Abstract
Background
Truncus arteriosus (TA) or persistent arterial trunk describes the presence of a solitary arterial trunk arising from the base of the heart, supported by a common ventriculoarterial junction. The trunk gives rise to the coronary arteries, systemic arteries, and at least one pulmonary artery. Truncus arteriosus is a rare congenital cardiac disease, and even rarer is the absence of ventricular septal defect.
Case summary
We report a case of a 2-day-old infant who presented with cyanosis and a cardiac murmur. He was diagnosed with TA with intact ventricular septum (IVS), as well as crossed pulmonary arteries which was identified on pre-operative imaging. We describe the surgical management and short-term follow-up.
Discussion
Our clinical case demonstrates a unique diagnosis and management of TA with IVS identified by pre-operative imaging with good surgical outcome.
Keywords: Congenital heart surgery, Truncus arteriosus, Crossed pulmonary arteries, Intact ventricular septum, Case report
Learning points.
Truncus arteriosus with intact ventricular septum is exceedingly rare. It is important to identify the intact interventricular septum on pre-operative imaging; this will aid in better planning for surgical management with resection of the fibrous tissue separating the right and left half of the truncal valve to allow baffling the truncal valve to the left ventricle.
The crossed branch pulmonary arteries present challenge to surgeons during distal anastomosis of the right ventricle to pulmonary artery conduit. Our patient had a length of main pulmonary artery to which the conduit could be anastomosed.
Introduction
Truncus arteriosus (TA) is a rare congenital cardiac disease with a reported incidence of 6–10 per 100 000 live births.1 It is described as the presence of a common ventriculoarterial junction that gives rise to a solitary arterial trunk, with this single outlet giving rise to the coronary arteries, systemic arteries, and at least one pulmonary artery. The original classification by Collet and Edwards accounted for aortic dominance and the arrangement of the origin on the pulmonary arteries.2 Van Praagh later modified the classification to include patients with intact ventricular septum (IVS).3
The majority of previously described cases of TA with IVS is described post-mortem.4 There have been previously reported cases with the trunk arising exclusively from either the right or left ventricle.5 Carr et al. described a case in which the common truncal valve was positioned over the right and left ventricles with the cusps of the leaflets attached to the upper edge of the ventricular septum which resulted in an IVS during diastole but some septal deficiency seen when the truncal valve opens during ventricular systole.6 There have been two reports of a variant of TA with IVS described in which the truncal valve is divided into right and left orifices by a plane of tissue arising from the crest of the muscular septum.7,8
Crossed pulmonary arteries are another rare congenital cardiac disease where the pulmonary arteries cross each other while coursing to their respective lungs. We report a case of TA with IVS, as well as crossed pulmonary arteries, which was identified on pre-operative imaging and the subsequent surgical management.
Timeline
| Birth | Ex 41 weeks, normal vaginal delivery, birth weight 3.45 kg |
| Commenced on continuous positive pressure ventilation (CPAP) from birth due to increased work of breathing and desaturation | |
| 12 h | Commenced on prostaglandin and transferred to a tertiary centre for further management (no echocardiogram able to be performed prior to transfer) |
| 6 days | Commenced on regular diuretics (furosemide and spironolactone 1 mg/kg twice daily) |
| Semi-electively intubated for respiratory distress | |
| 9 days | Surgical correction of truncus arteriosus |
| 12 days (Day 3 post-op) | Delayed chest closure |
| 14 days (day 5 post-op) | Extubated to CPAP |
| 23 days (day 14 post-op) | Transferred to peripheral centre for ongoing management, feeding establishment |
| 9 weeks (7 weeks post-op) | Cardiology outpatient follow-up |
| Moderate truncal regurgitation and mild stenosis |
Case presentation
The male patient was born at 41 weeks gestation in a regional centre, weighing 3.8 kg via emergency lower caesarean section due to meconium stained liquor and non-reassuring cardiotocography trace. Resuscitation at birth was not required and Apgar scores were normal. He was noted to be cyanosed with increased work of breathing and an ejection systolic murmur at 24 h of age. The chest X-ray demonstrated cardiomegaly. He was commenced on continuous positive pressure ventilation (CPAP) and a prostaglandin infusion prior to transfer to our tertiary centre.
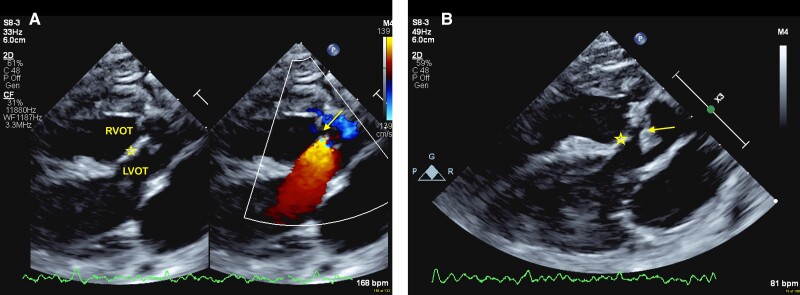
The echocardiogram confirmed the diagnosis of TA with IVS and a single short common pulmonary trunk (CPT) from the left side of the truncus which is classified as type 1b according to Collet and Edwards’ classification; this patient additionally had crossed pulmonary arteries. There was a dysplastic quadricuspid truncal valve with moderate regurgitation and moderate to severe stenosis (mean gradient 41 mmHg, peak gradient 73 mmHg). The common truncal valve appeared to sit over both ventricles, with a plane of tissue arising from the crest of the ventricular septum to the base of the valve leaflets separating the right and left ventricular outflows with no interventricular communication (Figure 1 and see Supplementary material online, Video S1). This was confirmed pre-operatively with trans-oesophageal echocardiogram (see Supplementary material online, Videos S2 and S3). There was biventricular hypertrophy with normal biventricular systolic function. There was a right aortic arch with mirror image branching of head and neck vessels.
Figure 1.
Transthoracic echocardiogram parasternal long-axis view demonstrating a plane of tissue (★) from the ventricular crest up to the common truncal valve (arrows), septating the right and left ventricular outflow tracts (RVOT, LVOT) with no interventricular communication seen on colour flow Doppler (A, colour flow Doppler; B, 2D Echocardiogram).
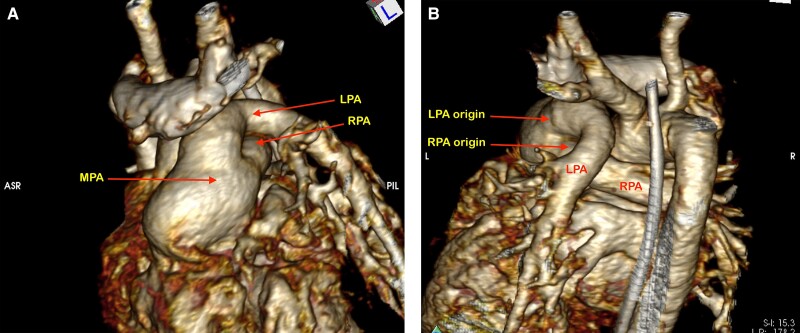
A computed tomography (CT) angiogram confirmed the anatomy of the pulmonary arteries and aortic arch (Figure 2, see Supplementary material online, Video S4). The right pulmonary artery origin was more leftward from the CPT and passed antero-inferior to the left pulmonary artery as it coursed to the right. This did not have any consequence on surgical management as there was a short common pulmonary artery trunk. Genetic testing confirmed 22q11 microdeletion consistent with DiGeorge syndrome.
Figure 2.
Computed tomography angiogram demonstrating truncus arteriosus type 1B with a short dilated main pulmonary artery (MPA) and crossing branch pulmonary arteries. (A) Anterior view and (B) posterior view. The right pulmonary artery (RPA) crosses anterior and inferior to the left pulmonary artery (LPA).
Surgical technique
The operation was performed via median sternotomy. Cardiopulmonary bypass was established after heparinisation, aorto-bicaval cannulation, and occlusion of the branch pulmonary arteries using snares. Cardiac arrest was induced using Del Nido cardioplegia delivered to the truncal root. A vent was placed through the foramen ovale. The common arterial trunk was transected and the main pulmonary artery (MPA) ostium excised; additional Del Nido cardioplegia was delivered directly to the coronary ostia.
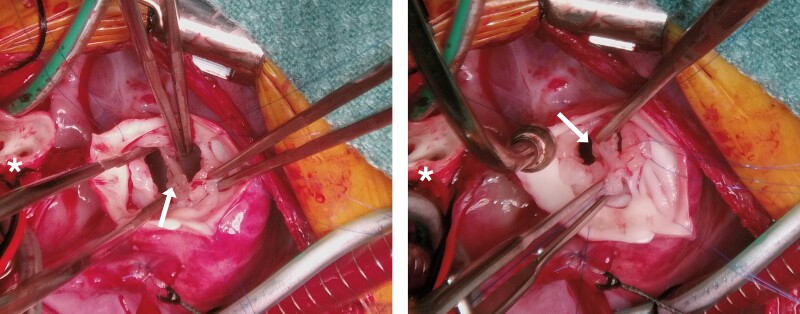
There was a fibrous membrane visible that closed the ventricular septal defect up to the level of the truncal valve (Figure 3). This fibrous tissue was in the midline of the truncal valve. This tissue was resected, effectively creating a ventricular septal defect (VSD), so that the left ventricle could be baffled to the aorta later. The quadricuspid truncal valve was then repaired by supporting two leaflets to their adjacent leaflet, creating a functionally bicuspid valve.
Figure 3.
Intraoperative photos. The truncal root has been transected. The main pulmonary artery ostium is visible to the left (*). A fibrous membrane closing the ventricular septal defect up to the level of the truncal valve can be seen (arrows). This was resected so that the left ventricle could be baffled to the aorta.
A 12 mm Contegra Conduit (Medtronic, Minneapolis, USA) was anastomosed to the distal MPA, and a right ventriculotomy was made. The created VSD was closed through the right ventriculotomy using a GORE-TEX patch (Gore Medical, Delaware, USA).
The distal aorta to truncal root anastomosis was then performed. The vent was removed, leaving a small atrial communication to aid post-operative management of ventricular diastolic dysfunction. The aortic cross-clamp was removed and the proximal Contegra Conduit to right ventricle anastomosis completed. The patient was weaned and separated from cardiopulmonary bypass without issue. Post-operative trans-oesophageal echocardiogram demonstrated the VSD patch baffling the left ventricle to the truncal valve which was functionally bicuspid with mild regurgitation and flow acceleration; there was no residual VSD (see Supplementary material online, Videos S5 and S6). The skin was closed using a GORE-TEX patch over the open sternum. A delayed sternal closure was performed on Day 2 post-operation.
Follow-up
The post-operative course was uncomplicated, and the intensive care unit length of stay was 5 days. The initial post-operative echocardiogram demonstrated biventricular hypertrophy with a degree of diastolic dysfunction and adequate systolic function. He had mild truncal valve stenosis (Vmax 2.4 m/s) and mild truncal valve regurgitation. The right ventricle to pulmonary artery conduit was unobstructed with no regurgitation.
Following the initial recovery, the patient was transferred to the referring hospital for management of feed establishment and diuresis. At routine follow-up 6 weeks post-operation, the patient was doing well and gaining weight. The echocardiogram demonstrated a mildly dilated left ventricle with normal biventricular systolic function. There was now moderate truncal regurgitation and mild truncal stenosis (Vmax 3.2 m/s, mean gradient 26 mmHg, peak gradient 41 mmHg).
Discussion
Truncus arteriosus is a rare congenital anomaly and exceedingly rare in the absence of any interventricular shunting. There have been previous reports describing the common arterial trunk arising exclusively from the right or left ventricle; these however are often diagnosed post-mortem.4 The previous reports by McElhinney et al.7 and Garg et al.8 described the surgical correction by obliterating the right half of the truncal valve with variable clinical outcome. Our approach differs to this in that the fibrous membrane separating the right and left half of the truncal valve was resected to allow the left ventricle to be baffled to the aorta with a good clinical outcome for the patient. The additional feature in our patient was the presence of crossed pulmonary arteries. This can sometimes present a challenge to surgeons during distal anastomosis of the right ventricle to pulmonary artery conduit. Our patient had a length of MPA to which the conduit could be anastomosed. The diagnosis of TA with IVS was able to be identified on pre-operative imaging and confirmed with trans-oesophageal echocardiogram. This was important to allow for surgical planning and improved outcome for the patient.
Conclusion
Our clinical case demonstrates a unique diagnosis and management of TA with IVS identified by pre-operative imaging with good surgical outcome.
Supplementary Material
Contributor Information
Hannah Davidson, Heart Centre for Children, The Children’s Hospital at Westmead, Cnr Hawkesbury Rd & Hainsworth St, Westmead NSW 2145, Australia; Discipline of Child and Adolescent Health, The Children's Hospital at Westmead Clinical School, Sydney Medical School, Haweksbury Road, Sydney, Westmead NSW 2145, Australia.
Michael Seco, Heart Centre for Children, The Children’s Hospital at Westmead, Cnr Hawkesbury Rd & Hainsworth St, Westmead NSW 2145, Australia; Discipline of Child and Adolescent Health, The Children's Hospital at Westmead Clinical School, Sydney Medical School, Haweksbury Road, Sydney, Westmead NSW 2145, Australia.
Hiroko Asakai, Heart Centre for Children, The Children’s Hospital at Westmead, Cnr Hawkesbury Rd & Hainsworth St, Westmead NSW 2145, Australia.
Matthew Liava’a, Heart Centre for Children, The Children’s Hospital at Westmead, Cnr Hawkesbury Rd & Hainsworth St, Westmead NSW 2145, Australia.
Lead author biography
 Dr Hannah Davidson is a Paediatric Cardiology Trainee with the Royal Australian College of Physicians and in her final of training at The Children’s Hospital at Westmead in Sydney, Australia.
Dr Hannah Davidson is a Paediatric Cardiology Trainee with the Royal Australian College of Physicians and in her final of training at The Children’s Hospital at Westmead in Sydney, Australia.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports.
Additional pre-operative and post-operative echocardiography loops are included in supplementary data. Supplementary echocardiogram loops included more common TA anatomy (type 2a) for comparisoni (Supplementary material online, Videos S7–S9).
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The patient’s guardian provided written informed consent for the inclusion of the clinical details and photography in this case report in accordance with the Conclusion (COPE) guidelines.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval: This case report has been approved by the Sydney Children’s Hospital Network Human Research Ethics Committee.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
References
- 1. Reller MD, Strickland MJ, Riehle-Colarusso T, Mahle WT, Correa A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998–2005. J Pediatr 2008;153:807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Collett RW, Edwards JE. Persistent truncus arteriosus; a classification according to anatomic types. Surg Clin North Am 1949;29:1245–1270. [DOI] [PubMed] [Google Scholar]
- 3. Van Praagh R, Van Praagh S. The anatomy of common aorticopulmonary trunk (truncus arteriosus communis) and its embryologic implications. A study of 57 necropsy cases. Am J Cardiol 1965;16:406–425. [DOI] [PubMed] [Google Scholar]
- 4. Spicer DE, Steffensen TS. A rare presentation of common arterial trunk with intact ventricular septum. J Cardiovasc Dev Dis 2020;7:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zeevi B, Dembo L, Berant M. Rare variant of truncus arteriosus with intact ventricular septum and hypoplastic right ventricle. Br Heart J 1992;68:214–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carr I, Bharati S, Kusnoor VS, Lev M. Truncus arteriosus communis with intact ventricular septum. Br Heart J 1979;42:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McElhinney DB, Reddy VM, Brook MM, Hanley FL. Repair of truncus arteriosus with intact ventricular septum (Van Praagh type B2) in a neonate. J Thorac Cardiovasc Surg 1997;114:134–138. [DOI] [PubMed] [Google Scholar]
- 8. Garg P, Mishra A, Shah R, Parmar D, Anderson RH. Neonatal repair of common arterial trunk with intact ventricular septum. World J Pediatr Congenit Heart Surg 2015;6:93–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.