Abstract
Background
The Quantra QPlus System is a cartridge-based device with a unique ultrasound technology that can measure the viscoelastic properties of whole blood during coagulation. These viscoelastic properties correlate directly with hemostatic function. The primary objective of this study was to assess blood product utilization in cardiac surgery patients before and after the implementation of the Quantra QPlus System.
Methods
Yavapai Regional Medical Center implemented the Quantra QPlus System to aid in their efforts to reduce the transfusion of allogenic blood products and improve outcomes in patients undergoing cardiac surgery. A total of 64 patients were enrolled prior to the utilization of the Quantra (pre-Quantra cohort), and 64 patients were enrolled after (post-Quantra cohort). The pre-Quantra cohort had been managed via standard laboratory assays along with physician discretion for transfusion decisions. The utilization of blood products and frequency of transfusions were compared and analyzed between the two cohorts. (using the Student’s t-test)
Results
The implementation of the Quantra resulted in a change in the pattern of blood product utilization leading to a demonstrated decrease in the amount of blood products transfused and the associated costs. The amount of FFP transfused was significantly decreased by 97% (P = 0.0004), whereas cryoprecipitate decreased by 67% (P = 0.3134), platelets decreased by 26% (P = 0.4879), and packed red blood cells decreased by 10% (P = 0.8027) however these trends did not reach statistical significance. The acquisition cost of blood products decreased by 41% for total savings of roughly $40,682.
Conclusions
Use of the Quantra QPlus System has the potential to improve patient blood management and decrease costs.
Study registered at ClinicalTrials.gov
Keywords: Cardiac surgery; Blood management; Point-of-care testing, Quantra, Transfusion
Introduction
Cardiac surgery is associated with perioperative blood loss and a high risk of allogeneic blood product transfusion [1]. Adverse clinical outcomes are associated with high blood product transfusion requirements and reoperation for bleeding [2]. Although various allogeneic blood components and pharmacological agents are available to treat coagulopathic bleeding perioperatively, accurate and timely tests to determine their indications and dosing are needed. This is crucial to avoid unnecessary transfusions, reduce blood product waste, decrease health care costs, and improve patient outcomes.
Patient blood management contributes to the maintenance of hemostasis, minimizing bleeding and decreasing the need for transfusion. Historically, clinicians have relied on conventional laboratory tests to identify the causes of coagulopathy. Conventional tests require blood samples to be transported to the lab which leads to long turnaround times. Further, these tests cannot detect important coagulation defects such as excessive fibrinolysis, platelet dysfunction, or specific coagulation factor deficiencies [3]. As a result, empiric therapy based on clinical judgment is often employed. This strategy can lead to underuse of blood components in some patients, resulting in excessive blood loss and possibly re-exploration, and to overuse of blood components in others, exposing them to unnecessary risks [4]. Measurement of activated clotting time (ACT) has been implemented at the point-of-care however this testing is limited to monitoring of heparin concentrations.
Clinical guidelines developed by the American Society of Anesthesiologists for patient blood management recommend the use of viscoelastic testing (VET) devices in conjunction with goal-directed treatment algorithms to aid in the management of coagulopathic bleeding [5]. Goal directed transfusion algorithms that incorporate whole blood testing, such as with viscoelastic devices, are recommended to reduce periprocedural bleeding and transfusion in cardiac surgical patients in guidelines set by the Society of Thoracic Surgeons (Class I, Level B-R) [6]. Several prospective randomized studies in cardiac surgery have demonstrated the effectiveness of VET in reducing postoperative hemorrhage and certain blood product transfusions [7, 8]. Two technologies have emerged at the forefront of whole blood VET: thromboelastography (TEG System; Haemonetics Corporation, Braintree, MA) and rotational thromboelastometry (ROTEM; Instrumentation Laboratory, Bedford, MA). Both use an adaptation of the classic methodology first reported by Hartert that involves a pin suspended in a cup containing a blood sample [9]. These tests have often been described as point-of-care however, few have been utilized at the bedside of the cardiac patient due to sample processing and pipetting requirements. Recently, cartridge-based versions of these devices have been introduced to address these limitations (Haemonetics’ TEG 6 S; Werfen, ROTEM sigma) [10].
The Quantra QPlus System (HemoSonics, LLC, Durham, NC) is a cartridge-based VET system that uses a novel ultrasound technology [11, 12]. The system has been cleared by the FDA for use in cardiac surgery and its performance relative to other VET platforms has been established [13–17]. The primary objective of this study was to assess the clinical outcomes in cardiac surgery patients undergoing cardiopulmonary bypass before and after the implementation of the Quantra QPlus System at Yavapai Regional Medical Center (YRMC). We hypothesized that the post-Quantra cohort would utilize fewer blood products during surgery and throughout the hospital stay and this would lead to lower costs associated with blood transfusions.
Methods
Study Design
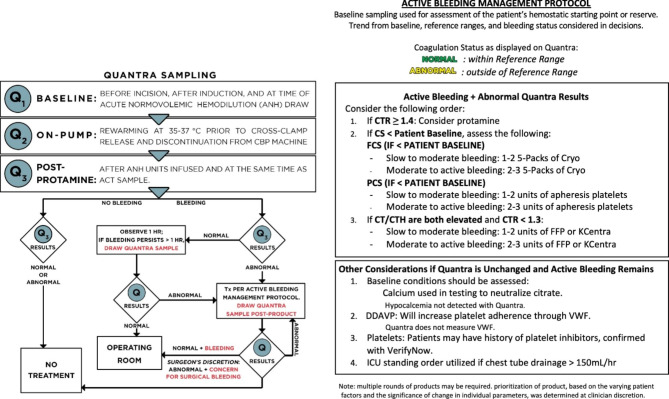
This study was conducted with the approval of WCG IRB (Protocol 1,287,400). The data was collected from January 2019 through April 2021. All consecutive patients undergoing cardiac surgical procedures at YRMC during a 9-month period from January 2019 to September 2019 were included in the pre-Quantra cohort (n = 64). During that 9-month time-period, utilization of blood products was guided by the institution’s standard of care. Beginning in October 2019, a Quantra-guided transfusion algorithm was instituted at YRMC for the management of patients undergoing cardiac surgical procedures. To allow sufficient time to implement the algorithm and to adjust Quantra trigger values, the post-Quantra cohort period began in May 2020 and concluded in April 2021. During this period, 65 patients underwent a cardiac surgical procedure in which the utilization of blood products was guided by the Quantra QPlus System using an institutional-specific treatment guide (Fig. 1).
Fig. 1.
Quantra Treatment Guide
Yavapai Regional Medical Center’s Quantra treatment guide utilized in cardiac surgical cases in the post-Quantra group. ACT: activated clotting time; CS: clot stiffness; CT: clot time; CTH: clot time with heparinase; CTR: Clot Time Ratio; DDAVP: desmopressin; FCS: fibrinogen contribution to clot stiffness; ICU: intensive care unit; ml: milliliter; PCS: platelet contribution to clot stiffness; Q1: baseline sample drawn in the operating room; Q2: sample taken while patient on cardiopulmonary bypass and body temperature at 35–37 degrees prior to cross clamp release; Q3: sample taken post protamine administration and after acute normovolemic hemodilution; Q4: Sampling done postoperatively; VET: viscoelastic testing
During both time periods, there were no major changes/innovations in the surgical procedures themselves that would affect the use of blood products or the decision to return a patient to the operating room. A single surgeon and surgical team at YRMC were responsible for the care of all surgical patients.
For each enrolled patient, the following baseline characteristics were recorded in the study database: gender; age; height/weight/BMI; preoperative medications relevant to coagulation status taken within 5 days of surgery start; preoperative comorbidities; and preoperative diagnosis. Surgery details including surgery type, duration of surgery, time on bypass, and cross-clamp time were also recorded. The following laboratory values were collected preoperatively, during surgery, and postoperatively in the intensive care unit (ICU): platelet count, hemoglobin (Hb) and hematocrit, prothrombin time/international normalized ratio (INR), and partial thromboplastin time. The Quantra QPlus System was used at the point-of-care to evaluate the viscoelastic properties of whole blood by means of the following functional parameters: Clot Time (CT), Clot Time with Heparinase (CTH), Clot Stiffness (CS), Fibrinogen Contribution to Clot Stiffness (FCS), Platelet Contribution to Clot Stiffness (PCS), and Clot Time Ratio (CTR). Quantra parameter results were collected at the following time points: baseline (before incision after induction of anesthesia), on pump (during rewarming), post protamine and after acute normovolemic hemodilution, and postoperatively in the ICU if active bleeding was observed. Decisions to administer blood products were guided by the Quantra Treatment Guide (Fig. 1). Blood products given during surgery and throughout hospital stay were recorded as units received of packed red blood cells (pRBC), fresh frozen plasma (FFP), platelets and cryoprecipitate. For cryoprecipitate, one “unit” or bag consisted of a 5-pack.
Statistical methods
Statistical analysis was performed using SAS version 9.4. P-values less than 0.05 were considered statistically significant. Categorical variables were summarized as frequencies with percentages and continuous variables as means with standard deviations (SD) or medians with interquartile ranges as appropriate.
The following parameters evaluated in the pre-Quantra and post-Quantra cohorts were compared using the student t-test or z-test: baseline patient characteristics, surgery information, number of units of blood products administered and cumulative costs of transfused blood products. Additionally, the risk analysis as well as the odds ratio were calculated relating the probability of a patient needing more than one blood product postoperatively given the Quantra was not used.
A single patient was identified as an outlier by the Grubb’s test using maximum normalized residuals. This patient in the post-Quantra cohort underwent surgical treatment of an acute myocardial infarction in which the number of blood products utilized in the perioperative and post-operative periods far exceeded that of any other patients in both cohorts. This patient was removed from the study and replaced with the next consecutive patient undergoing cardiac surgery in the post-Quantra cohort.
Results
Patient characteristics and surgery information
A total of 128 patients were included in this study with 64 patients included in the pre-Quantra cohort and 64 patients included in the post-Quantra cohort. There was no significant difference between the baseline demographics, comorbidities, laboratory values, or preoperative anticoagulant use in the 5 days preceding surgery in the pre- and post-Quantra cohorts (Table 1). Additionally, there was no significant difference in surgery type, mean surgery duration, number of deaths at discharge, or reoperation rates for bleeding between the pre- and post-Quantra cohorts (Table 2).
Table 1.
Baseline Characteristics of the Two Cohorts
| Pre-Quantra | Post-Quantra | P-Value | |||
|---|---|---|---|---|---|
| N | 64 | 64 | |||
| Women (n, %) | 19 | 29.69% | 19 | 29.23% | 0.9522 |
| Age (mean, SD) | 71.59 | 8.46 | 70.55 | 9.13 | 0.4279 |
| Hypertension (n, %) | 35 | 54.69% | 49 | 76.56% | 0.0155* |
| Diabetes (n, %) | 13 | 20.31% | 17 | 26.56% | 0.5313 |
| COPD (n, %) | 6 | 9.38% | 5 | 7.81% | > 0.999 |
| Kidney disease (n, %) | 2 | 3.13% | 9 | 14.06% | 0.0585 |
| Dyslipidemia (n, %) | 20 | 31.25% | 13 | 20.31% | 0.2254 |
| Anemia (n, %) | 1 | 1.56% | 3 | 4.69% | 0.6115 |
| Platelets (x1000/uL) (mean, SD) | 197.53 | 53.70 | 210.20 | 51.53 | 0.5602 |
| INR (mean, SD) | 1.12 | 0.11 | 1.09 | 0.17 | 0.2661 |
| aPTT (s) (mean, SD) | 32.35 | 7.22 | 35.64 | 16.00 | 0.1476 |
| Hemoglobin (g/L) | 13.94 | 2.29 | 14.15 | 1.88 | 0.5904 |
| Hematocrit (%) (mean, SD) | 42.31 | 6.40 | 42.61 | 5.56 | 0.7820 |
| Prehospital Medications1 | |||||
| Aspirin (n, %) | 12 | 18.75% | 16 | 24.62% | 0.4179 |
| Clopidogrel (n, %) | 1 | 1.56% | 1 | 1.56% | 0.9920 |
| Phytonadione (n, %) | 1 | 1.56% | 0 | 0.0% | 0.3125 |
| LMWH (n, %) | 11 | 17.19% | 16 | 24.62% | 0.2983 |
1] Prehospital medication is defined as a medication received within the 5 days leading up to surgery
*Statistically significant for two-tailed hypothesis using alpha 0.05
dL: deciliter; g: grams; INR: international normalized ratio; LMWH: low molecular weight heparin; N: total number of patients in cohort; n: total number of patients that received prehospital medication; SD: standard deviation; uL: microliters
Table 2.
Surgery Types and Duration
| Pre-Quantra | Post-Quantra | P-Value | |||
|---|---|---|---|---|---|
| CABG (n, %) | 30 | 48.88% | 37 | 56.92% | 0.2543 |
| CABG + Aortic valve replacement (n, %) | 4 | 6.25% | 7 | 10.77% | 0.3576 |
| CABG + Mitral valve replacement (n, %) | 4 | 6.25% | 2 | 3.08% | 0.3898 |
| Aortic valve replacement (only) (n, %) | 10 | 15.63% | 12 | 18.46% | 0.6672 |
| Mitral valve replacement (only) (n, %) | 10 | 15.63% | 5 | 7.70% | 0.1585 |
| Other (n, %) | 6 | 9.38% | 1 | 1.54% | 0.0523 |
| Surgery Duration by Group (min) | |||||
| All surgeries (mean, SD) | 207.81 | 53.39 | 200.69 | 48.15 | 0.5940 |
| CABG (mean, SD) | 195.47 | 30.54 | 199.84 | 35.23 | 0.5940 |
| CABG + Aortic valve replacement (mean, SD) | 231.00 | 8.04 | 271.86 | 46.83 | 0.1249 |
| CABG + Mitral valve replacement (mean, SD) | 269.00 | 67.74 | 298.00 | 22.63 | 0.6051 |
| Aortic valve replacement (mean, SD) | 190.20 | 47.57 | 157.92 | 24.04 | 0.0518 |
| Mitral valve replacement (mean, SD) | 182.20 | 44.13 | 174.00 | 18.23 | 0.7007 |
| Other (mean, SD) | 285.33 | 81.57 | 186.00 | 0 | 0.1534 |
| All surgeries perfusion time (min) (mean, SD) | 103.19 | 30.94 | 96.58 | 31.89 | 0.3140 |
| Reoperation for bleeding (n, %) | 2 | 3.13 | 3 | 4.69 | > 0.999 |
| Deaths (n, %) | 4 | 6.25 | 4 | 6.25 | 1.00 |
| Total hospital stay (days) (median, range) | 8.28 | 4.2, 30.0 | 7.31 | 4.1, 28.5 | 0.8874 |
Total hospital stay includes time patients were in the ICU plus progressive care
Deaths refer to mortality status at discharge
*Statistically significant p < 0.05
Other procedures consisted of double valve and ascending aortic repair
CABG: coronary artery bypass grafting; min: minute; n: total number of patients in surgery category; SD: standard deviation
Blood product utilization
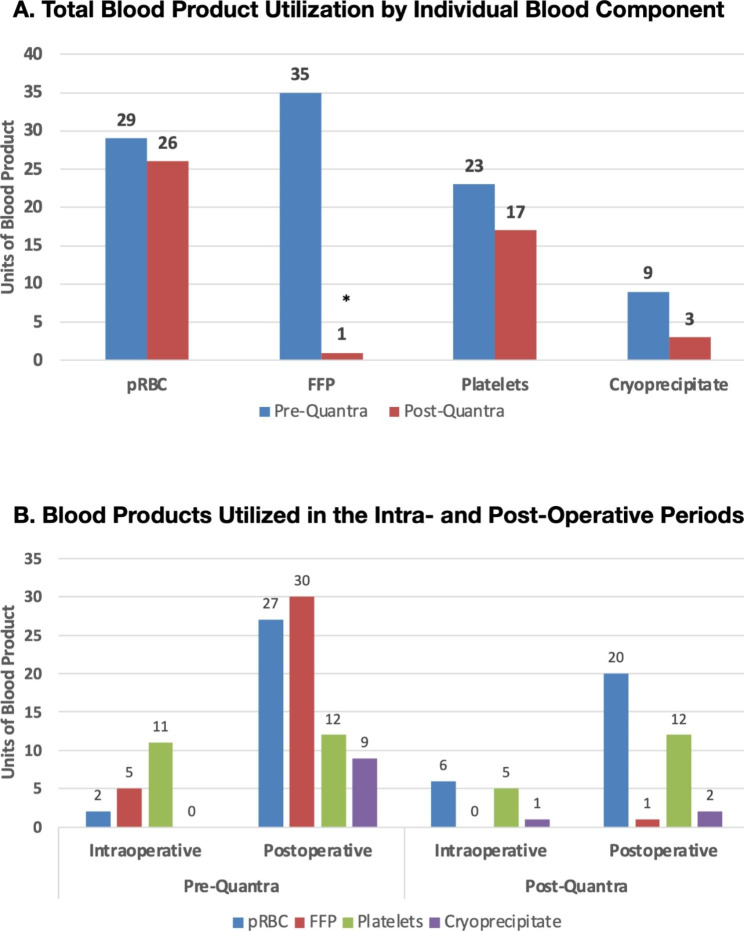
Blood product utilization was investigated as both total units of blood products administered across all patients and number of patients receiving blood products during the perioperative and postoperative periods. The total usage of blood products of all types was greater in the pre-Quantra cohort (Pre) compared to the post-Quantra cohort (Post) (Fig. 2A; Table 3 A). This was statistically significant for fresh frozen plasma (FFP) in which 35 units were transfused in the pre-Quantra cohort versus 1 unit in the post-Quantra cohort. Similarly, 9 units of cryoprecipitate were transfused pre-Quantra compared to 3 units in the post-Quantra cohort. By examining the total number of patients in each cohort that received any type of blood product, it is of note, that 29.7% of the post-Quantra cohort required blood products compared to 34.8% of the pre-Quantra cohort (Table 3B). The number of patients receiving at least one unit of pRBC, platelets or cryoprecipitate was similar across both cohorts however significantly more patients in the pre-Quantra cohort received FFP compared to the post-Quantra cohort (14 vs. 1).
Fig. 2.
Blood Product Utilization A. Total units of blood products utilized during the hospital stay for the two study cohorts. B. Utilization of blood products in the intra- and post-operative time periods
*Statistically significant p < 0.05
FFP: Fresh frozen plasma; pRBC: Packed red blood cells
Table 3.
Blood Product Utilization
| Pre-Quantra | Post-Quantra | P Value | |
|---|---|---|---|
| A. Blood products administered (mean units, range) | |||
| pRBC | 0.453 (0, 5) | 0.406 (0, 6) | 0.8027 |
| FFP | 0.547 (0, 6) | 0.016 (0, 1) | 0.0004* |
| Platelets | 0.359 (0, 4) | 0.266 (0, 2) | 0.4879 |
| Cryoprecipitate | 0.141 (0, 5) | 0.0467 (0, 1) | 0.3134 |
| Total, all products | 1.50 (0, 19) | 0.734 (0, 7) | 0.0933 |
| B. Patients receiving blood products (N, %) | |||
| pRBC | 13 (20.3) | 13 (20.3) | 1.00 |
| FFP | 14 (21.9) | 1 (1.6) | 0.0004* |
| Platelets | 10 (15.6) | 12 (18.8) | 0.6394 |
| Cryoprecipitate | 3 (4.7) | 3 (4.7) | 1.00 |
| Total, any product | 22 (34.4) | 19 (29.7) | 0.5689 |
A: Comparison of total number of units of blood products (all types) utilized across all patients intraoperatively and postoperatively. B: Number of patients receiving at least one unit of blood products, *Statistically significant p < 0.05
FFP: Fresh frozen plasma; pRBC: Packed red blood cells
Fig. 2B depicts the relative utilization of blood products in the intra- and post-operative time frames. In both study cohorts, the majority of blood products were administered in the post-operative period.
Odds ratio analysis
The odds ratio (OR) of a patient receiving a given blood product was also analyzed for each study cohort. The OR a patient would receive fresh frozen plasma was significantly increased in the pre-Quantra cohort (Table 4). Equally, the OR a patient would receive more than one unit of fresh frozen plasma was significantly increased in the pre-Quantra cohort (Table 4).
Table 4.
Odds of Receiving Blood Products in the Pre-Quantra and Post-Quantra Cohorts
| pRBC | FFP | Platelets | Cryoprecipitate | |
|---|---|---|---|---|
| OR (CI) | OR (CI) | OR (CI) | OR (CI) | |
| Pre-Quantra |
1)1.00 (0.42, 2.37) 2)1.58 (0.53, 4.74) |
1)17.64 (2.24, 138.75)* 2) 37.04 (2.16, 636.0)* |
1)0.80 (0.32, 2.02) 2)1.93 (0.61, 6.12) |
1)1.00 (0.19, 5.15) 2)7.34 (0.37, 145.1) |
| Post-Quantra |
1)1.00 (0.42, 2.37) 2)0.63 (0.21, 1.89) |
1)0.06 (0.01, 0.45) * 2)0.03 (0.02, 0.46)* |
1)1.25 (0.50, 3.131) 2)0.52 (0.16, 1.64) |
1)1.00 (0.19, 5.15) 2)0.14 (0.07, 2.69) |
1): Odds patient will receive a given blood product. 2): Odds patient will receive more than one unit of a specified blood product
*Statistically significant p < 0.05
CI: Confidence Interval based on alpha 0.05; FFP: Fresh frozen plasma; OR: Odds ratio; pRBC: Packed red blood cells
Blood product cost analysis
To compare the costs associated with blood product transfusions between the two cohorts, the following activity-based costs were utilized which incorporate the direct acquisition cost of the product as well as associated indirect costs [18]: pRBC $1112/unit; FFP $578/unit, platelets $1486/unit, and cryoprecipitate $1463/unit. The total cost of each blood product (pRBC, FFP, platelets and cryoprecipitate) was calculated for each cohort along with the total cost of blood products utilized in each cohort. The overall cost of all blood product transfusions combined was higher in the pre-Quantra cohort than the post-Quantra cohort ($99,823 vs. $59,141) (Table 5).
Table 5.
Cost Comparison of Total Blood Products Utilized
| Pre-Quantra | Post-Quantra | Difference (Pre - Post) | |
|---|---|---|---|
| pRBC (total) | $32,248 | $28,912 | $3,336 |
| FFP (total) | $20,230 | $578 | $19,652 |
| Platelets (total) | $34,178 | $25,262 | $10,402 |
| Cryoprecipitate (total) | $13,167 | $4,389 | $8,778 |
| Total Cost | $99,823 | $59,141 | $40,682 |
Tab 5 the total cost of each blood product was calculated for each cohort along with the total cost of all blood products utilized in each cohort
FFP: Fresh frozen plasma; pRBC: Packed red blood cells
Discussion
The use of viscoelastic testing devices as part of a goal-directed treatment approach to manage perioperative bleeding has been recommended by several international organizations and clinical guidelines, including the Society of Cardiovascular Anesthesiologists (SCA), the American Society of Anesthesiologists (ASA), the STS/SCA/AmSECT/SABM Update to the Clinical Practice Guidelines on Patient Blood Management, and the European EACTS/EACTA guidelines for cardiac surgery, among others [1, 5, 6, 19]. The aim of this study was to determine the impact of the implementation of the Quantra system on blood transfusions in patients undergoing a cardiac surgery requiring cardiopulmonary bypass at an institution that previously did not use viscoelastic testing. This was a retrospective analysis of how blood product utilization and cost changed after Quantra was introduced. This is one of the first studies demonstrating the clinical performance and utility of the Quantra QPlus System in the cardiac patient population.
Prior to the introduction of the Quantra, the institution’s standard of care for guiding the utilization of blood products relied on results from a panel of routine coagulation tests performed in the hospital laboratory which included aPTT, PT/INR, platelet count, and fibrinogen level. This testing was routinely performed during the last preoperative visit and repeated during the intraoperative phase if coagulopathic bleeding was suspected.
Across pre- and post-Quantra cohorts, patient baseline characteristics and procedural details were similar with approximately half of each cohort undergoing a CABG procedure. Additionally, there was no significant difference in preoperative medications between the pre- and post-Quantra cohorts, defined as patients required to remain on anticoagulant medications within 5 days of surgery.
For all types of blood products, the total number of units administered in the post-Quantra cohort was less than in the pre-Quantra cohort, however, the decrease reached statistical significance for FFP in which 34 units were given in the pre-Quantra cohort whereas only 1 unit was transfused in the post-Quantra cohort. The total reduction in blood product utilization was reflected in lower transfusion costs, with reported saving of over $40,000, representing a 41% reduction in costs vs. the pre-Quantra time period. In contrast, the number of patients receiving any amount of pRBC, platelets or cryoprecipitate was similar across both cohorts suggesting that the number of units of product each patient received tended to be less in the post-Quantra cohort. For FFP, 14 patients received product pre-Quantra and only 1 patient post-Quantra. These findings demonstrate a change in the pattern of blood product administration away from FFP, which is consisted with similar studies on the impact of viscoelastic testing devices in transfusion practices [20, 21]. The use of FFP is associated with several risks including allergic reactions, transfusion-related acute lung injury and transfusion-related circulatory overload [1].
Overall, these results indicate that the use of the Quantra has improved our institution’s transfusion practice by providing a targeted and optimized approach to blood product utilization. The system quantifies and trends (based on serial measurements) the function of the enzymatic clotting factors, presence of residual heparin, as well as the relative function of platelets and fibrinogen, which allows for a streamlined goal-directed treatment algorithm. Additionally, since the system was operated at the point of care and no sample manipulations are required, results were available in less than 15 min, which allowed to rapidly restore hemostatic balance in these patients. The results presented in Fig. 2 further suggest that improved assessment and treatment of coagulopathies in the intra-operative time-period led to reduced blood product transfusions in the post-operative setting.
There were several limitations to this study. The study was conducted at a single institution with a single surgical team involved in the care of patients during the interoperative and immediate postoperative periods therefore, the sample size is limited and results may not account for potential differences in clinical and/or transfusion practices across the diversity of teams and institutions that exist. The post-Quantra time-period was 3 months longer than the pre-Quantra time-period reflecting the additional time it took to attain a similar number of surgical cases due to a reduction in monthly surgical volumes brought on by the COVID-19 pandemic.
The majority of cardiac surgeries performed by this surgical team at this institution were elective, which enabled implementation of the standard recommended time for discontinuing antiplatelet medications prior to surgery for most cases. Results may not be generalizable to an institution with a large number of emergent cases and will require future studies to explore this. This study is one of the first descriptions of improved transfusion outcomes utilizing the Quantra in a facility that did not previously use viscoelastic testing. Further, this study was not intended to provide a comprehensive analysis of the economic impact of implementing Quantra at the point-of-care in a cardiac surgery program. Future studies should be conducted to evaluate the benefits or advantages of the Quantra in comparison with other viscoelastic testing platforms.
Conclusion
After the introduction of Quantra in the operating room as a component of an active bleeding management protocol for cardiac surgery patients, a reduction in blood product transfusions was observed. Most strikingly was the reduction in utilization of fresh frozen plasma for this cohort of patients. The reduction in transfusions resulted in a cost savings from the required blood products, but also an improvement in patient blood management and care.
Acknowledgements
The authors thank Missy Vadnais, PhD, VMD for assistance with data analysis and manuscript preparation.
Abbreviations
- CT
Clot time
- CTH
Clot Time with Heparinase
- CS
Clot Stiffness
- FCS
Fibrinogen Contribution to Clot Stiffness
- FFP
Fresh frozen plasma
- PCS
Platelet Contribution to Clot Stiffness
- pRBC
Packed red blood cells
- VET
Viscoelastic testing
- YRMC
Yavapai Regional Medical Center
Author contributions
PT designed the study. PT and EB wrote the main manuscript text. JT and SA contributed to the study. All authors reviewed the manuscript.
Funding
HemoSonics, LLC (Durham, NC, USA) provided funding for IRB fees and data extraction.
Data Availability
All data generated or analyzed during this study are included in this published article or are available from the corresponding author on reasonable request.
Declarations
Competing interests
Pierre Tibi, MD. reports funding from HemoSonics LLC in the way of speakers fees.
Ethical approval
Institutional review board (IRB) approval was obtained with a waiver of informed consent by WCG IRB (Protocol 1287400).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Raphael J, Mazer CD, et al. Society of Cardiovascular Anesthesiologists clinical practice improvement advisory for management of perioperative bleeding and hemostasis in cardiac surgery patients. J Cardiothorac Vasc Anesth. 2019;33(11):2877–99. doi: 10.1053/j.jvca.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Murphy GJ, Reeves BC et al. (2007). Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2017; 116(22), 2544–2552. [DOI] [PubMed]
- 3.Kozek-Langenecker S. Management of massive operative blood loss. Minerva Anesthesiol. 2007;73:401–15. [PubMed] [Google Scholar]
- 4.Despotis G, Eby C, Lublin DM. A review of transfusion risks and optimal management of perioperative bleeding with cardiac surgery. Transfusion. 2008;48:2S–30S. doi: 10.1111/j.1537-2995.2007.01573.x. [DOI] [PubMed] [Google Scholar]
- 5.American Society of Anesthesiologists Task Force on Perioperative Blood Management Practice guidelines for perioperative blood management: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management. Anesthesiology. 2015;122:241–75. doi: 10.1097/ALN.0000000000000463. [DOI] [PubMed] [Google Scholar]
- 6.Tibi P, McClure RS, Huang J, et al. STS/SCA/AmSECT/SABM update to the clinical practice guidelines on patient blood management. J Cardiothorac Vasc Anesth. 2021;35:2569–91. doi: 10.1053/j.jvca.2021.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Karkouti K, Callum J, Wijeysundera DN, et al. TACS investigators. Point-of-care hemostatic testing in cardiac surgery: a stepped-wedge clustered Randomized Controlled Trial. Circulation. 2016;134:1152–62. doi: 10.1161/CIRCULATIONAHA.116.023956. [DOI] [PubMed] [Google Scholar]
- 8.Weber CF, Görlinger K, Meininger D, et al. Point-of-care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology. 2012;117:531–47. doi: 10.1097/ALN.0b013e318264c644. [DOI] [PubMed] [Google Scholar]
- 9.Hochleitner G, Sutor K, Levett C, Leyser H, Schlimp CJ, Solomon C. Revisiting Hartert’s 1962 calculation of the physical Constants of Thrombelastography. Clin Appl Thromb Hemost. 2017;23:201–10. doi: 10.1177/1076029615606531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curry NS, Davenport R, Pavord S, et al. The use of viscoelastic haemostatic assays in the management of major bleeding: a british Society for Haematology Guideline. Br J Haematol. 2018;182:789–806. doi: 10.1111/bjh.15524. [DOI] [PubMed] [Google Scholar]
- 11.Corey FS, Walker WF. Sonic estimation of elasticity via resonance: a New Method of assessing Hemostasis. Ann Biomed Eng. 2016;44:1405–24. doi: 10.1007/s10439-015-1460-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrante EA, Blasier KR, Givens TB, Lloyd CA, Fischer TJ, Viola F. A Novel device for the evaluation of hemostatic function in critical care settings. Anesth Analg. 2016;123:1372–9. doi: 10.1213/ANE.0000000000001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groves DS, Winegar DA, Fernandez LG, Huffmyer JL, Viola F. Comparison of coagulation parameters in arterial and venous blood in cardiac surgery measured using the Quantra system. J Cardiothorac Vasc Anesth. 2019;33:976–84. doi: 10.1053/j.jvca.2018.08.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huffmyer JL, Fernandez LG, Haghighian C, Terkawi AS, Groves DS. Comparison of SEER sonorheometry with rotational thromboelastometry and laboratory parameters in cardiac surgery. Anesth Analg. 2016;123:1390–9. doi: 10.1213/ANE.0000000000001507. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds PS, Middleton P, McCarthy H, Spiess BD. A comparison of a new ultrasound-based whole blood viscoelastic test (SEER sonorheometry) versus thromboelastography in cardiac surgery. Anesth Analg. 2016;123:1400–7. doi: 10.1213/ANE.0000000000001362. [DOI] [PubMed] [Google Scholar]
- 16.Naik BI, Durieux ME, Knisely A, et al. SEER sonorheometry versus rotational thromboelastometry in large volume blood loss spine surgery. Anesth Analg. 2016;123:1380–9. doi: 10.1213/ANE.0000000000001509. [DOI] [PubMed] [Google Scholar]
- 17.Baryshnikova E, Di Dedda U, Ranucci M. A comparative study of SEER sonorheometry versus standard coagulation tests, rotational thromboelastometry, and multiple electrode aggregometry in cardiac surgery. J Cardiothorac Vasc Anesth. 2019;33:1590–8. doi: 10.1053/j.jvca.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Zbrozek A, Magee G. Cost of bleeding in trauma and complex cardiac surgery. Clin Ther. 2015;37:1966–74. doi: 10.1016/j.clinthera.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Pagano D, Milojevic M, Meesters MI, et al. 2017 EACTS/EACTA guidelines on patient blood management for adult cardiac surgery. Eur J Cardiothorac Surg. 2018;53:79–111. doi: 10.1093/ejcts/ezx325. [DOI] [PubMed] [Google Scholar]
- 20.Pearse BL, Smith I, Faulke D, Wall D, Fraser JF, Ryan EG, Drake L, Rapchuk IL, Tesar P, Ziegenfuss M, Fung YL. Protocol guided bleeding management improves cardiac surgery patient outcomes. Vox Sang. 2015 Oct;109(3):267–79. [DOI] [PubMed]
- 21.Weber CF, Görlinger K, Meininger D, et al. Point-of-care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology. 2012;117(3):531–47. doi: 10.1097/ALN.0b013e318264c644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or are available from the corresponding author on reasonable request.