Abstract
Background:
Coronary artery calcium (CAC) can be identified on non-gated chest CTs, but this finding is not consistently incorporated into care. A deep learning algorithm enables opportunistic CAC screening of non-gated chest CTs. Our objective was to evaluate the impact of notifying clinicians and patients of incidental CAC on statin initiation.
Methods:
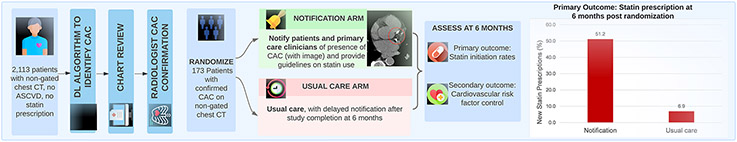
NOTIFY-1 was a randomized quality improvement project in the Stanford healthcare system. Patients without known atherosclerotic cardiovascular disease (ASCVD) or prior statin prescription were screened for CAC on a prior non-gated chest CT from 2014-2019 using a validated deep learning algorithm with radiologist confirmation. Patients with incidental CAC were randomized to notification of the primary care clinician and patient versus usual care. Notification included a patient-specific image of CAC and guideline recommendations regarding statin use. The primary outcome was statin prescription within 6 months.
Results:
Among 2,113 patients who met initial clinical inclusion criteria, CAC was identified by the algorithm in 424 patients. After additional exclusions following chart review, a radiologist confirmed CAC among 173 of 194 patients (89.2%) who were randomized to notification or usual care. At 6 months, the statin prescription rate was 51.2% (44/86) in the notification arm versus 6.9% (6/87) with usual care (p<0.001). There was also more coronary artery disease testing in the notification arm (15.1% [13/86] vs. 2.3% [2/87], p=0.008).
Conclusions:
Opportunistic CAC screening of prior non-gated chest CTs followed by clinician and patient notification led to a significant increase in statin prescriptions. Further research is needed to determine whether this approach can reduce ASCVD events.
Over 700,000 Americans have a first acute myocardial infarction or die from coronary artery disease annually.1 Many events could be avoided with greater application of preventive interventions among individuals at increased risk, including statin therapy. Multiple analyses have demonstrated sub-optimal statin therapy rates compared with guideline recommendations.2-4 Strategies to promote shared decision-making discussions between patients and clinicians are needed to address patient-level factors that may influence decisions to initiate statin therapy.5
Coronary artery calcium (CAC) testing is a promising approach to identify high-risk individuals and motivate adoption of preventive interventions. The presence of CAC is a strong predictor of atherosclerotic cardiovascular disease (ASCVD) events.6 The 2018 American College of Cardiology (ACC)/American Heart Association (AHA) Guidelines on Management of Blood Cholesterol include a IIa recommendation for initiating statin treatment among patients with CAC≥100, CAC≥75th percentile for age and sex, or CAC>0 with age ≥55.7 However, traditional CAC testing, which is performed on ECG-gated, non-contrast computed tomography (CT) scans, is rarely performed. However, over 19 million non-gated, non-contrast chest CT scans are performed annually for reasons other than to measure CAC.8 We previously developed a deep learning algorithm to estimate CAC score (DL-CAC) on non-gated, non-contrast chest CT scans to efficiently implement opportunistic screening for incidental CAC.9 For detecting CAC, the DL-CAC algorithm demonstrated a sensitivity of 82–94%, a specificity of 79%-100%, and a positive predictive value of 87–100% across 4 external datasets.
With no prior randomized studies using incidental CAC, the impact of notifying clinicians and patients regarding the presence of incidental CAC is unknown. We designed a randomized quality improvement project, NOTIFY-1, to understand whether notifying primary care clinicians and patients of incidental CAC would impact statin prescription rates among statin-naïve patients without known ASCVD.
METHODS
This was a prospective, randomized quality improvement project comparing notification of incidental CAC on prior non-gated chest CT scans with usual care among statin-naïve patients without known ASCVD in the Stanford Healthcare System. This project was motivated by institutional efforts to increase primary prevention statin therapy among high-risk patients. The QI project was deemed exempt from human subjects research requirements by the Stanford Institutional Review Board. The project was registered on clinicaltrials.gov (NCT04789278).10 To protect confidentiality, de-identified data are available from the corresponding author only on reasonable request.
Study Population
Patients under 85 years old with any amount of CAC on a non-contrast, non-gated chest CT scan between 2014-2019 were considered for inclusion. Patients required a Stanford primary care clinician or a Stanford endocrinologist with whom they had a prior encounter (in-person or telehealth) in the prior 2 years. Patients with an ASCVD diagnosis, prior statin prescription, or prior invasive coronary angiography or coronary CT angiography were excluded. Patients with dementia, hospice enrollment, metastatic cancer or non-metastatic cancer receiving treatment, or other advanced diseases with limited life expectancy were also excluded. Exclusions were identified in two stages: first via structured electronic health record (EHR) data review, and second via manual chart review (see Figure 1 for Study Flow Diagram). Inclusion and exclusion criteria are listed in Supplement Table S1.
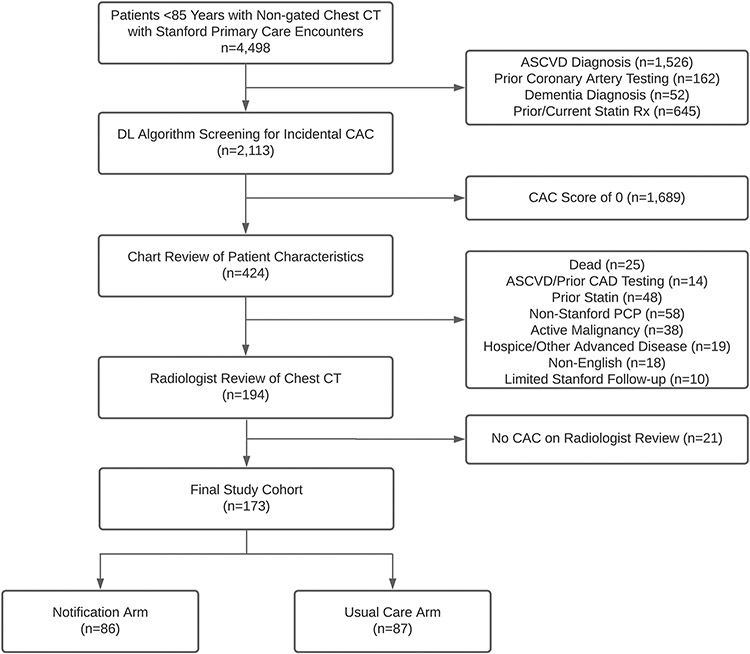
Figure 1. Study Flow Diagram.
Abbreviations: ASCVD: atherosclerotic cardiovascular disease; CAC: Coronary artery calcium; CT: computed tomography.
Identification of CAC
Following the initial exclusions using structured EHR data, the DL-CAC algorithm identified potentially eligible patients using the most recent non-gated chest CT scan between 2014-2019. The algorithm calculated the DL-CAC score in Agatston units; patients with a DL-CAC score of 0 were excluded. Following manual chart review of exclusion criteria, a radiologist reviewed each chest CT to confirm the presence of CAC.
Notification
Eligible patients with confirmed CAC were randomized 1:1 to notification or usual care via permuted block randomization using an online randomization module by study personnel. Patients randomized to notification first had an EHR message sent to their primary care clinician or endocrinologist. The clinician message (Supplement Figure S1) notified them regarding the presence of CAC on the prior non-gated chest CT. The letter included an axial image of the patient’s chest CT with a circle around the CAC and a reference to the 2018 American College of Cardiology (ACC)/American Heart Association (AHA) Guidelines on Management of Blood Cholesterol, which included a IIa recommendation for statin treatment of patients with CAC.7 The letter to clinicians stated that their patient would be contacted in 2 weeks with the same information unless they thought patient notification would be clinically inappropriate and recommended against it. Before the start of the project, we held educational sessions regarding CAC with primary care leadership and a subset of primary care clinicians in the Stanford Healthcare System.
Two weeks after clinician notification, messages were sent to patients via the EHR patient portal. These messages (Supplement Figure S2) noted the increased cardiovascular risk associated with the presence of CAC, included the same CT image with CAC encircled as in the clinician notification, and recommended discussion of risk-reducing interventions with their clinician including statin use. The notification did not include the DL-CAC score. If patients did not open their message within 2 weeks, we sent the same letter via postal mail.
The EHR was reviewed for documentation of any discussions regarding incidental CAC or statins. For patients without any discussion with their care team or prescription of a statin within 3 months, we resent messages once to both the clinician and patient.
This project used an adaptive two-stage design. In the first stage, 50 patients were randomized to notification versus usual care. In the second stage, the remainder of the cohort was randomized. This allowed potential modification to the protocol based on feedback from patients and clinicians. There were no modifications to the intervention after the first stage. Therefore, both stages were pooled for the analysis.
If the intervention increased statin rates at 6 months and was deemed acceptable by primary care at the end of this project, we planned to also send notifications to the usual care arm following the 6-month follow-up.
Outcomes
The primary outcome was the statin prescription rate within 6 months of randomization. We evaluated statin prescription rates at 6 weeks, 3 months, and 6 months post-randomization. As secondary outcomes, we captured discussions of statin therapy, aspirin rates, anti-hypertensive therapy rates, individual biological cardiovascular risk factors, and cardiovascular resource use. The biological cardiovascular risk factors extracted from the EHR were last systolic blood pressure, lipid levels (total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglycerides), hemoglobin A1c, and body mass index during follow-up. Using lipid measurements, systolic blood pressure, and antihypertensive therapy rates during follow-up, we calculated the 10-year risk of atherosclerotic cardiovascular disease events using the ACC/AHA Pooled Cohort Equations. For resource use, we captured the number of primary care encounters (in-person or telehealth), total cardiology encounters, new cardiology encounters, and CAD testing. CAD testing included coronary CT angiograms, CAC scans, stress tests, and invasive coronary angiograms. We evaluated diagnostic testing as a pooled composite outcome and as individual outcomes. All outcomes were assessed via the EHR by a non-blinded investigator (SN).
Statistical Analysis
The power analysis found that 150 patients would provide 87% power for a 20% absolute difference in statin prescription rates. All analyses were conducted with the intention-to-treat principle. The primary analysis compared statin prescription rates at 6 months across arms using Fisher’s exact test. The primary outcome was also compared across pre-specified subgroups: above and below median age, sex, ethnicity/race, low-density lipoprotein cholesterol level >100mg/dL or ≤100mg/dL, time since last primary care/endocrinology visit stratified at the median, antihypertensive medication use, and the DL-CAC score stratified at the study median. Heterogeneity in the effect across subgroups was tested via the Tarone test.
For secondary outcomes, we compared continuous variables (laboratory values, systolic blood pressure, and 10-year ASCVD risk) using analysis of covariance with adjustment for baseline value and age. For binary outcomes (lab testing, CAD testing, and new cardiology encounters), we used logistic regression with adjustment for age. For the ordinal resource use outcomes (number of primary care encounters, cardiology encounters, and blood pressure medications), we used negative binomial regression with adjustment for age and baseline frequency (e.g., number of cardiology encounters in the prior year). Adjustment variables were identified a priori. We repeated all analyses for secondary outcomes without adjustment.
There were missing values for baseline and follow-up lab values, baseline and follow-up 10-year ASCVD risk, and follow-up body mass index. For missing data, we performed a complete case analysis and two imputation analyses: last observation carried forward and multiple imputation with chained equations. With multiple imputation with chained equations, we imputed 100 datasets using missing variables, randomization allocation, baseline values, and comorbidities. Further statistical analysis details are included in the supplement.
Statistical significance was evaluated at a two-sided p-value threshold of 0.05. All statistical analyses were performed in STATA v16 (StataCorp LLC).
RESULTS
There were 2,113 patients with non-gated, non-contrast chest CTs between 2014-2019 after excluding patients with prior diagnoses of ASCVD or dementia, statin prescription, or coronary angiography (Figure 1). Among this cohort, 424 (20.1%) were identified as having CAC on non-gated chest CT by the DL-CAC algorithm. Of these, an additional 230 patients were excluded based on manual chart review (non-Stanford primary care [n=58], prior statin [n=48], metastatic cancer or non-metastatic cancer receiving treatment [n=38], death [n=25], hospice/advanced disease [n=19], non-English speaking [n=18], ASCVD/prior coronary testing [n=14], and limited Stanford follow-up [n=10]). Of the remaining 194 patients, a radiologist confirmed CAC among 173 (89.2%) patients, and these patients were randomized between March 30, 2021 and June 7, 2021.
Baseline Characteristics
Patient characteristics were balanced across both arms (Table 1). The median patient age was 70.8 (IQR: 64.0-75.8). The median 10-year pooled cohort equation risk for an ASCVD event was 14.6% (IQR: 8.7%-27.7%) among the 149 patients in whom the risk could be calculated. The 10-year risk was ≥7.5% for 94.0% of patients (140/149). The median time from chest CT to notification was 857 days (IQR: 641-1,069 days). The most common indication for the CT was to evaluate a pulmonary nodule (63/173; 36.4%) followed by lung cancer screening (22/173; 12.7%). Most radiology reports noted CAC in the body of the report (146/173; 84.4%) but only 3 reports (1.7%) noted CAC in the final impression.
Table 1.
Patient Characteristics
| Notification Arm n=86 |
Usual Care Arm n=87 |
|
|---|---|---|
| Age | 70.9 (64.0-75.8) | 70.8 (64.0-76.0) |
| Female Sex | 44 (51%) | 47 (54%) |
| Race | ||
| Asian | 9 (10%) | 11 (13%) |
| Black | 4 (5%) | 3 (3%) |
| White | 65 (76%) | 67 (77%) |
| Other | 7 (8%) | 5 (6%) |
| Unknown | 1 (1%) | 1 (1%) |
| Ethnicity | ||
| Hispanic | 5 (6%) | 6 (7%) |
| Non-Hispanic | 80 (93%) | 78 (90%) |
| Unknown | 1 (1%) | 3 (3%) |
| Clinician | ||
| Stanford Primary Care | 84 (98%) | 84 (97%) |
| Endocrinology | 2 (2%) | 3 (3%) |
| Time from Primary Care/Endocrinology Visit, days | 115 (44-304) | 170 (43-506) |
| Prior Cardiology Encounter, % | 6 (7%) | 7 (8%) |
| Chest CT Scan Indication | ||
| Pulmonary Nodule Evaluation | 23 (27%) | 40 (46%) |
| Lung Cancer Screening | 12 (14%) | 10 (11%) |
| Parenchymal Lung Disease Evaluation | 4 (5%) | 2 (2%) |
| Malignant Disease Evaluation | 7 (8%) | 4 (5%) |
| Pleural Effusion Evaluation | 0 (0%) | 2 (2%) |
| Non-specific Symptom Evaluation | 8 (9%) | 9 (10%) |
| Other Indications | 32 (37%) | 20 (23%) |
| Time from Chest CT, days | 848 (623-1056) | 882 (646-1105) |
| DL-CAC Score | 18 (7-112) | 20 (3-70) |
| DL-CAC >0 to <100 | 63 (73%) | 69 (79%) |
| DL-CAC ≥100 | 23 (27%) | 18 (21%) |
| CAC Description in Radiology Report | ||
| No CAC Noted | 6 (7%) | 11 (13%) |
| Mild | 53 (62%) | 52 (60%) |
| Moderate | 16 (19%) | 15 (17%) |
| Severe | 6 (7%) | 4 (5%) |
| Noted, No Qualitative Description | 5 (6%) | 5 (6%) |
| CAC Mentioned in Report Impression | 1 (1%) | 2 (2%) |
| Vital Signs | ||
| Body Mass Index, kg/m2 | 25.3 (22.5-29.4) | 25.9 (22.8-29.2) |
| Systolic Blood Pressure, mmHg | 128 (120-142) | 129 (119-146) |
| Diastolic Blood Pressure, mmHg | 78 (72-84) | 76 (72-84) |
| ASCVD Risk, 10-year | 16.1% (8.1%-29.7%) | 13.6% (9.0%-26.5%) |
| Medications | ||
| Antihypertensive, % | 33 (38%) | 39 (45%) |
| Aspirin, % | 12 (14%) | 12 (14%) |
| Comorbid Conditions | ||
| Atrial Fibrillation, % | 9 (10%) | 6 (7%) |
| Cancer, % | 35 (41%) | 34 (39%) |
| Chronic Kidney Disease, % | 14 (16%) | 7 (8%) |
| Chronic Obstructive Pulmonary Disease, % | 16 (19%) | 20 (23%) |
| Connective Tissue Disease, % | 12 (14%) | 5 (6%) |
| Depression, % | 28 (33%) | 19 (22%) |
| Diabetes Mellitus, % | 14 (16%) | 6 (7%) |
| Hypertension, % | 43 (50%) | 44 (51%) |
| Hypothyroidism, % | 28 (33%) | 17 (20%) |
| Liver Disease, % | 14 (16%) | 13 (15%) |
| Other Lung Disease, % | 17 (20%) | 16 (18%) |
| Smoking, Current % | 9 (11%) | 9 (10%) |
| Laboratory Values | ||
| Creatinine, mg/dL | 0.9 (0.8-1.0) | 0.8 (0.7-0.9) |
| Missing Creatinine | 0 (0%) | 1 (1%) |
| Hemoglobin A1c, % | 5.6 (5.4-5.8) | 5.6 (5.4-5.8) |
| Missing Hemoglobin A1c | 32 (37%) | 32 (37%) |
| High-Density Lipoprotein, mg/dL | 60 (49-77) | 60 (44-79) |
| Missing High-Density Lipoprotein | 9 (10%) | 15 (17%) |
| Low-density Lipoprotein (LDL), mg/dL | 115 (98-133) | 116 (100-128) |
| LDL <70mg/dL | 5 (6%) | 4 (5%) |
| LDL 70-99 mg/dL | 17 (20%) | 13 (15%) |
| LDL 100-129 mg/dL | 30 (35%) | 38 (44%) |
| LDL ≥130 mg/dL | 25 (29%) | 17 (20%) |
| Missing LDL | 9 (10%) | 15 (17%) |
| Triglyceride, mg/dL | 94 (69-133) | 94 (63-145) |
| Missing Triglyceride | 9 (10%) | 13 (15%) |
Abbreviations: anti-HTN: anti-hypertensive; ASCVD: atherosclerotic cardiovascular disease; CAC: Coronary artery calcium; CT: computed tomography; LDL: low-density lipoprotein. Continuous variables displayed as median (interquartile range) and categorical variables displayed as frequency (percentage).
Notification was sent to all 86 patients in the notification arm following notification of their clinicians. No clinicians objected to notifying their patients. Two endocrinologists requested the clinician notification be sent to the patient’s primary care clinician outside of the Stanford Healthcare System.
Primary Outcome
The notification arm was more likely to receive a statin prescription than the usual care arm (Table 2). Six months post randomization, statins were prescribed to 51.2% (44/86) of the notification arm versus 6.9% (6/87) of the usual care arm (p<0.001) (Figure 2). Among patients prescribed statins in the notification arm, 72.7% (32/44) received a moderate-intensity statin prescription and 18.2% (8/44) received high-intensity statin therapy.
Table 2.
Outcomes Across Arms
| Notification Arm N=86 |
Usual Care N=87 |
p-value | |
|---|---|---|---|
| Statin Discussion/Prescription, 3 months | 53 (64.6%) | 7 (8.4%) | <0.001 |
| Statin Discussion/Prescription, 6 months | 67 (77.9%) | 10 (12.0%) | <0.001 |
| Statin Prescription, 6 weeks | 7 (35.0%) | 0 (0.0%) | 0.008 |
| Statin Prescription, 3 months | 32 (39.0%) | 4 (4.8%) | <0.001 |
| Statin Prescription, 6 months | 44 (51.2%) | 6 (6.9%) | <0.001 |
| Statin Intensity | <0.001 | ||
| High-Intensity | 8 (9.3%) | 3 (3.4%) | |
| Moderate-Intensity | 32 (37.2%) | 3 (3.4%) | |
| Low-Intensity | 4 (4.7%) | 0 (0.0%) | |
| No Statin | 42 (48.8%) | 81 (93.1%) | |
| Secondary Outcomes*,† | |||
| Aspirin Treatment, 6 months | 16 (18.6%) | 17 (19.5%) | 0.848 |
| New Aspirin Treatment, 6 months‡ | 7/74 (9.5%) | 7/75 (9.3%) | 0.879 |
| Number of anti-hypertensives, 6 months | 0 (0-1) | 0 (0-2) | 0.985 |
| Hemoglobin A1c Measured, follow-up | 29 (33.7%) | 23 (26.4%) | 0.211 |
| Hemoglobin A1c, % (Non-missing), follow-up | 5.7 (0.7) | 5.5 (0.5) | 0.107 |
| Lipids Measured, follow-up§ | 50 (58.1%) | 29 (33.3%) | 0.002 |
| Low-Density Lipoprotein (LDL), mg/dL (Non-missing), follow-up | 97.2 (30.3) | 115.3 (29.4) | 0.005 |
| LDL <70mg/dL | 9 (10.5%) | 2 (2.3%) | |
| LDL 70-99 mg/dL | 20 (23.3%) | 6 (6.9%) | |
| LDL 100-129 mg/dL | 12 (14.0%) | 12 (13.8%) | |
| LDL ≥130 mg/dL | 9 (10.5%) | 9 (10.3%) | |
| Missing LDL | 36 (41.9%) | 58 (66.7%) | |
| High-Density Lipoprotein, mg/dL (Non-missing), follow-up | 64.2 (21.6) | 61.7 (22.5) | 0.872 |
| Triglycerides, mg/dL (Non-missing), follow-up | 87.1 (40.7) | 123.4 (70.8) | 0.009 |
| Systolic Blood Pressure Measured, follow-up§ | 69 (80.2%) | 64 (73.6%) | 0.287 |
| Systolic Blood Pressure, mmHg (Non-missing), follow-up | 131.3 (17.4) | 128.9 (15.0) | 0.374 |
| Body Mass Index Measured, follow-up§ | 66 (76.7%) | 63 (72.4%) | 0.486 |
| Body Mass Index, kg/m2 (Non-Missing), follow-up | 25.5 (5.1) | 26.7 (5.6) | 0.630 |
Abbreviations: ASCD: atherosclerotic cardiovascular disease.
For secondary outcomes of lab values, vitals, anti-hypertensives, and aspirin treatment, statistical testing is adjusted for baseline value and age. For lab testing, statistical testing is adjusted for age. Results with imputation via last observation carried forward and multiple imputation available in Supplement Table 2.
Aspirin and number of antihypertensives based on assessment at 6 months post-notification. All other outcomes based on last assessment during the 6-month follow-up period.
New aspirin treatment among those not on treatment at baseline.
Defined as a measurement during the 6-month follow-up period.
Figure 2. The NOTIFY-1 Quality Improvement Project.
Abbreviations: ASCVD: atherosclerotic cardiovascular disease; CAC: coronary artery calcium; CT: computed tomography; DL: deep learning.
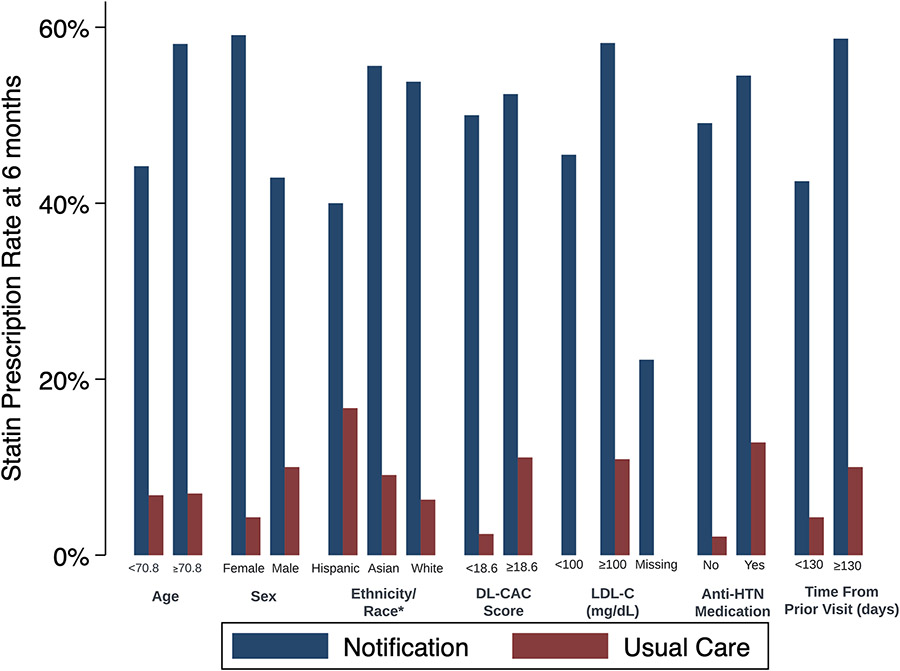
Figure 3 displays statin rates stratified by patient characteristics. There was no significant effect modification across the pre-specified subgroups (Supplement Table S2). Statins were prescribed to 59.1% of women in the notification group compared with 42.9% of men (p=0.108 for difference in the effect of notification across sex).
Figure 3. Sub-group Analyses of Statin Prescription Rates at 6 Months.
Abbreviations: anti-HTN: antihypertensive; CAC: coronary artery calcium; LDL: low density lipoprotein; Rx: prescription.
This figure demonstrates results of the primary analysis, statin prescription rates at 6 months, stratified by key patient characteristics. For the ethnicity/race subgroup analysis, we first classified patients with Hispanic ethnicity. Among non-Hispanic patients, we made subgroups based on race. We excluded subgroups with fewer than 10 individuals across arms for confidentiality. There was no significant heterogeneity in the treatment effect across patient subgroups (Supplement Table S2). The statin prescription rate was 0% for patients in the usual care arm with LDL-C <100 mg/dL or with missing LDL-C. Testing for heterogeneity of treatment effect using continuous variables (for age, DL-CAC score, and time since last visit) are listed in Supplement Table S2.
The notification arm was more likely to have discussions about statin therapy with clinicians. Among patients in the notification arm, 77.9% (67/86) had a documented statin discussion or new statin prescription compared with 12.0% (10/87) in the usual care arm (p<0.001).
Secondary Clinical Outcomes
At 6 months post-randomization, the aspirin treatment rate was similar across notification and usual care arms (18.6% vs. 19.5%, p=0.848, respectively) (Table 2). There was also no significant difference in the change in number of antihypertensives at 6 months.
Lipid levels were checked during follow-up among 58.1% (50/86) of the notification arm compared with 33.3% (29/87) of the usual care arm (p=0.002). Among those with repeat lipid testing, low-density lipoprotein cholesterol levels were lower in the notification arm vs. usual care (97.2 mg/dL [SD: 30.3] vs. 115.3 mg/dL [SD: 29.4], p=0.005, respectively). These results were consistent after imputing missing values (Supplement Table S3). There was no significant difference between groups in the change in hemoglobin A1c levels or systolic blood pressure during follow-up. The results were similar without adjustment for age (Supplement Table S4).
Healthcare Utilization
Over the 6-month follow-up period, there were more primary care encounters per patient in the notification arm compared with the usual care arm (2.2 [SD: 2.2] vs. 1.4 [SD: 1.6]; p=0.011). There was no significant difference in the number of cardiology encounters per patient across arms, but there were more patients with new cardiology encounters in the notification arm (16.3% vs. 4.6%, p=0.015).
There was an increase in CAD testing in the notification arm (Table 3). Among the notification arm, 15.1% (13/86) underwent testing for coronary artery disease compared with 2.3% (2/87) among the usual care arm (p=0.008). The largest absolute difference was among non-invasive stress testing (11.6% vs. 2.3%, p=0.018). No patients underwent invasive coronary angiography over the 6-month follow-up. The results were similar without adjustment (Supplement Table S5).
Table 3.
Healthcare Utilization Over 6 Months Stratified Across Arms*
| Notification Arm N=86 |
Usual Care N=87 |
p-value | |
|---|---|---|---|
| Primary Care/Endocrinology Encounters, count per patient† | 2.2 (2.2) | 1.4 (1.6) | 0.011 |
| Cardiology Encounters, count per patient | 0.4 (0.8) | 0.2 (1.0) | 0.143 |
| New Cardiology Encounters, patients (%) | 14 (16.3%) | 4 (4.6%) | 0.015 |
| Coronary Artery Disease Testing, patients (%)‡ | 13 (15.1%) | 2 (2.3%) | 0.008 |
| ECG-gated CAC Scans, patients (%) | 3 (3.5%) | 0 (0.0%) | 0.121 |
| Coronary CT Angiography, patients (%) | 1 (1.2%) | 0 (0.0%) | 0.497 |
| Invasive Coronary Angiography, patients (%) | 0 (0.0%) | 0 (0.0%) | --- |
| Stress Tests, patients (%) | 10 (11.6%) | 2 (2.3%) | 0.018 |
| Resting Echocardiograms, patients (%) | 5 (5.8%) | 7 (8.0%) | 0.766 |
Abbreviations: CAC: coronary artery calcium; CT: computed tomography; ECG: electrocardiogram.
The number of primary care/endocrinology visits and number of cardiology encounters was adjusted for baseline frequency and age. The new cardiology encounter and coronary artery disease testing outcomes were adjusted for baseline age. Unadjusted analyses available in Supplement Table 4.
Primary care encounters for patients with Stanford primary care clinician; primary care and endocrinology encounters for patients without Stanford primary care clinician.
Coronary artery disease testing includes ECG-gated CAC scans, coronary CT angiography, invasive coronary angiography, and stress tests (e.g., echo, nuclear, or treadmill stress tests).
DISCUSSION
In this randomized quality improvement project, notifying clinicians and patients about the presence of incidental CAC on previously performed non-gated chest CT scans led to a significant increase in statin prescription rates. The effect of notification was consistent across patient subgroups. The novel DL-CAC algorithm allowed rapid screening of thousands of previous CT scans for the presence of CAC. This demonstrates the potential of using a deep learning algorithm to perform opportunistic screening for incidental CAC in the millions of non-gated chest CT scans performed annually for other reasons.8
A general concern with diagnostic imaging is incidental findings.11, 12 CAC is a unique incidental finding because it can be leveraged to improve population health. The presence or absence of CAC improves risk assessment for a high-prevalence, preventable condition – ASCVD.8, 13-17 Currently, most non-gated chest CT reports do not focus on the presence or severity of CAC.18-20 CAC was mentioned in the initial CT report for 90% of our cohort, but CAC was included in the final impression in only 2% of reports and none of the patients was taking a statin. This suggests identifying CAC in the radiology report may not be as effective at promoting statin therapy as targeted clinician and patient notification tied to actionable recommendations. Extracting and disseminating this valuable, often unused finding with actionable information about its significance could promote implementation of lifestyle and pharmacologic interventions that substantially reduce cardiovascular morbidity.21
There is increasing interest in leveraging imaging data that is incidental to the clinical indication of the test to better understand a patient’s risk of future events.22 This has been commonly termed opportunistic screening, which contrasts with systematic screening of a segment of the population with a test such as mammography. Given the imaging has already been performed, the cost of extracting this data is low. However, there is still an impetus to demonstrate the potential value of the extracted data. This project is an example of how opportunistic screening with previously performed imaging can be leveraged to improve quality of care.
Notifying patients regarding CAC may have multiple potential benefits. Not only does CAC improve risk estimation above existing risk equations, but the image of calcification also has unique motivating effects. The power of visualizing one’s atherosclerosis has also been demonstrated for carotid disease.23 We leveraged that power by providing a personalized image of each patient’s CAC. Although 94% of our cohort met guideline criteria for taking a statin based on their 10-year ASCVD risk, none of these patients were on statins at baseline. The notification and visual demonstration of patient-specific CAC led to a substantial increase in statin rates as compared with no notification. These results are overall consistent with the Early Identification of Subclinical Atherosclerosis Using Non-Invasive Imaging Research (EISNER) trial, in which patients with visualized CAC had improvements in risk factor control and statin adherence compared with those without CAC.24 The Danish Cardiovascular Screening Trial (DANCAVAS) further strengthened the evidence for CAC testing.25 Among male participants randomized to a cardiovascular screening invitation, the most frequent positive finding was an elevated CAC score. In this trial, screening led to an increase in lipid-lowering drugs and antiplatelets and, among those between 65-69 years old, a reduction in all-cause mortality. Our opportunistic screening approach is distinct from EISNER and DANCAVAS by leveraging imaging data that already exists.
Our project does not separate out the effect of notification alone versus notification with the CAC image. Notifying patients of their elevated risk, based on their 10-year ASCVD risk alone, may have led to an increase in statin rates. However, prior efforts to notify clinicians of elevated cardiovascular risk based on risk equations have traditionally demonstrated smaller effect sizes.26-30 Therefore, we tested a novel approach of notifying patients regarding incidental CAC with personalized images of their CAC.
While opportunistic CAC screening may have multiple potential clinical benefits, there are important considerations before a health system implements such a program. First, there is a fine balance between motivating action and excessively increasing patient anxiety. To ensure access to treatment and counseling, all patients in our project had an active relationship with a health system clinician and messages were sent to both patients and clinicians. Additional qualitative research with patients and their caregivers regarding appropriate wording of notifications and the appropriateness of direct patient notification will be important. Second, clinicians need to feel responsible for the patient’s cardiovascular health and feel capable of acting on the results. Prior surveys estimated over half of referring clinicians are unaware of the significance of CAC.31 We conducted limited educational sessions prior to launching the project for a subset of clinicians who might be notified. More extensive health system education may have augmented the effectiveness of our intervention. Primary care clinicians raised no concerns about the notifications to their patients, possibly due to the education about the intervention before its launch. Ensuring clinicians are aligned with the intervention efforts is likely an important element to implementation success.
Our intervention integrated automated processes – the DL-CAC algorithm and the automated EHR review – with manual processes – manual chart review and radiologist review for the presence of CAC. Our project used strict screening to evaluate the efficacy of our approach; future studies can increase automation to facilitate large-scale implementation of opportunistic screening programs. Manual chart review could be largely replaced with expansion of the automated EHR review and simplifying the exclusion criteria. There is a critical balance between considerations of patient autonomy to know their data and avoiding unnecessary anxiety by not screening for CAC in patients unlikely to benefit from preventive therapies (e.g., patients in hospice). Additionally, manual radiologist review of images may be less critical at higher thresholds where a small absolute error in the DL-CAC estimate would have minimal impact on the implications of notification.
Our project was restricted to patients with CAC who had no known ASCVD and were statin naïve. We therefore excluded patients with ASCVD who already had a Class I guideline recommendation for statin therapy.7 We also applied additional exclusion criteria, such as requiring a Stanford primary care clinician or endocrinologist, for this proof-of-concept study. However, this limited the total eligible population for notification substantially. In future studies, the population eligible for opportunistic screening and notification will be expanded. Opportunistic screening could be broadened to include patients with known ASCVD given nearly 50% are not on statin therapy.32 Even for patients on a statin, identification of CAC may lead to dose intensification, improved medication adherence, or lifestyle changes. Increasing the eligible population may reduce the per-patient effect of notification on statin rates; for example, patients without a primary care clinician in the health care system may be less likely to receive statin therapy after notification. However, even if the per-patient effect is smaller, expanding the intervention would still increase the population-level benefit at a low incremental cost.
Our approach used the DL-CAC algorithm retrospectively and had a radiologist confirm the binary presence of CAC. The algorithm could also be applied prospectively to assist radiologists in consistently identifying and quantifying CAC. Quantification of CAC severity may further improve the impact of notification, especially among those at highest risk. Future work will include expanding the DL-CAC algorithm to apply to low-dose chest CTs used for lung cancer screening and contrast chest CTs. Applying this approach to broader populations and more CT scans can potentiate the potential impact of opportunistic CAC screening but will require further evaluation.
On average, the DL-CAC scores in our cohort were low. However, the presence of any CAC increases risk compared with patients without CAC; for this reason, the ACC/AHA 2018 Guidelines on Blood Cholesterol have a IIa recommendation for considering statin therapy among patients ≥55 years old and for patients with a CAC score ≥75th percentile for their age, sex, and race. For a 54-year-old woman, a CAC score of 1 would be ≥75th percentile.33 We did not observe differences in the effect of notification on statin rates between those with higher vs. lower CAC scores, but our CAC range was relatively narrow. Notifying patients with higher CAC scores and more pronounced calcification images may lead to larger increases in statin prescription rates.
Notifications also increased clinical encounters and testing. While the increase in primary care encounters and lipid testing are expected and potentially desirable, the benefit of increased cardiology encounters and CAD testing is unclear. There may also be a distinction between dedicated CAC scans, which could be used to validate the results of the DL-CAC algorithm, with non-invasive stress testing and coronary CT angiograms used to detect obstructive coronary disease but not recommended by guidelines in an asymptomatic cohort. Our results contrast with the EISNER trial, which found no significant increase in testing among the cohort randomized to a CAC scan.24 These differences may be explained by the differences between unexpected opportunistic screening and patients being actively enrolled and educated in advance of their gated CAC scan as part of a systematic screening program. Additional education for clinicians and patients may mitigate the observed increase in testing rates. We did not collect data regarding the patient experience and any potential anxiety of those notified. Further research regarding the impact on both healthcare resource utilization and the patient experience will be important steps prior to scaling this intervention.
Limitations
There are important limitations to our intervention. First, while we demonstrated a significant increase in statin rates from notifying patients about their subclinical atherosclerosis, the impact on clinical outcomes among this select cohort is unknown. Future studies to demonstrate whether notification reduces ASCVD events would be valuable. Second, this was performed at a single center; the generalizability to other health systems or to patients who did not meet our inclusion criteria is uncertain. Third, data on persistence of statin therapy beyond 6 months is critical to better understanding the impact of the intervention. We are continuing to follow patients and will plan to subsequently report outcomes at 12 months including statin treatment and downstream testing rates. Fourth, our intervention notified both clinicians and patients to facilitate shared decision-making; we did not investigate the impact of notifying only the clinician or only the patient. Finally, the investigators were not blinded to allocation when evaluating outcomes.
Conclusion
Notifying clinicians and their patients about the presence of incidental CAC detected on previously acquired non-gated chest CT scans led to a significant increase in statin prescription rates as compared with no notification. These findings illustrate the opportunity to use an automated method to screen non-gated chest CTs to identify patients with subclinical coronary atherosclerosis and the power of notification to motivate them and their clinicians to initiate preventive interventions.
Supplementary Material
Clinical Perspective.
What is New?
In this randomized quality improvement project, statin-naïve patients with CAC on prior non-gated CT scans were identified via a deep learning algorithm.
Notification of patients and their clinicians regarding incidental CAC with a personalized image of the CAC increased statin prescription rates.
What are the clinical implications?
Opportunistic CAC screening on prior chest CT scans is a potential approach to identify patients at high risk of atherosclerotic cardiovascular disease events who would benefit from preventive interventions.
The impact of such a screening strategy on clinical events is unknown and should be tested in a prospective randomized clinical trial.
Acknowledgements:
The authors would like to thank the patients participating in the NOTIFY-1 project and Stanford primary care clinicians.
Sources of funding:
This work was supported by the Stanford University Human-Centered Artificial Intelligence Seed Grant. ATS receives research support from the National Heart, Lung, and Blood Institute (1K23HL151672-01). FR receives research support from the National Heart, Lung, and Blood Institute (5K01HL144607-02), the American Heart Association/Robert Wood Johnson Harold Amos Medical Faculty Development Program, and Grant #2022051 from the Doris Duke Charitable Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
ABBREVIATIONS
- ASCVD
atherosclerotic cardiovascular disease
- CAC
coronary artery calcium
- CAD
coronary artery disease
- CT
computed tomography
- DL-CAC
deep learning based coronary artery calcium
- EHR
electronic health record
Footnotes
Disclosures: Dr. Rodriguez received consulting fees from Novo Nordisk, Novartis, and HealthPals. Mr. Eng and Mr. Khandwala are employees of Bunkerhill.
REFERENCES
- 1.Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, Elkind MSV, Evenson KR, Eze-Nliam C, Ferguson JF, Generoso G, Ho JE, Kalani R, Khan SS, Kissela BM, Knutson KL, Levine DA, Lewis TT, Liu J, Loop MS, Ma J, Mussolino ME, Navaneethan SD, Perak AM, Poudel R, Rezk-Hanna M, Roth GA, Schroeder EB, Shah SH, Thacker EL, VanWagner LB, Virani SS, Voecks JH, Wang NY, Yaffe K and Martin SS. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation. 2022;145:e153–e639. [DOI] [PubMed] [Google Scholar]
- 2.Metser G, Bradley C, Moise N, Liyanage-Don N, Kronish I and Ye S. Gaps and Disparities in Primary Prevention Statin Prescription During Outpatient Care. Am J Cardiol. 2021;161:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nanna MG, Navar AM, Wang TY, Mi X, Virani SS, Louie MJ, Lee LV, Goldberg AC, Roger VL, Robinson J and Peterson ED. Statin Use and Adverse Effects Among Adults >75 Years of Age: Insights From the Patient and Provider Assessment of Lipid Management (PALM) Registry. J Am Heart Assoc. 2018. May 8;7(10):e008546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saeed A, Zhu J, Thoma F, Marroquin O, Aiyer A, Agarwala A, Virani SS, Gulati M, Lee JS, Reis S, Saba S, Ballantyne C and Mulukutla S. Cardiovascular Disease Risk-Based Statin Utilization and Associated Outcomes in a Primary Prevention Cohort: Insights From a Large Health Care Network. Circ Cardiovasc Qual Outcomes. 2021;14:e007485. [DOI] [PubMed] [Google Scholar]
- 5.Clough JD, Martin SS, Navar AM, Lin L, Hardy NC, Rogers U and Curtis LH. Association of Primary Care Providers' Beliefs of Statins for Primary Prevention and Statin Prescription. J Am Heart Assoc. 2019;8:e010241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND and Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–45. [DOI] [PubMed] [Google Scholar]
- 7.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr., Sperling L, Virani SS and Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285–e350. [DOI] [PubMed] [Google Scholar]
- 8.Budoff MJ, Lutz SM, Kinney GL, Young KA, Hokanson JE, Barr RG, Steiner R, Nath H, Lopez-Garcia C, Duca LM, Rahmani S, Osawa K, Regan EA, Li D and Casaburi R. Coronary Artery Calcium on Noncontrast Thoracic Computerized Tomography Scans and All-Cause Mortality. Circulation. 2018;138:2437–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eng D, Chute C, Khandwala N, Rajpurkar P, Long J, Shleifer S, Khalaf MH, Sandhu AT, Rodriguez F, Maron DJ, Seyyedi S, Marin D, Golub I, Budoff M, Kitamura F, Takahashi MS, Filice RW, Shah R, Mongan J, Kallianos K, Langlotz CP, Lungren MP, Ng AY and Patel BN. Automated coronary calcium scoring using deep learning with multicenter external validation. NPJ Digit Med. 2021;4:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinicaltrials.gov. Incidental Coronary Calcification Quality Improvement Project (ICC QI). https://clinicaltrials.gov/ct2/show/NCT04789278. Accessed April 1, 2022.
- 11.O'Sullivan JW, Muntinga T, Grigg S and Ioannidis JPA. Prevalence and outcomes of incidental imaging findings: umbrella review. BMJ. 2018;361:k2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganguli I, Simpkin AL, Lupo C, Weissman A, Mainor AJ, Orav EJ, Rosenthal MB, Colla CH and Sequist TD. Cascades of Care After Incidental Findings in a US National Survey of Physicians. JAMA Netw Open. 2019;2:e1913325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiles C, Duan F, Gladish GW, Ravenel JG, Baginski SG, Snyder BS, DeMello S, Desjardins SS, Munden RF and Team NS. Association of Coronary Artery Calcification and Mortality in the National Lung Screening Trial: A Comparison of Three Scoring Methods. Radiology. 2015;276:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haller C, Vandehei A, Fisher R, Boster J, Shipley B, Kaatz C, Harris J, Shin SR, Townsend L, Rouse J, Davis S, Aden J and Thomas D. Incidence and Implication of Coronary Artery Calcium on Non-gated Chest Computed Tomography Scans: A Large Observational Cohort. Cureus. 2019;11:e6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes-Austin JM, Dominguez A 3rd, Allison MA, Wassel CL, Rifkin DE, Morgan CG, Daniels MR, Ikram U, Knox JB, Wright CM, Criqui MH and Ix JH. Relationship of Coronary Calcium on Standard Chest CT Scans With Mortality. JACC Cardiovasc Imaging. 2016;9:152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mets OM, Vliegenthart R, Gondrie MJ, Viergever MA, Oudkerk M, de Koning HJ, Mali WP, Prokop M, van Klaveren RJ, van der Graaf Y, Buckens CF, Zanen P, Lammers JW, Groen HJ, Isgum I and de Jong PA. Lung cancer screening CT-based prediction of cardiovascular events. JACC Cardiovasc Imaging. 2013;6:899–907. [DOI] [PubMed] [Google Scholar]
- 17.Xie X, Zhao Y, de Bock GH, de Jong PA, Mali WP, Oudkerk M and Vliegenthart R. Validation and prognosis of coronary artery calcium scoring in nontriggered thoracic computed tomography: systematic review and meta-analysis. Circ Cardiovasc Imaging. 2013;6:514–21. [DOI] [PubMed] [Google Scholar]
- 18.Balakrishnan R, Nguyen B, Raad R, Donnino R, Naidich DP, Jacobs JE and Reynolds HR. Coronary artery calcification is common on nongated chest computed tomography imaging. Clin Cardiol. 2017;40:498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirsch J, Martinez F, Lopez D, Novaro GM and Asher CR. National trends among radiologists in reporting coronary artery calcium in non-gated chest computed tomography. Int J Cardiovasc Imaging. 2017;33:251–257. [DOI] [PubMed] [Google Scholar]
- 20.Phillips WJ, Johnson C, Law A, Turek M, Small AR, Inacio JR, Dent S, Ruddy T, Beanlands RS, Chow BJW and Small GR. Reporting of coronary artery calcification on chest CT studies in breast cancer patients at high risk of cancer therapy related cardiac events. Int J Cardiol Heart Vasc. 2018;18:12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Munoz D, Smith SC Jr., Virani SS, Williams KA Sr., Yeboah J and Ziaeian B. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74:1376–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pickhardt PJ, Graffy PM, Perez AA, Lubner MG, Elton DC, Summers RM. Opportunistic Screening at Abdominal CT: Use of Automated Body Composition Biomarkers for Added Cardiometabolic Value. Radiographics. 2021;41(2):524–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naslund U, Ng N, Lundgren A, Fharm E, Gronlund C, Johansson H, Lindahl B, Lindahl B, Lindvall K, Nilsson SK, Nordin M, Nordin S, Nyman E, Rocklov J, Vanoli D, Weinehall L, Wennberg P, Wester P, Norberg M and group Vt. Visualization of asymptomatic atherosclerotic disease for optimum cardiovascular prevention (VIPVIZA): a pragmatic, open-label, randomised controlled trial. Lancet. 2019;393:133–142. [DOI] [PubMed] [Google Scholar]
- 24.Rozanski A, Gransar H, Shaw LJ, Kim J, Miranda-Peats L, Wong ND, Rana JS, Orakzai R, Hayes SW, Friedman JD, Thomson LE, Polk D, Min J, Budoff MJ and Berman DS. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J Am Coll Cardiol. 2011;57:1622–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindholt JS, Søgaard R, Rasmussen LM, Mejldal A, Lambrechtsen J, Steffensen FH, Frost L, Egstrup K, Urbonaviciene G, Busk M, Diederichsen ACP. Five-Year Outcomes of the Danish Cardiovascular Screening (DANCAVAS) Trial. N Engl J Med. 2022. Oct 13;387(15):1385–1394. [DOI] [PubMed] [Google Scholar]
- 26.Benner JS, Erhardt L, Flammer M, Moller RA, Rajicic N, Changela K, Yunis C, Cherry SB, Gaciong Z, Johnson ES, Sturkenboom MC, Garcia-Puig J, Girerd X and Investigators RO. A novel programme to evaluate and communicate 10-year risk of CHD reduces predicted risk and improves patients' modifiable risk factor profile. Int J Clin Pract. 2008;62:1484–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobson TA, Gutkin SW and Harper CR. Effects of a global risk educational tool on primary coronary prevention: the Atherosclerosis Assessment Via Total Risk (AVIATOR) study. Curr Med Res Opin. 2006;22:1065–73. [DOI] [PubMed] [Google Scholar]
- 28.Karmali KN, Persell SD, Perel P, Lloyd-Jones DM, Berendsen MA and Huffman MD. Risk scoring for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2017;3:CD006887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Persell SD, Brown T, Lee JY, Shah S, Henley E, Long T, Luther S, Lloyd-Jones DM, Jean-Jacques M, Kandula NR, Sanchez T and Baker DW. Individualized Risk Communication and Outreach for Primary Cardiovascular Disease Prevention in Community Health Centers: Randomized Trial. Circ Cardiovasc Qual Outcomes. 2015;8:560–6. [DOI] [PubMed] [Google Scholar]
- 30.Persell SD, Lloyd-Jones DM, Friesema EM, Cooper AJ and Baker DW. Electronic health record-based patient identification and individualized mailed outreach for primary cardiovascular disease prevention: a cluster randomized trial. J Gen Intern Med. 2013;28:554–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uretsky S, Chokshi N, Kobrinski T, Agarwal SK, Po JR, Awan H, Jagarlamudi A, Gudiwada SP, D'Avino RC and Rozanski A. The interplay of physician awareness and reporting of incidentally found coronary artery calcium on the clinical management of patients who underwent noncontrast chest computed tomography. Am J Cardiol. 2015;115:1513–7. [DOI] [PubMed] [Google Scholar]
- 32.Nelson AJ, Haynes K, Shambhu S, Eapen Z, Cziraky MJ, Nanna MG, Calvert SB, Gallagher K, Pagidipati NJ and Granger CB. High-Intensity Statin Use Among Patients With Atherosclerosis in the U.S. J Am Coll Cardiol. 2022;79:1802–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McClelland RL, Chung H, Detrano R, Post W and Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2006;113:30–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.