N | Y | Y | Y | NM | N | N | Extremely low |
| Li 21 | Y | N | N | PY | Y | Y | N | N | Y | N | Y | Y | N | N | PY | N | Extremely low |
| Item reporting rate (%) | 100 | 0 | 0 | 100 | 100 | 71.4 | 0 | 71.4 | 100 | 0 | 100 | 85.7 | 71.4 | 42.9 | 85.7 | 14.3 | |
Item reporting rate = (number of fully reported studies with items + number of partially reported studies)/total number of included studies] × 100%.
Abbreviations: Y, yes; PY, partly yes; N, no; NM, not analyzed.
Results of Report Quality Evaluation
The PRISMA statement was applied to evaluate the included studies, and the PRISMA scores of each study were distributed between 16 and 22. Among them, there were 2 studies with scores of 21 to 2715,18 and the reports were relatively complete; The remaining 5 studies16,17,19-21 had scores between 15 and 21, and the reports had certain defects. The specific results are shown in Table 3.
Table 3.
PRISMA Statement Score Results for Inclusion.
| Article structure | PRISMA entry | Full report |
Selected reports |
Not reported |
|||
|---|---|---|---|---|---|---|---|
| Number of literature | Percentage (100%) | Number of literature | Percentage (100%) | Number of literature | Percentage (100%) | ||
| Title | Title | 7 | 100 | 0 | 0 | 0 | 0 |
| Abstract | Structured summary | 0 | 0 | 7 | 100 | 0 | 0 |
| Preface | Theoretical foundation | 7 | 100 | 0 | 0 | 0 | 0 |
| Purpose | 1 | 14 | 6 | 86 | 0 | 0 | |
| Methods | Programs and registration | 0 | 0 | 0 | 0 | 7 | 100 |
| Inclusion criteria | 0 | 0 | 7 | 100 | 0 | 0 | |
| Information source | 7 | 100 | 0 | 0 | 0 | 0 | |
| Search | 1 | 14 | 6 | 86 | 0 | 0 | |
| Study selection | 7 | 100 | 0 | 0 | 0 | 0 | |
| Data extraction method | 5 | 71 | 0 | 0 | 2 | 29 | |
| Data entry | 0 | 0 | 7 | 100 | 0 | 0 | |
| Bias in individual studies | 7 | 100 | 0 | 0 | 0 | 0 | |
| Summary effect indicators | 7 | 100 | 0 | 0 | 0 | 0 | |
| Result synthesis | 4 | 57 | 3 | 43 | 0 | 0 | |
| Study bias | 4 | 57 | 0 | 0 | 3 | 43 | |
| Other analysis | 4 | 57 | 0 | 0 | 3 | 43 | |
| Results | Study selection | 4 | 57 | 0 | 0 | 3 | 43 |
| Research characteristics | 7 | 100 | 0 | 0 | 0 | 0 | |
| Risk of study bias for inclusion | 5 | 71 | 0 | 0 | 2 | 29 | |
| Individual study results | 7 | 100 | 0 | 0 | 0 | 0 | |
| Consolidated results | 7 | 100 | 0 | 0 | 0 | 0 | |
| Inter-study bias | 5 | 71 | 0 | 0 | 2 | 29 | |
| Other analysis | 4 | 57 | 0 | 0 | 3 | 43 | |
| Discussion | Summary of strength of evidence | 1 | 14 | 6 | 86 | 0 | 0 |
| Limitations | 4 | 57 | 3 | 43 | 0 | 0 | |
| Conclusion | 7 | 100 | 0 | 0 | 0 | 0 | |
| Funding | Financial support | 0 | 0 | 0 | 0 | 7 | 100 |
Results of Evidence Quality Rating
The quality of evidence was evaluated by the GRADE system for the outcome indicators of the included studies. The specific results are shown in Table 4.
Table 4.
Inclusion of Literature Outcome Measure GRADE Score.
| Closing indicators | First author | Evidence quality evaluation factors (points) | Quality of Evidence | ||||
|---|---|---|---|---|---|---|---|
| Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | |||
| Clinical validity | Zhang et al 18 | −1a | 0 | 0 | 0 | −1d | Low |
| Cardiotoxicity | Yu et al 15 | −1a | 0 | 0 | −1c | −1d | Extremely low |
| Zhang et al 18 | −1a | 0 | 0 | 0 | −1d | Low | |
| Abnormal electrocardiogram rate | Yu et al 15 | −1a | 0 | 0 | 0 | −1d | Low |
| Long et al 16 | −1a | 0 | 0 | −1c | −1d | Extremely low | |
| Ma et al 17 | −1a | 0 | 0 | 0 | −1d | Low | |
| Ma et al 19 | −1a | 0 | 0 | 0 | −1d | Low | |
| Yang et al 20 | −1a | 0 | 0 | 0 | −1d | Low | |
| Li 21 | −1a | 0 | 0 | 0 | 0 | Moderate | |
| Left ventricular ejection fraction (LVEF) | Yu et al 15 | −1a | 0 | 0 | 0 | −1d | Low |
| Long et al 16 | −1a | −1b | 0 | 0 | −1d | Extremely low | |
| Ma et al 17 | −1a | 0 | 0 | 0 | −1d | Low | |
| Ma et al 19 | −1a | −1b | 0 | 0 | −1d | Extremely low | |
| Li 21 | −1a | −1b | 0 | 0 | −1d | Extremely low | |
| Left ventricular end-diastolic internal diameter (LVIDD) | Ma et al 17 | −1a | 0 | 0 | −1c | −1d | Extremely low |
| Yang et al 20 | −1a | 0 | 0 | −1c | −1d | Extremely low | |
| Left ventricular end-systolic internal diameter (LVISD) | Ma et al 17 | −1a | −1b | 0 | −1c | −1d | Extremely low |
| Yang et al 20 | −1a | 0 | 0 | −1c | −1d | Extremely low | |
| Left ventricular short axis shortening rate (FS) | Yang et al 20 | −1a | 0 | 0 | −1c | −1d | Extremely low |
| Creatine kinase isoenzyme-MB (CK-MB) | Yu et al 15 | −1a | 0 | 0 | 0 | −1d | Low |
| Long et al 16 | −1a | −1b | 0 | −1c | −1d | Extremely low | |
| Ma et al 19 | −1a | 0 | 0 | −1c | −1d | Extremely low | |
| Li 21 | −1a | −1b | 0 | 0 | −1d | Extremely low | |
| Troponin I (cTnI) | Yu et al 15 | −1a | 0 | 0 | 0 | −1d | Low |
| Ma et al 17 | −1a | −1b | 0 | 0 | −1d | Extremely low | |
| Li 21 | −1a | −1b | 0 | 0 | −1d | Extremely low | |
| B-type natriuretic peptide (BNP) | Long et al 16 | −1a | −1b | 0 | −1c | −1d | Extremely low |
| Ma et al 19 | −1a | 0 | 0 | −1c | −1d | Extremely low | |
a indicates the risk of bias in the random method, allocation concealment, blind method and loss of follow-up. b indicates that there was large heterogeneity between studies and no reasonable measures were taken to reduce heterogeneity, such as subgroup analysis or sensitivity analysis. c indicates that the sample size is small and the confidence interval is wide. d indicates that the funnel plot was asymmetric, or there were fewer than 9 included studies, or all of them were positive results, so the possibility of publication bias was high.
Key Outcome Indicators
Due to the low quality of evidence for most outcome measures, only descriptive analysis of outcome measures was performed and no further pooled analysis was performed.
Clinical effectiveness rate
Zhang et al 18 showed that in terms of clinical efficacy in the treatment of acute leukemia, the combination of chemotherapeutic drugs with Shenmai injection group had a significant advantage compared with the chemotherapy application group alone, and the difference was statistically significant.
Cardiotoxicity
Two meta-analyses15,18 were performed to report the incidence of cardiotoxicity. Yu et al 15 showed that herbal injection could effectively reduce cardiotoxicity due to anthracyclines; Zhang et al 18 showed that Shenmai injection could alleviate the cardiotoxicity caused by the application of anthracyclines in patients with acute leukemia.
Electrocardiogram abnormality rate
Six meta-analyses15-17,19-21 showed that the herbal injection group had a statistically significant advantage over the control group in reducing the abnormal ECG rate due to anthracyclines. Notably, in the study of Li, 21 the evidence quality of this outcome index was medium.
Left ventricular ejection fraction (LVEF)
The left ventricular ejection fraction (LVEF) was reported in 5 meta-analyses.15-17,19,21 All 5 studies showed: the results indicated that the herbal injection group had a statistically significant advantage over the control group in improving the reduction in LVEF values due to anthracyclines. Among them, Ma et al 19 showed that: the group of Shenmai injection combined with dexrazoxane was superior to the group of dexrazoxane alone.
Discussion
Compared with other types of Chinese medicine, Chinese medicine injections have received more international recognition because of their exact efficacy, rapid absorption and no obvious side effects, 22 as reflected in the increasing number of relevant English publications on Chinese medicine injections. However, anthracyclines-induced cardiotoxicity has attracted much attention in recent years. Traditional Chinese medicine injections have been used for relatively short time to prevent and treat anthracyclines-induced cardiotoxicity, and there is a lack of large sample size and high-quality RCTs. At present, according to the meta-analysis, TCM injections have certain advantages in the prevention and treatment of anthracyclines-induced cardiotoxicity, but their credibility needs to be further verified by evidence. Of the 2 most commonly used tools for evaluating the methodological quality of meta-analyses, AMSTAR 2, as a newly developed systematic evaluation methodological quality evaluation tool on the basis of the first edition, has a good consistency and practicability among evaluators; while the PRISMA statement, as an item in the system review and meta-analysis priority report, plays an important role in improving and enhancing the reporting quality of the system review and meta-analysis. Therefore, in this study, the above 2 assessment tools were used to evaluate the methodological quality of the included studies, and the GRADE evaluation system was used to assess the evidence level of outcome indicators, in order to increase the reference value of this study. It was found through assessment that the existing studies failed to prove the effectiveness of TCM injection in preventing and treating anthracyclines-induced cardiotoxicity, because the methodological quality and evidence quality of each study were relatively low.
Problems
Aspects of the AMSTAR 2 tool
The AMSTAR 2 tool assessed the methodological quality of the included studies, and the results showed that the methodological quality of all studies was extremely low because of the following deficiencies in key entries: None of the 7 studies had a detailed study plan in advance or were enrolled in advance; 7 studies have retrieved at least 2 databases and provided search terms, but most of the studies did not retrieve the references, clinical trial registry and gray literature involved in the original study, which makes the search results not comprehensive enough; None of the 7 studies provided a specific list of excluded articles, which reduced the reliability of literature retrieval results and might be biased; In some studies, the risk of bias and publication bias for inclusion in the original study were not fully considered, and the possible impact of bias on the study results was not fully discussed.12,23
PRISMA statement aspects
The main causes of deficiencies include: failure to provide the study protocol and register it in advance; failure to provide a specific search method for the database, making the search process non-repeatable; failure to detail individual data entries; failure to conduct a sound assessment of publication bias or conduct other assessments; failure to assess the level of evidence for each primary outcome in detail, reducing the reliability of the outcome; failure to state source of funding.13,24
Aspects of GRADE evaluation
The reasons for the low quality of evidence include: the lack of clarity in the implementation of blinding and allocation concealment in most of the included original studies, leading to a greater risk of bias in the meta-analysis; the large heterogeneity in the merging of data for most outcome indicators along with the problem that the studies did not adopt subgroup analysis or sensitivity analysis to reduce heterogeneity; the small sample size in the original studies.The small sample size in the original study or the small number of clinical trials included resulted in a wide confidence region or a large publication bias. 14
Suggestion
It is recommended that future clinical trials should strictly follow the principles of randomization, set reasonable controls and implement blinded trials, 25 and appropriately expand the sample size to obtain more high-quality randomized controlled trials, so as to pave the way for the inclusion of high-quality original studies in subsequent systematic evaluation and meta-analysis. At the same time, in the current environment of low quality of systematic evaluation in TCM, it is recommended that all personnel engaged in systematic evaluation of TCM should conduct a comprehensive and systematic study to fully understand and master the concepts and methods of systematic evaluation and meta-analysis. The aim is to develop a better system of systematic evaluation of TCM and to provide higher quality evidence-based recommendations for the development of TCM. 26
Limitation
This study has certain limitations: (1) Because there were many intervention measures in some articles,15,21 this study did not perform further quantitative synthesis of data; (2) some of the outcome indicators have the problem of large heterogeneity, and this study did not perform further analysis of this component of the indicators; (3) GRADE grading evaluation is a relatively more subjective process, so different researchers may have different evaluation results.
Conclusion
At present, the methodological quality and evidence quality of meta-analysis/systematic evaluation related to the prevention and treatment of anthracycline-induced cardiotoxicity by herbal injections is low, and the effectiveness of herbal injections against anthracycline-induced cardiotoxicity needs further validation by high-quality evidence-based evidence.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by the following foundation projects: The National Famous Old Chinese Medicine Experts' Inheritance Studio Construction Project (National Chinese Medicine Human Education Letter [2022] No. 75); The National Famous Chinese Medicine Inheritance Studio Construction Project (National Chinese Medicine Human Education Letter [2022] No. 245); The National TCM Talent Support Programme: TCM Inheritance and Innovation “Hundred Million” Talent Project - QI Huang Scholars (National Chinese Medicine Human Education Letter [2018] No. 284).
ORCID iD: Chenchen Li  https://orcid.org/0000-0003-3848-4162
https://orcid.org/0000-0003-3848-4162
References
- 1.Curigliano G, Cardinale D, Dent S, et al. Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA Cancer J Clin. 2016;66(4):309-325. doi: 10.3322/caac.21341 [DOI] [PubMed] [Google Scholar]
- 2.Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur J Heart Fail. 2016;37(36):2768-2801. doi: 10.1093/eurheartj/ehw211 [DOI] [PubMed] [Google Scholar]
- 3.Zhang YF, Cui J.Research progress of chemotherapeutic drug-induced cardiotoxicity. Chinese J Clin Oncol. 2018;45(24): 1243-1247. doi: 10.3969/j.issn.1000-8179.2018.24.056 [DOI] [Google Scholar]
- 4.Ma J, Qin SK, Shen ZX.Guidelines for the prevention and treatment of cardiotoxicity induced by anthracyclines. Chin Clin Oncol. 2013;18(10):925-934. doi: 10.3969/j.issn.1009-0460.2013.10.014 [DOI] [Google Scholar]
- 5.Lv J, Ding N, Zhu JQ, et al. Research progress on cardiotoxicity and prevention of anthracyclines. Chin J Hosp Pharm. 2008;2008(15):1302-1303. doi: 10.3321/j.issn:1001-5213.2008.15.026 [DOI] [Google Scholar]
- 6.Tebbi CK, London WB, Friedman D, et al. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin’s disease. J Clin Oncol. 2007;25(5):493-500. doi: 10.1200/JCO.2005.02.3879 [DOI] [PubMed] [Google Scholar]
- 7.Shaikh F, Dupuis LL, Alexander S, Gupta A, Mertens L, Nathan PC.Cardioprotection and second malignant neoplasms associated with dexrazoxane in children receiving anthracycline chemotherapy: a systematic review and meta-analysis. J Natl Cancer Inst. 2016;108(4):djv357. doi: 10.1093/jnci/djv357 [DOI] [PubMed] [Google Scholar]
- 8.Payne DL, Nohria A.Prevention of chemotherapy induced cardiomyopathy. Curr Heart Fail Rep. 2017;14(5):398-403. doi: 10.1007/s11897-017-0353-9 [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Cui X, Yan Y, et al. Research progress of cardioprotective agents for prevention of anthracycline cardiotoxicity. Am J Transl Res. 2016;8(7):2862-2875. [PMC free article] [PubMed] [Google Scholar]
- 10.Baskaran UL, Sabina EP.Clinical and experimental research in antituberculosis drug-induced hepatotoxicity: a review. J Integr Med. 2017;15(1):27-36. doi: 10.1016/S2095-4964(17)60319-4 [DOI] [PubMed] [Google Scholar]
- 11.Slostad B, Khalsa T, Young K, Guerra H, Bhagra A.A case-based approach to integrative medicine for cardiovascular disease prevention. J Integr Med. 2020;18(2):159-162. doi: 10.1016/j.joim.2019.12.001 [DOI] [PubMed] [Google Scholar]
- 12.Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6: e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383-394. doi: 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 15.Yu R, Wang J, Peng GC, et al. A systematic review of the efficacy of Chinese medicine injections in preventing and treating anthracyclines-induced cardiotoxicity. Chin Tradit Herb Drugs. 2021;52(10):3051-3060. [Google Scholar]
- 16.Long YY, Xu Y, Tang XQ, et al. Meta-analysis of Shenqi Fuzheng Injection in prevention and treatment of anthracyclines cardiotoxicity. Lab Med Clin. 2019;16(11):1550-1554. doi: 10.3969/j.issn.1672-9455.2019.11.023 [DOI] [Google Scholar]
- 17.Ma T, Zhu MJ, Wang YX, et al. Meta-analysis and GRADE evaluation of Shenmai injection combined with dexpropylimine in preventing and treating anthracycline cardiotoxicity. J Tradit Chin Med. 2021;62(18):1598-1605. doi: 10.13288/j.11-2166/r.2021.18.008 [DOI] [Google Scholar]
- 18.Zhang D, Wu JR, Liu S, et al. Meta-analysis on randomized controlled trials of Shenmal injection in adjuvant treatment of acute leukemia. Chin J Pharmacoepidemiol. 2017;26(9): 620-626. [Google Scholar]
- 19.Ma T, Zhu MJ, Wang YX, et al. Meta-analysis of Xinmailong injection in the treatment of cardiac toxicity after anthracycline chemotherapy. Tradit Chin Drug Res Clin Pharmacol. 2021;32(6):886-893. doi: 10.19378/j.issn.1003-9783.2021.06.020 [DOI] [Google Scholar]
- 20.Yang M, Lu J, Mou JJ, et al. Systemic evaluation of Shengmai injection in preventing and treating cardiotoxicity of anthracyclines antitumor drugs. Chin J Pharmacovigil. 2012;9(11): 666-669. doi: 10.3969/j.issn.1672-8629.2012.11.009 [DOI] [Google Scholar]
- 21.Li TH.Anthracycline-induced cardiotoxicity and TCM syndrome type correlation and TCM prevention and treatment of clinical research. Liaoning Univ Trad Chinese Med. 2019;28-37. [Google Scholar]
- 22.Wang X, Zhang Y, Xu J.Constructing post-marketing re-evaluation indicator system for traditional Chinese medicine injection by Delphi method. Chin J Hosp Pharm. 2021;41(1): 85-88. doi: 10.13286/j.1001-5213.2021.01.17 [DOI] [Google Scholar]
- 23.Zhang FY, Shen AM, Zeng XT, et al. An Introduction to AMSTAR 2: a critical appraisal tool for systematic reviews. Chin J Evid Cardiovasc Med. 2018;10(01):14-18. doi: 10.3969/j.issn.1674-4055.2018.01.03 [DOI] [Google Scholar]
- 24.Zeng XT, Li S, Ma Z, et al. The eighth of meta-analysis series: reporting specifications of meta-analysis. Chin J Evid Cardiovasc Med. 2012;4(6):500-503. [Google Scholar]
- 25.Li J.Implementation methods of common design schemes in clinical medical research lecture 1: randomized controlled trials. Chin J Pract Pediatr. 2008; 23(1):73-80. doi: 10.3969/j.issn.1005-2224.2008.01.025 [DOI] [Google Scholar]
- 26.Li Q, Xia Y, Mou YJ, et al. Reevaluation on quality of TCM systematic reviews and meta-analyses documents published in domestic Chinese journals. Mod Chin Clin Med. 2012; 19(3):28-33. doi: 10.3969/j.issn.1672-2205.2012.03.008 [DOI] [Google Scholar]