Abstract
The opioid overdose death toll in the United States is an ongoing public health crisis. We characterized the magnitude and duration of respiratory depression, the leading cause of death in opioid overdose cases, induced by heroin or fentanyl and the development of tolerance in male and female rats. We used whole-body plethysmography to first establish dose-response curves by recording breathing for 60 minutes post-intravenous opioid injection. We then tested the development of respiratory tolerance to acute heroin or fentanyl over several weeks and to chronic fentanyl with acute fentanyl or heroin challenge. Heroin and fentanyl each provoked dose-dependent respiratory depression. Heroin caused prolonged (45–60 minute) respiratory depression in female and male rats, characterized by decreased frequency, tidal volume, and minute ventilation and increased inspiratory time and apneic pause. Fentanyl produced similar changes with a shorter duration (10–15 minutes). High-dose heroin or fentanyl produced robust respiratory depression that was slightly more severe in females and, when given intermittently (acute doses 2 to 3 weeks apart), did not lead to tolerance. In contrast, chronic fentanyl delivered with an osmotic minipump resulted in tolerance to acute fentanyl and heroin, characterized by a shorter duration of respiratory depression. This effect persisted during withdrawal in males only. Our model and experimental design will allow for investigation of the neurobiology of opioid-induced respiratory depression and for testing potential therapeutics to reverse respiratory depression or stimulate breathing.
SIGNIFICANCE STATEMENT
Fentanyl was more potent and had shorter duration in producing respiratory depression than heroin in both sexes, whereas female rats were more sensitive than males to heroin-induced respiratory depression. Tolerance/cross-tolerance develops in chronic fentanyl administration but is minimized with long interadministration intervals.
Introduction
The United States and other countries continue to face a deadly opioid overdose epidemic. In 2019, 1.2% of the world population misused opioids, and opioids were present in over 70% of overdose deaths (https://www.unodc.org/res/wdr2021/field/WDR21_Booklet_3.pdf). From 2019 to 2020 (during the COVID-19 pandemic), the Center for Disease Control reported a 31% increase (up to 28.3 per 100,000) in the age-adjusted rate of overdose deaths, with 75% of those deaths involving an opioid (https://www.cdc.gov/drugoverdose/deaths/index.html). In 2018, the opioid-involved death rate was 2 to 3 times higher for men than women (Wilson et al., 2020), which may reflect the higher prevalence of opioid use in men (Walter et al., 2022); however, the incidence of opioid-involved deaths has increased at twice the rate in women (Back et al., 2011). The primary cause of death from opioid overdose is respiratory depression and hypoxia.
Breathing ultimately involves the exchange of oxygen (O2) and carbon dioxide (CO2) in lung alveoli and is subject to both voluntary and involuntary control (Pattinson, 2008). Involuntary ventilation is controlled mainly by the brainstem, which integrates central and peripheral sensory inputs. In rats, the medullary pre-Bötzinger complex, coupled with the retrotrapezoid/parafacial respiratory group, receives inputs from the pontine Kölliker-Fuse/parabrachial complex. Together, this network of pattern generators and nuclei in surrounding regions produce and modulate respiratory drive and rhythm (Janczewski and Feldman, 2006; Smith et al., 2007; Saunders and Levitt, 2020). This rhythm-generation network drives and coordinates respiratory muscle activity via spinal and cranial motor neurons, with inputs from forebrain and chemoreceptors that primarily sense blood CO2 concentration (Smith et al., 2007; Pattinson, 2008; Koo and Eikermann, 2011). Carotid and aortic bodies in the periphery sense changes in blood CO2 as well as O2 levels and relay this information to the respiratory network via the nuclei tractus solitarius (Nattie, 1999; Prabhakar, 2016). Stimulation of the respiratory network changes breathing rhythms/patterns as well as the rate and depth of breathing.
Opioids induce respiratory depression via direct actions on breathing circuits in the brainstem, with additional blunting of central and peripheral chemosensitivity to hypoxia (lack of O2) and hypercapnia (abundance of CO2) and actions on respiratory muscle motor neurons (Pattinson, 2008; Koo and Eikermann, 2011;). In the brainstem, opioids bind to μ-opioid receptors (MORs), activating Gi-protein-mediated inhibitory intracellular pathways that reduce neuronal excitability and disrupt respiratory rhythms (Watson et al., 1980; Wamsley, 1983; Romberg et al., 2003; Waldhoer et al., 2004). MOR activation decreases breathing frequency by affecting inhalation and exhalation times and by increasing the “apneic pause” between exhalation and inhalation. Opioids also reduce the amount of air exchanged per breath (tidal volume) and CO2 sensitivity (Shook et al., 1990). At higher doses, opioids cause sedation by decreasing the activity of cortical regions, some of which regulate voluntary breathing (Pattinson et al., 2009; Montandon and Horner, 2019).
The effects of opioids may differ in men and women. Although opioid analgesia studies report either no sex differences or greater analgesia in women, in the few clinical studies that have specifically investigated sex differences in opioid-induced respiratory depression, women exhibited greater respiratory depressant effects than men (Dahan et al., 1998; Sarton et al., 1999). Animal studies of opioid analgesia have reported either no sex differences or less analgesia in females (Craft, 2003). Marchette et al. (2021) showed a rightward shift of heroin-induced dose-response analgesia in female rats. Female rats also required 3 times more heroin to reach comparable levels of opioid dependence and withdrawal-induced hyperalgesia than male rats (Marchette et al., 2021; Carmack et al., 2022). These findings suggest that female rats are less sensitive than male rats to some effects of opioids, but sex differences in opioid-induced respiratory depression remain to be determined.
The present study characterized the effects of two commonly misused opioids, heroin and fentanyl, on ventilation in adult female and male Long-Evans rats. We investigated the ventilatory effects of acute and repeated administration of these opioids and the occurrence of tolerance to chronic fentanyl, using minute ventilation (tidal volume * frequency) and peak inspiratory flow as representative measures of breathing rhythm and flow. We hypothesized that 1) following chronic exposure to fentanyl, acute doses of fentanyl and heroin would cause blunted respiratory depression or tolerance; (2) with intermittent repeated exposure, fentanyl and heroin would not produce tolerance-subjected respiratory depression, provided sufficient time was given between acute opioid exposures; and 3) female rats would be less sensitive to opioid-induced respiratory depression than males.
Material and Methods
Animals
Eight-week-old male (n = 91) and female (n = 85) Long-Evans rats were obtained from Charles River (Kingston, NY). Rats were group housed and kept on a 12-hour light/12-hour dark cycle (lights on at 6 AM) at 21 ± 2°C with free access to food and water, except during the tests. Separate cohorts were used for each of the three studies described below. All procedures were performed per the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the National Institute on Drug Abuse, Intramural Research Program, Animal Care and Use Committee.
Drugs
Diamorphine hydrochloride (heroin) was diluted in 0.9% sterile saline for i.v. injections (300–1200 μg/kg, 1 mL/kg). Fentanyl citrate was diluted in 0.9% sterile saline for i.v. injections (12.5–50 μg/kg, 1 mL/kg) and s.c. osmotic pump delivery (0.06 mg/kg/d for 7 days, 1 mL/kg/d). Both were obtained from the National Institute on Drug Abuse drug supply program (Research Triangle Institute, Research Triangle Park, NC) and dispensed by the National Institute on Drug Abuse Intramural Research Program Pharmacy. Naloxone was diluted in 0.9% sterile saline for s.c. injections (1 mg/mL/kg; Tocris Bioscience, Bio-Techne, Minneapolis, MN).
Intravenous Catheter Surgery
Rats were implanted with an indwelling silastic catheter (Dow Corning, Midland, MI) into the right jugular vein (Vendruscolo et al., 2011) under general anesthesia (2% to 3% isoflurane in O2). The analgesic meloxicam (1 mg/kg, 1 mg/mL, s.c.; Hospira, Lake Forest, IL) was given just prior to anesthetization and surgery. The catheter was anchored to the vein with suture threads and passed subcutaneously to exit dorsally on the back of the rat. Catheters were flushed daily with 0.3 mL of a 0.9% sterile saline solution containing the anticoagulant heparin (30 USP units/mL; Hospira, Lake Forest, IL) and the antibiotic gentamicin (4.25 mg/mL; Hospira, Lake Forest, IL).
Naloxone-Precipitated Withdrawal Test
To evaluate whether chronic fentanyl administration produced signs of opioid dependence, rats received naloxone hydrochloride (1 mg/kg, s.c.; MOR antagonist) and were immediately placed in a Plexiglas box (40 cm × 40 cm × 40 cm) in front of a mirror and observed for 10 minutes. Signs of opioid withdrawal included escape attempts (jumps), wet dog shakes, defecation/diarrhea (quantified by weight loss), teeth chattering, swallowing movements, salivation, ptosis, genital grooming, hyperirritability/vocalization upon touch, and abnormal posture (Vendruscolo et al., 2011; modified from Gellert and Holtzman, 1978; Schulteis et al., 1999). Naloxone-precipitated withdrawal score was calculated as the average sum of observed signs determined by two experienced scorers.
Plethysmography Apparatus
Ventilation was noninvasively monitored using four whole-body plethysmograph chambers (SCIREQ, Montreal, QC, Canada) that measured 20 cm in diameter, 15 cm in height, and 3 L in volume. Each chamber contained a tower attachment (15 cm) through which a 30-cm length of Tygon tubing was protected by a metal spring and connected to a liquid swivel. This swivel was connected to the rat’s catheter to permit intravenous drug injection without opening the chamber and to allow the rat to move and turn freely within the chamber. The plethysmograph detected changes in pressure and airflow within the chamber in relation to a reference chamber, and a software (iox 2.10.0.40 by emka TECHNOLOGIES) calculated eight ventilatory parameters based on the data (Table 1). Ventilatory parameters were continuously monitored during the 2.5-hour sessions.
TABLE 1.
Definition of ventilatory measures
| Abbreviation | Parameter (Unit) | Description |
|---|---|---|
| Ti | Inspiratory time (msec) | The time from start to end of inspiration |
| Te | Expiratory time (msec) | The time from start of expiration to beginning of next inspiration |
| f | Breathing frequency (bpm) | The breath-by-breath rate of breathing, computed on inspiration and expiration times (Ti + Te) |
| TV | Tidal volume (mL) | The amount of air breathed in during inspiration |
| MV | Minute ventilation (mL/min) | The amount volume breathed in one minute, computed on a breath-by-breath basis (TV * f) |
| PIF | Peak inspiratory flow (mL/sec) | The maximum negative flow during one breath |
| RT | Relaxation time (msec) | The time to expire a defined percentage of tidal volume |
| AP (Pau) | Apneic pause | A unitless indicator of bronchoconstriction, computed as (Te/RT – 1) |
Of the eight calculated parameters we measured, we chose the following to be presented here: minute ventilation, tidal volume, inspiratory time, peak inspiratory flow, and apneic pause [calculated as (expiratory time/relaxation time) – 1]. These parameters are those that give a fuller understanding of the effects of the opioids on ventilatory timing and mechanics (Getsy et al., 2022a–f; Lewis et al., 2022; Seckler et al., 2022). Minute ventilation provides a general assessment of how opioids affect the total volume of air breathed per minute and is the multiplicative index of frequency of breathing and tidal volume. To further the analyses, we also determined 1) inspiratory time to characterize the effects of the opioids on ventilatory timing patterns, 2) tidal volume to better understand the potential consequences to arterial blood-gas chemistry (pH, pCO2, pO2, sO2) since tidal volume is a definitive index of the volume of air reaching alveoli, 3) peak inspiratory flow to provide direct information as to the ability of the opioids to compromise the efficacy of inspiration, and 4) apneic pause as it represents the central delay in transitioning from inspiration to expiration, a hallmark of opioid-induced respiratory depression (Getsy et al., 2022a–f; Lewis et al., 2022; Seckler et al., 2022).
Experiment 1. Dose-Response Curve of Heroin- or Fentanyl-Induced Respiratory Depression
Following intravenous catheter implantation and recovery, the rats were habituated for 2 hours to the plethysmography chambers and given a bolus injection of saline (1 mL/kg, i.v.) at the halfway point (i.e., 1 hour). Breathing was not monitored during habituation. During testing, rats were acclimated to the chambers for 40 minutes, and then baseline data were collected for 20 minutes. The rats then received a bolus injection of fentanyl (0, 12, 25, or 50 µg/kg, 1 mL/kg, i.v.) or heroin (0, 300, 600, or 1200 µg/kg, 1 mL/kg, i.v.). Ventilation was then monitored for 60 minutes postadministration. The same procedure was repeated for 3 consecutive weeks, with a single opioid exposure per week, until all the rats received every dose of either fentanyl or heroin in a within-subjects Latin-square design over 4 weeks (n = 10 to 11/sex per drug; Fig. 1). The doses of heroin and fentanyl were 10 times higher than the doses we have used for operant self-administration studies (Wade et al., 2015). The rats weighed 262 ± 7.5 g (mean ± S.E.) and 205 ± 3.1 g (male and female, respectively) at the start of the study. By the end of the study, males gained 146 g and females 75 g on average.
Fig. 1.
Experimental timelines. HAB, habituation; SWT, Somatic withdrawal test.
Experiment 2. Measurement of Respiratory Depression during Intermittent Multiweek Acute Administration
Following intravenous catheter implantation, rats were habituated to the plethysmography chambers as described above. During testing, rats were acclimated to the chambers for 40 minutes, and then baseline data were collected for 20 minutes. The rats then received a bolus injection of heroin (600 μg/kg, i.v.) or fentanyl (25 μg/kg, i.v.). Ventilation was then monitored for 60 minutes postadministration. The same procedure was repeated 2 and 5 weeks later for a total of three injections of the same drug and dose per animal (n = 8–11/sex; Fig. 1). A control group received saline in the first two tests and heroin or fentanyl in the third test. The rats weighed 318 ± 3.0 g (mean ± S.E.) and 224 ± 1.9 g (male and female, respectively) at the start of the study. By the end of the study, female rats gained on average 100 g and male rats 241 g.
Experiment 3. Effect of Chronic Fentanyl on Acute Opioid-Induced Respiratory Depression
Rats were implanted with an intravenous catheter and a subcutaneous osmotic minipump in the same surgery. For the minipump implantation, a small incision was made on the dorsal neck region or lower back of the rat to allow the insertion of an osmotic pump (ALZET model 2ML1, DURECT Corporation, ALZET Osmotic Pumps, Cupertino, CA). The incision was closed with veterinary adhesive. Rats received continuous subcutaneous infusion of fentanyl (0.06 mg/kg/d for 7 days) or saline (1 mL/kg/d for 7 days). The dose of chronic fentanyl was based on the amount of fentanyl taken in extended access (12 hours) operant self-administration studies (Wade et al., 2015). Seventy-two hours postimplant, rats were evaluated for signs of opioid dependence by the naloxone-precipitated withdrawal test described above. On day 5 postimplant, the rats were habituated for 2 hours to the plethysmography chambers and given a single bolus injection of saline (1 mL/kg, i.v.) at the halfway point (i.e., 1 hour). On day 6 postimplant, rats were acclimated to the chambers for 40 minutes, and then baseline data were collected for 20 minutes. The rats then received a bolus injection of heroin (600 μg/kg, i.v.) or fentanyl (25 μg/kg, i.v.). Ventilation was then monitored for 60 minutes postadministration. The same procedure was repeated the following day (test 2) with the drug not given in test 1 (e.g., heroin in test 1 and fentanyl in test 2). The pump was removed at day 7 postimplant under isoflurane anesthesia. The same procedure was employed during chronic fentanyl protracted withdrawal (tests 3 and 4 on days 4 and 5 postremoval). Each rat received a total of two injections of both heroin and fentanyl (n = 10/sex; Fig. 1). The rats weighed 307 ± 9.8 g (mean ± S.E.) and 210 ± 6.2 g (male and female, respectively) at the start of the study. At the end of the experiment, female rats gained on average 11 g, whereas male rats gained 24 g on average.
Statistical Analysis
A custom-made application (rvent_app) was used to import, bin, compile, plot, and export Excel datasheets from the .txt files generated and calculated using iox 2.10.0.40 software (emka TECHNOLOGIES, Paris, France). Prism 8 software (GraphPad, San Diego, CA) was used for figure preparation. Statistica 13 software (TIBCO Software, Palo Alto, CA) was used for statistical analyses. All data are aggregated into 5-minute bins (indicated by period onset, e.g., value at 10 minutes is the average from 10 to 15 minutes postadministration). Data are expressed as mean ± S.D. percentage of baseline values. Baseline values were calculated from the average predrug baseline values recorded between −20 to−5 minutes for each individual rat (). Outliers were identified in Prism using the ROUT test with the maximum desired false discovery rate (Q) set to 1%. Excluded outliers were replaced by the average of temporally proximal values for each animal. The whole data set of an individual rat for a given variable was excluded if >25% of the data were identified as outliers (i.e., three or more data points). The exclusion criterion was applied to each variable separately. Average raw data are shown in Supplemental Tables 1–4. Values of P < 0.05 were considered significant. For statistical purposes, percentage of baseline analyses included 60-minute postinjection data but not baseline. An “overshoot” (rebound effect) was defined as a significant difference from vehicle in the opposite direction from respiratory depression.
Experiment 1
To determine the maximum effect of escalating doses of fentanyl and heroin, we compared the lowest or highest values (depending on the direction of respiratory depression), in the percentage of baseline, within 25 minutes postadministration using repeated-measures (RM)-ANOVA for each drug separately, with sex as a between-subjects factor and dose as a within-subjects factor (Latin-square design; descriptive statistics in Supplemental Tables 5 and 6). To determine the duration (defined as the end of the period) of the effect of fentanyl and heroin, we used the average baseline and the 60-minute postadministration raw data. We analyzed each dose separately using one-way ANOVA, with time as a within-subjects factor. To determine the dose-response curve of fentanyl and heroin over time, we used three-way RM-ANOVA, with sex as a between-subjects factor and test and dose as within-subjects factors. Dunnett or Duncan’s post hoc comparisons tests were used when appropriate.
Experiment 2
To characterize fentanyl-induced respiratory depression following repeated administration, we used three-way RM-ANOVA, with sex as a between-subjects factor and test and time as within-subjects factors. We also compared test 3 of a body-weight control group (opioid-naive rats given fentanyl in test 3 only) and the experimental group using a three-way RM-ANOVA, with sex and group as between-subjects factors and time as a within-subjects factor. To determine the maximum effect of fentanyl, we compared the lowest (or highest, depending on the direction of respiratory depression) values within 25 minutes postadministration using two-way ANOVA, with sex and test as between-subjects factors. These analyses were repeated for heroin, including a heroin body-weight control group. Duncan’s post hoc comparisons test was used when appropriate (descriptive statistics in Supplemental Tables 7 and 8).
Experiment 3
To test the effect of chronic fentanyl treatment on naloxone-induced somatic signs of withdrawal, we used two-way ANOVA, with sex and group as independent factors. To determine the maximum effect of acute fentanyl combined with chronic fentanyl exposure, we compared the lowest or highest values (depending on the direction of respiratory depression) within 25 minutes postadministration using two-way ANOVA, with sex and group as between-subjects factors. To determine whether tolerance to respiratory effects of acute fentanyl develops with chronic exposure to fentanyl, we used three-way RM-ANOVA, where sex and group were between-subjects factors and time was a within-subjects factor. To determine the duration (defined as the end of the period) of the effect of fentanyl and heroin, we used the average baseline and the 60-minute postadministration raw data. We analyzed each dose separately using one-way ANOVA, where time was a within-subjects factor. Due to unstable baseline in the saline-treated male group in the 20 minutes prior to drug administration during the chronic fentanyl tests, the baseline window was shifted to −40 to−20 minutes for all rats. This analysis was repeated for cross-tolerance with acute heroin in the same rats (descriptive statistics in Supplemental Tables 9 and 10). These analyses were repeated to determine the maximum effect and duration of effect of acute fentanyl (or heroin) during abstinence from chronic fentanyl exposure, as well as to determine whether tolerance to respiratory effects of acute fentanyl (or cross-tolerance to heroin) is maintained during abstinence (descriptive statistics in Supplemental Tables 11 and 12). Duncan’s post hoc comparisons test was used when appropriate.
Results
Experiment 1: Dose-Response Curve of Heroin and Fentanyl
Dose-Response Curve of Heroin and Fentanyl: Comprehensive Time Course
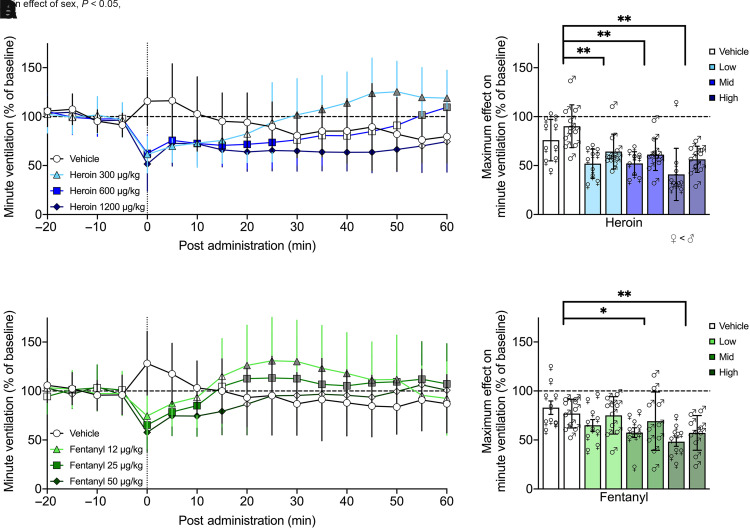
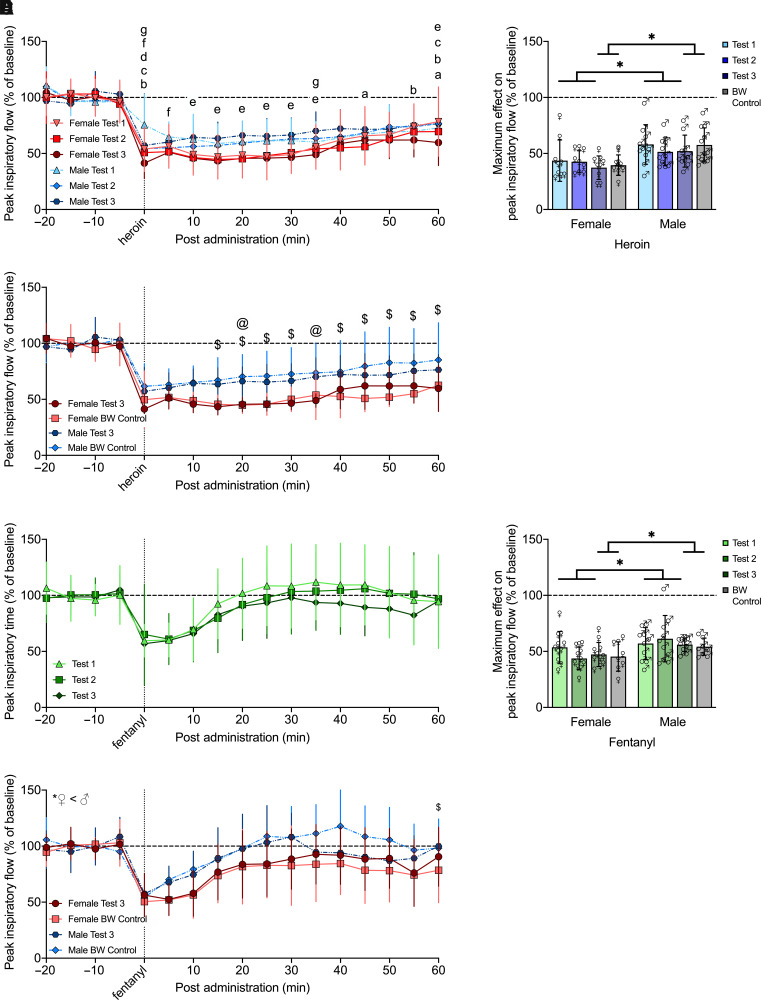
The decreases in minute ventilation and peak inspiratory flow that were elicited by an intravenous dose of heroin (0, 300, 600, or 1200 µg/kg) 60 minutes postadministration in female and male rats are shown in Figs. 2A and 3A. Each dose of heroin elicited decreases in minute ventilation and peak inspiratory flow, with no significant difference between males and females (P = 0.062, P = 0.306).
Fig. 2.
Minute ventilation was robustly decreased across several doses of heroin and fentanyl. Minute ventilation was decreased by a single i.v. dose of heroin (300, 600, or 1200 µg/kg; n = 10 female, 11 male) or fentanyl (12, 25, or 50 µg/kg; n = 10 female, 10 male) compared with vehicle. Each dose of heroin elicited decreases in minute ventilation, with higher doses producing prolonged effects for up to 50 min and no difference between males and females over time (A). Minute ventilation maximally decreased to ∼50% of baseline following heroin administration, which was significantly different from vehicle for all doses; overall, females had significantly lower values than males (B). Fentanyl administration elicited shorter-lasting decreases in minute ventilation, with higher doses producing prolonged effects for up to 15 minutes and no difference between the sexes (C). Maximum decreases following fentanyl administration were independent of sex, with decreases to ∼60% of baseline that were significantly different from vehicle for the middle and high doses (D). Data are expressed as mean ± S.D. in percentage of baseline in 5-minute bins over −20 to 60 minutes postadministration (A and C) or lowest 5-minute bin value within 25 minutes postadministration (B and D). Dashed line indicates average baseline measure. Filled-in symbols indicate significant difference, P < 0.05, from vehicle group, with gray color indicating overshoot/recovery (i.e., not respiratory depression). *P < 0.05; **P < 0.001, compared with vehicle. ♀, female; ♂, male.
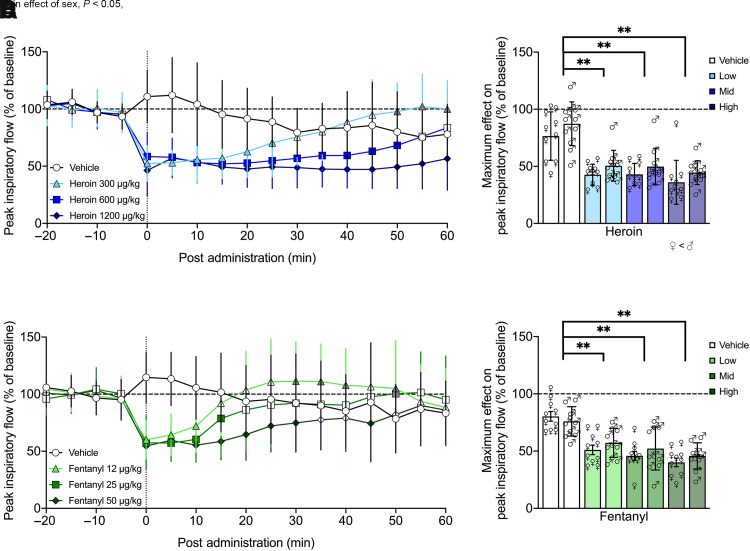
Fig. 3.
Peak inspiratory flow was robustly decreased across several doses of heroin and fentanyl. I.v. doses of heroin (300, 600, or 1200 µg/kg; n = 10 female, 11 male) or fentanyl (12, 25, or 50 µg/kg; n = 10 female, 10 male) decreased peak inspiratory flow. Each dose of heroin elicited decreases in peak inspiratory flow, with higher doses producing prolonged effects for up to 60 minutes and no difference between males and females over time (A). Peak inspiratory flow was maximally decreased to ∼45% of baseline following heroin administration, which was significantly different from vehicle for all doses; overall, females had significantly lower values than males (B). Fentanyl administration elicited shorter-lasting decreases in minute ventilation, with higher doses producing prolonged effects for up to 30 minutes and no difference between the sexes (C). There were also robust decreases in peak inspiratory flow following fentanyl independent of sex, with drops to ∼50% of baseline that were significantly different from vehicle for all doses (D). Data are expressed as mean ± S.D. in percentage of baseline in 5-minute bins over −20 to 60 minutes postadministration (A and C) or lowest 5-minute bin value within 25 minutes postadministration (B and D). Dashed line indicates average baseline measure. Filled-in symbols indicate significant difference, P < 0.05, from vehicle group, with gray color indicating overshoot/recovery (i.e., not respiratory depression). *P < 0.05; **P < 0.001, compared with vehicle. ♀, female; ♂, male.
Three-way RM-ANOVA for minute ventilation from 0 to 60 minutes post-heroin administration showed a main effect of dose (F3,57 = 12.117, P < 0.0001; dose 1200 < 0) and time (F12,228 = 9.834, P < 0.0001) but not sex (F1,19 = 3.917, P = 0.062) nor a sex × dose (F3,57 = 0.699, P = 0.556) or sex × time (F12,228 = 1.333, P = 0.201) interaction, indicating a decrease in minute ventilation following the heroin injection, which dissipated over time independently of sex. There was a dose × time interaction (F36,684 = 15.062, P < 0.0001), indicating the duration of effect was dose dependent. Post hoc comparisons to vehicle indicated a significant decrease in minute ventilation from 0 to 15 minutes [overshoot (values above vehicle and baseline) from 30 to 60 minutes] for the lowest dose (P < 0.05), from 0 to 25 minutes (overshoot from 55 to 60 minutes) for the middle dose (P < 0.05), and from 0 to 50 minutes for the highest dose (P < 0.05).
Similar effects were observed for peak inspiratory flow, with a main effect of dose (F3,57 = 22.363, P < 0.0001; doses 300, 600, 1200 < 0) and time (F12,228 = 7.960, P < 0.0001) but not sex (F1,19 = 1.109, P = 0.306) nor a sex × dose (F3,57 = 0.718, P = 0.546) or sex × time (F12,228 = 1.400, P = 0.167) interaction, indicating a decrease in peak inspiratory flow upon heroin injection, which dissipated over time independently of sex. There was a dose × time interaction (F36,684 = 15.558, P < 0.0001), indicating the duration of effect was dose dependent. Post hoc comparisons to vehicle indicated a significant decrease in peak inspiratory flow from 0 to 25 minutes (overshoot at 50–60 minutes) for the lowest dose (P < 0.05), which extended to 0–50 minutes for the middle dose (P < 0.05) and 0–60 minutes for the highest dose (P < 0.05).
Fentanyl (0, 12, 25, or 50 µg/kg, i.v.) also elicited decreases in minute ventilation and peak inspiratory flow in both female and male rats, as show in Figs. 2C and 3C. There was no significant difference between males and females in either parameter (P = 0.327, P = 0.823). Three-way RM-ANOVA for minute ventilation from 0 to 60 minutes post-fentanyl administration showed a main effect of dose (F3,54 = 2.915, P = 0.042; no significant post hoc differences) and time (F12,216 = 8.570, P < 0.0001) but not sex (F1,18 = 0.944, P = 0.344), nor was there a sex × dose (F3,54 = 2.181, P = 0.101) or sex × time (F12,216 = 0.724, P = 0.727) interaction, indicating a decrease in minute ventilation following the fentanyl injection, which dissipated over time independently of sex. There was a dose × time interaction (F36,648 = 9.535, P < 0.0001), indicating the duration of effect was dose dependent. Post hoc comparisons to vehicle indicated a significant decrease in minute ventilation from 0 to 5 minutes (overshoot at 20–50 minutes) for the lowest dose (P < 0.05), from 0 to 10 minutes (overshoot at 20–30, 40–60 minutes) for the middle dose (P < 0.05), and from 0 to 15 minutes for the highest dose (P < 0.05).
Effects on peak inspiratory flow were similar, with a main effect of dose (F3,54 = 6.675, P = 0.001; dose 50 < 0) and time (F12,216 = 9.932, P < 0.0001) but not sex (F1,18 = 0.033, P = 0.857), nor was there a sex × dose (F3,54 = 0.861, P = 0.467) or sex × time (F12,216 = 0.719, P = 0.732) interaction, indicating a decrease in peak inspiratory flow following the fentanyl injection, which dissipates over time independently of sex. There was a dose × time interaction (F36,648 = 8.215, P < 0.0001), indicating the duration of effect was dose dependent. Post hoc comparisons to vehicle indicated a significant decrease in peak inspiratory flow from 0 to 10 minutes (overshoot at 30–40, 50 minutes) for the lowest dose (P < 0.05), from 0 to 15 minutes (overshoot at 50 minutes) for the middle dose (P < 0.05), and from 0 to 30 and 45 minutes for the highest dose (P < 0.05). Effects on tidal volume, frequency, inspiratory time, and apneic pause were similar, with some additional sex × time interactions immediately upon heroin injection only, as show in Supplemental Figs. 1 and 2 and Supplemental Table 5.
Dose-Response Curve of Heroin and Fentanyl: Duration of Effect
The duration of effect on minute ventilation and peak inspiratory flow elicited by an i.v. dose of heroin (0, 300, 600, or 1200 µg/kg) or fentanyl (0, 12, 25, or 50 µg/kg) within 60 minutes postadministration was generally similar or slightly longer lasting in female compared with male rats. The results of one-way ANOVA from the 20-minute averaged baseline to 60 minutes postadministration separated by dose and sex are shown in Table 2. Heroin significantly decreased minute ventilation for at least 25 minutes (low dose) and up to 65 minutes (whole observation window, high dose) compared with baseline. Heroin significantly decreased peak inspiratory flow for at least 40 minutes (low dose) and up to 65 minutes (high dose).
TABLE 2.
Dose-response curve duration of effect in minutes
| Duration (Min) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Drug, Sex | Dose (µg/kg) | Minute Ventilation | Tidal Volume | Frequency of Breathing | Peak Inspiratory Flow | Inspiratory Time | Apneic Pause | |
| Heroin, female | 0 | 0 | 0 | 5a | 0 | 0 | NA | |
| 300 | 25b | 5b | 25b,c | 40b | 35a | NA | ||
| 600 | 45b | 5b | 50b,c | 55b | 60a | 50a | ||
| 1200 | 65b | 5b | 60b,c | 65b | 60a | 65a | ||
| Heroin, male | 0 | 0 | 0 | 5a | 0 | 0 | 0 | |
| 300 | 15b | 0 | 40b | 35b | 50a | 25a | ||
| 600 | 40b | 0 | 65b | 65b | 65a | 65a | ||
| 1200 | 65b | 5b | 65b | 65b | 65a | 65a | ||
| Fentanyl, female | 0 | 5a | NA | 5a | NA | NA | NA | |
| 12.5 | 10b | NA | 15b | 15b | 15a | 10a | ||
| 25 | 15b | 5b | 5b,c | 20b | 20a | 20a | ||
| 50 | 30b | 5b | 60b,c | 65b | 60a | 50a | ||
| Fentanyl, male | 0 | 0 | 0 | 5a | 0 | 5b | 0 | |
| 12.5 | 0 | 0 | 0 | 10b | 15a | 10a | ||
| 25 | 10b | 5b | NA | 20b | 35a | 25a | ||
| 50 | 10b | 5b | NA | 50b | 60a | 30a | ||
NA, not applicable.
Post hoc comparisons following one-way ANOVA separated by sex and dose.
aP < 0.05, increased from baseline (direction of respiratory depression for inspiratory time and apneic pause).
bP < 0.05, decreased from baseline (direction of respiratory depression for minute ventilation, tidal volume, frequency, and peak inspiratory flow).
cOnset delayed by 5–10 minutes.
The duration of effect of fentanyl was expectedly shorter. Fentanyl significantly decreased minute ventilation for at least 5 minutes (low dose) and up to 25 minutes (high dose). Peak inspiratory flow was significantly decreased following fentanyl for at least 15 minutes (low dose) and up to 65 minutes (high dose).
Dose-Response Curve of Heroin and Fentanyl: Maximum Effect
The maximum decreases in minute ventilation elicited by an i.v. dose of heroin (0, 300, 600, or 1200 µg/kg) within 25 minutes postadministration in female and male rats are shown in Fig. 2B. Two-way RM-ANOVA showed a main effect of sex (F1,19 = 7.055, P = 0.016; female < male) and dose (F3,57 = 16.749, P < 0.0001; doses 300, 600, 1200 < 0) but no interaction (F3,57 = 0.688, P = 0.563). Main effect post hoc comparisons indicated that each dose of heroin elicited decreases in minute ventilation (P < 0.0001), with a greater overall effect in females. Maximum decreases in peak inspiratory flow within the same parameters are shown in Fig. 3B. Two-way RM-ANOVA showed a main effect of sex (F1,19 = 5.196, P = 0.034; female < male) and dose (F3, 57 = 35.083, P < 0.0001; doses 300, 600, 1200 < 0) but no interaction (F3, 57 = 0.066, P = 0.978). Main effect post hoc comparisons indicated that each dose of heroin elicited decreases in peak inspiratory flow (P < 0.0001), with a greater overall effect in females. There was also a greater maximum decrease in tidal volume and frequency for females but no difference between males and females for inspiratory time or apneic pause and an overall effect of dose for all parameters (Supplemental Figs. 1 and 2).
Maximum decreases in minute ventilation elicited by an i.v. dose of fentanyl (0, 12.5, 25, or 50 µg/kg) within 25 minutes postadministration in female and male rats are shown in Fig. 2D. Two-way RM-ANOVA showed a main effect of dose (F3,54 = 10.285, P < 0.0001; doses 25, 50 < 0) but neither a main effect of sex (F1,18 = 0.994, P = 0.332) nor a sex × dose interaction (F3,54 = 1.326, P = 0.275), indicating no difference between males and females. Post hoc comparisons indicated the two higher doses of fentanyl elicited decreases in minute ventilation (P < 0.05). Maximum decreases in peak inspiratory flow within the same parameters are shown in Fig. 3D. Two-way RM-ANOVA showed a main effect of dose (F3,54 = 41.847, P < 0.0001) but neither sex (F1,18 = 0.622, P = 0.441) nor an interaction (F3,54 = 1.207, P = 0.316), indicating no difference between males and females. Post hoc comparisons indicated that each dose of fentanyl elicited decreases in peak inspiratory flow (P < 0.0001). There was an overall effect of dose and no differences between males and females for tidal volume, frequency, inspiratory time, or apneic pause (Supplemental Figs. 1 and 2).
Experiment 2: Intermittent (Multiweek Acute) Administration
Intermittent (Multiweek Acute) Administration: Effects on Minute Ventilation
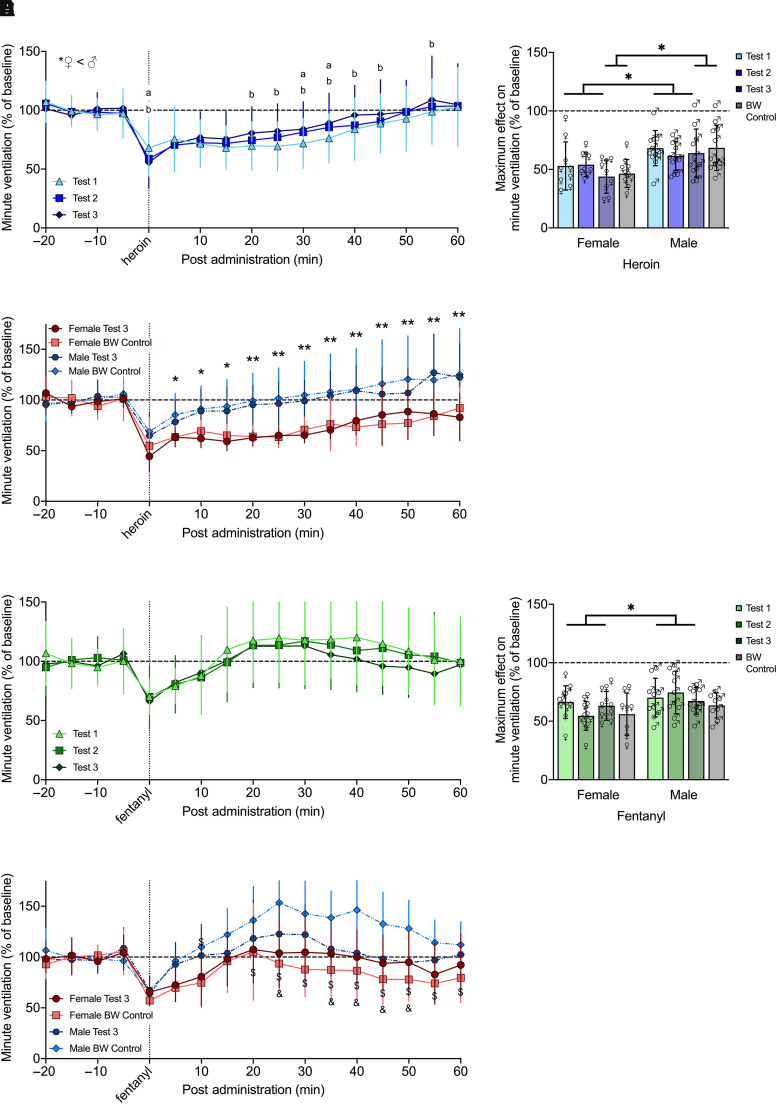
The effects of repeated (separated by 2 to 3 weeks) i.v. injections (tests 1, 2, and 3) of heroin (600 µg/kg) and fentanyl (25 µg/kg), doses that elicited robust respiratory depression in experiment 1 and were 10 times higher than those used for optimal self-administration (Wade et al., 2015), on minute ventilation for up to 60 minutes postadministration in male and female rats are shown in Fig. 4. Heroin and fentanyl elicited pronounced decreases in minute ventilation in female and male rats, with greater heroin-induced respiratory depression in females. To account for potential body weight effects on respiration, data from test 3 was also compared with a control group that received saline in the first two tests (data not shown) and heroin or fentanyl in the third test.
Fig. 4.
Effects of intermittent (2 to 3 weeks apart) acute administration of heroin or fentanyl on minute ventilation. Rats received i.v. injections (tests 1, 2, and 3) of heroin (600 µg/kg; n = 9 females, 11 males) or fentanyl (25 µg/kg; n = 11 females, 10 males) over 5 weeks. During test 3, a body-weight control group received heroin (n = 10 females, 12 males) or fentanyl (n = 8 females, 8 males). The rats exhibited minimal tolerance to heroin administered after several weeks, although the first dose induced greater decreases in minute ventilation at some of the later time points, and females had significantly greater decreases than males. aP < 0.05, test 1 versus test 2. bP < 0.05, test 1 versus test 3 (A). Heroin-induced maximal decrease in minute ventilation was significantly lower in females (∼50%) compared with males (∼65%) across test and age-matched groups. *P < 0.05, males versus females (B). For age-matched groups (test 3 versus body-weight control), females had significantly greater decreases than males, but there were no differences in the effects of heroin *P < 0.05; **P < 0.01, males versus females (C). The rats exhibited no tolerance to fentanyl administered after several weeks, with no difference between males and females (D). Fentanyl-induced maximal decrease in minute ventilation was significantly lower in females (∼60%) compared with males (∼70%) across test; however, there was no difference between sexes for age-matched groups. *P < 0.05, males versus females (E). For age-matched groups, females had significantly greater decreases than males, driven by an overshoot in the male body-weight control group, which was higher than the control females and test 3 males at several of the later time points. $P < 0.05, control males versus females. &P < 0.05, control males versus test 3 males (F). Data are expressed as mean ± S.D. in percentage of baseline in 5-minute bins over −20 to 60 minutes postadministration (A, C, D and F) or lowest value within 25 minutes postadministration (B and E). Dashed line indicates average baseline measure. ♀, female; ♂, male; BW, body weight.
For heroin, three-way RM-ANOVA over 60 minutes postadministration showed a main effect of time (F12,216 = 58.316, P < 0.000) and sex (F1,18 = 15.343, P = 0.001), with a greater decrease in minute ventilation in females than males. There was also a time × test interaction (F24,432 = 2.371, P < 0.000) but no main effect of test (F2,36 = 0.545, P = 0.584). Post hoc comparisons indicated the initial drug effect (minute 0) was lesser in test 1 versus tests 2 and 3, yet some later time points showed greater decreases in test 1 versus test 2 (minutes 30–35) and versus test 3 (minutes 20–45, 55; Fig. 4A).
Maximum decreases in minute ventilation elicited by heroin within 25 minutes postadministration are shown in Fig. 4B. Two-way RM-ANOVA across test showed a main effect of sex (F1,18 = 10.694, P < 0.000; female < male) but neither a main effect of test (F2,36 = 0.903, P = 0.414) nor a test × sex interaction (F2,36 = 0.821, P = 0.448), indicating that maximal decreases in minute ventilation were not subject to tolerance. There was a greater overall effect of heroin in females. In comparing the maximum effect of age-matched groups (test 3 versus body-weight control), the two-way ANOVA showed a main effect of sex (F1,38 = 15.459, P < 0.0001; female < male) but neither a main effect of group (F1,38 = 0.443, P = 0.510) nor a group × sex interaction (F1,38 = 0.032, P = 0.859).
Comparison of age-matched groups over 60 minutes postadministration via three-way RM-ANOVA showed a main effect of time (F12,456 = 45.603, P < 0.0001) and sex (F1,38 = 19.989, P < 0.0001), with a greater decrease in females than males, and a sex × time interaction (F12,456 = 2.607, P = 0.002). Post hoc comparisons indicated that females had significantly greater decreases than males from minutes 5 to 60. However, there was no main effect of group (F1,38 = 0.160, P = 0.691), indicating no difference in the effects of heroin between these age-matched groups (Fig. 4C).
For fentanyl, three-way RM-ANOVA showed a main effect of time (F12,228 = 32.662, P < 0.0001) but not sex (F1,19 = 1.040, P = 0.321) or test (F2,38 = 0.464, P = 0.632) nor a test × sex interaction (F24,456 = 0.859, P = 0.659). These results indicate a lack of prolonged tolerance to fentanyl and no difference between sexes (Fig. 4D).
Maximum decreases in minute ventilation elicited by fentanyl within 25 minutes postadministration is shown in Fig. 4E. Two-way RM-ANOVA across test showed a main effect of sex (F1,19 = 5.742, P = 0.027) but neither a main effect of test (F2,38 = 0.445, P = 0.644) nor a test × sex interaction (F2,38 = 2.357, P = 0.108), indicating that maximal decreases in minute ventilation were not subject to tolerance. There was a greater overall effect of heroin in females. In comparing the maximum effect of age-matched groups, the two-way ANOVA showed no main effect of sex (F1,33 = 1.759, P = 0.194) or group (F1,33 = 1.554, P = 0.221) nor a sex × group interaction (F1,33 = 0.137, P = 0.714).
Comparison of age-matched groups via three-way RM-ANOVA showed a main effect of time (F12,396 = 35.784, P < 0.0001) and sex (F1,33 = 12.200, P = 0.001), with a greater decrease in minute ventilation in females than males when given fentanyl. There was also a sex × group interaction (F1,33 = 4.374, P = 0.044; female control < male control), a time × sex interaction (F12,396 = 3.063, P < 0.0001), and an overall interaction (F12,396 = 2.948, P = 0.001). Post hoc comparisons indicated that the male body-weight control group was significantly higher than the female body-weight control group at minute 10 (during respiratory depression) and from minutes 20 to 60 (above/near baseline i.e., during overshoot/recovery period). The male body-weight control group was also significantly higher than the male test 3 group from minutes 25, 35 to 50 (overshoot/recovery; Fig. 4F).
Intermittent (Multiweek Acute) Administration: Effects on Peak Inspiratory Flow
Analyses on time course, maximum effect, and duration of effect were repeated for peak inspiratory flow, as shown in Fig. 5. Heroin and fentanyl elicited pronounced decreases in peak inspiratory flow in female and male rats. Observationally, the duration of effect of heroin far exceeded that of fentanyl in both sexes, whereas the maximal effect was similar for both opioids.
Fig. 5.
Effects of intermittent (2 to 3 weeks apart) acute administration of heroin or fentanyl on peak inspiratory flow did not result in tolerance and were stronger in females than males. Rats received i.v. injections (tests 1, 2, and 3) of heroin (600 µg/kg; n = 9 females, 11 males) or fentanyl (25 µg/kg; n = 11 females, 10 males), with each test separated by 2 to 3 weeks. During test 3, a control group received heroin (n = 10 females, 12 males) or fentanyl (n = 8 females, 8 males). Heroin induced greater decreases in peak inspiratory flow over time in females, most notably in test 3, with potential sensitization due to greater initial decrease in both males and females in test 3 and in males in test 2. aP < 0.05, female test 1 versus test 2. bP < 0.05, female test 1 versus test 3. cP < 0.05, female test 2 versus test 3. dP < 0.05, test 1 female versus male. eP < 0.05, test 3 female versus male. fP < 0.05, male test 1 versus test 2. gP < 0.05, male test 1 versus test 3 (A). Maximal decrease in peak inspiratory flow was significantly lower in females (∼40%) compared with males (∼55%) following heroin administration across test and age-matched groups. *P < 0.05, males versus females (B). For age-matched groups, females had significantly greater decreases than males, mostly driven by differences between male and female controls, with no overall group differences in the effects of heroin. $P < 0.05, control females versus males. @P < 0.05, test 3 females versus males (C). The rats exhibited no tolerance to fentanyl administered after several weeks, with no difference between males and females (D). Maximal decrease in peak inspiratory flow was significantly lower in females (∼45%) than males (∼55%) following fentanyl administration across test and age-matched groups. *P < 0.05, males versus females. *P < 0.05, males versus females (E). For age-matched groups, females had significantly greater decreases than males, with no group-specific differences. $P < 0.05, control females versus males; &P < 0.05, test 3 females versus males (F). Data are expressed as mean ± S.D. in percentage of baseline in 5-minute bins over −20 to 60 minutes postadministration (A, C, D, and F) or lowest value within 25 minutes postadministration (B and E). Dashed line indicates average baseline measure. ♀, female; ♂, male; BW, body weight.
For heroin, three-way RM-ANOVA showed a main effect of time (F12,204 = 33.650, P < 0.0001) and sex (F1,17 = 6.430, P = 0.021), with a greater decrease in peak inspiratory flow in females than males, but there was no main effect of test (F2,34 = 0.174, P = 0.841). There was a time × sex interaction (F12,204 = 2.969, P = 0.001), time × test interaction (F24,408 = 2.635, P < 0.0001) and time × sex × test interaction (F24,408 = 1.834, P = 0.010). Post hoc comparisons indicated potential sensitization to early effects of heroin, with a greater initial (minute 0) decrease during test 3 in both males and females. In test 3, female peak inspiratory flow was lower than that of males for 30 minutes (Fig. 5A).
Maximum decreases in peak inspiratory flow elicited by heroin within 25 minutes postadministration is shown in Fig. 5B. Two-way RM-ANOVA across test showed a main effect of sex (F1,17 = 11.217, P = 0.004) but neither a main effect of test (F2,34 = 0.880, P = 0.424) nor a sex × test interaction (F2,34 = 0.258, P = 0.774), indicating that maximal decreases in peak inspiratory flow were not subject to tolerance. There was a greater overall effect of heroin in females. Results were similar in comparing age-matched groups as the two-way ANOVA showed a main effect of sex (F1,37 = 16.823, P < 0.0001) but no group effect (F1,37 = 0.940, P = 0.339) nor group × test interaction (F1,37 = 0.172, P = 0.681), indicating that maximal decreases in peak inspiratory flow were greater in females and not subject to tolerance.
Comparison of age-matched groups via three-way RM-ANOVA showed a main effect of time (F12,444 = 16.391, P < 0.0001) and sex (F1,37 = 14.513, P = 0.001), with a greater decrease in females than males, but not group (F1,37 = 0.199, P = 0.658). There was a sex × group × time interaction (F12,444 = 1.776, P = 0.050), with post hoc comparisons indicating significantly greater decreases in peak inspiratory flow in the female body-weight control group compared to that of males (minutes 15–60, excluding minute 35) as well as significantly greater decreases in peak inspiratory flow during test 3 in females compared to males (minutes 20, 35) that received heroin (Fig. 5C).
For fentanyl, three-way RM-ANOVA showed a main effect of time (F12,228 = 33.893, P < 0.000) but neither sex (F1,19 = 1.125, P = 0.302) nor test (F2,38 = 0.947, P = 0.397) and no sex × test interaction (F24,456 = 0.786, P = 0.756; Fig. 5D).
For the maximum effect, two-way RM-ANOVA showed a main effect of sex (F1,19 = 7.455, P = 0.013) but not test (F2,38 = 0.480, P = 0.622) and no sex × test interaction (F2,38 = 1.647, P = 0.206), indicating that maximal decreases in peak inspiratory flow due to fentanyl were greater in females and not subject to tolerance (Fig. 5E). Results were similar in comparing age-matched groups as two-way ANOVA showed a main effect of sex (F1,33 = 7.491, P = 0.010) but no group effect (F1,33 = 0.322, P = 0.574) nor a group × test interaction (F1,33 = 0.0004, P = 0.984), further confirming that the maximum effect on peak inspiratory flow was greater in females and not subject to tolerance.
Finally, comparison of age-matched groups given fentanyl via three-way RM-ANOVA showed a main effect of time (F12,396 = 34.671, P < 0.0001) and sex (F1,33 = 5.411, P = 0.026) but not group (F1,33 = 0.011, P = 0.916) and no group × test interaction (F12,396 = 1.418, P = 0.155), indicating greater decreases in females than males but no difference in the effects of fentanyl on peak inspiratory flow between age-matched groups (Fig. 5F).
There was an overall effect of sex (greater effect on females) and no differences between tests for tidal volume, frequency, or inspiratory time and no effect of sex nor test on apneic pause on the maximum effect of heroin and fentanyl (Supplemental Figs. 3 and 4).
Experiment 3: Chronic Subcutaneous Fentanyl Administration with Acute Intravenous Opioid Challenges
Chronic Fentanyl: Naloxone-Precipitated Signs of Withdrawal
Continuous exposure to fentanyl for 4 days led to increased signs of naloxone-precipitated withdrawal, which were scored as outlined in Methods (F1,39 = 90.61, P < 0.0001; chronic fentanyl > chronic saline), independently of sex (mean ± S.D., female chronic saline = 7.9 ± 4.0; male chronic saline = 10.6 ± 7.2; female chronic fentanyl = 30.9 ± 5.2; male chronic fentanyl = 29.0 ± 10.8).
Chronic Fentanyl: Time-Course of Acute Opioid Effect with Fentanyl on Board
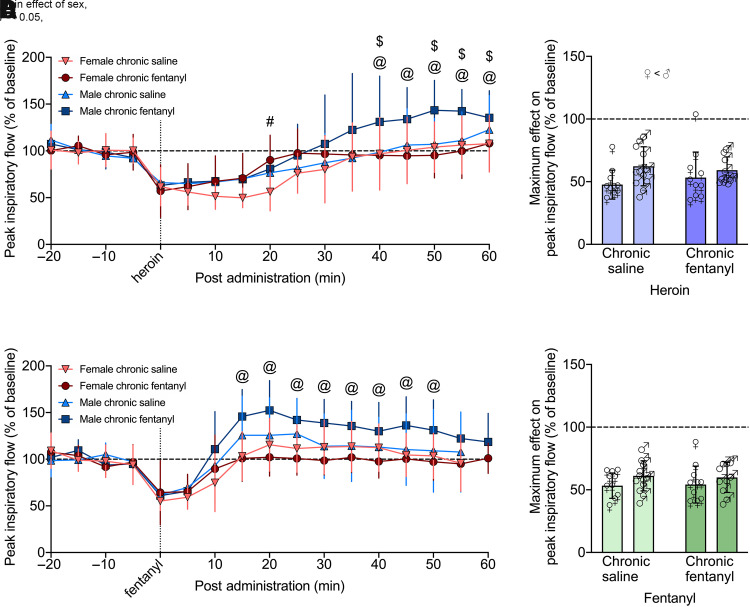
In rats chronically exposed to saline or fentanyl, the decreases in minute ventilation and peak inspiratory flow elicited by an i.v. dose of heroin (600 µg/kg) 60 minutes postadministration are shown in Figs. 6A and 7A. Heroin elicited decreases in minute ventilation and peak inspiratory flow that were longer lasting in the chronic fentanyl group, with no significant difference between males and females (P = 0.31, P = 0.84).
Fig. 6.
Chronic fentanyl exposure led to tolerance to acute heroin and fentanyl through shortened duration of effect on minute ventilation. Rats, under continuous exposure to saline (n = 10 females, 12 males) or fentanyl (n = 11 females, 10 males), received an i.v. injection of heroin (600 µg/kg) or fentanyl (25 µg/kg) during two tests 24 hour apart. Heroin elicited decreases in minute ventilation that were longer-lasting in the saline-exposed group, with no significant difference between males and females (A). Maximal decreases in minute ventilation was similar across chronic drug treatment and sex (B). Fentanyl elicited decreases in minute ventilation that were longer lasting in the saline-exposed group and were similar in female and male rats. (C). As with heroin, maximal decreases in minute ventilation from fentanyl were similar across chronic drug treatment and sex (D). Data are expressed as mean ± S.D. in percentage of baseline in 5-minute bins over −20 to 60 minutes postadministration (A and C) or as the lowest value within 25 minutes postadministration (B and E). Dashed line indicates average baseline measure. *P < 0.05, from saline-treated group. ♀, female; ♂, male.
Fig. 7.
Chronic exposure to fentanyl led to tolerance to acute heroin and fentanyl through shortened duration of effect on peak inspiratory flow. Rats, under continuous exposure to saline (n = 10 females, 12 males) or fentanyl (n = 11 females, 10 males), received an i.v. injection of heroin (600 µg/kg) or fentanyl (25 µg/kg) during two tests 24 hour apart. Heroin elicited decreases in peak inspiratory flow that lasted 45 minutes longer in the saline-treated group versus the chronic fentanyl group, with no sex differences (A). Maximal decrease in peak inspiratory flow following heroin administration was similar across chronic drug treatment and sex (B). Fentanyl elicited decreases in peak inspiratory flow that lasted 10 minutes longer in the saline- versus fentanyl-treated group, with no difference between males and females (C). Maximal decrease in peak inspiratory flow following fentanyl administration was similar across chronic drug treatment and sex (D). Data are expressed as mean ± S.D. in percentage of baseline in 5-minutes bins over −20 to 60 minutes postadministration (A and C) or as the lowest value within 25 minutes postadministration (B and E). Dashed line indicates average baseline measure, *P < 0.05, from saline-treated group. ♀, female; ♂, male.
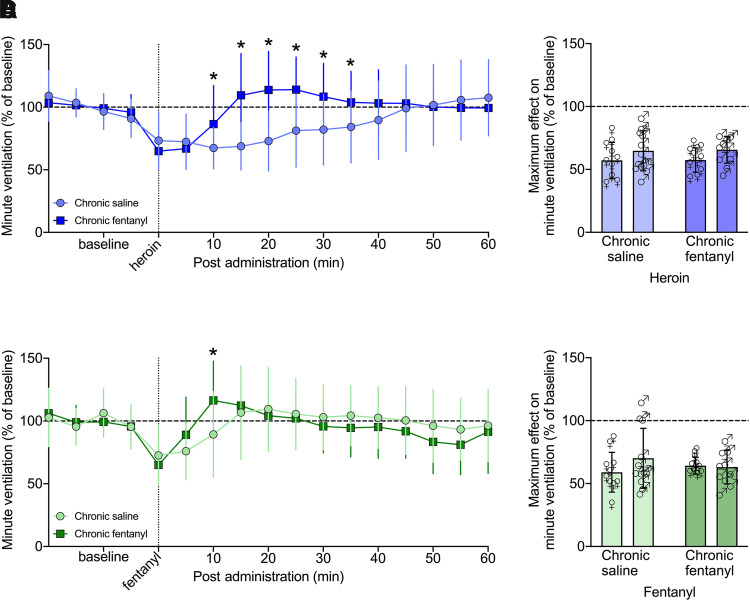
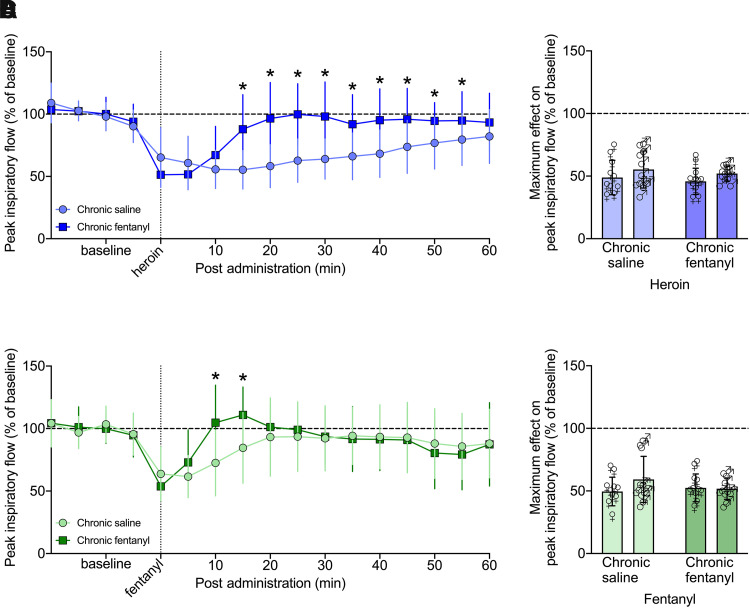
Three-way RM-ANOVA for minute ventilation from 0 to 60 minutes post-heroin administration showed a main effect of group (F1,39 = 4.998, P = 0.031; chronic fentanyl > chronic saline) and time (F12,468 = 20.218, P < 0.0001) but not sex (F1,39 = 1.075, P = 0.306), indicating a decrease in minute ventilation following heroin injection that dissipated over time independently of sex. There was a group × time interaction (F12,468 = 12.425, P < 0.0001), and post hoc comparisons showed that chronic fentanyl exposure increased minute ventilation from 10 to 35 minutes compared with saline exposure (P < 0.05).
Similar effects were observed for peak inspiratory flow, with a main effect of group (F1,39 = 18.365, P = 0.0001; chronic fentanyl > chronic saline) and time (F12,468 = 14.808, P < 0.0001) but not sex (F1,39 = 0.043, P = 0.837), indicating a sex-independent decrease in peak inspiratory flow following the heroin injection that dissipated over time. There was a group × time interaction (F12,468 = 9.579, P < 0.0001), and post hoc comparisons to the saline-exposed group indicated a significant increase in peak inspiratory flow from 15 to 55 minutes in the chronic fentanyl group (P < 0.05).
Fentanyl (25 µg/kg, i.v.) also elicited decreases in minute ventilation and peak inspiratory flow in both female and male rats, as shown in Figs. 6C and 7C. Three-way RM-ANOVA for minute ventilation from 0 to 60 minutes post-fentanyl administration showed a main effect of time (F12,468 = 16.652, P < 0.0001) but not sex (F1,39 = 0.002, P = 0.963). There was a sex × group interaction (F1,39 = 4.143, P = 0.048), but post hoc tests did not indicate any significant differences, indicating a decrease in minute ventilation following the fentanyl injection, which dissipated over time largely independently of sex. There was a group × time interaction (F12,468 = 4.594, P < 0.0001). Post hoc comparisons to the saline group indicated a significant increase in minute ventilation at 10 minutes in the chronic fentanyl group (P < 0.05).
Effects on peak inspiratory flow were similar to minute ventilation, with a main effect of time (F12,468 = 18.714, P < 0.0001) but not sex (F1,39 = 0.341, P = 0.563). There was a group × sex interaction (F1,39 = 4.146, P = 0.049), but post hoc comparisons did not yield any significant differences, indicating a decrease in peak inspiratory flow following the fentanyl injection which dissipated over time largely independently of sex. There was a group × time interaction (F12,468 = 5.734, P < 0.0001). Post hoc comparison with the saline group indicated a significant increase in peak inspiratory flow from 10 to 15 minutes following the acute fentanyl injection in the chronic fentanyl group (P < 0.05). Effects on tidal volume, frequency, inspiratory time, and apneic pause were similar to those on minute ventilation and peak inspiratory flow, as show in Supplemental Figs. 5 and 6.
Chronic Fentanyl: Maximum Effect of Acute Opioid with Fentanyl on Board
The maximum decrease in minute ventilation elicited by heroin (600 µg/kg, i.v.) within 25 minutes postadministration in female and male rats is shown in Fig. 6B. Two-way RM-ANOVA did not show a main effect of sex (F1,39 = 3.90, P = 0.052) or group (F1,39 = 0.019, P = 0.89) and no sex × group interaction (F1,39 = 0.006, P = 0.94). The maximum decrease in minute ventilation elicited by fentanyl (25 µg/kg, i.v.) within 25 minutes postadministration in female and male rats is shown in Fig. 6D. Two-way RM-ANOVA did not show a main effect of sex (F1,39 = 0.984, P = 0.33) or group (F1,39 = 0.031, P = 0.86) and no sex × group interaction (F1,39 = 1.485, P = 0.23).
Maximum decreases in peak inspiratory flow elicited by heroin (600 µg/kg, i.v.) within 25 minutes postadministration in female and male rats are shown in Fig. 7B. Two-way RM-ANOVA did not show a main effect of sex (F1,39 = 3.011, P = 0.09) or group (F1,39 = 0.743, P = 0.39) and no sex × group interaction (F1,39 = 0.0003, P = 0.99). Maximum decreases in peak inspiratory flow elicited by fentanyl (25 µg/kg, i.v.) within 25 minutes postadministration in female and male rats are shown in Fig. 7D. Two-way RM-ANOVA did not show a main effect of sex (F1,39 = 1.242, P = 0.27) or group (F1,39 = 0.308, P = 0.58) and no sex × group interaction (F1,39 = 1.622, P = 0.21).
Chronic Fentanyl: Duration of Effect of Acute Opioid with Fentanyl on Board
The duration of the decrease in minute ventilation and peak inspiratory flow elicited by an i.v. heroin or fentanyl injection was analyzed by one-way ANOVA separated by group and sex (Table 3). The analysis was separated by sex since there were significant sex effects on the three-way ANOVA (Supplemental Table 9). In females, heroin caused a reduction of minute ventilation that lasted 40 minutes in the saline-treated group and 25 minutes in the chronic fentanyl-treated group. Fentanyl lasted 15 minutes in the saline-treated group and 5 minutes in the chronic fentanyl-treated group. In males, heroin’s effects lasted 25 minutes (chronic saline group) and 10 minutes (chronic fentanyl group), whereas fentanyl lasted 10 minutes (chronic saline group) and 5 minutes (chronic fentanyl group). Similar effects were seen in peak inspiratory flow, with heroin lasting 65 minutes in the chronic saline group and 15 minutes in the chronic fentanyl group, in both males and females. Fentanyl lasted 10 minutes in males and females in the chronic fentanyl group and lasted 20 (female) or 15 (male) minutes in the chronic saline group.
TABLE 3.
Duration of effect of acute heroin or fentanyl with chronic fentanyl exposure in minutes
| Duration (Min) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Acute Drug | Chronic Treatment | Sex | Minute Ventilation | Tidal Volume | Frequency | Peak Inspiratory Flow | Inspiratory Time | Apneic Pause |
| Heroin | Fentanyl | Female | 10a | 10a | 0 | 15a | 15b | 10b |
| Male | 10a | 5a | NA | 15a | 25b | 15b | ||
| Saline | Female | 40a | 0 | 50a,c | 65a | 65b | 15b | |
| Male | 25a | 0 | 65a | 65a | 65b | 25b | ||
| Fentanyl | Fentanyl | Female | 5a | 5a | 0 | 10a | 10b | 10b |
| Male | 5a | 5a | 0 | 10a | 10b | 10b | ||
| Saline | Female | 15a | NA | 20a | 20a | 45b | 15b | |
| Male | 10a | 5a | 15a | 15a | 20b | 5b | ||
NA, not applicable.
Post hoc comparisons following one-way ANOVA separated by sex and dose.
aP < 0.05, decreased from baseline (direction of respiratory depression for minute ventilation, tidal volume, frequency, and peak inspiratory flow).
bP < 0.05, increased from baseline (direction of respiratory depression for inspiratory time and apneic pause).
cOnset delayed by 5 minutes.
Chronic Fentanyl: Time-Course of Acute Opioid Effects in Fentanyl Withdrawal/Abstinence
The decreases in minute ventilation and peak inspiratory flow elicited by heroin (600 µg/kg, i.v.) during the 60 minutes postadministration in female and male rats tested during withdrawal/abstinence (4 to 5 days) after chronic exposure to saline or fentanyl are shown in Figs. 8A and 9A.
Fig. 8.
Withdrawal from chronic fentanyl exposure affected minute ventilation overshoot response to acute heroin and fentanyl in males but not females. After 4 to 5 days of withdrawal/abstinence from continuous exposure to saline (n = 10 females, 12 males) or fentanyl (n = 10 females, 10 males), rats received an i.v. injection of heroin (600 µg/kg; n = 9 females, 11 males) or fentanyl (25 µg/kg; n = 11 females, 10 males) during two tests 24 hour apart. Heroin induced similar respiratory depression across both sex and treatment, but males in withdrawal from chronic fentanyl had significantly higher overshoot effects than saline-treated males and chronic fentanyl-treated females (A). Maximal decrease in minute ventilation was similar (∼65%) across chronic drug treatment and sex (B). Male rats have significantly higher overshoot effects from fentanyl administration compared with females following a brief respiratory depression, with no differences among treatment groups (C). Maximal decrease in peak inspiratory flow following fentanyl administration was similar (∼70%) across chronic drug treatment and sex (D). Data are expressed as mean ± S.D. in percentage of baseline in 5-minute bins over −20 to 60 minutes postadministration (A and C) or as the lowest value within 25 minutes postadministration (B and D). Dashed line indicates average baseline measure, *P < 0.05, male versus female. $P < 0.05, male chronic fentanyl versus saline. @P < 0.05, male chronic fentanyl versus female chronic fentanyl.
Fig. 9.
Withdrawal from chronic fentanyl exposure affected peak inspiratory flow overshoot response to acute heroin and fentanyl in males but not females. After 4 to 5 days of withdrawal/abstinence from continuous exposure to saline (n = 10 females, 12 males) or fentanyl (n = 10 females, 10 males), rats received an i.v. injection of heroin (600 µg/kg; n = 9 females, 11 males) or fentanyl (25 µg/kg; n = 11 females, 10 males) during two tests 24 hour apart. Heroin induced similar respiratory depression across both sex and treatment; however, males in withdrawal from chronic fentanyl had significantly higher overshoot effects than saline-“withdrawing” males and fentanyl-withdrawing females (A). Maximal decrease in minute ventilation was similar (∼55%) across chronic drug treatment and sex (B). During withdrawal, chronic fentanyl-treated male rats had significantly higher overshoot effects from acute fentanyl administration compared with chronic fentanyl-treated females, following a brief respiratory depression, whereas there were no differences compared with or among saline treatment (C). Maximal decrease in peak inspiratory flow following fentanyl administration was similar (∼55%) across chronic drug treatment and sex (D). Data are expressed as mean ± S.D. in percentage of baseline in 5-minute bins over −20 to 60 minutes postadministration (A and C) or as the lowest value within 25 minutes postadministration (B and D). Dashed line indicates average baseline measure, $P < 0.05, male chronic fentanyl versus saline. #P < 0.05, female chronic fentanyl versus saline. @P < 0.05, chronic fentanyl female versus male.
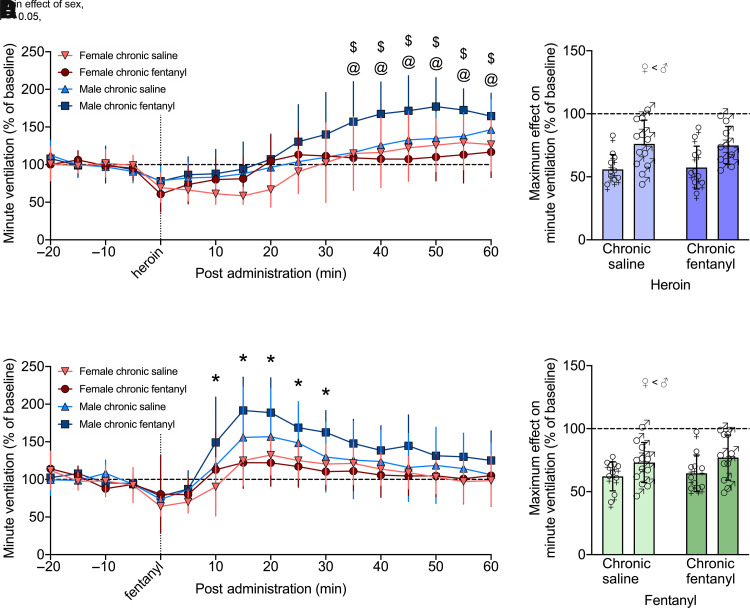
Three-way RM-ANOVA for minute ventilation from 0 to 60 minutes post-heroin administration showed a main effect of sex (F1,38 = 9.276, P = 0.004; female < male) and time (F12,456 = 54.112, P < 0.0001), indicating a decrease in minute ventilation following the heroin injection that dissipated over time. There was a group × time × sex interaction (F12,456 = 4.504, P < 0.0001), indicating that the duration of effect was group and sex dependent. Post hoc comparisons indicated a significant increase (overshoot) in minute ventilation from 35 to 65 minutes in males in the chronic fentanyl-exposed group when compared with chronic fentanyl-exposed females and saline-exposed males at the same time points (P < 0.05).
Similar effects were observed for peak inspiratory flow, with a main effect of time (F12,456 = 53.332, P < 0.0001), indicating a decrease in peak inspiratory flow following heroin injection that dissipated over time. There was a sex × group × time interaction (F12,456 = 4.122, P < 0.0001), indicating that the duration of effect is group and sex dependent. Post hoc comparisons indicated a significant increase (overshoot) in peak inspiratory flow from 40 to 60 minutes in males in the chronic fentanyl-exposed group when compared with chronic fentanyl-exposed females and saline-exposed males at the same time points (P < 0.05).
Fentanyl (25 µg/kg, i.v.) also elicited decreases in minute ventilation and peak inspiratory flow in both female and male rats (Figs. 8C and 9C). Three-way RM-ANOVA for minute ventilation from 0 to 60 minutes post-fentanyl administration showed a main effect of sex (F1,38 = 7.647, P = 0.009; female < male) and time (F12,456 = 41.865, P < 0.000), indicating a decrease in minute ventilation following the fentanyl injection that dissipated over time independently of group. There was a sex × time interaction (F12,456 = 3.583, P < 0.0001). Post hoc comparisons showed that males and females differed from 10 to 30 minutes postadministration (overshoot; P < 0.05).
Effects on peak inspiratory flow showed a main effect of sex (F1,38 = 7.240, P = 0.011; female < male) and a main effect of time (F12,456 = 52.406, P < 0.000), indicating a decrease in peak inspiratory flow after fentanyl injection that dissipated over time. There was a group × sex × time interaction (F12,456 = 2.296, P = 0.008). Post hoc comparisons indicated that chronic fentanyl-exposed males had higher peak inspiratory flow than chronic fentanyl-exposed females from 15 to 55 minutes post-fentanyl administration (overshoot; P < 0.05). Effects on tidal volume, frequency, inspiratory time, and apneic pause were similar, as show in Supplemental Figs. 7 and 8.
Chronic Fentanyl: Maximum Effect of Acute Opioid in Fentanyl Withdrawal/Abstinence
The maximum decrease in minute ventilation elicited by heroin (600 µg/kg, i.v.) within 25 minutes postadministration in female and male rats is shown in Fig. 8B. The two-way RM-ANOVA showed a main effect of sex (F1,38 = 14.569, P = 0.001; female < male) but not of group (F1,38 = 0.001, P = 0.981) and no interaction (F1,38 = 0.078, P = 0.781). The maximum decrease in minute ventilation elicited by fentanyl (25 µg/kg, i.v.) within 25 minutes postadministration in female and male rats is shown in Fig. 8D. The two-way RM-ANOVA showed a main effect of sex (F1,38 = 6.545, P = 0.015; female < male) but not of group (F1,38 = 0.445, P = 0.509) and no interaction (F1,38 = 0.027, P = 0.870).
The maximum decrease in peak inspiratory flow elicited by heroin (600 µg/kg, i.v.) within 25 minutes postadministration in female and male rats is shown in Fig. 9B. The two-way RM-ANOVA showed a main effect of sex (F1,38 = 4.867, P = 0.034; female < male) but not group (F1,38 = 0.069, P = 0.794) and no interaction (F1,38 = 0.872, P = 0.356). The maximum decrease in peak inspiratory flow elicited by fentanyl (25 µg/kg, i.v.) within 25 minutes postadministration in female and male rats is shown in Fig. 9D. The two-way RM-ANOVA did not show a main effect of sex (F1,38 = 3.192, P = 0.082) or group (F1,38 = 0.002, P = 0.963) and no interaction (F1,38 = 0.081, P = 0.778).
Chronic Fentanyl: Duration of Acute Opioid Effects in Fentanyl Withdrawal/Abstinence
The duration of the decrease in minute ventilation and peak inspiratory flow elicited by a heroin or fentanyl injection was analyzed by one-way ANOVA separated by group and sex (Table 4). The analysis was separated by sex since there were significant sex effects on the three-way ANOVA (Supplemental Table 11). Shorter duration of effect was observed in post hoc comparisons in the group with chronic fentanyl exposure. Specifically in females, heroin caused a reduction of minute ventilation that lasted 25 minutes in the saline-treated group and 10 minutes in the chronic fentanyl group. Fentanyl lasted 10 minutes in the saline group and 0 minutes in the chronic fentanyl group. In males, heroin effects lasted 10 minutes (saline) and 0 minutes (chronic fentanyl), whereas fentanyl lasted 5 minutes in the saline group and 0 minutes in the chronic fentanyl group. Similar effects were seen in peak inspiratory flow, with heroin lasting 30 minutes (male) to 35 minutes (female) in the saline group and 20 minutes (male and female) in the chronic fentanyl group. Fentanyl lasted 10 minutes (male and female) in the chronic fentanyl group and 10 minutes (male) to 15 minutes (female) in the saline group.
TABLE 4.
Duration of effect of acute heroin and fentanyl following chronic fentanyl withdrawal in minutes
| Duration (Min) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Acute Drug | Chronic Treatment | Sex | Minute Ventilation | Tidal Volume | Frequency | Peak Inspiratory Flow | Inspiratory Time | Apneic Pause |
| Heroin | Fentanyl | Female | 10a | 5a | 15a,b | 20a | 20b,c | NA |
| Male | 0 | 0 | 0 | 20a | 40b | NA | ||
| Saline | Female | 25a | 5a | 20a,b | 35a | 45c | 20c | |
| Male | 10a | 5a | NA | 30a | 50c | NA | ||
| Fentanyl | Fentanyl | Female | 0 | 5a | 10a | 10a | 15c | 5b,c |
| Male | 0 | 0 | 5a,b | 10a | 15c | 10c | ||
| Saline | Female | 10a | 0 | 15a | 15a | 15c | 15c | |
| Male | 5a | 5a | 5a,b | 10a | 15c | 10c | ||
NA, not applicable.
Post hoc comparisons following one-way ANOVA separated by sex and dose.
aP < 0.05, decreased from baseline (direction of respiratory depression for minute ventilation, tidal volume, frequency, and peak inspiratory flow).
bLate onset.
cP < 0.05, increased from baseline (direction of respiratory depression for inspiratory time and apneic pause).
Discussion
The present study characterized the ventilatory depression induced by heroin and fentanyl in female and male Long-Evans rats across several doses and exposure regimens. We found that heroin, compared with vehicle, induced prolonged (45–60 minutes) respiratory depression of greater magnitude in females than males that was characterized by 1) decreases in frequency, tidal volume, minute ventilation, and peak inspiratory flow, and 2) increased inspiratory time and apneic pause. Fentanyl produced the same pattern of changes in breathing but with a shorter duration (15 minutes) and fewer differences between sexes. The maximum effect elicited by each dose of fentanyl and heroin was similar, but the duration of the effect increased with the dose. The repeated injections of single doses of heroin or fentanyl given 2 or 3 weeks apart elicited similar levels of respiratory depression, indicative of a lack of tolerance to respiratory depression, consistent with our initial hypothesis. However, chronic continuous exposure to fentanyl (subcutaneous) led to marked tolerance to acute fentanyl exposure and cross-tolerance to acute heroin exposure. This was characterized by a shorter duration of respiratory depression rather than a reduction in the maximum effect. Contrary to our initial hypothesis, females were more sensitive to the respiratory effects of heroin, and fentanyl to a lesser extent, than males as reflected by greater maximum effect on minute ventilation and peak inspiratory flow for both drugs and longer-lasting respiratory depressing effects (Figs. 4 and 5).
Opioid-induced respiratory depression is characterized by irregular, slow, shallow breaths (Pattinson, 2008). The rats in our study received relatively high doses of opioids (10 times higher than those that are typically used for intravenous self-administration studies) (Wade et al., 2015). Rhythm generation is particularly sensitive to opioids, and changes in respiratory pattern are observed at lower doses than changes in tidal volume (Hurle et al., 1982; Shook et al., 1990; Stucke et al., 2015). Note that most flow control parameters, such as tidal volume, were affected for fewer than 5 minutes, whereas the effect on most rhythm generation parameters, such as inspiratory time, lasted longer.
We observed respiratory depression following an intravenous infusion of either heroin or fentanyl. Heroin exhibited a longer period of effect than fentanyl. This is because intravenous fentanyl is cleared by rapid tissue distribution [even more pronounced in females (Ohtsuka et al., 2007)] and metabolism to norfentanyl (Schneider and Brune, 1986; Feierman, 2000; Smith, 2011). This metabolite exhibits no opioid-like activity, although it remains possible that norfentanyl elicits changes in breathing and cardiovascular parameters. In contrast, heroin is sequentially cleared by formation of MOR-active metabolites, which maintain the opioid effect of intravenous heroin for much longer (Rook et al., 2006). Although heroin is essentially a prodrug, with good central nervous system penetration, its MOR affinity is ∼10 times lower than that of its metabolites, and it is rapidly converted (t1/2 ∼3 minutes) into 6-monoacetylmorphine, which has similar MOR potency to morphine (Selley et al., 2001). Previous studies in our laboratory suggest that 6-monoacetylmorphine plays an important role in the immediate action of heroin (Schlosburg et al., 2013). 6-Monoacetylmorphine is then converted to morphine (t1/2 ∼20–30 minutes) (Darke and Duflou, 2016), and morphine is cleared by glucuronidation (t1/2 ∼3 to 4 hours) (Ritter et al., 2020). These differences in drug metabolism and distribution are reflected in our study by the prolonged effects of heroin over fentanyl.
Sex as a biologic variable is an important consideration for preclinical research (Clayton and Collins, 2014). To our knowledge, no previous nonhuman primate or rat studies have specifically compared opioid-induced respiratory depression between sexes. In mice, the direction of the difference on opioid-induced respiratory depression between sexes is genotype dependent (Alhaddad et al., 2013; Bubier et al., 2020). We observed significant differences in baseline respiration between males and females, which prompted us to transform our data based on each individual rat’s baseline breathing. However, we observed no sex differences in the duration or magnitude of respiratory depression in the fentanyl dose-response curve and few sex differences following intermittent fentanyl administration [namely peak inspiratory flow (test 3 over time), apneic pause (over time), and tidal volume (max effect)] and on the effect of chronic fentanyl administration on acute fentanyl-induced respiratory depression. These results are consistent with the lack of sex differences on the pharmacokinetic data following 30 µg/kg i.v. fentanyl administration to male and female rats of a similar weight range as those used here (Ohtsuka et al., 2007). For heroin, we observed an overall greater respiratory depression in female rats.
Studies of sex differences in opioid analgesia are mixed, with studies reporting either no sex differences or greater analgesia in male rodents and primates, which is the inverse of human studies (Craft, 2003; Pattinson, 2008). For pharmacokinetics, several animal studies reported no sex differences in plasma levels of morphine in rats or rhesus monkeys (Cicero et al., 1997; Negus and Mello, 1999), but female rats had higher levels of morphine-3-glucuronide than males following morphine administration (Doyle and Murphy, 2018). Another factor that can influence the response to opioids is the genetic background since different rat strains show different antinociception at the same doses (Woolfolk and Holtzman, 1995; Morgan et al., 1999). Altogether, our results suggest that female Long-Evans rats might have a narrower therapeutic window for heroin since more heroin is necessary to produce the same level of analgesia compared with males (Marchette et al., 2021), but the same dose of heroin leads to greater respiratory depression (present data).
We observed no evidence of tolerance in our within-subjects dose-response function when doses were administered 1 week apart. We also found a general lack of tolerance to respiratory depression that was induced by repeated single opioid injections given 2 to 3 weeks apart from each other (intermittent acute exposure), with some exceptions. Intermittent acute heroin induced decreases in frequency of breathing and increases in inspiratory time that were less severe in males compared with females. Intermittent acute fentanyl induced increases in inspiratory time that were less severe independently of sex. Unlike the intermittent administration studies (experiment 2), we observed robust tolerance when rats were chronically exposed to fentanyl (subcutaneous mini pumps, experiment 3). Chronic fentanyl exposure induced tolerance to an acute intravenous injection of fentanyl and cross-tolerance to heroin (Figs. 6 and 7). Although there is no change to the maximum effect, the duration of respiratory depression is two- to threefold shorter when fentanyl was on board.
Tolerance to respiratory depression may develop more slowly than tolerance to analgesia, resulting in a higher risk of overdose in people with experience using opioids (White and Irvine, 1999). Male mice subjected to continuous morphine exposure first developed tolerance to the analgesic effects and later to respiratory depressive effects (Hill et al., 2016). This slower rate in the development of tolerance to respiratory depression than analgesia may also occur in humans as opioid-tolerant individuals can experience acute respiratory depression even when administering their usual prescribed doses of injectable and oral opioids (Jolley et al., 2015). However, chronic use of fentanyl did lead to tolerance to respiratory depression in humans, but apneic events were still present with higher doses (Algera et al., 2021).
Naloxone (Narcan) is currently the only available drug to reverse opioid overdose. Although highly successful in reversing opioid overdose prior to the synthetic opioid era (Moss et al., 2020), the use of naloxone as a rescue agent has several drawbacks. Naloxone is a potent and competitive MOR antagonist that can rapidly reverse analgesia and precipitate opioid withdrawal in opioid-dependent individuals (Moss et al., 2020; Purssell et al., 2020) and is aversive/dysphoric in humans and rodents (Dahan et al., 2018; Carmack et al., 2019). The short half-life of naloxone increases the risk of secondary overdose from renarcotization by longer-acting opioids and may require higher, repeated doses to combat high-affinity MOR agonists, such as carfentanil (Moss and Carlo, 2019; Kang et al., 2022). Based on the present data, we will be able to test novel experimental compounds for their ability to reverse respiratory depression in a within-subjects design.
In summary, our findings indicate that repeated heroin or fentanyl administration reliably produces robust dose-dependent respiratory depression, and chronic continuous administration of opioids produces tolerance to the respiratory depressant effects of opioids. Females displayed more severe heroin-induced respiratory depression than males, with some evidence of greater magnitude of fentanyl-induced respiratory depression. Such data combined with decreased sensitivity to opioid-induced analgesia could suggest a decreased “therapeutic window” in female rats. Repeated intermittent administration of single doses at 1 week and greater intervals produced little tolerance in female or male Long-Evans rats. Thus, future studies with within-subjects designs, as outlined here with intermittent opioid administration, should allow better understanding of the magnitude of, and temporal changes in, ventilatory parameters that are elicited by fentanyl and heroin and will serve as an invaluable tool to search for novel compounds to combat the opioid overdose death crisis.
Acknowledgments
The authors thank Michael Arends for proofreading the manuscript and Claudio Zanettini for developing and providing support for the custom-made program for data extraction. All drugs used in this study were obtained from the National Institute on Drug Abuse Drug Supply Program.
Abbreviations
- CO2
carbon dioxide
- MOR
μ-opioid receptor
- O2
oxygen
- RM-ANOVA
repeated-measures ANOVA
Authorship Contributions
Participated in research design: Marchette, Carlson, Volkow, Hampson, Lewis, L.F. Vendruscolo, Koob.
Conducted experiments: Marchette, Carlson, Frye, Hastings, J.C.M. Vendruscolo, Mejias-Torres.
Performed data analysis: Marchette, Carlson, Frye, Hastings, L.F. Vendruscolo.
Wrote or contributed to the writing of the manuscript: Marchette, Carlson, Frye, Hastings, Hampson, Volkow, Lewis, L.F. Vendruscolo, Koob.
Footnotes
This work was supported by National Institutes of Health National Institute on Drug Abuse [Grant 1 U01-DA051373-01] (to S.J.L.) and Intramural Research Program. R.C.N.M. is a fellow of the Center for Compulsive Behavior of the National Institutes of Health.
The authors have declared no conflict of interest exists.
R.C.N.M and E.R.C. contributed equally to this work as first authors.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Algera MH, Olofsen E, Moss L, Dobbins RL, Niesters M, van Velzen M, Groeneveld GJ, Heuberger J, Laffont CM, Dahan A (2021) Tolerance to Opioid-Induced Respiratory Depression in Chronic High-Dose Opioid Users: A Model-Based Comparison With Opioid-Naïve Individuals. Clin Pharmacol Ther 109:637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaddad HCisternino SSaubamea BSchlatter JChiadmi FRisède PSmirnova MCochois-Guégan VTournier NBaud FJ, et al. (2013) Gender and strain contributions to the variability of buprenorphine-related respiratory toxicity in mice. Toxicology 305:99–108. [DOI] [PubMed] [Google Scholar]
- Back SE, Payne RL, Wahlquist AH, Carter RE, Stroud Z, Haynes L, Hillhouse M, Brady KT, Ling W (2011) Comparative profiles of men and women with opioid dependence: results from a national multisite effectiveness trial. Am J Drug Alcohol Abuse 37:313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubier JA, He H, Philip VM, Roy T, Hernandez CM, Bernat R, Donohue KD, O’Hara BF, Chesler EJ (2020) Genetic variation regulates opioid-induced respiratory depression in mice. Sci Rep 10:14970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmack SA, Keeley RJ, Vendruscolo JCM, Lowery-Gionta EG, Lu H, Koob GF, Stein EA, Vendruscolo LF (2019) Heroin addiction engages negative emotional learning brain circuits in rats. J Clin Invest 129:2480–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmack SAVendruscolo JCMAdrienne McGinn MMiranda-Barrientos JRepunte-Canonigo VBosse GDMercatelli DGiorgi FMFu YHinrich AJ, et al. (2022) Corticosteroid sensitization drives opioid addiction. Mol Psychiatry 27:2492–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Nock B, Meyer ER (1997) Sex-related differences in morphine’s antinociceptive activity: relationship to serum and brain morphine concentrations. J Pharmacol Exp Ther 282:939–944. [PubMed] [Google Scholar]
- Clayton JA, Collins FS (2014) Policy: NIH to balance sex in cell and animal studies. Nature 509:282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM (2003) Sex differences in opioid analgesia: “from mouse to man”. Clin J Pain 19:175–186. [DOI] [PubMed] [Google Scholar]
- Dahan A, Sarton E, Teppema L, Olievier C (1998) Sex-related differences in the influence of morphine on ventilatory control in humans. Anesthesiology 88:903–913. [DOI] [PubMed] [Google Scholar]
- Dahan A, van der Schrier R, Smith T, Aarts L, van Velzen M, Niesters M (2018) Averting opioid-induced respiratory depression without affecting analgesia. Anesthesiology 128:1027–1037. [DOI] [PubMed] [Google Scholar]
- Darke S, Duflou J (2016) The toxicology of heroin-related death: estimating survival times. Addiction 111:1607–1613. [DOI] [PubMed] [Google Scholar]
- Doyle HH and Murphy AZ (2018) Sex-dependent influences of morphine and its metabolites on pain sensitivity in the rat. Physiol Behav 187:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierman DE (2000) The effect of paracetamol (acetaminophen) on fentanyl metabolism in vitro. Acta Anaesthesiol Scand 44:560–563. [DOI] [PubMed] [Google Scholar]
- Gellert VF, Holtzman SG (1978) Development and maintenance of morphine tolerance and dependence in the rat by scheduled access to morphine drinking solutions. J Pharmacol Exp Ther 205:536–546. [PubMed] [Google Scholar]
- Getsy PM, Baby SM, Gruber RB, Gaston B, Lewis THJ, Grossfield A, Seckler JM, Hsieh Y-H, Bates JN, Lewis SJ (2022a) S-Nitroso-L-Cysteine Stereoselectively Blunts the Deleterious Effects of Fentanyl on Breathing While Augmenting Antinociception in Freely-Moving Rats. Front Pharmacol 13:892307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getsy PMBaby SMMay WJBates JNEllis CRFeasel MGWilson CGLewis THJGaston BHsieh Y-H, et al. (2022b) L-cysteine methyl ester overcomes the deleterious effects of morphine on ventilatory parameters and arterial blood-gas chemistry in unanesthetized rats. Front Pharmacol 13:968378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getsy PM, Baby SM, May WJ, Lewis THJ, Bates JN, Hsieh Y-H, Gaston B, Lewis SJ (2022c) L-NAC reverses of the adverse effects of fentanyl infusion on ventilation and blood-gas chemistry. Biomed Pharmacother 153:113277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getsy PMBaby SMMay WJYoung APGaston BHodges MRForster HVBates JNWilson CGLewis THJ, et al. (2022d) D-Cysteine Ethyl Ester Reverses the Deleterious Effects of Morphine on Breathing and Arterial Blood-Gas Chemistry in Freely-Moving Rats. Front Pharmacol 13:883329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getsy PMYoung APBates JNBaby SMSeckler JMGrossfield AHsieh Y-HLewis THJJenkins MWGaston B, et al. (2022e) S-nitroso-L-cysteine stereoselectively blunts the adverse effects of morphine on breathing and arterial blood gas chemistry while promoting analgesia. Biomed Pharmacother 153:113436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getsy PM, Young AP, Grossfield A, Seckler JM, Wilson CG, Gaston B, Bates JN, Lewis SJ (2022f) D-cysteine ethyl ester and D-cystine dimethyl ester reverse the deleterious effects of morphine on arterial blood-gas chemistry and Alveolar-arterial gradient in anesthetized rats. Respir Physiol Neurobiol 302:103912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RLyndon AWithey SRoberts JKershaw YMacLachlan JLingford-Hughes AKelly EBailey CHickman M, et al. (2016) Ethanol reversal of tolerance to the respiratory depressant effects of morphine. Neuropsychopharmacology 41:762–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlé MA, Mediavilla A, Flórez J (1982) Morphine, pentobarbital and naloxone in the ventral medullary chemosensitive areas: differential respiratory and cardiovascular effects. J Pharmacol Exp Ther 220:642–647. [PubMed] [Google Scholar]
- Janczewski WA, Feldman JL (2006) Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol 570:407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolley CJ, Bell J, Rafferty GF, Moxham J, Strang J (2015) Understanding heroin overdose: A study of the acute respiratory depressant effects of injected pharmaceutical heroin. PLoS One 10:e0140995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YO’Conor KAKelleher ACRamsey JBakhoda AEisenberg SMZhao WStodden TPearson TDGuo M, et al. (2022) Naloxone’s dose-dependent displacement of [11C]carfentanil and duration of receptor occupancy in the rat brain. Sci Rep 12:6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo CY, Eikermann M (2011) Respiratory effects of opioids in perioperative medicine. Open Anesthesiol J 5:23–34. [Google Scholar]
- Lewis THJ, May WJ, Young AP, Bates JN, Baby SM, Getsy PM, Ryan RM, Hsieh Y-H, Seckler JM, Lewis SJ (2022) The ventilatory depressant actions but not the antinociceptive effects of morphine are blunted in rats receiving intravenous infusion of L-cysteine ethyl ester. Biomed Pharmacother 156:113939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchette RCN, Gregory-Flores A, Tunstall BJ, Carlson ER, Jackson SN, Sulima A, Rice KC, Koob GF, Vendruscolo LF (2021) κ-Opioid receptor antagonism reverses heroin withdrawal-induced hyperalgesia in male and female rats. Neurobiol Stress 14:100325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon G, Horner RL (2019) Electrocortical changes associating sedation and respiratory depression by the opioid analgesic fentanyl. Sci Rep 9:14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Cook CD, Picker MJ (1999) Sensitivity to the discriminative stimulus and antinociceptive effects of μ opioids: role of strain of rat, stimulus intensity, and intrinsic efficacy at the μ opioid receptor. J Pharmacol Exp Ther 289:965–975. [PubMed] [Google Scholar]
- Moss RB, Carlo DJ (2019) Higher doses of naloxone are needed in the synthetic opiod era. Subst Abuse Treat Prev Policy 14:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss RB, Pryor MM, Baillie R, Kudrycki K, Friedrich C, Reed M, Carlo DJ (2020) Higher naloxone dosing in a quantitative systems pharmacology model that predicts naloxone-fentanyl competition at the opioid mu receptor level. PLoS One 15:e0234683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie E (1999) CO2, brainstem chemoreceptors and breathing. Prog Neurobiol 59:299–331. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK (1999) Opioid antinociception in ovariectomized monkeys: comparison with antinociception in males and effects of estradiol replacement. J Pharmacol Exp Ther 290:1132–1140. [PubMed] [Google Scholar]
- Ohtsuka H, Fujita K, Kobayashi H (2007a) Pharmacokinetics of fentanyl in male and female rats after intravenous administration. Arzneimittelforschung 57:260–263. [DOI] [PubMed] [Google Scholar]
- Pattinson KTS (2008) Opioids and the control of respiration. Br J Anaesth 100:747–758. [DOI] [PubMed] [Google Scholar]
- Pattinson KTS, Governo RJ, MacIntosh BJ, Russell EC, Corfield DR, Tracey I, Wise RG (2009) Opioids depress cortical centers responsible for the volitional control of respiration. J Neurosci 29:8177–8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR (2016) O2 and CO2 Detection by the Carotid and Aortic Bodies. In: Chemosensory Transduction (Zufall F, Munger SD eds) pp 321–338, Academic Press, Elsevier, London. [Google Scholar]
- Purssell RGodwin JMoe JBuxton JCrabtree AKestler ADeWitt CScheuermeyer FErdelyi SBalshaw R, et al. (2021) Comparison of rates of opioid withdrawal symptoms and reversal of opioid toxicity in patients treated with two naloxone dosing regimens: a retrospective cohort study. Clin Toxicol (Phila) 59:38–46. [DOI] [PubMed] [Google Scholar]
- Ritter J, Flower R, Henderson G, Loke YK, MacEwan D, Rang HP (2020) Rang & Dale’s Pharmacology, 9th ed, Elsevier, London. [Google Scholar]
- Romberg R, Sarton E, Teppema L, Matthes HWD, Kieffer BL, Dahan A (2003) Comparison of morphine-6-glucuronide and morphine on respiratory depressant and antinociceptive responses in wild type and μ-opioid receptor deficient mice. Br J Anaesth 91:862–870. [DOI] [PubMed] [Google Scholar]
- Rook EJ, Huitema ADR, Van Den Brink W, Van Ree JM, Beijnen JH(2006) Population Pharmacokinetics of Heroin and its Major Metabolites. Clinical Pharmacokinetics 45:401–417. [DOI] [PubMed] [Google Scholar]
- Sarton E, Teppema L, Dahan A (1999) Sex differences in morphine-induced ventilatory depression reside within the peripheral chemoreflex loop. Anesthesiology 90:1329–1338. [DOI] [PubMed] [Google Scholar]
- Saunders SE, Levitt ES (2020) Kölliker-Fuse/Parabrachial complex mu opioid receptors contribute to fentanyl-induced apnea and respiratory rate depression. Respir Physiol Neurobiol 275:103388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Vendruscolo LF, Bremer PT, Lockner JW, Wade CL, Nunes AAK, Stowe GN, Edwards S, Janda KD, Koob GF (2013) Dynamic vaccine blocks relapse to compulsive intake of heroin. Proc Natl Acad Sci USA 110:9036–9041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E, Brune K (1986) Opioid activity and distribution of fentanyl metabolites. Naunyn Schmiedebergs Arch Pharmacol 334:267–274. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Heyser CJ, Koob GF (1999) Differential expression of response-disruptive and somatic indices of opiate withdrawal during the initiation and development of opiate dependence. Behav Pharmacol 10:235–242. [DOI] [PubMed] [Google Scholar]
- Seckler JM, Grossfield A, May WJ, Getsy PM, Lewis SJ (2022) Nitrosyl factors play a vital role in the ventilatory depressant effects of fentanyl in unanesthetized rats. Biomed Pharmacother 146:112571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selley DE, Cao C-C, Sexton T, Schwegel JA, Martin TJ, Childers SR (2001) μ Opioid receptor-mediated G-protein activation by heroin metabolites: evidence for greater efficacy of 6-monoacetylmorphine compared with morphine. Biochem Pharmacol 62:447–455. [DOI] [PubMed] [Google Scholar]
- Shook JE, Watkins WD, Camporesi EM (1990) Differential roles of opioid receptors in respiration, respiratory disease, and opiate-induced respiratory depression. Am Rev Respir Dis 142:895–909. [DOI] [PubMed] [Google Scholar]
- Smith HS (2011) The metabolism of opioid agents and the clinical impact of their active metabolites. Clin J Pain 27:824–838. [DOI] [PubMed] [Google Scholar]
- Smith JC, Abdala APL, Koizumi H, Rybak IA, Paton JFR (2007) Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol 98:3370–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucke AG, Miller JR, Prkic I, Zuperku EJ, Hopp FA, Stuth EAE (2015) Opioid-induced Respiratory Depression Is Only Partially Mediated by the preBötzinger Complex in Young and Adult Rabbits In Vivo. Anesthesiology 122:1288–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Schlosburg JE, Misra KK, Chen SA, Greenwell TN, Koob GF (2011) Escalation patterns of varying periods of heroin access. Pharmacol Biochem Behav 98:570–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO, Koob GF (2015) Compulsive-like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology 40:421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhoer M, Bartlett SE, Whistler JL (2004) Opioid receptors. Annu Rev Biochem 73:953–990. [DOI] [PubMed] [Google Scholar]
- Walter LA, Bunnell S, Wiesendanger K, McGregor AJ (2022) Sex, gender, and the opioid epidemic: Crucial implications for acute care. AEM Educ Train 6 (Suppl 1):S64–S70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley JK (1983) Opioid receptors: autoradiography. Pharmacol Rev 35:69–83. [PubMed] [Google Scholar]
- Watson SJ, Akil H, Walker JM (1980) Anatomical and biochemical studies of the opioid peptides and related substances in the brain. Peptides 1 (Suppl 1):11–20.7243606 [Google Scholar]
- White JM, Irvine RJ (1999) Mechanisms of fatal opioid overdose. Addiction 94:961–972. [PubMed] [Google Scholar]
- Wilson N, Kariisa M, Seth P, Smith H 4th, Davis NL (2020) Drug and Opioid-Involved Overdose Deaths - United States, 2017-2018. MMWR Morb Mortal Wkly Rep 69:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfolk DR, Holtzman SG (1995) Rat strain differences in the potentiation of morphine-induced analgesia by stress. Pharmacol Biochem Behav 51:699–703. [DOI] [PubMed] [Google Scholar]