Abstract
Background
There are ongoing efforts to examine the effect of 5-HT1A receptor partial agonists as an add-on therapy for several symptoms of schizophrenia. By conducting a systematic review and meta-analysis, we evaluated whether augmentation with 5-hydroxtrypatamine (5-HT)1A partial agonists of the azapirone class improves psychotic symptoms and attention/processing speed, a key domain of cognition, in patients with schizophrenia.
Methods
A literature search was performed from 1987 to February 25, 2022, to identify randomized controlled trials. The standardized mean difference (SMD) with 95% confidence intervals (CI) was calculated when there were 2 or more studies. Seven studies, involving 435 patients, met the inclusion criteria.
Results
Random-effects model meta-analyses revealed that add-on therapy with buspirone or tandospirone had a significant beneficial effect on overall psychotic symptoms (SMD = –1.13, 95% CI = –1.98 to –0.27) and positive symptoms (SMD = –0.72, 95% CI =–1.31 to –0.12), while the effect on negative symptoms did not reach statistical significance (SMD = –0.93, 95% CI = –1.90 to 0.04). A significant positive effect was also observed on attention/processing speed (SMD = 0.37, 95% CI = 0.12 to 0.61).
Conclusions
These findings support the idea that some compounds that stimulate 5-HT1A receptors provide an effective pharmacologic enhancer in the treatment of schizophrenia. Further clinical trials are warranted to determine the benefits of the adjunctive use of 5-HT1A partial agonists in ameliorating symptoms and improving functional outcomes in patients with schizophrenia or other psychiatric disorders.
Keywords: 5-HT1A receptor partial agonist, psychopathology, cognitive dysfunction, atypical antipsychotic drugs, schizophrenia
Significance Statement.
In view of the insufficient efficacy of current antipsychotic drugs in ameliorating psychiatric symptoms and cognitive impairments in some patients with schizophrenia, further efforts are required to develop new treatment approaches. The add-on prescription of serotonin 5-HT1A receptor partial agonists (e.g., buspirone, tandospirone) has been suggested to solve part of these problems. In connection with this, the current study examined the possible benefits of the adjunctive use of buspirone or tandospirone for potentiating treatment of psychotic symptoms and disturbances of attention/processing speed, a core domain of cognitive function, in patients with schizophrenia. Results of a meta-analysis showed that augmentation therapy with the above 5-HT1A partial agonists is beneficial in alleviating overall symptoms, particularly delusions and hallucinations, as well as improving attention/processing speed in patients receiving ongoing treatment with existing antipsychotic drugs. These findings may help in the development of effective strategies to further improve functional outcomes in patients with schizophrenia.
INTRODUCTION
Schizophrenia is a severe psychiatric disease with a lifetime prevalence of approximately 0.30%–0.66% (McGrath et al., 2008) and is typically diagnosed in the late teen years to early 30s (McGrath et al., 2008). The illness is characterized by positive (hallucinations, delusions) and negative (withdrawal, apathy, anhedonia) symptoms (Crow 1982; Schultz and Andreasen 1999) as well as disturbances in several domains of cognitive function (e.g., attention/processing speed, verbal memory, and working memory) that adversely affect functional outcomes (Heaton et al., 2001; Keefe et al., 2005; Kiwanuka et al., 2014; Sumiyoshi et al., 2016; Galderisi et al., 2020).
The pathophysiology of schizophrenia has been suggested to include abnormally low prefrontal dopamine (DA) activity (causing negative symptoms) leading to excessive activity in mesolimbic DA neurons (causing positive symptoms) (Davis et al., 1991; Deutch 1992; Weinberger and Gallhofer 1997). The role of DA transmission is relevant to the fact that antipsychotic drugs have been standard care for patients with schizophrenia (Keepers et al., 2020). For example, typical antipsychotic drugs (TAPDs), such as chlorpromazine, haloperidol, and perphenazine, are thought to be beneficial for positive symptoms through antagonism at DA-D2 receptors (Seeman, 2002; Gründer et al., 2009). Meanwhile, TAPDs have been associated with a limited benefit for negative symptoms and cognitive impairment as well as a high incidence of extrapyramidal symptoms (EPS) (Meltzer 2013, 2017; Meltzer and Gadaleta 2021). On the other hand, it has been suggested that atypical antipsychotic drugs (AAPDs), with clozapine as the prototype, may be more effective than typical AAPDs in treating psychotic and mood symptoms as well as cognitive impairment, and have reduced risk of causing EPSs (Meltzer 2013, 2017; Meltzer and Gadaleta 2021).
The distinct properties of AAPDs have been discussed in relation to their high affinity for serotonin (5-hydroxtrypatamine [5-HT]) receptor subtypes (Sumiyoshi 2008; Meltzer and Gadaleta 2021). For example, a relatively high affinity for 5-HT2A receptors vs D2 receptors may be related to the difference between AAPDs and TAPDs (Meltzer and Massey, 2011). This notion has been supported by in vivo experiments with rodents (Stockmeier et al., 1993; Sumiyoshi et al., 1993, 1994a, 1994b, 1995) and may explain the mechanisms of some of the AAPDs currently used, for example, risperidone, olanzapine, and quetiapine (Meltzer et al., 2003; Sumiyoshi et al., 2003a, 2003b, 2006; Araki et al., 2006). In spite of these observations, further efforts are needed to develop novel strategies to overcome unmet needs in the treatment of schizophrenia (Leucht et al., 2009; Meltzer et al., 2012; Meltzer 2013).
Among the 5-HT receptor subtypes, 5-HT1A receptors are thought to mediate the efficacy of several antipsychotic drugs, including AAPDs (Meltzer and Sumiyoshi 2008; Newman-Tancredi and Albert 2012; Sumiyoshi 2020). 5-HT1A receptors are widely located in brain areas governing cognitive and emotional processes, for example, the frontal cortex, hippocampus, and amygdala (Le François et al., 2008). For example, positron emission tomography studies (e.g. Tauscher et al., 2002) have shown an increase in cortical 5-HT1A receptor binding sites in schizophrenia, consistent with observations in studies that used postmortem brain tissues (Burnet et al., 1996; Simpson et al., 1996; Sumiyoshi et al., 1996; Burnet et al., 1997). The increased density of 5-HT1A receptors may represent upregulation secondary to diminished 5-HT1A receptor stimulation, as has been discussed (Hashimoto et al., 1991; Burnet et al., 1996, 1997; Simpson et al., 1996; Sumiyoshi et al., 1996). These considerations are consistent with the idea that most AAPDs act as partial agonists at 5-HT1A receptors, either directly (e.g., aripiprazole, lurasidone, brexpiprazole) or indirectly (e.g., risperidone, olanzapine) (Meltzer and Sumiyoshi 2008; Meltzer and Massey 2011; Gener et al., 2019). The 5-HT1A partial agonist actions of AAPDs provide a preferential increase in extracellular concentrations of DA and acetylcholine in the prefrontal cortex relative to subcortical areas (Li et al., 1998; Ichikawa et al., 2001; Masana et al., 2011). As enhancement of prefrontal DA activity is thought to regulate DA activity in mesolimbic DA neurons (Davis et al., 1991; Deutch, 1992), 5-HT1A partial agonism may alleviate psychotic symptoms, including positive symptoms, and cognitive impairment of schizophrenia (Ichikawa et al., 2001; Meltzer and Sumiyoshi, 2008; Newman-Tancredi and Kleven, 2011; Newman-Tancredi and Albert, 2012; Sumiyoshi, 2020).
Azapirone derivatives, for example, buspirone, tandospirone, gepirone, and ipsapirone, are 5-HT1A receptor partial agonists (Matheson et al., 1994; Newman-Tancredi and Kleven, 2011). Several studies have explored whether augmentation therapy with buspirone or tandospirone improves psychotic symptoms and cognitive function in patients with schizophrenia (Goff et al., 1991; Sirota et al., 2001; Sumiyoshi et al., 2001, 2007; Piškulić et al., 2009; Ghaleiha et al., 2010; Sheikhmoonesi et al., 2015; Boustani et al., 2018; Wang et al., 2019). In a meta-analysis reported in 2013, the addition of buspirone or tandospirone to ongoing treatment with antipsychotic drugs was found to improve overall psychopathology, especially positive but not negative symptoms (Kishi et al., 2013). Since then, additional findings have accumulated from relevant studies (Sheikhmoonesi et al., 2015; Boustani et al., 2018; Wang et al., 2019), including 1 study with a relatively large number of participants (Wang et al., 2019). Thus, it would now seem both timely and worthwhile to provide an up-to-date evaluation on the efficacy of the adjunctive use of 5-HT1A partial agonists in the treatment of schizophrenia.
Targeting 5-HT1A receptors may also be relevant to cognitive enhancement in schizophrenia. Accordingly, several clinical trials have been conducted to determine whether add-on therapy with buspirone or tandospirone can improve cognitive function (Sumiyoshi et al., 2001, 2007; Piškulić et al., 2009; Wang et al., 2019), including studies reporting negative findings (Piškulić et al., 2009). In this context, undertaking a meta-analysis can be extremely useful by increasing the statistical power for group comparisons (Cohn and Becker, 2003). Indeed, by combining these studies and conducting a meta-analysis, it will help determine whether augmentation therapy with 5-HT1A partial agonists in patients treated with antipsychotic drugs can become a therapeutic option to alleviate cognitive impairments in schizophrenia.
Therefore, the goal of this study was to examine the efficacy of 5-HT1A receptor partial agonists of the azapirone class as an add-on therapy in the treatment of schizophrenia by performing a systematic review and meta-analysis of randomized controlled trials (RCTs). The main aim was to evaluate the effect of adjunct therapy on overall psychopathology, as well as positive and negative symptoms, in view of an increasing interest in this issue (Zheng et al., 2018). There was also a focus on attention/processing speed, a central construct of cognitive function in schizophrenia that has been suggested to reflect a composite of other cognitive domains (Reichenberg, 2010). Specifically, the Digit Symbol Substitution Test (DSST) (Jaeger 2018), a representative measure of attention/processing speed, has been used across studies with buspirone (Sumiyoshi et al., 2007; Wang et al., 2019). Treatment with antipsychotic drugs is common in patients with schizophrenia or schizoaffective disorder, and these drugs ameliorate the psychotic symptoms of both disorders (Keepers et al., 2020). Therefore, studies focusing on either of these schizophrenia-spectrum disorders were included in the present meta-analysis. We hypothesized that the addition of buspirone or tandospirone to ongoing treatment with antipsychotic drugs would be beneficial for ameliorating psychotic symptoms and improving attention/processing speed in patients with schizophrenia.
METHODS
Inclusion/Exclusion Criteria and Search Strategies
We conducted the current meta-analysis based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The inclusion criteria were as follows: (1) RCTs, (2) human studies, (3) studies that targeted patients with schizophrenia or schizoaffective disorder, (4) studies that evaluated the effect of psychopathology and/or attention/processing speed, (5) studies that provided sufficient data to evaluate effect sizes, (6) studies written in English, (7) and studies with a duration of the drug administration ≥4 weeks. The following exclusion criteria were applied: (1) any other study types, (2) non-human studies, (3) studies that targeted patients with psychopathologies other than schizophrenia or schizoaffective disorder (e.g., bipolar disorder), (4) studies that did not evaluate the effect of psychopathology and/or attention/processing speed, (5) studies that provided insufficient data to evaluate effect sizes, (6) studies not written in English, and (7) studies with a duration of drug administration <4 weeks.
R.Y. and A.W. independently conducted literature searches using PubMed, the Cochrane Library, and PsycINFO from 1987 until February 25, 2022, using the following keywords: “alnespirone” OR” binospirone” OR “buspirone” OR “enilospirone” OR “eptapirone” OR “gepirone” OR “ipsapirone” OR “revospirone” OR “tandospirone” OR “zalospirone” AND “schizophrenia”. Additional studies were obtained by scanning the reference lists of the included studies and previous reviews. T.S. approved the final list of included studies.
Data Extraction and Quality Assessment
The information for each study was independently extracted by R.Y. and A.W. with coding discrepancies resolved by T.S. When the data were not fully described in the published article, the corresponding authors were contacted and asked to provide additional information. If there was no response to our queries, we tried to obtain the necessary information by measuring the length of graphs showing non-tabulated results. If none of these methods proved feasible, then the studies were excluded from the analysis. The outcome measures were classified into general psychopathology, positive symptoms, negative symptoms, and attention/processing speed. The Cochrane risk of bias tool was used to evaluate the methodological quality of each RCT (Higgins et al., 2011).
R.Y. and A.W. independently assessed the following characteristics of each trial: (1) random sequence generation, (2) blinding of participants and personnel, (3) blinding of outcome assessment, (4) incomplete outcome data, (5) selective reporting, and (6) other potential sources of bias. The assessment was conducted by evaluating what was reported in the selected articles and accessing and evaluating the study protocols where available. If necessary, any disagreements were thereafter resolved by T.S.
Statistical Analysis
We based the analyses on intent-to-treat or modified intent-to-treat data (i.e., at least 1 dose or at least 1 follow-up assessment); no data from observed cases analysis were included. Statistical analyses were performed using Review Manager 5.3 for Windows. The effect size was calculated based on the difference in the change of baseline scores between the experimental vs control conditions. When no data on the mean change from the baseline were available, we calculated the mean change and SD based on the assumption that the correlation between the scores at follow-up and those at the baseline was 0.5. For continuous data, the standardized mean difference (SMD = Hedges’ g as an effect size measure) was used, considering the correction for small sample bias (Lakens, 2013). If data for 2 or more outcome measures were provided, we selected a single outcome based on the focus of the meta-analysis (Scammacca et al., 2014). We used a fixed-effects model if homogeneity (P ≥ .05) was found, and a random-effects model (DerSimonian and Laird 1986) if not (P< .05). Because a meta-analysis requires at least 2 studies in theory (Pigott 2012), meta-analyses were performed when the mean effect was evaluated in at least 2 studies. Finally, funnel plots were visually inspected to explore the possibility of publication bias.
RESULTS
Systematic Review
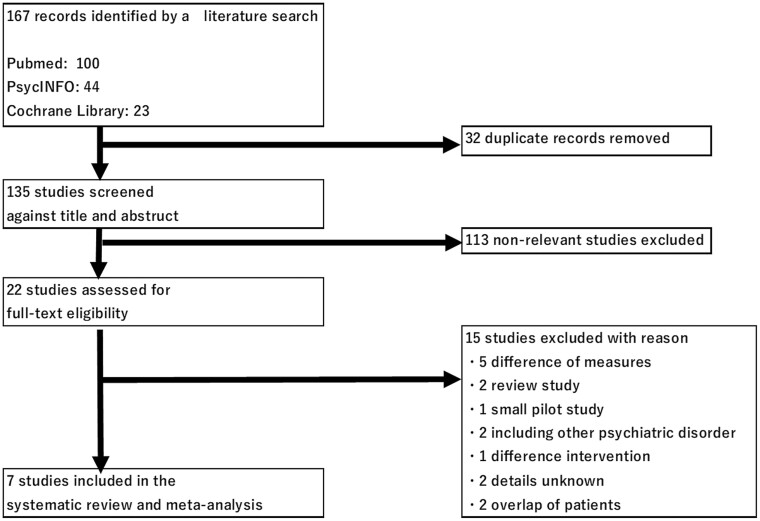
Figure 1 shows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses study selection flowchart. The initial search yielded 167 potential articles. After removing duplicates, 135 articles were screened. The 7 studies included in the systematic review encompassed 435 participants (experimental group, n = 221; control group, n = 214). Characteristics of the included studies are shown in Table 1. These studies were conducted in the United States, Australia, Japan, China, and Iran. Three of the 7 studies were on inpatients. Sample sizes ranged from 9 to 99 and 9 to 97 participants in each of the groups. Six studies included only schizophrenia patients, while 1 study (Piškulić et al., 2009) included schizophrenia (89.5%) and schizoaffective disorder (10.5%) patients. The mean age ranged from 27.8 to 46.6 years (the experimental group) and 31.8 to 47.3 years (the control group). The proportion of men ranged from 56.2% to 80.0%. The mean duration of illness ranged from 6.3 to 19.0 years. The daily dose of azapirone 5-HT1A receptor partial agonists (buspirone or tandospirone) ranged from 21.6 to 60 mg/d, while the mean duration of its use ranged from 6 to 24 weeks. AAPDs were used in most of the studies (Sumiyoshi et al., 2007; Piškulić et al., 2009; Ghaleiha et al., 2010; Boustani et al., 2018; Wang et al., 2019), specifically, risperidone (Sumiyoshi et al., 2007; Piškulić et al., 2009; Ghaleiha et al., 2010; Boustani et al., 2018; Wang et al., 2019), olanzapine (Sumiyoshi et al., 2007; Piškulić et al., 2009; Wang et al., 2019), clozapine (Sumiyoshi et al., 2007; Piškulić et al., 2009; Wang et al., 2019), quetiapine (Piškulic et al., 2009; Wang et al., 2019), ziprasidone (Sumiyoshi et al., 2007; Wang et al., 2019), amisulpride (Piškulić et al., 2009), and aripiprazole (Wang et al., 2019). Treatment with TAPDs was ongoing in 2 studies (Sumiyoshi et al., 2001; Sheikhmoonesi et al., 2015). Sumiyoshi et al. (2001) used haloperidol, sulpiride, and pimozide, while Sheikhmoonesi et al. (2015) used haloperidol, chlorpromazine, perphenazine, fluphenazine, thiothixene, and trifloprerazine. The mean symptom severity score ranged from 47.49 to 114.95, as measured by the Positive and Negative Syndrome Scale, or from 16.8 to 20.6, as measured by the Brief Psychiatric Rating Scale. Two studies (Sumiyoshi et al., 2007; Wang et al., 2019) assessed speed of processing using the DSST (Jaeger 2018).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) study selection flowchart.
Table 1.
Selected Characteristics of the Included Studies
| Study, country | Total (n) | Men/women (%) | Drug | n | Age (mean ± SD. y) | Duration (wk) | Dose (mg/d) | Out/inpatient | Duration of illness (y) | Symptom severity (BPRS or PANSS) | Concomitant txt | Attention/processing speed | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Sumiyoshi et al. (2001) Japan | 26 | 57.7/42.3 | Tan Pbo |
15 11 |
Tan: 27.8 ± 6.30, Pbo: 31.8 ± 9.40 |
6 | 30 (fixed) | Outpatient | Tan: 6.3 ± 4.30, Pbo: 7.5 ± 5.40 |
BPRS Tan: 16.8 ± 9.00 Pbo: 18.9 ± 8.70 |
Typical antipsychotic | |
| 2 | Sumiyoshi et al. (2007) USA | 73 | 56.2/43.8 | Bus Pbo |
36 37 |
Bus: 40.5 ± 11.80, Pbo: 39.7 ± 12.50 |
24 | 30 (fixed) | Outpatient | Bus: 19.0 ± 11.20 Pbo: 19.0 ± 13.50 |
BPRS Bus: 20.6 ± 8.00 Pbo: 20.0 ± 8.60 |
Atypical antipsychotic | Digital Symbol Substitution Test |
| 3 | Piškulić et al. (2009) Australia | 18 | 77.8/22.2 | Bus Pbo |
9 9 |
Bus: 43.4 ± 10.30 Pbo: 37.2 ± 13.70 |
6 | 21.6 ± 3.75 | Outpatient | Bus: 15.2 ± 10.20 Pbo: 11.7 ± 9.40 |
PANSS Bus:52.8 ± 9.30 Pbo:55.4 ± 14.90 |
Atypical antipsychotic | |
| 4 | Ghaleiha et al. (2010) Iran | 46 | 67.4/32.6 | Bus Pbo |
23 23 |
Bus: 32.86 ± 5.81 Pbo: 33.30 ± 6.86 |
8 | 60 (fixed) |
Inpatient | – | PANSS Bus:112.85 ± 8.57 Pbo:112.85 ± 8.57 |
Atypical antipsychotic | |
| 5 | Sheikhmoonesi et al. (2015) lran | 50 | 80.0/20.0 | Bus Pbo |
25 25 |
Bus:46.68 ± 9.46 Pbo:47.32 ± 10.58 |
6 | 30 (fixed) |
Inpatient | – | PANSS Bus:82.92 ± 3.07 Pbo:78.04 ± 3.21 |
Typical antipsychotic | |
| 6 | Boustani et al. (2018) lran | 40 | 65.0/35.0 | Bus Pbo |
20 20 |
Bus: 33.85 ± 8.03 Pbo: 33.60 ± 8.05 |
6 | 40 (fixed) |
Inpatient | Bus:8.80 ± 4.45 Pbo: 7.85 ± 3.24 |
PANSS Bus:113.95 ± 10.21 Pbo:114.95 ± 11.03 |
Atypical antipsychotic | |
| 7 | Wang et al. (2019) China | 196 | 70.9/29.1 | Bus Con |
99 97 |
Bus: 39.81 ± 10.11 Con: 39.02 + 9.56 |
24 | 30 (fixed) | – | Bus: 12.01 ± 8.87 Con: 11.24 + 8.28 |
PANSS Bus: 48.03 ± 12.95 Con: 47.49 ± 12.32 |
Atypical antipsychotic | Digital Symbol Substitution Test |
Abbreviations: BPRS, Brief Psychiatric Rating Scale; Bus, buspirone; Con, control; Pbo, placebo; PANSS, Positive and Negative Syndrome Scale; Tan, tandospirone; txt, treatment.
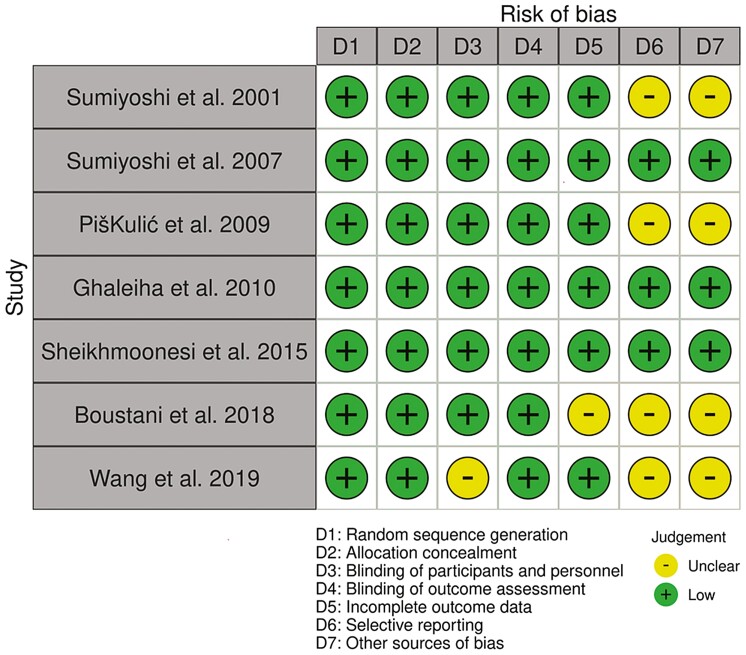
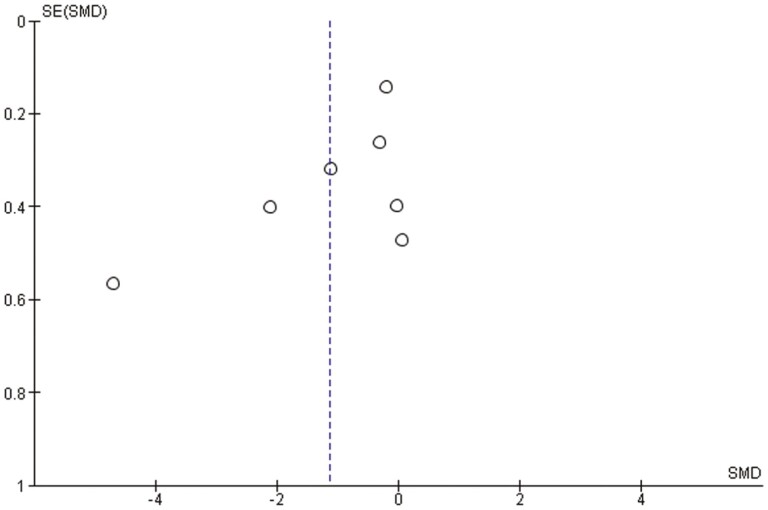
The summary for the risk of bias is shown in Figure 2. Four studies had an unclear risk of bias in relation to either the blinding of participants and personnel, incomplete outcome assessment, selective reporting, and other sources of bias. For the Cochrane risk of bias assessment, there were interrater discrepancies in random sequence generation, allocation concealment, blinding of participants and personnel, and other sources of bias. In addition, we found little indication of publication bias for the outcomes. Visual inspection of the funnel plot for overall psychopathology suggested symmetry (Figure 3).
Figure 2.
Risk of bias.
Figure 3.
Funnel plot for overall psychopathology.
Meta-Analysis
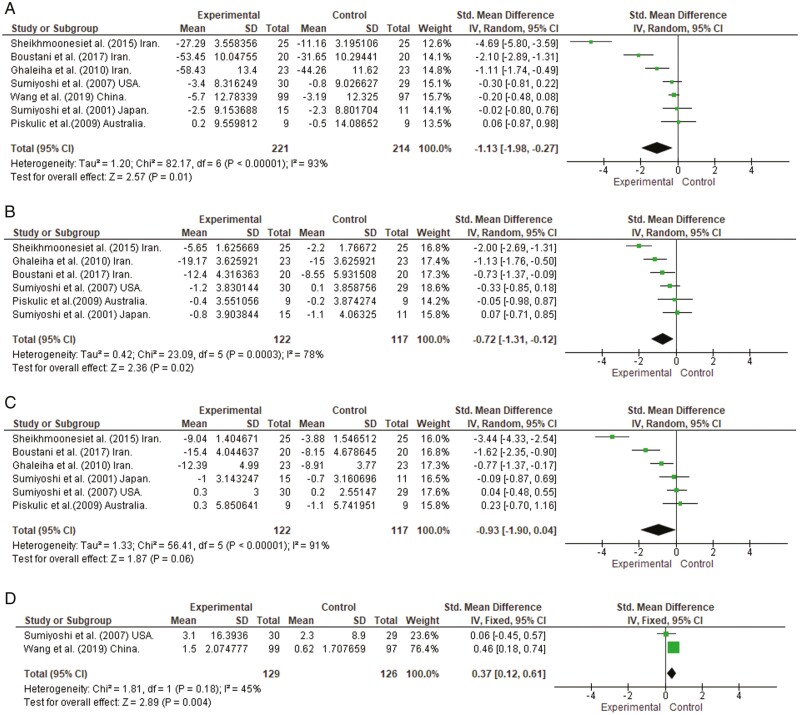
A significant effect for the addition of 5-HT1A partial agonists was observed on overall psychopathology (SMD = −1.13, 95% CI = −1.98 to −0.27, P = .01, I2 = 93%1; Figure 4A). We also performed a meta-analysis on positive and negative symptoms, respectively, in view of their examinations in 6 studies (Sumiyoshi et al., 2001, 2007; Piškulić et al., 2009; Ghaleiha et al., 2010; Sheikhmoonesi et al., 2015; Boustani et al., 2018). A significant effect was observed on positive symptoms (SMD = −0.72, 95% CI = −1.31 to −0.12, P = .02, I2 = 78%; see Figure 4B). On the other hand, the improvement in negative symptoms did not reach a statistically significant level (SMD = –0.93, 95% CI = –1.90 to 0.04, P = .06, I2 = 91%; see Figure 4C). Results from the meta-analysis on attention/processing speed in 2 studies that used the DSST (Sumiyoshi et al., 2007; Wang et al., 2019) showed a significant improvement in patients given buspirone (SMD = 0.37, 95% CI = 0.12 to 0.61, P = .004, I2 = 45%; see Figure 4D).
Figure 4.
Mean effect of 5-hydroxtrypatamine (5-HT)1A receptor partial agonists of the azapirone class as an add-on therapy on overall psychopathology (A), Positive symptoms (B), Negative symptoms (C), and Attention/processing speed (D).
DISCUSSION
This study confirmed the benefits of the adjunctive use of buspirone or tandospirone, azapirone derivatives, for potentiating treatment of psychotic symptoms as well as attention/processing speed in patients with schizophrenia. The ability of these 5-HT1A partial agonists to alleviate psychotic symptoms, observed here, is consistent with the results from a previous meta-analysis (Kishi et al., 2013) that used a smaller number of participants (n = 163 vs 435 in the current study). Moreover, the merit of the present analysis was to additionally suggest a potentially beneficial effect of the addition of 5-HT1A partial agonists to improve attention/processing speed in patients with schizophrenia.
Contrary to the results from some previous studies (Ghaleiha et al., 2010; Sheikhmoonesi et al., 2015; Boustani et al., 2018), the current meta-analysis did not find a significant effect of adjunct therapy with 5-HT1A partial agonists on negative symptoms. This may have been caused by variance in the severity of psychotic symptoms. Thus, studies with a relatively severe negative symptom level at baseline (Ghaleiha et al., 2010; Sheikhmoonesi et al., 2015; Boustani et al., 2018) reported a significant effect, while other studies in which patients had less severe symptoms (Sumiyoshi et al., 2001; Sumiyoshi et al., 2007; Piškulić et al., 2009) did not. It is thus possible that the clinical merits of the addition of 5-HT1A partial agonists is more evident in patients who are likely to benefit more from the restoration of dopaminergic activity in the cortex by means of stimulation of 5-HT1A receptors, as has been suggested (Ichikawa and Meltzer 1999; Millan 2000). It is noteworthy that some AAPDs have pronounced direct agonist effects at 5-HT1A receptors, for example, aripiprazole, brexpiprazole, cariprazine and vilazodone, whereas others have little activity at these receptors, for example, risperidone or olanzapine (although they can indirectly activate 5-HT1A receptors, as mentioned earlier). Such diversity in receptor profiles of ongoing antipsychotic drugs could influence the response of patients to adjunct treatment with azapirone 5-HT1A partial agonists.
Another important finding of the present study was the ability of augmentation therapy with 5-HT1A partial agonists to improve attention/processing speed in patients with schizophrenia. This domain of cognitive functioning plays a central role in facilitating higher cognitive operations, including perceptual processes, encoding and retrieval operations, transformation of information, and decision processes (Reichenberg, 2010). Specifically, performance on the DSST has been indicated to represent a composite of cognitive domains (Keefe et al., 2006; Jaeger, 2018). The current results also support the assertion that treatment with several antipsychotic drugs with high affinities for 5-HT1A receptors may be particularly beneficial for improving social and work outcomes that are closely linked to cognitive function (Sumiyoshi and Higuchi, 2013; Sumiyoshi et al., 2016; Sumiyoshi, 2020). Further study is warranted to determine if augmentation therapy with 5-HT1A partial agonists can also improve higher functional outcomes, such as social functioning.
The mechanisms by which the stimulation of 5-HT1A receptors ameliorate psychotic and cognitive symptoms may be related with several neural substrates (Lehmann and Ban, 1997; Sumiyoshi, 2008; Newman-Tancredi and Albert, 2012; Sumiyoshi and Higuchi, 2013). For example, in vivo microdialysis studies have reported that the ability of AAPDs to increase extracellular DA concentrations in the prefrontal cortex is mediated by 5-HT1A receptors (Li et al., 1998; Ichikawa et al., 2001; Bortolozzi et al., 2010). Moreover, chronic treatment with buspirone has been shown to enhance neurogenesis in rodents (Grabiec et al., 2009). In addition, our group previously observed that tandospirone ameliorates insufficient energy metabolism in rats treated neonatally with an N-methyl-D-aspartic acid receptor blocker, a putative animal model of cognitive impairment in schizophrenia (Uehara et al., 2014). In this context, it is of note that SEP-363856, a non–D2 receptor-binding drug that selectively acts on 5-HT1A receptors and trace amine-associated receptors (Dedic et al., 2019), has been reported to ameliorate psychotic symptoms in patients with schizophrenia (Koblan et al., 2020). These observations from basic and clinical studies may help elucidate the neural mechanisms underlying the benefits of some azapirone compounds as well as antipsychotic drugs with noticeable 5-HT1A partial agonist actions.
When interpreting the data presented here, it may be necessary to consider that buspirone and tandospirone exhibit certain binding affinities for D2 receptors, with Ki values of 1700 nM and 240 nM, respectively (Hamik et al., 1990). However, the doses of buspirone and tandospirone used in the studies included in our meta-analysis are small to moderate (i.e., 21.6-60 mg/d). Therefore, the activity of these azapirone compounds at D2 receptors should be relatively small, although we cannot entirely exclude the possibility that increased D2 antagonism (especially with buspirone) may have contributed to the improved treatment of psychotic symptoms.
Finally, the implications of the present findings for treatment with 5-HT1A partial agonists deserve some discussion. The results of this study are in line with those that have shown the ability of lurasidone, an antipsychotic drug with potent 5-HT1A partial agonist and D2 antagonist actions, to treat psychotic symptoms and improve cognitive function in patients with schizophrenia (Corponi et al., 2019; Meltzer et al., 2020). Possibly, the addition of 5-HT1A partial agonists of the azapirone class, for example, buspirone and tandospirone, to a limited dose of lurasidone may be beneficial for patients who have adverse reactions (e.g., EPS) when treated with a higher dose of the drug. This concept may be relevant to the clinical observation that clozapine, with the higher 5-HT1A/D2 ratio of binding affinity than that of lurasidone (Ishibashi et al., 2010; Meltzer and Gadaleta 2021), is associated with minimal incidence of extrapyramidal side effects (Miller, 2000). Furthermore, our findings are also in line with the antipsychotic efficacy of SEP-363856, a novel agent that exhibits 5-HT1A agonist actions but lacks a noticeable affinity for D2 receptors (Dedic et al., 2019; Koblan et al., 2020). In sum, the results of the present meta-analysis may pave the way for the development of more efficacious and safer compounds for the treatment of schizophrenia, and other psychiatric diseases as well.
Limitations
This study has several limitations that should be noted. First, the number of studies included in the meta-analysis on attention/processing speed was small. To fully validate the potential merit of 5-HT1A partial agonists for this domain of cognition, further clinical trials are needed. Second, most studies included in this meta-analysis focused on short-term (6-8 weeks) outcomes, possibly underestimating potential long-term benefits of the 5-HT1A partial agonists used in these studies. Third, this meta-analysis included a study with a larger number of participants (Wang et al., 2019) compared with the rest of the studies, which requires caution in interpreting the results. Fourth, this meta-analysis was based on a larger number of patients with buspirone as an add-on therapy compared with those with tandospirone. This may be explained, at least in part, by the fact that tandospirone is available only in Japan, unlike the case for buspirone. Finally, as this meta-analysis aimed to examine the effect of 5-HT1A receptor partial agonists of the azapirone class as an add-on therapy, other 5-HT1A partial agonists, such as lurasidone, which by itself is an antipsychotic drug, were not included.
CONCLUSIONS
The results of this meta-analysis suggest that augmentation with 5-HT1A partial agonists of the azapirone class produces beneficial effects on overall psychopathology, positive symptoms, and attention/processing speed in schizophrenia patients. Further investigations are warranted to determine if adjunctive use of 5-HT1A partial agonists or treatment with antipsychotic drugs with a high affinity for 5-HT1A receptors, or a combination thereof, would be efficacious for improving other symptom-related aspects and functional outcomes.
Acknowledgments
This study was supported by AMED Grants (21he2202007, 22dk0307114) the Japan Society for the Promotion of Science KAKENHI No. 20H03610, Intramural Research Grant (2-3, 3-1) for Neurological and Psychiatric Disorders of the National Center of Neurology and Psychiatry, and the Japan Health Research Promotion Bureau Grants (2020-B-08, 2021-B-01) to T.S.
Contributor Information
Risa Yamada, Department of Preventive Intervention for Psychiatric Disorders, National Institute of Mental Health, National Center of Neurology and Psychiatry, Kodaira, Tokyo, Japan; Department of Psychiatry, National Center Hospital of Neurology and Psychiatry, Kodaira, Tokyo, Japan.
Ayumu Wada, Department of Preventive Intervention for Psychiatric Disorders, National Institute of Mental Health, National Center of Neurology and Psychiatry, Kodaira, Tokyo, Japan; Department of Psychiatry, National Center Hospital of Neurology and Psychiatry, Kodaira, Tokyo, Japan; Department of Brain Bioregulatory Science, The Jikei University School of Medicine, Minato-ku, Tokyo, Japan.
Andrew Stickley, Department of Preventive Intervention for Psychiatric Disorders, National Institute of Mental Health, National Center of Neurology and Psychiatry, Kodaira, Tokyo, Japan.
Yuma Yokoi, Department of Educational Promotion, Clinical Research and Education Promotion Division, National Center of Neurology and Psychiatry, National Center Hospital, Kodaira, Tokyo, Japan.
Tomiki Sumiyoshi, Department of Preventive Intervention for Psychiatric Disorders, National Institute of Mental Health, National Center of Neurology and Psychiatry, Kodaira, Tokyo, Japan; Department of Psychiatry, National Center Hospital of Neurology and Psychiatry, Kodaira, Tokyo, Japan; Department of Brain Bioregulatory Science, The Jikei University School of Medicine, Minato-ku, Tokyo, Japan.
Author Contributions
R.Y. conducted the statistical analyses, reviewed the literature, and wrote the first and subsequent drafts of the manuscript. T.S. and A.W. critically reviewed these drafts. R.Y., A.W., and T.S. developed the study concept and hypothesis, managed the data collection, contributed to the interpretation of the results, and assisted in writing the manuscript. Y.Y. and A.S. contributed to the interpretation of the results, revised drafts of the manuscript, and provided feedback with particular expertise. All authors contributed to and have approved the final version of the manuscript.
Interest Statement
The authors declare no conflict of interest directly related to the current study.
References
- Araki T, Yamasue H, Sumiyoshi T, Kuwabara H, Suga M, Iwanami A, Kato N, Kasai K (2006) Perospirone in the treatment of schizophrenia: effect on verbal memory organization. Prog Neuropsychopharmacol Biol Psychiatry 30:204–208. [DOI] [PubMed] [Google Scholar]
- Bortolozzi A, Masana M, Díaz-Mataix L, Cortés R, Scorza MC, Gingrich JA, Toth M, Artigas F (2010) Dopamine release induced by atypical antipsychotics in prefrontal cortex requires 5-HT1A receptors but not 5-HT2A receptors. Int J Neuropsychopharmacol 13:1299–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boustani H, Pakseresht S, Haghdoust M-R, Malekpour N (2018) The effect of adding buspirone to atypical antipsychotics in treating the negative symptoms of schizophrenic patients. Minerva Psichiatrica 59:22–28. [Google Scholar]
- Burnet PW, Eastwood SL, Harrison PJ (1996) 5-HT1A and 5-HT2A receptor mRNAs and binding site densities are differentially altered in schizophrenia. Neuropsychopharmacology 15:442–455. [DOI] [PubMed] [Google Scholar]
- Burnet PW, Eastwood SL, Harrison PJ (1997) [3H]WAY-100635 for 5-HT1A receptor autoradiography in human brain: a comparison with [3H]8-OH-DPAT and demonstration of increased binding in the frontal cortex in schizophrenia. Neurochem Int 30:565–574. [DOI] [PubMed] [Google Scholar]
- Cohn LD, Becker BJ (2003) How meta-analysis increases statistical power. Psychol Methods 8:243–253. [DOI] [PubMed] [Google Scholar]
- Corponi F, Fabbri C, Bitter I, Montgomery S, Vieta E, Kasper S, Pallanti S, Serretti A (2019) Novel antipsychotics specificity profile: a clinically oriented review of lurasidone, brexpiprazole, cariprazine and lumateperone. Eur Neuropsychopharmacology 29:971–985. [DOI] [PubMed] [Google Scholar]
- Crow TJ (1982) Neurohumoural and structural changes in schizophrenia: two dimensions of pathology. Prog Brain Res 55:407–417. [DOI] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M (1991) Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry 148:1474–1486. [DOI] [PubMed] [Google Scholar]
- Dedic N, Jones PG, Hopkins SC, Lew R, Shao L, Campbell JE, Spear KL, Large TH, Campbell UC, Hanania T, Leahy E, Koblan KS (2019) SEP-363856, a novel psychotropic agent with a unique, non-D2 receptor mechanism of action. J Pharmacol Exp Ther 371:1–14. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188. [DOI] [PubMed] [Google Scholar]
- Deutch AY (1992) The regulation of subcortical dopamine systems by the prefrontal cortex: interactions of central dopamine systems and the pathogenesis of schizophrenia. J Neural Transm Suppl 36:61–89. [DOI] [PubMed] [Google Scholar]
- Galderisi S, et al. ; Italian Network for Research on Psychoses (2020) The interplay among psychopathology, personal resources, context-related factors and real-life functioning in schizophrenia: stability in relationships after 4 years and differences in network structure between recovered and non-recovered patients. World Psychiatry 19:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gener T, Tauste Campo A, Alemany-González M, Nebot P, Delgado-Sallent C, Chanovas J, Puig MV (2019) Serotonin 5-HT1A, 5-HT2A and dopamine D2 receptors strongly influence prefronto-hippocampal neural networks in alert mice: contribution to the actions of risperidone. Neuropharmacology 158:107743. [DOI] [PubMed] [Google Scholar]
- Ghaleiha A, Noorbala AA, Farnaghi F, Hajiazim M, Akhondzadeh S (2010) A double-blind, randomized, and placebo-controlled trial of buspirone added to risperidone in patients with chronic schizophrenia. J Clin Psychopharmacol 30:678–682. [DOI] [PubMed] [Google Scholar]
- Goff DC, Midha KK, Brotman AW, McCormick S, Waites M, Amico ET (1991) An open trial of buspirone added to neuroleptics in schizophrenic patients. J Clin Psychopharmacol 11:193–197. [PubMed] [Google Scholar]
- Grabiec M, Turlejski K, Djavadian R (2009) Reduction of the number of new cells reaching olfactory bulbs impairs olfactory perception in the adult opossum. Acta Neurobiol Exp 69:168–176. [DOI] [PubMed] [Google Scholar]
- Gründer G, Hippius H, Carlsson A (2009) The “atypicality” of antipsychotics: a concept re-examined and re-defined. Nat Rev Drug Discov 8:197–202. [DOI] [PubMed] [Google Scholar]
- Hamik A, Oksenberg D, Fischette C, Peroutka SJ (1990) Analysis of tandospirone (SM-3997) interactions with neurotransmitter receptor binding sites. Biol Psychiatry 28:99–109. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Nishino N, Nakai H, Tanaka C (1991) Increase in serotonin 5-HT1A receptors in prefrontal and temporal cortices of brains from patients with chronic schizophrenia. Life Sci 48:355–363. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Gladsjo JA, Palmer BW, Kuck J, Marcotte TD, Jeste DV (2001) Stability and course of neuropsychological deficits in schizophrenia. Arch Gen Psychiatry 58:24–32. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj 343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa J, Ishii H, Bonaccorso S, Fowler WL, O’Laughlin IA, Meltzer HY (2001) 5-HT2A and D2 receptor blockade increases cortical DA release via 5-HT1A receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. J Neurochem 76:1521–1531. [DOI] [PubMed] [Google Scholar]
- Ichikawa J, Meltzer HY (1999) Relationship between dopaminergic and serotonergic neuronal activity in the frontal cortex and the action of typical and atypical antipsychotic drugs. Eur Arch Psychiatry Clin Neurosci 249:90–98. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Horisawa T, Tokuda K, Ishiyama T, Ogasa M, Tagashira R, Matsumoto K, Nishikawa H, Ueda Y, Toma S, Oki H, Tanno N, Saji I, Ito A, Ohno Y, Nakamura M (2010) Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J Pharmacol Exp Ther 334:171–181. [DOI] [PubMed] [Google Scholar]
- Jaeger J (2018) Digit symbol substitution test: the case for sensitivity over specificity in neuropsychological testing. J Clin Psychopharmacol 38:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RS, Eesley CE, Poe MP (2005) Defining a cognitive function decrement in schizophrenia. Biol Psychiatry 57:688–691. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Bilder RM, Harvey PD, Davis SM, Palmer BW, Gold JM, Meltzer HY, Green MF, Miller DD, Canive JM, Adler LW, Manschreck TC, Swartz M, Rosenheck R, Perkins DO, Walker TM, Stroup TS, McEvoy JP, Lieberman JA (2006) Baseline neurocognitive deficits in the CATIE schizophrenia trial. Neuropsychopharmacology 31:2033–2046. [DOI] [PubMed] [Google Scholar]
- Keepers GA, Fochtmann LJ, Anzia JM, Benjamin S, Lyness JM, Mojtabai R, Servis M, Walaszek A, Buckley P, Lenzenweger MF, Young AS, Degenhardt A, Hong SH; (Systematic Review) (2020) The American Psychiatric Association Practice Guideline for the treatment of patients with schizophrenia. Am J Psychiatry 177:868–872. [DOI] [PubMed] [Google Scholar]
- Kishi T, Meltzer HY, Iwata N (2013) Augmentation of antipsychotic drug action by azapirone 5-HT1A receptor partial agonists: a meta-analysis. Int J Neuropsychopharmacol 16:1259–1266. [DOI] [PubMed] [Google Scholar]
- Kiwanuka JN, Strauss GP, McMahon RP, Gold JM (2014) Psychological predictors of functional outcome in people with schizophrenia. Schizophr Res 157:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koblan KS, Kent J, Hopkins SC, Krystal JH, Cheng H, Goldman R, Loebel A (2020) A non–D2-receptor-binding drug for the treatment of schizophrenia. N Engl J Med 382:1497–1506. [DOI] [PubMed] [Google Scholar]
- Lakens D (2013) Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol 4:863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le François B, Czesak M, Steubl D, Albert PR (2008) Transcriptional regulation at a HTR1A polymorphism associated with mental illness. Neuropharmacology 55:977–985. [DOI] [PubMed] [Google Scholar]
- Lehmann HE, Ban TA (1997) The history of the psychopharmacology of schizophrenia. Can J Psychiatry 42:152–162. [DOI] [PubMed] [Google Scholar]
- Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM (2009) Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet 373:31–41. [DOI] [PubMed] [Google Scholar]
- Li XM, Perry KW, Wong DT, Bymaster FP (1998) Olanzapine increases in vivo dopamine and norepinephrine release in rat prefrontal cortex, nucleus accumbens and striatum. Psychopharmacology 136:153–161. [DOI] [PubMed] [Google Scholar]
- Masana M, Bortolozzi A, Artigas F (2011) Selective enhancement of mesocortical dopaminergic transmission by noradrenergic drugs: therapeutic opportunities in schizophrenia. Int J Neuropsychopharmacol 14:53–68. [DOI] [PubMed] [Google Scholar]
- Matheson GK, Pfeifer DM, Weiberg MB, Michel C (1994) The effects of azapirones on serotonin1A neurons of the dorsal raphe. Gen Pharmacol 25:675–683. [DOI] [PubMed] [Google Scholar]
- McGrath J, Saha S, Chant D, Welham J (2008) Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev 30:67–76. [DOI] [PubMed] [Google Scholar]
- Meltzer HY (2013) Update on typical and atypical antipsychotic drugs. Annu Rev Med 64:393–406. [DOI] [PubMed] [Google Scholar]
- Meltzer HY (2017) New trends in the treatment of schizophrenia. CNS Neurol Disord Drug Targets 16:900–906. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Gadaleta E (2021) Contrasting typical and atypical antipsychotic drugs. Focus (Am Psychiatr Publ) 19:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, Li Z, Kaneda Y, Ichikawa J (2003) Serotonin receptors: their key role in drugs to treat schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 27:1159–1172. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Massey BW (2011) The role of serotonin receptors in the action of atypical antipsychotic drugs. Curr Opin Pharmacol 11:59–67. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Massey BW, Horiguchi M (2012) Serotonin receptors as targets for drugs useful to treat psychosis and cognitive impairment in schizophrenia. Curr Pharm Biotechnol 13:1572–1586. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Share DB, Jayathilake K, Salomon RM, Lee MA (2020) Lurasidone improves psychopathology and cognition in treatment-resistant schizophrenia. J Clin Psychopharmacol 40:240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, Sumiyoshi T (2008) Does stimulation of 5-HT1A receptors improve cognition in schizophrenia? Behav Brain Res 195:98–102. [DOI] [PubMed] [Google Scholar]
- Millan MJ (2000) Improving the treatment of schizophrenia: focus on serotonin 5-HT1A receptors. J Pharmacol Exp Ther 295:853–861. [PubMed] [Google Scholar]
- Miller DD (2000) Review and management of clozapine side effects. J Clin Psychiatry 61:14–17; discussion 18–19. [PubMed] [Google Scholar]
- Newman-Tancredi A, Albert PR (2012) Gene polymorphism at serotonin 5-HT1A receptors: moving towards personalized medicine for psychosis and mood deficits. In: Schizophrenia research: recent advances mental illnesses and treatments (Sumiyoshi T, ed), pp 337–358. New York: Nova Publishers. [Google Scholar]
- Newman-Tancredi A, Kleven MS (2011) Comparative pharmacology of antipsychotics possessing combined dopamine D2 and serotonin 5-HT1A receptor properties. Psychopharmacology 216:451–473. [DOI] [PubMed] [Google Scholar]
- Pigott T (2012) Advances in meta-analysis. Berlin: Springer. [Google Scholar]
- Piškulić D, Olver JS, Maruff P, Norman TR (2009) Treatment of cognitive dysfunction in chronic schizophrenia by augmentation of atypical antipsychotics with buspirone, a partial 5-HT1A receptor agonist. Hum Psychopharmacol 24:437–446. [DOI] [PubMed] [Google Scholar]
- Reichenberg A (2010) The assessment of neuropsychological functioning in schizophrenia. Dialogues Clin Neurosci 12:383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammacca N, Roberts G, Stuebing KK (2014) Meta-analysis with complex research designs: dealing with dependence from multiple measures and multiple group comparisons. Rev Educ Res 84:328–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz SK, Andreasen NC (1999) Schizophrenia. Lancet 353:1425–1430. [DOI] [PubMed] [Google Scholar]
- Seeman P (2002) Atypical antipsychotics: mechanism of action. Can J Psychiatry 47:27–38. [PubMed] [Google Scholar]
- Sheikhmoonesi F, Zarghami M, Bahari Saravi SF, Khalilian A, Ala S (2015) A triple-blinded, randomized, placebo-controlled trial to examine the efficacy of buspirone added to typical antipsychotic drugs in patients with chronic schizophrenia. J Res Med Sci 20:140–145. [PMC free article] [PubMed] [Google Scholar]
- Simpson MD, Lubman DI, Slater P, Deakin JF (1996) Autoradiography with [3H]8-OH-DPAT reveals increases in 5-HT1A receptors in ventral prefrontal cortex in schizophrenia. Biol Psychiatry 39:919–928. [DOI] [PubMed] [Google Scholar]
- Sirota P, Epstein B, Benatov R, Sousnostzky M, Kindler S (2001) An open study of buspirone augmentation of neuroleptics in patients with schizophrenia. J Clin Psychopharmacol 21:454–455. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, DiCarlo JJ, Zhang Y, Thompson P, Meltzer HY (1993) Characterization of typical and atypical antipsychotic drugs based on in vivo occupancy of serotonin2 and dopamine2 receptors. J Pharmacol Exp Ther 266:1374–1384. [PubMed] [Google Scholar]
- Sumiyoshi T (2008) Possible dose-side effect relationship of antipsychotic drugs: relevance to cognitive function in schizophrenia. Expert Rev Clin Pharmacol 1:791–802. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T (2020) Cognitive enhancement in schizophrenia by buspirone: role of serotonin1A receptor agonism. Schizophr Res 215:455–456. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Higuchi Y (2013) Facilitative effect of serotonin(1A) receptor agonists on cognition in patients with schizophrenia. Curr Med Chem 20:357–362. [PubMed] [Google Scholar]
- Sumiyoshi T, Kido H, Sakamoto H, Urasaki K, Suzuki K, Yamaguchi N, Mori H, Shiba K, Yokogawa K, Ichimura F (1993) Time course of dopamine-D2 and serotonin-5-HT2 receptor occupancy rates by haloperidol and clozapine in vivo. Jpn J Psychiatry Neurol 47:131–137. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Kido H, Sakamoto H, Urasaki K, Suzuki K, Yamaguchi N, Mori H, Shiba K (1994a) Time course of dopamine1,2 and serotonin2 receptor binding of antipsychotics in vivo. Pharmacol Biochem Behav 49:165–169. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Jayathilake K, Meltzer HY (2003a) A comparison of two doses of melperone, an atypical antipsychotic drug, in the treatment of schizophrenia. Schizophr Res 62:65–72. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Jayathilake K, Meltzer HY (2003b) The effect of melperone, an atypical antipsychotic drug, on cognitive function in schizophrenia. Schizophr Res 59:7–16. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Kido H, Sakamoto H, Urasaki K, Suzuki K, Yamaguchi N, Mori H, Shiba K, Yokogawa K (1994b) In vivo dopamine-D2 and serotonin-5-HT2 receptor binding study of risperidone and haloperidol. Pharmacol Biochem Behav 47:553–557. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Matsui M, Nohara S, Yamashita I, Kurachi M, Sumiyoshi C, Jayathilake K, Meltzer HY (2001) Enhancement of cognitive performance in schizophrenia by addition of tandospirone to neuroleptic treatment. Am J Psychiatry 158:1722–1725. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Park S, Jayathilake K, Roy A, Ertugrul A, Meltzer HY (2007) Effect of buspirone, a serotonin1A partial agonist, on cognitive function in schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res 95:158–168. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Stockmeier CA, Overholser JC, Dilley GE, Meltzer HY (1996) Serotonin1A receptors are increased in postmortem prefrontal cortex in schizophrenia. Brain Res 708:209–214. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi C, Sumiyoshi T, Roy A, Jayathilake K, Meltzer HY (2006) Atypical antipsychotic drugs and organization of long-term semantic memory: multidimensional scaling and cluster analyses of category fluency performance in schizophrenia. Int J Neuropsychopharmacol 9:677–683. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Suzuki K, Sakamoto H, Yamaguchi N, Mori H, Shiba K, Yokogawa K (1995) Atypicality of several antipsychotics on the basis of in vivo dopamine-D2 and serotonin-5HT2 receptor occupancy. Neuropsychopharmacology 12:57–64. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, et al. (2016) Cognitive insight and functional outcome in schizophrenia; a multi-center collaborative study with the specific level of functioning scale-Japanese version. Schizophr Res Cogn 6:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauscher J, Kapur S, Verhoeff NP, Hussey DF, Daskalakis ZJ, Tauscher-Wisniewski S, Wilson AA, Houle S, Kasper S, Zipursky RB (2002) Brain serotonin 5-HT1A receptor binding in schizophrenia measured by positron emission tomography and [11C]WAY-100635. Arch Gen Psychiatry 59:514–520. [DOI] [PubMed] [Google Scholar]
- Uehara T, Matsuoka T, Sumiyoshi T (2014) Tandospirone, a 5-HT1A partial agonist, ameliorates aberrant lactate production in the prefrontal cortex of rats exposed to blockade of N-methy-D-aspartate receptors; Toward the therapeutics of cognitive impairment of schizophrenia. Front Behav Neurosci 8:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yang X, Song X, Zhao L, Wei J, Wang J, Tian H, Zheng C, Wei M, Wang Q, Guo W, Deng W, Li T, Ma X (2019) Co-treatment of buspirone with atypical antipsychotic drugs (AAPDs) improved neurocognitive function in chronic schizophrenia. Schizophr Res 209:135–140. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Gallhofer B (1997) Cognitive function in schizophrenia. Int Clin Psychopharmacol 12:S29–S36. [DOI] [PubMed] [Google Scholar]
- Zheng W, Li XH, Cai DB, Yang XH, Ungvari GS, Ng CH, Ning YP, Xiang YT (2018) Adjunctive azapirone for schizophrenia: a meta-analysis of randomized, double-blind, placebo-controlled trials. Eur Neuropsychopharmacol 28:149–158. [DOI] [PubMed] [Google Scholar]