Abstract
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has resulted in more than 670 million infections and almost 7 million deaths globally. The emergence of numerous SARS-CoV-2 has heightened public concern regarding the future course of the epidemic. Currently, the SARS-CoV-2 Omicron variant has rapidly become globally dominant in the COVID-19 pandemic due to its high infectivity and immune evasion. Consequently, vaccination implementation is critically significant. However, growing evidence suggests that COVID-19 vaccination may cause new-onset autoimmune diseases, including autoimmune glomerulonephritis, autoimmune rheumatic diseases, and autoimmune hepatitis. Nevertheless, the causal relationship between COVID-19 vaccines and these autoimmune diseases remains to be demonstrated. In this review, we provide evidence that vaccination induces autoimmunity and summarize possible mechanisms of action, such as molecular mimicry, activation by bystanders, and adjuvants. Our objective is not to refute the importance of vaccines, but to raise awareness about the potential risks of COVID-19 vaccination. In fact, we believe that the benefits of vaccination far outweigh the possible risks and encourage people to get vaccinated.
Keywords: Autoimmune diseases, COVID-19, SARS-CoV-2, Vaccines, Vaccination
1. Introduction
Coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In March 2020, the World Health Organization declared COVID-19 a global pandemic [1]. As of 31 January 2023, the Johns Hopkins University Center for Systems Science and Engineering reported that the worldwide number of confirmed infections has exceeded 670 million worldwide, resulting in nearly 7 million deaths [2]. Since the outbreak began, national governments have adopted various strategies to manage the pandemic, including strict quarantine, complete lockdowns, and mask-wearing, which have effectively reduced the spread of SARS-CoV-2. However, given the tradeoff between infection control measures and socioeconomic development, the development and production of vaccines will now take precedence over other actions [3].
Vaccines are indispensable in preventing and controlling over 20 life-threatening diseases, such as diphtheria, tetanus, pertussis, influenza, and measles [4]. Moreover, vaccines are critical tools in the fight against the COVID-19 pandemic. The World Health Organization reports that there are currently 176 vaccine candidates in clinical development, including Protein subunit (PS), Viral Vector (non-replicating) (VVnr), DNA, Inactivated Virus (IV), RNA, Viral Vector (replicating) (VVr), Virus Like Particle (VLP), VVr + Antigen Presenting Cell (VVr + APC), Live Attenuated Virus (LAV), VVnr + Antigen Presenting Cell (VVnr + APC), and Bacterial antigen-spore expression vector (BacAg-SpV) [5]. Most vaccines developed by researchers worldwide are currently administered by intramuscular (IM) injection. However, COVID-19 intranasal (IN) vaccines are being developed to provide protective mucosal immunity in addition to eliciting antibody-mediated and cell-mediated immunity [6]. Unfortunately, several SARS-CoV-2 variants have emerged globally, including Alpha (B.1.1.7) [7], Beta (B.1.351) [8], Gamma (P.1) [9], Delta(B.1.617.2) [10] and Omicron (B.1.1.529) [11], and others. Undoubtedly, the emergence of variants poses a huge challenge to vaccine development.
Although vaccination against COVID-19 has proven to be effective in reducing disease severity and mortality, some people are hesitant to get vaccinated due to concerns about side effects [12]. Recently, reports have emerged suggesting that COVID-19 vaccines may cause rare autoimmune diseases, including autoimmune glomerulonephritis [13], autoimmune rheumatic diseases [14], and autoimmune hepatitis [15]. These adverse events have increased public skepticism about vaccination. However, the causal relationship between COVID-19 vaccination and these autoimmune phenomena needs further investigation. Previous studies have suggested that vaccines can trigger autoimmunity through mechanisms such as molecular mimicry, bystander activation, anti-idiotypic network, and epitope spreading [16]. Based on current evidence, we provide a summary of the rare autoimmune diseases that may be induced by COVID-19 vaccines and discuss possible underlying mechanisms.
2. Methods
This review aims to provide a comprehensive analysis of the emergence of rare autoimmune diseases following COVID-19 vaccination. A systematic PubMed search was conducted to identify relevant literature on COVID-19 vaccination and new-onset autoimmune phenomena published up to 1st February 2023. Inclusion criteria encompassed case reports, case series, original articles, letters to the editor, and reviews. Additionally, the references and related citations for the resulting articles were examined for potential inclusion. Search terms utilized included: “COVID-19 Vaccine”, “SARS-CoV-2 vaccine”, “autoimmune diseases”, “IgA glomerulo-nephritis”, “membranous nephropathy”, “membranous nephropathy”, “lupus nephritis”, “systemic lupus erythematosus”, “focal segmental glomerulosclerosis”, ”ANCA-associated nephritis”, “thrombotic microangiopathy”, “autoimmune rheumatic diseases”, “Rheumatoid arthritis”, “antiphospholipid syndrome”, “adult-onset Still’s disease”, “ANCA associated vasculitis”, “giant cell arteritis”, “Sjogren’s syndrome”, “Behcet disease”, “autoimmune hepatitis”, “type 1 diabetes mellitus”, “autoimmune hemolytic anemia”, “vaccine-induced immune thrombocytopenia and thrombosis”, “myocarditis”, “alopecia areata”, “autoimmune thyroid diseases”, “Guillain-barre syndrome”, “molecular mimicry”, “adjuvants”, “Bystander Activation”, “epitope diffusion”, and “polyclonal activation”.
3. Results
To facilitate comprehension, we organized our findings into distinct categories. Specifically, these categories comprise Immune-mediated nephropathy, autoimmune rheumatic diseases, autoimmune hepatitis, type 1 diabetes mellitus, autoimmune hemolytic anemia, as well as additional autoimmune conditions.
3.1. Immune-mediated nephropathy
3.1.1. IgA nephropathy(IgAN)
IgA nephropathy (IgAN) is the most prevalent primary form of glomerulonephritis worldwide, and its clinical presentation ranges from asymptomatic microscopic hematuria to rapidly progressive glomerulonephritis [17]. Reports have emerged on the potential of COVID-19 vaccination to induce IgAN, as well as relapse of previously treated cases. Among the published cases, 39 individuals experienced new-onset IgAN following vaccination, with a male predominance of 61.5% (24/39) [[18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41]].(Table 1 ). The vast majority of these cases (approximately 90%) involved mRNA vaccines, while only three were associated with Viral vector vaccines, and one with an inactivated vaccine. The mRNA vaccine’s lipid nanoparticle formulation may activate CD4+ and CD8 T+ cells at a higher rate. In most instances (14/39), patients reported experiencing symptoms within 1 to 2 days after receiving the second dose, with gross hematuria being the most common initial symptom following vaccination (excluding cases with missing data), followed by albuminuria. Notably, the majority of these individuals had a history of microscopic hematuria or occult blood before vaccination and developed macroscopic hematuria shortly after receiving the vaccine. This suggests that these individuals may have had a pre-existing, undiagnosed IgAN and that the vaccine may have hastened disease progression as a predisposing factor.
Table 1.
Reported cases of de novo IgAN following COVID-19 vaccination.
| Authors | Age | sex | PMH | Timeline of symptom onset | Type of Vaccine | Begin of symptoms | Laboratory features | treatment | outcome |
|---|---|---|---|---|---|---|---|---|---|
| Abdel-Qader et al. | 12 | M | N | <1 day after 1st dose | mRNA | GH;AKI; proteinuria | Scr:1.77mg/dl; proteinuria :1.7 g/l; RBC: 1920/ul; Renal biospy: IgAN | steroid | R |
| Mohamed et al. | 50 | M | seasonal allergy | 2 wk after 1st dose | mRNA | skin rash; proteinuria | UPCR:1.1 g/day; Renal biospy: IgAN Skin biopsy:IgA vasculitis; | steroid | R |
| Niel et al. | 13 | F | N | <1 day after 1st dose | mRNA | MH;asthenia; Muscle pain; fever | SCr: 3.57 mg/dl; proteinuria:3.9 g/l; Renal biospy: IgAN | steroid; HD | R |
| Okada et al. | 17 | F | AH | 4 days after 1st dose | mRNA | GH; proteinuria | SCr: 0.58 mg/dl; Renal biospy: IgAN | Conservative | CR |
| Fujita et al. | 40 | F | Occult blood | 9 days after 1st dose | mRNA | GH;Fever; Chills; shivering; back pain; general malaise; | SCr: 0.86 mg/dl; RBC: 100/HPF; ALB: >3 g/dl; Renal biospy: IgAN | TPE | CR |
| Yokote et al. | 36 | F | MH;RA Proteinuria; |
11 days after 1st dose | mRNA | GH | SCr: 0.9 mg/dl; UPCR: 15.6 g/g; RBC:>100/HPF;Renal biospy: IgAN | Steroid; immunosuppressive | R |
| 19 | M | MH | 18 h after 2nd dose | mRNA | GH | SCr: 0.97 mg/dl; RBC: 50–99/HPF; UPCR: 1.5 g/g; Renal biospy: IgAN | RASi | R | |
| Anderegg et al. | 39 | M | HTN | Immediate after 2nd dose | mRNA | Fever;GH flu-like symptoms | AKI;Renal biospy: IgAN | Steroid; immunosuppressive | R |
| 81 | M | N | after 2nd dose | mRNA | flu-like symptoms | Renal biospy: IgAN | Steroid; immunosuppressive plasmapheresis | R | |
| Klomjit et al. | 38 | M | NA | 2 wk after 2nd dose | mRNA | UK | Scr:1.6mg/dL; protein:0.32g/d; RBC:51-100/HPF; Renal biospy: IgAN | Conservative | NA |
| 44 | F | NA | 2 wk after 1st dose | mRNA | UK | Scr:2.5mg/dL; protein:14 g/d; RBC:21-30 /HPF; ALB:3.7g/dl; Renal biospy: IgAN | steroid | NR | |
| 66 | F | NA | 2 wk after 1st dose | mRNA | UK | Scr:1.5mg/dL; protein:1.2 g/d; RBC:51–100 /HPF; ALB:4.1g/dl; Renal biospy: IgAN | steroid | R | |
| 62 | M | NA | 6 wk after 1st dose | mRNA | UK | Scr:2.2mg/dL protein:0.9 g/d RBC:31–40 /HPF ALB:4.2g/dl Renal biospy: IgAN |
Conservative | R | |
| Lo et al. | 28 | F | MH | 3 h after 2nd dose | mRNA | GH | SCr: 72 μmol/L; UPCR: 320mg/mmol Renal biospy: IgAN |
Conservative | CR |
| Hanna et al. | 17 | M | N | <1 day after 2nd dose | mRNA | GH;AKI Proteinuria; |
SCr: 1.78 mg/dl; ALB: 3.8 g/dl; Renal biospy: IgAN | steroid | R |
| Nihei et al. | 28 | F | GH; proteinuria | 7 days after 2nd dose | mRNA | GH; fever | RBC: >100/HPF; UPCR: 0.13 g/g; Renal biospy: IgAN | Conservative | R |
| Abramson et al. | 30 | M | N | 1day after 2nd dose | mRNA | GH;proteinuria | SCr: 1.02 mg/dl; UPCR: 0.8 g/g; RBC: >30/HPF; Renal biospy: IgAN | RASi | PR |
| Tan et al. | 41 | F | GDM | 1day after 2nd dose | mRNA | Headache; GH generalized myalgia | RBC: >200; UPCR: 7.58g/g; SCr:541mmol/L; Renal biospy: IgAN | steroid; immunosuppressive | NA |
| 60 | F | N | 1day after 2nd dose | mRNA | GH | RBC: >200 ll; UPCR: 2.03 g/g; SCr:153mmol/L; Renal biospy: IgAN | steroid;TPE Immunosuppressive |
NA | |
| Park et al. | 50 | M | HTN | 1day after 2nd dose | mRNA | NA | SCr: 1.54 mg/dl; Renal biospy: IgAN | RASi | PR |
| 22 | F | N | 2day after 2nd dose | mRNA | NA | SCr: 0.8mg/dl; Renal biospy: IgAN | Conservative | R | |
| 39 | F | N | 2day after 2nd dose | mRNA | NA | SCr: 0.8 mg/dl; Renal biospy: IgAN | Conservative | R | |
| 67 | M | HTN | 1mo after 2nd dose | mRNA | NA | SCr: 2.90mg/dl; Renal biospy: IgAN | steroid; | PR | |
| Lim et al. | 42 | F | N | 1day after 2nd dose | mRNA | GH; proteinuria | SCr: 0.47 mg/dl; UTP :1.7 g/d; Renal biospy: IgAN | RASi | PR |
| Uchiyama et al. | 15 | M | MH | 1day after 2nd dose | mRNA | GH;Fever; myalgia | SCr: 0.97 mg/dl; numerous RBC; Renal biospy: IgAN | Conservative | R |
| 18 | M | MH | 2 day after 2nd dose | mRNA | GH | SCr: 0.82 mg/dl; numerous RBC; Renal biospy: IgAN | Conservative | R | |
| Kudose et al. | 50 | F | HTN; Obesity; APS | 2 day after 2nd dose | mRNA | GH;fever generalized body aches | SCr: 1.7 mg/dl; UPCR: 2 g/g, RBC: >50/HPF Renal biospy: IgAN | Conservative | R |
| 19 | M | MH | 2 day after 2nd dose | mRNA | GH | SCr: 1.2 mg/dl; numerous RBC Renal biospy: IgAN |
Conservative | R | |
| Horino et al. | 17 | M | MH | 2 day after 2nd dose | mRNA | fever;GH headache | SCr: 0.70 mg/dl; RBC: >100/HPF; Renal biospy: IgAN | Tonsillectomy; steroid | PR |
| Srinivasan et al. | 35 | M | Nephrolithiasis UC | 2 day after 2nd dose | mRNA | GH;foamy urine | SCr: 1.3 mg/dl; UPCR: 0.656 g/g Renal biospy: IgAN |
immunosuppressive | PR |
| Morisawa et al. | 16 | M | AH | 2 day after 2nd dose | mRNA | Fever; GH | SCr: 1.1 mg/dl; UPCR:0.28 g/g Renal biospy: IgAN |
Steroid; immunosuppressive | R |
| 13 | F | AH | 2 day after 2nd dose | mRNA | Fever; GH | SCr: 0.54 mg/dl; UPCR: 1.99 g/g; Renal biospy: IgAN | Conservation | R | |
| Alonso et al. | 30 | M | MPGN type 1; CKD;KT | 34 day after 2nd dose | mRNA | NA | SCr: 1.65 mg/dl; hematuria: 150/ml; Renal biospy: IgAN | Steroid | NR |
| Mokos et al. | 73 | M | HTN; KT; AAN | 5 wk after 2nd dose | Viral vector | lower leg edema | SCr: 1.67 mg/dl; UTP: 1.4 g/d, RBC: 3–5/HPF Renal biospy: IgAN | RASi | NR |
| Fenoglio et al. | 74 | M | UK | 6 wk after 1st dose | Viral vector | RF;NS | Renal biospy: IgAN | Steroid; hemodialysis | NA |
| 79 | M | UK | 61 days after 1st dose | Viral vector | RF;NS | Renal biospy: IgAN | Steroid; immunosuppressive | NA | |
| Chen et al. | 55 | M | N | <1day after 1st dose | mRNA | AKI;general malaise abnormal liver function; nausea;vomiting | Scr: 1.94 mg/dl; UTP:11.57 g; Renal biospy: IgAN | Conservation | NR |
| Ran et al. | 58 | M | N | 2 days after 1st dose | inactivated | GH;dizziness; generalized body aches; lower limb edema | Scr: 7.45 mg/dL; RBC:>100/HPF; UTP:3.79g; Renal biospy: IgAN | Steroid;dialysis | NA |
| Nakatani et al. | 47 | M | HTN | 19 days after 1st dose | mRNA | purpuric eruption | Proteinuria:3 +; occult blood :3 + Skin biopsy:IgA vasculitis Renal biospy: IgAN |
Steroid | PR |
F, female; M, male; IgAN, IgA nephropathy; F, female; M, male; HTP, hypertension; MH, microscopic hematuria; AH, asymptomatic hematuria; GH, gross hematuria; AKI, acute kidney injury; CKD, Chronic Kidney Disease; NS, nephrotic syndrome;RF, renal failure; KT, kidney transplantation; AAN, aristolochic acid nephropath; APS,antiphospholipid syndrome;UC, ulcerative colitis; MPGN type 1;membranous proliferative glomerulonephritis type 1; RF;rheumatoid arthritis; GDM, gestational diabetes mellitus; Scr, Serum creatinine; ALB, serum albumin; RBC, red blood cell; UTP,24-hurine protein;UPCR, urine protein-to-creatinine ratio; HD, hemodialysis; RASi,renin-angiotensin-aldosterone system inhibition;TPE, Therapeutic plasma exchange;N,none; CR, complete remission; PR, partial remission; NA, non-applicable; NR, no response;R, response.
Among this group of patients, we also observed several noteworthy phenomena. For instance, Mokos et al. reported the first instance of IgAN in a renal transplant recipient following the administration of the adenovirus vector SARS-CoV-2 vaccine, which suggests a heightened possibility of adverse reactions in immunocompromised solid organ recipients [37]. Additionally, Nakatani et al. reported the first case of new-onset IgA vasculitis with IgAN soon after receiving the mRNA vaccine, which highlights the need for careful follow-up to prevent new glomerulonephritis in the presence of significant purpura following vaccination [41].
The majority of the reported cases recover spontaneously within a short period of time with conservative treatment, although some cases may necessitate steroids, immunosuppressants, and dialysis to achieve remission. Fortunately, patients who did not exhibit obvious symptoms before vaccination or only had asymptomatic microscopic hematuria may be less likely to require additional treatment. Therefore, individuals in this group need not worry excessively about receiving the COVID-19 vaccine.
3.1.2. Membranous nephropathy(MN)
Membranous nephropathy (MN) is a glomerular disease characterized by the thickening of the glomerular capillary walls and accounts for approximately 30% of cases of nephrotic syndrome in adults [42]. This review identified 18 cases of de novo MN after COVID-19 vaccination [13,25,[43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53]](Table 2 ), with a male-to-female ratio of 17:5. The first reported case of new-onset MN following COVID-19 vaccination was by Gueguen et al. [46] Of these 18 cases, 14 cases (9 after the second dose) occurred following mRNA vaccines, 3 cases after viral vector vaccines, and 1 case after the first dose of the inactivated vaccine. Interestingly, mRNA vaccines appear to induce new-onset MN more frequently than viral vector vaccines and inactivated vaccines, which may be attributed to the higher immunogenicity of mRNA [54]. Most patients did not have a history of autoimmune or other glomerulonephritis and only developed nephrotic syndrome after vaccination. Immunotherapy was administered to all cases (except for those without a stated treatment regimen or effect), leading to clinical remission. M-type phospholipase A2 receptor (PLA2R) is a major target antigen involved in idiopathic membranous nephropathy in adults [55]. Of the patients in this group, three were PLA2R antibody-positive, suggesting a possible loss of tolerance to PLAR2 antigen following COVID-19 vaccination [47,48,50]. However, further studies are needed to elucidate the underlying mechanisms of COVID-19 vaccination and new-onset MN.
Table 2.
Reported cases of de novo MN after COVID-19 vaccination.
| Authors | Age | sex | PMH | Timeline of symptom onset | Type of Vaccine | Begin of symptoms | Laboratory features | treatment | outcome |
|---|---|---|---|---|---|---|---|---|---|
| Fenoglio et al. | 82 | M | N | after 2nd dose | mRNA | NS | Renal biospy: MN | rituximab | NA |
| 67 | F | N | after 2nd dose | mRNA | NS | Renal biospy: MN | rituximab | NA | |
| 82 | F | N | after 2nd dose | mRNA | NS | Renal biospy: MN | Steroid | NA | |
| Caza et al. | 54 | M | NA | 1 days after 2nd dose | mRNA | NS | Scr:1.3; Proteinuria :3+ ALB:3.4; Hematuria:positive Renal biospy: MN |
Steroid; Rituximab | NR |
| 68 | M | NA | 4 wk after 1st dose | Viral vector | NS;AKI; CKD | Scr:3.3; Proteinuria:0.6 ALB:3.2; Renal biospy: MN |
diuretic | NR | |
| 47 | M | NA | 6 days after 2 nd dose | mRNA | NS | Scr:0.7;Proteinuria:2.7 ALB:2.3; Hematuria:positive Renal biospy: MN |
N | PR | |
| Thammathiwat et al. | 53 | M | N | 1 wk after 1st dose | Inactivated | Intermittent lower extremity edema; foamy urine | Scr: 1.5 mg/dL;Proteinuria:3+ RBC:2+; ALB :2.3 g/dL; UPCR:13.4 g/g; Renal biospy: MN |
Steroid; immunosuppressive | CR |
| Da et al. | 70 | M | N | 1 wk after 1st dose | mRNA | generalized edema | ALB: 1.7g/L; Scr:1.29mg/dL; cholesterol :9.24mmol/L; Renal biospy: MN | Irbesartan; diuretic; warfarin | NR |
| Klomjit et al. | 50 | F | NA | 4 wk after 2nd dose | mRNA | Joint pain proteinuria | Scr:0.7mg/dL; protein:6.5 g/d RBC:3-10 /HPF; ALB:3.5g/dl |
Conservative | R |
| Gueguen et al. | 76 | M | HTP; UV-treated cutaneous mycosis fungoid | 4days after 1st dose | mRNA | edema | ALB: 1.6g/L Scr:0.86mg/dL |
rituximab | PR |
| Rashid et al. | 56 | M | HTP | 1 month after 1st dose | mRNA | Fatigue; exertional dyspnea; lower urinary tract symptoms | Scr:13.96mg/dL ALB: 2.2g/L Hematuria:2+ Renal biopsy : MN with positive PLA2R |
Rituximab; HD | R |
| Psyllaki et al. | 68 | M | N | 1 wk after 1st dose | mRNA | lower extremities edema | ALB: 2.9g/L eGFR 70 mL/min/1.73 m2 Renal biopsy : MN with positive PLA2R |
Rituximab | PR |
| Chavarot et al. | 66 | M | HTP;Left nephrectomy; | 8 wk after 2nd dose | mRNA | NA | Scr:1.36mg/dL immunohistochemistry:MN | Conservative | NA |
| Paxton et al. | 22 | M | eczema epilepsy | 1 month after 2nd dose | mRNA | lower limb oedema lethargy | Scr:0.72mg/dL;ALB: 8g/L Proteinuria: 7 g/d UPCR:700.4 mg/mmol Renal biopsy : MN with positive PLA2R |
Rituximab | PR |
| Saigal et al. | 32 | M | NA | 2 wk after dose | Viral vector | NS | NA | Steroid; cyclophosphamide | PR |
| 47 | M | NA | 11 days after dose | Viral vector | Isolated proteinuria | NA | RASi | CR | |
| Fornara et al. | 52 | F | NA | 49 days after dose | mRNA | NA | Scr:0.6mg/dL | RASi | stable |
| GARG et al. | 50 | F | N | 5 wk after 2nd dose | mRNA | edema; proteinuria | Proteinuria:8,720 mg/g; Rena biopsy:MN (NELL-1 positive) | conservative | R |
F, female; M, male;membranous nephropathy,MN; HTP, hypertension; AKI, acute kidney injury; CKD, Chronic Kidney Disease; NS, nephrotic syndrome;Scr, Serum creatinine; ALB, serum albumin; RBC, red blood cell; UTP,24-hurine protein;UPCR,urine protein-to-creatinine ratio; HD, hemodialysis; RASi, renin-angiotensin-aldosterone system inhibition;N,none; CR, complete remission; PR, partial remission; NA, non-applicable; NR, no response;R, response.
3.1.3. Lupus nephritis (LN)
Systemic lupus erythematosus (SLE) is an autoimmune disease with a range of variable features, yet its pathogenesis and precise mechanism remain unclear. Lupus nephritis(LN), one of the most severe organ complications of SLE, is a form of glomerulonephritis that is histologically classified into six distinct classes [56]. To date, at least 12 cases of new-onset SLE induced by COVID-19 vaccination have been reported in the literature, of which two were male and the rest were female [[57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68]] (Table 3 ). Most cases developed two to three weeks after vaccination (Table 3). Although the exact evidence to elucidate the relationship between SLE and COVID-19 vaccine is not available, there are some potential mechanisms between COVID-19 vaccination and SLE, including molecular mimicry [69], type 1 interferons and inflammatory mediators production via stimulation of toll-like receptors(TLR) [70] and vaccine adjuvants triggering autoimmune events [71], among others. Previous studies have reported flares of SLE triggered by other vaccines such as hepatitis-B, and HPV [72,73]. Therefore, the possibility of a similar effect cannot be denied.
Table 3.
Reported cases of SLE after COVID-19 vaccination.
| Author | Age | Gender | PMH | Timeline of symptom onset | Type of Vaccine | Scoring of SLE | Serology profile | Diagnose |
|---|---|---|---|---|---|---|---|---|
| Patil | 22 | F | infective jaundice (non-B) | 2 wk after 1st dose | Viral vector | 22 | ANA,anti-dsDNA | SLE with anemia of chronic diseases |
| Nune | 24 | M | N | 2 wk after 2nd dose | mRNA | 21 | ANA,anti-dsDNA, low C3 and C4 | SLE withn ecrotizing lymphadenitis |
| Hidaka | 53 | F | Bronchial asthma, Vogt–Koyanagi–Harada disease, Hashimoto disease | few days after 1st and 2nd dose | mRNA | 20 | ANA, low C3 and C4 | SLE with Evans syndrome |
| Gamonal | 27 | F | N | 3 wk after 2nd dose | Viral vector | 21 | ANA,anti-dsDNA, anti-Sm,anti-SSA, low C3 and C4 | SLE with AA |
| Rios | 42 | F | three pregnancies with two spontan-eous abortions | 2 wk after 1st dose | mRNA | 18 | ANA,anti-dsDNA, low C4 | SLE with secondary APS |
| Wang | 37 | F | N | 2 wk after 1st dose | Viral vector | 12 | ANA, anti-dsDNA, low C3 | SLE with acrocyanosis |
| Mousa | 22 | F | N | 1 wk after 1st dose | mRNA | 25 | ANA, anti-dsDNA, low C3 and C4 | SLE with acute pancreatitis vasculitic rash |
| Raviv | 24 | M | N | 2 days after 1st dose | mRNA | 21 | ANA,anti-Rib-P, anti-chromatin (nucleosomal), low C3 | SLE |
| Báez | 27 | F | type 1 diabetes mellitus | 2 wk after 2nd dose | mRNA | 19 | ANA,anti-SSA,anti-SSB, anti-dsDNA,low C4 | SLE |
| Lemoine | 68 | M | N | 2 days after 1st dose | mRNA | 12 | ANA,anti-dsDNA, p-ANCA with anti-MPO specificity | SLE |
| Khanna | 18 | F | autism | 1 wk after 1st dose | mRNA | 19 | ANA,anti-RNP, anti-Sm, low C3 and C4 | SLE |
| Ghang | 20 | F | N | 10 days after 1st dose | mRNA | 14 | ANA,anti-SSA, anti-SSB | SLE |
F, female; M, male; ANA,anti-nuclear antibody; anti-dsDNA,antibody recognizing double-strandded DNA; anti-SSA,anti-Sjgren syndrome A antibody; anti-SSB,anti-Sjgren syndrome B antibody; anti-RNP,anti-ribonuclear protein antibody;anti-Sm,anti-Smith antibody; anti-Rib-P, anti-ribosomal P protein antibody; C3, complement 3; C4,complement 4; SLE, systemic lupus Erythematosus; AA,alopecia areata;APS, antiphospholipid syndrome; PMH, past medical history. Scoring of SLE is based on the 2019 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) classification criteria.
To date, only three cases of new-onset LN following COVID-19 vaccination have been reported [[74], [75], [76]](Table 4 ), with two cases following viral vector vaccination and one case following mRNA vaccination. The age range of the cases varied widely (14 to 60 years), and initial symptoms were diverse. Nevertheless, the serological profiles were generally similar, showing elevated ANA titers, positive anti-dsDNA antibody, and low complement levels. All three patients received immunosuppressive therapy, resulting in varying degrees of remission.
Table 4.
Reported cases of LN after COVID-19 vaccination.
| Authors | Age | sex | PMH | Timeline of symptom onset | Type of Vaccine | Begin of symptoms | Serology profile | treatment | outcome |
|---|---|---|---|---|---|---|---|---|---|
| Zavala-Miranda et al. | 23 | F | N | 1 wk after 1st dose | Viral vector | eyelid edema foamy urine hair loss | ANA;anti-dsDNA; low C3 and C4 | mycophenolate mofetil; glucocorticoids; hydroxychloroquine; diuretics | R |
| Kim et al. | 60 | F | N | after 2nd dose | Viral vector | asthenic;did not eat well | ANA; anti-dsDNA; anti-Sm;low C3 and C4 | methylprednisolone; cyclophosphamide; Prednisolone; hydroxychloroquine | R |
| Nelson et al. | 14 | M | N | 2 days afte 3rd dose | mRNA | non-photosensitive facial rash | ANA;anti-dsDNA; anti-Sm;anti-RNP low C3 and C4 | Prednisone; mycophenolate mofetil; Losartan; hydroxychloroquine | R |
F, female; M, male; ANA,anti-nuclear antibody; anti-dsDNA,antibody recognizing double-strandded DNA; anti-RNP,anti-ribonuclear protein antibody;anti-Sm,anti-Smith antibody; N,none;NA, non-applicable; R, response.
While these findings suggest that the COVID-19 vaccine may trigger the onset of LN in susceptible populations, a definitive cause-effect relationship between COVID-19 vaccination and LN has not yet been established.
3.1.4. Focal segmental glomerulosclerosis(FSGS)
Focal segmental glomerulosclerosis (FSGS) is a heterogenous syndrome that arises from podocyte injury caused by various factors, and it represents a major cause of kidney disease worldwide [77]. Recently, five cases of de novo FSGS after COVID-19 vaccination have been reported [13,[78], [79], [80], [81]] (Table 5 ), with four of the cases following mRNA vaccine and one following the viral vector vaccine. The majority of patients were women (3/5, 60%) and below 30 years of age (4/5,80%). Nephrotic syndromes characterized by edema and proteinuria developed in almost all patients after vaccination, and renal biopsy confirmed FSGS. In addition to conservative treatment, all five patients received steroid therapy, and three also received immunosuppressive therapy. Most cases responded well to regular immunosuppression.However, Jha et al. reported a case of a 21-year-old male patient who developed de novo FSGS after ChAdOx1 nCoV-19 vaccination and did not respond to steroid + tacrolimus + rituximab therapy, unlike the other four patients [78]. It is difficult to establish a definite causal relationship between COVID-19 vaccination and FSGS, due to the small number of reported cases. Nevertheless, previous studies have suggested that mRNA vaccines may elicit T cell responses, and T-cell-mediated immune response could potentially trigger podocyte damage [82].
Table 5.
Reported cases of FSGS after COVID-19 vaccination.
| Authors | Age | sex | PMH | Timeline of symptom onset | Type of Vaccine | Begin of symptoms | Laboratory features | treatment | outcome |
|---|---|---|---|---|---|---|---|---|---|
| Jha et al. | 21 | M | N | 12 days after 1st dose | Viral vector | facial puffiness; lower limb swelling | Scr:1.06mg/dl;protein: 4+; ALB:0.9 g/dl; Renal biospy: FSGS | steroid; tacrolimus; rituximab | NR |
| Dormann et al. | 20 | F | Vegan diet | 5 days after 1st dose | mRNA | generalized edema | Scr:0.47 mg/dl;proteinuria; UPCR: 10.3 g/g;ALB: 120 mg/dl; Renal biospy: FSGS | prednisolon;diuretic; lipid-lowering; vitamin D substitution | PR |
| Fenoglio et al. | 24 | F | N | after 2nd dose | mRNA | NS | Renal biospy: FSGS | Glucocorticoids | NA |
| Lim et al. | 29 | M | N | 7 days after 1st dose | mRNA | edema; decreased urine output | Scr:1.24mg/dL; protein:4+; ALB:2.5g/dL; UPCR: 6.12 g/g; C3:144mg/dL;C4:65 mg/dL Renal biospy: FSGS | prednisolone; diuretics; angiotensin receptor blocker;statin | R |
| Marega et al. | 80 | F | TTP;HT; HTP;IHD | 2 wk after 2nd dose | mRNA | weight increased; generalized edema | Scr :2 mg/dL;ALB: 2 g/dL proteinuria :300 mg/dL;MH; PLA2R Ab:Positive; kidney biopsy :FSGS superimposed on MN | Steroids; cyclophosphamide | R |
F, female; M, male; focal segmental glomerulosclerosis, FSGS; IHD,ischemic heart disease;TTP, thrombotic thrombocytopenic purpura; HT, hypothyroidism;HTP, hypertension;MH,Microscopic hematuria;NS, nephrotic syndrome; PLA2R,phospholipase A2 receptor; Scr, Serum creatinine; ALB, serum albumin; UPCR,urine protein-to-creatinine ratio;C3, complement C3; C4, complement C4; N,none; R,remission; PR, partial remission; NA, non-applicable; NR, no response.
Overall, these findings suggest that COVID-19 vaccination may induce FSGS in some susceptible individuals. Further studies are needed to determine the underlying mechanisms and to identify risk factors associated with FSGS after COVID-19 vaccination.
3.1.5. Others
In addition to the aforementioned adverse events induced by COVID-19 vaccination, other autoimmune manifestations have also been reported in some cases, including ANCA-associated nephritis, thrombotic microangiopathy (TMA), and others.
Kim et al. reported a case of a 72-year-old woman who presented with anorexia, abdominal pain, and fever after receiving the mRNA vaccine. Laboratory tests showed occult blood (2+), proteinuria (2+), microscopic hematuria, and elevated serum creatinine levels (1.25 mg/dL). Serological tests revealed positive ANCA titers and antibodies against MPO. Based on renal biopsy results, ANCA-associated pauci-immune crescentic glomerulonephritis was diagnosed. After high-dose steroid treatment and plasmapheresis, the patient’s symptoms and renal function improved [83].
Fabritiis et al. reported a case of a 35-year-old previously healthy man who developed moderate fatigue one day after receiving the first dose of mRNA vaccine, coupled with dysgeusia, myalgia, pharyngodynia, and foamy urine. Laboratory tests showed nephrotic proteinuria, microhematuria. Kidney biopsy showed some ultrastructural alterations suggestive of an initial phase of renal TMA and complete remission of proteinuria and microhematuria was achieved in four weeks after high-dose steroid treatment [84]. However, Bitzan et al. reported five cases of TMA after influenza vaccination before [85]. To date, there are few reports of thrombotic microangiopathy following COVID-19 vaccination, suggesting that the underlying pathogenic relationship remains to be explored.
3.2. Autoimmune rheumatic diseases (ARDs)
3.2.1. Rheumatoid arthritis (RA)
Rheumatoid arthritis (RA) is an inflammatory autoimmune disease characterized by the presence of rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPAs) that cause inflammation [86]. Seven cases of de novo RA following COVID-19 vaccination have been reported, with a higher incidence in women (4/7, 57%) [14,[87], [88], [89], [90], [91]]. Four of these were reported after the mRNA vaccine and one after the Viral vector vaccine. Yonezawa et al. reported the first case of de novo seropositive RA after COVID-19 mRNA vaccination [87]. In most cases, patients presented with early symptoms of arthritis, such as morning stiffness, swelling, and pain. Serological tests showed positive RF and ACPA. Treatment included steroids, with four cases treated with methotrexate, two with hydroxychloroquine, and one with tocilizumab. Most patients responded well to the treatment and achieved varying degrees of clinical remission. However, Nahra et al. reported that symptoms recurred in patients after the prednisone dosage was reduced to less than 10mg per day [88].
3.2.2. Antiphospholipid syndrome(APS)
Antiphospholipid syndrome(APS)is a unique form of autoantibody-induced thrombophilia characterized by recurrent thrombosis and pregnancy complications. Lupus anticoagulant is the most significant predictor of APS-related features [92]. Moreno-Torres et al. recently reported a case of APS following an mRNA COVID-19 vaccine. The patient, a 27-year-old woman with a previous history of selective immunoglobulin A deficiency and paucisymptomatic COVID-19, presented with fever, digital ischemia, and abdominal pain 36 hours after receiving the first dose of BNT162b2 mRNA vaccine. Laboratory tests showed the presence of lupus anticoagulant. The patient was treated with low-dose prednisone, hydroxychloroquine, adjusted low-molecular-weight-heparin (LMWH) and thrice-weekly hemodialysis [93].
In another case, Molina-Rios et al. reported a 42-year-old woman with a history of three pregnancies, two of which resulted in spontaneous abortions. She presented with inflammatory polyarthralgia, bilateral synovitis, and bilateral Achilles tendon enthesopathy two weeks after receiving the first dose of the mRNA vaccine. Combined with laboratory data and imaging findings, SLE and secondary APS were considered [94].
3.2.3. Adult-onset Still’s disease (AOSD)
Adult-onset Still’s disease (AOSD) is a rare auto-inflammatory disorder of unknown etiology characterized by a high spiking fever, arthralgia, evanescent rash, and striking leucocytosis with neutrophilia [95]. A total of twenty-two cases of new-onset AOSD were reported in patients presenting with macroscopic hematuria following COVID-19 vaccination, with a male-to-female ratio of 4:7 [[96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110]]. Ten cases were reported following the mRNA vaccine, with five after the second dose, and the remainder following the viral vector vaccination, with one case reported after the second dose. Fever was the first symptom in all patients after vaccination, which was sometimes accompanied by other symptoms, such as sore throat, arthritis, headache, and others. As the disease progressed, most patients developed skin rashes. Laboratory tests showed elevated leukocytosis and CRP in most patients, while some patients showed an increase in liver enzymes. The vast majority of patients respond to steroid therapy, with a few requiring tocilizumab therapy. However, Gasparotto et al. reported a case of a 50-year-old female who did not go into remission after steroid and anti-IL1 treatment [101].
The exact pathogenesis of AOSD is not fully understood, but previous studies have shown that innate immune system activation is involved, with Toll-like receptors (TLRs) possibly activating the innate immune system with subsequent cytokine overproduction, especially ongoing aberrant IL-1, IL-18, IL-6, and TNF-α [95].
3.2.4. ANCA-associated vasculitis (AAV) and Giant cellarteritis(GCA)
Antineutrophil cytoplasmic autoantibodies (ANCA) associated vasculitis (AAV) is a rare autoimmune disorder that involves severe small-vessel vasculitis and is characterized by a loss of tolerance to neutrophil primary granule proteins such as leukocyte proteinase 3 (PR3-ANCA) or myeloperoxidase (MPO-ANCA). AAV is classified into three types based on clinical features, namely granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and eosinophilic GPA (EGPA) [111]. A total of 17 new-onset AAV cases have been reported, including 5 cases of PR3-ANCA vasculitis, 9 cases of MPO-ANCA vasculitis, and 3 cases of dual-positive MPO- and PR3-ANCA vasculitis [[112], [113], [114], [115], [116], [117], [118], [119], [120], [121], [122], [123], [124], [125], [126], [127], [128]]. The majority of cases were women (11/17, 64.7%). Of these 17 cases, 13 cases were reported following the mRNA vaccines, 3 were reported following viral vector vaccines, and 1 was reported following inactivated vaccines. All patients received steroid therapy, while some also received hemodialysis (2/17,11.8%), plasmapheresis (7/17,41.2%), cyclophosphamide (11/17,64.7%), and rituximab(6/17,35.3%). Christodoulou et al. reported the first case of a healthy patient who developed de novo MPO-ANCA vasculitis shortly after COVID-19 vaccination and responded to steroids and cyclophosphamide but relapsed immediately after COVID-19 infection [121]. This finding suggests that both SARS-CoV-2 infection and vaccination may induce vasculitis and involve similar immune mechanisms.
Furthermore, So et al. have reported a new case of new-onset MPA following COVID-19 vaccination. A 42-year-old healthy male presented with general weakness, shortness of breath, edema, gross hematuria, and significant weight loss after receiving a second dose of mRNA vaccine. Elevated MPO antibodies and histological findings lead to the diagnosis of MPA. After receiving glucocorticoid therapy, rituximab, and plasma exchange, the patient’s symptoms improved [129]. However, the exact mechanism by which COVID-19 vaccines induce AAV is not yet well understood.
Giant cell arteritis (GCA) is a rare inflammatory disease affecting medium and large blood vessels, with clinical manifestations including headache, scalp tenderness, jaw and tongue pain, and visual disturbances [130]. There were five new-onset cases of GCA reported, with three patients developing symptoms after the mRNA vaccine and one patient after the first dose of the viral vector vaccine [[131], [132], [133], [134], [135]]. Four (80%) patients presented with headaches, while two (40 %) patients presented with jaw claudication. Scalp tenderness was observed in two (40%) cases. Most patients responded to steroid therapy. Mungmungpuntipantip et al. suggest that COVID-19 vaccination may increase blood viscosity, which may lead to inflammation resulting in arteritis [136]. However, the underlying pathogenesis of GCA remains unclear and may be associated with COVID-19 vaccines.
3.2.5. Sjogren’s syndrome (SS) and Behçet's disease (BD)
Ramos-Casals et al. documented the first case of subclinical Sjogren’s syndrome (SS) occurring 10 days after the first dose of the ChAdOx1 nCoV-19 vaccine (Oxford/AstraZeneca). A 55-year-old male with a history of colon carcinoma presented with widespread petechiae, bleeding gums, and hematuria following vaccination. Laboratory tests revealed a platelet count of 3 x 109/L, positive IgG antibodies, ANA, and anti-Ro52 antibodies [137].
Tagini et al. described the first case of a new-onset Behçet's disease (BD) developing 15 days after the second dose of the SARS-CoV-2 mRNA-1273 vaccine. A woman in her late 20s with a medical history of polycystic ovary syndrome presented with a 1-week history of general malaise and her first episode of painful oral and genital ulcers after vaccination. BD was suspected based on clinical presentation, and the patient rapidly recovered after receiving prednisone 1 mg/kg/day [138].
3.3. Autoimmune hepatitis (AIH)
Autoimmune hepatitis (AIH) is a chronic liver disease characterized by elevated serum transaminase levels, elevated immunoglobulin G levels, the presence of autoantibodies, and interface hepatitis on liver histology [139]. The first case of AIH following COVID-19 vaccination was reported by Bril et al. in a 35-year-old female who developed AIH one week after receiving the first dose of COVID-19 vaccine (Pfizer-BioNTech) in her third month postpartum [140]. Although immunoglobulin G levels were not elevated as typically seen in AIH, histology showed the presence of eosinophils, which are also observed in liver damage caused by drugs or toxins. However, both conditions have also been reported in AIH [141,142]. To date, COVID-19 vaccines that induce AIH include mRNA vaccines, viral vector vaccines, and inactivated vaccines, with mRNA vaccines accounting for the majority and inactivated vaccines accounting for the least [15,140,[143], [144], [145], [146], [147], [148], [149], [150], [151], [152], [153], [154], [155], [156], [157], [158], [159], [160], [161], [162], [163], [164], [165], [166], [167], [168]]. Clayton-Chubb et al. reported for the first time a case of adenovirus-based vaccine eliciting AIH. A 36-year-old male physician with a history of hypertension but no history of liver disease developed AIH following the first dose of the ChAdOx1 nCoV-19 vaccine (Oxford-AstraZeneca) [152]. Mekritthikrai et al. reported the first case of AIH flare-up after receiving an inactivated COVID-19 vaccine. A 52-year-old woman without previous liver disease was diagnosed with AIH after receiving 2 doses of inactivated COVID-19 vaccine (CoronaVac) [157].
Although an increasing number of reports have linked COVID-19 vaccination to the development of AIH, the mechanisms remain unclear. Vojdani et al. found that antibodies to the SARS-CoV-2 spike protein cross-reacted with transglutaminase 3 (TTG3), transglutaminase 2(TTG2), and other proteins, suggesting that SARS-CoV-2 may trigger autoimmunity [169]. Furthermore, previous reports described a relationship between vaccination and the development of AIH, including influenza vaccines [170], which suggest that COVID-19 vaccines may also induce AIH. The cases presented above indicate that different types of vaccines have different potentials for inducing autoimmunity, and the mechanisms by which they promote autoimmunity may also differ slightly. For mRNA vaccines, adjuvant activity and binding to pattern recognition receptors (PRRs), such as Toll-like receptors, may trigger T and B cell immune responses [171,172]. For viral vector vaccines, adjuvants or binding to model receptors, particularly TLR9, may be involved [173]. Inactivated vaccines may exert immune effects through adjuvants and molecular mimicry [157].
3.4. Type 1 diabetes mellitus (T1DM)
Type 1 diabetes mellitus (T1DM) is an autoimmune endocrine disorder characterized by symptoms such as polyuria, nocturia, enuresis, lethargy, polydipsia, weight loss, and abdominal pain [174]. Out of the 13 cases analyzed (6 men and 7 women), approximately 54% did not have any medical history of autoimmune diseases, while 15% of patients had a history of vitiligo, type 2 diabetes mellitus, and Hashimoto’s thyroiditis [[175], [176], [177], [178], [179], [180], [181], [182], [183]]. While 10 patients received the mRNA vaccine, 2 received the inactivated vaccine. Almost all of the patients exhibited classic symptoms of diabetes, such as thirst, polyuria, polydipsia, and fatigue, and showed remission after insulin treatment, with one patient getting worse after medical nutrition therapy.
Tang et al. reported the first case of a 50-year-old healthy male who developed fulminant type 1 diabetes mellitus (FT1DM) after receiving an inactivated COVID-19 vaccine. The patient experienced fever and an abrupt onset of polydipsia and polyuria 5 days after vaccination. Laboratory tests at presentation revealed hyperglycemia, ketosis, metabolic acidosis, and a positive result for susceptibility human leukocyte antigen (HLA) alleles for FT1DM (DQB1*02:03/03:03 and DRB1*09:01/09:01). However, the patient’s islet function was almost completely lost 4 weeks after initial presentation. Tang et al. suggested that genetic susceptibility and autoimmunity might be involved in the pathogenesis of FT1DM, indicating that vaccination might trigger autoimmunity in individuals with susceptible genetic backgrounds and cause FT1DM [176]. Additionally, Sasaki et al. proposed that there might not be a single mechanism responsible for the loss of islet function and the development of hyperglycemia associated with Covid-19 vaccination, as new-onset T1DM patients with positive autoantibodies developed symptoms 4-7 weeks after vaccination, while patients with negative autoantibodies showed symptoms within a week after vaccination. The type of vaccine may also be a contributing factor to the difference in onset times [181].
MDA5 is a crucial innate pathogen recognition protein that has been shown to play a role in the immune response to COVID-19 mRNA vaccines. According to Sakurai et al., MDA5 recognizes RNA from these vaccines and triggers the synthesis of type I interferons. This immune response may interfere with insulin production, proinsulin conversion, and mitochondrial function in pancreatic β-cells, leading to the development of diabetes [180].
3.5. Autoimmune hemolytic anemia(AIHA)
To date, studies on vaccine-induced autoimmune hematologic disorders have been focused on cases of vaccine-associated thrombosis with thrombocytopenia (VITT), while reports of other vaccine-related hematologic diseases are uncommon. Autoimmune hemolytic anemia (AIHA) is one such rare disorder characterized by the accelerated destruction of autologous red blood cells (RBCs) due to autoantibodies [184].
Of the 9 new-onset cases of AIHA reported, of which 6 patients developed symptoms following the mRNA vaccine, and 3 patients developed symptoms after the viral vector vaccine [[185], [186], [187], [188], [189], [190]]. In laboratory tests, all the patients showed decreased levels of Hb and haptoglobin, increased levels of LDH and bilirubin, and a positive direct antiglobulin test (DAT). Treatment involved steroid therapy for all patients, while 4 (44%) patients were treated with rituximab or immune globulin, and 2 (22%) patients received supportive RBC transfusions. One patient received plasmapheresis or mycophenolate mofetil.
The mechanism by which COVID-19 vaccines induce AIHA remains unclear. Angileri et al. proposed that molecular mimicry may be involved. Ankyrin-1 (ANK-1) is an erythrocyte membrane protein that is essential for erythrocyte differentiation and function. They found that ANK-1 shares a putative immunogenic antigenic epitope (amino acids LLLQY) with 100% identity with Spike’s predicted immunogenic epitope 750-SNLLLQYGSFCTQL-763 for B cells. Thus, the viral spike glycoprotein encoded by mRNA vaccines could induce the production of antibodies against spike protein, which may cross-react with ANK-1 RBC protein through molecular mimicry, leading to AIHA [191].
3.6. Others
In addition to the adverse events previously described, COVID-19 vaccination has also been associated with various autoimmune manifestations. These include vaccine-induced immune thrombocytopenia and thrombosis (VITT), myocarditis, alopecia areata(AA), autoimmune thyroid diseases, and Guillain-barre syndrome (GBS). Pavord et al. conducted a prospective cohort study involving 294 patients with suspected VITT. The syndrome is characterized by thrombosis, thrombocytopenia, elevated D-dimer, and positive platelet factor 4 (PF4) antibodies, which were observed 5 to 30 days after SARS-CoV-2 vaccination [192]. Myocarditis is another rare complication reported after COVID-19 vaccination. Fichadiya et al. reported a case of a 60-year-old healthy male with no past medical history who developed heart failure (NHYA class 4) symptoms and suspected myocarditis four weeks after receiving the first dose of COVID-19 vaccine (mRNA1273, Moderna) [193]. Alopecia Areata(AA), classified as an autoimmune disease with an unknown cause, has also been linked to COVID-19 vaccination. Lee et al. reported a case of an 80-year-old man who developed AA after the first dose of the COVID-19 vaccine(BNT162b2) [194]. Additionally, several thyroid disorders such as Graves’ disease, subacute thyroiditis, and others, have been reported following COVID-19 vaccination [195]. Guillain-Barré syndrome (GBS) is an acute disorder of the nervous system and is one of the autoimmune neurological diseases. McKean and Chircop reported a case of a 48- year- old man with dyslipidemia who developed GBS following the first dose of the COVID-19 vaccine (Vaxzevria) [196].
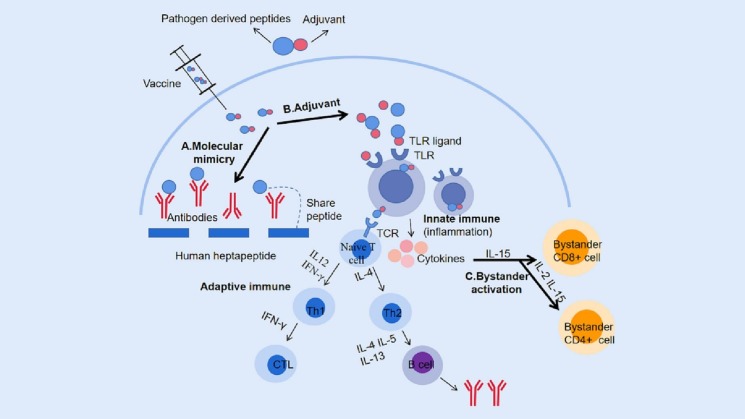
Recent findings suggest that the incidence of autoimmune diseases triggered by COVID-19 vaccines is on the rise, highlighting the pressing need to identify high-risk and vulnerable populations. Furthermore, it is essential to gather more evidence to confirm the link between COVID-19 vaccines and autoimmunity. To this end, several mechanisms have been proposed to elucidate the causal relationship, which will be detailed in the subsequent section (Fig. 1 ).
Fig. 1.
Schematic illustration of mechanisms inducing autoimmune diseases following COVID-19 vaccination. A. Following vaccination, vaccine antigens can trigger an immune response in the body. However, due to the presence of a heptapeptide that is shared between the SARS-CoV-2 spike glycoprotein and human proteins, vaccine antigens may also attack human proteins with similar structures via the molecular mimicry pathway. B. Adjuvants in vaccines can act as ligands for pattern recognition receptors (PRRs), such as toll-like receptors (TLR), bind to them to mobilize innate immune cells and secrete massive cytokines, and induce an innate immune response. Additionally, adjuvants also enhance the induction of adaptive immune responses to vaccine antigens. Upon binding to T-cell receptors (TCRs), antigens activate naive T-cells, which differentiate into Th1 or Th2 cells under the influence of different cytokines. Th1 cells primarily stimulate cellular responses, including the production of cytotoxic T lymphocytes (CTLs) that can eliminate infected cells, while Th2 cells promote humoral responses, such as B-cell proliferation, differentiation, and secretion of neutralizing antibodies. C. During the innate immune response following vaccination, the immune system produces a large number of cytokines, which may induce autoimmunity through the bystander activation pathway. This includes the activation of bystander CD8+T cells primarily under the action of IL-15 and the activation of CD4+T cells primarily under the influence of IL-2. PRRs, pattern recognition receptors; TLR, Toll-like receptor; TCR, T cell receptor; CTL, Cytotoxic T lymphocyte.
4. Mechanisms of COVID-19 vaccine-induced autoimmune diseases
4.1. Molecular mimicry and immune cross-reaction
The term molecular mimicry refers to the structural similarity between specific human proteins and certain disease-causing elements contained in vaccines. This similarity may result in immune cross-reaction, where the immune system mistakes human proteins for pathogens and attacks them, leading to autoimmune diseases [69]. Segal et al. have suggested several examples of vaccine-induced autoimmune diseases associated with molecular mimicry and immunological cross-reaction. These include influenza (H1N1) vaccines and Guillain-Barre syndrome(GBS), hepatitis B virus vaccines and multiple sclerosis (MS), and human papillomaviruses (HPV) and systemic lupus erythematosus (SLE) [69]. The mechanisms of molecular mimicry and immune cross-reaction may also play an important role in inducing autoimmune diseases following post-SARS-CoV-2 vaccination. Kanduc et al. have analyzed abundant heptapeptide sharing between SARS-CoV-2 spike glycoprotein and human proteins [197]. Therefore, antibodies produced by the body against the SARS-CoV-2 spike protein after vaccination may cross-react with the host and cause autoimmune diseases.
4.2. Adjuvants
Adjuvants are substances that accelerate, prolong, or enhance antigen-specific immune responses without having any specific antigenicity. They stimulate the immune system and increase the response to vaccines [198]. Toll-like receptors (TLRs) are a group of PRRs present on the innate immune system cells that recognize pathogens and initiate a response to infection. Adjuvants act as TLR ligands, binding to TLRs and leading to the initiation of innate immune responses, followed by adaptive immune responses [199]. However, adaptive antibody responses can also occur even in the absence of TLR ligands, as noted by Gavin et al. [200]. The concept of ”ASIA-Autoimmune/inflammatory Syndrome Induced by Adjuvants” has been proposed by Shoenfeld and Agmon-Levin, suggesting that adjuvants can trigger immune-mediated diseases [201]. Nevertheless, more studies are needed to confirm the role of adjuvants in the development of COVID-19 vaccine-mediated autoimmune diseases.
4.3. Bystander activation
Bystander activation refers to the cytokine-dependent, T and B cell receptor-independent activation of T and B cells [202,203]. In viral infections, Tough et al. reported for the first time that T-cell proliferation is driven by cytokines instead of TCRs and that memory cells may require minimal TCR ligation or be completely independent of TCRs. They require only intermittent exposure to IFN I released during viral infection to promote the long-term memory carried by CD8+ cells [204]. Additionally, Boyman suggested that bystander activation of CD4+ T cells may involve IL-2 and other cytokines [205]. In adjuvant-containing vaccines or adjuvants alone, T cells can be activated via bystander pathways; however, the migration of these activated auto-reactive cells to the site of inflammation and the mass secretion of cytokines may be sufficient to induce autoimmune pathologies, such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and type 1 diabetes [202]. Therefore, it is highly probable that bystander activation is involved in the autoimmunity phenomena that occur following COVID-19 vaccination.
4.4. Others
In addition to the mechanisms discussed above, two other mechanisms involved in the development of vaccine-induced autoimmune diseases are epitope diffusion and polyclonal activation of B cells. Epitope spreading(ES) refers to an immune response to an epitope that is different from the dominant epitope and has no cross-reaction. This immune response can spread to different epitopes on the same protein (intramolecular ES) or to epitopes on other proteins (intermolecular ES) [206]. Studies by Cornaby et al. have shown that intermolecular and intramolecular B cell ES contribute to the development of numerous autoimmune diseases, including rheumatoid arthritis, systemic lupus erythematosus, Graves’ disease, and Multiple sclerosis [207].
Polyclonal activation and proliferation of B cells are induced by long-lasting or constant immune system activation [208]. In some cases, this activation leads to the formation of circulating immune complexes, which can eventually cause damage to self-tissues.
5. Conclusion
Since the outbreak of COVID-19, its impact on the global economy, politics, and other aspects has been significant. In December 2021, the SARS-CoV-2 Omicron variant quickly became the dominant strain in the COVID-19 pandemic. Notably, Omicron contains numerous mutations in the spike protein that confer high immune evasion, and Shen’s research suggests that a booster dose of the COVID-19 vaccine is critical for generating neutralizing antibody responses against Omicron [209]. Given Omicron’s high infectivity, although it is less pathogenic than previous strains, a large number of cases would still overwhelm healthcare systems [210]. In this case, further optimization of the COVID-19 vaccine, such as changing the route of administration or adjusting vaccination strategies (e.g., adopting sequential immunization), is of great significance for controlling infections and alleviating public health pressure [211]. However, it is important not to ignore the potential side effects of vaccination.
In this comprehensive review, we have discussed rare autoimmune diseases that may potentially arise following COVID-19 vaccination, such as autoimmune glomerulonephritis, autoimmune rheumatic diseases, and autoimmune hepatitis, among others. However, the true incidence of these diseases after vaccination remains difficult to determine, as not all cases are or will be reported. Further exploration is necessary to establish a causal relationship between COVID-19 vaccines and the aforementioned autoimmune diseases.
It is important to emphasize that vaccines are generally safe and necessary for disease prevention. The benefits of COVID-19 vaccination significantly outweigh the theoretical risks, and we strongly encourage worldwide vaccination to build immune protection in the population. Nevertheless, it is our responsibility to remain vigilant and actively understand the serious adverse events associated with COVID-19 vaccines, critically evaluate vaccine safety, and increase public and healthcare worker awareness regarding vaccination. This will enable prompt identification, diagnosis, and treatment of these autoimmune diseases following vaccination through the recognition of their clinical and laboratory features.
Authors' contributions
Study concept and design: Q.G.L. and X.X.L. Literature search and data collection: M.G. Drafting of the manuscript: M.G., X.X.L. and Q.G.L. X.M.C. and Q.G.L. made critical revisions of the review. All authors read and approved the final manuscript. Q.G.L. takes responsibility for the integrity of the content and and the accuracy of analysis.
Funding
This study was supported by National Natural Science Foundation of China (81830019), Beijing Natural Science Foundation (7202188) and Haihe Laboratory of Cell Ecosystem Innovation Fund (22HHXBSS00002).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- 1.https://www.who.int/director-general/speeches/detail/who-director-general-s-openingremarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed 1 January 2023)
- 2.https://coronavirus.jhu.edu (accessed 31 January 2023)
- 3.Coping with COVID Nat Immunol. 2021;22:255. doi: 10.1038/s41590-021-00900-w. [DOI] [PubMed] [Google Scholar]
- 4.https://www.who.int/health-topics/vaccines-and-immunization#tab=tab_1 (accessed 1 January 2023)
- 5.https://www.who.int/teams/blueprint/covid-19/covid-19-vaccine-tracker-and-landscape (accessed 31 January 2023)
- 6.Dhama K., Dhawan M., Tiwari R., Emran T.B., Mitra S., Rabaan A.A., et al. COVID-19 intranasal vaccines: current progress, advantages, prospects, and challenges. Hum Vaccines Immunother. 2022;18:2045853. doi: 10.1080/21645515.2022.2045853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The COVID-19 Genomics UK (COG-UK) consortium, Volz E., Mishra S., Chand M., Barrett J.C., Johnson R., et al. Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature. 2021;593:266–269. doi: 10.1038/s41586-021-03470-x. [DOI] [PubMed] [Google Scholar]
- 8.Tegally H., Wilkinson E., Giovanetti M., Iranzadeh A., Fonseca V., Giandhari J., et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature. 2021;592:438–443. doi: 10.1038/s41586-021-03402-9. [DOI] [PubMed] [Google Scholar]
- 9.Faria N.R., Mellan T.A., Whittaker C., Claro I.M., Candido D.S., Mishra S., et al. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science. 2021;372:815–821. doi: 10.1126/science.abh2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novelli G., Colona V., Pandolfi P. A focus on the spread of the delta variant of SARS-CoV-2 in India. Indian J Med Res. 2021;153:537. doi: 10.4103/ijmr.ijmr_1353_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao S., Guo H., Luo G. Omicron variant (B.1.1.529) of SARS-CoV-2, a global urgent public health alert! J Med Virol. 2022;94:1255–1256. doi: 10.1002/jmv.27491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tibbetts J.H. Will a COVID vaccine be accepted? BioScience. 2020:biaa133. doi: 10.1093/biosci/biaa133. [DOI] [Google Scholar]
- 13.Fenoglio R., Lalloni S., Marchisio M., Oddone V., De Simone E., Del Vecchio G., et al. New Onset Biopsy-Proven Nephropathies after COVID Vaccination. Am J Nephrol. 2022;53:325–330. doi: 10.1159/000523962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safary A., Esalatmanesh K., Eftekharsadat A.T., Jafari Nakjavani M.-R., Khabbazi A. Autoimmune inflammatory rheumatic diseases post-COVID-19 vaccination. Int Immunopharmacol. 2022;110 doi: 10.1016/j.intimp.2022.109061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Londoño M.-C., Gratacós-Ginès J., Sáez-Peñataro J. Another case of autoimmune hepatitis after SARS-CoV-2 vaccination – still casualty? J Hepatol. 2021;75:1248–1249. doi: 10.1016/j.jhep.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martino M.D., Chiappini E., Galli L. 2013. Vaccines and Autoimmunity. [DOI] [PubMed] [Google Scholar]
- 17.Lai K.N., Tang S.C.W., Schena F.P., Novak J., Tomino Y., Fogo A.B., et al. IgA nephropathy. Nat Rev Dis Primer. 2016;2:16001. doi: 10.1038/nrdp.2016.1. [DOI] [PubMed] [Google Scholar]
- 18.Abdel-Qader D.H., Hazza Alkhatatbeh I., Hayajneh W., Annab H., Al Meslamani A.Z., Elmusa R.A. IgA nephropathy in a pediatric patient after receiving the first dose of Pfizer-BioNTech COVID-19 vaccine. Vaccine. 2022;40:2528–2530. doi: 10.1016/j.vaccine.2022.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohamed M.M.B., Wickman T.J., Fogo A.B., Velez J.C.Q. De Novo immunoglobulin A vasculitis following exposure to SARS-CoV-2 immunization. Ochsner J. 2021;21:395–401. doi: 10.31486/toj.21.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niel O., Florescu C. IgA nephropathy presenting as rapidly progressive glomerulonephritis following first dose of COVID-19 vaccine. Pediatr Nephrol. 2022;37:461–462. doi: 10.1007/s00467-021-05351-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okada M., Kikuchi E., Nagasawa M., Oshiba A., Shimoda M. An adolescent girl diagnosed with IgA nephropathy following the first dose of the COVID-19 vaccine. CEN Case Rep. 2022;11:376–379. doi: 10.1007/s13730-021-00679-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujita Y., Yoshida K., Ichikawa D., Shibagaki Y., Yazawa M. Abrupt worsening of occult IgA nephropathy after the first dose of SARS-CoV-2 vaccination. CEN Case Rep. 2022;11:302–308. doi: 10.1007/s13730-021-00670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokote S., Ueda H., Shimizu A., Okabe M., Yamamoto K., Tsuboi N., et al. IgA nephropathy with glomerular capillary IgA deposition following SARS-CoV-2 mRNA vaccination: a report of three cases. CEN Case Rep. 2022;11:499–505. doi: 10.1007/s13730-022-00707-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderegg M.A., Liu M., Saganas C., Montani M., Vogt B., Huynh-Do U., et al. De novo vasculitis after mRNA-1273 (Moderna) vaccination. Kidney Int. 2021;100:474–476. doi: 10.1016/j.kint.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klomjit N., Alexander M.P., Fervenza F.C., Zoghby Z., Garg A., Hogan M.C., et al. COVID-19 vaccination and glomerulonephritis. Kidney Int Rep. 2021;6:2969–2978. doi: 10.1016/j.ekir.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lo W.K., Chan K.W. Gross haematuria after mRNA COVID -19 vaccination in two patients with histological and clinical diagnosis of IgA nephropathy. Nephrology. 2022;27:110–111. doi: 10.1111/nep.13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanna C., Herrera Hernandez L.P., Bu L., Kizilbash S., Najera L., Rheault M.N., et al. IgA nephropathy presenting as macroscopic hematuria in 2 pediatric patients after receiving the Pfizer COVID-19 vaccine. Kidney Int. 2021;100:705–706. doi: 10.1016/j.kint.2021.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nihei Y., Kishi M., Suzuki H., Koizumi A., Yoshida M., Hamaguchi S., et al. IgA nephropathy with gross hematuria following COVID-19 mRNA vaccination. Intern Med. 2022;61:1033–1037. doi: 10.2169/internalmedicine.8787-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abramson M., Mon-Wei Yu S., Campbell K.N., Chung M., Salem F. IgA nephropathy after SARS-CoV-2 vaccination. Kidney Med. 2021;3:860–863. doi: 10.1016/j.xkme.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park K., Miyake S., Tai C., Tseng M., Andeen N.K., Kung V.L. Letter regarding: “A Case of Gross Hematuria and IgA Nephropathy Flare-Up Following SARS-CoV-2 Vaccination.”. Kidney Int Rep. 2021;6:2246–2247. doi: 10.1016/j.ekir.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim J.-H., Kim M.-S., Kim Y.-J., Han M.-H., Jung H.-Y., Choi J.-Y., et al. New-onset kidney diseases after COVID-19 vaccination: a case series. Vaccines. 2022;10:302. doi: 10.3390/vaccines10020302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kudose S., Friedmann P., Albajrami O., D’Agati V.D. Histologic correlates of gross hematuria following Moderna COVID-19 vaccine in patients with IgA nephropathy. Kidney Int. 2021;100:468–469. doi: 10.1016/j.kint.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horino T., Sawamura D., Inotani S., Ishihara M., Komori M., Ichii O. Newly diagnosed IgA nephropathy with gross haematuria following COVID-19 vaccination. QJM Int J Med. 2022;115:28–29. doi: 10.1093/qjmed/hcab305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srinivasan V., Geara A.S., Han S., Hogan J.J., Coppock G. Need for symptom monitoring in IgA nephropathy patients post COVID-19 vaccination. Clin Nephrol. 2022;97:193–194. doi: 10.5414/CN110689. [DOI] [PubMed] [Google Scholar]
- 35.Morisawa K., Honda M. Two patients presenting IgA nephropathy after COVID-19 vaccination during a follow-up for asymptomatic hematuria. Pediatr Nephrol. 2022;37:1695–1696. doi: 10.1007/s00467-022-05518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alonso M., Villanego F., Segurado Ó., Vigara L.A., Orellana C., Quiros P., et al. IgA de novo en trasplante renal tras vacunación frente a SARS-CoV-2. Nefrología. 2021 doi: 10.1016/j.nefro.2021.11.002. S0211699521002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mokos M., Bašić-Jukić N. IgA nephropathy following SARS-CoV -2 vaccination in a renal transplant recipient with a history of aristolochic acid nephropathy. Ther Apher Dial. 2022;26:667–668. doi: 10.1111/1744-9987.13765. [DOI] [PubMed] [Google Scholar]
- 38.Fenoglio R., Lalloni S., Marchisio M., Oddone V., De Simone E., Del Vecchio G., et al. New onset biopsy-proven nephropathies after COVID vaccination. Am J Nephrol. 2022;53:325–330. doi: 10.1159/000523962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y.-S., Yang C.-W., Tsai C.-C., Ang M.-D., Chou S.-F., Chiang W.-C., et al. Newly-diagnosed immunoglobulin A nephropathy with increased plasma galactose-deficient-IgA 1 antibody associated with mRNA COVID-19 vaccination: a case report. J Int Med Res. 2022;50 doi: 10.1177/03000605221129674. 030006052211296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ran E., Wang M., Wang Y., Liu R., Yi Y., Liu Y. New-onset crescent IgA nephropathy following the CoronaVac vaccine: A case report. Medicine (Baltimore) 2022;101 doi: 10.1097/MD.0000000000030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakatani S., Mori K., Morioka F., Hirata C., Tsuda A., Uedono H., et al. New-onset kidney biopsy-proven IgA vasculitis after receiving mRNA-1273 COVID-19 vaccine: case report. CEN Case Rep. 2022;11:358–362. doi: 10.1007/s13730-021-00677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ronco P., Beck L., Debiec H., Fervenza F.C., Hou F.F., Jha V., et al. Membranous nephropathy. Nat Rev Dis Primer. 2021;7:69. doi: 10.1038/s41572-021-00303-z. [DOI] [PubMed] [Google Scholar]
- 43.Caza T.N., Cassol C.A., Messias N., Hannoudi A., Haun R.S., Walker P.D., et al. Glomerular Disease in Temporal Association with SARS-CoV-2 Vaccination: A Series of 29 Cases. Kidney360. 2021;2:1770–1780. doi: 10.34067/KID.0005372021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thammathiwat T., Chompuk L., Worawichawong S., Boonpucknavig V., Sirilak S., Pongcharoen S., et al. Membranous nephropathy following full-dose of inactivated SARS-CoV-2 virus vaccination: a case report and literature review. Vaccines. 2022;11:80. doi: 10.3390/vaccines11010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Da Y., Goh G.H., Khatri P. A case of membranous nephropathy following Pfizer–BioNTech mRNA vaccination against COVID-19. Kidney Int. 2021;100:938–939. doi: 10.1016/j.kint.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gueguen L., Loheac C., Saidani N., Khatchatourian L. Membranous nephropathy following anti–COVID-19 mRNA vaccination. Kidney Int. 2021;100:1140–1141. doi: 10.1016/j.kint.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rashid W., Mousa H., Khan J., Ijaz F., Ezell G.D. A case of membranous nephropathy hypothesized to be associated with COVID-19 vaccine. Cureus. 2022 doi: 10.7759/cureus.24245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Psyllaki A., Stavrakaki I., Androvitsanea A., Gakiopoulou H., Petrakis I., Stylianou K. Two cases of glomerular involvement after vaccination against COVID-19: epiphenomenon or causality? Clin Kidney J. 2022;15:574–575. doi: 10.1093/ckj/sfab252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chavarot N., Padden M., Amrouche L., Malard S., Scemla A., Sberro-Soussan R., et al. De novo posttransplant membranous nephropathy following BNT162b2 mRNA COVID-19 vaccine in a kidney transplant recipient. Am J Transplant. 2022;22:3188–3189. doi: 10.1111/ajt.17166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paxton L., McMahon L., Wong L. De novo PLA2R positive membranous nephropathy following BNT162b2 mRNA COVID-19 vaccine. Intern Med J. 2022;52:2191–2192. doi: 10.1111/imj.15915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saigal M., Taduri G., Gudditi S., Herur S., Alaparthi P., Kinjarapu S. POS-872 Post covid vaccination- new onset glomerulonephritis- a mere co incidence or a impending reality?? Kidney Int Rep. 2022;7:S377. doi: 10.1016/j.ekir.2022.01.910. [DOI] [Google Scholar]
- 52.Fornara L., Musetti G., Guglielmetti G.E. De novo glomerulonephritides following bnt162b2 covid-19 vaccine: a case series. Nephrol. Dialysis Transplant. 2022 doi: 10.1093/ndt/gfac067.007. [DOI] [Google Scholar]
- 53.Garg A., Androga A.L., Grande P.J. POS-897 a case of de novo nell-1 membranous nephropathy post covid-19 vaccination. Kidney Int Rep. 2022;7:S389. doi: 10.1016/j.ekir.2022.01.936. [DOI] [Google Scholar]
- 54.McDonald I., Murray S.M., Reynolds C.J., Altmann D.M., Boyton R.J. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. Npj Vaccines. 2021;6:74. doi: 10.1038/s41541-021-00336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Debiec H., Ronco P. PLA 2 R autoantibodies and PLA 2 R glomerular deposits in membranous nephropathy. N Engl J Med. 2011;364:689–690. doi: 10.1056/NEJMc1011678. [DOI] [PubMed] [Google Scholar]
- 56.Anders H.-J., Saxena R., Zhao M., Parodis I., Salmon J.E., Mohan C. Lupus nephritis. Nat Rev Dis Primer. 2020;6:7. doi: 10.1038/s41572-019-0141-9. [DOI] [PubMed] [Google Scholar]
- 57.Patil S., Patil A. Systemic lupus erythematosus after COVID-19 vaccination: A case report. J Cosmet Dermatol. 2021;20:3103–3104. doi: 10.1111/jocd.14386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nune A., Iyengar K.P., Ish P., Varupula B., Musat C.A., Sapkota H.R. The Emergence of new-onset SLE following SARS-CoV-2 vaccination. QJM Int J Med. 2021;114:739–740. doi: 10.1093/qjmed/hcab229. [DOI] [PubMed] [Google Scholar]
- 59.Hidaka D., Ogasawara R., Sugimura S., Fujii F., Kojima K., Nagai J., et al. New-onset Evans syndrome associated with systemic lupus erythematosus after BNT162b2 mRNA COVID-19 vaccination. Int J Hematol. 2022;115:424–427. doi: 10.1007/s12185-021-03243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gamonal S.B.L., Marques N.C.V., Pereira H.M.B., Gamonal A.C.C. New-onset systemic lupus erythematosus after ChAdOX1 nCoV-19 and alopecia areata after BNT162b2 vaccination against SARS-CoV-2. Dermatol Ther. 2022:35. doi: 10.1111/dth.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Molina-Rios S., Rojas-Martinez R., Estévez-Ramirez G.M., Medina Y.F. Systemic lupus erythematosus and antiphospholipid syndrome after COVID-19 vaccination. A case report. Mod Rheumatol Case Rep. 2023;7:43–46. doi: 10.1093/mrcr/rxac018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang H., Sun Y., Lan C.E. Systemic lupus erythematosus with acrocyanosis after AstraZeneca COVID -19 vaccination. Kaohsiung J Med Sci. 2022;38:1230–1231. doi: 10.1002/kjm2.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alrashdi Mousa N., Saleh A.M., Khalid A., Alshaya A.K., Alanazi S.M.M. Systemic lupus erythematosus with acute pancreatitis and vasculitic rash following COVID-19 vaccine: a case report and literature review. Clin Rheumatol. 2022;41:1577–1582. doi: 10.1007/s10067-022-06097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raviv Y., Betesh-Abay B., Valdman-Grinshpoun Y., Boehm-Cohen L., Kassirer M., Sagy I. First presentation of systemic lupus erythematosus in a 24-year-old male following mRNA COVID-19 vaccine. Case Rep Rheumatol. 2022 doi: 10.1155/2022/9698138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Báez-Negrón L., Vilá L.M. New-onset systemic lupus erythematosus after mRNA SARS-CoV-2 vaccination. Case Rep Rheumatol. 2022;2022:1–4. doi: 10.1155/2022/6436839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lemoine C., Padilla C., Krampe N., Doerfler S., Morgenlander A., Thiel B., et al. Systemic lupus erythematous after Pfizer COVID-19 vaccine: a case report. Clin Rheumatol. 2022;41:1597–1601. doi: 10.1007/s10067-022-06126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khanna U., Oprea Y., Mir A., Halverstam C. New diagnosis of systemic lupus erythematosus after COVID-19 vaccination: A case report and review of literature. JAAD Case Rep. 2022;30:30–34. doi: 10.1016/j.jdcr.2022.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ghang B., Kim S., Kim J. Autoimmune rheumatic disease after SARS-CoV-2 vaccination. J Med Virol. 2022;94:5618–5620. doi: 10.1002/jmv.28092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Segal Y., Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol. 2018;15:586–594. doi: 10.1038/cmi.2017.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Teijaro J.R., Farber D.L. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21:195–197. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guimarães L.E. Vaccines, adjuvants and autoimmunity. Pharmacol Res. 2015 doi: 10.1016/j.phrs.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Agmon-Levin N., Zafrir Y., Paz Z., Shilton T., Zandman-Goddard G., Shoenfeld Y. Ten cases of systemic lupus erythematosus related to hepatitis B vaccine. Lupus. 2009;18:1192–1197. doi: 10.1177/0961203309345732. [DOI] [PubMed] [Google Scholar]
- 73.Geier D.A., Geier M.R. Quadrivalent human papillomavirus vaccine and autoimmune adverse events: a case–control assessment of the vaccine adverse event reporting system (VAERS) database. Immunol Res. 2017;65:46–54. doi: 10.1007/s12026-016-8815-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zavala-Miranda M.F., González-Ibarra S.G., Pérez-Arias A.A., Uribe-Uribe N.O., Mejia-Vilet J.M. New-onset systemic lupus erythematosus beginning as class V lupus nephritis after COVID-19 vaccination. Kidney Int. 2021;100:1340–1341. doi: 10.1016/j.kint.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim H.J. New-onset class III lupus nephritis with multi-organ involvement after COVID-19 vaccination. Kidney Int. 2022 doi: 10.1016/j.kint.2022.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nelson M.C., Rytting H., Greenbaum L.A., Goldberg B. Presentation of SLE after COVID vaccination in a pediatric patient. BMC Rheumatol. 2022;6:81. doi: 10.1186/s41927-022-00313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rosenberg A.Z., Kopp J.B. Focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2017;12:502–517. doi: 10.2215/CJN.05960616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jha V., Akal R., Sharma A., Mahapatra D. Post covishield (ChAdOx1 nCoV-19) vaccination: New onset focal segmental glomerulosclerosis resistant to steroid and calcineurin inhibitor. Indian J Nephrol. 2022;32:378. doi: 10.4103/ijn.ijn_23_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dormann H., Knüppel-Ruppert A., Amann K., Erley C. Nephrotic syndrome after vaccination against COVID-19: three new cases from Germany. Dtsch Ärztebl Int. 2021 doi: 10.3238/arztebl.m2021.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lim C.A., Lee H.S., Yoon S., Kim E.J., Seo J.W., Koo J.-R., et al. Focal segmental glomerulosclerosis following the Pfizer-BioNTech COVID-19 vaccine. Kidney Res Clin Pract. 2022;41:263–266. doi: 10.23876/j.krcp.21.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marega A., Pizzolitto S. De novo double glomerulopathy (membranous nephropathy, MN and collapsing focal segmental glomerulosclerosis, cfsgs) associated to positive myeloperoxidase-o (mpo) antibody following pfizer – biontech mrna vaccination covid 19. Nephrol. Dialysis Transplant. 2022 doi: 10.1093/ndt/gfac067.029. [DOI] [Google Scholar]
- 82.Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L.M., Vormehr M., et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 83.Kim B.C., Kim H.S., Han K.H., Han S.Y., Jo H.A. A case report of MPO-ANCA-associated vasculitis following heterologous mRNA1273 COVID-19 booster vaccination. J Korean Med Sci. 2022;37 doi: 10.3346/jkms.2022.37.e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Fabritiis M., Angelini M.L., Fabbrizio B., Cenacchi G., Americo C., Cristino S., et al. Renal thrombotic microangiopathy in concurrent COVID-19 vaccination and infection. Pathogens. 2021;10:1045. doi: 10.3390/pathogens10081045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bitzan M., Zieg J. Influenza-associated thrombotic microangiopathies. Pediatr Nephrol. 2018;33:2009–2025. doi: 10.1007/s00467-017-3783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smolen J.S., Aletaha D., Barton A., Burmester G.R., Emery P., Firestein G.S., et al. Rheumatoid arthritis. Nat Rev Dis Primer. 2018;4:18001. doi: 10.1038/nrdp.2018.1. [DOI] [PubMed] [Google Scholar]
- 87.Yonezawa H., Ohmura S., Ohkubo Y., Miyamoto T. New-onset seropositive rheumatoid arthritis following COVID-19 vaccination in a patient with seronegative status. Intern Med. 2022;61:3449–3452. doi: 10.2169/internalmedicine.0257-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nahra V., Makandura M., Mattar M., Anthony D.D. A case series on the COVID-19 vaccines and possible immune-related adverse events: a new challenge for the rheumatologists. Cureus. 2022 doi: 10.7759/cureus.29660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morikawa M.M., Harada M., Kishimoto E., Suzuki K., Nakagawa E., Hiramatsu T., et al. BNT162b2 coronavirus disease-2019 vaccination accelerated rheumatoid arthritis disease activity in chronic eosinophilic pneumonia: A case report. Medicine (Baltimore) 2022;101 doi: 10.1097/MD.0000000000030806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baimukhamedov C., Makhmudov S., Botabekova A. Seropositive rheumatoid arthritis after vaccination against SARS-CoV-2 infection. Int J Rheum Dis. 2021;24:1440–1441. doi: 10.1111/1756-185X.14220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Watanabe T., Minaga K., Hara A., Yoshikawa T., Kamata K., Kudo M. Case report: new-onset rheumatoid arthritis following COVID-19 vaccination. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.859926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ruiz-Irastorza G., Crowther M., Branch W., Khamashta M.A. Antiphospholipid syndrome. The Lancet. 2010;376:1498–1509. doi: 10.1016/S0140-6736(10)60709-X. [DOI] [PubMed] [Google Scholar]
- 93.Moreno-Torres V., Gutiérrez Á., Valdenebro M., Ortega A., Cítores M.-J., Montero E. Catastrophic antiphospholipid syndrome triggered by mRNA COVID-19 vaccine. Clin Exp Rheumatol. 2021 doi: 10.55563/clinexprheumatol/s3sbgu. [DOI] [PubMed] [Google Scholar]
- 94.Molina-Rios S., Rojas-Martinez R., Estévez-Ramirez G.M., Medina Y.F. Systemic lupus erythematosus and antiphospholipid syndrome after COVID-19 vaccination. A case report. Mod Rheumatol Case Rep. 2023;7:43–46. doi: 10.1093/mrcr/rxac018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sfriso P., Bindoli S., Galozzi P. Adult-onset still’s disease: molecular pathophysiology and therapeutic advances. Drugs. 2018;78:1187–1195. doi: 10.1007/s40265-018-0956-9. [DOI] [PubMed] [Google Scholar]
- 96.Sharabi A., Shiber S., Molad Y. Adult-onset still’s disease following mRNA COVID-19 vaccination. Clin Immunol. 2021;233 doi: 10.1016/j.clim.2021.108878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Risal U., Subedee A., Pangeni R., Pandey R., Pandey S., Adhikari S., et al. Case report: adult onset still’s disease after vaccination against Covid-19. Wellcome Open Res. 2022;6:333. doi: 10.12688/wellcomeopenres.17345.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Matsuda M., Funakubo Asanuma Y., Yokota K., Sakai S., Yazawa H., Maruyama T., et al. New-onset adult-onset still’s disease following COVID-19 vaccination: three case reports and a literature review. Intern Med. 2023;62:299–305. doi: 10.2169/internalmedicine.0590-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Park S.Y., Lee K.-H. Adult-onset still’s disease after BNT162b2 mRNA COVID-19 vaccine. J Korean Med Sci. 2021;36 doi: 10.3346/jkms.2021.36.e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weng J., Zhou L.L., Hahn T., Shojania K., Dutz J. Adult-onset still disease after ChAdOx1 nCOV-19 vaccination. J Rheumatol. 2023;50:290–291. doi: 10.3899/jrheum.220219. [DOI] [PubMed] [Google Scholar]
- 101.Gasparotto M., Bindoli S., Padoan R., Cozzi G., Depascale R., Zanatta E., et al. New onset and flare of rheumatic diseases following COVID-19 vaccination are mild and respond well to treatment: 9-month follow-up data from a single centre cohort. Clin Exp Rheumatol. 2021 doi: 10.55563/clinexprheumatol/vx44zn. [DOI] [PubMed] [Google Scholar]
- 102.Albertino L.F., Moulaz I.R., Zogheib T.F., Valentim M.Z.C., Machado K.L.L.L. Adult-onset Still’s disease after ChAdOx1 nCoV-19 vaccine: a possible association. Autopsy Case Rep. 2022;12 doi: 10.4322/acr.2021.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sweeney A., Tracey G., Garnham K. Adult-onset Still disease post-adenovirus vector COVID -19 vaccine. Intern Med J. 2021;51:2144–2145. doi: 10.1111/imj.15563. [DOI] [PubMed] [Google Scholar]
- 104.Padiyar S., Kamath N., Mathew J., Chandu A.S., Deodhar D., Shastry B.A., et al. New-onset Adult-onset Still’s disease-like syndrome after ChAdOx1 nCoV-19 vaccination—a case series with review of literature. Clin Rheumatol. 2022;41:1569–1575. doi: 10.1007/s10067-022-06065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Winichakoon P., Chanloung W., Nantsupawat T., Louthrenoo W. Adult-onset still’s disease-like syndrome following COVID-19 vaccination: a case report and review of the literature. Vaccines. 2022;10:1022. doi: 10.3390/vaccines10071022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Leone F., Cerasuolo P.G., Bosello S.L., Verardi L., Fiori E., Cocciolillo F., et al. Adult-onset Still’s disease following COVID-19 vaccination. Lancet Rheumatol. 2021;3:e678–e680. doi: 10.1016/S2665-9913(21)00218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Magliulo D., Narayan S., Ue F., Boulougoura A., Badlissi F. Adult-onset Still’s disease after mRNA COVID-19 vaccine. Lancet Rheumatol. 2021;3:e680–e682. doi: 10.1016/S2665-9913(21)00219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.AlQudari E.A., Alabdan L.I., Alkhathami A.A., Alotaibi M.D., Alhamzi H.A. Adult-onset Still’s disease after the ChAdOx1 nCoV-19 vaccine. Cureus. 2022 doi: 10.7759/cureus.21279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Baicus C., Delcea C., Pinte L., Dan G.A. Hyper-inflammation after COVID-19 mARN vaccination: at the crossroads of multisystem inflammatory disease and adult-onset Still’s disease. Does terminology matter? Rom J Intern Med. 2022;60:3–5. doi: 10.2478/rjim-2021-0035. [DOI] [PubMed] [Google Scholar]
- 110.Chua X.-H., Lin W.-L., Lee Y.-T. Adult-onset Still’s disease following coronavirus 2 (SARS-CoV-2) vaccination: a case report. Vaccines. 2022;10:1687. doi: 10.3390/vaccines10101687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kitching A.R., Anders H.-J., Basu N., Brouwer E., Gordon J., Jayne D.R., et al. ANCA-associated vasculitis. Nat Rev Dis Primer. 2020;6:71. doi: 10.1038/s41572-020-0204-y. [DOI] [PubMed] [Google Scholar]
- 112.Yadav R., Shah S., Chhetri S. ANCA-associated vasculitis following Johnson and Johnson COVID-19 vaccine. Ann Med Surg. 2022;79 doi: 10.1016/j.amsu.2022.104123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Suzuki M., Sekiguchi Y., Sasaki M., Inaba S., Oyama S., Inoue Y., et al. Antineutrophil cytoplasmic antibody-associated vasculitis after COVID-19 vaccination with Pfizer-BioNTech. Intern Med. 2022;61:2925–2929. doi: 10.2169/internalmedicine.9807-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim B.C., Kim H.S., Han K.H., Han S.Y., Jo H.A. A case report of MPO-ANCA-associated vasculitis following heterologous mRNA1273 COVID-19 booster vaccination. J Korean Med Sci. 2022;37 doi: 10.3346/jkms.2022.37.e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Al-Yafeai Z., Horn B.J.M., Terraccaine W., Jose A., Krishnan P. 2022. A Case of Antineutrophil Cytoplasmic Antibodies (ANCA)-Associated Vasculitis Post COVID-19 Vaccination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Uddin K., Mohamed K.H., Agboola A.A., Naqvi W.A., Hussaini H., Mohamed A.S., et al. Antineutrophil cytoplasmic antibody (ANCA)-associated renal vasculitis following COVID-19 VACCINATION: A CASE REPORT AND LITERATURE REVIEW. Cureus. 2022 doi: 10.7759/cureus.30206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shakoor M.T., Birkenbach M.P., Lynch M. ANCA-associated vasculitis following Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021;78:611–613. doi: 10.1053/j.ajkd.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sekar A., Campbell R., Tabbara J., Rastogi P. ANCA glomerulonephritis after the moderna COVID-19 vaccination. Kidney Int. 2021;100:473–474. doi: 10.1016/j.kint.2021.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ma Y., Huang T., Xu G. ANCA-associated vasculitis following the CoronaVac vaccination. Ther Adv Chronic Dis. 2022;13 doi: 10.1177/20406223221125708. 204062232211257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Villa M., Díaz-Crespo F., Pérez de José A., Verdalles Ú., Verde E., Almeida Ruiz F., et al. A case of ANCA-associated vasculitis after AZD1222 (Oxford–AstraZeneca) SARS-CoV-2 vaccination: casualty or causality? Kidney Int. 2021;100:937–938. doi: 10.1016/j.kint.2021.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Christodoulou M., Iatridi F., Chalkidis G., Lioulios G., Nikolaidou C., Badis K., et al. ANCA-associated vasculitis may result as a complication to both SARS-CoV-2 infection and vaccination. Life. 2022;12:1072. doi: 10.3390/life12071072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Prabhahar A., Naidu G.S.R.S.N.K., Chauhan P., Sekar A., Sharma A., Sharma A., et al. ANCA-associated vasculitis following ChAdOx1 nCoV19 vaccination: case-based review. Rheumatol Int. 2022;42:749–758. doi: 10.1007/s00296-021-05069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Baier E., Olgemöller U., Biggemann L., Buck C., Tampe B. Dual-positive MPO- and PR3-ANCA-associated vasculitis following SARS-CoV-2 mRNA booster vaccination: a case report and systematic review. Vaccines. 2022;10:653. doi: 10.3390/vaccines10050653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Obata S., Hidaka S., Yamano M., Yanai M., Ishioka K., Kobayashi S. MPO-ANCA-associated vasculitis after the Pfizer/BioNTech SARS-CoV-2 vaccination. Clin Kidney J. 2022;15:357–359. doi: 10.1093/ckj/sfab181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Feghali E.J., Zafar M., Abid S., Santoriello D., Mehta S. De-novo antineutrophil cytoplasmic antibody-associated vasculitis following the mRNA-1273 (Moderna) vaccine for COVID-19. Cureus. 2021 doi: 10.7759/cureus.19616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dube G.K., Benvenuto L.J., Batal I. Antineutrophil cytoplasmic autoantibody–associated glomerulonephritis following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int Rep. 2021;6:3087–3089. doi: 10.1016/j.ekir.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Takenaka T., Matsuzaki M., Fujiwara S., Hayashida M., Suyama H., Kawamoto M. Myeloperoxidase anti-neutrophil cytoplasmic antibody positive optic perineuritis after mRNA coronavirus disease-19 vaccine. QJM Int J Med. 2021;114:737–738. doi: 10.1093/qjmed/hcab227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Anderegg M.A., Liu M., Saganas C., Montani M., Vogt B., Huynh-Do U., et al. De novo vasculitis after mRNA-1273 (Moderna) vaccination. Kidney Int. 2021;100:474–476. doi: 10.1016/j.kint.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.So D., Min K.-W., Jung W.Y., Han S.-W., Yu M.-Y. Microscopic polyangiitis following mRNA COVID-19 vaccination: a case report. J Korean Med Sci. 2022;37 doi: 10.3346/jkms.2022.37.e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lazarewicz K., Watson P. Giant cell arteritis. BMJ. 2019 doi: 10.1136/bmj.l1964. [DOI] [PubMed] [Google Scholar]
- 131.Cadiou S., Perdriger A., Ardois S., Albert J.-D., Berthoud O., Lescoat A., et al. SARS-CoV-2, polymyalgia rheumatica and giant cell arteritis: COVID-19 vaccine shot as a trigger? Comment on: “Can SARS-CoV-2 trigger relapse of polymyalgia rheumatica?” by Manzo et al. Joint Bone Spine 2021;88:105150. Joint Bone Spine. 2022;89:105282. doi: 10.1016/j.jbspin.2021.105282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sauret A., Stievenart J., Smets P., Olagne L., Guelon B., Aumaître O., et al. Case of giant cell arteritis after SARS-CoV-2 vaccination: a particular phenotype? J Rheumatol. 2022;49 doi: 10.3899/jrheum.210724. 120.1-120. [DOI] [PubMed] [Google Scholar]
- 133.Gambichler T., Krogias C., Tischoff I., Tannapfel A., Gold R., Susok L. Bilateral giant cell arteritis with skin necrosis following SARS-CoV-2 vaccination. Br J Dermatol. 2022:186. doi: 10.1111/bjd.20824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mejren A., Sørensen C., Gormsen L., Tougaard R., Nielsen B. Large-vessel giant cell arteritis after COVID-19 vaccine. Scand J Rheumatol. 2022;51:154–155. doi: 10.1080/03009742.2021.1961401. [DOI] [PubMed] [Google Scholar]
- 135.Anzola A.M., Trives L., Martínez-Barrio J., Pinilla B., Álvaro-Gracia J.M., Molina-Collada J. New-onset giant cell arteritis following COVID-19 mRNA (BioNTech/Pfizer) vaccine: a double-edged sword? Clin Rheumatol. 2022;41:1623–1625. doi: 10.1007/s10067-021-06041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mungmungpuntipantip R., Wiwanitkit V. COVID-19 vaccination and large-vessel giant cell arteritis. Scand J Rheumatol. 2022;51:329. doi: 10.1080/03009742.2021.1995985. [DOI] [PubMed] [Google Scholar]
- 137.Ramos-Casals M., Sainz-de-la-Maza M., Muxí A. COVID-19 vaccination unveiling subclinical Sjögren’s syndrome. Clin Exp Rheumatol. 2021;39:228–229. doi: 10.55563/clinexprheumatol/u1v6z1. [DOI] [PubMed] [Google Scholar]
- 138.Tagini F., Carrel L., Fallet B., Gachoud D., Ribi C., Monti M. Behçet’s-like adverse event or inaugural Behçet’s disease after SARS-CoV-2 mRNA-1273 vaccination? Rheumatology. 2022;61:e112–e113. doi: 10.1093/rheumatology/keab751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mieli-Vergani G., Vergani D., Czaja A.J., Manns M.P., Krawitt E.L., Vierling J.M., et al. Autoimmune hepatitis. Nat Rev Dis Primer. 2018;4:18017. doi: 10.1038/nrdp.2018.17. [DOI] [PubMed] [Google Scholar]
- 140.Bril F., Al Diffalha S., Dean M., Fettig D.M. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: causality or casualty? J Hepatol. 2021;75:222–224. doi: 10.1016/j.jhep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tiniakos D.G., Brain J.G., Bury Y.A. Role of histopathology in autoimmune hepatitis. Dig Dis. 2015;33:53–64. doi: 10.1159/000440747. [DOI] [PubMed] [Google Scholar]
- 142.Hartl J., Miquel R., Zachou K., Wong G.-W., Asghar A., Pape S., et al. Features and outcome of AIH patients without elevation of IgG. JHEP Rep. 2020;2 doi: 10.1016/j.jhepr.2020.100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rocco A., Sgamato C., Compare D., Nardone G. Autoimmune hepatitis following SARS-CoV-2 vaccine: May not be a casuality. J Hepatol. 2021;75:728–729. doi: 10.1016/j.jhep.2021.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Goulas A., Kafiri G., Kranidioti H., Manolakopoulos S. A typical autoimmune hepatitis (AIH) case following Covid-19 mRNA vaccination. More than a coincidence? Liver Int. 2022;42:254–255. doi: 10.1111/liv.15092. [DOI] [PubMed] [Google Scholar]
- 145.Ghielmetti M., Schaufelberger H.D., Mieli-Vergani G., Cerny A., Dayer E., Vergani D., et al. Acute autoimmune-like hepatitis with atypical anti-mitochondrial antibody after mRNA COVID-19 vaccination: A novel clinical entity? J Autoimmun. 2021;123 doi: 10.1016/j.jaut.2021.102706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pinazo-Bandera J.M., Hernández-Albújar A., García-Salguero A.I., Arranz-Salas I., Andrade R.J., Robles-Díaz M. Acute hepatitis with autoimmune features after COVID-19 vaccine: coincidence or vaccine-induced phenomenon? Gastroenterol Rep. 2022;10:goac014. doi: 10.1093/gastro/goac014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rela M., Jothimani D., Vij M., Rajakumar A., Rammohan A. Auto-immune hepatitis following COVID vaccination. J Autoimmun. 2021;123 doi: 10.1016/j.jaut.2021.102688. [DOI] [PubMed] [Google Scholar]
- 148.Shahrani S., Sooi C.Y., Hilmi I.N., Mahadeva S. Autoimmune hepatitis (AIH) following coronavirus (COVID -19) vaccine—No longer exclusive to mRNA vaccine? Liver Int. 2022;42:2344–2345. doi: 10.1111/liv.15350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Garrido I., Lopes S., Simões M.S., Liberal R., Lopes J., Carneiro F., et al. Autoimmune hepatitis after COVID-19 vaccine – more than a coincidence. J Autoimmun. 2021;125 doi: 10.1016/j.jaut.2021.102741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Avci E., Abasiyanik F. Autoimmune hepatitis after SARS-CoV-2 vaccine: New-onset or flare-up? J Autoimmun. 2021;125 doi: 10.1016/j.jaut.2021.102745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Erard D., Villeret F., Lavrut P.-M., Dumortier J. Autoimmune hepatitis developing after COVID 19 vaccine: Presumed guilty? Clin Res Hepatol Gastroenterol. 2022;46 doi: 10.1016/j.clinre.2021.101841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Clayton-Chubb D., Schneider D., Freeman E., Kemp W., Roberts S.K. Autoimmune hepatitis developing after the ChAdOx1 nCoV-19 (Oxford-AstraZeneca) vaccine. J Hepatol. 2021;75:1249–1250. doi: 10.1016/j.jhep.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Izagirre A., Arzallus T., Garmendia M., Torrente S., Castiella A., Zapata E.M. Autoimmune hepatitis following COVID-19 vaccination. J Autoimmun. 2022;132 doi: 10.1016/j.jaut.2022.102874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tan C.K., Wong Y.J., Wang L.M., Ang T.L., Kumar R. Autoimmune hepatitis following COVID-19 vaccination: True causality or mere association? J Hepatol. 2021;75:1250–1252. doi: 10.1016/j.jhep.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kang S.H., Kim M.Y., Cho M.Y., Baik S.K. Autoimmune hepatitis following vaccination for SARS-Cov-2 in Korea: coincidence or autoimmunity? J Korean Med Sci. 2022;37 doi: 10.3346/jkms.2022.37.e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fimiano F., D’Amato D., Gambella A., Marzano A., Saracco G.M., Morgando A. Autoimmune hepatitis or drug-induced autoimmune hepatitis following Covid-19 vaccination? Liver Int. 2022;42:1204–1205. doi: 10.1111/liv.15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mekritthikrai K., Jaru-Ampornpan P., Komolmit P., Thanapirom K. Autoimmune hepatitis triggered by COVID-19 vaccine: the first case from inactivated vaccine. ACG Case Rep J. 2022;9 doi: 10.14309/crj.0000000000000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vuille-Lessard É., Montani M., Bosch J., Semmo N. Autoimmune hepatitis triggered by SARS-CoV-2 vaccination. J Autoimmun. 2021;123 doi: 10.1016/j.jaut.2021.102710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hasegawa N., Matsuoka R., Ishikawa N., Endo M., Terasaki M., Seo E., et al. Autoimmune hepatitis with history of HCV treatment triggered by COVID-19 vaccination: case report and literature review. Clin J Gastroenterol. 2022;15:791–795. doi: 10.1007/s12328-022-01654-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Camacho-Domínguez L., Rodríguez Y., Polo F., Restrepo Gutierrez J.C., Zapata E., Rojas M., et al. COVID-19 vaccine and autoimmunity. A new case of autoimmune hepatitis and review of the literature. J Transl Autoimmun. 2022;5 doi: 10.1016/j.jtauto.2022.100140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mathew M., John S.B., Sebastian J., Ravi M.D. COVID-19 vaccine triggered autoimmune hepatitis: case report. Eur. J Hosp Pharm. 2022 doi: 10.1136/ejhpharm-2022-003485. ejhpharm-2022-003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ghorbani H., Rouhi T., Vosough Z., Shokri-shirvani J. Drug-induced hepatitis after Sinopharm COVID-19 vaccination: A case study of a 62-year-old patient. Int J Surg Case Rep. 2022;93 doi: 10.1016/j.ijscr.2022.106926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zin Tun G.S., Gleeson D., Al-Joudeh A., Dube A. Immune-mediated hepatitis with the Moderna vaccine, no longer a coincidence but confirmed. J Hepatol. 2022;76:747–749. doi: 10.1016/j.jhep.2021.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Palla P., Vergadis C., Sakellariou S., Androutsakos T. Letter to the editor: Autoimmune hepatitis after COVID-19 vaccination: A rare adverse effect? Hepatology. 2022;75:489–490. doi: 10.1002/hep.32156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ferronato M., Lenzi M., Muratori L. Liver injury with autoimmune features after vaccination against SARS-CoV-2: The verdict is still open. Eur J Intern Med. 2022 doi: 10.1016/j.ejim.2022.09.029. S095362052200351X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhou T., Fronhoffs F., Dold L., Strassburg C.P., Weismüller T.J. New-onset autoimmune hepatitis following mRNA COVID-19 vaccination in a 36-year-old woman with primary sclerosing cholangitis – should we be more vigilant? J Hepatol. 2022;76:218–220. doi: 10.1016/j.jhep.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.López Romero-Salazar F., Veras Lista M., Gómez-Domínguez E., Ibarrola-Andrés C., Muñoz Gómez R., Fernández Vázquez I. SARS-CoV-2 vaccine, a new autoimmune hepatitis trigger? Rev Esp Enfermedades Dig. 2022 doi: 10.17235/reed.2022.8820/2022. [DOI] [PubMed] [Google Scholar]
- 168.McShane C., Kiat C., Rigby J., Crosbie Ó. The mRNA COVID-19 vaccine – A rare trigger of autoimmune hepatitis? J Hepatol. 2021;75:1252–1254. doi: 10.1016/j.jhep.2021.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vojdani A., Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217 doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Muratori P., Serio I., Lalanne C., Lenzi M. Development of autoimmune hepatitis after influenza vaccination; trigger or killer? Clin Res Hepatol Gastroenterol. 2019;43:e95–e96. doi: 10.1016/j.clinre.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 171.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines — a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Talotta R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to “potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases”. Clin Immunol. 2021;224 doi: 10.1016/j.clim.2021.108665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Teijaro J.R., Farber D.L. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21:195–197. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Syed F.Z. Type 1 Diabetes Mellitus. Ann Intern Med. 2022;175 doi: 10.7326/AITC202203150. ITC33–48. [DOI] [PubMed] [Google Scholar]
- 175.Patrizio A., Ferrari S.M., Antonelli A., Fallahi P. A case of Graves’ disease and type 1 diabetes mellitus following SARS-CoV-2 vaccination. J Autoimmun. 2021;125 doi: 10.1016/j.jaut.2021.102738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tang X., He B., Liu Z., Zhou Z., Li X. Fulminant type 1 diabetes after COVID-19 vaccination. Diabetes Metab. 2022;48 doi: 10.1016/j.diabet.2022.101324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sato T., Kodama S., Kaneko K., Imai J., Katagiri H. Type 1 diabetes mellitus associated with nivolumab after second SARS-CoV-2 vaccination. Japan. Emerg Infect Dis. 2022;28:1518–1520. doi: 10.3201/eid2807.220127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yano M., Morioka T., Natsuki Y., Sasaki K., Kakutani Y., Ochi A., et al. New-onset Type 1 diabetes after COVID-19 mRNA vaccination. Intern Med. 2022;61:1197–1200. doi: 10.2169/internalmedicine.9004-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Moon H., Suh S., Park M.K. Adult-onset Type 1 diabetes development following COVID-19 mRNA vaccination. J Korean Med Sci. 2023;38 doi: 10.3346/jkms.2023.38.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sakurai K., Narita D., Saito N., Ueno T., Sato R., Niitsuma S., et al. Type 1 diabetes mellitus following COVID-19 RNA-based vaccine. J Diabetes Investig. 2022;13:1290–1292. doi: 10.1111/jdi.13781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sasaki H., Itoh A., Watanabe Y., Nakajima Y., Saisho Y., Irie J., et al. Newly developed type 1 diabetes after coronavirus disease 2019 vaccination: A case report. J Diabetes Investig. 2022;13:1105–1108. doi: 10.1111/jdi.13757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Aydoğan B.İ., Ünlütürk U., Cesur M. Type 1 diabetes mellitus following SARS-CoV-2 mRNA vaccination. Endocrine. 2022;78:42–46. doi: 10.1007/s12020-022-03130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bleve E., Venditti V., Lenzi A., Morano S., Filardi T. COVID-19 vaccine and autoimmune diabetes in adults: report of two cases. J Endocrinol Invest. 2022;45:1269–1270. doi: 10.1007/s40618-022-01796-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Brodsky R.A. Warm autoimmune hemolytic anemia. N Engl J Med. 2019;381:647–654. doi: 10.1056/NEJMcp1900554. [DOI] [PubMed] [Google Scholar]
- 185.De Bruyne S., Van Landeghem S., Schauwvlieghe A., Noens L. Life-threatening autoimmune hemolytic anemia following mRNA COVID-19 vaccination: don’t be too prudent with the red gold. Clin Chem Lab Med CCLM. 2022;60:e125–e128. doi: 10.1515/cclm-2022-0118. [DOI] [PubMed] [Google Scholar]
- 186.Jaydev F., Kumar V., Khatri J., Shahani S., Beganovic S. A Case of autoimmune hemolytic anemia after the first dose of COVID-19 mRNA-1273 vaccine with undetected pernicious anemia. Case Rep Hematol. 2022;2022:1–5. doi: 10.1155/2022/2036460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fatima Z., Reece B.R.A., Moore J.S., Means R.T. Autoimmune hemolytic anemia after mRNA COVID Vaccine. J Investig Med High Impact Case Rep. 2022;10 doi: 10.1177/23247096211073258. 232470962110732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Murdych T.M. A case of severe autoimmune hemolytic anemia after a receipt of a first dose of SARS-CoV-2 vaccine. Int J Lab Hematol. 2022:44. doi: 10.1111/ijlh.13653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gadi S.R.V., Brunker P.A.R., Al-Samkari H., Sykes D.B., Saff R.R., Lo J., et al. Severe autoimmune hemolytic anemia following receipt of SARS-CoV -2 mRNA vaccine. Transfusion (Paris) 2021;61:3267–3271. doi: 10.1111/trf.16672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Abasszade J.H., La P.B.D., Shelmerdine E., Nalpantidis A., Curtin N., Grigoriadis G., et al. Severe autoimmune haemolytic anaemia following SARS-CoV-2 vaccination in patients with treatment naïve B-cell neoplasms: a case series. Pathology (Phila) 2022;54:802–805. doi: 10.1016/j.pathol.2022.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Angileri F., Légaré S., Marino Gammazza A., Conway de Macario E., Macario A.J.L., Cappello F. Is molecular mimicry the culprit in the autoimmune haemolytic anaemia affecting patients with COVID-19? Br J Haematol. 2020:190. doi: 10.1111/bjh.16883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pavord S., Scully M., Hunt B.J., Lester W., Bagot C., Craven B., et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021;385:1680–1689. doi: 10.1056/NEJMoa2109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Fichadiya H., Singh V., Almeligy A., Shah A., Fichadiya H. An interesting case of myocarditis in a 60 year old male following administration of first dose of covid 19 MRNA vaccine. J Am Coll Cardiol. 2022;79:2377. doi: 10.1016/S0735-1097(22)03368-X. [DOI] [Google Scholar]
- 194.May Lee M., Bertolani M., Pierobon E., Lotti T., Feliciani C., Satolli F. Alopecia areata following COVID -19 vaccination: vaccine-induced autoimmunity? Int J Dermatol. 2022;61:634–635. doi: 10.1111/ijd.16113. [DOI] [PubMed] [Google Scholar]
- 195.Caron P. Autoimmune and inflammatory thyroid diseases following vaccination with SARS-CoV-2 vaccines: from etiopathogenesis to clinical management. Endocrine. 2022;78:406–417. doi: 10.1007/s12020-022-03118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.McKean N., Chircop C. Guillain-Barré syndrome after COVID-19 vaccination. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-244125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kanduc D., Shoenfeld Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: implications for the vaccine. Immunol Res. 2020;68:310–313. doi: 10.1007/s12026-020-09152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Israeli E., Agmon-Levin N., Blank M., Shoenfeld Y. Adjuvants and autoimmunity. Lupus. 2009;18:1217–1225. doi: 10.1177/0961203309345724. [DOI] [PubMed] [Google Scholar]
- 199.Agmon-Levin N., Paz Z., Israeli E., Shoenfeld Y. Vaccines and autoimmunity. Nat Rev Rheumatol. 2009;5:648–652. doi: 10.1038/nrrheum.2009.196. [DOI] [PubMed] [Google Scholar]
- 200.Gavin A.L., Hoebe K., Duong B., Ota T., Martin C., Beutler B., et al. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Perricone C., Colafrancesco S., Mazor R.D., Soriano A., Agmon-Levin N., Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) 2013: Unveiling the pathogenic, clinical and diagnostic aspects. J Autoimmun. 2013;47:1–16. doi: 10.1016/j.jaut.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 202.Pacheco Y., Acosta-Ampudia Y., Monsalve D.M., Chang C., Gershwin M.E., Anaya J.-M. Bystander activation and autoimmunity. J Autoimmun. 2019;103 doi: 10.1016/j.jaut.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 203.Kim T.-S., Shin E.-C. The activation of bystander CD8+ T cells and their roles in viral infection. Exp Mol Med. 2019;51:1–9. doi: 10.1038/s12276-019-0316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tough D.F., Borrow P., Sprent J. Induction of Bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 205.Boyman O. Bystander activation of CD4 + T cells: HIGHLIGHTS. Eur J Immunol. 2010;40:936–939. doi: 10.1002/eji.201040466. [DOI] [PubMed] [Google Scholar]
- 206.Powell A.M., Black M.M. Epitope spreading: protection from pathogens, but propagation of autoimmunity?: Epitope spreading. Clin Exp Dermatol. 2001;26:427–433. doi: 10.1046/j.1365-2230.2001.00852.x. [DOI] [PubMed] [Google Scholar]
- 207.Cornaby C., Gibbons L., Mayhew V., Sloan C.S., Welling A., Poole B.D. B cell epitope spreading: Mechanisms and contribution to autoimmune diseases. Immunol Lett. 2015;163:56–68. doi: 10.1016/j.imlet.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 208.Dörner T., Giesecke C., Lipsky P.E. Mechanisms of B cell autoimmunity in SLE. Arthritis Res Ther. 2011;13:243. doi: 10.1186/ar3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Shen X. Boosting immunity to Omicron. Nat Med. 2022;28:445–446. doi: 10.1038/s41591-022-01727-0. [DOI] [PubMed] [Google Scholar]
- 210.Vogel L. An early look at Omicron. Can Med Assoc J. 2022;194:E58. doi: 10.1503/cmaj.1095982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Li M., Wang H., Tian L., Pang Z., Yang Q., Huang T., et al. COVID-19 vaccine development: milestones, lessons and prospects. Signal Transduct Target Ther. 2022;7:146. doi: 10.1038/s41392-022-00996-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.