Abstract
Purpose
Premature infants at risk of retinopathy of prematurity (ROP) miss placental transfer of the carotenoids lutein (L) and zeaxanthin (Z) during the third trimester. We previously demonstrated that prenatal L and Z supplementation raised carotenoid levels in infants at birth in the Lutein and Zeaxanthin in Pregnancy (L-ZIP) study (NCT03750968). Based on their antioxidant effects and bioavailability, we hypothesized that prenatal maternal supplementation with macular carotenoids would reduce the risk of ROP. To test this hypothesis, we utilized “macular pigment mice” genetically engineered to take up L and Z into the retina in a model of oxygen-induced retinopathy (OIR).
Methods
Pregnant Bco2−/− mice were divided into nine experimental subgroups based on the type of supplementation (L, Z, or placebo) and on the maternal supplementation start date corresponding to the three trimesters of human fetal development (E0, E11, and P1). Pups and nursing mothers were exposed to 75% O2 for 5 days (P7–P12) and returned to room air for 5 days (P12–P17). Pups were killed at P12 and P17, and their retinas were analyzed for vaso-obliteration and intravitreal neovascularization.
Results
Pups of pregnant mice supplemented with L or Z had significant reductions in areas of vaso-obliteration and intravitreal neovascularization compared to placebo. Prenatal carotenoid supplementation starting at E0 or E11 was significantly more protective against OIR than postnatal supplementation starting at P1.
Conclusions
Prenatal supplementation with L and Z was beneficial in a mouse OIR model. We recommend testing prenatal L and Z supplementation in future human clinical trials to prevent ROP.
Keywords: Bco2 knockout mice, carotenoids, lutein, oxygen-induced retinopathy, oxidative stress, retinopathy of prematurity, zeaxanthin
Retinopathy of prematurity (ROP), a retinal vasoproliferative disease that affects premature babies, is one of the most common causes of childhood blindness.1 The pathogenesis of ROP is multifactorial, and its major risk factors are low birth weight, young gestational age, oxygen stresses, and poor nutrition. ROP has been described by a two-phase hypothesis based on animal models of oxygen-induced retinopathy (OIR). The first phase is characterized by disruption of normal retinal vascular growth caused by fluctuations in oxygenation, hyperoxia-induced damage to newly developed capillaries, oxidative stress, and other preterm complications. This is followed by hypoxia-induced neovascularization that may lead to retinal detachment and vision loss.2,3 In a normal human fetus, retinal development starts from the optic nerve and proceeds toward the periphery at ∼16 weeks of gestation and is completed around 36 weeks of gestation.4 Preterm infants with an immature cardiopulmonary system may require oxygen therapy, but oxygen stresses can lead to areas of avascular retina, which can become hypoxic as the infants are transitioned to room air. This is believed to lead to hypoxia-induced pathological vasoproliferation.5
Oxidative stress is a major risk factor in the development and progression of ROP6 because life-saving therapies in neonatal intensive care units may expose premature infants to high oxygenation, intense light, multiple medications, and stressful conditions. Also preterm babies are more susceptible to oxidative stress because the protective antioxidant system of the immature retina has insufficient ability to quench reactive oxygen species.7 Lutein (L) and zeaxanthin (Z) are natural antioxidants commonly present in human diets, including fruits, vegetables, and egg yolks. L, Z, and their ocular metabolite meso-zeaxanthin (MZ) are collectively known as the macular pigment (MP) and are preferentially localized in the foveal region of the human macula, an area specialized for central and distinct spatial vision. MP protects the macula from light-induced oxidative damage through its blue-light-filtering properties and antioxidant and anti-inflammatory activities.8,9 Macular carotenoids with conjugated double-bond structures are strong scavengers of singlet oxygen and peroxyl radicals.10 Carotenoids react with reactive oxygen species (ROS) via oxidation, electron transfer, or hydrogen abstraction.11 The retina is a potential site for the generation of ROS where the outer segments of retinal photoreceptors are rich in polyunsaturated fatty acids that are susceptible to photo-oxidation.8 The anti-inflammatory activity of macular carotenoids acts via blocking the translocation of nuclear factor kappa B (NF-κB) to the nucleus. Carotenoids are able to interact with the NF-κB pathway and thus inhibit the downstream production of inflammatory cytokines such as interleukin-8 or prostaglandin E2.12
L and Z may play a crucial role in prenatal life, as evidenced by their presence in the placenta and cord blood at the time of birth due to placental transfer of carotenoids from the mother to the fetus, especially during the third trimester.13 The presence of macular carotenoids in prenatal eyes as early as 17 to 22 weeks of gestation suggests a potential role in the development of the primate fovea and other important retinal structures.14–16 The MP carotenoids continue to accumulate in the human retina from birth until at least 7 years of age.17 L and Z are the most abundant dietary carotenoids in breast milk, and their deficiency may negatively influence an infant's health.18 Furthermore, it has been observed that the mother's carotenoid status may be depleted during pregnancy as she transfers these nutrients to her child.19 Premature babies miss the third-trimester placental transfer of carotenoids, and they may rapidly become further carotenoid depleted due to their exposure to severe oxidative stress.13 Moreover, currently available prenatal vitamins and premature infant nutritional formulations typically do not have any added L or Z,20 but we have recently shown that L (10 mg/d) and Z (2 mg/d) in human prenatal supplements safely increased the systemic and ocular carotenoid status of the mother and her newborn infant (NCT03750968).21
Published clinical studies of the potential benefit of L and Z supplements for the prevention of ROP have focused on postnatal infant supplementation to preemies at concentrations comparable to an Age-Related Eye Disease Study 2 (AREDS2) dose on a milligram-per-kilogram basis. These studies did not show a statistically significant decrease in disease progression,22–24 but we speculate that these interventions may have been administered too late. Therefore, we hypothesize that prenatal maternal supplementation with macular carotenoids given to the mother at an AREDS2 dose could alleviate ROP pathology at a rate better than shown by prior postnatal studies.
Before proceeding to human clinical trials of prenatal L and Z for prevention of ROP, it is important to perform efficacy tests in a suitable animal model that actively accumulates dietary carotenoids into the retina. The mouse OIR model is a well-established, consistent, and economical model to mimic aspects of the two-phased hypothesis: phase I, in which there is damage to newly developed capillaries, sometimes termed vaso-obliteration (VO); and phase II, in which there is intravitreal neovascularization (INV). Retinal vascular development occurs ex utero in mice because they are born with incomplete retina vasculature at full-term birth. Until recently, however, the potential efficacy of carotenoids against mouse OIR was not testable because wild-type (WT) mice do not accumulate retinal carotenoids even in the face of high-dose supplementation. To address this problem of poor retinal uptake of carotenoids, we previously developed transgenic “macular pigment mice” whose xanthophyll cleavage enzyme, beta-carotene oxygenase 2 (Bco2), has been knocked out. Bco2−/− mice readily accumulate L and Z in their retinas in response to oral supplementation.25,26 Here, we used these macular pigment mice to test the relative efficacies of prenatal and postnatal L and Z in a mouse model of OIR.
Materials and Methods
Materials
L and Z high-performance liquid chromatography (HPLC) standards (≥98% pure) were provided by Kemin Health (Des Moines, IA, USA) and DSM Nutritional Products (Kaiseraugst, Switzerland), respectively. L, Z, and placebo Actilease beadlets were supplied by DSM Nutritional Products. L and Z Actilease beadlets were mixed with a previously reported base diet (TestDiet; Land O'Lakes Purina Feed, Richmond, IN, USA) at a dosage of 1 g/kg (∼2.6 mg per mouse per day).27 All organic solvents were of HPLC grade and were purchased from Thermo Fisher Scientific (Waltham, MA, USA).
Animals
Bco2−/− breeding pairs on a C57BL/6J background were received from Case Western Reserve University (Cleveland, OH, USA). Bco2−/− mice were provided food and water ad libitum and kept on a 12-hour light/dark cycle. Animal experiments were designed and performed according to the guidelines and regulations of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Experimental animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Utah. Pregnant mice were supplemented with L and Z through Actilease beadlets in a base diet as described above, providing in utero supplementation to fetuses. Pups were nourished postnatally through maternal milk.
Oxygen-Induced Retinopathy
Female Bco2−/− mice were mated with male Bco2−/− mice, and pregnancy was confirmed based on the presence of a vaginal plug at embryonic day 0 (E0). Pregnant Bco2−/− mice were divided into three experimental groups based on the start date of supplementation (E0, E11, and postnatal day 1 [P1]), dates chosen to be comparable to the beginnings of the three trimesters of human pregnancy.28 Each experimental group was further divided into three supplementation subgroups (L, Z, or placebo diets). At every evaluation time point (P12 and P17), each subgroup had at least three pups from at least two different litters. OIR was induced in newborn mice as previously described.29 Briefly, on P7, when the inner vascular plexus covers the retinal extent, Bco2−/− mouse litters from all three groups along with their nursing mothers were exposed to 75% oxygen for 5 days (until P12) in a ProOx model P360 hyperoxia chamber (BioSpherix, Parish, NY, USA) to initiate OIR and then returned to room air (21% oxygen) for another 5 days (until P17) for vascular regrowth and INV until the pups were killed at P12 or P17. A schematic summary of the research protocol is provided in Supplementary Table A. Although the number of pups per litter tends to be smaller whenever carotenoid supplementation is started prenatally in Bco2−/− mice, Supplementary Figure A shows that pups in our various subgroups had identical weight gain at P7, P12, and P17.
Mouse pups were killed with CO2 gas, and the whole eyes (right and left) were enucleated and fixed in a single Eppendorf tube filled with 4% paraformaldehyde for 1 hour. The eyes were dissected under a stereo zoom microscope (SMZ-168; Motic, Hong Kong) to separate the retina from the retinal pigment epithelium/choroid. Each retina was washed three times with PBS for 5 to 10 minutes. The separated retinal tissues were treated with blocking solution for 1 hour at room temperature. The blocking solution consisted of 10% Gibco Normal Goat Serum (PCN5000; Thermo Fisher Scientific), 80% 1× PBS prepared from 10× PBS (46-013-CM; Corning, Corning, NY, USA), and 10% Triton X-100 prepared from Triton X-100 (T8787; Sigma-Aldrich, St. Louis, MO, USA).
Separated retinas were then stained overnight (8–12 hours) at 4°C. The staining solution consisted of 5% Gibco Normal Goat Serum, 84.5% 1× PBS prepared from 10× PBS, 10% Triton X-100 prepared from Triton X-100, and 0.5% Invitrogen Isolectin GS-IB4 from Griffonia simplicifolia, Alexa Fluor 568 Conjugate (I21412; Thermo Fisher Scientific). The retina was divided into four quadrants by four radial cuts that were made from the edge to the center and then mounted on a glass slide with a few drops of Fluoromount-G mounting medium (Southern Biotech, Birmingham, AL, USA). Both eyes were mounted on a labeled glass slide adjacent to each other. Retinal flatmount images were captured via a confocal laser scanning microscope (Fluoview FV1000; Olympus, Tokyo, Japan). The central avascular areas (blue marking) and the total retinal areas were measured manually using Image J (National Institutes of Health, Bethesda, MD, USA). The areas of INV (green marking) and the total retinal area were quantified with Image J and Image-Pro Plus (Media Cybernetics, Rockville, MD, USA), and the results were expressed as a percentage of total retinal area.29,30
Extraction of Retinal Carotenoids
Retinal carotenoids were extracted from pooled retinas of P17 mouse pups whose mothers had started carotenoid supplementation at P1 (n = 4 pairs of retina). The samples were placed in 1 mL tetrahydrofuran + 0.1% butylated hydroxytoluene and 200 µL ethanol and sonicated in an ice-cold water bath for 10 minutes. The extract was then vortexed and centrifuged at 2500 g for 5 minutes, and the supernatant was collected in a separate tube. The extraction step was repeated two to three more times until the solvent turned colorless. All supernatants were pooled and then evaporated under nitrogen gas at 45°C on an evaporator system (Glas-Col, Terre Haute, IN, USA). The dried residue was dissolved in HPLC mobile phase which was then centrifuged at 2500 g for 5 minutes, and the supernatant was injected into an HPLC system for separation and quantification as previously described.26
Statistics
Data are presented as mean ± SD. Statistical comparisons were made using a one-way ANOVA followed by Student's t-tests and Tukey's multiple comparison tests. Both eyes were imaged, but for scoring and statistical analysis we typically chose one randomly selected eye per pup unless that eye had an unusable image.
Results
Retinal Bioavailability of Maternally Administered Carotenoids in Bco2−/− Mouse Pups
WT mice do not accumulate orally administered L or Z in their retinas even in the setting of high-dose supplementation, but we have previously demonstrated that Bco2−/− “macular pigment mice” take up L and Z into their retinas when they are provided in Actilease beadlets mixed with their normal diet at a dose of 2.6 mg/d/mouse.25 It has not been shown, however, that maternally administered L or Z can be delivered to their pups’ retinas. The retinas of newborn P1 mouse pups are too small to detect carotenoid levels in the range necessary for HPLC to prove placental carotenoid transmission, so we measured levels from larger P17 mouse retinas to confirm retinal delivery via breastmilk. Figure 1 examines retinal carotenoid bioavailability in pups nursed by their Bco2−/− and WT mouse mothers who were fed with placebo-, L-, or Z-supplemented chow starting at P1 and then subjected to our standard OIR protocol. At P17, WT mouse pups never had any detectable retinal carotenoids in any of the supplementation groups. P17 Bco2−/− mouse pups had no detectable retinal carotenoids when their mothers were fed placebo beadlet chow, whereas L and Z supplementation resulted in retinal accumulation of 103 pg/mouse and 125 pg/mouse for L and Z, respectively.
Figure 1.
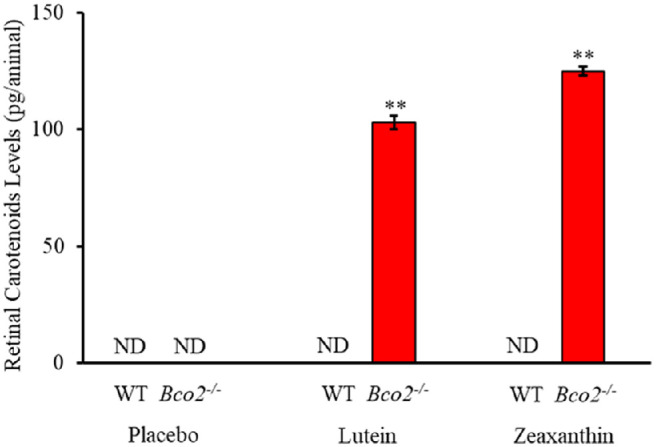
Retinal carotenoids in WT and Bco2−/− mice subjected to OIR where the nursing mother along with her pups were fed with L, Z, or placebo diets starting at postnatal day 1 (P1) and killed on P17. Retinal carotenoids were never detectable in WT mice. Values indicate means ± SD for three batches of eight pooled eyes for each subgroup. ND, not detectable. **P < 0.01 compared to the placebo-fed group and to the WT controls.
OIR Studies in Bco2−/− Mouse Pups
Nursing mothers along with their litters were subjected to a hyperoxic environment (75% O2) from P7 to P12 in the OIR chamber causing VO in the central retina of the pups. The central avascular retinal area was measured at P12. Figure 2 shows that pups of Bco2−/− mothers fed prenatally with a placebo diet starting at E0 (equivalent to the beginning of the first trimester of human pregnancy) exhibited a significantly higher percentage of VO (28.3%) compared to the L-fed group (10.7%) and the Z-fed group (8.6%). Likewise, pups of Bco2−/− mothers fed with the placebo diet starting at E11 (equivalent to the beginning of the second trimester of human pregnancy) had a significantly higher percentage of VO (27.7%) relative to the L-fed group (12.6%) and the Z-fed group (10.3%) (Fig. 3). When maternal carotenoid supplementation was initiated postnatally at P1, a time point in mouse retinal development equivalent to the beginning of the third trimester of human pregnancy, L and Z were still significantly protective against OIR VO (26.2%, 20.2%, and 18.9% for placebo, L, and Z, respectively), but with less efficacy (Fig. 4). A replot of the data from these experiments in Figure 5 demonstrates that prenatally initiated supplementation with L or Z was always significantly better than postnatally initiated supplementation. There was a consistent trend that Z supplementation was superior to L supplementation, but this was not statistically significant.
Figure 2.
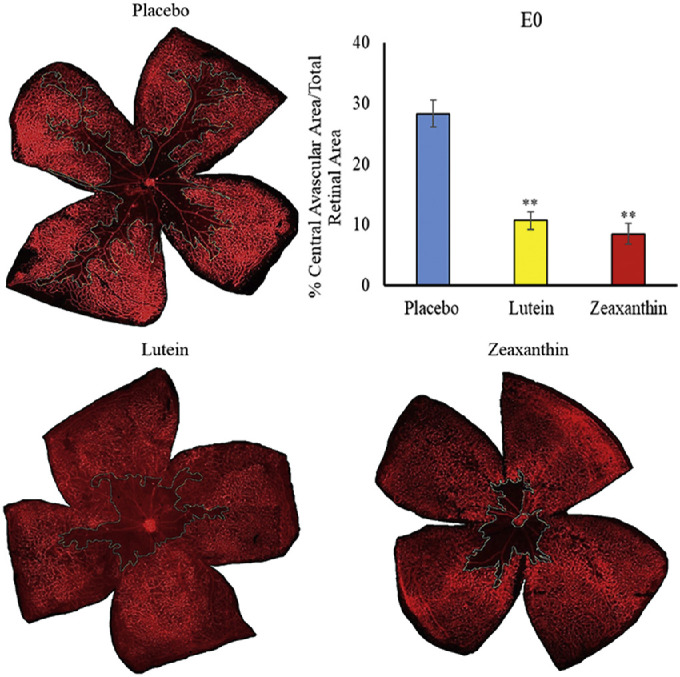
Representative lectin-stained flatmounts of retinas from Bco2−/− mouse pups killed at P12 showing VO after treatment under our OIR protocol. Their mothers started supplementation with L, Z, or placebo diets at E0. The blue marking shows the central avascular area. **P < 0.01 compared to the placebo group. The placebo group included a total of six pups from two litters; the L group, a total of four pups from four litters; and the Z group, a total of four pups from three litters. Refer to Supplementary Figure B for unmarked versions of the retinal flatmounts.
Figure 3.
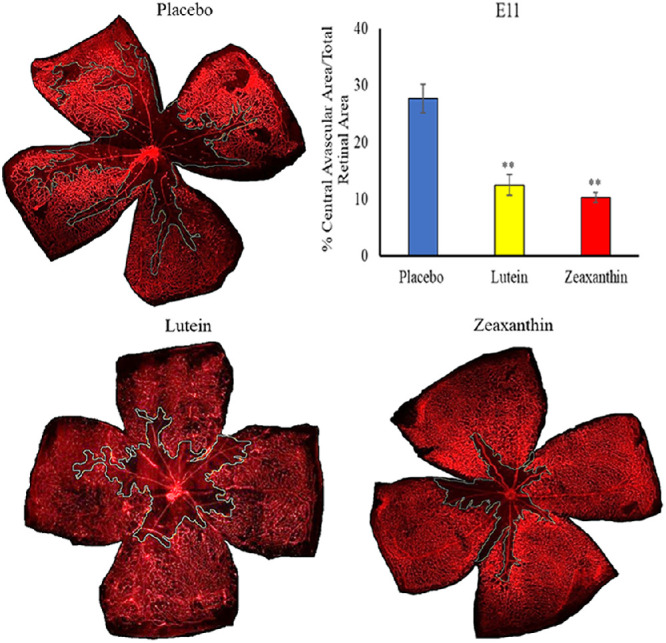
Representative lectin-stained flatmounts of retinas from Bco2−/− mouse pups killed at P12 showing VO after treatment under our OIR protocol. Their mothers started supplementation with L, Z, or placebo diets at E11. The blue marking shows the central avascular area. **P < 0.01 compared to the placebo group. The placebo group included a total of five pups from two litters; the L group, a total of five pups from three litters; and the Z group, a total of five pups from three litters Refer to Supplementary Figure C for unmarked versions of the retinal flatmounts.
Figure 4.
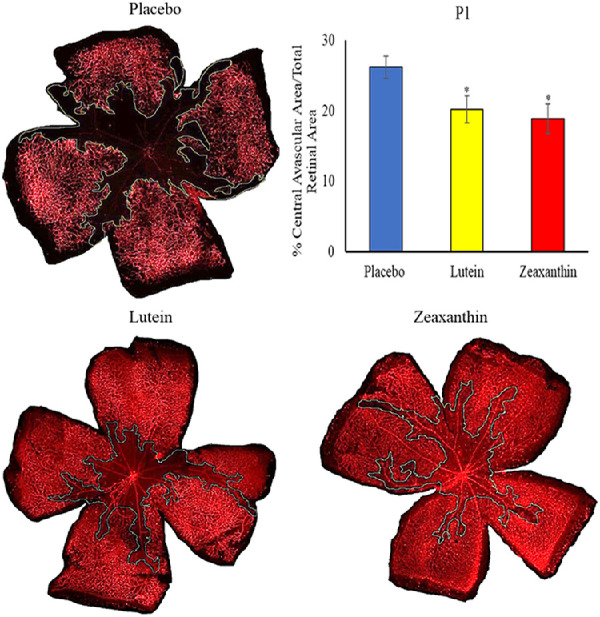
Representative lectin-stained flatmounts of retinas from Bco2−/− mouse pups killed at P12 showing VO after treatment under our OIR protocol. Their mothers started supplementation with L, Z, or placebo diets at P1. The blue marking shows the central avascular area. *P < 0.05 compared to the placebo group. The placebo group included a total of six pups from two litters; the L group, a total of five pups from two litters; and the Z group, a total of five pups from two litters. Refer to Supplementary Figure D for unmarked versions of the retinal flatmounts.
Figure 5.
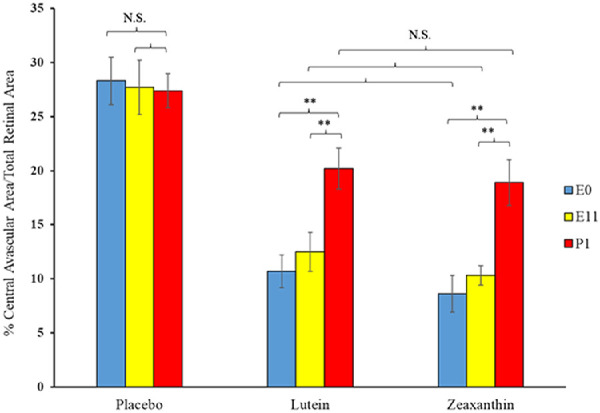
Replot of the VO results from the OIR experiments of Figures 2 to 4. Pregnant Bco2−/− mice started L, Z, or placebo supplementation on E0, E11, or P1, time points in mouse gestation corresponding to the three trimesters of retinal development during human pregnancy. **P < 0.01 compared to the postnatal group P1. NS, not significant.
When nursing Bco2−/− mothers along with their litters were moved from the hyperoxia environment (75% O2) on P12 to room air (21% O2) for 5 days, neovascular tufts formed corresponding to the second phase of human ROP. Figures 6 to 8 show the INV area of retinal flatmounts of P17 Bco2−/− mouse pups whose mothers were fed with placebo, L, or Z diets starting at E0, E11, or P1. Compared to the placebo-fed groups, which demonstrated 8.3% INV, groups fed prenatal supplementation of L and Z starting at E0 had significantly reduced retinal INV at 4.5% and 3.0%, respectively (Fig. 6). Likewise, prenatal supplementation of L and Z at E11 to pregnant mothers showed significantly reduced retinal INV areas at 5.0% and 4.1%, respectively, compared to the placebo-fed groups at 7.9% (Fig. 7). Pups of Bco2−/− mouse mothers postnatally fed with the placebo diet starting at P1 had a significantly higher percentage of INV (7.6%) compared to the L-fed group (5.7%) and the Z-fed group (5%), respectively (Fig. 8). Similar to our VO experiments, a replot of the data from these INV experiments in Figure 9 demonstrates that prenatally initiated supplementation with L or Z was always better than postnatally initiated supplementation, but the improvement was statistically significant only if carotenoid supplementation was initiated at E0. Again, there was a consistent trend that Z supplementation was superior to L supplementation, but this was not statistically significant.
Figure 6.
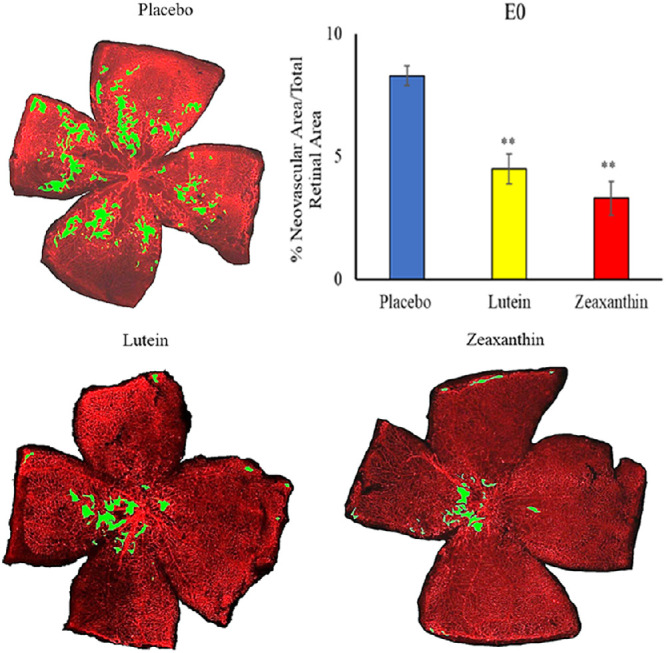
Representative lectin-stained flatmounts of retinas from Bco2−/− mouse pups killed at P17 showing INV after treatment under our OIR protocol. Their mothers started supplementation with L, Z, or placebo diets at E0. The green marking shows the INV area. **P < 0.01 compared to the placebo group. The placebo group included a total of five pups from three litters; the L group, a total of three pups from three litters; and the Z group, a total of three pups from three litters. Refer to Supplementary Figure E for unmarked versions of the retinal flatmounts.
Figure 8.
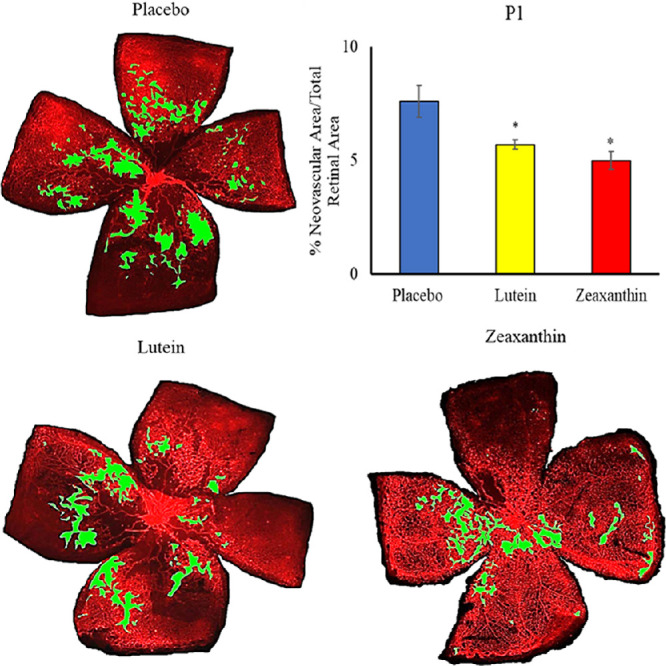
Representative lectin-stained flatmounts of retinas from Bco2−/− mouse pups killed at P17 showing INV after treatment under our OIR protocol. Their mothers started supplementation with L, Z, or placebo diets at P1. The green marking shows the INV area. **P < 0.05 compared to the placebo group. The placebo group included a total of five pups from two litters; the L group, a total of five pups from two litters; and the Z group, a total of five pups from two litters. Refer to Supplementary Figure G for unmarked versions of the retinal flatmounts.
Figure 7.
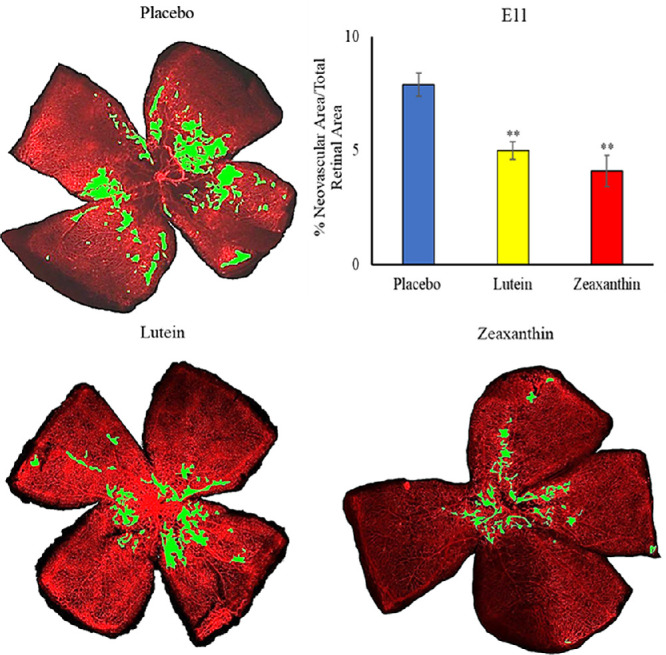
Representative lectin-stained flatmounts of retinas from Bco2−/− mouse pups killed at P17 showing INV after treatment under our OIR protocol. Their mothers started supplementation with L, Z, or placebo diets at E11. The green marking shows the INV area. **P < 0.01 compared to the placebo group. The placebo group included a total of five pups from two litters; the L group, a total of four pups from two litters; and the Z group, a total of four pups from two litters. Refer to Supplementary Figure F for unmarked versions of the retinal flatmounts.
Figure 9.
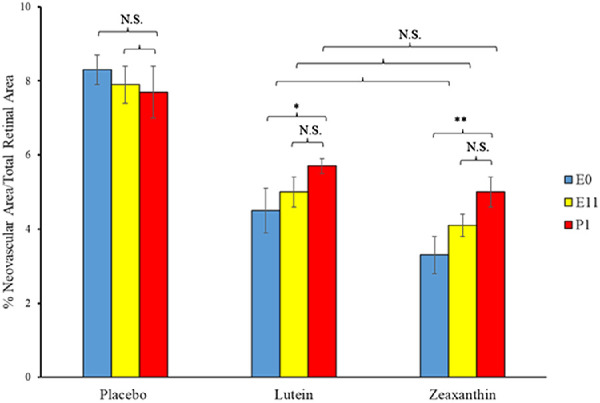
Replot of the INV results from the OIR experiments of Figures 6 to 8. Pregnant Bco2−/− mice started L, Z, or placebo supplementation on E0, E11, or P1, time points in mouse gestation corresponding to the three trimesters of retinal development during human pregnancy. *P < 0.05, **P < 0.01 compared to the P1 postnatal group. NS, not significant.
Discussion
In designing this study, we hypothesized that maternal prenatal supplementation with eye-protective carotenoids may help to prevent or ameliorate human ROP and tested this hypothesis in a mouse model of OIR. This hypothesis was based on the specific prenatal deposition of the macular carotenoids in the developing human retina, their well-known antioxidant properties, and the high levels of oxidative stress in premature infants who have not benefited from third-trimester placental transfer of L and Z from the mother to her child. Recent recognition that WT mice are a suboptimal model for diet-based carotenoid interventions against ocular disease guided us to use Bco2−/− mice that reproducibly deposit L and Z in their retinas when supplemented with these MP carotenoids.25
Excessive oxygen or fluctuations in oxygen levels are believed to be involved in the first phase of ROP where there is delayed normal retinal vascular growth and damage to existing capillaries. When premature babies are transferred to room air after oxygen therapy, the avascular retina becomes hypoxic, releases even more ROS, and upregulates angiogenic factors leading to pathological intravitreal neovascularization characterized by neovascular tufts at the junction of the avascular and vascular zones.31
Minimizing ROS and oxidative stress-induced damage in preterm infants through maternal prenatal supplementation with carotenoids could be an effective nutritional therapy against ROP. The macular antioxidant carotenoids L and Z protect the retina from oxidative stress and light-induced damage and can enhance visual performance in adults.32,33 These eye-protective nutrients begin to accumulate in the developing retina during the second trimester of pregnancy and are actively transported in such large amounts from mother to infant during the third trimester that the mother's systemic carotenoid levels may even decline. We have shown both cross-sectionally and in the recently completed Lutein and Zeaxanthin in Pregnancy (L-ZIP) randomized clinical trial that the mother's ocular and systemic carotenoid status strongly influences her newborn's ocular and systemic carotenoid status.21 Moreover, L and Z supplementation at AREDS2 dosages was safe and well tolerated during pregnancy.
The present study demonstrates that Bco2−/− mouse pups whose mothers received oral MP carotenoid supplementation accumulated L and Z in their retinas, whereas WT pups did not. Both prenatal and postnatal supplementation significantly inhibited hyperoxia-induced VO and hypoxia-induced INV OIR in Bco2−/− pups, but prenatal supplementation was more effective than postnatal initiation of supplementation in this mouse model of OIR. Both macular carotenoids attenuated OIR pathology, but they did not affect normal retinal vascular growth or development. Not only were L and Z effective in protecting against OIR pathology in the hyperoxia phase (P7-P12), but they also extended protection into the hypoxia phase (P12–P17). Z was somewhat more effective than L in retinal protection against OIR complications in our Bco2−/− mice, possibly because Z has better bioavailability relative to L in mice26 and because Z has higher antioxidant activity than L due to its longer conjugated double-bond structure.10 Similarly, dietary supplementation with Z in Japanese quail exhibited a better photo-protective effect than L against light-induced photoreceptor damage.34
The molecular mechanisms underlying the protective effect of carotenoids against OIR still have to be studied in detail. L and Z may protect against and ameliorate OIR by controlling vessel loss in an oxygen-stressed environment by reducing the hypoxic stimulus for pathological INV. Studies on the apoE−/− mouse model for AMD-like retinal degeneration and on the streptozotocin-induced diabetic retinopathy mouse model have shown that L treatment downregulates vascular endothelial growth factor expression and elevates antioxidant enzyme levels.35,36
Previously published studies of the potential benefits of L and Z for the prevention and treatment of ROP and OIR have had disappointing results. Even though an AREDS2-type dosage of ∼0.14 mg/kg/d was used in human premature infant studies, supplementation was started only after birth, and systemic bioavailability of the formulation was not confirmed.22–24 Fu et al.37 used WT mice supplemented daily by intraperitoneal administration of 0.2 mg/kg of L in 10% DMSO from P7 to P11 in a mouse model of OIR and found no significant changes in retinal VO or INV area relative to control administration of 10% DMSO. Our contrasting positive results presumably arise from starting our intervention prenatally and the use of Bco2−/− mice that are known to take up carotenoids into their retinas because their highly active xanthophyll cleavage enzyme has been knocked out.
The results of this animal study, coupled with the L-ZIP clinical study results showing the safety and biological efficacy of carotenoid supplementation during pregnancy, suggest that the potential benefits of L and Z against ROP should be revisited, this time in a prospective, randomized clinical trial of prenatal maternal supplementation as opposed to the prior human and animal studies that have focused on postnatal supplementation. Even though there are currently no prenatal vitamins or postnatal premature infant formulas on the market with significant market share that have added L and Z, they can be readily prepared and appear to be safe and well tolerated. ROP continues to be a major public health problem worldwide, and a proven, low-risk, relatively inexpensive nutritional intervention would be welcome.
Supplementary Material
Acknowledgments
The authors thank Johannes von Lintig, PhD, from Case Western Reserve University for generously providing the Bco2−/− founder mice. We also thank Eric Kunz, Lara Carroll, and Punyavathi Arunkumar for their expertise and assistance in flatmounts, imaging, and scoring.
Supported by grants from the National Eye Institute, National Institutes of Health (EY11600 and EY14800 to PSB; EY15130 and EY17011 to MEH); and by an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology and Visual Sciences.
Disclosure: R. Arunkumar, None; B. Li, None; E.K. Addo, None; M.E. Hartnett, None; P.S. Bernstein, None
References
- 1. Kim SJ, Port AD, Swan R, Campbell JP, Chan RVP, Chiang MF.. Retinopathy of prematurity: A review of risk factors and their clinical significance. Surv Ophthalmol. 2018; 63(5): 618–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hartnett ME. Advances in understanding and management of retinopathy of prematurity. Surv Ophthalmol. 2017; 62(3): 257–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hartnett ME, Penn JS.. Mechanisms and management of retinopathy of prematurity. N Engl J Med. 2012; 367(26): 2515–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hughes S, Yang H, Chan-Ling T. Vascularization of the human fetal retina: Roles of vasculogenesis and angiogenesis. Invest Ophthalmol Vis Sci. 2000; 41(5): 1217–1228. [PubMed] [Google Scholar]
- 5. Country MW. Retinal metabolism: A comparative look at energetics in the retina. Brain Res. 2017; 1672: 50–57. [DOI] [PubMed] [Google Scholar]
- 6. Wang H, Zhang SX, Hartnett ME.. Signaling pathways triggered by oxidative stress mediate features of severe retinopathy of prematurity. JAMA Ophthalmol. 2013; 131(1): 80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saugstad OD. Bronchopulmonary dysplasia—oxidative stress and antioxidants. Semin Neonatol. 2003; 8(1): 39–49. [DOI] [PubMed] [Google Scholar]
- 8. Arunkumar R, Gorusupudi A, Bernstein PS.. The macular carotenoids: A biochemical overview. Biochim Biophys Acta Mol Cell Biol Lipids. 2020; 1865(11): 158617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bernstein PS, Arunkumar R.. The emerging roles of the macular pigment carotenoids throughout the lifespan and in prenatal supplementation. J Lipid Res. 2021; 62: 100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li B, Ahmed F, Bernstein PS.. Studies on the singlet oxygen scavenging mechanism of human macular pigment. Arch Biochem Biophys. 2010; 504(1): 56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Britton G. Structure and properties of carotenoids in relation to function. FASEB J. 1995; 9(15): 1551–1558. [PubMed] [Google Scholar]
- 12. Kaulmann A, Bohn T.. Carotenoids, inflammation, and oxidative stress—implications of cellular signaling pathways and relation to chronic disease prevention. Nutr Res. 2014; 34(11): 907–929. [DOI] [PubMed] [Google Scholar]
- 13. Connor SL, Connor WE, Bezzerides EA, Wang Y.. The maternal transfer of lutein and zeaxanthin into the fetus during pregnancy. FASEB J. 2008; 22(suppl 1): 451.4. [Google Scholar]
- 14. Bone RA, Landrum JT, Fernandez L, Tarsis SL.. Analysis of the macular pigment by HPLC: Retinal distribution and age study. Invest Ophthalmol Vis Sci. 1988; 29(6): 843–849. [PubMed] [Google Scholar]
- 15. Kiely M, Cogan PF, Kearney PJ, Morrissey PA.. Concentrations of tocopherols and carotenoids in maternal and cord blood plasma. Eur J Clin Nutr. 1999; 53(9): 711–715. [DOI] [PubMed] [Google Scholar]
- 16. Thoene M, Anderson-Berry A, Van Ormer M, et al.. Quantification of lutein + zeaxanthin presence in human placenta and correlations with blood levels and maternal dietary intake. Nutrients. 2019; 11(1): 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bernstein PS, Sharifzadeh M, Liu A, et al.. Blue-light reflectance imaging of macular pigment in infants and children. Invest Ophthalmol Vis Sci. 2013; 54(6): 4034–4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xue Y, Campos-Giménez E, Redeuil KM, et al.. Concentrations of carotenoids and tocopherols in breast milk from urban Chinese mothers and their associations with maternal characteristics: A cross-sectional study. Nutrients. 2017; 9(11): 1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Picone S, Ritieni A, Fabiano A, et al.. Lutein levels in arterial cord blood correlate with neuroprotein activin A in healthy preterm and term newborns: A trophic role for lutein? Clin Biochem. 2018; 52: 80–84. [DOI] [PubMed] [Google Scholar]
- 20. Chan GM, Chan MM, Gellermann W, et al.. Resonance Raman spectroscopy and the preterm infant carotenoid status. J Pediatr Gastroenterol Nutr. 2013; 56(5): 556–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Addo EK, Gorusupudi A, Allman S, Bernstein PS.. The Lutein and Zeaxanthin in Pregnancy (L-ZIP) study—carotenoid supplementation during pregnancy: Ocular and systemic effects—study protocol for a randomized controlled trial. Trials. 2021; 22(1): 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dani C, Lori I, Favelli F, et al.. Lutein and zeaxanthin supplementation in preterm infants to prevent retinopathy of prematurity: A randomized controlled study. J Matern Fetal Neonatal Med. 2012; 25(5): 523–527. [DOI] [PubMed] [Google Scholar]
- 23. Rubin LP, Chan GM, Barrett-Reis BM, et al.. Effect of carotenoid supplementation on plasma carotenoids, inflammation and visual development in preterm infants. J Perinatol. 2012; 32(6): 418–424. [DOI] [PubMed] [Google Scholar]
- 24. Manzoni P, Guardione R, Bonetti P, et al.. Lutein and zeaxanthin supplementation in preterm very low-birth-weight neonates in neonatal intensive care units: A multicenter randomized controlled trial. Am J Perinatol. 2013; 30(1): 25–32. [DOI] [PubMed] [Google Scholar]
- 25. Li B, Vachali PP, Shen Z, et al.. Retinal accumulation of zeaxanthin, lutein, and β-carotene in mice deficient in carotenoid cleavage enzymes. Exp Eye Res. 2017; 159: 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arunkumar R, Gorusupudi A, Li B, et al.. Lutein and zeaxanthin reduce A2E and iso-A2E levels and improve visual performance in Abca4–/–/Bco2–/– double knockout mice. Exp Eye Res. 2021; 209: 108680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lindshield BL, King JL, Wyss A, et al.. Lycopene biodistribution is altered in 15,15′-carotenoid monooxygenase knockout mice. J Nutr. 2008; 138(12): 2367–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stahl A, Connor KM, Sapieha P, et al.. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci. 2010; 51(6): 2813–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smith LE, Wesolowski E, McLellan A, et al.. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994; 35(1): 101–111. [PubMed] [Google Scholar]
- 30. Connor KM, Krah NM, Dennison RJ, et al.. Quantification of oxygen-induced retinopathy in the mouse: A model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc. 2009; 4(11): 1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saito Y, Uppal A, Byfield G, Budd S, Hartnett ME.. Activated NAD(P)H oxidase from supplemental oxygen induces neovascularization independent of VEGF in retinopathy of prematurity model. Invest Ophthalmol Vis Sci. 2008; 49(4): 1591–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bernstein PS, Li B, Vachali PP, et al.. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog Retin Eye Res. 2016; 50: 34–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li B, Rognon GT, Mattinson T, et al.. Supplementation with macular carotenoids improves visual performance of transgenic mice. Arch Biochem Biophys. 2018; 649: 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thomson LR, Toyoda Y, Delori FC, et al.. Long term dietary supplementation with zeaxanthin reduces photoreceptor death in light-damaged Japanese quail. Exp Eye Res. 2002; 75(5): 529–542. [DOI] [PubMed] [Google Scholar]
- 35. Fernández-Robredo P, Sádaba LM, Salinas-Alamán A, Recalde S, Rodríguez JA, García-Layana A.. Effect of lutein and antioxidant supplementation on VEGF expression, MMP-2 activity, and ultrastructural alterations in apolipoprotein E-deficient mouse. Oxid Med Cell Longev. 2013; 2013: 213505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sharavana G, Baskaran V.. Lutein downregulates retinal vascular endothelial growth factor possibly via hypoxia inducible factor 1 alpha and X-box binding protein 1 expression in streptozotocin induced diabetic rats. J Funct Foods. 2017; 31: 97–103. [Google Scholar]
- 37. Fu Z, Meng SS, Burnim SB, Smith LE, Lo AC.. Lutein facilitates physiological revascularization in a mouse model of retinopathy of prematurity. Clin Exp Ophthalmol. 2017; 45(5): 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


