Abstract
Aberrant post-translational glycosylation is a well-established hallmark of cancer. Altered core fucosylation mediated by α-(1,6)-fucosyltransferase (Fut8) is one of the key changes in tumor glycan patterns that contributes to neoplastic transformation, tumor metastasis, and immune evasion. Increased Fut8 expression and activity are associated with many types of human cancers, including lung, breast, melanoma, liver, colorectal, ovarian, prostate, thyroid, and pancreatic cancer. In animal models, inhibition of Fut8 activity by gene knockout, RNA interference, and small analogue inhibitors led to reduced tumor growth/metastasis, downregulation of immune checkpoint molecules PD-1, PD-L1/2, and B7-H3, and reversal of the suppressive state of tumor microenvironment. Although the biologics field has long benefited tremendously from using FUT8−/− Chinese hamster ovary cells to manufacture IgGs with greatly enhanced effector function of antibody-dependent cellular cytotoxicity for therapy, it is only in recent years that the roles of Fut8 itself in cancer biology have been studied. Here, we summarize the pro-oncogenic mechanisms involved in cancer development that are regulated by Fut8-mediated core fucosylation, and call for more research in this area where modifying the activity of this sole enzyme responsible for core fucosylation could potentially bring rewarding surprises in fighting cancer, infections, and other immune-related diseases.
Keywords: glycosylation, core fucosylation, Fut8, cancer, immunotherapy
Core fucosylation catalyzed by Fut8 plays a critical role in growth factor signaling, cancer cell metastasis, and immune function regulation. Apart from industrial attention on knocking out FUT8 in CHO cells for producing afucosylated therapeutic IgGs with enhanced ADCC/ADCP, the enzyme itself is a potential therapeutic target for cancer immunotherapy.
INTRODUCTION
Recently, an all-embracing term, AntibodyPlus, was proposed by the Chinese Antibody Society for any therapeutics with an antibody component carrying an effector module, such as antibody-drug conjugates (ADCs), bispecific antibodies, chimeric antigen receptor T cells, as well as many other complex modalities [1]. As glycosylation on the fragment crystallizable (Fc) region of immunoglobulin G (IgG) significantly influences the interaction of antibodies with Type I and Type II Fc receptors [2], glyco-engineered antibodies having enhanced effector functions are certainly the simplest form in the realm of AntibodyPlus, and they are among the forerunners as the next generation antibody therapeutics.
As of 30 June 2022, 162 antibodies or Fc-fusion proteins have been approved by regulatory agencies across the globe [3]. The great majority of marketed therapeutic antibodies are produced in the Food and Drug Administration (FDA)-approved, industry-preferred Chinese hamster ovary (CHO) cells [3]. Although CHO cells have highly appealing advantages in supporting large-scale serum-free fermentation to produce high quality antibody products, the glycosylation profiles of CHO cells are, however, different from those of human origin. For example, human proteins have terminal sialic acid attached to galactose in α-2,6-, α-2,3-, and α-2,8-linkages, predominantly α-2,6-linkage, whereas CHO cells lack α-2,6-sialyltransferase (St6gal1) and only express α-2,3-sialyltransferase [4, 5]. Sialylation plays critical roles in the half-life and efficacy of therapeutic glycoproteins. The asialoglycoprotein receptor on hepatocytes recognizes exposed terminal galactose on non-sialylated N-glycans and mediates uptake and quick clearance of asialylated proteins from circulation [6, 7]. The conventional CHO-K1 cells not transfected with exogenous ST6GAL1, such as CHOZN® GS CHO cells, have low abundance of complex glycans, and low to undetectable sialylated glycans, on glycoproteins including antibodies [8]. On the contrary, hypersialylation of IgG can significantly increase serum half-life by up to 9-fold [9]. Moreover, the anti-inflammatory activity of sialylated IgG has been broadly described [10–14], and α-2,6-linked sialic acid on intravenous immunoglobulin (IVIG) and on its recombinant equivalent is the active moiety that mediates IVIG’s anti-inflammatory effects [10, 15]. Thus, continuous efforts in glyco-engineering of CHO host cell lines are necessary for the development of “bio-better” protein therapeutics with human-like glycoprofiles and desired bioactivities [8].
Apart from sialylation on antibody products, IgG fucosylation is another major focal point of the biopharma industry. For the purpose of this review, we will first introduce the industrial application of targeting α-(1,6)-fucosyltransferase (FUT8) in the producing cell lines for manufacturing afucosylated therapeutic antibodies whose mechanisms of actions (MOAs) rely on antibody-dependent cellular cytotoxicity and/or phagocytosis (ADCC and ADCP, respectively); then, we will discuss the recent findings on the unexpected roles of Fut8 itself in various aspects of immuno-oncology. Technologies centered on Fut8 modulation for CHO cell glyco-engineering could potentially be extended to new therapeutic and diagnostic avenues.
FUT8 DEPLETION IN ANTIBODY-PRODUCING CELLS FOR ENHANCED THERAPEUTIC INDEX OF ANTI-CANCER AFUCOSYLATED IGG
Application of afucosylated IgG is disease dependent
In cancer biology, ADCC and ADCP are oftentimes the dominant MOA for therapeutic IgG1 antibodies against tumor cells. Naturally, over 90–95% of normal human IgG1 in the serum have core fucosylation (Fig. 1) in the two biantennary complex-type N-linked oligosaccharides in their Fc region [16]. Yet, in a “less-is-more” fashion, human IgG1 lacking core fucose demonstrates 50-fold increased affinity toward the low affinity activating FcγR, hFcγRIIIA, and therefore exhibits 50–100-fold enhanced ADCC, resulting in much better therapeutic outcomes in cancer treatment [17]. Our own work also showed that afucosylated IgG possessing higher effector functions through the activating FcγRIIIA or FcγRIV in various mammals is a cross-species phenomenon [18]. This knowledge greatly facilitates the efficacy, toxicity, and MOA studies of the next-generation afucosylated therapeutic IgG and Fc fusion proteins directly in underrepresented animal models of human diseases.
Figure 1.
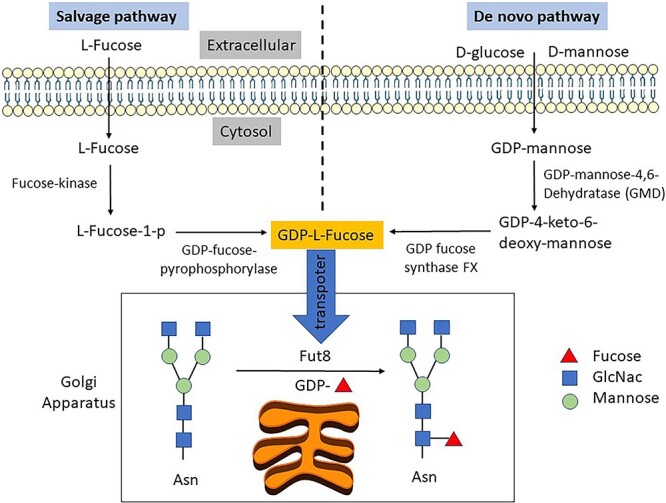
De novo and salvage pathways for GDP-L-fucose synthesis and core fucosylation by Fut8. GDP-L-fucose is generated in the cytosol by the more dominant de novo pathway and the salvage pathway. In the de novo pathway, D-glucose or D-mannose is synthesized into GDP-mannose, which is further transformed into GDP-L-fucose by GDP-mannose 4,6-dehydratase (GMD) and GDP-L-fucose synthase (also referred to as the FX protein, or more formally, GDP-4-keto-6-deoxy-D-mannose-3,5-epimerase-4-reductase). In the salvage pathway, free L-fucose derived from extracellular or lysosomal sources is reused and transformed by fucose kinase and GDP-fucose-pyrophosphorylase into GDP-L-fucose. In the Golgi apparatus, Fut8 transfers the fucose residue from GDP-L-fucose onto the innermost GlcNAc of an N-glycan to form an α-1,6 linkage in the process of core fucosylation, as opposed to terminal fucosylation (the addition of fucose to the terminal ends of glycans by other fucosyltransferases).
Many underrepresented mammalian models are highly valuable for infectious disease research. For example, ferrets are a well-established model for evaluating antiviral therapies and studying the pathogenesis and transmission of human respiratory viruses, including influenza [19, 20] and SARS-CoV-2 [21, 22]. Our functional identification of previously uncharacterized hFcγRIIIA homologues in ferrets, rabbits, and hamsters [18], and a mFcγRIV homologue in guinea pigs [23] that are hypersensitive to afucosylated IgG supports studies on the application of afucosylated neutralizing antibodies against Ebola [24] and on the mechanisms of enhanced disease severity by low-fucosylated IgG in dengue [25, 26] and COVID-19 [27–29].
Although more research is warranted on the supplementation of the exogenously transferred, or on the mitigation of the endogenously produced, afucosylated IgG in various disease settings, so far there are already seven afucosylated or low-fucosylated therapeutic hIgG1 approved by FDA/EMA [30], and > 20 are in clinical trials for cancer treatment [31]. For example, the anti-CD20 Obinutuzumab (or GA101) has only a < 30% reduction in fucosylation than Rituximab, but it has increased cell-mediated cytotoxicity and has been approved for treating patients with chronic lymphocytic leukemia or follicular lymphoma [31, 32]. Another example is Mogamulizumab (or KW-0761), an afucosylated IgG1 against CC chemokine receptor 4. Mogamulizumab is approved in Japan for the treatment of hematologic malignancies and cutaneous T-cell lymphoma (CTCL) [31]. We also reported in animal models that simple glyco-engineering and isotype switch of an otherwise non-effective anti-CD39 mouse IgG1 into the afucosylated mIgG2c format could impart this antibody, as compared with the mIgG1/mIgG2c counterparts with wild-type glycan, the strongest anti-tumor activities via mFcγRIV-mediated depletion of suppressive cells and inhibition of angiogenesis in the tumor microenvironment (TME) [33]. Hence, antibody afucosylation can significantly increase the therapeutic index, and reduce regimen dose-associated costs and side effects in cancer treatment. It has been proposed by the antibody therapeutics field that as long as the MOA is via ADCC or ADCP, future therapeutic IgG1 antibodies should all be afucosylated [34].
FUT8 targeting in antibody-producing cells facilitated by the use of fucose-binding lectins
The central piece of all the above successes is the technological ability to completely knock out the gene coding for Fut8, the sole enzyme in the fucosyltransferase family that catalyzes core fucosylation, i.e., the addition of α-1,6-fucose to the innermost N-acetyl-D-glucosamine (GlcNAc) residue of N-glycans (Fig. 1) [35]. Although the Japanese scientists first used the homologous recombination method to generate FUT8−/− CHO cells, they had to spend 1.5 years and screen >12 000 clones to finally obtain a CHO cell line with double allele knockout of FUT8 [36]. Nowadays, knocking out FUT8 in CHO cells and in established hybridoma cells with genome editing tools (TALEN or CRISPR), coupled with negative selection with fucose-binding lectins, can be achieved in merely a few weeks. FUT8 deficiency does not adversely affect the growth characteristics of CHO cell lines for antibody production, nor the in vivo half-life and complement-dependent cytotoxicity of the antibody products [36].
Traditionally, Lens culinaris agglutinin (LCA) has been used as a fucose-binding lectin for negative enrichment of FUT8−/− cells. The mature 245 aa LCA consists of α and β chains folding together as one subunit, and two subunits form into a dimer with a M.W. of 46 kDa [37]. LCA shows affinity to the core-fucosylated, agalactosylated, bi-antennary N-glycan (Ka = 1.1 × 105 M−1) [38]. More recently, a novel lectin (PhoSL) from the mushroom Pholiota squarrosa has been identified, which consists of only 40 amino acids (APVPVTKLVCDGDTYKCTAYLDFGDGRWVAQWDTNVFHTG) [39]. PhoSL binds exclusively to core α-1,6-fucosylated N-glycans (Ka = 1.2–5.0 × 105 M−1) and not to other types of fucosylated oligosaccharides, such as α-1,2-, α-1,3-, or α-1,4-fucosylated glycans [39]. Unlike LCA that recognizes only mono- and biantennary oligosaccharides, PhoSL binds not only to mono- or biantennary oligosaccharides but also to tri- or tetra-antennary oligosaccharides [39]. Our own practice also confirmed that PhoSL fused with hIgG1 Fc or mIgG2c Fc and expressed in FUT8−/− CHO cells is a potent reagent for staining cell surface α-1,6-fucosylated glycans (Supplementary Fig. 1), possibly due to its small size, substrate linkage specificity, and broad antennary structural profiles, as well as avidity enhancement by trimerization [40]. Afucosylated recombinant PhoSL-Fc expressed in FUT8−/− CHO cells has no non-specific binding to itself (via the glycan moiety of the Fc). Therefore, it should have wide applications as an essential reagent in monitoring cell surface α-1,6-fucosylated glycans as a surrogate for cellular Fut8 activities, as well as in detecting α-1,6-fucosylated biomarkers in diseases, especially in tumorigenesis [41].
ROLE OF FUT8 IN IMMUNE MODULATION AND CANCER DEVELOPMENT
Fut8 is widely expressed in mammalian tissues, but its expression profile is altered under pathological conditions. The upregulation of Fut8 mRNA, protein, and activity in various human cancers, including liver [42, 43], lung [44–46], breast [47–50], melanoma [51], colorectal [52–54], ovarian [55, 56], prostate [57, 58], thyroid [59], and pancreatic cancer [60] has been extensively reported. For example, in breast cancer patients, high Fut8 protein expression is correlated with lymphatic metastasis and stage status, whereas reduced Fut8 expression is correlated with disease-free survival and favorable overall survival (OS) [50]. A meta-analysis showed that lower Fut8 expression level was associated with OS in non-small cell lung cancer (NSCLC), breast cancer, diffuse large B cell lymphoma, and glioma, and with disease-free survival in NSCLC, breast cancer, and colorectal cancer, as well as with relapse-free survival in pancreatic ductal adenocarcinoma [61]. In terms of MOA, Fut8-mediated abnormal core fucosylation could affect growth factor signaling and intercellular communication, tumor cell–matrix interaction, migration, invasion, and metastasis, as well as immune regulation. Thus, manipulating Fut8 expression and activity could become a novel strategy to synergize with the current antibody-based cancer therapy.
Fut8 maintains high expression and protein stability of immune checkpoint molecules
Immune checkpoint inhibitors (ICIs) targeting programmed cell death protein 1 (PD-1) or cytotoxic T lymphocyte antigen-4 (CTLA-4), or both have revolutionized cancer therapy, and provided significant therapeutic benefits for subsets of cancer patients [62], which ultimately led to the 2018 Nobel Prize in Physiology or Medicine jointly awarded to Drs James P. Allison and Tasuku Honjo. In the era of antibody-based immunotherapy, many biopharmaceutical companies strived to push for their anti-PD-1, anti-PD-L1, anti-CTLA-4, and other ICIs onto the market. However, systemic administration of antagonist-type antibodies to block ligand engagement usually requires high dosages in vivo, accompanied with not only huge price tags but also oftentimes undesirable immune-related adverse events, some of which are severe and long-lasting [62, 63]. Current clinical trials are focused on enhancing antitumor therapeutic index through combinations of multiple ICIs and with chemotherapy that can cause tumor cell death via different MOAs. Despite all the success of ICI therapy, the basic mechanisms that govern the high expression of the inhibitory receptors on chronically activated T cells and on tumor cells remain to be elucidated. For instance, to date, most published papers studied PD-1 regulation mainly at the transcriptional level, and there were few studies focusing on PD-1 glycosylation, which may regulate its expression and function [64].
By using CRISPR-mediated gene knockout, Okada et al. reported that genes regulating the core fucosylation pathway are required for the expression of PD-1 [64]. In the murine PD-1 extracellular domain, all four N-glycosylation sites are highly core-fucosylated, but N49 and N74 sites are involved in the core fucosylation-dependent expression of PD-1. The absence of core fucosylation significantly enhances FBXO38 E3 ligase-mediated ubiquitination of PD-1, leading to its degradation in the proteasome [65, 66]. Particularly, PD-1 down-modulation can be reproduced solely by knocking out FUT8 [64]. Likewise, a metabolic fucosylation inhibitor 2-fluoro-L-fucose (2F-Fuc) also suppressed cell surface PD-1 expression, enhancing T cell proliferation and cytokine production [64]. Furthermore, blocking Fut8-dependent core fucosylation with 2F-Fuc strengthened anti-tumor immunity in a B16-OVA tumor model with adoptive transfer of OT-I anti-OVA CD8+ T cells [64, 65]. Similarly, genetic ablation of Fut8 in OT-I mice prevented the outgrowth of E.G7-OVA tumor cells by activating CD8+ CTLs [65]. When clinical lung carcinoma tissues were treated with Fut8 shRNA lentivirus, PD-1 expression detected by immunohistochemistry (IHC) was significantly decreased upon Fut8 knockdown, whereas granzyme B expression by IHC was dramatically increased [65]. Taken together, results from different groups suggested that inhibition of Fut8 could enhance CTL-mediated cytotoxicity via downregulated PD-1 expression.
Like PD-1, PD-L1 and PD-L2 are also strongly core-fucosylated [67, 68]. Their cell surface expression is stringently regulated by glycosylation and ubiquitination [67–69]. In lung adenocarcinoma, the level of core fucosylation is highly associated with lung tumor T stage and Tumor, Node, and Metastasis stage [65]. Simultaneous high expression of both Fut8 and PD-L1 correlated significantly with lower OS rate in lung adenocarcinoma patients [65]. Similarly, Fut8-mediated core fucosylation stabilizes PD-L2 by blocking ubiquitin-dependent lysosomal degradation, promoting its binding to PD-1 for immune evasion [68]. Therefore, it has been proposed that small-molecule inhibitors and natural food compounds may be used to target PD-1/PD-L1/2 glycosylation and ubiquitination, as an alternative means of mAb-based ICIs to downregulate PD-1/PD-L1/2 for cancer therapy [67–72].
Besides the immune checkpoint ligands of PD-1 (PD-L1/2), B7 homolog 3 protein (B7-H3, or CD276) is also an important immune checkpoint member of the B7 superfamily, and is preferentially expressed on a wide range of human solid tumors, which often correlates with negative prognosis and poor clinical outcomes in patients [73]. For instance, B7-H3 is highly expressed in triple-negative breast cancer patients, most of which are refractory to conventional ICI therapies. Huang et al. reported that Fut8 catalyzes the core fucosylation of this heavily glycosylated protein, and maintains its high expression [48]. Kaplan–Meier survival analysis revealed that the OS of patients with both Fut8highB7-H3high expression is significantly shorter than that of patients with both Fut8lowB7-H3low expression [48]. Knockdown of Fut8 or using core fucosylation inhibitor 2F-Fuc reversed B7-H3-mediated immunosuppression, enhanced T cell proliferation and activation, and together with anti-PD-L1, improved the therapeutic efficacy in B7-H3-positive TNBC tumors [48]. As current ICI therapies are only effective in subsets of patients with certain types of cancer, down-modulation of immune checkpoint molecules like PD-1/PD-L1/2, B7-H3, and possibility others through inhibiting Fut8 expression or activity constitutes a novel strategy of ICI, and may synergize with other approved ICIs.
Fut8 deregulates EGFR signaling, promotes a pro-oncogenic TME, and drives cancer cell metastasis
Cancer-associated fibroblasts (CAFs) are one of the pro-oncogenic components in the tumor stroma [74], and have been reported to support tumor progression by a variety of mechanisms [75]. Deregulated signaling of epidermal growth factor receptor (EGFR) has been observed in CAFs of many types of cancers [76–78]. Li et al. reported that Fut8-dependent EGFR core fucosylation enhances the cancer-promoting capacity of CAFs in NSCLC [46]. Fut8 is overexpressed in the CAFs of most lung adenocarcinoma cases, and mediates high levels of EGFR core fucosylation that is necessary for the cancer-promoting capacity of CAFs [46]. In CAFs, downregulation of Fut8 by shRNA led to reduced phosphorylation of EGFR and its downstream molecules such as ERK, AKT, and JAK, resulting in delayed growth of inoculated A549 tumors in nude mice [46].
Using a systems-based glycoproteomic analysis of matched primary and metastatic melanoma samples, Agrawal et al. identified 114 core-fucosylated membrane proteins common to three metastatic melanoma cell lines, most of which are involved in cell invasion and migration [51]. Among them, neural cell adhesion molecule L1 (L1CAM) was found to be a mediator of the pro-invasive effects of Fut8. L1CAM is a highly glycosylated protein known to regulate cell attachment, invasion, and migration in several cancers [79]. Cleavage of L1CAM by plasmin inhibits its ability to mediate cell invasion and metastatic outgrowth [80]. Higher levels of L1CAM cleavage were observed in Fut8-silenced cells, whereas Fut8 overexpression reduced L1CAM cleavage [51]. Thus, Fut8 drives cancer cell invasion and tumor metastasis, in part due to reduced cleavage of core-fucosylated L1CAM [51].
Similar glycoproteomics analysis was performed on two highly invasive breast cancer cell lines, and novel Fut8 targets and signaling networks critical for breast cancer cell invasiveness were identified [49]. Particularly, core fucosylation of integrin αvβ5 might promote breast cancer cell adhesion to vitronectin; and core fucosylation of interleukin-6 cytokine family signal transducer (IL6ST) could enhance cellular signaling to IL-6 and oncostatin M, two cytokines implicated in the breast cancer epithelial–mesenchymal transition (EMT) and metastasis [49]. In support of this notion, Fut8 ablation in MDA-MB-231 or Hs578T breast carcinoma cells significantly suppressed their migration and invasiveness [49]. In addition, heightened core fucosylation of TGF-β1 receptors (TGF-βRI and TGF-βRII) was found to accelerate EMT and promote breast cancer metastasis via E-cadherin downregulation and vimentin upregulation [47]. FUT8 is the only fucosyltransferase gene that is induced by TGF-β1, representing a pathological feed-forward regulatory mechanism linking Fut8 with EMT and breast cancer metastasis [47].
Despite remarkable advances in ICIs that have gained the FDA approval, outcomes for metastatic melanoma patients remain poor. In TNBC, most patients do not even respond to anti-PD-1/PD-L1 immunotherapy. Lack of response, initial response followed by relapse with treatment-refractory disease, and adverse side effects are common occurrences [81]. Therefore, molecules in common pathways essential for tumor metastasis, such as Fut8, are potential new targets for the development of innovative anti-cancer drugs. Table 1 summarizes Fut8-dependent regulation of molecules involved in tumorigenesis and resistance to cancer immunotherapy.
Table 1.
Correlation of higher Fut8 expression/activity with increase in levels of molecules involved in tumorigenesis and resistance to cancer immunotherapy
| Molecules | Mechanims via Fut8-dependent core fucosylation | References | |
|---|---|---|---|
| Immune-checkpoints | PD-1 ↑ | Prevents ubiquitin-mediated degradation in proteasome | [64–66] |
| PD-L1 ↑ | Prevents ubiquitin-mediated degradation in proteasome | [67, 69, 70] | |
| PD-L2 ↑ | Prevents ubiquitin-mediated degradation in proteasome | [68] | |
| B7-H3 ↑ | Prevents ubiquitin-mediated degradation in proteasome | [48] | |
| Growth factors | EGFR ↑ | Enhances the cancer-promoting capacity of CAFs | [46] |
| TGF-βRI/II ↑ | Accelerates EMT and promote metastasis | [47] | |
| Adhesion & signaling molecules | L1CAM ↑ | Blocks the cleavage of L1CAM by plasmin | [51] |
| integrin αvβ5 ↑ | Promotes cancer cell adhesion to vitronectin | [49] | |
| IL6ST ↑ | Enhances cellular signaling to IL-6 and oncostatin M, and promotes EMT and metastasis | [49] | |
↑ indicates increase either in protein levels or activities. EMT: epithelial–mesenchymal transition.
INHIBITION OF FUCOSYLATION AS A NEW CANCER THERAPY
In the Golgi apparatus, Fut8 catalyzes core fucosylation using guanosine 5′-diphospho-β-L-fucose (GDP-fucose) as a fucose donor. GDP-fucose is generated in the cytosol by two distinct pathways from L-fucose, i.e., the more dominant de novo synthesis pathway and the salvage pathway (Fig. 1). The de novo pathway accounts for 90% of GDP-fucose synthesis, whereas the salvage pathway utilizes free fucose derived from extracellular or lysosomal sources to synthesize GDP-fucose [82].
One strategy to develop fucosylation inhibitors is to use L-fucose analogues to deplete cells of GDP-fucose, which is used by all the fucosyltransferases to incorporate fucose into cellular glycans via the salvage pathway. Several analogues have shown inhibitory activities, e.g., 2-fluorofucose (2F-Fuc), 5-alkynylfucose, and their corresponding peracetylated derivatives [83]. Among them, 2F-Fuc is orally bioavailable and exhibited significant in vivo activities in inhibiting fucosylation of endogenously produced antibodies, afforded complete protection from tumor engraftment in a syngeneic tumor vaccine model, and delayed the outgrowth of tumor xenografts in immune-deficient mice [80], as well as in various other mouse tumor models [47, 84, 85]. Multiple MOAs, directly and indirectly on immune cells, tumor cells, and TME are involved. Besides the generation of afucosylated antibodies directly in vivo for enhanced ADCC/ADCP, adoptive transfer of T cells from inhibitor-treated tumor-bearing mice to untreated tumor-bearing mice was sufficient to delay tumor growth, suggesting enhanced T cell-mediated cellular immunity against tumor after inhibition of fucosylation [86]. In addition, diminished cell surface fucosylation reduced neutrophil extravasation from the bloodstream into the extravascular space [86], as fucose-containing polysaccharide sialyl-Lewis X (sLeX) on circulating neutrophils mediates binding to E-selectin (CD62) and P-selectin (CD62P) on endothelial cell membranes for extravasation [87]. Neutrophil counts in circulation were significantly increased in six of seven inhibitor-treated subjects [83]. In a First-In-Human, First-In-Class, Phase I trial of patients with advanced solid tumors, the fucosylation inhibitor SGN-2FF demonstrated proof-of-mechanism and preliminary antitumor activity but was associated with thromboembolic events leading to study termination [88]. More recently, newer inhibitors have been developed. For instance, A2FF1P and B2FF1P have four to seven times higher potency than SGN-2FF, possibly due to better retainment inside the cell, and more efficient conversion of GDP-Fuc2F [89]. The two inhibitors Fucotrim I and Fucotrim II were also reported to be more potent than SGN-2FF [90]. As fucosylation is part of the house-keeping glycosylation process, too-high doses or too-long treatment with fucosylation inhibitors will certainly have side effects. For cancer therapy, proper drug doses and treatment window are critical to achieve the best outcomes. The safety profiles of these new inhibitors await further clinical trials.
As all fucosyltransferases use GDP-fucose as a substrate, it should be anticipated that fucose-containing glycans other than the α-(1,6) fucosyl linkage would also be inhibited by these first-generation inhibitors. Future generations of fucosylation inhibitors most likely would need to be more tumor-specific, for example, targeting Fut8 and core fucosylation. Recent advances in deciphering the crystal structure of human Fut8 enzyme in complex with GDP and a biantennary complex N-glycan (G0) (PDB: 6TKV) have provided a structural basis for the rational design of small inhibitors blocking the enzymatic activity of Fut8 [91–93]. Fut8 inhibitor monotherapy and in combination with other ICIs for cancer treatment would be exciting areas to explore in the coming years.
CONCLUSION AND FUTURE PERSPECTIVE
As illustrated (Fig. 2), inhibition of fucosylation in general, and Fut8-dependent core fucosylation in particular, has the potential to enhance immune-mediated antitumor activity through modulation of both antibody-dependent cell-mediated cytotoxic responses and cell-mediated antitumor immunity across a broad range of cancers. This strategy also aims to restore proper tissue homeostasis and keep cancerous cells in check by reducing their metastatic potential.
Figure 2.
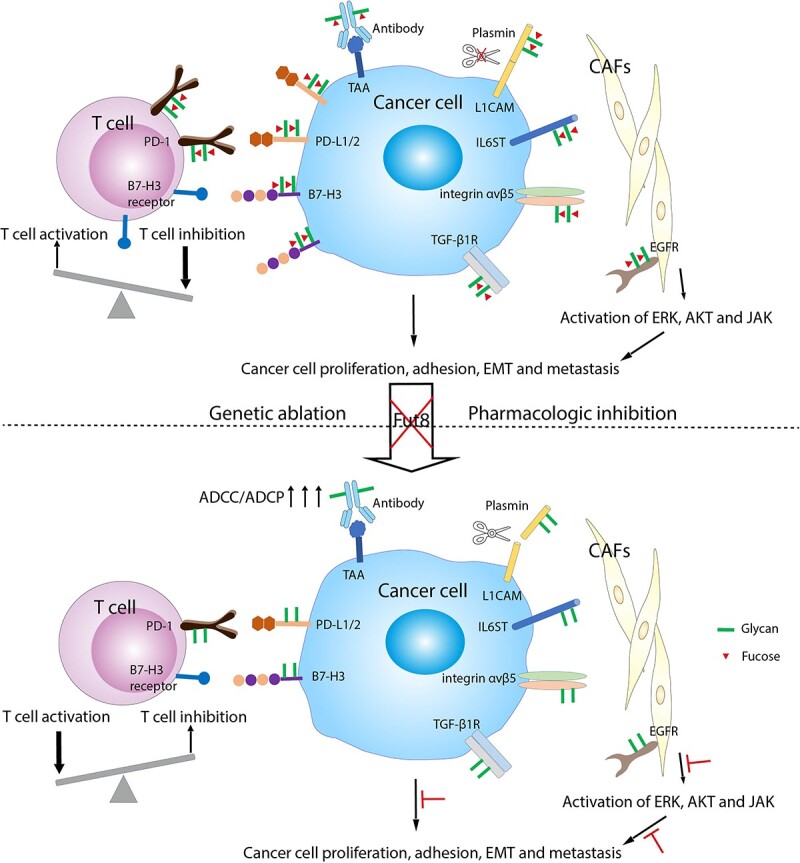
Pro-oncogenic mechanisms by Fut8-driven aberrant core fucosylation in cancer development, and their reversion by Fut8 inhibition. (Upper panel) Enhanced Fut8 expression and activities in TME inhibits T cell activation, drives cancer cell proliferation, EMT, and metastasis in multiple pathways. Core fucosylation on immune checkpoint molecules PD-1/L1/2 and B7-H3 stabilizes their protein expression by dampening ubiquitin-mediated degradation. T cells in the TME are thus more suppressed via inhibitory signals from PD-1 and an unknown receptor for B7-H3. On the cancer cell side, heightened core fucosylation on TGF-β1 receptors, integrin αvβ5 and IL6ST, for example, allows transmission of stronger signals for cancer cell growth, adhesion, and EMT. Higher levels of core fucosylation on L1CAM, which regulates cell attachment, invasion, and migration, protect the molecule from plasmin-mediated cleavage and promote cancer cell invasion and metastatic outgrowth. Although hosts may develop antibodies (Abs) recognizing tumor-associated antigens (TAA), their ADCC/ADCP activities are restricted by the core fucose on the N-glycan of the Abs. On CAFs, Fut8-dependent EGFR core fucosylation enhances the cancer-promoting capacity of CAFs by increased activation of ERK, AKT, and JAK pathways downstream of EGFR phosphorylation. (Lower panel) Upon Fut8 inhibition with genetic ablation, shRNA interference, or via pharmacological inhibitors, the above pro-oncogenic signaling can be reversed. Reduced core fucosylation leads to more degradation of PD-1/L1/2 and B7-H3, favoring T cell activation with more secretion of cytokines and granzyme B for direct cancer cell lysis. Afucosylated Abs can exert higher ADCC/ADCP activities against cancer cells. Reduced core fucosylation on TGF-β1 receptors, integrin αvβ5, IL6ST, L1CAM, and EGFR also leads to less cancer cell growth, EMT, and metastasis through various signaling pathways.
Although we did not touch in great details in this review on the roles of fucosylated biomarkers for cancer diagnosis and immunotherapy monitoring, this topic is yet another uncharted area that knowledge and reagents centered on modulating fucosylation and their catalyzing enzymes can contribute to cancer treatment. For instance, the level of core fucosylation of N-glycan in α-fetoprotein increases in hepatocellular carcinomas and carcinoma metastatic to the liver, but not in benign liver diseases, such as acute viral hepatitis, chronic hepatitis, or liver cirrhosis [94]. In another example, fucosylated α1-acid glycoprotein can serve as a biomarker to predict prognosis following tumor immunotherapy of cancer patients [95, 96]. Interested readers are advised to refer to the two excellent reviews on this topic [41, 97].
After decades of tumbling for the right strategy in cancer treatment, the recent success of immunotherapy with ICIs owes much to the paradigm shift from immune enhancement to immune normalization [98]. The concept of immune normalization is explained using proper flow and drainage of a pipe as an analogy for the antitumor immune response. When there is a blockage and insufficient flow of the pipe (antitumor immune responses), the immune enhancement approach is to increase the pressure of the flow with the risk of breaking the pipe (adverse effects). In contrast, the immune normalization approach is to unblock the pipe and restore the flow [98]. Similarly, aberrant glycosylation associated with cancer development, exemplified by heightened fucosylation in tumor sites and accompanied fucosylated biomarkers [41, 97], signals various forms of “pipe blockage”. Strategies targeting aberrant fucosylation, and especially the Fut8 enzyme, are to “decongest the pipe” and normalize the antitumor immune responses.
In terms of the modalities of targeting drugs, small chemical inhibitors or natural compounds that inhibit Fut8 enzymatic activities are relatively easy to develop or screen against, but their safety profiles and effects on the cellular fucosylation in normal tissues should be carefully weighed. Fut8 is a type II transmembrane protein mainly localized in the Golgi, but it is also found to be partially displayed on the cell surface [99], due to membrane trafficking. This opens the possibility of antibody-mediated, tumor site-specific targeting of cell surface Fut8 for lysosomal degradation [100] or trogocytosis, both of which can effectively reduce the cell surface protein content. Interestingly, for the latter strategy, compared with the wild-type antibody, afucosylated antibody demonstrates much higher trogocytosis activities through the activating FcγRs [33].
Started from about two decades ago in the seminal work of ablating its gene in antibody-producing cells for enhanced effector functions of therapeutic IgG1 antibodies [36], Fut8 has now transited from a supporting role in the grand drama of cancer immunotherapy to being a rising star entering the center of the stage. Undoubtedly, more synergies toward immune normalization are expected by fucosylation/Fut8 inhibitors and ICIs in cancer treatment in the near future.
Supplementary Material
Contributor Information
Changchuin Mao, Antagen Pharmaceuticals, Inc., Canton, MA 02021, USA.
Jun Li, Department of Biological Sciences, Florida International University, Miami, FL 33199, USA.
Lili Feng, Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, Shandong, 250117, P. R. China.
Wenda Gao, Antagen Pharmaceuticals, Inc., Canton, MA 02021, USA.
AUTHORS’ CONTRIBUTIONS
C.M. generated data presented in the Supplementary Figure on PhoSL-hIgG1 characterization and usage. J.L. and W.G. conceived the idea of screening Fut8 inhibitors from libraries of natural compounds, which formed the basis and initiated this work. W.G. wrote the manuscript, and L.F. made the illustrations for both figures. All the authors approved the final version of the manuscript.
FUNDING
This study was supported by Small Business Innovative Research grants 75N93022C00011 and 75N93022C00020 to Antagen.
CONFLICT OF INTEREST STATEMENT
C.M. is employed by Antagen Pharmaceuticals, Inc. W.G. is a co-founder and equity holder of Antagen. Antagen owns proprietary technologies to develop afucosylated antibodies and fusion proteins in its FUT8−/− CHO cell lines. J.L. and L.F. claim no conflict of interest.
ETHICS AND CONSENT STATEMENT
No patient consent is required.
ANIMAL ETHICS STATEMENT
Not applicable.
References
- 1. Zhu, Y, Wang, SS, Zhou, ZSet al. The emergence of AntibodyPlus: the future trend of antibody-based therapeutics. Antib Ther 2022; 5: 280–7. 10.1093/abt/tbac024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pincetic, A, Bournazos, S, DiLillo, DJet al. Type-I and type-II fc receptors regulate innate and adaptive immunity. Nat Immunol 2014; 15: 707–16. 10.1038/ni.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lyu, X, Zhao, Q, Hui, Jet al. The global landscape of approved antibody therapies. Antib Ther 2022; 5: 233–57. 10.1093/abt/tbac021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Takeuchi, M, Takasaki, S, Miyazaki, Het al. Comparative study of the asparagine-linked sugar chains of human erythropoietins purified from urine and the culture medium of recombinant Chinese hamster ovary cells. J Biol Chem 1988; 263: 3657–63. [PubMed] [Google Scholar]
- 5. Santell, L, Ryll, T, Etcheverry, Tet al. Aberrant metabolic sialylation of recombinant proteins expressed in Chinese hamster ovary cells in high productivity cultures. Biochem Biophys Res Commun 1999; 258: 132–7. 10.1006/bbrc.1999.0550. [DOI] [PubMed] [Google Scholar]
- 6. Schwartz, AL, Ashwell, G. The hepatic asialoglycoprotein receptor. CRC Crit Rev Biochem 1984; 16: 207–33. 10.3109/10409238409108716. [DOI] [PubMed] [Google Scholar]
- 7. Weigel, PH. Galactosyl and N-acetylgalactosaminyl homeostasis: a function for mammalian asialoglycoprotein receptors. BioEssays News Rev Mol Cell Dev Biol 1994; 16: 519–24. 10.1002/bies.950160713. [DOI] [PubMed] [Google Scholar]
- 8. Lin, N, Mascarenhas, J, Sealover, NRet al. Chinese hamster ovary (CHO) host cell engineering to increase sialylation of recombinant therapeutic proteins by modulating sialyltransferase expression. Biotechnol Prog 2015; 31: 334–46. 10.1002/btpr.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bas, M, Terrier, A, Jacque, Eet al. Fc sialylation prolongs serum half-life of therapeutic antibodies. J Immunol 2019; 202: 1582–94. 10.4049/jimmunol.1800896. [DOI] [PubMed] [Google Scholar]
- 10. Kaneko, Y, Nimmerjahn, F, Ravetch, JV. Anti-inflammatory activity of immunoglobulin G resulting from fc sialylation. Science 2006; 313: 670–3. 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 11. Anthony, RM, Ravetch, JV. A novel role for the IgG fc glycan: the anti-inflammatory activity of sialylated IgG fcs. J Clin Immunol 2010; 30: S9–14. 10.1007/s10875-010-9405-6. [DOI] [PubMed] [Google Scholar]
- 12. Washburn, N, Schwab, I, Ortiz, Det al. Controlled tetra-fc sialylation of IVIg results in a drug candidate with consistent enhanced anti-inflammatory activity. Proc Natl Acad Sci U S A 2015; 112: E1297–306. 10.1073/pnas.1422481112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ohmi, Y, Ise, W, Harazono, Aet al. Sialylation converts arthritogenic IgG into inhibitors of collagen-induced arthritis. Nat Commun 2016; 7: 11205. 10.1038/ncomms11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pagan, JD, Kitaoka, M, Anthony, RM. Engineered sialylation of pathogenic antibodies in vivo attenuates autoimmune disease. Cell 2018; 172: 564–77.e13. 10.1016/j.cell.2017.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anthony, RM, Wermeling, F, Karlsson, MCIet al. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci U S A 2008; 105: 19571–8. 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Haan, N, Reiding, KR, Driessen, Get al. Changes in healthy human IgG fc-glycosylation after birth and during early childhood. J Proteome Res 2016; 15: 1853–61. 10.1021/acs.jproteome.6b00038. [DOI] [PubMed] [Google Scholar]
- 17. Shields, RL, Lai, J, Keck, Ret al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem 2002; 277: 26733–40. 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 18. Mao, C, Near, R, Zhong, Xet al. Cross-species higher sensitivities of FcγRIIIA/FcγRIV to afucosylated IgG for enhanced ADCC. Antib Ther 2021; 4: 159–70. 10.1093/abt/tbab016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Albrecht, RA, Liu, W-C, Sant, AJet al. Moving forward: recent developments for the ferret biomedical research model. MBio 2018; 9: e01113–8. 10.1128/mBio.01113-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wong, J, Layton, D, Wheatley, AKet al. Improving immunological insights into the ferret model of human viral infectious disease. Influenza Other Respi Viruses 2019; 13: 535–46. 10.1111/irv.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim, Y-I, Kim, S-G, Kim, S-Met al. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe 2020; 27: 704–9.e2. 10.1016/j.chom.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beale, DJ, Shah, R, Karpe, AVet al. Metabolic profiling from an asymptomatic ferret model of SARS-CoV-2 infection. Metabolites 2021; 11: 327. 10.3390/metabo11050327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mao, C, Near, R, Gao, W. Identification of a Guinea pig Fcγ receptor that exhibits enhanced binding to Afucosylated human and mouse IgG. J Infect Dis Med 2017; 01: 102. 10.4172/2576-1420.1000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wec, AZ, Bornholdt, ZA, He, Set al. Development of a human antibody cocktail that deploys multiple functions to confer pan-ebolavirus protection. Cell Host Microbe 2019; 25: 39–48.e5. 10.1016/j.chom.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang, TT, Sewatanon, J, Memoli, MJet al. IgG antibodies to dengue enhanced for FcγRIIIA binding determine disease severity. Science 2017; 355: 395–8. 10.1126/science.aai8128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bournazos, S, Vo, HTM, Duong, Vet al. Antibody fucosylation predicts disease severity in secondary dengue infection. Science 2021; 372: 1102–5. 10.1126/science.abc7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Larsen, MD, deGraaf, EL, Sonneveld, MEet al. Afucosylated IgG characterizes enveloped viral responses and correlates with COVID-19 severity. Science 2021; 371: eabc8378. 10.1126/science.abc8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoepel, W, Chen, H-J, Geyer, CEet al. High titers and low fucosylation of early human anti-SARS-CoV-2 IgG promote inflammation by alveolar macrophages. Sci Transl Med 2021; 13: eabf8654. 10.1126/scitranslmed.abf8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chakraborty, S, Gonzalez, JC, Sievers, BLet al. Early non-neutralizing, afucosylated antibody responses are associated with COVID-19 severity. Sci Transl Med 2022; 14: eabm7853. 10.1126/scitranslmed.abm7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Golay, J, Andrea, AE, Cattaneo, I. Role of fc core fucosylation in the effector function of IgG1 antibodies. Front Immunol 2022; 13: 929895. 10.3389/fimmu.2022.929895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pereira, NA, Chan, KF, Lin, PCet al. The “less-is-more” in therapeutic antibodies: afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. MAbs 2018; 10: 693–711. 10.1080/19420862.2018.1466767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mössner, E, Brünker, P, Moser, Set al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood 2010; 115: 4393–402. 10.1182/blood-2009-06-225979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang, H, Feng, L, deAndrade, MPet al. Glycoengineered anti-CD39 promotes anticancer responses by depleting suppressive cells and inhibiting angiogenesis in tumor models. J Clin Invest 2022; 132: e157431. 10.1172/JCI157431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mimura, Y, Katoh, T, Saldova, Ret al. Glycosylation engineering of therapeutic IgG antibodies: challenges for the safety, functionality and efficacy. Protein Cell 2018; 9: 47–62. 10.1007/s13238-017-0433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schneider, M, Al-Shareffi, E, Haltiwanger, RS. Biological functions of fucose in mammals. Glycobiology 2017; 27: 601–18. 10.1093/glycob/cwx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yamane-Ohnuki, N, Kinoshita, S, Inoue-Urakubo, Met al. Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol Bioeng 2004; 87: 614–22. 10.1002/bit.20151. [DOI] [PubMed] [Google Scholar]
- 37. Foriers, A, Lebrun, E, Van Rapenbusch, Ret al. The structure of the lentil (Lens culinaris) lectin. Amino acid sequence determination and prediction of the secondary structure. J Biol Chem 1981; 256: 5550–60. [PubMed] [Google Scholar]
- 38. Tateno, H, Nakamura-Tsuruta, S, Hirabayashi, J. Comparative analysis of core-fucose-binding lectins from Lens culinaris and Pisum sativum using frontal affinity chromatography. Glycobiology 2009; 19: 527–36. 10.1093/glycob/cwp016. [DOI] [PubMed] [Google Scholar]
- 39. Kobayashi, Y, Tateno, H, Dohra, Het al. A novel core fucose-specific lectin from the mushroom Pholiota squarrosa. J Biol Chem 2012; 287: 33973–82. 10.1074/jbc.M111.327692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yamasaki, K, Yamasaki, T, Tateno, H. The trimeric solution structure and fucose-binding mechanism of the core fucosylation-specific lectin PhoSL. Sci Rep 2018; 8: 7740. 10.1038/s41598-018-25630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liao, C, An, J, Yi, Set al. FUT8 and protein core fucosylation in tumors: from diagnosis to treatment. J Cancer 2021; 12: 4109–20. 10.7150/jca.58268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Noda, K, Miyoshi, E, Uozumi, Net al. Gene expression of alpha1-6 fucosyltransferase in human hepatoma tissues: a possible implication for increased fucosylation of alpha-fetoprotein. Hepatology 1998; 28: 944–52. 10.1002/hep.510280408. [DOI] [PubMed] [Google Scholar]
- 43. Cheng, L, Gao, S, Song, Xet al. Comprehensive N-glycan profiles of hepatocellular carcinoma reveal association of fucosylation with tumor progression and regulation of FUT8 by microRNAs. Oncotarget 2016; 7: 61199–214. 10.18632/oncotarget.11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen, C-Y, Jan, Y-H, Juan, Y-Het al. Fucosyltransferase 8 as a functional regulator of nonsmall cell lung cancer. Proc Natl Acad Sci U S A 2013; 110: 630–5. 10.1073/pnas.1220425110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Honma, R, Kinoshita, I, Miyoshi, Eet al. Expression of fucosyltransferase 8 is associated with an unfavorable clinical outcome in non-small cell lung cancers. Oncology 2015; 88: 298–308. 10.1159/000369495. [DOI] [PubMed] [Google Scholar]
- 46. Li, F, Zhao, S, Cui, Yet al. α1,6-Fucosyltransferase (FUT8) regulates the cancer-promoting capacity of cancer-associated fibroblasts (CAFs) by modifying EGFR core fucosylation (CF) in non-small cell lung cancer (NSCLC). Am J Cancer Res 2020; 10: 816–37. [PMC free article] [PubMed] [Google Scholar]
- 47. Tu, C-F, Wu, M-Y, Lin, Y-Cet al. FUT8 promotes breast cancer cell invasiveness by remodeling TGF-β receptor core fucosylation. Breast Cancer Res 2017; 19: 111. 10.1186/s13058-017-0904-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huang, Y, Zhang, H-L, Li, Z-Let al. FUT8-mediated aberrant N-glycosylation of B7H3 suppresses the immune response in triple-negative breast cancer. Nat Commun 2021; 12: 2672. 10.1038/s41467-021-22618-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tu, C-F, Li, F-A, Li, L-Het al. Quantitative glycoproteomics analysis identifies novel FUT8 targets and signaling networks critical for breast cancer cell invasiveness. Breast Cancer Res 2022; 24: 21. 10.1186/s13058-022-01513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yue, L, Han, C, Li, Zet al. Fucosyltransferase 8 expression in breast cancer patients: a high throughput tissue microarray analysis. Histol Histopathol 2016; 31: 547–55. 10.14670/HH-11-693. [DOI] [PubMed] [Google Scholar]
- 51. Agrawal, P, Fontanals-Cirera, B, Sokolova, Eet al. A systems biology approach identifies FUT8 as a driver of melanoma metastasis. Cancer Cell 2017; 31: 804–19.e7. 10.1016/j.ccell.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Muinelo-Romay, L, Vázquez-Martín, C, Villar-Portela, Set al. Expression and enzyme activity of alpha(1,6)fucosyltransferase in human colorectal cancer. Int J Cancer 2008; 123: 641–6. 10.1002/ijc.23521. [DOI] [PubMed] [Google Scholar]
- 53. Noda, M, Okayama, H, Kofunato, Yet al. Prognostic role of FUT8 expression in relation to p53 status in stage II and III colorectal cancer. PLoS One 2018; 13: e0200315. 10.1371/journal.pone.0200315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Osumi, D, Takahashi, M, Miyoshi, Eet al. Core fucosylation of E-cadherin enhances cell-cell adhesion in human colon carcinoma WiDr cells. Cancer Sci 2009; 100: 888–95. 10.1111/j.1349-7006.2009.01125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lv, X, Song, J, Xue, Ket al. Core fucosylation of copper transporter 1 plays a crucial role in cisplatin-resistance of epithelial ovarian cancer by regulating drug uptake. Mol Carcinog 2019; 58: 794–807. 10.1002/mc.22971. [DOI] [PubMed] [Google Scholar]
- 56. Takahashi, T, Ikeda, Y, Miyoshi, Eet al. alpha1,6fucosyltransferase is highly and specifically expressed in human ovarian serous adenocarcinomas. Int J Cancer 2000; 88: 914–9. . [DOI] [PubMed] [Google Scholar]
- 57. Wang, X, Chen, J, Li, QKet al. Overexpression of α (1,6) fucosyltransferase associated with aggressive prostate cancer. Glycobiology 2014; 24: 935–44. 10.1093/glycob/cwu051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Höti, N, Yang, S, Hu, Yet al. Overexpression of α (1,6) fucosyltransferase in the development of castration-resistant prostate cancer cells. Prostate Cancer Prostatic Dis 2018; 21: 137–46. 10.1038/s41391-017-0016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ito, Y, Miyauchi, A, Yoshida, Het al. Expression of alpha1,6-fucosyltransferase (FUT8) in papillary carcinoma of the thyroid: its linkage to biological aggressiveness and anaplastic transformation. Cancer Lett 2003; 200: 167–72. 10.1016/s0304-3835(03)00383-5. [DOI] [PubMed] [Google Scholar]
- 60. Tada, K, Ohta, M, Hidano, Set al. Fucosyltransferase 8 plays a crucial role in the invasion and metastasis of pancreatic ductal adenocarcinoma. Surg Today 2020; 50: 767–77. 10.1007/s00595-019-01953-z. [DOI] [PubMed] [Google Scholar]
- 61. Ma, M, Han, G, Wang, Yet al. Role of FUT8 expression in clinicopathology and patient survival for various malignant tumor types: a systematic review and meta-analysis. Aging 2020; 13: 2212–30. 10.18632/aging.202239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jacob, JB, Jacob, MK, Parajuli, P. Review of immune checkpoint inhibitors in immuno-oncology. Adv Pharmacol 2021; 91: 111–39. 10.1016/bs.apha.2021.01.002. [DOI] [PubMed] [Google Scholar]
- 63. Johnson, DB, Nebhan, CA, Moslehi, JJet al. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol 2022; 19: 254–67. 10.1038/s41571-022-00600-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Okada, M, Chikuma, S, Kondo, Tet al. Blockage of core fucosylation reduces cell-surface expression of PD-1 and promotes anti-tumor immune responses of T cells. Cell Rep 2017; 20: 1017–28. 10.1016/j.celrep.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 65. Zhang, N, Li, M, Xu, Xet al. Loss of core fucosylation enhances the anticancer activity of cytotoxic T lymphocytes by increasing PD-1 degradation. Eur J Immunol 2020; 50: 1820–33. 10.1002/eji.202048543. [DOI] [PubMed] [Google Scholar]
- 66. Meng, X, Liu, X, Guo, Xet al. FBXO38 mediates PD-1 ubiquitination and regulates anti-tumour immunity of T cells. Nature 2018; 564: 130–5. 10.1038/s41586-018-0756-0. [DOI] [PubMed] [Google Scholar]
- 67. Li, C-W, Lim, S-O, Xia, Wet al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat Commun 2016; 7: 12632. 10.1038/ncomms12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Xu, Y, Gao, Z, Hu, Ret al. PD-L2 glycosylation promotes immune evasion and predicts anti-EGFR efficacy. J Immunother Cancer 2021; 9: e002699. 10.1136/jitc-2021-002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Horita, H, Law, A, Hong, Set al. Identifying regulatory posttranslational modifications of PD-L1: a focus on monoubiquitinaton. Neoplasia 2017; 19: 346–53. 10.1016/j.neo.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hsu, J-M, Li, C-W, Lai, Y-Jet al. Posttranslational modifications of PD-L1 and their applications in cancer therapy. Cancer Res 2018; 78: 6349–53. 10.1158/0008-5472.CAN-18-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hu, X, Wang, J, Chu, Met al. Emerging role of ubiquitination in the regulation of PD-1/PD-L1 in cancer immunotherapy. Mol Ther 2021; 29: 908–19. 10.1016/j.ymthe.2020.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cha, J-H, Yang, W-H, Xia, Wet al. Metformin promotes antitumor immunity via endoplasmic-reticulum-associated degradation of PD-L1. Mol Cell 2018; 71: 606–20.e7. 10.1016/j.molcel.2018.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Picarda, E, Ohaegbulam, KC, Zang, X. Molecular pathways: targeting B7-H3 (CD276) for human cancer immunotherapy. Clin Cancer Res 2016; 22: 3425–31. 10.1158/1078-0432.CCR-15-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. LeBleu, VS, Kalluri, R. A peek into cancer-associated fibroblasts: origins, functions and translational impact. Dis Model Mech 2018; 11: dmm029447. 10.1242/dmm.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Feng, B, Wu, J, Shen, Bet al. Cancer-associated fibroblasts and resistance to anticancer therapies: status, mechanisms, and countermeasures. Cancer Cell Int 2022; 22: 166. 10.1186/s12935-022-02599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pisano, A, Santolla, MF, De Francesco, EMet al. GPER, IGF-IR, and EGFR transduction signaling are involved in stimulatory effects of zinc in breast cancer cells and cancer-associated fibroblasts. Mol Carcinog 2017; 56: 580–93. 10.1002/mc.22518. [DOI] [PubMed] [Google Scholar]
- 77. Luo, H, Yang, G, Yu, Tet al. GPER-mediated proliferation and estradiol production in breast cancer-associated fibroblasts. Endocr Relat Cancer 2014; 21: 355–69. 10.1530/ERC-13-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yi, Y, Zeng, S, Wang, Zet al. Cancer-associated fibroblasts promote epithelial-mesenchymal transition and EGFR-TKI resistance of non-small cell lung cancers via HGF/IGF-1/ANXA2 signaling. Biochim Biophys Acta Mol Basis Dis 2018; 1864: 793–803. 10.1016/j.bbadis.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 79. Altevogt, P, Doberstein, K, Fogel, M. L1CAM in human cancer. Int J Cancer 2016; 138: 1565–76. 10.1002/ijc.29658. [DOI] [PubMed] [Google Scholar]
- 80. Valiente, M, Obenauf, AC, Jin, Xet al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 2014; 156: 1002–16. 10.1016/j.cell.2014.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zaretsky, JM, Garcia-Diaz, A, Shin, DSet al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med 2016; 375: 819–29. 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Becker, DJ, Lowe, JB. Fucose: biosynthesis and biological function in mammals. Glycobiology 2003; 13: 41R–53R. 10.1093/glycob/cwg054. [DOI] [PubMed] [Google Scholar]
- 83. Okeley, NM, Alley, SC, Anderson, MEet al. Development of orally active inhibitors of protein and cellular fucosylation. Proc Natl Acad Sci U S A 2013; 110: 5404–9. 10.1073/pnas.1222263110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhou, Y, Fukuda, T, Hang, Qet al. Inhibition of fucosylation by 2-fluorofucose suppresses human liver cancer HepG2 cell proliferation and migration as well as tumor formation. Sci Rep 2017; 7: 11563. 10.1038/s41598-017-11911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Park, S, Lim, J-M, Chun, JNet al. Altered expression of fucosylation pathway genes is associated with poor prognosis and tumor metastasis in non-small cell lung cancer. Int J Oncol 2020; 56: 559–67. 10.3892/ijo.2019.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Okeley, NM, Heiser, RA, Zeng, Wet al. Abstract 5551: SGN-2FF: a small-molecule inhibitor of fucosylation modulates immune cell activity in preclinical models and demonstrates pharmacodynamic activity in early phase 1 analysis. Cancer Res 2018; 78: 5551. 10.1158/1538-7445.AM2018-5551. [DOI] [Google Scholar]
- 87. Malý, P, Thall, A, Petryniak, Bet al. The alpha(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell 1996; 86: 643–53. 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- 88. Do, KT, Chow, LQM, Reckamp, Ket al. First-in-human, first-in-class, phase I trial of the fucosylation inhibitor SGN-2FF in patients with advanced solid Tumors. Oncologist 2021; 26: 925–e1918. 10.1002/onco.13911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pijnenborg, JFA, Visser, EA, Noga, Met al. Cellular fucosylation inhibitors based on fluorinated fucose-1-phosphates*. Chem 2021; 27: 4022–7. 10.1002/chem.202005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Pijnenborg, JFA, Rossing, E, Merx, Jet al. Fluorinated rhamnosides inhibit cellular fucosylation. Nat Commun 2021; 12: 7024. 10.1038/s41467-021-27355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. García-García, A, Ceballos-Laita, L, Serna, Set al. Structural basis for substrate specificity and catalysis of α1,6-fucosyltransferase. Nat Commun 2020; 11: 973. 10.1038/s41467-020-14794-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Järvå, MA, Dramicanin, M, Lingford, JPet al. Structural basis of substrate recognition and catalysis by fucosyltransferase 8. J Biol Chem 2020; 295: 6677–88. 10.1074/jbc.RA120.013291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Boruah, BM, Kadirvelraj, R, Liu, Let al. Characterizing human α-1,6-fucosyltransferase (FUT8) substrate specificity and structural similarities with related fucosyltransferases. J Biol Chem 2020; 295: 17027–45. 10.1074/jbc.RA120.014625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Aoyagi, Y, Suzuki, Y, Igarashi, Ket al. The usefulness of simultaneous determinations of glucosaminylation and fucosylation indices of alpha-fetoprotein in the differential diagnosis of neoplastic diseases of the liver. Cancer 1991; 67: 2390–4. . [DOI] [PubMed] [Google Scholar]
- 95. Yazawa, S, Takahashi, R, Yokobori, Tet al. Fucosylated glycans in α1-acid glycoprotein for monitoring treatment outcomes and prognosis of cancer patients. PLoS One 2016; 11: e0156277. 10.1371/journal.pone.0156277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yokobori, T, Yazawa, S, Asao, Tet al. Fucosylated α1-acid glycoprotein as a biomarker to predict prognosis following tumor immunotherapy of patients with lung cancer. Sci Rep 2019; 9: 14503. 10.1038/s41598-019-51021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bastian, K, Scott, E, Elliott, DJet al. FUT8 alpha-(1,6)-Fucosyltransferase in cancer. Int J Mol Sci 2021; 22: 455. 10.3390/ijms22010455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sanmamed, MF, Chen, L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell 2018; 175: 313–26. 10.1016/j.cell.2018.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tomida, S, Takata, M, Hirata, Tet al. The SH3 domain in the fucosyltransferase FUT8 controls FUT8 activity and localization and is essential for core fucosylation. J Biol Chem 2020; 295: 7992–8004. 10.1074/jbc.RA120.013079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhao, L, Zhao, J, Zhong, Ket al. Targeted protein degradation: mechanisms, strategies and application. Signal Transduct Target Ther 2022; 7: 113–3. 10.1038/s41392-022-00966-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


