Cryotherapy is the treatment of choice for actinic keratosis (AK) and has high efficacy rates (1, 2). However, owing to the inconsistency of cryotherapy, a range of lesion cure rates and various adverse events have been reported (3). Based on the cell death temperature of keratinocytes, treatment parameters, including freeze time, number of freeze-thaw cycles, and margins, have been suggested in the literature to optimize the effects of cryotherapy (4). However, since manual control of cryotherapy cannot be performed with an appropriate and consistent intensity, it is difficult to follow the exact treatment time and to ascertain whether the intended temperature has been attained. Therefore, it is also difficult to predict outcomes and adverse events. To overcome these limitations, temperature- and time-controlled cryotherapy devices (TCD, Cutis-CoolTM; RecensMedical, Ulsan, Korea), which use carbon dioxide as a cryogen, have been developed recently. The TCD enables precise and consistent cryotherapy to be performed by setting the treatment parameters, such as the number of freezing cycles, skin surface temperature and spraying time. The TCD administers cryotherapy in a freezing area of approximately 10-mm diameter for a set time at a constant intensity to reach the set skin surface temperature (5). (Video S1). The aim of this study was to evaluate the efficacy and safety of the TCD for mild facial AK and establish cornerstone cryotherapy parameters for mild facial AK.
MATERIALS AND METHODS
This study retrospectively analysed the medical records and clinical photographs of patients diagnosed with mild facial AK and treated with the TCD who visited the Kyungpook National University Hospital, a tertiary referral centre in South Korea, between May 2020 and November 2021. The patients were diagnosed clinically or histologically with mild facial AK (slightly palpable and more felt than seen) (6) and treated with the TCD at –20°C for 20 s in a single freeze-thaw cycle. Freezing areas overlapped by 20% while sufficiently covering the entire target lesion. After 4 weeks of treatment, the treatment response of lesions was evaluated using medical records and clinical photographs. Complete remission was defined as the disappearance of the lesion on skin biopsy or no lesion remaining on the clinical photographs, with a smooth surface when touched by a finger. The treatment was repeated every 4 weeks until complete remission was achieved. Four weeks after the first treatment (4WFT), the cure rate and adverse events were assessed. The cure rate, recurrence rate, adverse events, and cosmetic outcomes were assessed 12 and 24 weeks after the last treatment (12WLT and 24WLT, respectively). All adverse events that occurred were recorded as responses to open-ended questions, and pain after treatment was assessed using a visual analogue scale (VAS 0–10). Cosmetic outcomes were assessed by the patients themselves and by 2 dermatologists using clinical photographs and graded as follows: excellent: no or mild redness or change in pigmentation; good: moderate redness or a change in pigmentation; fair: slight-to-moderate scarring, atrophy, or induration; poor: extensive scarring, atrophy, or induration. Patients who did not visit the hospital for regular follow-ups or for whom clinical photographs were not taken were excluded. This study was approved by the Institutional Review Board of Kyungpook National University Hospital (KNUH 2022-02-003).
RESULTS
Twenty-three patients with a total of 38 lesions were included in this retrospective study. The mean age of the patients was 75.7 years, and Fitzpatrick skin photo-types III (8.7%) and IV (91.3%) were predominant. The mean ± standard deviation (SD) treatment number was 1.13 ± 0.5. Of the total 38 lesions, 3 were retreated, 2 of which were treated 3 times in total and 1 lesion was treated 2 times in total. Complete remission rates at 4WFT, 12WLT, and 24WLT were 92.1%, 97.4%, and 94.6%, respectively (Fig. 1). Recurrence occurred in only 1 lesion (2.6%) at 12WLT and in 2 lesions (5.4%) at 24WLT. Serious adverse events were not observed. Only pain (100%; mean ± SD VAS score, 3.84 ± 1.76) during or immediately after the treatment, erythema, and hypopigmentation were observed. The erythema rate decreased significantly from 31.6% at 4WFT to 5.3% at 12WLT to 0.0% at 24WLT, whereas the hypopigmentation rate decreased slightly from 23.7% to 23.7% to 10.0% (Fig. 2). As for the cosmetic outcomes at 24WLT, 91.3% of the patient responses were good or excellent (34.8% and 56.5%, respectively), and 100% of the investigator assessments demonstrated good or excellent results (30.4% and 69.6%, respectively).
Fig. 1.
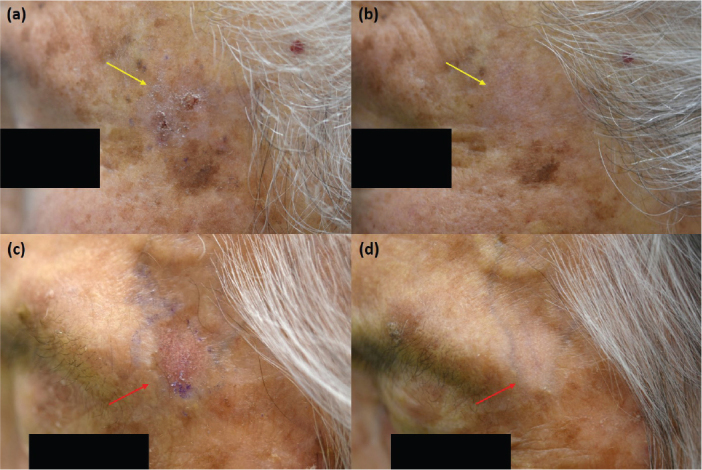
(a) Solitary hyperkeratotic walnut-sized mild actinic keratosis on the left temple before treatment. (b) Complete lesion remission 24 weeks after single treatment (yellow arrow). (c) Solitary hyperkeratotic erythematous bean-sized mild actinic keratosis on the left temple before treatment. (d) Complete lesion remission 24 weeks after single treatment (red arrow).
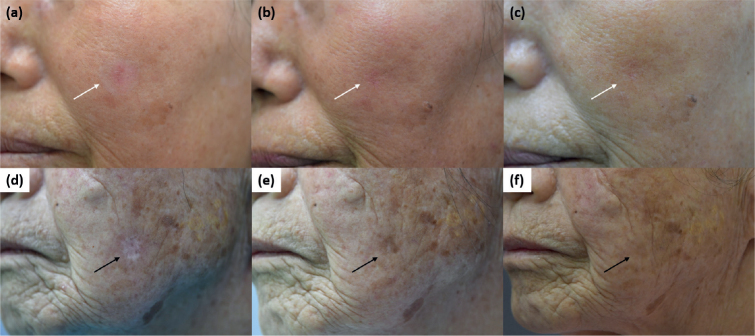
Fig. 2.
(a) Erythema at the maximal state 4 weeks after the first treatment. Improvement of erythema at (b) 12 weeks and (c) 24 weeks after the last treatment (white arrow). (d) Hypopigmentation 4 weeks after the first treatment. Improvement of hypopigmentation at (e) 12 weeks and (f) 24 weeks after the last treatment (black arrow).
DISCUSSION
Cryotherapy parameters for AK differ depending on the clinical grade of the lesions (1). Mild or moderate AK corresponding to grades I and II in Olsen’s classification is generally treated with a freezing time of 5–20 s, with 1 freeze-thaw cycle and a margin of < 2 mm (1, 4). For thicker and larger lesions, a longer freezing time is required (1). However, the manual cryotherapy makes it difficult to follow the guidelines because of its inconsistent intensity. Moreover, despite the fact that low temperature is an important mechanism of action in cryotherapy, there is no standard treatment temperature for cryotherapy, and a few studies have reported varying treatment responses (cure rate 66.7–100%) (Table I; 7–14). However, with the TCD, it was possible to deliver precise and consistent cryotherapy by controlling the treatment temperature and time. Therefore, we attempted to obtain effective and safe treatment parameters using the TCD. In an unpublished pilot study, we noticed that performing cryotherapy at –20°C for 20 s in a single freeze-thaw cycle is more effective than performing at other parameters, while minimizing the side-effects of treating mild facial AK. Therefore, we attempted to confirm that these parameters are effective and safe for the treatment of mild facial AK. The current study confirmed that the parameters were sufficiently effective, and the cure rates were not inferior to those reported in previous studies (Table I). However, care must be taken when comparing the cure rates of the current study with those of previous studies. Each previous study had a different follow-up period (minimum 1 week; maximum 8.5 years; mean ± SD 11.38 ± 25.4 months), did not specify the freezing method or time, and had varying severities of AK (Table I).
Table I.
Summary of recent studies on the efficacy of cryotherapy in actinic keratosis
| Study | Severity of AK | Freeze time | Tempera-ture | Freeze-thaw cycle, n | Follow-up time | Cure rate (%) |
|---|---|---|---|---|---|---|
| Lubritz et al. (7), 1982 | N/S | N/S | N/S | N/S | 1–8.5 years | 98.8 |
| Szeimies et al. (8), 2002 | Mild, moderate, severe | Mean of 24 s | N/S | 2 | 12 weeks | 75.3 |
| Freeman et al. (9), 2003 | Mild, moderate | Based on the diameter of lesion:< 10 mm, mean of 12 s 10–20 mm, mean of 16 s > 20 mm, mean of 26 s | N/S | 1 | 12 weeks | 68.3 |
| Thai et al. (2), 2004 | Mild, moderate | 2 s–1 min 30 s | N/S | 1 | 12 weeks | 67.2 |
| Morton et al. (10), 2006 | Mild, moderate | Mean of 16 s | N/S | 2 | 12 weeks, 24 weeks | 75.0, 85.0 |
| Krawtchenko et al. (11), 2007 | N/S | 20–40 s | N/S | 1 | 6 weeks | 68.0 |
| Kaufmann et al. (12), 2008 | Mild, moderate | Mean of 20 s | N/S | 2 | 24 weeks | 88.0 |
| Hauschild et al. (13), 2009 | Mild, moderate | 5–10 s | N/S | 1 | 12 weeks | 76.6 |
| Goldberg et al. (3), 2010 | N/S | 5–10 s | –5 °C | 1 | 1 week, 6 weeks | 66.7, 100.0 |
| Zane et al. (14), 2014 | Mild, moderate, severe | 10–20 s | N/S | 1 | 12 weeks, 1 year | 78.2, 66.8 |
| Current study | Mild | 20 s | –20 °C | 1 | 12 weeks, 24 weeks | 97.4, 94.6 |
N/S: not specified.
As cryotherapy intentionally damages tissue for secondary healing, it has potential complications and adverse events (4). During cryotherapy, localized burning pain occurs immediately and usually ends within 1 h, followed by erythema and oedema (4). After 12–36 h, serous or haemorrhagic vesicles develop, and after 1–2 weeks, crust forms (1, 4). Owing to the susceptibility of melanocytes to freezing, hypopigmentation or hyperpigmentation can develop (15). In the current study, it is noteworthy that less hyper/hypopigmentation occurred after this treatment despite Fitzpatrick skin phototypes III–VI, which are prone to develop post-inflammatory pigmentation. The TCD resulted in only mild adverse events, including pain, erythema, and hypopigmentation, which were all acceptable to the patients and investigators. No serious adverse events were observed, suggesting that TCD treatment at –20 °C for 20 s is safer and more predictable than the existing cryotherapy methods.
In conclusion, TCD treatment at –20°C for 20 s is an effective, safe, and predictable treatment for mild facial AK. This study will help establish standard cryotherapy parameters for AK with other severities or at other sites.
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Arisi M, Guasco Pisani EG, Calzavara-Pinton P, Zane C. Cryotherapy for actinic keratosis: basic principles and literature review. Clin Cosmet Investig Dermatol 2022; 15: 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thai KE, Fergin P, Freeman M, Vinciullo C, Francis D, Spelman L, et al. A prospective study of the use of cryosurgery for the treatment of actinic keratoses. Int J Dermatol 2004; 43: 687–692. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg LH, Kaplan B, Vergilis-Kalner I, Landau J. Liquid nitrogen: temperature control in the treatment of actinic keratosis. Dermatol Surg 2010; 36: 1956–1961. [DOI] [PubMed] [Google Scholar]
- 4.Zimmerman EE, Crawford P. Cutaneous cryosurgery. Am Fam Phys 2012; 86: 1118–1124. [PubMed] [Google Scholar]
- 5.Kwack MH, Lee S, Lee EH, Ha GU, Kim GH, Lee WJ. Effect of a precision cryotherapy device with temperature adjustability on pigmentation. Indian J Dermatol 2022; 67: 204.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olsen EA, Abernethy ML, Kulp-Shorten C, Callen JP, Glazer SD, Huntley A, et al. A double-blind, vehicle-controlled study evaluating masoprocol cream in the treatment of actinic keratoses on the head and neck. J Am Acad Dermatol 1991; 24: 738–743. [DOI] [PubMed] [Google Scholar]
- 7.Lubritz RR, Smolewski SA. Cryosurgery cure rate of actinic keratoses. J Am Acad Dermatol 1982; 7: 631–632. [DOI] [PubMed] [Google Scholar]
- 8.Szeimies RM, Karrer S, Radakovic-Fijan S, Tanew A, Calzabara-Pinton PG, Zane C, et al. Photodynamic therapy using topical methyl 5-aminolevulinate compared with cryotherapy for actinic keratosis: a prospective, randomized study. J Am Acad Dermatol 2002; 47: 258–262. [PubMed] [Google Scholar]
- 9.Freeman M, Vinciullo C, Francis D, Spelman L, Ngyuen R, Fergin P, et al. A comparison of photodynamic therapy using topical methyl aminolevulinate (Metvix) with single cycle cryotherapy in patients with actinic keratosis: a prospective, randomized study. J Dermatolog Treat 2003; 14: 99–106. [DOI] [PubMed] [Google Scholar]
- 10.Morton C, Campbell S, Gupta G, Keohane S, Lear J, Zaki I, et al. Intraindividual, right-left comparison of topical methyl aminolaevulinate-photodynamic therapy and cryotherapy in subjects with actinic keratosis: a multicentre, randomized controlled study. Br J Dermatol 2006; 155: 1029–1036. [DOI] [PubMed] [Google Scholar]
- 11.Krawtchenko N, Roewert-Huber J, Ulrich M, Mann I, Sterry W, Stockfleth E. A randomized study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol 2007; 157: 34–40. [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann R, Spelman L, Weightman W, Reifenberger J, Szeimies RM, Verhaeghe E, et al. Multicentre intraindividual randomized trial of topical methyl aminolaevulinate-photodynamic therapy vs. cryotherapy for multiple actinic keratoses on the extremities. Br J Dermatol 2008; 158: 994–999. [DOI] [PubMed] [Google Scholar]
- 13.Hauschild A, Stockfleth E, Popp G, Borrosch F, Brüning H, Dominicus R, et al. Optimization of photodynamic therapy with a novel self-adhesive 5-aminolaevulinic acid patch: results of two randomized controlled phase III studies. Br J Dermatol 2009; 160: 1066–1074. [DOI] [PubMed] [Google Scholar]
- 14.Zane C, Facchinetti E, Rossi MT, Specchia C, Ortel B, Calzavara-Pinton P. Cryotherapy is preferable to ablative CO2 laser for the treatment of isolated actinic keratoses of the face and scalp: a randomized clinical trial. Br J Dermatol 2014; 170: 1114–1121. [DOI] [PubMed] [Google Scholar]
- 15.Cook DK, Georgouras K. Complications of cutaneous cryotherapy. Med J Aust 1994; 161: 210–213. [DOI] [PubMed] [Google Scholar]