Abstract
Dentate granule cells (GCs) have been characterized as unilaterally projecting neurons within each hippocampus. Here, we describe a unique class, the commissural GCs, which atypically project to the contralateral hippocampus in mice. Although commissural GCs are rare in the healthy brain, their number and contralateral axon density rapidly increase in a rodent model of temporal lobe epilepsies. In this model, commissural GC axon growth appears together with the well-studied hippocampal mossy fiber sprouting and may be important for the pathomechanisms of epilepsy. Our results augment the current view on hippocampal GC diversity and demonstrate powerful activation of a commissural wiring program in the adult brain.
Keywords: hippocampal granule cell, contralateral projection, commissural axon, sprouting, circuit formation, adult brain
Significance Statement.
Neuronal connections between the two hippocampi are composed mainly of axons of mossy cells and CA3 pyramidal cells and to lesser degree axons of CA1 pyramidal cells and different interneurons. Here, we show that dentate granule cells also project to the contralateral hippocampus and that the density of commissural granule cell axons rapidly increases in a rodent model of temporal lobe epilepsy. Our results reveal a striking capacity for commissural axon growth in the adult brain and open up multiple questions for future research regarding possible mechanisms of adult-brain circuit formation and epilepsy.
Introduction
In contrast to during brain development, circuit formation in the adult brain is generally thought to be absent. However, research on neurological disorders, adult-born, grafted, and reprogrammed neurons, and innate behaviors has shown that the adult brain retains a considerable capacity for axon growth and circuit formation (1). Understanding the underlying mechanisms or identifying new forms of circuit formation in adults would facilitate insights into the organization of brain circuits in health and diseases.
The hippocampal dentate gyrus is one brain region where circuit formation in adults can be routinely observed, either as circuit integration of adult-born immature granule cells (GCs) (2) or epilepsy-associated local mossy fiber sprouting by mature GCs (3). The circuit formed by adult-born GCs is virtually identical to that formed by GCs during development: GCs project their axons, the mossy fibers, through the hilus to ipsilateral CA3 area, and form synapses on different glutamatergic and GABAergic cell types. By contrast, during mossy fiber sprouting, new GC axons mostly grow into the molecular layer of dentate gyrus and create a local feedback circuit. Thus, target specification is different between adult-born and sprouting adult mossy fibers. Adult-born GCs likely utilize similar cues to those during development, whereas target specification during sprouting remains comparably less understood (4).
In this study, we followed up on an observation we made while studying mossy fibers in the intrahippocampal kainic acid (KA) injection model of temporal lobe epilepsy (4, 5). Specifically, signatures of sprouting became apparent in the associational/commissural pathway. Such a locale would be unexpected for sprouted (as well as for developmentally established and adult-born) GC axons and represent a previously undisclosed axon targeting. Meanwhile, it remained possible that these axons originated from cell types other than GCs. Using the Rbp4-Cre transgenic mouse line with a genetic access restricted to GCs in the hippocampus, we tested the hypothesis that sprouting axons in the associational/commissural pathway originated from GCs. We show that some GCs atypically project to the contralateral hippocampus in the healthy mouse brain and that the number of such commissural GCs as well as their contralateral axon density rapidly increases in a rodent model of temporal lobe epilepsy.
Results
Commissural GC projections in saline- and KA-injected mouse brain
To study control and sprouting mossy fibers, we performed unilateral intrahippocampal saline and KA (known to induce mossy fiber sprouting within 1–2 weeks) microinjections, respectively, in mice. Hereafter, we refer to these specific procedures as saline or KA, for short (note that although bilateral hippocampal or intraperitoneal KA injections can be also used to induce mossy fiber sprouting, as shown by many studies, the term KA should be unambiguous in this current study). To enable GC-specific labeling, we used the Rbp4-Cre transgenic mouse line. In published reports, this line appeared to selectively express Cre in the dentate gyrus, as opposed to other hippocampal areas (6, Fig.2; 7, Fig.S7E). To verify this, we crossed Rbp4-Cre with the Ai14 reporter line and saw that Cre expression was highly specific to GCs, with ∼30% of GCs expressing Cre (Fig. S1). To begin, we coinjected AAV.cDIO-EGFP (EGFP is to be expressed in GCs in a Cre-dependent manner) and KA (70 nl, 5 mM) or saline (equal volume to KA; used as control) into the left ventral dentate gyrus (ipsilateral side). Two weeks later, we prepared 50-µm-thick whole-brain serial sections and looked for EGFP+ axons in the ipsilateral (left) and contralateral (right) hemispheres.
Fig. 2.
Retrograde labeling identifies commissural GCs. A) Experimental design (also see main text). B) Confocal images show retrogradely labeled, EGFP+ cells in GC layer (GCL) in the left hippocampus after ipsilateral saline or KA injection. Note the (i) presence of retrogradely labeled mossy cells (which also project to the contralateral hippocampus) and their axons in the inner molecular layer after saline injection and (ii) lack of mossy cells and axons and GCL dispersion (these are known effects of KA) after KA injection; retrogradely labeled CA3 pyramidal cells (which also project to the contralateral hippocampus) are visible in both images. C and D) Confocal image and morphological reconstruction of retrogradely labeled commissural GCs after saline (cell S1 is also shown in B, left) and KA injection (cell KA1–8 are also shown in B, right), respectively. E) Plot shows the number of retrogradely labeled commissural GCs in the ipsilateral (with respect to saline or KA injection) left hippocampus. Each data point represents the total number of commissural GCs detected in one animal. Data represent mean ± SEM (Mann–Whitney test). F) Plot shows the septo-temporal distribution of retrogradely labeled commissural GCs after ipsilateral saline and KA injection. For averaging, individual distributions were aligned to the ipsilateral (saline and KA) injection sites.
We made two observations. First, we found EGFP+ axons entering the ipsilateral associational/commissural pathway and appearing on the contralateral side after both saline and KA (Fig. 1A). In the contralateral side, the axons appeared to terminate in the hippocampal CA1-CA3 and subiculum areas (Fig. 1B). These findings were unexpected because contralateral GC projections have not been described before in naïve or epileptic brain. Second, we found visibly more EGFP+ commissural axons after KA. To quantify this, we reconstructed all EGFP+ axons on the contralateral side starting from the midline, which revealed significantly more EGFP+ axons after KA (total axon length, saline: 2.74 ± 0.45 cm, n = 5 mice; KA: 11.78 ± 2.36 cm, n = 5 mice; data represent mean ± SEM; P = 0.007, Mann–Whitney test) (Fig. 1C). The increased axon length after KA suggested robust growth of new commissural GC fibers within 2 weeks after injection. To further investigate this phenotype, we also considered an alternative scenario whereby a potentially increased EGFP transfection efficacy after KA (e.g. due to cellular changes) labeled more GCs, including already existing commissural GCs. For this, we calculated the ratio of EGFP+ versus DAPI+ labeled cells in the transfected area of the ipsilateral GC layer after saline and KA; however, rather than increased, EGFP transfection efficacy was decreased after KA (EGFP+/DAPI+ cells, saline: 34 ± 0.48%, n = 5 mice and KA: 20 ± 2.3%, n = 5 mice), suggesting that the increased contralateral axon length after KA appeared despite less ipsilateral neurons was labeled.
Fig. 1.
Commissural GC projections in the contralateral hippocampus. A) Tiled confocal image shows EGFP+ GCs at the ipsilateral injection site (left hippocampus), as well as EGFP+ axons entering the commissural/associational pathway (a), before entering the contralateral hippocampus (b), and in the contralateral hippocampal CA3 (c) and CA1 (d) areas. The bottom left inset shows the experimental design (also see main text). DG, dentate gyrus. B) Reconstruction of contralateral EGFP+ axons from saline- and KA-injected mice. Each image shows axons reconstructed from 20 to 26 consecutive, 50-µm-thick sections from one animal, labeled with sample ID and total axon length. C) Quantification of total contralateral EGFP+ axon length. Each data point represents the total axon length traced from one animal, as in B). Data represent mean ± SEM (Mann–Whitney test).
Retrograde labeling identifies commissural GCs
Next, we aimed to identify GCs that project to the contralateral side. The previous experiment did not allow separate visualization of such cells, because the somato-dendritic domain of all Cre-expressing GCs was EGFP labeled independent of their axonal targeting (see ipsilateral side in Fig. 1A). Thus, to specifically label contralaterally projecting cells, we injected retroAAV.EGFP into the right (contralateral) ventral hippocampus and saline or KA together with AAV.cDIO-mCherry (mCherry is to be expressed in GCs in a Cre-dependent manner to visualize the injection site) into the left (ipsilateral) hippocampus with symmetrical injection coordinates (Fig. 2A).
Two weeks later, we prepared serial sections from the left (ipsilateral) hippocampus and looked for retrogradely labeled EGFP+ cells. A caveat of this approach is that other contralaterally projecting neurons, such as mossy and CA3 pyramidal cells, were also retrogradely labeled; nonetheless, we could alleviate this issue by restricting our analysis into the GC layer. Again, we made two observations. First, we found EGFP+ cells after both saline and KA (Fig. 2B), whose localization and dendritic morphology resembled typical GCs (Fig. 2C and D). Second, consistent with the increased length of commissural projections after KA, we detected significantly more retrogradely labeled GC cells after KA (120.8 ± 26.2 cells, n = 9 mice), approximately five to six times more, than after saline (22.1 ± 6.1 cells, n = 8 mice) (P = 0.0001, Mann–Whitney test) (Fig. 2E). As expected from a KA-induced effect, most EGFP+ GCs appeared around the injection site (Fig. 2F). However, unexpectedly, a similar pattern was apparent after saline injection as well, raising the possibility that the injection procedure itself contributed to the detection of commissural GCs. Thus, as a control experiment, we injected retroAAV.EGFP contralaterally without any ipsilateral injection in wild-type mice, in which EGFP+ GCs were still visible in similar number and distribution as after ipsilateral saline injection (Fig. S2). Note that meanwhile these experiments identified commissural GCs projecting from the left to the right hippocampus, commissural GCs projecting from the right to the left hippocampus were also detectable in similar number and distribution (Fig. S3).
Together, these results provided support for the notion that some GCs atypically project to the contralateral hippocampus in naïve mice and established that the number of such commissural GCs significantly increases after KA. In addition, the distribution of commissural GCs in ipsilateral saline- and noninjected mice suggested that the contralateral axons of commissural GCs are organized in a laminar fashion; i.e. they projected to the same septo-temporal level where their somata were located.
Finally, in addition to the KA model, we looked for retrogradely labeled GCs in the Id2 overexpression (OE) model of local (ipsilateral) mossy fiber sprouting (5). If in addition to local GC sprouting Id2 OE induced commissural GC growth, then the number of retrogradely labeled GCs should increase, similar to the KA model; however, the number of retrogradely labeled GCs did not increase in the Id2 OE model (3.2 ± 2.6 cells, n = 4 mice) compared to saline-injected controls (12.5 ± 0.5 cells, n = 2 mice), suggesting that Id2 OE alone does not have the capacity to induce commissural GC axon growth.
Anterograde labeling identifies contralateral targets of commissural GCs
To identify neurons targeted by commissural GCs, we used a two-component trans-synaptic labeling approach. The first component, AAV.cDIO-WGA_Flpe, was injected into the left (ipsilateral) dentate gyrus (WGA_Flpe is to be expressed in GCs in a Cre-dependent manner and transported to target cells trans-synaptically). The second component, AAV.fDIO-mRuby, was injected into the right (contralateral) hippocampus (mRuby is to be expressed in a WGA_Flpe-dependent manner). In theory, only those cells should express mRuby in the right hippocampus, which received WGA_Flpe from commissural GCs in the left hippocampus. As a limitation of this approach, multicomponent expression systems may display unwanted transcription in the first, second, or both components, resulting in false-positive labeling. To mitigate this issue, we first tested our system in wild-type animals, lacking Cre. In the contralateral dentate, CA3-CA2, and CA1-subiculum areas, we found an average of 92 ± 6.2, 55 ± 2.9, and 58 ± 2.5 mRuby+ cells, respectively, in 50-µm-thick sections (n = 5 mice), which we then considered as baselines (Fig. S4).
Next, we performed the same experiment but now in Rbp4-Cre mice and together with ipsilateral saline or KA injection (Fig. 3A). In both conditions, this experiment revealed trans-synaptically labeled mRuby+ cells in the contralateral CA3-CA1 and subiculum areas (Fig. 3B), where most commissural GC axons terminated (Fig. 1B). For the accurate estimation of mRuby+ cell numbers, we subtracted the above-derived baselines from the cell counts (for nonnormalized data, see Fig. S4). The number of mRuby+ cells was significantly increased after KA, most prominently in CA1 and subiculum (saline vs. KA, n = 5 and 5 mice; dentate: 1.5 ± 3.4 vs. 16 ± 4.8 cells, CA3-CA2: 26 ± 4.9 vs. 44 ± 5.2 cells, CA1-subiculum: 18 ± 3.5 vs. 81 ± 6.5 cells, per 50-µm-thick sections) (Fig. 3C). Because mRuby+ cells resided in the principal cell layers, glutamatergic cells likely represented the major targets of commissural GCs. To examine this possibility, we immunostained sections with AMPA-type glutamate receptor subunit 2/3 (GluR2/3), Wfs1, and parvalbumin (PV) antibodies and tested the colocalization of these markers with trans-synaptically activated mRuby in CA3 pyramidal neurons (note, however, that GluR2/3 is also expressed by some GABAergic interneuron types) (8, 9), CA1 pyramidal neurons (7), and GABAergic PV interneurons (10), respectively, after KA (Fig. 3D). We found that most mRuby+ cells expressed GluR2/3+ in CA3 (saline: 269/269 cells, 3 mice, 100%; KA: 226/226 cells, 3 mice, 100%) and Wfs1 in CA1 (saline: 237/294 cells, 3 mice, 81 ± 1.4%; KA: 156/204 cells, 2 mice, 77 ± 1.4%), but not PV (saline: 0/370 cells, 3 mice, 0%; KA, 1/367 cells, 3 mice, 0.27%; intriguingly, this also suggested that PV neurons were not prone to nonspecific mRuby expression) (Fig. 3E). To further investigate the identity of targeted neurons, we filled mRuby+ cells with biocytin during electrophysiological recordings and reconstructed them afterwards. In agreement with the immunostaining, these mRuby+ cells displayed pyramidal cell morphology (Fig. 3F).
Fig. 3.
Trans-synaptic labeling identifies postsynaptic targets of commissural GCs. A) Experimental design (also see main text). B) Confocal images show mRuby+ cells in the contralateral hippocampus after ipsilateral saline or KA injection. The CA3 and CA1 areas are shown in higher magnification in both panels. SO, stratum oriens; SP, stratum pyramidale; SL, stratum lucidum; SR, stratum radiatum. C) Quantification of mRuby+ cells in contralateral hippocampal DG, CA3-CA2, and CA1-subiculum areas after ipsilateral saline or KA injection. Each point represents data from one animal. Data were normalized by subtracting mRuby+ cell counts obtained from wild-type animals [see main text; for nonnormalized data, see Fig. S4; two-way ANOVA, Farea(2, 16) = 56, P < 0.0001; Ftreatment(1, 8) = 38, P = 0.0003; Farea×treatment(2, 16) = 24, P < 0.0001; adjusted P-values (FDR) of post hoc analyses are indicated in the figure]. D) Confocal images show immunostaining for mRuby and DAPI as well as immunostaining for either GluR2/3 (left, in contralateral CA3), Wfs1 (middle, in contralateral CA1; note that Wfs1 immuno-positivity was mostly apparent in the superficial, but not deep, CA1 pyramidal cells), or PV (right, in contralateral CA3 and CA1) in 50-µm-thick sections after saline (top row) and KA injections (bottom row). Insets show magnification of the highlighted areas (the three staining methods are shown separately). E) Bar plots show the coexpression of mRuby with GluR2/3 (in CA3), or Wfs1 (in CA1), or PV (in CA3 and CA1). F) Reconstruction of trans-synaptically labeled mRuby+ neurons after saline and KA injections reveals pyramidal cell morphology. Dendrites and axons are shown in black and red or magenta, respectively. G) The upper left image shows reconstruction of anterogradely labeled EGFP+ axons in the contralateral hippocampus in a 50-µm-thick section after ipsilateral saline injection. The electron microscopy images show EGFP+ synapses (green and red arrowheads) in the CA3 (stratum oriens) and CA1 (stratum radium) area, as well as EGFP nonlabeled synapses (yellow and blue arrowheads), for comparison. In the lower right image, the asterisk labels a putative interneuron dendrite; synapses between interneuron dendrites and adjacent EGFP+ processes were not present.
Finally, we used electron microscopy to visualize synaptic contacts made by anterogradely labeled (from the left, ipsilateral dentate gyrus) EGFP+ axons in the contralateral hippocampal CA1 and CA3 areas after ipsilateral saline injection. This analysis revealed structurally intact synapses abundantly filled with synaptic vesicles and multiple release sites juxtaposing postsynaptic densities in dendritic spines (Fig. 3G), but not on interneuron dendrites, suggesting that commissural GCs established functional synapses with postsynaptic target cells. In addition, we found that EGFP+ synapses in the CA1 area were comparably larger (1–3 µm in diameter) than surrounding EGFP nonlabeled, presumed Schaffer collateral synapses (∼≤1 µm) (Figs. 3G and S5). Together, these results showed that commissural GCs targeted glutamatergic cells in the contralateral hippocampus.
Electrophysiological and morphological characterization of commissural GCs
Thus far, we made multiple observations showing more commissural GCs, contralaterally projecting GC axons, and trans-synaptically labeled contralateral hippocampal cells after KA. Consistent with one other, these observations suggested that commissural GCs represent a unique GC class. To test if additional distinctions could be recognized in the biophysical, morphological, and transcriptomic domains, we employed a multimodal cell characterization approach (5, 10–12) after retroAAV.EGFP labeling (Fig. 4A).
Fig. 4.
Electrophysiological and morphological characterization of commissural GCs. A) Experimental design (also see main text). B) Example electrophysiological traces in response to 1.5-s-long depolarizing and hyperpolarizing current injections recorded from GCs after saline or KA. C) Quantification of resting membrane potential, input resistance, and capacitance of saline–noncommissural, saline–commissural, KA–noncommissural, and KA–commissural GCs [two-way ANOVA, resting membrane potential: Ftreatment(1, 189) = 75, P < 0.0001; Fcommissural(1, 189) = 2.1, P = 0.15; Ftreatment×commissural(1, 189) = 0.017, P = 0.90; input resistance: Ftreatment(1, 189) = 19, P < 0.0001; Fcommissural(1, 189) = 0.081, P = 0.78; Ftreatment×commissural(1, 189) = 0.71, P = 0.40; capacitance: Ftreatment(1, 189) = 23, P < 0.0001; Fcommissural(1, 189) = 0.25, P = 0.62; Ftreatment×commissural(1, 189) = 0.039, P = 0.84; post hoc analyses: for all noncommissural vs. commissural comparisons, the P-values were >0.05, indicated as nonsignificant or “ns” in figure; for all noncommissural vs. noncommissural and commissural vs. commissural comparisons between saline and KA, the P-values were <0.01; each circle represents a single cell; data represent mean ± SEM]. D) Quantification of action potential firing (AP count) in response to depolarizing current injections recorded from GCs after ipsilateral saline [left panel, two-way ANOVA, Ftreatment(1, 109) = 0.077, P = 0.78; Fcurrent(11, 1,177) = 65, P < 0.0001; Ftreatment×current(11, 1,177) = 0.29, P = 0.99] or KA injection [right panel, Ftreatment(1, 79) = 0.38, P = 0.54; Fcurrent(11, 854) = 180, P < 0.0001; Ftreatment×current(11, 854) = 1.7, P = 0.073]. E) Morphological reconstruction of retrogradely labeled EGFP+ commissural GCs, which were filled with biocytin during electrophysiological recordings. Arrowheads indicate ispilaterally sprouting axons.
Two weeks after injections, we prepared 300-µm-thick brain slices and performed electrophysiological patch-clamp recordings from EGFP+ commissural and neighboring EGFP– noncommissural GCs after saline or KA; from a subset of cells, we also collected the mRNA after recordings for subsequent single-cell sequencing. We refer to the four groups as saline–noncommissural (n = 103 cells/6 mice), saline–commissural (n = 8 cells/6 mice), KA–noncommissural (n = 49 cells/12 mice), and KA–commissural (n = 33 cells/12 mice) GCs (Fig. 4B). Limited by their overall number (Fig. 2E and F) (∼3–5 cells in 300-µm-thick slices, but not all accessible with the patch pipette), the number of saline–commissural GCs remained relatively low compared to other groups.
With regard to electrophysiological properties, GCs from KA-injected mice had more depolarized resting membrane potential and higher capacitance and lower input resistance; however, independently of the treatment, there was no difference between commissural and noncommissural GCs (resting membrane potential, saline–noncommissural: −80 ± 0.8 mV; saline–commissural: −78 ± 2.4 mV; KA–noncommissural: −66 ± 0.95 mV; KA–commissural: −64 ± 1.4 mV; capacitance, saline–noncommissural: 46 ± 1.2 pF; saline–commissural: 47 ± 3.9 pF; KA–noncommissural: 59 ± 2.1 pF; KA–commissural: 61 ± 2.6 pF; input resistance, saline–noncommissural: 188 ± 6.9 MOhm; saline–commissural: 196 ± 29 MOhm; KA–noncommissural: 140 ± 5.8 MOhm; KA–commissural: 125 ± 10 MOhm) (Fig. 4C). In addition, current injection-evoked action potential firing was enhanced in GCs after KA, but not different between commissural and noncommissural GCs (Fig. 4D). Thus, while the biophysical and AP firing properties reflected KA effects, e.g. (4, 5, 13, 14), they did not reveal commissural GC-specific features.
We also analyzed the local axonal morphology of commissural GCs, which could not be visualized in our previous experiment (see Fig. 2C and D). Because KA also induces ipsilateral mossy fiber sprouting in GCs, one key question was whether commissural GCs sprouted new ipsilateral axons in addition to the contralateral ones. To answer this question, we reconstructed biocytin-filled commissural GCs after the recordings (Fig. 4E). Due to severance of long axons in brain slices, the recovery of GC axons beyond the hilus was limited. However, in four out of seven commissural GCs, we confirmed the presence of ipsilateral mossy fiber sprouting, suggesting that contralateral and ipsilateral sprouting phenotypes were not distinctive markers for the commissural and noncommissural GC populations.
Transcriptomic characterization of commissural GCs
Next, we analyzed single-cell RNAseq data from 27 saline–noncommissural, 5 saline–commissural, 36 KA–noncommissural, and 27 KA–commissural GCs 2 weeks after injections. An advantage of this time point is the insights into potentially lasting transcriptomic changes in commissural GCs whose contralateral axons have been already established (a prerequisite for retrograde labeling). However, insights into the potentially transient induction phase (most likely within 1–2 days after KA, when contralateral axons are not yet established) and molecular programs that control commissural axon growth would be limited (see Discussion).
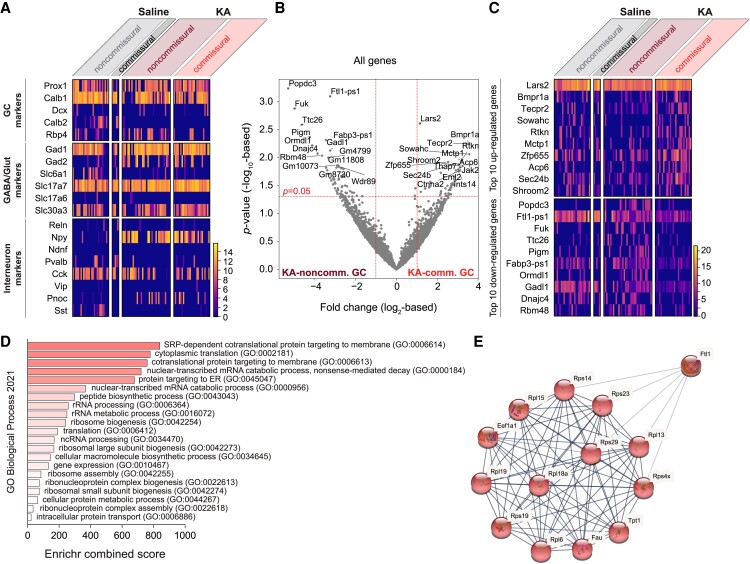
First, we examined the expression of known GC markers (Fig. 5A). Independently of commissural projections or KA treatment, cells expressed mature GC markers Calb1 and Prox1 (15, 16) but lacked immature GC markers Dcx or Calb2 (17, 18). In addition, cells expressed Gad1 (glutamate decarboxylase 1) (19), Slc17a7 (vesicular glutamate transporter 1) (20), and Slc30a3 (zinc transporter 3, ZnT-3) (21). Congruent with our previous observation (Figs. 2C and D and S1), only ∼30% of the cells expressed Rbp4, further indicating that commissural GCs are not restricted to the Rbp4-expressing GC population. Furthermore, we analyzed genes with known lasting expression changes after KA. Following previously described patterns, Npy (22) and Pnoc (23, 24) were up-regulated, whereas Cck was down-regulated (22), both in KA–commissural and KA–noncommissural GCs. Other interneuronal markers (e.g. Reln, Ndnf, Pvalb, Vip, and Sst) were not or infrequently detected (Fig. 5A). Thus, commissural and noncommissural GCs did not differ by known markers.
Fig. 5.
Transcriptomic characterization of commissural GCs. A) Heat map shows expression GC, GABAergic versus glutamatergic, and interneuron marker genes. Scale shows log10-based gene expression level. B) Volcano plot shows differential gene expression between KA–noncommissural and KA–commissural GCs. C) Heat map shows expression of the top 10 up- and down-regulated genes in KA–commissural GCs compared to KA–noncommissural GCs. D) GO enrichment analysis based on genes, whose expression level was >2-fold higher in KA–commissural GCs compared to noncommissural GCs. Enriched biological processes (FDR < 0.005 for all; also see Fig. S7) are ranked by decreasing Enrichr combined score from bottom to top. The analysis of genes whose expression level was >2-fold lower in KA–commissural GCs compared to noncommissural GCs did not reveal significantly enriched biological processes (see Fig. S7). E) Plot shows the only enriched protein cluster identifiable by STRING cluster analysis based on the top 150 up-regulated genes in KA–commissural GCs compared to KA–noncommissural GCs (also see Fig. S8). Proteins in this cluster are related to ribosome function. STRING analysis of the 150 down-regulated genes did not reveal identifiable clusters (see Fig. S9).
To analyze transcriptomic profiles more broadly, we performed differential gene expression analysis between KA–commissural and KA–noncommissural GCs (although the low number of commissural GCs hindered such a comparison after saline, we presumed that unique properties of the commissural GCs should be similar both after saline and KA). This comparison revealed differentially regulated genes between the two groups (Figs. 5B and S6). Although the expression of several genes were significantly different by single comparison (P < 0.05), none of them were by multiple comparisons (false discovery rate or FDR < 0.05 and fold-change > 2 or |log2FC| > 1). In addition, top ranking genes did not display a clear distinguishing pattern, suggesting that commissural GCs cannot be identified by specific marker expression (Fig. 5C).
To further examine the transcriptomic profile of commissural GCs, we performed gene ontology (GO) enrichment analysis based on genes, whose expression level was >2-fold higher or lower than in noncommissural GCs. Using Enrichr (25), we found significant enrichment of multiple translation and protein transport-related biological pathways in commissural GCs (Fig. 5D). These pathways shared several genes which encoded ribosomal proteins (such as ribosomal protein large subunit, RPL, and ribosomal protein small subunit, RPS) (Fig. S7). As an independent approach, using STRING, we analyzed protein interactions based on the top 150 up- and down-regulated genes. Consistent with the Enrichr results, this analysis also revealed a significantly enriched cluster of ribosome proteins in commissural GCs (Figs. 5E and S8). While both Enrichr and STRING revealed significant enrichments, neither approach suggested de-regulation of molecular pathways (Figs. S7 and S9).
Discussion
In the present study, we systematically characterized GCs targeting the contralateral hippocampus. Our current results suggest three conclusions that have implications not only for GC diversity and circuit formation in the adult brain but potentially also for the pathophysiology of epilepsy.
First, we described a rare type of hippocampal GCs that atypically project to the contralateral hippocampus in naïve mice. Previously, GCs had been extensively characterized as unilaterally projecting neurons within each hippocampus, critically involved in certain forms of learning and memory (26–28). While most GCs are located in the dentate GC layer and relatively homogenous, other atypical GC types have been also described, such as semilunar GCs in the inner molecular layer (29–34) and GCs in CA3 (35). Unlike these other types, commissural GCs are embedded in the GC layer. Using retrograde tracing, we identified ∼20 commissural GCs in each dentate gyrus. As a caveat, this cell count was based on retrograde tracer injections at a single level of the septo-temporal axis of the hippocampus and therefore does not account for all commissural GCs in the entire hippocampus; in addition, imperfect axonal uptake, retrograde transport, and/or expression of the tracer may limit the efficacy of retrograde labeling. Thus, the actual number of commissural GCs is likely higher, possibly a few hundred or thousand in each dentate gyrus. In the contralateral hippocampus, commissural GC axons mostly innervated the CA3-CA1 and subiculum areas, and using trans-synaptic labeling, we showed that the major postsynaptic targets are pyramidal neurons. As an additional caveat, the anterograde axon and trans-synaptic cell tracing experiments were based on the ∼30% of GCs that express Cre in the Rbp4-Cre transgenic line, although we also showed that commissural GCs are not restricted to Rbp4-expressing GC population. Thus, the total length of commissural GC axons and the number of innervated neurons are also likely higher. Irrespectively, our results indicate that commissural GCs are uniquely positioned to mediate interhemispheric information transfer within the hippocampal GC population.
To gain further insights into the identity of these cells, we used multimodal electrophysiological, morphological, and transcriptomic analyses, which revealed that commissural GCs are principally very similar to other mature GCs. Apart from their commissural axons, our analyses did not reveal unique features that could be used as biomarker for this atypical GC population. Nonetheless, we detected several differentially regulated genes and, based on these, significant enrichment of ribosome- and translation-related biological processes in commissural GCs, feasibly reflecting an increased cellular load to support the biogenesis and/or maintenance of long commissural axons.
Second, in contrast with a prevalent hypothesis that posits broad axon growth inhibition in the adult central nervous system (36, 37), our results revealed robust growth of contralateral projections after KA treatment. Specifically, we found that the number of retrogradely labeled commissural GCs, length of anterogradely labeled contralateral GC axons, and the number of trans-synaptically labeled contralateral hippocampal neurons increased by ∼5–6-, 4.3-, and 2- to 10-fold, respectively. With regard to previous reports on inter-hippocampal projections, contralateral sprouting of commissurally projecting hippocampal PV (38) and CA3 pyramidal neurons (39) and of an unidentified cell type (40) was observed in epilepsy models. Independently of epilepsy, human embryonic stem cells grafted into the mouse and rat dentate gyrus grew new commissural axons into the contralateral hippocampus (41). Thus, our finding together with other evidence indicates that the adult brain retains a capacity for inter-hippocampal axon growth and/or sprouting and that such processes are exempt from growth inhibition.
Given that most GCs activate a unilateral wiring program for local mossy fiber sprouting after KA, the commissural growth of axons may be controlled by additional wiring mechanisms (e.g. for midline crossing and for contralateral target specification), the identification of which remains an important goal. Similarly to local mossy fiber sprouting (5), commissural axon growth feasibly starts within 1–2 days after KA injection. Induction mechanisms may be active only transiently, constrained by KA availability and/or neuronal sensitivity to KA. For example, we previously identified Id2 as a key regulator of local mossy fiber sprouting based on its exceptionally strong up-regulation 1 day after KA (5); however, 2 weeks later, its expression was decreased to the level of a much less likely candidate. Thus, assuming that commissural fiber growth is controlled transcriptionally, it is possible that our transcriptomic data (2 weeks after KA) reflected molecular states associated with maintenance rather than induction. Currently, the major limitation of exploring the molecular mechanisms of induction in greater detail is the inability to access commissural GCs shortly after KA: retrograde labeling does not solve this problem, because commissural axons are not yet established in the contralateral hippocampus.
Third, commissural GC projections may contribute to epilepsy. In the unilateral intrahippocampal KA microinjection model, spontaneous behavioral seizures may develop several weeks after injections (42–44). Approximately 60% of the seizures start in the KA-injected (ipsilateral) hippocampus, whereas the remainder starts in the noninjected (contralateral) hippocampus, bilaterally, or elsewhere (42). Independently of the locus of seizure onset, closed-loop (seizure-triggered) activation of local inhibitory PV interneurons in the hippocampus contralateral to KA microinjection effectively reduced seizure duration and/or completely stopped seizures (45), suggesting that contralateral excitatory neurons (major targets of PV neurons) and their commissural connections are important for seizure activity. Consistent with this, corpus callosum bisection may lead to seizure reduction and/or freedom in pharmacologically intractable seizures (46). Here, we showed that commissural GCs contribute to an inter-hippocampal excitatory-to-excitatory circuit. By directly targeting pyramidal cells in the contralateral hippocampus, commissural GCs bypass several feedback and feedforward inhibitory microcircuits which could control seizures (47), suggesting that excessive activation of this cell type may facilitate the generation and/or generalization of seizures (48).
Conclusion
In this study, we characterized commissural GCs that atypically project to the contralateral hippocampus. Multiple questions arise regarding this cell population, for example, how commissural GCs contribute to information processing in the healthy brain and if they play a role in epilepsy. Although we showed that commissural GC form synapses on contralateral pyramidal cells, the physiological properties of these synapses as well as the conditions under which they are activated remain to be elucidated. Furthermore, deciphering molecular mechanisms that control contralateral axon growth in the adult brain will likely have important implications for understanding the molecular logic of adult brain circuits. A critical next step to addressing these problems is to gain more specific access to commissural GCs, allowing higher throughput molecular and activity readouts as well as activity manipulations. The anterograde and retrograde approaches employed in our study have had limited capacity to achieve these goals. Finally, the question whether commissural GCs are present in species other than mice remains open.
Materials and methods
All mouse protocols and husbandry practices were approved by the Veterinary Office of Zürich Kanton. For comprehensive description of (i) mouse breeding and husbandry, (ii) stereotactic injection, (iii) histology and neuroanatomy, (iv) image analysis, (v) in vitro electrophysiology, (vi) single-cell RNA sequencing and bioinformatics, and (vii) statistical analysis, see Appendix S1.
Supplementary Material
Acknowledgments
We thank Jean-Charles Paterna and Melanie Rauch (Viral Vector Facility, University of Zürich/ETH Zürich) for discussions and virus production.
Contributor Information
Matteo Egger, Laboratory of Neural Connectivity, Brain Research Institute, Faculties of Medicine and Science, University of Zürich, Zürich 8057, Switzerland; Adaptive Brain Circuits in Development and Learning (AdaBD), University Research Priority Program (URPP), University of Zürich, Zürich 8057, Switzerland.
Wenshu Luo, Laboratory of Neural Connectivity, Brain Research Institute, Faculties of Medicine and Science, University of Zürich, Zürich 8057, Switzerland.
Natalia Cruz-Ochoa, Laboratory of Neural Connectivity, Brain Research Institute, Faculties of Medicine and Science, University of Zürich, Zürich 8057, Switzerland; Adaptive Brain Circuits in Development and Learning (AdaBD), University Research Priority Program (URPP), University of Zürich, Zürich 8057, Switzerland.
David Lukacsovich, Laboratory of Neural Connectivity, Brain Research Institute, Faculties of Medicine and Science, University of Zürich, Zürich 8057, Switzerland.
Csaba Varga, Laboratory of Neural Connectivity, Brain Research Institute, Faculties of Medicine and Science, University of Zürich, Zürich 8057, Switzerland.
Lin Que, Laboratory of Neural Connectivity, Brain Research Institute, Faculties of Medicine and Science, University of Zürich, Zürich 8057, Switzerland.
Gyula Maloveczky, Laboratory of Neural Connectivity, Brain Research Institute, Faculties of Medicine and Science, University of Zürich, Zürich 8057, Switzerland.
Jochen Winterer, Laboratory of Neural Connectivity, Brain Research Institute, Faculties of Medicine and Science, University of Zürich, Zürich 8057, Switzerland.
Rashmit Kaur, Laboratory of Neural Connectivity, Brain Research Institute, Faculties of Medicine and Science, University of Zürich, Zürich 8057, Switzerland.
Tamás Lukacsovich, Laboratory of Neural Connectivity, Brain Research Institute, Faculties of Medicine and Science, University of Zürich, Zürich 8057, Switzerland.
Csaba Földy, Laboratory of Neural Connectivity, Brain Research Institute, Faculties of Medicine and Science, University of Zürich, Zürich 8057, Switzerland; Adaptive Brain Circuits in Development and Learning (AdaBD), University Research Priority Program (URPP), University of Zürich, Zürich 8057, Switzerland.
Supplementary material
Supplementary material is available at PNAS Nexus online.
Funding
This study received funding from the Swiss National Science Foundation (310030_188506), the Novartis Stiftung für medizinisch-biologische Forschung (20A022), the Dr. Eric Slack-Gyr-Stiftung, and the University Research Priority Program AdaBD (Adaptive Brain Circuits in Development and Learning) of University of Zurich.
Author contributions
W.L., M.E., and C.F. designed research; M.E., W.L., N.A.C.-O., D.L., C.V., L.Q., G.M., J.W., R.K., and T.L. performed research and analyzed data; W.L. and C.F. wrote the paper.
Data availability
The RNA sequencing data have been deposited to National Center for Biotechnology Information Gene Expression Omnibus (NCBI GEO, GSE214905).
References
- 1. Seng C, Luo W, Földy C. 2022. Circuit formation in the adult brain. Eur J Neurosci. 56(3):4187–4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Denoth-Lippuner A, Jessberger S. 2021. Formation and integration of new neurons in the adult hippocampus. Nat Rev Neurosci. 22(4):223–236. [DOI] [PubMed] [Google Scholar]
- 3. 2012. Jasper's basic mechanisms of the epilepsies [internet]. 4th edition. Bethesda: (MD: ): National Center for Biotechnology Information (US). [PubMed] [Google Scholar]
- 4. Luo W, et al. 2022. Pcdh11x controls target specification of mossy fiber sprouting. Front Neurosci. 16:888362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luo W, et al. 2021. Recurrent rewiring of the adult hippocampal mossy fiber system by a single transcriptional regulator, Id2. Proc Natl Acad Sci U S A. 118(40):e2108239118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gerfen CR, Paletzki R, Heintz N. 2013. GENSAT BAC Cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron 80(6):1368–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cembrowski MS, et al. 2016. Spatial gene-expression gradients underlie prominent heterogeneity of CA1 pyramidal neurons. Neuron 89(2):351–368. [DOI] [PubMed] [Google Scholar]
- 8. He Y, Janssen WG, Vissavajjhala P, Morrison JH. 1998. Synaptic distribution of GluR2 in hippocampal GABAergic interneurons and pyramidal cells: a double-label immunogold analysis. Exp Neurol. 150(1):1–13. [DOI] [PubMed] [Google Scholar]
- 9. Cox DJ, Racca C. 2013. Differential dendritic targeting of AMPA receptor subunit mRNAs in adult rat hippocampal principal neurons and interneurons. J Comp Neurol. 521(9):1954–2007. [DOI] [PubMed] [Google Scholar]
- 10. Que L, Lukacsovich D, Luo W, Földy C. 2021. Transcriptional and morphological profiling of parvalbumin interneuron subpopulations in the mouse hippocampus. Nat Commun. 12(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Földy C, et al. 2016. Single-cell RNAseq reveals cell adhesion molecule profiles in electrophysiologically defined neurons. Proc Natl Acad Sci U S A. 113(35):E5222–E5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Winterer J, et al. 2019. Single-cell RNA-Seq characterization of anatomically identified OLM interneurons in different transgenic mouse lines. Eur J Neurosci. 50(11):3750–3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Young CC, et al. 2009. Upregulation of inward rectifier K+ (Kir2) channels in dentate gyrus granule cells in temporal lobe epilepsy. J Physiol. 587(Pt 17):4213–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Janz P, et al. 2017. Synaptic remodeling of entorhinal input contributes to an aberrant hippocampal network in temporal lobe epilepsy. Cereb Cortex. 27(3):2348–2364. [DOI] [PubMed] [Google Scholar]
- 15. Celio MR. 1990. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience 35(2):375–475. [DOI] [PubMed] [Google Scholar]
- 16. Iwano T, Masuda A, Kiyonari H, Enomoto H, Matsuzaki F. 2012. Prox1 postmitotically defines dentate gyrus cells by specifying granule cell identity over CA3 pyramidal cell fate in the hippocampus. Development 139(16):3051–3062. [DOI] [PubMed] [Google Scholar]
- 17. Brandt MD, et al. 2003. Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol Cell Neurosci. 24(3):603–613. [DOI] [PubMed] [Google Scholar]
- 18. von Bohlen Und Halbach O. 2007. Immunohistological markers for staging neurogenesis in adult hippocampus. Cell Tissue Res. 329(3):409–420. [DOI] [PubMed] [Google Scholar]
- 19. Sloviter RS, et al. 1996. Basal expression and induction of glutamate decarboxylase and GABA in excitatory granule cells of the rat and monkey hippocampal dentate gyrus. J Comp Neurol. 373(4):593–618. [DOI] [PubMed] [Google Scholar]
- 20. Gómez-Lira G, Lamas M, Romo-Parra H, Gutiérrez R. 2005. Programmed and induced phenotype of the hippocampal granule cells. J Neurosci. 25(30):6939–6946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wenzel HJ, Cole TB, Born DE, Schwartzkroin PA, Palmiter RD. 1997. Ultrastructural localization of zinc transporter-3 (ZnT-3) to synaptic vesicle membranes within mossy fiber boutons in the hippocampus of mouse and monkey. Proc Natl Acad Sci U S A. 94(23):12676–12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gall C, Lauterborn J, Isackson P, White J. 1990. Seizures, neuropeptide regulation, and mRNA expression in the hippocampus. Prog Brain Res. 83:371–390. [DOI] [PubMed] [Google Scholar]
- 23. Hunsberger JG, Bennett AH, Selvanayagam E, Duman RS, Newton SS. 2005. Gene profiling the response to kainic acid induced seizures. Brain Res Mol Brain Res. 141(1):95–112. [DOI] [PubMed] [Google Scholar]
- 24. Armagan G, et al. 2012. Kainic acid-induced changes in the opioid/nociceptin system and the stress/toxicity pathways in the rat hippocampus. Neurochem Int. 60(6):555–564. [DOI] [PubMed] [Google Scholar]
- 25. Xie Z, et al. 2021. Gene set knowledge discovery with Enrichr. Curr Protoc. 1(3):e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blaabjerg M, Zimmer J. 2007. The dentate mossy fibers: structural organization, development and plasticity. Prog Brain Res. 163:85–107. [DOI] [PubMed] [Google Scholar]
- 27. Josselyn SA, Tonegawa S. 2020. Memory engrams: recalling the past and imagining the future. Science 367(6473):eaaw4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hainmueller T, Bartos M. 2020. Dentate gyrus circuits for encoding, retrieval and discrimination of episodic memories. Nat Rev Neurosci. 21(3):153–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Williams PA, Larimer P, Gao Y, Strowbridge BW. 2007. Semilunar granule cells: glutamatergic neurons in the rat dentate gyrus with axon collaterals in the inner molecular layer. J Neurosci. 27(50):13756–13761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Larimer P, Strowbridge BW. 2010. Representing information in cell assemblies: persistent activity mediated by semilunar granule cells. Nat Neurosci. 13(2):213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rovira-Esteban L, et al. 2020. Semilunar granule cells are the primary source of the perisomatic excitatory innervation onto parvalbumin-expressing interneurons in the dentate gyrus. eNeuro 7(4):ENEURO.0323-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Erwin SR, et al. 2020. A sparse, spatially biased subtype of mature granule cell dominates recruitment in hippocampal-associated behaviors. Cell Rep. 31(4):107551. [DOI] [PubMed] [Google Scholar]
- 33. Gupta A, et al. 2020. Dendritic morphology and inhibitory regulation distinguish dentate semilunar granule cells from granule cells through distinct stages of postnatal development. Brain Struct Funct. 225(9):2841–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Afrasiabi M, Gupta A, Xu H, Swietek B, Santhakumar V. 2022. Differential activity-dependent increase in synaptic inhibition and parvalbumin interneuron recruitment in dentate granule cells and semilunar granule cells. J Neurosci. 42(6):1090–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Szabadics J, Varga C, Brunner J, Chen K, Soltesz I. 2010. Granule cells in the CA3 area. J Neurosci. 30(24):8296–8307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yiu G, He Z. 2006. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 7(8):617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schwab ME. 2010. Functions of Nogo proteins and their receptors in the nervous system. Nat Rev Neurosci. 11(12):799–811. [DOI] [PubMed] [Google Scholar]
- 38. Christenson Wick Z, Leintz CH, Xamonthiene C, Huang BH, Krook-Magnuson E. 2017. Axonal sprouting in commissurally projecting parvalbumin-expressing interneurons. J Neurosci Res. 95(12):2336–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Siddiqui AH, Joseph SA. 2005. CA3 Axonal sprouting in kainate-induced chronic epilepsy. Brain Res. 1066(1–2):129–146. [DOI] [PubMed] [Google Scholar]
- 40. Davenport CJ, Brown WJ, Babb TL. 1990. Sprouting of GABAergic and mossy fiber axons in dentate gyrus following intrahippocampal kainate in the rat. Exp Neurol. 109(2):180–190. [DOI] [PubMed] [Google Scholar]
- 41. Steinbeck JA, Koch P, Derouiche A, Brüstle O. 2012. Human embryonic stem cell-derived neurons establish region-specific, long-range projections in the adult brain. Cell Mol Life Sci. 69(3):461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bragin A, Engel J Jr, Wilson CL, Vizentin E, Mathern GW. 1999. Electrophysiologic analysis of a chronic seizure model after unilateral hippocampal KA injection. Epilepsia 40(9):1210–1221. [DOI] [PubMed] [Google Scholar]
- 43. Ben-Ari Y. 2002. Kainate and temporal lobe epilepsies: 3 decades of progress. In: Noebels JL. Avoli M. Rogawski MA. Olsen RW, Delgado-Escueta AV, editors. Jasper's basic mechanisms of the epilepsies [internet]. 4th ed. Bethesda: (MD: ): National Center for Biotechnology Information (US). p. 501–526. [PubMed] [Google Scholar]
- 44. Jagirdar R, Drexel M, Bukovac A, Tasan RO, Sperk G. 2016. Expression of class II histone deacetylases in two mouse models of temporal lobe epilepsy. J Neurochem. 136(4):717–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Krook-Magnuson E, Armstrong C, Oijala M, Soltesz I. 2013. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat Commun. 4:1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wada JA. 2005. Callosal bisection and transcallosal secondary antiepileptogenesis. Epilepsia. 46(Suppl 1):2–6. [DOI] [PubMed] [Google Scholar]
- 47. Paz JT, Huguenard JR. 2015. Microcircuits and their interactions in epilepsy: is the focus out of focus? Nat Neurosci. 18(3):351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fei F, et al. 2022. Discrete subicular circuits control generalization of hippocampal seizures. Nat Commun. 13(1):5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA sequencing data have been deposited to National Center for Biotechnology Information Gene Expression Omnibus (NCBI GEO, GSE214905).