Abstract
Digital health technologies can provide continuous monitoring and objective, real-world measures of Parkinson’s disease (PD), but have primarily been evaluated in small, single-site studies. In this 12-month, multicenter observational study, we evaluated whether a smartwatch and smartphone application could measure features of early PD. 82 individuals with early, untreated PD and 50 age-matched controls wore research-grade sensors, a smartwatch, and a smartphone while performing standardized assessments in the clinic. At home, participants wore the smartwatch for seven days after each clinic visit and completed motor, speech and cognitive tasks on the smartphone every other week. Features derived from the devices, particularly arm swing, the proportion of time with tremor, and finger tapping, differed significantly between individuals with early PD and age-matched controls and had variable correlation with traditional assessments. Longitudinal assessments will inform the value of these digital measures for use in future clinical trials.
Subject terms: Parkinson's disease, Parkinson's disease
Introduction
Parkinson’s disease (PD) is the world’s fastest-growing neurological disorder1. Despite increasing prevalence and substantial investment from private and public funders, however, therapeutic breakthroughs have been scant this century, especially for early disease2. While rating scales have improved, they still provide subjective, episodic, and largely insensitive assessments contributing to large, lengthy, expensive trials that are prone to failure3,4. Moreover, scales like the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Part III have high inter-observer variability and have faced challenges in detecting disease progression in neuroprotective trials5. In addition, these scales may not accurately assess the patient experience. Better measures could lead to more efficient, patient-centric, and timely evaluation of therapies.
Digital tools can provide objective, sensitive, real-world measures of PD4,6–8. A smartphone research application, previously used in phase 1 and 2 clinical trials, differentiated individuals with early PD from age-matched controls through finger tapping and detected tremor not apparent to investigators9,10. Similarly, a smartwatch measured tremor and detected motor fluctuations and dyskinesias11.
Despite this promising pilot data, few studies have assessed multiple digital devices in a multicenter study that replicates a clinical trial setting in individuals with early, untreated PD. We sought to evaluate the ability of research-grade wearable sensors, a smartwatch and a smartphone to assess key features of PD. We used a platform specifically designed to incorporate several assessments that map directly onto the MDS-UPDRS, providing an objective digital analog to subjective clinical measurements. We aimed to determine the specific disease features these digital tools can detect, whether the measures differed between individuals with early PD and age-matched controls, and how well the digital measures correlated with traditional ones. Here, we report the results of the baseline analyses of a 12-month longitudinal study, focused on the smartphone application and smartwatch results from the first in clinic visit and at-home passive monitoring period following that visit.
Results
Study participants
Eighty-two individuals with early, untreated PD and 50 age-matched controls completed informed consent to participate in this WIRB-Copernicus Group (WCG)TM Institutional Review Board approved study at 17 research sites between June 2019 and December 2020 (Supplemental Fig. 1). Participants with PD were more likely to be men and were similar to those in the PPMI study (Table 1)3,12–15.
Table 1.
Baseline characteristics of research participants in this study and de novo Parkinson’s disease participants in the Parkinson’s Progression Markers Initiative.
| Characteristic | PD cohort (n = 82) | Control cohort (n = 50) | p value | PPMI de novo cohort (n = 423) | |
|---|---|---|---|---|---|
| Demographic characteristics | Age, y | 63.3 (9.4) | 60.2 (9.9) | 0.07 | 61.7 (9.7) |
| Male, n (%) | 46 (56) | 18 (36) | 0.03 | 277 (65) | |
| Race, n (%) | 0.81 | ||||
| White | 78 (95) | 48 (96) | 391 (92) | ||
| Black or African American | 0 (0.0) | 0 (0) | 6 (1) | ||
| Asian | 3 (4) | 1 (2) | 8 (2) | ||
| Not specified | 1 (1) | 1 (2) | 18 (4) | ||
| Hispanic or Latino, n (%) | 3 (4) | 1 (2) | 0.99 | 9 (2) | |
| Education >12 Years, n (%) | 78 (95) | 48 (96) | 0.99 | 347 (82) | |
| Clinical characteristics | Right or mixed handedness, n (%) | 74 (90) | 47 (94) | 0.53 | 385 (91) |
| Parkinson’s disease duration, months | 10.0 (7.3) | N/A | N/A | 6.7 (6.5) | |
| Hoehn & Yahr, n (%) | <0.001 | ||||
| Stage 0 | 0 (0) | 49 (100) | 0 (0) | ||
| Stage 1 | 19 (23) | 0 (0) | 185 (44) | ||
| Stage 2 | 62 (76) | 0 (0) | 236 (56) | ||
| Stage 3-5 | 1 (1) | 0 (0) | 2 (0.5) | ||
| MDS-UPDRS | |||||
| Total Score | 35.2 (12.4) | 5.9 (5.3) | <0.001 | 32.4 (13.1) | |
| Part I | 5.5 (3.6) | 2.8 (2.6) | <0.001 | 5.6 (4.1) | |
| Part II | 5.6 (3.8) | 0.4 (1.0) | <0.001 | 5.9 (4.2) | |
| Part III | 24.1 (10.2) | 2.7 (3.5) | <0.001 | 20.9 (8.9) | |
| Montreal Cognitive Assessment | 27.6 (1.4) | 28.1 (1.5) | 0.04 | 27.1 (2.3) | |
| Parkinson’s Disease Quality of Life Questionnaire | 7.7 (6.7) | N/A | N/A | N/A | |
| Geriatric Depression Scale (Short Version) | 1.6 (1.9) | 1.0 (1.2) | 0.05 | 2.3 (2.4) | |
| REM Sleep Behavior Disorder Questionnaire | 4.4 (3.1) | 2.7 (2.0) | <0.001 | 4.1 (2.7) | |
| Epworth Sleepiness Scale | 4.9 (3.2) | 4.6 (3.7) | 0.66 | 5.8 (3.5) | |
| Scale for Outcomes in Parkinson’s Disease for Autonomic Symptoms | 9.1 (5.1) | 5.3 (4.2) | <0.001 | 9.5 (6.2) |
PD Parkinson’s disease, PPMI Parkinson’s Progression Markers Initiative, N/A Not available, MDS-UPDRS Movement Disorder Society-Unified Parkinson’s Disease Rating Scale.
Results are mean (standard deviation) for continuous measures and n (%) for categorical measures.
One control cohort participant is missing the Hoehn & Yahr and MDS-UPDRS scores and one additional is missing the MDS-UPDRS part III and total scores.
Gait
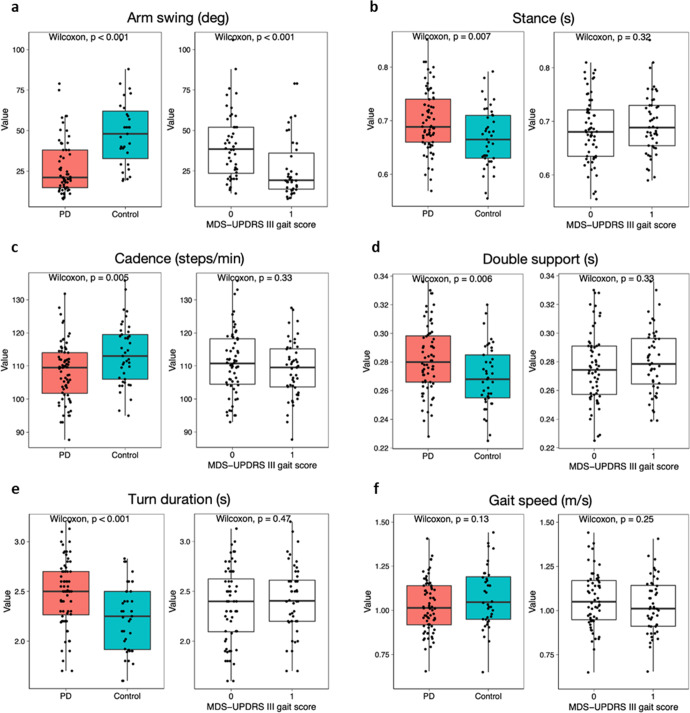
Smartphone data were available for gait analysis for 72 participants with PD and 41 controls; smartwatch data were available for at least 59 PD participants and at least 31 controls. Based on data from the baseline clinic visit, one gait parameter from the smartwatch and five from the phone best differentiated those with PD from controls. The magnitude of arm swing (Fig. 1a), as measured by the smartwatch, was smaller in PD than controls (27.8 [17.0] degrees vs 49.2 [21.8] degrees; P < 0.001). The smartphone detected increased stance time, slower gait cadence, increased total double support time, and increased turn duration among PD participants (Fig. 1b–e). Gait speed (Fig. 1f) did not differ between the two groups (1.03 [0.15] m/s vs 1.05 [0.24] m/s; P = 0.13). The smartphone also detected increased initial double support time in individuals with PD (data not shown). Only the magnitude of arm swing (P < 0.001) and stride length variability (P = 0.01) showed separation between controls and PD when grouped based on the MDS-UPDRS part III gait score (item 3.10). The gait differences observed between PD participants and controls were generally smaller among women than men (data not shown). Gait parameters derived from the smartwatch and smartphone showed moderate to very strong correlations (0.36 < r < 0.79) with comparable metrics from the research-grade wearable sensors.
Fig. 1. Comparisons of gait features derived from a smartphone and smartwatch.
Box plots for (panel a) Arm swing (deg), (panel b) Stance (s), (panel c) Cadence (steps/min), (panel d) Double support (s), (panel e) Turn duration (s), and (panel f) Gait speed (m/s) between those with and without Parkinson’s disease and by MDS-UPDRS part III gait item 3.10. MDS-UPDRS Movement Disorder Society-Unified Parkinson’s Disease Rating Scale.
Psychomotor function
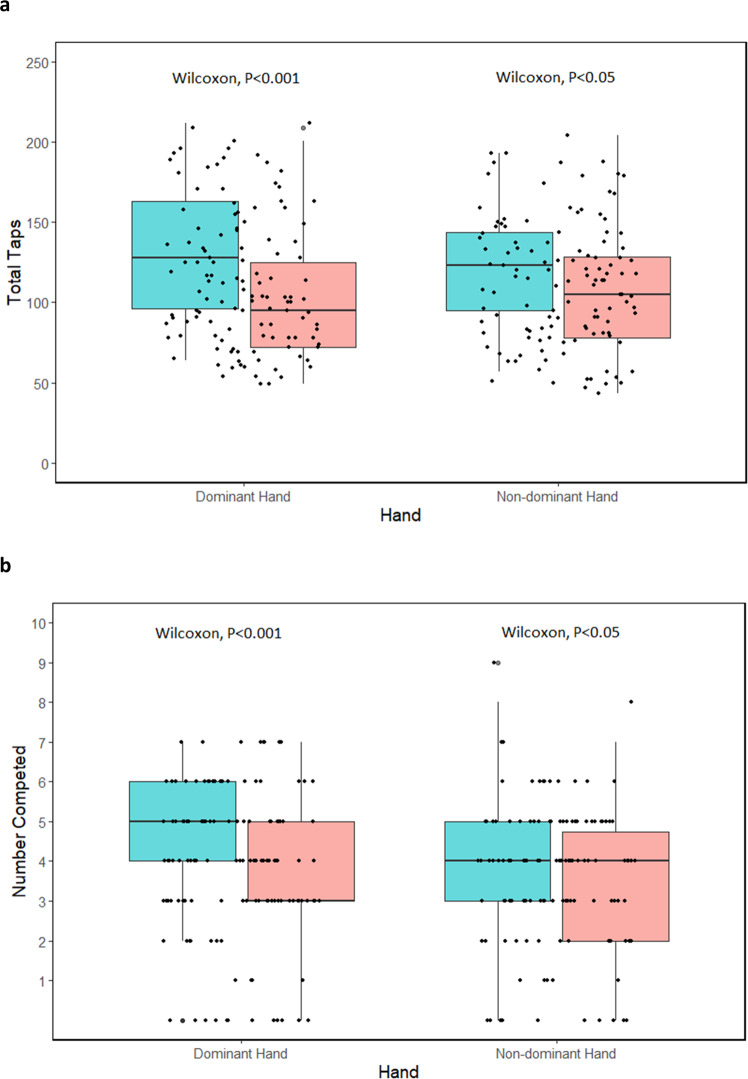
Smartphone data were available for analysis for 78 participants with PD and 45 controls for finger-tapping, and 82 participants with PD and 49 controls for the fine motor task. Finger tapping in the dominant and nondominant hand was slower in PD participants than controls (104.5 [40.5] taps per 30 s vs 130.2 [40.9] taps per 30 s; P < 0.001; and 106.4 [39.9] taps vs 122.2 (34.6) taps; P = 0.02, respectively) (Fig. 2a). Significant differences in total taps were also seen in PD participants when looking at most affected versus least affected side (98.6 [37.9] versus 112.3 [42.2]; P < 0.05). The inter-tap interval, or time between each tap, was longer in individuals with PD than controls in their dominant (169.9 [68.2] ms vs 137.3 (38.4) ms; P = .008) and nondominant hand (173.0 [67.7] ms vs 141.9 (32.1) ms; P = 0.02). The inter-tap interval coefficient of variation was also greater in PD in the dominant (50.4% [0.2] vs 32.1% [0.1]; P < 0.001) and non-dominant hand (50.8% [0.2] vs 36.2% [0.1]; P < 0.001). Slower tapping speeds very weakly correlated with higher scores on the MDS-UPDRS finger tapping for the right (r = −0.19) and left (r = −0.10) hands. The number complete in the fine-motor test was significantly less in individuals with PD than controls in their dominant (3.4 [1.7] vs 4.6 [1.9]; P < 0.001) and non-dominant hand (3.4 [1.7] vs 4.0 [1.9]; P < 0.05) (Fig. 2b). There were no significant differences in PD participants in total complete when examining most affected versus least affected side.
Fig. 2. Comparisons of psychomotor function derived from a smartphone.
Box plots for (panel a) number of total finger taps and (panel b) number of objects successfully manipulated on a smartphone between those with and without Parkinson’s disease by dominant and non-dominant hand.
Tremor
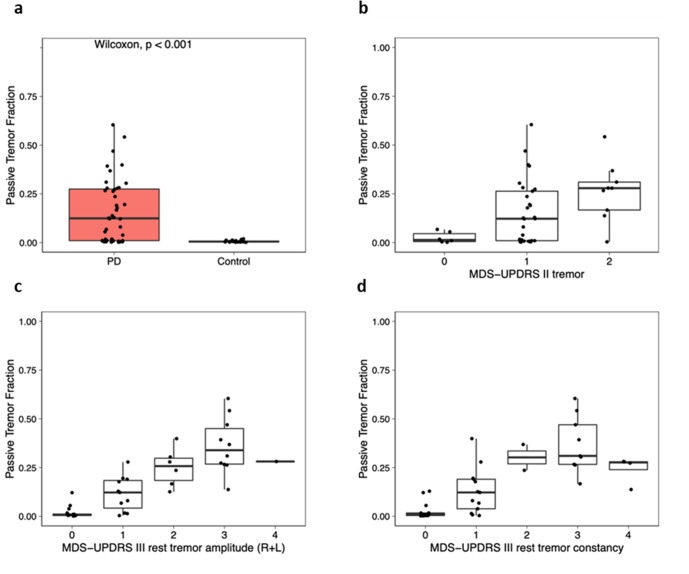
Passive tremor classification data from the smartwatch were available for 44 participants with PD and 22 controls for the at-home monitoring period following the baseline visit. The proportion of time with tremor (“passive tremor fraction”) was significantly higher among participants with PD (15.9% (16.3)) compared to controls (0.6% (0.5); P < 0.001) (Fig. 3a). Among PD participants, the tremor fraction measured correlated moderately with self-reported tremor severity (MDS-UPDRS part II, item 10, r = 0.43, p = 0.003), very strongly with clinician-reported upper extremity rest tremor amplitude (MDS-UPDRS part III, item 17, r = 0.86, p < 0.001), and strongly with rest tremor constancy (MDS-UPDRS part III, item 18, r = 0.79, p < 0.001, Fig. 3b–d).
Fig. 3. Comparisons of tremor scores derived from continuous passive tremor data from a smartwatch.
Box plots for passive tremor fraction between (panel a) those with and without Parkinson’s disease and by (panel b) MDS-UPDRS part II tremor, (panel c) MDS-UPDRS part III rest tremor amplitude (right + left), and (panel d) MDS-UPDRS part III rest tremor constancy. MDS-UPDRS Movement Disorder Society-Unified Parkinson’s Disease Rating Scale.
Cognition
Data on smartphone cognitive tests were available for 82 participants with PD and 49 controls. PD participants performed worse on the Trail Making Test Part A (54.5 (23.8) vs 48.0 (36.0) seconds; P < 0.05) and had fewer correct responses (18.3 (8.2) vs 20.4 (8.9); P = 0.05) on the Symbol Digit Modalities Test than controls. Higher scores on the Montreal Cognitive Assessment correlated weakly with decreased time to complete Trails A (r = −0.20, P = 0.14) and Trails B (r = −0.38, P < 0.01), more correct matches on the Symbol Digital Modalities Test (r = 0.25, P < 0.05), and the proportion of correct answers on the Visuospatial Working Memory Test (r = 0.18, P = 0.16).
Speech
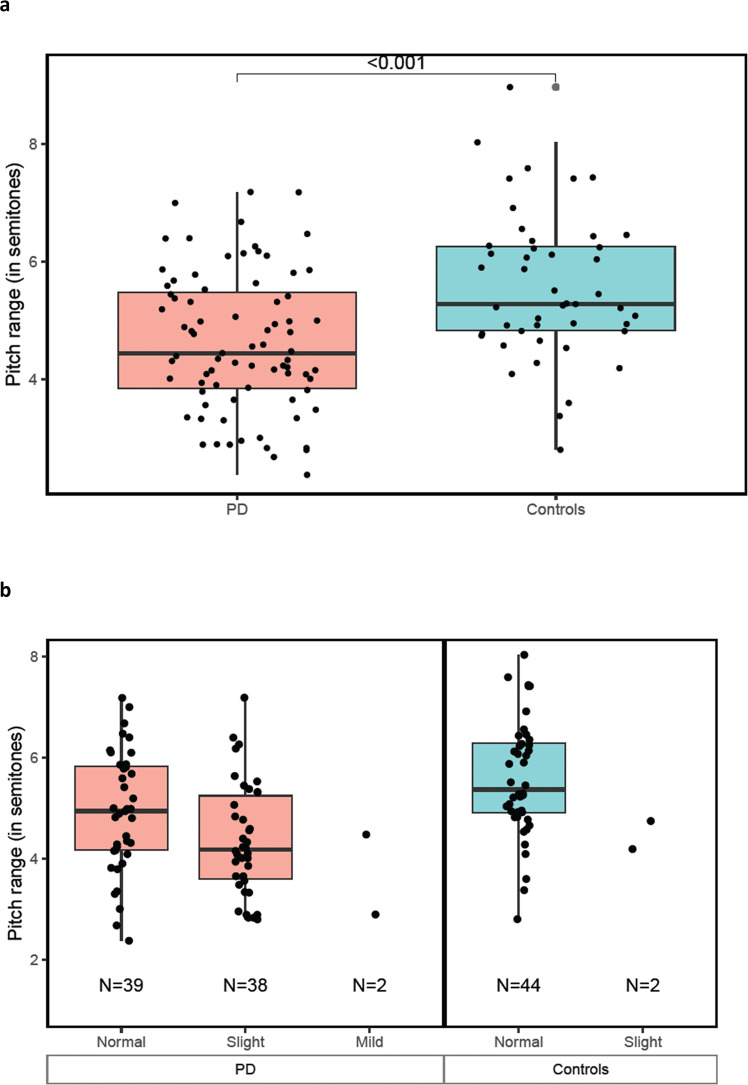
Baseline reading task data were available from 79 PD participants and 46 controls. Phonation task data were analyzed for 53 PD participants and 41 controls. In the reading task, the average pitch range (measured in semitones) for PD participants was reduced compared to controls (4.6 [1.2] vs 5.6 [1.2]; P = 0.00004) (Fig. 4). This was also true for individuals with PD who were rated as having “normal” speech on the MDS-UPDRS, (4.9 [1.2] vs 5.6 [1.2]; p = 0.015). Speech from reading and phonation tasks differed between those with and without PD (Supplemental Table 1). The most distinctive speech features varied with sex. For women, the median pause duration in the reading task best distinguished PD from controls (AUC = 0.72). For men, this distinction was best made by pitch semitone range in the reading task (AUC = 0.79).
Fig. 4. Comparisons of speech performance derived from a smartphone.
Box plots for pitch range (in semitones) between (panel a) those with and without Parkinson’s disease and by (panel b) MDS-UPDRS speech item 3.1. MDS-UPDRS Movement Disorder Society-Unified Parkinson’s Disease Rating Scale.
Discussion
In this multicenter study, a commercially available smartwatch and a smartphone research application captured key motor and non-motor features of early, untreated PD. These measures differed from age-matched controls, had variable correlation with traditional measures, and offer the promise of objective, real-world measures of the disease for use in future studies.
Compared to other digital studies in PD, this study has several strengths. The study population, which came from a network that often conducts PD trials, consisted of individuals with early, untreated PD, a target population for disease-modifying therapies. This study focused on deriving key features from a specialized software battery of assessments installed on commercially available devices, which have several advantages16,17. The devices are familiar to many, user friendly, have standardized software upgrades, enable remote data capture, and can inform individuals of results11. Devices and data plans were provided to minimize the effects of variable access to technology or the internet based on socioeconomic status or geographic location. Perhaps reflecting these advantages, interest among sites and participants was high, and enrollment was completed in a timely manner despite the COVID-19 pandemic. Finally, using a combination of devices both in the clinic and at home, the study collected a wide range of digital measures, which longitudinally evaluate a broad spectrum of motor and non-motor symptoms.
The study results are largely consistent with previous studies using other digital devices. For example, the reduced arm swing is a common early feature of PD that has been detected by sensing cameras and may even be a prodromal feature that can be measured with wearable sensors18,19. Similarly, slower finger tapping, increased tremor, worse cognition, and speech changes in PD compared to controls have all been demonstrated by different devices9,11,20–23.
Consistent with a previous study, digital devices were more sensitive than rating scales for some measures24. For example, the smartphone application detected abnormalities in speech even when it was rated “normal” by investigators. The better sensitivity of digital measures may explain the variable correlation with traditional clinical measures. For instance, the “inter-tap interval coefficient of variation” is not reliably weighed on the MDS-UPDRS, which does not separate speed from dysrhythmia. This study, like others, also found differences by sex, including in speech, and shows the value of connected speech tasks25,26. More research is needed, including longitudinal analysis from this study, to determine which measures are most sensitive to change over time.
This study was limited by missing data, wearing of the watch on different sides, lack of familiarity with some tasks, the homogeneous study population, and questions about the meaningfulness of the measures. Digital devices provide large volumes of data, but like imaging studies, can be prone to missing data27. For some assessments, data from more than half of the participants were not available. The biggest loss was due to device permissions restrictions, which were inadvertently turned off in some devices, impeding the transfer of passive data from the smartwatch to the analytical database. This shortcoming limits statistical power and highlights the need for rigorous data management and monitoring throughout the study.
In WATCH-PD, the smartwatch was worn on only one wrist. The side worn by PD participants (more affected side) and controls (individual preference) differed and at times was inconsistent with actual use. Standardizing wear for all participants to one side may be simpler and provide more consistent data. Some app tasks were also new to participants. This novelty may have contributed to more modest correlations than seen in previous studies and could have benefitted from practice prior to the baseline assessment9.
Like many other digital studies and PD trials, participants in this study were overwhelmingly white and well-educated, and thus not representative of the general population28–30. Empowering participants with data and active study roles and ensuring that investigators and sites are trustworthy, accessible, and representative of the broader population can make research more inclusive and equitable31–33. Given that this study evaluated individuals with early PD who had mild motor impairment and excluded those with cognitive impairment, the digital tasks may not be feasible in more advanced populations.
Regulators in Europe and the U.S. have recently accepted measures of gait speed in Duchenne muscular dystrophy and moderate to vigorous physical activity in idiopathic pulmonary fibrosis as endpoints for clinical trials34,35. These endpoints, which could be derived from this cohort, may also be valuable in PD. Other measures not included in this study, like step counts or words spoken, may also be collected by digital technologies and are not easily measured clinically. For use as endpoints in clinical trials, digital measures should be sensitive to change. Longitudinal data from this study are forthcoming and will help determine each measure’s sensitivity to change. Most importantly, digital measures must be meaningful. To determine which measures are meaningful, the voice of the patient must be included. The U.S. Food and Drug Administration has undertaken such efforts for idiopathic pulmonary fibrosis, and we are conducting qualitative research with participants from this study to understand what digital measures are relevant to their symptoms36.
This multicenter study in early, untreated PD provides valuable data on multiple digital measures derived from widely available devices. We were able to detect motor and non-motor features that differed between individuals with early PD and age-matched controls. In some cases, the digital devices were more sensitive than clinician-dependent rating scales. Longitudinal analysis as well as participant input will help identify potential digital measures to evaluate much-needed therapies for this rapidly growing population.
Methods
Study design
WATCH-PD (Wearable Assessment in The Clinic and at Home in PD)(NCT03681015) is a 12-month, multicenter observational study that evaluated the ability of digital devices to assess disease features and progression in persons with early, untreated PD.
Ethics
The WCGTM Institutional Review Board approved the procedures used in the study, and there was full compliance with human experimentation guidelines.
Setting
The study population was recruited from clinics, study interest registries, and social media and enrolled at 17 Parkinson Study Group research sites. All participants with PD had diagnoses confirmed clinically by a movement disorders specialist with approximately half undergoing a screening dopamine transporter imaging (DaTscan) to confirm diagnosis via imaging. Participants were evaluated in clinic and at home. In-person visits occurred at screening/baseline and then at months 1, 3, 6, 9, and 12. Due to the COVID-19 pandemic, most month-3 visits were converted to remote visits via video or phone, and participants could elect to complete additional visits remotely.
Participants
We sought to evaluate a population similar to the Parkinson’s Progression Markers Initiative (PPMI)37, which is a target population of trials evaluating disease-modifying therapies. For those with PD, the principal inclusion criteria were age 30 or greater at diagnosis, disease duration less than two years, and Hoehn & Yahr stage two or less. Exclusion criteria included baseline use of dopaminergic or other PD medications and an alternative parkinsonian diagnosis. Control participants without PD or other significant neurologic diseases were age-matched to the PD cohort. All participants provided written informed consent before study participation.
Data sources/measurement
Supplemental Table 2 outlines the study’s gait, motor function, tremor, cognitive, and speech assessments.
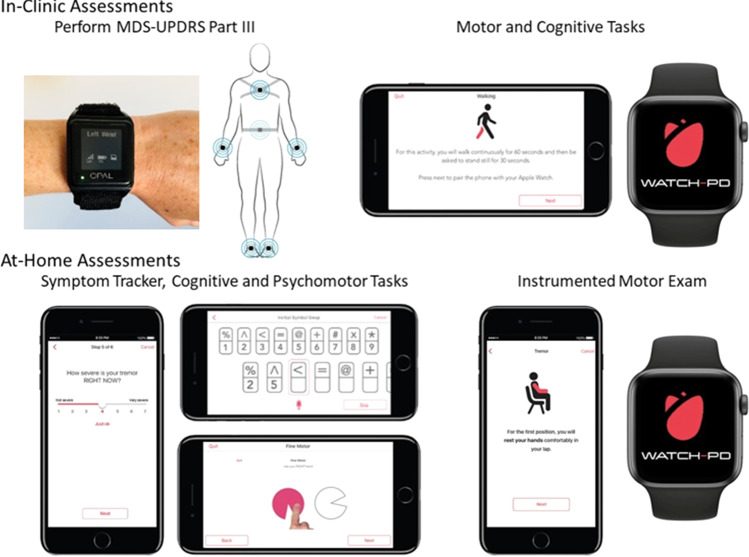
This study used three devices (Fig. 5): research-grade wearable “Opal” sensors (APDM Wearable Technologies, a Clario Company), an Apple Watch 4 or 5, and an iPhone 10 or 11 (Apple, Inc.) running a smartphone application specifically for PD (BrainBaseline™). Raw mobility and speech signals were recorded from Apple’s native accelerometer (100 Hz sampling rate) and microphone (32 kHz sampling rate) hardware configurations.
Fig. 5. Digital devices evaluated in-clinic and at-home during the study.
MDS-UPDRS Movement Disorder Society-Unified Parkinson’s Disease Rating Scale. Copyrighted images of BrainBaseline’s Movement Disorders Mobile Application were reprinted with permission by Clinical ink (Horsham, PA). Copyrighted images of APDM Wearable Technology were reprinted with permission by APDM Wearable Technologies, a Clario Company (Portland, OR).
During in-clinic visits, six wearable sensors with an accelerometer, gyroscope, and magnetometer were placed on the sternum, lower back, and on each wrist and foot. Smartphone application tasks were conducted at each clinic visit and at home every two weeks on the smartphone. The smartphone was worn in a lumbar sport pouch during gait and balance tests.
Gait features were extracted from the smartwatch and smartphone using software developed in-house. Gait bouts were identified after turns, and gait features during each bout were extracted using open-source GaitPy38,39. Arm swing features were calculated using rotational velocity from the smartwatch. Movement data was collected from the wearable sensors using Mobility Lab software (APDM Wearable Technologies, a Clario Company), and measures were extracted using custom algorithms written in Python (Wilmington, DE), available from the authors upon request.
After each in-person visit, participants wore the smartwatch on their more affected side and tracked symptoms on the smartphone daily for at least one week. Accelerometry data and tremor scores were collected from the smartwatch via Apple’s Movement Disorders Application Programming Interface (developer.apple.com/documentation/coremotion/getting_movement_disorder_symptom_data). Tremor analysis was performed on participants with at least 24 h of passive data over two weeks after baseline. The Movement Disorders API (open source code available at https://github.com/ResearchKit/mPower) generates tremor classification scores (none, slight, mild, moderate, strong, or unknown) for each 1-minute period, and the fraction of time spent in each category was calculated for each participant.
Using the BrainBaseline™ App, a cognitive and psychomotor battery was administered via the smartphone that included the Trail Making Test, modified Symbol Digit Modalities Test, Visuospatial Working Memory Task, and two timed fine motor tests (Supplemental Fig. 1)40–42.
Speech tasks included phonation, reading and a diadochokinetic task (not analyzed here)43. Phonation and reading files were processed using custom Python code (available from the authors upon request) with features computed using the Parselmouth interface to Praat and the Librosa library44,45. Common speech endpoints, such as jitter, shimmer, pitch statistics and Mel Frequency Cepstral Coefficients (MFCC), were computed. Speech segmentation was performed and used to extract time-related features for reading tasks46.
Participants also completed traditional rating scales including the MDS-UPDRS Parts I-III, Montreal Cognitive Assessment 8.1, Modified Hoehn and Yahr, Geriatric Depression Scale, REM Sleep Behavior Disorder Questionnaire, Epworth Sleepiness Scale, Scale for Outcomes in Parkinson’s Disease for Autonomic Symptoms, and the Parkinson’s Disease Questionnaire-847–54.
Study size
The study was powered to detect a mean change over 12 months for a digital endpoint with superior responsiveness to MDS-UPDRS Part III. The mean change in part III from baseline to year one in individuals with early, untreated PD in the PPMI study was 6.9 with a standard deviation of 7.03. Allowing for up to half of the participants to begin dopaminergic therapy over 12 months and 15% drop out, the study aimed to recruit at least 75 participants with PD to yield 30 participants completing the study off medication. The study had more than 95% power to detect a true change of 6.9 units using a one-sample t-test and a two-tailed 5% significance.
Statistical methods
For each gait feature, PD participants were compared to controls, and to gait scores from the MDS-UPDRS, using the Wilcoxon rank-sum test. The relationships between the gait features derived from smartwatch, smartphone and comparable features from the APDM sensors were estimated using linear regression. The relationship between the passive tremor fraction and tremor scores from the MDS-UPDRS was estimated using linear regression. Pearson correlations were determined to assess relationships between the digital cognitive battery and traditional cognitive measures. For each speech feature, normality was assessed using the Shapiro-Wilk test and transformed if necessary55. Features within each speech task with greater than 90% correlation were eliminated and remaining features were analyzed, with sensitivity analysis to understand sex differences. The AUC metric was used to determine how well each feature separated PD and control participants, and was computed for all participants by sex24,56.
Statistical analyses were performed using SAS v9.4, R v4.1, and Python 3.8. P-values < 0.05 were considered statistically significant. Results are considered exploratory and no adjustment for multiple comparisons were made.
Missing data
If a participant had missing data for an outcome or as part of a necessary algorithm, that person was excluded for that analysis. Values of zero (i.e., did not attempt the task) were also excluded. Detailed reasons for missing data are outlined in Supplemental Table 3.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
Funding for the study was contributed by Biogen, Takeda, and the members of the Critical Path for Parkinson’s Consortium 3DT Initiative, Stage 2. Biogen and Takeda contributed to the following activities: design and conduct of the study; analysis and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication. The authors acknowledge Odinachi Oguh, MD for assistance in the conduct of the study.
Author contributions
R.D. and J.E. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: J.C., P.B., E.R.D. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: J.L.A., T.K., B.T., P.A., J.E., K.P.K., R.D. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: T.K., B.T., P.A., K.P.K, J.E. Obtained funding: Dorsey. Administrative, technical, or material support: T.K., R.A., R.R., N.Z., B.T., P.O'., R.D.L., J.C., J.E., A.B. Supervision: Adams, T.K., J.C., J.E., R.D.
Data availability
The data are available to members of the Critical Path for Parkinson’s Consortium 3DT Initiative Stage 2. For those who are not a part of 3DT Stage 2, a proposal may be made to the WATCH-PD Steering Committee (via the corresponding author) for de-identified baseline datasets.
Code availability
Custom Python code used for feature extraction is available from the authors upon request.
Competing interests
Dr. Adams has received compensation for consulting services from VisualDx and the Huntington Study Group; and research support from Biogen, Biosensics, Huntington Study Group, Michael J. Fox Foundation, National Institutes of Health/National Institute of Neurological Disorders and Stroke, NeuroNext Network, and Safra Foundation. Ms. Tairmae Kangarloo, Dr. Brian Tracey, Dr. Dmitri Volfson, Dr. Neta Zach, and Dr. Robert Latzman are employees of and own stock in Takeda Pharmaceuticals, Inc. Dr. Josh Cosman is an employee of and owns stock in AbbVie Pharmaceuticals. Dr. Jeremey Edgerton, Dr. Krishna Praneeth Kilambi, and Katherine Fisher are employees of and own stock in Biogen Inc. Dr. Peter R. Bergethon was an employee of Biogen during a portion of this study. He has no conflicts or interests at the present time. Dr. Dorsey has received compensation for consulting services from Abbott, Abbvie, Acadia, Acorda, Bial-Biotech Investments, Inc., Biogen, Boehringer Ingelheim, California Pacific Medical Center, Caraway Therapeutics, Curasen Therapeutics, Denali Therapeutics, Eli Lilly, Genentech/Roche, Grand Rounds, Huntington Study Group, Informa Pharma Consulting, Karger Publications, LifeSciences Consultants, MCM Education, Mediflix, Medopad, Medrhythms, Merck, Michael J. Fox Foundation, NACCME, Neurocrine, NeuroDerm, NIH, Novartis, Origent Data Sciences, Otsuka, Physician’s Education Resource, Praxis, PRIME Education, Roach, Brown, McCarthy & Gruber, Sanofi, Seminal Healthcare, Spark, Springer Healthcare, Sunovion Pharma, Theravance, Voyager and WebMD; research support from Biosensics, Burroughs Wellcome Fund, CuraSen, Greater Rochester Health Foundation, Huntington Study Group, Michael J. Fox Foundation, National Institutes of Health, Patient-Centered Outcomes Research Institute, Pfizer, PhotoPharmics, Safra Foundation, and Wave Life Sciences; editorial services for Karger Publications; stock in Included Health and in Mediflix, and ownership interests in SemCap. Ms. Kostrzebski holds stock in Apple, Inc. Dr. Espay has received grant support from the NIH and the Michael J Fox Foundation; personal compensation as a consultant/scientific advisory board member for Neuroderm, Neurocrine, Amneal, Acadia, Acorda, Bexion, Kyowa Kirin, Sunovion, Supernus (formerly, USWorldMeds), Avion Pharmaceuticals, and Herantis Pharma; personal compensation as honoraria for speakership for Avion; and publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer. He cofounded REGAIN Therapeutics (a biotech start-up developing nonaggregating peptide analogues as replacement therapies for neurodegenerative diseases) and is co-owner of a patent that covers synthetic soluble nonaggregating peptide analogues as replacement treatments in proteinopathies. Dr. Spindler has received compensation for consulting services from Medtronic, and clinical trial funding from Abbvie, Abbott, US WorldMeds, Praxis, and Takeda. Dr. Wyant receives research funding from the National Institutes of Health/National Institute of Neurological Disorders and Stroke NeuroNEXT Network, Eli Lilly, and the Farmer Family Foundation. She also receives royalties from UpToDate. Joan Severson, Allen Best, David Anderson, Michael Merickel, Daniel Jackson Amato, and Brian Severson are employees of Clinical Ink, who acquired the BrainBaseline Platform in 2021 from Digital Artefacts. Joan Severson and Allen Best have financial interests in Clinical Ink.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Jamie L. Adams, Email: jamie_adams@urmc.rochester.edu
the Parkinson Study Group Watch-PD Study Investigators and Collaborators:
Jamie L. Adams, Christopher Tarolli, Emma Waddell, Stella Jensen-Roberts, Julia Soto, Penelope Hogarth, Mastura Wahedi, Katrina Wakeman, Alberto J. Espay, Julia Brown, Christina Wurzelbacher, Steven A. Gunzler, Elisar Khawam, Camilla Kilbane, Meredith Spindler, Megan Engeland, Arjun Tarakad, Matthew J. Barrett, Leslie J. Cloud, Virginia Norris, Zoltan Mari, Kara J. Wyant, Kelvin Chou, Angela Stovall, Cynthia Poon, Tanya Simuni, Kyle Tingling, Nijee Luthra, Caroline Tanner, Eda Yilmaz, Danilo Romero, Karen Thomas, Leslie Matson, Lisa Richardson, Michelle Fullard, Jeanne Feuerstein, Erika Shelton, David Shprecher, Michael Callan, Andrew Feigin, Caitlin Romano, Martina Romain, Michelle Shum, Erica Botting, Leigh Harrell, Claudia Rocha, Ritesh Ramdhani, Joshua Gardner, Ginger Parker, Victoria Ross, Steve Stephen, Katherine Fisher, Jeremy Edgerton, Jesse Cedarbaum, Robert Rubens, Jaya Padmanabhan, Diane Stephenson, Brian Severson, Michael Merickel, Daniel Jackson Amato, and Thomas Carroll
Supplementary information
The online version contains supplementary material available at 10.1038/s41531-023-00497-x.
References
- 1.GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16:877–897. doi: 10.1016/S1474-4422(17)30299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorsey, E. R., Sherer, T., Okun, M. S. & Bloem, B. R. Ending Parkinson’s Disease: A Prescription for Action. (PublicAffairs, 2020).
- 3.Simuni T, et al. Longitudinal change of clinical and biological measures in early Parkinson’s disease: Parkinson’s Progression Markers Initiative cohort. Mov. Disord. 2018;33:771–782. doi: 10.1002/mds.27361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorsey ER, Papapetropoulos S, Xiong M, Kieburtz K. The first frontier: digital biomarkers for neurodegenerative disorders. Digital Biomark. 2017;1:6–13. doi: 10.1159/000477383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Post B, Merkus MP, de Bie RMA, de Haan RJ, Speelman JD. Unified Parkinson’s disease rating scale motor examination: are ratings of nurses, residents in neurology, and movement disorders specialists interchangeable? Mov. Disord. 2005;20:1577–1584. doi: 10.1002/mds.20640. [DOI] [PubMed] [Google Scholar]
- 6.Zhan A, et al. Using smartphones and machine learning to quantify Parkinson disease severity: the mobile Parkinson disease score. JAMA Neurol. 2018;75:876–880. doi: 10.1001/jamaneurol.2018.0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braybrook M, et al. An ambulatory tremor score for Parkinson’s disease. J. Parkinson’s Dis. 2016;6:723–731. doi: 10.3233/JPD-160898. [DOI] [PubMed] [Google Scholar]
- 8.Maetzler W, Domingos J, Srulijes K, Ferreira JJ, Bloem BR. Quantitative wearable sensors for objective assessment of Parkinson’s disease. Mov. Disord. 2013;28:1628–1637. doi: 10.1002/mds.25628. [DOI] [PubMed] [Google Scholar]
- 9.Lipsmeier F, et al. Evaluation of smartphone-based testing to generate exploratory outcome measures in a phase 1 Parkinson’s disease clinical trial. Mov. Disord. 2018;33:1287–1297. doi: 10.1002/mds.27376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pagano G, et al. A phase II study to evaluate the safety and efficacy of Prasinezumab in early Parkinson’s disease (PASADENA): rationale, design, and baseline data. Front. Neurol. 2021;12:705407. doi: 10.3389/fneur.2021.705407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powers R, et al. Smartwatch inertial sensors continuously monitor real-world motor fluctuations in Parkinson’s disease. Sci. Transl. Med. 2021;13:eabd7865. doi: 10.1126/scitranslmed.abd7865. [DOI] [PubMed] [Google Scholar]
- 12.Simuni T, et al. Baseline prevalence and longitudinal evolution of non-motor symptoms in early Parkinson’s disease: the PPMI cohort. J. Neurol. Neurosurg. Psychiatry. 2018;89:78–88. doi: 10.1136/jnnp-2017-316213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chahine LM, et al. Predicting progression in Parkinson’s disease using baseline and 1-year change measures. J. Parkinson’s Dis. 2019;9:665–679. doi: 10.3233/JPD-181518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simuni T, et al. Correlates of excessive daytime sleepiness in de novo Parkinson’s disease: a case control study. Mov. Disord. 2015;30:1371–1381. doi: 10.1002/mds.26248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chahine LM, et al. Cognition among individuals along a spectrum of increased risk for Parkinson’s disease. PLoS One. 2018;13:e0201964. doi: 10.1371/journal.pone.0201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mancini M, Horak FB. Potential of APDM mobility lab for the monitoring of the progression of Parkinson’s disease. Expert Rev. Med. Devices. 2016;13:455–462. doi: 10.1586/17434440.2016.1153421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahadevan N, et al. Development of digital biomarkers for resting tremor and bradykinesia using a wrist-worn wearable device. npj Digital Med. 2020;3:5. doi: 10.1038/s41746-019-0217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ospina BM, et al. Objective arm swing analysis in early-stage Parkinson’s disease using an RGB-D camera (Kinect®) J. Parkinson’s Dis. 2018;8:563–570. doi: 10.3233/JPD-181401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirelman A, et al. Arm swing as a potential new prodromal marker of Parkinson’s disease. Mov. Disord. 2016;31:1527–1534. doi: 10.1002/mds.26720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Omberg L, et al. Remote smartphone monitoring of Parkinson’s disease and individual response to therapy. Nat. Biotechnol. 2022;40:480–487. doi: 10.1038/s41587-021-00974-9. [DOI] [PubMed] [Google Scholar]
- 21.Rusz J, et al. Automated speech analysis in early untreated Parkinson’s disease: relation to gender and dopaminergic transporter imaging. Eur. J. Neurol. 2022;29:81–90. doi: 10.1111/ene.15099. [DOI] [PubMed] [Google Scholar]
- 22.Šimek M, Rusz J. Validation of cepstral peak prominence in assessing early voice changes of Parkinson’s disease: effect of speaking task and ambient noise. J. Acoust. Soc. Am. 2021;150:4522. doi: 10.1121/10.0009063. [DOI] [PubMed] [Google Scholar]
- 23.Whitfield JA, Gravelin AC. Characterizing the distribution of silent intervals in the connected speech of individuals with Parkinson disease. J. Commun. Disord. 2019;78:18–32. doi: 10.1016/j.jcomdis.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, et al. An intelligent mobile-enabled system for diagnosing Parkinson disease: development and validation of a speech impairment detection system. JMIR Med. Inform. 2020;8:e18689. doi: 10.2196/18689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeancolas L, et al. X-Vectors: new quantitative biomarkers for early Parkinson’s disease detection from speech. Front. Neuroinform. 2021;15:578369. doi: 10.3389/fninf.2021.578369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hlavnička J, et al. Automated analysis of connected speech reveals early biomarkers of Parkinson’s disease in patients with rapid eye movement sleep behaviour disorder. Sci. Rep. 2017;7:12. doi: 10.1038/s41598-017-00047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulugeta G, Eckert MA, Vaden KI, Johnson TD, Lawson AB. Methods for the analysis of missing data in FMRI studies. J. Biom. Biostat. 2017;8:335. doi: 10.4172/2155-6180.1000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorsey ER, et al. The use of smartphones for health research. Acad. Med. 2017;92:157–160. doi: 10.1097/ACM.0000000000001205. [DOI] [PubMed] [Google Scholar]
- 29.Schneider MG, et al. Minority enrollment in Parkinson’s disease clinical trials. Parkinsonism Relat. Disord. 2009;15:258–262. doi: 10.1016/j.parkreldis.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Luca DG, et al. Minority enrollment in Parkinson’s disease clinical trials: meta-analysis and systematic review of studies evaluating treatment of neuropsychiatric symptoms. J. Parkinson’s Dis. 2020;10:1709–1716. doi: 10.3233/JPD-202045. [DOI] [PubMed] [Google Scholar]
- 31.Gilmore-Bykovskyi A, Jackson JD, Wilkins CH. The urgency of justice in research: beyond COVID-19. Trends Mol. Med. 2021;27:97–100. doi: 10.1016/j.molmed.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warren RC, Forrow L, Hodge DA, Truog RD. Trustworthiness before trust — Covid-19 vaccine trials and the black community. N. Engl. J. Med. 2020;383:e121. doi: 10.1056/NEJMp2030033. [DOI] [PubMed] [Google Scholar]
- 33.Adrissi J, Fleisher J. Moving the dial toward equity in Parkinson’s disease clinical research: a review of current literature and future directions in diversifying PD clinical trial participation. Curr. Neurol. Neurosci. Rep. 2022;22:475–483. doi: 10.1007/s11910-022-01212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Servais L, et al. First regulatory qualification of a novel digital endpoint in Duchenne muscular dystrophy: a multi-stakeholder perspective on the impact for patients and for drug development in neuromuscular diseases. Digi. Biomark. 2021;5:183–190. doi: 10.1159/000517411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inacio, P. Bellerophon, FDA agree on design of phase 3 INOpulse trial. Pulmonary Fibrosis News (2020). https://pulmonaryfibrosisnews.com/2020/03/13/bellerophon-fda-agree-on-design-of-phase-3-inopulse-trial/.
- 36.The voice of the patient: idiopathic pulmonary fibrosis. U.S. Food and Drug Administration Patient-Focused Drug Development Initiative (2015). https://www.fda.gov/files/about%20fda/published/The-Voice-of-the-Patient–Idiopathic-Pulmonary-Fibrosis.pdf.
- 37.Parkinson Progression Marker Initiative. The Parkinson Progression Marker Initiative (PPMI) Prog. Neurobiol. 2011;95:629–635. doi: 10.1016/j.pneurobio.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Gohary M, et al. Continuous monitoring of turning in patients with movement disability. Sensors. 2014;14:356–369. doi: 10.3390/s140100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Czech M, Patel S. GaitPy: an open-source python package for gait analysis using an accelerometer on the lower back. J. Open Source Softw. 2019;4:1778. doi: 10.21105/joss.01778. [DOI] [Google Scholar]
- 40.Bowie CR, Harvey PD. Administration and interpretation of the trail making test. Nat. Protoc. 2006;1:2277–2281. doi: 10.1038/nprot.2006.390. [DOI] [PubMed] [Google Scholar]
- 41.Hockey A, Geffen G. The concurrent validity and test–retest reliability of a visuospatial working memory task. Intelligence. 2004;32:591–605. doi: 10.1016/j.intell.2004.07.009. [DOI] [Google Scholar]
- 42.Smith, A. Symbol Digit Modalities Test. (Western Psychological Services, 1973).
- 43.Rusz J, Cmejla R, Ruzickova H, Ruzicka E. Quantitative acoustic measurements for characterization of speech and voice disorders in early untreated Parkinson’s disease. J. Acoust. Soc. Am. 2011;129:350–367. doi: 10.1121/1.3514381. [DOI] [PubMed] [Google Scholar]
- 44.McFee, B. et al. librosa: audio and music signal analysis in Python. Proceedings of the 14th Python in Science Conference, 18–24 (2015).
- 45.Jadoul Y, Thompson B, de Boer B. Introducing Parselmouth: a Python interface to Praat. J. Phon. 2018;71:1–15. doi: 10.1016/j.wocn.2018.07.001. [DOI] [Google Scholar]
- 46.Gorman K, Howell J, Wagner M. Prosodylab-aligner: a tool for forced alignment of laboratory speech. Can. Acoust. 2011;39:192–193. [Google Scholar]
- 47.Peto V, Jenkinson C, Fitzpatrick R. PDQ-39: a review of the development, validation and application of a Parkinson’s disease quality of life questionnaire and its associated measures. J. Neurol. 1998;245:S10–S14. doi: 10.1007/PL00007730. [DOI] [PubMed] [Google Scholar]
- 48.Goetz CG, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 49.Nasreddine ZS, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 50.Goetz CG, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov. Disord. 2004;19:1020–1028. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- 51.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin. Gerontologist: J. Aging Ment. Health. 1986;5:165–173. doi: 10.1300/J018v05n01_09. [DOI] [Google Scholar]
- 52.Stiasny-Kolster K, et al. The REM sleep behavior disorder screening questionnaire–a new diagnostic instrument. Mov. Disord. 2007;22:2386–2393. doi: 10.1002/mds.21740. [DOI] [PubMed] [Google Scholar]
- 53.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 54.Visser M, Marinus J, Stiggelbout AM, Van Hilten JJ. Assessment of autonomic dysfunction in Parkinson’s disease: the SCOPA-AUT. Mov. Disord. 2004;19:1306–1312. doi: 10.1002/mds.20153. [DOI] [PubMed] [Google Scholar]
- 55.Royston P. Remark AS R94: a remark on algorithm AS 181: the W-test for normality. J. R. Stat. Soc. Ser. C. (Appl. Stat.) 1995;44:547–551. [Google Scholar]
- 56.Tsanas A, Little MA, McSharry PE, Ramig LO. Accurate telemonitoring of Parkinson’s disease progression by noninvasive speech tests. IEEE Trans. Biomed. Eng. 2010;57:884–893. doi: 10.1109/TBME.2009.2036000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available to members of the Critical Path for Parkinson’s Consortium 3DT Initiative Stage 2. For those who are not a part of 3DT Stage 2, a proposal may be made to the WATCH-PD Steering Committee (via the corresponding author) for de-identified baseline datasets.
Custom Python code used for feature extraction is available from the authors upon request.