Abstract
Study Objectives
Obstructive sleep apnea (OSA) is reported to be highly prevalent among Aboriginal Australians. However, no studies have assessed the implementation and efficacy of continuous positive airway pressure (CPAP) therapy in this population. Hence, we compared the clinical, self-reported perception of sleep quality and polysomnographic (PSG) characteristics among Aboriginal patients with OSA.
Methods
Adult Aboriginal Australians who underwent both diagnostic (Type 1 and 2) and in-lab CPAP implementation studies were included.
Results
Total of 149 patients were identified (46% female, median age 49 years, body mass index 35 kg/m2). The OSA severity was 6% mild, 26% moderate, and 68% severe on the diagnostic PSG. On application of CPAP, there were significant improvements in; total arousal index (diagnostic 29 to 17/h on CPAP), total apnea–hypopnea index (AHI) (diagnostic 48 to 9/h on CPAP), non-rapid eye movement AHI (diagnostic 47 to 8/h on CPAP), rapid eye movement (REM) AHI (diagnostic 56 to 8/h on CPAP) and oxygen saturation (SpO2) nadir (diagnostic 77% to 85% on CPAP) (p < 0.001 for each). Following a single night of CPAP, 54% of patients reported sleeping “better than normal” compared to 12% following the diagnostic study (p = 0.003). In multivariate regression models, males had a significantly lesser change in REM AHI than females (5.7 events/hour less change (IQR 0.4, 11.1), p = 0.029).
Conclusions
There is substantial improvement in several sleep-related domains on the application of CPAP among Aboriginal patients with a good initial acceptance of treatment. Whether the positive impact observed in this study translates to better sleep health outcomes with long-term adherence to CPAP therapy is yet to be assessed.
Keywords: Adherence, architecture, first nations, indigenous, oxygen saturation, positive airway pressure, rapid eye movement, sex, sleep apnea, sleep quality
Introduction
Obstructive sleep apnea (OSA) is estimated to affect 425 million people worldwide, imposing a significant global health problem [1]. Undiagnosed OSA may increase the risks of cardiovascular disease and all-cause mortality [2,3]. Moreover, untreated sleep-related disorders are associated with the development of hypertension and type 2 diabetes mellitus (T2DM), with both observational and experimental data showing that OSA is more prevalent in those with T2DM compared to the general population [4,5]. Therapeutic interventions for sleep disorders, including continuous positive airway pressure (CPAP) therapy may have a beneficial effect among patients with cardiovascular disease, hypertension, and diabetes [4,5].
In Australia, 3.3% of the population self-identify as of Aboriginal descent (henceforth, Aboriginal Australians are respectfully represented as Aboriginal people/patients/population and for other Indigenous people globally as Indigenous people/patients/population), while in the Northern Territory (NT) of Australia, Aboriginal people make up approximately 30% of the NT population, the highest proportion compared to all other Australian States and Territories [6]. Overall, Aboriginal Australians are more likely to report having chronic health conditions, such as diabetes, coronary artery disease, and hypertension compared to non-Aboriginal Australians, with an estimated gap in life expectancy of 19–21 years [7–10]. This trend is even more evident among Aboriginal Australians who reside remotely, such as those residing in the NT of Australia [11–18].
Nevertheless, studies investigating the sleep health profile of Aboriginal Australians have been sparse [19]. However, the limited data that exists in the current literature suggests that sleep disorders, in particular OSA, are highly prevalent among Aboriginal Australians at a rate 1.8 times higher than their non-Aboriginal counterparts [20]. Additionally, previous studies have demonstrated that women have more severe OSA during rapid eye movement (REM) sleep stages compared to males [21, 22]. Moreover, recent studies from the Top End, NT of Australia have reported that Aboriginal Australians demonstrate a higher overall severity of OSA, in the presence of concurrent medical comorbidities, such as hypertension, cardiovascular disease, chronic renal disease, and diabetes [23,24]. From a global perspective, there are only a small number of studies that have investigated OSA among First Nations Indigenous peoples [25,26]. However, studies from the New Zealand First Nations Māori have demonstrated a higher proportion of poor sleep quality and higher levels of OSA [27–29].
Despite emerging evidence in the literature to suggest sleep disorders including OSA could be highly prevalent among Aboriginal Australians and other First Nations Indigenous people globally [19–23, 25–34], there is little published data demonstrating the efficacy of CPAP therapy in the treatment of OSA as well as the effect of CPAP therapy on Aboriginal/Indigenous peoples sleep architecture, including if there is a difference in response to therapeutic interventions (CPAP) between sexes. Furthermore, low awareness of sleep health and lower health self-efficacy have been suggested to hinder successful acclimatization to CPAP therapy for OSA in Indigenous populations [35,36]. Studies in the past have used questionnaires to assess the first impression following CPAP implementation among CPAP-naïve adults in other non-Aboriginal/Indigenous ethnic population to predict CPAP adherence [37]. Hence, it may be worthwhile to investigate how Aboriginal Australians perceive their sleep quality and other related outcomes upon implementation of CPAP therapy. We hypothesized that among adult Aboriginal Australian patients, CPAP therapy would have a positive impact on sleep architecture, OSA severity, and perception of sleep quality in comparison to what is observed during a diagnostic sleep study. Furthermore, we hypothesized that, there could be differences in the response to CPAP between males and females. Therefore, the aims of this study were to compare self-reported sleep perception and polysomnographic (PSG) parameters between initial diagnostic PSG and following implementation of CPAP therapy, and the impact of CPAP therapy between males and females, among those Aboriginal adults diagnosed to have OSA in the top end health service (TEHS) region of the NT of Australia.
Method
Setting
This study was conducted at the Royal Darwin Hospital and Darwin Respiratory and Sleep Health (DRSH) based at the Darwin Private Hospital (DPH) in the TEHS region of the NT of Australia. The NT is an Australian federal territory occupying the central-northern region of Australia. It is the least populated, and least dense state or territory in Australia, with a population density of just 0.18 people/km2 [6]. The Top End region (TEHS) covers approximately 35% or 475 338 km2 of the total area of the NT, with an estimated population of 195 000 people, representing 79% of the total NT population. The vast majority (80%) of Aboriginal people living in the NT reside in remote or very remote communities as defined by the Australian Statistical Geographic Standard (ASGS level 4 and level 5) [6,38,39].
Study participants
All adult Aboriginal patients residing in the TEHS region over 18 years of age identified to have OSA on a diagnostic PSG (either during an unmonitored level 2 or in-lab monitored diagnostic sleep study [level 1]) and subsequently underwent an in-lab monitored CPAP implementation study between 2012 and 2020 were included.
Diagnostic sleep study and CPAP implementation data
All diagnostic and PAP implementation studies were performed at the DRSH/DPH sleep center, currently an accredited sleep service facility by the National Association of Testing Authorities, Australia, and Australasian Sleep Association. Diagnostic sleep studies (PSGs) were performed as either Level 1 studies (in-Lab, with a sleep technologist in attendance) or as Level 2 sleep studies (unattended). If both Type 1 and Type 2 diagnostic studies were available, Type 1 studies took precedence, or the study that had higher session quality for recorded channels. Presence of OSA was determined if the total apnea–hypopnea index (AHI) was >5/hour and was categorized into four groups: AHI ≤ 5 (normal range), AHI = 5–15 (mild sleep apnea), AHI = 15–30 (moderate sleep apnea), AHI > 30 (severe sleep apnea). More details on setting, and sleep study protocol (PSG) are available from previous reports from our center [21]. For the purpose of this study, only patients who underwent in-lab monitored CPAP implementation study were included to compare the parameters between diagnostic and CPAP implementation studies. At the DRSH center, all PSGs are performed, analyzed, and scored manually by qualified registered polysomnographic technologists utilizing American Academy of Sleep Medicine recommendations. The CPAP titration studies were undertaken as per the standard recommended guidelines and were manually titrated by sleep technologists during the overnight CPAP study [40].
Clinical data
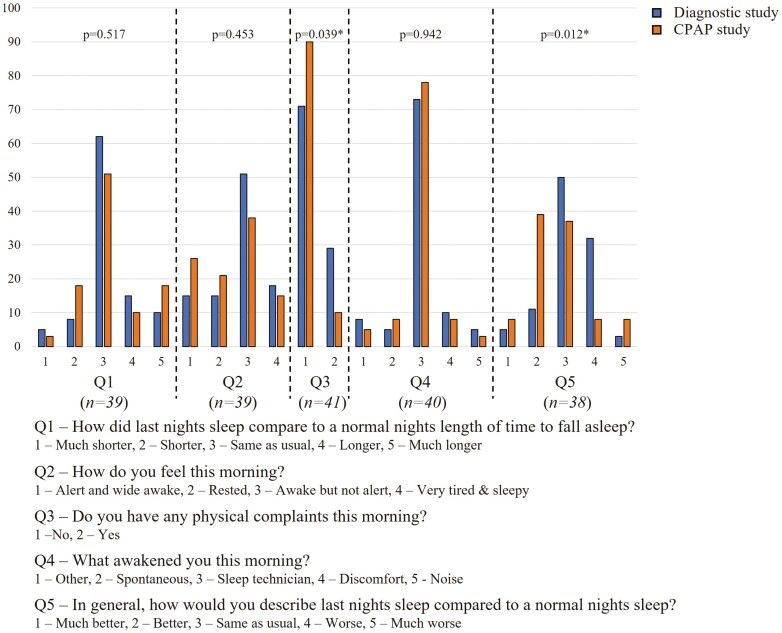
As per standard protocol at DRSH, all patients are administered a detailed questionnaire prior to undergoing both diagnostic and CPAP implementation studies. The questionnaire was designed to provide information on self-identified Aboriginal status, age, sex, and medical comorbid conditions. For non-English speaking patients, native language interpreters, or family members assisted with the questionnaire when available. Height, weight, and body mass index (BMI) were measured. Symptoms related to sleep disorders were also recorded, including, self-reported Epworth Sleepiness Scale score, alcohol, and smoking history. Patients' usual place of residence was identified by postcode or suburb and categorized as regional, remote, or very remote according to the Australian statistical geographic standard [39]. Furthermore, as per the usual practice in this center, all patients are advised to complete a detailed questionnaire regarding their self-reported sleep perception/quality on the night of undergoing both diagnostic (Type 1 and 2) and CPAP implementation study (Figure 1 in results section).
Figure 1.
Self-reported questionnaire answers related to sleep following diagnostic and CPAP studies.
Statistical analysis
Statistical analysis was conducted in Stata/SE 15. The assumption of normality was checked using Shapiro–Wilk test and found to be non-normally distributed. Thus, continuous data were described with median and interquartile range (IQR) and differences between diagnostic and CPAP studies tested via a matched Wilcoxon signed-rank test, while differences between male/female or by the severity of OSA were assessed via Kruskal–Wallis rank sum test. Categorical data were described with count and percentages and were analyzed via a chi-squared (χ2). The participants’ characteristics were compared according to diagnostic and treatment (CPAP) studies, OSA severity categories, and sex. Multivariate quantile regression adjusting for age, sex, BMI, and baseline (diagnostic study) values of the dependent parameter were utilized to investigate what factors influenced the response of PSG parameters to CPAP. Changes between diagnostic and CPAP studies on self-reported questionnaire data were tested via McNemar’s exact test for binary outcomes, Stuart–Maxwell test for >2 category outcomes, or Wilcoxon paired sign-rank test for continuous outcomes, with patients who had a Type 2 diagnostic study excluded from these analyses. Improvements in perceived sleep quality were assessed as higher scores on the question “In general, how would you describe last night's sleep compared to a normal night's sleep?” post CPAP compared to post-diagnostic studies. Univariate logistic regression reporting odds ratios (95% confidence intervals) was utilized to assess the association between demographic factors, or PSG improvements following CPAP and self-reported improvement in sleep quality. Statistical significance was set at p < 0.05 throughout.
Ethics
This study was approved by the Human Research Ethics governance/committee of the NT, TEHS, and Menzies School of Health Research (Reference: HREC 2020-3932). The authors acknowledge the rights of Aboriginal people involved in this study, and as such conducted and reported according to strengthening and reporting of health research involving Indigenous people [41], including consultation with local institute Aboriginal Australian representative.
Results
Clinical data
Of the total 855 Aboriginal patients recorded to have undergone a diagnostic PSG, 649 (76%) were diagnosed to have OSA, of them, 149 patients (46% female) were identified to have undergone both a diagnostic PSG (Level 1 (50%) or Level 2 (50%)) and a subsequent in-lab CPAP implementation study with a median 54 days between studies. The majority of patients resided in outer regional areas (73%), were categorized as obese (81%) and were current smokers (68%) (Table 1). Hypertension, heart disease, and diabetes were common comorbid conditions (49%, 30%, and 29%, respectively). Female patients had a significantly higher BMI than male patients, and a higher proportion resided in very remote locations.
Table 1.
Baseline characteristics and medical comorbid conditions among study participants with OSA
| Clinical parameters | Patients (n = 149) |
Male (n = 81) |
Female (n = 68) |
P-value |
|---|---|---|---|---|
| Age (years) | 48.89 (39.13, 57.08) | 46.76 (38.08, 57.68) | 49.7 (43.18, 56.3) | 0.358 |
| ASGS 3 (Regional) | 108 (72%) | 65 (80%) | 43 (63%) | 0.073 |
| ASGS 4 (Remote) | 10 (7%) | 4 (5%) | 6 (9%) | |
| ASGS 5 (Very remote) | 31 (21%) | 12 (15%) | 19 (28%) | |
| Height (cm) | 169 (163.03, 176) | 175.5 (171, 178.5) | 163 (157.15, 167) | <0.001* |
| Weight (kg) | 100.55 (88, 118.25) | 104 (90, 115.95) | 96.5 (84.5, 119) | 0.328 |
| BMI (kg/m2) | 35.16 (30.72, 41.98) | 34.3 (29.7, 39.29) | 37.77 (31.72, 46.33) | 0.002* |
| Underweight (<18.5 kg/m2) | 1 (1%) | 0 (0%) | 1 (2%) | 0.004* |
| Normal weight (18.5–24.9 kg/m2) | 4 (3%) | 3 (4%) | 1 (2%) | |
| Overweight (25–29.9 kg/m2) | 23 (16%) | 19 (24%) | 4 (6%) | |
| Obese (>30 kg/m2) | 117 (81%) | 57 (72%) | 60 (91%) | |
| Neck circumference (cm) | 43 (41, 48) | 45 (42, 48) | 42.75 (39, 45) | <0.001* |
| ESS | 10 (6, 14) | 10 (6, 14) | 10 (8, 14) | 0.476 |
| Current smoker | 97 (68%) | 51 (65%) | 46 (71%) | 0.318 |
| Former smoker | 18 (13%) | 8 (10%) | 10 (15%) | |
| Never smoker | 27 (19%) | 18 (23%) | 9 (14%) | |
| Ever alcohol | 107 (75%) | 60 (77%) | 47 (72%) | 0.527 |
| Hypertension | 69 (49%) | 36 (47%) | 33 (52%) | 0.570 |
| Heart disease | 42 (30%) | 22 (29%) | 20 (31%) | 0.729 |
| Diabetes | 41 (29%) | 20 (26%) | 21 (33%) | 0.373 |
| Dyslipidaemia | 39 (28%) | 19 (25%) | 20 (31%) | 0.385 |
| Chronic Kidney disease | 18 (13%) | 7 (9%) | 11 (17%) | 0.206 |
| COPD | 15 (11%) | 5 (6%) | 10 (16%) | 0.102 |
| Hypothyroidism | 9 (6%) | 3 (4%) | 6 (9%) | 0.300 |
| Time between diagnostic and CPAP (days) | 54 (34, 115) | 43 (31, 76) | 74 (39, 189) | 0.004* |
P-value obtained via Kruskal–Wallis’s test (continuous parameters) or chi-squared test (categorical parameters) using Fishers exact test if categories had <10 patients.
Data displayed as median (IQR) or number (%).
PSG outcomes for diagnostic and PAP implementation
The majority of patients on the diagnostic PSG demonstrated the presence of severe OSA (AHI >30/h [68%]), with a low median sleep efficiency (81%) and a low portion of sleep time spent in REM sleep (median 16.5%) (Table 2). On application of CPAP, total AHI significantly improved (median change per patient 32.5 [IQR 14.9, 52.6]), resulting in 81% of patients improving by at least one OSA severity category, and 27% of patients' OSA fully resolving. REM sleep latency was shortened by a median of 15 minutes, and the proportion of time in REM sleep improved by a median of 5.3%. All oxygen desaturation parameters significantly improved, with the greatest improvement coming in oxygen saturation (SpO2) nadir (median change of 8% [IQR 2, 13.5]). Significant differences in the level of change from diagnostic to CPAP study for some parameters were noted between females and males. Females showed a significant improvement in REM sleep latency (median reduction of 24 minutes) while males showed no such significant improvement (median reduction of 2 minutes) (difference between median change for females vs. males, p = 0.048). Females also showed significantly greater improvements in REM AHI compared to males (median change 41.5 vs. 28.3, respectively, p = 0.029) (Table 3).
Table 2.
Diagnostic and CPAP study PSG parameters
| PSG data | Total | Female | Male | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Diagnostic study (n = 149) | CPAP study (n = 149) |
P-value | Diagnostic study (n = 68) | CPAP study (n = 68) |
P-value | Diagnostic study (n = 81) | CPAP study (n = 81) |
P-value | |
| Sleep latency | 14.45 (4.05, 40.5) | 13 (6.45, 27) | 0.260 | 19.5 (4.75, 32.75) | 15.85 (7.8, 29.25) | 0.783 | 12.75 (3.45, 41.75) | 9.9 (5, 21.7) | 0.178 |
| REM latency | 114.5 (82.5, 184.75) | 86.75 (58, 145.75) | 0.008* | 127 (87, 198) | 91.25 (62.75, 128.25) | 0.002* | 106 (76.5, 164.5) | 80.75 (53.25, 150.75) | 0.556 |
| Total sleep time | 384.5 (331, 445) | 347.25 (294.5, 398) | <0.001* | 385.25 (330.5, 450.5) | 345.25 (296, 398) | <0.001* | 382.8 (331, 441.5) | 349.5 (294.5, 399.75) | <0.001* |
| WASO | 66.75 (33.65, 110.25) | 73.3 (37.75, 113.05) | 0.843 | 67 (36.85, 103.5) | 71.25 (37.75, 108.2) | 0.961 | 65.9 (31.6, 117) | 74.05 (39, 116.25) | 0.801 |
| Sleep efficiency | 81.1 (69.8, 88.2) | 79.6 (70.25, 87.8) | 0.373 | 81.15 (70.1, 87.55) | 78.85 (70.8, 88.15) | 0.608 | 81.1 (69.7, 88.2) | 80.9 (69.9, 87.05) | 0.533 |
| N1 stage (%) | 12.5 (7.3, 20.9) | 9.25 (5.95, 14.95) | <0.001* | 10.2 (6.4, 16.9) | 8 (4.5, 11.15) | 0.009* | 15.3 (8.1, 26.1) | 11.45 (7, 17.7) | 0.001* |
| N2 stage (%) | 56 (50.3, 65.5) | 49.7 (41.7, 55.1) | <0.001* | 57.7 (48.25, 64.75) | 47.25 (38.5, 55.65) | <0.001* | 54.3 (50.5, 67.4) | 50.45 (45.35, 54.3) | <0.001* |
| N3 stage (%) | 34.867 (0.7, 15.8) | 17.5 (9.1, 24.65) | <0.001* | 10.2 (4.15, 18.2) | 21.9 (15.5, 28.55) | <0.001* | 4.6 (0, 10.7) | 12.95 (5.55, 21.25) | <0.001* |
| REM (%) | 16.5 (10.2, 21.6) | 22.1 (16.15, 28.4) | <0.001* | 17.65 (11.45, 21.6) | 21.5 (15.7, 28.35) | 0.005* | 15.6 (6.9, 21.8) | 23.1 (16.35, 28.45) | <0.001* |
| RAI | 16.6 (8.4, 38.7) | 4 (1.65, 10.55) | <0.001* | 14.55 (6.9, 30.1) | 2.75 (0.95, 5.4) | <0.001* | 24.6 (10.9, 45.3) | 6.55 (2.55, 18.7) | <0.001* |
| SAI | 2.7 (1.1, 5.9) | 7.25 (4.45, 10.35) | <0.001* | 3.3 (1.8, 5.75) | 6 (4.45, 9.85) | <0.001* | 2 (0.9, 5.9) | 7.5 (4.3, 10.6) | <0.001* |
| TAI | 28.5 (17.6, 48.9) | 16.55 (11.3, 25.55) | <0.001* | 26.15 (15, 42.3) | 13.45 (10.3, 19.25) | <0.001* | 31.5 (18.9, 55.7) | 21.1 (13.7, 31.7) | <0.001* |
| Total AHI | 47.6 (23.7, 70.7) | 8.8 (4.4, 20.2) | <0.001* | 34.4 (19.6, 62.5) | 6.5 (3.15, 14.25) | <0.001* | 54.9 (32.4, 75.8) | 12.1 (5.5, 26.7) | <0.001* |
| NREM AHI | 46.5 (21.15, 71.2) | 8.4 (3.8, 21.8) | <0.001* | 29.35 (15.4, 62.7) | 5.75 (2, 12.6) | <0.001* | 55 (28.8, 76.15) | 11.8 (5, 30.6) | <0.001* |
| REM AHI | 56.1 (25.7, 72.3) | 8.2 (2, 19.6) | <0.001* | 61.3 (34.6, 76.7) | 5.75 (1.2, 19.55) | <0.001* | 47.2 (21.2, 68.6) | 8.6 (2.55, 19.6) | <0.001* |
| OSA | 149 (100%) | 107 (72%) | <0.001* | 68 (100%) | 43 (63%) | <0.001* | 81 (100%) | 64 (79%) | <0.001* |
| Mild | 9 (6%) | 58 (54%) | <0.001* | 8 (12%) | 28 (65%) | <0.001* | 1 (1%) | 30 (47%) | <0.001* |
| Moderate | 38 (26%) | 26 (24%) | 0.826 | 20 (29%) | 10 (23%) | 0.477 | 18 (22%) | 16 (25%) | 0.695 |
| Severe | 102 (68%) | 23 (21%) | <0.001* | 40 (59%) | 5 (12%) | <0.001* | 62 (77%) | 18 (28%) | <0.001* |
| SpO2 (wake) | 95 (93, 96) | 96 (94, 97) | <0.001* | 95 (93, 96) | 96 (94, 97) | 0.001* | 94 (93, 96) | 96 (94, 97) | <0.001* |
| SpO2 (NREM) | 93 (92, 94.5) | 95 (94, 97) | <0.001* | 93.5 (92, 95) | 95 (94, 97) | <0.001* | 93 (91, 94) | 95 (94, 96) | <0.001* |
| SpO2 (REM) | 93 (89, 94) | 95 (94, 97) | <0.001* | 93 (89, 94.5) | 95 (94, 97) | <0.001* | 93 (90, 94) | 96 (94, 97) | <0.001* |
| SpO2 (Total) | 93 (92, 95) | 95 (94, 97) | <0.001* | 94 (92, 95) | 95 (94, 97) | <0.001* | 93 (92, 94.5) | 95 (94, 96) | <0.001* |
| SpO2 (Nadir) | 77 (69, 82) | 85 (79, 90) | <0.001* | 78.5 (70.5, 83) | 85 (80, 90) | <0.001* | 77 (68, 81) | 85 (78, 89) | <0.001* |
P-value obtained via Wilcoxon signed-rank test, or equality of proportions z-test. Data displayed as median (IQR) or number (%).
Table 3.
Changes in PSG parameters from diagnostic to CPAP studies
| PSG data | Total cohort (n = 149) |
Females (n = 68) |
Males (n = 81) |
P-value |
|---|---|---|---|---|
| Sleep latency | 0 (−20.5, 11) | 2 (−22.95, 16) | −1 (−18.5, 7) | 0.285 |
| REM latency | −15 (−82.5, 31) | −23.75 (−87.5, 17.5) | −2 (−53, 48) | 0.048* |
| Total sleep time | −36.7 (−111.4, 22.9) | −33 (−111.25, 17.75) | −42 (−121.3, 25.25) | 0.905 |
| WASO | −4 (−41, 48) | −7.6 (−43.95, 47.75) | −4 (−34.1, 48) | 0.706 |
| Sleep efficiency | −0.9 (−10.4, 7.65) | −0.9 (−9.35, 7.9) | −0.95 (−10.5, 7.2) | 0.969 |
| N1 stage (%) | −2.5 (−9.05, 1.9) | −2.55 (−7.9, 2.1) | −2.5 (−12.2, 1.8) | 0.349 |
| N2 stage (%) | −8.65 (−19.9, 0.25) | −10.05 (−20.8, −0.75) | −5.2 (−19.55, 2.22) | 0.270 |
| N3 stage (%) | 34.877.65 (0.65, 15.1) | 10.25 (1.65, 17.85) | 6.55 (0, 13.75) | 0.110 |
| REM (%) | 5.3 (−3, 14.75) | 3 (−3.6, 11.85) | 6.9 (−2.7, 16.85) | 0.137 |
| RAI | −10.65 (−29.75, −4) | −9.5 (−24.8, −3.9) | −13.55 (−34, −4.2) | 0.390 |
| SAI | 3.2 (0.6, 6.8) | 3 (−0.3, 7.2) | 3.45 (1.3, 6.55) | 0.208 |
| TAI | −10.2 (−27.8, −1.4) | −9.2 (−26.45, −2.15) | −10.45 (−31.8, −0.35) | 0.971 |
| Total AHI | −32.5 (−52.6, −14.9) | −26.05 (−55.05, −12.7) | −38.6 (−52.4, −18.5) | 0.270 |
| NREM AHI | −28.7 (−49.3, −11.8) | −19.45 (−49.85, −10.1) | −33.6 (−48.5, −15.2) | 0.308 |
| REM AHI | −34.9 (−63, −15.5) | −41.5 (−68.45, −22.65) | −28.3 (−56.7, −10.1) | 0.029* |
| SpO2 (wake) | 1 (0, 2) | 1 (0, 2) | 1 (0, 2) | 0.145 |
| SpO2 (NREM) | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) | 0.245 |
| SpO2 (REM) | 3 (1, 6) | 3 (1, 6) | 3 (1, 6) | 0.948 |
| SpO2 (Total) | 2 (0.5, 3) | 2 (0, 3) | 2 (1, 3) | 0.473 |
| SpO2 (Nadir) | 8 (2, 13.5) | 8.5 (1.5, 15) | 8 (2.5, 13) | 0.888 |
P-value obtained via Kruskal–Wallis’s rank sum test. Data displayed as median (IQR).
Regression analysis post-CPAP implementation
In multivariate regression models (adjusting for age, sex, BMI, and the diagnostic PSG value for each tested parameter) the diagnostic PSG value of the parameter consistently showed the strongest association with change in that parameter in the CPAP study (Table 4). However, age, sex, and BMI were also noted to have significant effects on various parameters. Increasing age was associated with an increase in WASO in the CPAP compared to the diagnostic PSG studies [1.99 (95% CI 1.09, 2.9)], and a decrease in total sleep time, sleep efficiency, and SpO2 during non-rapid eye movement and REM stages. Increasing BMI was associated with decreases in non-rapid eye movement stage 2 sleep and increases in REM AHI. Male sex was also associated with increases in REM AHI and TAI. The previously noted difference in REM sleep latency between males and females was not apparent in multivariate models.
Table 4.
Multivariate regression results for parameter effects on delta values from diagnostic to CPAP studies
| PSG data | Diagnostic PSG value | Age | BMI | Sex |
|---|---|---|---|---|
| Sleep latency | −0.97 (−1.05, −0.89)* | 0.11 (−0.17, 0.4) | −0.1 (−0.51, 0.3) | −4.99 (−12.08, 2.1) |
| REM latency | −0.78 (−0.94, −0.62)* | −0.54 (−1.74, 0.65) | 0.14 (−1.55, 1.84) | 8.45 (−21.54, 38.43) |
| Total sleep time | −0.85 (−1.04, −0.67)* | −1.44 (−2.86, −0.02)* | −0.23 (−2.28, 1.81) | 3.16 (−30.96, 37.29) |
| WASO | −0.87 (−1.04, −0.69)* | 1.99 (1.09, 2.9)* | 0.9 (−0.33, 2.12) | 7.06 (−14.2, 28.32) |
| Sleep efficiency | −0.8 (−0.99, −0.61)* | −0.42 (−0.65, −0.2)* | −0.14 (−0.46, 0.18) | 0.11 (−5.4, 5.62) |
| N1 stage (%) | −0.85 (−0.94, −0.76)* | 0.06 (−0.04, 0.16) | −0.05 (−0.19, 0.1) | 1.77 (−0.82, 4.36) |
| N2 stage (%) | −0.88 (−1.01, −0.75)* | −0.08 (−0.27, 0.11) | −0.27 (−0.54, −0.01)* | 2.04 (−2.62, 6.7) |
| N3 stage (%) | −0.56 (−0.81, −0.31)* | −0.1 (−0.3, 0.1) | −0.09 (−0.37, 0.2) | −5.52 (−10.72, −0.33)* |
| REM (%) | −0.98 (−1.23, −0.74)* | −0.01 (−0.19, 0.16) | 0.21 (−0.05, 0.46) | 1.48 (−2.9, 5.87) |
| RAI | −0.83 (−0.89, −0.77)* | −0.04 (−0.16, 0.09) | −0.07 (−0.25, 0.11) | 1.77 (−1.38, 4.91) |
| SAI | −0.77 (−0.99, −0.56)* | −0.03 (−0.11, 0.04) | −0.08 (−0.18, 0.03) | 0.68 (−1.16, 2.52) |
| TAI | −0.84 (−0.91, −0.77)* | −0.08 (−0.23, 0.06) | −0.12 (−0.33, 0.09) | 5.01 (1.36, 8.66)* |
| Total AHI | −0.83 (−0.91, −0.74)* | 0.05 (−0.15, 0.25) | −0.03 (−0.34, 0.27) | 1.32 (−3.83, 6.47) |
| NREM AHI | −0.8 (−0.89, −0.71)* | −0.02 (−0.25, 0.21) | −0.05 (−0.39, 0.3) | 1.78 (−4.13, 7.68) |
| REM AHI | −0.94 (−1.01, −0.86)* | 0.17 (−0.05, 0.38) | 0.34 (0.02, 0.66)* | 5.71 (0.35, 11.06)* |
| SpO2 (wake) | −0.56 (−0.71, −0.41)* | −0.02 (−0.05, 0.01) | −0.01 (−0.06, 0.03) | 0.17 (−0.6, 0.95) |
| SpO2 (NREM) | −0.54 (−0.65, −0.42)* | −0.04 (−0.07, 0)* | −0.04 (−0.09, 0.02) | −0.17 (−1.04, 0.7) |
| SpO2 (REM) | −0.83 (−0.92, −0.74)* | −0.07 (−0.11, −0.03)* | −0.05 (−0.11, 0.02) | −0.48 (−1.51, 0.54) |
| SpO2 (Total) | −0.55 (−0.67, −0.44)* | −0.03 (−0.06, 0.01) | −0.04 (−0.09, 0.02) | −0.05 (−0.89, 0.8) |
| SpO2 (Minimum) | −0.6 (−0.77, −0.44)* | −0.02 (−0.14, 0.1) | −0.08 (−0.26, 0.11) | −1.16 (−4.22, 1.89) |
Data reported as beta (95% CI).
* P-value <0.05. Male sex used as reference.
Self-reported sleep perception data
Approximately half (54%) of the patients who had a Type 1 diagnostic study completed the post-study questionnaire for both diagnostic and CPAP study nights. Patients self-reported similar sleep onset latency for both diagnostic and CPAP studies (median 30 minutes [IQR 15, 45] for diagnostic and 25 minutes [IQR 15, 45] for CPAP, p = 0.472), with most reporting this to be either the same as usual (62% and 51% for diagnostic and CPAP, respectively) or longer than usual (26% and 28%, respectively) (Figure 1). Self-reported total sleep time was also similar between diagnostic and CPAP studies (median 6 h (IQR 5, 7) for both, p = 0.879), as was the number of overnight awakenings (median 4 awakenings (IQR 3, 5) vs. 3 (IQR 2, 5), respectively, p = 0.718). Significantly fewer patients reported awakening with physical complaints in the morning following CPAP (10% vs. 29%, p = 0.039). A significantly higher proportion of patients reported their sleep to be better than usual following CPAP compared to the diagnostic study (54% vs. 12%, p = 0.012).
Twenty-one patients (55%) reported greater sleep quality following their CPAP study compared to their diagnostic study, while nine reported the same level of quality and eight reported reduced quality. In univariate logistic regression, greater improvements in WASO between diagnostic and CPAP studies were associated with increased odds of reporting improved sleep quality [OR 1.01 (95% CI 1, 1.03), p = 0.035]. There were no significant associations between self-reported sleep quality improvement and any demographic factors, nor any other PSG variable change between diagnostic and CPAP studies.
Discussion
The current study is the first to quantitatively demonstrate the efficacy of CPAP therapy among an adult Aboriginal Australian population. This study has demonstrated that Aboriginal Australians show substantial improvement in several sleep-related domains on application of CPAP therapy for OSA, as assessed on both PSG and self-reported parameters.
Although few studies in the past have examined data on CPAP therapy among Aboriginal/Indigenous people [35,36,42,43], no studies have explored the initial impact on PSG parameters alongside self-reported sleep quality upon application of CPAP therapy. Exploring these aspects in the current study is a step forward in our understanding of the direct efficacy and acceptability of CPAP therapy in Aboriginal/Indigenous people and could be considered an invaluable addition to the existing literature.
As mentioned above, studies have demonstrated there are differences in the way OSA manifests in females compared to males, with the suggestion that these changes may reflect differences between sexes in upper airway function during sleep in patients with OSA [23,24]. Moreover, evidence in the literature suggests that there are significant differences in several sleep-related parameters/architecture between sexes, especially across ages [44]. The current study adds to this body of evidence, showing a significantly higher AHI during REM sleep stages among females than males in the diagnostic studies (median 61 vs. 47), in addition to demonstrating a significantly greater improvement in AHI during REM sleep on initiation of CPAP therapy (median change diagnostic to CPAP 42 vs. 28). Even after adjusting for age, sex, BMI, and baseline values of AHI during REM sleep, females had a significantly greater change than males (mean difference in improvement 5.7 events/h). However, despite the difference in REM AHI improvement between males and females, there was no significant difference in the level of improvement for SpO2 during REM sleep. It is plausible that this may be related to underlying pulmonary diseases and ventilatory impairment [45–52], giving rise to persistent hypoxemia in males. However, in our study, less males had a diagnosis of COPD than females, though this did not meet statistical significance (p = 0.102). Males showed a significantly greater improvement in non-rapid eye movement 3 sleep percentage than females in the multivariate models indicating a greater effect on sleep quality, although no significant differences in WASO nor sleep efficiency were noted.
In addition to sex, both age and BMI were noted to have significant effects on several parameters in response to CPAP therapy. Previous reports have indicated that both BMI and increasing age are associated with lower oxygen saturation and higher AHI parameters, with an increased BMI additionally associated with CPAP failure rates [53–55]. Consistent with what is observed in non-Aboriginal/Indigenous patients, in our study, Aboriginal patients with a larger BMI showed a significantly reduced effect of CPAP on REM sleep AHI. Whilst there is a paucity of data on older patients with sleep apnea, our data demonstrated that older patients showed detriments in WASO, sleep efficiency, and total sleep time on the application of CPAP compared to the diagnostic study. However, it should be acknowledged that these patients, whilst considered elderly for Indigenous Australians, are relatively young compared to non-Aboriginal Australians, with the majority of the Aboriginal population studying under 60 years of age.
In this study, we also assessed and compared the self-reported perception of sleep quality following the diagnostic and CPAP implementation study. Our study suggests that there is an initial recognition of treatment benefits with a significant proportion of patients reporting their sleep quality to be better than usual following CPAP application (54% vs. 12%, p = 0.012). There were also significantly fewer patients who reported awakening with physical complaints in the morning following CPAP. Longer-term studies are needed to assess if Aboriginal patients reporting immediate symptomatic benefits with CPAP are more likely to adhere to long-term CPAP therapy, and to have an improved quality of life [56].
The authors acknowledge that the number of study participants who underwent in-lab CPAP implementation study was significantly lower in comparison to the number of Aboriginal patients diagnosed to have OSA during this study period. Several factors may have influenced this effect. The vast majority of Aboriginal Australians reside in remote and rural communities in the TEHS region; hence remoteness and geographical isolation could be a barrier to undergoing in-lab CPAP implementation studies. Due to the lack of a standalone, publicly funded sleep lab in the TEHS region, the service delivery model of this center is to facilitate both diagnostic and CPAP implementation via unmonitored (level 2) home diagnostic study and auto-PAP trials during respiratory/sleep outreach visits to remote communities [12], or alternatively providing an auto-PAP trial to patients lodged in hostels during their visit to Darwin city or opportunistically, while the patient is admitted to hospital [21]. In addition to this geographical barrier is the cost of treatment, competing health priorities, lack of access to reliable electricity (in-home trials), and language and cultural barriers that limit effective communication between healthcare professionals and their patients [20]. Moreover, knowledge and self-awareness of medical conditions can also be poor among Aboriginal people residing in remote localities [57]. As can be seen in the response to the question “In general, how would you describe last nights sleep compared to a normal nights sleep?,” more than one-third of patients reported their sleep to be worse than usual following the diagnostic study. Given this, it is possible that those who didn’t follow up and have a subsequent CPAP study are those who had a similar experience of worse sleep quality and considered further follow-up and management of the condition to be potentially harmful to their health and thus not worthwhile. Nonetheless, it is important to recognize that despite 30% of the studied population residing in remote or very remote locations, the median time between diagnostic and CPAP study was just 53 days. Whilst the time lapse between diagnostic and CPAP implementation studies in Australia is highly variable and often center dependent, some international studies have reported wait times of up to 280 days prior to CPAP implementation studies [58]. This suggests that there is adequate availability of resources to address sleep health issues among Aboriginal Australians, including those residing in remote communities, and remoteness in isolation may be less of a barrier to CPAP studies in the NT Australia population [56,59]. However, other social determinants as barriers to providing timely and appropriate care need to be explored further. Moreover, developing culturally appropriate pictorial education resources for both patients and community Aboriginal health practitioners, including, exploring alternate therapeutic interventions in the management of OSA such as weight loss strategies, positional therapy, and surgical interventions may be worthwhile [12,60–62].
There have been only a limited number of studies investigating long-term adherence to CPAP therapy among Aboriginal Australians; however, limited studies published in the literature suggest a lack of knowledge [57] and awareness about OSA and its treatment, as well as a sense of shame about being diagnosed with a sleep disorder [43]. As 30% of this cohort is living in remote and very remote locations, there is also less of an opportunity for ongoing education, mask fitting, and access to daytime CPAP desensitization programs which may also limit the ability of Aboriginal patients to successfully acclimatize to therapy [63–65]. Nonetheless, in the presence of a higher burden of chronic medical comorbidities among Aboriginal Australians [66–68], untreated OSA may further perpetuate long-term adverse health consequences [69,70]. Hence, it is paramount that ongoing research explores the determinants of implementation and adherence to CPAP therapy, identifying both barriers and enablers that are culturally and clinically relevant [61,62], in order to close the sleep health gap among Aboriginal people.
Limitations of the study
The study results are limited to TEHS-specific NT Aboriginal populations and the results cannot be generalized to other Australian Aboriginal populations or Indigenous groups globally. Post-sleep study questionnaires were also not available for all patients. In this study, all patients underwent level-1 in-lab CPAP implementation studies. It is speculative if the outcomes would have been different if the study included patients undergoing unmonitored auto-PAP trials. In our center, remote residing patients are offered auto-PAP trials during community outreach visits, and as such a different cohort may have been captured in this study who preferred in-lab CPAP titration trials compared to in community auto-PAP trials. Moreover, in this study we did not assess long-term CPAP adherence data among those reporting improvement in sleep quality following CPAP implementation. Furthermore, due to lack of similar studies in other Indigenous population we were unable we compare our study finding to other Indigenous groups.
Conclusion
This study demonstrates that application of CPAP significantly improves self-reported sleep quality and multiple domains of sleep parameters include AHI and oxygen saturations among Aboriginal Australians diagnosed with OSA. Further studies are needed to investigate the acceptability and long-term adherence of CPAP therapy, including other sleep health related parameters in this population.
Acknowledgments
We would like to thank our respiratory clinical nurse consultants, Mrs Raelene Messenger and Mrs Siji Issac from the respiratory chronic disease unit, at the RDH, including, rural and remote community Aboriginal health workers and RDH patients travel division for coordinating care for Aboriginal people living in the remote and rural communities in the TEHS, NT region. We also would like to thank all the sleep technologists at DRSH/DPH in conducting diagnostic and CPAP implementation studies, including traveling to remote Aboriginal communities to conduct level 2 studies during respiratory and sleep outreach visits. We also extend our sincere appreciation to our Aboriginal health worker, Mr Izaak Thomas (Australian Indigenous Luritja descendent) from the respiratory chronic respiratory disease coordination division in reviewing this research, addressing much needed data in the diagnosis and management of adult Aboriginal patients with sleep disorders and for the appropriateness and respect in relation to the Aboriginal context represented in this study.
Contributor Information
Matthew Lindfield, Department of Respiratory and Sleep Medicine, Royal Darwin Hospital, Darwin, NT, Australia; Department of Respiratory and Sleep Medicine, The Canberra Hospital, Canberra, ACT, Australia.
Timothy P Howarth, Darwin Respiratory and Sleep Health, Darwin Private Hospital, Darwin, NT, Australia; College of Health and Human Sciences, Charles Darwin University, Darwin, NT, Australia; Department of Applied Physics, University of Eastern Finland, Kuopio, Finland.
Ara J Perez, Darwin Respiratory and Sleep Health, Darwin Private Hospital, Darwin, NT, Australia.
Jessie Crespo, Darwin Respiratory and Sleep Health, Darwin Private Hospital, Darwin, NT, Australia.
Charmain B Atos, Darwin Respiratory and Sleep Health, Darwin Private Hospital, Darwin, NT, Australia.
Hsin-Chia C Huang, Department of Respiratory and Sleep Medicine, The Canberra Hospital, Canberra, ACT, Australia; College of Health and Medicine, Australian National University, ACT, Australia.
Subash S Heraganahally, Department of Respiratory and Sleep Medicine, Royal Darwin Hospital, Darwin, NT, Australia; Darwin Respiratory and Sleep Health, Darwin Private Hospital, Darwin, NT, Australia; College of Medicine and Public Health, Flinders University, Adelaide, SA, Australia.
Conflicts of interest
All authors declare no conflicts of interest for this study.
Research conducted at
Respiratory and Sleep Medicine, Royal Darwin Hospital, Darwin, Northern Territory, Australia and Darwin Respiratory and Sleep Health, Darwin Private Hospital, Darwin, Northern Territory, Australia. 0810.
Funding
Nil to declare
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
REFERENCES
- 1. Benjafield AV, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–698. doi: 10.1016/S2213-2600(19)30198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Punjabi NM, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6(8):e1000132. doi: 10.1371/journal.pmed.1000132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marshall NS, et al. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31(8):1079–1085 [PMC free article] [PubMed] [Google Scholar]
- 4. Schipper SBJ, et al. Sleep disorders in people with type 2 diabetes and associated health outcomes: a review of the literature. Diabetologia. 2021;64(11):2367–2377. doi: 10.1007/s00125-021-05541-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kapa S, et al. Sleep apnea and hypertension: interactions and implications for management. Hypertension. 2008;51(3):605–608. doi: 10.1161/HYPERTENSIONAHA.106.076190 [DOI] [PubMed] [Google Scholar]
- 6. Australian Bureau of Statistics. Estimates of Aboriginal and Torres Strait Islander Australians. Canberra: Australian Bureau of Statistics; 2016. [Google Scholar]
- 7. Vos T, et al. Burden of disease and injury in Aboriginal and Torres Strait Islander Peoples: the Indigenous health gap. Int J Epidemiol. 2009;38(2):470–477. doi: 10.1093/ije/dyn240 [DOI] [PubMed] [Google Scholar]
- 8. Hill K, et al. Excess Indigenous mortality: are Indigenous Australians more severely disadvantaged than other Indigenous populations? Int J Epidemiol. 2007;36(3):580–589. doi: 10.1093/ije/dym011 [DOI] [PubMed] [Google Scholar]
- 9. Australian Indigenous HealthInfoNet (2022). Overview of Aboriginal and Torres Strait Islander health status 2021. Perth: Australian Indigenous HealthInfoNet. [Google Scholar]
- 10. Bramley D, et al. Indigenous disparities in disease-specific mortality, a cross-country comparison: New Zealand, Australia, Canada, and the United States. N Z Med J. 2004;117(1207):U1215. [PubMed] [Google Scholar]
- 11. Andreasyan K, et al. Patterns of mortality in Indigenous adults in the Northern Territory, 1998-2003: are people living in more remote areas worse off? Med J Aust. 2009;190(6):307–311. doi: 10.5694/j.1326-5377.2009.tb02418.x [DOI] [PubMed] [Google Scholar]
- 12. Kruavit A, et al. Chronic respiratory disease in the regional and remote population of the northern territory top end: a perspective from the specialist respiratory outreach service. Aust J Rural Health. 2017;25:275–284. doi: 10.1111/ajr.12349 [DOI] [PubMed] [Google Scholar]
- 13. Heraganahally SS, et al. Chronic obstructive pulmonary disease in aboriginal patients of the northern territory of australia: a landscape perspective. Int J Chron Obstruct Pulmon Dis. 2019;14:2205–2217. doi: 10.2147/COPD.S213947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mehra S, et al. Bronchiectasis among Australian Aboriginal and Non-Aboriginal patients in the regional and remote population of the Northern Territory of Australia. Rural Remote Health. 2021;21(2):6390. doi: 10.22605/RRH6390 [DOI] [PubMed] [Google Scholar]
- 15. Heraganahally SS, et al. Chronic obstructive pulmonary disease with and without bronchiectasis in Aboriginal Australians – a comparative study. Int Med J. 2020;50(12):1505–1513. doi: 10.1111/imj.14718 [DOI] [PubMed] [Google Scholar]
- 16. Heraganahally SS, et al. Comparison of clinical manifestation among Australian Indigenous and non-indigenous patients presenting with pleural effusion. Intern Med J. 2022;52(7):1232–1241. doi: 10.1111/imj.15310 [DOI] [PubMed] [Google Scholar]
- 17. Heraganahally SS, et al. Comparison and outcomes of emergency department presentations with respiratory disorders among australian indigenous and non-indigenous patients. BMC Emerg Med. 2022;22:11. doi: 10.1186/s12873-022-00570-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heraganahally SS, et al. Utility and outcomes among indigenous and non-indigenous patients requiring domiciliary oxygen therapy in the regional and rural australian population. Aust J Rural Health. 2021;29(6):918–926. doi: 10.1111/ajr.12782 [DOI] [PubMed] [Google Scholar]
- 19. Blunden S, et al.; Australasian Sleep Association Indigenous Sleep Health Working Party. Australasian Sleep Association Indigenous Sleep Health Working Party. Sleep health and its implications in First Nation Australians: a systematic review. Lancet Reg Health West Pac. 2022;21:100386. doi: 10.1016/j.lanwpc.2022.100386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Woods CE, et al. Sleep disorders in Aboriginal and Torres Strait Islander people and residents of regional and remote Australia. J Clin Sleep Med. 2015;11(11):1263–1271. doi: 10.5664/jcsm.5182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mehra S, et al. Gender differences in the clinical and polysomnographic characteristics among australian aboriginal patients with obstructive sleep apnea. Nat Sci Sleep. 2020;12:593–602. doi: 10.2147/NSS.S258330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O’Connor C, et al. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161(5):1465–1472. doi: 10.1164/ajrccm.161.5.9904121 [DOI] [PubMed] [Google Scholar]
- 23. Heraganahally SS, et al. Sleep apnoea among Australian Aboriginal and Non- Aboriginal patients in the Northern Territory of Australia– a comparative study. Sleep. 2020;43(3). doi: 10.1093/sleep/zsz248 [DOI] [PubMed] [Google Scholar]
- 24. Heraganahally SS, et al. Obstructive sleep apnoea and cardiac disease among aboriginal patients in the northern territory of australia. Heart Lung Circ. 2021;30:S1443–9506(21)00044-5. doi: 10.1016/j.hlc.2021.01.007 [DOI] [PubMed] [Google Scholar]
- 25. Woods CE, et al. Obstructive sleep apnoea in adult indigenous populations in high-income countries: an integrative review. Sleep Breath. 2015;19(1):45–53. doi: 10.1007/s11325-014-1032-7 [DOI] [PubMed] [Google Scholar]
- 26. Dosman JA, et al. Obesity, sex, snoring and severity of osa in a first nation community in saskatchewan, Canada. Clocks Sleep. 2022;4(1):100–113. doi: 10.3390/clockssleep4010011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paine SJ, et al. Prevalence and consequences of insomnia in New Zealand: disparities between Maori and non-Maori. Aust N Z J Public Health. 2005;29(1):22–28. doi: 10.1111/j.1467-842x.2005.tb00743.x [DOI] [PubMed] [Google Scholar]
- 28. Paine SJ, et al. Racial discrimination and ethnic disparities in sleep disturbance: the 2002/03 New Zealand Health survey. Sleep. 2016;39(2):477–485. doi: 10.5665/sleep.5468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mihaere KM, et al. Obstructive sleep apnea in New Zealand adults: prevalence and risk factors among Māori and non-Māori. Sleep. 2009;32(7):949–956. doi: 10.1093/sleep/32.7.949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. La-Grappe D, et al. Sleep disorders among Aboriginal Australians with Machado-Joseph Disease: quantitative results from a multiple methods study to assess the experience of people living with the disease and their caregivers. Neurobiol Sleep Circadian Rhythms. 2022;12. doi: 10.1016/j.nbscr.2022.100075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Howarth TP, et al. Sleep quality and obstructive sleep apnoea in Indigenous and non-Indigenous Australian children. Sleep Med. 2022;98:68–78. doi: 10.1016/j.sleep.2022.06.014 [DOI] [PubMed] [Google Scholar]
- 32. Deacon-Crouch M, et al. Is sleep duration associated with overweight/obesity in Indigenous Australian adults? BMC Public Health. 2020;20:1229. doi: 10.1186/s12889-020-09287-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yiallourou SR, et al. Sleep quantity and quality and cardiometabolic risk factors in Indigenous Australians. J Sleep Res. 2021;30(2):e13067. doi: 10.1111/jsr.13067 [DOI] [PubMed] [Google Scholar]
- 34. Deacon-Crouch M, et al. Association between indigenous status and Body Mass Index (BMI) in Australian adults: does sleep duration affect the relationship? PLoS One. 2022;17(2):e0263233. doi: 10.1371/journal.pone.0263233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bakker JP, et al. Ethnic disparities in CPAP adherence in New Zealand: effects of socioeconomic status, health literacy and self-efficacy. Sleep. 2011;34:1595–1603. doi: 10.5665/sleep.1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Campbell A, et al. Ethnicity and socioeconomic status predict initial continuous positive airway pressure compliance in New Zealand adults with obstructive sleep apnoea. Intern Med J. 2012;42:e95–101. doi: 10.1111/j.1445-5994.2010.02360.x [DOI] [PubMed] [Google Scholar]
- 37. Balachandran JS, et al. A brief survey of patients’ first impression after CPAP titration predicts future CPAP adherence: a pilot study. J Clin Sleep Med. 2013;9(3):199–205. doi: 10.5664/jcsm.2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. The 2017-18 annual report for the Department of Health and the Health Services. Northern Territory Government 2018. Available from: department of Health website: www.health.nt.gov.au [Accessed October 2022].
- 39. Australian Bureau of Statistics. (2013a). Australian Statistical Geography Standard (ASGS): Volume 5—Remoteness Structure, July 2011. ABS cat. no. 1270.0.55.005. Canberra: Australian Bureau of Statistics. [Google Scholar]
- 40. Kushida CA, et al.; Positive Airway Pressure Titration Task ForcePositive Airway Pressure Titration Task Force. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157–171. [PMC free article] [PubMed] [Google Scholar]
- 41. National Health and Medical Research Council. Ethical conduct in research with Aboriginal and Torres Strait Islander Peoples and communities: guidelines for researchers and stakeholders. Canberra: Commonwealth of Australia; 2018. [Google Scholar]
- 42. Marchildon GP, et al. Exploring policy driven systemic inequities leading to differential access to care among Indigenous populations with obstructive sleep apnea in Canada. Int J Equity Health. 2015;14:148. doi: 10.1186/s12939-015-0279-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Woods C, et al. Barriers and enablers to successful uptake of Continuous Positive Airway Pressure (CPAP) treatment for obstructive sleep apnoea for aboriginal and torres strait islander people. J Sleep Disor: Treat Care. 2016;5:3. doi: 10.4172/2325-9639.1000179 [DOI] [Google Scholar]
- 44. Carrier J, et al. Sex differences in age-related changes in the sleep-wake cycle. Front Neuroendocrinol. 2017;47:66–85. doi: 10.1016/j.yfrne.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 45. Schubert J, et al. Prevalence and nature of lung function abnormalities among Indigenous Australians referred to specialist respiratory outreach clinics in the Northern Territory. Int Med J. 2019;49:217–224. doi: 10.1111/imj.14112 [DOI] [PubMed] [Google Scholar]
- 46. Heraganahally SS, et al. Critical analysis of spirometric patterns in correlation to chest computed tomography among adult Indigenous Australians with chronic airway diseases. Expert Rev Respir Med. 2021;15(9):1229–1238. doi: 10.1080/17476348.2021.1928496 [DOI] [PubMed] [Google Scholar]
- 47. Howarth TP, et al. Comparison of diffusing capacity of carbon monoxide (DLCO) and total lung capacity (TLC) between Indigenous Australians and Australian Caucasian adults. PLoS One. 2021;16(4):e0248900. doi: 10.1371/journal.pone.0248900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Heraganahally SS, et al. Sex differences in pulmonary function parameters among Indigenous Australians with and without chronic airway disease. PLoS One. 2022;17(2):e0263744. doi: 10.1371/journal.pone.0263744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Heraganahally SS, et al. Lung function parameters among Australian Aboriginal “Apparently Healthy” Adults: an Australian Caucasian and Global Lung Function Initiative (GLI-2012) various ethnic norms comparative study. Expert Rev Respir Med. 2020;23:1–11. doi: 10.1080/17476348.2021.1847649 [DOI] [PubMed] [Google Scholar]
- 50. Heraganahally S, et al. Implications of using the GLI-2012, GOLD and Australian COPD-X recommendations in assessing the severity of airflow limitation on spirometry among an Indigenous population with COPD: an Indigenous Australians perspective study. BMJ Open Respir Res. 2021;8:e001135. doi: 10.1136/bmjresp-2021-001135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Heraganahally SS, et al. The effects of inhaled airway directed pharmacotherapy on decline in lung function parameters among indigenous australian adults with and without underlying airway disease. Int J Chron Obstruct Pulmon Dis. 2021;16:2707–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sze DFL, et al. Differences in the spirometry parameters between indigenous and non-indigenous patients with COPD: a matched control study. Int J Chron Obstruct Pulmon Dis. 2022;17:869–881. doi: 10.2147/COPD.S361839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yalim SD, The impact of age, gender and body mass index on the polysomnography variables. J Turk Sleep Med. 2021;2:159–165. doi: 10.4274/jtsm.galenos.2021.47966 [DOI] [Google Scholar]
- 54. Slouka D, et al. Risk factors for failure of continuous positive airway pressure treatment in patients with obstructive sleep apnoea. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2018 Jun;162(2):134–138. doi: 10.5507/bp.2017.056 [DOI] [PubMed] [Google Scholar]
- 55. Glasser M, et al. Sleep Apnoea in older people. Breathe. 2011;3(7):250–256. doi: 10.1183/20734735.021910 [DOI] [Google Scholar]
- 56. Heraganahally SS, et al. Outcome of public hospital-funded continuous positive airway therapy device for patients with obstructive sleep apnoea: an australian perspective study. Sleep Vigilance. 2020;4:195–204. doi: 10.1007/s41782-020-00114-4 [DOI] [Google Scholar]
- 57. Pal A, et al. COPD disease knowledge, self-awareness and reasons for hospital presentations among a predominately Indigenous Australian cohort – a study to explore preventable hospitalization. BMJ Open Resp Res. 2022;9:e001295. doi: 10.1136/bmjresp-2022-001295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Flemons WW, et al. Access to diagnosis and treatment of patients with suspected sleep apnea. pulmonary perspective. Am J Respir Crit Care Med. 2004;169:668–672. doi: 10.1164/rccm.200308-1124PP [DOI] [PubMed] [Google Scholar]
- 59. Heraganahally SS, et al. Obstructive sleep apnoea and adherence to continuous positive airway therapy among Australian women. Intern Med J. 2022;52(3):440–450. doi: 10.1111/imj.15076 [DOI] [PubMed] [Google Scholar]
- 60. Garg H, et al. Positional sleep apnea among regional and remote australian population and simulated positional treatment effects. Nat Sci Sleep. 2020;12:1123–1135. doi: 10.2147/NSS.S286403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Heraganahally SS, et al. Validity of the New “Top End Sleepiness Scale” (TESS) against the STOP-Bang Tool in Predicting Obstructive Sleep Apnoea among Indigenous Australian Adults. Int Med J. 2021. doi: 10.1111/imj.15633. [DOI] [PubMed] [Google Scholar]
- 62. Benn E, et al. The Top End Sleepiness Scale (TESS): a new tool to assess subjective daytime sleepiness among indigenous australian adults. Nat Sci Sleep. 2021;13:315–328. doi: 10.2147/NSS.S298409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gracey M. Why closing the Aboriginal health gap is so elusive. Intern Med J. 2014;44(11):1141–1143. doi: 10.1111/imj.12577 [DOI] [PubMed] [Google Scholar]
- 64. Jones MP, et al. Effects of turnover and stability of health staff on quality of care in remote communities of the Northern Territory, Australia: a retrospective cohort study. BMJ Open. 2021;11(10):e055635e055635. doi: 10.1136/bmjopen-2021-055635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Heraganahally SS, et al. A cost-effective novel innovative box (C-Box) to prevent cockroach infestation of continuous positive airway pressure equipment: a Unique Problem in Northern Tropical Australia. Am J Trop Med Hyg. 2019;101(4):937–940. doi: 10.4269/ajtmh.19-0434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Heraganahally SS, et al. Chest computed tomography findings among adult Indigenous Australians in the Northern Territory of Australia. J Med Imaging Radiat Oncol. 2022;66(3):337–344. doi: 10.1111/1754-9485.13295 [DOI] [PubMed] [Google Scholar]
- 67. Seyedshahabedin MM, et al. Flexible bronchoscopy indications and outcomes between Indigenous and non-Indigenous patients in the Northern Territory of Australia. Int Med J. 2022. doi: 10.1111/imj.15865 [DOI] [PubMed] [Google Scholar]
- 68. Heraganahally SS, et al. The prevalence of bronchodilator responsiveness “asthma” among adult indigenous Australians referred for lung function testing in the top end northern territory of Australia. J Asthma Allergy. 2022;15:1305–1319. doi: 10.2147/JAA.S376213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Knauert M, et al. Clinical consequences and economic costs of untreated obstructive sleep apnea syndrome. World J Otorhinolaryngol Head Neck Surg. 2015;1(1):17–27. doi: 10.1016/j.wjorl.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kendzerska T, et al. Untreated obstructive sleep apnea and the risk for serious long-term adverse outcomes: a systematic review. Sleep Med Rev. 2014;18:49–59. doi: 10.1016/j.smrv.2013.01.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.