Abstract
Spinal cord injury (SCI) severely diminishes quality of life and presents patients with a substantial financial burden. The lack of a curative treatment has guided efforts toward identifying potential regenerative treatments. Neural stem/progenitor cell (NSPC) transplantation represents a promising strategy for the regeneration of the injured spinal cord due to the ability of these cells to replace neural cells lost post-injury. However, the transplant-derived oligodendrocytes and neurons need to be able to associate and integrate within the appropriate endogenous circuits to guarantee optimal functional recovery. To date, the integration of these transplant-derived cells has lacked specificity and remains a challenge. As such, it appears that the transplanted cells will require additional guidance cues to instruct the cells where to integrate. In the present review, we propose a variety of combinatorial techniques that can be used in conjunction with NSPC transplantation to direct the cells toward particular circuits of interest. We begin by introducing distinct molecular signatures that assist in the formation of specific circuits during development, and highlight how favorable molecular cues can be incorporated within the cells and their environment to guide the grafted cells. We also introduce alternative methods including task-specific rehabilitation, galvanotaxis, and magnet-based tools, which can be applied to direct the integration of the grafted cells toward the stimulated circuits. Future research examining these combinatorial efforts may serve to improve outcomes following SCI.
Keywords: spinal cord injury, stem cell transplantation, integration, differentiation
Significance Statement.
There is an urgent need to develop novel stem cell therapies for spinal cord injury that can succeed in clinical trials and enter the clinical setting. In the present review, we argue that integration is an important challenge related to neural stem/progenitor cell transplantation. We suggest using combinatorial approaches incorporating neural stem/progenitor cell transplantation with molecular guidance cues, task-specific rehabilitation, galvanotaxis, or magnet-based tools to optimize integration in the appropriate circuits.
Introduction
Traumatic spinal cord injury (SCI) is followed by considerable sensorimotor impairment, which reduces quality of life and ultimately contributes to high rates of mortality.1 It is estimated that up to 500 000 people are affected by SCI every year worldwide.2 This presents the healthcare system with significant financial burdens, as the cost of care for each individual with an SCI can range between $1.1 and 4.6 million USD.1 From a pathophysiological perspective, SCI is followed by several maladaptive processes, including demyelination, neuronal degeneration, astrogliosis, and cell death.3 Although there are some pharmacological and surgical interventions available that aim to mitigate these mechanisms, there are no curative treatments for SCI.1 As such, cell transplantation has emerged as a potential regenerative therapy.
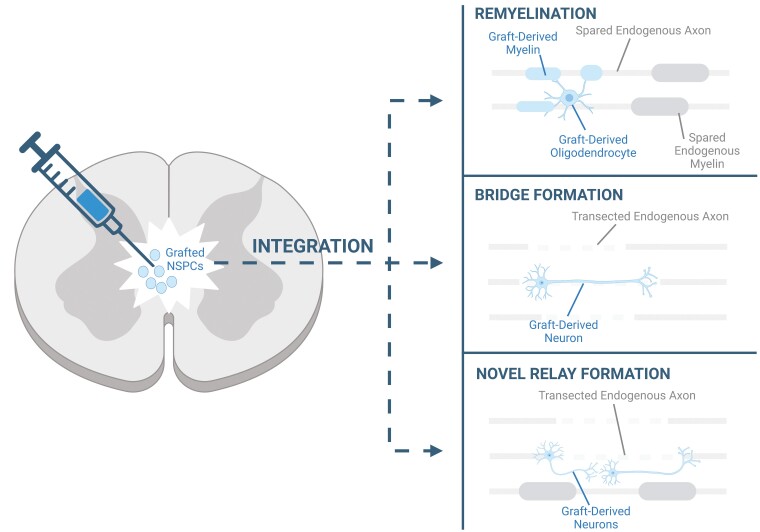
Several cell types have been transplanted for SCI, including mesenchymal stem cells, Schwann cells, oligodendrocyte precursor cells, olfactory ensheathing cells, and neural stem/progenitor cells (NSPCs).4 Of these various sources, NSPCs represent the most attractive cell therapy for SCI treatment, given that they are capable of targeting the wide range of pathophysiological consequences seen following injury. NSPCs have been shown to target the inflammatory response, reduce astrogliosis, provide neurotrophic support, and promote angiogenesis.5-7 In addition, following delivery into the spinal cord, NSPCs can migrate around the cord and differentiate into neurons, oligodendrocytes, and astrocytes, thus giving them the unique advantage of being able to replace the neural cells that have been lost post-SCI. Importantly, the graft-derived oligodendrocytes and neurons can integrate within the endogenous circuits and thereby contribute to regeneration. Specifically, the transplant-derived oligodendrocytes can associate with the spared endogenous axons by contributing to remyelination,8 whereas the transplant-derived neurons can restore connectivity in the spinal cord by replacing the neurons that have been lost within the neural pathways or by giving rise to novel relays that bypass the damaged pathways (Fig. 1).9 In this regard, a number of studies have shown that transplant-derived cells are capable of functionally integrating within the endogenous circuitry post-SCI.10-13
Figure 1.
Summary of the ways in which NSPC-derived cells can integrate within endogenous circuits. Following transplantation into the injured spinal cord, grafted NSPCs can differentiate into oligodendrocytes that can remyelinate the spared endogenous axons. In addition, NSPCs can differentiate into neurons, which can contribute to bridge formation across the damaged circuit or contribute to the formation of a novel relay around the lesion site.
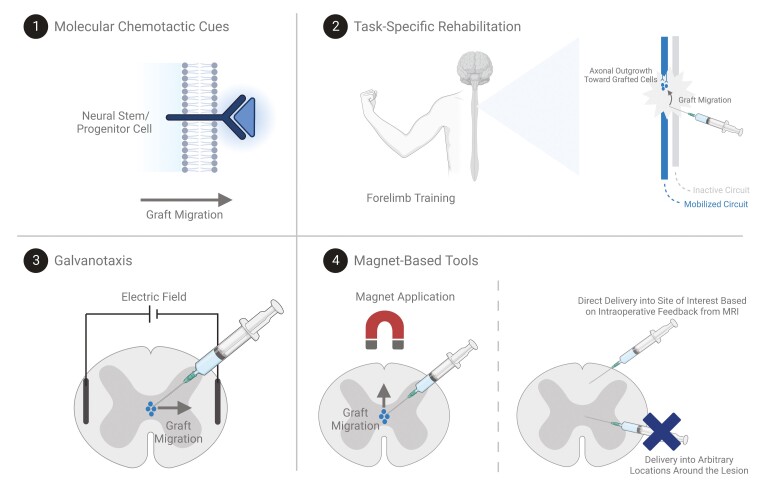
However, the specificity with which the NSPC-derived oligodendrocytes and neurons choose their targets and integrate within specific host neural networks remains unclear and introduces additional challenges.14,15 Ensuring that the cell grafts migrate toward and integrate within the appropriate endogenous circuits is paramount to ensuring optimal functional recovery, as aberrant integration can lead to potential side effects. Moreover, the integration of the cells should align with the deficits and therapeutic goals of the patient. Notably, different patients experience variable extents of injury and may have different priorities in the functions that they aim to recover. For some patients, recovering hand function is the main goal, whereas for others, regaining walking ability or trunk stability is a greater priority.16 In this regard, additional extrinsic guidance cues may help in instructing the grafted cells to target the most desirable neural networks, as opposed to those that do not align with the individual’s therapeutic aims. In the present review, we will highlight a number of strategies that can be combined with NSPC transplantation to promote targeted cell integration following transplantation (Fig. 2).
Figure 2.
Schematic summarizing potential combinatorial methods that may help in optimizing NSPC migration and integration. Distinct molecular signatures can be engineered within the NSPCs or the spinal cord microenvironment to drive chemotactic migration toward the sites of interest. Task-specific rehabilitation paradigms may promote integration of NSPC-derived cells within the mobilized circuits by promoting axonal outgrowth from the activated circuits toward the grafted cells and recruiting graft migration. Electric fields may help in promoting graft migration along the electric field. Finally, magnet-based tools can be used to encourage the transplanted cells to migrate in particular directions and to optimize the cell delivery site based on visual feedback from MRI technologies.
Molecular Guidance Cues
One strategy by which targeted NSPC integration can be optimized involves mimicking the molecular cues that are known to instruct neural circuit formation during development. Notably, the adult spinal cord is comprised of an intricate network of neural circuits responsible for heterogenous roles ranging from voluntary movement to somatosensory and autonomic functions. The specificity in the generation of these distinct pathways arises as a result of various combinations of chemotropic guidance cues.17-20 These guidance cues selectively instruct particular neural cells to form connections with their appropriate targets by acting as attractive or repulsive molecules, thereby fine-tuning their growth and migration.21,22 As such, these unique molecular patterns may be helpful in the context of promoting targeted graft integration. Specifically, NSPCs and their engraftment niche can be carefully engineered to make them more conducive to driving graft integration within anticipated circuits. For example, NSPCs can be manipulated to express molecules that are favorable in the development of the anticipated circuits, or these molecules can be injected into the spinal cord microenvironment in order to create a favorable chemotactic gradient. The following section summarizes various molecular guidance cues that contribute to the formation of unique motor and sensory pathways, and subsequently highlights how different molecules have been applied in the context of SCI regeneration.
The main circuit responsible for voluntary motor functions in humans is the corticospinal tract (CST). It descends from the motor cortex and travels in the spinal cord until it reaches an appropriate exit point where it enters the periphery to innervate muscles. As the motor neuron descends in the spinal cord, it encounters a series of pathfinding cues that refine its location and guide it to grow in a caudal direction. The Wnt family of proteins is thought to be one of the cues that guide axonal elongation in the CST. In the spinal cord, there is a decreasing gradient of Wnt expression in the rostrocaudal direction. These environmental cues mediate axonal growth via the repulsive Ryk receptor found on CST axons. Specifically, the interaction between high levels of Wnt in the rostral cord and the Ryk receptor on these axons is thought to cause repulsion and to drive the axons away toward the caudal cord. When investigating these interactions in mice, it was found that blocking the Ryk receptor with anti-Ryk antibodies inhibited the growth of these axons in the caudal direction, thus reinforcing the importance of these molecular cues in the development of the CST.23 Another important guidance cue that has been found to be implicated in CST outgrowth is IGF-I. In contrast to the repulsive mechanisms associated with Wnt-Ryk interactions, IGF-I provides an attractive cue upon interaction with the IGF-IR receptor found on motor neurons and ultimately promotes CST growth. Blocking this interaction using a competitive IGF-IR inhibitor, or an anti-IGF-IR antibody, inhibits axonal extension in these motor neurons both in vitro and in vivo.24 In addition, Özdinler and Macklis found that BDNF is also implicated in CST development by promoting arborization and branching.24 As the CST descends further, it eventually reaches the level at which it must exit toward the periphery. The level at which a particular CST axon exits the cord is determined by the interaction between EphA4 receptors and their ephrin-A ligands.25 In this regard, it was found that hindlimb CST axons terminated prematurely in the cervical cord and did not elongate properly into the lumbar cord in EphA4 mutant mice. This suggested that EphA4 and ephrins provide inhibitory cues that propel the CST away in a caudal direction. Indeed, it has been found that neurons innervating the hindlimbs express higher amounts of the ephrin ligands than those innervating the forelimbs, thus explaining how these distinct neurons are guided toward the appropriate exit points toward the periphery.26 On the other hand, the exit of the CST is also governed by netrins, which act through a number of different receptors to refine CST positioning and limb innervation.27 This is supported by the finding that motor neurons aberrantly escape the spinal cord and can be found in the periphery in netrin mutant mice.28
Several molecular guidance cues have also been identified in the sensory systems that govern touch, temperature, pain, and proprioception. In these circuits, a sensory neuron enters the spinal cord from the periphery and either ascends in the spinal cord or synapses on a second-order neuron that ascends toward the brain. As the axons from the dorsal root ganglion (DRG) enter the spinal cord, they encounter repulsive cues from netrin-1. In this context, netrin-1 acts via the Unc5c receptors found on the DRG axons and facilitates repulsion away from the ventral cord toward the dorsal areas. Netrin-1 mutant mice display disorganized and aberrant axonal trajectories, whereas the introduction of netrin-1 to the dorsal spinal cord inhibits the dorsal projection of the DRG axons.29 These mechanisms are responsible for directing the sensory circuits to the dorsal spinal cord. However, sensory circuits are further refined by additional cues that distinguish between the proprioceptive pathways and the nociceptive pathways. In this regard, the cutaneous nociceptive axons are responsive to LysoPtdGlc, which is found in the dorsal cord and selectively acts on their GPR55 receptors in a repulsive manner to drive them in the ventral direction. When these interactions between LysoPtdGlc-GPR55 are interrupted, the nociceptive axons begin to grow within the proprioceptive areas.30 On the other hand, proprioceptive neurons express the plexin A1 receptor, which is implicated in regulating the dorsoventral positioning of the proprioceptive neurons through the interaction with Sema6d.31 Once the incoming sensory axons identify the correct target location in the cord they can ascend. In this regard, it is hypothesized that netrin-1 may be implicated in the rostral ascent of the sensory axons, as the application of netrin-1 to DRG axons in vitro has been shown to inhibit their growth.32
Understanding the molecular landscape during development provides an important context for researchers because it provides a framework that researchers can aim to mimic during cell transplantation. Strategic incorporation of these molecular patterns may be advantageous because NSPCs, as well as the NSPC-derived oligodendrocytes and neurons, may be responsive to these chemotactic instructions, thus influencing their target choices. Similarly, genetically engineered cells or particular environmental molecular gradients may be capable of mobilizing the spared axons residing in particular circuits to undergo elongation toward the graft, thus improving graft-host interactions. In this regard, incorporating GDNF with Schwann cell transplantation has been shown to promote propriospinal axon extension toward the grafted cells, whereas incorporation of other factors including NGF, BDNF, and neurotrophins in fibroblasts has also been shown to promote endogenous axonal outgrowth within distinct spinal pathways.33-38 Importantly, there is also some evidence suggesting that overexpression of factors such as polysialic acid and GDNF in NSPCs may increase the migration and integration of the grafted cells.39,40 However, there have been no studies comparing the effect of different molecular cues on driving targeted NSPC integration within specific circuits of interest. For example, taking advantage of the Wnt-Ryk interactions may promote NSPC integration within the CST, whereas other signals, such as the interactions between netrin-1 and Unc5c, may be relevant for the guidance of NSPCs toward the sensory circuitry. Therefore, future investigations related to this combinatorial approach are warranted.
Task-Specific Rehabilitation
The combination of NSPC transplantation with task-specific rehabilitation is another strategy that may help in guiding graft integration within the appropriate circuits. Rehabilitation is considered a gold standard for SCI management given that it is a cost-effective and non-invasive option for patients.1,41 Importantly, it drives neural activity within the particular circuits that are engaged during the motor task. This is particularly relevant in the context of SCI regeneration, as there is an increasing body of literature demonstrating that activity-dependent processes are capable of driving adaptive reorganization within the nervous system. Notably, neural activity induces synaptic strengthening and neuroplasticity within the active circuits in a Hebbian “use it or lose it” manner.42-44 For example, targeted activation of the CST has been shown to induce axonal sprouting and outgrowth in this circuit post-SCI.45 On the other hand, targeted neuronal activity has also been shown to regulate oligodendrocyte recruitment and myelination.46,47 Optogenetic methods have demonstrated that activated fibers have thicker myelin and greater myelin protein expression compared to non-stimulated control fibers in the premotor cortex and subcortical white matter.48 Axonal activity has also been shown to influence myelin repair within the injured spinal cord, whereby stimulation of the dorsal CST enhances CST remyelination following SCI, whereas inhibition of this circuit reduced myelination.49 As such, targeted activation of particular circuits induces adaptive changes that may be favorable when combined with NSPC transplantation. Notably, selective activation of particular circuits during a specific rehabilitation paradigm may induce the mobilized circuits to undergo sprouting and growth into the graft, ultimately leading to synaptogenesis and graft-host interactions. On the other hand, this neural activity may also serve to guide NSPC migration and to recruit the NSPC-derived cells toward the mobilized circuits through the secretion of neurotrophic factors, which are known to act as chemoattractants in the spinal cord.50,51 Similarly, active endogenous neurons may be more likely to become myelinated by graft-derived oligodendrocytes, as opposed to neurons that are not active. Therefore, researchers may be able to guide where the NSPCs integrate by carefully choosing an appropriate rehabilitation paradigm that mobilizes the circuits of interest.
To date, only a few studies have combined NSPC transplantation with rehabilitation in the context of SCI. Of these studies, one demonstrated that mice that received treadmill training following thoracic SCI exhibited higher NSPC survival and neuronal differentiation compared to untrained mice.52 Similar results were seen in a rat model of cervical SCI, which additionally displayed that oligodendrocyte differentiation and locomotor recovery were enhanced in the group that received both NSPC transplantation and treadmill training compared to NSPCs alone.53 Although neither of these studies specifically investigated the effect of rehabilitation on graft integration, they demonstrate that rehabilitation has a positive impact on transplanted NSPCs. However, the extent to which this combinatorial approach may influence graft integration will likely depend on the type and specificity of the rehabilitation paradigm that is incorporated. Task-specific rehabilitation strategies—in contrast to more arbitrary rehabilitation paradigms—would be ideal to achieve greater precision in the circuits that are being mobilized. Various task-specific training methods exist, and choosing the appropriate method that aligns with the individual’s therapeutic priorities is vital. For example, the recovery of hand function is the main therapeutic priority for many individuals with cervical SCI, and even modest improvements in hand function would have a tremendous impact on their quality of life.16 For these individuals, upper limb tasks may be favorable. In animal models, reaching tasks have been developed as skilled motor tasks for forelimb rehabilitation.54-57 On the other hand, improving bowel, bladder, and sexual functions is an important objective for other individuals. Therefore, these individuals may benefit from task-specific rehabilitation such as body-weight supported treadmill training, which has been shown to promote several outcomes including urinary incontinence, bladder capacity, voiding efficiency, and defecation.58 The specificity of the assigned rehabilitation paradigm will likely distinguish between the physical therapy regimens that successfully influence graft integration vs. those that do not. In this regard, one recent study by the Tuszynski group found that task-specific forelimb training was associated with significantly greater axonal outgrowth from the grafted neural cells, as well as improved host corticospinal growth into the grafted cells, thus suggesting that targeted rehabilitation improved graft integration into the CST.59 Nonetheless, the effects of different types of task-specific rehabilitation on guiding graft integration into distinct circuits have not been thoroughly investigated in the context of SCI.
Galvanotaxis
Galvanotaxis is an emerging technology that may be able to influence NSPC migration along a particular desired route. Galvanotaxis refers to the movement of cells in response to electrical fields. These electrical fields are known to arise during development and to influence the migration of endogenous NSPCs. NSPCs have also been found to be responsive to galvanotaxis in vitro.60 Therefore, this suggests that grafted NSPCs may be sensitive to externally applied electrical fields post-SCI. External electrical fields can be applied in vivo through the implantation of electrodes into specified neuroanatomical locations that align with the anticipated direction of movement. Importantly, a few studies have successfully assessed the role of galvanotaxis in influencing grafted NSPC behavior and migration. In one study, murine NSPCs were transplanted onto the corpus callosum into an area where the cells experience inherent migratory cues. In this study, activation of the external electrical fields overrode the endogenous migratory cues and ultimately influenced the migration of the cells.61 Similarly, external electrical fields have also been found to exhibit an effect on the migration patterns of human NSPCs following transplantation around the rostral migratory stream in the brain. Notably, the rostral migratory stream is an area in which the cells have an inherent migratory pattern toward the olfactory bulb. However, the application of an external electrical field was capable of driving the cells to migrate against their inherent program, upstream toward the subventricular zone.62 As such, there are some preliminary results supporting the potential of this technology in guiding NSPC migration. This may be advantageous in the context of optimizing integration because it would ensure that the graft-derived oligodendrocytes and neurons would be in close proximity to the networks of interest upon differentiation. Furthermore, this technology has been tested in 2 clinical trials, thus demonstrating that it is relevant for application in humans.63,64 However, there are some limitations related to the invasiveness and high costs associated with this strategy. In addition, the efficacy of galvanotaxis in guiding NSPC migration has not been tested in the spinal cord. Therefore, future investigations will be necessary to assess whether external electrical fields would be capable of driving NSPC migration toward particular spinal circuits with sufficient neuroanatomical precision.
Magnet-Based Techniques
An alternative method that aims to direct graft migration and integration involves incorporating magnetic tools. This technique is advantageous because it is minimally invasive and does not require the implantation of electrodes. Instead, focused magnetic fields can be applied externally to guide the movement of transplanted cells that have been pre-labeled with magnetic particles. Therefore, the magnetic fields can exert an effect not only on the magnetized NSPCs but also on the NSPC-derived mature cells which, depending on the labeling strategy, can preserve the magnetic label upon differentiation. Importantly, magnetically labeled NSPCs have been shown to be responsive to magnetic fields in vitro.65,66 Moreover, magnetic fields are capable of influencing NSPC migration in vivo as well. In one study assessing ischemic stroke, magnetically labeled NSPCs were injected into the tail vein of rats, and the application of a magnet was found to increase the distribution of the cells toward the brain.66 On the other hand, the application of a magnetic field has also been shown to influence the migration of cells within neural tissue. Following transplantation into the lateral ventricles, magnetized NSPCs displayed an inherent migratory pattern along the rostral migratory stream toward the olfactory bulb as well as along the white matter. However, these migration patterns changed when a magnetic field was applied, whereby the cells switched the direction of their inherent movement toward the magnet.67 Therefore, these findings suggest that these magnet-based methods could be used in SCI to direct transplanted cells toward specific circuits. Nonetheless, the capacity of this technology in targeting precise spinal circuits will require further research efforts. Moreover, although preliminary studies suggest that the introduction of a magnetic label does not affect cell viability, differentiation, or axonal outgrowth, it will be important to verify that the magnetic labeling strategies do not change the physiological properties of the cells and do not introduce any toxic effects.65,68
Another magnet-based approach that aims to optimize NSPC integration involves incorporating magnetic resonance imaging (MRI) intraoperatively during transplantation. Specifically, this method aims to utilize MRI-compatible injection platforms to provide visual feedback for the delivery of an injection cannula containing magnetically labeled NSPCs toward a particular anatomical location of interest. Therefore, this technique is distinct from the other combinatorial approaches mentioned previously, as it focuses on delivering the cells directly to the site of interest, rather than delivering the cells into arbitrary locations around the injury, and requiring the cells to be subjected to additional migratory cues to move toward the spinal circuits that require regeneration in vivo. Importantly, this minimally invasive method has been successfully utilized to transplant NSPCs into the putamen in baboons, and even into the ventral horn of the spinal cord in minipigs.69,70 However, this strategy requires the development of MRI-injection platforms, which may not be the most practical and may introduce limitations related to the translation of this technology.
Additional Considerations
Combinatorial strategies, such as molecular cues, task-specific rehabilitation, galvanotaxis, and magnet-based techniques may improve graft-host interactions by directing NSPC migration, instructing NSPC-derived oligodendrocytes and neurons which circuits they should target, and encouraging endogenous circuits to elongate toward the grafted cells. However, even if the grafted cells are successfully guided toward a particular circuit, the integration may still be suboptimal if the differentiation of the NSPCs is inappropriate. Optimal NSPC integration is contingent upon the ability of the NSPCs to differentiate into the appropriate mature cell types that match the circuit of interest. Notably, following transplantation, various injury-related signaling cascades skew NSPCs to differentiate into astrocytes rather than oligodendrocytes or neurons.40 Therefore, additional efforts may be required to enhance the differentiation of the cells toward a mature phenotype that is appropriate for the nature of the injury to induce task-specific recovery. In this regard, biomarker and MRI assessments can be used to distinguish and allocate patients to distinct NSPC treatments. In patients that experience greater extents of myelin damage, biasing NSPC differentiation along the oligodendrogenic lineage may be helpful to increase the amount of integrating oligodendrocytes.71 On the other hand, NSPCs can be biased toward a motor neuron fate for patients that display corticospinal damage, or they can be specified into sensory neurons for patients that exhibit injuries in the sensory pathways. Ultimately, these biasing methods can help to ensure that the final cell phenotypes match the circuit of interest in order to optimize NSPC integration.15,72
Conclusion
NSPC therapies have been investigated extensively in pre-clinical animal studies and several past, as well as upcoming, clinical trials have been initiated.4,73 However, no cell-based SCI treatment has successfully entered a clinical setting to date. This lack of success may be attributable to the inability of the cells to appropriately integrate within the endogenous circuitry. In the present review, we highlighted several approaches that could be combined with NSPC transplantation to drive grafted cells to integrate within anticipated circuits. These approaches include incorporating distinct molecular cues, task-specific rehabilitation, electric fields, and magnet-based strategies. Implementing circuit-specific regeneration paradigms may improve patient-specific care and optimally improve the quality of life of patients. Nonetheless, the role of these combinatorial approaches on NSPC integration has been insufficiently investigated in the context of SCI and therefore requires further research.
Acknowledgments
M.G.F. is supported by the Robert Campeau Family Foundation/Dr. C.H. Tator Chair in Brain and Spinal Cord Research at UHN. All figures were made using BioRender. We would like to thank Dr. Tim Worden for editing the manuscript.
Contributor Information
Katarzyna Pieczonka, Division of Genetics and Development, Krembil Brain Institute, University Health Network, Toronto, ON, Canada; Institute of Medical Science, Faculty of Medicine, University of Toronto, Toronto, ON, Canada.
Michael G Fehlings, Division of Genetics and Development, Krembil Brain Institute, University Health Network, Toronto, ON, Canada; Institute of Medical Science, Faculty of Medicine, University of Toronto, Toronto, ON, Canada; Division of Neurosurgery and Spinal Program, Department of Surgery, University of Toronto, Toronto, ON, Canada.
Funding
K.P. is supported by the CIHR Canada Graduate Scholarships Doctoral Research Award.
Conflict of Interest
M.G.F. acknowledges founding Inteligex, a regenerative neuroscience biotechnology company. Inteligex does not have current products in clinical trials and is non-revenue producing. K.P. declared no potential conflicts of interest.
Author Contributions
K.P.: conception and design, financial support, provision of study material or patients, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript. M.F.: conception and design, financial support, administrative support, provision of study material or patients, final approval of manuscript.
Data Availability
Data are available from the corresponding authors on reasonable request.
References
- 1. Ahuja CS, Wilson JR, Nori S, et al. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3:17018. https://doi.org/ 10.1038/nrdp.2017.18 [DOI] [PubMed] [Google Scholar]
- 2. Katoh H, Yokota K, Fehlings MG.. Regeneration of spinal cord connectivity through stem cell transplantation and biomaterial scaffolds. Front Cell Neurosci. 2019;13:248. https://doi.org/ 10.3389/fncel.2019.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anjum A, Yazid MD, Fauzi Daud M, et al. Spinal cord injury: pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int J Mol Sci . 2020;21:7533. https://doi.org/ 10.3390/ijms21207533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahuja CS, Mothe A, Khazaei M, et al. The leading edge: emerging neuroprotective and neuroregenerative cell-based therapies for spinal cord injury. Stem Cells Transl Med. 2020;9:1509-1530. https://doi.org/ 10.1002/sctm.19-0135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lu P, Jones LL, Snyder EY, Tuszynski MH.. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181:115-129. https://doi.org/ 10.1016/s0014-4886(03)00037-2 [DOI] [PubMed] [Google Scholar]
- 6. Nori S, Okada Y, Yasuda A, et al. Grafted human-induced pluripotent stem-cell–derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc Natl Acad Sci USA. 2011;108:16825-16830. https://doi.org/ 10.1073/pnas.1108077108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karova K, Wainwright JV, Machova-Urdzikova L, et al. Transplantation of neural precursors generated from spinal progenitor cells reduces inflammation in spinal cord injury via NF-κB pathway inhibition. J Neuroinflamm. 2019;16:12. https://doi.org/ 10.1186/s12974-019-1394-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG.. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. 2006;26:3377-3389. https://doi.org/ 10.1523/JNEUROSCI.4184-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Assinck P, Duncan GJ, Hilton BJ, Plemel JR, Tetzlaff W.. Cell transplantation therapy for spinal cord injury. Nat Neurosci. 2017;20:637-647. https://doi.org/ 10.1038/nn.4541 [DOI] [PubMed] [Google Scholar]
- 10. Bonner JF, Connors TM, Silverman WF, et al. Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal cord. J Neurosci. 2011;31:4675-4686. https://doi.org/ 10.1523/JNEUROSCI.4130-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kadoya K, Lu P, Nguyen K, et al. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat Med. 2016;22:479-487. https://doi.org/ 10.1038/nm.4066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spruance VM, Zholudeva LV, Hormigo KM, et al. Integration of transplanted neural precursors with the injured cervical spinal cord. J Neurotrauma. 2018;35:1781-1799. https://doi.org/ 10.1089/neu.2017.5451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ceto S, Sekiguchi KJ, Takashima Y, et al. Neural stem cell grafts form extensive synaptic networks that integrate with host circuits after spinal cord injury. Cell Stem Cell. 2020;27:430-440.e5.e5. https://doi.org/ 10.1016/j.stem.2020.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bonner JF, Steward O.. Repair of spinal cord injury with neuronal relays: from fetal grafts to neural stem cells. Brain Res. 2015;1619;115-123. https://doi.org/ 10.1016/j.brainres.2015.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McIntyre WB, Pieczonka K, Khazaei M, Fehlings MG.. Regenerative replacement of neural cells for treatment of spinal cord injury. Expert Opin Biol Ther. 2021:1-17. https://doi.org/ 10.1080/14712598.2021.1914582 [DOI] [PubMed] [Google Scholar]
- 16. Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371-1383. https://doi.org/ 10.1089/neu.2004.21.1371 [DOI] [PubMed] [Google Scholar]
- 17. Canty AJ, Murphy M.. Molecular mechanisms of axon guidance in the developing corticospinal tract. Prog Neurobiol. 2008;85:214-235. https://doi.org/ 10.1016/j.pneurobio.2008.02.001 [DOI] [PubMed] [Google Scholar]
- 18. Bonanomi D. Axon pathfinding for locomotion. Semin Cell Dev Biol. 2019;85:26-35. https://doi.org/ 10.1016/j.semcdb.2017.11.014 [DOI] [PubMed] [Google Scholar]
- 19. Chen Z. Common cues wire the spinal cord: axon guidance molecules in spinal neuron migration. Semin Cell Dev Biol. 2019;85:71-77. https://doi.org/ 10.1016/j.semcdb.2017.12.012 [DOI] [PubMed] [Google Scholar]
- 20. Chédotal A. Roles of axon guidance molecules in neuronal wiring in the developing spinal cord. Nat Rev Neurosci. 2019;20:380-396. https://doi.org/ 10.1038/s41583-019-0168-7 [DOI] [PubMed] [Google Scholar]
- 21. Robichaux MA, Cowan CW.. Signaling mechanisms of axon guidance and early synaptogenesis. Curr Top Behav Neurosci. 2014;16:19-48. https://doi.org/ 10.1007/7854_2013_255 [DOI] [PubMed] [Google Scholar]
- 22. Almeida RG. The rules of attraction in central nervous system myelination. Front Cell Neurosci. 2018;12:367. https://doi.org/ 10.3389/fncel.2018.00367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu Y, Shi J, Lu C-C, et al. Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nat Neurosci. 2005;8:1151-1159. https://doi.org/ 10.1038/nn1520 [DOI] [PubMed] [Google Scholar]
- 24. Özdinler PH, Macklis JD.. IGF-I specifically enhances axon outgrowth of corticospinal motor neurons. Nat Neurosci. 2006;9:1371-1381. https://doi.org/ 10.1038/nn1789 [DOI] [PubMed] [Google Scholar]
- 25. Eberhart J, Swartz M, Koblar SA, et al. Expression of EphA4, ephrin-A2 and ephrin-A5 during axon outgrowth to the hindlimb indicates potential roles in pathfinding. Dev Neurosci. 2000;22:237-250. https://doi.org/ 10.1159/000017446 [DOI] [PubMed] [Google Scholar]
- 26. Canty AJ, Greferath U, Turnley AM, Murphy M.. Eph tyrosine kinase receptor EphA4 is required for the topographic mapping of the corticospinal tract. Proc Natl Acad Sci U S A. 2006;103:15629-15634. https://doi.org/ 10.1073/pnas.0607350103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Poliak S, Morales D, Croteau L-P, et al. Synergistic integration of Netrin and ephrin axon guidance signals by spinal motor neurons. eLife. 2015;4:e10841. https://doi.org/ 10.7554/eLife.10841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garrett AM, Jucius TJ, Sigaud LPR, et al. Analysis of expression pattern and genetic deletion of Netrin5 in the developing mouse. Front Mol Neurosci. 2016;9(3). 10.3389/fnmol.2016.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Masuda T, Watanabe K, Sakuma C, et al. Netrin-1 acts as a repulsive guidance cue for sensory axonal projections toward the spinal cord. J Neurosci. 2008;28:10380-10385. https://doi.org/ 10.1523/JNEUROSCI.1926-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guy AT, Nagatsuka Y, Ooashi N, et al. NEURONAL DEVELOPMENT. Glycerophospholipid regulation of modality-specific sensory axon guidance in the spinal cord. Science. 2015;349:974-977. https://doi.org/ 10.1126/science.aab3516 [DOI] [PubMed] [Google Scholar]
- 31. Yoshida Y, Han B, Mendelsohn M, Jessell TM.. PlexinA1 signaling directs the segregation of proprioceptive sensory axons in the developing spinal cord. Neuron. 2006;52:775-788. https://doi.org/ 10.1016/j.neuron.2006.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Watanabe K, Tamamaki N, Furuta T, et al. Dorsally derived netrin 1 provides an inhibitory cue and elaborates the “waiting period” for primary sensory axons in the developing spinal cord. Development. 2006;133:1379-1387. https://doi.org/ 10.1242/dev.02312 [DOI] [PubMed] [Google Scholar]
- 33. Senut MC, Tuszynski MH, Raymon HK, et al. Regional differences in responsiveness of adult CNS axons to grafts of cells expressing human neurotrophin 3. Exp Neurol. 1995;135:36-55. https://doi.org/ 10.1006/exnr.1995.1064 [DOI] [PubMed] [Google Scholar]
- 34. Nakahara Y, Gage FH, Tuszynski MH.. Grafts of fibroblasts genetically modified to secrete NGF, BDNF, NT-3, or basic FGF elicit differential responses in the adult spinal cord. Cell Transplant. 1996;5:191-204. https://doi.org/ 10.1177/096368979600500209 [DOI] [PubMed] [Google Scholar]
- 35. Tuszynski MH, Grill R, Jones LL, et al. NT-3 gene delivery elicits growth of chronically injured corticospinal axons and modestly improves functional deficits after chronic scar resection. Exp Neurol. 2003;181:47-56. https://doi.org/ 10.1016/s0014-4886(02)00055-9 [DOI] [PubMed] [Google Scholar]
- 36. Iannotti C, Li H, Yan P, et al. Glial cell line-derived neurotrophic factor-enriched bridging transplants promote propriospinal axonal regeneration and enhance myelination after spinal cord injury. Exp Neurol. 2003;183:379-393. https://doi.org/ 10.1016/s0014-4886(03)00188-2 [DOI] [PubMed] [Google Scholar]
- 37. Taylor L, Jones L, Tuszynski MH, Blesch A.. Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord. J Neurosci. 2006;26:9713-9721. https://doi.org/ 10.1523/JNEUROSCI.0734-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Deng L-X, Deng P, Ruan Y, et al. A novel growth-promoting pathway formed by GDNF-overexpressing Schwann cells promotes propriospinal axonal regeneration, synapse formation, and partial recovery of function after spinal cord injury. J Neurosci. 2013;33:5655-5667. https://doi.org/ 10.1523/JNEUROSCI.2973-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Glaser T, Brose C, Franceschini I, et al. Neural cell adhesion molecule polysialylation enhances the sensitivity of embryonic stem cell-derived neural precursors to migration guidance cues. Stem Cells. 2007;25:3016-3025. https://doi.org/ 10.1634/stemcells.2007-0218 [DOI] [PubMed] [Google Scholar]
- 40. Khazaei M, Ahuja CS, Nakashima H, et al. GDNF rescues the fate of neural progenitor grafts by attenuating Notch signals in the injured spinal cord in rodents. Sci Transl Med. 2020;12:eaau3538. https://doi.org/ 10.1126/scitranslmed.aau3538 [DOI] [PubMed] [Google Scholar]
- 41. Bilchak JN, Caron G, Côté M-P.. Exercise-induced plasticity in signaling pathways involved in motor recovery after spinal cord injury. Int J Mol Sci. 2021;22:4858. https://doi.org/ 10.3390/ijms22094858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hubel DH, Wiesel TN.. Receptive fields of cells in striate cortex of very young, visually inexperienced kittens. J Neurophysiol. 1963;26:994-1002. https://doi.org/ 10.1152/jn.1963.26.6.994 [DOI] [PubMed] [Google Scholar]
- 43. Wiesel TN, Hubel DH.. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:1003-1017. https://doi.org/ 10.1152/jn.1963.26.6.1003 [DOI] [PubMed] [Google Scholar]
- 44. Sandrow-Feinberg HR, Houlé JD.. Exercise after spinal cord injury as an agent for neuroprotection, regeneration and rehabilitation. Brain Res. 2015;1619:12-21. https://doi.org/ 10.1016/j.brainres.2015.03.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jack AS, Hurd C, Forero J, et al. Cortical electrical stimulation in female rats with a cervical spinal cord injury to promote axonal outgrowth. J Neurosci Res. 2018;96:852-862. https://doi.org/ 10.1002/jnr.24209 [DOI] [PubMed] [Google Scholar]
- 46. Monje M. Myelin plasticity and nervous system function. Annu Rev Neurosci. 2018;41:61-76. https://doi.org/ 10.1146/annurev-neuro-080317-061853 [DOI] [PubMed] [Google Scholar]
- 47. Stadelmann C, Timmler S, Barrantes-Freer A, Simons M.. Myelin in the central nervous system: structure, function, and pathology. Physiol Rev. 2019;99:1381-1431. https://doi.org/ 10.1152/physrev.00031.2018 [DOI] [PubMed] [Google Scholar]
- 48. Gibson EM, Purger D, Mount CW, et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344:1252304. https://doi.org/ 10.1126/science.1252304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Luo M, Yin Y, Li D, et al. Neuronal activity-dependent myelin repair promotes motor function recovery after contusion spinal cord injury. Brain Res Bull. 2021;166:73-81. https://doi.org/ 10.1016/j.brainresbull.2020.11.009 [DOI] [PubMed] [Google Scholar]
- 50. Keeler BE, Liu G, Siegfried RN, et al. Acute and prolonged hindlimb exercise elicits different gene expression in motoneurons than sensory neurons after spinal cord injury. Brain Res. 2012;1438:8-21. https://doi.org/ 10.1016/j.brainres.2011.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Keefe KM, Sheikh IS, Smith GM.. Targeting neurotrophins to specific populations of neurons: NGF, BDNF, and NT-3 and their relevance for treatment of spinal cord injury. Int J Mol Sci. 2017;18:548. https://doi.org/ 10.3390/ijms18030548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hwang DH, Shin HY, Kwon MJ, et al. Survival of neural stem cell grafts in the lesioned spinal cord is enhanced by a combination of treadmill locomotor training via insulin-like growth factor-1 signaling. J Neurosci. 2014;34:12788-12800. https://doi.org/ 10.1523/JNEUROSCI.5359-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Younsi A, Zheng G, Scherer M, et al. Treadmill training improves survival and differentiation of transplanted neural precursor cells after cervical spinal cord injury. Stem Cell Res. 2020;45:101812. https://doi.org/ 10.1016/j.scr.2020.101812 [DOI] [PubMed] [Google Scholar]
- 54. Montoya CP, Campbell-Hope LJ, Pemberton KD, Dunnett S.. The “staircase test”: a measure of independent forelimb reaching and grasping abilities in rats. J Neurosci Methods. 1991. https://doi.org/ 10.1016/0165-0270(91)90048-5 [DOI] [PubMed] [Google Scholar]
- 55. Metz GA, Whishaw IQ.. Skilled reaching an action pattern: stability in rat (Rattus norvegicus) grasping movements as a function of changing food pellet size. Behav Brain Res. 2000;116:111-122. https://doi.org/ 10.1016/s0166-4328(00)00245-x [DOI] [PubMed] [Google Scholar]
- 56. Wang D, Ichiyama RM, Zhao R, Andrews MR, Fawcett JW.. Chondroitinase combined with rehabilitation promotes recovery of forelimb function in rats with chronic spinal cord injury. J Neurosci. 2011;31:9332-9344. https://doi.org/ 10.1523/JNEUROSCI.0983-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fenrich KK, May Z, Torres-Espín A, et al. Single pellet grasping following cervical spinal cord injury in adult rat using an automated full-time training robot. Behav Brain Res. 2016;299:59-71. https://doi.org/ 10.1016/j.bbr.2015.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hubscher CH, Herrity AN, Williams CS, et al. Improvements in bladder, bowel and sexual outcomes following task-specific locomotor training in human spinal cord injury. PLoS One. 2018;13:e0190998. https://doi.org/ 10.1371/journal.pone.0190998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lu P, Freria CM, Graham L, et al. Rehabilitation combined with neural progenitor cell grafts enables functional recovery in chronic spinal cord injury. 2022. https://insight.jci.org/articles/view/158000/figure/3. Accessed August 24, 2022. [DOI] [PMC free article] [PubMed]
- 60. Babona-Pilipos R, Popovic MR, Morshead CM.. A galvanotaxis assay for analysis of neural precursor cell migration kinetics in an externally applied direct current electric field. J Vis Exp. 2012:4193. https://doi.org/ 10.3791/4193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Iwasa SN, Rashidi A, Sefton E, et al. Charge-balanced electrical stimulation can modulate neural precursor cell migration in the presence of endogenous electric fields in mouse brains. eNeuro. 2019;6. https://doi.org/ 10.1523/ENEURO.0382-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Feng J-F, Liu J, Zhang L, et al. Electrical guidance of human stem cells in the rat brain. Stem Cell Rep. 2017;9:177-189. https://doi.org/ 10.1016/j.stemcr.2017.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shapiro S, Borgens R, Pascuzzi R, et al. Oscillating field stimulation for complete spinal cord injury in humans: a phase 1 trial. J Neurosurg Spine. 2005;2:3-10. https://doi.org/ 10.3171/spi.2005.2.1.0003 [DOI] [PubMed] [Google Scholar]
- 64. Walters BC. Oscillating field stimulation in the treatment of spinal cord injury. PM&R. 2010;2:S286-S291. https://doi.org/ 10.1016/j.pmrj.2010.10.014 [DOI] [PubMed] [Google Scholar]
- 65. Hamasaki T, Tanaka N, Ishida O, et al. Characterization of labeled neural progenitor cells for magnetic targeting. Neuroreport. 2005;16(15):1641-1645. [PubMed] [Google Scholar]
- 66. Song M, Kim Y-J, Kim Y, et al. Using a neodymium magnet to target delivery of ferumoxide-labeled human neural stem cells in a rat model of focal cerebral ischemia. Hum Gene Ther. 2010;21:603-610. https://doi.org/ 10.1089/hum.2009.144 [DOI] [PubMed] [Google Scholar]
- 67. Yun S, Shin T-H, Lee J-H, et al. Design of magnetically labeled cells (Mag-Cells) for in vivo control of stem cell migration and differentiation. Nano Lett. 2018;18:838-845. https://doi.org/ 10.1021/acs.nanolett.7b04089 [DOI] [PubMed] [Google Scholar]
- 68. Hamasaki T, Tanaka N, Kamei N, et al. Magnetically labeled neural progenitor cells, which are localized by magnetic force, promote axon growth in organotypic cocultures. Spine. 2007;32:2300-2305. https://doi.org/ 10.1097/BRS.0b013e318154c651 [DOI] [PubMed] [Google Scholar]
- 69. Lamanna JJ, Urquia LN, Hurtig CV, et al. Magnetic resonance imaging-guided transplantation of neural stem cells into the porcine spinal cord. Stereotact Funct Neurosurg. 2017;95:60-68. https://doi.org/ 10.1159/000448765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Malloy KE, Li J, Choudhury GR, et al. Magnetic resonance imaging-guided delivery of neural stem cells into the basal ganglia of nonhuman primates reveals a pulsatile mode of cell dispersion. Stem Cells Transl Med. 2017;6:877-885. https://doi.org/ 10.5966/sctm.2016-0269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nagoshi N, Khazaei M, Ahlfors J-E, et al. Human spinal oligodendrogenic neural progenitor cells promote functional recovery after spinal cord injury by axonal remyelination and tissue sparing. Stem Cells Transl Med. 2018;7:806-818. https://doi.org/ 10.1002/sctm.17-0269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hejrati N, McIntyre WB, Pieczonka K, et al. Chapter 31 - Translational research in spinal cord injury – what is in the future? In: Fehlings MG, Kwon BK, Vaccaro AR, et al. eds. Neural Repair and Regeneration After Spinal Cord Injury and Spine Trauma. Academic Press; 2022:587-602. [Google Scholar]
- 73. Sugai K, Sumida M, Shofuda T, et al. First-in-human clinical trial of transplantation of iPSC-derived NS/PCs in subacute complete spinal cord injury: study protocol. Regen Ther. 2021;18:321-333. https://doi.org/ 10.1016/j.reth.2021.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the corresponding authors on reasonable request.