A new cesarean scar ectopic pregnancy clinical classification system based on anterior myometrial thickness and gestational sac diameter with recommended surgical strategy for each classification type resulted in high treatment success rates.
OBJECTIVE:
To establish a new cesarean scar ectopic pregnancy clinical classification system with recommended individual surgical strategy and to evaluate its clinical efficacy in treatment of cesarean scar ectopic pregnancy.
METHODS:
This retrospective cohort study included patients with cesarean scar ectopic pregnancy in Qilu Hospital in Shandong, China. From 2008 to 2015, patients with cesarean scar ectopic pregnancy were included to determine risk factors for intraoperative hemorrhage during cesarean scar ectopic pregnancy treatment. Univariable analysis and multivariable logistic regression analyses were used to explore the independent risk factors for hemorrhage (300 mL or greater) during a cesarean scar ectopic pregnancy surgical procedure. The model was internally validated with a separate cohort. Receiver operating characteristic curve methodology was used to identify optimal thresholds for the identified risk factors to further classify cesarean scar ectopic pregnancy risk, and the recommended operative treatment was established for each classification group by expert consensus. A final cohort of patients from 2014 to 2022 were classified according to the new classification system, and the recommended surgical procedure and clinical outcomes were abstracted from the medical record.
RESULTS:
Overall, 955 patients with first-trimester cesarean scar ectopic pregnancy were included; 273 were used to develop a model to predict intraoperative hemorrhage with cesarean scar ectopic pregnancy, and 118 served as an internal validation group for the model. Anterior myometrium thickness at the scar (adjusted odds ratio [aOR] 0.51, 95% CI 0.36–0.73) and average diameter of the gestational sac or mass (aOR 1.10, 95% CI 1.07–1.14) were independent risk factors for intraoperative hemorrhage of cesarean scar ectopic pregnancy. Five clinical classifications of cesarean scar ectopic pregnancy were established on the basis of the thickness and gestational sac diameter, and the optimal surgical option for each type was recommended by clinical experts. When the classification system was applied to a separate cohort of 564 patients with cesarean scar ectopic pregnancy, the overall success rate of recommended first-line treatment with the new classification grouping was 97.5% (550/564). No patients needed to undergo hysterectomy. Eighty-five percent of patients had a negative serum β-hCG level within 3 weeks after the surgical procedure; 95.2% of patients resumed their menstrual cycles within 8 weeks.
CONCLUSION:
Anterior myometrium thickness at the scar and the diameter of the gestational sac were confirmed to be independent risk factors for intraoperative hemorrhage during cesarean scar ectopic pregnancy treatment. A new clinical classification system based on these factors with recommended surgical strategy resulted in high treatment success rates with minimal complications.
Cesarean scar ectopic pregnancy is a complication in which an early pregnancy implants in the scar from a prior cesarean delivery.1,2 The incidence of cesarean scar ectopic pregnancy has increased gradually worldwide, likely as a result of high cesarean delivery rates and increasing recognition of this condition through reliable diagnostic imaging. Reported estimates of incidence range from 1 in 1,800 to 1 in 2,226 of overall pregnancies.3,4 Without appropriate treatment, cesarean scar ectopic pregnancy presents substantial risk of severe morbidity such as life-threatening hemorrhage, uterine rupture, placenta accreta spectrum, hysterectomy with subsequent loss of fertility, and even maternal mortality.2,5,6
Transvaginal ultrasonography is the best and first-line imaging modality to diagnose cesarean scar ectopic pregnancy.6,7 In 2000, Vial et al8 reported that the implantation patterns of cesarean scar ectopic pregnancy can be categorized as either endogenic or exogenic according to ultrasonographic appearance.1,9 Since then, several classification systems for cesarean scar ectopic pregnancy have been proposed.10–12 However, these classification systems did not present quantitative ultrasonographic measurements based on risk factors for intraoperative hemorrhage during cesarean scar ectopic pregnancy treatment and did not suggest specific clinical treatment options based on classification type.
Many different treatment options for cesarean scar ectopic pregnancy, such as surgical, medical, and minimally invasive therapies, have been described.2 Undoubtedly, treatment decisions should be individually tailored according to the severity of symptoms, future family-planning wishes, physician experience, and institutional resources.13 However, there is still no consensus on the optimal surgical treatment strategy for cesarean scar ectopic pregnancy. Therefore, our aim was to create a practical cesarean scar ectopic pregnancy classification system to guide optimal surgical treatment selection with the goal of improving the treatment success rate and reducing the incidence of severe complications.
METHODS
This retrospective cohort study included patients diagnosed with cesarean scar ectopic pregnancy who were admitted to Qilu Hospital of Shandong University (Shandong, China) from January 2008 to June 2022. Data were obtained from their electronic medical records. This study was approved by the ethics committee of Qilu Hospital of Shandong University (No. 2016039).
The diagnosis of cesarean scar ectopic pregnancy was based on clinical history, pelvic examination, serum β-hCG level, and transvaginal ultrasound examination. Transvaginal ultrasound diagnosis of cesarean scar ectopic pregnancy was made when the following criteria were met: 1) empty uterine cavity; 2) empty cervical canal; 3) presence of a gestational sac, with or without fetal cardiac activity, in the anterior part of the uterine isthmus; 4) absence or thinning of myometrium at the level of the bladder; and 5) peritrophoblastic or periplacental flow surrounding the cesarean scar ectopic pregnancy appearing on Doppler flow ultrasonogram.3,7,8,14 All diagnoses of cesarean scar ectopic pregnancy were made by at least two experienced gynecologic ultrasonography physicians to ensure accuracy of diagnosis. This study included only patients in the first trimester because those in the second trimester required more detailed assessment of placental invasion and had a higher risk of other complications. We excluded patients with serious systemic diseases, such as heart disease, thrombocytopenia, and systemic lupus erythematosus because these conditions may affect treatment options and efficacy, and gestational trophoblastic disease. Patients for whom clinical data were incomplete or lost to follow-up were also excluded.
Our aims were 1) to identify risk factors for intraoperative hemorrhage during cesarean scar ectopic pregnancy treatment to develop a classification system with paired appropriate surgical management and 2) to evaluate outcomes when the classification system with recommended surgical treatment was in use. For the first aim, patients who underwent surgical resection after pretreatment with methotrexate, mifepristone, or uterine artery embolization who were admitted from January 2008 to December 2015 were identified and allocated to the modeling group. Data from the modeling group were analyzed to identify independent risk factors for intraoperative hemorrhage (300 mL or greater) and to establish a nomogram risk assessment model. One hundred eighteen patients who underwent treatment protocols similar to those of the modeling group admitted from December 2015 to June 2022 were allocated to a validation group to evaluate model performance.
After identification of the optimal cutoff values for the independent risk factors for intraoperative hemorrhage using the modeling group, a new clinical classification system with a suggested surgical strategy for each classification of cesarean scar ectopic pregnancy was proposed by our group. From July 2014 to June 2022, 564 patients with cesarean scar ectopic pregnancy who underwent surgical resection according to the new classification system were identified, and outcome data were analyzed to evaluate the clinical efficacy and safety of the new cesarean scar ectopic pregnancy classification system.
Baseline patient characteristics were abstracted from medical records, including gravidity, parity, number of prior cesareans, time interval since last cesarean, gestational age, average diameter of the gestational sac or mass, anterior myometrium thickness at the cesarean scar site, serum β-hCG level, duration of vaginal bleeding before the surgical procedure, gestational sac or mass type (heterogeneous mass composed of different components)15 assessed with ultrasonography, presence or absence of fetal cardiac activity, uterine arteriovenous fistula (also known as uterine arteriovenous malformation, an abnormal connection between arteries and veins bypassing the capillary system),16 and previous cesarean scar ectopic pregnancy treatment failure.
The primary outcome for model development was intraoperative hemorrhage, defined as blood loss of 300 mL or more during the cesarean scar ectopic pregnancy surgical procedure. Secondary outcomes of surgical treatment included procedure duration, hospitalization cost, and other major complications. Major complications were hysterectomy or estimated blood loss greater than 1,000 mL.17 For evaluation of the new classification system with recommended surgical management, successful treatment of cesarean scar ectopic pregnancy was defined as 1) complete resection of the products of conception, 2) no need to shift to a second-line surgical strategy, 3) no major complications, 4) no readmission for additional treatment, and 5) serum β-hCG levels returning to normal within 4 weeks.
Detailed information about how the surgeries for each classification type of cesarean scar ectopic pregnancy were approached is presented in Appendix 1, available online at http://links.lww.com/AOG/D51. After being discharged, the patients were asked to have their blood β-hCG levels tested once a week until levels returned to normal. Ultrasound examination was also recommended at 2 weeks after the surgical procedure to confirm that all products of conception were removed and to monitor the mass size until complete resolution. Time of resumption of menstrual cycles was also recorded.
All statistical analyses were performed with SPSS 26.0 and R 4.0.3. The normality of continuous variables was determined by the Shapiro-Wilk test in SPSS. Normally distributed quantitative variables were presented as mean±SD; nonnormally distributed variables were presented as median (interquartile range). Qualitative variables were expressed as counts and percentages. Continuous variables were tested by the Student t test (normal), Mann-Whitney U test (nonnormal), or Kruskal-Wallis H test (more than two groups, nonnormal), and qualitative variables were tested by the χ2 test. The univariable analysis was used to identify predictors associated with intraoperative hemorrhage. Variables with P<.05 were considered for inclusion in the multivariable logistic regression analysis (forward stepwise selection) to identify the independent risk factors for intraoperative hemorrhage. Based on the regression coefficients of independent variables, a nomogram diagram model was established. A receiver operating characteristic (ROC) curve was used to test discriminative power, and calibration curves were created. The optimal cutoff values of gestational sac diameter and anterior myometrial thickness to predict intraoperative hemorrhage were evaluated by ROC curve. P<.05 was considered statistically significant.
RESULTS
Overall, 1,038 patients with cesarean scar ectopic pregnancy were identified over the study period. After application of the exclusion criteria, a total of 955 patients with cesarean scar ectopic pregnancy were included (Fig. 1).
Fig. 1. Flowchart of patients included in this study.
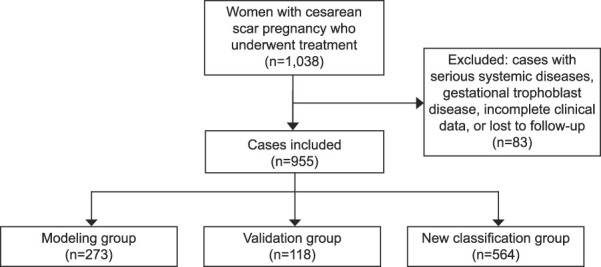
Ban. Cesarean Scar Ectopic Pregnancy Classification System. Obstet Gynecol 2023.
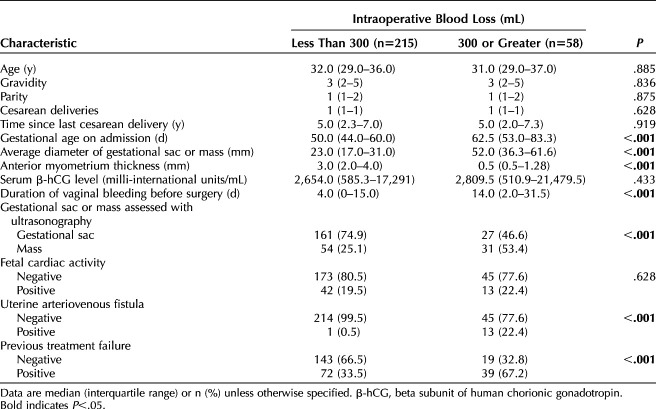
To identify risk factors for intraoperative hemorrhage during cesarean scar ectopic pregnancy surgical resection and to establish a risk prediction model, 273 patients with cesarean scar ectopic pregnancy from January 2008 to December 2015 were included in the modeling group, and 118 patients from December 2015 to June 2022 were enrolled in the model validation group. Among the patients in the initial modeling group, 58 (21.2%) experienced intraoperative hemorrhage (300 mL or greater).
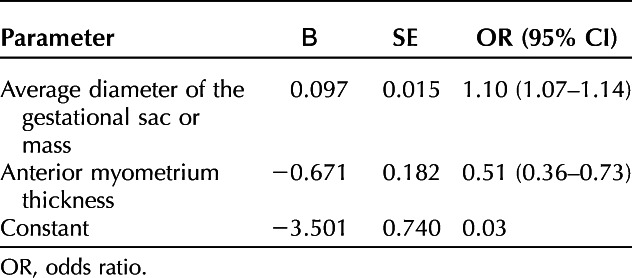
The results of univariable analyses of baseline characteristics between those experiencing hemorrhage and those who did not are presented in Table 1. No significant differences in age, gravidity, parity, number of cesarean deliveries, time interval from previous cesarean, serum β-hCG levels, and fetal cardiac activity were observed. However, there were significant differences in gestational age, diameter of gestational sac, thickness of anterior myometrium, duration of vaginal bleeding before the surgical procedure, gestational sac or mass type assessed with ultrasonography, uterine arteriovenous fistula, and previous cesarean scar ectopic pregnancy treatment failure between the groups (Table 1). In multivariable modeling, diameter of gestational sac (adjusted odds ratio 1.10, 95% CI 1.07–1.14) and anterior myometrium thickness (adjusted odds ratio 0.51, 95% CI 0.36–0.73) were independent risk factors for intraoperative hemorrhage (Table 2). With these two independent risk factors, a nomogram prediction model was established (Appendix 2, available online at http://links.lww.com/AOG/D51).The area under the ROC curve for the model was 0.92 (95% CI 0.88–0.96), the sensitivity was 86.2%, and the specificity was 88.8%. In the validation cohort, discrimination was similarly good, with an area under the ROC curve of 0.87 (95% CI 0.77–0.97) for the risk assessment model, a sensitivity of 88%, and a specificity of 81.7% (Appendix 3, available online at http://links.lww.com/AOG/D51). The nomogram calibration curves for the validation group are included in Appendix 3 (http://links.lww.com/AOG/D51), exhibiting close approximation between predicted and observed probability.
Table 1.
Baseline Characteristics of Patients With Intraoperative Hemorrhage Compared With a Control Group Without Hemorrhage in the Modeling Group (N=273)
Table 2.
Independent Risk Factors for Intraoperative Hemorrhage of Cesarean Scar Ectopic Pregnancy in the Modeling Group by Multivariable Logistic Regression Analysis
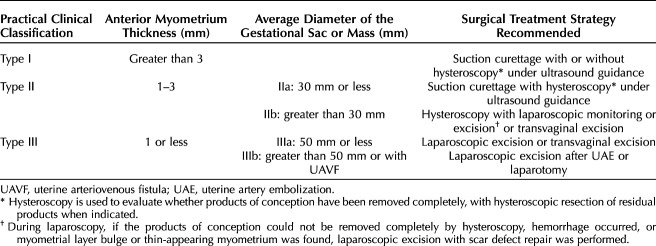
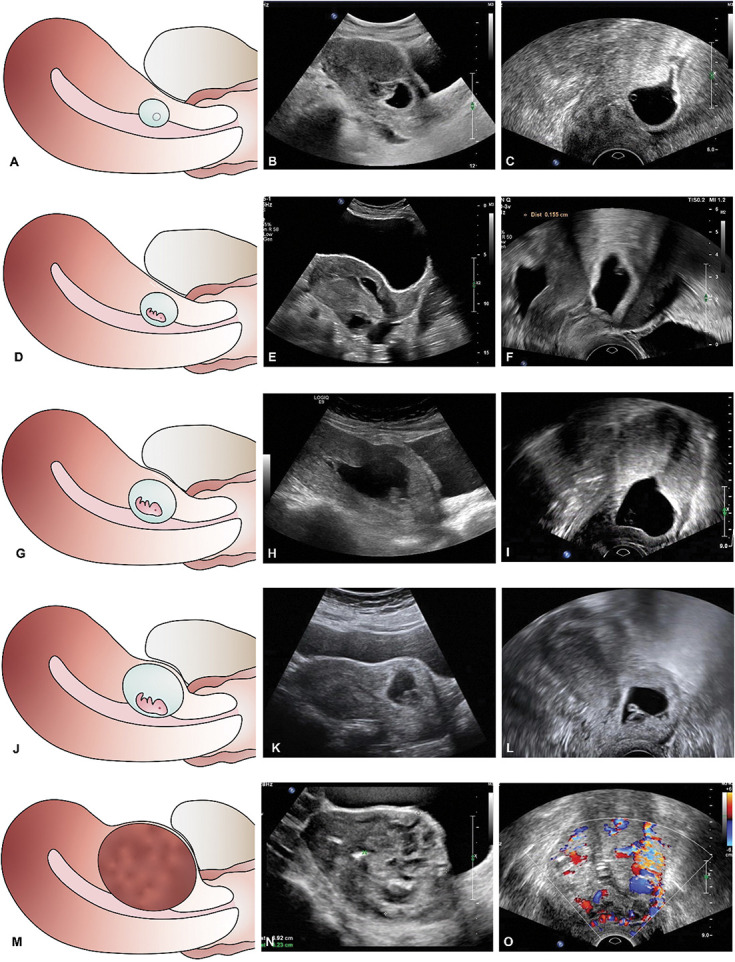
With the use of ROC curve methodology, appropriate cut points for anterior myometrial thickness and gestational sac diameter were selected for classification of patients with cesarean scar ectopic pregnancy according to risk of intraoperative hemorrhage (Appendix 4, available online at http://links.lww.com/AOG/D51). An anterior myometrial thickness of 1.3 mm resulted in a sensitivity of 75.9%, specificity of 88.4%, positive likelihood ratio of 6.5, and negative likelihood ratio of 0.3 for the prediction of intraoperative hemorrhage. To better classify patients with myometrial thickness greater than 1.0 mm, another ROC curve was created showing that with a cutoff value of 2.8 mm, the sensitivity was 76.2% and specificity was 66%. On the basis of these twooptimizing thresholds of anterior myometrial thickness, for more practical clinical diagnosis and application, cesarean scar ectopic pregnancy was classified into three types: type I (thickness greater than 3 mm), type II (thickness 1–3 mm), and type III (thickness 1 mm or less) (Table 3, Fig. 2).
Table 3.
New Clinical Classification of Cesarean Scar Ectopic Pregnancy and Recommended Individual Surgical Treatment Strategy
Fig. 2. Description of new clinical classification system for cesarean scar pregnancy. A–C. Type I is defined as the implantation of a gestational sac within the cesarean scar, with anterior myometrium thickness greater than 3 mm regardless of the size of the gestational sac. D–F. Type IIa is defined as anterior myometrium thickness between 1 and 3 mm and average diameter of the gestational sac or mass 30 mm or less. G–I. Type IIb is defined as anterior myometrium thickness between 1 and 3 mm and average diameter of the gestational sac or mass greater than 30 mm. J–L. In type IIIa, the gestational sac bulges out under the cesarean scar, with anterior myometrium thickness 1 mm or less and average diameter of the gestational sac or mass 50 mm or less. M–O. Type IIIb is defined as anterior myometrium thickness 1 mm or less and average diameter of the gestational sac or mass greater than 50 mm.
Ban. Cesarean Scar Ectopic Pregnancy Classification System. Obstet Gynecol 2023.
Gestational sac diameter was also analyzed to optimize thresholds to further subclassify cesarean scar ectopic pregnancy types II and III. Using ROC curve methodology, we identified a cutoff value of 33.8 mm as an optimal threshold for subclassifying cesarean scar ectopic pregnancy type II. Therefore, cesarean scar ectopic pregnancy type II was classified into two subtypes: type IIa (diameter 30 mm or less) (Table 3 and Fig. 2D–F) and type IIb (diameter greater than 30 mm) (Table 3 and Fig. 2G–I). For those in the cesarean scar ectopic pregnancy type III classification, a cutoff value of 50.3 mm optimized sensitivity and sensitivity. Therefore, cesarean scar ectopic pregnancy type III was classified into two subtypes: type IIIa (diameter 50 mm or less) (Table 3 and Fig. 2J–L) and type IIIb (diameter greater than 50 mm) (Table 3 and Fig. 2M–O).
Each classification group was matched with a recommended surgical strategy according to the clinical experience of our group (Table 3). To evaluate the success of the new clinical classification system with planned surgical strategy for the management of cesarean scar ectopic pregnancy, outcomes of 564 patients who underwent surgical resection directly according to the new classification system from July 2014 to June 2022 were examined.
Baseline characteristics of patients with cesarean scar ectopic pregnancy in the new classification group are presented in Appendix 5, available online at http://links.lww.com/AOG/D51. When the patients in the five classification groups were compared, significant differences were observed in time interval since last cesarean, gestational age, sac diameter, anterior myometrial thickness, serum β-hCG levels before the surgical procedure, duration of vaginal bleeding, gestational sac or mass types assessed with ultrasonography, fetal cardiac activity, uterine arteriovenous fistula, and previous treatment failure.
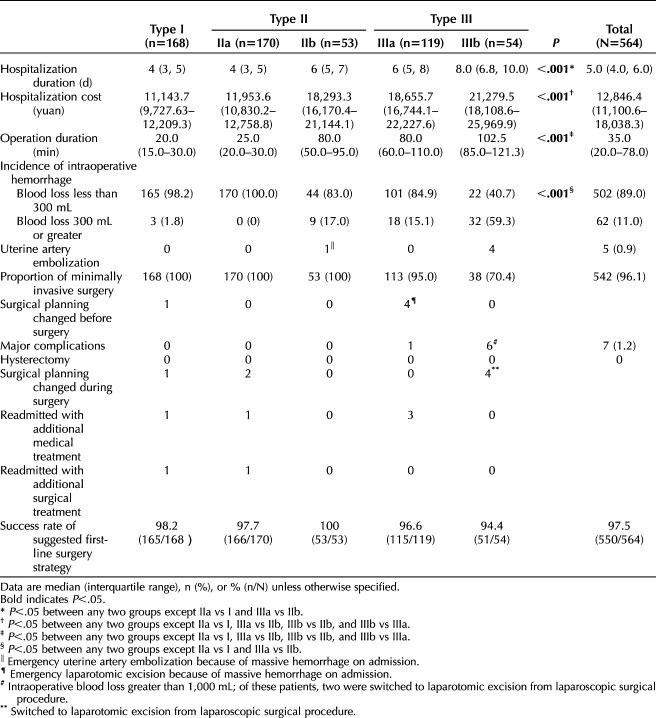
The overall success rate was 97.5% (550/564 successful). Success rates for treatment of cesarean scar ectopic pregnancy by classification group are shown in Table 4. Across the five classification groups, significant differences in hospitalization duration, hospitalization cost, surgical procedure duration, and incidence of intraoperative hemorrhage were observed.
Table 4.
Description of Outcomes and Treatment Efficacy for Patients Using the New Classification System to Determine Operative Management
All cases followed the recommended first-line surgical treatment strategy, except five in which the surgeon selected a different surgical plan before the procedure and seven in which the surgeon selected a different surgical plan during the procedure (Table 4). Detailed information about these cases can be found in Appendix 6, available online at http://links.lww.com/AOG/D51.
All patients recovered well after surgical treatment, except for seven patients who were readmitted because of an inadequate decline in serum β-hCG levels and unexpected vaginal bleeding. Among these seven patients, five required additional medical treatment and two underwent additional surgical treatment of a persistent mass at the cesarean scar site (Table 4). There were no cases of uterine rupture or hysterectomy.
One patient with type IIb underwent uterine artery embolization treatment because of acute heavy vaginal bleeding before the surgical procedure. Four patients with type IIIb underwent uterine artery embolization before their surgical procedures to prevent hemorrhage during the cesarean scar ectopic pregnancy surgical procedure (Table 4).
Of the new classification cohort, 85% of patients had a negative serum β-hCG level within 3 weeks after the surgery and 95.2% of patients resumed their menstrual cycles within 8 weeks (Table 4).
DISCUSSION
In this study, based on independent risk factors for hemorrhage during cesarean scar ectopic pregnancy surgical procedures, a new system was created to classify cesarean scar ectopic pregnancy into five types, with a recommended surgical treatment strategy for each type based on expert opinion. Our cesarean scar ectopic pregnancy classification system uses precise quantitative indicators of anterior myometrial thickness and gestational sac diameter to determine the optimal first-line surgical treatment approach to avoid insufficient therapy and to achieve success with the least invasive treatments.
In the present study, anterior myometrial thickness and gestational sac diameter were identified as independent risk factors for intraoperative hemorrhage of cesarean scar ectopic pregnancy. Previous studies have shown that the risk factors for hemorrhage during cesarean scar ectopic pregnancy treatment include not only thin anterior myometrial thickness and large gestational sac diameter but also greater gestational age, high β-hCG levels, and abundant blood supply.18,19 The reasons for different parameters being identified may be different treatment options, but all of these results suggest the importance of anterior myometrial thickness and gestational sac diameter in determining risk and cesarean scar ectopic pregnancy treatment.
We found that the overall success rate of the recommended first-line surgical treatment according to the new cesarean scar ectopic pregnancy classification system was 97.5%. The key to good clinical prognosis of cesarean scar ectopic pregnancy lies in early precise diagnosis with classification and optimal individual management. At present, several classification systems for cesarean scar ectopic pregnancy have been reported in the literature. The system of Vial et al8 was demonstrated first and has been widely used for the diagnosis and treatment of cesarean scar ectopic pregnancy. Zhang et al11 classified cesarean scar ectopic pregnancy into risky and stable types based on the gestational sac location and remaining myometrial thickness.11 One recent study categorized patients with cesarean scar ectopic pregnancy into four grades according to conditions of retained myometrium.12 However, these previous studies were limited by a lack of clear quantitative indicators that could be used to classify cesarean scar ectopic pregnancy and the absence of optimal clinical treatment options for each type. In contrast, the new cesarean scar ectopic pregnancy clinical classification in our study was established according to precise quantitative indicators of anterior myometrial thickness and gestational sac diameter with a recommendation for optimal first-line surgical treatment strategy.
In our study, the success rate of recommended first-line surgical treatment for type I was 98.21% (165/168), with shorter hospitalization duration, lower hospitalization cost, and shorter surgical procedure duration than the other groups, suggesting that this minimally invasive procedure is effective, safe, and economical for cesarean scar ectopic pregnancy type I. Recently, the implementation and potential benefits of hysteroscopic removal of first-trimester cesarean scar ectopic pregnancy were reported, which were consistent with our result because the cesarean scar ectopic pregnancy type referred to in their study could be classified as type I in our classification system.20,21
Patients with cesarean scar ectopic pregnancy type IIIb may have the highest risk of intraoperative hemorrhage, so laparoscopic excision after uterine artery embolization or laparotomy directly was recommended. In keeping with this result, one study implied that for type II of the Vial et al8 classification, laparotomy could be an effective and reasonable treatment for deep-implantation cesarean scar ectopic pregnancy.22
For other classification groups with higher risk of intraoperative hemorrhage, determination of the optimal management strategy warrants further research and assessment of our developed classification system in other cohorts before widespread adoption. It is noteworthy that treatment decisions should also be made according to the clinical condition of patients and the resources available at an institution. For patients with cesarean scar ectopic pregnancy and heavy vaginal bleeding, efficient emergency procedures are necessary for treatment. Patients with high-risk types of cesarean scar ectopic pregnancy need to be referred to larger centers with an adequate supply of blood products and rich clinical experience in the treatment of cesarean scar ectopic pregnancy.
The main strength of our study is the identification of anterior myometrial thickness and gestational sac diameter as independent risk factors for intraoperative hemorrhage of cesarean scar ectopic pregnancy in a large cohort of patients. This model was validated with a separate cohort of patients. Based on these two risk factors, a new five-category clinical classifications of cesarean scar ectopic pregnancy was developed, and the recommended optimal surgical strategy for each type was established. The overall success rate of recommended first-line treatment in the new classification grouping of 564 patients was found to be high with the least invasive treatments and minimal complications.
There were several limitations in this study. First, this was a single-center study and performed over a long time period in which familiarity with the diagnosis and surgical proficiency likely improved and contributed to better outcomes regardless of treatment choice. Second, this study included only patients with cesarean scar ectopic pregnancy in the first trimester. For patients with cesarean scar ectopic pregnancy greater than 12 weeks, more detailed assessment of placental invasion is needed, and risks of other complications are higher. Another limitation is a lack of long-term follow-up data, such as the outcome of the subsequent pregnancy and the probability of recurrent cesarean scar ectopic pregnancy.
In conclusion, we have created a new clinical classification system based on risk factors with optimal surgical strategies proposed. The new classification system has been evaluated and found to have high success rates for cesarean scar ectopic pregnancy treatment. Continued research is required to validate the new classification system in other populations.
Footnotes
This study was supported by grants from the National Natural Science Foundation of China (No. 81601315) and Natural Science Foundation of Shandong Province (No. ZR2022MH192).
Financial Disclosure The authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/D52.
Figure.
No available caption
REFERENCES
- 1.Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol 2006;107:1373–81. doi: 10.1097/01.AOG.0000218690.24494.ce [DOI] [PubMed] [Google Scholar]
- 2.Miller R, Timor-Tritsch IE, Gyamfi-Bannerman C. Society for Maternal-Fetal Medicine (SMFM) consult series #49: cesarean scar pregnancy. Am J Obstet Gynecol 2020;222:B2–14. doi: 10.1016/j.ajog.2020.01.030 [DOI] [PubMed] [Google Scholar]
- 3.Jurkovic D, Hillaby K, Woelfer B, Lawrence A, Salim R, Elson CJ. First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment cesarean section scar. Ultrasound Obstet Gynecol 2003;21:220–7. doi: 10.1002/uog.56 [DOI] [PubMed] [Google Scholar]
- 4.Seow KM, Huang LW, Lin YH, Yan-Sheng Lin M, Tsai YL, Hwang JL. Cesarean scar pregnancy: issues in management. Ultrasound Obstet Gynecol 2004;23:247–53. doi: 10.1002/uog.974 [DOI] [PubMed] [Google Scholar]
- 5.Xie RH, Guo X, Li M, Liao Y, Gaudet L, Walker M, et al. Risk factors and consequences of undiagnosed cesarean scar pregnancy: a cohort study in China. BMC Pregnancy Childbirth 2019;19:383. doi: 10.1186/s12884-019-2523-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Timor-Tritsch IE, Monteagudo A, Cali G, D'Antonio F, Kaelin Agten A. Cesarean scar pregnancy: diagnosis and pathogenesis. Obstet Gynecol Clin North Am 2019;46:797–811. doi: 10.1016/j.ogc.2019.07.009 [DOI] [PubMed] [Google Scholar]
- 7.Timor-Tritsch IE, Monteagudo A, Santos R, Tsymbal T, Pineda G, Arslan AA. The diagnosis, treatment, and follow-up of cesarean scar pregnancy. Am J Obstet Gynecol 2012;207:44.e1–13. doi: 10.1016/j.ajog.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 8.Vial Y, Petignat P, Hohlfeld P. Pregnancy in a cesarean scar. Ultrasound Obstet Gynecol 2000;16:592–3. doi: 10.1046/j.1469-0705.2000.00300-2.x [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez N, Tulandi T. Cesarean scar pregnancy: a systematic review. J Minim Invasive Gynecol 2017;24:731–8. doi: 10.1016/j.jmig.2017.02.020 [DOI] [PubMed] [Google Scholar]
- 10.Hwang JH, Lee JK, Oh MJ, Lee NW, Hur JY, Lee KW. Classification and management of cervical ectopic pregnancies: experience at a single institution. J Reprod Med 2010;55:469–76. [PubMed] [Google Scholar]
- 11.Zhang H, Huang J, Wu X, Fan H, Li H, Gao T. Clinical classification and treatment of cesarean scar pregnancy. J Obstet Gynaecol Res 2017;43:653–61. doi: 10.1111/jog.13267 [DOI] [PubMed] [Google Scholar]
- 12.Lin SY, Hsieh CJ, Tu YA, Li YP, Lee CN, Hsu WW, Shih JC. New ultrasound grading system for cesarean scar pregnancy and its implications for management strategies: an observational cohort study. PLoS One 2018;13:e0202020. doi: 10.1371/journal.pone.0202020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Timor-Tritsch IE, Monteagudo A, Cali G, D'Antonio F, Agten AK. Cesarean scar pregnancy: patient counseling and management. Obstet Gynecol Clin North Am 2019;46:813–28. doi: 10.1016/j.ogc.2019.07.010 [DOI] [PubMed] [Google Scholar]
- 14.Fylstra DL. Ectopic pregnancy within a cesarean scar: a review. Obstet Gynecol Surv 2002;57:537–43. doi: 10.1097/00006254-200208000-00024 [DOI] [PubMed] [Google Scholar]
- 15.Liu D, Yang M, Wu Q. Application of ultrasonography in the diagnosis and treatment of cesarean scar pregnancy. Clin Chim Acta 2018;486:291–7. doi: 10.1016/j.cca.2018.08.012 [DOI] [PubMed] [Google Scholar]
- 16.Timor-Tritsch IE, Haynes MC, Monteagudo A, Khatib N, Kovacs S. Ultrasound diagnosis and management of acquired uterine enhanced myometrial vascularity/arteriovenous malformations. Am J Obstet Gynecol 2016;214:731.e1–10. doi: 10.1016/j.ajog.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 17.Birch Petersen K, Hoffmann E, Rifbjerg Larsen C, Nielsen HS. Cesarean scar pregnancy: a systematic review of treatment studies. Fertil Steril 2016;105:958–67. doi: 10.1016/j.fertnstert.2015.12.130 [DOI] [PubMed] [Google Scholar]
- 18.Wang Q, Ma H, Peng H, He L, Bian C, Zhao X. Risk factors for intra-operative haemorrhage and bleeding risk scoring system for caesarean scar pregnancy: a case-control study. Eur J Obstet Gynecol Reprod Biol 2015;195:141–5. doi: 10.1016/j.ejogrb.2015.06.023 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Zhang Z, Liu X, Zhang L, Hong F, Lu M. Risk factors for massive hemorrhage during the treatment of cesarean scar pregnancy: a systematic review and meta-analysis. Arch Gynecol Obstet 2021;303:321–8. doi: 10.1007/s00404-020-05877-9 [DOI] [PubMed] [Google Scholar]
- 20.Chueh HY, Pai AHY, Su YY, Hsu CC, Chang FY, Yen CF. Hysteroscopic removal, with or without laparoscopic assistance, of first-trimester cesarean scar pregnancy. Fertil Steril 2022;117:643–5. doi: 10.1016/j.fertnstert.2021.11.027 [DOI] [PubMed] [Google Scholar]
- 21.Qu W, Li H, Zhang T, Zhang Y, Ban Y, Li N, et al. Comparison of different treatment strategies in the management of endogenic caesarean scar pregnancy: a multicentre retrospective study. BMC Pregnancy Childbirth 2022;22:404. doi: 10.1186/s12884-022-04633-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun YY, Xi XW, Yan Q, Qiao QQ, Feng YJ, Zhu YP. Management of type II unruptured cesarean scar pregnancy: comparison of gestational mass excision and uterine artery embolization combined with methotrexate. Taiwan J Obstet Gynecol 2015;54:489–92. doi: 10.1016/j.tjog.2015.08.002 [DOI] [PubMed] [Google Scholar]