Abstract
Allogeneic hematopoietic stem cell transplantation is an effective treatment to cure inborn errors of immunity. Remarkable progress has been achieved thanks to the development and optimization of effective combination of advanced conditioning regimens and use of immunoablative/suppressive agents preventing rejection as well as graft versus host disease. Despite these tremendous advances, autologous hematopoietic stem/progenitor cell therapy based on ex vivo gene addition exploiting integrating γ-retro- or lenti-viral vectors, has demonstrated to be an innovative and safe therapeutic strategy providing proof of correction without the complications of the allogeneic approach. The recent advent of targeted gene editing able to precisely correct genomic variants in an intended locus of the genome, by introducing deletions, insertions, nucleotide substitutions or introducing a corrective cassette, is emerging in the clinical setting, further extending the therapeutic armamentarium and offering a cure to inherited immune defects not approachable by conventional gene addition. In this review, we will analyze the current state-of-the art of conventional gene therapy and innovative protocols of genome editing in various primary immunodeficiencies, describing preclinical models and clinical data obtained from different trials, highlighting potential advantages and limits of gene correction.
Keywords: [Primary immunodeficiency, Inborn errors of immunity, Hematopoietic stem cell transplantation, Gene therapy, Lentiviral vectors, Retroviral vectors, Gene editing, CRISPR/Cas9]
Highlights
-
•
Gene therapies for inborn errors of immunity achieved remarkable safety and efficacy results.
-
•
Genome editing is a promising therapy for inborn errors of immunity.
-
•
New safety issues need to be addressed for appropriate risk/benefit assessments.
-
•
Sustainability and accessibility of these therapies are current issues.
1. Introduction
Inborn errors of immunity (IEI) are an heterogenous group of rare inherited disorders caused by defects in genes involved in innate and acquired immune functions. Advances in high-throughput DNA sequencing and availability of novel bioinformatic tools have recently broadened the group of inherited immune defects, which has currently reached a total number of 485 inborn errors of immunity [1], [2], [3]. These discoveries, while broaden the comprehension of the molecular and functional mechanisms underlying immune defects, they encourage the development of disease-specific therapies implementing gene specific therapeutic approaches. Supportive therapies including antibiotics, immunoglobulin replacement, immunosuppressive agents and corticosteroids are administered to prevent or treat infections and immune dysregulation, however long-term immunosuppressive treatment can be life threating. Allogeneic Hematopoietic Stem Cell Transplantation (HSCT) represents the treatment of choice for the cure of adaptive immune defects and selected innate immune deficiencies [4]. Severity of the clinical phenotype, patient age at transplant, and more importantly availability of a suitable donor limit HSCT success [5], [6], [7]. To overcome these hurdles, advances in HLA typing technology have been developed in the last decade to implement donors’ selection [7], [8]. HLA identical sibling (Matched Sibling Donor, MSD) availability represents the first bone marrow donor choice; matched related donor (MRD) can be also considered. To reduce risk of graft versus host disease (GvHD) and graft rejection, manipulation of the stem cell sources has been introduced with promising results in case of matched unrelated donors (MUD/MMUD), improving outcomes in IEI patients treated post-transplant with cyclophosphamide as GvHD prophylaxis [7], [9], [10].
Optimal conditioning regimen and consideration of patient-specific factors [5] are associated with better transplant outcome. To this regard, joint EBMT/ESID inborn errors working party (IEWP) has recently designed HSCT guidelines recommending six conditioning protocols with different myeloablative potential according to the degree of chimerism needed and the distinct disease background [7]. Remarkably, to limit conditioning-related organ toxicities and better preserve tissue integrity alternative conditioning regimen based on monoclonal antibodies alone, radiolabeled or drug-conjugated targeting hematopoietic cells are advancing towards the clinical setting [11], [12], [13], [14]], [15], [16], [17], [18]. While all these novel approaches have contributed to improve the efficacy and safety of HSCT, concerns related to its morbidity remain particularly in older patients. Transplantation of autologous gene corrected hematopoietic stem/progenitor cells (HSPCs) exploiting viral vectors (hereinafter referred as viral mediated gene addition) has dramatically changed the therapeutic scenario allowing to treat an ever-growing number of IEI by gene therapy (GT) [19], [20], [21], [22], [23]. Over the past twenty years, the success of clinical trials indicates viral mediated gene addition as the best-established therapeutic alternative to HSCT allowing to overcome the limits of donor availability and eliminating the risk of GvHD. However, viral mediated gene addition poses novel challenges related to the genetic engineering procedures that require ex vivo culture and manipulation impacting on HSPC stemness and genomic integrity[19], [20], [21], [22]. To this regard, well controlled monitoring and long-term studies of patients treated in various viral mediated gene addition clinical trials including IEIs, metabolic and lysosomal storage diseases will allow to further understand the robustness of this therapeutic correction. Gene editing (GE) extends the current armamentarium of GT, enabling site-specific deletions, insertions or nucleotide substitutions to suppress or restore gene function. Importantly, conversely to semi-random integration of the therapeutic sequence upon viral mediated gene addition, GE allows the integration of the therapeutic transgene under the control of its endogenous promoter or in a safe harbor genomic region [23], [24]. Several GE tools are emerging as novel techniques for a precise modification of a targeted DNA sequence, promising more potential advantages than the viral mediated gene addition [25], [26]. However, GE may open to the potential risk of undesired genetic changes [27], [28], and transient toxicity due to the innate cellular response, which in turn impacts on cell growth, differentiation, and functionality [23].
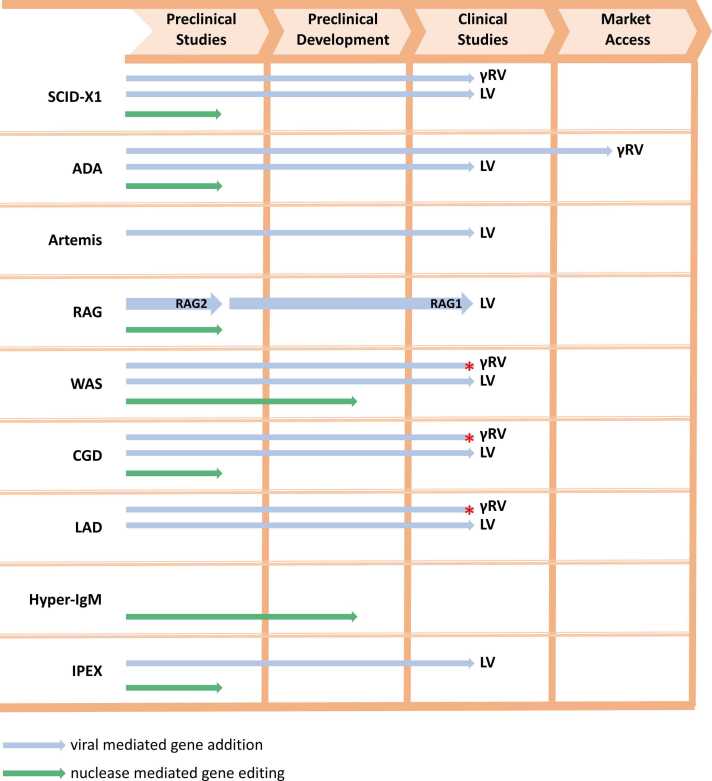
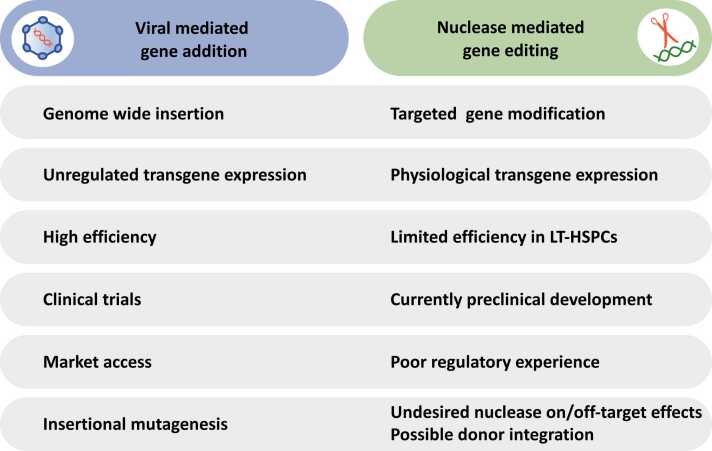
Here, we review the state-of the art and progress of viral mediated gene addition and targeted GE of mutant allele(s), focusing on preclinical data and ongoing GT clinical trials to treat some IEIs (Fig. 1), highlighting success and limits of the current therapeutic approaches (Fig. 2).
Fig. 1.
Schematic representation of the most advanced preclinical and clinical steps. Blue arrows indicate LV mediated gene addition, while green arrows indicated GE strategies. Red asterisks show clinical trials with a completed or unknown status not verified in more than two years.
Fig. 2.
Advantages and pitfalls of viral mediated gene addition and GE tools in IEIs. LT-HSPCs, long-term hematopoietic stem and progenitor cells.
2. Viral mediated gene addition and gene editing tools
In the last decades, the tumultuous evolution of biotechnological tools enabled efficient gene transfer into and GE of human hematopoietic cells, such as T cells and HSPCs, and allowed the development of GT medicinal products, many of which reached advanced stage of clinical testing and, in some cases, commercialization [19].
2.1. Viral vectors
Integration of the therapeutic sequence allows permanent cell marking upon division and across their progeny, replacing the function of the mutant gene or providing new properties to the engineered cells. Integrating γ-retro- (γRV) and lenti- (LV) viral vectors were developed by engineering the Moloney Murine Leukemia Virus (MMLV) and the Human Immunodeficiency Virus 1, respectively [29], [30]. Both vectors integrate semi-randomly into the host cell genome upon transduction. Viral vector production requires transfection into packaging cell lines of plasmids encoding for essential viral sequences (packaging and envelope plasmids) and transcribing the viral RNA genome (transfer plasmid) [31], [32]. In the last decades, removal of pathogenic and dispensable elements from the viral genome, as well as spatial segregation of sequences among transfected plasmids, virtually abrogated the risk of generating replication-competent viruses. Differently from γRV, LV transduce non-dividing cells [33], resulting in robust gene transfer in long-term repopulating HSPCs and permanent marking of hematopoiesis. The development of optimized culture protocols, as well as the usage of transduction enhancers, improved therapeutic efficacy of gene transfer by increasing transduction efficiency and better preserving stem cell properties [34], [35], [36], [37], [38], [39].
Both γRV and LV have been extensively used in (pre)clinical studies, with the latter showing an excellent safety profile due to its tendency to integrate in gene body rather than nearby promoters and enhancers [40]. Leukemogenesis was observed in patients transplanted with HSPCs engineered by γRV, mostly due to hyperactivation of protooncogenes nearby the vector integration site by viral long-terminal repeats (LTRs) [41], [42], [43], [44], [45], [46]. Vectors devoid of promoter/enhancer sequences in the LTRs (self-inactivating - SIN - vectors) improved safety of viral mediated gene addition [47], [48], [49], albeit requiring addition of an internal promoter to express the therapeutic transgene. Aberrant cell transcription driven by the transgene promoter, dysregulated expression of the transgene, generation of chimeric vector-host cell transcripts and knock-out of endogenous genes might still promote clonal expansion with either benign or malignant outcomes [50], [51], [52], [53], [54], [55], [56], [57]. Disease physiopathology [58], patient-specific characteristics and conditioning regimen may also influence viral mediated gene addition outcomes in terms of safety and efficacy. These observations highlight the need for careful design of the therapeutic strategy and support the rationale for GE in specific diseases, particularly those in which tight control of transgene expression is mandatory. Importantly, genotoxicity issues were mostly confined to trials using early generation non-SIN γRV. On the contrary, consistent data have shown persistent highly polyclonal repopulation and multilineage reconstitution by LV-engineered HSPCs in patients [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69].
2.2. Search and cut (and paste): programmable nucleases for gene editing
Programmable nucleases are chimeric molecules composed of: i) a DNA binding element, which dictates nuclease specificity for the intended DNA sequence, and ii) an endonuclease, which catalyzes the formation of a double strand break (DSB) [70]. DNA DSBs trigger a complex DNA damage response (DDR), which recruits the DNA repair machinery and orchestrate transcriptional programs dictating alternative cell fates. DNA repair can occur either by error-prone pathways (non-homologous end joining; NHEJ), which rejoin DNA ends by installing indels at the DSB site, or by high-fidelity pathways (e.g., homology-directed repair; HDR), which are mostly restricted to S/G2 cell-cycle phases. The presence of a DNA sequence homologous to the DSB site is required for templating HDR. Therapeutic GE strategies exploiting both pathways have been tested in human cells [71]. NHEJ-based GE mostly allows abrogation of gene function by disrupting a coding sequence, or perturbation of gene regulation by targeting promoter/enhancer sequences. HDR-based GE, instead, enables site-specific integration of short- or long-range therapeutic sequences either at disease-causing genes, genomic safe harbors (e.g., the Adeno-Associated Virus Site 1, AAVS1), or favorable loci for transgene expression.
During the last decades, several efforts have been pursued to discover novel programmable nucleases and to optimize the existing ones. Ideal features of nucleases are: i) broad genome accessibility; ii) small size; iii) accuracy and precision; iv) high cutting efficiency with low/absent cytotoxicity. The main classes of programmable nucleases are Zinc Finger Nucleases (ZFNs), Transcription Activator-Like Effector Nucleases (TALENs) and Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/Cas systems. Whereas ZFNs and TALENs are exclusively based on protein architectures, CRISPR/Cas systems require short RNA (guide RNA, gRNA) to direct Cas endonuclease activity to the target site.
ZFNs are composed by an unspecific FokI endonuclease domain and an array of ZF DNA binding domains (DBD), linked by a flexible peptide whose sequence and structure must be optimized for efficient cleavage and specificity [72]. Each ZFN recognizes a 9- to 18-base pairs (bp) DNA sequence [73]. Binding of two ZFN monomers to the DNA is required for effective DNA cleavage upon FokI dimerization [74], [75], [76], [77], [78]. Recently, FokI engineering and reduction of ZF DBD affinity for binding further improved ZFN specificity, reaching up to undetectable off-target activity [74]. Optimized ZFNs proved versatile, very efficient, and highly specific in seminal studies, achieving successful HSPC gene correction in preclinical models [18], [75], [76], [77]. However, the laborious engineering needed to redirect ZFN specificity have limited their widespread implementation.
TALENs are composed by an array of TALE DBD flanked by N- and C-terminal domains, the latter fused to FokI by a flexible peptide linker [78]. As for ZFNs, a pair of TALENs must bind in head-to-head orientation spaced by a short DNA sequence. Shortened version of the C-terminal domain and obligate heterodimeric FokI increase TALEN specificity [79], [80], [81]. Differently from ZFNs, instead, a simple recognition code associates each TALE DBD with one nucleotide, allowing more straightforward design of custom TALEN pairs targeting the intended DNA sequence [82]. TALENs proved efficient and highly precise when targeting disease-causing genes in human hematopoietic cells for the treatment of PID [83], [84]. Yet, the high homology among DNA sequences comprised within the TALEN represents a limiting issue for its assembly and expression, although several strategies were developed to simplify such process.
CRISPR/Cas systems originate from archaea and bacteria, where they function as defense against exogenous agents by exerting an acquired immunity-like function [85], [86], [87], [88]. In 2012, CRISPR/Cas9 system from Streptococcus pyogenes were adapted to eukaryotic cells [89]. Cas9 endonuclease is tethered to the intended target site by a single gRNA, which is made of an invariable RNA sequence linked to a 17- to 20-bp spacer, dictating Cas9 specificity through gRNA complementary to the target DNA. Upon recognition of the 5’-NGG protospacer adjacent motif (PAM) at the 3’ end of the protospacer, Cas9 binds and catalyzes the formation of a blunt-end DNA DSB 3-bp upstream from the PAM [90], [91]. In the last decade, tremendous efforts allowed to discover and/or engineer numerous CRISPR/Cas systems with different origin, complexity, activity, specificity, and PAM requirements, unprecedentedly expanding the GE toolbox and broadening genome accessibility [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102]. Since swapping the gRNA spacer is the only change required to redirect Cas specificity to another intended sequence, CRISPR/Cas systems are currently the most versatile GE tool. For these reasons, GE with CRISPR/Cas for hematological diseases is rapidly advancing to the clinic and demonstrated promising efficacy results in early clinical studies for the treatment of hemoglobinopathies [103].
3. Viral mediated gene addition and gene editing approaches for IEIs
3.1. Severe Combined Immunodeficiencies (SCID)
3.1.1. X-linked SCID-X1 (SCID-X1)
X-linked severe combined immunodeficiency (SCID-X1) is a rare, life-threatening IEI caused by mutations of the IL2RG gene, which encodes the common cytokine receptor γ chain (γc), a common receptor subunit for a number of cytokines essential for the development of T lymphocytes and NK cells [104], [105]. Accordingly, SCID-X1 patients are characterized by the absence of T cells and NK cells and partially functional B cells leading to the failure of both cellular and humoral immune responses.
Allogeneic HSCT is the standard treatment for SCID-X1 patients with a survival rate up to 87% with no differences in patients without or with conditioning [106]. Despite conditioning-related toxicity and the strong selective advantage of functional cells, conditioning resulted in superior graft function, especially for NK and B cell compartments [107], [108]. Whenever MRD are lacking, matched unrelated HSCT may be a valid alternative option, despite the lower survival rate [109]. Improved outcomes have been recently reported in patients with various immune defects including SCID-X1 receiving MUD and MRD transplantation with TCRα/β − CD19 depletion and T replete marrow -PT-Cy approaches[9], [110], [111], [112]. However, autologous transplantation of ex vivo genetically modified HSPCs would be a valuable option with no risk of GvHD [113]. The first two clinical trials began in Paris [41], [114] and in London [115] with a total of 20 SCID-X1 patients transplanted with autologous HSPCs transduced with IL2RG-expressing MLV-derived γRV with intact LTR regions, showing long-lasting correction, and functional and polyclonal T cell recovery in 18/20 patients [116], [117]. However, only low levels of corrected B and NK cells were found, probably due to the absence of conditioning. Despite the evident clinical benefit, six patients developed T-cell acute lymphoblastic leukemia after GT, which remitted in five of them upon chemotherapy while one patient died. Further investigations showed that the major trigger of the hematological malignancy was vector integration in oncogenes (LMO2, CCND2) due to the enhancer activity of U3 LTR upon γRV integration nearby their promoters [42], [45], [118]. Moreover, the deregulated expression of gamma chain might favor lymphoproliferation contributing to malignant transformation, unraveling the physiological role of IL2RG chain during cell cycle[119]. Thus, SIN-γRV were next developed encoding the IL2RG cDNA under the control of the ubiquitous human elongation factor 1α (EF1α) short promoter (EFS) and carrying the deletion of the LTR U3 enhancer region to abolish any LTR-driven transactivation activity [120]. This vector was used in a multicenter clinical study (ClinicalTrials.gov number: NCT01410019, NCT01175239, NCT01129544) initially including 9 SCID-X1 patients showing an overall survival of 89%, outstanding T cell recovery and confirming the improved safety of SIN-γRV after a median follow-up of 7.9 years [121], [122]. In contrast, reconstitution of B cell number and function was partial and transient, likely due to low engraftment of transduced progenitors. Of note, improved gene marking in B cell compartment was achieved when SIN-γRV GT was combined with busulfan-based reduced intensity conditioning (RIC) [122], in line with similar findings observed in HSCT context [106], [123].
To further improve the safety of gene addition approaches, SIN-LV vector carrying a codon-optimized (co)IL2RG cDNA have been developed and used in six clinical trials active in Europe, US and China (ClinicalTrials.gov number: NCT01512888, NCT03315078, NCT03311503, NCT03601286, NCT03217617, NCT04286815), most of them combined with RIC. These clinical trials are confirming the safety and efficacy of SIN-LV GT even in older SCID-X1 patients [124]. Recently, long-term surveillance of LV integration site landscape showed the emergence of dominant High Mobility Group AT-hook 2 gene (HMGA2) integration site clones in progenitor and myeloid lineages of all 8 treated SCID-X1 patients (ClinicalTrials.gov Identifier: NCT01306019), in the absence of altered hematopoiesis or emergence of leukemic clones [125]. The activation of HMGA2 in patients cells, also detected in two SCID-X1 patients treated with γRV gene addition [126], confers an additional growth advantage to HSPCs expressing IL2RG transgene [125], supporting clonal expansion. Thus, long-term studies are required to comprehensively assess safety of these approaches taking also in consideration the putative role of deregulated IL2RG transgene expression to leukemogenesis [53], [55].
In this context, HDR-mediated GE may be a valuable option. Recently, independent groups developed ZFN- and CRISPR/Cas9-based approaches targeting the exon 5 or the intron 1 of IL2RG using integrase defective LV (IDLV) or adeno-associated viral vector serotype 6 (AAV6) as HDR donor templates carrying the corrective IL2RG partial cDNA [18], [127], [128]. Mice transplanted with IL2RG-edited HSPCs showed multilineage reconstitution of the hematopoietic system, development of functional edited T cells and editing efficiencies up to 10–20% in long-term human xenografts. Given the selective advantage of gene corrected lymphoid progeny, the current HSPC GE efficiencies match the threshold required for clinical benefit, as predicted by mouse studies and GT clinical trials [18], [124].
In summary, LV mediated gene addition may position itself ahead of HSCT, as long as it is presumed to be safer. Milder conditioning, faster engraftment and the absence of GvHD must be balanced by cell collection hurdles, particularly in very young patients [123]. Facing the future, GE represents a new promising therapeutic option given the strong selective advantage of corrected cells and the low therapeutic threshold necessary to cure the disease that can be achieved by current GE platform [18], [128].
3.1.2. Adenosine deaminase SCID (ADA-SCID)
Adenosine deaminase (ADA) deficiency, the most common SCID form [129], [130], [131], [132], [133], is caused by defects in ADA gene encoding an enzyme of the purine metabolism converting adenosine and 2′deoxyadenosine into inosine and 2′deoxyinosine. Absence of this enzyme causes the accumulation of toxic metabolites which severely affects development and function of lymphoid and NK cells [129], [134]. Thus, ADA-SCID patients have profound lymphocytopenia, impaired cellular and humoral immunity and, in turn, high risk of opportunistic infections [134], [135]. Moreover, autoimmune manifestations (i.e., hemolytic anemia and immune thrombocytopenia) may develop especially in delayed and late onset forms [136]. Because of the ubiquitous expression of ADA enzyme, other non-immunological manifestations may occur, including failure to thrive, hepatic disorders, renal disease, skeletal alterations, and neurological/cognitive/behavioral deficits [132], [137], [138], [139].
Without treatment patients die by the age of 2 years [135], and early intervention is critical. Current options include enzyme replacement therapy (ERT) with polyethylene glycol-conjugated bovine enzyme (PEG-ADA), HSCT, and viral mediated gene addition. Consensus guidelines indicated to initiate ERT immediately upon diagnosis to reduce the levels of metabolites and to increase the number of lymphocytes, and now recommend it as a bridge therapy to HSCT or GT [140] because of suboptimal immune reconstitution and long-term complications [141].
The treatment of choice is HSCT with MSD/matched family donor (MFD) resulting in high overall survival rate (86% MSD, 81% MFD) [142]. According to European recommendations, when an MSD/MFD is not available, patients can be treated by viral mediated gene addition, which reduces the risk of GvHD and circumvents donor availability limitations. Poor long-term efficacy was observed in first clinical trials, which were based on the transplant of γRV-transduced T cells [143], [144], [145], umbilical cord blood [146] or bone marrow cells [147] in not conditioned ADA patients who continued to receive PEG-ADA. Poor long-term engraftment of infused cells was likely due to the absence of conditioning and the detrimental PEG-ADA effect on the survival advantage of corrected cells. Consistently, subsequent γRV mediated gene addition protocols including RIC and ERT-discontinuation showed long-term engraftment of corrected cells, robust immune cell reconstitution with reduced rate of infections, and effective metabolic detoxification [148], [149], [150], [151], [152]. The valuable outcome in ADA patients prompted the Milan’s team at the San Raffaele Telethon Institute for Gene Therapy (SR-Tiget, Italy) to collect all data for the market approval of ex vivo GT for ADA [151]. In 2016, Strimvelis (autologous CD34 + cells transduced to express ADA, GSK2696273) was the first ex vivo HSPC GT receiving regulatory approval in EU as advanced therapy medicinal product (ATMP) [153]. This milestone was crucial for the future commercialization of new medicines for orphan diseases. Reports on safety data of γRV mediated gene addition showed absence of leukoproliferative events [148], [150], [152], [154], [155], [156], [157] despite the presence in the peripheral blood of hematopoietic clones carrying vector integrations close to proto-oncogenes (LMO2, MECOM) of unknown clinical significance [157]. However, 1 out of 36 patients treated with Strimvelis developed lymphoid T-cell leukemia 4.7 years after GT, likely due to an insertional event[158]. Considering the safer profile of SIN-LV, novel vector mediated gene addition platforms were developed showing a safe and effective profile in 50 ADA-SCID patients treated in US and UK with autologous CD34+ HSPCs transduced with EFS-ADA LV [48], [64], [120], [159]. An overall survival of 100% up to 24 and 36 months, sustained ADA expression, metabolic correction, and functional immune reconstitution was reported in 48 out of 50 treated patients in absence of autoimmunity, GvHD and clonal expansion. Moreover, similar outcomes were observed between the fresh and the cryopreserved formulations of transduced HSPCs [64]. These excellent results prompt a reconsideration of the hierarchy of treatment options, which needs to be supported by long-term studies comparing HSCT and GT outcomes. A recent report comparing HSCT and viral mediated gene addition outcomes, preceded or not by ERT, in 131 ADA-SCID patients [160] showed higher five-year overall and event-free survival rates in patients receiving ERT-GT (100%, 75.3%) than ERT-HSCT (79.6%, 73%) and HSCT (72.5%, 49.5%). Excellent and comparable survival rates were found between viral mediated gene addition and patients treated by MSD-HSCT and without infection. Moreover, alternative donor HSCT (particularly HLA-matched unrelated donor and cord blood) for young infants without infection should be considered an alternative when MSD/MFD HSCT and viral mediated gene addition are not available [160].
Because of the strong selective advantage of ADA positive cells and the potential risk of insertional mutagenesis of integrating vectors, GE approaches have been considered in recent years. Some attempts, mostly in cell lines, were based on ZFN platform to insert and correct ADA gene by HDR [161] or CRISPR/Cas9 system to correct the nonsense Q3X (ADA c7C>T) point mutation endemic in patients of Somali origin [162]. Thus, further studies are needed to assess the feasibility of GE for the treatment of ADA.
3.1.3. ARTEMIS-deficient SCID (ART-SCID; OMIM)
Artemis (DCLREIC encoding the Artemis protein), an endonuclease of the family of metallo-β lactamase, mediates hairpin opening during the DSB repair of the V(D)J recombination process [163]. Mutations in this gene lead to a form of SCID characterized by the absence or very low number of T and B cells in the presence of NK cells (T-B-NK+ SCID, ART-SCID), but also Omenn cases [164], [165]. This defect is characterized by sensitivity to ionizing radiation due to the impairment of the predominant DSB repair pathway, NHEJ. Artemis loss-of-function mutations often comprise large deletions in the first four exons or non-sense founder mutation, as found in Navajo and Apache Native Americans [164]. Missense mutations and in-frame deletions in the highly conserved residues such as H35, D165 and H228 can also abolish Artemis’ protein function [166].
HSCT is the treatment of choice, and its success is reported to be 85% in case of MSD and drops to 65% for haplo-identical transplants [167]. Compared to other SCID forms, ART-SCID is the most difficult type of SCID to treat with allogeneic HSCT due to high rate of complications related to conditioning with alkylating drugs and radiations [168].
Autologous transplant of gene corrected HSPCs offers an attractive therapeutic alternative. However, because of Artemis role during NHEJ process, its expression needs a tight regulation. Consistently, in vitro and in vivo studies have shown loss of viability, perturbed cell cycle, increased DNA damage and apoptosis when high levels of protein were induced in HSPCs. To generate SIN vectors, various promoters including cytomegalovirus, EF1α promoter and the endogenous DCLREIC promoter 1-kb sequence upstream the translation start site were tested [169]. In vitro and in vivo studies showed immune reconstitution when hematopoietic cells were transduced with a SIN-LV carrying its endogenous promoter. Moreover, although woodchuck post-transcriptional regulatory element (WPRE) increases transgene expression and stability, its inclusion was found to cause toxicity in case of Artemis expression [170], [171]. A SIN-LV carrying DCLREIC under EF1α promoter but lacking WPRE was developed demonstrating physiological expression of the gene and stable correction in primary and secondary transplants with no evidence of oncogenic potential [172]. These promising data prompted to translate this novel platform (G2ARTE LV) to the clinical setting and a new phase I/II study was opened on October 2021 (ClinicalTrials.gov Identifier: NCT05071222).
The first clinical trial (ClinicalTrials.gov Identifier: NCT03538899) started in 2018 using a LV expressing Artemis driven by its endogenous promoter (drug: AProArt) and recruiting two groups of patients: the first including newly diagnosed patients and a second group of patients previously treated with HSCT. Short term follow-up of three patients pretreated with low dose of busulfan and receiving AProArt showed multilineage vector integration and in vitro response to mitogen. [173]. The clinical benefit of this approach was recently confirmed in 10 treated patients with a median follow-up of 31.2 months showing genetically corrected and functional T and B cells without evidence of clonal expansion. Development of autoimmune hemolytic anemia occurred in four of nine patients and resolved with and without treatments[174]. ART-SCID might benefit of GT treatment but also of non-genotoxic conditioning. To this regard, clinical trials are now ongoing to test feasibility and efficacy of biological conditioning. In a phase I dose escalation trial using AMG191 targeting CD117 (c-KIT) as the sole condition, two ART-SCID patients were included (ClinicalTrials.gov Identifier: NCT02963064). Preliminary data indicate engraftment of donor cells, however the donor chimerism in granulocytes remains low with a level of 3% and 7% at week 52 in patient 1 and 2, respectively [175].
3.1.4. Recombination Activating Genes (RAG) deficiencies
The Recombination Activating Genes 1 and 2 (RAG1 and RAG2) are the first players of the V(D)J recombination, the process responsible for the assembly of T cell receptor and B cell receptor and leading to the development of T and B cells [176]. Mutations of these genes cause a broad spectrum of clinical phenotypes including T-B-SCID caused by null mutations, while hypomorphic mutations lead to Omenn syndrome, atypical SCID, combined immunodeficiency with granulomas and/or autoimmunity. HSCT is the treatment of choice, however less satisfactory outcomes are obtained in hypomorphic conditions in which inflammation and autoimmune manifestations may be present [177]. HSCT outcome depends on availability of matched donors as haploidentical transplantation is associated with less favorable outcome while higher risk of GvHD and poor B cell reconstitution are observed in the context of absent conditioning. Of note, RIC or myeloablative conditioning are associated with better T and B cell reconstitution [167], [178], [179].
GT is a valid option especially for hypomorphic patients who are diagnosed late in the course of the disease and presenting with severe clinical conditions. Preclinical studies have highlighted benefits and limits of viral mediate gene addition approaches [180], [181], [182]. Safety concerns due to vector genotoxicity or deregulated RAG1 transgene expression causing lymphoproliferation were reported in γRV studies [183], whereas poor immune reconstitution and immune dysregulation were achieved in mice treated with LV carrying human co-hRAG1 driven by various promoters [182], [184], [185]. However, good level of lymphoid reconstitution and function were achieved in preclinical gene addition studies exploiting a LV carrying the co-hRAG1 driven by the synthetic promoter MND derived from a γRV promoter [181], [186]. Of note, MND is a strong trans-activating promoter, and recent description of myelodysplastic syndrome in three patients suffering from cerebral adrenoleukodystrophy and receiving a LV expressing the therapeutic gene under control of the same promoter (https://www.fda.gov/media/159129/download) is raising safety concerns and force to careful long-term studies. Results from preclinical models have led to a phase I/II clinical trial to cure null RAG1 children up to 24 months of age (ClinicalTrials.gov Identifier: NCT04797260) and the first patient has been recently treated in Leiden University Medical Center (https://www.lumc.nl/over-het-lumc/nieuws/2022/Juni/Eerste-patient-in-Nederland-succesvol-behandeld-met-stamcelgentherapie/?setlanguage=English&setcountry=en) and his follow up is still ongoing. To avoid deregulated expression and achieve a physiological correction of RAG genes recapitulating their expression kinetics during early T and B cell development, various attempts to establish a GE procedure for RAG1 deficiency using TALEN or CRISPR/Cas9 platforms combined with AAV6 are currently at preclinical level [187], [188].
With regard to RAG2 deficiency, preclinical viral mediated gene addition studies based on γRV [189] and SIN-LV [190] showed stable immune reconstitution in the absence of detectable toxicity in Rag2-/- mouse model. Moreover, viral mediated gene addition exploiting the UCOE-RAG2co LV in the Omenn syndrome Rag2R229Q/R229Q mouse model demonstrated the efficacy and feasibility of GT even in the context of inflammatory conditions [191]. Despite these promising data, up to date any clinical translation was pursued. Recently, induced pluripotent stem cells (iPSCs) carrying RAG2 mutation was exploited as a platform to assess the efficacy of CRISPR/Cas9 in combination with AAV6 to correct mutation and function [192]. Promising data were obtained laying ground towards its application to human hematopoietic stem cells. Nevertheless, there remains to be addressed the minimal dose of edited HSPCs required to correct the disease especially in hypomorphic conditions in which selective advantage of corrected cells is hindered by the competition of lymphoid progenitor derived from uncorrected lymphocyte progenitors with edited hematopoietic cells. Data from preclinical models could be instrumental to provide the feasibility and efficacy of HDR mediated editing to cure this complex and heterogenous immunodeficiency.
3.2. Other PIDs
3.2.1. Wiskott-Aldrich syndrome (WAS)
Wiskott-Aldrich Syndrome (WAS) is a severe X-linked PID caused by mutations in the WAS gene which encodes the WAS protein (WASp), a key regulator of cytoskeleton reorganization also involved in signaling transduction in all hematopoietic cells [193]. Recently, WASp roles in the nucleus have been described [194], [195], [196], highlighting its multifaceted roles and explaining the complexity of clinical manifestations. The absence of WASp impairs both innate and adaptive immunity as well as platelet number and function resulting in the full-blown WAS characterized by immunodeficiency, thrombocytopenia, eczema and increased risk of autoimmune manifestations and malignancies [197]. Residual WASp expression is associated with a milder form of the disease named X-linked thrombocytopenia, with longer life expectancy [197]. Of note, all WAS mutations cause micro-thrombocytopenia likely due to ineffective thrombocytopoiesis and/or enhanced platelet clearance due to intrinsic platelet defects or immune-mediated elimination [198], [199], [200].
Allogeneic HSCT is the treatment of choice and long-term correction is achieved upon immuno-ablation and myeloablation prior HSCT [201], [202]. While early studies reported high overall survival rates mostly limited to HSCT with HLA-identical sibling donors, recently good outcomes were observed in case of unrelated and alternative donors [203], [204], [205], [206]. A recent and large retrospective study on 176 patients (2006–2017) confirmed that age older than 5 years is a risk for poor outcome, while donor source and conditioning did not influence overall survival and chronic GvHD-free survival rates. Nevertheless, RIC based on treosulfan resulted in increased incidence of graft failure and lower myeloid donor chimerism [207].
Viral mediated gene addition approaches have been developed over years as alternative treatment options. Remarkably WAS represents an ideal candidate for GT considering the selective advantage of hematopoietic cells expressing WASp over the negative ones. The first GT clinical trial in WAS was based on mobilized autologous HSPCs transduced with a γRV expressing WAS under a viral promoter. Despite excellent outcomes in terms of immune and platelet reconstitution, all patients developed acute leukemia due to insertional mutagenesis close to the oncogenes (LMO2, MDS1and MN1) [43], [208], [209]. Extensive preclinical studies were then conducted to develop a safer platform based on a SIN-LV encoding the human WAS cDNA under the control of a fragment of the human endogenous promoter. This vector was used in the clinical trials performed in Milan [60], Paris and London [210], and Boston [211] showing a safe profile with stable polyclonal vector integration pattern [62], [210], [212], [213] and robust engraftment of gene corrected cells leading to improvement in infectious, eczema, autoimmune and bleeding events. Despite normalization of platelet volume, granule content and function, only partial correction of platelet numbers was achieved in most of treated patients [198], [212] likely due to the RIC that prevents full chimerism necessary for platelet correction [203], [214]. However, the risk of acute and chronic toxicities discourage the use of a more intensive conditioning regimen. On the same line, the use of stronger promoters may enhance WASp expression in platelets [215], [216], though they need to be carefully balanced with genotoxicity.
GE may represent a therapeutic option to correct the physiological regulation of WASp expression. The first evidence for the feasibility of GE was reported by Laskowski et al. who delivered ZFN targeting intron 1 and a corrective plasmid spanning exon 2 to exon 12 in iPSCs derived from a WAS patient. Edited iPSCs expressed WASp and differentiated into functional T and NK cells [217]. With the aim to correct all mutations scattered throughout the WAS gene, a GE platform, based on CRISPR/Cas9 and AAV6 donor delivering the coWAS cDNA in frame with the translation start codon in exon 1, was tested in HSPCs from healthy donors and WAS patients [218]. Edited HSPCs derived from patients preserved the engraftment and multilineage differentiation capacity in vivo showing improvement of immune cell and platelet abnormalities [218]. These promising results prompted to invest in preclinical efficacy and safety studies for the clinical translation of GE platform for the treatment of WAS. However, some disease-specific aspects need to be considered: i) despite the regulated WASp expression achievable by GE and the selective advantage of corrected cells, full chimerism of corrected cells is required to ensure proper immune cell and platelet reconstitution; ii) the excellent efficacy and safety of current therapeutic strategies will limit the number of patients treatable by GE.
3.2.2. Chronic Granulomatous Disease (CGD)
Chronic granulomatous disease (CGD) is a severe immunodeficiency caused by mutations in genes encoding for gp91phox, p22phox, p47phox, and p67phox subunits of the nicotinamide adenine dinucleotide phosphate oxidase (NADPH) enzyme complex. Defects in any of these subunits result in defective production of microbicidal reactive oxygen species [219]. Mutations in CYBB gene coding the gp91phox cause X-linked CGD form, accounting for approximately 65% of CGD, whereas mutations in the other subunits are responsible for the autosomal CGD forms. The disease is characterized by recurrent bacterial and fungal infections and high rate of inflammatory complications including inflammatory bowel disease, granuloma formation in liver, lungs and skin. Allogeneic HSCT allows correction of the immunodeficiency and improves survival. High myeloid chimerism is needed raising concerns on the effect of conditioning regimen in patients suffering from continuous infections [220], [221]. Additionally, bone marrow donor availability is restricted by the fact that female carriers of X-linked CGD may develop autoimmune manifestations not related to degree of lyonization [222], [223].
GT clinical trials started in 1990 using γRV in the absence of conditioning with no clinical benefit caused by lack of engraftment of gene corrected cells [224]. Next, clinical trials exploiting γRV carrying the transgene under a potent LTR enhancer/promoter element from the Spleen Focus Forming Virus and administering myeloablative conditioning achieved improved neutrophil function with clearance of infections [225], [226]. However, all treated individuals developed myeloproliferative disease due to insertional oncogenesis [44], [227]. More recently, a LV carrying CYBB cDNA under the control of engineered chimeric promoter phagocyte specific obtained 33% of superoxide production by mean fluorescent intensity and reduction of cytochrome-c levels [63].
To overcome deregulated expression and achieve more physiological regulation of the gene, De Ravin and colleagues proposed a GE platform targeting the genomic safe harbor AAVS1, followed by transduction with a rAAV6 containing the CYBB cDNA [228]. High HDR in HSPCs and in vivo persistence of edited cells with restored physiological expression of gp91phox in edited derived phagocytes were achieved targeting a point mutation in the exon 7 of CYBB and using a single-stranded oligodeoxynucleotide (ssODN) donor template in presence of transient inhibition of p53-binding protein 1 [229], [230].
GE strategy based on HDR gene correction was also developed to correct autosomal CGD, due to homozygous deletion of two nucleotide in the exon 2 of NCF1, which has two pseudogenes NCF1A and NCF1C. Restoration of oxidase function in 6% of myeloid cells differentiated from p47-CGD iPSCs was achieved by electroporating ZFNs targeting NCF1 exon 2 and both pseudogenes in the presence of a corrective cassette delivered by rAAV6 [231]. More recently, Schambach’s team obtained promising results in p47 deficient iPSCs targeting the corrective cassette into AAVS1. His group identified the micro-RNA 223 promoter as the most efficient one to achieve NADPH oxidase activity similar to wild-type cells [232].
Overall, these data although promising need further studies to understand the impact of the disease background on the efficacy and safety of various GT approaches. To this regard, preclinical LV mediated GT data in a mouse model of X-CGD showed inflammatory stress hematopoiesis that might influence the fitness of gene-corrected cells while increasing the risk of myeloid transformation of HSPC derived corrected clones [233].
3.2.3. Leukocyte Adhesion Deficiency (LAD1)
Leukocyte adhesion deficiency (LAD1) belongs to the group of IEIs affecting the leukocyte and adhesion cascade [234]. Mutations in the integrin subunit beta 2 (ITGB2) causing dysfunctional β-integrin molecules (CD18) activity led to impaired leukocytes migration and adhesion [235]. β2 integrin forms heterodimeric cell surface receptors with the four α subunits CD11a, CD11b, CD11c and CD11d. The complex CD11a/CD18 forms a heterodimer called LFA-1, which binds six ligands and mediates leukocyte arrest and adhesion of leukocytes to the endothelium. LAD1 patients present delayed umbilical cord detachment at birth and develop recurrent skin and mucosal infections characterized by the presence of pus. Patients are characterized by severe inflammation with elevated levels of cytokines at the site of inflammation that can be treated with Ustekinumab, a monoclonal antibody binding the common p40 subunit of IL-12 and IL-23 [235], [236]. The severity of the disease correlates with the expression of LFA-1 on leukocytes (<10%), and patients expressing very low level of CD18 (less than 2%) have a very high rate of early deaths, whereas partial CD18 deficiency is characterized by increased infections with early mortality [235], [237].
Allogeneic HSCT is the treatment of choice. A recent study performed on a large cohort of 84 LAD patients reported improvement of survival in patients receiving matched sibling or 10/10 matched donors; nevertheless, graft failure and GvHD remain relevant issues [238], [239].
Gene addition is an attractive therapeutic alternative and indeed in 1992 GT was performed in two affected children using a γRV with no clinical benefit [240]. Data from a canine LAD model treated with foamy viral vector (FV) mediated gene addition carrying CD18 under control of the MSCV internal promoter demonstrated the therapeutic potential of this strategy [241]. However, this platform is not suitable for translation to the clinical setting. A phase I/II study of ex vivo LV mediated gene addition exploiting a therapeutic vector carrying CD18 under the control of a chimeric promoter (Chim.hCD18-LV) sponsored by Rocket Pharmaceutical has been recently opened (ClinicalTrials.gov Identifier: NCT03825783; ClinicalTrials.gov Identifier: NCT03812263). Preliminary data show improvement of the clinical phenotype [242], but long-term follow up studies are needed to evaluate the efficacy of this treatment because of the severe inflammation and infection that characterize this disease [234].
3.2.4. Hyper-IgM due to defects in CD40L deficiency (HIGM1)
X-linked hyper-immunoglobulin M (IgM) syndrome (X-HIGM or HIGM1) is caused by mutations in CD40L molecule, a type II transmembrane glycoprotein of the tumor necrosis factor superfamily mainly expressed by activated CD4+ T cells, with some level of expression in other hematopoietic cell types including B cells, NK, CD8+ T cells and basophils [243], [244], [245]. CD40L once engaged its cognate receptor CD40 expressed on the surface of antigen presenting cells triggers B cell activation promoting proliferation, germinal center formation, antibody affinity maturation, class switching and long-term memory responses[244]. The immune phenotype of HIGM1 patients reflects the broad spectrum of action of this molecule. Patients suffer from hypogammaglobulinemia with normal to elevated levels of IgM and markedly reduced number of switched memory B cells. Although the clinical phenotype may be variable, patients if left untreated may succumb early in life for bacterial, viral, and opportunistic agents [246], [247]. Neutropenia, autoimmunity and severe biliary tract disease, liver failure secondary to Cryptosporidium infection and cancers have been reported [248]. Long-term survival is poor despite immunoglobulin supplementation and antibiotic prophylaxis.
Allogeneic HSCT is the only curative option currently available with variable success depending on the preexisting clinical conditions, the donor cell sources, and conditioning regimens [249], [250], [251], [252], [253]. A recent study performed on a cohort of 130 patients receiving transplant between 1993 and 2015 reported an overall survival of 78.2% and event-free survival of 58.1% with a better survival in patients undergoing transplant in year 2000. Better outcome is observed in patients treated early in life and in the absence of preexisting lung and liver comorbidities. Event-free survival was reported in MSD and when transplant was performed upon myeloablative conditioning [250], [251], [252], [254].
Severe complications and adverse events upon HSCT prompted to explore gene correction as therapeutic alternative to treat patients in critical conditions and with no donors. Preclinical viral mediated gene addition studies using γRV demonstrated to be effective in correcting the deficiency, but transgene overexpression caused thymic lymphoproliferative disease or hyperplastic B cell expansion [50], [51]. Conversely, natural and cell specific regulation using pre-mRNA trans-splicing or vector with sCD40L promoter showed promising data demonstrating that a tight and more physiological expression of the molecule can prevent immune dysregulation and clonal expansion [255], [256]. However, both approaches were far from their applicability in the human clinical setting.
To achieve a physiological gene expression, GE is a promising tool. TALEN and CRISPR/Cas9 platforms integrating a full-length wild-type copy of the cDNA into the 5’ untranslated region of the endogenous gene in primary patient T lymphocytes [84] or in CD34+ HSPCs [83] provided promising data showing improved activation-dependent expression and function, setting the stage of GE feasibility and efficacy in HIGM1. Recently, a “one-size-fits-all” gene edited strategy, integrating a corrective cassette including the 5’-truncated cDNA and all downstream exons and the cognate 3’UTR, within the first intron of the human CD40L gene has shown high efficiency of correction in patient T cells and HSPCs, which are predicted to be sufficient for rescuing the disease phenotype in animal models [257]. Importantly, the editing template includes the presence of a selector cassette NGFR (C-terminal truncated low affinity NGFR receptor), which allows to enrich the fraction of edited T cells.
Overall, these studies pave the way towards clinical translation indicating HDR-edited T cells as preferred option to treat HIGM patients, also based on the long-term follow up of clinical studies with engineered T cells [258]. However, a more tailored approach must be considered in this disease. The limited in vivo persistence of edited T cells clones might require repeated administrations or a dose escalation design, and the relative expansion of edited CD4+ T cells in response to antigen leading to acute inflammatory reaction (Immune reconstitution inflammatory syndrome) can be controlled by the depletion of EGFR+ cells further corroborating the use of a selector cassette.
3.2.5. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX)
IPEX syndrome is a severe and uncurable immunodeficiency characterized by immune dysregulation caused by mutations in the FOXP3 gene that lead to loss of suppressive function of CD4+ CD25+ thymic derived T regulatory (Treg) cells [259]. Pharmacological treatment and allogeneic HSCT are currently proposed, however two independent studies have recently reported a progressive decline of disease-free survival in patients treated with immunosuppressive agents (73% of survival at 15 years after HSCT and a disease-free survival of 60%) [260], [261], [262]. Pretransplant conditioning limits HSCT applicability in patients with advanced organ damage.
Transplant of corrected autologous HSPCs could be a therapeutic option for patients with moderate and late onset disease also taking in consideration that a fraction of cells carrying the wild-type form of FOXP3 allows better disease survival after HSCT and prevent autoimmunity in carriers [263], [264], [265]. In this scenario, several gene transfer approaches to correct FOXP3 gene in HSPCs and T cells including viral mediated gene addition and GE have been established and optimized [266]. Based on promising evidence that γRV-mediated ectopic expression of FOXP3 in T cells can induce suppressive function to T effector cells in the mouse model, LV mediated gene addition was proposed [266], [267]. To this end, generation of stable CD4+ T cells expressing FOXP3 was obtained using a LV carrying the gene under EF1α promoter and expressing truncated NGFR surface marker to monitor corrected cells [268], [269]. Transduced and expanded CD4+ T cells display in vitro and in vivo functional properties and phenotypic stability of naturally occurring Treg cells. In vivo humanized mouse models further confirmed these results showing maintenance of immune response against antigens and immune surveillance [270]. A first phase I dose escalation clinical trial has been recently opened (ClinicalTrials.gov Identifier: NCT05241444) to establish safety and feasibility of administering autologous CD4^LVFOXP3 in pediatric and young adults with progressive history of IPEX disease. This clinical trial will allow to determine the therapeutic dose (single or multiple dose administration) and the in vivo persistence of expanded and transduced T cells. Viral mediated gene addition is constrained by the evidence that ectopic constitutive FOXP3 expression alters engraftment and T cell lineage differentiation [271], thus indicating the need to preserve gene regulation. To this purpose CRISPR/Cas9 gene correction strategy targeting FOXP3 locus in the presence of the full length FOXP3 cDNA delivered by AAV6 was tested in T cells and HSPCs from cord blood, showing lower expression and suppressive function than normal [272], [273].
4. Challenges toward clinical application of gene editing
Current preclinical and short-term clinical data support feasibility, efficacy, and safety of GE approaches for hematological diseases, including IEIs. Yet, several challenges remain to be addressed, and thus should be considered when moving GE toward clinical testing. These aspects should not preclude its further development but rather be instrumental for identifying technological and knowledge gaps and for appropriate risk/benefit assessments on case-by-case bases.
GE generates heterogenous genetic outcomes, some of which may bear genotoxic potential. Biochemical or biological specificity assays and bioinformatic tools can efficiently predict and validate nuclease off-target sites, based on their homology with the target sequence [274], [275], [276], [277], [278], [279]. Combining data from two or more specificity assays can instruct the choice between different nucleases or gRNAs. The occurrence and the proportion of editing events at validated off-target sites can be monitored in the cell product, in xenograft models and potentially in patients to rule out aberrant expansion of clones bearing such events. Other complex and potentially more catastrophic outcomes of DNA repair have been reported upon editing, such as deletion of long sequences flanking the target site, translocations, aneuploidy, partial loss of chromosome arm and chromothripsis [280], [281], [282], [283], [284], [285]. In the context of gene correction approaches, integration of transcriptionally competent viral sequences from the HDR template may pose an additional safety concern[286]. The development of novel technologies and preclinical models informing on the genotoxic profile of a specific GE strategy would be highly relevant. Similarly, GE strategies decreasing the occurrence of unintended outcomes or purging out cells carrying aberrant repair events are needed. Yet, biological and clinical significance of unintended GE outcomes, either on- or off-target, likely depends on several factors, including the genomic region neighboring the target site, the patient’s genetic background and the disease pathophysiology, and thus should be carefully weighed on case-by-case bases.
Optimization of programmable nucleases enables efficient DNA cleavage at the target site, supporting high levels of NHEJ-mediated gene disruption in both preclinical and clinical studies in human T cells and HSPCs. Engagement of the HDR machinery is required to achieve targeted integration of a therapeutic sequence but confined to S/G2 cell-cycle phases [287]. Activated T cells are permissive to HDR editing, reaching high proportion of cells carrying the desired modification [84], [257], [288], [289], [290]. Conversely, long-term repopulating HSPCs are much less proficient for HDR. Hence, substantial tailoring of editing and culture conditions is required to reach therapeutically relevant levels of gene correction in preclinical studies [291], [292], [293]. Current efficiencies mostly fulfil the expected therapeutic threshold for diseases in which engraftment of few functional HSPCs may suffice to rescue the pathophysiological defect [18], [83], [128], [218], [229], [257], usually because of selective advantage of the edited progeny. The choice of HDR template delivery platform (e.g., AAV, IDLV, ssODN), as well as the length of the template itself, extensively influence the efficiency of targeted integration due to: i) different number of DNA copies reaching the nucleus at the time of DNA repair; ii) variable kinetics of template availability, dilution and disposal; iii) competence for different DNA repair pathways; iv) inherent toxicity of the delivery vehicle [286], [294], [295], [296]. Strategies further enhancing HDR efficiency across different cell types, regardless of the chosen delivery platform, comprise i) manipulation of DNA repair pathways to disfavor NHEJ; ii) tethering HDR-enhancing proteins or NHEJ-inhibiting ones to the nuclease; iii) cell-cycle synchronization; iv) transient cell cycle progression and activation of the HDR machinery [83], [230], [297], [298], [299], [300], [301], [302], [303], [304], [305]. This last approach currently appears the most effective in long-term repopulating HSPCs [303], [304], enabling up to 30% HDR editing in mobilized peripheral blood derived HSPCs without compromising stem cell properties. Enrichment of edited HSPCs by selector expression or in marker-free settings remains an option when aiming to high proportion of corrected cells [306], [307]. Its clinical implementation, however, is constrained by the complexity of the procedure and the high number of cells needed at the beginning of the process to compensate for the lower number of long-term engrafting clones in the enriched fraction.
GE entails several components and procedures that may hamper cell functionality and lead to cell death. Different cell types have their own degree of tolerance for each step of the genetic manipulation process, such as electroporation, induction of DNA breaks, and sensing of exogenous nucleic acids and viral vectors. These inputs may trigger cell responses that eventually cumulates and converge to common outcomes [308]. Culture per se may alter cell properties and the addition of small molecules and cytokines to the medium, co-culture with supportive cells and 3D-culture systems promotes maintenance of the stem cell phenotype in T cells and HSPCs [77], [302], [309], [310], [311], [312], [313], while enabling activation, proliferation and efficient genetic modification. Shortened ex vivo processes would minimize manufacturing costs and, most importantly, improves quality of the manufactured cell product. On a different perspective, further development of culture systems able to expand by symmetric self-renewal ex vivo engineered human HSPCs bearing long-term repopulation potential would be highly valuable for specific applications. Hematopoietic cells promptly recognize and are highly susceptible to exogenous molecules, particularly those mimicking pathogen-like structures, and DNA damage. Exogenous nucleic acids can elicit potent intracellular responses that converge on the secretion of cytokines and type I interferons (IFNs) and results in the induction of inflammatory transcriptional programs [314], [315]. Sensing of both gRNAs and mRNAs encoding for the editing tool may promote activation of such responses, leading to cell differentiation, exhaustion, and apoptosis, ultimately impacting on editing efficiency and tolerability [18], [316], [317]. mRNA engineering and purification bypass these issues, while improving editing efficiency. Electroporation of Cas and gRNA as ribonucleoprotein complex is currently the preferred option for ex vivo transient expression of the nuclease because of the lower toxicity, shorter half-life and thus reduced off-target activity [318]. Naldini’s team has previously showed that human HSPCs are vulnerable to nuclease-induced DNA DSBs [296], [303]. Whereas even one or few breaks suffice for p53-dependent DDR activation, the extent and duration of this response correlate with the number of induced DNA DSBs. Thus, depending on nuclease specificity, the consequences of p53 activation can range from minor reduction of clonogenic potential up to the induction of inflammatory programs, sharp decrease of cell viability and nearly complete loss of stem cell properties [296]. Viral vectors, the most common vehicles to deliver long DNA templates for HDR editing, may also converge on p53 activation upon recognition of viral structures and replication intermediates [286], [294], [295], [296], [303], [319]], [320]. Triggering of DDR by AAV dramatically shrinks size and clonality of hematopoietic edited grafts [303]. HSPC clones overcoming the cumulative p53 induction by DSB and AAV eventually recover and preserve physiologic multilineage differentiation, self-renewal and clonal dynamics. Transient p53 inhibition during editing procedure supports more robust polyclonality of the grafts without perturbing clonal behavior, and thus improves efficiency of editing and alleviates the risk of oligoclonal reconstitution. The use of alternative viral vectors (e.g., IDLV) [35], [286] or, prospectively, non-viral mediated delivery of naked DNA templates [290] may improve tolerance to HDR editing.
Process development of GE ATMPs should consider the aforementioned pitfalls and elaborate complex studies and assays to carefully scrutinize each parameter influencing safety and efficacy of the final product. Furthermore, GE requires multiple components and complex processes to be carried on under good-manufacturing practice (GMP) and GMP-like conditions to ensure fulfilment of regulatory agency requirements. The overall costs for research, process development and clinical testing of GE ATMPs are extremely high and require unprecedented efforts in terms of economic resources and time [321]. While GE processes may be similar among different IEIs, thus being an asset for potential investors, reagents and assays are mostly disease specific, allowing only partial saving on costs. These observations are even more relevant for gene correction approaches for rare and ultra-rare diseases. In this case, return on investments of pharma companies is constrained, among other factors, by the low number of treated patients and the unaffordability of such exorbitant costs in low-/middle-income countries. Even in high-income countries, reimbursement of these therapies by governments or direct payment by public or private entities is necessary to allow patients’ access to these treatments. The recent withdrawal from investments on gene therapy for IEIs and hemoglobinopathies by some pharma companies, particularly in Europe, highlights the challenge of providing access to these treatments [322], even after approval by regulatory agencies. Preclusion to access to these lifesaving cures raises significant ethical concerns and warns against economic sustainability of future GE based therapies[323].
5. Future perspectives and conclusion
Viral mediated gene addition therapies have proven clinical benefit for the treatment of several IEIs, achieving remarkable results in terms of safety and overall survival. A recent meta-analysis shows that viral mediated gene addition clinical studies for 5 different IEIs allowed to treat 224 patients often lacking MSD, reaching > 94% overall survival [158]. These results support the contention that viral mediated gene addition fairly competes with HSCT, and possibly outperforms it, in terms of both early-transplant mortality and 5-year survival. Follow-up studies are needed to stringently validate this observation and perform comparative risk/benefit assessment for HSCT and GT. The use of latest-generation LV, shorter culture protocols, accurate design of the therapeutic cassette and improved manufacturing of the cell product guarantee higher quality, efficacy and safety of GT ATMPs. Long-term studies monitoring vector integration sites in the genomic DNA of circulating hematopoietic subpopulations remain instrumental to assess clonal composition and HSPC activity in patients receiving GT [19], [324], [325]. Dominant clonal expansion has been recently described even in LV mediated GT, despite most likely being unrelated to vector integration [52], [326], [327], [328]. Yet, these findings highlight once more the importance of implementing advanced vector engineering, careful evaluation of promoter choice and investigation of risk factors predisposing to cancer development upon GT.
Parallel to the tremendous advances of GT in treating various blood diseases including IEIs, targeted GE represents the next frontier for the cure of monogenic disorders. Despite the promise of a more precise gene correction, major challenges for its safe application remain the poor permissiveness of long-term repopulating HSPCs to HDR and the potential cyto-/geno-toxic effects of GE. A joint effort at multiple levels (research, clinic, pharmaceutical companies, regulatory agencies, governments) should be done, however, to overcome the current challenges by filling knowledge and technological gaps in the field and by smoothing the path towards development, testing and commercialization. Novel GE tools (base and prime editing) are emerging as a platform to correct small pathogenic variants. Yet, their application is currently narrowed to diseases for which mutation-specific correction is sustainable for drug development, limiting their applicability to few IEIs[329]. Further development of these tools may allow targeted integration of long-range therapeutic sequences minimizing DNA breaks [330], [331], [332], [333], with the goal of reducing genetic heterogeneity within the engineered cell product and possibly decreasing toxicity. Novel conditioning regimens targeting bone marrow niche and sparing non-hematopoietic organs are now emerging in the clinical setting contributing to a safer use of autologous HSCT (NCT02963064), eventually coupled with GT. To this regard, a very innovative approach coupling transient expression of engraftment effectors in donor HSPCs with bone marrow mobilization has been recently tested in murine and xenograft models [334] paving the way towards a novel nongenotoxic conditioning. The combination of increased engraftment with low dose chemotherapy including immunodepleting agents will allow to further increase the strength of GT. In the long run, in vivo vector mediated gene addition and GE of long-term HSPCs, either in the bone marrow niche or in circulation during mobilization, would possibly bypass most issues related to HSCT and economic sustainability of these therapies, beyond broadening access to these treatments in low-/middle-income countries. Yet, these improvements will likely come at the cost of lower efficiency, substantial bystander modification of non-hematopoietic cells, and potential concerns related to off-target effects. Ultimately, advancement in the manufacturing capacity as well as systemic revision of the current regulation and cost-benefit models for the development and commercialization of novel ATMPs are highly needed to ensure access of IEI patients to these life-saving cures.
Competing interests
All authors are inventors of patents on gene editing owned and managed by the San Raffaele Scientific Institute and Telethon Foundation.
Acknowledgements
This work was supported by grants to: A.V. from Fondazione Telethon (SR-Tiget Core Grant number E2), Italian Ministry (PRIN-2017 20175XHBPN) and EDSCIDPROG Erare3 JTC 2017; M.C.C from Italian Ministry of Health (GR-2019-12369050); S.F. from European Hematology Association (EHA, Junior Research Grant 2022).
References
- 1.Tangye S.G., Al-Herz W., Bousfiha A., Cunningham-Rundles C., Franco J.L., Holland S.M., Klein C., Morio T., Oksenhendler E., Picard C., Puel A., Puck J., Seppänen M.R.J., Somech R., Su H.C., Sullivan K.E., Torgerson T.R., Meyts I. Human inborn errors of immunity: 2022 update on the classification from the international union of immunological societies expert committee. J. Clin. Immunol. 2022;1(2022):1–35. doi: 10.1007/S10875-022-01289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tangye S.G., Al-Herz W., Bousfiha A., Cunningham-Rundles C., Franco J.L., Holland S.M., Klein C., Morio T., Oksenhendler E., Picard C., Puel A., Puck J., Seppänen M.R.J., Somech R., Su H.C., Sullivan K.E., Torgerson T.R., Meyts I. The Ever-Increasing Array of Novel Inborn Errors of Immunity: an Interim Update by the IUIS Committee. J. Clin. Immunol. 2021;41:666–679. doi: 10.1007/S10875-021-00980-1/TABLES/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castagnoli R., Delmonte O.M., Notarangelo L.D. Congenital and acquired defects of immunity: an ever-evolving story. Pediatr. Allergy Immunol. 2022;33:61–64. doi: 10.1111/PAI.13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snowden J.A., Sánchez-Ortega I., Corbacioglu S., Basak G.W., Chabannon C., de la Camara R., Dolstra H., Duarte R.F., Glass B., Greco R., Lankester A.C., Mohty M., Neven B., de Latour R.P., Pedrazzoli P., Peric Z., Yakoub-Agha I., Sureda A., Kröger N. Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022. Bone Marrow Transplant. 2022:1217–1239. doi: 10.1038/s41409-022-01691-w. 2022 57:8. 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Stoep M.Y.E.C., Oostenbrink L.V.E., Bredius R.G.M., Moes D.J.A.R., Guchelaar H.J., Zwaveling J., Lankester A.C. Therapeutic drug monitoring of conditioning agents in pediatric allogeneic stem cell transplantation; where do we stand? Front Pharm. 2022;13:719. doi: 10.3389/FPHAR.2022.826004/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lankester A.C., Neven B., Mahlaoui N., von Asmuth E.G.J., Courteille V., Alligon M., Albert M.H., Serra I.B., Bader P., Balashov D., Beier R., Bertrand Y., Blanche S., Bordon V., Bredius R.G., Cant A., Cavazzana M., Diaz-de-Heredia C., Dogu F., Ehlert K., Entz-Werle N., Fasth A., Ferrua F., Ferster A., Formankova R., Friedrich W., Gonzalez-Vicent M., Gozdzik J., Güngör T., Hoenig M., Ikinciogullari A., Kalwak K., Kansoy S., Kupesiz A., Lanfranchi A., Lindemans C.A., Meisel R., Michel G., Miranda N.A.A., Moraleda J., Moshous D., Pichler H., Rao K., Sedlacek P., Slatter M., Soncini E., Speckmann C., Sundin M., Toren A., Vettenranta K., Worth A., Yeşilipek M.A., Zecca M., Porta F., Schulz A., Veys P., Fischer A., Gennery A.R. Hematopoietic cell transplantation in severe combined immunodeficiency: the SCETIDE 2006-2014 European cohort. J. Allergy Clin. Immunol. 2022;149:1744–1754. doi: 10.1016/j.jaci.2021.10.017. e8. [DOI] [PubMed] [Google Scholar]
- 7.Lankester A.C., Albert M.H., Booth C., Gennery A.R., Güngör T., Hönig M., Morris E.C., Moshous D., Neven B., Schulz A., Slatter M., Veys P. EBMT/ESID inborn errors working party guidelines for hematopoietic stem cell transplantation for inborn errors of immunity. Bone Marrow Transplant. 2021:2052–2062. doi: 10.1038/s41409-021-01378-8. 2021 56:9. 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prem S., Remberger M., Alotaibi A., Lam W., Law A.D., Kim D., Michelis F.V., Al-Shaibani Z., Lipton J.H., Mattsson J., Viswabandya A., Kumar R., Ellison C. Relationship between certain HLA alleles and the risk of cytomegalovirus reactivation following allogeneic hematopoietic stem cell transplantation. Transpl. Infect. Dis. 2022;24 doi: 10.1111/TID.13879. [DOI] [PubMed] [Google Scholar]
- 9.Neven B., Diana J.S., Castelle M., Magnani A., Rosain J., Touzot F., Moreira B., Fremond M.L., Briand C., Bendavid M., Levy R., Morelle G., Vincent M., Magrin E., Bourget P., Chatenoud L., Picard C., Fischer A., Moshous D., Blanche S. Haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide for primary immunodeficiencies and inherited disorders in children. Biol. Blood Marrow Transpl. 2019;25:1363–1373. doi: 10.1016/J.BBMT.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Giardino S., Bagnasco F., Falco M., Miano M., Pierri F., Risso M., Terranova P., di Martino D., Massaccesi E., Ricci M., Chianucci B., Dell’Orso G., Sabatini F., Podestà M., Lanino E., Faraci M. Haploidentical stem cell transplantation after TCR-αβ+ and CD19+ cells depletion in children with congenital non-malignant disease. Transpl. Cell Ther. 2022;28:394.e1–394.e9. doi: 10.1016/J.JTCT.2022.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Kwon H.S., Logan A.C., Chhabra A., Pang W.W., Czechowicz A., Tate K., Le A., Poyser J., Hollis R., Kelly B.V., Kohn D.B., Weissman I.L., Prohaska S.S., Shizuru J.A. Anti-human CD117 antibody-mediated bone marrow niche clearance in nonhuman primates and humanized NSG mice. Blood. 2019;133:2104–2108. doi: 10.1182/BLOOD-2018-06-853879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palchaudhuri R., Saez B., Hoggatt J., Schajnovitz A., Sykes D.B., Tate T.A., Czechowicz A., Kfoury Y., Ruchika F.N.U., Rossi D.J., Verdine G.L., Mansour M.K., Scadden D.T. Non-genotoxic conditioning for hematopoietic stem cell transplantation using a hematopoietic-cell-specific internalizing immunotoxin. Nat. Biotechnol. 2016:738–745. doi: 10.1038/nbt.3584. 2016 34:7. 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulz A.S., Glatting G., Hoenig M., Schuetz C., Gatz S.A., Grewendorf S., Sparber-Sauer M., Muche R., Blumstein N., Kropshofer G., Suttorp M., Bunjes D., Debatin K.M., Reske S.N., Friedrich W. Radioimmunotherapy-based conditioning for hematopoietic cell transplantation in children with malignant and nonmalignant diseases. Blood. 2011;117:4642–4650. doi: 10.1182/BLOOD-2010-06-284349. [DOI] [PubMed] [Google Scholar]
- 14.Gao C., Schroeder J.A., Xue F., Jing W., Cai Y., Scheck A., Subramaniam S., Rao S., Weiler H., Czechowicz A., Shi Q. Nongenotoxic antibody-drug conjugate conditioning enables safe and effective platelet gene therapy of hemophilia A mice. Blood Adv. 2019;3:2700–2711. doi: 10.1182/BLOODADVANCES.2019000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czechowicz A., Palchaudhuri R., Scheck A., Hu Y., Hoggatt J., Saez B., Pang W.W., Mansour M.K., Tate T.A., Chan Y.Y., Walck E., Wernig G., Shizuru J.A., Winau F., Scadden D.T., Rossi D.J. Selective hematopoietic stem cell ablation using CD117-antibody-drug-conjugates enables safe and effective transplantation with immunity preservation. Nat. Commun. 2019:1–12. doi: 10.1038/s41467-018-08201-x. 2019 10:1. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saha A., Hyzy S., Lamothe T., Hammond K., Clark N., Lanieri L., Bhattarai P., Palchaudhuri R., Gillard G.O., Proctor J., Riddle M.J., Panoskaltsis-Mortari A., MacMillan M.L., Wagner J.E., Kiem H.P., Olson L.M., Blazar B.R. A CD45-targeted antibody-drug conjugate successfully conditions for allogeneic hematopoietic stem cell transplantation in mice. Blood. 2022;139:1743–1759. doi: 10.1182/BLOOD.2021012366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castiello M.C., Bosticardo M., Sacchetti N., Calzoni E., Fontana E., Yamazaki Y., Draghici E., Corsino C., Bortolomai I., Sereni L., Yu H.H., Uva P., Palchaudhuri R., Scadden D.T., Villa A., Notarangelo L.D. Efficacy and safety of anti-CD45–saporin as conditioning agent for RAG deficiency. J. Allergy Clin. Immunol. 2021;147:309–320. doi: 10.1016/J.JACI.2020.04.033. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiroli G., Ferrari S., Conway A., Jacob A., Capo V., Albano L., Plati T., Castiello M.C., Sanvito F., Gennery A.R., Bovolenta C., Palchaudhuri R., Scadden D.T., Holmes M.C., Villa A., Sitia G., Lombardo A., Genovese P., Naldini L. Preclinical modeling highlights the therapeutic potential of hematopoietic stem cell gene editing for correction of SCID-X1. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aan0820. [DOI] [PubMed] [Google Scholar]
- 19.Naldini L., Cicalese M.P., Bernardo M.E., Gentner B., Gabaldo M., Ferrari G., Aiuti A. The EHA research roadmap: hematopoietic stem cell gene therapy. Hemasphere. 2022;6 doi: 10.1097/HS9.0000000000000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox T.A., Booth C. Gene therapy for primary immunodeficiencies. Br. J. Haematol. 2021;193:1044–1059. doi: 10.1111/BJH.17269. [DOI] [PubMed] [Google Scholar]
- 21.Kohn L.A., Kohn D.B. Gene therapies for primary immune deficiencies. Front Immunol. 2021;12:451. doi: 10.3389/FIMMU.2021.648951/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrari G., Thrasher A.J., Aiuti A. Gene therapy using haematopoietic stem and progenitor cells. Nat. Rev. Genet. 2020:216–234. doi: 10.1038/s41576-020-00298-5. 2020 22:4. 22. [DOI] [PubMed] [Google Scholar]
- 23.Ferrari S., Vavassori V., Canarutto D., Jacob A., Castiello M.C., Javed A.O., Genovese P. Gene editing of hematopoietic stem cells: hopes and hurdles toward clinical translation. Front Genome Ed. 2021;3:9. doi: 10.3389/fgeed.2021.618378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doudna J.A. The promise and challenge of therapeutic genome editing. Nature. 2020:229–236. doi: 10.1038/s41586-020-1978-5. 2020 578:7794. 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anzalone A. v, Randolph P.B., Davis J.R., Sousa A.A., Koblan L.W., Levy J.M., Chen P.J., Wilson C., Newby G.A., Raguram A., Liu D.R. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature. 2019;576 doi: 10.1038/s41586-019-1711-4. 7785. 576 (2019) 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komor A.C., Kim Y.B., Packer M.S., Zuris J.A., Liu D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature. 2015;533 doi: 10.1038/nature17946. 7603. 533 (2016) 420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee B.C., Lozano R.J., Dunbar C.E. Understanding and overcoming adverse consequences of genome editing on hematopoietic stem and progenitor cells. Mol. Ther. 2021;29:3205–3218. doi: 10.1016/J.YMTHE.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgio G., Teboul L. Anticipating and identifying collateral damage in genome editing. Trends Genet. 2020;36:905–914. doi: 10.1016/J.TIG.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maier P., von Kalle C., Laufs S. Retroviral vectors for gene therapy. Future Microbiol. 2010;5:1507–1523. doi: 10.2217/fmb.10.100. [DOI] [PubMed] [Google Scholar]
- 30.Naldini L., Trono D., Verma I.M. Lentiviral vectors, two decades later. Science. 1979;353(2016):1101–1102. doi: 10.1126/science.aah6192. [DOI] [PubMed] [Google Scholar]
- 31.Merten O.W., Hebben M., Bovolenta C. Production of lentiviral vectors. Mol. Ther. Methods Clin. Dev. 2016;3:16017. doi: 10.1038/MTM.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinn P.L., Sauter S.L., McCray P.B. Gene Therapy Progress and Prospects: development of improved lentiviral and retroviral vectors – design, biosafety, and production. Gene Ther. 2005:1089–1098. doi: 10.1038/sj.gt.3302570. 2005 12:14. 12. [DOI] [PubMed] [Google Scholar]
- 33.Naldini L. Lentiviruses as gene transfer agents for delivery to non-dividing cells. Curr. Opin. Biotechnol. 1998;9:457–463. doi: 10.1016/S0958-1669(98)80029-3. [DOI] [PubMed] [Google Scholar]
- 34.Petrillo C., Cesana D., Piras F., Bartolaccini S., Naldini L., Montini E., Kajaste-Rudnitski A. Cyclosporin A and rapamycin relieve distinct lentiviral restriction blocks in hematopoietic stem and progenitor cells. Mol. Ther. 2015;23:352–362. doi: 10.1038/MT.2014.193/ATTACHMENT/B168BE26-A9B6-4B8C-8024-C05E4E7237C9/MMC1.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petrillo C., Thorne L.G., Unali G., Schiroli G., Giordano A.M.S., Piras F., Cuccovillo I., Petit S.J., Ahsan F., Noursadeghi M., Clare S., Genovese P., Gentner B., Naldini L., Towers G.J., Kajaste-Rudnitski A. Cyclosporine H overcomes innate immune restrictions to improve lentiviral transduction and gene editing in human hematopoietic stem cells. Cell Stem Cell. 2018;23:820–832. doi: 10.1016/j.stem.2018.10.008. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang C.X., Sather B.D., Wang X., Adair J., Khan I., Singh S., Lang S., Adams A., Curinga G., Kiem H.P., Miao C.H., Rawlings D.J., Torbett B.E. Rapamycin relieves lentiviral vector transduction resistance in human and mouse hematopoietic stem cells. Blood. 2014;124:913–923. doi: 10.1182/BLOOD-2013-12-546218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heffner G.C., Bonner M., Christiansen L., Pierciey F.J., Campbell D., Smurnyy Y., Zhang W., Hamel A., Shaw S., Lewis G., Goss K.A., Garijo O., Torbett B.E., Horton H., Finer M.H., Gregory P.D., Veres G. Prostaglandin E2 increases lentiviral vector transduction efficiency of adult human hematopoietic stem and progenitor cells. Mol. Ther. 2018;26:320–328. doi: 10.1016/J.YMTHE.2017.09.025/ATTACHMENT/5BA6193F-304F–44F9-9015-F768B732BA69/MMC1.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soldi M., Sergi Sergi L., Unali G., Kerzel T., Cuccovillo I., Capasso P., Annoni A., Biffi M., Rancoita P.M.V., Cantore A., Lombardo A., Naldini L., Squadrito M.L., Kajaste-Rudnitski A. Laboratory-scale lentiviral vector production and purification for enhanced Ex vivo and in vivo genetic engineering. Mol. Ther. Methods Clin. Dev. 2020;19:411–425. doi: 10.1016/j.omtm.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Höfig I., Atkinson M.J., Mall S., Krackhardt A.M., Thirion C., Anastasov N. Poloxamer synperonic F108 improves cellular transduction with lentiviral vectors. J. Gene Med. 2012;14:549–560. doi: 10.1002/JGM.2653. [DOI] [PubMed] [Google Scholar]
- 40.Montini E., Cesana D., Schmidt M., Sanvito F., Ponzoni M., Bartholomae C., Sergi L.S., Benedicenti F., Ambrosi A., di Serio C., Doglioni C., von Kalle C., Naldini L. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat. Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- 41.Cavazzana-Calvo M., Hacein-Bey S., de Saint Basile G., Gross F., Yvon E., Nusbaum P., Selz F., Hue C., Certain S., Casanova J.L., Bousso P., le Deist F., Fischer A. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 1979;(2000) doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 42.Hacein-Bey-Abina S., von Kalle C., Schmidt M., McCormack M.P., Wulffraat N., Leboulch P., Lim A., Osborne C.S., Pawliuk R., Morillon E., Sorensen R., Forster A., Fraser P., Cohen J.I., de Saint Basile G., Alexander I., Wintergerst U., Frebourg T., Aurias A., Stoppa-Lyonnet D., Romana S., Radford-Weiss I., Gross F., Valensi F., Delabesse E., Macintyre E., Sigaux F., Soulier J., Leiva L.E., Wissler M., Prinz C., Rabbitts T.H., le Deist F., Fischer A., Cavazzana-Calvo M. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 1979;302(2003):415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 43.Braun C.J., Boztug K., Paruzynski A., Witzel M., Schwarzer A., Rothe M., Modlich U., Beier R., Göhring G., Steinemann D., Fronza R., Ball C.R., Haemmerle R., Naundorf S., Kühlcke K., Rose M., Fraser C., Mathias L., Ferrari R., Abboud M.R., Al-Herz W., Kondratenko I., Maródi L., Glimm H., Schlegelberger B., Schambach A., Albert M.H., Schmidt M., van Kalle C., Klein C. Gene therapy for Wiskott-Aldrich syndrome-long-term efficacy and genotoxicity. Sci. Transl. Med. 2014;6 doi: 10.1126/scitranslmed.3007280. [DOI] [PubMed] [Google Scholar]
- 44.Stein S., Ott M.G., Schultze-Strasser S., Jauch A., Burwinkel B., Kinner A., Schmidt M., Krämer A., Schwäble J., Glimm H., Koehl U., Preiss C., Ball C., Martin H., Göhring G., Schwarzwaelder K., Hofmann W.K., Karakaya K., Tchatchou S., Yang R., Reinecke P., Kühlcke K., Schlegelberger B., Thrasher A.J., Hoelzer D., Seger R., von Kalle C., Grez M. Genomic instability and myelodysplasia with monosomy 7 consequent to EVI1 activation after gene therapy for chronic granulomatous disease. Nat. Med. 2010:198–204. doi: 10.1038/nm.2088. 2010 16:2. 16. [DOI] [PubMed] [Google Scholar]
- 45.Howe S.J., Mansour M.R., Schwarzwaelder K., Bartholomae C., Hubank M., Kempski H., Brugman M.H., Pike-Overzet K., Chatters S.J., de Ridder D., Gilmour K.C., Adams S., Thornhill S.I., Parsley K.L., Staal F.J.T., Gale R.E., Linch D.C., Bayford J., Brown L., Quaye M., Kinnon C., Ancliff P., Webb D.K., Schmidt M., von Kalle C., Gaspar H.B., Thrasher A.J. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J. Clin. Invest. 2008;118:3143–3150. doi: 10.1172/JCI35798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Modlich U., Bohne J., Schmidt M., von Kalle C., Knöss S., Schambach A., Baum C. Cell-culture assays reveal the importance of retroviral vector design for insertional genotoxicity. Blood. 2006;108:2545–2553. doi: 10.1182/blood-2005-08-024976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cesana D., Ranzani M., Volpin M., Bartholomae C., Duros C., Artus A., Merella S., Benedicenti F., Sergi Sergi L., Sanvito F., Brombin C., Nonis A., di Serio C., Doglioni C., von Kalle C., Schmidt M., Cohen-Haguenauer O., Naldini L., Montini E. Uncovering and dissecting the genotoxicity of self-inactivating lentiviral vectors in vivo. Mol. Ther. 2014;22:774–785. doi: 10.1038/mt.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zufferey R., Dull T., Mandel R.J., Bukovsky A., Quiroz D., Naldini L., Trono D. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J. Virol. 1998;72:9873–9880. doi: 10.1128/JVI.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montini E., Cesana D., Schmidt M., Sanvito F., Bartholomae C.C., Ranzani M., Benedicenti F., Sergi L.S., Ambrosi A., Ponzoni M., Doglioni C., di Serio C., von Kalle C., Naldini L. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J. Clin. Investig. 2009;119:964–975. doi: 10.1172/JCI37630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sacco M.G., Ungari M., Catò E.M., Villa A., Strina D., Notarangelo L.D., Jonkers J., Zecca L., Facchetti F., Vezzoni P. Lymphoid abnormalities in CD40 ligand transgenic mice suggest the need for tight regulation in gene therapy approaches to hyper immunoglobulin M (IgM) syndrome. Cancer Gene Ther. 2000;7:1299–1306. doi: 10.1038/sj.cgt.7700232. [DOI] [PubMed] [Google Scholar]
- 51.Brown M.P., Topham D.J., Sangster M.Y., Zhao J., Flynn K.J., Surman S.L., Woodland D.L., Doherty P.C., Farr A.G., Pattengale P.K., Brenner M.K. Thymic lymphoproliferative disease after successful correction of CD40 ligand deficiency by gene transfer in mice. Nat. Med. 1998;4:1253–1260. doi: 10.1038/3233. [DOI] [PubMed] [Google Scholar]
- 52.Cavazzana-Calvo M., Payen E., Negre O., Wang G., Hehir K., Fusil F., Down J., Denaro M., Brady T., Westerman K., Cavallesco R., Gillet-Legrand B., Caccavelli L., Sgarra R., Maouche-Chrétien L., Bernaudin F., Girot R., Dorazio R., Mulder G.-J., Polack A., Bank A., Soulier J., Larghero J., Kabbara N., Dalle B., Gourmel B., Socie G., Chrétien S., Cartier N., Aubourg P., Fischer A., Cornetta K., Galacteros F., Beuzard Y., Gluckman E., Bushman F., Hacein-Bey-Abina S., Leboulch P. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature. 2010:318–7322. doi: 10.1038/nature09328. 2010 467:7313. 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woods N.B., Bottero V., Schmidt M., von Kalle C., Verma I.M. Gene therapy: therapeutic gene causing lymphoma. Nature. 2006;440:1123. doi: 10.1038/4401123a. [DOI] [PubMed] [Google Scholar]
- 54.Almarza D., Bussadori G., Navarro M., Mavilio F., Larcher F., Murillas R. Risk assessment in skin gene therapy: viral–cellular fusion transcripts generated by proviral transcriptional read-through in keratinocytes transduced with self-inactivating lentiviral vectors. Gene Ther. 2011:674–681. doi: 10.1038/gt.2011.12. 2011 18:7. 18. [DOI] [PubMed] [Google Scholar]
- 55.Ginn S.L., Liao S.H., Dane A.P., Hu M., Hyman J., Finnie J.W., Zheng M., Cavazzana-Calvo M., Alexander S.I., Thrasher A.J., Alexander I.E. Lymphomagenesis in SCID-X1 mice following lentivirus-mediated phenotype correction independent of insertional mutagenesis and γc overexpression. Mol. Ther. 2010;18:965–976. doi: 10.1038/MT.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moiani A., Paleari Y., Sartori D., Mezzadra R., Miccio A., Cattoglio C., Cocchiarella F., Lidonnici M.R., Ferrari G., Mavilio F. Lentiviral vector integration in the human genome induces alternative splicing and generates aberrant transcripts. J. Clin. Invest. 2012;122:1653–1666. doi: 10.1172/JCI61852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cesana D., Sgualdino J., Rudilosso L., Merella S., Naldini L., Montini E. Whole transcriptome characterization of aberrant splicing events induced by lentiviral vector integrations. J. Clin. Invest. 2012;122:1667–1676. doi: 10.1172/JCI62189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martins V.C., Busch K., Juraeva D., Blum C., Ludwig C., Rasche V., Lasitschka F., Mastitsky S.E., Brors B., Hielscher T., Fehling H.J., Rodewald H.R. Cell competition is a tumour suppressor mechanism in the thymus. Nature. 2014:465–470. doi: 10.1038/nature13317. 2014 509:7501. 509. [DOI] [PubMed] [Google Scholar]
- 59.Biffi A., Montini E., Lorioli L., Cesani M., Fumagalli F., Plati T., Baldoli C., Martino S., Calabria A., Canale S., Benedicenti F., Vallanti G., Biasco L., Leo S., Kabbara N., Zanetti G., Rizzo W.B., Mehta N.A.L., Cicalese M.P., Casiraghi M., Boelens J.J., del Carro U., Dow D.J., Schmidt M., Assanelli A., Neduva V., di Serio C., Stupka E., Gardner J., von Kalle C., Bordignon C., Ciceri F., Rovelli A., Roncarolo M.G., Aiuti A., Sessa M., Naldini L. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 1979;341(2013) doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- 60.Aiuti A., Biasco L., Scaramuzza S., Ferrua F., Cicalese M.P., Baricordi C., Dionisio F., Calabria A., Giannelli S., Castiello M.C., Bosticardo M., Evangelio C., Assanelli A., Casiraghi M., di Nunzio S., Callegaro L., Benati C., Rizzardi P., Pellin D., di Serio C., Schmidt M., von Kalle C., Gardner J., Mehta N., Neduva V., Dow D.J., Galy A., Miniero R., Finocchi A., Metin A., Banerjee P.P., Orange J.S., Galimberti S., Valsecchi M.G., Biffi A., Montini E., Villa A., Ciceri F., Roncarolo M.G., Naldini L. Lentiviral hematopoietic stem cell gene therapy in patients with wiskott-aldrich syndrome. Science. 2013;1979 doi: 10.1126/science.1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scala S., Basso-Ricci L., Dionisio F., Pellin D., Giannelli S., Salerio F.A., Leonardelli L., Cicalese M.P., Ferrua F., Aiuti A., Biasco L. Dynamics of genetically engineered hematopoietic stem and progenitor cells after autologous transplantation in humans. Nat. Med. 2018 doi: 10.1038/s41591-018-0195-3. [DOI] [PubMed] [Google Scholar]
- 62.Ferrua F., Cicalese M.P., Galimberti S., Giannelli S., Dionisio F., Barzaghi F., Migliavacca M., Bernardo M.E., Calbi V., Assanelli A.A., Facchini M., Fossati C., Albertazzi E., Scaramuzza S., Brigida I., Scala S., Basso-Ricci L., Pajno R., Casiraghi M., Canarutto D., Salerio F.A., Albert M.H., Bartoli A., Wolf H.M., Fiori R., Silvani P., Gattillo S., Villa A., Biasco L., Dott C., Culme-Seymour E.J., van Rossem K., Atkinson G., Valsecchi M.G., Roncarolo M.G., Ciceri F., Naldini L., Aiuti A. Lentiviral haemopoietic stem/progenitor cell gene therapy for treatment of Wiskott-Aldrich syndrome: interim results of a non-randomised, open-label, phase 1/2 clinical study. Lancet Haematol. 2019;6:e239–e253. doi: 10.1016/S2352-3026(19)30021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kohn D.B., Booth C., Kang E.M., Pai S.Y., Shaw K.L., Santilli G., Armant M., Buckland K.F., Choi U., de Ravin S.S., Dorsey M.J., Kuo C.Y., Leon-Rico D., Rivat C., Izotova N., Gilmour K., Snell K., Dip J.X.B., Darwish J., Morris E.C., Terrazas D., Wang L.D., Bauser C.A., Paprotka T., Kuhns D.B., Gregg J., Raymond H.E., Everett J.K., Honnet G., Biasco L., Newburger P.E., Bushman F.D., Grez M., Gaspar H.B., Williams D.A., Malech H.L., Galy A., Thrasher A.J., Buckland K.F., Bauser C.A., Honnet G., Grez M., Gaspar H.B., Galy A., Thrasher A.J. Lentiviral gene therapy for X-linked chronic granulomatous disease. Nat. Med. 2020 doi: 10.1038/s41591-019-0735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kohn D.B., Booth C., Shaw K.L., Xu-Bayford J., Garabedian E., Trevisan V., Carbonaro-Sarracino D.A., Soni K., Terrazas D., Snell K., Ikeda A., Leon-Rico D., Moore T.B., Buckland K.F., Shah A.J., Gilmour K.C., de Oliveira S., Rivat C., Crooks G.M., Izotova N., Tse J., Adams S., Shupien S., Ricketts H., Davila A., Uzowuru C., Icreverzi A., Barman P., Campo Fernandez B., Hollis R.P., Coronel M., Yu A., Chun K.M., Casas C.E., Zhang R., Arduini S., Lynn F., Kudari M., Spezzi A., Zahn M., Heimke R., Labik I., Parrott R., Buckley R.H., Reeves L., Cornetta K., Sokolic R., Hershfield M., Schmidt M., Candotti F., Malech H.L., Thrasher A.J., Gaspar H.B. Autologous ex vivo lentiviral gene therapy for adenosine deaminase deficiency. New Engl. J. Med. 2021;384:2002–2013. doi: 10.1056/NEJMOA2027675/SUPPL_FILE/NEJMOA2027675_DATA-SHARING.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Six E., Guilloux A., Denis A., Lecoules A., Magnani A., Vilette R., Male F., Cagnard N., Delville M., Magrin E., Caccavelli L., Roudaut C., Plantier C., Sobrino S., Gregg J., Nobles C.L., Everett J.K., Hacein-Bey-Abina S., Galy A., Fischer A., Thrasher A.J., André I., Cavazzana M., Bushman F.D. Clonal tracking in gene therapy patients reveals a diversity of human hematopoietic differentiation programs. Blood. 2020;135:1219–1231. doi: 10.1182/BLOOD.2019002350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Río P., Navarro S., Wang W., Sánchez-Domínguez R., Pujol R.M., Segovia J.C., Bogliolo M., Merino E., Wu N., Salgado R., Lamana M.L., Yañez R.M., Casado J.A., Giménez Y., Román-Rodríguez F.J., Álvarez L., Alberquilla O., Raimbault A., Guenechea G., Lozano M.L., Cerrato L., Hernando M., Gálvez E., Hladun R., Giralt I., Barquinero J., Galy A., García de Andoín N., López R., Catalá A., Schwartz J.D., Surrallés J., Soulier J., Schmidt M., Díaz de Heredia C., Sevilla J., Bueren J.A. Successful engraftment of gene-corrected hematopoietic stem cells in non-conditioned patients with Fanconi anemia. Nat. Med. 2019:1396–1401. doi: 10.1038/s41591-019-0550-z. 2019 25:9. 25. [DOI] [PubMed] [Google Scholar]
- 67.Thompson A.A., Walters M.C., Kwiatkowski J., Rasko J.E.J., Ribeil J.A., Hongeng S., Magrin E., Schiller G.J., Payen E., Semeraro M., Moshous D., Lefrere F., Puy H., Bourget P., Magnani A., Caccavelli L., Diana J.S., Suarez F., Monpoux F., Brousse V., Poirot C., Brouzes C., Meritet J.F., Pondarré C., Beuzard Y., Chrétien S., Lefebvre T., Teachey D.T., Anurathapan U., Ho P.J., von Kalle C., Kletzel M., Vichinsky E., Soni S., Veres G., Negre O., Ross R.W., Davidson D., Petrusich A., Sandler L., Asmal M., Hermine O., de Montalembert M., Hacein-Bey-Abina S., Blanche S., Leboulch P., Cavazzana M. Gene therapy in patients with transfusion-dependent β-thalassemia. New Engl. J. Med. 2018 doi: 10.1056/NEJMoa1705342. [DOI] [PubMed] [Google Scholar]
- 68.Mamcarz E., Zhou S., Lockey T., Abdelsamed H., Cross S.J., Kang G., Ma Z., Condori J., Dowdy J., Triplett B., Li C., Maron G., Aldave Becerra J.C., Church J.A., Dokmeci E., Love J.T., da Matta Ain A.C., van der Watt H., Tang X., Janssen W., Ryu B.Y., de Ravin S.S., Weiss M.J., Youngblood B., Long-Boyle J.R., Gottschalk S., Meagher M.M., Malech H.L., Puck J.M., Cowan M.J., Sorrentino B.P. Lentiviral gene therapy combined with low-dose busulfan in infants with SCID-X1. New Engl. J. Med. 2019;380:1525–1534. doi: 10.1056/NEJMoa1815408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marktel S., Scaramuzza S., Cicalese M.P., Giglio F., Galimberti S., Lidonnici M.R., Calbi V., Assanelli A., Bernardo M.E., Rossi C., Calabria A., Milani R., Gattillo S., Benedicenti F., Spinozzi G., Aprile A., Bergami A., Casiraghi M., Consiglieri G., Masera N., D’Angelo E., Mirra N., Origa R., Tartaglione I., Perrotta S., Winter R., Coppola M., Viarengo G., Santoleri L., Graziadei G., Gabaldo M., Valsecchi M.G., Montini E., Naldini L., Cappellini M.D., Ciceri F., Aiuti A., Ferrari G. Intrabone hematopoietic stem cell gene therapy for adult and pediatric patients affected by transfusion-dependent ß-thalassemia. Nat. Med. 2019;25:234–241. doi: 10.1038/s41591-018-0301-6. [DOI] [PubMed] [Google Scholar]
- 70.Carroll D. Genome engineering with targetable nucleases. Annu Rev. Biochem. 2014;83:409–439. doi: 10.1146/annurev-biochem-060713-035418. [DOI] [PubMed] [Google Scholar]
- 71.Porteus M.H. A new class of medicines through DNA Editing. New Engl. J. Med. 2019;380:947–959. doi: 10.1056/NEJMra1800729. [DOI] [PubMed] [Google Scholar]
- 72.Händel E.M., Alwin S., Cathomen T. Expanding or restricting the rarget site repertoire of zinc-finger nucleases: the inter-domain linker as a major determinant of target site selectivity. Mol. Ther. 2009;17:104–111. doi: 10.1038/mt.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gaj T., Gersbach C.A., Barbas C.F. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31:397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miller J.C., Patil D.P., Xia D.F., Paine C.B., Fauser F., Richards H.W., Shivak D.A., Bendaña Y.R., Hinkley S.J., Scarlott N.A., Lam S.C., Reik A., Zhou Y., Paschon D.E., Li P., Wangzor T., Lee G., Zhang L., Rebar E.J. Enhancing gene editing specificity by attenuating DNA cleavage kinetics. Nat. Biotechnol. 2019;37:945–952. doi: 10.1038/s41587-019-0186-z. [DOI] [PubMed] [Google Scholar]
- 75.Maier D.A., Brennan A.L., Jiang S., Binder-Scholl G.K., Lee G., Plesa G., Zheng Z., Cotte J., Carpenito C., Wood T., Kaye Spratt S., Ando D., Gregory P., Holmes M.C., Perez E.E., Riley J.L., Carroll R.G., June C.H., Levine B.L. Efficient clinical scale gene modification via zinc finger nuclease-targeted disruption of the HIV co-receptor CCR5. Hum. Gene Ther. 2013;24:245–258. doi: 10.1089/hum.2012.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tebas P., Stein D., Tang W.W., Frank I., Wang S.Q., Lee G., Spratt S.K., Surosky R.T., Giedlin M.A., Nichol G., Holmes M.C., Gregory P.D., Ando D.G., Kalos M., Collman R.G., Binder-Scholl G., Plesa G., Hwang W.T., Levine B.L., June C.H. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N. Engl. J. Med. 2014;370:901–910. doi: 10.1056/NEJMoa1300662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Genovese P., Schiroli G., Escobar G., di Tomaso T., Firrito C., Calabria A., Moi D., Mazzieri R., Bonini C., Holmes M.C., Gregory P.D., van der Burg M., Gentner B., Montini E., Lombardo A., Naldini L. Targeted genome editing in human repopulating haematopoietic stem cells. Nature. 2014;510:235–240. doi: 10.1038/nature13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Joung J.K., Sander J.D. TALENs: a widely applicable technology for targeted genome editing. Nat. Rev. Mol. Cell Biol. 2013;14:49–55. doi: 10.1038/nrm3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bedell V.M., Wang Y., Campbell J.M., Poshusta T.L., Starker C.G., Krug R.G., Tan W., Penheiter S.G., Ma A.C., Leung A.Y.H., Fahrenkrug S.C., Carlson D.F., Voytas D.F., Clark K.J., Essner J.J., Ekker S.C. In vivo genome editing using a high-efficiency TALEN system. Nature. 2012;491:114–118. doi: 10.1038/nature11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hockemeyer D., Wang H., Kiani S., Lai C.S., Gao Q., Cassady J.P., Cost G.J., Zhang L., Santiago Y., Miller J.C., Zeitler B., Cherone J.M., Meng X., Hinkley S.J., Rebar E.J., Gregory P.D., Urnov F.D., Jaenisch R. Genetic engineering of human pluripotent cells using TALE nucleases. Nat. Biotechnol. 2011;29:731–734. doi: 10.1038/nbt.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cade L., Reyon D., Hwang W.Y., Tsai S.Q., Patel S., Khayter C., Joung J.K., Sander J.D., Peterson R.T., Yeh J.R.J. Highly efficient generation of heritable zebrafish gene mutations using homo-and heterodimeric TALENs. Nucleic Acids Res. 2012;40:8001–8010. doi: 10.1093/nar/gks518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moscou M.J., Bogdanove A.J. A simple cipher governs DNA recognition by TAL effectors. Science. 1979;(2009) doi: 10.1126/science.1178817. [DOI] [PubMed] [Google Scholar]
- 83.Kuo C.Y., Long J.D., Campo-Fernandez B., de Oliveira S., Cooper A.R., Romero Z., Hoban M.D., Joglekar A.V., Lill G.R., Kaufman M.L., Fitz-Gibbon S., Wang X., Hollis R.P., Kohn D.B. Site-specific gene editing of human hematopoietic stem cells for X-linked hyper-IgM syndrome. Cell Rep. 2018;23:2606–2616. doi: 10.1016/j.celrep.2018.04.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hubbard N., Hagin D., Sommer K., Song Y., Khan I., Clough C., Ochs H.D., Rawlings D.J., Scharenberg A.M., Torgerson T.R. Targeted gene editing restores regulated CD40L function in X-linked hyper-IgM syndrome. Blood. 2016;127:2513–2522. doi: 10.1182/blood-2015-11-683235. [DOI] [PubMed] [Google Scholar]
- 85.Jansen R., van Embden J.D.A., Gaastra W., Schouls L.M. Identification of genes that are associated with DNA repeats in prokaryotes. Mol. Microbiol. 2002;43:1565–1575. doi: 10.1046/j.1365-2958.2002.02839.x. [DOI] [PubMed] [Google Scholar]
- 86.Sorek R., Kunin V., Hugenholtz P. CRISPR - a widespread system that provides acquired resistance against phages in bacteria and archaea. Nat. Rev. Microbiol. 2008;6:181–186. doi: 10.1038/nrmicro1793. [DOI] [PubMed] [Google Scholar]
- 87.van der Oost J., Jore M.M., Westra E.R., Lundgren M., Brouns S.J.J. CRISPR-based adaptive and heritable immunity in prokaryotes. Trends Biochem Sci. 2009;34:401–407. doi: 10.1016/j.tibs.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 88.Bhaya D., Davison M., Barrangou R. CRISPR-cas systems in bacteria and archaea: versatile small RNAs for adaptive defense and regulation. Annu Rev. Genet. 2011;45:273–297. doi: 10.1146/annurev-genet-110410-132430. [DOI] [PubMed] [Google Scholar]
- 89.Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J.A., Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 1979;337(2012):816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hsu P.D., Scott D.A., Weinstein J.A., Ran F.A., Konermann S., Agarwala V., Li Y., Fine E.J., Wu X., Shalem O., Cradick T.J., Marraffini L.A., Bao G., Zhang F. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 2013;31:827–832. doi: 10.1038/nbt.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sternberg S.H., Redding S., Jinek M., Greene E.C., Doudna J.A. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature. 2014;507:62–67. doi: 10.1038/NATURE13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zetsche B., Gootenberg J.S., Abudayyeh O.O., Slaymaker I.M., Makarova K.S., Essletzbichler P., Volz S.E., Joung J., van der Oost J., Regev A., Koonin E.V., Zhang F. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-cas system. Cell. 2015;163:759–771. doi: 10.1016/j.cell.2015.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Slaymaker I.M., Gao L., Zetsche B., Scott D.A., Yan W.X., Zhang F. Rationally engineered Cas9 nucleases with improved specificity. Science. 1979;351(2016):84–88. doi: 10.1126/science.aad5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ran F.A., Hsu P.D., Lin C.Y., Gootenberg J.S., Konermann S., Trevino A.E., Scott D.A., Inoue A., Matoba S., Zhang Y., Zhang F. Double nicking by RNA-guided CRISPR cas9 for enhanced genome editing specificity. Cell. 2013;154:1380–1389. doi: 10.1016/j.cell.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hou Z., Zhang Y., Propson N.E., Howden S.E., Chu L.F., Sontheimer E.J., Thomson J.A. Efficient genome engineering in human pluripotent stem cells using Cas9 from Neisseria meningitidis. Proc. Natl. Acad. Sci. USA. 2013 doi: 10.1073/pnas.1313587110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Müller M., Lee C.M., Gasiunas G., Davis T.H., Cradick T.J., Siksnys V., Bao G., Cathomen T., Mussolino C. Streptococcus thermophilus CRISPR-Cas9 systems enable specific editing of the human genome. Mol. Ther. 2016;24:636–644. doi: 10.1038/mt.2015.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Walton R.T., Christie K.A., Whittaker M.N., Kleinstiver B.P. Unconstrained genome targeting with near-PAMless engineered CRISPR-Cas9 variants. Science. 1979;368(2020):290–296. doi: 10.1126/science.aba8853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kleinstiver B.P., Pattanayak V., Prew M.S., Tsai S.Q., Nguyen N.T., Zheng Z., Joung J.K. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature. 2016;529:490–495. doi: 10.1038/nature16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Casini A., Olivieri M., Petris G., Montagna C., Reginato G., Maule G., Lorenzin F., Prandi D., Romanel A., Demichelis F., Inga A., Cereseto A. A highly specific SpCas9 variant is identified by in vivo screening in yeast. Nat. Biotechnol. 2018 doi: 10.1038/nbt.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kleinstiver B.P., Prew M.S., Tsai S.Q., Topkar V.V., Nguyen N.T., Zheng Z., Gonzales A.P.W., Li Z., Peterson R.T., Yeh J.R.J., Aryee M.J., Joung J.K. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature. 2015 doi: 10.1038/nature14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hu J.H., Miller S.M., Geurts M.H., Tang W., Chen L., Sun N., Zeina C.M., Gao X., Rees H.A., Lin Z., Liu D.R. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity. Nature. 2018;556:57–63. doi: 10.1038/NATURE26155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nishimasu H., Shi X., Ishiguro S., Gao L., Hirano S., Okazaki S., Noda T., Abudayyeh O.O., Gootenberg J.S., Mori H., Oura S., Holmes B., Tanaka M., Seki M., Hirano H., Aburatani H., Ishitani R., Ikawa M., Yachie N., Zhang F., Nureki O. Engineered CRISPR-Cas9 nuclease with expanded targeting space. Science. 2018;361:1259–1262. doi: 10.1126/SCIENCE.AAS9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Frangoul H., Altshuler D., Cappellini M.D., Chen Y.-S., Domm J., Eustace B.K., Foell J., de la Fuente J., Grupp S., Handgretinger R., Ho T.W., Kattamis A., Kernytsky A., Lekstrom-Himes J., Li A.M., Locatelli F., Mapara M.Y., de Montalembert M., Rondelli D., Sharma A., Sheth S., Soni S., Steinberg M.H., Wall D., Yen A., Corbacioglu S. CRISPR-Cas9 gene editing for sickle cell disease and β-thalassemia. N. Engl. J. Med. 2020;384:252–260. doi: 10.1056/NEJMOA2031054. [DOI] [PubMed] [Google Scholar]
- 104.M. Cavazzana, E. Six, C. Lagresle-Peyrou, I. André-Schmutz, S. Hacein-Bey-Abina, Gene Therapy for X-Linked Severe Combined Immunodeficiency: Where Do We Stand?, Https://Home.Liebertpub.Com/Hum. 27 (2016) 108–116. https://doi.org/10.1089/HUM.2015.137. [DOI] [PMC free article] [PubMed]
- 105.Noguchi M., Yi H., Rosenblatt H.M., Filipovich A.H., Adelstein S., Modi W.S., McBride O.W., Leonard W.J. Interleukin-2 receptor γ chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–157. doi: 10.1016/0092-8674(93)90167-O. [DOI] [PubMed] [Google Scholar]
- 106.Lankester A.C., Neven B., Mahlaoui N., von Asmuth E.G.J., Courteille V., Alligon M., Albert M.H., Serra I.B., Bader P., Balashov D., Beier R., Bertrand Y., Blanche S., Bordon V., Bredius R.G., Cant A., Cavazzana M., Diaz-de-Heredia C., Dogu F., Ehlert K., Entz-Werle N., Fasth A., Ferrua F., Ferster A., Formankova R., Friedrich W., Gonzalez-Vicent M., Gozdzik J., Güngör T., Hoenig M., Ikinciogullari A., Kalwak K., Kansoy S., Kupesiz A., Lanfranchi A., Lindemans C.A., Meisel R., Michel G., Miranda N.A.A., Moraleda J., Moshous D., Pichler H., Rao K., Sedlacek P., Slatter M., Soncini E., Speckmann C., Sundin M., Toren A., Vettenranta K., Worth A., Yeşilipek M.A., Zecca M., Porta F., Schulz A., Veys P., Fischer A., Gennery A.R. Hematopoietic cell transplantation in severe combined immunodeficiency: the SCETIDE 2006-2014 European cohort. J. Allergy Clin. Immunol. 2022;149:1744–1754. doi: 10.1016/J.JACI.2021.10.017. e8. [DOI] [PubMed] [Google Scholar]
- 107.Buckley R.H. Transplantation of hematopoietic stem cells in human severe combined immunodeficiency: longterm outcomes. Immunol. Res. 2011;49:25–43. doi: 10.1007/S12026-010-8191-9/FIGURES/7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mazzolari E., Forino C., Guerci S., Imberti L., Lanfranchi A., Porta F., Notarangelo L.D. Long-term immune reconstitution and clinical outcome after stem cell transplantation for severe T-cell immunodeficiency. J. Allergy Clin. Immunol. 2007;120:892–899. doi: 10.1016/J.JACI.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 109.Haddad E., Logan B.R., Griffith L.M., Buckley R.H., Parrott R.E., Prockop S.E., Small T.N., Chaisson J., Dvorak C.C., Murnane M., Kapoor N., Abdel-Azim H., Hanson I.C., Martinez C., Bleesing J.J.H., Chandra S., Smith A.R., Cavanaugh M.E., Jyonouchi S., Sullivan K.E., Burroughs L., Skoda-Smith S., Haight A.E., Tumlin A.G., Quigg T.C., Taylor C., Dávila Saldaña B.J., Keller M.D., Seroogy C.M., Desantes K.B., Petrovic A., Leiding J.W., Shyr D.C., Decaluwe H., Teira P., Gillio A.P., Knutsen A.P., Moore T.B., Kletzel M., Craddock J.A., Aquino V., Davis J.H., Yu L.C., Cuvelier G.D.E., Bednarski J.J., Goldman F.D., Kang E.M., Shereck E., Porteus M.H., Connelly J.A., Fleisher T.A., Malech H.L., Shearer W.T., Szabolcs P., Thakar M.S., vander Lugt M.T., Heimall J., Yin Z., Pulsipher M.A., Pai S.Y., Kohn D.B., Puck J.M., Cowan M.J., O’Reilly R.J., Notarangelo L.D. SCID genotype and 6-month posttransplant CD4 count predict survival and immune recovery. Blood. 2018;132:1737–1749. doi: 10.1182/BLOOD-2018-03-840702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kurzay M., Hauck F., Schmid I., Wiebking V., Eichinger A., Jung E., Boekstegers A., Feuchtinger T., Klein C., Albert M.H. T-cell replete haploidentical bone marrow transplantation and post-transplant cyclophosphamide for patients with inborn errors. Haematologica. 2019;104:E478–E482. doi: 10.3324/HAEMATOL.2018.215285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shah R.M., Elfeky R., Nademi Z., Qasim W., Amrolia P., Chiesa R., Rao K., Lucchini G., Silva J.M.F., Worth A., Barge D., Ryan D., Conn J., Cant A.J., Skinner R., Abd Hamid I.J., Flood T., Abinun M., Hambleton S., Gennery A.R., Veys P., Slatter M. T-cell receptor αβ+ and CD19+ cell-depleted haploidentical and mismatched hematopoietic stem cell transplantation in primary immune deficiency. J. Allergy Clin. Immunol. 2018;141:1417–1426. doi: 10.1016/J.JACI.2017.07.008. e1. [DOI] [PubMed] [Google Scholar]
- 112.Balashov D., Shcherbina A., Maschan M., Trakhtman P., Skvortsova Y., Shelikhova L., Laberko A., Livshits A., Novichkova G., Maschan A. Single-center experience of unrelated and haploidentical stem cell transplantation with TCRαβ and CD19 depletion in children with primary immunodeficiency syndromes. Biol. Blood Marrow Transpl. 2015;21:1955–1962. doi: 10.1016/J.BBMT.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 113.Hacein-Bey S., saint Basile G.D., Lemerle J., Fischer A., Cavazzana-Calvo M. γc gene transfer in the presence of stem cell factor, FLT-3L, Interleukin-7 (IL-7), IL-1α, and IL-15 Cytokines Restores T-Cell Differentiation From γc(−) X-Linked Severe Combined Immunodeficiency Hematopoietic Progenitor Cells in Murine Fetal Thymic Organ Cultures. Blood. 1998;92:4090–4097. doi: 10.1182/BLOOD.V92.11.4090. [DOI] [PubMed] [Google Scholar]
- 114.Hacein-Bey-Abina S., le Deist F., Carlier F., Bouneaud C., Hue C., de Villartay J.-P., Thrasher A.J., Wulffraat N., Sorensen R., Dupuis-Girod S., Fischer A., Davies E.G., Kuis W., Leiva L., Cavazzana-Calvo M. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. New Engl. J. Med. 2002;346:1185–1193. doi: 10.1056/NEJMOA012616. [DOI] [PubMed] [Google Scholar]
- 115.Gaspar H.B., Parsley K.L., Howe S., King D., Gilmour K.C., Sinclair J., Brouns G., Schmidt M., von Kalle C., Barington T., Jakobsen M.A., Christensen H.O., Al Ghonaium A., White H.N., Smith J.L., Levinsky P.R.J., Ali P.R.R., Kinnon P.C., Thrasher P.A.J. Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector. Lancet. 2004;364:2181–2187. doi: 10.1016/S0140-6736(04)17590-9. [DOI] [PubMed] [Google Scholar]
- 116.Gaspar H.B., Cooray S., Gilmour K.C., Parsley K.L., Adams S., Howe S.J., Al Ghonaium A., Bayford J., Brown L., Davies E.G., Kinnon C., Thrasher A.J. Immunodeficiency: Long-term persistence of a polyclonal t cell repertoire after gene therapy for X-linked severe combined immunodeficiency. Sci. Transl. Med. 2011;3 doi: 10.1126/SCITRANSLMED.3002715/SUPPL_FILE/3-97RA79_SM.PDF. [DOI] [PubMed] [Google Scholar]
- 117.Hacein-Bey-Abina S., Hauer J., Lim A., Picard C., Wang G.P., Berry C.C., Martinache C., Rieux-Laucat F., Latour S., Belohradsky B.H., Leiva L., Sorensen R., Debré M., Casanova J.L., Blanche S., Durandy A., Bushman F.D., Fischer A., Cavazzana-Calvo M. Efficacy of gene therapy for X-linked severe combined immunodeficiency. New Engl. J. Med. 2010;363:355–364. doi: 10.1056/NEJMOA1000164/SUPPL_FILE/NEJMOA1000164_DISCLOSURES.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hacein-Bey-Abina S., Garrigue A., Wang G.P., Soulier J., Lim A., Morillon E., Clappier E., Caccavelli L., Delabesse E., Beldjord K., Asnafi V., MacIntyre E., Dal Cortivo L., Radford I., Brousse N., Sigaux F., Moshous D., Hauer J., Borkhardt A., Belohradsky B.H., Wintergerst U., Velez M.C., Leiva L., Sorensen R., Wulffraat N., Blanche S., Bushman F.D., Fischer A., Cavazzana-Calvo M. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Investig. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Amorosi S., Russo I., Amodio G., Garbi C., Vitiello L., Palamaro L., Adriani M., Vigliano I., Pignata C. The cellular amount of the common gamma-chain influences spontaneous or induced cell proliferation. J. Immunol. 2009;182:3304–3309. doi: 10.4049/JIMMUNOL.0802400. [DOI] [PubMed] [Google Scholar]
- 120.Zychlinski D., Schambach A., Modlich U., Maetzig T., Meyer J., Grassman E., Mishra A., Baum C. Physiological promoters reduce the genotoxic risk of integrating gene vectors. Mol. Ther. 2008;16:718–725. doi: 10.1038/MT.2008.5. [DOI] [PubMed] [Google Scholar]
- 121.Hacein-Bey-Abina S., Pai S.Y., Gaspar H.B., Armant M., Berry C.C., Blanche S., Bleesing J., Blondeau J., de Boer H., Buckland K.F., Caccavelli L., Cros G., de Oliveira S., Fernández K.S., Guo D., Harris C.E., Hopkins G., Lehmann L.E., Lim A., London W.B., van der Loo J.C.M., Malani N., Male F., Malik P., Marinovic M.A., McNicol A.M., Moshous D., Neven B., Oleastro M., Picard C., Ritz J., Rivat C., Schambach A., Shaw K.L., Sherman E.A., Silberstein L.E., Six E., Touzot F., Tsytsykova A., Xu-Bayford J., Baum C., Bushman F.D., Fischer A., Kohn D.B., Filipovich A.H., Notarangelo L.D., Cavazzana M., Williams D.A., Thrasher A.J. A modified γ-retrovirus vector for X-linked severe combined immunodeficiency. New Engl. J. Med. 2014;371:1407–1417. doi: 10.1056/NEJMoa1404588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pai S.Y., Thrasher A.J. Gene therapy for X-linked severe combined immunodeficiency: Historical outcomes and current status. J. Allergy Clin. Immunol. 2020;146:258–261. doi: 10.1016/J.JACI.2020.05.055. [DOI] [PubMed] [Google Scholar]
- 123.Miggelbrink A.M., Logan B.R., Buckley R.H., Parrott R.E., Dvorak C.C., Kapoor N., Abdel-Azim H., Prockop S.E., Shyr D., Decaluwe H., Hanson I.C., Gillio A., Dávila Saldaña B.J., Eibel H., Hopkins G., Walter J.E., Whangbo J.S., Kohn D.B., Puck J.M., Cowan M.J., Griffith L.M., Haddad E., O’Reilly R.J., Notarangelo L.D., Pai S.Y. B-cell differentiation and IL-21 response in IL2RG/JAK3 SCID patients after hematopoietic stem cell transplantation. Blood. 2018;131:2967–2977. doi: 10.1182/BLOOD-2017-10-809822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.de Ravin S.S., Wu X., Moir S., Anaya-O’Brien S., Kwatemaa N., Littel P., Theobald N., Choi U., Su L., Marquesen M., Hilligoss D., Lee J., Buckner C.M., Zarember K.A., O’Connor G., McVicar D., Kuhns D., Throm R.E., Zhou S., Notarangelo L.D., Hanson I.C., Cowan M.J., Kang E., Hadigan C., Meagher M., Gray J.T., Sorrentino B.P., Malech H.L. Lentiviral hematopoietic stem cell gene therapy for X-linked severe combined immunodeficiency. Sci. Transl. Med. 2016;8 doi: 10.1126/scitranslmed.aad8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.de Ravin S.S., Liu S., Sweeney C.L., Brault J., Whiting-Theobald N., Ma M., Liu T., Choi U., Lee J., O’Brien S.A., Quackenbush P., Estwick T., Karra A., Docking E., Kwatemaa N., Guo S., Su L., Sun Z., Zhou S., Puck J., Cowan M.J., Notarangelo L.D., Kang E., Malech H.L., Wu X. Lentivector cryptic splicing mediates increase in CD34+ clones expressing truncated HMGA2 in human X-linked severe combined immunodeficiency. Nat. Commun. 2022;13 doi: 10.1038/S41467-022-31344-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang G.P., Ciuffi A., Leipzig J., Berry C.C., Bushman F.D. HIV integration site selection: analysis by massively parallel pyrosequencing reveals association with epigenetic modifications. Genome Res. 2007;17:1186–1194. doi: 10.1101/GR.6286907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Urnov F.D., Miller J.C., Lee Y.L., Beausejour C.M., Rock J.M., Augustus S., Jamieson A.C., Porteus M.H., Gregory P.D., Holmes M.C. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature. 2005;435:646–651. doi: 10.1038/nature03556. [DOI] [PubMed] [Google Scholar]
- 128.Pavel-Dinu M., Wiebking V., Dejene B.T., Srifa W., Mantri S., Nicolas C.E., Lee C., Bao G., Kildebeck E.J., Punjya N., Sindhu C., Inlay M.A., Saxena N., DeRavin S.S., Malech H., Roncarolo M.G., Weinberg K.I., Porteus M.H. Gene correction for SCID-X1 in long-term hematopoietic stem cells. Nat. Commun. 2019;10 doi: 10.1038/s41467-019-09614-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Flinn A.M., Gennery A.R. Adenosine deaminase deficiency: a review. Orphanet J. Rare Dis. 2018;13:1–7. doi: 10.1186/S13023-018-0807-5/METRICS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ryser O., Morell A., Hitzig W.H. Primary immunodeficiencies in Switzerland: first report of the national registry in adults and children. J. Clin. Immunol. 1988;8:479–485. doi: 10.1007/BF00916954. [DOI] [PubMed] [Google Scholar]
- 131.Yee A., de Ravin S.S., Elliott E., Ziegler J.B. Severe combined immunodeficiency: a national surveillance study. Pedia Allergy Immunol. 2008;19:298–302. doi: 10.1111/J.1399-3038.2007.00646.X. [DOI] [PubMed] [Google Scholar]
- 132.Verbsky J.W., Baker M.W., Grossman W.J., Hintermeyer M., Dasu T., Bonacci B., Reddy S., Margolis D., Casper J., Gries M., DeSantes K., Hoffman G.L., Brokopp C.D., Seroogy C.M., Routes J.M. Newborn screening for severe combined immunodeficiency; the Wisconsin experience (2008-2011) J. Clin. Immunol. 2012;32:82–88. doi: 10.1007/S10875-011-9609-4. [DOI] [PubMed] [Google Scholar]
- 133.Vogel B.H., Bonagura V., Weinberg G.A., Ballow M., Isabelle J., Diantonio L., Parker A., Young A., Cunningham-Rundles C., Fong C.T., Celestin J., Lehman H., Rubinstein A., Siegel S., Weiner L., Saavedra-Matiz C., Kay D.M., Caggana M. Newborn screening for SCID in New York State: experience from the first two years. J. Clin. Immunol. 2014;34:289–303. doi: 10.1007/S10875-014-0006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bradford K.L., Moretti F.A., Carbonaro-Sarracino D.A., Gaspar H.B., Kohn D.B. Adenosine deaminase (ADA)-deficient severe combined immune deficiency (SCID): molecular pathogenesis and clinical manifestations. J. Clin. Immunol. 2017;37:626–637. doi: 10.1007/S10875-017-0433-3. [DOI] [PubMed] [Google Scholar]
- 135.Hershfield M.S. Adenosine deaminase deficiency: clinical expression, molecular basis, and therapy. Semin Hematol. 1998;35:291–298. [PubMed] [Google Scholar]
- 136.Sauer A.V., Brigida I., Carriglio N., Aiuti A. Autoimmune dysregulation and purine metabolism in adenosine deaminase deficiency. Front Immunol. 2012;3 doi: 10.3389/FIMMU.2012.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sauer A. v, Hernandez R.J., Fumagalli F., Bianchi V., Poliani P.L., Dallatomasina C., Riboni E., Politi L.S., Tabucchi A., Carlucci F., Casiraghi M., Carriglio N., Cominelli M., Forcellini C.A., Barzaghi F., Ferrua F., Minicucci F., Medaglini S., Leocani L., la Marca G., Notarangelo L.D., Azzari C., Comi G., Baldoli C., Canale S., Sessa M., D’Adamo P., Aiuti A. Alterations in the brain adenosine metabolism cause behavioral and neurological impairment in ADA-deficient mice and patients. Sci. Rep. 2017;7 doi: 10.1038/SREP40136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nikolajeva O., Worth A., Hague R., Martinez-Alier N., Smart J., Adams S., Davies E.G., Gaspar H.B. Adenosine deaminase deficient severe combined immunodeficiency presenting as atypical haemolytic uraemic syndrome. J. Clin. Immunol. 2015;35:366–372. doi: 10.1007/S10875-015-0158-0. [DOI] [PubMed] [Google Scholar]
- 139.Sauer A. v, Aiuti A. New insights into the pathogenesis of adenosine deaminase-severe combined immunodeficiency and progress in gene therapy. Curr. Opin. Allergy Clin. Immunol. 2009;9:496–502. doi: 10.1097/ACI.0B013E3283327DA5. [DOI] [PubMed] [Google Scholar]
- 140.Ghimenton E., Flinn A., Lum S.H., Leahy T.R., Nademi Z., Owens S., Williams E., Flood T., Hambleton S., Slatter M., Gennery A.R. Hematopoietic cell transplantation for adenosine deaminase severe combined immunodeficiency-improved outcomes in the modern era. J. Clin. Immunol. 2022;42:819–826. doi: 10.1007/S10875-022-01238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chan B., Wara D., Bastian J., Hershfield M.S., Bohnsack J., Azen C.G., Parkman R., Weinberg K., Kohn D.B. Long-term efficacy of enzyme replacement therapy for Adenosine deaminase (ADA)-deficient Severe Combined Immunodeficiency (SCID. Clin. Immunol. 2005;117:133–143. doi: 10.1016/J.CLIM.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 142.Hassan A., Booth C., Brightwell A., Allwood Z., Veys P., Rao K., Hönig M., Friedrich W., Gennery A., Slatter M., Bredius R., Finocchi A., Cancrini C., Aiuti A., Porta F., Lanfranchi A., Ridella M., Steward C., Filipovich A., Marsh R., Bordon V., Al-Muhsen S., Al-Mousa H., Alsum Z., Al-Dhekri H., Al Ghonaium A., Speckmann C., Fischer A., Mahlaoui N., Nichols K.E., Grunebaum E., al Zahrani D., Roifman C.M., Boelens J., Davies E.G., Cavazzana-Calvo M., Notarangelo L., Gaspar H.B. Outcome of hematopoietic stem cell transplantation for adenosine deaminase-deficient severe combined immunodeficiency. Blood. 2012:3615–3624. doi: 10.1182/BLOOD-2011-12-396879. [DOI] [PubMed] [Google Scholar]
- 143.Bordignon C., Notarangelo L.D., Nobili N., Ferrari G., Casorati G., Panina P., Mazzolari E., Maggioni D., Rossi C., Servida P., Ugazio A.G., Mavilio F. Gene therapy in peripheral blood lymphocytes and bone marrow for ADA- immunodeficient patients. Science. 1979;270(1995):470–475. doi: 10.1126/science.270.5235.470. [DOI] [PubMed] [Google Scholar]
- 144.Blaese R.M., Culver K.W., Miller A.D., Carter C.S., Fleisher T., Clerici M., Shearer G., Chang L., Chiang Y., Tolstoshev P., Greenblatt J.J., Rosenberg S.A., Klein H., Berger M., Mullen C.A., Ramsey W.J., Muul L., Morgan R.A., Anderson W.F. T lymphocyte-directed gene therapy for ADA- SCID: initial trial results after 4 years. Science. 1995;270:475–480. doi: 10.1126/SCIENCE.270.5235.475. [DOI] [PubMed] [Google Scholar]
- 145.Onodera M., Ariga T., Kawamura N., Kobayashi I., Ohtsu M., Yamada M., Tame A., Furuta H., Okano M., Matsumoto S., Kotani H., McGarrity G.J., Michael Blaese R., Sakiyama Y. Successful peripheral T-lymphocyte–directed gene transfer for a patient with severe combined immune deficiency caused by adenosine deaminase deficiency. Blood. 1998;91:30–36. doi: 10.1182/BLOOD.V91.1.30. [DOI] [PubMed] [Google Scholar]
- 146.Kohn D.B., Weinberg K.I., Nolta J.A., Heiss L.N., Lenarsky C., Crooks G.M., Hanley M.E., Annett G., Brooks J.S., El-Khoureiy A., Lawrence K., Wells S., Moen R.C., Bastian J., Williams-Herman D.E., Elder M., Wara D., Bowen T., Hershfield M.S., Mullen C.A., Blaese R.M., Parkman R. Engraftment of gene–modified umbilical cord blood cells in neonates with adenosine deaminase deficiency. Nat. Med. 1995:1017–1023. doi: 10.1038/nm1095-1017. 1995 1:10. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hoogerbrugge P.M., van Beusechem V.W., Fischer A., Debree M., le Deist F., Perignon J.L., Morgan G., Gaspar B., Fairbanks L.D., Skeoch C.H., Moseley A., Harvey M., Levinsky R.J., Valerio D. Bone marrow gene transfer in three patients with adenosine deaminase deficiency. Gene Ther. 1996;3:179–183. [PubMed] [Google Scholar]
- 148.Aiuti A., Slavin S., Aker M., Ficara F., Deola S., Mortellaro A., Morecki S., Andolfi G., Tabucchi A., Carlucci F., Marinello E., Cattaneo F., Vai S., Servida P., Miniero R., Roncarolo M.G., Bordignon C. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 1979;296(2002):2410–2413. doi: 10.1126/science.1070104. [DOI] [PubMed] [Google Scholar]
- 149.Gaspar H.B., Cooray S., Gilmour K.C., Parsley K.L., Zhang F., Adams S., Bjorkegren E., Bayford J., Brown L., Davies E.G., Veys P., Fairbanks L., Bordon V., Petropolou T., Kinnon C., Thrasher A.J. Hematopoietic stem cell gene therapy for adenosine deaminase-deficient severe combined immunodeficiency leads to long-term immunological recovery and metabolic correction. Sci. Transl. Med. 2011;3 doi: 10.1126/SCITRANSLMED.3002716. [DOI] [PubMed] [Google Scholar]
- 150.Shaw K.L., Garabedian E., Mishra S., Barman P., Davila A., Carbonaro D., Shupien S., Silvin C., Geiger S., Nowicki B., Smogorzewska E.M., Brown B., Wang X., de Oliveira S., Choi Y., Ikeda A., Terrazas D., Fu P.Y., Yu A., Fernandez B.C., Cooper A.R., Engel B., Podsakoff G., Balamurugan A., Anderson S., Muul L., Jagadeesh G.J., Kapoor N., Tse J., Moore T.B., Purdy K., Rishi R., Mohan K., Skoda-Smith S., Buchbinder D., Abraham R.S., Scharenberg A., Yang O.O., Cornetta K., Gjertson D., Hershfield M., Sokolic R., Candotti F., Kohn D.B. Clinical efficacy of gene-modified stem cells in adenosine deaminase-deficient immunodeficiency. J. Clin. Invest. 2017;127:1689–1699. doi: 10.1172/JCI90367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.M.P. Cicalese, F. Ferrua, L. Castagnaro, R. Pajno, F. Barzaghi, S. Giannelli, F. Dionisio, I. Brigida, M. Bonopane, M. Casiraghi, A. Tabucchi, F. Carlucci, E. Grunebaum, M. Adeli, R.G. Bredius, J.M. Puck, P. Stepensky, I. Tezcan, K. Rolfe, E. de Boever, R.R. Reinhardt, J. Appleby, F. Ciceri, M.G. Roncarolo, A. Aiuti, Update on the safety and efficacy of retroviral gene therapy for immunodeficiency due to adenosine deaminase deficiency, Blood. (2016). [DOI] [PMC free article] [PubMed]
- 152.Cicalese M.P., Ferrua F., Castagnaro L., Rolfe K., de Boever E., Reinhardt R.R., Appleby J., Roncarolo M.G., Aiuti A. Gene therapy for adenosine deaminase deficiency: a comprehensive evaluation of short- and medium-term safety. Mol. Ther. 2018;26:917–931. doi: 10.1016/J.YMTHE.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Aiuti A., Roncarolo M.G., Naldini L. Gene therapy for ADA-SCID, the first marketing approval of an ex vivo gene therapy in Europe: paving the road for the next generation of advanced therapy medicinal products. EMBO Mol. Med. 2017;9:737–740. doi: 10.15252/EMMM.201707573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Aiuti A., Cattaneo F., Galimberti S., Benninghoff U., Cassani B., Callegaro L., Scaramuzza S., Andolfi G., Mirolo M., Brigida I., Tabucchi A., Carlucci F., Eibl M., Aker M., Slavin S., Al-Mousa H., Al Ghonaium A., Ferster A., Duppenthaler A., Notarangelo L., Wintergerst U., Buckley R.H., Bregni M., Marktel S., Valsecchi M.G., Rossi P., Ciceri F., Miniero R., Bordignon C., Roncarolo M.-G. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N. Engl. J. Med. 2009;360:447–458. doi: 10.1056/NEJMOA0805817. [DOI] [PubMed] [Google Scholar]
- 155.Gaspar H.B., Bjorkegren E., Parsley K., Gilmour K.C., King D., Sinclair J., Zhang F., Giannakopoulos A., Adams S., Fairbanks L.D., Gaspar J., Henderson L., Xu-Bayford J.H., Davies E.G., Veys P.A., Kinnon C., Thrasher A.J. Successful reconstitution of immunity in ADA-SCID by stem cell gene therapy following cessation of PEG-ADA and use of mild preconditioning. Mol. Ther. 2006;14:505–513. doi: 10.1016/j.ymthe.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 156.Candotti F., Shaw K.L., Muul L., Carbonaro D., Sokolic R., Choi C., Schurman S.H., Garabedian E., Kesserwan C., Jagadeesh G.J., Fu P.Y., Gschweng E., Cooper A., Tisdale J.F., Weinberg K.I., Crooks G.M., Kapoor N., Shah A., Abdel-Azim H., Yu X.J., Smogorzewska M., Wayne A.S., Rosenblatt H.M., Davis C.M., Hanson C., Rishi R.G., Wang X., Gjertson D., Yang O.O., Balamurugan A., Bauer G., Ireland J.A., Engel B.C., Podsakoff G.M., Hershfield M.S., Blaese R.M., Parkman R., Kohn D.B. Gene therapy for adenosine deaminase-deficient severe combined immune deficiency: clinical comparison of retroviral vectors and treatment plans. Blood. 2012;120:3635–3646. doi: 10.1182/BLOOD-2012-02-400937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Reinhardt B., Habib O., Shaw K.L., Garabedian E., Carbonaro-Sarracino D.A., Terrazas D., Fernandez B.C., de Oliveira S., Moore T.B., Ikeda A.K., Engel B.C., Podsakoff G.M., Hollis R.P., Fernandes A., Jackson C., Shupien S., Mishra S., Davila A., Mottahedeh J., Vitomirov A., Meng W., Rosenfeld A.M., Roche A.M., Hokama P., Reddy S., Everett J., Wang X., Luning Prak E.T., Cornetta K., Hershfield M.S., Sokolic R., de Ravin S.S., Malech H.L., Bushman F.D., Candotti F., Kohn D.B. Long-term outcomes after gene therapy for adenosine deaminase severe combined immune deficiency. Blood. 2021;138:1304–1316. doi: 10.1182/BLOOD.2020010260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tucci F., Galimberti S., Naldini L., Valsecchi M.G., Aiuti A. A systematic review and meta-analysis of gene therapy with hematopoietic stem and progenitor cells for monogenic disorders. Nat. Commun. 2022:1–13. doi: 10.1038/s41467-022-28762-2. 2022 13:1. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Carbonaro D.A., Zhang L., Jin X., Montiel-Equihua C., Geiger S., Carmo M., Cooper A., Fairbanks L., Kaufman M.L., Sebire N.J., Hollis R.P., Blundell M.P., Senadheera S., Fu P.Y., Sahaghian A., Chan R.Y., Wang X., Cornetta K., Thrasher A.J., Kohn D.B., Gaspar H.B. Preclinical demonstration of lentiviral vector-mediated correction of immunological and metabolic abnormalities in models of adenosine deaminase deficiency. Mol. Ther. 2014;22:607–622. doi: 10.1038/MT.2013.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cuvelier G.D.E., Logan B.R., Prockop S.E., Buckley R.H., Kuo C.Y., Griffith L.M., Liu X., Yip A., Hershfield M.S., Ayoub P.G., Moore T.B., Dorsey M.J., O’Reilly R.J., Kapoor N., Pai S.Y., Kapadia M., Ebens C.L., Forbes Satter L.R., Burroughs L.M., Petrovic A., Chellapandian D., Heimall J., Shyr D.C., Rayes A., Bednarski J.J., Chandra S., Chandrakasan S., Gillio A.P., Madden L., Quigg T.C., Caywood E.H., Dávila Saldaña B.J., DeSantes K., Eissa H., Goldman F.D., Rozmus J., Shah A.J., vander Lugt M.T., Thakar M.S., Parrott R.E., Martinez C., Leiding J.W., Torgerson T.R., Pulsipher M.A., Notarangelo L.D., Cowan M.J., Dvorak C.C., Haddad E., Puck J.M., Kohn D.B. Outcomes following treatment for ADA-deficient severe combined immunodeficiency: a report from the PIDTC. Blood. 2022;140:685–705. doi: 10.1182/BLOOD.2022016196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Joglekar A. v, Hollis R.P., Kuftinec G., Senadheera S., Chan R., Kohn D.B. Integrase-defective lentiviral vectors as a delivery platform for targeted modification of adenosine deaminase locus. Mol. Ther. 2013;21:1705–1717. doi: 10.1038/MT.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Calero-Garcia M., Carmo M., Thrasher A., Gaspar H.B. 140. Point mutation correction for ADA SCID. Mol. Ther. 2016;24:S57. doi: 10.1016/S1525-0016(16)32949-5. [DOI] [Google Scholar]
- 163.Bassing C.H., Swat W., Alt F.W. The mechanism and regulation of chromosomal V(D)J recombination. Cell. 2002;109:S45–S55. doi: 10.1016/S0092-8674(02)00675-X. [DOI] [PubMed] [Google Scholar]
- 164.Moshous D., Callebaut I., de Chasseval R., Corneo B., Cavazzana-Calvo M., le Deist F., Tezcan I., Sanal O., Bertrand Y., Philippe N., Fischer A., de Villartay J.P. Artemis, a novel DNA double-strand break repair/V(D)J recombination protein, is mutated in human severe combined immune deficiency. Cell. 2001;105:177–186. doi: 10.1016/S0092-8674(01)00309-9. [DOI] [PubMed] [Google Scholar]
- 165.Ege M., Ma Y., Manfras B., Kalwak K., Lu H., Lieber M.R., Schwarz K., Pannicke U. Omenn syndrome due to ARTEMIS mutations. Blood. 2005;105:4179–4186. doi: 10.1182/BLOOD-2004-12-4861. [DOI] [PubMed] [Google Scholar]
- 166.Felgentreff K., Lee Y.N., Frugoni F., Du L., van der Burg M., Giliani S., Tezcan I., Reisli I., Mejstrikova E., de Villartay J.P., Sleckman B.P., Manis J., Notarangelo L.D. Functional analysis of naturally occurring DCLRE1C mutations and correlation with the clinical phenotype of ARTEMIS deficiency. J. Allergy Clin. Immunol. 2015;136:140–150. doi: 10.1016/j.jaci.2015.03.005. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schuetz C., Neven B., Dvorak C.C., Leroy S., Ege M.J., Pannicke U., Schwarz K., Schulz A.S., Hoenig M., Sparber-Sauer M., Gatz S.A., Denzer C., Blanche S., Moshous D., Picard C., Horn B.N., de Villartay J.P., Cavazzana M., Debatin K.M., Friedrich W., Fischer A., Cowan M.J. SCID patients with ARTEMIS vs RAG deficiencies following HCT: increased risk of late toxicity in ARTEMIS-deficient SCID. Blood. 2014;123:281–289. doi: 10.1182/BLOOD-2013-01-476432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Neven B., Ferrua F. Hematopoietic stem cell transplantation for combined immunodeficiencies, on behalf of IEWP-EBMT. Front Pedia. 2020;7:552. doi: 10.3389/FPED.2019.00552/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Punwani D., Kawahara M., Yu J., Sanford U., Roy S., Patel K., Carbonaro D.A., Karlen A.D., Khan S., Cornetta K., Rothe M., Schambach A., Kohn D.B., Malech H.L., McIvor R.S., Puck J.M., Cowan M.J. Lentivirus mediated correction of artemis-deficient severe combined immunodeficiency. 2017;28:112–124. doi: 10.1089/HUM.2016.064. 〈Https://Home.Liebertpub.Com/Hum〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Multhaup M., Karlen A.D., Swanson D.L., Wilber A., Somia N.V., Cowan M.J., McIvor R.S. Cytotoxicity associated with artemis overexpression after lentiviral vector-mediated gene transfer. 2010;21:865–875. doi: 10.1089/HUM.2009.162. 〈Https://Home.Liebertpub.Com/Hum〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.M.M. Multhaup, K.M. Podetz-Pedersen, A.D. Karlen, E.R. Olson, R. Gunther, N.V. Somia, B.R. Blazar, M.J. Cowan, R.S. McIvor, Role of Transgene Regulation in Ex Vivo Lentiviral Correction of Artemis Deficiency, Https://Home.Liebertpub.Com/Hum. 26 (2015) 232–243. https://doi.org/10.1089/HUM.2014.062. [DOI] [PMC free article] [PubMed]
- 172.Charrier S., Lagresle-Peyrou C., Poletti V., Rothe M., Cédrone G., Gjata B., Mavilio F., Fischer A., Schambach A., de Villartay J.P., Cavazzana M., Hacein-Bey-Abina S., Galy A. Biosafety studies of a clinically applicable lentiviral vector for the gene therapy of artemis-SCID. Mol. Ther. Methods Clin. Dev. 2019;15:232–245. doi: 10.1016/j.omtm.2019.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cowan M.J., Yu J., Facchino J., Chag S., Fraser-Browne C., Long-Boyle J., Kawahara M., Sanford U., Oh J., Teoh S., Punwani D., Dara J., Dvorak C.C., Broderick L., Hu D., Miller H.K., Petrovic A., Malech H.L., McIvor R.S., Puck J. Early outcome of a phase I/II clinical trial ( NCT03538899) of gene-corrected autologous CD34+ hematopoietic cells and low-exposure busulfan in newly diagnosed patients with artemis-deficient severe combined immunodeficiency (ART-SCID. Biol. Blood Marrow Transplant. 2020;26:S88–S89. doi: 10.1016/j.bbmt.2019.12.589. [DOI] [Google Scholar]
- 174.Cowan M.J., Yu J., Facchino J., Fraser-Browne C., Sanford U., Kawahara M., Dara J., Long-Boyle J., Oh J., Chan W., Chag S., Broderick L., Chellapandian D., Decaluwe H., Golski C., Hu D., Kuo C.Y., Miller H.K., Petrovic A., Currier R., Hilton J.F., Punwani D., Dvorak C.C., Malech H.L., McIvor R.S., Puck J.M. Lentiviral gene therapy for artemis-deficient SCID. New Engl. J. Med. 2022;387:2344–2355. doi: 10.1056/NEJMOA2206575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Agarwal R., Dvorak C.C., Prohaska S., Long-Boyle J., Kwon H.-S., (Wes) J., Brown M., Weinberg K.I., Le A., Guttman-Klein A., Logan A.C., Weissman I.L., Digiusto D., Cowan M.J., Parkman R., Roncarolo M.G., Shizuru J.A. Toxicity-free hematopoietic stem cell engraftment achieved with Anti-CD117 monoclonal antibody conditioning. Biol. Blood Marrow Transplant. 2019;25:S92. doi: 10.1016/J.BBMT.2018.12.172. [DOI] [Google Scholar]
- 176.Notarangelo L.D., Kim M.S., Walter J.E., Lee Y.N. Human RAG mutations: biochemistry and clinical implications. Nat. Rev. Immunol. 2016:234–246. doi: 10.1038/nri.2016.28. 2016 16:4. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Villa A., Notarangelo L.D. RAG gene defects at the verge of immunodeficiency and immune dysregulation. Immunol. Rev. 2019;287:73–90. doi: 10.1111/IMR.12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Neven B., Leroy S., Decaluwe H., le Deist F., Picard C., Moshous D., Mahlaoui N., Debré M., Casanova J.L., Dal Cortivo L., Madec Y., Hacein-Bey-Abina S., de Saint Basile G., de Villartay J.P., Blanche S., Cavazzana-Calvo M., Fischer A. Long-term outcome after hematopoietic stem cell transplantation of a single-center cohort of 90 patients with severe combined immunodeficiency. Blood. 2009;113:4114–4124. doi: 10.1182/BLOOD-2008-09-177923. [DOI] [PubMed] [Google Scholar]
- 179.Pai S.-Y., Logan B.R., Griffith L.M., Buckley R.H., Parrott R.E., Dvorak C.C., Kapoor N., Hanson I.C., Filipovich A.H., Jyonouchi S., Sullivan K.E., Small T.N., Burroughs L., Skoda-Smith S., Haight A.E., Grizzle A., Pulsipher M.A., Chan K.W., Fuleihan R.L., Haddad E., Loechelt B., Aquino V.M., Gillio A., Davis J., Knutsen A., Smith A.R., Moore T.B., Schroeder M.L., Goldman F.D., Connelly J.A., Porteus M.H., Xiang Q., Shearer W.T., Fleisher T.A., Kohn D.B., Puck J.M., Notarangelo L.D., Cowan M.J., O’Reilly R.J. Transplantation outcomes for severe combined immunodeficiency, 2000–2009. New England Journal of Medicine. 2014:434–446. doi: 10.1056/NEJMOA1401177/SUPPL_FILE/NEJMOA1401177_DISCLOSURES.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Villa A., Capo V., Castiello M.C. Innovative cell-based therapies and conditioning to cure RAG deficiency. Front Immunol. 2020;11:3035. doi: 10.3389/FIMMU.2020.607926/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Garcia-Perez L., van Eggermond M., van Roon L., Vloemans S.A., Cordes M., Schambach A., Rothe M., Berghuis D., Lagresle-Peyrou C., Cavazzana M., Zhang F., Thrasher A.J., Salvatori D., Meij P., Villa A., van Dongen J.J.M., Zwaginga J.J., van der Burg M., Gaspar H.B., Lankester A., Staal F.J.T., Pike-Overzet K. Successful preclinical development of gene therapy for recombinase-activating gene-1-deficient SCID. Mol. Ther. Methods Clin. Dev. 2020;17:666–682. doi: 10.1016/j.omtm.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pike-Overzet K., Rodijk M., Ng Y.Y., Baert M.R.M., Lagresle-Peyrou C., Schambach A., Zhang F., Hoeben R.C., Hacein-Bey-Abina S., Lankester A.C., Bredius R.G.M., Driessen G.J.A., Thrasher A.J., Baum C., Cavazzana-Calvo M., van Dongen J.J.M., Staal F.J.T. Correction of murine Rag1 deficiency by self-inactivating lentiviral vector-mediated gene transfer. Leukemia. 2011:1471–1483. doi: 10.1038/leu.2011.106. 2011 25:9. 25. [DOI] [PubMed] [Google Scholar]
- 183.Lagresle-Peyrou C., Yates F., Malassis-Séris M., Hue C., Morillon E., Garrigue A., Liu A., Hajdari P., Stockholm D., Danos O., Lemercier B., Gougeon M.L., Rieux-Laucat F., de Villartay J.P., Fischer A., Cavazzana-Calvo M. Long-term immune reconstitution in RAG-1-deficient mice treated by retroviral gene therapy: a balance between efficiency and toxicity. Blood. 2006;107:63–72. doi: 10.1182/BLOOD-2005-05-2032. [DOI] [PubMed] [Google Scholar]
- 184.van Til N.P., Sarwari R., Visser T.P., Hauer J., Lagresle-Peyrou C., van der Velden G., Malshetty V., Cortes P., Jollet A., Danos O., Cassani B., Zhang F., Thrasher A.J., Fontana E., Poliani P.L., Cavazzana M., Verstegen M.M.A., Villa A., Wagemaker G. Recombination-activating gene 1 (Rag1)-deficient mice with severe combined immunodeficiency treated with lentiviral gene therapy demonstrate autoimmune Omenn-like syndrome. J. Allergy Clin. Immunol. 2014;133:1116–1123. doi: 10.1016/j.jaci.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 185.van Til N.P., Cortes P., Danos O., Cassani B., Poliani P.L., Villa A., Wagemaker G. Reply. J. Allergy Clin. Immunol. 2014;134:243–244. doi: 10.1016/j.jaci.2014.04.032. [DOI] [PubMed] [Google Scholar]
- 186.Challita P.-M., Skelton D., El-Khoueiry A., Yu X.-J., Weinberg K., Kohn D.B. Multiple modifications in cis elements of the long terminal repeat of retroviral vectors lead to increased expression and decreased DNA methylation in embryonic carcinoma cells. J. Virol. 1995;69:748–755. doi: 10.1128/JVI.69.2.748-755.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hwu P.W.-L., Chien Y.H., Lee N.C., Tseng S.H., Wang A., Wang J., Leonardi E.T., Schilling T., Ozdas S., Tai C.H. ESGCTCollaborative virtual congress19–22 october 2021abstracts. 2021;32:A1–A152. doi: 10.1089/HUM.2021.29180.ABSTRACTS. 〈Https://Home.Liebertpub.Com/Hum〉 [DOI] [Google Scholar]
- 188.ASGCT annual meeting abstracts. Mol. Ther. 2021;29:1–427. doi: 10.1016/J.YMTHE.2021.04.019. [DOI] [Google Scholar]
- 189.Yates F., Malassis-Séris M., Stockholm D., Bouneaud C., Larousserie F., Noguiez-Hellin P., Danos O., Kohn D.B., Fischer A., de Villartay J.-P., Cavazzana-Calvo M. Gene therapy of RAG-2-/- mice: sustained correction of the immunodeficiency. Blood. 2002;100:3942–3949. doi: 10.1182/blood-2002-03-0782. [DOI] [PubMed] [Google Scholar]
- 190.van Til N.P., de Boer H., Mashamba N., Wabik A., Huston M., Visser T.P., Fontana E., Poliani P.L., Cassani B., Zhang F., Thrasher A.J., Villa A., Wagemaker G. Correction of murine Rag2 severe combined immunodeficiency by lentiviral gene therapy using a codon-optimized RAG2 therapeutic transgene. Mol. Ther. 2012;20:1968–1980. doi: 10.1038/mt.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Capo V., Castiello M.C., Fontana E., Penna S., Bosticardo M., Draghici E., Poliani L.P., Sergi Sergi L., Rigoni R., Cassani B., Zanussi M., Carrera P., Uva P., Dobbs K., Sacchetti N., Notarangelo L.D., van Til N.P., Wagemaker G., Villa A. Efficacy of lentivirus-mediated gene therapy in an Omenn syndrome recombination-activating gene 2 mouse model is not hindered by inflammation and immune dysregulation. J. Allergy Clin. Immunol. 2018;142:928–941. doi: 10.1016/j.jaci.2017.11.015. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gardner C.L., Pavel-Dinu M., Dobbs K., Bosticardo M., Reardon P.K., Lack J., DeRavin S.S., Le K., Bello E., Pala F., Delmonte O.M., Malech H., Montel-Hagan A., Crooks G., Acuto O., Porteus M.H., Notarangelo L.D. Gene editing rescues In vitro T cell development of RAG2-deficient induced pluripotent stem cells in an artificial thymic organoid system. J. Clin. Immunol. 2021:852–862. doi: 10.1007/S10875-021-00989-6. 2021 41:5. 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Massaad M.J., Ramesh N., Geha R.S. Wiskott-Aldrich syndrome: a comprehensive review. Ann. N. Y Acad. Sci. 2013;1285:26–43. doi: 10.1111/NYAS.12049. [DOI] [PubMed] [Google Scholar]
- 194.Yuan B., Zhou X., Suzuki K., Ramos-Mandujano G., Wang M., Tehseen M., Cortés-Medina L.V., Moresco J.J., Dunn S., Hernandez-Benitez R., Hishida T., Kim N.Y., Andijani M.M., Bi C., Ku M., Takahashi Y., Xu J., Qiu J., Huang L., Benner C., Aizawa E., Qu J., Liu G.H., Li Z., Yi F., Ghosheh Y., Shao C., Shokhirev M., Comoli P., Frassoni F., Yates J.R., Fu X.D., Esteban C.R., Hamdan S., Li M., Izpisua Belmonte J.C. Wiskott-Aldrich syndrome protein forms nuclear condensates and regulates alternative splicing. Nat. Commun. 2022;13 doi: 10.1038/S41467-022-31220-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Schrank B.R., Aparicio T., Li Y., Chang W., Chait B.T., Gundersen G.G., Gottesman M.E., Gautier J. Nuclear ARP2/3 drives DNA break clustering for homology-directed repair. Nature. 2018;559:61–66. doi: 10.1038/S41586-018-0237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sadhukhan S., Sarkar K., Taylor M., Candotti F., Vyas Y.M. Nuclear role of WASp in gene transcription is uncoupled from its ARP2/3-dependent cytoplasmic role in actin polymerization. J. Immunol. 2014;193:150–160. doi: 10.4049/JIMMUNOL.1302923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mallhi K.K., Petrovic A., Ochs H.D. Hematopoietic stem cell therapy for wiskott-aldrich syndrome: improved outcome and quality of life. J. Blood Med. 2021;12:435–447. doi: 10.2147/JBM.S232650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sereni L., Castiello M.C., Marangoni F., Anselmo A., di Silvestre D., Motta S., Draghici E., Mantero S., Thrasher A.J., Giliani S., Aiuti A., Mauri P., Notarangelo L.D., Bosticardo M., Villa A. Autonomous role of Wiskott-Aldrich syndrome platelet deficiency in inducing autoimmunity and inflammation. J. Allergy Clin. Immunol. 2018;142:1272–1284. doi: 10.1016/J.JACI.2017.12.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Marathe B.M., Prislovsky A., Astrakhan A., Rawlings D.J., Wan J.Y., Strom T.S. Antiplatelet antibodies in WASP(-) mice correlate with evidence of increased in vivo platelet consumption. Exp. Hematol. 2009;37:1353–1363. doi: 10.1016/J.EXPHEM.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shcherbina A., Rosen F.S., Remold-O’Donnell E. Pathological events in platelets of Wiskott-Aldrich syndrome patients. Br. J. Haematol. 1999;106:875–883. doi: 10.1046/J.1365-2141.1999.01637.X. [DOI] [PubMed] [Google Scholar]
- 201.Pai S.Y., Notarangelo L.D. Hematopoietic cell transplantation for Wiskott-Aldrich syndrome: advances in biology and future directions for treatment. Immunol. Allergy Clin. North Am. 2010;30:179–194. doi: 10.1016/J.IAC.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lorenzi R., Brickell P.M., Katz D.R., Kinnon C., Thrasher A.J. Wiskott-Aldrich syndrome protein is necessary for efficient IgG-mediated phagocytosis. Blood. 2000;95:2943–2946. doi: 10.1182/BLOOD.V95.9.2943.009K17_2943_2946. [DOI] [PubMed] [Google Scholar]
- 203.Burroughs L.M., Petrovic A., Brazauskas R., Liu X., Griffith L.M., Ochs H.D., Bleesing J.J., Edwards S., Dvorak C.C., Chaudhury S., Prockop S.E., Quinones R., Goldman F.D., Quigg T.C., Chandrakasan S., Smith A.R., Parikh S., Dávila Saldaña B.J., Thakar M.S., Phelan R., Shenoy S., Forbes L.R., Martinez C., Chellapandian D., Shereck E., Miller H.K., Kapoor N., Barnum J.L., Chong H., Shyr D.C., Chen K., Abu-Arja R., Shah A.J., Weinacht K.G., Moore T.B., Joshi A., DeSantes K.B., Gillio A.P., Cuvelier G.D.E., Keller M.D., Rozmus J., Torgerson T., Pulsipher M.A., Haddad E., Sullivan K.E., Logan B.R., Kohn D.B., Puck J.M., Notarangelo L.D., Pai S.Y., Rawlings D.J., Cowan M.J. Excellent outcomes following hematopoietic cell transplantation for Wiskott-Aldrich syndrome: a PIDTC report. Blood. 2020;135:2094–2105. doi: 10.1182/BLOOD.2019002939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ngwube A., Hanson I.C., Orange J., Rider N.L., Seeborg F., Shearer W., Noroski L., Nicholas S., Forbes L., Leung K., Sasa G., Naik S., Hegde M., Omer B., Ahmed N., Allen C., Gottschalk S., Wu M.F., Liu H., Brenner M., Heslop H., Krance R., Martinez C. Outcomes after allogeneic transplant in patients with wiskott-aldrich syndrome. Biol. Blood Marrow Transpl. 2018;24:537–541. doi: 10.1016/J.BBMT.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 205.Elfeky R.A., Furtado-Silva J.M., Chiesa R., Rao K., Amrolia P., Lucchini G., Gilmour K., Adams S., Bibi S., Worth A., Thrasher A.J., Qasim W., Veys P. One hundred percent survival after transplantation of 34 patients with Wiskott-Aldrich syndrome over 20 years. J. Allergy Clin. Immunol. 2018;142:1654–1656. doi: 10.1016/J.JACI.2018.06.042. e7. [DOI] [PubMed] [Google Scholar]
- 206.Shekhovtsova Z., Bonfim C., Ruggeri A., Nichele S., Page K., Alseraihy A., Barriga F., de Toledo Codina J.S., Veys P., Boelens J.J., Mellgren K., Bittencourt H., O’Brien T., Shaw P.J., Chybicka A., Volt F., Giannotti F., Gluckman E., Kurtzberg J., Gennery A.R., Rocha V. A risk factor analysis of outcomes after unrelated cord blood transplantation for children with Wiskott-Aldrich syndrome. Haematologica. 2017;102:1112–1119. doi: 10.3324/HAEMATOL.2016.158808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Albert M.H., Slatter M.A., Gennery A.R., Güngör T., Bakunina K., Markovitch B., Hazelaar S., Sirait T., Courteille V., Aiuti A., Aleinikova O.V., Balashov D., Bernardo M.E., Bodova I., Bruno B., Cavazzana M., Chiesa R., Fischer A., Hauck F., Ifversen M., Kałwak K., Klein M.A., Kulagin A., Kupesiz A., Kuskonmaz B., Lindemans C.A., Locatelli F., Lum S.H., Maschan A., Meisel R., Moshous D., Porta F., Sauer M.G., Sedlacek P., Schulz A., Suarez F., Vallée T.C., Winiarski J.H., Zecca M., Neven B., Veys P., Lankester A.C. Hematopoietic stem cell transplantation for Wiskott-Aldrich syndrome: an EBMT Inborn Errors Working Party analysis. Blood. 2022:2066–2079. doi: 10.1182/BLOOD.2021014687. [DOI] [PubMed] [Google Scholar]
- 208.Boztug K., Schmidt M., Schwarzer A., Banerjee P.P., Díez I.A., Dewey R.A., Böhm M., Nowrouzi A., Ball C.R., Glimm H., Naundorf S., Kühlcke K., Blasczyk R., Kondratenko I., Maródi L., Orange J.S., von Kalle C., Klein C. Stem-cell gene therapy for the Wiskott-Aldrich syndrome. N. Engl. J. Med. 2010;363:1918–1927. doi: 10.1056/NEJMoa1003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Cavazzana M., Bushman F.D., Miccio A., André-Schmutz I., Six E. Gene therapy targeting haematopoietic stem cells for inherited diseases: progress and challenges. Nat. Rev. Drug Discov. 2019;18:447–462. doi: 10.1038/S41573-019-0020-9. [DOI] [PubMed] [Google Scholar]
- 210.Hacein-Bey Abina S., Gaspar H.B., Blondeau J., Caccavelli L., Charrier S., Buckland K., Picard C., Six E., Himoudi N., Gilmour K., McNicol A.M., Hara H., Xu-Bayford J., Rivat C., Touzot F., Mavilio F., Lim A., Treluyer J.M., Héritier S., Lefrère F., Magalon J., Pengue-Koyi I., Honnet G., Blanche S., Sherman E.A., Male F., Berry C., Malani N., Bushman F.D., Fischer A., Thrasher A.J., Galy A., Cavazzana M. Outcomes following gene therapy in patients with severe Wiskott-Aldrich syndrome. JAMA. 2015;313:1550–1563. doi: 10.1001/JAMA.2015.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chu J.I., Henderson L.A., Armant M., Male F., Dansereau C.H., MacKinnon B., Burke C.J., Cavanaugh M.E., London W.B., Barlan I.B., Özen A., Baris S., Karakoc-Aydiner E., Kiykim A., Hanson I.C., Despotovic J.M., Saitoh A., Takachi T., Imai K., King A., Arredondo S., Ritz J., Bushman F.D., Galy A., Notarangelo L.D., Williams D.A., Pai S.-Y. Gene therapy using a self-inactivating lentiviral vector improves clinical and laboratory manifestations of wiskott-aldrich syndrome. Blood. 2015;126 doi: 10.1182/BLOOD.V126.23.260.260. 260–260. [DOI] [Google Scholar]
- 212.Magnani A., Semeraro M., Adam F., Booth C., Dupré L., Morris E.C., Gabrion A., Roudaut C., Borgel D., Toubert A., Clave E., Abdo C., Gorochov G., Petermann R., Guiot M., Miyara M., Moshous D., Magrin E., Denis A., Suarez F., Lagresle C., Roche A.M., Everett J., Trinquand A., Guisset M., Bayford J.X., Hacein-Bey-Abina S., Kauskot A., Elfeky R., Rivat C., Abbas S., Gaspar H.B., Macintyre E., Picard C., Bushman F.D., Galy A., Fischer A., Six E., Thrasher A.J., Cavazzana M. Long-term safety and efficacy of lentiviral hematopoietic stem/progenitor cell gene therapy for Wiskott-Aldrich syndrome. Nat. Med. 2022;28:71–80. doi: 10.1038/S41591-021-01641-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Labrosse R., Chu J., Armant M., van der Spek J., Miggelbrink A., Fong J., Everett J.K., Raymond H., Kessler L., Dansereau C., Mackinnon B., Koo S., Morris E., London W.B., Ozen A., Baris S., Despotovic J.M., Forbes L., Saitoh A., Takachi T., King A., Nguyen T.M.A.T., Vu V.D.U., Bushman F.D., Galy A., Notarangelo L., Williams D.A., Pai S.-Y. Outcome of hematopoietic stem cell gene therapy for wiskott-aldrich syndrome. Blood. 2019;134 doi: 10.1182/BLOOD-2019-126161. 4629–4629. [DOI] [PubMed] [Google Scholar]
- 214.Moratto D., Giliani S., Bonfim C., Mazzolari E., Fischer A., Ochs H.D., Cant A.J., Thrasher A.J., Cowan M.J., Albert M.H., Small T., Pai S.Y., Haddad E., Lisa A., Hambleton S., Slatter M., Cavazzana-Calvo M., Mahlaoui N., Picard C., Torgerson T.R., Burroughs L., Koliski A., Neto J.Z., Porta F., Qasim W., Veys P., Kavanau K., Hönig M., Schulz A., Friedrich W., Notarangelo L.D. Long-term outcome and lineage-specific chimerism in 194 patients with Wiskott-Aldrich syndrome treated by hematopoietic cell transplantation in the period 1980-2009: an international collaborative study. Blood. 2011;118:1675–1684. doi: 10.1182/BLOOD-2010-11-319376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Astrakhan A., Sather B.D., Ryu B.Y., Khim S., Singh S., Humblet-Baron S., Ochs H.D., Miao C.H., Rawlings D.J. Ubiquitous high-level gene expression in hematopoietic lineages provides effective lentiviral gene therapy of murine Wiskott-Aldrich syndrome. Blood. 2012;119:4395–4407. doi: 10.1182/BLOOD-2011-03-340711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Singh S., Khan I., Khim S., Seymour B., Sommer K., Wielgosz M., Norgaard Z., Kiem H.P., Adair J., Liggitt D., Nienhuis A., Rawlings D.J. Safe and effective gene therapy for murine wiskott-aldrich syndrome using an insulated lentiviral vector. Mol. Ther. Methods Clin. Dev. 2016;4:1–16. doi: 10.1016/J.OMTM.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Laskowski T.J., van Caeneghem Y., Pourebrahim R., Ma C., Ni Z., Garate Z., Crane A.M., Li X.S., Liao W., Gonzalez-Garay M., Segovia J.C., Paschon D.E., Rebar E.J., Holmes M.C., Kaufman D., Vandekerckhove B., Davis B.R. Gene Correction of iPSCs from a wiskott-aldrich syndrome patient normalizes the lymphoid developmental and functional defects. Stem Cell Rep. 2016;7:139–148. doi: 10.1016/J.STEMCR.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rai R., Romito M., Rivers E., Turchiano G., Blattner G., Vetharoy W., Ladon D., Andrieux G., Zhang F., Zinicola M., Leon-Rico D., Santilli G., Thrasher A.J., Cavazza A. Targeted gene correction of human hematopoietic stem cells for the treatment of Wiskott - Aldrich Syndrome. Nat. Commun. 2020:1–15. doi: 10.1038/s41467-020-17626-2. 2020 11:1. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Segal B.H., Leto T.L., Gallin J.I., Malech H.L., Holland S.M. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine. 2000;79:170–200. doi: 10.1097/00005792-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 220.Morillo-Gutierrez B., Beier R., Rao K., Burroughs L., Schulz A., Ewins A.M., Gibson B., Sedlacek P., Krol L., Strahm B., Zaidman I., Kalwak K., Talano J.A., Woolfrey A., Fraser C., Meyts I., Müller I., Wachowiak J., Bernardo M.E., Veys P., Sykora K.W., Gennery A.R., Slatter M. Treosulfan-based conditioning for allogeneic HSCT in children with chronic granulomatous disease: a multicenter experience. Blood. 2016;128:440–448. doi: 10.1182/BLOOD-2016-03-704015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Güngör T., Teira P., Slatter M., Stussi G., Stepensky P., Moshous D., Vermont C., Ahmad I., Shaw P.J., da Cunha J.M.T., Schlegel P.G., Hough R., Fasth A., Kentouche K., Gruhn B., Fernandes J.F., Lachance S., Bredius R., Resnick I.B., Belohradsky B.H., Gennery A., Fischer A., Gaspar H.B., Schanz U., Seger R., Rentsch K., Veys P., Haddad E., Albert M.H., Hassan M., Fraser C.J., Wawer A., Kalwak K., Duffiner U. Reduced-intensity conditioning and HLA-matched haemopoietic stem-cell transplantation in patients with chronic granulomatous disease: a prospective multicentre study. Lancet. 2014;383:436–448. doi: 10.1016/S0140-6736(13)62069-3. [DOI] [PubMed] [Google Scholar]
- 222.Battersby A.C., Braggins H., Pearce M.S., Cale C.M., Burns S.O., Hackett S., Hughes S., Barge D., Goldblatt D., Gennery A.R. Inflammatory and autoimmune manifestations in X-linked carriers of chronic granulomatous disease in the United Kingdom. J. Allergy Clin. Immunol. 2017;140:628–630. doi: 10.1016/j.jaci.2017.02.029. e6. [DOI] [PubMed] [Google Scholar]
- 223.Marciano B.E., Zerbe C.S., Falcone E.L., Ding L., DeRavin S.S., Daub J., Kreuzburg S., Yockey L., Hunsberger S., Foruraghi L., Barnhart L.A., Matharu K., Anderson V., Darnell D.N., Frein C., Fink D.L., Lau K.P., Long Priel D.A., Gallin J.I., Malech H.L., Uzel G., Freeman A.F., Kuhns D.B., Rosenzweig S.D., Holland S.M. X-linked carriers of chronic granulomatous disease: Illness, lyonization, and stability. J. Allergy Clin. Immunol. 2018;141:365–371. doi: 10.1016/j.jaci.2017.04.035. [DOI] [PubMed] [Google Scholar]
- 224.Malech H.L., Maples P.B., Whiting-Theobald N., Linton G.F., Sekhsaria S., Vowells S.J., Li F., Miller J.A., Decarlo E., Holland S.M., Leitman S.F., Carter C.S., Butz R.E., Read E.J., Fleisher T.A., Schneiderman R.D., van Epps D.E., Spratt S.K., Maack C.A., Rokovich J.A., Cohen L.K., Gallin J.I. Prolonged production of NADPH oxidase-corrected granulocytes after gene therapy of chronic granulomatous disease. Proc. Natl. Acad. Sci. USA. 1997;94:12133. doi: 10.1073/PNAS.94.22.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kang E.M., Marciano B.E., Deravin S., Zarember K.A., Holland S.M., Malech H.L. Chronic granulomatous disease: Overview and hematopoietic stem cell transplantation. J. Allergy Clin. Immunol. 2011;127:1319–1326. doi: 10.1016/j.jaci.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kang E.M., Choi U., Theobald N., Linton G., Long Priel D.A., Kuhns D., Malech H.L. Retrovirus gene therapy for X-linked chronic granulomatous disease can achieve stable long-term correction of oxidase activity in peripheral blood neutrophils. Blood. 2010;115:783–791. doi: 10.1182/BLOOD-2009-05-222760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Siler U., Paruzynski A., Holtgreve-Grez H., Kuzmenko E., Koehl U., Renner E., Alhan C., de Loosdrecht A., Schwäble J., Pfluger T., Tchinda J., Schmugge M., Jauch A., Naundorf S., Kühlcke K., Notheis G., Güngor T., Kalle C., Schmidt M., Grez M., Seger R., Reichenbach J. Successful combination of sequential gene therapy and rescue Allo-HSCT in two children with X-CGD - importance of timing. Curr. Gene Ther. 2015;15:416–427. doi: 10.2174/1566523215666150515145255. [DOI] [PubMed] [Google Scholar]
- 228.de Ravin S.S., Reik A., Liu P.Q., Li L., Wu X., Su L., Raley C., Theobald N., Choi U., Song A.H., Chan A., Pearl J.R., Paschon D.E., Lee J., Newcombe H., Koontz S., Sweeney C., Shivak D.A., Zarember K.A., Peshwa M.V., Gregory P.D., Urnov F.D., Malech H.L. Targeted gene addition in human CD34 + hematopoietic cells for correction of X-linked chronic granulomatous disease. Nat. Biotechnol. 2016;34:424–429. doi: 10.1038/nbt.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.de Ravin S.S., Li L., Wu X., Choi U., Allen C., Koontz S., Lee J., Theobald-Whiting N., Chu J., Garofalo M., Sweeney C., Kardava L., Moir S., Viley A., Natarajan P., Su L., Kuhns D., Zarember K.A., Peshwa M.V., Malech H.L. CRISPR-Cas9 gene repair of hematopoietic stem cells from patients with X-linked chronic granulomatous disease. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aah3480. [DOI] [PubMed] [Google Scholar]
- 230.de Ravin S.S., Brault J., Meis R.J., Liu S., Li L., Pavel-Dinu M., Lazzarotto C.R., Liu T., Koontz S.M., Choi U., Sweeney C.L., Theobald N., Lee G.H., Clark A.B., Burkett S.S., Kleinstiver B.P., Porteus M.H., Tsai S., Kuhns D.B., Dahl G.A., Headey S., Wu X., Malech H.L. Enhanced homology-directed repair for highly efficient gene editing in hematopoietic stem/progenitor cells. Blood. 2021;137:2598–2608. doi: 10.1182/BLOOD.2020008503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Merling R.K., Kuhns D.B., Sweeney C.L., Wu X., Burkett S., Chu J., Lee J., Koontz S., di Pasquale G., Afione S.A., Chiorini J.A., Kang E.M., Choi U., de Ravin S.S., Malech H.L. Gene-edited pseudogene resurrection corrects p47phox-deficient chronic granulomatous disease. Blood Adv. 2017;1:270–278. doi: 10.1182/BLOODADVANCES.2016001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.D. Klatt, E. Cheng, Di. Hoffmann, G. Santilli, A.J. Thrasher, C. Brendel, A. Schambach, Differential Transgene Silencing of Myeloid-Specific Promoters in the AAVS1 Safe Harbor Locus of Induced Pluripotent Stem Cell-Derived Myeloid Cells, Https://Home.Liebertpub.Com/Hum. 31 (2020) 199–210. https://doi.org/10.1089/HUM.2019.194. [DOI] [PMC free article] [PubMed]
- 233.Jofra Hernández R., Calabria A., Sanvito F., de Mattia F., Farinelli G., Scala S., Visigalli I., Carriglio N., de Simone M., Vezzoli M., Cecere F., Migliavacca M., Basso-Ricci L., Omrani M., Benedicenti F., Norata R., Rancoita P.M.V., di Serio C., Albertini P., Cristofori P., Naldini L., Gentner B., Montini E., Aiuti A., Mortellaro A. Hematopoietic tumors in a mouse model of X-linked chronic granulomatous disease after lentiviral vector-mediated gene therapy. Mol. Ther. 2021;29:86–102. doi: 10.1016/J.YMTHE.2020.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ley K., Laudanna C., Cybulsky M.I., Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. 2007 7:9. [DOI] [PubMed] [Google Scholar]
- 235.Etzioni A. Defects in the leukocyte adhesion cascade. Clin. Rev. Allergy Immunol. 2009;38:54–60. doi: 10.1007/S12016-009-8132-3. 2009 38:1. [DOI] [PubMed] [Google Scholar]
- 236.Moutsopoulos N.M., Zerbe C.S., Wild T., Dutzan N., Brenchley L., DiPasquale G., Uzel G., Axelrod K.C., Lisco A., Notarangelo L.D., Hajishengallis G., Notarangelo L.D., Holland S.M. Interleukin-12 and interleukin-23 blockade in leukocyte adhesion deficiency type 1. N. Engl. J. Med. 2017;376:1141–1146. doi: 10.1056/NEJMOA1612197/SUPPL_FILE/NEJMOA1612197_DISCLOSURES.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hanna S., Etzioni A. Leukocyte adhesion deficiencies. Ann. N. Y Acad. Sci. 2012;1250:50–55. doi: 10.1111/J.1749-6632.2011.06389.X. [DOI] [PubMed] [Google Scholar]
- 238.Bakhtiar S., Shadur B., Stepensky P. The evidence for allogeneic hematopoietic stem cell transplantation for congenital neutrophil disorders: a comprehensive review by the inborn errors working party group of the EBMT. Front Pedia. 2019;7:436. doi: 10.3389/FPED.2019.00436/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Bakhtiar, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT S., Salzmann-Manrique, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT E., Blok, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT H.-J., Eikema, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT D.-J., Hazelaar, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT S., Ayas, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT M., Toren, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT A., Goldstein, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT G., Moshous, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT D., Locatelli, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT F., Merli, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT P., Michel, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT G., Öztürk, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT G., Schulz, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT A., Heilmann, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT C., Ifversen, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT M., Wynn, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT R.F., Aleinikova, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT O., Bertrand, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT Y., Tbakhi, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT A., Veys, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT P., Karakukcu, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT M., Kupesiz, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT A., Ghavamzadeh, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT A., Handgretinger, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT R., Unal, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT E., Perez-Martinez, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT A., Gokce, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT M., Porta, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT F., Aksu, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT T., Karasu, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT G., Badell, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT I., Ljungman, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT P., Skorobogatova, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT E., Yesilipek, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT A., Zuckerman, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT T., Bredius, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT R.R.G., Stepensky, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT P., Shadur, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT B., Slatter, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT M., Gennery, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT A.R., Albert, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT M.H., Bader, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT P., Lankester, on behalf of the P.D.W.P. and the I.E.W.P. of EBMT A. Allogeneic hematopoietic stem cell transplantation in leukocyte adhesion deficiency type I and III. Blood Adv. 2021;5:262–273. doi: 10.1182/BLOODADVANCES.2020002185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bauer T.R., Hickstein D.D. Gene therapy for leukocyte adhesion deficiency. Curr. Opin. Mol. Ther. 2000;2:383–388. [PubMed] [Google Scholar]
- 241.Bauer T.R., Tuschong L.M., Calvo K.R., Shive H.R., Burkholder T.H., Karlsson E.K., West R.R., Russell D.W., Hickstein D.D. Long-term follow-up of foamy viral vector-mediated gene therapy for canine leukocyte adhesion deficiency. Mol. Ther. 2013;21:964–972. doi: 10.1038/mt.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kohn D.B., Booth C., Sevilla J., Rao G.R., Almarza E., Terrazas D., Nicoletti E., Fernandes A., Kuo C., de Oliveira S., Moore T.B., Law K.M., Beard B.C., Choi G., Zeini M., Mesa-Núñez C., Thrasher A.J., Bueren J.A., Schwartz J.D. A phase 1/2 study of lentiviral-mediated ex-vivo gene therapy for pediatric patients with severe leukocyte adhesion deficiency-I (LAD-I): interim results. Blood. 2021;138:2932. doi: 10.1182/BLOOD-2021-151898. [DOI] [Google Scholar]
- 243.Armitage R.J., Fanslow W.C., Strockbine L., Sato T.A., Clifford K.N., Macduff B.M., Anderson D.M., Gimpel S.D., Davis-Smith T., Maliszewski C.R., Clark E.A., Smith C.A., Grabstein K.H., Cosman D., Spriggs M.K. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992;357:80–82. doi: 10.1038/357080a0. 1992 357:6373. [DOI] [PubMed] [Google Scholar]
- 244.van Kooten G., Banchereau J. CD40-CD40 ligand. J. Leukoc. Biol. 2000;67:2–17. doi: 10.1002/JLB.67.1.2. [DOI] [PubMed] [Google Scholar]
- 245.Roy M., Waldschmidt T., Aruffo A., Ledbetter J.A., Noelle R.J. The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells. J. Immunol. 1993;151:2497–2510. [PubMed] [Google Scholar]
- 246.Davies E.G., Thrasher A.J. Update on the hyper immunoglobulin M syndromes. Br. J. Haematol. 2010;149:167–180. doi: 10.1111/J.1365-2141.2010.08077.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Qamar N., Fuleihan R.L. The hyper IgM syndromes. Clin. Rev. Allergy Immunol. 2013;46:120–130. doi: 10.1007/S12016-013-8378-7. 2013 46:2. [DOI] [PubMed] [Google Scholar]
- 248.Hayward A.R., Levy J., Facchetti F., Notarangelo L., Ochs H.D., Etzioni A., Bonnefoy J.Y., Cosyns M., Weinberg A. Cholangiopathy and tumors of the pancreas, liver, and biliary tree in boys with X-linked immunodeficiency with hyper-IgM. J. Immunol. 1997;158 [PubMed] [Google Scholar]
- 249.C. Aroline, T. Homas, G. Eneviève De S. Aint, B. Asile, F. Rançoise, L.E.D. Eist, M. Alika, B. Enkerrou, S. Téphane, B. Lanche, Correction of X-Linked Hyper-IgM Syndrome by Allogeneic Bone Marrow Transplantation, Https://Doi.Org/10.1056/NEJM199508173330705. 333 (1995) 426–429. https://doi.org/10.1056/NEJM199508173330705.
- 250.Gennery A.R., Slatter M.A., Grandin L., Taupin P., Cant A.J., Veys P., Amrolia P.J., Gaspar H.B., Davies E.G., Friedrich W., Hoenig M., Notarangelo L.D., Mazzolari E., Porta F., Bredius R.G.M., Lankester A.C., Wulffraat N.M., Seger R., Güngör T., Fasth A., Sedlacek P., Neven B., Blanche S., Fischer A., Cavazzana-Calvo M., Landais P. Transplantation of hematopoietic stem cells and long-term survival for primary immunodeficiencies in Europe: Entering a new century, do we do better. J. Allergy Clin. Immunol. 2010;126:602–610. doi: 10.1016/j.jaci.2010.06.015. e11. [DOI] [PubMed] [Google Scholar]
- 251.Gennery A.R., Khawaja K., Veys P., Bredius R.G.M., Notarangelo L.D., Mazzolari E., Fischer A., Landais P., Cavazzana-Calvo M., Friedrich W., Fasth A., Wulffraat N.M., Matthes-Martin S., Bensoussan D., Bordigoni P., Lange A., Pagliuca A., Andolina M., Cant A.J., Davies E.G. Treatment of CD40 ligand deficiency by hematopoietic stem cell transplantation: a survey of the European experience, 1993-2002. Blood. 2004;103:1152–1157. doi: 10.1182/BLOOD-2003-06-2014. [DOI] [PubMed] [Google Scholar]
- 252.de la Morena M.T., Leonard D., Torgerson T.R., Cabral-Marques O., Slatter M., Aghamohammadi A., Chandra S., Murguia-Favela L., Bonilla F.A., Kanariou M., Damrongwatanasuk R., Kuo C.Y., Dvorak C.C., Meyts I., Chen K., Kobrynski L., Kapoor N., Richter D., DiGiovanni D., Dhalla F., Farmaki E., Speckmann C., Español T., Shcherbina A., Hanson I.C., Litzman J., Routes J.M., Wong M., Fuleihan R., Seneviratne S.L., Small T.N., Janda A., Bezrodnik L., Seger R., Raccio A.G., Edgar J.D.M., Chou J., Abbott J.K., van Montfrans J., González-Granado L.I., Bunin N., Kutukculer N., Gray P., Seminario G., Pasic S., Aquino V., Wysocki C., Abolhassani H., Dorsey M., Cunningham-Rundles C., Knutsen A.P., Sleasman J., Costa Carvalho B.T., Condino-Neto A., Grunebaum E., Chapel H., Ochs H.D., Filipovich A., Cowan M., Gennery A., Cant A., Notarangelo L.D., Roifman C.M. Long-term outcomes of 176 patients with X-linked hyper-IgM syndrome treated with or without hematopoietic cell transplantation. J. Allergy Clin. Immunol. 2017;139:1282–1292. doi: 10.1016/j.jaci.2016.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Mitsui-Sekinaka K., Imai K., Sato H., Tomizawa D., Kajiwara M., Nagasawa M., Morio T., Nonoyama S. Clinical features and hematopoietic stem cell transplantations for CD40 ligand deficiency in Japan. J. Allergy Clin. Immunol. 2015;136:1018–1024. doi: 10.1016/j.jaci.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 254.Ferrua F., Galimberti S., Courteille V., Slatter M.A., Booth C., Moshous D., Neven B., Blanche S., Cavazzana M., Laberko A., Shcherbina A., Balashov D., Soncini E., Porta F., Al-Mousa H., Al-Saud B., Al-Dhekri H., Arnaout R., Formankova R., Bertrand Y., Lange A., Smart J., Wolska-Kusnierz B., Aquino V.M., Dvorak C.C., Fasth A., Fouyssac F., Heilmann C., Hoenig M., Schuetz C., Kelečić J., Bredius R.G.M., Lankester A.C., Lindemans C.A., Suarez F., Sullivan K.E., Albert M.H., Kałwak K., Barlogis V., Bhatia M., Bordon V., Czogala W., Alonso L., Dogu F., Gozdzik J., Ikinciogullari A., Kriván G., Ljungman P., Meyts I., Mustillo P., Smith A.R., Speckmann C., Sundin M., Keogh S.J., Shaw P.J., Boelens J.J., Schulz A.S., Sedlacek P., Veys P., Mahlaoui N., Janda A., Davies E.G., Fischer A., Cowan M.J., Gennery A.R. Hematopoietic stem cell transplantation for CD40 ligand deficiency: Results from an EBMT/ESID-IEWP-SCETIDE-PIDTC study. J. Allergy Clin. Immunol. 2019;143:2238–2253. doi: 10.1016/j.jaci.2018.12.1010. [DOI] [PubMed] [Google Scholar]
- 255.Romero Z., Torres S., Cobo M., Mũoz P., Unciti J.D., Martín F., Molina I.J. A tissue-specific, activation-inducible, lentiviral vector regulated by human CD40L proximal promoter sequences. Gene Ther. 2010;18:364–371. doi: 10.1038/gt.2010.144. 2011 18:4. [DOI] [PubMed] [Google Scholar]
- 256.Tahara M., Pergolizzi R.G., Kobayashi H., Krause A., Luettich K., Lesser M.L., Crystal R.G. Trans-splicing repair of CD40 ligand deficiency results in naturally regulated correction of a mouse model of hyper-IgM X-linked immunodeficiency. Nat. Med. 2004;10:835–841. doi: 10.1038/nm1086. 2004 10:8. [DOI] [PubMed] [Google Scholar]
- 257.Vavassori V., Mercuri E., Marcovecchio G.E., Castiello M.C., Schiroli G., Albano L., Margulies C., Buquicchio F., Fontana E., Beretta S., Merelli I., Cappelleri A., Rancoita P.M., Lougaris V., Plebani A., Kanariou M., Lankester A., Ferrua F., Scanziani E., Cotta‐Ramusino C., Villa A., Naldini L., Genovese P. Modeling, optimization, and comparable efficacy of T cell and hematopoietic stem cell gene editing for treating hyper‐IgM syndrome. EMBO Mol. Med. 2021;13 doi: 10.15252/emmm.202013545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Oliveira G., Ruggiero E., Stanghellini M.T.L., Cieri N., D’Agostino M., Fronza R., Lulay C., Dionisio F., Mastaglio S., Greco R., Peccatori J., Aiuti A., Ambrosi A., Biasco L., Bondanza A., Lambiase A., Traversari C., Vago L., von Kalle C., Schmidt M., Bordignon C., Ciceri F., Bonini C. Tracking genetically engineered lymphocytes long-term reveals the dynamics of t cell immunological memory. Sci. Transl. Med. 2015;7 doi: 10.1126/SCITRANSLMED.AAC8265/SUPPL_FILE/7-317RA198_SM.PDF. [DOI] [PubMed] [Google Scholar]
- 259.Bacchetta R., Barzaghi F., Roncarolo M.G. From IPEX syndrome to FOXP3 mutation: a lesson on immune dysregulation. Ann. N. Y Acad. Sci. 2018;1417:5–22. doi: 10.1111/NYAS.13011. [DOI] [PubMed] [Google Scholar]
- 260.Chan A.Y., Leiding J.W., Liu X., Logan B.R., Burroughs L.M., Allenspach E.J., Skoda-Smith S., Uzel G., Notarangelo L.D., Slatter M., Gennery A.R., Smith A.R., Pai S.Y., Jordan M.B., Marsh R.A., Cowan M.J., Dvorak C.C., Craddock J.A., Prockop S.E., Chandrakasan S., Kapoor N., Buckley R.H., Parikh S., Chellapandian D., Oshrine B.R., Bednarski J.J., Cooper M.A., Shenoy S., Davila Saldana B.J., Forbes L.R., Martinez C., Haddad E., Shyr D.C., Chen K., Sullivan K.E., Heimall J., Wright N., Bhatia M., Cuvelier G.D.E., Goldman F.D., Meyts I., Miller H.K., Seidel M.G., vander Lugt M.T., Bacchetta R., Weinacht K.G., Andolina J.R., Caywood E., Chong H., de la Morena M.T., Aquino V.M., Shereck E., Walter J.E., Dorsey M.J., Seroogy C.M., Griffith L.M., Kohn D.B., Puck J.M., Pulsipher M.A., Torgerson T.R. Hematopoietic cell transplantation in patients with primary immune regulatory disorders (PIRD): a primary immune deficiency treatment consortium (PIDTC) survey. Front. Immunol. 2020;11:239. doi: 10.3389/FIMMU.2020.00239/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Passerini L., Barzaghi F., Curto R., Sartirana C., Barera G., Tucci F., Albarello L., Mariani A., Testoni P.A., Bazzigaluppi E., Bosi E., Lampasona V., Neth O., Zama D., Hoenig M., Schulz A., Seidel M.G., Rabbone I., Olek S., Roncarolo M.G., Cicalese M.P., Aiuti A., Bacchetta R. Treatment with rapamycin can restore regulatory T-cell function in IPEX patients. J. Allergy Clin. Immunol. 2020;145:1262–1271. doi: 10.1016/j.jaci.2019.11.043. e13. [DOI] [PubMed] [Google Scholar]
- 262.Barzaghi F., Amaya Hernandez L.C., Neven B., Ricci S., Kucuk Z.Y., Bleesing J.J., Nademi Z., Slatter M.A., Ulloa E.R., Shcherbina A., Roppelt A., Worth A., Silva J., Aiuti A., Murguia-Favela L., Speckmann C., Carneiro-Sampaio M., Fernandes J.F., Baris S., Ozen A., Karakoc-Aydiner E., Kiykim A., Schulz A., Steinmann S., Notarangelo L.D., Gambineri E., Lionetti P., Shearer W.T., Forbes L.R., Martinez C., Moshous D., Blanche S., Fisher A., Ruemmele F.M., Tissandier C., Ouachee-Chardin M., Rieux-Laucat F., Cavazzana M., Qasim W., Lucarelli B., Albert M.H., Kobayashi I., Alonso L., Diaz De Heredia C., Kanegane H., Lawitschka A., Seo J.J., Gonzalez-Vicent M., Diaz M.A., Goyal R.K., Sauer M.G., Yesilipek A., Kim M., Yilmaz-Demirdag Y., Bhatia M., Khlevner J., Richmond Padilla E.J., Martino S., Montin D., Neth O., Molinos-Quintana A., Valverde-Fernandez J., Broides A., Pinsk V., Ballauf A., Haerynck F., Bordon V., Dhooge C., Garcia-Lloret M.L., Bredius R.G., Kałwak K., Haddad E., Seidel M.G., Duckers G., Pai S.Y., Dvorak C.C., Ehl S., Locatelli F., Goldman F., Gennery A.R., Cowan M.J., Roncarolo M.G., Bacchetta R. Long-term follow-up of IPEX syndrome patients after different therapeutic strategies: An international multicenter retrospective study. J. Allergy Clin. Immunol. 2018;141:1036–1049. doi: 10.1016/j.jaci.2017.10.041. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zemmour D., Charbonnier L.M., Leon J., Six E., Keles S., Delville M., Benamar M., Baris S., Zuber J., Chen K., Neven B., Garcia-Lloret M.I., Ruemmele F.M., Brugnara C., Cerf-Bensussan N., Rieux-Laucat F., Cavazzana M., André I., Chatila T.A., Mathis D., Benoist C. Single-cell analysis of FOXP3 deficiencies in humans and mice unmasks intrinsic and extrinsic CD4+ T cell perturbations. Nat. Immunol. 2021;22:607–619. doi: 10.1038/s41590-021-00910-8. 2021 22:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Seidel M.G., Fritsch G., Lion T., Jürgens B., Heitger A., Bacchetta R., Lawitschka A., Peters C., Gadner H., Matthes-Martin S. Selective engraftment of donor CD4+25high FOXP3-positive T cells in IPEX syndrome after nonmyeloablative hematopoietic stem cell transplantation. Blood. 2009;113:5689–5691. doi: 10.1182/BLOOD-2009-02-206359. [DOI] [PubMed] [Google Scholar]
- 265.di Nunzio S., Cecconi M., Passerini L., McMurchy A.N., Baron U., Turbachova I., Vignola S., Valencic E., Tommasini A., Junker A., Cazzola G., Olek S., Levings M.K., Perroni L., Roncarolo M.G., Bacchetta R. Wild-type FOXP3 is selectively active in CD4+CD25hi regulatory T cells of healthy female carriers of different FOXP3 mutations. Blood. 2009;114:4138–4141. doi: 10.1182/BLOOD-2009-04-214593. [DOI] [PubMed] [Google Scholar]
- 266.Borna S., Lee E., Sato Y., Bacchetta R. Towards gene therapy for IPEX syndrome. Eur. J. Immunol. 2022;52:705–716. doi: 10.1002/EJI.202149210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Fontenot J.D., Gavin M.A., Rudensky A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. 2003 4:4. [DOI] [PubMed] [Google Scholar]
- 268.Passerini L., Mel E.R., Sartirana C., Fousteri G., Bondanza A., Naldini L., Roncarolo M.G., Bacchetta R. CD4+ T cells from IPEX patients convert into functional and stable regulatory T cells by FOXP3 gene transfer. Sci. Transl. Med. 2013;5 doi: 10.1126/SCITRANSLMED.3007320/SUPPL_FILE/5-215RA174_SM.PDF. [DOI] [PubMed] [Google Scholar]
- 269.Allan S.E., Alstad A.N., Merindol N., Crellin N.K., Amendola M., Bacchetta R., Naldini L., Roncarolo M.G., Soudeyns H., Levings M.K. Generation of potent and stable human CD4+ T regulatory cells by activation-independent expression of FOXP3. Mol. Ther. 2008;16:194–202. doi: 10.1038/sj.mt.6300341. [DOI] [PubMed] [Google Scholar]
- 270.Sato Y., Passerini L., Piening B.D., Uyeda M.J., Goodwin M., Gregori S., Snyder M.P., Bertaina A., Roncarolo M.G., Bacchetta R. Human‐engineered Treg‐like cells suppress FOXP3–deficient T cells but preserve adaptive immune responses in vivo. Clin. Transl. Immunol. 2020;9 doi: 10.1002/CTI2.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Santoni De Sio F.R., Passerini L., Valente M.M., Russo F., Naldini L., Roncarolo M.G., Bacchetta R. Ectopic FOXP3 expression preserves primitive features of human hematopoietic stem cells while impairing functional T cell differentiation. Sci. Rep. 2017;7:1–10. doi: 10.1038/s41598-017-15689-8. 2017 7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Honaker Y., Hubbard N., Xiang Y., Fisher L., Hagin D., Sommer K., Song Y., Yang S.J., Lopez C., Tappen T., Dam E.M., Khan I., Hale M., Buckner J.H., Scharenberg A.M., Torgerson T.R., Rawlings D.J. Gene editing to induce FOXP3 expression in human CD4+ T cells leads to a stable regulatory phenotype and function. Sci. Transl. Med. 2020;12 doi: 10.1126/SCITRANSLMED.AAY6422/SUPPL_FILE/AAY6422_SM.PDF. [DOI] [PubMed] [Google Scholar]
- 273.Goodwin M., Lee E., Lakshmanan U., Shipp S., Froessl L., Barzaghi F., Passerini L., Narula M., Sheikali A., Lee C.M., Bao G., Bauer C.S., Miller H.K., Garcia-Lloret M., Butte M.J., Bertaina A., Shah A., Pavel-Dinu M., Hendel A., Porteus M., Roncarolo M.G., Bacchetta R. CRISPR-based gene editing enables FOXP3 gene repair in IPEX patient cells. Sci. Adv. 2020;6 doi: 10.1126/SCIADV.AAZ0571/SUPPL_FILE/AAZ0571_TABLE_S3.XLSX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Haeussler M., Schönig K., Eckert H., Eschstruth A., Mianné J., Renaud J.B., Schneider-Maunoury S., Shkumatava A., Teboul L., Kent J., Joly J.S., Concordet J.P. Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR. Genome Biol. 2016;17 doi: 10.1186/s13059-016-1012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Labun K., Montague T.G., Krause M., Torres Cleuren Y.N., Tjeldnes H., Valen E. CHOPCHOP v3: expanding the CRISPR web toolbox beyond genome editing. Nucleic Acids Res. 2019;47:W171–W174. doi: 10.1093/nar/gkz365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Kim D., Bae S., Park J., Kim E., Kim S., Yu H.R., Hwang J., il Kim J., Kim J.S. Digenome-seq: genome-wide profiling of CRISPR-Cas9 off-target effects in human cells. Nat. Methods. 2015;12:237–243. doi: 10.1038/nmeth.3284. [DOI] [PubMed] [Google Scholar]
- 277.Tsai S.Q., Nguyen N.T., Malagon-Lopez J., Topkar V.V., Aryee M.J., Joung J.K. CIRCLE-seq: a highly sensitive in vitro screen for genome-wide CRISPR-Cas9 nuclease off-targets. Nat. Methods. 2017;14:607–614. doi: 10.1038/nmeth.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Gabriel R., Lombardo A., Arens A., Miller J.C., Genovese P., Kaeppel C., Nowrouzi A., Bartholomae C.C., Wang J., Friedman G., Holmes M.C., Gregory P.D., Glimm H., Schmidt M., Naldini L., von Kalle C. An unbiased genome-wide analysis of zinc-finger nuclease specificity. Nat. Biotechnol. 2011;29:816–823. doi: 10.1038/nbt.1948. [DOI] [PubMed] [Google Scholar]
- 279.Tsai S.Q., Zheng Z., Nguyen N.T., Liebers M., Topkar V. v, Thapar V., Wyvekens N., Khayter C., Iafrate A.J., Le L.P., Aryee M.J., Joung J.K. GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat. Biotechnol. 2015;33:187–198. doi: 10.1038/nbt.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Kosicki M., Tomberg K., Bradley A. Repair of double-strand breaks induced by CRISPR–Cas9 leads to large deletions and complex rearrangements. Nat. Biotechnol. 2018 doi: 10.1038/nbt.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Leibowitz M.L., Papathanasiou S., Doerfler P.A., Blaine L.J., Sun L., Yao Y., Zhang C.Z., Weiss M.J., Pellman D. Chromothripsis as an on-target consequence of CRISPR–Cas9 genome editing. Nat. Genet. 2021;53:895–905. doi: 10.1038/s41588-021-00838-7. 2021 53:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Nahmad A.D., Reuveni E., Goldschmidt E., Tenne T., Liberman M., Horovitz-Fried M., Khosravi R., Kobo H., Reinstein E., Madi A., Ben-David U., Barzel A. Frequent aneuploidy in primary human T cells after CRISPR–Cas9 cleavage. Nat. Biotechnol. 2022:1–7. doi: 10.1038/s41587-022-01377-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Adikusuma F., Piltz S., Corbett M.A., Turvey M., McColl S.R., Helbig K.J., Beard M.R., Hughes J., Pomerantz R.T., Thomas P.Q. Large deletions induced by Cas9 cleavage. Nature. 2018;560:E8–E9. doi: 10.1038/s41586-018-0380-z. [DOI] [PubMed] [Google Scholar]
- 284.Papathanasiou S., Markoulaki S., Blaine L.J., Leibowitz M.L., Zhang C.Z., Jaenisch R., Pellman D. Whole chromosome loss and genomic instability in mouse embryos after CRISPR-Cas9 genome editing. Nat. Commun. 2021;12:1–7. doi: 10.1038/s41467-021-26097-y. 2021 12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Alanis-Lobato G., Zohren J., McCarthy A., Fogarty N.M.E., Kubikova N., Hardman E., Greco M., Wells D., Turner J.M.A., Niakan K.K. Frequent loss of heterozygosity in CRISPR-Cas9-edited early human embryos. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/PNAS.2004832117/SUPPL_FILE/PNAS.2004832117.SD01.XLSX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Ferrari S., Jacob A., Cesana D., Laugel M., Beretta S., Varesi A., Unali G., Conti A., Canarutto D., Albano L., Calabria A., Vavassori V., Cipriani C., Castiello M.C., Esposito S., Brombin C., Cugnata F., Adjali O., Ayuso E., Merelli I., Villa A., di Micco R., Kajaste-Rudnitski A., Montini E., Penaud-Budloo M., Naldini L. Choice of template delivery mitigates the genotoxic risk and adverse impact of editing in human hematopoietic stem cells. Cell Stem Cell. 2022;29:1428–1444. doi: 10.1016/J.STEM.2022.09.001. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.San Filippo J., Sung P., Klein H. Mechanism of eukaryotic homologous recombination. Annu Rev. Biochem. 2008;77:229–257. doi: 10.1146/annurev.biochem.77.061306.125255. [DOI] [PubMed] [Google Scholar]
- 288.Roth T.L., Puig-Saus C., Yu R., Shifrut E., Carnevale J., Li P.J., Hiatt J., Saco J., Krystofinski P., Li H., Tobin V., Nguyen D.N., Lee M.R., Putnam A.L., Ferris A.L., Chen J.W., Schickel J.N., Pellerin L., Carmody D., Alkorta-Aranburu G., del Gaudio D., Matsumoto H., Morell M., Mao Y., Cho M., Quadros R.M., Gurumurthy C.B., Smith B., Haugwitz M., Hughes S.H., Weissman J.S., Schumann K., Esensten J.H., May A.P., Ashworth A., Kupfer G.M., Greeley S.A.W., Bacchetta R., Meffre E., Roncarolo M.G., Romberg N., Herold K.C., Ribas A., Leonetti M.D., Marson A. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature. 2018 doi: 10.1038/s41586-018-0326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Eyquem J., Mansilla-Soto J., Giavridis T., van der Stegen S.J.C., Hamieh M., Cunanan K.M., Odak A., Gönen M., Sadelain M. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543:113–117. doi: 10.1038/nature21405. 2017 543:7643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Shy B.R., Vykunta V.S., Ha A., Talbot A., Roth T.L., Nguyen D.N., Pfeifer W.G., Chen Y.Y., Blaeschke F., Shifrut E., Vedova S., Mamedov M.R., Chung J.-Y.J., Li H., Yu R., Wu D., Wolf J., Martin T.G., Castro C.E., Ye L., Esensten J.H., Eyquem J., Marson A. High-yield genome engineering in primary cells using a hybrid ssDNA repair template and small-molecule cocktails. Nat. Biotechnol. 2022;2022:1–11. doi: 10.1038/s41587-022-01418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Bak R.O., Dever D.P., Porteus M.H. CRISPR/Cas9 genome editing in human hematopoietic stem cells. Nat. Protoc. 2018;13:358–376. doi: 10.1038/nprot.2017.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Lattanzi A., Camarena J., Lahiri P., Segal H., Srifa W., Vakulskas C.A., Frock R.L., Kenrick J., Lee C., Talbott N., Skowronski J., Cromer M.K., Charlesworth C.T., Bak R.O., Mantri S., Bao G., DiGiusto D., Tisdale J., Wright J.F., Bhatia N., Roncarolo M.G., Dever D.P., Porteus M.H. Development of β-globin gene correction in human hematopoietic stem cells as a potential durable treatment for sickle cell disease. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abf2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Ferrari S., Beretta S., Jacob A., Cittaro D., Albano L., Merelli I., Naldini L., Genovese P. BAR-Seq clonal tracking of gene-edited cells. Nat. Protoc. 2021;16:2991–3025. doi: 10.1038/s41596-021-00529-x. [DOI] [PubMed] [Google Scholar]
- 294.Pattabhi S., Lotti S.N., Berger M.P., Singh S., Lux C.T., Jacoby K., Lee C., Negre O., Scharenberg A.M., Rawlings D.J. In vivo outcome of homology-directed repair at the HBB gene in HSC using alternative donor template delivery methods. Mol. Ther. Nucleic Acids. 2019;17:277–288. doi: 10.1016/j.omtn.2019.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Romero Z., Lomova A., Said S., Miggelbrink A., Kuo C.Y., Campo-Fernandez B., Hoban M.D., Masiuk K.E., Clark D.N., Long J., Sanchez J.M., Velez M., Miyahira E., Zhang R., Brown D., Wang X., Kurmangaliyev Y.Z., Hollis R.P., Kohn D.B. Editing the sickle cell disease mutation in human hematopoietic stem cells: comparison of endonucleases and homologous donor templates. Mol. Ther. 2019;27:1389–1406. doi: 10.1016/j.ymthe.2019.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Schiroli G., Conti A., Ferrari S., della Volpe L., Jacob A., Albano L., Beretta S., Calabria A., Vavassori V., Gasparini P., Salataj E., Ndiaye-Lobry D., Brombin C., Chaumeil J., Montini E., Merelli I., Genovese P., Naldini L., di Micco R. Precise gene editing preserves hematopoietic stem cell function following transient p53-mediated DNA damage response. Cell Stem Cell. 2019;24:551–565. doi: 10.1016/j.stem.2019.02.019. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Maruyama T., Dougan S.K., Truttmann M., Bilate A.M., Ingram J.R., Ploegh H.L. Inhibition of non-homologous end joining increases the efficiency of CRISPR/Cas9-mediated precise [TM: inserted] genome editing. Nature. 2015;33:538–542. doi: 10.1038/nbt.3190.Inhibition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Chu V.T., Weber T., Wefers B., Wurst W., Sander S., Rajewsky K., Kühn R. Increasing the efficiency of homology-directed repair for CRISPR-Cas9-induced precise gene editing in mammalian cells. Nat. Biotechnol. 2015;33:543–548. doi: 10.1038/nbt.3198. [DOI] [PubMed] [Google Scholar]
- 299.Gutschner T., Haemmerle M., Genovese G., Draetta G.F., Chin L. Post-translational regulation of Cas9 during G1 enhances homology-directed repair. Cell Rep. 2016;14:1555–1566. doi: 10.1016/j.celrep.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 300.Charpentier M., Khedher A.H.Y., Menoret S., Brion A., Lamribet K., Dardillac E., Boix C., Perrouault L., Tesson L., Geny S., de Cian A., Itier J.M., Anegon I., Lopez B., Giovannangeli C., Concordet J.P. CtIP fusion to Cas9 enhances transgene integration by homology-dependent repair. Nat. Commun. 2018;9 doi: 10.1038/s41467-018-03475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Jayavaradhan R., Pillis D.M., Goodman M., Zhang F., Zhang Y., Andreassen P.R., Malik P. CRISPR-Cas9 fusion to dominant-negative 53BP1 enhances HDR and inhibits NHEJ specifically at Cas9 target sites. Nat. Commun. 2019;10 doi: 10.1038/s41467-019-10735-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Charlesworth C.T., Camarena J., Cromer M.K., Vaidyanathan S., Bak R.O., Carte J.M., Potter J., Dever D.P., Porteus M.H. Priming human repopulating hematopoietic stem and progenitor cells for Cas9/sgRNA gene targeting. Mol. Ther. Nucleic Acids. 2018;12:89–104. doi: 10.1016/j.omtn.2018.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Ferrari S., Jacob A., Beretta S., Unali G., Albano L., Vavassori V., Cittaro D., Lazarevic D., Brombin C., Cugnata F., Kajaste-Rudnitski A., Merelli I., Genovese P., Naldini L. Efficient gene editing of human long-term hematopoietic stem cells validated by clonal tracking. Nat. Biotechnol. 2020;38:1298–1308. doi: 10.1038/s41587-020-0551-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Shin J.J., Schröder M.S., Caiado F., Wyman S.K., Bray N.L., Bordi M., Dewitt M.A., Vu J.T., Kim W.T., Hockemeyer D., Manz M.G., Corn J.E. Controlled cycling and quiescence enables efficient HDR in engraftment-enriched adult hematopoietic stem and progenitor cells. Cell Rep. 2020;32 doi: 10.1016/J.CELREP.2020.108093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Lomova A., Clark D.N., Campo-Fernandez B., Flores-Bjurström C., Kaufman M.L., Fitz-Gibbon S., Wang X., Miyahira E.Y., Brown D., DeWitt M.A., Corn J.E., Hollis R.P., Romero Z., Kohn D.B. Improving gene editing outcomes in human hematopoietic stem and progenitor cells by temporal control of DNA repair. Stem Cells. 2019;37:284–294. doi: 10.1002/stem.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Dever D.P., Bak R.O., Reinisch A., Camarena J., Washington G., Nicolas C.E., Pavel-Dinu M., Saxena N., Wilkens A.B., Mantri S., Uchida N., Hendel A., Narla A., Majeti R., Weinberg K.I., Porteus M.H. CRISPR/Cas9 β-globin gene targeting in human haematopoietic stem cells. Nature. 2016;539:384–389. doi: 10.1038/nature20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Agudelo D., Duringer A., Bozoyan L., Huard C.C., Carter S., Loehr J., Synodinou D., Drouin M., Salsman J., Dellaire G., Laganière J., Doyon Y. Marker-free coselection for CRISPR-driven genome editing in human cells. Nat. Methods. 2017;14:615–620. doi: 10.1038/nmeth.4265. 2017 14:6. [DOI] [PubMed] [Google Scholar]
- 308.Piras F., Kajaste-Rudnitski A. Antiviral immunity and nucleic acid sensing in haematopoietic stem cell gene engineering. Gene Ther. 2020;28:16–28. doi: 10.1038/s41434-020-0175-3. 2020 28:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Fares I., Chagraoui J., Gareau Y., Gingras S., Ruel R., Mayotte N., Csaszar E., Knapp D.J.H.F., Miller P., Ngom M., Imren S., Roy D.C., Watts K.L., Kiem H.P., Herrington R., Iscove N.N., Humphries R.K., Eaves C.J., Cohen S., Marinier A., Zandstra P.W., Sauvageau G. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science. 2014;1979(345):1509–1512. doi: 10.1126/science.1256337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Boitano A.E., Wang J., Romeo R., Bouchez L.C., Parker A.E., Sutton S.E., Walker J.R., Flaveny C.A., Perdew G.H., Denison M.S., Schultz P.G., Cooke M.P. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;1979(329):1345–1348. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Hoggatt J., Singh P., Sampath J., Pelus L.M. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113:5444–5455. doi: 10.1182/blood-2009-01-201335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Bai T., Li J., Sinclair A., Imren S., Merriam F., Sun F., O’Kelly M.B., Nourigat C., Jain P., Delrow J.J., Basom R.S., Hung H.C., Zhang P., Li B., Heimfeld S., Jiang S., Delaney C. Expansion of primitive human hematopoietic stem cells by culture in a zwitterionic hydrogel. Nat. Med. 2019;25:1566–1575. doi: 10.1038/s41591-019-0601-5. [DOI] [PubMed] [Google Scholar]
- 313.Crippa S., Conti A., Vavassori V., Ferrari S., Beretta S., Rivis S., Bosotti R., Scala S., Pirroni S., Jofra-Hernandez R., Santi L., Basso-Ricci L., Merelli I., Genovese P., Aiuti A., Naldini L., di Micco R., Bernardo M.E. Mesenchymal stromal cells improve the transplantation outcome of CRISPR-Cas9 gene-edited human HSPCs. Mol. Ther. 2022 doi: 10.1016/J.YMTHE.2022.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Essers M.A.G., Offner S., Blanco-Bose W.E., Waibler Z., Kalinke U., Duchosal M.A., Trumpp A. IFNα activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 315.Liu J., Guo Y.M., Hirokawa M., Iwamoto K., Ubukawa K., Michishita Y., Fujishima N., Tagawa H., Takahashi N., Xiao W., Yamashita J., Ohteki T., Sawada K. A synthetic double-stranded RNA, poly I: C, induces a rapid apoptosis of human CD34+ cells. Exp. Hematol. 2012 doi: 10.1016/j.exphem.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 316.Hendel A., Bak R.O., Clark J.T., Kennedy A.B., Ryan D.E., Roy S., Steinfeld I., Lunstad B.D., Kaiser R.J., Wilkens A.B., Bacchetta R., Tsalenko A., Dellinger D., Bruhn L., Porteus M.H. Chemically modified guide RNAs enhance CRISPR-Cas genome editing in human primary cells. Nat. Biotechnol. 2015;33:985–989. doi: 10.1038/nbt.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Mu W., Tang N., Cheng C., Sun W., Wei X., Wang H. In vitro transcribed sgRNA causes cell death by inducing interferon release. Protein Cell. 2019;10:461–465. doi: 10.1007/S13238-018-0605-9/FIGURES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Cromer M.K., Vaidyanathan S., Ryan D.E., Curry B., Lucas A.B., Camarena J., Kaushik M., Hay S.R., Martin R.M., Steinfeld I., Bak R.O., Dever D.P., Hendel A., Bruhn L., Porteus M.H. Global transcriptional response to CRISPR/Cas9-AAV6-based genome editing in CD34+ hematopoietic stem and progenitor cells. Mol. Ther. 2018 doi: 10.1016/j.ymthe.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Pavani G., Laurent M., Fabiano A., Cantelli E., Sakkal A., Corre G., Lenting P.J., Concordet J.-P., Toueille M., Miccio A., Amendola M. Ex vivo editing of human hematopoietic stem cells for erythroid expression of therapeutic proteins. Nat. Commun. 2020;11:3778. doi: 10.1038/s41467-020-17552-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Piras F., Riba M., Petrillo C., Lazarevic D., Cuccovillo I., Bartolaccini S., Stupka E., Gentner B., Cittaro D., Naldini L., Kajaste‐Rudnitski A. Lentiviral vectors escape innate sensing but trigger p53 in human hematopoietic stem and progenitor cells. EMBO Mol. Med. 2017;9:1198–1211. doi: 10.15252/emmm.201707922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.R.C. Wilson, D. Carroll, The Daunting Economics of Therapeutic Genome Editing, Https://Home.Liebertpub.Com/Crispr. 2 (2019) 280–284. https://doi.org/10.1089/CRISPR.2019.0052. [DOI] [PubMed]
- 322.Aiuti A., Pasinelli F., Naldini L. Ensuring a future for gene therapy for rare diseases. Nat. Med. 2022;2022:1–4. doi: 10.1038/s41591-022-01934-9. [DOI] [PubMed] [Google Scholar]
- 323.Urnov F.D. Imagine CRISPR cures. Mol. Ther. 2021;29:3103–3106. doi: 10.1016/j.ymthe.2021.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.del Core L., Cesana D., Gallina P., Secanechia Y.N.S., Rudilosso L., Montini E., Wit E.C., Calabria A., Grzegorczyk M.A. Normalization of clonal diversity in gene therapy studies using shape constrained splines. Sci. Rep. 2022;12:1–14. doi: 10.1038/s41598-022-05837-0. 2022 12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Cesana D., Calabria A., Rudilosso L., Gallina P., Benedicenti F., Spinozzi G., Schiroli G., Magnani A., Acquati S., Fumagalli F., Calbi V., Witzel M., Bushman F.D., Cantore A., Genovese P., Klein C., Fischer A., Cavazzana M., Six E., Aiuti A., Naldini L., Montini E. Retrieval of vector integration sites from cell-free DNA. Nat. Med. 2021;27:1458–1470. doi: 10.1038/s41591-021-01389-4. [DOI] [PubMed] [Google Scholar]
- 326.Goyal S., Tisdale J., Schmidt M., Kanter J., Jaroscak J., Whitney D., Bitter H., Gregory P.D., Parsons G., Foos M., Yeri A., Gioia M., Voytek S.B., Miller A., Lynch J., Colvin R.A., Bonner M. Acute myeloid leukemia case after gene therapy for sickle cell disease. New Engl. J. Med. 2022;386:138–147. doi: 10.1056/NEJMOA2109167/SUPPL_FILE/NEJMOA2109167_DISCLOSURES.PDF. [DOI] [PubMed] [Google Scholar]
- 327.Kanter J., Walters M.C., Krishnamurti L., Mapara M.Y., Kwiatkowski J.L., Rifkin-Zenenberg S., Aygun B., Kasow K.A., Pierciey F.J., Bonner M., Miller A., Zhang X., Lynch J., Kim D., Ribeil J.-A., Asmal M., Goyal S., Thompson A.A., Tisdale J.F. Biologic and clinical efficacy of lentiglobin for sickle cell disease. ew Engl. J. Med. 2022;386:617–628. doi: 10.1056/NEJMOA2117175/SUPPL_FILE/NEJMOA2117175_DATA-SHARING.PDF. [DOI] [PubMed] [Google Scholar]
- 328.de Ravin S.S., Liu S., Sweeney C.L., Brault J., Whiting-Theobald N., Ma M., Liu T., Choi U., Lee J., O’Brien S.A., Quackenbush P., Estwick T., Karra A., Docking E., Kwatemaa N., Guo S., Su L., Sun Z., Zhou S., Puck J., Cowan M.J., Notarangelo L.D., Kang E., Malech H.L., Wu X. Lentivector cryptic splicing mediates increase in CD34+ clones expressing truncated HMGA2 in human X-linked severe combined immunodeficiency. Nat. Commun. 2022;13:1–15. doi: 10.1038/s41467-022-31344-x. 2022 13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Anzalone A.V., Koblan L.W., Liu D.R. Genome editing with CRISPR–Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 2020;38:824–844. doi: 10.1038/s41587-020-0561-9. 2020 38:7. [DOI] [PubMed] [Google Scholar]
- 330.Anzalone A.V., Gao X.D., Podracky C.J., Nelson A.T., Koblan L.W., Raguram A., Levy J.M., Mercer J.A.M., Liu D.R. Programmable deletion, replacement, integration and inversion of large DNA sequences with twin prime editing. Nat. Biotechnol. 2021;40:731–740. doi: 10.1038/s41587-021-01133-w. 2021 40:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Saito M., Ladha A., Strecker J., Faure G., Neumann E., Altae-Tran H., Macrae R.K., Zhang F. Dual modes of CRISPR-associated transposon homing. Cell. 2021;184:2441–2453. doi: 10.1016/j.cell.2021.03.006. e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Strecker J., Ladha A., Gardner Z., Schmid-Burgk J.L., Makarova K.S., Koonin E. v, Zhang F. RNA-guided DNA insertion with CRISPR-associated transposases. Science. 2019;1979(364):48–53. doi: 10.1126/SCIENCE.AAX9181/SUPPL_FILE/AAX9181_STRECKER_SM.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Karvelis T., Druteika G., Bigelyte G., Budre K., Zedaveinyte R., Silanskas A., Kazlauskas D., Venclovas Č., Siksnys V. Transposon-associated TnpB is a programmable RNA-guided DNA endonuclease. Nature. 2021;7886(599):692–696. doi: 10.1038/s41586-021-04058-1. 2021 599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Omer-Javed A., Pedrazzani G., Albano L., Ghaus S., Latroche C., Manzi M., Ferrari S., Fiumara M., Jacob A., Vavassori V., Nonis A., Canarutto D., Naldini L. Mobilization-based chemotherapy-free engraftment of gene-edited human hematopoietic stem cells. Cell. 2022;185:2248–2264. doi: 10.1016/J.CELL.2022.04.039. e21. [DOI] [PMC free article] [PubMed] [Google Scholar]