Background:
The relation between ventricular arrhythmia and fibrosis in mitral valve prolapse (MVP) is reported, but underlying valve-induced mechanisms remain unknown. We evaluated the association between abnormal MVP-related mechanics and myocardial fibrosis, and their association with arrhythmia.
Methods:
We studied 113 patients with MVP with both echocardiogram and gadolinium cardiac magnetic resonance imaging for myocardial fibrosis. Two-dimensional and speckle-tracking echocardiography evaluated mitral regurgitation, superior leaflet and papillary muscle displacement with associated exaggerated basal myocardial systolic curling, and myocardial longitudinal strain. Follow-up assessed arrhythmic events (nonsustained or sustained ventricular tachycardia or ventricular fibrillation).
Results:
Myocardial fibrosis was observed in 43 patients with MVP, predominantly in the basal-midventricular inferior-lateral wall and papillary muscles. Patients with MVP with fibrosis had greater mitral regurgitation, prolapse, and superior papillary muscle displacement with basal curling and more impaired inferior-posterior basal strain than those without fibrosis (P<0.001). An abnormal strain pattern with distinct peaks pre–end-systole and post–end-systole in inferior-lateral wall was frequent in patients with fibrosis (81 versus 26%, P<0.001) but absent in patients without MVP with basal inferior-lateral wall fibrosis (n=20). During median follow-up of 1008 days, 36 of 87 patients with MVP with >6-month follow-up developed ventricular arrhythmias associated (univariable) with fibrosis, greater prolapse, mitral annular disjunction, and double-peak strain. In multivariable analysis, double-peak strain showed incremental risk of arrhythmia over fibrosis.
Conclusions:
Basal inferior-posterior myocardial fibrosis in MVP is associated with abnormal MVP-related myocardial mechanics, which are potentially associated with ventricular arrhythmia. These associations suggest pathophysiological links between MVP-related mechanical abnormalities and myocardial fibrosis, which also may relate to ventricular arrhythmia and offer potential imaging markers of increased arrhythmic risk.
Keywords: arrhythmia, echocardiography, fibrosis, magnetic resonance imaging, mechanics, mitral valve prolapse, papillary muscles
CLINICAL PERSPECTIVE.
This study sought the association between mitral valve prolapse–related abnormal mechanics and left ventricular fibrosis using speckle-tracking echocardiography and cardiac magnetic resonance imaging with late gadolinium enhancement in 113 patients with mitral valve prolapse. Cardiac magnetic resonance imaging with late gadolinium enhancement revealed 43 patients (38%) had fibrosis predominantly in the basal-midventricular inferior-lateral wall and papillary muscles. Fibrosis was related to greater prolapse, papillary muscle displacement and mitral regurgitation, presence of mitral annular disjunction, ventricular mechanical dispersion, and the speckle-tracking echocardiography–derived unique longitudinal strain pattern characterized by double peaks before and after end-systole, named double-peak strain, indicating late-systolic inferobasal lengthening. Furthermore, outcomes in 87 patients showed potential association between mitral valve prolapse–related abnormal mechanics represented by double-peak strain and ventricular arrhythmias even after adjustment for fibrosis and arrhythmic history. These associations suggest pathophysiological links between mitral valve prolapse–related mechanical abnormalities and myocardial fibrosis, which has been related to ventricular arrhythmias. These findings provide potential imaging markers to identify patients at risk of fibrosis and ventricular arrhythmias and motivate large-scale prospective study to introduce this concept into clinical practice.
See Editorial by Lancellotti and Yun Yun
Mitral valve prolapse (MVP) is common, affecting up to 3% of the population.1,2 In MVP, myxomatous thickening and elongation of the mitral valve leaflets causes superior leaflet displacement into the left atrium with progressive mitral regurgitation (MR). The chronic volume overload drives cardiac remodeling with left ventricular (LV) eccentric hypertrophy and dysfunction.1,3 Timely mitral valve repair generally reduces MR, remodeling, and adverse outcomes,3 although residual LV dysfunction can remain.4 However, there is increasing concern regarding a malignant phenotype with increased risk of sudden cardiac death regardless of MR severity or LV remodeling5–7 and its incidence is reported up to 1.9%/year.8–11 The lack of preventive strategies for malignant MVP highlights the critical need to understand mechanisms of life-threatening arrhythmias in MVP.5,6,9–16
Myocardial fibrosis is known to play a role in MVP-related sudden cardiac death.11,13,17–19 Autopsy data show papillary muscle (PM) and inferobasal myocardial fibrosis in the vast majority of patients with MVP with sudden cardiac death.13,20 Late gadolinium enhancement cardiac magnetic resonance imaging (LGE-CMR) has related PM fibrosis to arrhythmia,17,18 showing LV fibrosis in 37% of patients with MVP versus 7% with equivalent MR without MVP,19 predominantly basal or midventricular inferolateral fibrosis near the PMs. However, it remains unknown why fibrosis is more common in MVP and what mechanisms predispose to fibrosis and arrhythmia. We hypothesize that myocardial fibrosis relates to MVP-induced excessive stress and deformation of the LV myocardium through abnormal MVP-PM mechanics. MVP-specific abnormal motions include excessive superior displacement of the PMs paralleling superior leaflet prolapse, with associated exaggerated inward excursion (curling) of the basal myocardium.21,22
This study evaluated the relationship between LV fibrosis and abnormal MVP-related mechanics and LV deformation from standard and speckle-tracking echocardiography; and the association of abnormal mechanics and fibrosis with arrhythmic events.
Methods
The data supporting the findings are available from the corresponding author upon reasonable request.
Study Population
Patients with MVP who underwent cardiac magnetic resonance imaging and echocardiography and met the MVP criteria by both modalities within 6 months at 2 institutions (Massachusetts General Hospital, Boston, and Weill-Cornell Medical Center, New York) were identified from databases. Exclusion criteria were: (1) other cardiac diseases including prior myocardial infarction/revascularization, ≥moderate aortic valve or congenital heart diseases, (2) regional wall motion abnormalities interpreted as hypo- or a-kinesis, which was unlike MVP-specific exaggerated motion, (3) inadequate image quality for strain analyses, and (4) age <18 years. The inclusion and exclusion process of study subjects is described in Figure S1. Controls (n=20) without cardiovascular diseases and risk factors were patients who underwent screening echocardiography before non-cardiac surgery or chemotherapy without structural or functional cardiac abnormalities. To establish that abnormalities in LV and papillary mechanics linking MVP to inferior-lateral fibrosis were related to MVP, 20 patients without MVP with fibrosis in basal inferior-lateral wall were included from Massachusetts General Hospital–Magnetic Resonance Imaging database. Institutional ethics committees’ approvals waived informed consent for this retrospective noninvasive study.
Echocardiographic Measurements
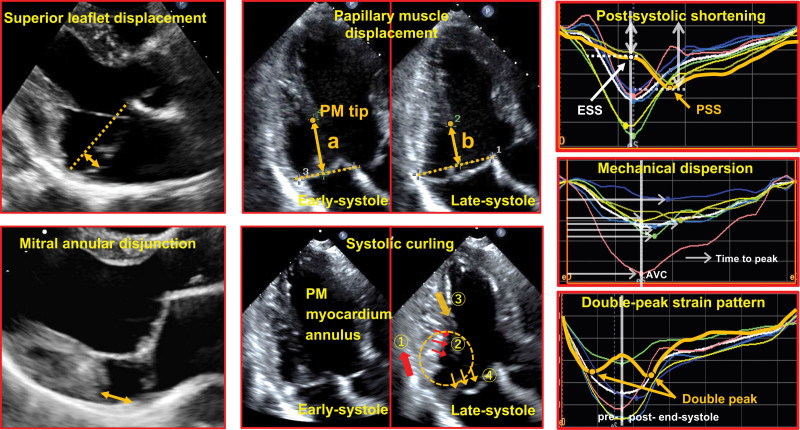
All subjects underwent routine 2-dimensional echocardiography using commercially available scanners (iE33/EPIQ7, Philips Healthcare; Vivid 7/E9/E95, GE Healthcare; Acuson SC2000, Siemens). Measurements were blinded to clinical information (Figure 1). Prolapse was measured as superior systolic leaflet displacement in the parasternal long-axis view from the mitral annular line to the most superior point of the maximally prolapsed leaflet.23,24 Mitral annular disjunction (MAD) was present if the posterior myocardial end was separated from the posterior leaflet-left atrial junction.14,15,25 PM displacement was measured as the change in distance between PM tip and annular line from early to late systole, obtained if visualized throughout systole.22,26 Systolic curling was exaggerated apical systolic motion of the posterior MV annulus and inward excursion of the adjacent posterobasal myocardium, associated with superior displacement of the connected leaflet and PM structures (Figure 1; Video S1).14,25 MR severity was quantified and graded by an integrated approach by current guidelines.27
Figure 1.
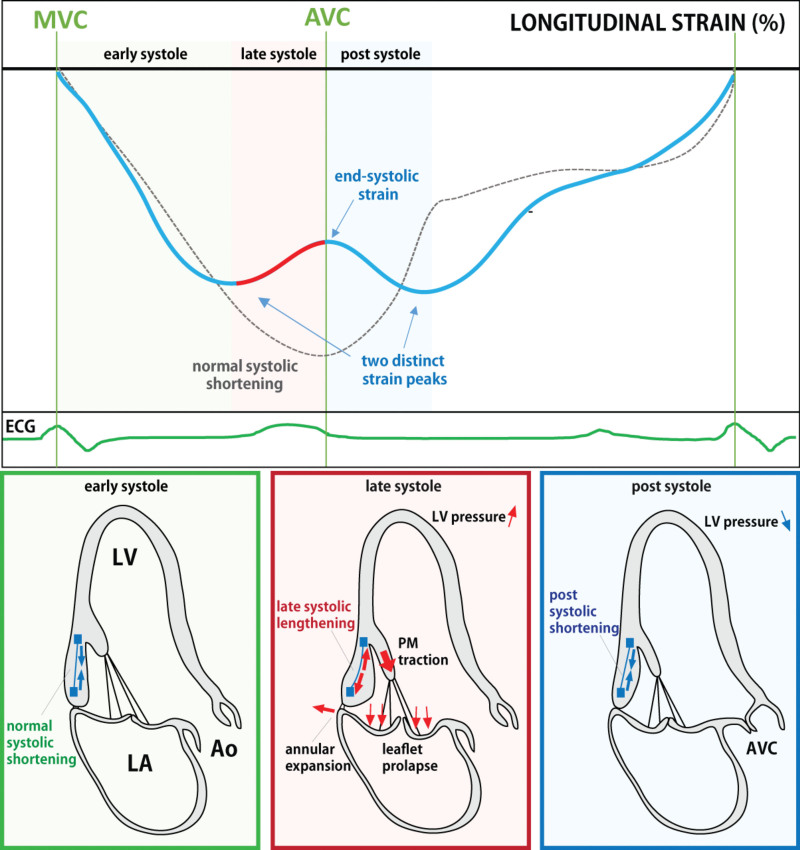
Mitral valve prolapse–related echocardiographic measurements and strain parameters. Superior leaflet displacement is measured as the distance from the most superior point of a prolapsing leaflet to the mitral annular line in systole (left upper, bidirectional arrow). Papillary muscle (PM) displacement is measured as the difference between distance from PM tip to mitral annular line in early systole and late systole (right upper, bidirectional arrows). Mitral annular disjunction is evaluated as separation between the mitral posterior leaflet-left atrial wall junction and the end of the posterior myocardium (left lower, bidirectional arrow). Systolic curling (right lower) is characterized by exaggerated apical systolic motion of the posterior mitral annulus (① single red arrow) and inward excursion of the adjacent posterobasal myocardium (② triple red arrows) associated with papillary muscle traction (③ orange single arrow) tugged by superior leaflet displacement (④triple orange arrows). Orange dotted circle shows how these structures appear to move as a mechanically-linked (curling) loop in systole. Postsystolic shortening (PSS: orange arrow) is shown after aortic valve closure (end-systole) in upper panel. PSS is calculated as %([PSS–end-systolic strain]/PSS). Middle, Time to peak in each regional strain (white arrows). Mechanical dispersion is defined as standard deviation of time to peak in 18 segments. Lower, Double-peak strain pattern as distinct pre–end-systolic and post–end-systolic strain peaks (orange arrows). AVC indicates aortic valve closure; and ESS, end-systolic strain.
Speckle-Tracking Echocardiographic Strain
Myocardial strain was analyzed on 2-dimensional images using vendor-independent software (2-dimensional Cardiac Performance Analysis, TOMTEC-ARENA 2.30.02, Unterschleissheim, Germany) by an experienced observer blinded to CMR and clinical findings. The LV endocardium was manually traced in apical 2-, 3-, and 4-chamber views at end-systole for automatic speckle-tracking analysis throughout 1 cardiac cycle. Endocardium was carefully verified and traced to avoid including non-muscle segments, particularly in patients with MAD (Figure S2; Video S2). End-systole was determined by aortic valve closure or smallest LV cavity size by M-mode. Six segmental longitudinal strain (LS) curves were generated in each view for 18 total curves/patient, with manual adjustments if tracking was deemed suboptimal. If tracking remained unsatisfactory (due to image quality or multiple premature contractions), subjects were excluded. All 18 segmental end-systolic strains were averaged for global LS. Absolute strain values are used to avoid confusion. If peak segmental LS occurred after aortic valve closure, postsystolic shortening index (PSI) was calculated as %([peak LS–end-systolic LS]/peak LS; Figure 1).28 If peak segmental LS occurred at or before end-systole, segmental PSI was regarded as 0. LV mechanical dispersion (MD) was defined as the SD of time from onset of systole to peak LS in all 18 segments as %ECG R-R interval (Figure 1).15,29
We recognized a previously undescribed strain pattern in the basal inferior-lateral wall of patients with MVP with 2 distinct LS peaks, one before and the second after end-systole (Figure 1). Of note, this is different from the postsystolic contraction known to occur with myocardial ischemia and in non-MVP fibrosis,28 which may pause briefly, creating a second peak of strain rate.30 In contrast, in MVP, there can be a second peak of actual strain after a late-systolic decrease in strain that physically indicates actual segmental lengthening so that strain is lower in late systole.
Cardiac Magnetic Resonance
Cine-CMR was performed using commercial 1.5-3.0 Tesla scanners (Signa HDxt 1.5T, GE Healthcare; Avanto 1.5T/Skyra 3T/Trim Trio 3T, Siemens) using a steady-state free precession sequence. LGE-CMR was performed 10 to 30 minutes after intravenous injection of gadolinium (0.10–0.2 mmol/kg) using a standard 2-dimensional gradient echo or 3-dimensional SSFP inversion recovery sequence tailored to null viable myocardium (typical inversion time ≈300 ms). Cine- and LGE-CMR were obtained in matching short- and long-axis planes (typical slice thickness 6.0mm, interslice gap 4.0mm), with short-axis images from mitral annulus to apex and standard 2-, 3- and 4-chamber long-axis orientations.
LGE-CMR identified focal fibrosis by established criteria as localized hyperenhancement31–34 scored as a binary variable in a conventional 17-segment AHA model, as was PM fibrosis with established methods used by our group.35 LGE was quantitatively measured with full-width-at-half-maximal method.35
Clinical Data and Arrhythmic Events
Patient age, sex, cardiovascular risk factors, and clinical outcomes were obtained from electronic records. Clinical follow-up was calculated from the initial imaging time. The primary outcome of significant arrhythmic events was defined as occurrence of nonsustained ventricular tachycardia (VT), sustained VT or ventricular fibrillation detected on ECG, 24-hour Holter, uninterrupted mobile telemetry, implantable loop recorder, or implantable pacemaker or cardiac defibrillator. Nonsustained VT was defined as ≧3 ventricular beats with ≧120 bpm and sustained VT as lasting ≧30 seconds. Premature ventricular contractions, couplets, and triplets were not included as significant arrhythmic events regardless of burden. Patients were censored at the time of MV surgery on follow-up.
Statistical Analyses
Continuous variables were expressed as mean±SD or median and interquartile range according to distribution. Categorical data were expressed as number and percentage of total patients in each group. T test or Wilcoxon rank-sum test was used for differences in continuous variables between 2 groups when normally or non-normally distributed, respectively. Statistical significance among 3 groups was determined by 1-way ANOVA and post hoc Tukey-Kramer tests when normally distributed or Kruskal-Wallis test when not. Normality was confirmed using the Kolmogorov-Smirnov test. Categorical variables were compared by using χ2 tests, or Fisher exact test with fewer than 5 expected outcomes per cell. Univariable and multivariable regression analysis explored independent determinants of LV fibrosis. Variables with univariate P≦0.10 were included in multivariable analysis. A 2-sided P<0.05 was considered significant. Kaplan-Meier survival analysis was used for time to arrhythmic events. Six months was set as a minimal follow-up duration. The log-rank test evaluated differences between 2 groups. Cox proportional hazard models evaluated the hazard risk for arrhythmic events while adjusting for confounders. To avoid overfitting the data, and because myocardial fibrosis is an established factor for arrhythmic events,36 the different components of MVP-related motion and mechanics were included together with fibrosis in separate multivariable models for arrhythmic events. The proportional hazard assumptions were verified with scaled Schoenfeld residuals. Supplemental analysis of arrhythmic events in patients without fibrosis was done similarly to explore the effect of the double-peak strain pattern alone. In the 58 patients (67% of the cohort with outcome data) who had dedicated long-term electrocardiographic monitoring, subgroup analysis of the relation between fibrosis, MVP-related mechanical abnormalities, and arrhythmic events was likewise done, including univariable and multivariable Cox proportional hazard analysis. For evaluation of intra- and inter-observer reliability of key measurements, 15 subjects were randomly selected. Two independent observers performed measurement blinded to each other. The intraclass and interclass correlation coefficient was used for numeric variables and the kappa statistic was for categorical variables. Analyses were performed using commercial software (JMP, version 16, SAS, Cary, NC, or IBM SPSS version 25, ICM Corporation, Armonk, NY).
Results
Study Population
From 193 patients with MVP with both echocardiography and LGE-CMR within 6 months, 113 were included (62 from Massachusetts General Hospital and 51 from Weill-Cornell). The rest were excluded because of other cardiac diseases (n=30), regional wall motion abnormalities (n=1), postoperative status (n=5), absence of echocardiographic images necessary for analyses (n=30), or insufficient speckle-tracking image quality (n=14). Median time between echocardiography and CMR was 28 (interquartile range, 4–75) days. CMR indications included evaluation of ventricular arrhythmia (n=48), question of cardiomyopathy (n=42), valve or aortic disease (n=17), coronary artery disease (n=3), and others (n=3).
Baseline Characteristics
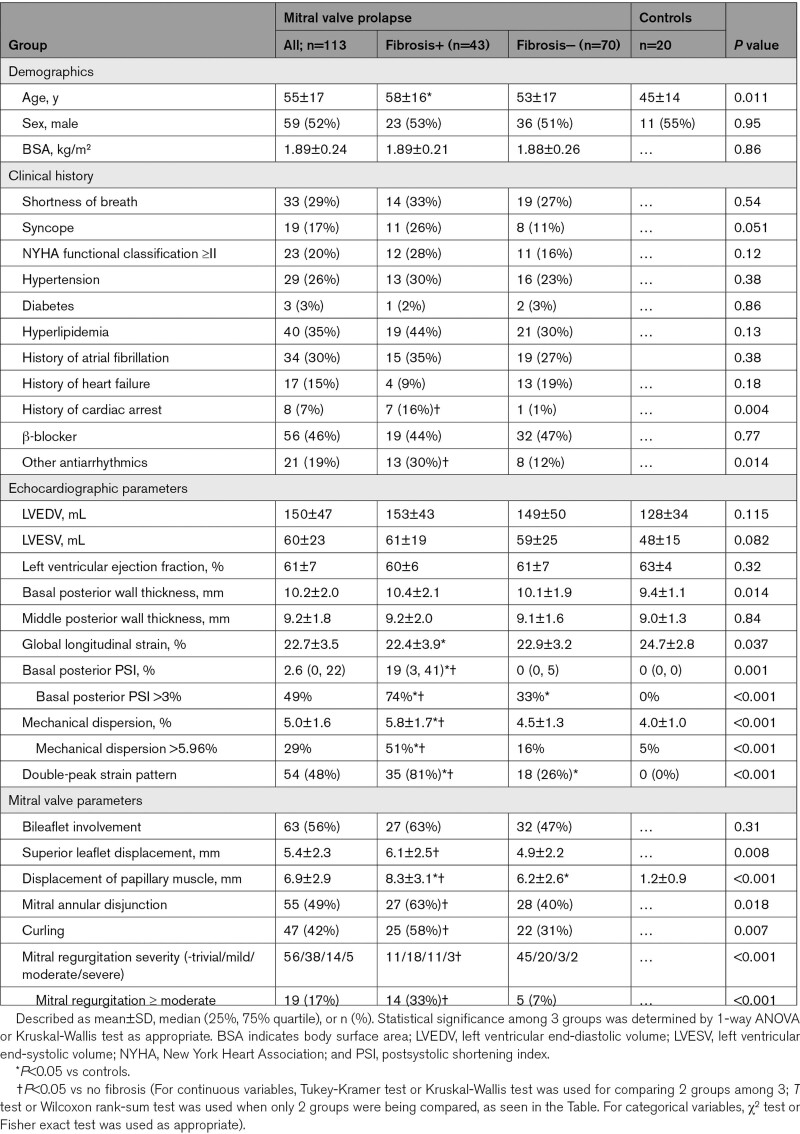
Compared with healthy controls, patients with MVP had lower global LS and higher postsystolic shortening and MD despite similar LV ejection fraction (Table 1). Nineteen patients with MVP (17%) had ≥moderate MR. Systolic PM displacement could be measured in 79 (70%) of patients with MVP, with a mean of 6.9±2.9 mm. Significant postsystolic shortening was evident in the basal posterior segments of patients with MVP only; its index (PSI) exceeded 3% (2 SD in controls) in 49% of patients with MVP. MD exceeded 5.96% of the R-R interval (2 SD in controls) in 29% of patients with MVP.
Table 1.
Clinical and Echocardiographic Parameters in Patients With Mitral Valve Prolapse With Fibrosis, Those Without Fibrosis, and Controls
Myocardial Mechanics in Patients With MVP With Versus Without Fibrosis
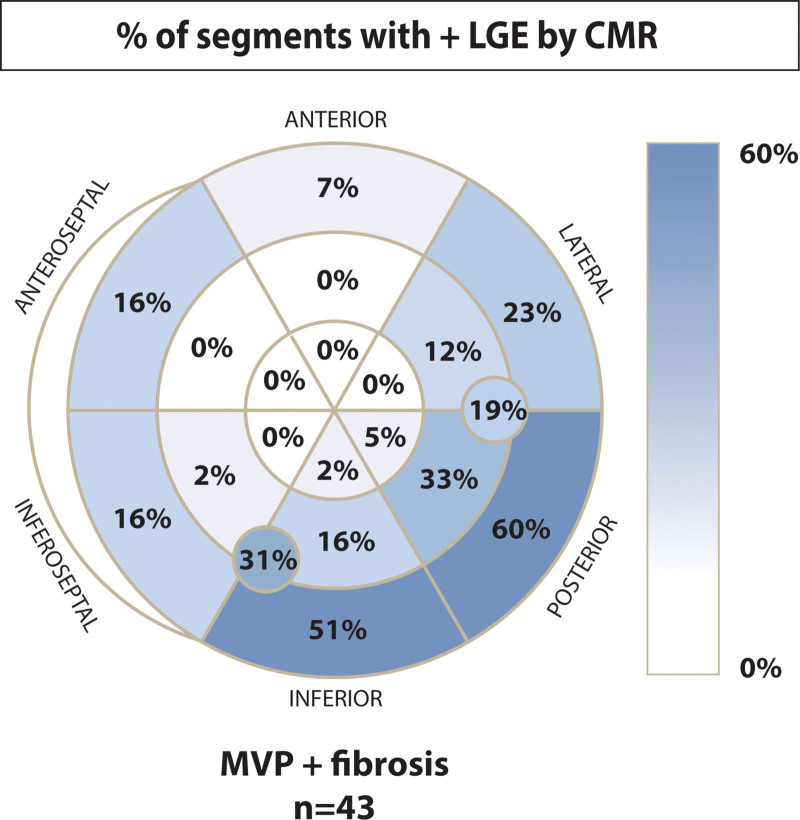
Myocardial fibrosis was identified by LGE in 43 patients with MVP (38%), predominantly localized to the LV basal and midventricular inferior-lateral wall or PMs (95%, Figure 2). Most patients had isolated midwall fibrosis (58%) or a mixed midwall with subendocardial or subepicardial pattern (9%). Fibrosis in either PM was noted in 16 patients. A median value of LGE volume in the myocardium was 3.5% (interquartile range, 1.6–6.5) in patients with MVP with fibrosis.
Figure 2.
Regional distribution of myocardial fibrosis in mitral valve prolapse (MVP). Fibrosis was predominantly localized in basal-middle lateral to inferoseptal wall adjacent to papillary muscles (91%). The percentage of fibrosis in papillary muscle was described in the circles in the middle inferior and posterior-lateral segments. Fibrosis developed in either papillary muscle in 45% of patients with fibrosis. CMR indicates cardiac magnetic resonance imaging; and LGE, late gadolinium enhancement.
Patients with MVP with and without LV fibrosis had similar age, sex, LV volumes, LV ejection fraction, basal and mid-LV wall thickness, global LS, and prevalence of bileaflet prolapse (Table 1). However, patients with MVP with fibrosis had greater superior leaflet and PM displacement, a higher prevalence of MAD, curling, and greater MR severity than those without fibrosis (33% versus 7% with ≥moderate MR). Furthermore, the prevalence of fibrosis increased with increasing MR severity, which is in line with prior data (Figure S3).19 Strain showed significantly larger and more frequent abnormal basal posterior PSI and MD in patients with than without fibrosis, consistent with more abnormal myocardial mechanics. Basal posterior PSI and MD were not significantly different between patients with MVP without fibrosis and normal controls.
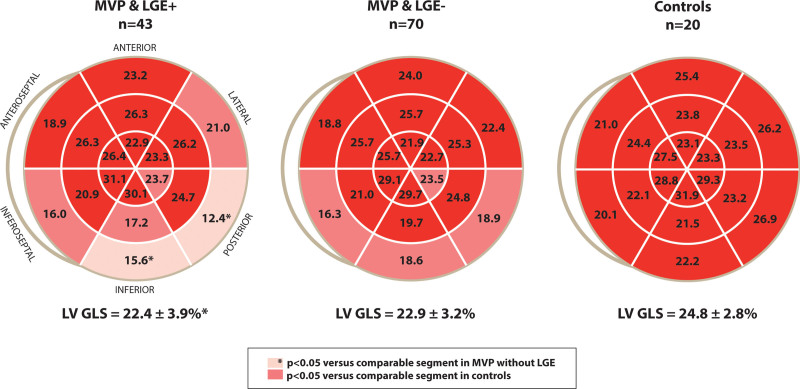
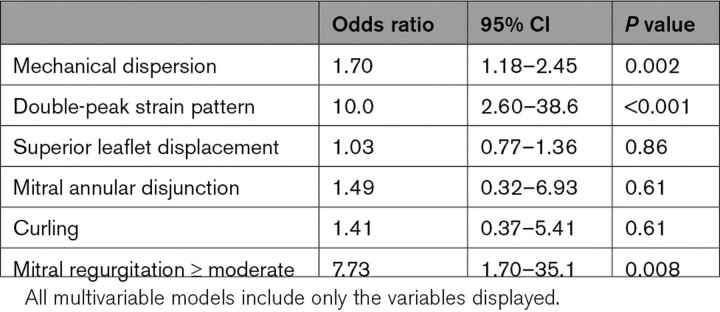
An abnormal LS pattern was observed in a subset of patients with MVP (53 (47%)), particularly in the basal inferior to lateral wall, characterized by a double strain peak, as described above (Figure 1). The prevalence of a double-peak strain pattern was higher in patients with MVP with than without fibrosis (81% versus 26%, P<0.001). Patients with MVP with fibrosis had lower regional end-systolic LS in the basal inferior and posterior LV segments than patients with MVP lacking fibrosis (Figure 3). These segments are consistent with the LGE localization of fibrosis (Figure 2). In multivariable analysis, fibrosis was independently associated with MR severity, MD, and a double-peak strain pattern (Table 2). Importantly, comparing patients with MVP with fibrosis and patients without MVP with fibrosis with a similar basal localization, LS in the non-MVP group did not show a double-peak strain pattern despite significantly decreased global LS (Table S1 and Figure S4).
Figure 3.
Regional longitudinal strain in patients with mitral valve prolapse (MVP) with and without fibrosis, and in controls. Basal posterior to inferoseptal wall showed impaired longitudinal strain in patients with MVP regardless of fibrosis compared with controls. Impaired strain in patients with MVP with fibrosis was more pronounced in basal inferior to posterior and midventricular inferior walls. LGE indicates late gadolinium enhancement; and LVGLS, left ventricular global longitudinal strain.
Table 2.
Multivariable Logistic Regression Analysis for Presence of Left Ventricular Fibrosis
Ventricular Arrhythmic Events
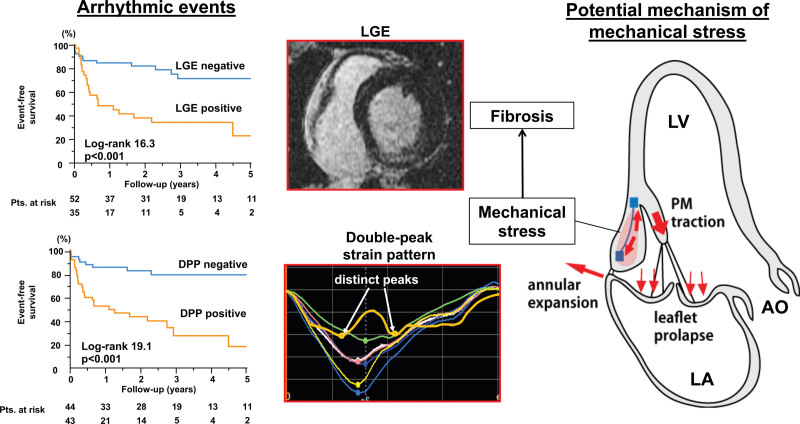
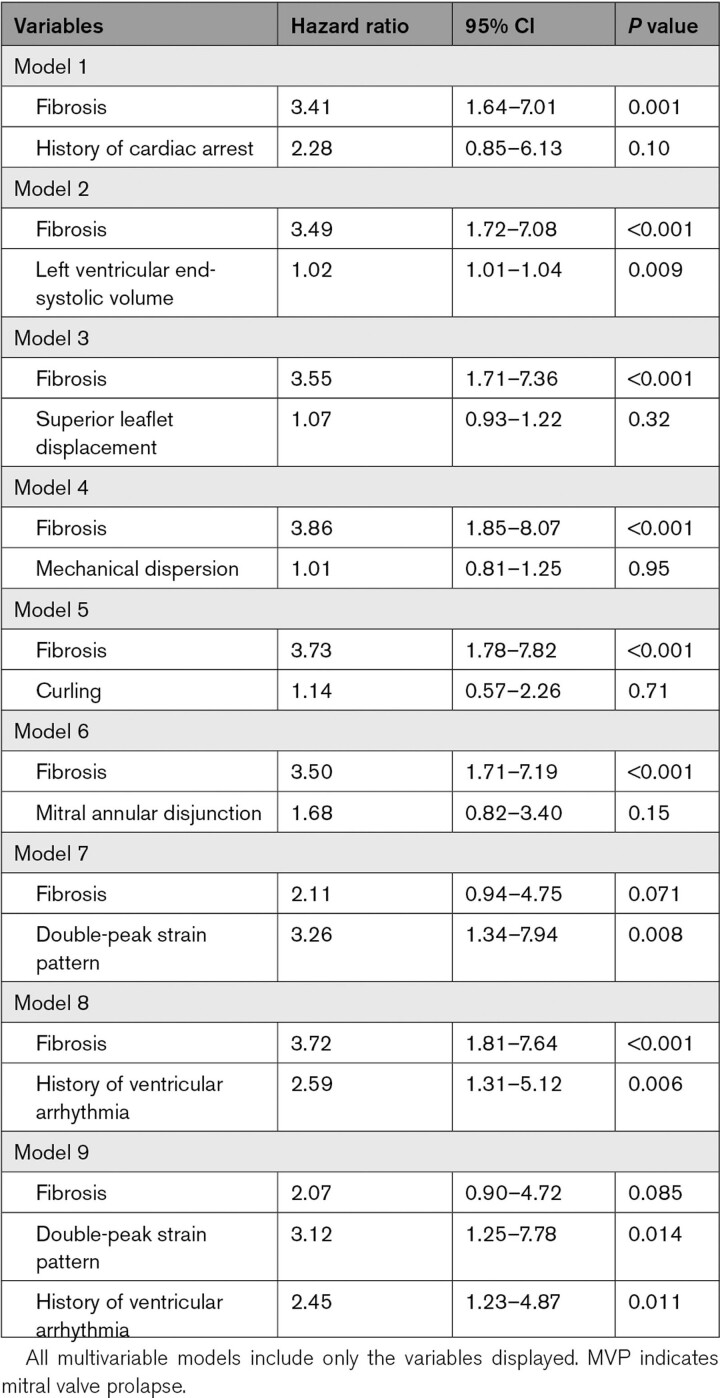
During a median follow-up of 1008 days (interquartile range, 495–1731) after the initial imaging, arrhythmic events were documented in 36 of 87 patients with MVP with >6-month follow-up (5 sustained VT and 31 nonsustained VT; no ventricular fibrillation or cardiac arrest). In univariable Cox proportional hazard analysis, arrhythmic events were associated with myocardial fibrosis and the double-peak strain pattern as well as LV volumes, superior leaflet and PM displacement, MAD, and basal posterior PSI (Table S2). Kaplan-Meier survival curves show that both presence of fibrosis and double-peak strain distinguished well between patients with MVP with versus without arrhythmic events on follow-up (both: P<0.001; Figure 4). Importantly, in multivariable Cox proportional hazard analysis, speckle-tracking analysis-derived MVP-related mechanics (double-peak strain pattern) were independently associated with arrhythmic events, beyond the contribution of fibrosis (Table 3). Other variables determined in univariable analysis except LV end-systolic volume lost their significant impact on arrhythmic events after adjustment on fibrosis. The double-peak strain pattern also had significantly independent association with arrhythmic events after adjusting for history of ventricular arrhythmic events (hazard ratio, 3.12 [95% CI, 1.25–7.78]; P=0.014, Table 3). Supplemental analysis of arrhythmic events in patients without fibrosis showed a significant effect of the double-peak strain pattern (Figure S5). In the 58 patients with dedicated long-term electrocardiographic monitoring, subgroup analysis showed the same relationship between fibrosis, MVP-related mechanical abnormalities, and arrhythmic events as in the entire group (Table S3).
Figure 4.
Abnormal valve-related mechanics relate to myocardial fibrosis and ventricular arrhythmia in mitral valve prolapse. Mitral valve prolapse potentially provokes mechanical stress on basal wall adjacent to papillary muscle (right schema), which relates to fibrosis and abnormal myocardial deformation (middle). Both fibrosis and the Double-peak strain pattern (DPP) were associated with ventricular arrhythmic events (left, Kaplan-Meier curves). AO indicates aorta; LA, left atrium; LGE, late gadolinium enhancement; LV, left ventricle; PM, papillary muscle.
Table 3.
Multivariable Cox Proportional Hazard Analysis for Arrhythmic Events in Patients With MVP
Intrareliability and inter-reliability were assessed for curling, MAD, and double-peak strain pattern with κ statistics, and global LS and MD with intraclass and interclass correlation coefficient, demonstrating good to excellent correlation (κ statistics, 0.732–0.865; intraclass and interclass correlation coefficient, 0.749–0.908, Table S4).
Discussion
There is increasing recognition of myocardial fibrosis in MVP,9,13,19,20,37 associated with ventricular arrhythmias8–10,13,18 and possibly ventricular dysfunction despite valve repair,4 but the mechanism of such fibrosis is unknown. This study investigated abnormal myocardial mechanics in MVP by 2-dimensional and speckle-tracking echocardiography and their relationship with myocardial fibrosis by CMR and the clinical endpoint of ventricular arrhythmia. Key findings are that (1) abnormal myocardial mechanics in MVP, with regionally decreased LS, occur in the basal inferior-posterior LV; (2) we now recognize an aberrant double-peak strain curve in the region with reduced myocardial strain; and (3) these abnormal mechanics are significantly associated with myocardial fibrosis in the same region. Additionally, abnormal myocardial mechanics appear to convey incremental risk of ventricular arrhythmia in MVP beyond that associated with fibrosis alone.
Regional Myocardial Mechanics and Fibrosis
Our study observed regional myocardial fibrosis in 38% of patients with MVP, with predominant basal inferior-posterior localization similar to a recent report.19 The imaging and strain findings are part of growing evidence for abnormal mechanics in MVP that can potentially provide insights into the mechanism of fibrosis through valve-induced forces reflected by abnormal motion. (1) Particularly novel is that patients with MVP with fibrosis had more abnormal MVP mechanics compared with patients without fibrosis, including greater superior leaflet and PM displacement and a higher prevalence of curling motion of valve-linked basal structures, suggesting a potential pathophysiological link to fibrosis development. (2) Moreover, there was a strong association between myocardial fibrosis and regions of abnormal myocardial strain with a double-peak strain pattern, consistent with another report of postsystolic shortening in that region.38 Fibrosis is known to cause abnormal strain, but several points suggest the abnormal myocardial mechanics are not explained by fibrosis alone: First, abnormal basal inferior-posterior strain is noted in patients with MVP without fibrosis. Second, no double-peak strain pattern was observed in patients without MVP with basal inferior-lateral fibrosis (Table S1; Figure S4).
Myocardial Mechanics
Fukuda and colleagues also showed reduced basal LV strain in MVP, possibly due to increased valve-related forces opposing basal longitudinal shortening.39 Interestingly, they showed basal strain was normalized after MV repair; in another study, repair also normalized the exaggerated superior PM displacement that parallels leaflet prolapse.26,40 Regarding the double-peak strain pattern, we can hypothesize a mechanism illustrated in Figure 5: in early systole (left), myocardial contraction and shortening is expected to be normal in MVP. As LV pressure rises in mid- to late-systole, leaflet prolapse, and valve-related abnormalities become most pronounced. The basal LV myocardium is temporarily stretched by the interplay of annular expansion and leaflet displacement with papillary muscle traction.22,41 These forces cause lengthening of the basal myocardium towards end-systole (middle), reflecting the valve-induced force transmitted to the myocardium. Segmental lengthening is reflected in decreased strain, the fractional change in segmental length relative to baseline. After aortic valve closure, LV pressure falls with relief of prolapse and traction on the attached wall segments. Postsystolic myocardial shortening (passive recoil or a delayed active component) causes a second strain peak (right).
Figure 5.
Proposed pathophysiological mechanisms underlying the observed double-peak strain curve in patients with mitral valve prolapse (MVP). The myocardium normally starts to shorten in early systole (lower left panel). In mid- to late-systole with increasing left ventricular (LV) pressure, leaflet prolapse begins along with papillary muscle traction and annular expansion. The related forces integrate to produce abnormal stretch of the basal myocardium, which visually protrudes into the LV cavity (lower middle). This stretching has the potential to oppose longitudinal shortening toward the apex, resulting in an observed late-systolic decrease in longitudinal strain (change in length relative to early systolic baseline). This can be also thought of as prolapse-induced force directed away from the apex and therefore capable of reducing basal myocardial shortening strain. Postsystole, with decreasing LV pressure, the previously stretched basal myocardium is relieved from the abnormal MVP-related forces, which enables it to shorten again and create the second distinct postsystolic strain peak (lower right). AO indicates aorta; AVC, aortic valve closure; LA, left atrium; LGE, late gadolinium enhancement; LV, left ventricle; MVC, mitral valve closure; and PM, papillary muscle.
At a more basic level, it is possible that valve-induced myocardial stretch can decrease myofilament overlap and therefore longitudinal shortening.42 After systole, valve-induced traction resolves and myofilament overlap is restored, allowing resumed contraction and a second strain peak.42 Valve-induced myocardial stretch can likewise lead to stretch-induced arrhythmias from electrical and mechanical dispersion, as shown experimentally.43
Importantly, this double-peak of strain is not the same as postsystolic shortening, which is observed in ischemic myocardium.28 As noted above, Weidemann et al30 evaluated patients without MVP with fibrosis and demonstrated postsystolic shortening in fibrotic segments with a double-peak of strain rate that reflects a slight transient delay in shortening. In contrast, the double-peak strain pattern we observed in patients with MVP showed an actual late-systolic decrease in strain that indicates late-systolic myocardial lengthening, not just a shortening delay. As indicated in Figure 5, this abnormal late-systolic decrease in strain suggests the possibility that it is produced by the abnormal late-systolic mechanics specific to MVP. Indeed, the double-peak strain pattern was also related to other MVP-specific parameters that include superior leaflet and PM displacement, basal LV curling, and MAD (Figure S6). This is encouraging in terms of the concept that MAD may exert its influence on the LV through abnormal mechanics—increased systolic annular expansion, which will increase force exerted on the papillary muscles and underlying myocardium.26,40,44 Of note, the important tissue Doppler Pickelhaube sign described by Muthukumar et al45 likewise indicates abnormal valve-related ventricular mechanics in arrhythmogenic MVP. Prospective study is warranted to correlate that sign with double-peak strain, which additionally demonstrates late-systolic inferobasal lengthening to support mechanistic hypotheses for myocardial fibrosis in MVP.
Our study also showed patients with MVP with fibrosis had more severe MR, with moderate or severe MR in 33% of those with fibrosis versus 7% without. This suggests that MR may synergistically contribute to the development of fibrosis in MVP, consistent with other recent studies.19,37 It remains unclear why all patients with MVP and abnormal basal LV mechanics do not have evidence of fibrosis. A shorter time burden of stress and inherent (genetic) susceptibility might play a role. A recent study discovered ciliary defects can cause MVP and suggested they may predispose to LV fibrosis through abnormal mechanosensing and dysregulated extracellular matrix synthesis. Genotype may therefore impact profibrotic susceptibility in MVP.46
Clinical Implications
In the era of evidence-based guidelines for treating hemodynamically severe MR, there is increasing concern for a malignant MVP phenotype that can persist despite valve repair and for which no intervention or preventive measure is currently available. Understanding the mechanisms underlying arrhythmias and residual contractile dysfunction in MVP is critical to develop optimal therapeutic strategies.4 CMR studies have related ventricular arrhythmias to myocardial fibrosis. Han, Fulton and colleagues, for example, related PM fibrosis and PM origin of ventricular arrhythmia.18,47,48 Premature ventricular contractions develop more commonly in the basal inferior wall, suggesting a treatable substrate for electrical instability.49 Our findings provide markers from noninvasively determined myocardial mechanics that indicate increased likelihood of inferobasal fibrosis. Although the evaluation of arrhythmia in our cohort was retrospective and might have missed subclinical arrhythmia in a subset of patients, our study provides notable data that these mechanical markers are associated with a greater propensity for ventricular arrhythmias than fibrosis alone (Figure 4; Table 3). This is consistent with other evidence that mechanical abnormalities by themselves can trigger arrhythmias.41 Dejgaard and colleagues related arrhythmias to annular disjunction with potentially altered valvular-ventricular interactions.14,50 Although how MAD affects arrhythmia has not been elucidated,11,51 our data also showed the significant association of MAD with fibrosis and arrhythmic events, with the arrhythmic association only weakened in multivariable models by the inclusion of the strongly cross-correlated variables of fibrosis and abnormal strain mechanics. Ermakov et al15 reported higher MD related to more arrhythmic events in patients with MVP. The discrepancy with the current study which did not show significance in MD for arrhythmic events could be caused by different definition of arrhythmic events: our study did not include premature ventricular contractions alone as an arrhythmic outcome.
Most arrhythmic events in our cohort were nonsustained VT. Of note, Essayagh et al51 reported the risk of mortality and serious arrhythmic events was increased with nonsustained VT ≥120 bpm, categorized as a moderate level of ventricular arrhythmia that increased morality and future serious arrhythmic events. This supports the definition of nonsustained ≥120 bpm in the current study as a reasonable outcome for potentially predicting clinically relevant arrhythmic events.
In our study, the double-peak strain pattern remained a strong and independent risk factor for arrhythmia, which generated the hypothesis relating MVP-associated abnormal mechanics with ventricular arrhythmias beyond the relation with fibrosis alone. The evaluation of abnormal mechanics has the potential to improve risk stratification compared with fibrosis alone (Figure 4; Table 3). Further prospective and systematic studies are needed to confirm prognostic value of this pattern and to evaluate post-interventional changes in the abnormal myocardial mechanics in MVP and their impact on risk of arrhythmia. However, MV interventions may not relieve preformed fibrosis and stretch-induced electrical dispersion, which would indicate the value of earlier intervention. The role of medications with antifibrotic properties to prevent or reverse myocardial fibrosis in patients with MVP remains to be determined. Current guidelines do not include indications for CMR and preventing arrhythmias in MVP, which also posed limitations in our study. Appreciating the findings in this study has the potential to indicate the need for large-scale prospective study with the possibility to improve practice guidelines.
Limitations
We acknowledge this retrospective study is prone to an inherent risk of imaging selection bias related to CMR indication as well as monitoring ascertainment bias. However: (1) We do not believe those biases should affect the mechanistic association between fibrosis and evidence of abnormal mechanics. (2) Both fibrosis and double-peak strain pattern remained significantly associated with arrhythmic events even in multivariable analyses adjusting for the CMR indication to evaluate ventricular arrhythmic events (Table 3). (3) The history of prior arrhythmias could be a confounder for prognostic analyses. However, it is also important to evaluate whether prior arrhythmias can drive further arrhythmias, as shown previous studies.52,53 Indeed, the history of arrhythmias was significantly associated with future events in our study, as in others reporting risk of malignant MVP. Importantly, fibrosis and double-peak strain pattern remained significant factors for arrhythmias after adjusting for prior arrhythmias. Of note, unadjusted potential confounders might affect multivariable models to seek factors associated with fibrosis and arrhythmic events. Those unsolved limitations emphasize the need for a well-planned prospective study. Furthermore, (4) in current practice, CMR is not performed in all patients with MVP: Most importantly, we think our findings will provide a foundation for upcoming prospective studies that are needed to identify those patients with MVP at greatest risk of both fibrosis and serious ventricular arrhythmia. Although we limited the imaging acquisition window between echo and CMR to <6 months, there remains a possibility that LV properties and function relating to mechanics and tissue characterization have changed during this interval.
Other potential limitations are considered as follows. CMR feature-tracking could not be retrospectively standardized; increasing its temporal resolution can be an area of future investigation. LGE was also performed using different vendor platforms and field strengths, which entailed use of CMR at 2 separate tertiary care referral sites. Although it might impact fibrosis detectability, similar multicenter protocols have been used to validate prognostic value of cardiac magnetic resonance imaging with late gadolinium enhancement,54,55 and the data were interpreted in a blinded manner by experienced observers. Therefore, we think the site-specific differences in acquisition protocols were not a major source of bias in this study.
Finally, the retrospective nature of the arrhythmia assessment in our cohort remains an important limitation, as subclinical arrhythmic events might have been missed. Prospective studies are needed to overcome these issues and further explore the relationship between mechanics, fibrosis, and arrhythmia in MVP. Nevertheless, the 67% of patients with dedicated long-term electrocardiographic monitoring showed no difference in the relationship between fibrosis, MVP-related mechanical abnormalities, and arrhythmic events compared with that in the entire group (Table S3). We also note that while small sample size and low event rates constrain statistical power, important relationships between mitral valve mechanics and fibrosis/arrhythmia could still be demonstrated.
Technical factors such as vendor differences can impact speckle-tracking echocardiography, which is nevertheless considered a powerful tool regardless of vendor.56 Of note, this study only detected focal myocardial fibrosis (by LGE), in contrast with diffuse fibrosis, only detectable by T1 mapping (extracellular volume),57 which was not measured. It will be of interest to look at diffuse changes in future studies. Finally, this retrospective study was not designed to observe the natural history of fibrosis in MVP, and therefore could not prove causality between fibrosis and abnormal LV mechanics, despite suggestive evidence. This can be studied more effectively in prospective cohorts with serial imaging and in experimental models.46
Conclusions
Basal inferior-lateral myocardial fibrosis in patients with MVP is associated with abnormal MVP-related myocardial mechanics shown by altered motion patterns. Fibrosis is strongly associated with a double-peak strain pattern of the basal inferior-lateral LV, with LS peaks before and after end-systole, as well as with increased superior leaflet and PM displacement with exaggerated curling motion of the basal myocardium. In contrast, the double-peak strain pattern was not found in patients without MVP with basal inferior-lateral fibrosis. These associations suggest a potential mechanism for fibrosis based on the interaction of valve-induced forces with the connected myocardium.58 These abnormal myocardial mechanics also could be associated with ventricular arrhythmia, with an independent and incremental risk compared with fibrosis alone. Our findings suggest a potential pathophysiological link between MVP-related mechanical abnormalities and basal wall myocardial fibrosis and generate the hypothesis for association of MVP-related abnormal mechanics with ventricular arrhythmia, which might be potential imaging markers of increased arrhythmic risk.59,60
Article Information
Sources of Funding
Supported in part by grants from the Ellison Foundation, Boston, MA, American Heart Association (AHA) grant no. 963783/Levine/2022, AHA Postdoctoral Fellowship no. 905880/van Kampen/2021, American Society of Echocardiography Pamela Douglas Research Scholarship (Dr Nagata), National Institutes of Health (NIH) R01s HL128099 and HL141917 (Dr Levine), R01 HL128708 (Dr Weinsaft), K23 HL140092 (Dr Kim), R01HL130539 (Dr Neilan), R01HL103723 (Dr Hung), R01s HL131546 and HL149696 (Dr Norris), F31HL167482 (J.E. Morningstar) and R01 HL153447 (Dr Delling). Work at the Medical University of South Carolina was performed in a facility constructed with support from NIH grant C06 RR018823, Extramural Research Facilities Program, National Center for Research Resources, with funding from NIH GM103444, HL122906, and HL162913 (Dr Norris).
Disclosures
None.
Supplemental Material
Tables S1–S4
Figures S1–S6
Videos S1–S2
Supplementary Material
Nonstandard Abbreviations and Acronyms
- LGE-CMR
- late gadolinium enhancement cardiac magnetic resonance imaging
- LS
- longitudinal strain
- LV
- left ventricular
- MAD
- mitral annular disjunction
- MD
- mechanical dispersion
- MR
- mitral regurgitation
- MVP
- mitral valve prolapse
- PM
- papillary muscle
- PSI
- postsystolic shortening index
- VT
- ventricular tachycardia
Y. Nagata and P.B. Bertrand contributed equally and are joint first authors.
R.A. Norris, J.W. Weinsaft, and R.A. Levine contributed equally and are joint last authors.
This manuscript was sent to Linda D. Gillam, MD, MPH, Senior Guest Editor, for review by expert referees, editorial decision, and final disposition
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCIMAGING.122.014963.
Contributor Information
Yasufumi Nagata, Email: nyasufumi1979@yahoo.co.jp.
Philippe B. Bertrand, Email: philippe.bertrand@zol.be.
Vinit Baliyan, Email: VBALIYAN@mgh.harvard.edu.
Jonathan Kochav, Email: jonathan.kochav@gmail.com.
Ruth D. Kagan, Email: rdk9009@nyp.org.
Kristian Ujka, Email: kristianujka@hotmail.com.
Hassan Alfraidi, Email: hassan.alfraidi@mail.mcgill.ca.
Antonia van Kampen, Email: AVANKAMPEN@mgh.harvard.edu.
Jordan E. Morningstar, Email: morningj@musc.edu.
Jacob P. Dal-Bianco, Email: JDALBIANCO@mgh.harvard.edu.
Serguei Melnitchouk, Email: smelnitchouk@mgh.harvard.edu.
Godtfred Holmvang, Email: GHOLMVANG@mgh.harvard.edu.
Michael A. Borger, Email: michael.borger@helios-gesundheit.de.
Reece Moore, Email: moorere.3@gmail.com.
Lanqi Hua, Email: LHUA1@mgh.harvard.edu.
Razia Sultana, Email: rsultana9231@gmail.com.
Brian Yum, Email: yum.brian@gmail.com.
J. Luis Guerrero, Email: JGUERRERO@mgh.harvard.edu.
Tomas G. Neilan, Email: tneilan@mgh.harvard.edu.
Michael H. Picard, Email: mhpicard@mgh.harvard.edu.
Jiwon Kim, Email: jik9027@med.cornell.edu.
Francesca N. Delling, Email: Francesca.Delling@ucsf.edu.
Judy Hung, Email: jhung@partners.org.
Russell A. Norris, Email: norrisra@musc.edu.
Jonathan W. Weinsaft, Email: jww2001@med.cornell.edu.
References
- 1.Parwani P, Avierinos JF, Levine RA, Delling FN. Mitral valve prolapse: multimodality imaging and genetic insights. Prog Cardiovasc Dis. 2017;60:361–369. doi: 10.1016/j.pcad.2017.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine RA, Hagege AA, Judge DP, Padala M, Dal-Bianco JP, Aikawa E, Beaudoin J, Bischoff J, Bouatia-Naji N, Bruneval P, et al. ; Leducq Mitral Transatlantic Network. Mitral valve disease--morphology and mechanisms. Nat Rev Cardiol. 2015;12:689–710. doi: 10.1038/nrcardio.2015.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P, et al. ; ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation. 2014;129:2440–2492. doi: 10.1161/CIR.0000000000000029 [DOI] [PubMed] [Google Scholar]
- 4.Quintana E, Suri RM, Thalji NM, Daly RC, Dearani JA, Burkhart HM, Li Z, Enriquez-Sarano M, Schaff HV. Left ventricular dysfunction after mitral valve repair--the fallacy of “normal” preoperative myocardial function. J Thorac Cardiovasc Surg. 2014;148:2752–2760. doi: 10.1016/j.jtcvs.2014.07.029 [DOI] [PubMed] [Google Scholar]
- 5.Chesler E, King RA, Edwards JE. The myxomayous mitral valve and sudden death. Circulation. 1983;67:632–639. doi: 10.1161/01.cir.67.3.632 [DOI] [PubMed] [Google Scholar]
- 6.Andrew F Anita LT, James BA, William FM, Virmani R. Comparison of cardiac findings in patients with mitral valve prolapse who die suddenly to those who have congestive heart failure from mitral regurgitation and to those with fatal noncardiac conditions. Am J Cardiol. 1993;70:234–239. doi: 10.1016/0002-9149(92)91281-8 [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Friera L, Salguero R, Vannini L, Arguelles AF, Arribas F, Solis J. Mechanistic insights of the left ventricle structure and fibrosis in the arrhythmogenic mitral valve prolapse. Glob Cardiol Sci Pract. 2018;2018:4. doi: 10.21542/gcsp.2018.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han HC, Ha FJ, Teh AW, Calafiore P, Jones EF, Johns J, Koshy AN, O’Donnell D, Hare DL, Farouque O, et al. Mitral valve prolapse and sudden cardiac death: a systematic review. J Am Heart Assoc. 2018;7:e010584. doi: 10.1161/JAHA.118.010584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller MA, Dukkipati SR, Turagam M, Liao SL, Adams DH, Reddy VY. Arrhythmic mitral valve prolapse: JACC review topic of the week. J Am Coll Cardiol. 2018;72:2904–2914. doi: 10.1016/j.jacc.2018.09.048 [DOI] [PubMed] [Google Scholar]
- 10.Basso C, Iliceto S, Thiene G, Perazzolo Marra M. Mitral valve prolapse, ventricular arrhythmias, and sudden death. Circulation. 2019;140:952–964. doi: 10.1161/CIRCULATIONAHA.118.034075 [DOI] [PubMed] [Google Scholar]
- 11.Nalliah CJ, Mahajan R, Elliott AD, Haqqani H, Lau DH, Vohra JK, Morton JB, Semsarian C, Marwick T, Kalman JM, et al. Mitral valve prolapse and sudden cardiac death: a systematic review and meta-analysis. Heart. 2019;105:144–151. doi: 10.1136/heartjnl-2017-312932 [DOI] [PubMed] [Google Scholar]
- 12.Sriram CS, Syed FF, Ferguson ME, Johnson JN, Enriquez-Sarano M, Cetta F, Cannon BC, Asirvatham SJ, Ackerman MJ. Malignant bileaflet mitral valve prolapse syndrome in patients with otherwise idiopathic out-of-hospital cardiac arrest. J Am Coll Cardiol. 2013;62:222–230. doi: 10.1016/j.jacc.2013.02.060 [DOI] [PubMed] [Google Scholar]
- 13.Basso C, Perazzolo Marra M, Rizzo S, De Lazzari M, Giorgi B, Cipriani A, Frigo AC, Rigato I, Migliore F, Pilichou K, et al. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation. 2015;132:556–566. doi: 10.1161/CIRCULATIONAHA.115.016291 [DOI] [PubMed] [Google Scholar]
- 14.Dejgaard LA, Skjolsvik ET, Lie OH, Ribe M, Stokke MK, Hegbom F, Scheirlynck ES, Gjertsen E, Andresen K, Helle-Valle TM, et al. The mitral annulus disjunction arrhythmic syndrome. J Am Coll Cardiol. 2018;72:1600–1609. doi: 10.1016/j.jacc.2018.07.070 [DOI] [PubMed] [Google Scholar]
- 15.Ermakov S, Gulhar R, Lim L, Bibby D, Fang Q, Nah G, Abraham TP, Schiller NB, Delling FN. Left ventricular mechanical dispersion predicts arrhythmic risk in mitral valve prolapse. Heart. 2019;105:1063–1069. doi: 10.1136/heartjnl-2018-314269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noseworthy PA, Asirvatham SJ. The knot that binds mitral valve prolapse and sudden cardiac death. Circulation. 2015;132:551–552. doi: 10.1161/CIRCULATIONAHA.115.017979 [DOI] [PubMed] [Google Scholar]
- 17.Han Y, Peters DC, Kissinger KV, Goddu B, Yeon SB, Manning WJ, Nezafat R. Evaluation of papillary muscle function using cardiovascular magnetic resonance imaging in mitral valve prolapse. Am J Cardiol. 2010;106:243–248. doi: 10.1016/j.amjcard.2010.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fulton BL, Liang JJ, Enriquez A, Garcia FC, Supple GE, Riley MP, Schaller RD, Dixit S, Callans DJ, Marchlinski FE, et al. Imaging characteristics of papillary muscle site of origin of ventricular arrhythmias in patients with mitral valve prolapse. J Cardiovasc Electrophysiol. 2018;29:146–153. doi: 10.1111/jce.13374 [DOI] [PubMed] [Google Scholar]
- 19.Kitkungvan D, Nabi F, Kim RJ, Bonow RO, Khan MA, Xu J, Little SH, Quinones MA, Lawrie GM, Zoghbi WA, et al. Myocardial fibrosis in patients with primary mitral regurgitation with and without prolapse. J Am Coll Cardiol. 2018;72:823–834. doi: 10.1016/j.jacc.2018.06.048 [DOI] [PubMed] [Google Scholar]
- 20.Garbi M, Lancellotti P, Sheppard MN. Mitral valve and left ventricular features in malignant mitral valve prolapse. Open Heart. 2018;5:e000925. doi: 10.1136/openhrt-2018-000925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilbert BW, Schatz RA, Vonramm OT, Behar VS, Kisslo JA. Mitral valve prolapse - two-dimensional echocardiographic and angiographic correlation. Circulation. 1976;54:716–723. doi: 10.1161/01.cir.54.5.716 [DOI] [PubMed] [Google Scholar]
- 22.Sanfilippo AJ, Harrigan P, Popovic AD, Weyman AE, Levine RA. Papillary muscle traction in mitral valve prolapse: quantitation by two-dimensional echocardiography. J Am Coll Cardiol. 1992;19:564–571. doi: 10.1016/s0735-1097(10)80274-8 [DOI] [PubMed] [Google Scholar]
- 23.Levine RA, Stathogiannis E, Newell JB, Harrigan P, Weyman AE. Reconsideration of echocardiographic standards for mitral valve prolapse: lack of association between leaflet displacement isolated to the apical four chamber view and independent echocardiographic evidence of abnormality. J Am Coll Cardiol. 1988;11:1010–1019. doi: 10.1016/s0735-1097(98)90059-6 [DOI] [PubMed] [Google Scholar]
- 24.Lee AP, Hsiung MC, Salgo IS, Fang F, Xie JM, Zhang YC, Lin QS, Looi JL, Wan S, Wong RH, et al. Quantitative analysis of mitral valve morphology in mitral valve prolapse with real-time 3-dimensional echocardiography: importance of annular saddle shape in the pathogenesis of mitral regurgitation. Circulation. 2013;127:832–841. doi: 10.1161/CIRCULATIONAHA.112.118083 [DOI] [PubMed] [Google Scholar]
- 25.Perazzolo Marra M, Basso C, De Lazzari M, Rizzo S, Cipriani A, Giorgi B, Lacognata C, Rigato I, Migliore F, Pilichou K, et al. Morphofunctional abnormalities of mitral annulus and arrhythmic mitral valve prolapse. Circ Cardiovasc Imaging. 2016;9:e005030. doi: 10.1161/CIRCIMAGING.116.005030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hei S, Iwataki M, Jang JY, Kuwaki H, Mahara K, Fukuda S, Kim YJ, Nabeshima Y, Onoue T, Nagata Y, et al. Possible mechanism of late systolic mitral valve prolapse: systolic superior shift of leaflets secondary to annular dilatation that causes papillary muscle traction. Am J Physiol Circ Physiol. 2019;316:H629–H638. doi: 10.1152/ajpheart.00618.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American society of echocardiography developed in collaboration with the society for cardiovascular magnetic resonance. J Am Soc Echocardiogr. 2017;30:303–371. doi: 10.1016/j.echo.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 28.Asanuma T, Nakatani S. Myocardial ischaemia and post-systolic shortening. Heart. 2015;101:509–516. doi: 10.1136/heartjnl-2013-305403 [DOI] [PubMed] [Google Scholar]
- 29.Prihadi EA, Vollema EM, Ng ACT, Ajmone Marsan N, Bax JJ, Delgado V. Determinants and prognostic implications of left ventricular mechanical dispersion in aortic stenosis. Eur Heart J Cardiovasc Imaging. 2019;20:740–748. doi: 10.1093/ehjci/jez004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weidemann F, Niemann M, Herrmann S, Kung M, Stork S, Waller C, Beer M, Breunig F, Wanner C, Voelker W, et al. A new echocardiographic approach for the detection of non-ischaemic fibrosis in hypertrophic myocardium. Eur Heart J. 2007;28:3020–3026. doi: 10.1093/eurheartj/ehm454 [DOI] [PubMed] [Google Scholar]
- 31.Kim J, Kochav JD, Gurevich S, Afroz A, Petashnick M, Volo S, Diaz B, Okin PM, Horn E, Devereux RB, et al. Left ventricular geometric remodeling in relation to non-ischemic scar pattern on cardiac magnetic resonance imaging. Int J Cardiovasc Imaging. 2014;30:1559–1567. doi: 10.1007/s10554-014-0487-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinsaft JW, Kim J, Medicherla CB, Ma CL, Codella NC, Kukar N, Alaref S, Kim RJ, Devereux RB. Echocardiographic algorithm for post-myocardial infarction LV thrombus: a gatekeeper for thrombus evaluation by delayed enhancement CMR. JACC Cardiovasc Imaging. 2016;9:505–515. doi: 10.1016/j.jcmg.2015.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heitner JF, Kim RJ, Kim HW, Klem I, Shah DJ, Debs D, Farzaneh-Far A, Polsani V, Kim J, Weinsaft J, et al. Prognostic value of vasodilator stress cardiac magnetic resonance imaging: a multicenter study with 48000 patient-years of follow-up. JAMA Cardiol. 2019;4:256–264. doi: 10.1001/jamacardio.2019.0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neilan TG, Coelho-Filho OR, Danik SB, Shah RV, Dodson JA, Verdini DJ, Tokuda M, Daly CA, Tedrow UB, Stevenson WG, et al. CMR quantification of myocardial scar provides additive prognostic information in nonischemic cardiomyopathy. JACC Cardiovasc imaging. 2013;6:944–954. doi: 10.1016/j.jcmg.2013.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chinitz JS, Chen D, Goyal P, Wilson S, Islam F, Nguyen T, Wang Y, Hurtado-Rua S, Simprini L, Cham M, et al. Mitral apparatus assessment by delayed enhancement CMR: relative impact of infarct distribution on mitral regurgitation. JACC Cardiovasc Imaging. 2013;6:220–234. doi: 10.1016/j.jcmg.2012.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mavrogeni S, Petrou E, Kolovou G, Theodorakis G, Iliodromitis E. Prediction of ventricular arrhythmias using cardiovascular magnetic resonance. Eur Heart J Cardiovasc Imaging. 2013;14:518–525. doi: 10.1093/ehjci/jes302 [DOI] [PubMed] [Google Scholar]
- 37.Constant Dit Beaufils AL, Huttin O, Jobbe-Duval A, Senage T, Filippetti L, Piriou N, Cueff C, Venner C, Mandry D, Sellal JM, et al. Replacement myocardial fibrosis in patients with mitral valve prolapse: relation to mitral regurgitation, ventricular remodeling, and arrhythmia. Circulation. 2021;143:1763–1774. doi: 10.1161/CIRCULATIONAHA.120.050214 [DOI] [PubMed] [Google Scholar]
- 38.Huttin O, Pierre S, Venner C, Voilliot D, Sellal JM, Aliot E, Sadoul N, Juilliere Y, Selton-Suty C. Interactions between mitral valve and left ventricle analysed by 2D speckle tracking in patients with mitral valve prolapse: one more piece to the puzzle. Eur Heart J Cardiovasc Imaging. 2017;18:323–331. doi: 10.1093/ehjci/jew075 [DOI] [PubMed] [Google Scholar]
- 39.Fukuda S, Song JK, Mahara K, Kuwaki H, Jang JY, Takeuchi M, Sun BJ, Kim YJ, Miyamoto T, Oginosawa Y, et al. Basal left ventricular dilatation and reduced contraction in patients with mitral valve prolapse can be secondary to annular dilatation: preoperative and postoperative speckle-tracking echocardiographic study on left ventricle and mitral valve annulus interaction. Circ Cardiovasc Imaging. 2016;9:e005113. doi: 10.1161/CIRCIMAGING.115.005113 [DOI] [PubMed] [Google Scholar]
- 40.Hei S, Iwataki M, Jang JY, Kuwaki H, Fukuda S, Kim YJ, Toki M, Onoue T, Hayashi A, Nishino S, et al. Relations of augmented systolic annular expansion and leaflet/papillary muscle dynamics in late-systolic mitral valve prolapse evaluated by echocardiography with a speckle tracking analysis. Int Heart J. 2020;61:970–978. doi: 10.1536/ihj.20-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Charles CG, Gareth Tobler H Marc CP, Ishik CT, Adrian A Benditt DG. Electrophysiologic effects of papillary muscle traction in the intact heart. Circulation. 1986;73:1013–1021. doi: 10.1161/01.cir.73.5.1013 [DOI] [PubMed] [Google Scholar]
- 42.Niederer SA, Smith NP. The role of the Frank-Starling law in the transduction of cellular work to whole organ pump function: a computational modeling analysis. PLoS Comput Biol. 2009;5:e1000371. doi: 10.1371/journal.pcbi.1000371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keurs HEDJTK. Electromechanical coupling in the cardiac myocyte; stretch-arrhythmia feedback. Pflugers Arch. 2011;462:165–175. doi: 10.1007/s00424-011-0944-3 [DOI] [PubMed] [Google Scholar]
- 44.Clavel MA, Mantovani F, Malouf J, Michelena HI, Vatury O, Jain MS, Mankad SV, Suri RM, Enriquez-Sarano M. Dynamic phenotypes of degenerative myxomatous mitral valve disease: quantitative 3-dimensional echocardiographic study. Circ Cardiovasc Imaging. 2015;8:e002989. doi: 10.1161/CIRCIMAGING.114.002989 [DOI] [PubMed] [Google Scholar]
- 45.Muthukumar L, Rahman F, Jan MF, Shaikh A, Kalvin L, Dhala A, Jahangir A, Tajik AJ. The pickelhaube sign: novel echocardiographic risk marker for malignant mitral valve prolapse syndrome. JACC Cardiovasc Imaging. 2017;10:1078–1080. doi: 10.1016/j.jcmg.2016.09.016 [DOI] [PubMed] [Google Scholar]
- 46.Toomer KA, Yu M, Fulmer D, Guo L, Moore KS, Moore R, Drayton KD, Glover J, Peterson N, Ramos-Ortiz S, et al. Primary cilia defects causing mitral valve prolapse. Sci Transl Med. 2019;11:eaax0290. doi: 10.1126/scitranslmed.aax0290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Enriquez A, Shirai Y, Huang J, Liang J, Briceno D, Hayashi T, Muser D, Fulton B, Han Y, Perez A. Papillary muscle ventricular arrhythmias in patients with arrhythmic mitral valve prolapse: electrophysiologic substrate and catheter ablation outcomes. J Cardiovasc Electrophysiol. 2019;30:827–835. doi: 10.1111/jce.13900 [DOI] [PubMed] [Google Scholar]
- 48.Han Y, Peters DC, Salton CJ, Bzymek D, Nezafat R, Goddu B, Kissinger KV, Zimetbaum PJ, Manning WJ, Yeon SB. Cardiovascular magnetic resonance characterization of mitral valve prolapse. JACC Cardiovasc Imaging. 2008;1:294–303. doi: 10.1016/j.jcmg.2008.01.013 [DOI] [PubMed] [Google Scholar]
- 49.Syed FF, Ackerman MJ, McLeod CJ, Kapa S, Mulpuru SK, Sriram CS, Cannon BC, Asirvatham SJ, Noseworthy PA. Sites of successful ventricular fibrillation ablation in bileaflet mitral valve prolapse syndrome. Circ Arrhythm Electrophysiol. 2016;9:e004005. doi: 10.1161/CIRCEP.116.004005 [DOI] [PubMed] [Google Scholar]
- 50.Lee AP, Jin CN, Fan Y, Wong RHL, Underwood MJ, Wan S. Functional implication of mitral annular disjunction in mitral valve prolapse: a quantitative dynamic 3D echocardiographic study. JACC Cardiovasc Imaging. 2017;10:1424–1433. doi: 10.1016/j.jcmg.2016.11.022 [DOI] [PubMed] [Google Scholar]
- 51.Essayagh B, Sabbag A, Antoine C, Benfari G, Yang LT, Maalouf J, Asirvatham S, Michelena H, Enriquez-Sarano M. Presentation and outcome of arrhythmic mitral valve prolapse. J Am Coll Cardiol. 2020;76:637–649. doi: 10.1016/j.jacc.2020.06.029 [DOI] [PubMed] [Google Scholar]
- 52.Buxton AE, Lee KL, Hafley GE, Pires LA, Fisher JD, Gold MR, Josephson ME, Lehmann MH, Prystowsky EN; Investigators M. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: lessons from the MUSTT study. J Am Coll Cardiol. 2007;50:1150–1157. doi: 10.1016/j.jacc.2007.04.095 [DOI] [PubMed] [Google Scholar]
- 53.Prinz C, Schwarz M, Ilic I, Laser KT, Lehmann R, Prinz EM, Bitter T, Vogt J, van Buuren F, Bogunovic N, et al. Myocardial fibrosis severity on cardiac magnetic resonance imaging predicts sustained arrhythmic events in hypertrophic cardiomyopathy. Can J Cardiol. 2013;29:358–363. doi: 10.1016/j.cjca.2012.05.004 [DOI] [PubMed] [Google Scholar]
- 54.Antiochos P, Ge Y, Steel K, Bingham S, Abdullah S, Mikolich JR, Arai AE, Bandettini WP, Patel AR, Farzaneh-Far A, et al. ; SPINS Study Investigators. Imaging of clinically unrecognized myocardial fibrosis in patients with suspected coronary artery disease. J Am Coll Cardiol. 2020;76:945–957. doi: 10.1016/j.jacc.2020.06.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kochav JD, Kim J, Judd R, Kim HW, Klem I, Heitner J, Shah D, Shenoy C, Farzaneh-Far A, Polsani V, et al. Ischemia-mediated dysfunction in subpapillary myocardium as a marker of functional mitral regurgitation. JACC Cardiovasc Imaging. 2021;14:826–839. doi: 10.1016/j.jcmg.2021.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mirea O, Pagourelias ED, Duchenne J, Bogaert J, Thomas JD, Badano LP, Voigt JU, Force EA-A-IST. Variability and reproducibility of segmental longitudinal strain measurement: a report from the EACVI-ASE strain standardization task force. JACC Cardiovasc Imaging. 2018;11:15–24. doi: 10.1016/j.jcmg.2017.01.027 [DOI] [PubMed] [Google Scholar]
- 57.Bui AH, Roujol S, Foppa M, Kissinger KV, Goddu B, Hauser TH, Zimetbaum PJ, Ngo LH, Manning WJ, Nezafat R, et al. Diffuse myocardial fibrosis in patients with mitral valve prolapse and ventricular arrhythmia. Heart. 2017;103:204–209. doi: 10.1136/heartjnl-2016-309303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park MH, van Kampen A, Melnitchouk S, Wilkerson RJ, Nagata Y, Zhu Y, Wang H, Pandya PK, Morningstar JE, Borger MA, et al. Native and post-repair residual mitral valve prolapse increases forces exerted on the papillary muscles: a possible mechanism for localized fibrosis?. Circ Cardiovasc Interv. 2022;15:e011928. doi: 10.1161/CIRCINTERVENTIONS.122.011928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Delling FN, Noseworthy PA, Adams DH, Basso C, Borger M, Bouatia-Naji N, Elmariah S, Evans F, Gerstenfeld E, Hung J, et al. Research opportunities in the treatment of mitral valve prolapse: JACC expert panel. J Am Coll Cardiol. 2022;80:2331–2347. doi: 10.1016/j.jacc.2022.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morningstar JE, Gensemer C, Morre R, Fulmer D, Beck TC, Wang C, Moore K, Guo L, Sieg F, Nagata Y, et al. Mitral valve prolapse induces regionalized myocardial fibrosis. J Am Heart Assoc. 2021;10:e022332. doi: 10.1161/JAHA.121.022332 [DOI] [PMC free article] [PubMed] [Google Scholar]