The levonorgestrel 52-mg intrauterine device significantly improved heavy menstrual bleeding in a population that included participants with severe obesity and nulliparous participants.
OBJECTIVE:
To evaluate heavy menstrual bleeding treatment outcomes with levonorgestrel 52-mg intrauterine device (IUD) use in participants without body mass index (BMI) or parity restrictions.
METHODS:
Investigators included participants aged 18–50 years with no pelvic or systemic pathology causing heavy menstrual bleeding at 29 U.S. centers in a prospective trial. Participants had up to three screening cycles with menstrual product collection for alkaline hematin blood-loss measurements. Investigators enrolled those with two menses with blood loss of 80 mL or more (values averaged for baseline blood loss), placed the IUD, and followed participants for up to six 28-day cycles. Participants collected any menstrual products used during cycles 3 and 6 for blood-loss measurement. We evaluated outcomes in participants with at least one follow-up assessment for the primary outcome of median absolute blood-loss change and, secondarily, treatment success, defined as the proportion with a final measured blood loss less than 80 mL and at least 50% reduction from baseline. We evaluated exploratory outcomes of differences in blood-loss changes by BMI and parity using Wilcoxon rank sum test.
RESULTS:
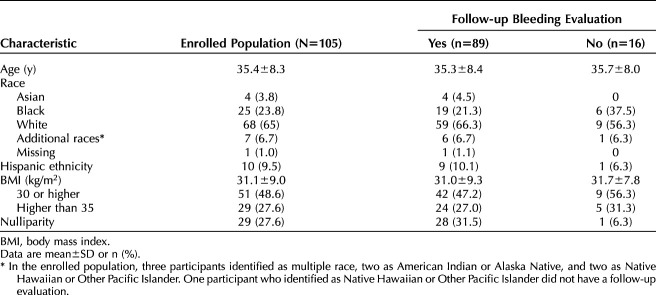
Of 105 enrolled participants, 47 (44.8%) had obesity (BMI 30.0 or higher) and 29 (27.6%) were nulliparous. Baseline mean blood loss ranged from 73 to 520 mL (median 143 mL, interquartile range 112–196 mL). Eighty-nine (84.8%) had at least one evaluable follow-up evaluation. Participants had median (interquartile range) absolute blood-loss decreases at cycles 3 (n=86) and 6 (n=81) of 93.3% (86.1–97.7%) and 97.6% (90.4–100%), respectively. At cycle 6, participants without obesity (n=43) and with obesity (n=38) had similar median [interquartile range] decreases (97.6% [91.8–100%] and 97.5% [90.3–100%], respectively; P=.89), with comparable findings for nulliparous (n=25) and parous (n=56) participants (97.0% [91.7–99.1%] and 98.1% [89.9–100%], respectively; P=.43). Treatment success occurred in 81.8% (95% CI 74.2–89.4%) of 99 participants, excluding those with no outcomes due to lost to follow-up or consent withdrawal, and did not vary by BMI or parity. The most common adverse events leading to discontinuation were bleeding or cramping (n=6 [5.7%]) and expulsion (n=5 [4.8%]).
CONCLUSION:
This levonorgestrel 52-mg IUD reduces blood loss by more than 90% over 6 months compared with baseline for most users with heavy menstrual bleeding.
FUNDING SOURCE:
Medicines360.
CLINICAL TRIAL REGISTRATION:
In clinical practice, heavy menstrual bleeding refers to excessive blood loss that interferes with quality of life, a definition first proposed in 2007 by the National Institute for Health and Care Excellence in the United Kingdom.1 However, when defining treatment options, regulatory agencies consider only flow, with a blood loss of 80 mL or more considered heavy menstrual bleeding.2 Average menstrual blood loss is between 30 and 40 mL per cycle.2
Using the PALM-COEIN criteria,3 patients with heavy menstrual bleeding with no known causes, such as uterine pathology or coagulopathies, are considered to have abnormal uterine bleeding-endometrial. First-line medical treatment options for heavy menstrual bleeding due to abnormal uterine bleeding-endometrial currently include systemic or local (eg, intrauterine) progestins, non-steroidal anti-inflammatory drugs, and antifibrinolytics.4 The levonorgestrel 52-mg intrauterine device (IUD) is the most effective heavy menstrual bleeding treatment option,4–6 with clinical trials demonstrating an approximate 70% reduction in blood loss during the first 3 months after placement with a further reduction with continued use.5 In the United States, two levonorgestrel 52-mg IUDs are currently marketed, only one of which (Mirena) is currently approved by the U.S. Food and Drug Administration for treatment of heavy menstrual bleeding; specifically, the label indicates treatment for heavy menstrual bleeding for up to 5 years in patients “who choose to use intrauterine contraception as their method of contraception.”7 This approval was granted based on a clinical trial in parous patients with body mass indexes (BMIs) (calculated as weight in kilograms divided by height in meters squared) of 35 or lower and those desiring the IUD for contraception.8 We lack rigorous data with quantitative blood-loss measurements of treatment outcomes in patients with higher BMIs, nulliparous patients, and patients not desiring the IUD for contraception.
In this study, which we performed as part of the clinical development program for the other levonorgestrel 52-mg IUD (Liletta), we conducted a phase 3 trial to evaluate heavy menstrual bleeding treatment in a population that included participants with these characteristics.
METHODS
This study was a multicenter, phase 3, open-label clinical trial conducted at 29 clinical sites in the United States to assess the Liletta levonorgestrel 52-mg IUD for heavy menstrual bleeding treatment. The study was approved by a central (Advarra) or local IRB for each study site, as applicable. All participants signed written informed consent before study procedures were initiated. The study was registered with ClinicalTrials.gov, identifier number NCT03642210.
Investigators invited healthy, nonpregnant, nulliparous and parous women aged 18–50 years (inclusive) who reported regular heavy menses for most menses when not using hormonal contraception or a copper IUD to participate from October 2018 to December 2020. Exclusion criteria ensured good general health, non-perimenopausal or menopausal status, and no structural, infectious, medical, drug, or premalignant or malignant causes of heavy menstrual bleeding, and did not include any restrictions on weight or BMI (Appendix 1, available online at http://links.lww.com/AOG/D92). Participants who were heterosexually active agreed to use a nonhormonal contraceptive method during screening.
After signing informed consent, participants entered a screening phase that included menstrual blood-loss evaluation in up to three cycles to establish a diagnosis of heavy menstrual bleeding and confirm eligibility. At the initial screening visit, investigators obtained demographic information, which included race as required for regulatory approval studies. Investigators assessed participants’ medical history, including medication use, a urine pregnancy test, and blood testing to excluded systemic or hormonal heavy menstrual bleeding causes in line with the entry criteria. Participants had pelvic examinations, including a Pap test if clinically indicated, and Chlamydia and gonorrhea testing if not performed and documented within the preceding 30 days. Participants without documentation of a recent normal uterine ultrasound examination result underwent transvaginal ultrasonography to assess for exclusionary findings. Participants then had an endometrial biopsy performed, unless a normal biopsy result was documented within the preceding 6 months. After completion of all evaluations, participants received study-specific menstrual products (Appendix 2, available online at http://links.lww.com/AOG/D92) and a paper diary to record daily vaginal bleeding. Study staff instructed participants to use only the menstrual products provided by the study during all blood-loss assessments. Participants collected products during menses and kept them in a supplied large keg, which they brought to each screening visit.
Screening cycle assessments occurred in up to three cycles, during which participants collected menstrual products for alkaline hematin testing. Participants were scheduled to attend a visit within 5 days of the end of menses to provide collected menstrual products and have a serum sample obtained for alkaline hematin blood-loss calculations, although the visit could occur up to 21 days after menses. At each visit, study staff assessed the menstrual products, reviewed diaries, and provided additional menstrual products for the next cycle, if indicated. Investigators and staff could opt to skip a cycle once during screening if a participant stated all menstrual products were not collected that cycle or if the cycle had less flow than a typical heavy cycle. After the first cycle, participants with menstrual blood loss less than 60 mL were considered to screen failures. Participants with menstrual blood loss between 60 mL and 79 mL in the first cycle who had menstrual blood loss less than 80 mL in the second cycle were also considered screen failures. Participants could enroll once they had two cycles with menstrual blood loss of 80 mL or more.
Enrollment (IUD placement) could occur anytime the investigator was reasonably certain the participant was not pregnant, and participants were followed for up to six 28-day cycles. If IUD placement occurred after the first 7 days of menses onset in a participant who was not using permanent contraception, the participant was asked to use a barrier method or remain heterosexually abstinent for the first 7 days. Participants were instructed to not use menstrual cups at any time after IUD placement and continue daily diary use through completion of follow-up.
Because regular cycles were not expected after levonorgestrel 52-mg IUD placement, blood-loss assessments (collection of study specific menstrual products) were performed over 28-day intervals during cycles 3 (days 57–84) and 6 (days 141–168) of IUD use. Follow-up visits were scheduled 4–6 weeks after IUD placement and within 5 days (maximum 21 days) of completion of menstrual product collection. At each visit, investigators performed a urine pregnancy test and pelvic examination to confirm IUD presence, reviewed diaries, collected the kegs with menstrual products (if any blood loss occurred), and dispensed new menstrual products, if needed. During the visits after cycles 3 and 6, blood was collected to coordinate with the menstrual products for alkaline hematin testing.
Any participant who experienced IUD expulsion during study follow-up could choose to have the IUD replaced one time, only if pregnancy could be excluded and replacement occurred within 2 weeks of expulsion and at least 7 days before treatment cycle 3 or 6. The IUD was removed during follow-up on participant request or when clinically indicated. At the end of the six-cycle treatment phase, unless medically contraindicated, participants could opt to keep the IUD or have it removed by a study investigator. All participants who had IUD removal during the study were contacted 7–10 days later to assess for any IUD- or IUD removal–related adverse events.
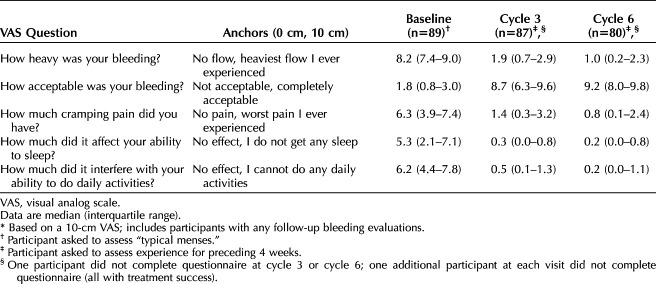
We evaluated participants' subjective assessments of changes in menstrual bleeding severity, dysmenorrhea, and daily activities using 10-cm visual analog scale questionnaires at enrollment and at the cycles 3 and 6 visits. The enrollment questionnaire asked participants to answer based on their typical menses, and the follow-up questionnaires asked participants to consider their experience over the preceding 4 weeks. Questions assessed bleeding heaviness, bleeding acceptability, cramping, interference with daily activities, and effect of bleeding on ability to sleep.
We evaluated outcomes in participants with at least one follow-up assessment. Menstrual blood loss volume was assessed by a central laboratory (KCAS Bioanalytical & Biomarker Services, Shawnee, Kansas) using alkaline hematin testing9 that included all submitted menstrual products (study specific and non-study specific). Baseline menstrual blood loss was the average of the two or three screening cycles required to achieve two cycles of 80 mL or more based only on study-specific menstrual product evaluation. The primary outcome (treatment success) was defined as menstrual blood loss during IUD treatment less than 80 mL and more than 50% reduction from baseline during the prior 28-day cycle of treatment (cycle 3 or cycle 6). We assessed median absolute change in blood loss overall, as well as exploratory evaluations in subgroups by obesity status and parity, using Wilcoxon rank sum test. We secondarily assessed continuation rates and adverse events leading to discontinuation. Data were analyzed using SAS 9.3.
The sample size was estimated based on an expected successful treatment rate of 80% or greater8 for the entire study cohort such that the lower bound of the 95% CI would be within 10% from the point estimate (ie, 70% or higher). A sample of 85 participants provided a 71.5% lower bound of the 95% CI for an expected successful treatment rate of 80% or higher based on normal approximation. To account for early discontinuations in up to 15% of enrolled participants, we targeted IUD placement in approximately 100 participants.
RESULTS
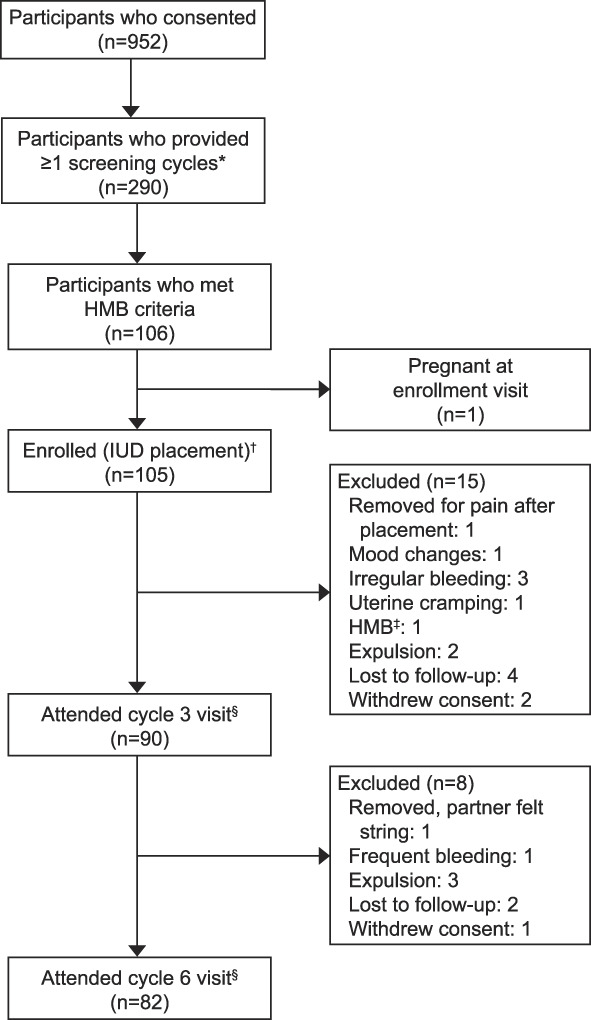
We consented 952 participants, of whom 290 had one or more screening cycles with menstrual product collection. Of the 106 who met eligibility criteria, one had a positive pregnancy test at the enrollment visit, resulting in 105 (36.2%) who were enrolled, all of whom had successful IUD placement. Characteristics of participants who underwent IUD placement are presented in Table 1. Twenty-three (21.9%) participants discontinued for reasons of expulsion (n=5, 4.8%), bleeding complaint (n=4, 3.8%), withdrawal of consent (n=3, 2.9%), lost to follow-up (6, 5.7%), participant request during cycle 2 due to subjective lack of efficacy (n=1, 1.0%), uterine pain immediately after placement (n=1, 1.0%), uterine cramping (n=1, 1.0%), mood changes (n=1, 1.0%), and partner feeling the threads (n=1, 1.0%). Participant flow through the study is presented in Figure 1.
Table 1.
Characteristics of Participants in a Phase 3 Study Evaluating a Levonorgestrel 52-mg Intrauterine Device for Heavy Menstrual Bleeding Treatment
Fig. 1. Study flow for participants in a phase 3 study evaluating a levonorgestrel 52-mg intrauterine device (IUD) for heavy menstrual bleeding (HMB) treatment. *Menstrual product collection for alkaline hematin analysis. †First participant enrolled (IUD placed) January 2018; last participant completed follow-up August 2021. ‡Subjective lack of efficacy after first cycle. §One attended visit but did not supply bleeding outcome.
Creinin. Levonorgestrel 52-mg IUD for HMB. Obstet Gynecol 2023.
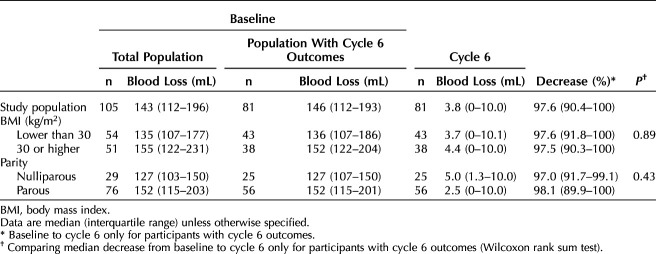
Eighty-nine (84.8%) and 81 (77.1%) participants provided bleeding outcomes at cycles 3 and 6, respectively, with 89 (84.8%) providing at least one follow-up cycle for evaluation of the primary outcome. Baseline menstrual blood loss was 165±79 mL (range 73–520 mL) for the enrolled population and 161±74 mL (range 73–520) for the 89 participants with follow-up evaluations. Treatment success occurred in 81 participants, which is 91.0% (95% CI 85.1–97.0%) of the 89 participants with any follow-up bleeding evaluations, 81.8% (95% CI 74.2–89.4%) of the 99 participants excluding those with no outcomes due to lost to follow-up or consent withdrawal, and 77.1% (95% CI 69.1–85.2%) of the enrolled population. Limiting the screening and follow-up alkaline hematin analyses data only to study-specific menstrual products did not change the overall outcome, with success in 82 of 88 participants (93.2%, 95% CI 87.9–98.4%). Treatment success rates did not differ by obesity status (BMI less than 30 vs 30 or higher) or parity (Table 2) or when evaluating outcomes by BMI 35 or lower compared with higher than 35 (Appendix 3, available online at http://links.lww.com/AOG/D92).
Table 2.
Treatment Success Rate Over Six Cycles in a Phase 3 Study Evaluating a Levonorgestrel 52-mg Intrauterine Device for Heavy Menstrual Bleeding Treatment (n=89)
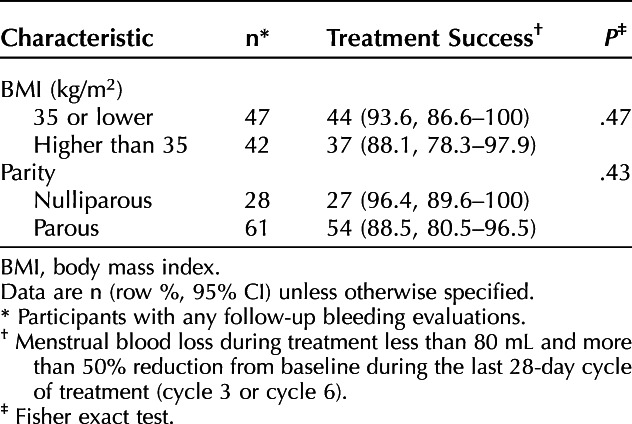
The median (interquartile range) percentage decrease in blood loss for participants with follow-up bleeding evaluations was 93.3% (86.1–97.7%) by cycle 3 and 97.6% (90.4–100%) by cycle 6. For the 79 participants evaluated in the sixth cycle, 15 (19.0%) reported no bleeding or spotting and 23 (29.1%) reported spotting only. Median decrease in blood loss by cycle 6 did not differ by obesity status or parity (Table 3) or when evaluating outcomes by BMI 35 or lower compared with and higher than 35 (Appendix 3, http://links.lww.com/AOG/D92).
Table 3.
Change in Blood Loss Over Six Cycles in a Phase 3 Study Evaluating a Levonorgestrel 52-mg Intrauterine Device for Heavy Menstrual Bleeding Treatment
Nine (8.6%, 95% CI 3.2–13.9%) participants experienced expulsion (eight complete, one partial), for which four had reinsertion and provided bleeding outcomes for the remainder of the study; the five who discontinued due to expulsion had complete expulsion. Six of the nine expulsions occurred during the first 90 days after placement. Seven of the nine participants with expulsion had obesity, and eight of the nine were parous. One participant who had a complete expulsion became pregnant with fertilization date, as determined by the site investigator, after the expulsion occurred. No serious adverse events were reported. Changes in participants’ subjective assessment of changes in menstrual bleeding severity, dysmenorrhea, and daily activities are presented in Table 4.
Table 4.
Subjective Assessment* of Changes in Menstrual Bleeding Severity, Dysmenorrhea, and Daily Activities With a Levonorgestrel 52-mg Intrauterine Device for Heavy Menstrual Bleeding Treatment
DISCUSSION
We demonstrated a significant and rapid decrease in uterine bleeding after placement of a levonorgestrel 52-mg IUD in participants with confirmed heavy menstrual bleeding. Within three cycles, the median blood loss decreased by more than 90%. Whereas prior trials had excluded participants with higher BMIs (higher than 35) and nulliparous participants, we included participants with these characteristics. Our findings showed no difference between bleeding outcomes based on BMI or parity, although our study was underpowered for these subgroups. The effects on participant quality of life measures were substantial (Table 4).
Results of our study add to the growing literature on the safety and efficacy of levonorgestrel 52-mg IUDs for heavy menstrual bleeding treatment. We report an overall efficacy of approximately 80% over the six study cycles, the same rate reported in the prior U.S. phase 3 evaluation of Mirena in a parous population with a mean BMI of 27.2±3.9.8 Prior randomized trials have demonstrated the equivalence of Mirena and Liletta in studies using pictorial bleeding assessment chart blood-loss evaluations10 or Wyatt Pictograms11 for evaluating blood loss. The trial using the pictorial bleeding assessment chart randomized participants equally with 12 months of follow-up; mean population blood-loss decreases were identical (78–79%) in the two groups. This pictorial bleeding assessment chart study is the basis for approval of Liletta (known as Levosert, Donasert, Avibela and other names outside the United States) for heavy menstrual bleeding by the European Medicines Agency and other regulatory authorities. However, in the United States, a prospective trial that used alkaline hematin testing was required for regulatory approval.
Although bleeding decreased substantially in participants with follow-up bleeding data, 14 (13.3%) discontinued early due to expulsion or IUD-related complaints (Fig. 1). Our findings related to expulsion are important for clinicians to understand and convey during counseling. The expulsion rate of 9% within six cycles is higher than is typically seen with levonorgestrel 52-mg IUD use for contraception, with expulsion rates of 1.6% through 6 months and 4.1% through 8 years of use.12,13 Two recent retrospective studies have compared expulsion rates in levonorgestrel 52-mg IUD users desiring contraception or with subjective heavy menstrual bleeding.14,15 A Brazilian analysis reported identical expulsion rates of 5.6% over an average follow-up duration of 45 months among contraceptors (n=5,655) and those with subjective heavy menstrual bleeding (n=548).14 However, a much larger retrospective study that used data from three integrated health care systems found an adjusted hazard ratio of 2.84 (95% CI 2.66–3.03) for expulsion among patients with a listed heavy menstrual bleeding diagnosis (n=31,600) compared with those without the diagnosis (n=197,234).15 Recent studies have shown higher expulsion rates in IUD users who are parous12,16 and have obesity.12 The prior phase 3 levonorgestrel 52-mg IUD heavy menstrual bleeding study performed in the United States reported a 6% expulsion rate at 6 months.8 Our study included participants with higher BMIs than the prior U.S. trial, which did not enroll participants with BMIs higher than 35.0; more than 25% of enrollees in our study exceeded that BMI. Almost all of the expulsions that occurred in our study were in parous participants with obesity. These data show that expulsion risk in patients with subjective heavy menstrual bleeding is different than those with heavy menstrual bleeding defined by quantitative methods for regulatory approval and that IUD users with obesity with very heavy bleeding have a much greater risk of expulsion.
The demographics of the study population include relatively high proportions of participants in racial and ethnic minority groups, those with obesity, and nulliparous participants, meaning the results are likely widely applicable. Still, the number of participants with obesity and nulliparous participants were sufficient only for exploratory analyses in these populations, because the overall study was underpowered for these specific assessments. This study used thorough evaluations during screening to ensure participants had no organic, hematologic, or iatrogenic causes of heavy menstrual bleeding. Whereas, during screening cycles, participants collected menstrual products only during menstrual bleeding, they collected products during an entire 28-day period during the treatment cycles. Even with this requirement to collect menstrual products for more days, participants had significantly less bleeding or spotting, resulting in more than a 90% decrease in flow within three cycles. A limitation of the study was that the alkaline hematin process was validated for specific menstrual products, and participants did not always use only the study products. Exclusion of these cycles could result in falsely low blood-loss evaluations during IUD use, so we included all measured blood loss regardless of menstrual product type. Moreover, our sensitivity analysis showed no difference in success rates or decrease in blood loss when the analysis was restricted just to approved products.
This study demonstrates rapid decrease in blood loss in study participants with objectively proven heavy menstrual bleeding with no known causes, such as uterine pathology or coagulopathies. Our study included nulliparous participants and participants with severe obesity, populations who have been historically excluded in prior trials. Our results suggest that efficacy is maintained in populations that include patients with these characteristics and expand the generalizability of the levonorgestrel 52-mg IUD as a highly effective treatment for heavy menstrual bleeding.
Authors' Data Sharing Statement
Will individual participant data be available (including data dictionaries)? No
What data in particular will be shared? Not available
What other documents will be available? Not available
When will data be available (start and end dates)? Not applicable
By what access criteria will data be shared (including with whom, for what types of analyses, and by what mechanism)? Not applicable
Footnotes
Supported by Medicines360. The Sponsor, Medicines360, designed the study and oversaw its conduct, including funding the trial and providing all study product free of charge to participants.
Financial Disclosure Mitchell D. Creinin has received speaking honoraria from Gedeon Richter, Mayne and Organon. He has served on the Advisory Boards for Evofem, Fuji Pharma, Gedeon Richter, GlaxoSmithKline, Mayne, Merck & Co., OLIC, Organon, and Searchlight. He has been a Consultant for Estetra SRL, Libbs, Mayne, and Medicines360, and his university department receives contraceptive research funding for Dr. Creinin from Chemo Research SL, Evofem, HRA Pharma, Medicines360 (includes support for medical and safety oversight of this study), Merck & Co., and Sebela. Kurt T. Barnhart has served on the Advisory Board for Merck & Co. His university department receives contraceptive research funding from Bayer HealthCare, Medicines360, Merck & Co., and Sebela. Lori M. Gawron's university department receives contraceptive research funding from Bayer HealthCare, Cooper Surgical, Medicines360, Merck & Co., Sebela, Femasys, and Teva. David Eisenberg has served on the Advisory Board for Merck & Co. He has been a consultant for Evofem Biosciences and FemaSys. He has received honoraria from Omnia Education. His university department receives contraceptive research funding from Evofem Biosciences, Medicines360, Merck & Co., Myovant and Sebela. R. Garn Mabey has received research funding from Allergan, Medicines360, Mithra, and Sebela. Jeffrey T. Jensen has received payments for consulting from Bayer Healthcare, Evofem, Hope Medicine, Foundation Consumer Healthcare, Mayne Pharma, ViiV Healthcare, and TherapeuticsMD. OHSU has received research support from AbbVie, Bayer Healthcare, Daré, Estetra SPRL, Hope Medicine, Medicines360, Merck, Myovent, and Sebela. These companies and organizations may have a commercial or financial interest in the results of this research and technology. These potential conflicts of interest have been reviewed and managed by OHSU.
Presented at the American College of Obstetricians and Gynecologists' Annual Clinical and Scientific Meeting, May 6–8, 2022, San Diego, California.
The authors thank the participating investigators and coordinators at the 29 study centers for conduct of the clinical trial and submission of data.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/D93.
Figure.
No available caption
REFERENCES
- 1.National Collaborating Centre for Women's and Children's Health (UK). Heavy menstrual bleeding. RCOG Press; 2007. [PubMed] [Google Scholar]
- 2.Hallberg L, Högdahl AM, Nilsson L, Rybo G. Menstrual blood loss--a population study. Variation at different ages and attempts to define normality. Acta Obstet Gynecol Scand 1966;45:320–51. doi: 10.3109/00016346609158455 [DOI] [PubMed] [Google Scholar]
- 3.Munro MG, Critchley HOD, Fraser IS, FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynecol Obstet 2018;143:393–408. doi: 10.1002/ijgo.12666 [DOI] [PubMed] [Google Scholar]
- 4.Bofill Rodriguez M, Dias S, Jordan V, Lethaby A, Lensen SF, Wise MR, et al. Interventions for heavy menstrual bleeding; overview of Cochrane reviews and network meta-analysis. The Cochrane Database of Systematic Reviews 2022, Issue 5. Art. No.: CD013180. doi: 10.1002/14651858.CD013180.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bitzer J, Heikinheimo O, Nelson AL, Calaf-Alsina J, Fraser IS. Medical management of heavy menstrual bleeding: a comprehensive review of the literature. Obstet Gynecol Surv 2015;70:115–30. doi: 10.1097/OGX.0000000000000155 [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Liu J, Peng S, Zheng Y. LNG-IUS vs. medical treatments for women with heavy menstrual bleeding: a systematic review and meta-analysis. Front Med (Lausanne) 2022;9:948709. doi: 10.3389/fmed.2022.948709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bayer HealthCare Pharmaceuticals. Mirena prescribing information. Accessed July 16, 2022. https://labeling.bayerhealthcare.com/html/products/pi/Mirena_PI.pdf
- 8.Kaunitz AM, Bissonnette F, Monteiro I, Lukkari-Lax E, Muysers C, Jensen JT. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol 2010;116:625–32. doi: 10.1097/AOG.0b013e3181ec622b [DOI] [PubMed] [Google Scholar]
- 9.Shaw ST, Jr, Aaronson DE, Moyer DL. Quantitation of menstrual blood loss--further evaluation of the alkaline hematin method. Contraception 1972;5:497–513. doi: 10.1016/0010-7824(72)90015-7 [DOI] [PubMed] [Google Scholar]
- 10.Mawet M, Nollevaux F, Nizet D, Wijzen F, Gordenne V, Tasev N, et al. Impact of a new levonorgestrel intrauterine system, Levosert(®), on heavy menstrual bleeding: results of a one-year randomised controlled trial. Eur J Contraception Reprod Health Care 2014;19:169–79. doi: 10.3109/13625187.2014.894184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ilyin AB, Khasanov AA, Suturina LV, Borisova NI, Reshetov ZS, Foidart JM, et al. Comparison of two levonorgestrel-releasing intrauterine systems for the treatment of heavy menstrual bleeding: a randomised, controlled, phase 3 trial. Eur J Contraception Reprod Health Care 2021;26:491–8. doi: 10.1080/13625187.2021.1942447 [DOI] [PubMed] [Google Scholar]
- 12.Gilliam ML, Jensen JT, Eisenberg DL, Thomas MA, Olariu A, Creinin MD. Relationship of parity and prior cesarean delivery to levonorgestrel 52 mg intrauterine system expulsion over 6 years. Contraception 2021;103:444–9. doi: 10.1016/j.contraception.2021.02.013 [DOI] [PubMed] [Google Scholar]
- 13.Creinin MD, Schreiber CA, Turok DK, Cwiak C, Chen BA, Olariu AI. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol 2022;227:871.e1–7. doi: 10.1016/j.ajog.2022.05.022 [DOI] [PubMed] [Google Scholar]
- 14.Furlani RM, Garcia E, Castro S, Machado HC, Bahamondes L, Monteiro I. Expulsion rates of the levonorgestrel 52 mg intrauterine system are similar among women with heavy menstrual bleeding and users for contraception. Contraception 2022;105:75–9. doi: 10.1016/j.contraception.2021.09.001 [DOI] [PubMed] [Google Scholar]
- 15.Getahun D, Fassett MJ, Gatz J, Armstrong MA, Peipert JF, Raine-Bennett T, et al. Association between menorrhagia and risk of intrauterine device-related uterine perforation and device expulsion: results from the Association of Uterine Perforation and Expulsion of Intrauterine Device study. Am J Obstet Gynecol 2022;227:59.e1–9. doi: 10.1016/j.ajog.2022.03.025 [DOI] [PubMed] [Google Scholar]
- 16.Madden T, McNicholas C, Zhao Q, Secura GM, Eisenberg DL, Peipert JF. Association of age and parity with intrauterine device expulsion. Obstet Gynecol 2014;124:718–26. doi: 10.1097/aog.0000000000000475 [DOI] [PMC free article] [PubMed] [Google Scholar]