Abstract
Study Objectives
The purpose of this study was to (1) estimate trauma associated sleep disorder (TASD) prevalence among post-9/11 era veterans and to describe differences in service and comorbid mental health clinical characteristics among individuals with and without probable TASD, and (2) estimate TASD prevalence and characteristics of reported traumatic experiences stratified by sex.
Methods
We used cross-sectional data from the post-deployment mental health study of post-9/11 veterans, which enrolled and collected baseline data from 2005 to 2018. We classified veterans as having probable TASD using self-reported measures: traumatic experiences from the traumatic life events questionnaire (TLEQ) and items from the Pittsburgh sleep quality index with Addendum for posttraumatic stress disorder (PTSD) mapped to TASD diagnostic criteria and ascertained mental health diagnoses (PTSD, major depressive disorder [MDD]) via Structured Clinical Interview for DSM-IV. We calculated effect sizes as prevalence ratios (PR) for categorical variables and Hedges’ g for continuous variables.
Results
Our final sample included 3618 veterans (22.7% female). TASD prevalence was 12.1% (95% CI: 11.1% to 13.2%) and sex-stratified prevalence was similar for female and male veterans. Veterans with TASD had a much higher comorbid prevalence of PTSD (PR: 3.72, 95% CI: 3.41 to 4.06) and MDD (PR: 3.93, 95% CI: 3.48 to 4.43). Combat was the highest reported most distressing traumatic experience among veterans with TASD (62.6%). When stratifying by sex, female veterans with TASD had a wider variety of traumatic experiences.
Conclusions
Our results support the need for improved screening and evaluation for TASD in veterans, which is currently not performed in routine clinical practice.
Keywords: veterans, military, combat, trauma, trauma associated sleep disorder, nightmares, parasomnias, sleep–wake disorders
Statement of Significance.
This is the first study to investigate the newly proposed diagnosis of trauma associated sleep disorder (TASD) in a large, diverse cohort of post-9/11 veterans and describe the characteristics of those with TASD. A better understanding of the prevalence of TASD and associated comorbidities helps to contextualize the problem of disorder poses. Additionally, understanding this severe nocturnal disorder among veterans is important for policymakers and funding organizations in allocating resources. Some misclassification of TASD cases may be present; however, we used items from validated self-reported clinical instruments linked to TASD diagnostic criteria to limit such misclassification. Future research should investigate the association between using these instruments to evaluate nightmares and disruptive nocturnal behaviors and the clinical diagnosis of TASD.
Introduction
Trauma-associated nightmares are one of the most commonly reported symptoms following a traumatic experience [1, 2]. While trauma-associated nightmares that are associated with posttraumatic stress disorder (PTSD) may be more severe and distressing, trauma survivors (including combat veterans) with and without PTSD commonly report trauma-associated nightmares accompanied by disruptive nocturnal behaviors [3–6]. These behaviors include sympathetic activation, dream enactment behavior, and rapid eye movement (REM) sleep without atonia which distinguishes trauma-related nightmares from idiopathic nightmares and nightmare disorder [7]. These differences fueled the proposal of a new parasomnia diagnosis that captures these distinct phenomena: trauma associated sleep disorder (TASD) [7–9].
The newly proposed TASD diagnosis is defined using the following criteria: (1) onset of symptoms after combat or other traumatic experience, (2) history of altered dream mentation that is related to a prior traumatic experience, (3) self or witness reports of disruptive nocturnal behaviors, (4) symptoms of autonomic hyperarousal or polysomnographic monitoring that demonstrates at least one of the following: tachycardia, tachypnea, or diaphoresis, and (5) absence of electroencephalogram epileptiform activity on polysomnography and the disturbance is not better explained by another sleep disorder, medical disorder, medication, or substance use [9]. Additionally, diagnostic criteria note that individuals with TASD frequently have comorbid insomnia and/or obstructive sleep apnea and that onset of TASD is typically close in temporal proximity to trauma exposure [9].
There has been some debate regarding the overlap between symptoms of TASD and other parasomnias such as REM behavior disorder and whether these represent the same underlying diagnosis [10–13]. However, REM behavior disorder is relatively rare (0.5%–1.25%) in the general population with symptoms typically beginning in late adulthood, being most prevalent (2%) in older adults and predominantly affecting males [14–18]. Among those with an idiopathic REM behavior disorder, the condition can phenoconvert to a neurodegenerative disease at a rate of 6.3% per year, or 73.5% after 12 years [19]. The same phenoconversion to neurodegenerative disease has not been reported in those followed with TASD [9, 12, 13, 20]. Additionally, the onset of REM behavior disorder is insidious and trauma is not a reported risk factor for this sleep disorder [21]. This contrasts with TASD, which has primarily been recorded in a much younger population, is specifically linked to a traumatic experience, and does not appear to share the same neurodegenerative process. REM behavior disorder can be effectively treated with clonazepam or melatonin, whereas these same medications are ineffective in treating TASD [13, 21]. Finally, trauma-related nightmares are a prevalent feature of TASD, but they are less prevalent in REM behavior disorder [13]. Among those with REM behavior disorder who experience nightmares, these nightmares are more often characterized by confrontation with unfamiliar people and animals rather than traumatic experience reenactment [13, 22].
Although TASD has been primarily studied in active duty service members and veterans, the prevalence of TASD in this population remains unclear [7, 8, 20]. Additionally, TASD cases studied and reported on have primarily been in male service members or veterans and potential sex-based differences in TASD presentation remain unclear. Therefore, the primary purpose of our study was to estimate TASD prevalence in a large, diverse cohort of post-9/11 era veterans and describe differences in service and comorbid mental health clinical characteristics among individuals with and without probable TASD. The secondary purpose of our study was to estimate TASD prevalence and characteristics of reported traumatic experiences stratified by sex.
Methods
For this descriptive study, we used cross-sectional data from the Post-Deployment Mental Health (PDMH) study [23]; a multi-site study of post-9/11 US military veterans (including National Guard members and Reservists) conducted through the Department of Veterans Affairs at the Veterans Integrated Services Network (VISN) 6 Mid-Atlantic Mental Illness Research, Education, and Clinical Center. The local review boards at each of the participating PDMH study sites approved the study protocol.
Participants, recruitment, and enrollment
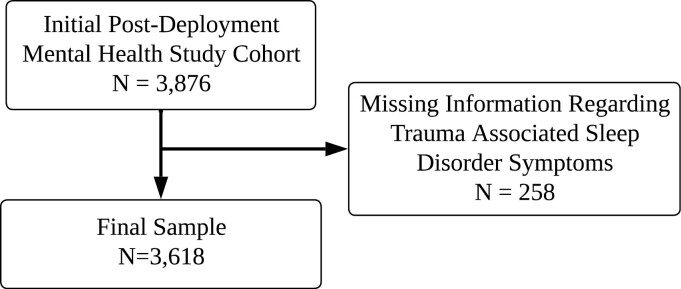
Individuals were eligible to participate in the PDMH study if they had prior US military service (i.e. Veterans, active duty personnel) after September 11, 2001, and/or Reserve status (National Guard members and Reservists) on or after September 11, 2001. The PDMH study excluded individuals if: (1) English was not their primary language, (2) they had difficulty comprehending the informed consent form or process, or (3) they were unable to travel to one of the four participating data-collection sites. Veterans were not required to be deployed, nor were they required to be enrolled in the Veterans Affairs healthcare system to enroll in the PDMH study. A total of 3876 post-9/11 veterans enrolled in the PDMH study from 2005 to 2018. During the data-collection visit, veterans used a computer to enter self-reported data into a database hosted on a website that could be simultaneously accessed by research staff and the study participant. Research staff was available nearby during data collection. Participants had the option of declining any questions they did not want to answer, resulting in some missing data. For the present analysis, we excluded veterans enrolled in the PDMH sample who were missing data regarding TASD symptoms.
The PDMH study started oversampling on sex in 2015 to increase the number of female veterans in the cohort, which resulted in the cohort matching regional veteran sex distribution. The PDMH cohort has a higher proportion of veterans with a service-connected disability and a more diverse racial makeup than post-9/11 veterans nationally [23, 24]. Beyond these differences, the PDMH cohort is similar to post-9/11 veterans nationally in terms of age, military branch, rank, and current military status [23, 24]. The cohort is also similar to post-9/11 veterans nationally in regard to mental health diagnoses for PTSD, depression, and alcohol abuse/dependence [23]. The Veterans Affairs Mid-Atlantic Mental Illness Research, Education, and Clinical Center Workgroup have published an in-depth description of study methods that includes additional details regarding the overall cohort, recruitment, enrollment, and an in-depth review of data collection and management procedures elsewhere [23].
Measures
Demographic and military service characteristics
The PDMH study includes a battery of questions regarding demographic, medical, mental health, and military service characteristics. The following items were included in the analyses for the current project: age, sex, race, service-connected condition prevalence, number of tours served, highest rank, current or most recent military service branch, current military service status, service in war/operation zones, combat scenarios, wounded or injured in a war zone, and awarded medals.
Combat exposure
Combat exposure was assessed using the Combat Exposure Scale, which is a 7-item self-report questionnaire assessing wartime psychological trauma. Items are rated on a single 5-point scale assessing the frequency of exposure (1 = “no” or “never” to 5 = “26 + times” or “51 + times”) to each of seven combat situations, such as firing rounds at the enemy and being on dangerous duty [25]. The Combat Exposure Scale has demonstrated good reliability and validity [23, 26].
Trauma-related sleep disturbances
Sleep disturbances related to trauma exposure were assessed using the Pittsburgh Sleep Quality Index-Addendum for PTSD (PSQI-A). The PSQI-A is a 7-item self-report measure designed to reflect the unique sleep-related clinical presentation of individuals having trauma exposure and/or symptoms of PTSD. The PSQI-A asked participants, “During the past month, how often have you had trouble sleeping because you….” The PSQI-A then asks participants to rate the frequency of seven disruptive nocturnal behaviors: hot flashes, nervousness, nightmares of trauma, other nightmares, panic, night terrors, and acting out dreams. Response options (and associated scores) for these items are: not during the past month (0), less than once a week (1), once or twice a week (2), or ≥3 times a week (3). The PSQI-A has established psychometric properties in samples of women (with and without PTSD diagnoses) [27] and in a sample of military veterans [28]. The PSQI-A has satisfactory internal consistency (Cronbach α = 0.85) and adequate psychometric properties [27].
Trauma history
The Traumatic Life Events Questionnaire (TLEQ) was used to assess the frequency, severity, and characteristics of lifetime traumatic experiences. The TLEQ is a 22-item questionnaire that provides an assessment of prior traumatic life experiences as well as time, since those experiences are across a wide range of categories and are reliable and widely validated in adults, including combat veterans [29]. In the PDMH study, the TLEQ was modified to ascertain the temporal relationship between trauma exposure and military service (before, during, or after) consistent with other studies [30].
Mental health clinical characteristics
PTSD and major depressive disorder (MDD) diagnoses were derived from the Structured Clinical Interview for DSM-IV (SCID) which was used to assess all Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) Axis I psychiatric diagnoses, except for childhood-onset disorders, sexual disorders, sleep disorders, and dementias. Licensed doctoral-level clinical psychologists supervised the administration of the SCID. Interrater reliability across interviewers was excellent for all Axis I diagnoses (Fleiss’ κ = 0.94) [23]. To account for changes in PTSD diagnostic criteria that changed from DSM-IV to the fifth edition (DSM-5), the Veterans Integrated Services Network 6 Mid-Atlantic Mental Illness Research, Education, and Clinical Center Workgroup developed a DSM-5 PTSD SCID supplement (described in detail elsewhere) [23] and added it to the PDMH baseline data collection in 2011. In the current study, we use the PTSD diagnosis variable based on the SCID (DSM-IV-TR) for those diagnosed before the 2011 update. Starting from 2011 and onwards, we used the PTSD diagnosis that was based on the DSM-5. Differences in PTSD prevalence using DSM-IV versus DSM-5 criteria have been reported as minimal [31, 32].
In addition to MDD diagnosis, we report depressive symptoms as measured by the Beck Depression Inventory-II (BDI-II) [33]. The BDI-II is a widely used 21-item instrument designed to measure the severity of depression among both clinical and nonclinical adult populations. Respondents endorse one of four statements reflecting increasing severity for each of the 21 symptoms of depression. Respondents score each item on a 4-point scale from 0 to 3, with total scores ranging from 0 to 63. The BDI-II has demonstrated excellent psychometric properties across a range of populations and clinical settings [34].
Suicidality was assessed using the Beck Scale for Suicide Ideation[35], a 21-item scale of suicidality that includes items about deterrents, suicidal plans, and the patient’s level of revealing suicidality to others. Respondents score each Beck Scale For Suicide Ideation item on a 3-point scale from 0 to 2 with higher scores indicating higher severity. The Beck Scale For Suicide Ideation has demonstrated adequate psychometric properties [35–37]. Violent behavior was assessed from a single item included as part of demographic questionnaires for the study: “During the past 30 days, have you had trouble controlling violent behavior (e.g. hitting someone)?” [38]
Trauma associated sleep disorder
In the current study, we focus on self-reported symptoms that align with a diagnosis of TASD. To identify veterans with probable cases of TASD, we used a combination of questions from the TLEQ and the PSQI-A. Table 1 presents a comparison of TASD's proposed diagnostic criteria and how we used the TLEQ and the PSQI-A to reflect the criteria A–D. To assess the presence of prior traumatic experiences, we calculated the sum of all traumatic experiences from the TLEQ for everyone in our cohort. We considered individuals reporting ≥1 traumatic experience(s) from the TLEQ as meeting TASD diagnostic criteria A. We outline the specific PSQI-A items used to determine TASD Diagnostic criteria B–D in Table 1. PSQI-A items counted toward their respective diagnostic criteria if a participant scored the item ≥2 (“once or twice a week” or “three or more times a week”). Criteria B and C each have a list of multiple associated symptoms and both require at least one of those symptoms to be present for individuals to meet those criteria. We treated the PSQI-A items we linked to each of these criteria accordingly, requiring participants to report a score of ≥2 on at least one of the PSQI-A questions linked to each respective criterion. A diagnosis of TASD requires the absence of polysomnography electroencephalogram epileptiform activity (criteria E) [9], which were not available as part of the PDMH study. We classified participants as having probable TASD only if they met the indications for criteria A (TLEQ), B, C, and D (PSQI-A) as described above. We classified individuals failing to meet one or more of these four criteria as negative for probable TASD.
Table 1.
Operationalization of trauma associated sleep disorder diagnostic criteria used to identify veterans with probable trauma associated sleep disorder
| Trauma associated sleep disorder diagnostic criteria | Measure used to assess | Measure indications for meeting criteria |
|---|---|---|
| A. Onset of symptoms after combat or other traumatic experience | TLEQ | Prior traumatic experience |
| B. History of altered dream mentation that is related to prior traumatic experience | PSQI-A | Trouble sleeping because of memories or nightmares of a traumatic experience† |
| C. Self or witness reports of disruptive nocturnal behaviors involving one of the following: 1.Abnormal vocalizations a.moaning, screaming, or yelling 2.Abnormal motor behaviors in sleep: a.tossing, turning, or thrashing b.combative behaviors such as striking bedpartner |
PSQI-A | Trouble sleeping because of one or more of the following: 1.Episodes of terror or screaming during sleep without fully awakening† 2.Episodes of “acting out” dreams, such as kicking, running, or screaming† |
| D. Symptoms of autonomic hyperarousal or polysomnographic monitoring that demonstrates at least one of the following associated with dream mentation: 1.Tachycardia, 2.Tachypnea, or 3.Diaphoresis |
PSQI-A | Trouble sleeping because of one or more of the following: 1.Feeling hot flashes† 2.Feeling general nervousness† 3.Severe anxiety or panic, not related to traumatic memories† |
| E. Absence of EEG epileptiform activity on polysomnography and the disturbance is not better explained by another sleep disorder, medical disorder, medication, or substance use* | Polysomnography | Not available in this study |
† Occurring ≥1 time per week in the past month (scoring PSQI-A item ≥2).
* Sensitivity analyses were performed to remove individuals with a history of self-reported epilepsy in the past 12 months and substance use.
‡EEG = electroencephalogram; TLEQ = Traumatic Life Events Questionnaire; PSQI-A = Pittsburgh Sleep Quality Index with Addendum for Posttraumatic Stress Disorder.
Statistical approach
We conducted all analyses in SAS 9.4 (Cary, NC). To achieve our primary purpose, we estimated the prevalence of probable TASD and described the characteristics of veterans in the PDMH cohort stratified by probable TASD status. We summarized demographic, service, and clinical characteristics of interest (PTSD, MDD, and trauma exposure variables) stratified by probable TASD status. In addition, we calculated probable TASD prevalence and described characteristics of associated traumatic experiences by sex. In the text, we present effect sizes as prevalence ratios (PRs) for categorical variables and Hedges’ g for continuous variables with their respective 95% confidence intervals (CI). Effect sizes represent the magnitude of observed conditions or characteristic prevalence for those with probable TASD relative to those without. Effect sizes reported when describing sex-based represent the magnitude of observed condition prevalence of females with probable TASD relative to males with probable TASD. We use the common interpretation of Hedges’ g values as small (0.2), medium (0.5), and large (0.8) differential associations between groups [39]. Because the purpose of our study was descriptive rather than focused on a predictive or causal inference question, all analyses are unadjusted outside of sex-based stratification as described above [40]. Finally, we conducted 2 sensitivity analyses. First, we conducted a sensitivity analysis for our probable TASD prevalence estimates, removing individuals with a self-reported history of seizures, convulsions, blackouts, or fainting spells (including epilepsy) within the past year, a history of ≥1 traumatic brain injury (TBI), and endorsed substance use (Drug Abuse Screening Test-20 score ≥6) [23, 41–44]. Second, we conducted a sensitivity analysis further excluding individuals with self-reported use of medications that have been associated with experiencing nightmares and/or dream enactment behaviors (antidepressants, antimicrobials, beta-blocker antihypertensives, and dopamine agonists) [45–51].
Results
Participants and probable trauma associated sleep disorder prevalence
Figure 1 shows the flow of participants from the initial to final sample. The final sample consisted of 3618 veterans from the PDMH study registry, with 6.7% of the original sample excluded for missing data regarding TASD symptoms. Overall estimated prevalence of probable TASD was 12.1% (95% CI: 11.1% to 13.2%). When stratifying by sex, probable TASD prevalence was 12.0% (95% CI: 9.8% to 14.3%) for female veterans and 12.2% (95% CI: 11.0% to 13.4%) for male veterans. We present the distribution of TASD diagnostic criteria and the linked PSQI-A items for criteria B–D stratified by probable TASD status in Supplementary Tables S1, S2, and S3.
Figure 1.
Study sample flow diagram.
Our first sensitivity analysis removed 72 veterans with a self-reported history of seizures, convulsions, blackouts, or fainting spells (including epilepsy) within the past year, 201 veterans with a history of ≥1 TBI, and 192 veterans with substance use. Out of those removed, 71 were probable TASD cases (22.8% of cases from primary analysis). This first sensitivity analysis resulted in a similar estimated probable TASD prevalence for the overall group (10.8%, 95% CI: 9.7% to 11.8%). Sex-stratified prevalence estimates from this sensitivity analysis were also similar for female veterans (10.4%, 95% CI: 8.2% to 12.6%) and male veterans (10.9%, 95% CI: 9.6% to 12.1%). Our second sensitivity analysis removed the same veterans from the first sensitivity analysis and an additional 680 veterans with self-reported use of medications commonly associated with nightmares and/or dream enactment behaviors: antidepressants (n = 521), antimicrobials (n = 3), beta-blocker antihypertensives (n = 75), and dopamine agonists (n = 22). Out of the additional veterans removed in this second sensitivity analysis, 134 were probable TASD cases (30.5% of cases from primary analysis). Our second sensitivity analysis resulted in attenuated estimates of probable TASD (8.1%, 95% CI: 7.0% to 9.2%) compared to our primary analysis results. Sex-stratified prevalence estimates from the second sensitivity analysis also differed for female veterans (6.5%, 95% CI: 4.5% to 8.6%) and male veterans (8.5%, 95% CI: 7.3% to 9.8%) compared to estimates from our primary analysis.
Demographic and service characteristics
We describe demographic and service characteristics stratified by TASD symptom status in Table 2. Age differences were small (g: 0.13, 95% CI: 0.03 to 0.23), with those with probable TASD being slightly younger. Although our sample had a similar overall frequency of White (48.0%, 95% CI: 45.7% to 49.7%) and Black or African American (47.3%, 95% CI: 45.7% to 49.0%) participants, there was a slightly higher proportion of Black or African American participants among those with probable TASD compared to those without (PR: 1.09, 95% CI: 1.00 to 1.21).
Table 2.
Post-deployment mental health registry demographics and service characteristics stratified by probable trauma associated sleep disorder prevalence
| Probable Trauma Associated Sleep Disorder | |||
|---|---|---|---|
| Yes (N = 439) |
No (N = 3179) |
Total (N = 3618) |
|
| Age | |||
| Mean (SD) | 36.9 (9.80) | 38.2 (10.48) | 38.1 (10.41) |
| Median (Range) | 34.0 (21.0, 64.0) | 37.0 (19.0, 72.0) | 36.0 (19.0, 72.0) |
| Female, n (%) | 99 (22.6%) | 724 (22.8%) | 823 (22.7%) |
| Race, n (%) | |||
| White | 186 (43.1%) | 1536 (48.7%) | 1722 (48.0%) |
| Black or African American | 222 (51.4%) | 1475 (46.8%) | 1697 (47.3%) |
| Two or more racial identities | 11 (2.5%) | 53 (1.7%) | 64 (1.8%) |
| Asian or Pacific Islander | 3 (0.7%) | 54 (1.7%) | 57 (1.6%) |
| American Indiana or Alaska Native | 10 (2.3%) | 36 (1.1%) | 46 (1.3%) |
| Missing | 7 | 25 | 32 |
| Service-connected for ≥1 condition, n (%) | 328 (75.2%) | 1864 (58.9%) | 2192 (60.9%) |
| Missing | 3 | 16 | 19 |
| Service-connected percentage | |||
| Mean (SD) | 64.6 (28.21) | 51.2 (28.88) | 53.2 (29.17) |
| Median (Range) | 70.0 (0.0, 100.0) | 50.0 (0.0, 170.0) | 50.0 (0.0, 170.0) |
| Missing | 114 | 1327 | 1441 |
| Tours served | |||
| Mean (SD) | 1.7 (1.54) | 1.5 (1.34) | 1.5 (1.37) |
| Median (Range) | 1.0 (0.0, 19.0) | 1.0 (0.0, 23.0) | 1.0 (0.0, 23.0) |
| Missing | 3 | 40 | 43 |
| Highest rank, n (%) | |||
| Enlisted, Up to E-4 | 282 (64.4%) | 1770 (55.7%) | 2052 (56.8%) |
| Enlisted, E-5 & above | 139 (31.7%) | 1117 (35.2%) | 1256 (34.8%) |
| Warrant Officer | 4 (0.9%) | 46 (1.4%) | 50 (1.4%) |
| Officer | 13 (3.0%) | 242 (7.6%) | 255 (7.1%) |
| Missing | 1 | 4 | 5 |
| Current or most recent service branch, n(%) | |||
| Army | 206 (47.1%) | 1138 (36.0%) | 1344 (37.4%) |
| Army Reserve | 46 (10.5%) | 323 (10.2%) | 369 (10.3%) |
| Army National Guard | 74 (16.9%) | 542 (17.2%) | 616 (17.1%) |
| Navy | 45 (10.3%) | 434 (13.7%) | 479 (13.3%) |
| Navy Reserve | 7 (1.6%) | 71 (2.2%) | 78 (2.2%) |
| Air Force | 12 (2.7%) | 198 (6.3%) | 210 (5.8%) |
| Air Force Reserve | 2 (0.5%) | 46 (1.5%) | 48 (1.3%) |
| Air National Guard | 4 (0.9%) | 39 (1.2%) | 43 (1.2%) |
| Marines | 36 (8.2%) | 279 (8.8%) | 315 (8.8%) |
| Marine Reserves | 2 (0.5%) | 35 (1.1%) | 37 (1.0%) |
| Coast Guard or Coast Guard Reserves | 0 (0.0%) | 15 (0.5%) | 15 (0.4%) |
| Unknown Guard/Reserves | 3 (0.7%) | 39 (1.2%) | 42 (1.2%) |
| Missing | 2 | 20 | 22 |
| Military status, n(%) | |||
| Discharged | 294 (67.0%) | 1753 (55.2%) | 2047 (56.6%) |
| Retired a | 72 (16.4%) | 546 (17.2%) | 618 (17.1%) |
| Reserves | 43 (9.8%) | 482 (15.2%) | 525 (14.5%) |
| National Guard | 34 (7.7%) | 389 (12.2%) | 423 (11.7%) |
| Other | 60 (13.7%) | 352 (11.1%) | 412 (11.4%) |
| Active Duty | 17 (3.9%) | 156 (4.9%) | 173 (4.8%) |
| Inactive National Guard b | 6 (1.4%) | 21 (0.7%) | 27 (0.7%) |
| Missing | 0 | 1 | 1 |
| Served in war/operation zone, n(%) | 402 (91.8%) | 2426 (76.4%) | 2828 (78.3%) |
| Missing | 1 | 5 | 6 |
| Unit type(s) during war zone service, n(%) d | |||
| Combat Unit | 178 (44.3%) | 821 (33.8%) | 999 (35.3%) |
| Combat Support Unit | 235 (58.5%) | 1224 (50.5%) | 1459 (51.6%) |
| Fired weapon in combat situation, n(%) | 253 (57.9%) | 896 (28.2%) | 1149 (31.8%) |
| Missing | 2 | 4 | 6 |
| Ever under enemy fire, n(%) | 332 (75.8%) | 1745 (55.0%) | 2077 (57.5%) |
| Missing | 1 | 5 | 6 |
| Wounded or injured in war zone, n(%) | 167 (38.1%) | 444 (14.0%) | 611 (16.9%) |
| Missing | 1 | 6 | 7 |
| Awarded medals, n(%) | 313 (71.6%) | 2103 (66.2%) | 2416 (66.9%) |
| Missing | 2 | 4 | 6 |
aSurvey option added November 2011; n missing for this option = 1751.
bSurvey option added November 2008; n missing for this option = 646.
cSurvey option added November 2008.
dDenominator includes only those indicating war/operation zone service.
Veterans with probable TASD had a higher proportion of having at least one service-connected condition (PR: 1.28, 95% CI: 1.20 to 1.36). Among those with service-connected conditions in our sample, those with probable TASD also had a moderately higher service-connected percentage (g: 0.47, 95% CI: 0.35 to 0.59), implying more severe or a higher number of service-connected conditions resulting in disability linked to military service. Those with probable TASD were also more likely to have enlisted and have a lower highest rank achievement (E-4 or below; PR: 1.16, 95% CI: 1.07 to 1.25) and less likely to have achieved the highest rank as a Commissioned Officer (O-1 or higher; PR: 0.39, 95% CI: 0.23 to 0.67). Individuals with probable TASD were also more likely to indicate the Army as their current or most recent branch (PR: 1.31, 95% CI: 1.17 to 1.46) and to have been discharged from the military (PR: 1.21, 95% CI: 1.13 to 1.31).
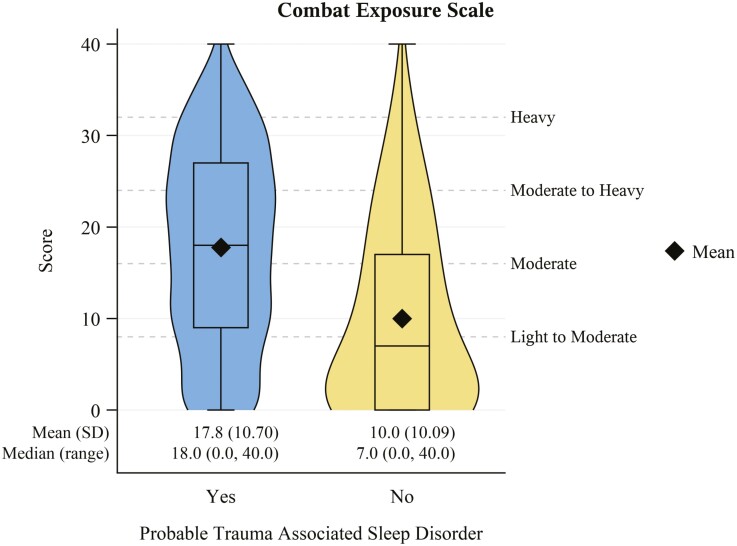
Although most of the individuals in the PDMH cohort reported serving in a war/operation zone, veterans with probable TASD reported war/operation zone service at a higher frequency (PR: 1.20, 95% CI: 1.16 to 1.24). Veterans in the cohort with probable TASD were also more likely to report service in a combat unit (PR: 1.31, 95% CI: 1.16 to 1.48) or combat support unit (PR: 1.16, 95% CI: 1.06 to 1.27) during war zone service and less likely to serve in a combat service support unit (PR: 0.73, 95% CI: 0.59 to 0.89). This group was also more likely to have fired a weapon in a combat situation (PR: 2.05, 95% CI: 1.86 to 2.26), have received enemy fire (PR: 1.38, 95% CI: 1.30 to 1.47), or been wounded in a war zone (PR: 2.73, 95% CI: 2.35 to 3.16). The Combat Exposure Scale (Figure 2) was also notably higher among those with probable TASD compared to those without (g: 0.77, 95% CI: 0.67 to 0.87).
Figure 2.
Combat exposure scale score distribution stratified by probable trauma associated sleep disorder status. Scores of 0-8 = light exposure; 9-16 = light to moderate exposure; 17-24 = moderate exposure; 25-32 = moderate to heavy exposure; 33-41 = heavy exposure. N missing = 7 (2 with probable trauma associated sleep disorder; 5 without).
Traumatic experience characteristics
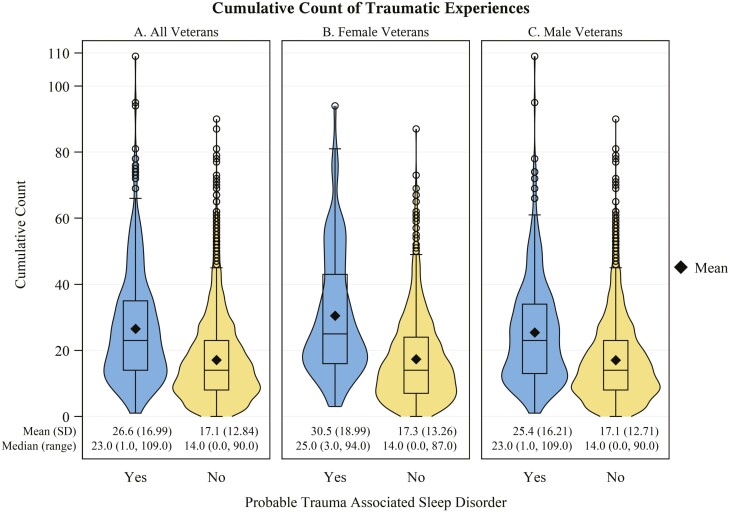
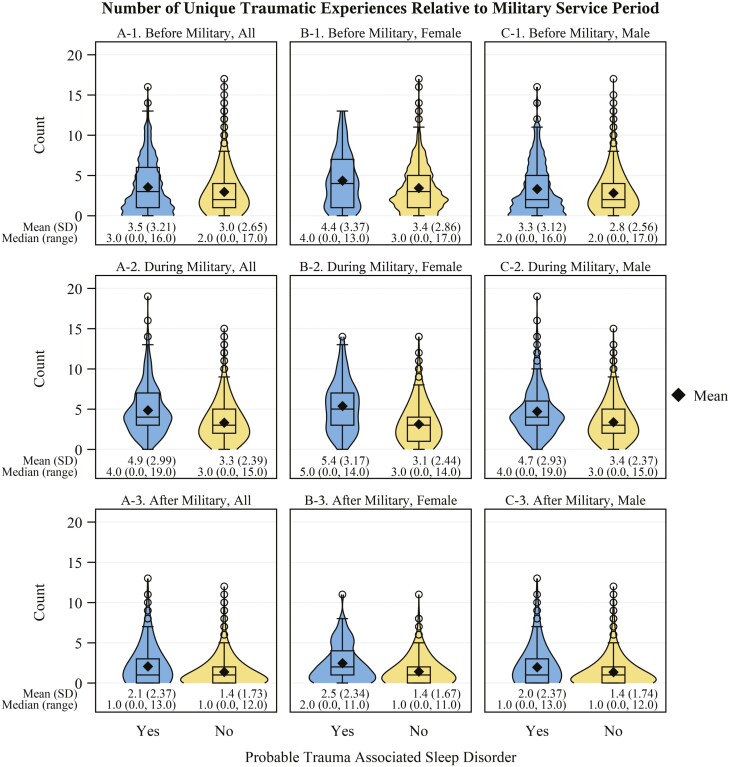
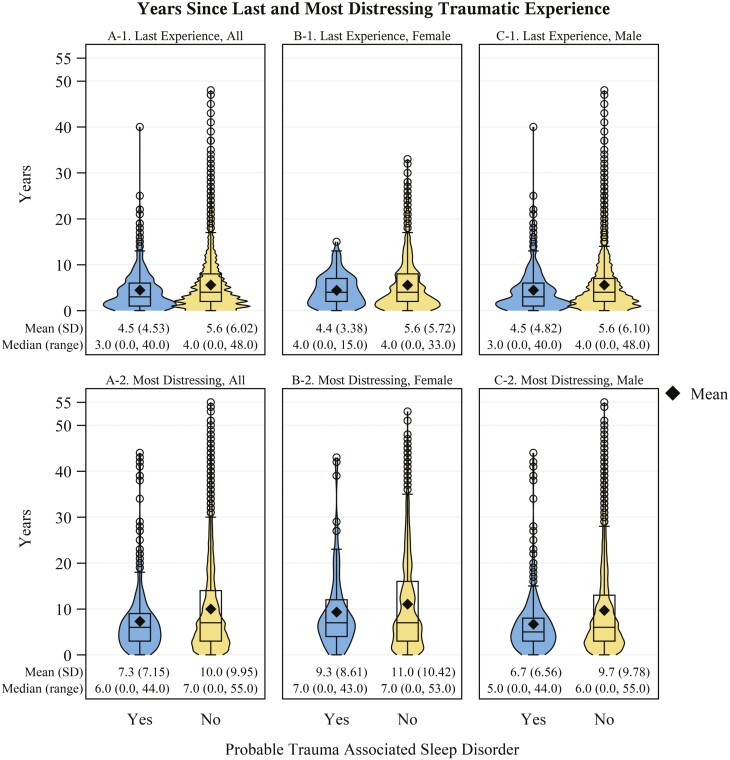
Veterans with probable TASD had a higher cumulative count of traumatic experiences (Figure 3A) compared to those without (g: 0.71, 95% CI: 0.61 to 0.81). These veterans also had a higher number of unique traumatic experiences before (g: 0.18, 95% CI: 0.08 to 0.28), during (g: 0.65, 95% CI: 0.55 to 0.75), and after (g: 0.38, 95% CI: 0.28 to 0.48) military service (Figure 4A) than those without probable TASD. Those with probable TASD also had a shorter time (in years) since the most recent traumatic experience (g: 0.19, 95% CI: 0.09 to 0.29; Figure 5A-1) and time since most distressing traumatic experience (g: 0.28, 95% CI: 0.18 to 0.38; Figure 5A-2). In Table 3, we describe the distribution of reported traumatic events that cause the most distress stratified by TASD symptom status. Individuals with probable TASD were nearly 2 times as likely to report combat or warfare as the traumatic event that causes the most distress compared to those without probable TASD (PR: 1.87, 95% CI: 1.71 to 2.03). Those with probable TASD also had a higher proportion of veterans reporting a traumatic event that they cannot talk about the traumatic event that causes the most distress (PR: 3.96, 95% CI: 2.65 to 5.90).
Figure 3.
Cumulative count of traumatic experiences stratified by probable trauma associated sleep disorder status. Panel A: All veterans in the sample; Panel B: Female veterans; Panel C: Male veterans. Frequency for each traumatic experience type is counted on a scale of 0 (never) to 6 (more than 5 times). N missing = 61 (0 with TASD; 61 without).
Figure 4.
Number of unique traumatic experiences relative to military service period by probable trauma associated sleep disorder status. Panel Column A: All veterans in the sample; Panel Column B: Female veterans; Panel Column C: Male veterans. Panel row 1: Before military service; Panel row 2: During military service; Panel row 3: After military service. Instances, where the same repeated traumatic event type occurred more than once during the specified period, are not reflected. N missing = 61 (0 with TASD; 61 without).
Figure 5.
Years since traumatic experience distribution stratified by probable trauma associated sleep disorder status. Panel Column A: All veterans in the sample; Panel Column B: Female veterans; Panel Column C: Male veterans. Panel row 1: Years since last traumatic experience; Panel row 2: Years since most distressing traumatic experience. N missing = A-1. 63 (1 with trauma associated sleep disorder; 62 without); A-2. 87 (3 with trauma associated sleep disorder; 84 without).
Table 3.
Post-deployment mental health registry trauma characteristics stratified by probable trauma associated sleep disorder prevalence
| Probable Trauma Associated Sleep Disorder | |||
|---|---|---|---|
| Yes (N = 439) |
No (N = 3179) |
Total (N = 3618) |
|
| Traumatic event that causes the most distress, n(%) | |||
| Combat or warfare | 275 (62.6%) | 1067 (33.6%) | 1342 (37.1%) |
| Illness | 46 (10.5%) | 955 (30.1%) | 1001 (27.7%) |
| Accident | 12 (2.7%) | 304 (9.6%) | 316 (8.7%) |
| Some “other” traumatic event | 21 (4.8%) | 211 (6.6%) | 232 (6.4%) |
| Attack | 28 (6.4%) | 197 (6.2%) | 225 (6.2%) |
| Childhood violence | 5 (1.1%) | 155 (4.9%) | 160 (4.4%) |
| Unwanted sexual contact: before adulthood | 7 (1.6%) | 108 (3.4%) | 115 (3.2%) |
| A traumatic event you cannot talk about | 35 (8.0%) | 64 (2.0%) | 99 (2.7%) |
| None of these events happened to me | 1 (0.2%) | 67 (2.1%) | 68 (1.9%) |
| Unwanted sexual contact: as an adult | 9 (2.1%) | 48 (1.5%) | 57 (1.6%) |
| Missing | 0 | 3 | 3 |
We describe the sex-stratified distribution of reported traumatic events that cause the most distress by TASD symptom status in Table 4. Combat or warfare was the most prevalent event identified by veterans with probable TASD among both females (41.4%, 95% CI: 31.7% to 51.1%) and males (68.8%, 95% CI: 63.9% to 73.7%). However, female veterans were more likely to have their most distressing traumatic event be related to an attack (PR: 1.91, 95% CI: 0.91 to 4.00), unwanted sexual contact (before adulthood: 7.1%, 95% CI: 2.0% to 12.1% vs. 0.0%; as an adult: PR: 6.87, 95% CI: 1.75 to 26.97), or a traumatic event they cannot talk about (PR: 2.29, 95% CI: 1.21 to 4.33). Female veterans (Figure 3B) with probable TASD had a higher mean cumulative count of traumatic events versus male veterans (Figure 3C) with probable TASD (g: 0.3, 95% CI: 0.08 to 0.52). Female veterans with probable TASD also had a higher mean number of unique traumatic experience types before (Figures 4B-1 and 4C-1; g: 0.35, 95% CI: 0.12 to 0.58), during (Figures 4B-2 and 4C-2; g: 0.23, 95% CI: 0.01 to 0.45), and after (Figures 4B-3 and 4C-3; g: 0.21, 95% CI: −0.01 to 0.43) military service compared to male veterans with probable TASD. Female veterans showed a longer time since their most distressing traumatic experience compared to male veterans with probable TASD (Figures 5B-2 and 5C-2; g: 0.37, 95% CI: 0.14 to 0.60).
Table 4.
Post-deployment mental health registry trauma characteristics stratified by sex and probable trauma associated sleep disorder prevalence
| Sex | ||||||
|---|---|---|---|---|---|---|
| Female | Male | |||||
| Probable Trauma Associated Sleep Disorder | Probable Trauma Associated Sleep Disorder | |||||
| Yes (N = 99) |
No (N = 724) |
Total (N = 823) |
Yes (N = 340) |
No (N = 2455) |
Total (N = 2795) |
|
| Traumatic event that causes the most distress, n(%) | ||||||
| Combat or warfare | 41 (41.4%) | 115 (15.9%) | 156 (19.0%) | 234 (68.8%) | 952 (39.5%) | 1186 (42.5%) |
| Illness | 11 (11.1%) | 260 (35.9%) | 271 (32.9%) | 35 (10.3%) | 695 (28.8%) | 730 (26.1%) |
| Accident | 5 (5.1%) | 56 (7.7%) | 61 (7.4%) | 7 (2.1%) | 248 (10.3%) | 255 (9.1%) |
| Some “other” traumatic event | 4 (4.0%) | 46 (6.4%) | 50 (6.1%) | 17 (5.0%) | 165 (6.8%) | 182 (6.5%) |
| Attack | 10 (10.1%) | 71 (9.8%) | 81 (9.8%) | 18 (5.3%) | 126 (5.2%) | 144 (5.2%) |
| Childhood violence | 1 (1.0%) | 39 (5.4%) | 40 (4.9%) | 4 (1.2%) | 116 (4.8%) | 120 (4.3%) |
| Unwanted sexual contact: before adulthood | 7 (7.1%) | 67 (9.3%) | 74 (9.0%) | 0 (0.0%) | 41 (1.7%) | 41 (1.5%) |
| A traumatic event you cannot talk about | 14 (14.1%) | 20 (2.8%) | 34 (4.1%) | 21 (6.2%) | 44 (1.8%) | 65 (2.3%) |
| None of these events happened to me | 0 (0.0%) | 11 (1.5%) | 11 (1.3%) | 1 (0.3%) | 56 (2.3%) | 57 (2%) |
| Unwanted sexual contact: as an adult | 6 (6.1%) | 39 (5.4%) | 45 (5.5%) | 3 (0.9%) | 9 (0.4%) | 12 (0.4%) |
| Missing | 0 | 0 | 0 | 0 | 3 | 3 |
Mental health clinical characteristics
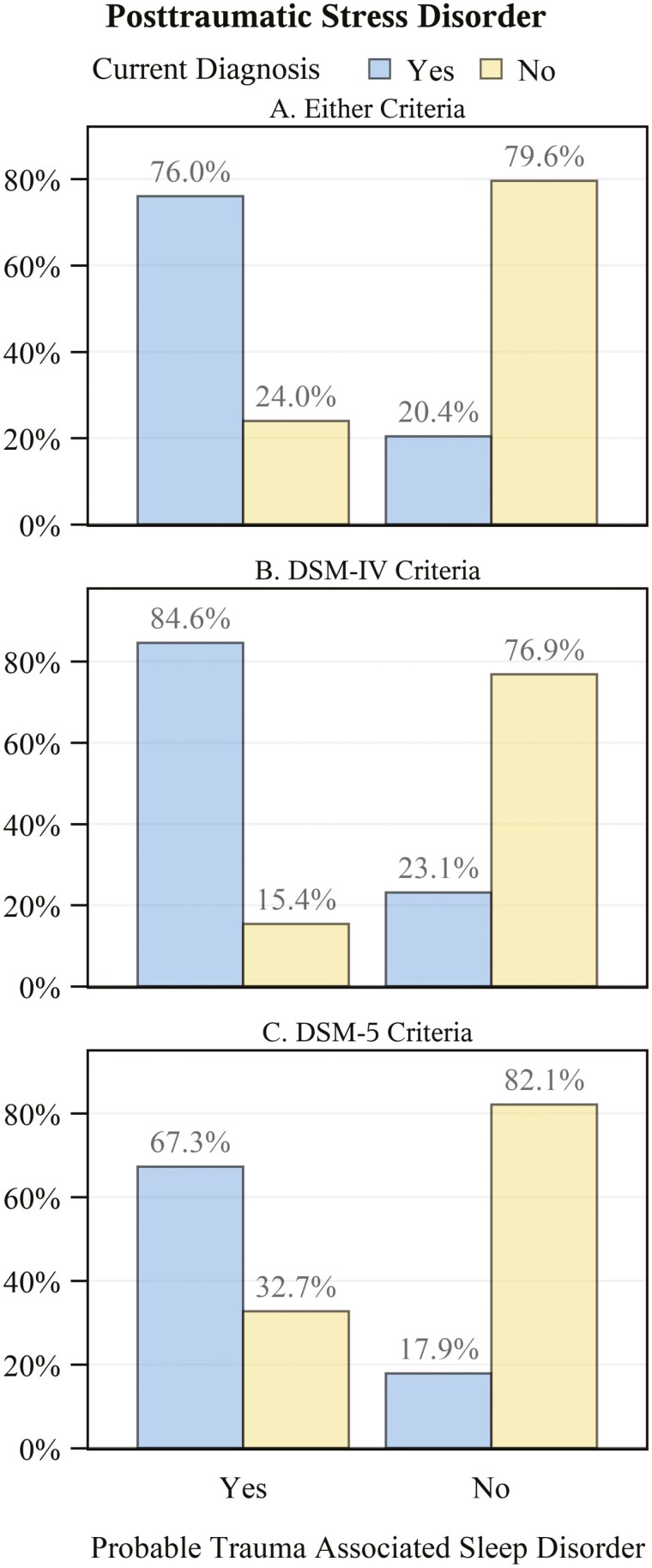
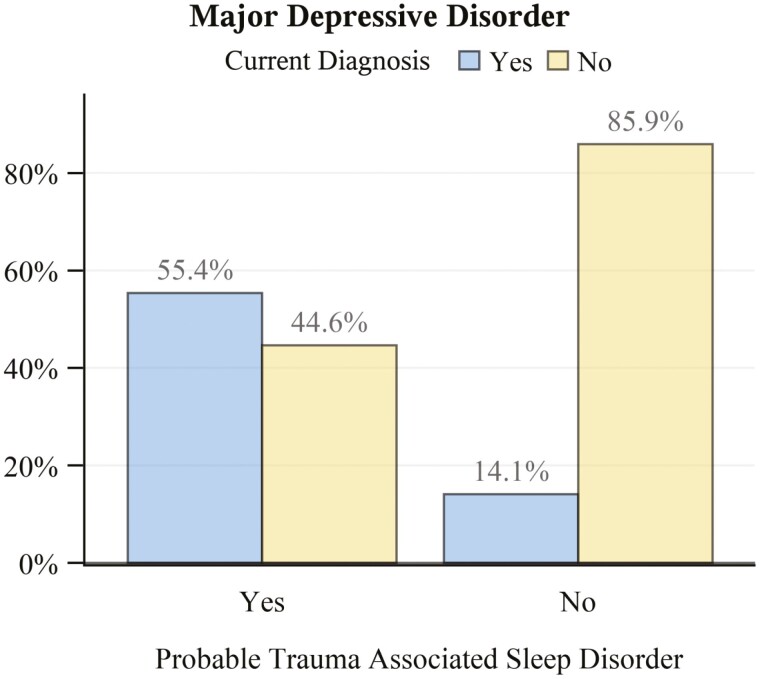
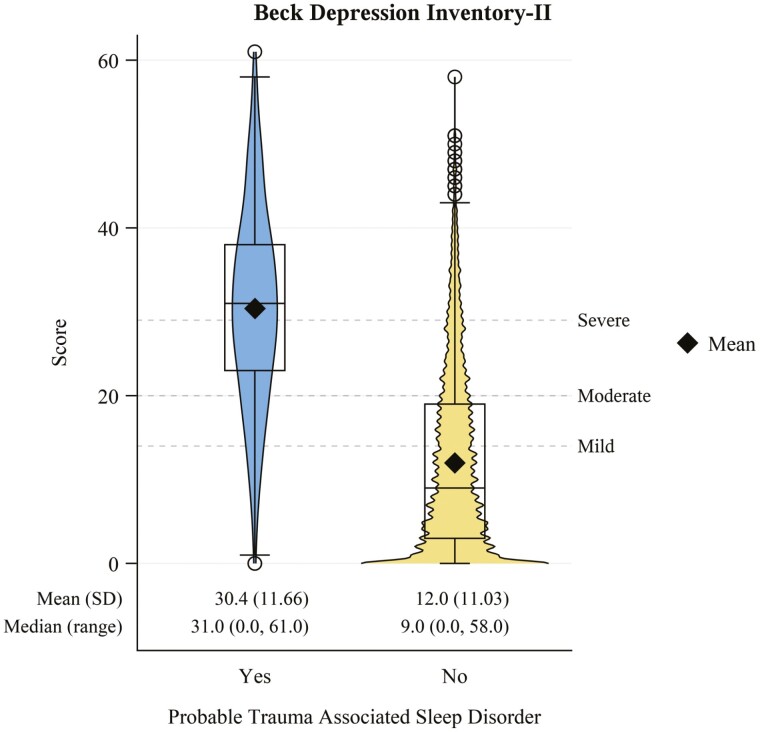
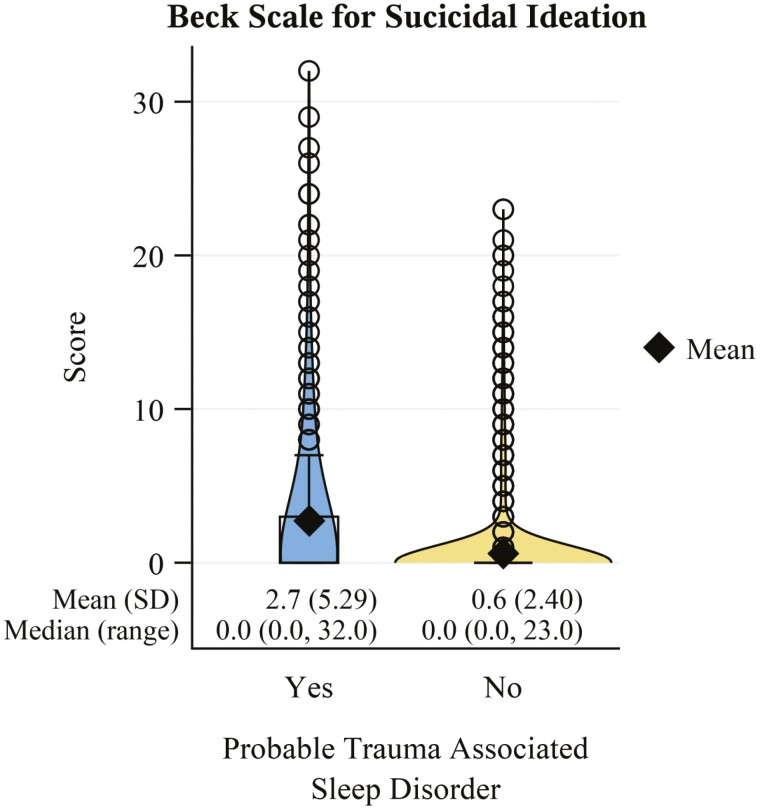
Veterans with probable TASD had a nearly 4-fold higher comorbid prevalence of current PTSD (Figure 6A; PR: 3.72, 95% CI: 3.41 to 4.06). This estimate was similar when stratifying by DSM-IV (Figure 6B; PR: 3.66, 95% CI: 3.3 to 4.07) versus DSM-5 (Figure 6C; PR: 3.76, 95% CI: 3.28 to 4.33) diagnostic criteria. Those with probable TASD also had a nearly 3-fold higher prevalence of lifetime PTSD (PR: 2.71. 95% CI: 2.55 to 2.89). Veterans with probable TASD also had an approximately 4-times higher comorbid prevalence of current MDD (Figure 7; PR: 3.93, 95% CI: 3.48 to 4.43) versus those without TASD. BDI-II scores (Figure 8) were consistent with this finding, being notably higher among those with probable TASD than those without (g: 1.66, 95% CI: 1.55 to 1.77). Beck Scale for Suicide Ideation scores (Figure 9) were also notably higher among those with probable TASD (g: 0.72, 95% CI: 0.62 to 0.82). Those with probable TASD also reported a 5-times higher frequency of trouble controlling violent behavior within the past 30 days (PR: 5.00, 95% CI: 4.07 to 6.15) compared to veterans without probable TASD (28.4% vs. 5.7%; overall 8.4%).
Figure 6.
Frequency of current posttraumatic stress disorder diagnosis by probable trauma associated sleep disorder status. N missing = 7 (1 with probable trauma associated associated sleep disorder; 6 without). Panel A: All veterans regardless of which diagnostic criteria were used; Panel B: Limited to those diagnosed with DSM-IV criteria; Panel C: Limited to those diagnosed with DSM-5 criteria.
Figure 7.
Frequency of current major depressive disorder diagnosis by probable trauma associated sleep disorder status. N missing = 10 (2 with probable trauma associated sleep disorder; 8 without).
Figure 8.
Beck Depression Inventory-II score distribution stratified by probable trauma associated sleep disorder status.
Figure 9.
Beck Scale for Suicide Ideation score distribution stratified by probable trauma associated sleep disorder status. N missing = 5 (2 with probable trauma associated sleep disorder; 3 without).
Discussion
Our study is the first to estimate the prevalence of probable TASD in post-9/11 veterans and describe the demographic and clinical characteristics of those with and without probable TASD using a large, diverse cohort that is more representative of US post-9/11 veterans nationally than in previous studies. We found that more than 1 out of 10 post-9/11 veterans (12.1%, 95% CI: 11.1% to 13.2%) report symptoms consistent with TASD diagnostic criteria. There did not appear to be a clinically relevant difference between prevalence estimates among male and female veterans when stratifying by sex. Nightmares and disruptive nocturnal behaviors remain underdiagnosed due to inconsistent screening and evaluation for these conditions in clinical settings [52]. Our findings suggest the importance of evaluating veterans for not only nightmares but also TASD.
Demographic and service characteristics of veterans with a probable trauma associated sleep disorder
Demographic characteristics were relatively similar between those with and without probable TASD, having only minor differences in the distribution of age. Likewise, we observed small differences between those with and without probable TASD regarding race, with a slightly higher proportion of Black or African American individuals among those with probable TASD. In contrast, large differences in service-related characteristics were observed among those with probable TASD compared to those without. Veterans classified as having probable TASD were more likely to list Army as their current or most recent branch than other branches. This finding is consistent with other studies that have found sleep disorders, including insomnia and obstructive sleep apnea, are generally more frequently diagnosed in those who have served in the Army and may be potentially related to this branch’s higher rate of deployments and associated combat exposure [53, 54].
Although there was a high rate of operational/war zone service in our overall cohort (78%), 92% of individuals with probable TASD reported this as part of their service background and had higher exposure to combat-related trauma or stress compared to those without probable TASD across all measures in our study. Indeed, nearly two-thirds of those with probable TASD reported combat or warfare as the traumatic event that causes them the most distress; the same was true for only one-third of veterans without probable TASD. Combat is the traumatic event that has been typically associated with TASD [7, 8]. While some of those with probable TASD reporting a different traumatic event type as their most distressing may have also had prior trauma exposure related to combat, our results imply that other types of traumatic events may be an inciting event for up to one-third of TASD cases in post-9/11 veterans. These results support prior findings that while combat is frequently the inciting traumatic experience in veterans, other traumatic experiences can also result in TASD [7, 8, 20]. Our results also suggest that noncombat-related traumatic experiences in nonveteran populations may have the potential to result in TASD; however, TASD has only been studied in veterans and has yet to be studied in nonveterans.
Sex-stratified trauma characteristics
We found no clinically meaningful difference between the prevalence of probable TASD among female and male post-9/11 veterans when stratifying our cohort by sex. Notably, probable TASD prevalence was similar across sex groups despite female veterans with probable TASD reporting a higher number of cumulative lifetime traumatic experiences and a higher count of unique traumatic experiences at all periods relative to military service. This similarity in prevalence between sexes provides support that TASD is not a disorder that is unique to males. Indeed, a recent case series reporting polysomnography characteristics of veterans with suspected TASD also included female veterans [20]. Time since the last traumatic experience for individuals with probable TASD was similar between sexes in our study, while male veterans reported their most distressing traumatic event was more recent by a small to moderate amount. The observed longer time since most distressing traumatic event among female veterans with probable TASD is likely related to this group having a higher overall frequency of cumulative lifetime traumatic experiences and a higher number of unique traumatic experience types before and during military service.
Female and male veterans with probable TASD also differed in the type of trauma they reported as the most distressing. Combat or warfare was the highest reported most distressing traumatic event among both females and males with probable TASD, but was notably more prevalent among males. Female veterans in our study reported a wider range of traumatic experience types as their most distressing, with a higher frequency reporting their most distressing experience being related to attacks, sexual assault, and traumatic events they cannot talk about. This is consistent with other studies that have reported a higher rate of combat or warfare trauma exposure among male veterans and more varied traumatic experiences among female veterans [55–58]. Overall, these findings further support the assertion that combat-related trauma is not the only form of trauma that can result in TASD. Our sex-specific findings also highlight areas of future study, such as the effect of cumulative traumatic experience exposures on the risk of TASD and the relationship between TASD and complex trauma.
Currently, the diagnosis of TASD is based on proposed diagnostic criteria and there is no ordinal measure of TASD symptom severity. Prior research suggests that current ordinal scales of severity for other trauma-related disorders (e.g. PTSD) may fail to adequately capture symptom severity in women, especially Black or African American women [59]. Careful consideration of the intersectionality of race and gender is warranted as standardized tools for screening and diagnosis of TASD become available that may include ordinal scales to categorize TASD symptom severity.
Mental health clinical characteristics
The veterans with probable TASD in our study were much more likely to have comorbid mental health disorders including MDD, PTSD, suicidal ideation, and self-reported trouble controlling violent behavior than those without probable TASD. Prior clinical reports have found that patients diagnosed with TASD also frequently had PTSD [8, 11]. While this finding is not necessarily surprising, given that trauma is an inherent characteristic of both TASD and PTSD, the finding that 23% of veterans with TASD did not have PTSD further supports that these are likely two distinct, but overlapping clinical disorders [7]. While nightmares frequently occur in PTSD, the occurrence of disruptive nocturnal behaviors (e.g. thrashing, dream enactment behaviors, and vocalizations), that appear to be more frequent on polysomnography than previously reported, are a symptom and objective finding that distinguishes TASD and PTSD [20].
The finding that suicidal ideation was higher in veterans with TASD is consistent with a recent study of veteran suicides that that found the peak incidence of suicide was between 00:00 and 03:00 hr and posited that this window of peak suicidality may coincide with the causes of nocturnal distress and wakefulness caused by sleep disorders [60]. TASD is a severe sleep disorder and the associated nocturnal distress and wakefulness highlight the need to determine appropriate treatments for this complex sleep disorder. Given the high incidence of suicides among veterans, this is especially important considering preliminary evidence suggests treating sleep disorders may help reduce suicidal ideation [61].
Veterans with TASD were also 5-times more likely to indicate that they had trouble controlling violent behavior (e.g. hitting someone) in the past 30 days. The question available regarding violent behavior was somewhat limited and did not ask if this behavior occurred while awake or asleep. Therefore, the higher prevalence of self-reported trouble controlling violent behavior we observed may be related to the underlying nature of TASD (e.g. hitting or striking a bed partner while asleep). Other studies have reported higher rates of violence and aggression among veterans with mental health disorders among post-9/11 veterans, such as PTSD [62, 63]. One study that included a randomly selected group of post-9/11 veterans from the National Post-Deployment Adjustment Survey found that veterans with PTSD (n = 1090) at baseline were more likely to report severe violent behavior (involving a weapon; 20% vs. 5%) or other violent behavior (48% vs. 21%) [63]. On face value, there appear to be different rates of violence among those with and without probable TASD versus those without in our study; however, differences in the wording of questions used to ascertain violent behavior, stratification by weapon use, 30-day retrospective versus 12-month retrospective recall, and the high prevalence of PTSD among those with probable TASD in our study make direct comparison difficult.
Contrasting trauma associated sleep disorder and REM behavior disorder
Some have argued that TASD and REM behavior disorder represent the same condition[10–13]. However, probable TASD prevalence in our study was higher than estimates of REM behavior disorder in veteran populations. Part of this difference may be from the use of clinical instruments to render the diagnosis. Using International Classification of Diseases (ICD) Codes, one study reported the prevalence of a combined group of parasomnias (including the ICD code for REM behavior disorder) at 0.3% among veterans accessing clinical care through the Department of Veterans Affairs from 2000 to 2010 [64]. When limiting to only veterans with ≥1 sleep disorder diagnosis, the estimated prevalence from the same study was still only 3% [64]. A more recent study estimated the prevalence of REM behavior disorder at 9%; however, this was in a sample of primarily male veterans being referred for polysomnography with a suspected sleep disorder (n = 394) rather than a more generalizable sample [65]. In addition, the authors in that study elected to label participants as having REM behavior disorder even in cases where TASD diagnosis criteria were convincingly met [12, 65]. Our study estimated probable TASD prevalence in a large sample of male and female veterans, which is more representative of post-9/11 veterans nationally than in previous studies [23, 24]. While the PDMH study began oversampling female veterans in 2015 [23], estimates when stratifying by sex were similar, indicating oversampling did not impact the overall probable TASD prevalence in our study. The finding that there was no difference in prevalence between male and female veterans is distinctly different than REM behavior disorder, a parasomnia that predominantly impacts males [17, 18]. In addition, although the wording of the question may explain away at least part of the higher difficulty controlling violent behavior we observed among those with probable TASD, this finding conflicts with prior research reporting that no differences in violent daytime behaviors exist between those with REM behavior disorder and controls [66]. The differences in estimated probable TASD prevalence in our study and estimated REM behavior disorder in the studies discussed may be related to the high rate of combat exposure and/or operation or war zone service among post-9/11 veterans and provides a further basis for future studies to determine the differences between TASD and REM behavior disorder in the post-9/11 veteran population [67].
Sensitivity analyses
Results from our first sensitivity analysis that excluded veterans with self-reported epilepsy in the past 12 months, history of TBI, and substance use found no meaningful difference in estimated prevalence compared to our primary analysis. In contrast, our estimated prevalence of probable TASD attenuated when additionally excluding veterans with self-reported use of antidepressants, antimicrobials, beta-blocker antihypertensives, and dopamine agonists. This second sensitivity analysis removed a sizable proportion of the sample from our analysis, including 53.3% of probable TASD cases resulting in wider confidence intervals (less precise estimates). This attenuation seems linked to the high comorbidity of MDD with TASD, as the category resulting in the highest number of exclusions in this second sensitivity analysis was antidepressant medication (n = 521). Indeed, we have previously demonstrated that another trauma-incited condition (i.e. PTSD) and depression are highly comorbid in returning post-9/11 veterans and load together onto a higher-order factor [38]. Although antidepressant medication has been associated with nightmares, this potential side effect from antidepressants is relatively rare in randomized trials of these medications. For example, adult randomized trials of duloxetine had a higher incidence of “abnormal dreams (including nightmare)” but that incidence was <2% and was not limited to nightmares [68]. Likewise, randomized trials of bupropion resulted in the incidence of abnormal dreams at 3% in the treatment arm versus 2% in the placebo arm and only in those being treated for seasonal affective disorder specifically [69]. Furthermore, while selective serotonin reuptake inhibitors and selective norepinephrine reuptake inhibitors are associated with dream enactment behavior resulting from a lack of atonia during REM sleep, this is also an uncommon side effect [51]. Because of the high prevalence of comorbid MDD among those with probable TASD and the fact that the overwhelming majority of individuals taking antidepressant medications do not experience nightmares or dream enactment behaviors as an adverse event, we posit that the overall and sex-stratified prevalence estimates from our second sensitivity analysis are overly conservative and underestimate the prevalence of probable TASD.
Strengths and limitations
Our study is the largest to date to evaluate a cohort of veterans for symptoms of TASD using validated clinical instruments and correlating these self-reported symptoms with the proposed diagnostic criteria for TASD. However, our study does have some limitations. We relied on self-reported items from validated questionnaires to determine a probable TASD diagnosis and did not have a clinical evaluation or diagnostic testing that included polysomnography. The PSQI-A does not specifically link the questions regarding hot flashes, general nervousness, and severe anxiety/panic unrelated to trauma memories (that we mapped to criterion D) to being associated with dream mentation. However, we determined probable TASD using a combination of self-reported criteria rather than this single criterion alone and all questions from the PSQI-A mapped to these criteria were required to be reported at a frequency of ≥1 time per week (response of “once or twice a week” or more frequent) to count toward TASD criteria. Despite our efforts to avoid misclassification of probable TASD, the absence of sleep-specific clinical interviews and video polysomnography, as well as the limitations of available questionnaires may have resulted in the misclassification of some veterans. We did perform sensitivity analysis excluding individuals from our cohort with indications of self-reported epilepsy in the past 12 months, history of TBI, and substance use and found no meaningful difference in estimated prevalence. We also performed a second sensitivity analysis that further excluded individuals with self-reported use of medications that have been associated with nightmares as well as dream enactment behaviors. We consider the results from this second sensitivity analysis to be overly conservative of probable TASD prevalence, but we present them for the reader to consider.
Although we had information regarding the time since traumatic experiences, we did not have data regarding the time since the onset of sleep symptoms measured by the PSQI-A. There is a note that accompanies the proposed TASD diagnostic criteria indicating that onset of disruptive nighttime behaviors is typically in close temporal proximity to trauma exposure. However, this accompanying note provides no time cutoff for what is considered “close temporal proximity” and the core proposed TASD diagnostic criteria used for determining probable TASD are agnostic regarding this accompanying note [9]. Importantly, this accompanying note is specific to the timing of TASD incidence rather than prevalence (which was the focus of our study). Other studies have reported a long-standing prevalence of trauma-associated sleep disturbances among trauma survivors. In a study of avalanche survivors with and without PTSD 16 years after avalanche exposure, survivors were 2.7 to 3.1 times more likely to report trauma-related nightmares compared to those in towns unexposed to avalanche-related traumatic experiences [70]. Thus, prevalent cases of TASD may persist long after TASD incidence and its associated inciting traumatic event. Additionally, some cases of TASD may have a more delayed onset symptom presentation.
Another limitation is that symptom validity was not sampled and prior research suggests that some individuals will over-report self-reported symptoms to varying degrees, even in research contexts [71–74]. We also did not have details regarding subthreshold PTSD diagnosis widely available in our cohort to allow for more detailed reporting of PTSD and TASD overlap.
Conclusion
More than 1 out of 10 post-9/11 veterans met self-reported diagnostic criteria for TASD. Probable TASD prevalence was similar when stratifying by sex, highlighting that the sleep disorder is not a male-only phenomenon nor is TASD a parasomnia that disproportionately impacts male veterans. Combat or war-related trauma was the highest reported most distressing traumatic experience overall and when stratifying by sex, although other types of trauma were also associated with cases of probable TASD. This was especially true for female veterans with probable TASD, who reported a higher frequency and wider variety of traumatic experiences than male veterans with probable TASD. Our results further support the assertion that TASD is a distinct parasomnia that can have overlapping symptoms or be comorbid with other disorders. Further our results support the need for improved screening and evaluation for TASD in veterans, which is currently not routine in clinical practice, and the investigation of neurobiological mechanisms that may explain the effect of trauma, cumulative trauma, and/or complex trauma resulting in TASD.
Supplementary Material
Acknowlegments
The VA Mid-Atlantic Mental Illness Research, Education, and Clinical Center Workgroup for this publication includes Jean C. Beckham, PhD, Patrick S. Calhoun, PhD, Eric Dedert, PhD, Eric B. Elbogen, PhD, John A. Fairbank, PhD, Robin A. Hurley, MD, Jason D. Kilts, PhD, Nathan A. Kimbrel, PhD, Angela Kirby, MS, Sarah L. Martindale, Ph.D, Christine E. Marx, MD, MS, Scott D. McDonald, PhD, Scott D. Moore, MD, PhD, Rajendra A. Morey, MD, MS, Jennifer C. Naylor, PhD, Jared Rowland, PhD, Robert D. Shura, PsyD, Cindy Swinkels, PhD, Larry A. Tupler, PhD, Elizabeth E. Van Voorhees, PhD, Ruth Yoash-Gantz, PsyD. We also wish to acknowledge John Taylor Freeman, PhD for his assistance.
Contributor Information
Kenneth A Taylor, Duke University School of Medicine, Orthopaedic Surgery, Durham, NC, USA; Duke University School of Medicine, Duke Clinical Research Institute, Durham, NC, USA.
Vincent Mysliwiec, University of Texas Health Science Center at San Antonio, Department of Psychiatry and Behavioral Sciences, San Antonio, TX, USA.
Nathan A Kimbrel, Durham Veterans Affairs (VA) Healthcare System, Durham, NC, USA; VA Mid-Atlantic Mental Illness Research, Education and Clinical Center, Durham, NC, USA; VA Health Services Research and Development, Center of Innovation to Accelerate Discovery and Practice Transformation, Durham, NC, USA; Duke University School of Medicine, Psychiatry and Behavioral Sciences, Durham, NC, USA.
Ann V Augustine, Durham Veterans Affairs (VA) Healthcare System, Durham, NC, USA; Duke University School of Medicine, Neurology, Durham, NC, USA.
Christi S Ulmer, Duke University School of Medicine, Psychiatry and Behavioral Sciences, Durham, NC, USA; Durham Veterans Affairs (VA) Healthcare System, Center of Innovation to Accelerate Discovery and Practice Transformation (ADAPT), Durham, NC, USA.
The VA Mid-Atlantic MIRECC Registry Workgroup:
Jean C Beckham, Patrick S Calhoun, Eric Dedert, Eric B Elbogen, John A Fairbank, Robin A Hurley, Jason D Kilts, Nathan A Kimbrel, Angela Kirby, Sarah L Martindale, Christine E Marx, Scott D McDonald, Scott D Moore, Rajendra A Morey, Jennifer C Naylor, Jared Rowland, Robert D Shura, Cindy Swinkels, Larry A Tupler, Elizabeth E Van Voorhees, and Ruth Yoash-Gantz
Funding
This work was supported by the Durham Center of Innovation to Accelerate Discovery and Practice Transformation (ADAPT), (CIN 13-410) at the Durham VA Health Care System. Preparation of this work was also supported by the Durham Veterans Affairs Healthcare System and the Department of Veterans Affairs Office of Research and Development. The contents do not represent the views of the US Department of Veterans Affairs or the United States Government.
Data Availability
To protect the privacy and protected health information of individuals that participated in the study the data underlying this article cannot be shared publicly. The data will be shared upon reasonable request to the VA Mid-Atlantic Mental Illness Research, Education, and Clinical Center Workgroup and SAS code will be shared upon reasonable request to the corresponding author.
References
- 1. Lavie P. Sleep disturbances in the wake of traumatic events. N Engl J Med. 2001;345(25):1825–1832. doi: 10.1056/nejmra012893. [DOI] [PubMed] [Google Scholar]
- 2. Worley CB, et al. Epidemiology of disturbing dreams in a diverse US sample. Sleep Med. 2021;83:5–12. doi: 10.1016/j.sleep.2021.04.026. [DOI] [PubMed] [Google Scholar]
- 3. Hefez A, et al. Long-term effects of extreme situational stress on sleep and dreaming. Am J Psychiatry. 1987;144(3):344–347. doi: 10.1176/ajp.144.3.344. [DOI] [PubMed] [Google Scholar]
- 4. Mellman TA, et al. Sleep events among veterans with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152(1):110–115. doi: 10.1176/ajp.152.1.110. [DOI] [PubMed] [Google Scholar]
- 5. Ross RJ, et al. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146(6):697–707. doi: 10.1176/ajp.146.6.697. [DOI] [PubMed] [Google Scholar]
- 6. van der Kolk B, et al. Nightmares and trauma: a comparison of nightmares after combat with lifelong nightmares in veterans. Am J Psychiatry. 1984;141(2):187–190. doi: 10.1176/ajp.141.2.187. [DOI] [PubMed] [Google Scholar]
- 7. Mysliwiec V, et al. Trauma associated sleep disorder: a parasomnia induced by trauma. Sleep Med Rev. 2018;37:94–104. doi: 10.1016/j.smrv.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 8. Mysliwiec V, et al. Trauma associated sleep disorder: a proposed parasomnia encompassing disruptive nocturnal behaviors, nightmares, and REM without atonia in trauma survivors. J Clin Sleep Med. 2014;10(10):1143–1148. doi: 10.5664/jcsm.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brock MS, et al. Trauma associated sleep disorder: clinical developments 5 years after discovery. Curr Psychiatry Rep. 2019;21(9):80. doi: 10.1007/s11920-019-1066-4. [DOI] [PubMed] [Google Scholar]
- 10. Mellman TA. A time to recognize trauma-related sleep disorder or a time to pause and consider? Sleep. 2020;43(6). doi: 10.1093/sleep/zsaa069. [DOI] [PubMed] [Google Scholar]
- 11. Feemster JC, et al. Trauma-associated sleep disorder: a posttraumatic stress/rem sleep behavior disorder mash-up? J Clin Sleep Med. 2019;15(2):345–349. doi: 10.5664/jcsm.7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mysliwiec V, Brock MS.. Time to recognize trauma associated sleep disorder as a distinct parasomnia. Sleep. 2020;43(3). doi: 10.1093/sleep/zsaa019. [DOI] [PubMed] [Google Scholar]
- 13. Rachakonda TD, et al. Trauma-associated sleep disturbances: a distinct sleep disorder?. Curr Sleep Med Rep. 2018;4(2):143–148. doi: 10.1007/s40675-018-0119-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haba-Rubio J, Frauscher B, Marques-Vidal P, et al. Prevalence and determinants of rapid eye movement sleep behavior disorder in the general population. Sleep. 2018;41(2). doi: 10.1093/sleep/zsx197. [DOI] [PubMed] [Google Scholar]
- 15. Kang SH, et al. REM sleep behavior disorder in the Korean elderly population: prevalence and clinical characteristics. Sleep. 2013;36(8):1147–1152. doi: 10.5665/sleep.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sasai-Sakuma T, Takeuchi N, Asai Y, Inoue Y, Inoue Y.. Prevalence and clinical characteristics of REM sleep behavior disorder in Japanese elderly people. Sleep. 2020;43(8). doi: 10.1093/sleep/zsaa024. [DOI] [PubMed] [Google Scholar]
- 17. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 18. Boeve BF, et al. Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov Disord. 2001;16(4):622–630. doi: 10.1002/mds.1120. [DOI] [PubMed] [Google Scholar]
- 19. Postuma RB, et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain. 2019;142(3):744–759. doi: 10.1093/brain/awz030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brock MS, et al. Clinical and polysomnographic features of trauma-associated sleep disorder. J Clin Sleep Med. 2022;18(12):2775–2784. doi: 10.5664/jcsm.10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dauvilliers Y, et al. REM sleep behaviour disorder. Nat Rev Dis Primers. 2018;4(1):19. doi: 10.1038/s41572-018-0016-5. [DOI] [PubMed] [Google Scholar]
- 22. Schenck CH. REM sleep behavior disorder. In: Chokroverty S, Billiard M, eds. Sleep Medicine: A Comprehensive Guide to Its Development, Clinical Milestones, and Advances in Treatment. Springer; New York; 2015:391–405. [Google Scholar]
- 23. Brancu M, Wagner HR, Morey RA, et al. The Post-Deployment Mental Health (PDMH) study and repository: a multi-site study of US Afghanistan and Iraq era veterans. Int J Methods Psychiatr Res. 2017;26(3):e1570. doi: 10.1002/mpr.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. U.S. Department of Veterans Affairs National Center for Veterans Analysis and Statistics. Key Statistics by Veteran Status and Period of Service.2018:1. August 24, 2018. https://www.va.gov/vetdata/docs/SpecialReports/KeyStats.pdf. Accessed September 27, 2022. [Google Scholar]
- 25. Keane TM, et al. Clinical evaluation of a measure to assess combat exposure. Psychol Assess. 1989;1:53–55. doi: 10.1037/1040-3590.1.1.53. [DOI] [Google Scholar]
- 26. Lund M, et al. The combat exposure scale: a systematic assessment of trauma in the vietnam war. J Clin Psychol. 1984;40(6):1323–1328. doi: . [DOI] [PubMed] [Google Scholar]
- 27. Germain A, et al. A brief sleep scale for Posttraumatic Stress Disorder: Pittsburgh Sleep Quality Index Addendum for PTSD. J Anxiety Disord. 2005;19(2):233–244. doi: 10.1016/j.janxdis.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 28. Insana SP, Hall M, Buysse DJ, Germain A.. Validation of the Pittsburgh Sleep Quality Index Addendum for posttraumatic stress disorder (PSQI-A) in U.S. male military veterans. J Trauma Stress. 2013;26(2):192–200. doi: 10.1002/jts.21793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kubany ES, Haynes SN, Leisen MB, et al. Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: the Traumatic Life Events Questionnaire. Psychol Assess. 2000;12(2):210–24. doi: 10.1037//1040-3590.12.2.210. [DOI] [PubMed] [Google Scholar]
- 30. Clancy CP, et al. Lifetime trauma exposure in veterans with military-related posttraumatic stress disorder: association with current symptomatology. J Clin Psychiatry. 2006;67(9):1346–1353. doi: 10.4088/jcp.v67n0904. [DOI] [PubMed] [Google Scholar]
- 31. Weathers FW. Redefining posttraumatic stress disorder for DSM-5. Curr Opin Psychol. 2017;14:122–126. doi: 10.1016/j.copsyc.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 32. Moshier SJ, et al. An Empirical Crosswalk for the PTSD Checklist: translating DSM-IV to DSM-5 Using a Veteran Sample. J Trauma Stress. 2019;32(5):799–805. doi: 10.1002/jts.22438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Beck AT, Steer RA, Brown GK.. BDI-II, Beck depression inventory: manual. 2nd ed. Psychological Corp.; Harcourt Brace; 1996:vi, 38 p. [Google Scholar]
- 34. Wang YP, et al. Psychometric properties of the Beck Depression Inventory-II: a comprehensive review. Braz J Psychiatry. 2013;35(4):416–431. doi: 10.1590/1516-4446-2012-1048. [DOI] [PubMed] [Google Scholar]
- 35. Beck AT, et al. Scale for Suicide Ideation: psychometric properties of a self-report version. J Clin Psychol. 1988;44(4):499–505. doi: . [DOI] [PubMed] [Google Scholar]
- 36. Brown GK, et al. Risk factors for suicide in psychiatric outpatients: a 20-year prospective study. J Consult Clin Psychol. 2000;68(3):371–377. doi: 10.1037/0022-006x.68.3.371. [DOI] [PubMed] [Google Scholar]
- 37. Cochrane-Brink KA, et al. Clinical rating scales in suicide risk assessment. Gen Hosp Psychiatry. 2000;22(6):445–451. doi: 10.1016/s0163-8343(00)00106-7. [DOI] [PubMed] [Google Scholar]
- 38. Kimbrel NA, Calhoun PS, Elbogen EB, Brancu M, Workgroup VAM-AMR, Beckham JC.. The factor structure of psychiatric comorbidity among Iraq/Afghanistan-era veterans and its relationship to violence, incarceration, suicide attempts, and suicidality. Psychiatry Res. 2014;220(1–2):397–403. doi: 10.1016/j.psychres.2014.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hedges LV. Distribution theory for glass’s estimator of effect size and related estimators. J Educ Stat. 1981;6(2):107–128. doi: 10.2307/1164588. [DOI] [Google Scholar]
- 40. Lesko CR, et al. A framework for descriptive epidemiology. Am J Epidemiol. 2022;191(12):2063–2070. doi: 10.1093/aje/kwac115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Skinner HA. The drug abuse screening test. Addict Behav. 1982;7(4):363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- 42. Gavin DR, et al. Diagnostic validity of the drug abuse screening test in the assessment of DSM-III drug disorders. Br J Addict. 1989;84(3):301–307. doi: 10.1111/j.1360-0443.1989.tb03463.x. [DOI] [PubMed] [Google Scholar]
- 43. Skinner HA. The Drug Abuse Screening Test-20—DAST-20. Toronto, Canada; Addiction Research Foundation; 1989. [Google Scholar]
- 44. Kulka RA, et al. Trauma and the Vietnam war generation. Brunner: /Mazel; 1990. [Google Scholar]
- 45. Foral P, et al. Medication-induced sleep disturbances. Consult Pharm. 2011;26(6):414–425. doi: 10.4140/TCP.n.2011.414. [DOI] [PubMed] [Google Scholar]
- 46. Pagel JF, et al. Drug induced nightmares—an etiology based review. Hum Psychopharmacol. 2003;18(1):59–67. doi: 10.1002/hup.465. [DOI] [PubMed] [Google Scholar]
- 47. Thompson DF, et al. Drug-induced nightmares. Ann Pharmacother. 1999;33(1):93–98. doi: 10.1345/aph.18150. [DOI] [PubMed] [Google Scholar]
- 48. Westerlund A. Central nervous system side-effects with hydrophilic and lipophilic beta-blockers. Eur J Clin Pharmacol. 1985;28(Suppl):73–76. doi: 10.1007/bf00543714. [DOI] [PubMed] [Google Scholar]
- 49. Kierlin L, et al. Parasomnias and antidepressant therapy: a review of the literature. Front Psychiatry. 2011;2:71. doi: 10.3389/fpsyt.2011.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tribl GG, et al. Dreaming under antidepressants: a systematic review on evidence in depressive patients and healthy volunteers. Sleep Med Rev. 2013;17(2):133–142. doi: 10.1016/j.smrv.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 51. Baltzan M, et al. Dream enactment behavior: review for the clinician. J Clin Sleep Med. 2020;16(11):1949–1969. doi: 10.5664/jcsm.8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Creamer JL, et al. Nightmares in United States military personnel with sleep disturbances. J Clin Sleep Med. 2018;14(3):419–426. doi: 10.5664/jcsm.6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Caldwell JA, et al. The association of insomnia and sleep apnea with deployment and combat exposure in the entire population of US army soldiers from 1997 to 2011: a retrospective cohort investigation. Sleep. 2019;42(8). doi: 10.1093/sleep/zsz112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Moore BA, Tison LM, Palacios JG, Peterson AL, Mysliwiec V.. Incidence of insomnia and obstructive sleep apnea in active duty United States military service members. Sleep. 2021;44(7). doi: 10.1093/sleep/zsab024. [DOI] [PubMed] [Google Scholar]
- 55. Portnoy GA, et al. Understanding gender differences in resilience among veterans: trauma history and social ecology. J Trauma Stress. 2018;31(6):845–855. doi: 10.1002/jts.22341. [DOI] [PubMed] [Google Scholar]
- 56. Hoge CW, et al. Commentary: women in combat and the risk of post-traumatic stress disorder and depression†. Int J Epidemiol. 2007;36(2):327–329. doi: 10.1093/ije/dym013. [DOI] [PubMed] [Google Scholar]
- 57. Klingensmith K, et al. Military sexual trauma in US veterans: results from the National Health and Resilience in Veterans Study. J Clin Psychiatry. 2014;75(10):e1133–e1139. doi: 10.4088/jcp.14m09244. [DOI] [PubMed] [Google Scholar]
- 58. Street AE, et al. A new generation of women veterans: stressors faced by women deployed to Iraq and Afghanistan. Clin Psychol Rev. 2009;29(8):685–694. doi: 10.1016/j.cpr.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 59. Morgan-Lopez AA, et al. Estimating posttraumatic stress disorder severity in the presence of differential item functioning across populations, comorbidities, and interview measures: Introduction to Project Harmony. J Trauma Stress. 2022;35(3):926–940. doi: 10.1002/jts.22800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McCarthy MS, Hoffmire C, Brenner LA, Nazem S.. Sleep and timing of death by suicide among U.S. Veterans 2006-2015: analysis of the American Time Use Survey and the National Violent Death Reporting System. Sleep. 2019;42(8). doi: 10.1093/sleep/zsz094. [DOI] [PubMed] [Google Scholar]
- 61. Trockel M, et al. Effects of cognitive behavioral therapy for insomnia on suicidal ideation in veterans. Sleep. 2015;38(2):259–265. doi: 10.5665/sleep.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Elbogen EB, et al. Assessing risk of violent behavior among veterans with severe mental illness. J Trauma Stress. 2008;21(1):113–117. doi: 10.1002/jts.20283. [DOI] [PubMed] [Google Scholar]
- 63. Elbogen EB, et al. Violent behaviour and post-traumatic stress disorder in US Iraq and Afghanistan veterans. Br J Psychiatry. 2014;204(5):368–375. doi: 10.1192/bjp.bp.113.134627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Alexander M, et al. The national veteran sleep disorder study: descriptive epidemiology and secular trends, 2000-2010. Sleep. 2016;39(7):1399–1410. doi: 10.5665/sleep.5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Elliott JE, Opel RA, Pleshakov D, et al. Posttraumatic stress disorder increases the odds of REM sleep behavior disorder and other parasomnias in Veterans with and without comorbid traumatic brain injury. Sleep. 2020;43(3). doi: 10.1093/sleep/zsz237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. D’Agostino A, et al. Challenging the myth of REM sleep behavior disorder: no evidence of heightened aggressiveness in dreams. Sleep Med. 2012;13(6):714–719. doi: 10.1016/j.sleep.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 67. Barone DA. Dream enactment behavior-a real nightmare: a review of post-traumatic stress disorder, REM sleep behavior disorder, and trauma-associated sleep disorder. J Clin Sleep Med. 2020;16(11):1943–1948. doi: 10.5664/jcsm.8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Eli Lilly and Company. Cymbalta (duloxetine delayed-relase capsules) [package insert].U.S. Food and Drug Administration; website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/021427s056lbl.pdf. Revised July 2021. Accessed October 26, 2022. [Google Scholar]
- 69. GlaxoSmithKline. Wellbutrin XL(bupropion hydrochloride extended-release) [package insert].U.S. Food and Drug Administration; website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/021515s044lbl.pdf. Revised March 2022. Accessed October 26, 2022. [Google Scholar]
- 70. Thordardottir EB, et al. The manifestations of sleep disturbances 16 years post-trauma. Sleep. 2016;39(8):1551–1554. doi: 10.5665/sleep.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Salinsky M, et al. Psychiatric comorbidity and traumatic brain injury attribution in patients with psychogenic nonepileptic or epileptic seizures: a multicenter study of US veterans. Epilepsia. 2018;59(10):1945–1953. doi: 10.1111/epi.14542. [DOI] [PubMed] [Google Scholar]
- 72. Armistead-Jehle P. Symptom validity test performance in U.S. veterans referred for evaluation of mild TBI. App Neuropsychol. 2010;17(1):52–59. doi: 10.1080/09084280903526182. [DOI] [PubMed] [Google Scholar]
- 73. Frueh BC, et al. Apparent symptom overreporting in combat veterans evaluated for ptsd. Clin Psychol Rev. 2000;20(7):853–885. doi: 10.1016/S0272-7358(99)00015-X. [DOI] [PubMed] [Google Scholar]
- 74. Jackson CE, et al. Reporting of symptoms associated with concussion by OEF/OIF/OND Veterans: comparison between research and clinical contexts. Brain Injury. 2017;31(4):485–492. doi: 10.1080/02699052.2017.1280740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
To protect the privacy and protected health information of individuals that participated in the study the data underlying this article cannot be shared publicly. The data will be shared upon reasonable request to the VA Mid-Atlantic Mental Illness Research, Education, and Clinical Center Workgroup and SAS code will be shared upon reasonable request to the corresponding author.