Abstract
Purpose
Dipeptidyl peptidase-4 (DPP-4) inhibitors increase endothelial progenitor cells (EPCs) in peripheral blood circulation. However, the underlying mechanisms and effects on vascular endothelial function remain unclear. We evaluated whether the DPP-4 inhibitor teneligliptin increases circulating EPCs by inhibiting stromal-derived factor-1α (SDF-1α) and improves flow-mediated vascular dilatation (FMD) in type 2 diabetes mellitus patients with acute coronary syndrome (ACS) or its risk factors.
Patients and Methods
This single-center, open-label, prospective, randomized controlled trial evaluated 17 patients (hemoglobin A1c ≤7.5% and peak creatinine phosphokinase <2000 IU/mL) with ACS or a history of ACS or multiple cardiovascular risk factors. Metabolic variables of glucose and lipids, circulating EPCs, plasma DPP-4 activity, and SDF-1α levels, and FMD were evaluated at baseline and 28 ± 4 weeks after enrollment. Patients were randomly assigned to either the teneligliptin (n = 8) or control (n = 9) groups.
Results
The DPP-4 activity (∆−509.5 ± 105.7 vs ∆32.8 ± 53.4 μU/mL) and SDF-1α levels (∆−695.6 ± 443.2 vs ∆11.1 ± 193.7 pg/mL) were significantly decreased after 28 weeks in the teneligliptin group than those in the control group. The number of EPCs showed an increasing trend in the teneligliptin treated group; albeit this did not reach statistical significance. Glucose and lipid levels were not significantly different between the groups before and after 28 weeks. However, FMD was significantly improved in the teneligliptin group when compared to the control group (∆3.8% ± 2.1% vs ∆−0.3% ± 2.9%, P=0.006).
Conclusion
Teneligliptin improved FMD through a mechanism other than increasing the number of circulating EPCs.
Keywords: endothelial progenitor cell, teneligliptin, DPP-4 inhibitor, flow-mediated dilation, type 2 diabetes mellitus
Introduction
Dipeptidyl peptidase-4 (DPP-4) inhibitors are antidiabetic drugs that reduce blood glucose levels by preventing the decomposition of incretin hormones, including glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP).1,2 They are widely used due to their low risk of hypoglycemia and improved safety.3 The American Diabetes Association and European Association for the Study of Diabetes have recommended DPP-4 inhibitors as second-line drugs for type 2 diabetes mellitus (T2DM) patients without atherosclerotic cardiovascular disease (ASCVD) or chronic kidney disease.4 DPP-4 inhibitors are third-line drugs for T2DM patients with ASCVD or high/very high cardiovascular risk, according to the most recent European Society of Cardiology guidelines.5 However, some studies have shown that they have pleiotropic effects in addition to reducing blood glucose levels. One study reported that the oral DPP-4 inhibitor sitagliptin increases plasma stromal-derived factor-1α (SDF-1α) levels, thereby increasing circulating endothelial progenitor cells (EPCs).6 In another study, sitagliptin increased the endothelial nitric oxide synthase expression and circulating EPC levels via the same pathway and improved blood flow by enhancing neovasculogenesis in a mouse model of hindlimb ischemia.7 As a result, we hypothesized that teneligliptin, a selective and long-lasting DPP-4 inhibitor produced in Japan that is effective in and well-tolerated by T2DM patients,8–10 would improve vascular endothelial function via the mobilization of EPCs, and thus may improve left ventricular (LV) function in T2DM patients with the acute coronary syndrome (ACS) or high cardiovascular risk. Herein, we aimed to evaluate whether the DPP-4 inhibitor teneligliptin increases the circulating levels of EPCs via the inhibition of SDF-1α metabolism in T2DM patients with ACS or high cardiovascular risk.
Patient and Methods
Study Protocols
In this single-center, open-label, prospective, randomized controlled trial, 18 patients were recruited between November 2015 and September 2018. All patients were evaluated at baseline and 28 ± 4 weeks after enrollment. The participants were randomly assigned to either the teneligliptin group (those who took teneligliptin) or the control group (those who did not take teneligliptin) via the envelope method. In the teneligliptin group, 20 mg of teneligliptin was administered daily. This study was conducted following the principles of the Declaration of Helsinki. The Institutional Review Board of the Jichi Medical University Saitama Medical Center approved this study protocol (S16-025), and eligible patients provided written informed consent before participation. This trial was registered as a randomized controlled trial titled “Effects of one of antidiabetic agents, teneligliptin, on the vascular endothelium function of ACS patients with diabetes” with the University Hospital Medical Information Network (UMIN), registration number UMIN000018936. This study was presented at the annual meeting of American Diabetes Association 2020, but has not been published yet except meeting’s abstract.11
Participant Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) hospitalization for ACS (peak creatinine phosphokinase (CK) level <2000 U/L); (2) history or high risk of cardiovascular disease defined as either i) patients with documented symptomatic atherosclerotic cardiovascular disease, including stroke, myocardial infarction, hospital admission for unstable angina, coronary artery bypass graft, percutaneous coronary intervention (with or without stenting), peripheral revascularization (angioplasty or surgery), symptomatic with documented hemodynamically significant carotid or peripheral vascular disease, or amputation secondary to vascular disease or ii) patients with two or more of the following risk factors at the time of screening: duration of T2DM ≥10 years, systolic blood pressure >140 mmHg while the patient took at least one antihypertensive drugs, current daily cigarette smoking, the history of micro- or macro-albuminuria, or the history of HDL-C of <1 mmol/L (<39 mg/dL);12 (3) casual blood glucose level ≥200 mg/dL and/or a hemoglobin A1c (HbA1c) level ≥6.5% and <7.5%; and (4) no use of other DPP-4 inhibitors, GLP-1 receptor agonists, thiazolidinediones, or insulin at baseline. One study reported that thiazolidinediones increased the number of EPCs; therefore, we excluded thiazolidinediones.13 The fulfillment of either (1) or (2) was required for inclusion. The exclusion criteria were as follows: (1) type 1 diabetes mellitus, (2) severe hyperglycemia that required the use of insulin and/or multiple antidiabetic drugs, (3) myocardial injury with a peak CK level ≥2000 U/L, (4) unstable or severe cardiovascular disease, (5) diabetic ketoacidosis and/or coma, (6) severe infection and/or severe wound and/or perioperative period, and (7) hypersensitivity to this drug. Due to the difficulty in patient recruitment, we changed some of the inclusion criteria during the study as follows: (1) peak CK level changed from 1000 to 2000 U/L; (2) HbA1c upper limit changed from 7.0% to 7.5%; (3) narrowing down the list of prohibited concomitant medications from all antidiabetic drugs to other DPP-4 inhibitors, GLP-1 receptor agonists, thiazolidinediones, and insulin, which may affect the circulation levels of EPCs; and (4) additional patients with a history or high risk of cardiovascular disease.
Study Endpoint
The primary endpoints of this study were temporal changes in the circulating levels of EPCs, plasma DPP-4 activity, and plasma SDF-1α levels from baseline to 28 weeks. The secondary endpoints were temporal changes in flow-mediated vascular dilatation (FMD), LV function of echocardiographic findings, plaque area in the non-treatment site if intravascular ultrasound was performed, blood glucose and lipid parameters, and urine 8-hydroxydeoxyguanosine from baseline to 28 weeks. No essential changes were made to the outcomes after the trial commencement.
Flow Cytometry
Three milliliters of whole blood containing a small amount of heparin were collected from each participant and sent to the SRL (Hachioji, Tokyo, Japan) to measure the number of EPCs. Briefly, EPCs were counted by measuring CD34 antibody-positive cells using a single-color flow cytometry assay, as previously described.14 Plasma levels of SDF-1α were measured using the Quantikine Human CXCL12/SDF-1α Immunoassay (R&D Systems, Minneapolis, MN, USA) with a commercially available enzyme-linked immunosorbent assay kit. Plasma DPP-4 activity was evaluated using the DPP-4 Activity Fluorometric Assay Kit (BioVision, San Francisco, CA, USA).
FMD
We measured FMD using standard techniques according to the guidelines for the ultrasound assessment of the brachial artery.15 Only two physicians (N. Akashi and T. Umemoto) obtained all FMD measurements. We evaluated the FMD of the brachial artery using UNEXEF 18G (UNEX Corporation, Nagoya, Japan) in the morning in the fasting state and before administering any medications. We used a 10-MHz linear array transducer probe, and the longitudinal vascular image of the right brachial artery was recorded. First, we measured the baseline artery diameter. After 5 min of suprasystolic cuff occlusion (50 mmHg above systolic blood pressure) on the right forearm, the cuff was deflated, and the peak artery diameter was measured. FMD was defined using the following equation: FMD (%) = (peak diameter – baseline diameter) / baseline diameter.16
Statistical Analysis
Data were expressed as percentages for categorical variables and as means ± standard deviations for continuous variables. Categorical variables were presented as numbers (percentages) and were compared using Pearson’s χ2 test or Fisher’s exact test for small samples. Normally distributed continuous variables were compared between the groups using an unpaired Student’s t-test. Continuous variables were compared using the Wilcoxon rank-sum test and did not follow a normal distribution. We compared the temporal changes in each variable for both primary and secondary endpoints from baseline to 28 weeks using an unpaired Student’s t-test and analysis of covariance, with the baseline values as covariates. Statistical significance was defined as a two-sided P <0.05. All analyses were performed using SAS 9.4 (SAS Institute, Cary, North Carolina, USA). Based on outsourcing agreements, a contract research organization performed the monitoring, data management, statistical analyses, and audits (Soiken Inc., Osaka, Japan).
Results
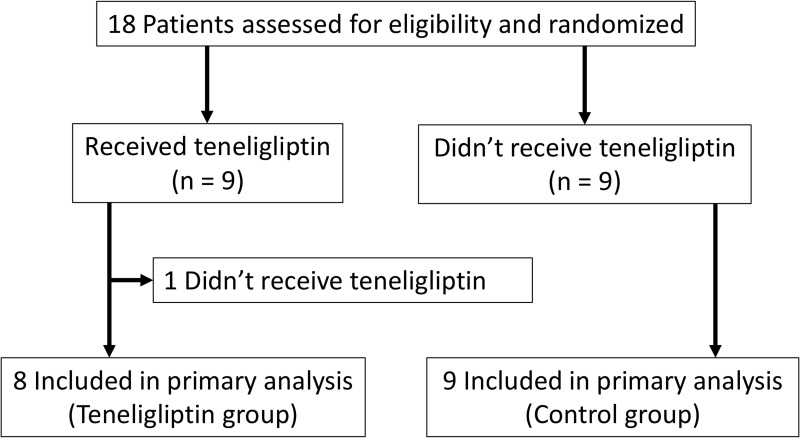
The flowchart for this study is shown in Figure 1. Eighteen patients were randomly assigned to either the teneligliptin (n = 9) or the control (n = 9) group. In the teneligliptin group, one patient declined teneligliptin treatment immediately after entry into this study after experiencing hypoglycemia with 0.25 mg repaglinide. Finally, 17 patients were assessed at baseline and 28 weeks after enrollment. The baseline patient characteristics are summarized in Table 1. The patient demographics, medical history, and primary diseases were not significantly different between the groups except for estimated glomerular filtration rate (88.7 ± 11.4 vs 68.6 ± 22.5 mL/min/1.73m2, P = 0.04).
Figure 1.
Study flow chart.
Table 1.
Baseline Patient Characteristics
| Teneligliptin Group (n = 8) | Control Group (n = 9) | P value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 71.8 ± 11.5 | 63.6 ± 13.8 | 0.21 |
| Female sex (%) | 3 (37.5) | 1 (11.1) | 0.24 |
| Medical history | |||
| Diabetes mellitus, n (%) | 8 (100.0) | 9 (100.0) | – |
| Hypertension, n (%) | 7 (87.5) | 7 (77.8) | 1 |
| Dyslipidemia, n (%) | 5 (62.5) | 7 (77.8) | 0.62 |
| Hyperuricemia, n (%) | 1 (12.5) | 0 (0.0) | 0.47 |
| Atrial fibrillation, n (%) | 1 (12.5) | 2 (22.2) | 0.55 |
| Chronic kidney disease, n (%) | 0 (0.0) | 1 (11.1) | 1 |
| Peripheral arterial disease, n (%) | 0 (0.0) | 1 (11.1) | 1 |
| Primary disease | |||
| Unstable angina pectoris, n (%) | 0 (0.0) | 4 (44.4) | 0.08 |
| Non ST-elevation myocardial infarction, n (%) | 4 (50.0) | 1 (11.1) | 0.13 |
| ST-elevation myocardial infarction, n (%) | 1 (12.5) | 2 (22.2) | 1 |
| Other, n (%) | 3 (37.5) | 2 (22.2) | 0.62 |
| Laboratory data | |||
| Fasting plasma glucose (mg/dL) | 128.1 ± 13.1 | 127.0 ± 30.3 | 0.92 |
| Hemoglobin A1c (%) | 6.8 ± 0.1 | 7.0 ± 0.2 | 0.07 |
| Triglyceride (mg/dL) | 98.7 ± 37.7 | 176.2 ± 121.0 | 0.13 |
| LDL-cholesterol (mg/dL) | 107.9 ± 38.3 | 91.7 ± 44.1 | 0.43 |
| HDL-cholesterol (mg/dL) | 53.0 ± 14.8 | 46.7 ± 11.2 | 0.33 |
| eGFR (mL/min/1.73m2) | 88.7 ± 11.4 | 68.6 ± 22.5 | 0.04 |
Abbreviation: LDL, low-density lipoprotein; HDL, high-density lipoprotein; eGFR, estimated glomerular filtration rate.
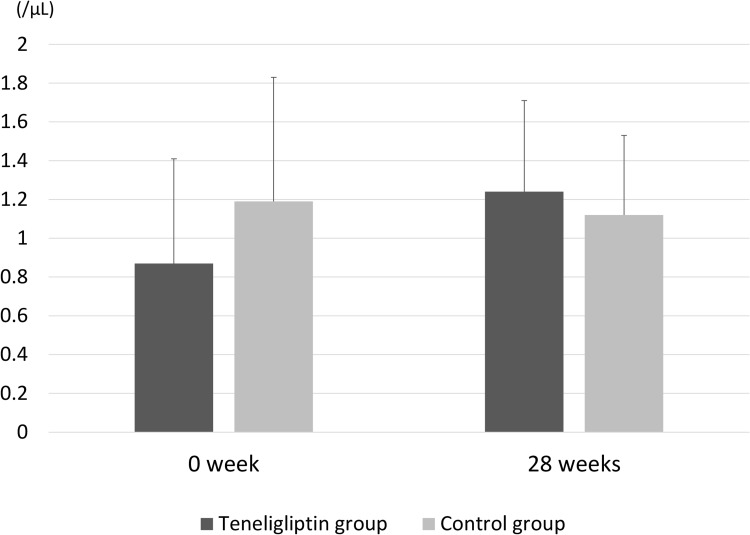
Table 2 shows comparisons of the primary endpoints between the groups. The temporal changes in plasma DPP-4 activity from baseline to 28 weeks were significantly reduced in the teneligliptin group compared with those in the control group (−509.5 ± 105.7 vs 32.8 ± 53.4 μU/mL, P <0.001). The temporal changes in the SDF-1α level were significantly reduced in the teneligliptin group compared with those in the control group (−695.6 ± 443.2 vs 11.1 ± 193.7 pg/mL, P = 0.001). The number of EPCs showed an increasing trend in the teneligliptin treated group; albeit this did not reach statistical significance (0.37 ± 0.45 vs −0.02 ± 0.51 /μL, P = 0.13). In the teneligliptin group, 28-week treatment showed a ~33% increase in the number of EPCs over the baseline numbers of EPCs.
Table 2.
Primary Endpoints in the Teneligliptin and Control Groups
| Variable | Teneligliptin Group (n = 8) | Control Group (n = 9) | Between-Group Difference | ||
|---|---|---|---|---|---|
| Mean ± SD | P value | Mean ± SD | P value | P value | |
| EPC (/μL) | |||||
| 0 W | 0.87 ± 0.54 | 1.19 ± 0.64 | 0.29 | ||
| 28 W | 1.24 ± 0.47 | 1.12 ± 0.41 | 0.61 | ||
| 28 W-0 W | 0.37 ± 0.45 | 0.06 | −0.02 ± 0.51 | 0.91 | 0.13 |
| DPP-4 activity (μU/mL) | |||||
| 0 W | 551.0 ± 112.2 | 656.0 ± 73.8 | 0.036 | ||
| 28 W | 41.5 ± 39.4 | 676.3 ± 58.4 | <0.001 | ||
| 28 W-0 W | −509.5 ± 105.7 | <0.001 | 32.8 ± 53.4 | 0.13 | <0.001 |
| SDF-1α (pg/mL) | |||||
| 0 W | 2495.9 ± 500.6 | 2406.6 ± 428.1 | 0.70 | ||
| 28 W | 1800.3 ± 250.5 | 2522.0 ± 332.0 | <0.001 | ||
| 28 W-0 W | −695.6 ± 443.2 | 0.003 | 11.1 ± 193.7 | 0.88 | 0.001 |
Abbreviations: EPC, endothelial progenitor cell; DPP-4, dipeptidyl peptidase-4; SDF-1α, stromal cell derived factor - 1α.
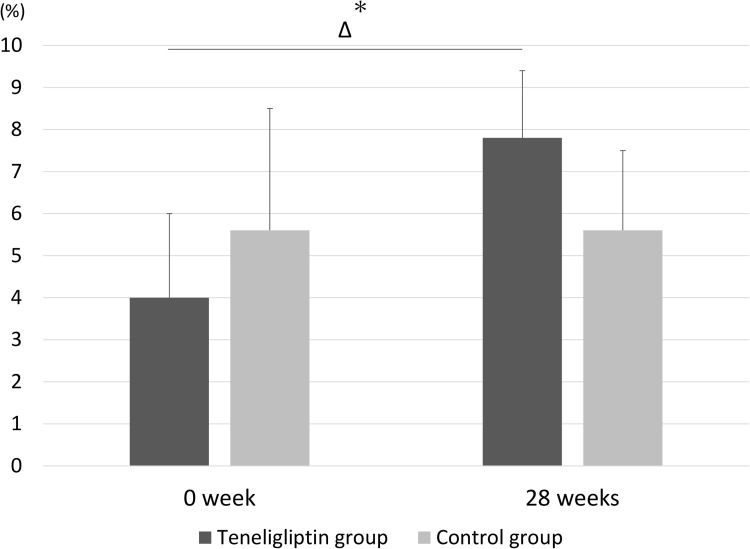
Table 3 shows a comparison of the secondary endpoints between the groups. In Table 3A, the temporal changes in FMD between baseline and 28 weeks were significantly increased in the teneligliptin group compared to those in the control group (3.8% ± 2.1% vs −0.3% ± 2.9%, P = 0.006). In Table 3B, although the temporal changes in HbA1c between baseline and 28 weeks were not significantly different between the groups, the HbA1c levels in the teneligliptin group were significantly higher at baseline than at 28 weeks (6.8 ± 0.1% vs 6.4 ± 0.4%, P = 0.012). The low-density lipoprotein (LDL)-cholesterol levels in the teneligliptin group were significantly higher at baseline than at 28 weeks (107.9 ± 38.3 vs 82.8 ± 23.4 mg/dL, P = 0.040). In contrast, the temporal changes between baseline and 28 weeks were not significantly different between the groups. Analysis of covariance revealed no significant differences in the temporal changes in plasma DPP-4 activity, SDF-1α level, or FMD between the groups (Table 4 and Table 5). Figure 2 shows a bar graph of the mean EPCs between baseline and 28 weeks in the two groups. Figure 3 shows a bar graph of the mean FMD between baseline and 28 weeks in the two groups. The results in Figures 2 and 3 are presented in Table 2 and Table 3.
Table 3.
Secondary Endpoints in the Teneligliptin and Control Groups
| Variable | Teneligliptin Group (n = 8) | Control Group (n = 9) | Between-Group Difference | ||
|---|---|---|---|---|---|
| Mean ± SD | P value | Mean ± SD | P value | P value | |
| A. Ultrasonographic findings | |||||
| Flow-mediated dilation (%) | |||||
| 0 W | 4.0 ± 2.0 (n = 8) | 5.6 ± 2.9 (n = 9) | 0.23 | ||
| 28 W | 7.8 ± 1.6 (n = 8) | 5.6 ± 1.9 (n = 8) | 0.025 | ||
| 28 W-0 W | 3.8 ± 2.1 (n = 8) | 0.001 | −0.3 ± 2.9 (n = 8) | 0.79 | 0.006 |
| Baseline diameter (mm) | |||||
| 0 W | 4.8 ± 0.7 (n = 8) | 4.4 ± 1.0 (n = 9) | 0.39 | ||
| 28 W | 4.1 ± 0.6 (n = 8) | 3.9 ± 0.8 (n = 8) | 0.66 | ||
| 28 W-0 W | −0.7 ± 0.2 (n = 8) | <0.001 | −0.4 ± 0.7 | 0.13 | 0.30 |
| E/A | |||||
| 0 W | 1.3 ± 1.2 (n = 7) | 1.3 ± 1.2 (n = 7) | 0.94 | ||
| 28 W | 1.1 ± 0.6 (n = 7) | 0.8 ± 0.1 (n = 6) | 0.36 | ||
| 28 W-0 W | −0.2 ± 0.6 (n = 7) | 0.40 | −0.1 ± 0.3 (n = 6) | 0.60 | 0.60 |
| E/e’ | |||||
| 0 W | 18.1 ± 9.3 (n = 8) | 18.9 ± 7.2 (n = 9) | 0.86 | ||
| 28 W | 17.6 ± 7.2 (n = 8) | 16.6 ± 10.2 (n = 8) | 0.83 | ||
| 28 W-0 W | −0.5 ± 4.4 (n = 8) | 0.74 | −1.2 ± 5.0 (n = 8) | 0.54 | 0.80 |
| Plaque area (mm2) | |||||
| 0 W | 7.0 ± 2.4 (n = 5) | 5.2 ± 1.2 (n = 2) | 0.39 | ||
| 28 W | 6.8 ± 2.5 (n = 4) | 4.7 ± 1.7 (n = 2) | 0.36 | ||
| 28 W-0 W | −0.3 ± 0.3 (n = 4) | 0.13 | −0.5 ± 0.5 (n = 2) | 0.37 | 0.51 |
| B. Metabolic variables | |||||
| Fasting plasma glucose (mg/dL) | |||||
| 0 W | 128.1 ± 13.1 (n = 8) | 127.0 ± 30.3 (n = 9) | 0.92 | ||
| 28 W | 121.3 ± 17.5 (n = 7) | 142.9 ± 42.5 (n = 8) | 0.23 | ||
| 28 W-0 W | −7.3 ± 10.0 (n = 7) | 0.10 | 24.4 ± 37.8 (n = 8) | 0.11 | 0.05 |
| Hemoglobin A1c (%) | |||||
| 0 W | 6.8 ± 0.1 (n = 8) | 7.0 ± 0.2 (n = 9) | 0.07 | ||
| 28 W | 6.4 ± 0.4 (n = 8) | 7.0 ± 0.7 (n = 8) | 0.08 | ||
| 28 W-0 W | −0.4 ± 0.3 (n = 8) | 0.012 | −0.1 ± 0.6 (n = 8) | 0.83 | 0.19 |
| Triglyceride (mg/dL) | |||||
| 0 W | 98.7 ± 37.7 (n = 7) | 176.2 ± 121.0 (n = 9) | 0.13 | ||
| 28 W | 149.6 ± 107.2 (n = 7) | 143.6 ± 77.4 (n = 8) | 0.90 | ||
| 28 W-0 W | 59.0 ± 93.4 (n = 6) | 0.18 | −8.1 ± 53.4 (n = 8) | 0.68 | 0.11 |
| LDL-cholesterol (mg/dL) | |||||
| 0 W | 107.9 ± 38.3 (n = 8) | 91.7 ± 44.1 (n = 9) | 0.43 | ||
| 28 W | 82.8 ± 23.4 (n = 8) | 87.9 ± 22.2 (n = 8) | 0.66 | ||
| 28 W-0 W | −25.1 ± 28.1 (n = 8) | 0.040 | −5.0 ± 29.4 (n = 8) | 0.65 | 0.18 |
| HDL-cholesterol (mg/dL) | |||||
| 0 W | 53.0 ± 14.8 (n = 8) | 46.7 ± 11.2 (n = 9) | 0.33 | ||
| 28 W | 50.9 ± 9.4 (n = 8) | 50.4 ± 10.5 (n = 8) | 0.92 | ||
| 28 W-0 W | −2.1 ± 9.8 (n = 8) | 0.56 | 1.8 ± 6.6 (n = 8) | 0.48 | 0.37 |
| 8-OHdG (ng/mg creatinine) | |||||
| 0 W | 11.3 ± 4.1 (n = 8) | 7.7 ± 2.5 (n = 9) | 0.042 | ||
| 28 W | 9.3 ± 2.4 (n = 8) | 7.6 ± 1.6 (n = 8) | 0.11 | ||
| 28 W-0 W | −1.9 ± 2.6 (n = 8) | 0.07 | −0.3 ± 2.2 (n = 8) | 0.67 | 0.21 |
Abbreviations: LDL, low-density lipoprotein; HDL, high-density lipoprotein; 8-OHdG, 8-hydroxydeoxyguanosine.
Table 4.
Primary Endpoint with an Analysis of Covariance Model
| Variable | n | Adjusted Mean (SE) | Adjusted Mean Difference (95% CI) | P value (Between-Group Difference) | |
|---|---|---|---|---|---|
| Teneligliptin Group (n = 8) | Control Group (n = 9) | ||||
| ΔEPC (/μL) | 16 | 0.29 (0.13) | 0.05 (0.13) | 0.24 (−0.16, 0.64) | 0.22 |
| ΔDPP-4 activity (μU/mL) | 16 | −544.9 (17.5) | 68.1 (17.5) | −612.9 (−669.8, −556.1) | <0.001 |
| ΔSDF-1α (pg/mL) | 16 | −700.0 (87.5) | 15.5 (87.5) | −715.6 (−983.0, −448.1) | <0.001 |
Abbreviations: EPC, endothelial progenitor cell; DPP-4, dipeptidyl peptidase-4; SDF-1α, stromal cell derived factor - 1α.
Table 5.
Secondary Endpoint with an Analysis of Covariance Model
| Variable | n | Adjusted Mean (SE) | Adjusted Mean Difference (95% CI) | P value (Between-Group Difference) | |
|---|---|---|---|---|---|
| Teneligliptin Group (n = 8) | Control Group (n = 9) | ||||
| Flow-mediated dilation (%) | 16 | 3.1 (0.6) | 0.4 (0.6) | 2.7 (0.7, 4.7) | 0.013 |
| Baseline diameter (mm) | 16 | −0.6 (0.2) | −0.5 (0.2) | −0.1 (−0.6, 0.4) | 0.57 |
| E/A | 13 | −0.1 (0.1) | −0.2 (0.1) | 0.1 (−0.1, 0.3) | 0.57 |
| E/e’ | 16 | −0.5 (1.7) | −1.2 (1.7) | 0.6 (−4.6, 5.9) | 0.79 |
| Plaque area (mm2) | 6 | −0.3 (0.2) | −0.6 (0.3) | 0.3 (−0.8, 1.4) | 0.44 |
| Fasting plasma glucose (mg/dL) | 15 | −7.7 (11.6) | 24.8 (10.8) | −32.5 (−67.8, 2.8) | 0.07 |
| Hemoglobin A1c (%) | 16 | −0.4 (0.2) | −0.1 (0.2) | −0.3 (−0.9, 0.3) | 0.30 |
| Triglyceride (mg/dL) | 14 | 52.1 (30.8) | −2.9 (26.5) | 55.0 (−36.9, 146.8) | 0.21 |
| LDL-cholesterol (mg/dL) | 16 | −20.7 (5.3) | −9.4 (5.3) | −11.3 (−27.5, 4.9) | 0.15 |
| HDL-cholesterol (mg/dL) | 16 | −1.2 (2.4) | 0.9 (2.4) | −2.1 (−9.5, 5.3) | 0.55 |
| DHLA (μg/mL) | 15 | 3.5 (3.0) | 5.4 (3.2) | −1.8 (−11.5, 7.9) | 0.69 |
| EPA (μg/mL) | 15 | 7.6 (10.7) | −16.9 (11.5) | 24.5 (−11.7, 60.7) | 0.17 |
| AA (μg/mL) | 15 | 14.6 (15.3) | 16.7 (16.4) | −2.0 (−51.8, 47.7) | 0.93 |
| DHA (μg/mL) | 15 | 8.9 (13.7) | −17.4 (14.7) | 26.3 (−19.5, 72.1) | 0.23 |
| RLP-cholesterol (mg/dL) | 15 | 3.5 (1.5) | 0.5 (1.6) | 3.0 (−2.0, 7.9) | 0.21 |
| Lipoprotein(a) (mg/dL) | 15 | −6.2 (1.4) | −0.7 (1.5) | −5.5 (−9.9, −1.1) | 0.018 |
| 8-OHdG (ng/mg creatinine) | 16 | −1.0 (0.5) | −1.3 (0.5) | 0.3 (−1.5, 2.1) | 0.71 |
Abbreviations: LDL, low-density lipoprotein; HDL, high-density lipoprotein; DHLA, dihomo-gamma-linolenic acid; EPA, eicosapentaenoic acid; AA, arachidonic acid; DHA, docosahexaenoic acid; RLP, remnant-like particle cholesterol; 8-OHdG, 8-hydroxydeoxyguanosine.
Figure 2.
Mean endothelial progenitor cells (EPCs) between baseline and 28 weeks in the teneligliptin and control groups. Temporal changes in EPC levels from baseline to 28 weeks were not significantly different between the groups (P = 0.13). The bar graph and error bars show the mean and standard deviation, respectively.
Figure 3.
Mean flow-mediated dilatation (FMD) between baseline and 28 weeks in the teneligliptin and control groups. Temporal changes in FMD from baseline to 28 weeks were significantly increased in the teneligliptin group (3.8% ± 2.1%) compared with those in the control group (−0.3% ± 2.9%). The bar graph and error bars show the mean and standard deviation, respectively. *P <0.05, compared to the control group.
Discussion
In this randomized study, the temporal changes in SDF-1α levels were significantly reduced in the teneligliptin group compared with those in the control group. In addition, the changes in plasma DPP-4 activity were significantly reduced in the teneligliptin group compared with those in the control group between baseline and 28 weeks. Although not statistically significant, the temporal changes in the circulating levels of EPCs in the teneligliptin group tended to be greater than those in the control group (P = 0.13). The improvement in FMD, the standard tool for assessing vascular endothelial function, was significant in the teneligliptin group when compared to that in the control group between baseline and 28 weeks. These results suggest that teneligliptin may improve endothelial function in T2DM patients with ACS or high cardiovascular risk via different ways from increasing the number of circulating EPCs.
Several trials have revealed that DPP-4 inhibitors could increase SDF-1 levels and lower blood glucose.17,18 The chemokine SDF-1 is roughly categorized into two alternative splicing variants (α and β), which are similar in amino acid sequences, except for the presence of four additional amino acids at the C-terminus of SDF-1β, which play an important role in tissue inflammation, vascular repair, and regeneration.19,20 Active SDF-1α is degraded to inactive forms by DPP-4; therefore, inhibition of DPP-4 could increase the plasma levels of active SDF-1α.21 This increase may have resulted in the mobilization of EPCs from the bone marrow. Fadini et al showed that the DPP-4 inhibitor sitagliptin increases the circulating EPC levels in patients with T2DM, possibly via an increase in SDF-1α levels.6 Conversely, our study demonstrated that teneligliptin therapy for approximately 28 weeks significantly decreased the plasma levels of SDF-1α compared with treatment without teneligliptin. These discrepant findings regarding SDF-1α levels may be due to differences in the methodology used for detecting SDF-1α.22–24 We used a commercially available kit from R&D Systems, an antibody that recognizes an epitope at the C-terminus of SDF-1α because it did not cross-react with SDF-1β. This commercial assay kit measures the total SDF-1α (both active and inactive forms). However, Fadini et al used a different assay kit from Bio-Rad Laboratories that uses an antibody against an epitope at the SDF-1α N-terminus, which contains the active receptor (CXCR4)-binding site, and only active SDF-1α was measured. In vivo, radiolabeling indicates that the half-life of SDF-1α is 25.8 ± 4.6 minutes,25 suggesting that the former kit (R&D systems), which was used for all SDF-1α measurements in our study, may be affected by inactive SDF-1α. Although details are not available, it is possible that the active plasma SDF-1α levels also increased after teneligliptin administration in this study, which might have improved vascular endothelial function.
Our study showed that teneligliptin therapy for approximately 28 weeks significantly improved FMD compared to therapy without teneligliptin. However, some previous studies have reported the inconsistent effects of DPP-4 inhibitors on FMD.26–31 Clinical research, including two independent prospective, randomized, crossover trials, demonstrated that sitagliptin attenuates endothelial function, as assessed by FMD for 6 weeks.27 However, a randomized prospective multicenter study demonstrated that sitagliptin improves endothelial function, as assessed by FMD, to the same extent as the α-glucosidase inhibitor voglibose for 12 weeks.28 In addition, a previous study showed that sitagliptin could improve endothelial dysfunction, as evaluated using the reactive hyperemia peripheral arterial tonometry index, in patients with DM and coronary artery disease for 6 months.32 One reason for these discrepancies is that the duration of treatment with DPP-4 inhibitors might have been too short of improving endothelial function. Specifically, at least 6 weeks or more of treatment with DPP-4 inhibitors may be needed to improve endothelial function.
Previous studies have shown that DPP-4 inhibitors increase circulating EPCs,6,7,23,33 which play a role in endothelial repair and angiogenesis.34 However, our study did not demonstrate significant differences in the temporal changes in circulating EPCs between the groups. The reason for the lack of a significant difference in the temporal changes in circulating EPCs may be the lack of statistical power because of the small number of patients. Although the difference was insignificant, the temporal changes in the EPCs in the teneligliptin group tended to be greater than those in the control group. Owing to the significant improvement in FMD observed in the teneligliptin group in this study, it is possible that EPCs were increased at the site of vascular injury, while the number of circulating EPCs did not significantly improve. Huang et al confirmed that the oral administration of DPP-4 inhibitors in mice increased EPCs and enhanced neovascularization at the site of hindlimb ischemia,7 suggesting that EPCs may be particularly increased at the site of vascular injury. In contrast, the existing large-scale clinical trials on DPP-4 inhibitors did not show significantly improvement in the incidence of cardiovascular events.35–37 However, these large-scale trials were designed to demonstrate noninferiority of DPP-4 inhibitors to placebo, it is possible that the follow-up period and the number of patients were not sufficient to establish superiority.
The relationship between ameliorating glucose and lipid metabolism and improving vascular endothelial function should also be discussed. Previous studies using FMD and plethysmography have shown that hyperglycemia, with or without DM, attenuates vascular endothelial function.38,39 Hyperglycemia induces further oxidative stress via activation of the polyol, hexosamine, protein kinase C, and advanced glycation end-product pathways, leading to vascular endothelial dysfunction.40 Furthermore, hyper LDL-cholesterolemia attenuates vascular endothelial function, and a meta-analysis of familial hypercholesterolemia showed that FMD was lower in familial hypercholesterolemia patients.41 The present study demonstrated significantly lower HbA1c and LDL-cholesterol levels in the teneligliptin group at 28 weeks than at baseline. Although the reason for the decline in LDL-cholesterol levels in the teneligliptin group is unknown, it is possible that the amelioration of glucose and lipid metabolism in this group contributed to improved vascular endothelial function.
A previous single-center randomized controlled trial showed that changes in FMD were significantly related to changes in circulating EPC levels in T2DM patients.42 However, in this trial, patients newly diagnosed with T2DM who were administered metformin after the diagnosis of T2DM were recruited. Compared to our study, which enrolled T2DM patients with ACS or high cardiovascular risk who used DPP-4 inhibitors, the strong correlation between the changes in FMD and the number of circulating EPCs might have been associated with differences in disease severity and drug treatments. Nevertheless, our finding that teneligliptin significantly improved temporal changes in FMD should be considered significant. Since our results did not show whether the DPP-4 inhibitor own effect or the hypoglycemic effect contributed to the improvement in FMD, future exploration is needed in the future.
The present study had several limitations. First, although we conducted a single-center, randomized controlled trial, there was an inherent risk of β error in the statistical analysis because of the small number of patients.43 Large-scale investigations are warranted in the future. Second, we could not perform sample size calculations because this study was a pilot study at the beginning of this study. We planned to include 25 patients in each group who were statistically comparable; however, we did not collect as many patients as expected. Third, since our study had three primary endpoints, there is a possibility of multiplicity issues caused by multiple statistical analyses and increased α error. This may affect the interpretation of the results. Fourth, selection bias may occur because the envelope method was used for patient assignment. Indeed, several values in the patient background differed between the two groups, and it cannot be ruled out that this may have influenced the results. Fifth, in general, EPCs are defined as CD34+/ KDR+ or CD34+/CD133+ cells with double staining of flow cytometry.44 It cannot be said that CD34 antibody-positive cells using a single-color flow cytometry assay that we used are EPCs. Sixth, the commercial assays for SDF-1α that we used might have been unable to distinguish between the active and inactive forms. Seventh, as there were no restrictions except for antidiabetic drugs, other medications (eg, statins and antihypertensive drugs) could have affected the FMD results. Finally, the study period was short (approximately 6 months). Longer treatment duration may be needed to show the cardiac vascular endothelial and diastolic functions in high cardiovascular risk individuals.
In conclusion, although the temporal changes in circulating EPCs were not significantly different, teneligliptin significantly improved the vascular endothelial function, as assessed by FMD, in T2DM patients with ACS or high cardiovascular risk. Thus, teneligliptin could improve vascular endothelial function in different ways by increasing the number of circulating EPCs.
Acknowledgments
We thank Ms. Tomoko Takahara, a technical assistant at the Division of Cardiovascular Medicine, Jichi Medical University Saitama Medical Center. The authors acknowledge the institutions and investigators involved in the study.
Funding Statement
This study was funded by Mitsubishi Tanabe Pharma Corporation, Tokyo, Japan.
Ethics Approval and Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (the institutional review board of Jichi Medical University / Date of approval: March 3, 2014 / Approval number: 13-112) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study.
Disclosure
Dr Naoyuki Akashi reports grants from Mitsubishi Tanabe Pharma Corporation, during the conduct of the study. The authors report no other potential conflicts of interest in this work.
References
- 1.Deacon CF. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes Obes Metab. 2011;13(1):7–18. doi: 10.1111/j.1463-1326.2010.01306.x [DOI] [PubMed] [Google Scholar]
- 2.Omar B, Ahren B. Pleiotropic mechanisms for the glucose-lowering action of DPP-4 inhibitors. Diabetes. 2014;63(7):2196–2202. doi: 10.2337/db14-0052 [DOI] [PubMed] [Google Scholar]
- 3.Chen XW, He ZX, Zhou ZW, et al. Clinical pharmacology of dipeptidyl peptidase 4 inhibitors indicated for the treatment of type 2 diabetes mellitus. Clin Exp Pharmacol Physiol. 2015;42(10):999–1024. doi: 10.1111/1440-1681.12455 [DOI] [PubMed] [Google Scholar]
- 4.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;61(12):2461–2498. doi: 10.1007/s00125-018-4729-5 [DOI] [PubMed] [Google Scholar]
- 5.Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2019;40:1–69. doi: 10.1093/eurheartj/ehz486 [DOI] [PubMed] [Google Scholar]
- 6.Fadini GP, Boscaro E, Albiero M, et al. The oral dipeptidyl peptidase-4 inhibitor sitagliptin increases circulating endothelial progenitor cells in patients with type 2 diabetes: possible role of stromal-derived factor-1 alpha. Diabetes Care. 2010;33(7):1607–1609. doi: 10.2337/dc10-0187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang CY, Shih CM, Tsao NW, et al. Dipeptidyl peptidase-4 inhibitor improves neovascularization by increasing circulating endothelial progenitor cells. Br J Pharmacol. 2012;167(7):1506–1519. doi: 10.1111/j.1476-5381.2012.02102.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goda M, Kadowaki T. Teneligliptin for the treatment of type 2 diabetes. Drugs Today. 2013;49(10):615–629. doi: 10.1358/dot.2013.49.10.2035882 [DOI] [PubMed] [Google Scholar]
- 9.Otsuki H, Kosaka T, Nakamura K, et al. Safety and efficacy of teneligliptin: a novel DPP-4 inhibitor for hemodialysis patients with type 2 diabetes. Int Urol Nephrol. 2014;46(2):427–432. doi: 10.1007/s11255-013-0552-6 [DOI] [PubMed] [Google Scholar]
- 10.Kadowaki T, Marubayashi F, Yokota S, Katoh M, Iijima H. Safety and efficacy of teneligliptin in Japanese patients with type 2 diabetes mellitus: a pooled analysis of two Phase III clinical studies. Expert Opin Pharmacother. 2015;16(7):971–981. doi: 10.1517/14656566.2015.1032249 [DOI] [PubMed] [Google Scholar]
- 11.Akashi N, Umemoto T, Yamada H, et al. 1096-P: DPP-4 inhibitor teneligliptin improves the vascular endothelial function via mechanism different from changes of EPCs levels and blood glucose metabolism. Diabetes. 2020;69(Supplement_1). doi: 10.2337/db20-1096-P [DOI] [Google Scholar]
- 12.Neal B, Perkovic V, de Zeeuw D, et al. Rationale, design, and baseline characteristics of the Canagliflozin Cardiovascular Assessment Study (CANVAS)--A randomized placebo-controlled trial. Am Heart J. 2013;166:217–223 e211. doi: 10.1016/j.ahj.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 13.Makino H, Okada S, Nagumo A, et al. Pioglitazone treatment stimulates circulating CD34-positive cells in type 2 diabetes patients. Diabetes Res Clin Pract. 2008;81(3):327–330. doi: 10.1016/j.diabres.2008.05.012 [DOI] [PubMed] [Google Scholar]
- 14.Ip SH, Rittershaus CW, Healey KW, et al. Rapid enumeration of T lymphocytes by a flow-cytometric immunofluorescence method. Clin Chem. 1982;28(9):1905–1909. doi: 10.1093/clinchem/28.9.1905 [DOI] [PubMed] [Google Scholar]
- 15.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–265. doi: 10.1016/s0735-1097(01)01746-6 [DOI] [PubMed] [Google Scholar]
- 16.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55(5):1075–1085. doi: 10.1161/hypertensionaha.110.150821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Busso N, Wagtmann N, Herling C, et al. Circulating CD26 is negatively associated with inflammation in human and experimental arthritis. Am J Pathol. 2005;166(2):433–442. doi: 10.1016/s0002-9440(10)62266-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Packer M. Have dipeptidyl peptidase-4 inhibitors ameliorated the vascular complications of type 2 diabetes in large-scale trials? The potential confounding effect of stem-cell chemokines. Cardiovasc Diabetol. 2018;17(1):9. doi: 10.1186/s12933-017-0648-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janowski M. Functional diversity of SDF-1 splicing variants. Cell Adh Migr. 2009;3(3):243–249. doi: 10.4161/cam.3.3.8260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo Q, Zhang B, Kuang D, Song G. Role of stromal-derived factor-1 in mesenchymal stem cell paracrine-mediated tissue repair. Curr Stem Cell Res Ther. 2016;11(7):585–592. doi: 10.2174/1574888x11666160614102629 [DOI] [PubMed] [Google Scholar]
- 21.Lovshin JA, Rajasekeran H, Lytvyn Y, et al. Dipeptidyl peptidase 4 inhibition stimulates distal tubular natriuresis and increases in circulating SDF-1α(1-67) in patients with type 2 diabetes. Diabetes Care. 2017;40(8):1073–1081. doi: 10.2337/dc17-0061 [DOI] [PubMed] [Google Scholar]
- 22.Aso Y, Jojima T, Iijima T, et al. Sitagliptin, a dipeptidyl peptidase-4 inhibitor, increases the number of circulating CD34⁺CXCR4⁺ cells in patients with type 2 diabetes. Endocrine. 2015;50(3):659–664. doi: 10.1007/s12020-015-0688-5 [DOI] [PubMed] [Google Scholar]
- 23.Dei Cas A, Spigoni V, Cito M, et al. Vildagliptin, but not glibenclamide, increases circulating endothelial progenitor cell number: a 12-month randomized controlled trial in patients with type 2 diabetes. Cardiovasc Diabetol. 2017;16(1):27. doi: 10.1186/s12933-017-0503-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park KS, Kwak S, Cho YM, et al. Vildagliptin reduces plasma stromal cell-derived factor-1α in patients with type 2 diabetes compared with glimepiride. J Diabetes Investig. 2017;8(2):218–226. doi: 10.1111/jdi.12572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Misra P, Lebeche D, Ly H, et al. Quantitation of CXCR4 expression in myocardial infarction using 99mTc-labeled SDF-1 alpha. J Nucl Med. 2008;49(6):963–969. doi: 10.2967/jnumed.107.050054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubota Y, Miyamoto M, Takagi G, et al. The dipeptidyl peptidase-4 inhibitor sitagliptin improves vascular endothelial function in type 2 diabetes. J Korean Med Sci. 2012;27(11):1364–1370. doi: 10.3346/jkms.2012.27.11.1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayaori M, Iwakami N, Uto-Kondo H, et al. Dipeptidyl peptidase-4 inhibitors attenuate endothelial function as evaluated by flow-mediated vasodilatation in type 2 diabetic patients. J Am Heart Assoc. 2013;2(1):e003277. doi: 10.1161/jaha.112.003277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura K, Oe H, Kihara H, et al. DPP-4 inhibitor and alpha-glucosidase inhibitor equally improve endothelial function in patients with type 2 diabetes: EDGE study. Cardiovasc Diabetol. 2014;13(1):110. doi: 10.1186/s12933-014-0110-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ida S, Murata K, Betou K, et al. Effect of trelagliptin on vascular endothelial functions and serum adiponectin level in patients with type 2 diabetes: a preliminary single-arm prospective pilot study. Cardiovasc Diabetol. 2016;15(1):153. doi: 10.1186/s12933-016-0468-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitao N, Miyoshi H, Furumoto T, et al. The effects of vildagliptin compared with metformin on vascular endothelial function and metabolic parameters: a randomized, controlled trial (Sapporo Athero-Incretin Study 3). Cardiovasc Diabetol. 2017;16(1):125. doi: 10.1186/s12933-017-0607-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tripolt NJ, Aberer F, Riedl R, et al. Effects of linagliptin on endothelial function and postprandial lipids in coronary artery disease patients with early diabetes: a randomized, placebo-controlled, double-blind trial. Cardiovasc Diabetol. 2018;17(1):71. doi: 10.1186/s12933-018-0716-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsubara J, Sugiyama S, Akiyama E, et al. Dipeptidyl peptidase-4 inhibitor, sitagliptin, improves endothelial dysfunction in association with its anti-inflammatory effects in patients with coronary artery disease and uncontrolled diabetes. Circ J. 2013;77(5):1337–1344. doi: 10.1253/circj.cj-12-1168 [DOI] [PubMed] [Google Scholar]
- 33.Fadini GP, Bonora BM, Cappellari R, et al. Acute effects of linagliptin on progenitor cells, monocyte phenotypes, and soluble mediators in type 2 diabetes. J Clin Endocrinol Metab. 2016;101(2):748–756. doi: 10.1210/jc.2015-3716 [DOI] [PubMed] [Google Scholar]
- 34.Fadini GP, Agostini C, Sartore S, Avogaro A. Endothelial progenitor cells in the natural history of atherosclerosis. Atherosclerosis. 2007;194(1):46–54. doi: 10.1016/j.atherosclerosis.2007.03.046 [DOI] [PubMed] [Google Scholar]
- 35.Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232–242. doi: 10.1056/NEJMoa1501352 [DOI] [PubMed] [Google Scholar]
- 36.Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–1326. doi: 10.1056/NEJMoa1307684 [DOI] [PubMed] [Google Scholar]
- 37.White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327–1335. doi: 10.1056/NEJMoa1305889 [DOI] [PubMed] [Google Scholar]
- 38.Williams SB, Goldfine AB, Timimi FK, et al. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation. 1998;97(17):1695–1701. doi: 10.1161/01.cir.97.17.1695 [DOI] [PubMed] [Google Scholar]
- 39.Title LM, Cummings PM, Giddens K, Nassar BA. Oral glucose loading acutely attenuates endothelium-dependent vasodilation in healthy adults without diabetes: an effect prevented by vitamins C and E. J Am Coll Cardiol. 2000;36(7):2185–2191. doi: 10.1016/s0735-1097(00)00980-3 [DOI] [PubMed] [Google Scholar]
- 40.Maruhashi T, Higashi Y. Pathophysiological association between diabetes mellitus and endothelial dysfunction. Antioxidants. 2021;10(8). doi: 10.3390/antiox10081306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masoura C, Pitsavos C, Aznaouridis K, et al. Arterial endothelial function and wall thickness in familial hypercholesterolemia and familial combined hyperlipidemia and the effect of statins. A systematic review and meta-analysis. Atherosclerosis. 2011;214(1):129–138. doi: 10.1016/j.atherosclerosis.2010.10.008 [DOI] [PubMed] [Google Scholar]
- 42.Liao YF, Chen LL, Zeng TS, et al. Number of circulating endothelial progenitor cells as a marker of vascular endothelial function for type 2 diabetes. Vasc Med. 2010;15(4):279–285. doi: 10.1177/1358863x10367537 [DOI] [PubMed] [Google Scholar]
- 43.Brown CG, Kelen GD, Ashton JJ, Werman HA. The beta error and sample size determination in clinical trials in emergency medicine. Ann Emerg Med. 1987;16(2):183–187. doi: 10.1016/s0196-0644(87)80013-6 [DOI] [PubMed] [Google Scholar]
- 44.Shantsila E, Watson T, Lip GY. Endothelial progenitor cells in cardiovascular disorders. J Am Coll Cardiol. 2007;49(7):741–752. doi: 10.1016/j.jacc.2006.09.050 [DOI] [PubMed] [Google Scholar]