Abstract
Introduction
Early detection of depression is a cost-effective way to prevent adverse outcomes on brain physiology, cognition, and health. Here we propose that loneliness and social adaptation are key factors that can anticipate depressive symptoms.
Methods
We analyzed data from two separate samples to evaluate the associations between loneliness, social adaptation, depressive symptoms, and their neural correlates.
Results
For both samples, hierarchical regression models on self-reported data showed that loneliness and social adaptation have negative and positive effects on depressive symptoms. Moreover, social adaptation reduces the impact of loneliness on depressive symptoms. Structural connectivity analysis showed that depressive symptoms, loneliness, and social adaptation share a common neural substrate. Furthermore, functional connectivity analysis demonstrated that only social adaptation was associated with connectivity in parietal areas.
Discussion
Altogether, our results suggest that loneliness is a strong risk factor for depressive symptoms while social adaptation acts as a buffer against the ill effects of loneliness. At the neuroanatomical level, loneliness and depression may affect the integrity of white matter structures known to be associated to emotion dysregulation and cognitive impairment. On the other hand, socio-adaptive processes may protect against the harmful effects of loneliness and depression. Structural and functional correlates of social adaptation could indicate a protective role through long and short-term effects, respectively. These findings may aid approaches to preserve brain health via social participation and adaptive social behavior.
Keywords: depressive symptoms, loneliness, social adaptation, brain health, neural correlates
1. Introduction
Depression is a potentially life-threatening disorder that affects hundreds of millions of people globally (World Health Organization, 2021; United Nations, 2022). It has well-known devastating effects on brain health, defined as the dynamic status of cognitive, emotional, and mental domains, affecting the integrity of brain structure and function (Chapman et al., 2021; Chen et al., 2022). Depressive symptomatology is often preceded by sub-clinical depression (SCD), a less severe yet debilitating expression of depressive symptoms (Zhang et al., 2022). SCD can often remain unidentified while negatively affecting brain physiology, cognition, and general health (Crockett et al., 2020). Thus, it is crucial to identify factors that can help prevent depressive symptoms and serve as early markers of permanent brain damage (Jiang et al., 2020; Zhang et al., 2022).
One early marker of depression is the experience of loneliness (Cacioppo et al., 2010; Erzen and Çikrikci, 2018), defined as emotional distress due to perceived isolation (Jeste et al., 2020). Loneliness is associated with increased depressive symptoms due to dysfunctional cognition, self-regulation (Tong et al., 2021; Steen et al., 2022), alterations in white matter (WM) structures associated with social cognition (Nakagawa et al., 2015) and cognitive control (Lam et al., 2021). However, the negative impact of loneliness can be minimized by social adaptation, defined as appropriate social functioning in line with social norms and expectations (Londono and McMillan, 2015; Neely-Prado et al., 2021; Schulte et al., 2022). Social adaptation can reduce depressive symptoms through increased social support (Berkman and Glass, 2000; Heinrichs et al., 2003; Cohen, 2020), enhanced self-esteem (Denissen et al., 2008; Joseph and De Guzman, 2021), exposure to stimulating environments (Jopp and Hertzog, 2010; Stevens et al., 2021), and enhancement of cognitive and brain reserve (Darwish et al., 2018; Zhao et al., 2020). Despite the above, little is known regarding the relationship between loneliness, social adaptation to sub-clinical depressive symptoms, and their association with brain pathophysiology.
Moreover, the experience of loneliness represents a decisive risk factor for depressive symptomatology and decreased brain health (Cacioppo et al., 2010; Erzen and Çikrikci, 2018). In a 5-year longitudinal study, it was found that loneliness predicted subsequent changes in depressive symptomatology but not vice versa (Cacioppo et al., 2010). The notion that loneliness can give rise to depressive symptoms is consistent with Beck’s cognitive model of depression (Beck, 2008; Disner et al., 2011). For example, loneliness is associated with low self-esteem and negative attributions about others and the world (Hawkley and Cacioppo, 2010; Cacioppo and Cacioppo, 2018). According to Beck (2008), such negative views about self, others, and the world are critical factors in the development and maintenance of depression (Disner et al., 2011; Jere et al., 2021; Marchetti and Pössel, 2022). In addition, negatively biased cognition and attention result in hypervigilance to threat and executive dysfunctions (Cacioppo, 2009; Hawkley and Cacioppo, 2010; Cacioppo et al., 2014), which are both considered characteristic depressive symptoms (De Zorzi et al., 2021). Loneliness also negatively effects mood via diminished social reward (Cacioppo et al., 2009). Diminished social reward ultimately reduces exposure to cognitively stimulating environments and participation in otherwise pleasurable activities, an essential aspect of depression (Cacioppo et al., 2014; Tan et al., 2020).
At a neurophysiological level, loneliness-related white matter (WM) alterations can help explain depressive symptomatology. A study found that loneliness (UCLA loneliness scale scores) in healthy individuals was associated with decreased WM density in crucial areas for social cognition and self-efficacy (i.e., insula, temporoparietal regions, prefrontal cortex) (Nakagawa et al., 2015). Decreased integrity in WM structures bridging these areas in depression is widely documented (Raikes et al., 2018; Vulser et al., 2018; Zhang et al., 2018; Shen et al., 2019; Liu et al., 2021). Additionally, loneliness is strongly associated with decreased connectivity of WM tracts between the insula and temporoparietal junction; these are critical nodes of the ventral attentional system (Tian et al., 2014). Attentional impairments within physiological and cognitive domains are significantly associated with depression (Keller et al., 2019). Despite the above, to our knowledge, there is no direct evidence of the relationship between brain structural correlates of loneliness and subclinical depressive symptoms.
Social adaptation can buffer against the impact of loneliness on depressive symptoms, thereby preserving brain health. Social adaptation encompasses various aspects of social functioning, such as interaction with others, interest in sociocultural activities, leisure time, and environmental mastery (Londono and McMillan, 2015; Neely-Prado et al., 2019; Schulte et al., 2022). These aspects may protect against depression-related impairments such as coping difficulties, affected cognition, negative mood, and helplessness. For example, contact with family and friends (social network) provides support resources that promote effective coping (Berkman and Glass, 2000; Cohen, 2004). Previous research shows that social support can foster brain health during depression, alleviating symptoms and enhancing self-efficacy (Panzarella et al., 2006; Alsubaie et al., 2019; Grey et al., 2020). Secondly, engagement in sociocultural activities and leisure time may protect against depressive symptoms by minimizing negative mood, preventing cognitive decline, and enhancing well-being (Fratiglioni et al., 2004; Jopp and Hertzog, 2010; Stevens et al., 2021). Lastly, manipulating the surrounding context to suit personal needs (an essential socio-adaptive process) allows control over depressive symptoms and recovery (Joseph and De Guzman, 2021).
From a neurocognitive perspective, social adaptation is sustained by social cognition and cognitive control processes. Social cognition is critical for interacting with others and navigating the social world (Frith and Frith, 2007, 2012; Gallotti et al., 2017), while cognitive control is critical to adjusting to the social context (Samadi and Sohrabi, 2016). Regarding brain structural connectivity, socio-cognitive processes, including face perception, empathy, and mentalizing (Wang et al., 2018), are associated with WM microstructure. For instance, for face perception, studies have shown that decreased microstructural integrity in the inferior longitudinal fasciculus (ILF) and inferior fronto-occipital fasciculus (IFOF) is associated with performance in face perception (Thomas et al., 2008; Tavor et al., 2014; Hodgetts et al., 2015) and emotion recognition tasks (Marstaller et al., 2016; Unger et al., 2016). Additionally, poor integrity in these tracts has been associated with low scores in empathy and mentalizing ability (Herbet et al., 2014; Parkinson and Wheatley, 2014). On the other hand, the integrity of the IFOF and IFL is also known to be linked to cognitive control processes such as attentional control (Leng et al., 2016; Sani et al., 2021), goal-directed behavior (Johnson et al., 2013; Conner et al., 2018), and emotion regulation (Liao et al., 2014; Graziano et al., 2022). Interestingly, emotion regulation processes have been consistently associated with the integrity of the arcuate fasciculus (Allan et al., 2016; David et al., 2020; Seyedmirzaei et al., 2022). For example, decreased integrity in the arcuate fasciculus is associated with aggression and anger problems in veterans (David et al., 2020). Another study showed that integrity of the arcuate fasciculus is positively correlated to performance in emotion regulation and alexithymia (inability to identify emotions) tasks, with those having better-preserved integrity showing better scores in the instruments (Seyedmirzaei et al., 2022).
Regarding brain activity, sociocognitive processes are known to be supported by parietal function (Bonini et al., 2022). Previous research shows that parietal areas are a crucial node involved in mentalizing ability and understanding the thoughts and actions of others (Lieberman, 2007; Uddin et al., 2007; Schurz et al., 2020). It is well known that activity in inferior parietal areas and the precuneus support psychological and social aspects of the mental self, such as self-referential judgments and self-appraisal (Uddin et al., 2007). Notably, an fMRI study showed that the precuneus is activated when making positive attributions about the self (Cabanis et al., 2013). This process implies a protective strategy to preserve and enhance a positive self-concept while deflecting negative feedback (Shepperd et al., 2008; Coleman, 2011). In brief, activity in the cuneus and parietal areas can support protective processes against depression. However, there is no empirical evidence about the role of parietal function in preventing and alleviating depressive symptomatology.
Despite the above, the behavioral and brain correlates by which loneliness and social adaptation explain depression are still unknown.
To cover this gap, this work aims to (1) evaluate the relationship between loneliness (socio-affective distress) and social adaptation (effective social functioning and engagement) on depressive symptoms; (2) assess the role of social adaptation against the ill effects of loneliness; and (3) examine how brain microstructural integrity and functional connectivity associated to lifestyle factors (loneliness and social adaptation) can explain depressive symptoms.
In line with previous evidence, we expect that (1) loneliness will be associated with increased severity of depressive symptoms while social adaptation will be associated with lower depressive symptoms; (2) social adaptation will decrease the magnitude of the relationship between loneliness and depressive symptoms; (3) the relationship between loneliness, social adaptation and depression is associated to brain white matter integrity and functional connectivity in areas sub serving socio-cognitive processes, cognitive control, and emotion regulation.
To test the relationship between loneliness, social adaptation and depressive symptoms, we analyzed data from an initial sample of 255 subjects who completed questionnaires tapping on each construct (loneliness, social adaptation and depressive symptoms). These initial results were validated on a second sample of 64 subjects (sample 2). Then, to assess the neurophysiological factors explaining the effects of loneliness and social adaptation on depressive symptoms, we analyzed brain scans (MRI and fMRI) belonging to sample 2.
Our findings can be of great use for interventions to enhance brain health through preventing and mitigating depression, increased engagement and social participation.
2. General protocol
Here we considered data from two separate samples from different studies before the pandemic. First, we considered self-report measures from a large sample (sample 1). To test the expected relationship between loneliness, social adaptation and depressive symptoms, we conducted a hierarchical multiple regression on self-report data. Then, we analyzed data from a smaller sample (sample 2) to evaluate the neurophysiological mechanism by which loneliness and social adaptation affect depressive symptoms factors; we analyzed structural (WM integrity) and FC correlates of self-report variables and their associations. Since subjects from sample 2 had also completed the same self-report instruments tapping on loneliness, social adaptation and depressive symptoms, we could replicate the hierarchical model conducted in the former sample (sample 1). All participants signed an informed consent according to the principles of the Declaration of Helsinki and received a payment as compensation for their collaboration and time. Below are the characteristics and specific procedures for each sample.
3. Sample 1
3.1. Procedure
Participants were contacted via telephone or social network media and invited to attend the laboratory to complete various scales tapping on depressive symptomatology, loneliness, and social adaptation.
The Universidad Diego Portales ethics committee approved every procedure of this research.
3.2. Participants
Two hundred and fifty-five participants between 18 and 46 years old [M = 31.11; SD = 8.16; female (n = 142)] were enrolled in this study. They had an average of 13 years of education (M = 13.62, SD = 3.08, range 2–23 years of education), equivalent to completed primary and secondary education, and no history of psychiatric or neurological conditions.
3.3. Self-report assessment
3.3.1. Beck depression inventory
The Beck depression inventory (BDI) is a 21-item scale that measures depressive symptoms and their severity. Each item comprises a set of 4 statements regarding the severity of a depressive symptom. The statements range from 0 (indicating low severity) to 3 (indicating high severity). Subjects must choose the statement (or statements) that best describe their state within the last week, including the day of evaluation. The total score lies between 0 and 63 and is obtained by adding the scores of all items. Here we used the Chilean adaptation of the BDI-IA (Valdés et al., 2017). This sample showed good reliability (α = 0.89). For a detailed account of the instrument (see Supplementary materials).
3.3.2. University of California loneliness scale
The University of California loneliness scale (UCLA) (Russell et al., 1980) is a widely used measure of the subject’s loneliness experience and satisfaction with social relationships. Here we used an abbreviated version of the UCLA (Hays and Dimatteo, 1987), comprising the following eight items: (1) “I lack companionship”; (2) “I feel left out”; (3) “I feel isolated from others”; (4) “I feel alone frequently” (5) “There is no one I can turn to”; (6) “I’m unhappy with being so withdrawn”; (7) “People are around me but not with me”; (8) “I am frequently in tune with people around.” Responses were recorded via a 4-point Likert scale, ranging from 1 (never) to 4 (always). The total score is obtained by inverting positive items (i.e., item 8) and summarizing the score of all items. Thus, larger scores indicate a more pronounced experience of loneliness. UCLA has shown good levels of reliability, as evidenced by an α coefficient of 0.89 (Russell et al., 1980). We used a shorter 8-item version that showed good reliability levels in our sample (α =0.84).
3.3.3. Social adaptation self-administered scale
The Social adaptation self-administered scale (SASS) (Bosc et al., 1997) is a 21-item scale that explores various aspects of social functioning, such as social ties, family relationships, work and leisure time, control of the environment, and sociocultural interest. The following are examples of items tapping on each aspect: social ties (i.e., “What value do you attach to the relationship with others?”); family relationships (i.e., “How frequently do you seek contact with your family members); control of the environment (i.e., “Do you feel able to organize your environment according to your wishes and needs?”); engagement to work (“How interested are you in your occupation?”), and leisure time (i.e., “Are you interested in hobbies and leisure activities?”). Responses are recorded via a 4-point Likert scale (from 0 to 3). The total score ranges from 0 to 60, corresponding to minimal and maximal social adjustment. Scores between 35 and 52 are considered normal (Bosc et al., 1997). The instrument showed good reliability in our sample (α = 0.73).
3.4. Data analyses
3.4.1. Self-report data
Descriptive data analysis for loneliness, social adaptation, and depressive symptomatology are displayed in Table 1. We also conducted correlation analyses, including sociodemographic data and variables of interest (see Supplementary Table S1).
Table 1.
Performance in self-report scales for Sample 1.
| N = 255 (142 female, 113 male) | |||
|---|---|---|---|
| Mean | SD | Range | |
| BDI | 8.67 | 8.17 | 0–45 |
| UCLA | 16.12 | 4.91 | 1–32 |
| SASS | 42.61 | 6.47 | 21–56 |
BDI, Beck Depression inventory; UCLA, University of California loneliness scale; SASS, Social adaptation Self-evaluation scale; IFS, INECO Frontal Screening.
Behavioral data were analyzed with R studio (R core team, 2020). We first conducted a hierarchical multiple regression to evaluate the effects of loneliness and social adaptation on depressive symptomatology. Hierarchical multiple regression allows the evaluation of the predictive value of different groups of independent variables entered at additional steps of the analysis while controlling for variables entered in previous steps (Petrocelli, 2003). This technique is also helpful to determine (how or if) newly added variables interact with previously added variables and improve the explained variance of the model. As for the present analysis, we first specified a base model including our control variables (age, gender, and years of education). We expected that these variables would not have any significant effect on depressive symptoms (BDI score). In a subsequent step, our measure of loneliness (UCLA score) was incorporated into the variables. For the last step, we specified a model that included the social adaptation measure (SASS score).
3.5. Results
3.5.1. Self-report measures
3.5.1.1. Hierarchical multiple regression
In the first step, we entered sociodemographic control variables. Results showed that age (β = 0.13, p = 0.41), gender (β = −0.19, p = 0.16), and years of education (β = −0.13, p = 0.42) did not have significant effects on depressive symptoms (BDI scores) (see Table 2). In the second step, loneliness (UCLA score) was added to the existing predictors. Results show that after controlling for age, gender, and years of education, only loneliness (β = 0.63, p < 0.001) emerged as a significant predictor of depressive symptomatology (BDI score) [F(4, 50) = 10.07, p < 0.001]. We entered the social adaptation measure (SASS scores) in the third step. This model revealed that after controlling for non-interest variables, both loneliness (β = 0.48, p < 0.001) and social adaptation (β = −0.26, p = 0.04) significantly predicted depressive symptomatology, positively and negatively, respectively [F(5, 49) = 9.43, p < 0.001]. It is important to note that after including social adaptation, the effect of loneliness decreases by 0.14 standardized units compared to the previous step (see Table 2). Further, after comparing R2 between the second and third steps, the latter explains 4% more variance (and nearly 50% of the total explained variance) associated with depressive symptoms. As stated above, we confirmed that associations between the variables of interest remained significant after controlling for age, gender, and years of education.
Table 2.
Multiple hierarchical regression output for Sample 1.
| Demographic | Loneliness | Social adaptation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Standardized Beta | Standardized CI | p | Standardized Beta | Standardized CI | p | Standardized Beta | Standardized CI | p | |
| Age | 0.13 | −0.18 to 0.44 | 0.411 | 0.04 | −0.21 to 0.29 | 0.752 | 0.08 | −0.17 to 0.32 | 0.531 |
| Gender | −0.19 | −0.46 to 0.08 | 0.166 | −0.44 | −0.23 to 0.22 | 0.980 | 0.00 | −0.21 to 0.22 | 0.988 |
| Years of education | −0.13 | −0.44 to 0.19 | 0.421 | −0.21 | −0.46 to 0.04 | 0.093 | −0-17 | −0.42 to 0.08 | 0.170 |
| Loneliness | 0.63 | 0.41–0.85 | <0.001 | 0.48 | 0.22–0.74 | <0.001 | |||
| Social adaptation | −0.26 | −0.51 to 0.01 | 0.044 | ||||||
| R2/R2 adjusted | 0.09/0.03 | 0.45/0.40 | 0.49/0.44 | ||||||
4. Sample 2
Data from this sample was used to (a) replicate the hierarchical model conducted in the previous sample; (b) and assess the neurophysiological factors associated with the observed association between the interest variables.
Testing took place on two separate occasions. On day 1, participants were invited to attend the laboratory to complete various scales tapping on depressive symptomatology, loneliness, and social adaptation. On day 2, participants attended an fMRI scanning session. Images were obtained using a Siemens Avanto 1.5 T scanner with a standard head coil.
The Universidad Diego Portales ethics committee approved every procedure of this research.
4.1. Participants
The sample consisted of Sixty-four healthy participants between 20 and 73 years old [M = 36.88; SD = 13.62; female (n = 38)]. Subjects were recruited by accessibility from the general population. They have an average of 11 years of education (M = 11.1, SD = 3.09, range 2–20 years), equivalent to completed primary and incomplete secondary education. The mean educational level of this sample is reflective of the Chilean population, as noted in the latest UN Human Development Report (United Nations Development Programme, 2022). Subjects had no history of psychiatric or neurological conditions.
All participants were screened for executive dysfunction as assessed by the INECO frontal screening (Torralva et al., 2009). The mean score for our sample was 20.92 points, just above the 18-point cut-off for the Chilean population (Ihnen et al., 2013).
4.2. Self-report assessment
As in study 1, depressive symptoms were assessed with the Beck depression inventory (BDI; 0.83); loneliness was measured with the UCLA loneliness scale (α = 0.85); and Social adaptation was measured with the Social adaptation self-administered scale (SASS, α = 0.70).
Descriptive data analysis for our variables of interest is displayed in Table 3. We also conducted correlation analyses, including sociodemographic data and variables of interest (see Supplementary Table S2).
Table 3.
Sample performance in self-report scales for Sample 2.
| N = 64 (37 female) | |||
|---|---|---|---|
| Mean | SD | Range | |
| BDI | 9.37 | 7.05 | 0–31 |
| UCLA | 8.65 | 5.27 | 0–24 |
| SASS | 42.27 | 7.12 | 27–55 |
4.3. Images data collection
Sixty-two subjects were recorded using MRI (Siemens Avanto, 1.5 T). The following parameters were applied in the T1 registers: TR = 1820 ms, TE = 3 ms, flip angle = 15 degrees, voxel size = 1 mm3, and the number of slices = 144. Instead, the recording parameters of resting state functional MRI (rfMRI) were TR = 3,300 ms, TE = 50 ms, flip angle = 90 degrees, voxel size = 3 × 3 × 3.75 mm3, number of slices = 36, number of volumes = 190. Diffusion weight images (DWI) were registered only in 60 subjects with the following parameters: TR = 5,600 ms, TE = 97 ms, flip angle = 90 degrees, in-plane resolution = 2 mm, slice thickness = 2 mm, b-value = 1,000 s/mm2 and 36 diffusion sampling directions.
4.3.1. DWI: Preprocessing and analysis
DWI was preprocessed using the qsiprep (version 0.15.4) standard pipeline (Cieslak et al., 2021) to improve replicability and robustness.1 DSI Studio (v2022.08.03)2 was implemented for structural connectivity analysis. The data was reconstructed using q-space diffeomorphic reconstruction in the MNI coordinates (Yeh and Tseng, 2011). The output resolution and diffusion sampling length ratio were set to 2 mm and 1.25, respectively. Then, tensor metrics were calculated to quantify the mean diffusivity MD, and overall mean squared displacement of the water molecules (Hecke et al., 2016). The MD index is related to the integrity of the WM tracts. Loss of integrity is represented by an increase in MD, where MD changes can precede the alterations of the gray matter volume (Suri et al., 2014). This index has been previously correlated with neuro-plastic changes induced by behavioral tasks (Nakagawa et al., 2016; Takeuchi et al., 2017; Takeuchi and Kawashima, 2018). The rest of the DTI indexes (i.e., fractional anisotropy, axial diffusivity and radial diffusivity) are linked to damage in white matter tracts (Song et al., 2003, 2005; Budde et al., 2007; Chang et al., 2017) which is not necessarily the case in our sample of healthy subjects. Thus, the MD metric may be more sensitive to subtle neuro-plastic changes for our sample. In addition, unlike other DTI indexes, MD is independent of the number of diffusion gradients (Giannelli et al., 2010). Despite the advantages of the MD index for our objectives, we also analyzed FA, AD and RD metrics (refer to Supplementary Figures S2–S4). A deterministic fiber tracking algorithm (Yeh et al., 2013) and a nonparametric Spearman partial correlation were used to individually relate the MD index and the variables of interest, BDI, UCLA, and SASS, respectively, through diffusion MRI connectometry (Yeh et al., 2016). Additionally, to investigate the common neural substrate between these variables in the 62 subjects with MRI, a principal component analysis (PCA) was used to join their variances. To this end, the three variables were scaled, and the loading of the first dimension (PCA1) by subject was correlated with MD. All analyses were corrected for age, sex, and years of education using a multiple regression model. The seeding region comprised the whole brain, excluding the cerebellum, and 10.000 seeds were distributed randomly. False connections were removed with topology-informed pruning (Yeh et al., 2019) with four iterations; for tract selections, a t-score threshold of 2.5 was set. Additionally, p values were corrected by the false discovery (FDR), calculated through 4,000 randomized permutations to obtain the null distribution of the track length. Thus, only tracts with p < 0.05 FDR corrected were selected. Diffusion connectometry was based on the human structural connectome (Yeh et al., 2018) to delimit the tracts.
4.3.2. rfMRI: Preprocessing and analysis
To improve replicability and robustness, the preprocessing of rfMRI was done using the fmriprep (version 22.0.2) standard pipeline (Esteban et al., 2019). In this algorithm, the head motion was estimated across full-time series, treated as a confound and expanded with the inclusion of temporal derivatives and quadratic terms (Satterthwaite et al., 2013). Then, framewise displacement was calculated as the absolute sum of relative motions (Jenkinson et al., 2002; Power et al., 2014). Motion outlier was fixed to a framewise displacement that exceeded a threshold of 0.5 mm, but none of the subjects in this study exceeded it. Furthermore, smoothing (with a gaussian kernel of 6x6x6 mm), denoising (linear regression of confounding effects of WM, cerebrospinal fluid, realignment, and scrubbing), band-pass filtering (0.001–0.009 Hz), and functional connectivity analysis were implemented in the CONN 20.b toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012) running on MATLAB R2018b. We independently associated the variables of interest (BDI, UCLA, SASS, and PCA1) with global correlation at the voxel level, which represents the average correlation coefficient between each voxel and all other voxels in the brain (Nieto-Castanon, 2020). All analyses were corrected for age, sex, and years of education using a multiple regression model. The multiple comparison problem was solved through the Gaussian random field theory (Worsley et al., 1996), which set the cluster size threshold at p < 0.05 FDR corrected and the voxel threshold at p < 0.001 uncorrected.
4.4. Results
4.4.1. Self-report measures
4.4.1.1. Hierarchical multiple regression
Hierarchical regression results are very similar to those from sample 2. In the first step, including only sociodemographic control variables results showed that age (β = −0.05, p = 0.45), gender (β = −0.06, p = 0.35), and years of education (β = −0.07, p = 0.30) did not have significant effects on depressive symptomatology (BDI scores, see Table 3). In the second step, loneliness (UCLA score) was added to de existing predictors. Results show that after controlling for non-interest variables, only loneliness (β = 0.50, p < 0.001) emerged as a significant predictor of depressive symptomatology (BDI score) [F(4, 249) = 21.74, p < 0.001]. We entered the social adaptation measure (SASS scores) in the third step. This model revealed that after controlling for non-interest variables, both loneliness (β = 0.31, p < 0.001) and social adaptation (β = −0.41, p < 0.001) significantly predicted depressive symptomatology positively and negatively, respectively, [F(5, 248) = 30.05, p < 0.001]. It is important to note that after including social adaptation, the effect of loneliness decreases by 0.19 standardized units compared to the previous step (see Table 4). Furthermore, after comparing R2 between the second and third steps, the latter explains 12% more variance associated with depressive symptomatology (38% explained variance). As stated above, we confirmed that associations between the variables of interest remained significant after controlling for age, gender, and years of education.
Table 4.
Multiple hierarchical regression output for Sample 2.
| Demographic | Loneliness | Social adaptation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Standardized Beta | Standardized CI | p | Standardized Beta | Standardized CI | p | Standardized Beta | Standardized CI | p | |
| Age | −0.05 | −0.18 to 0.08 | 0.455 | −0.06 | −0.17 to 0.05 | 0.287 | −0.05 | −0.15 to 0.05 | 0.335 |
| Gender | −0.06 | −0.18 to 0.07 | 0.353 | −0.05 | −0.16 to 0.06 | 0.353 | −0.05 | −0.14 to 0.05 | 0.365 |
| Years of education | −0.07 | −0.18 to 0.06 | 0.309 | −0.06 | −0.17 to 0.05 | 0.291 | 0.03 | −0.08 to 0.13 | 0.604 |
| Loneliness | 0.50 | 0.39–0.61 | <0.001 | 0.31 | 0.19–0.42 | <0.001 | |||
| Social adaptation | −0.41 | −0.52 to -0.29 | <0.001 | ||||||
| R2/R2 adjusted | 0.01/−0.00 | 0.26/0.25 | 0.38/0.36 | ||||||
4.4.1.2. Structural connectivity (DWI)
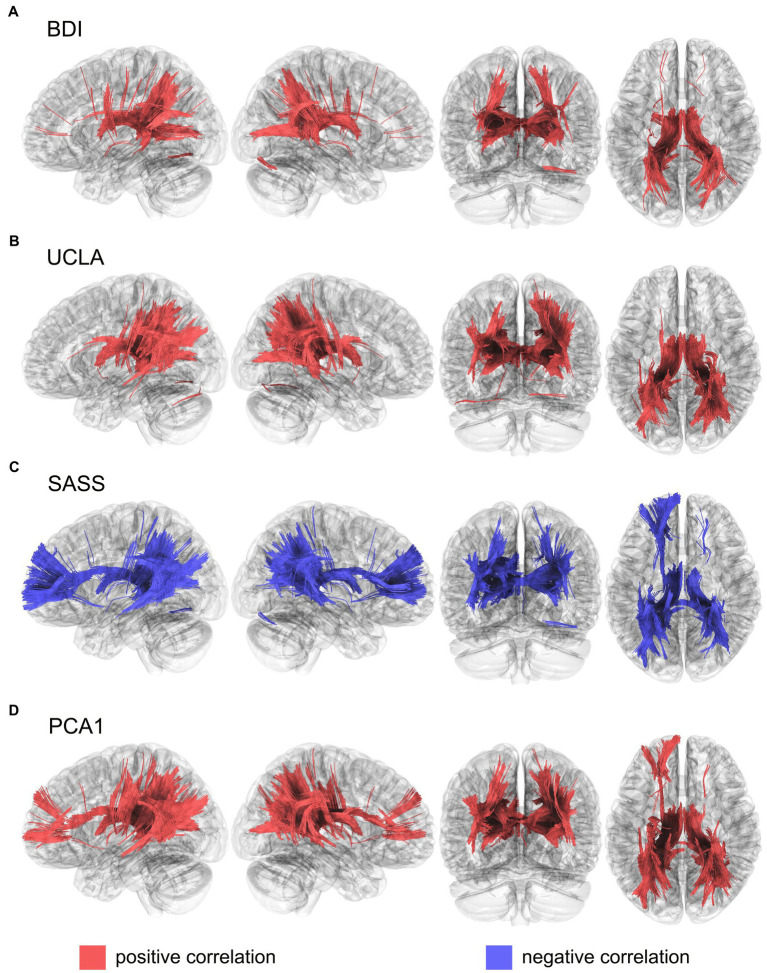
The BDI scores were positively correlated with the MD values of the fornix, corpus callosum, thalamic radiation superior and posterior, in both hemispheres, and the right segment of the inferior fronto-occipital fasciculus (Figure 1A). While UCLA scores were positively correlated with de MD index with the fornix, thalamic radiation superior and posterior, and corpus callosum in both hemispheres, and the right tracts of the inferior fronto-occipital, middle longitudinal, and corticopontine occipital tracts (Figure 1B). Instead, SASS showed a negative correlation in the tracts of the fornix, thalamic radiation superior, anterior, and posterior, corpus callosum, cortical tract posterior and inferior fronto-occipital fasciculus in both hemispheres, like so the left inferior longitudinal fasciculus, the left cingulum frontoparietal, and the left arcuate fasciculus (Figure 1C). To evaluate the common neural substrates for these three variables, we grouped their variance through the first dimension of a PCA (PCA1), which was able to explain 70.9% of the total variance (see Supplementary Figure S1). This PCA points to the different directionality of the interest variables, where BDI and UCLA were positively correlated, but SASS presented a negative correlation with both. Moreover, the PCA1 was positively correlated with the MD index of the fornix, thalamic radiation superior, anterior and posterior, corpus callosum, inferior fronto-occipital fasciculus in both hemispheres, and the left frontoparietal cingulum, left inferior longitudinal fasciculus, right arcuate fasciculus, right corticopontine occipital tract, and the right corticostriatal posterior tract (Figure 1D). Fractional anisotropy (FA), axial diffusivity (AD) and radial diffusivity (RD) showed similar patterns of results, although these appeared less pronounced (Supplementary Figures S2–S4).
Figure 1.
Structural connectivity correlates of (A). BDI, (B). UCLA, (C). SASS and (D). PCA1, calculated using mean diffusivity index from a diffusion tensor imaging model. Results were corrected with a p < 0.05 FDR. BDI, Beck depression inventory; SASS, social adaptation self-administered scale; UCLA, University of California loneliness scale; PCA1, first component from a principal component analysis that includes BDI, UCLA and SASS.
4.4.1.3. Functional connectivity
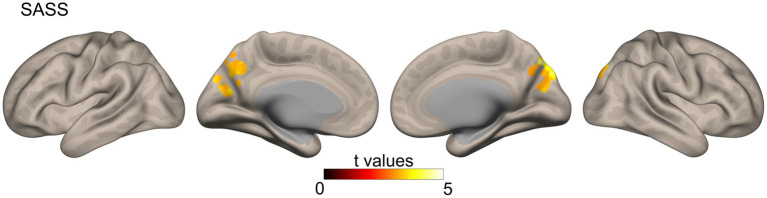
Similar to the correlational analysis performed on structural connectivity data, we analyzed the correlations between our variables of interest (BDI, UCLA, and SASS) and the functional connectivity data. However, only the SASS scores showed significant results in areas of the precuneus, cuneus, and lateral occipital cortex for both hemispheres (Figure 2).
Figure 2.
Functional connectivity correlates of SASS were calculated from a resting-state functional MRI sequence using a global correlation (connectivity between a specific voxel and the rest of the brain, measured through the average of their Pearson correlation coefficient r). Results were corrected with Gaussian random field theory (cluster size threshold: p < 0.05 FDR corrected, voxel threshold at p < 0.001 uncorrected). SASS, social adaptation self-administered scale.
5. Discussion
Here we used data from two separate studies to evaluate the effects of loneliness and social adaptation on depressive symptoms from a behavioral and neurobiological perspective. In sample 1, a hierarchical regression model confirmed that loneliness (UCLA scores) negatively affected depressive symptoms (BDI scores). The model also showed that the effects of loneliness decreased after including the social adaptation measure, which positively affected depressive symptoms. In sample 2, we replicated our hierarchical regression model, which yielded the same pattern of assocican reveal dynamic plastic adaptations to repeated stimuluastions. We then conducted DTI analysis on imaging data from subjects in sample 2. Results revealed that loneliness, social adaptation, and depressive symptoms shared an overlapping WM architecture. The structures comprising this typical architecture are known to support social cognition (Herbet et al., 2014; Parkinson and Wheatley, 2014), emotion regulation (Allan et al., 2016; Vulser et al., 2018; David et al., 2020), and cognitive control (Noble et al., 2013; Herbet et al., 2018; Li et al., 2018). After conducting a functional connectivity analysis, only social adaptation was associated with functional connectivity in crucial areas for social cognition. Our findings may contribute to the understanding of the association between these variables and brain health.
Our finding that loneliness is associated with increased depressive symptoms is consistent with previous research on the long-term effect of loneliness on depression. It is known that loneliness can increase the likelihood of suffering from depressive symptomatology through low social reward, negative mood, distorted cognitive schemas, and attentional dysfunctions (Cacioppo et al., 2010; Erzen and Çikrikci, 2018). First, the diminished social reward experienced in loneliness can limit social engagement and participation in cognitively stimulating and mood-lifting activities, thereby increasing negative mood and depressive symptoms (Cacioppo et al., 2014; Tan et al., 2020). Second, it is well known that loneliness is associated with low self-esteem and negative views about others and the world (Cacioppo, 2009; Hawkley et al., 2009). Negative beliefs about the self, others and the world are key risk factors for developing and maintaining depression (Beck, 2008; Disner et al., 2011). Such a negatively biased cognition is known to underlie the attentional and executive dysfunctions typically exhibited by lonely people (Cacioppo, 2009; Hawkley and Cacioppo, 2010; Cacioppo et al., 2014) and those suffering from depressive symptoms (De Zorzi et al., 2021). Our results suggest that loneliness may represent a risk factor warning about depressive symptoms and potential progression into more severe manifestation and impairment.
On the contrary, social adaptation or appropriate social functioning can offer a range of protective factors against depressive symptoms. Specifically, interaction with others lowers emotional distress, promotes reappraisal, and facilitates overall coping (Berkman and Glass, 2000; Hostinar and Gunnar, 2015; Szkody and McKinney, 2020). Additionally, engagement in sociocultural and leisure activities may improve mood, increase behavioral activation, and prevent maladaptive cognitive patterns. Consistent with the above, we found that (1) social adaptation (adaptive social functioning) was associated with lower depressive symptoms: and (2) that social adaptation weakened the association between loneliness and depression. These results suggest that socio-adaptive behavior mitigates depressive symptoms through enhanced coping ability, improving mood, promoting stimulating activities, and preventing social isolation. These processes may also explain the protective role of social adaptation against the ill effect of loneliness. It is also important to note that the association pattern between our variables was replicated in sample 2, which strengthens the validity of our results. Therefore, our findings provide robust evidence about the role of socio-adaptative skills as a buffer against loneliness and depressive symptomatology.
Regarding our neurophysiological findings, the WM correlates of depressive symptoms reported here are in line with previous studies showing that depression is related to poor WM integrity via increased MD values in the fornix, thalamic radiation, corpus callosum, and inferior fronto-occipital fasciculus (Raikes et al., 2018; Vulser et al., 2018; Shen et al., 2019). In the case of loneliness, our outcomes were in line with previous evidence of the association between UCLA scores and decreased WM (high MD values) in the fornix, superior and posterior thalamic radiation, corpus callosum, right inferior fronto-occipital fasciculus, middle longitudinal, and corticopontine occipital tracts (Tian et al., 2014; Nakagawa et al., 2015; Costanzo et al., 2022). Integrity in these fibers has been previously associated with attentional control (Tian et al., 2014) and socio-cognitive (Nakagawa et al., 2015) deficits in loneliness. As for social adaptation, results showed that performance in SASS was associated with preserved WM integrity (via low MD values) in tracts of the fornix, thalamic radiations (superior, anterior, and posterior), corpus callosum, posterior cortical tract, inferior fronto-occipital fasciculus, left inferior longitudinal fasciculus, left frontoparietal cingulum, and the left arcuate fasciculus. These tracts are known to support cognitive control (Noble et al., 2013; Herbet et al., 2018; Li et al., 2018) and socio-cognitive abilities (Thomas et al., 2008; Herbet et al., 2014; Parkinson and Wheatley, 2014; Tavor et al., 2014; Hodgetts et al., 2015; Marstaller et al., 2016; Unger et al., 2016). Cognitive control and social cognition are core aspects of social adaptation (Huepe et al., 2011; Samadi and Sohrabi, 2016; Neely-Prado et al., 2019). This is consistent with recent conceptualizations of social adaptation as a dynamic process encompassing the accommodation own thoughts and behaviors based on social norms and expectations (Londono and McMillan, 2015; Neely-Prado et al., 2021; Schulte et al., 2022).
Social adaptation also showed associations with functional connectivity. Specifically, SASS scores were positively associated with functional connectivity in the precuneus, cuneus, and lateral occipital cortices. These areas are critical nodes in the fronto-parietal mentalizing network (Lieberman, 2007; Uddin et al., 2007; Schurz et al., 2020) and are also crucial for self-appraisal processes (Uddin et al., 2007). Moreover, the function of the precuneus has been associated with the self-serving bias (Cabanis et al., 2013), a protective strategy for enhancing self-image (Shepperd et al., 2008; Coleman, 2011). One possible interpretation for the effects of protective strategy is that it serves as a “kickstart” adaptive process that will change brain structure and behavior. It is well known that functional connectivity can reveal dynamic plastic adaptations to repeated stimulus and behavior (i.e., experience-dependent plasticity) that could later develop into more stable structural changes (Ma et al., 2011; Urner et al., 2013; Guerra-Carrillo et al., 2014; Lea-Carnall et al., 2017). For example, studies have shown that training for motor skills or visual learning is associated with the increased strength of resting state functional connectivity relative to baseline (Ma et al., 2011; Urner et al., 2013). Following this line of thought, it is reasonable to think that the protective effects of social adaptation via functional processes (i.e., self-serving strategy) might kickstart functional and structural changes that can prevent and even override maladaptive cognition, behavior and brain health. Moreover, just as it happens with learning, depending on the frequency of stimulation (or repetition of conduct), these initial dynamic neuro-plastic adaptations can develop into more stable structural adaptations (i.e., WM integrity above). In other words, the initial neuroplastic changes at the functional level might foment longer-term protective adaptations that can persist for months or years (Islam et al., 2020; Wilde et al., 2021).
After grouping depressive symptoms, loneliness, and social adaptation into a single component reflecting their shared variance, we found that they were associated with a common WM substrate. This common substrate included the fornix, thalamic radiations (superior, anterior, and posterior), corpus callosum, inferior fronto-occipital fasciculus, left fronto-parietal cingulum, left Inferior longitudinal fasciculus, right arcuate fasciculus, right corticopontine occipital tract, and the right corticostriatal posterior tract. We found that integrity in these tracts was negatively associated with depressive symptoms and loneliness but positively associated with social adaptation. Previous research has shown that integrity within these tracts is associated with various processes, including social cognition (Herbet et al., 2014; Marstaller et al., 2016; Unger et al., 2016; Nakajima et al., 2018; Wang et al., 2018) executive functioning (Tian et al., 2014; Li et al., 2018; Zhang et al., 2022), emotion regulation (Liao et al., 2014; Allan et al., 2016; David et al., 2020; Graziano et al., 2022; Seyedmirzaei et al., 2022), attentional control (Johnson et al., 2013; Conner et al., 2018), and goal-directed behavior (Johnson et al., 2013; Conner et al., 2018). We suggest that reduced WM integrity associated with loneliness and depressive symptoms might reflect multi-domain deficits, including emotion dysregulation, attentional impairments, poor cognitive control, and socio-cognitive abilities. This kind of deficit has been associated with depressive psychopathology. Conversely, stronger structural connectivity related to social adaptation may suggest that proficient performance in auto-regulatory (Noble et al., 2013; Herbet et al., 2018; Li et al., 2018) and socio-cognitive (Thomas et al., 2008; Herbet et al., 2014; Parkinson and Wheatley, 2014; Tavor et al., 2014; Hodgetts et al., 2015; Marstaller et al., 2016; Unger et al., 2016) may counteract the maladaptive effects of loneliness and minimize depressive symptoms.
6. Conclusion
Depressive symptomatology is a highly prevalent illness with profound effects on brain health (Jesulola et al., 2018; Liew, 2019; Spellman and Liston, 2020). However, most research on depression has focused on genetic markers of the illness (Jesulola et al., 2018), while social and behavioral factors have received far less attention. Loneliness and social adaptability are potential early markers for depression due to their behavioral and neural associations with SCD. Furthermore, loneliness contributes to the long-term appearance of depressive symptoms. Instead, social adaptation may modulate the relationship between loneliness and depression symptoms by reducing their effects. Thus, this socio-adaptive mechanism may involve cognitive control, strategies to augment self-image, and improved social cognition in the long and short term. These processes might benefit brain health above and beyond reducing depressive symptomatology. Brain health is a complex concept encompassing aspects of the individual (i.e., brain physiology, cognitive function, mental health, socio-cognitive abilities) and contextual level (social engagement, environmental mastery) (Chapman et al., 2021; Chen et al., 2022). Our findings can serve as a basis for socio-adaptive-based interventions to promote brain health. These interventions can prevent and help manage the severe impact of depression on cognitive and brain integrity by actively fomenting socio-adaptive factors such as social connectedness, social participation, a sense of belonging, and control of the environment. Modification of negative schemas, facilitation of interaction-rich environments, monitoring advances in social participation, and fomenting control of the environment are some ways in which “socio-adaptive” based interventions can help improve the health of individuals and communities. These interventions would be of high value, significantly, when isolation and mental health are highly compromised.
7. Limitations
Although we refer to the long and short-term effects of social adaptation, this interpretation should be taken with caution due to the cross-sectional nature of this study. Future research should evaluate the long-term effects of socio-adaptive processes.
We acknowledge that the range of age and education within variables could have been more homogenous. However, these variables had no significant effects on the outcome variable (or associations with predictor variables) nor affected the main effects. Further, in the case of educational level, both samples are representative of the Chilean population (United Nations Development Programme, 2022).
Here we made an interesting interpretation of the short and long-term protective effects of social adaptation via functional and structural neuro-plastic changes. While previous research supports these processes, these interpretations should be taken cautiously. Further research should empirically assess whether repetition of the self-enhancement protective strategy can induce initial functional connectivity neuro-plastic changes and if there is a posterior change in long-term structural changes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Comite de etica Universidad Diego Portales, Santiago, Chile. The patients/participants provided their written informed consent to participate in this study.
Author contributions
DF-O and DH conceived and designed the project. DF-O, DH, and RGG contributed analysis tools and performed the analysis. DF-O, DH, RGG, JPMS, and AI conducted data analysis and interpretation. DF-O, DH, RGG, JPMS, MV, and AI participated in the drafting of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
David Huepe was supported by ANID/FONDECYT Regular (1201486, 1210195, 1210176, and 1220995). Agustin Ibañez is supported by CONICET; FONCYT-PICT (2017–1818, 2017–1820); ANID/FONDAP (15150012); ANID/PIA/ANILLOS ACT210096; and the Multi-Partner Consortium to Expand Dementia Research in Latin America (ReDLat), funded by the National Institutes of Aging of the National Institutes of Health under award number R01AG057234, and Alzheimer’s Association grant (SG-20-725707-ReDLat), the Rainwater Foundation, and the Global Brain Health Institute. The content is solely the responsibility of the authors and does not represent the official views of these institutions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2023.1096178/full#supplementary-material
References
- Allan C. L., Sexton C. E., Filippini N., Topiwala A., Mahmood A., Zsoldos E., et al. (2016). Sub-threshold depressive symptoms and brain structure: a magnetic resonance imaging study within the Whitehall II cohort. J. Affect. Disord. 204, 219–225. doi: 10.1016/j.jad.2016.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsubaie M. M., Stain H. J., Webster L. A. D., Wadman R. (2019). The role of sources of social support on depression and quality of life for university students. Int. J. Adolesc. Youth 24, 484–496. doi: 10.1080/02673843.2019.1568887 [DOI] [Google Scholar]
- Beck A. T. (2008). The evolution of the cognitive model of depression and its neurobiological correlates. Am. J. Psychiatr. 165, 969–977. doi: 10.1176/appi.ajp.2008.08050721 [DOI] [PubMed] [Google Scholar]
- Berkman L. F., Glass T. (2000). Social integration, social networks, social support, and health. Soc. Epidemiol. (pp. 137–173). [Google Scholar]
- Bonini L., Rotunno C., Arcuri E., Gallese V. (2022). Mirror neurons 30 years later: implications and applications. Trends Cogn. Sci. 26, 767–781. doi: 10.1016/j.tics.2022.06.003 [DOI] [PubMed] [Google Scholar]
- Bosc M., Dubini A., Polin V. (1997). Development and validation of a social functioning scale, the social adaptation self-evaluation scale. Eur. Neuropsychopharmacol. 7, S57–S70. doi: 10.1016/S0924-977X(97)00420-3 [DOI] [PubMed] [Google Scholar]
- Budde M. D., Joong H. K., Liang H. F., Schmidt R. E., Russell J. H., Cross A. H., et al. (2007). Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn. Reson. Med. 57, 688–695. doi: 10.1002/mrm.21200 [DOI] [PubMed] [Google Scholar]
- Cabanis M., Pyka M., Mehl S., Müller B. W., Loos-Jankowiak S., Winterer G., et al. (2013). The precuneus and the insula in self-attributional processes. Cogn. Affect. Behav. Neurosci. 13, 330–345. doi: 10.3758/s13415-012-0143-5 [DOI] [PubMed] [Google Scholar]
- Cacioppo J. T., Hawkley L. C. (2009). Perceived social isolation and cognition. Trends Cogn. Sci. 13, 447–454. doi: 10.1016/j.tics.2009.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo J. T., Cacioppo S. (2018). The growing problem of loneliness. Lancet 391:426. doi: 10.1016/S0140-6736(18)30142-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo J. T., Cacioppo S., Boomsma D. I. (2014). Evolutionary mechanisms for loneliness. Cogn. Emotion. 28, 3–21. doi: 10.1080/02699931.2013.837379, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo J. T., Hawkley L. C., Thisted R. A. (2010). Perceived social isolation makes me sad: 5-year cross-lagged analyses of loneliness and depressive symptomatology in the Chicago health, aging, and social relations study. Psychol. Aging 25, 453–463. doi: 10.1037/a0017216, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo J. T., Norris C. J., Decety J., Monteleone G., Nusbaum H. (2009). In the eye of the beholder: individual differences in perceived social isolation predict regional brain activation to social stimuli. J. Cogn. Neurosci. 21, 83–92. doi: 10.1162/jocn.2009.21007, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E. H., Argyelan M., Aggarwal M., Chandon T. S. S., Karlsgodt K. H., Mori S., et al. (2017). The role of myelination in measures of white matter integrity: combination of diffusion tensor imaging and two-photon microscopy of CLARITY intact brains. Neuro Image 147, 253–261. doi: 10.1016/j.neuroimage.2016.11.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman S. B., Fratantoni J. M., Robertson I. H., D’Esposito M., Ling G. S. F., Zientz J., et al. (2021). A novel brainhealth index prototype improved by telehealth-delivered training during COVID-19. Front. Public Health 182:641754. doi: 10.3389/fpubh.2021.641754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Demnitz N., Yamamoto S., Yaffe K., Lawlor B., Leroi I. (2022). Defining brain health: a concept analysis. Int. J. Geriatr. Psychiatry 37:5564. doi: 10.1002/gps.5564 [DOI] [PubMed] [Google Scholar]
- Cieslak M., Cook P. A., He X., Yeh F. C., Dhollander T., Adebimpe A., et al. (2021). QSIPrep: an integrative platform for preprocessing and reconstructing diffusion MRI data. Nat. Methods 18, 775–778. doi: 10.1038/s41592-021-01185-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. (2004). Social relationships and health. Am. Psychologist. 59, 676–684. doi: 10.1037/0003-066X.59.8.676 [DOI] [PubMed] [Google Scholar]
- Cohen S., M. G. (2020). “Social support, stress and the buffering hypothesis: a theoretical analysis” in Handbook of Psychology and Health, Vol. 4. eds. A. Baum, S. E. Taylor and J. E. Singer (Milton Park: Routledge; ) [Google Scholar]
- Coleman M. D. (2011). Emotion and the self-serving bias. Curr. Psychol. 30, 345–354. doi: 10.1007/s12144-011-9121-2 [DOI] [Google Scholar]
- Conner A. K., Briggs R. G., Sali G., Rahimi M., Baker C. M., Burks J. D., et al. (2018). A connectomic atlas of the human cerebrum—chapter 13: tractographic description of the inferior fronto-occipital fasciculus. Operat. Neurosurg. 15, S436–S443. doi: 10.1093/ons/opy267, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo A., van der Velpen I. F., Ikram M. A., Vernooij-Dassen M. J. F., Niessen W. J., Vernooij M. W., et al. (2022). Social health is associated with tract-specific brain white matter microstructure in community-dwelling older adults. Biol. Psychiatry Glob. Open Sci. doi: 10.1016/j.bpsgos.2022.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett M. A., Martínez V., Jiménez-Molina Á. (2020). Subthreshold depression in adolescence: gender differences in prevalence, clinical features, and associated factors. J. Affect. Disord. 272, 269–276. doi: 10.1016/j.jad.2020.03.111 [DOI] [PubMed] [Google Scholar]
- Darwish H., Farran N., Assaad S., Chaaya M. (2018). Cognitive reserve factors in a developing country: education and occupational attainment lower the risk of dementia in a sample of lebanese older adults. Front. Aging Neurosci. 10:277. doi: 10.3389/fnagi.2018.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S., Heesink L., Geuze E., Gladwin T., van Honk J., Kleber R., et al. (2020). Regions of white matter abnormalities in the arcuate fasciculus in veterans with anger and aggression problems. Brain Struct. Funct. 225, 1401–1411. doi: 10.1007/s00429-019-02016-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zorzi L., Ranfaing S., Honoré J., Sequeira H. (2021). Autonomic reactivity to emotion: a marker of sub-clinical anxiety and depression symptoms? Psychophysiology 58:e13774. doi: 10.1111/psyp.13774 [DOI] [PubMed] [Google Scholar]
- Denissen J. J. A., Penke L., Schmitt D. P., van Aken M. A. G. (2008). Self-esteem reactions to social interactions: evidence for sociometer mechanisms across days, people, and nations. J. Pers. Soc. Psychol. 95, 181–196. doi: 10.1037/0022-3514.95.1.181 [DOI] [PubMed] [Google Scholar]
- Disner S. G., Beevers C. G., Haigh E. A. P., Beck A. T. (2011). Neural mechanisms of the cognitive model of depression. Nat. Rev. Neurosci. 12, 467–477. doi: 10.1038/nrn3027 [DOI] [PubMed] [Google Scholar]
- Erzen E., Çikrikci Ö. (2018). The effect of loneliness on depression: a meta-analysis. Int. J. Soc. Psychiatry. 64, 427–435. doi: 10.1177/0020764018776349 [DOI] [PubMed] [Google Scholar]
- Esteban O., Markiewicz C. J., Blair R. W., Moodie C. A., Isik A. I., Erramuzpe A., et al. (2019). fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat. Methods 16, 111–116. doi: 10.1038/s41592-018-0235-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratiglioni L., Paillard-Borg S., Winblad B. (2004). An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 3, 343–353. doi: 10.1016/S1474-4422(04)00767-7 [DOI] [PubMed] [Google Scholar]
- Frith C. D., Frith U. (2007). Social cognition in humans. Curr. Biol. 17, R724–R732. doi: 10.1016/j.cub.2007.05.068 [DOI] [PubMed] [Google Scholar]
- Frith C. D., Frith U. (2012). Mechanisms of social cognition. Annu. Rev. Psychol. 63, 287–313. doi: 10.1146/annurev-psych-120710-100449 [DOI] [PubMed] [Google Scholar]
- Gallotti M., Fairhurst M. T., Frith C. D. (2017). Alignment in social interactions. Conscious. Cogn. 48, 253–261. doi: 10.1016/j.concog.2016.12.002 [DOI] [PubMed] [Google Scholar]
- Giannelli M., Cosottini M., Michelassi M. C., Lazzarotti G., Belmonte G., Bartolozzi C., et al. (2010). Dependence of brain DTI maps of fractional anisotropy and mean diffusivity on the number of diffusion weighting directions. J. Appl. Clin. Med. Phys. 11, 176–190. doi: 10.1120/jacmp.v11i1.2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano P. A., Garic D., Dick A. S. (2022). Individual differences in white matter of the uncinate fasciculus and inferior fronto-occipital fasciculus: possible early biomarkers for callous-unemotional behaviors in young children with disruptive behavior problems. J. Child Psychol. Psychiatry 63, 19–33. doi: 10.1111/jcpp.13444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey I., Arora T., Thomas J., Saneh A., Tohme P., Abi-Habib R. (2020). The role of perceived social support on depression and sleep during the COVID-19 pandemic. Psychiatry Res. 293:113452. doi: 10.1016/j.psychres.2020.113452, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra-Carrillo B., Mackey A. P., Bunge S. A. (2014). Resting-state fMRI: a window into human brain plasticity. Neuroscientist 20, 522–533. doi: 10.1177/1073858414524442 [DOI] [PubMed] [Google Scholar]
- Hawkley L. C., Cacioppo J. T. (2010). Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Ann. Behav. Med. 40, 218–227. doi: 10.1007/s12160-010-9210-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkley L. C., Thisted R. A., Cacioppo J. T. (2009). Loneliness predicts reduced physical activity: cross-sectional & longitudinal analyses. Health Psychol. 28, 354–363. doi: 10.1037/a0014400, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays R. D., Dimatteo M. R. (1987). A short-form measure of loneliness. J. Pers. Assess. 51, 69–81. doi: 10.1207/s15327752jpa5101_6 [DOI] [PubMed] [Google Scholar]
- Hecke V., Emsell W., Sunaert L. (2016). Diffusion Tensor Imaging: A Practical Handbook. Berlin: Springer. [Google Scholar]
- Heinrichs M., Baumgartner T., Kirschbaum C., Ehlert U. (2003). Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol. Psychiatry 54, 1389–1398. doi: 10.1016/S0006-3223(03)00465-7 [DOI] [PubMed] [Google Scholar]
- Herbet G., Lafargue G., Bonnetblanc F., Moritz-Gasser S., Menjot de Champfleur N., Duffau H. (2014). Inferring a dual-stream model of mentalizing from associative white matter fibres disconnection. Brain 137, 944–959. doi: 10.1093/brain/awt370 [DOI] [PubMed] [Google Scholar]
- Herbet G., Zemmoura I., Duffau H. (2018). Functional anatomy of the inferior longitudinal fasciculus: from historical reports to current hypotheses. Front. Neuroanat. 12:77. doi: 10.3389/fnana.2018.00077, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgetts C. J., Postans M., Shine J. P., Jones D. K., Lawrence A. D., Graham K. S. (2015). Dissociable roles of the inferior longitudinal fasciculus and fornix in face and place perception. elife 4:e07902. doi: 10.7554/eLife.07902, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar C. E., Gunnar M. R. (2015). Social support can buffer against stress and shape brain activity. AJOB Neurosci. 6, 34–42. doi: 10.1080/21507740.2015.1047054, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huepe D., Roca M., Salas N., Canales-Johnson A., Rivera-Rei Á. A., Zamorano L., et al. (2011). Fluid intelligence and psychosocial outcome: from logical problem solving to social adaptation. PLoS One 6:e24858. doi: 10.1371/journal.pone.0024858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihnen J., Antivilo A., Muñoz-Neira C., Slachevsky A. (2013). Chilean version of the INECO frontal screening (IFS-Ch): psychometric properties and diagnostic accuracy. Dementia Neuropsychologia 7, 40–47. doi: 10.1590/s1980-57642013dn70100007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M. R., Luo R., Valaris S., Haley E. B., Takase H., Chen Y. I., et al. (2020). Diffusion tensor-MRI detects exercise-induced neuroplasticity in the hippocampal microstructure in mice. Brain Plasticity 5, 147–159. doi: 10.3233/BPL-190090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuro Image 17, 825–841. doi: 10.1006/nimg.2002.1132 [DOI] [PubMed] [Google Scholar]
- Jere S., Patil A. P., Shidaganti G. I., Aladakatti S. S., Jayannavar L. (2021). Dataset for modeling Beck's cognitive triad to understand depression. Data Brief 38:107431. doi: 10.1016/j.dib.2021.107431, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste D. V., Lee E. E., Cacioppo S. (2020). Battling the modern behavioral epidemic of loneliness: suggestions for research and interventions. JAMA Psychiat. 77, 553–554. doi: 10.1001/jamapsychiatry.2020.0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesulola E., Micalos P., Baguley I. J. (2018). Understanding the pathophysiology of depression: from monoamines to the neurogenesis hypothesis model-are we there yet? Behav. Brain Res. 341, 79–90. [DOI] [PubMed] [Google Scholar]
- Jiang L., Zhang S., Wang Y., So K. -F., Ren C., Tao Q. (2020). Efficacy of light therapy for a college student sample with non-seasonal subthreshold depression: an RCT study. J. Affect. Disord. 277, 443–449. doi: 10.1016/j.jad.2020.08.055 [DOI] [PubMed] [Google Scholar]
- Johnson E. L., Munro S. E., Bunge S. A. (2013). Development of neural networks supporting goal-directed behavior. Minnesota Symposia Child Psychol. 37, 21–54. doi: 10.1002/9781118732373.ch2 [DOI] [Google Scholar]
- Jopp D. S., Hertzog C. (2010). Assessing adult leisure activities: an extension of a self-report activity questionnaire. Psychol. Assess. 22, 108–120. doi: 10.1037/a0017662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S., De Guzman R. (2021). Relationship between psychological well-being and depression among selected adolescents. Indian J. Positive Psychol. 12, 232–235. [Google Scholar]
- Keller A. S., Leikauf J. E., Holt-Gosselin B., Staveland B. R., Williams L. M. (2019). Paying attention to attention in depression. Transl. Psychiatry 9, 1–12. doi: 10.1038/s41398-019-0616-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J. A., Murray E. R., Yu K. E., Ramsey M., Nguyen T. T., Mishra J., et al. (2021). Neurobiology of loneliness: a systematic review. Neuropsychopharmacology 46, 1873–1887. doi: 10.1038/s41386-021-01058-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea-Carnall C. A., Trujillo-Barreto N. J., Montemurro M. A., El-Deredy W., Parkes L. M. (2017). Evidence for frequency-dependent cortical plasticity in the human brain. Proc. Natl. Acad. Sci. 114, 8871–8876. doi: 10.1073/pnas.1620988114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Y., Shi Y., Yu Q., Van Horn J. D., Tang H., Li J., et al. (2016). Phenotypic and genetic correlations between the lobar segments of the inferior fronto-occipital fasciculus and attention. Sci. Rep. 6:33015. doi: 10.1038/srep33015, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Tsapanou A., Qolamreza R. R., Gazes Y. (2018). White matter integrity mediates decline in age-related inhibitory control. Behav. Brain Res. 339, 249–254. doi: 10.1016/j.bbr.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M., Yang F., Zhang Y., He Z., Su L., Li L. (2014). White matter abnormalities in adolescents with generalized anxiety disorder: a diffusion tensor imaging study. BMC Psychiatry 14, 1–6. doi: 10.1186/1471-244X-14-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M. D. (2007). Social cognitive neuroscience: a review of core processes. Annu. Rev. Psychol. 58, 259–289. doi: 10.1146/annurev.psych.58.110405.085654 [DOI] [PubMed] [Google Scholar]
- Liew T. M. (2019). Depression, subjective cognitive decline, and the risk of neurocognitive disorders. Alzheimer’s Res. Ther. 11, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Kang L., Zhang A., Yang C., Liu M., Wang J., et al. (2021). Injuries in left corticospinal tracts, forceps major, and left superior longitudinal fasciculus (temporal) as the quality indicators for major depressive disorder. Neural Plast. 2021, 1–7. doi: 10.1155/2021/2348072, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londono Y., McMillan D. E. (2015). Psychosocial adaptation: an evolutionary concept analysis exploring a common multidisciplinary language. J. Adv. Nurs. 71, 2504–2519. doi: 10.1111/jan.12723 [DOI] [PubMed] [Google Scholar]
- Ma L., Narayana S., Robin D. A., Fox P. T., Xiong J. (2011). Changes occur in resting state network of motor system during 4 weeks of motor skill learning. NeuroImage 58, 226–233. doi: 10.1016/j.neuroimage.2011.06.014, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti I., Pössel P. (2022). Cognitive triad and depressive symptoms in adolescence: specificity and overlap. Child Psychiatry Hum. Dev., 1–9. doi: 10.1007/s10578-022-01323-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marstaller L., Burianová H., Reutens D. C. (2016). Individual differences in structural and functional connectivity predict speed of emotion discrimination. Cortex 85, 65–74. doi: 10.1016/j.cortex.2016.10.001 [DOI] [PubMed] [Google Scholar]
- Nakagawa S., Takeuchi H., Taki Y., Nouchi R., Kotozaki Y., Shinada T., et al. (2016). Basal ganglia correlates of fatigue in young adults. Sci. Rep. 6, 4–10. doi: 10.1038/srep21386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S., Takeuchi H., Taki Y., Nouchi R., Sekiguchi A., Kotozaki Y., et al. (2015). White matter structures associated with loneliness in young adults. Sci. Rep. 5, 1–11. doi: 10.1038/srep17001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima R., Yordanova Y. N., Duffau H., Herbet G. (2018). Neuropsychological evidence for the crucial role of the right arcuate fasciculus in the face-based mentalizing network: a disconnection analysis. Neuropsychologia, 115, 179–187. [DOI] [PubMed] [Google Scholar]
- Neely-Prado A., Navarrete G., Huepe D. (2019). Socio-affective and cognitive predictors of social adaptation in vulnerable contexts. PLoS One 14:8236. doi: 10.1371/journal.pone.0218236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely-Prado A., van Elk M., Navarrete G., Hola F., Huepe D. (2021). Social adaptation in context: the differential role of religiosity and self-esteem in vulnerable vs. non-vulnerable populations–a registered report study. Front. Psychol. 12:5257. doi: 10.3389/fpsyg.2021.519623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Castanon A. (2020). Handbook of Functional Connectivity Magnetic Resonance Imaging Methods in CONN. Hilbert Press. [Google Scholar]
- Noble K. G., Korgaonkar M. S., Grieve S. M., Brickman A. M. (2013). Higher education is an age-independent predictor of white matter integrity and cognitive control in late adolescence. Dev. Sci. 16, 653–664. doi: 10.1111/desc.12077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzarella C., Alloy L. B., Whitehouse W. G. (2006). Expanded hopelessness theory of depression: on the mechanisms by which social support protects against depression. Cogn. Ther. Res. 30, 307–333. doi: 10.1007/s10608-006-9048-3 [DOI] [Google Scholar]
- Parkinson C., Wheatley T. (2014). Relating anatomical and social connectivity: white matter microstructure predicts emotional empathy. Cereb. Cortex 24, 614–625. doi: 10.1093/cercor/bhs347 [DOI] [PubMed] [Google Scholar]
- Petrocelli J. V. (2003). Hierarchical multiple regression in counseling research: common problems and possible remedies. Meas. Eval. Couns. Dev. 36, 9–22. doi: 10.1080/07481756.2003.12069076 [DOI] [Google Scholar]
- Power J. D., Mitra A., Laumann T. O., Snyder A. Z., Schlaggar B. L., Petersen S. E. (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuro Image 84, 320–341. doi: 10.1016/j.neuroimage.2013.08.048, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- R core team . (2020). A language and environment for statistical computing. Available at: https://www.r-project.org/
- Raikes A. C., Bajaj S., Dailey N. S., Smith R. S., Alkozei A., Satterfield B. C., et al. (2018). Diffusion tensor imaging (DTI) correlates of self-reported sleep quality and depression following mild traumatic brain injury. Front. Neurol. 9:468. doi: 10.3389/fneur.2018.00468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D., Peplau L. A., Cutrona C. E. (1980). The revised UCLA loneliness scale: concurrent and discriminant validity evidence. J. Pers. Soc. Psychol. 39, 472–480. doi: 10.1037/0022-3514.39.3.472 [DOI] [PubMed] [Google Scholar]
- Samadi M., Sohrabi N. (2016). Mediating role of the social problem solving for family process, family content, and adjustment. Procedia Soc. Behav. Sci. 217, 1185–1188. doi: 10.1016/j.sbspro.2016.02.141 [DOI] [Google Scholar]
- Sani I., Stemmann H., Caron B., Bullock D., Stemmler T., Fahle M., et al. (2021). The human endogenous attentional control network includes a ventro-temporal cortical node. Nat. Commun. 12:360. doi: 10.1038/s41467-020-20583-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T. D., Elliott M. A., Gerraty R. T., Ruparel K., Loughead J., Calkins M. E., et al. (2013). An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuro Image 64, 240–256. doi: 10.1016/j.neuroimage.2012.08.052, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte M., Trujillo N., Rodriguez-Villagra O. A., Salas N., Ibañez A., Huepe D. (2022). The role of executive functions, social cognition and intelligence in predicting social adaptation of vulnerable populations. Sci. Rep. 12:18693. doi: 10.1038/s41598-022-21985-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurz M., Maliske L., Kanske P. (2020). Cross-network interactions in social cognition: a review of findings on task related brain activation and connectivity. Cortex 130, 142–157. doi: 10.1016/J.CORTEX.2020.05.006 [DOI] [PubMed] [Google Scholar]
- Seyedmirzaei H., Shafie M., Kargar A., Golbahari A., Bijarchian M., Ahmadi S., et al. (2022). White matter tracts associated with alexithymia and emotion regulation: a diffusion MRI study. J. Affect. Disord. 314, 271–280. doi: 10.1016/j.jad.2022.07.039 [DOI] [PubMed] [Google Scholar]
- Shen X., Adams M. J., Ritakari T. E., Cox S. R., McIntosh A. M., Whalley H. C. (2019). White matter microstructure and its relation to longitudinal measures of depressive symptoms in mid-and late life. Biol. Psychiatry 86, 759–768. doi: 10.1016/j.biopsych.2019.06.011, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepperd J., Malone W., Sweeny K. (2008). Exploring causes of the self-serving bias. Soc. Personal. Psychol. Compass 2, 895–908. doi: 10.1111/j.1751-9004.2008.00078.x [DOI] [Google Scholar]
- Song S. K., Sun S. W., Ju W. K., Lin S. J., Cross A. H., Neufeld A. H. (2003). Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuro Image 20, 1714–1722. doi: 10.1016/j.neuroimage.2003.07.005 [DOI] [PubMed] [Google Scholar]
- Song S. K., Yoshino J., Le T. Q., Lin S. J., Sun S. W., Cross A. H., et al. (2005). Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuro Image 26, 132–140. doi: 10.1016/j.neuroimage.2005.01.028 [DOI] [PubMed] [Google Scholar]
- Spellman T., Liston C. (2020). Toward circuit mechanisms of pathophysiology in depression. Am. J. Psychiatry. 177, 381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen O. D., Ori A. P. S., Wardenaar K. J., van Loo H. M. (2022). Loneliness associates strongly with anxiety and depression during the COVID pandemic, especially in men and younger adults. Sci. Rep. 12, 1–11. doi: 10.1038/s41598-022-13049-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M., Lieschke J., Cruwys T., Cárdenas D., Platow M. J., Reynolds K. J. (2021). Better together: how group-based physical activity protects against depression. Soc. Sci. Med. 286:114337. doi: 10.1016/j.socscimed.2021.114337 [DOI] [PubMed] [Google Scholar]
- Suri S., Topiwala A., Mackay C. E., Ebmeier K. P., Filippini N. (2014). Using structural and diffusion magnetic resonance imaging to differentiate the dementias. Curr. Neurol. Neurosci. Rep. 14:475. doi: 10.1007/s11910-014-0475-3 [DOI] [PubMed] [Google Scholar]
- Szkody E., McKinney C. (2020). Appraisal and social support as moderators between stress and physical and psychological quality of life. Stress. Health 36, 586–595. doi: 10.1002/smi.2957 [DOI] [PubMed] [Google Scholar]
- Takeuchi H., Kawashima R. (2018). Mean diffusivity in the dopaminergic system and neural differences related to dopaminergic system. Curr. Neuropharmacol. 16, 460–474. doi: 10.2174/1570159x15666171109124839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H., Taki Y., Sekiguchi A., Nouchi R., Kotozaki Y., Nakagawa S., et al. (2017). Mean diffusivity of basal ganglia and thalamus specifically associated with motivational states among mood states. Brain Struct. Funct. 222, 1027–1037. doi: 10.1007/s00429-016-1262-5 [DOI] [PubMed] [Google Scholar]
- Tan M., Shallis A., Barkus E. (2020). Social anhedonia and social functioning: loneliness as a mediator. Psych. J. 9, 280–289. doi: 10.1002/pchj.344 [DOI] [PubMed] [Google Scholar]
- Tavor I., Yablonski M., Mezer A., Rom S., Assaf Y., Yovel G. (2014). Separate parts of occipito-temporal white matter fibers are associated with recognition of faces and places. NeuroImage 86, 123–130. doi: 10.1016/j.neuroimage.2013.07.085 [DOI] [PubMed] [Google Scholar]
- Thomas C., Moya L., Avidan G., Humphreys K., Jung K. J., Peterson M. A., et al. (2008). Reduction in white matter connectivity, revealed by diffusion tensor imaging, may account for age-related changes in face perception. J. Cogn. Neurosci. 20, 268–284. doi: 10.1162/jocn.2008.20025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Liang S., Yuan Z., Chen S., Xu P., Yao D. (2014). White matter structure in loneliness: preliminary findings from diffusion tensor imaging. Neuro Report 25, 843–847. doi: 10.1097/WNR.0000000000000197 [DOI] [PubMed] [Google Scholar]
- Tong H., Hou W. K., Liang L., Li T. W., Liu H., Lee T. M. C. (2021). Age-related differences of rumination on the loneliness–depression relationship: evidence from a population-representative cohort. Innov. Aging 5:igab 034. doi: 10.1093/geroni/igab034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torralva R., Gleichgerrcht L., Manes F. (2009). INECO frontal screening (IFS): a brief, sensitive, and specific tool to assess executive functions in dementia. J. Int. Neuropsychol. Soc. 15, 777–786. doi: 10.1017/S1355617709990415 [DOI] [PubMed] [Google Scholar]
- Uddin L. Q., Iacoboni M., Lange C., Keenan J. P. (2007). The self and social cognition: the role of cortical midline structures and mirror neurons cristina albert trends in cognitive … the self and social cognition: the role of cortical midline structures and mirror neurons. Trends Cogn. Sci. 11, 153–157. doi: 10.1016/j.tics.2007.01.001 [DOI] [PubMed] [Google Scholar]
- Unger A., Alm K. H., Collins J. A., O'Leary J. M., Olson I. R. (2016). Variation in white matter connectivity predicts the ability to remember faces and discriminate their emotions. J. Int. Neuropsychol. Soc. 22, 180–190. doi: 10.1017/S1355617715001009, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations . (2022). COVID-19: Depression, anxiety soared 25 per cent in a year. Available at: https://news.un.org/en/story/2022/03/1113162
- United Nations Development Programme . (2022). Human development report 2021/2022. Available at: https://hdr.undp.org/system/files/documents/global-report-document/hdr2021-22pdf_1.pdf
- Urner M., Schwarzkopf D. S., Friston K., Rees G. (2013). Early visual learning induces long-lasting connectivity changes during rest in the human brain. NeuroImage 77, 148–156. doi: 10.1016/j.neuroimage.2013.03.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdés C., Morales-Reyes I., Pérez J. C., Medellín A., Rojas G., Krause M. (2017). Propiedades psicométricas del Inventario de Depresión de Beck IA para la población chilena. Rev. Med. Chil. 145, 1005–1012. doi: 10.4067/s0034-98872017000801005 [DOI] [PubMed] [Google Scholar]
- Vulser H., Paillère Martinot M.-L., Artiges E., Miranda R., Penttilä J., Grimmer Y., et al. (2018). Early variations in white matter microstructure and depression outcome in adolescents with subthreshold depression. Am. J. Psychiatr. 175, 1255–1264. doi: 10.1176/appi.ajp.2018.17070825 [DOI] [PubMed] [Google Scholar]
- Wang Y., Metoki A., Alm K. H., Olson I. R. (2018). White matter pathways and social cognition. Neurosci. Biobehav. Rev. 90, 350–370. doi: 10.1016/j.neubiorev.2018.04.015, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. (2012). Conn: a functional connectivity toolbox for correlated and Anticorrelated brain networks. Brain Connect. 2, 125–141. doi: 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- Wilde E. A., Hyseni I., Lindsey H. M., Faber J., McHenry J. M., Bigler E. D., et al. (2021). A preliminary DTI Tractography study of developmental neuroplasticity 5–15 years after early childhood traumatic brain injury. Front. Neurol. 12:4055. doi: 10.3389/fneur.2021.734055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (2021). Depression. Available at: https://www.who.int/news-room/fact-sheets/detail/depression
- Worsley K. J., Marrett S., Neelin P., Vandal A. C., Friston K. J., Evans A. C. (1996). A unified statistical approach for determining significant signals in images of cerebral activation. Hum. Brain Mapp. 4, 58–73. doi: [DOI] [PubMed] [Google Scholar]
- Yeh F. C., Badre D., Verstynen T. (2016). Connectometry: a statistical approach harnessing the analytical potential of the local connectome. Neuro Image 125, 162–171. doi: 10.1016/j.neuroimage.2015.10.053 [DOI] [PubMed] [Google Scholar]
- Yeh F. C., Panesar S., Barrios J., Fernandes D., Yeh F. C., Abhinav K., et al. (2019). Automatic removal of false connections in diffusion MRI Tractography using topology-informed pruning (TIP). Neurotherapeutics 16, 52–58. doi: 10.1101/338624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh F. C., Panesar S., Fernandes D., Meola A., Yoshino M., Fernandez-Miranda J. C., et al. (2018). Population-averaged atlas of the macroscale human structural connectome and its network topology. Neuro Image 178, 57–68. doi: 10.1016/j.neuroimage.2018.05.027, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh F. C., Tseng W. Y. I. (2011). NTU-90: a high angular resolution brain atlas constructed by q-space diffeomorphic reconstruction. Neuro Image 58, 91–99. doi: 10.1016/j.neuroimage.2011.06.021 [DOI] [PubMed] [Google Scholar]
- Yeh F. C., Verstynen T. D., Wang Y., Fernández-Miranda J. C., Tseng W. Y. I. (2013). Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS One 8:e80713. doi: 10.1371/journal.pone.0080713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. S., Liu X., Chen S., Ke Y., Qi S., Wei X., et al. (2022). Discriminating subclinical depression from major depression using multi-scale brain functional features: a radiomics analysis. J. Affect. Disord. 297, 542–552. doi: 10.1016/j.jad.2021.10.122 [DOI] [PubMed] [Google Scholar]
- Zhang P. W., Sweeney J. A., Jia Z., Gong Q. (2018). Brain structure alterations in depression: Psychoradiological evidence. CNS Neurosci. Ther. 24, 994–1003. doi: 10.1111/cns.12835, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Jiang W., Wang X., Cai Z., Liu Z., Liu G. (2020). Exercise, brain plasticity, and depression. CNS Neurosci. Ther. 26, 885–895. doi: 10.1111/cns.13385 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.