Abstract
Background:
This study compares outcomes of patients with preoperative atrial fibrillation (AF) undergoing coronary artery bypass grafting (CABG) with or without concomitant AF ablation in a nationally representative Medicare cohort.
Objectives:
This study examined early and late outcomes in CABG patients with a preoperative history of AF to determine the correlation between surgical AF ablation to mortality and stroke or systemic embolization.
Methods:
In the Medicare-linked Society of Thoracic Surgeons (STS) database, 361,138 patients underwent isolated CABG from 2006 to 2013; 34,600 (9.6%) had preoperative AF; 10,541 (30.5%) were treated with surgical ablation (Ablation) and 23,059 were not (No Ablation). Propensity score matching was performed using a hierarchical mixed model. Long-term survival was summarized using Kaplan-Meier curves and Cox regression models with robust variance estimation. The stroke or systemic embolization incidence was modeled using the Fine-Gray model. Median follow-up was 4 years.
Results:
Long term mortality in propensity score matched CABG patients (mean age 74, STS risk score 2.25) receiving Ablation versus No Ablation was similar (log-rank p=0.30). Stroke or systemic embolization occurred in 2.2% vs 2.1% at 30-days and 9.9% vs 12.0% at 5-years (Gray’s p=0.0091). Landmark analysis from 2 to 5 years showed lower mortality (hazard ratio 0.89, confidence interval 0.82–0.97; p=0.0358) and lower risk of stroke or systemic embolization (hazard ratio 0.73, confidence interval 0.61–0.87; p=0.0006) in the Ablation group.
Conclusion:
Concomitant Ablation in CABG patients with preoperative AF is associated with lower stroke or systemic embolization and mortality in patients who survive more than 2 years.
Graphical Abstract
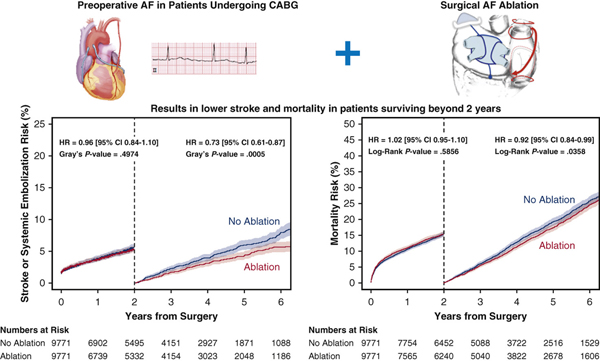
The Medicare-linked Society of Thoracic Surgeons database was queried for patients undergoing isolated CABG from 2006–2013 and 34,600 out of 361,138 patients (9%) had preoperative AF. The Cox Maze IV maze lesion set is shown. However, detailed lesion set information was not available in our study. A comparison was made of 10, 541 patients who had AF treated at the time of CABG, to the 23, 059 who did not. Propensity score matching was used to balance the groups. Endpoints of Stroke or systemic embolization and Mortality were analyzed. Landmark analysis from 2 to 5 years showed lower mortality (hazard ratio 0.89, confidence interval 0.82–0.97; p=0.0358) and lower risk of stroke or systemic embolization (hazard ratio 0.73, confidence interval 0.61–0.87; p=0.0006) in the surgical ablation group.
Introduction
The incidence of preoperative atrial fibrillation (AF) in patients undergoing coronary artery bypass grafting (CABG) ranges from 5 to 10%.1, 2 Preoperative AF is an independent risk factor for worse perioperative outcomes and decreased long-term survival in patients who undergo CABG when compared to patients without pre-operative AF.2 A national database study confirmed that preoperative AF is associated with worse long-term survival, and higher risk of stroke or systemic embolization after CABG.1
While AF ablation during CABG in patients with preoperative AF has been associated with higher freedom from AF,3–5 single-center studies have shown improvement in clinical outcomes after AF ablation during other cardiac surgery6–10 but few in a dedicated CABG cohort.3 In this study, we sought to examine early and late outcomes in CABG patients with a preoperative history of AF to determine the association between surgical AF ablation and mortality and stroke or systemic embolization.
Methods
Data were obtained from the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database (versions 2.52, 2.61, 2.73) on patients who were discharged between January 1, 2006-December 31, 2013 and could be linked to Centers for Medicare & Medicaid Services (CMS) data using a validated deterministic matching algorithm.11 Medicare recipients age ≥65 years old, with preoperative AF, undergoing isolated CABG as a first cardiac surgery were included in this study. After exclusions, the study population consisted of 34,600 patients with preoperative AF: 24,059 (69.5%) in the no surgical ablation (No Ablation) group and 10,541 (30.5%) in the Ablation group (Figure 1).
Figure 1.
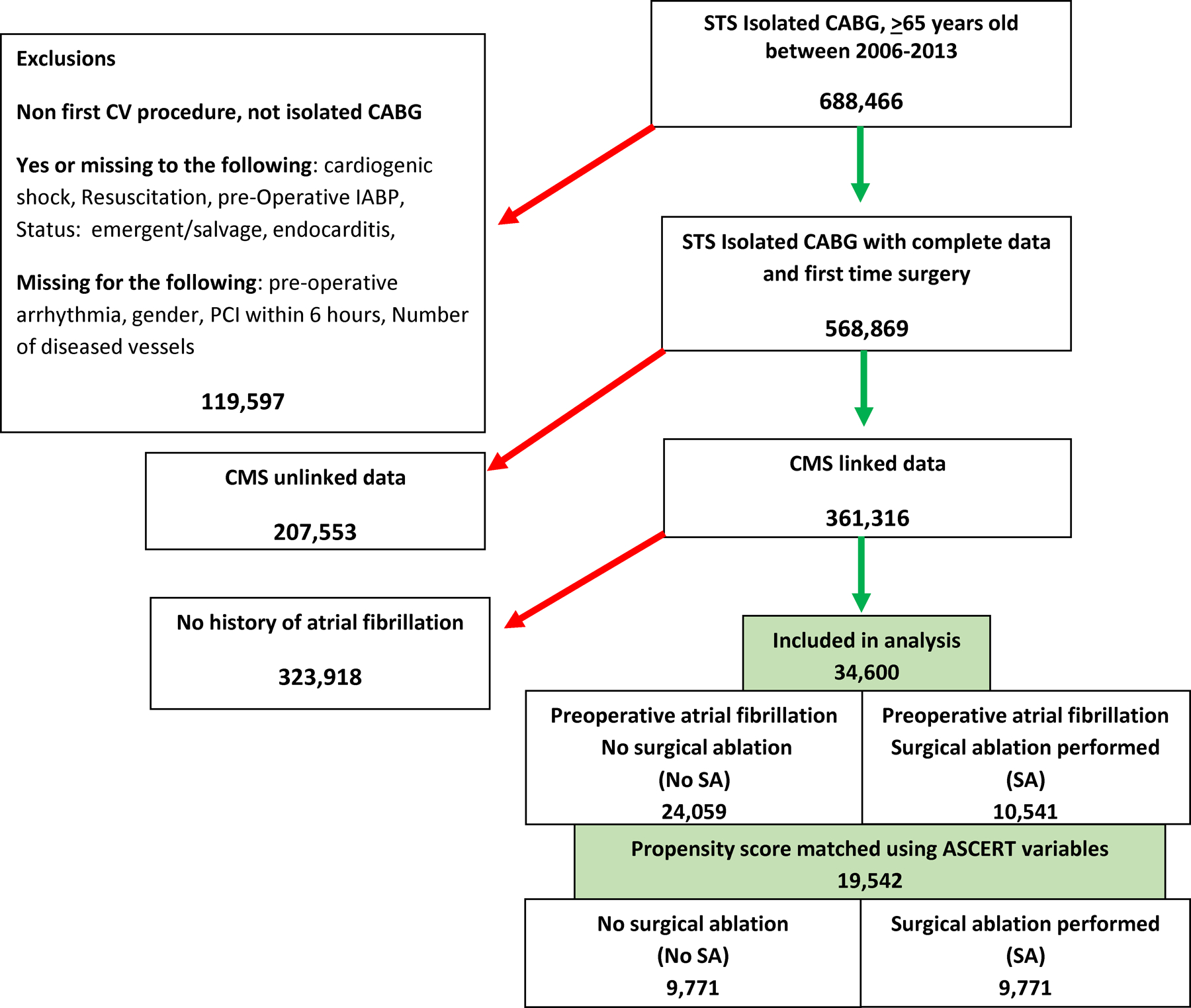
Flow diagram of study cohort.
The STS database was queried for isolated CABG patients between 2006 and 2013. Exclusions and numbers of patients removed are shown on the left. This resulted in 34,600 patients with preoperative AF who were divided into those with or without surgical ablation during the CABG procedure. CMS=Centers for Medicare and Medicaid Services, CABG= coronary artery bypass grafting, CV= cardiovascular, PCI= percutaneous coronary intervention, STS=Society of Thoracic Surgeons.
Endpoints
The primary outcome was all-cause mortality after CABG using STS registry data for in-hospital deaths and the linked Medicare Denominator File for post-discharge deaths.12 The secondary outcomes were stroke or systemic embolization (stroke, hemorrhagic stroke, transient ischemic attack, or systemic arterial embolism)13 and in-hospital major morbidity.
Incident stroke or systemic embolization was defined by using STS registry data to account for in-hospital strokes and Medicare Part A data to identify subsequent re-hospitalizations with stroke or systemic embolization as a primary diagnosis (International Classification of Diseases, 9th Revision codes: 433.1, 434.x1, 430, 431, 432.0, 432.1, 432.9, 444,x, 435.x).
In-hospital major morbidity, a previously defined composite14, 15, referred to any of the following post-procedure complications: permanent stroke, new cases of renal failure, prolonged ventilation (ventilation longer than 24 hours after surgery), reoperation for cardiac reasons (graft dysfunction, bleeding, valve dysfunction or other) and deep sternal wound infection.
Statistical analysis
Patients treated in a given hospital share some commonalities inherent to that hospital (compared to patients treated elsewhere) that may influence how they were treated and thus impact their outcomes above and beyond the effects of treatment itself. Therefore, propensity scores (PS) for surgical ablation were determined using a hierarchical mixed model that includes both patient and hospital characteristics as well as a random intercept (with hospitals as random effects) to holistically capture and control for any confounding due to inherent hospital-related commonalities. Patient variables were based in the validated ASCERT model 16 for predictors of long-term mortality after CABG (see list, Table 1). We also included region (Northeast, West, South, Midwest), hospital teaching status, hospital average annual volume of CABG in patients 65 and older and preoperative medications: aspirin, beta-blockers, angiotensin-converting enzyme inhibitors, statins, and anticoagulants. Consistent with the validated STS risk models16, missing values (less than 3%) were imputed with relevant groups-specific medians for continuous variables and most common category for categorical ones.
Table 1.
Baseline and operative characteristics of patients with preoperative atrial fibrillation in the no surgical ablation and surgical ablation groups.
| Unmatched | Propensity Score Matched | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Variable | No Ablation | Ablation | P-value | No Ablation | Ablation | P-value | |||||
| (N=24,059) | (N=10,541) | (N=9771) | (N=9771) | ||||||||
| Age, years | 75 | (71, 80) | 74 | (70, 78) | <0.0001 | 74 | (69, 79) | 74 | (70, 78) | 0.1049 | |
| Female | 6920 | (29) | 2561 | (24) | <0.0001 | 2370 | (24) | 2439 | (25) | 0.2729 | |
| Body Mass Index, kg/m2 | 28.10 | (25, 32) | 28.61 | (25, 33) | 0.954 | 28 | (25, 32) | 28 | (25, 32) | 0.9893 | |
| Height, centimeters | 173 | (165, 180) | 175 | (168, 180) | <0.0001 | 175 | (168, 180) | 175 | (168, 180) | 0.6838 | |
| Race Other | 428 | (1.8) | 208 | (2.0) | <0.0001 | 174 | (1.8) | 176 | (1.8) | 0.9835 | |
| Asian | 283 | (1.2) | 102 | (1.0) | 102 | (1.0) | 99 | (1.0) | |||
| Hispanic | 548 | (2.3) | 138 | (1.3) | 130 | (1.3) | 138 | (1.4) | |||
| Black | 751 | (3.1) | 217 | (2.1) | 204 | (2.1) | 210 | (2.2) | |||
| Caucasian | 21952 | (91.2) | 9848 | (93.4) | 9132 | (93.5) | 9122 | (93.4) | |||
| Missing | 97 | (0.4) | 28 | (0.3) | 29 | (0.3) | 26 | (0.3) | |||
| Current Smoking | 3111 | (12.9) | 1160 | (11.0) | <0.0001 | 1126 | (11.5) | 1108 | (11.3) | 0.6857 | |
| Diabetes | 9903 | (41.2) | 4164 | (39.5) | <0.0001 | 3832 | (39.2) | 3844 | (39.3) | 0.8209 | |
| Hypertension | 21615 | (89.8) | 9417 | (89.3) | 0.1523 | 8695 | (89.0) | 8728 | (89.3) | 0.4477 | |
| Dyslipidemia | 19776 | (82.4) | 8687 | (82.2) | <0.0001 | 8102 | (83.0) | 8063 | (82.5) | 0.4132 | |
| Peripheral vascular disease | 5355 | (22.3) | 1837 | (17.4) | <0.0001 | 1758 | (18.0) | 1760 | (18.0) | 0.9783 | |
| Renal failure (Glomerular filtration rate< 30 or dialysis) | 1625 | (6.8) | 423 | (4.0) | <0.0001 | 434 | (4.4) | 452 | (4.6) | 0.5319 | |
| Glomerular filtration rate | 61 | (49, 74) | 65 | (53, 75) | <0.0001 | 64.7 | (52, 80) | 64.7 | (52, 75) | 0.2077 | |
| Chronic lung disease | <0.0001 | 0.2990 | |||||||||
| Severe | 1644 | (6.8) | 552 | (5.2) | 533 | (5.5) | 532 | (504) | |||
| Moderate | 2179 | (9.1) | 788 | (7.5) | 707 | (7.2) | 733 | (7.5) | |||
| Cerebrovascular disease | 5735 | (23.8) | 2204 | (20.9) | <0.0001 | 2098 | (21.5) | 2077 | (21.3) | 0.5540 | |
| Transient ischemic attack | 1620 | (6.7) | 653 | (6.2) | 0.0627 | 651 | (6.7) | 608 | (6.2) | 0.2103 | |
| Previous carotid surgery | 1739 | (7.2) | 583 | (5.5) | <0.0001 | 554 | (5.7) | 557 | (5.7) | 0.9262 | |
| Carotid stenosis > 75% | 964 | (4.0) | 319 | (3.0) | <0.0001 | 315 | (3.2) | 305 | (3.1) | 0.6832 | |
| Immunosuppressive treatment | 993 | (4.1) | 342 | (3.2) | <0.0001 | 306 | (3.1) | 318 | (3.3) | 0.6272 | |
| Preoperative myocardial infarction | <0.0001 | 0.0015 | |||||||||
| < or = 6 hours | 47 | (0.2) | 12 | (0.1) | 8 | (0.1) | 12 | (0.1) | |||
| >6 hours but <24 hours | 212 | (0.9) | 43 | (0.4) | 43 | (0.4) | 42 | (0.4) | |||
| Between 1 and 21 days | 7476 | (31.1) | 2211 | (21.0) | 2031 | (20.8) | 2130 | (21.8) | |||
| No myocardial infarction | 11850 | (49.3) | 6350 | (60.2) | 5696 | (58.3) | 5822 | (59.6) | |||
| Coronary artery disease | |||||||||||
| Left main ≥ 50% | 8713 | (36.2) | 3360 | (31.9) | <0.0001 | 3174 | 32.5 | 3173 | 32.5 | 0.9838 | |
| Three vessels | 18524 | (77.0) | 7946 | (75.4) | 0.0043 | 7443 | (76.2) | 7416 | (75.9) | 0.8993 | |
| Two vessels | 4720 | (19.6) | 2225 | (21.1) | 1993 | (20.4) | 2014 | (20.61) | |||
| One vessel | 815 | (3.4) | 370 | (3.5) | 335 | (3.4) | 341 | (3.5) | |||
| Left ventricular ejection fraction ≥ 55 % | 10761 | (46) | 4971 | (48.2) | <0.0001 | 4631 | (48.8) | 4570 | (47.81) | 0.4303 | |
| NYHA class IV | 1796 | (25) | 608 | (22.5) | 0.0001 | 578 | (23.1) | 573 | (22.5) | 0.6947 | |
| Aortic stenosis, Moderate or Severe | 1124 | (4.7) | 383 | (3.6) | <0.0001 | 347 | (3.6) | 362 | (3.7) | 0.5651 | |
| Urgent procedure | 14303 | (59.5) | 5366 | (50.9) | <0.0001 | 5040 | (51.6) | 5047 | (51.7) | 0.9202 | |
| CHA2DS2-VASc by score | <0.0001 | 0.138 | |||||||||
| • 1–3 | 9560 | (65.5) | 5028 | (34.5) | 4608 | (50.1) | 4574 | (49.8) | |||
| • 4–6 | 13145 | (72.1) | 5088 | (27.9) | 4805 | (50.1) | 4785 | (49.9) | |||
| • 7–9 | 1354 | (76.1) | 425 | (23.9) | 358 | (46.5) | 412 | (53.5) | |||
| STS mortality risk score | 2.84 | (1.6, 4.6) | 2.19 | (1.4, 3.6) | <0.0001 | 2.25 | (1.4, 3.7) | 2.3 | (1.4, 3.7) | 0.9239 | |
| • Predicted major morbidity | 19.34 | (13.8, 28) | 16.58 | (12.1, 23.4) | <0.0001 | 16.77 | (12.2, 23.9) | 16.8 | (12.3, 23.8) | 0.9303 | |
| • Predicted stroke | 1.72 | (1.2, 2.6) | 1.44 | (1.02, 2.13) | <0.0001 | 1.46 | (1, 2.2) | 1.47 | (1, 2.2) | 0.7821 | |
| • Predicted renal failure | 4.69 | (2.7, 9) | 3.92 | (2.33, 7.25) | <0.0001 | 3.92 | (2.3, 7.5) | 3.97 | (2.4, 7.4) | 0.3675 | |
| • Predicted prolonged ventilation | 11.87 | (8.1, 18.3) | 9.89 | (6.9, 14.7) | <0.0001 | 10.05 | (7.1, 15.1) | 10 | (7, 15) | 0.9011 | |
| • Predicted deep sternal wound infection | 0.39 | (0.25, 0.64) | 0.35 | (0.24, 0.56) | <0.0001 | 0.35 | (0.24, 0.57) | 0.35 | (0.24, .056) | 0.5853 | |
| • Predicted reoperation | 6.98 | (5.5, 9.3) | 6.22 | (5, 8.1) | <0.0001 | 6.28 | (5.1, 8.2) | 6.28 | (5.06, 8.13) | 0.8795 | |
| Cardiopulmonary bypass time, minutes | 90 | (70, 115) | 107 | (84, 136) | <0.0001 | 90 | (70, 115) | 108 | (85, 137) | <0.0001 | |
NYHA= New York Heart Association, STS=Society of Thoracic Surgeons. Summaries reported for continuous variables are median (first quartile, third quartile); for variables with discrete distributions, values are n and (percentage). STS risk scores use the 2007 model.
A one-to-one optimal matching algorithm was used to obtain a matched sample to overcome differences in potential confounders between the two study groups. To assess balance, we compared the distribution of baseline characteristics before and after matching. Absolute values of standardized mean differences of less than 10% suggest adequate balance.17 Before matching, the standardized differences ranged between −23 and 14, while in the matched sample, they ranged between −1.7 and 2.4, which indicates that balance was achieved after matching (Figure 2). Since “use” versus “non-use’ of ablation could be influenced by unmeasured confounders that cannot be adjusted for using registry variables, we tested the association between ablation and the falsification endpoint of fracture. In addition, to account for possible residual confounding, we use multivariate COX-regression analysis in the matched sample (same covariates as ASCERT model) (16) for long-term outcomes. Results were virtually the same as univariate COX model (Supplemental Table 1). A null association between the exposure (Ablation) and a falsification suggested balance among unmeasured confounders.
Figure 2.
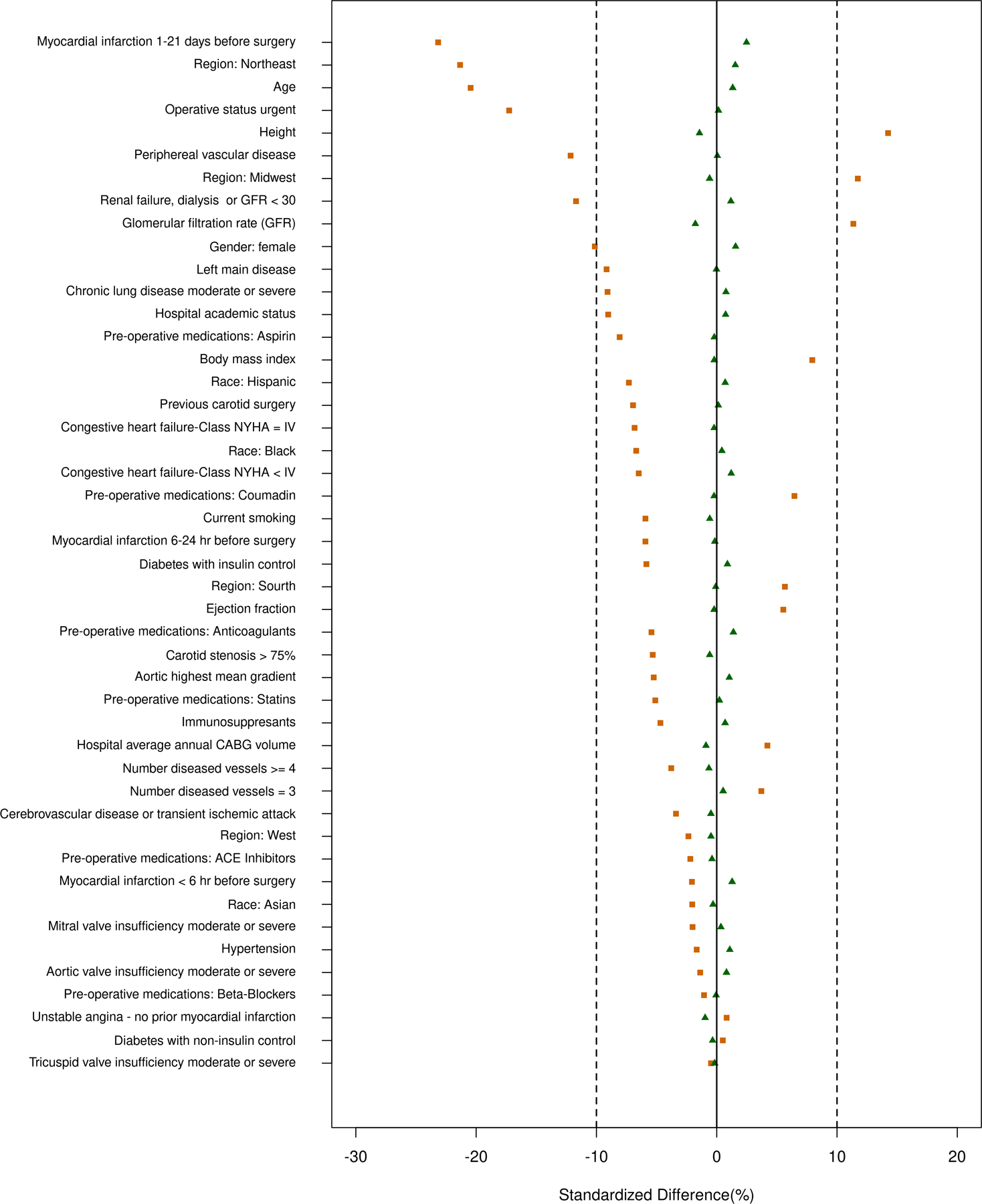
Propensity score match assessment of balance before and after matching.
A one-to-one optimal matching algorithm was used to overcome differences in potential confounders between the two study groups. We compared the distribution of baseline characteristics before (black squares) and after matching (triangles). Before matching, the standardized differences ranged between −23 and 14, while in the matched sample, they ranged between −1.7 and 2.4, which indicates balance was achieved.
The study groups were summarized before and after matching using medians and interquartile ranges (25th and 75th percentiles) for continuous variables and frequency counts and percentages for categorical variables and in-hospital outcomes. Differences in distributions between groups were evaluated with Wilcoxon and Pearson chi-square tests respectively.
For in-hospital outcomes, we computed odds ratios and 95% confidence intervals (CI) for the overall and matched sample using generalized estimated equations logistic regression to account for hospital clustering of patients.
Time-to-event analysis was used to compare long-term survival and stroke or systemic embolization occurrence by group. For survival, patient follow-up was censored at the end of study period (January 1st, 2014). Product-limit Kaplan Meier (KM) survival and failure estimates were computed for each group in the unmatched and matched samples and compared via log-rank tests. Cox proportional hazard regression models were used to compute hazard ratios for Ablation vs. No Ablation in both samples. Because patients from a given hospital share commonalities compared to patients from different hospitals, we used a robust sandwich variance estimation to account for hospital clustering of patients and computed 95% confidence intervals accordingly. The proportional hazard assumption was tested using log-log survival plots (log(-log) survival versus log-time) and interactions between study groups and log-time. While the assumption was met in the overall sample, both methods suggested a marginal violation of the assumption in the matched sample, with curves crossing two years after surgery and a statistically significant interaction with time (p-value=0.01). Given these results, HRs were computed in the unmatched and matched samples for the overall follow-up period, but we also performed a landmark analysis at 2 years to explore earlier vs. later effects of surgical ablation in survival. Both, KM survival curves and HRs were computed for each period.
For stroke or systemic embolization and the falsification endpoint of fracture, death was considered a competing risk. Follow-up was censored at death date, end of fee-for-service date or end of study period, whichever came first. Date for in-hospital strokes post-procedure was not available and surgery date was assigned as the event date (23% of all strokes). For regression analysis, the Fine and Gray method was used to calculate the sub-distribution hazard ratios. The proportional hazards assumption (accounting for competing risk of death) was tested by plotting Schoenfeld residuals18, 19 for each treatment group versus log-time and also with interaction terms between study groups and log-time in regression models. Results suggested a violation of the assumption in the matched sample (p-value for interaction with log-time in the matched sample was 0.0064 for stroke or systemic embolization). Therefore, a landmark analysis at 2 years was performed and CIF curves and sub-distribution HRs were computed for each period.
A p-value of <0.05 was considered statistically significant for all tests. All tests were 2-sided. Analyses were performed using SAS (version 9.4, SAS Institute, Cary, NC).
Results
Patient and Operative characteristics
Compared to patients treated with Ablation, patients in the No Ablation group were older, had a higher incidence of renal failure (glomerular filtration rate < 30 or dialysis), diabetes, chronic lung disease, lower ejection fraction and New York Heart Association Class IV; incidence of dyslipidemia and hypertension was similar. After PS-matching, the groups were statistically similar except for fewer myocardial infarctions in the Ablation group (40.4%) compared to No Ablation group (41.7%) (Table 1). Mean cardiopulmonary bypass time was longer in the Ablation group (113 ± 43 minutes vs 95 minutes ± 36 minutes, p<0.0001) for the unmatched and matched groups. There was no data available on the ablation lesion sets or management of the left atrial appendage (LAA).
Perioperative results
In the unmatched analysis, in-hospital outcomes were not statistically different. In the matched analysis, patients in the SA group had greater in-hospital mortality, prolonged ventilation and new renal failure compared to the No Ablation group. Sub-analysis of the operative mortality showed that patients with higher CHA2DS2-VASc (Congestive heart failure, Hypertension, Age ≥75, Diabetes, Stroke, VAScular disease, Age 65–74, Sex category) scores revealed higher operative mortality in patients with a CHA2DS2-VASc score of 7–9, and the lowest operative mortality in the Ablation group with a CHA2DS2-VASc score of 1–3 (Table 2).
Table 2.
In-hospital Outcomes by surgical ablation group in unmatched and propensity score matched patients.
| Unmatched | Propensity Score Matched | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Variable | No Ablation Group (N=24,059) |
Ablation Group (N=10,541) |
P-value | No Ablation Group (N=9771) |
Ablation Group (N=9771) |
P-value | Odds Ratio 95% CI |
P-value | ||||
| In-hospital mortality | 799 | (3.3) | 327 | (3.1) | 0.2910 | 245 | (2.5) | 309 | (3.2) | 0.0058 | 1.27, 10.7–1.51 | 0.0072 |
| Operative mortality | 962 | (4.0) | 390 | (3.7) | 0.1012 | 304 | (3.1) | 372 | (3.8) | 0.0165 | 1.21, 1.02–1.42 | 0.0244 |
| CHA2DS2-VASc 1–3 | 242 | (3.0) | 107 | (2.5) | 95 | (2.4) | 99 | (0.2) | ||||
| CHA2DS2-VASc 4–6 | 633 | (5.7) | 244 | (5.5) | 195 | (4.7) | 236 | (5.7) | ||||
| CHA2DS2-VASc 7–9 | 87 | (7.6) | 39 | (10.7) | 15 | (4.9) | 37 | (10.5) | ||||
| Composite Morbidity | 4756 (19.8) | 2043 (19.38) | 0.4104 | 1690 (17.3) | 1912 (19.6) | <.0001 | 1.16, 1.08–1.25 | <0.0001 | ||||
| - Re-operation for Bleeding/Tamponade, Graft, Valve, Other Cardiac Reason | 936 | (3.9) | 445 | (4.2) | 0.1497 | 386 | (4.0) | 420 | (4.3) | 0.2224 | 1.09, 0.95–1.25 | 0.2145 |
| - Deep Sternal Wound Infection | 103 | (0.4) | 44 | (0.4) | 0.8891 | 44 | (0.5) | 37 | (0.4) | 0.4371 | 0.84, 0.54–1.30 | 0.4359 |
| - Permanent Stroke | 474 | (2.0) | 191 | (1.8) | 0.3226 | 161 | (1.7) | 181 | (1.9) | 0.2761 | 1.13, 0.91–1.39 | 0.2757 |
| - Prolonged Ventilation | 3326 | (13.8) | 1398 | (13.3) | 0.1610 | 1104 | (11.3) | 1314 | (13.5) | <.0001 | 1.22, 1.12–1.33 | <0.0001 |
| - New Renal Failure | 1371 | (6.0) | 664 | (6.5) | 0.0838 | 480 | (5.1) | 617 | (6.5) | <.0001 | 1.32, 1.16–1.49 | <0.0001 |
CI=Confidence Interval, values are number of patients and (percentage).
Long-term results – Mortality and Stroke or systemic embolization
In the unmatched analysis, the 1-year overall Kaplan-Meier failure estimates (mortality incidence) was 10.8% (CI 10.2 to 11.4) in the Ablation group compared to 13.3% (CI 12.9 to13.8) in the No Ablation group. The 5-year overall mortality was 29.9% (CI 28.8 to 31.0) in the Ablation group compared to 37.1% (CI 36.3 to 37.8) in the No Ablation group. The hazard ratio for mortality (Figure 3A) was 0.78 (95% CI, 0.74 to 0.82). The 1-year CIF for stroke or systemic embolization was 3.7% (CI 3.4 to 4.2) in the Ablation group compared to 4.4% (CI 4.1 to 4.7) in the No Ablation group. The 5-year overall stroke or systemic embolization (Figure 4A) was 8.8% (CI 8.2 to 9.5) in the Ablation group compared to 10.7% (CI 10.2 to 11.2) in the No Ablation group. The HR was 0.84 (95% CI 0.77, 0.91).
Figure 3.
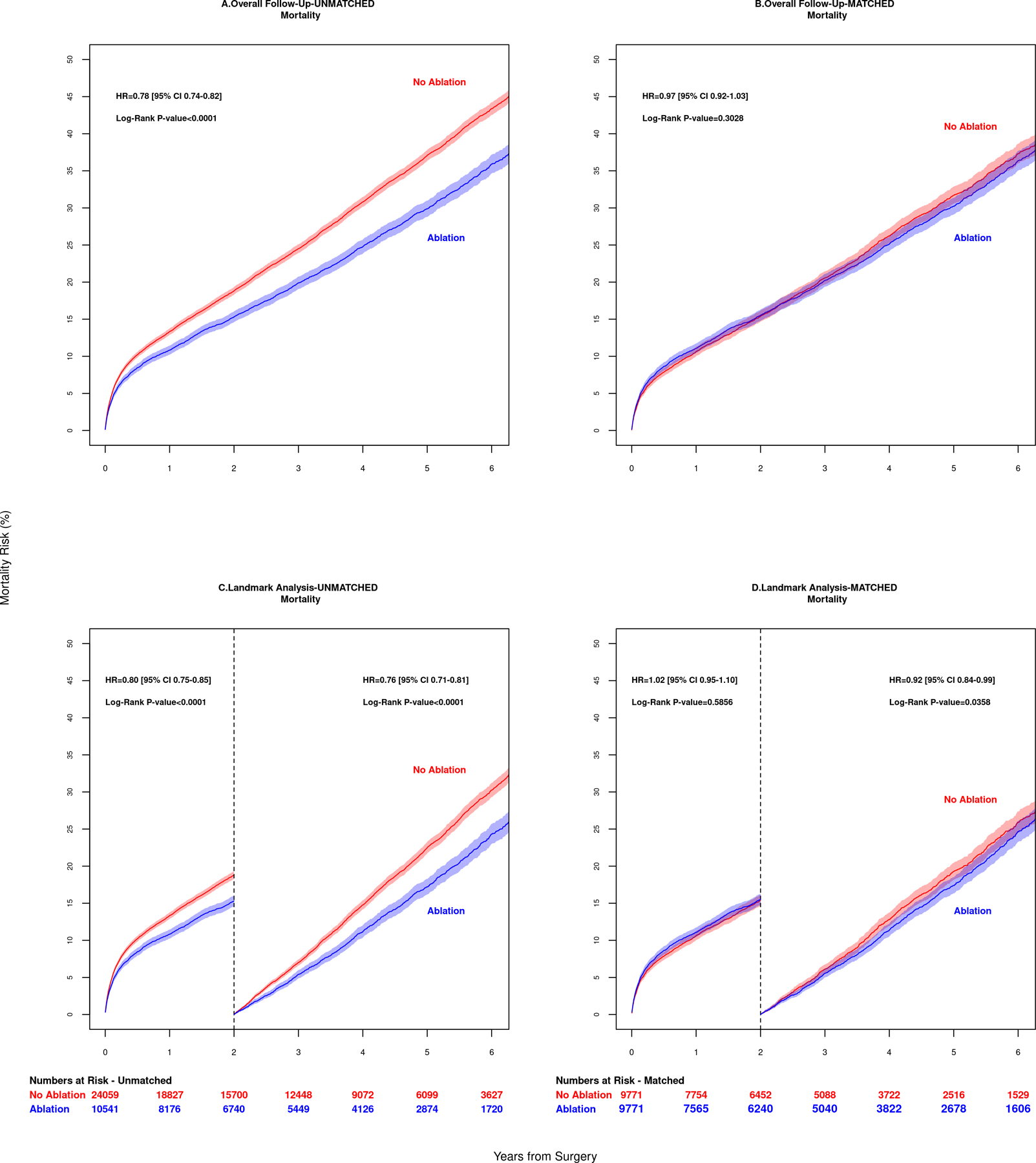
Mortality outcomes of patients in 6 year follow up are shown for (A) unadjusted overall data and (B) adjusted matched data. Mortality risk was similar between groups in the adjusted group, p= 0.303. Landmark Analysis results for patients surviving past 2 years are shown for unmatched data in panel (C) and matched data in panel (D). Patients who had surgical ablation of atrial fibrillation had a lower risk of death, p=0.0358.
Figure 4.
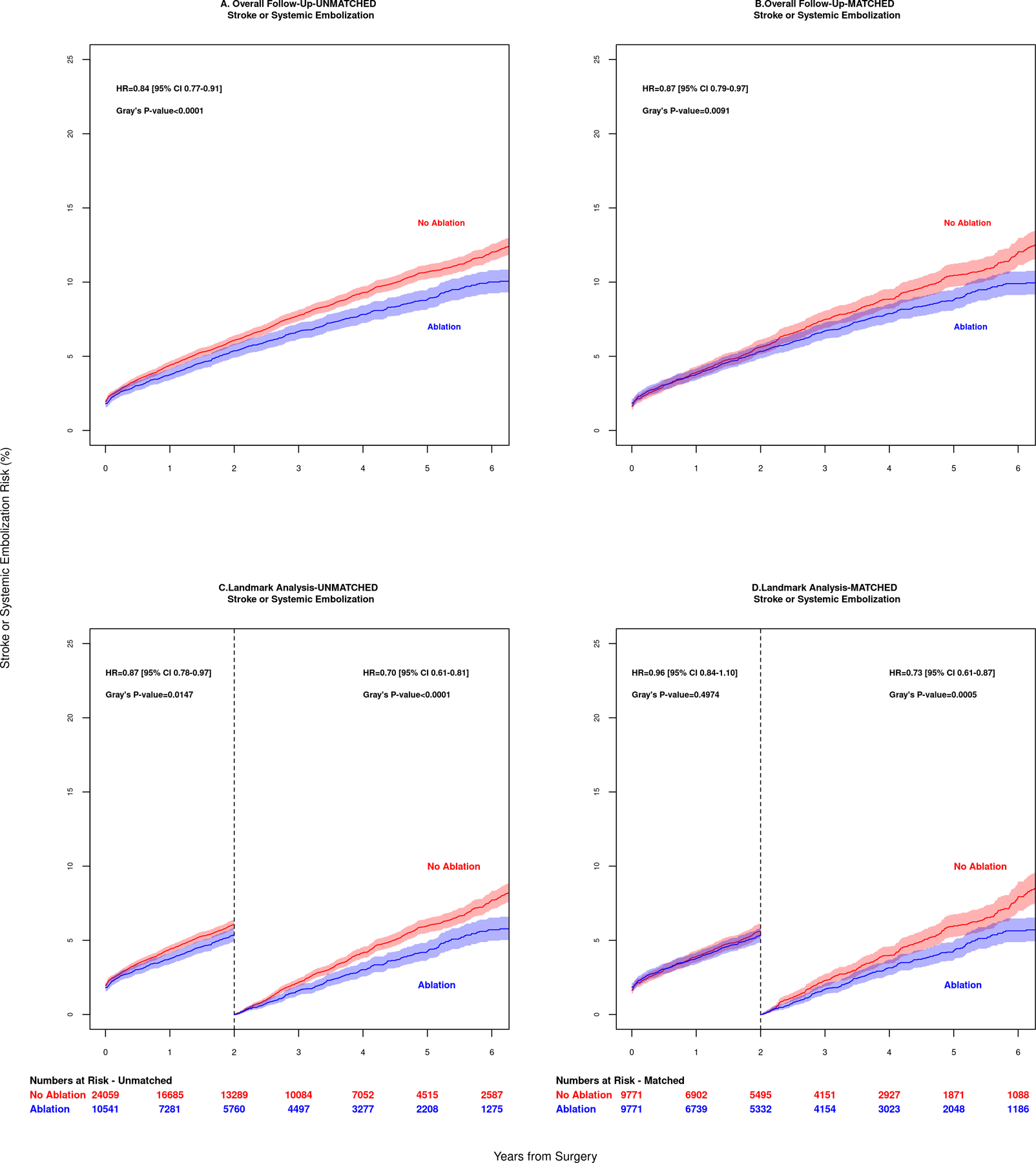
Stroke or systemic embolization outcomes of patients in 6 year follow up are shown for (A) unadjusted overall data and (B) adjusted matched data. Landmark analysis results for patients surviving beyond 2 years are shown for unmatched data in panel (C) and matched data in panel (D). Patients who had surgical ablation of atrial fibrillation had a lower risk of stroke or systemic embolic event, p=0.0005.
After PS-matching, the 1-year overall mortality incidence was 11.1% (CI 10.5 to 11.7) in the Ablation group compared to 10.6% (CI 10.0 to11.3) in the No Ablation group. The 5-year overall mortality was 30.2% (CI 29.1 to 31.4) in the Ablation group compared to 31.7% (CI 30.6 to 32.9) in the No Ablation group, log-rank p= 0.303 (Figure 3B). The 1-year CIF for stroke or systemic embolization was 3.8% (CI 3.4 to 4.2%) in the Ablation group compared to 3.9% (3.5 to 4.3%) in the No Ablation group. The 5-year overall stroke or systemic embolization was 8.8% (8.1 to 9.5%) in the Ablation group compared to 10.5% (9.7 to 11.2%) in the No Ablation group, log-rank p=0.0091 (Figure 4B).
2-Year Landmark Analysis – Mortality and Stroke or systemic embolization
After PS-matching, the difference in 2-year mortality was not statistically significant between Ablation and No Ablation groups (HR 1.00, 95% CI, 0.93 to 1.08; log-rank p=0.59). Among patients surviving past 2 years, mortality was lower in the Ablation group (HR 0.89, 95% CI, 0.82 to 0.97; log-rank p=0.04), Figures 3C and 3D.
Similarly, the difference in 2-year incidence of stroke or systemic embolization was not statistically significant between Ablation and No Ablation groups (HR 0.96, 95% CI 0.84 to 1.10; Gray’s test p=0.50). Among patients surviving past 2 years, incidence of stroke or systemic embolization was lower in the Ablation group (HR 0.73, 95% CI 0.61 to 0.87; Gray’s test p=0.0005), Figures 4C and 4D.
Discussion
This is the largest contemporary study of Ablation in CAB patients compared to those who had untreated AF. In this study Ablation was associated with a small but statistically significant higher operative mortality. In sub analysis of these patients, we determined the highest operative mortality was in the Ablation group with CHA2DS2-VASc scores of 7–9 and the lowest mortality was also in the Ablation group in patients with a CHA2DS2-VASc score of 1–3. Additionally, perioperative morbidity including prolonged ventilation and new renal failure was also higher. However, 2-year mortality did not differ by use versus non-use of Ablation. In patients who survived past 2 years, stroke or systemic embolization and mortality were lower in the Ablation group (Figure 5).
Figure 5.
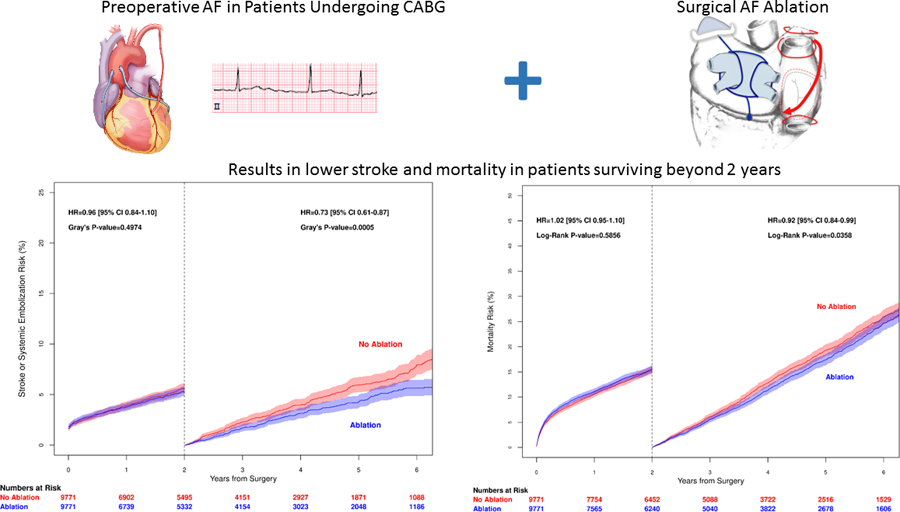
Patients undergoing isolated coronary artery bypass surgery with a history of preoperative atrial fibrillation (AF) may benefit from concomitant AF ablation. The Cox Maze IV maze lesion set is shown. However, detailed lesion set information was not available in our study. While early stroke and mortality were similar, stroke or systemic embolization and mortality for patients surviving more than 2 years was lower.
A previous single-center series demonstrated a reduction in stroke after concomitant surgical AF ablation during cardiac surgery.20 This current analysis is the first to show a long-term stroke or systemic embolization was lower after AF ablation in a CABG-only cohort. Because neither atria are opened routinely in CABG, the addition of AF ablation requires either omission of endocardial lesions or alteration of the CABG operation to allow for atrial access required for the Cox-maze procedure.21 While the exact lesion set is unknown in this dataset (data unavailable until 2014), many patients may have received an incomplete ablation set of the Cox-maze procedure, causing an underestimation of the efficacy of AF ablation.
The effect of AF ablation on operative mortality and long-term survival has not been established in CABG patients. A previous study of Medicare patients with preoperative AF undergoing CABG showed that the addition of AF ablation was associated with an increase in 30-day mortality that was not statistically significant (adjusted OR 1.15, p=0.19).22 However, another study of STS-CMS linked patients with preoperative AF demonstrated Ablation was associated with lower perioperative mortality 23 but analyzed patients undergoing multiple cardiac operations including mostly mitral valve surgery. A similar study, compiling data from seven centers, found improved long-term survival in patients with AF who had concomitant Ablation during CABG, valve, or CABG plus valve procedures.24
Our study found that the HR for AF ablation and mortality was not proportional over time, and that survival curves crossed at 2 years. Therefore, AF ablation was associated with a small, statistically significant higher operative mortality, that was mitigated over time and became equivalent through 2 years, and per the landmark analysis, significantly lower in patients surviving past 2 years. Moreover, patients with lower CHA2DS2-VASc scores had lower operative mortality when undergoing AF ablation, which is consistent with previous reports. On the other hand, patients with high CHA2DS2-VASc scores had lower operative mortality if AF ablation was performed, suggesting an important risk-benefit consideration.
Guidelines for the treatment of AF recommend concomitant ablation during CABG (class 1) in whom acceptable perioperative safety is expected.25 Our study supports the recommendations that AF ablation should be performed during CABG in most patients with preoperative AF because after 2 years it is associated with lower stroke and mortality. Future guideline recommendations should take into account higher risk patients (e.g. very elderly patients, high CHA2DS2-VASc). Our study also reveals that a many patients did not get treatment of AF during CABG surgery suggesting underutilization of surgical ablation of atrial fibrillation. The maze procedure has become less complex with modification of lesions, application of energy sources like cryothermy and radio-frequency, and devices to close the LAA.26 Unfortunately, there was no data available on the LAA, although some variation of LAA closure is typical with AF ablation. Recent data regarding LAA closure in a population of elderly patients from the STS database showed a lower risk of readmission for thromboembolism over 3 years in patients who did have surgical occlusion of the LAA.27 The evolution of these changes are not completely reflected in this current dataset; continued advancements may further lower the perioperative risks, improve the efficacy of AF ablation, and reduce AF under treatment.
There has been ongoing study of whether percutaneous coronary intervention and CABG are equivalent.28 Our data raises an important treatment implication for patients with CAD and AF. The improved late survival and lower risk of stroke and thromboembolic events shown in our study should be considered when evaluating CAD patients with concomitant AF and perhaps tip the treatment strategy toward surgery, especially in younger patients who may benefit the most.
Study Limitations
The linkage between STS and Medicare database was incomplete and may have led to unintended bias. We analyzed the unlinked-subjects who could not be linked and found no significant differences in baseline characteristic with the linked-subjects. This analysis is most generalizable to patients 65 years or older who are most susceptible to the added risks associated with extended operating times. Finally, absent from this study (due to lack of data availability) is the type and duration of preoperative AF (paroxysmal vs persistent), the AF lesions sets, and LAA management. Incomplete lesion sets of the Cox-maze procedure potentially lowered the effectiveness of AF ablation, and the omission of surgical LAA closure (associated with reduced risk of thromboembolism)27 both bias results to the null hypothesis.
In conclusion, AF ablation in CABG patients with preoperative AF is associated with lower stroke or systemic embolization at 5 years. The small but statistically significant higher perioperative risk should be considered in patients with vulnerabilities to increased operating time required for AF ablation. Lower mortality was observed in patients surviving past 2 years. Patients with ablation of AF had a lower operative mortality in the low CHA2DS2-VASc scores group but higher operative mortality in those with CHA2DS2-VASc scores 7–9 suggesting a risk-benefit consideration for high risk, elderly patients.
Supplementary Material
Video.
Dr. James L. Cox discusses the role of atrial fibrillation ablation during coronary artery bypass graft surgery.
Central Message:
Concomitant AF treatment during coronary artery bypass surgery is associated with fewer stroke and systolic embolic events as well as higher survival in patients who survive 2 years beyond surgery.
Perspective Statement:
Treating AF during cardiac surgery is a class I indication in patients with a suitable perioperative risk. Ablation in CABG patients with preoperative AF is associated with lower stroke and systemic embolization and mortality in patients who survive 2 years. AF ablation was associated with a higher operative mortality in patients with a CHA2DS2-VASc score of 7–9.
Central Picture:
Surgical ablation of AF in CABG patients is associated with lower mortality at 5 years.
Acknowledgement:
The authors thank Mr. and Mrs. Timothy Thoelecke for their financial support of the Bluhm Cardiovascular Institute, which made this project possible.
Dr. McCarthy: Atricure, speaker fees; Medtronic, speaker fees.
Dr. Friedman: Educational grants from Boston Scientific, Medtronic, Abbott; research grants: National Cardiovascular Data Registry funded by the National Institutes of Health T 32 training grant HL069749–13, Boston Scientific, Abbott, Medtronic, and Biosense Webster; consulting fees Abbott; supported by the Joseph C. Greenfield, Jr., M.D. Scholar in Cardiology Award
All other authors have no relevant disclosures.
Funding:
Institutional funding from Northwestern University, Chicago IL.
Abbreviations:
- AF
atrial fibrillation
- CABG
coronary artery bypass grafting
- CHA2DS2-VASc
Congestive heart failure, Hypertension, Age ≥75, Diabetes, Stroke, VAScular disease, Age 65–74, Sex category
- CI
confidence interval
- CIF
cumulative incidence function
- CMS
Centers for Medicare and Medicaid Services
- HR
hazard ratio
- KM
Kaplan Meier
- LAA
left atrial appendage
- PS
propensity score
- STS
Society of Thoracic Surgeons
Footnotes
Presented at the American Association for Thoracic Surgeons 98th Annual Meeting, Late Breaking Clinical Trials Session on May 1, 2018
References
- 1.Malaisrie SC, McCarthy PM, Kruse J, Matsouaka R, Andrei AC, Grau-Sepulveda MV, et al. Burden of preoperative atrial fibrillation in patients undergoing coronary artery bypass grafting. The Journal of thoracic and cardiovascular surgery 2018. [DOI] [PubMed]
- 2.Saxena A, Virk SA, Bowman S, Chan L, Jeremy R, Bannon PG. Preoperative atrial fibrillation portends poor outcomes after coronary bypass graft surgery: A systematic review and meta-analysis. The Journal of thoracic and cardiovascular surgery 2018;155:1524–1533 e1522. [DOI] [PubMed] [Google Scholar]
- 3.Damiano RJ, Jr.,Gaynor SL, Bailey M, Prasad S, Cox JL, Boineau JP, et al. The long-term outcome of patients with coronary disease and atrial fibrillation undergoing the Cox maze procedure. The Journal of thoracic and cardiovascular surgery 2003;126:2016–2021. [DOI] [PubMed] [Google Scholar]
- 4.Akpinar B, Sanisoglu I, Guden M, Sagbas E, Caynak B, Bayramoglu Z. Combined off-pump coronary artery bypass grafting surgery and ablative therapy for atrial fibrillation: early and mid-term results. The Annals of thoracic surgery 2006;81:1332–1337. [DOI] [PubMed] [Google Scholar]
- 5.Geidel S, Lass M, Krause K, Schneider C, Boczor S, Kuck KH, et al. Persistent atrial fibrillation ablation concomitant to coronary surgery. Thorac Cardiovasc Surg 2011;59:207–212. [DOI] [PubMed] [Google Scholar]
- 6.Lee R, McCarthy PM, Wang EC, Vaduganathan M, Kruse J, Malaisrie SC, et al. Midterm survival in patients treated for atrial fibrillation: a propensity-matched comparison to patients without a history of atrial fibrillation. The Journal of thoracic and cardiovascular surgery 2012;143:1341–1351; discussion 1350–1341. [DOI] [PubMed] [Google Scholar]
- 7.Attaran S, Saleh HZ, Shaw M, Bond L, Pullan MD, Fabri BM. Comparing the outcome of on-pump versus off-pump coronary artery bypass grafting in patients with preoperative atrial fibrillation. Interactive cardiovascular and thoracic surgery 2011;13:288–292. [DOI] [PubMed] [Google Scholar]
- 8.Ad N, Holmes SD, Massimiano PS, Pritchard G, Stone LE, Henry L. The effect of the Cox-maze procedure for atrial fibrillation concomitant to mitral and tricuspid valve surgery. The Journal of thoracic and cardiovascular surgery 2013. [DOI] [PubMed]
- 9.McCarthy PM, Manjunath A, Kruse J, Andrei AC, Li Z, McGee EC Jr.,, et al. Should paroxysmal atrial fibrillation be treated during cardiac surgery? The Journal of thoracic and cardiovascular surgery 2013;146:810–823. [DOI] [PubMed] [Google Scholar]
- 10.Musharbash FN, Schill MR, Sinn LA, Schuessler RB, Maniar HS, Moon MR, et al. Performance of the Cox-maze IV procedure is associated with improved long-term survival in patients with atrial fibrillation undergoing cardiac surgery. The Journal of thoracic and cardiovascular surgery 2018;155:159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobs JP, Edwards FH, Shahian DM, Haan CK, Puskas JD, Morales DL, et al. Successful linking of the Society of Thoracic Surgeons adult cardiac surgery database to Centers for Medicare and Medicaid Services Medicare data. The Annals of thoracic surgery 2010;90:1150–1156; discussion 1156–1157. [DOI] [PubMed] [Google Scholar]
- 12.Mann EAF, and Durham S Death Information in the Research Identifiable Medicare Data Vol 2017. 10/13/2016 ed. https://www.resdac.org/resconnect/articles/117: Research Data Assistance Center; 2016. [Google Scholar]
- 13.D’Agostino RS, Jacobs JP, Badhwar V, Paone G, Rankin JS, Han JM, et al. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2017 Update on Outcomes and Quality. The Annals of thoracic surgery 2017;103:18–24. [DOI] [PubMed] [Google Scholar]
- 14.Shahian DM, Edwards FH, Ferraris VA, Haan CK, Rich JB, Normand SL, et al. Quality measurement in adult cardiac surgery: part 1--Conceptual framework and measure selection. The Annals of thoracic surgery 2007;83:S3–12. [DOI] [PubMed] [Google Scholar]
- 15.O’Brien SM, Shahian DM, DeLong ER, Normand SL, Edwards FH, Ferraris VA, et al. Quality measurement in adult cardiac surgery: part 2--Statistical considerations in composite measure scoring and provider rating. The Annals of thoracic surgery 2007;83:S13–26. [DOI] [PubMed] [Google Scholar]
- 16.Shahian DM, O’Brien SM, Sheng S, Grover FL, Mayer JE, Jacobs JP, et al. Predictors of long-term survival after coronary artery bypass grafting surgery: results from the Society of Thoracic Surgeons Adult Cardiac Surgery Database (the ASCERT study). Circulation 2012;125:1491–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin PC. Using the Standardized Difference to Compare the Prevalence of a Binary Variable Between Two Groups in Observational Research. Communications in Statistics - Simulation and Computation 2009;38:1228–1234. [Google Scholar]
- 18.Fine JP, Gray RJ . A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association 1999;94:496–509. [Google Scholar]
- 19.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Annals of Statistics 1988;16:1141–1154. [Google Scholar]
- 20.Fukunaga S, Hori H, Ueda T, Takagi K, Tayama E, Aoyagi S. Effect of surgery for atrial fibrillation associated with mitral valve disease. The Annals of thoracic surgery 2008;86:1212–1217. [DOI] [PubMed] [Google Scholar]
- 21.Cox JL, Churyla A, Malaisrie SC, Kruse J, Pham DT, Kislitsina ON, et al. When Is a Maze Procedure a Maze Procedure? Can J Cardiol 2018;34:1482–1491. [DOI] [PubMed] [Google Scholar]
- 22.Rankin JS, Lerner DJ, Braid-Forbes MJ, Ferguson MA, Badhwar V. One-year mortality and costs associated with surgical ablation for atrial fibrillation concomitant to coronary artery bypass grafting. Eur J Cardiothorac Surg 2017;52:471–477. [DOI] [PubMed] [Google Scholar]
- 23.Badhwar V, Rankin JS, Ad N, Grau-Sepulveda M, Damiano RJ, Gillinov AM, et al. Surgical Ablation of Atrial Fibrillation in the United States: Trends and Propensity Matched Outcomes. The Annals of thoracic surgery 2017;104:493–500. [DOI] [PubMed] [Google Scholar]
- 24.Iribarne A, DiScipio AW, McCullough JN, Quinn R, Leavitt BJ, Westbrook BM, et al. Surgical Atrial Fibrillation Ablation Improves Long-Term Survival: A Multicenter Analysis. The Annals of thoracic surgery 2019;107:135–142. [DOI] [PubMed] [Google Scholar]
- 25.Badhwar V, Rankin JS, Damiano RJ, Jr., Gillinov AM, Bakaeen FG, Edgerton JR, et al. The Society of Thoracic Surgeons 2017 Clinical Practice Guidelines for the Surgical Treatment of Atrial Fibrillation. The Annals of thoracic surgery 2017;103:329–341. [DOI] [PubMed] [Google Scholar]
- 26.Cox JL, Malaisrie SC, Kislitsina ON, McCarthy PM. The electrophysiologic basis for lesions of the contemporary Maze operation. The Journal of thoracic and cardiovascular surgery 2019;157:584–590. [DOI] [PubMed] [Google Scholar]
- 27.Friedman DJ, Piccini JP, Wang T, Zheng J, Malaisrie SC, Holmes DR, et al. Association Between Left Atrial Appendage Occlusion and Readmission for Thromboembolism Among Patients With Atrial Fibrillation Undergoing Concomitant Cardiac Surgery. JAMA 2018;319:365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doenst T, Haverich A, Serruys P, Bonow RO, Kappetein P, Falk V, et al. PCI and CABG for Treating Stable Coronary Artery Disease: JACC Review Topic of the Week. Journal of the American College of Cardiology 2019;73:964–976. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


