Abstract
Background:
Branched-chain amino acids (BCAA: leucine, isoleucine, and valine) are essential amino acids involved in biological functions of brain development and recently linked with autism. However, their role in Attention Deficit Hyperactivity Disorder (ADHD) is not well-studied. We investigated individual and combined relationships of maternal plasma and newborn cord plasma BCAAs with childhood development of ADHD.
Methods:
We utilized the Boston Birth Cohort, a predominantly urban, low-income, US minority population. Child developmental outcomes were defined in three mutually exclusive groups—ADHD, neurotypical (NT), or other developmental disabilities based on physician diagnoses per ICD-9 or 10 in medical records. The final sample included 626 children (299 ADHD, 327 NT) excluding other developmental disabilities. BCAAs were measured by liquid chromatography–tandem mass spectrometry. We used factor analysis to create composite scores of maternal and cord BCAA, which we divided into tertiles. Logistic regressions analyzed relationships between maternal or cord BCAA tertiles with child ADHD risk, controlling for maternal race, age, parity, smoking, education, low birth weight, preterm birth, and child sex. Additionally, we analyzed maternal and cord plasma BCAAs jointly on child ADHD risk.
Results:
Adjusted logistic regression found significantly increased odds of child ADHD diagnosis for the second (OR 1.63, 95% CI: 1.04, 2.54, p=0.032) and third tertiles (OR 2.01, 95% CI: 1.28, 3.15, p=0.002) of cord BCAA scores compared to the first tertile. This finding held for the third tertile when further adjusting for maternal BCAA score. There was no significant association between maternal BCAA score and child ADHD risk, nor a significant interaction between maternal and cord BCAA scores.
Conclusions:
In this prospective US birth cohort, higher cord BCAA levels were associated with a greater risk of developing ADHD in childhood. These results have implications for further research into mechanisms of ADHD development and possible early life screening and interventions.
Keywords: ADHD, cord blood, metabolome, branched-chain amino acids
Background
Attention Deficit Hyperactivity Disorder (ADHD) is characterized by developmentally inappropriate levels of inattention and/or hyperactivity/impulsivity (Sharma & Couture, 2014). The prevalence of ADHD diagnosis has been on the rise in the past two decades (Xu, Strathearn, Liu, Yang, & Bao, 2018). Approximately 9.4% of children in the U.S. have received a diagnosis of ADHD according to the 2016 National Survey of Children’s Health(Danielson et al., 2018). While various behavioral and pharmacological treatments are available to manage ADHD symptoms, they are symptomatic management rather than cure; and they are costly and may be associated with side effects. ADHD symptoms often persist into adulthood and may continue to affect quality of life, requiring treatment (Childress & Berry, 2012). To halt and reverse ADHD trend, there is urgent need to develop primary prevention for ADHD. However, currently we do not have effective strategy to prevent ADHD, largely because its etiology is not well understood, underscoring the importance to advance this field.
Besides well-observed higher risk in males, there is growing recognition that many early life factors may affect the development of ADHD, including maternal stress, cholesterol levels, maternal acetaminophen use, and early childhood exposure to lead (Jensen, 2000; Ji et al., 2020; Ji, Hong, et al., 2018; Ji, Riley, et al., 2018; Ji et al., 2017; Okano, Ji, Riley, & Wang, 2019; Sciberras, Mulraney, Silva, & Coghill, 2017). Maternal obesity has been associated with increased risk of ADHD in children (Rivera, Christiansen, & Sullivan, 2015). In addition, the role of maternal obesity/diabetes in autism spectrum disorder (ASD) has been demonstrated in diverse populations (Li et al., 2016). The co-occurrence of ADHD and ASD diagnoses in children has been noted in the literature (Danielson et al., 2018; Davis & Kollins, 2012).
This study was motivated by a recent report that maternal plasma Branched Chain Amino Acid (BCAA) metabolites were associated with child ASD risk (Panjwani et al., 2019). BCAAs, which include leucine, isoleucine, and valine, are essential amino acids that are acquired from protein-containing foods, mainly dairy and meat for omnivores (Merz et al., 2018). BCAAs are known to play an important role for brain function. Excess levels of BCAAs in the brain are most associated with Maple Syrup Urine Disease (MSUD), which results in encephalopathy, cerebral edema, and death without proper treatment. In cross-sectional studies, low levels of BCAAs have been associated with ASD, epilepsy, and behavioral abnormalities (Sperringer, Addington, & Hutson, 2017; St-Jean et al., 2017).
However, the relationship between maternal BCAAs and ADHD is unknown. Further, there have been no studies to simultaneously analyzing maternal and cord plasma BCAA levels in relation to the risk of ADHD. This study aims to examine whether maternal and cord plasma BCAA levels may independently or jointly affect the development of ADHD in childhood. We are also interested in exploring whether BCAA-ADHD associations can be modified by known or suspected early life factors, including child sex, race/ethnicity, preterm birth, and maternal metabolic conditions.
Methods
Data Collection
This study utilized data from the mother-child dyads in the Boston Birth Cohort (BBC), an ongoing study at the Boston Medical Center (BMC) of a predominantly urban, low-income, US minority population. On a rolling basis, the mother-child dyads were recruited 24–72 hours post-partum from 1998 to 2015 and the children followed up prospectively from birth onwards up to 2017. The Institutional Review Boards of the Johns Hopkins Bloomberg School of Public Health and the Boston University Medical Center approved both the initial and follow-up studies.
The mothers provided written informed consent. Excluded were those mothers with multiple gestation pregnancies, pregnancies due to in vitro fertilization, and babies with chromosomal abnormalities or major birth defects. Maternal blood samples were collected at the time of enrollment during a non-fasted state. The umbilical cord blood samples were collected at birth. The samples were fractionated at the BMC and liquid chromatography–tandem mass spectrometry was used to quantitatively profile maternal and cord plasma metabolites at the Harvard-MIT Broad Institute Metabolite Profiling Laboratory. This study was limited to those mother-child dyads with metabolite measurements and children receiving continued care at the BMC. Medical records of mothers and children were reviewed with standardized abstraction forms and mothers’ sociodemographic information and stress levels were obtained via face-to-face standardized questionnaire interview. Further details of recruitment and follow-up for the BBC study have been previously published (Li et al., 2016).
Identification of Child Developmental Outcomes and Covariates
Child developmental outcomes were defined in three mutually exclusive groups—ADHD, other developmental disabilities, or neurotypical (NT), based on physician diagnoses per ICD-9 or 10 in child’s electronic medical records, including primary care and subspecialty care. Children diagnosed with ADHD were defined as having any of the following International Classification of Diseases (ICD) Ninth revision (ICD-9) (314.0, 314.00, 314.01, 314.1, 314.2, 314.8, or 314.9) or ICD-10 (F90.0, F90.1, F90.2, F90.8, or F90.9) codes. Children with other DD were defined as having ICD-9 (290-319) or ICD-10 (F01-F99) codes related to mental, behavioral and neurodevelopmental disorders, but excluding ADHD-related ICD codes. NT children were defined as children not having any of the ICD-9 (290-319) or ICD-10 (F01-F99) codes related to mental, behavioral and neurodevelopmental disorders. The final sample included 626 mother-children dyads (299 ADHD, 327 NT) after excluding other developmental disabilities (Figure S1).
Maternal covariates included: age at delivery; race-ethnicity (black, white, Hispanic, or other); parity (nulliparous vs multiparous); smoking during pregnancy (“never smoked,” “ever smoked,” or “continuous smoking”), and education (“high school or less” vs “some college or more”). Child covariates included: child’s sex (female vs. male); preterm (full term vs. preterm), and birthweight (non-low birthweight (≥2,500 g) vs. low birthweight).
Statistical Analysis
Our hypothesis consisted of 1) a positive association between fetal cord plasma BCAA concentrations and child ADHD risk, 2) a positive association between maternal plasma BCAA concentrations and child ADHD risk, and 3) a positive interaction between maternal plasma and cord plasma BCAA concentrations and child ADHD risk. First, baseline maternal and child characteristics for the ADHD and NT groups were compared utilizing t-tests for continuous variables and chi-squared tests for categorical variables (Table 1). The levels of metabolites were originally measured as peak intensities. Metabolite values below the limit of detection were imputed with one-half the minimum peak intensity. Then, they were inverse-normally transformed for analysis. We used factor analysis to create composite scores of cord and maternal BCAAs based on the three BCAAs using the Anderson–Rubin Method (Anderson & Rubin, 1956). We further stratified the maternal and cord BCAAs scores into tertiles. We utilized logistic regressions to analyze the relationships between maternal or cord BCAA tertiles, with the first tertile as the reference group, and child ADHD risk, respectively. We controlled for maternal race, age, parity, smoking, education, low birth weight, preterm birth and child sex. For comparison, we conducted a linear trend test between maternal or cord BCAA scores and child ADHD risk. Additionally, we analyzed cord and maternal plasma BCAA jointly on child risk of ADHD controlling for covariates. We also conducted subgroup analysis for the association between cord BCAA and child risk of ADHD by strata of potential confounders including acetaminophen score identified previously (constructed using acetaminophen metabolites via the Anderson–Rubin Method) (Anderson & Rubin, 1956; Ji et al., 2020).
Table 1.
Baseline maternal and perinatal characteristics among children with ADHD vs. Neurotypical (NT)
| Characteristics | Total | ADHD | NT | P-valuea |
|---|---|---|---|---|
| N | 626 | 299 | 327 | |
| Maternal age (years), mean (SD)b | 28.24 | 28.46 | 28.04 | 0.423 |
| Nulliparous, n (%) | 258 (41.21%) | 122 (40.80%) | 136 (41.59%) | 0.842 |
| Maternal Race or ethnicity, n (%)c | 0.027* | |||
| Black/AA | 406 (64.86%) | 184 (61.54%) | 222 (67.89%) | |
| White | 36 (5.75%) | 25 (8.36%) | 11 (3.36%) | |
| Hispanic | 134 (21.41%) | 69 (23.08%) | 65 (19.88%) | |
| Asian | 50 (7.99%) | 21 (7.02%) | 29 (8.87%) | |
| Maternal Education, n (%) | 0.398 | |||
| Below College Degree | 541 (86.42%) | 264 (88.29%) | 277 (84.71%) | |
| College Degree or Above | 81 (12.94%) | 33 (11.04%) | 48 (14.68%) | |
| Missing | 4 (0.64%) | 2 (0.67%) | 2 (0.61%) | |
| Maternal BMI, n (%) | ||||
| Mean (SD) | 26.45 (6.24) | 26.98 (6.39) | 25.97 (6.07) | 0.048* |
| <25 kg/m2 | 290 (46.33%) | 125 (41.81%) | 165 (50.46%) | 0.084 |
| 25-<30kg/m2 | 168 (26.84%) | 84 (28.09%) | 84 (25.69%) | |
| >=30 kg/m2 | 134 (21.41%) | 75 (25.08%) | 59 (18.04%) | |
| Missing | 34 (5.43%) | 15 (5.02%) | 19 (5.81%) | |
| Maternal diabetesd | 0.027* | |||
| No diabetes | 560 (89.46%) | 259 (86.62%) | 301 (92.05%) | |
| Diabetes | 66 (10.54%) | 40 (13.38%) | 26 (7.95%) | |
| Maternal smoking, n (%)e | <0.001** | |||
| Never | 523 (83.55%) | 233 (77.93%) | 290 (88.69%) | |
| Quit | 39 (6.23%) | 27 (9.03%) | 12 (3.67%) | |
| Continuous | 57 (9.11%) | 38 (12.71%) | 19 (5.81%) | |
| Missing | 7 (1.12%) | 1 (0.33%) | 6 (1.83%) | |
| Child Sex, n (%) | <0.001** | |||
| Male | 355 (56.71%) | 230 (76.92%) | 125 (38.23%) | |
| Female | 271 (43.29%) | 69 (23.08%) | 202 (61.77%) | |
| Gestational Age, n (%) | <0.001** | |||
| Term (>37 weeks) | 557 (88.98%) | 249 (83.28%) | 308 (94.19%) | |
| Late preterm (34-36 weeks) | 43 (6.87%) | 30 (10.03%) | 13 (3.98%) | |
| Early preterm (<34 weeks) | 26 (4.15%) | 20 (6.69%) | 6 (1.83%) | |
| Birthweight | 0.033* | |||
| >= 2,500g | 529 (84.50%) | 243 (81.27%) | 286 (87.46%) | |
| <= 2,500 g | 97 (15.50%) | 56 (18.73%) | 41 (12.54%) | |
| Cord Leucine (above median), n (%) | 323 (51.60%) | 170 (56.86%) | 153 (46.79%) | 0.012* |
| Cord Isoleucine (above median), n (%) | 323 (51.60%) | 169 (56.52%) | 154 (47.09%) | 0.018* |
| Cord Valine (above median), n (%) | 317 (50.64%) | 167 (55.85%) | 150 (45.87%) | 0.013* |
| Cord BCAA score (above median), n (%) | 318 (50.80%) | 165 (55.18%) | 153 (46.79%) | 0.036* |
| Mom Leucine (above median), n (%) | 228 (47.40%) | 98 (50.52%) | 130 (45.30%) | 0.261 |
| Mom Isoleucine (above median), n (%) | 225 (46.78%) | 96 (49.48%) | 129 (44.95%) | 0.328 |
| Mom Valine (above median), n (%) | 224 (46.57%) | 96 (49.48%) | 128 (44.60%) | 0.292 |
| Mom BCAA score (above median), n (%) | 226 (46.99%) | 96 (49.48%) | 130 (45.30%) | 0.367 |
P-values derived from t-test or chi-square; *p<0.05, **p<0.01
Maternal age at time of delivery
Black includes self-reported Black, African American, Haitian, Cape Verdean, and Caribbean race and ethnicities
Type II diabetes mellitus and/or gestational diabetes mellitus
Never smokers defined as mothers with no history of smoking six months prior to conception or during pregnancy; some smoking defined as mothers who smoked at some point in the window of six months prior to conception to delivery but did not smoke throughout that window; continuous is defined as mothers that smoked starting six months prior to and throughout pregnancy.
Results
A total of 626 mother-infant pairs with cord metabolites were included, of which 297 had any ADHD diagnosis and 329 were considered neurotypical (NT), without any developmental disability diagnosis. Baseline characteristics of this sample are reported in Table 1. Mothers of children with ADHD had a significantly higher BMI compared to mothers of NT children (26.98 vs. 25.97, p=0.048). The prevalence of diabetes was significantly higher among mothers of children with ADHD than of NT children (13.38% vs. 7.95%, p=0.027). Additionally, mothers of children with ADHD had a significantly higher rate of smoking continuously throughout pregnancy compared to those of NT children (12.71% vs. 5.81%, p<0.001).
Significantly more children diagnosed with ADHD were male (76.92% vs. 38.23%, p<0.001), born preterm (16.72% vs. 5.81%, p<0.001), and had low birthweight (18.73% vs. 12.54%, p=0.033). Further, more children with ADHD had cord blood levels of leucine, isoleucine, and valine as well an overall BCAA score above the median compared to NT children. Graphic plots display positive associations between cord leucine, isoleucine, valine as well as cord BCAA score with the risk of ADHD (Figure 1). The pattern holds when stratifying by child sex and maternal obesity/diabetes, though males and those with obese or diabetic mothers have a higher risk of ADHD overall. Of the 626 mother-infant pairs, 481 pairs had both maternal and cord metabolite data. For this subset, there was no significant difference between children with ADHD and NT children for maternal blood leucine, isoleucine, and valine levels, and overall maternal BCAA score above their respective medians.
Figure 1.
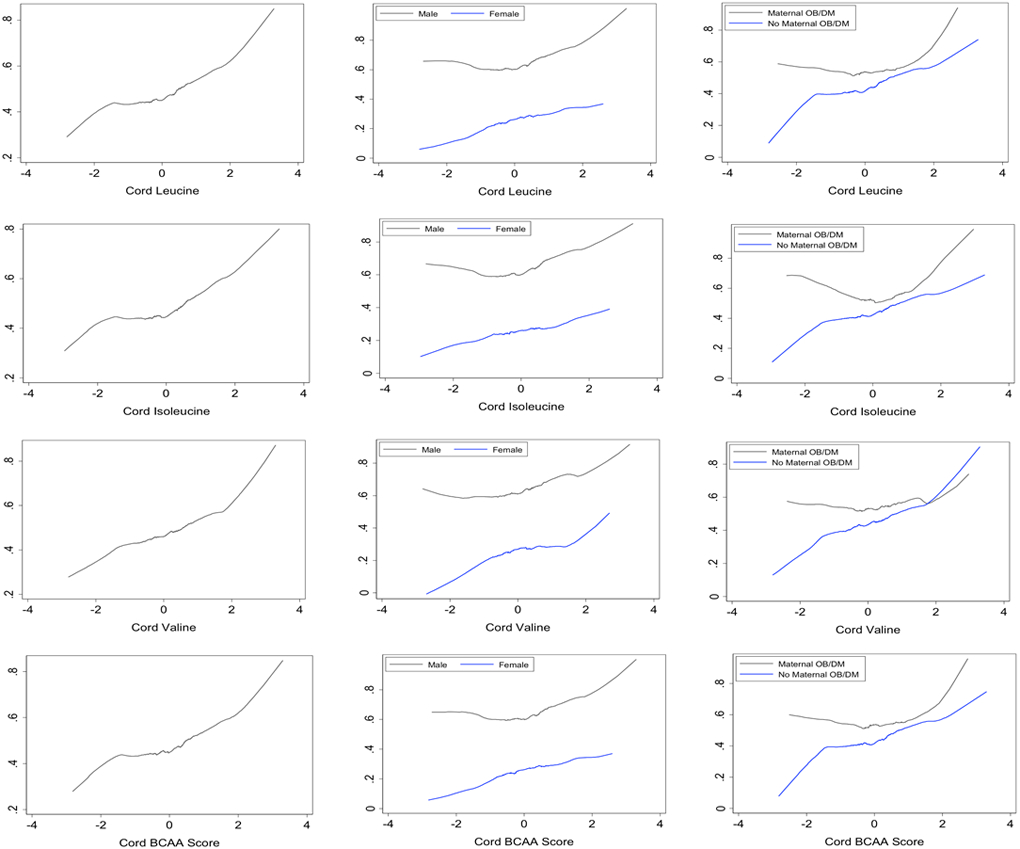
Overall, gender specific, and maternal obesity/diabetes specific associations between cord plasma branched chain amino acids (BCAAs) and risk of ADHD
Logistic regression with pertinent co-variables found that the third tertiles of cord leucine (OR 1.89, p=0.006), isoleucine (OR 1.89, p=0.005), and valine (OR 1.74, p=0.015) were associated with significantly higher odds of ADHD comparing to the first tertiles (Table 2). The risk of ADHD diagnosis was significantly higher for children with a cord BCAA score in the second (OR 1.63, p=0.032) and third tertile 3.04 (OR 2.01, p=0.002). This finding held when further adjusting for maternal BCAA score (OR 1.89, p=0.020) (Table 3). Linear trend tests also found significant positive associations between leucine, isoleucine, valine, and cord BCAA scores as continuous variables and the risk of ADHD.
Table 2.
Crude and adjusted associations between cord plasma branched chain amino acids (BCAAs) individually or as a composite score and the risk of ADHD diagnosis in childhood.
| NT | ADHD | Crude Logistic | Adjusted Logistic | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Total | N (%) | N (%) | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Cord Leucine | 626 | ||||||||
| T1 | 198 | 116 (58.59%) | 82 (41.41%) | Ref | Ref | ||||
| T2 | 213 | 114 (53.52%) | 99 (46.48%) | 1.22 | (0.83, 1.82) | 0.302 | 1.49 | (0.95, 2.32) | 0.080 |
| T3 | 215 | 97 (45.12%) | 118 (54.88%) | 1.72 | (1.17, 2.54) | 0.006 ** | 1.89 | (1.20, 2.95) | 0.006 ** |
| Linear trend test: p = 0.005 | |||||||||
| Cord Isoleucine | 626 | ||||||||
| T1 | 202 | 118 (58.42%) | 84 (41.58%) | Ref | Ref | ||||
| T2 | 205 | 112 (54.63%) | 93 (45.37%) | 1.17 | (0.79, 1.73) | 0.442 | 1.45 | (0.93, 2.27) | 0.104 |
| T3 | 219 | 97 (44.29%) | 122 (55.71%) | 1.77 | (1.20, 2.60) | 0.004 ** | 1.89 | (1.22, 2.95) | 0.005 ** |
| Linear trend test: p = 0.011 | |||||||||
| Cord Valine | 626 | ||||||||
| T1 | 201 | 115 (57.21%) | 86 (42.79%) | Ref | Ref | ||||
| T2 | 214 | 118 (55.14%) | 96 (44.86%) | 1.09 | (0.74, 1.60) | 0.671 | 1.10 | (0.71, 1.71) | 0.622 |
| T3 | 211 | 94 (44.55%) | 117 (54.45%) | 1.66 | (1.13, 2.46) | 0.010 * | 1.74 | (1.11, 2.72) | 0.015 * |
| Linear trend test: p = 0.008 | |||||||||
| Cord BCAA Score | 626 | ||||||||
| T1 | 198 | 118 (59.60%) | 80 (40.40%) | Ref | Ref | ||||
| T2 | 211 | 110 (52.13%) | 101 (47.87%) | 1.35 | (0.92, 2.00) | 0.129 | 1.63 | (1.04, 2.54) | 0.032 * |
| T3 | 217 | 99 (45.62%) | 118 (54.38%) | 1.75 | (1.19, 2.59) | 0.005 ** | 2.01 | (1.28, 3.15) | 0.002 ** |
| Linear trend test: p = 0.010 | |||||||||
Note: The adjusted model included maternal race, age, parity, smoking, education, low birth weight/preterm birth and child sex.
p<0.05
p<0.01
Table 3.
Associations of cord and maternal plasma branched amino acids score and the risk of ADHD, without and with adjustment for each other.
| NT | ADHD | Crude Logistic | Adjusted Logistic | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Total | N (%) | N (%) | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Associations of maternal BCAA score with ADHD, without adjustment for cord BCAA score | ||||||||||
| Maternal BCAA score only | 803 | |||||||||
| T1 | 271 | 186 (68.63%) | 85 (31.37%) | Ref | Ref | |||||
| T2 | 262 | 179 (68.32%) | 83 (31.68%) | 1.01 | (0.70, 1.46) | 0.938 | 0.93 | (0.62, 1.40) | 0.738 | |
| T3 | 270 | 177 (65.56%) | 93 (34.44%) | 1.15 | (0.80, 1.64) | 0.446 | 1.18 | (0.79, 1.75) | 0.422 | |
| Associations of cord and maternal BCAA score with ADHD after adjustment for each other | ||||||||||
| Maternal BCAA score | 481 | |||||||||
| T1 | 166 | 102 (61.45%) | 64 (38.55%) | Ref | Ref | |||||
| T2 | 155 | 94 (60.65%) | 61 (39.35%) | 1.00 | (0.64, 1.57) | 0.991 | 0.82 | (0.49, 1.37) | 0.445 | |
| T3 | 160 | 91 (56.88%) | 69 (43.13%) | 1.17 | (0.75, 1.83) | 0.481 | 1.04 | (0.63, 1.72) | 0.876 | |
| Cord BCAA score | 481 | |||||||||
| T1 | 167 | 110 (65.87%) | 57 (34.13%) | Ref | Ref | |||||
| T2 | 166 | 98 (69.04%) | 68 (40.96%) | 1.33 | (0.85, 2.07) | 0.215 | 1.57 | (0.95, 2.61) | 0.080 | |
| T3 | 148 | 79 (53.38%) | 69 (46.62%) | 1.68 | (1.06, 2.64) | 0.026 * | 1.89 | (1.11, 3.22) | 0.020 * | |
| Interaction between cord BCAA (continuous) and maternal BCAA (continuous): 1.00 (0.80, 1.26), p-value: 0.998 | ||||||||||
Note: The adjusted model included maternal race, age, parity, smoking, education, low birth weight/preterm birth and child sex.
p<0.05
p<0.01
In contrast, there was no significant association between maternal BCAA score tertiles and child risk of ADHD, either before or after adjusting for cord BCAA score. Further, there was no significant interaction between maternal and cord BCAA scores on child ADHD (Table 3).
We performed sensitivity analyses for cord BCAA scores and the risk of ADHD by multiple subgroups as demonstrated in Table S1. The significant linear positive association between cord BCAA and the risk of ADHD persisted for children with non-obese or non-diabetic mothers, those born at term, with black mothers, with a maternal HDL level below the median, and a cord acetaminophen score below the median. There was an interaction of maternal obesity or diabetes with cord BCAAs on the risk of child ADHD diagnosis. Subgroup analyses for maternal BCAA scores were also conducted but with no significant findings (Table S2).
Discussion
Main Findings
In this US prospective birth cohort, we found higher BCAAs levels in cord plasma were associated with a greater risk of ADHD diagnosis, even when adjusting for maternal plasma BCAA levels. In contrast, there was no significant association between maternal plasma BCAA scores and risk of ADHD diagnosis, either before or after adjusting for cord plasma BCAAs. We did not find a significant interaction between cord and maternal plasma BCAAs and the risk of ADHD.
Interpretation
Few studies have analyzed the relationship between maternal and cord levels of plasma BCAAs and the development of the ADHD. The exact mechanism of how BCAAs contribute to the ADHD is yet unknown. At the blood-brain barrier, BCAAs modify large, neutral amino acid transporters, such as LAT-1.(Fernstrom, 2005) BCAAs compete with aromatic amino acids at these transporters, including tyrosine and tryptophan. Tyrosine is the precursor for dopamine and norepinephrine and tryptophan is the precursor for serotonin. Therefore, increased plasma levels of BCAAs may lead to decreased tyrosine and tryptophan brain levels. This in turn results in decreased synthesis of dopamine, norepinephrine, and serotonin.(Fernstrom, 2005) Urinary metabolites of these neurotransmitters can be used as biomarkers of neurotransmitter activity in the brain. Some studies have shown decreased levels of urinary metabolites in ADHD patients, suggesting there may be an imbalance between brain concentrations of BCAAs and aromatic amino acids (Yamashita, Morinaga, & Yamamoto3, 2018).
One hypothesis of the development of ADHD is that some brain areas in individuals with ADHD have a reduced amount of dopamine (DA) and norepinephrine (NE).(Arnsten & Pliszka, 2011) Studies have shown deficits in certain areas of the brain, primarily in the activity and volume of the prefrontal cortex, caudate, and cerebellum. These areas are sensitive to the neurotransmitters such as DA and NE (Arnsten & Pliszka, 2011). This hypothesis is consistent with the drugs used to treat ADHD symptoms that increase the amount of DA and NE in the synaptic cleft in the prefrontal cortex, such as methylphenidate and amphetamines. However, other studies have found hyperactivity of DA and NE in patients with ADHD (Pliszka, 2005). These conflicting hypotheses point to a more complex etiology of ADHD that involve both hypo- and hyperactivity of neurotransmitters.
Our findings reveal that children with higher levels of BCAAs prenatally may be predisposed to developing ADHD. This would fit with the hypothesis of the development of ADHD, as higher levels of BCAAs at the beginning of life may alter the amount of neurotransmitter synthesis and create an imbalance. As we did not find a significant interaction between cord BCAAs and maternal BCAAs for developing ADHD, the risk modulation of higher prenatal BCAAs levels seem to not be related to the mother’s BCAA state. Rather, this suggests the fetus may regulate circulating levels of BCAAs through an endogenous process.
While BCAAs cannot be over-produced because they are essential amino acids, a decreased breakdown of BCAAs in the fetus may be contributing to higher levels. Those who have a higher risk to develop ADHD may have a defect in the enzymes in the catabolism pathway of BCAAs, such as branched-chain keto acid dehydrogenase that has been implicated in Maple Syrup Urine Disease (Blackburn et al., 2017). Interestingly, those with MSUD on dietary therapy have a cumulative lifetime incidence of 54% for developing ADHD (Muelly et al., 2013).
Strengths and Limitations
A major strength of this study is it utilizes a large prospective birth cohort with mother-child dyads design. Furthermore, this is the first study to examine the association of cord or maternal plasma metabolites of branched-chain amino acids with the risk of childhood ADHD. It has implications for understanding the neurodevelopmental mechanisms of ADHD as well as potential future diagnostics and treatment of the disorder.
However, this study also has its limitations. First, while the overall sample size for this study is relatively large, it is a small fraction of the total dataset from the Boston Birth Cohort, as only a subset of the cohort had maternal and cord metabolites. The sample size was further reduced in the stratifying analyses, specifically the pool of children with ADHD, diminishing the robustness of those results. Second, our main analysis focused on tertiles of BCAA scores. While the second and third tertiles had significant results, we were unable to perform more refined dose-response analyses due to the sample size constraints. Third, we only measured relative intensities instead of absolute concentrations of BCAA. As BCAAs are essential amino acids, further studies are needed to understand the optimal levels of BCAA for healthy neurodevelopment.
Fourth, the maternal blood samples for the BBC collected between 24 to 72 hours after delivery may reflect the stress-related changes or effects of medications around time of delivery that could affect protein homeostasis (Panjwani et al., 2019). Similarly, cord blood levels of proteins may also shift depending on peripartum events. Therefore, the time point used to assess levels of BCAAs used in this study provides only a snapshot and not a full window of the levels of BCAAs in both the mother and fetus that may affect neurodevelopment. Further, perinatal factors may have introduced bias to the BCAA levels at the time of collection, such as the mode of delivery, gestational age at delivery, maternal or fetal stress levels, and maternal medications during labor. Fifth, as the Boston Birth Cohort consists of a predominantly urban, low-income, and minority population, the results may not be easily extrapolated to other populations.
Due to observational nature of the study design, we should regard our findings as hypothesis generating, not causal. We cannot exclude the possibility that our findings could be due to uncontrolled confounders or a marker for other underlying pathway. Further, due to limited sample size, we were unable to adequately explore complex interactions among many potential covariables, such as maternal obesity and diabetes, cholesterol levels, maternal acetaminophen use, and preterm birth (Ji et al., 2020; Ji, Riley, et al., 2018; Ji et al., 2017; Posod et al., 2017). This awaits future studies with larger sample size and use of more sophisticated analytical tools.
This study prompts further investigation into the relationship between cord metabolites and the development of ADHD. Future directions include analyzing how BCAA scores trend from the time of birth to time of diagnosis. Another direction would be to study other cord metabolites related to how BCAAs alter neurotransmission, such as metabolic products of dopamine and norepinephrine. To understand if impaired breakdown of BCAAs may be contributing to higher levels of BCAAs associated with the risk for ADHD, levels of enzymes and substrates involved in the catabolism of BCAAs in cord blood could be analyzed.
Conclusions
Utilizing a prospective birth cohort study, we found an association between higher levels of BCAAs in cord blood but not maternal blood with greater risk of ADHD diagnosis in children. We did not find a significant interaction of cord BCAAs and maternal BCAAs and the risk of ADHD diagnosis. These results suggest a higher cord level of BCAAs may signal an underlying mechanism that could be contributing to the development of ADHD. Additional investigation is needed to confirm our findings and elucidate the mechanisms behind prenatal BCAA levels and the pathophysiology of ADHD. This line of investigation, if fruitful, may provide insight for further research into mechanisms of ADHD development and potential early life screening and interventions for ADHD.
Supplementary Material
Key Points and Relevance.
The etiology of Attention Deficit and Hyperactivity Disorder (ADHD) is not well known, though there is growing recognition that early life factors may play a role.
Branched chain amino acids (BCAAs) play an important role in brain function and higher maternal BCAA levels have been associated with risk of child Autism Spectrum Disorder.
In this study of a high-risk US birth cohort, we found higher cord plasma BCAA levels were associated with a greater risk of developing ADHD in childhood.
Though more investigation is needed, this finding provides insight for further research into mechanisms of ADHD development and potential screening and interventions for ADHD early in life.
Acknowledgments:
This study is supported in part by the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) under grant number R40MC27443, Autism Field-initiated Innovative Research Studies Program; and grant number UJ2MC31074, Autism Single Investigator Innovation Program. The Boston Birth Cohort (the parent study) was supported in part by the National Institutes of Health (NIH) grants (R21ES011666, R21HD066471, U01AI090727, R21AI079872, R01HD086013, 2R01HD041702, and R01HD098232). This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by HRSA, HHS or the U.S. Government. The funding agencies had no involvement in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The authors thank Linda Rosen of the Boston University Clinical Data Warehouse for assistance in obtaining relevant clinical information; the Clinical Data Warehouse service is supported by Boston University Clinical and Translational Institute and the National Institutes of Health Clinical and Translational Science Award (grant U54-TR001012). Neha Anand is supported in part by the TL1 Predoctoral Training Award.
References
- Anderson TW, & Rubin H (1956). Statistical inference in factor analysis. . Proceedings of the Berkely Symposium on Mathematical Statistics and Probability, 3(5), 111–150. [Google Scholar]
- Arnsten AF, & Pliszka SR (2011). Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol Biochem Behav, 99(2), 211–216. doi: 10.1016/j.pbb.2011.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn PR, Gass JM, Vairo FPE, Farnham KM, Atwal HK, Macklin S, … Atwal PS (2017). Maple syrup urine disease: mechanisms and management. Appl Clin Genet, 10, 57–66. doi: 10.2147/TACG.S125962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AC, & Berry SA (2012). Pharmacotherapy of attention-deficit hyperactivity disorder in adolescents. Drugs, 72(3), 309–325. doi: 10.2165/11599580-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Danielson ML, Bitsko RH, Ghandour RM, Holbrook JR, Kogan MD, & Blumberg SJ (2018). Prevalence of Parent-Reported ADHD Diagnosis and Associated Treatment Among U.S. Children and Adolescents, 2016. J Clin Child Adolesc Psychol, 47(2), 199–212. doi: 10.1080/15374416.2017.1417860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis NO, & Kollins SH (2012). Treatment for co-occurring attention deficit/hyperactivity disorder and autism spectrum disorder. Neurotherapeutics, 9(3), 518–530. doi: 10.1007/s13311-012-0126-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernstrom JD (2005). Branched-chain amino acids and brain function. J Nutr, 135(6 Suppl), 1539S–1546S. doi: 10.1093/jn/135.6.1539S [DOI] [PubMed] [Google Scholar]
- Jensen PS (2000). ADHD: current concepts on etiology, pathophysiology, and neurobiology. Child Adolesc Psychiatr Clin N Am, 9(3), 557–572, vii-viii. [PubMed] [Google Scholar]
- Ji Y, Azuine RE, Zhang Y, Hou W, Hong X, Wang G, … Wang X (2020). Association of Cord Plasma Biomarkers of In Utero Acetaminophen Exposure With Risk of Attention-Deficit/Hyperactivity Disorder and Autism Spectrum Disorder in Childhood. JAMA Psychiatry, 77(2), 180–189. doi: 10.1001/jamapsychiatry.2019.3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Hong X, Wang G, Chatterjee N, Riley AW, Lee L-C, … Wang X (2018). A Prospective Birth Cohort Study on Early Childhood Lead Levels and Attention Deficit Hyperactivity Disorder: New Insight on Sex Differences. The Journal of Pediatrics, 199, 124–131.e128. doi: 10.1016/j.jpeds.2018.03.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Riley AW, Lee LC, Hong X, Wang G, Tsai HJ, … Wang X (2018). Maternal Biomarkers of Acetaminophen Use and Offspring Attention Deficit Hyperactivity Disorder. Brain Sci, 8(7). doi: 10.3390/brainsci8070127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Riley AW, Lee LC, Volk H, Hong X, Wang G, … Wang X (2017). A Prospective Birth Cohort Study on Maternal Cholesterol Levels and Offspring Attention Deficit Hyperactivity Disorder: New Insight on Sex Differences. Brain Sci, 8(1). doi: 10.3390/brainsci8010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Fallin MD, Riley A, Landa R, Walker SO, Silverstein M, … Wang X (2016). The Association of Maternal Obesity and Diabetes With Autism and Other Developmental Disabilities. Pediatrics, 137(2), e20152206. doi: 10.1542/peds.2015-2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz B, Frommherz L, Rist MJ, Kulling SE, Bub A, & Watzl B (2018). Dietary Pattern and Plasma BCAA-Variations in Healthy Men and Women-Results from the KarMeN Study. Nutrients, 10(5). doi: 10.3390/nu10050623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muelly ER, Moore GJ, Bunce SC, Mack J, Bigler DC, Morton DH, & Strauss KA (2013). Biochemical correlates of neuropsychiatric illness in maple syrup urine disease. J Clin Invest, 123(4), 1809–1820. doi: 10.1172/JCI67217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano L, Ji Y, Riley AW, & Wang X (2019). Maternal psychosocial stress and children's ADHD diagnosis: a prospective birth cohort study. J Psychosom Obstet Gynaecol, 40(3), 217–225. doi: 10.1080/0167482X.2018.1468434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panjwani AA, Ji Y, Fahey JW, Palmer A, Wang G, Hong X, … Wang X (2019). Maternal Obesity/Diabetes, Plasma Branched-Chain Amino Acids, and Autism Spectrum Disorder Risk in Urban Low-Income Children: Evidence of Sex Difference. Autism Res, 12(10), 1562–1573. doi: 10.1002/aur.2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliszka SR (2005). The neuropsychopharmacology of attention-deficit/hyperactivity disorder. Biol Psychiatry, 57(11), 1385–1390. doi: 10.1016/j.biopsych.2004.08.026 [DOI] [PubMed] [Google Scholar]
- Posod A, Muller S, Komazec IO, Dejaco D, Peglow UP, Griesmaier E, … Kiechl-Kohlendorfer U (2017). Former very preterm infants show alterations in plasma amino acid profiles at a preschool age. Pediatr Res, 81(5), 787–794. doi: 10.1038/pr.2017.24 [DOI] [PubMed] [Google Scholar]
- Rivera HM, Christiansen KJ, & Sullivan EL (2015). The role of maternal obesity in the risk of neuropsychiatric disorders. Front Neurosci, 9, 194. doi: 10.3389/fnins.2015.00194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciberras E, Mulraney M, Silva D, & Coghill D (2017). Prenatal Risk Factors and the Etiology of ADHD-Review of Existing Evidence. Curr Psychiatry Rep, 19(1), 1. doi: 10.1007/s11920-017-0753-2 [DOI] [PubMed] [Google Scholar]
- Sharma A, & Couture J (2014). A review of the pathophysiology, etiology, and treatment of attention-deficit hyperactivity disorder (ADHD). Ann Pharmacother, 48(2), 209–225. doi: 10.1177/1060028013510699 [DOI] [PubMed] [Google Scholar]
- Sperringer JE, Addington A, & Hutson SM (2017). Branched-Chain Amino Acids and Brain Metabolism. Neurochem Res, 42(6), 1697–1709. doi: 10.1007/s11064-017-2261-5 [DOI] [PubMed] [Google Scholar]
- St-Jean A, Meziou S, Roy C, Ayotte P, Muckle G, & Lucas M (2017). Branched-chain and aromatic amino acids in relation to behavioral problems among young Inuit from Nunavik, Canada: a cohort study. Pediatr Res, 82(3), 416–422. doi: 10.1038/pr.2017.115 [DOI] [PubMed] [Google Scholar]
- Xu G, Strathearn L, Liu B, Yang B, & Bao W (2018). Twenty-Year Trends in Diagnosed Attention-Deficit/Hyperactivity Disorder Among US Children and Adolescents, 1997-2016. JAMA network open, 1(4), e181471–e181471. doi: 10.1001/jamanetworkopen.2018.1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Morinaga M, & Yamamoto3 T (2018). Insight into Neurodevelopmental Disorders Related to the Imbalance of Monoamine Neuroactive Metabolites and Essential Amino Acids. Biomed J Sci &Tech Res, 4(1). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


