Abstract—
Long COVID-19 is a chronic disease that continues to be studied. Data on epidemiology and the main symptoms typical for long COVID-19 are presented. Issues related to the pathogenesis of the disease are discussed. At the same time, special attention is paid to the inflammation process (including of the vascular wall endothelium), the state of the immune system (cytokine storm), the hemostasis system (the mechanism for the development of microangiopathy and thrombosis), and oxidative stress. During the analysis, a special place is given to central nervous system disorders (including organic brain damage) and disorders of cognitive functions. In addition, currently known complications from the cardiovascular system and respiratory organs are described. The treatment and rehabilitation of patients with long COVID-19 is not only a medical, but also a significant social problem.
Keywords: long COVID-19, immunity, hemostasis, inflammation, complications, central nervous system, cognitive functions, heart, vessels, lungs
INTRODUCTION
Long COVID-19 is a term for a chronic disease, the traits of which appear even after the relief of the main symptoms. It is also called post-acute COVID-19, ongoing symptomatic COVID-19, chronic COVID-19, post COVID-19 syndrome, and long-haul COVID-19. The terms post-acute sequelae of SARS-CoV-2 infection (PASC) and post acute COVID-19 (2.PASC) (Rubin, 2020), as well as long-haulers COVID-19, are rarely used. Consequently, long COVID-19 can be presented as a complex of different clinical symptoms in patients who were considered recovered from COVID-19 that can appear at least four weeks after the initial infection (Chippa et al., 2022). It should be noted that post-acute or long COVID is an increasingly common syndrome. It includes many debilitating symptoms that can last for several weeks or more (months, years), even after a mild disease (Dani et al., 2021). Long COVID-19 is conceptualized as a multiple organ disease with a wide spectrum of clinical manifestations that can indicate about pulmonary, cardiovascular, endocrine, hematological, renal, gastrointestinal, dermatological, immunological, psychiatric or neurological diseases. It is assumed that long COVID-19 is actually myalgic encephalomyelitis. “However, similarities between these two diseases are observed when comparing the International Consensus Criteria for the Diagnosis of Myalgic Encephalomyelitis with the symptoms described in long COVID-19” (Espinosa Rodríguez et al., 2021, p. 65).
EPIDEMIOLOGY OF LONG COVID-19
Post-COVID syndrome or long COVID-19 is developed in every third adult and every tenth child regardless of the severity of the disease (Pazuhina et al., 2022). At the same time, the symptoms can be caused by persistent chronic inflammation (for example, fatigue), consequences of organ damage (for example, pulmonary fibrosis, chronic kidney disease), hospitalization, and social isolation (for example, muscle atrophy, malnutrition) (Scordo et al., 2021).
There are millions of individuals with long COVID-19. Since the number of confirmed cases of COVID-19 infection worldwide by March, 2021 exceeded 110 million, it can be assumed that at least about 15 million individuals can have experienced the traits and symptoms of long COVID. It is difficult to imagine how many patients with long COVID-19 there currently are. Apparently, the given number of 15 million individuals should be at least doubled. While the pandemic lasts, this number will only increase.
DEFINITIONS AND SYMPTOM COMPLEX OF LONG COVID-19
The National Institute for Health and Care Excellence (NICE), the Scottish Intercollegiate Guidelines Network (SIGN), and the Royal College of General Practitioners (RCGP) distinguish three phases in the course of COVID-19 infection: acute COVID-19 (traits and symptoms of COVID-19 infection for up to 4 weeks); ongoing symptomatic COVID-19 (4–12 weeks); and post COVID-19 syndrome (traits and symptoms persist for more than 12 weeks) (Shah et al., 2021). The term long COVID-19 refers to the traits and symptoms that continue or develop after the acute phase of COVID-19 and includes both ongoing symptomatic COVID-19 and post COVID-19 syndrome. Chronic COVID and long-term COVID are other names used to describe the consequences of COVID-19 (Mahase et al., 2020; Callard and Perego, 2021).
According to some authors (López Castro, 2020), symptoms or abnormal clinical parameters that persist for more than two weeks after the beginning of COVID-19 disease and do not return to the initial healthy level can be considered as long-term consequences of the pathological process. Such shifts in the activity of the patient’s body are most often reported in survivors of severe and critical conditions. However long-term changes in the activity of different organs and systems are also observed in individuals with mild infection and even in those who did not require hospitalization.
It is noted (Silva Andrade et al., 2021) that the symptoms of long COVID-19 are not localized. They can be manifested at the level of the immune, pulmonary, circulatory, cardiovascular, gastrointestinal, and nervous systems. At the same time, multi-systemic lesions in long COVID-19 are accompanied by a cytokine storm, which leads to endothelial inflammation, microvascular thrombosis, and multiple organ failure (Silva Andrade et al., 2021).
It should be noted that most of the symptoms in long COVID-19 are similar to the symptoms that develop during the acute phase of the disease; of them, fatigue, dyspnea, cough, headache, brain fog, anosmia, and dysgeusia are most commonly reported (Garg et al., 2021). More diverse and debilitating symptoms, affecting the skin, respiratory, cardiovascular, digestive, and central nervous systems, are reported. With CNS damage, exacerbations of a psychoneurological nature or the emergence of new psychiatric disorders can occur, especially in patients with long COVID-19 who had an accompanying severe condition or were admitted to the intensive care unit (Wong et al., 2020; Garg et al., 2021).
The five most common symptoms in long COVID-19 are (López Castro, 2020): fatigue (58%), headache (44%), impaired attention (27%), hair loss (25%), and dyspnea (24%). According to (Okada et al., 2021), the loss of smell or taste is the second most common persistent symptom after fatigue 6 months after the beginning of COVID-19.
It was established (Yelin et al., 2021) that among hospitalized patients with COVID-19, radiological changes persisted in almost two-thirds of patients 90 days after discharge. A decrease in cognitive functions, a drop in the quality of life, and dyspnea were simultaneously detected. Muscle pain and difficulties in performing normal activities most affected the quality of life of patients.
When monitoring patients for almost 4 months after the beginning of symptoms (Xiong et al., 2021), the most common symptoms detected were fatigue and dyspnea.
The effect of COVID-19 on a cohort of 150 recovering patients that were treated as outpatients after mild and moderate episodes was studied (Carvalho-Schneider et al., 2021). Patients were contacted 30 and 60 days after symptom onset and asked to complete a simple questionnaire regarding their symptoms and general well-being. The study demonstrated that two-thirds of patients reported at least one symptom on both day 30 and day 60, and a third of test subjects felt as bad on day 60 as they did during the acute episode, or worse. The most common symptoms identified were: anosmia or ageusia (27.8% at day 30, 22% at day 60), followed by flu-like symptoms (36% at day 30, 21% at day 60)
A significant number of studies indicate that in addition to pneumonia and acute respiratory distress syndrome (ARDS), long COVID-19 leads to multisystem lesions in the form of myocarditis, arrhythmias, cardiac arrest, gastrointestinal symptoms, hypoxic brain injury, acute liver and kidney dysfunction (Garg et al., 2021). There are also reports about skin lesions in the form of urticaria, maculopapular rashes similar to frostbite of the fingers and toes (COVID feet), livedoid vasculopathy, varicella, and variceliform vesicles. Clinically, many of these skin lesions are most likely secondary relative to the occlusion of small and medium blood vessels due to the formation of microthrombi or due to viral immune antigen–antibody complexes. And the same explanation could be true for the possible hypoxic damage occurring simultaneously in other vital organs (in the brain, heart, lungs, and kidneys).
PATHOGENESIS OF LONG COVID-19
According to some data (Pierce et al., 2022), the pathogenesis of long COVID-19 includes four pathophysiological categories: virus-specific pathophysiological variations, oxidative stress, immunological abnormalities, and inflammatory damage. At the same time, recently it became known that the SARS-CoV-2 virus is not homogeneous in its genetic nature. Among the strains of RNA viruses, 9 new coronaviruses are noted, including α, β, γ, δ, and Omicron (BA.1, BA.2). The symptoms, severity and outcome of the pathological process largely depend on the strain of the virus that caused the COVID-19 disease.
The SARS-CoV-2 virus affects individuals of all ages, while the frequency of hospitalizations of COVID-19 patients increases with age. The older patients are, the more likely it is that they end up in the intensive care unit and the more often the symptoms typical for long COVID-19 appear in such patients (Verity et al., 2020). At the same time, women get sick with long COVID-19 1.5 times more often than men; however, this difference is not observed in individuals older than 80 years (Garg et al., 2020). At the same time, COVID-19 is more severe and with a higher mortality rate in men than in women. Apparently, this is associated with the effect of androgens, since their receptor activates cell surface receptors that mediate virus penetration into the cell (Nassau et al., 2022).
Ultrastructural studies demonstrate that SARS-CoV-2 damages the cells with viral distribution in the cytoplasm, and many viral particles are encapsulated in cytoplasmic vesicles. At the same time, SARS-CoV-2 replication triggers the release of pro-inflammatory cytokines and chemokines. Cell death is induced in the in the epithelium of the respiratory tract, alveolar epithelial and vascular endothelial cells, in which a high expression of angiotensin converting enzyme 2 (ACE2) is observed (Lucas et al., 2020; Yap et al., 2020). As is known, ACE2 is the most important element of the renin–angiotensin–aldosterone system (RAAS), which is involved in the regulation of arterial pressure, electrolyte homeostasis, cell growth and proliferation, inflammation. It is also contained in the extracellular matrix. In the acute phase, SARS-CoV-2 shows a particular affinity for ACE2 receptors that are detected in high concentrations in the lungs, liver, kidneys, and blood vessels. The virus primarily affects the respiratory system and, upon reaching the alveoli of the lungs, is attached to the host cells, especially type II pneumocytes. By damaging the alveoli, SARS-CoV-2 contributes to the destruction of the capillary endothelium, causing endotheliitis, which leads to the formation of microthrombi (Kuznik et al., 2020a; López Castro, 2020; Garg et al., 2020, 2021). Penetrating into the blood vessels, SARS-CoV-2 spreads throughout the body. At the same time, the virus stimulates the endothelium and elements of the blood, causing a significant release of cytokines and chemokines, which in severe cases leads to a cytokine storm (Kuznik et al., 2020a, 2020b; Khavinson et al., 2020, 2021). It is assumed that there is an association between mast cell activation syndrome and the intensity of the cytokine storm in patients with long COVID-19. At the same time, damage to organs caused by an excessive inflammatory response requires much more time for normalization of the damaged functions, which is a reason for the appearance of the main symptoms in long COVID-19 (López Castro, 2020).
It was established that general immune abnormalities in COVID-19 accompanied by a cytokine storm include progressive hypercytokinemia with an increase in the levels of TNF-α, IL-6, IL-1β, IL-10 and other pro-inflammatory chemokines, as well as activated macrophages, with an increase in the concentration and an increase in the activation of NF-κB observed in the cases of moderate and severe severity. At the same time, the inflammation and release of molecular patterns associated with the damage (that are also characteristic features for COVID-19) are initiated (Morris et al., 2021).
Interferons, especially IFN-1, play an important role in the pathogenesis of COVID-19. Coronaviruses are able to inhibit the production of interferon both in vitro and in vivo. It was established that a significant decrease in the effect of IFN-1 is detected in patients with severe COVID-19 compared with patients with mild or moderate cases (Hadjadj et al., 2020; Adeloye et al., 2021). The significance of natural killer (NK) lymphocytes in the pathogenesis of severe complications in COVID-19 (the content of which, especially in severe infections caused by SARS-CoV-2, drops significantly) should be also pointed out. Apparently, NK cells migrate to the lungs and other organs of patients infected with SARS-CoV-2 (Wang et al., 2020a). At the same time, the content of TNF-α secreted by monocytes increases with COVID-19 in the plasma of patients. In turn, a dysfunction of monocytes, which occurs with COVID-19, negatively affects the antiviral function of NK cells (Lubavina et al., 2021).
The formation of antigen–antibody complexes by the second or third week (when the humoral immunity is activated) is one of the most important manifestations of COVID-19 (Strauss et al., 2020). The emerging autoantibodies (aAB) are largely responsible for the pulmonary fibrosis and other long-term consequences of COVID-19 occurring in patients. At the same time, violations of the regulatory function of immunity (underlying the prothrombotic state) and the development of organ damage (observed in patients with long COVID-19) are observed (Zuo et al., 2020).
In order to estimate both the long-term effect of SARS-CoV-2 infection on the immune system and the presence of aAB, a prospective study of two groups of patients was conducted: with acute SARS-CoV-2 and previously infected with SARS-CoV-2 (a total of 80 individuals), as well as 39 COVID-19 free donors (Lingel et al., 2021). The level of aAB against cyclic citrullinated peptide (CCP), a specific predictor of rheumatoid arthritis, was significantly (p = 0.035) increased only in convalescents. A significant (up to critical numbers) increase in the level of tissue transglutaminase (TG), a specific predictor of celiac disease, was observed both in patients with acute COVID-19 and in convalescents (p = 0.002). An increased level of antibodies to both CCP and TG was detected 4–8 months after infection with SARS-CoV-2. Antibodies to TG were found predominantly in elderly patients against the background of a post-SARS-CoV-2-specific immune composition (R2 = 0.31; p = 0.044). Therefore, increased content of antibodies to both CCP and TG can persist for a long time after recovery, even from mild COVID-19.
It is most likely that hyperactivation of the immune system, accompanied by hyperinflammation and cytokine storm, as well as a simultaneous production of specific aABs against CNS tissues, is the main mechanism for the occurrence of encephalopathy, encephalitis, myelitis, and acute disseminated encephalomyelitis in COVID-19. aAB were mainly found in the cerebrospinal fluid of critically ill patients with COVID-19. Clarifying the contribution of neuroimmunological mechanisms, including the formation of aAB, to long-term consequences after COVID-19 disease (fatigue, memory impairment, sleep disturbance or anxiety) will require long-term clinical follow-up.
The content of aAB to different receptors was studied in 31 patients suffering from different symptoms of long COVID-19 after the acute phase of the disease (Wallukat et al., 2021). At the same time, from 2 to 7 different aAB to G protein-coupled receptors - functional autoantibodies (GPCR-fAAB) receptors were identified in all that acted as receptor agonists. Among them, there were both positive and negative aAB to GPCR-fAAB. Positive chronotropic GPCR-fAAB identified in the blood of patients with long COVID-19 are targeted at β2 adrenoreceptors (β2-fAAB), α1 adrenoreceptors (α1-fAAB), angiotensin II AT1 receptors (AT1-fAAB), and nociceptin-like opioid receptors (NOC-fAAB). The identified negative chronotropic GPCR-fAABs are targeted at the muscarinic M2 receptor (M2-fAAB), MAS receptor (MAS-fAAB), and ETA receptor (ETA-fAAB). It was suggested that a persistent presence of aAB to GPCR-fAAB underlies the pathogenesis of long COVID-19.
It is indicated (Bornstein et al., 2021) that there is a striking similarity between long COVID-19 and myalgic encephalomyelitis, also called chronic fatigue syndrome, associated with viral and autoimmune pathogenesis. According to the authors, antibodies to neurotransmitter receptors against β-adrenergic and muscarinic receptors should play a key role in the pathogenesis of both diseases. The authors (Bornstein et al., 2021) found the same increase in the detected aAB in both groups of patients. Extracorporeal apheresis using a special filter is efficient at significantly reducing the number of such antibodies, clearly reducing debilitating symptoms in patients with chronic fatigue syndrome. Presumably, this form of neuropheresis could be a promising therapeutic variant for patients with post-COVID-19 syndrome.
It is known that lupus anticoagulant (LA) is an antibody to phospholipids (aPL) that increases blood coagulation time. LA are found in patients with antiphospholipid syndrome, which in itself represents a significant risk factor for thrombosis. It can be assumed that the presence of LA is one of the traits of COVID-19 in patients with a severe and prolonged course of the disease, which certainly contributes to thrombosis. At the same time, patients with the highest titers or multiple positive results are at greatest risk of thrombosis due to aPL. Apparently, the recognition of a number of self-antigens (leading to antigen binding) underlies the effect of aAB in long COVID-19. At the same time, the important processes involved in inflammation, defense against pathogens, and coagulation are modified. With long COVID-19, the prevalence of aAB targeting immunomodulatory proteins, including cytokines, chemokines, complement components, and cell surface proteins, significantly increases. All this contributes not only to a more severe, but also a prolonged course of the pathological process (Chen et al., 2021).
In addition, SARS-CoV-2 contributes to a release of cellular ATP. An increased level of extracellular ATP activates purinergic receptors on immune cells, initiating a physiological pro-inflammatory immune response. Persistent viral infection stimulates the release of ATP even more leading to the activation of P2X7 purinergic receptors (P2X7Rs) and severe, but physiological inflammation. The disease progression determines long-term activation of P2X7Rs causing cell death and uncontrolled release of ATP, which causes cytokine storm and desensitization of all other purinergic receptors of the immune cells. This provokes immune paralysis with co-infections or secondary infections, designated as hyperinflammation (Dani et al., 2021; Estiri et al., 2021), which may be one of the reasons for the development of long COVID-19.
Four predictive risk factors for severe and long disease during the initial diagnosis of COVID-19 were identified (Su et al., 2022): type 2 diabetes, SARS-CoV-2 RNAemia, Epstein–Barr virus viremia, and the presence of specific aABs.
As already emphasized, the severe course of COVID-19, as well as the appearance of long COVID-19, occurs more often in elderly and senile individuals. The aging process, characterized by stable growth arrest (which is based on violations of pro-inflammatory and preventive functions), contributes not only to the pathogenesis of COVID-19, but also to the disease duration. It should be noted that elderly patients with COVID-19 are more likely to accumulate a high level of factors that contribute to cellular aging. At the same time, aging cells can contribute to an uncontrolled cytokine storm mediated by SARS-CoV-2, as well as a long-term excessive inflammatory response. Finally, aging cells contribute to the tissue damage leading to lung failure and multi-tissue dysfunction (Pezzini and Padovani, 2020).
Thus, the data presented indicate extreme complexity of the pathogenesis of long COVID-19. Inflammation, development of a cytokine storm, thrombus formation, appearance of aABs to one’s own tissues and receptor formations, as well as many other factors that are still poorly studied play an important role in this process.
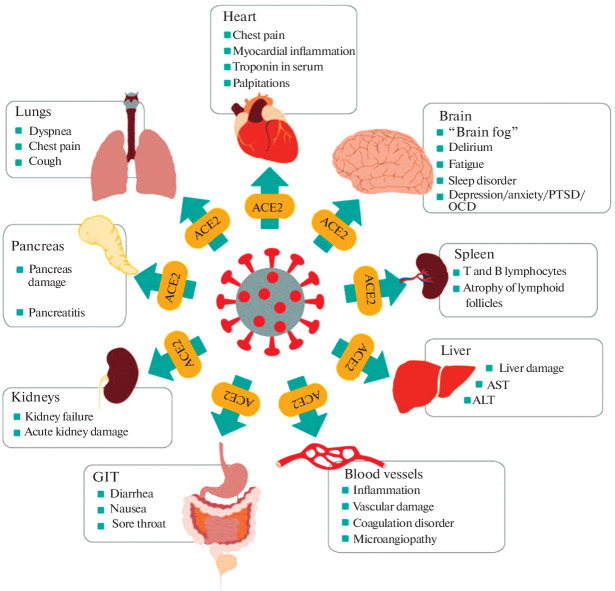
The following scheme reflecting multiple organ damage caused by coronavirus and leading to long COVID-19 is proposed (Chippa et al., 2022) (Fig. 1).
Fig. 1.
Multiple organ damage caused by coronavirus leading to long COVID (Chippa et al., 2022). It is clear from this scheme that there are no organs and systems, to which damage does not accompany long COVID-19.
CNS DAMAGE AND COGNITIVE FUNCTION DISORDERS
At present, it is undeniably proven that the SARS-CoV-2 virus enters the brain either through the bloodstream or through nerve endings, which is considered a direct route. Such conclusions are based on studies reporting the presence of viral material in the cerebrospinal fluid and brain cells. Moreover, SARS-CoV-2 infection can directly affect the nervous system in a cytopathic manner. At the same time, SARS-CoV-2 neuroinvasion can occur at the border of the neuro-mucosa by penetrating through the mucous and regional neural structures (Meinhardt et al., 2021). However, indirect mechanisms, including coagulation disorders and prolonged activation of the immune system, can lead to further tissue and organ damage (including the brain) observed in the course of the disease. At the same time, there are many neurological symptoms of varying severity (from headaches to life-threatening strokes) (Haidar et al., 2022). Furthermore, the SARS-CoV-2 virus leads to the activation of microglia and astrocytes with a release of neuroinflammatory response mediators (Tremblay et al., 2020). The activation of glia in patients with moderate and severe COVID-19 is accompanied by an increase in the concentration of plasma biomarkers of CNS damage (Kanberg et al., 2020).
A long course of COVID-19, accompanied by a sense of fear, can lead to a decrease in brain volume. Even the fear of getting sick is accompanied by a decrease in the volume of the right posterior cingulate gyrus (Takahashi et al., 2022). However, significant changes in the brain structure are observed with a long course of the disease. There is a significant loss of gray matter density and volume, especially in the parahippocampal gyrus associated with memory encoding, in the lateral orbitofrontal cortex involved in decision making, and in the insular lobe, which plays a role in the formation of emotions. In hospitalized patients on long-term therapy, a more pronounced loss of the gray matter in the cingulate cortex, central nucleus of the amygdala, and some regions of the hippocampus is observed. As is known, all of these regions are associated with memory and the formation of emotions. The consequences of COVID-19 for the brain were studied in 34 discharged patients without neurological manifestations (Tian et al., 2022). It was established that in severe cases, hypoperfusion of the cortical cerebral blood flow (observed at 3 months) was less pronounced at 10 months.
It is known that cytokine storm sharply activates the enzyme indoleamine-2,3-dioxygenase (IDO-1), which leads to an increase in kynurenine metabolites. Kynurenine is metabolized by IDO-1 in the brain with the formation of chemokines, which long-term can lead to brain damage. At the same time, the SARS-CoV-2 neuroinvasion increases the local level of angiotensin II due to the suppression of ACE2. Thus, angiotensin II can increase kynurenine metabolites, causing pro-oxidant and pro-inflammatory effects, which leads to cognitive function disorder, an increase in oxidative stress, and a decrease in brain-derived neurotrophic factor. In addition to long-term respiratory problems and chronic fatigue, patients experience problems with attention and memory, that can be accompanied by psychiatric or neurological complications. In individuals of all ages with COVID-19, a risk of any mental diagnosis during the year was 20–60% higher than in those who did not have this infection. These disorders can occur even after an asymptomatic course of infection and also after a mild course (Logue et al., 2021). According to (Mao et al., 2020), COVID-19 is associated with neurological manifestations in 36% of patients. At the same time, neurological manifestations can vary from mild headache or brain fog to more severe complications, including Guillain–Barré syndrome (Alberti et al., 2020), encephalitis (Moriguchi et al., 2020), and arterial and venous strokes (Kananeh et al., 2020). After analyzing 19 studies involving a total of 11 324 patients (Premraj et al., 2022) concluded that the prevalence of neurological symptoms after COVID-19 was manifested in the following order: fatigue (37%), brain fog (32%), memory problems (27%), impaired attention (22%), myalgia (18%), anosmia (12%), dysgeusia (11%), and headache (10%). Neuropsychiatric conditions included sleep disorders (31%), anxiety (23%), and depression (12%). In patients who had fatigue and neurological symptoms (dizziness, headache) at the beginning of the disease, subsequent cognitive function disorders developed more often.
Brain fog, which is also called thought fog or consciousness fogging, is one of the most common symptoms reported by long COVID patients. A sense of fog in the head after suffering from coronavirus can be caused by processes similar to those that occur in Alzheimer’s disease. Some patients who had coronavirus complain of mental confusion, problems with coordination, and difficulties when trying to concentrate. So-called taupathies (brain defects emerging as a result of the clumping of tau protein due to inflammatory processes as a result of the coronavirus) may be the reason for this. Similar defects appear in Alzheimer’s disease. These similarities lead to the hypothesis that neurological symptoms can arise as a result of neuroinflammation and immune cell dysfunction both in the periphery and in the CNS, as well as to the suggestion that the long-term consequences of long COVID-19 can lead to cognitive disorders in general. The conducted studies allow us to make a conclusion (Graham et al., 2021; Meier et al., 2021) that the methods and drugs used to combat the symptoms of Alzheimer’s disease can be used for the treatment of brain fog after coronavirus.
It was established that different types of neurological damage and diseases with a variety of manifestations can follow COVID-19: chemosensory disorders, muscle damage, encephalopathy, delirium, coma, meningitis, encephalitis, cerebrovascular diseases, and peripheral and central neuroimmunological disorders (Graham et al., 2021). Four types of such disorders can be distinguished: (1) neurological disorders, associated with the consequences of pulmonary diseases, and systemic inflammatory response syndrome and sepsis manifested along with them; (2) direct penetration of a virus into the CNS; (3) disorders caused by postinfectious immune-mediated complications, including Guillain-Barré syndrome or acute disseminated encephalomyelitis; (4) peripheral organ disorder, dysfunction or failure in a specific activity (Zhou et al., 2020; Logue et al., 2021). Indeed, the neurological complications of COVID-19 can originate from the interaction of these four types of damage.
It is known that ARDS in COVID-19 is a critical condition, often leading to long COVID-19. It was established that after suffering from ARDS, more than two thirds of patients reported clinically significant symptoms of fatigue a year after the beginning of the disease (Komaroff and Lipkin, 2021). At the same time, pains in different organs, sleep disturbances, the appearance of symptoms indicating vegetative dysfunction, worsening of the general condition after a slight increase in physical and/or cognitive activity, and the presence of neurocognitive disability are noted (Mantovani et al., 2021).
Several neuropsychiatric symptoms are reported (Ye et al., 2020): headache (44%), attention disorder (27%), and anosmia (21%). Other symptoms were also observed, including brain fog and neuropathies. The etiology of symptoms in patients with COVID-19 is complex and multifactorial. They can be associated with a direct effect of infection, cerebrovascular disease, including disease caused by hypercoagulation (Okada et al., 2021), physiological disorder (hypoxia), drug side effects, and the social aspects of a potentially fatal disease (Wong et al., 2020). Adults have a doubled risk of getting a new mental health diagnosis after COVID-19, with the most common conditions being anxiety disorders, dementia, and insomnia. In turn, sleep disorders can contribute to a more pronounced manifestation of mental disorders. Psychoneurological assistance is recommended to all patients recovering from COVID-19.
Hair loss is one of the symptoms of post-COVID-19; it can be considered as telogen outflow (diffuse hair loss) after severe systemic stress or infection. At the same time, the follicles prematurely pass from the active growth phase (anagen) to the resting phase (telogen) (Okada et al., 2021).
Chemosensory dysfunctions, including anosmia, hyposmia, ageusia, and hypogeusia, are frequent symptoms of SARS-CoV-2 infection.
Taste disorder in patients with COVID-19 is described as hypogeusia (a decrease in taste sensations); dysgeusia is a change or distortion of taste sensations; ageusia is a complete loss of the taste. The overall prevalence of taste disorders is 45%, including dysgeusia (38%), hypogeusia (35%), ageusia (24%), with an average duration of symptoms of 15 days, after which patients completely recovered, or the disease could become chronic (Singer-Cornelius et al., 2021). These results can reflect a neurotropic viral invasion and possible damage to the nervous system. Each individual taste sensory cell (sensors for sweet, bitter, acidic, salty, and umami) connects to each corresponding ganglion neuron in the taste bud. SARS-CoV-2 can penetrate these afferent neurons, and colonization by the virus permanently impairs sensory nerve conduction. In addition, the SARS-CoV-2 virus can enter the cells through the ACE2-expressing layered squamous epithelium of the dorsal tongue and filiform papillae near the taste buds and eventually progress to a taste cell infection (Okada et al., 2021).
It should be noted that the virus can enter the ACE2-expressing layered squamous epithelium of the dorsal tongue and filiform papillae near the taste buds and eventually progress to infection of the taste cells. It is suggested that the microvilli of taste sensory cells allow SARS-CoV-2 to enter the cells (Okada et al., 2021). When studying autopsy and ambulatory salivary gland samples with COVID-19, T-lymphocytic inflammation (CD3) with an increased content of B lymphocytes (CD20) and infiltration prevailed (Huang et al., 2021). At the same time, inflammatory and immune responses to SARS-CoV-2 in the taste receptors and salivary gland tissues can differ in different individuals, including depending on sex, age, and genetic variability (Okada et al., 2021). At the same time, patients report the loss of smell and taste more frequently than other symptoms (Agyeman et al., 2020). It was noted that the loss of smell in the subtype of omicron BA.2 is more common than in omicron BA.1.
The olfactory canal is the strongest candidate for the SARS-CoV-2 path of penetration into the CNS. In fact, there is a relationship between the olfactory pathway and subcortical and cortical structures, which can explain the olfactory disorders that often occur in patients with SARS-CoV-2. The fact that these neurons are constantly regenerating from undifferentiated basal cells is of a particular importance. The olfactory pathway begins at the top of the nasal cavity/mucosa, where the olfactory epithelium is located. The olfactory receptors are located on bipolar neurons that form small bundles that make up the olfactory nerve (DosSantos et al., 2020; Haidar et al., 2021, 2022). These anatomical peculiarities allow the virus to reach the brain and cerebrospinal fluid through the olfactory nerve and bulb within 7 days, causing inflammation and a demyelinating response (Bohmwald et al., 2018). Meta-analysis demonstrates that the prevalence of olfactory dysfunction ranges from 41.0 to 61.0%, and the prevalence of taste dysfunction from 38.2 to 49.0%. It is noteworthy that the loss of taste is consistently considered as a common symptom of long COVID-19, determined as the persistence of symptoms four weeks after the infection. In a retrospective cohort of 3737 patients who reported the anosmia and/or dysgeusia during the acute COVID-19, olfaction was restored in 68% and taste 73% during the six weeks after the appearance of the symptom (Agyeman et al., 2020; Nguyen et al., 2021).
Swallowing disorders (dysphagia) can occur with long COVID-19, which can be a consequence of both a primary lesion of the central and peripheral network of swallowing neurons and artificial lung ventilation. The clinical observations in COVID-19 (long COVID) indicate the persistence of dysphagia. Consequently, these patients also require long-term interventions to restore safe and sufficient oral nutrition.
PATHOLOGY OF THE CARDIOVASCULAR SYSTEM AND THROMBUS FORMATION
Cardiovascular problems, including stroke, thromboembolism, deep vein thrombosis, and myocardial infarction, are increasingly becoming an important cause of death during SARS-CoV-2 infection (Haidar et al., 2021). A study by a team of scientists from the Washington University School of Medicine in St. Louis and staff from the St. Louis Healthcare System for Veterans Affairs in the United States demonstrated that individuals who had COVID-19 even in a mild form were at an increased risk of developing cardiovascular diseases and dangerous conditions within one month to a year after infection. Cerebrovascular diseases, arrhythmias, ischemic and non-ischemic heart diseases, pericarditis, myocarditis, heart failure, thromboembolism, and stroke are all diseases of the cardiovascular system in those who had COVID-19 (even in a mild form). In a new study, American scientists (Xie et al., 2022) used the United States Department of Veterans Affairs database in order to track the consequences of the disease in 153 760 individuals who had COVID-19 of varying degree of severity. The authors estimated the risks of developing cardiovascular diseases in patients compared with a modern healthy cohort, beginning from 30 days after a positive test to the end of the study. Recovered patients had an increased risk of stroke, transient ischemic attacks, atrial fibrillation, pericarditis, and myocarditis. There was also a significant increase in the risk of myocardial infarction, ischemic cardiomyopathy, heart failure, cardiac arrest, pulmonary embolism, and deep vein thrombosis. The overall risk of a major adverse cardiovascular event (MACE) increased. Increased risks persisted in all subgroups when divided by age, sex, and the presence or absence of comorbidities: high blood pressure, diabetes, and hyperlipidemia. The risks were higher in individuals who had a severe acute phase of the infection; however, they were increased even in those who survived the disease without hospitalization. The reasons for an increase in the risks are unclear. The researchers put forward different hypotheses (from direct damage to cardiomyocytes by the virus and inflammation of endothelial cells to abnormal sustained activation of the immune system and autoimmune reactions). There are also supporters of the hypothesis about the insertion of the SARS-CoV-2 genome into DNA of infected cells with subsequent expression of chimeric proteins (Xie et al., 2022).
In summarizing studies (Adeloye et al., 2021), it was reported that cardiopulmonary symptoms, including chest pain, dyspnea, fatigue, and vegetative manifestations, particularly, postural orthostatic tachycardia, are common and to a large extent determine increased anxiety and significant disability subsequently. A number of cardiovascular disorders were reported in patients after the acute phase, including myocardial inflammation, myocardial infarction, right ventricular dysfunction, and arrhythmia. The pathophysiological mechanisms of delayed complications are still poorly studied; at the same time, there is a dissociation between ongoing symptoms and objective indices of cardiopulmonary health. It is expected that COVID-19 will change the long-term trajectory of many chronic heart diseases that often affect individuals with a risk for severe cardiovascular system damage.
Acute heart failure (AHF) is one of the main complications in all groups of patients after COVID-19. The recovery after AHF is slow and often accompanies long COVID-19 (Lu et al., 2022). The development of a stroke with COVID-19 is not rare, the probability of which increases with age, comorbidities, and severity of infection. There are also multiple cases of stroke in young (including previously healthy) patients with COVID-19. This suggests that the virus itself can be responsible for the initiation and exacerbation of thrombotic events.
Possible mechanisms contributing to the emergence of long COVID-19 include cell damage, prolonged viral shedding, a chronic immune inflammatory response, and a significant increase in coagulation activity (Estiri et al., 2021; Tran et al., 2022). The question of how and why hypercoagulation occurs with COVID-19 was studied in some detail and covered in a number of domestic reviews (Simarova et al., 2021; Slukhanchuk et al., 2021). However, information appeared recently highlighting the mechanism of development of hypercoagulability and thrombosis with COVID-19 in a new way. It was found that the level of aAB to phospholipids in the blood of patients with COVID-19 correlates with the expression of endothelial cell activation markers (cell adhesion molecules E-selectin, VCAM-1, and ICAM-1). At the same time, the activation of the endothelium often leads to vascular pathologies, including thrombosis. In the blood of patients with COVID-19, there was a high concentration of IgG and IgM antibodies to three types of phospholipids: cardiolipin, beta-2-glycoprotein I, and phosphatidylserine/prothrombin (PS/PT) that cover the vascular endothelium, and are also located in the brain and lungs. The presence of circulating aPL is the strongest factor capable of activating the endothelium in COVID-19. In this case, immune complexes are formed with activated negative surface of phospholipids, which leads to an increase in inflammation and the spontaneous formation of thrombi in arteries and veins that often accompany long COVID-19 (Shi et al., 2022).
The presence of deep and persistent endothelial dysfunction in patients with COVID-19 not only in the severe course of the disease, but also in patients of moderate severity, is of no doubt (Kuznik et al., 2020a; Haidar et al., 2022). At the same time, there are significant changes in the function of erythrocytes contributing to hypercoagulation and the development of thrombosis. These changes are characterized by increased ROS formation, which determines endothelial dysfunction through upregulation of vascular arginase-1 and oxidative stress in the acute phase. Moreover, erythrocytes of patients with COVID-19 induced the activation of arginase-1 and an increase in the oxidative stress in endothelial and smooth muscle cells. An excess of endothelial arginase-1 reduces the bioavailability of NO and inhibits endothelial NO synthase (eNOS), leading to a subsequent increase in ROS production contributing to thrombosis. However, it should be noted that IFNγ is one of the factors triggering erythrocyte-induced endothelial dysfunction (Mahdi et al., 2022).
New facts illuminating the mechanism of the occurrence of hypercoagulation do not reduce the significance of previously established ones: activation of the complement system and RAAS, development of the cytokine storm, an increase in microvesicles, the activation of platelets, and others.
It was established that the arteries of both the upper and lower extremities are mainly affected in COVID-19. Against the background of thrombosis, severe ischemia (a critical deterioration in blood supply) can be developed, and it can even lead to the amputation of a limb. Patients often successfully tolerate the peak of the disease itself, but later they encounter thrombosis if left without anticoagulant or antiplatelet therapy. Thus, a clinical experience with four patients affected by COVID-19 who underwent limb amputation due to acute irreversible ischemia has been reported (Ilonzo et al., 2021). Histological examination with hematoxylin/eosin revealed typical inflammatory cells and suggested a possible presence of infectious angiitis caused by COVID-19 in the described patients.
RESPIRATORY ORGAN DISORDERS
It is known that the upper and lower respiratory tracts are most susceptible to infection when inhaling the virus, the severity of lung damage is closely associated with the intensity of long COVID-19 infection accompanied by a gradual loss of lung function, which is associated with pulmonary interstitial fibrosis (Goh et al., 2020; Ilonzo et al., 2021; Wang et al., 2020a). Pulmonary complications that accompany prolonged COVID can certainly affect the quality of daily life of individuals who initially consider themselves recovered from the acute disease. Even the initial studies demonstrated that approximately 20% of infected patients require hospitalization, and 6% require intensive care and an invasive mechanical ventilation procedure. At the same time, rapid and progressive respiratory failure similar to ARDS occurred in 8.2% of the total number of cases (Torres-Castro et al., 2021). Moreover, severe ARDS under the influence of the SARS-CoV-2 virus caused more than three million deaths worldwide, despite the use of all possible therapeutic options for mechanical ventilation (Maracaja et al., 2021). It was established that the virus can affect the lungs in three ways: ARDS with diffuse alveolar damage; diffuse thrombotic alveolar microvascular occlusion; and inflammation of the respiratory tract associated with inflammatory mediators. Alveolar oxygenation disorder, hypoxemia and acidosis is a result of these processes and their combination. In the absence of efficient treatment, either death of the patient from respiratory failure or irreversible lung damage (if the patient recovers) are the consequences of impaired oxygenation (Calabrese et al., 2020; Wang et al., 2020b).
The available data indicate that lung damage with COVID-19 is accompanied by different pathophysiological phenomena: diffuse destruction of the alveolar epithelium, formation of a hyaline membrane, capillary damage and bleeding, fibrous proliferation of the alveolar septa, and pulmonary consolidation. The extensive damage to alveolar epithelial and endothelial cells with secondary fibroproliferation indicates the possibility of chronic vascular and alveolar remodeling leading to pulmonary fibrosis and/or pulmonary hypertension (Frija-Masson et al., 2020). This results in respiratory failure, pulmonary vascular disease leading to thromboembolism or focal pulmonary embolism, and pneumonia resulting in pulmonary fibrosis (Silva Andrade et al., 2021).
The picture of damage to lung tissue during virus introduction (Wang et al., 2020a) is as follows: SARS-CoV-2 directly attacks type 2 pneumocytes by binding to the ACE2 receptor on the cell surface and destroying them. The degree of cell damage depends not only on the effects of viral replication, but also on the release of pro-inflammatory cytokines, which leads to dysfunction of type 2 pneumocytes. These two effects are accompanied by cell dysfunction with subsequent cell death (necrosis) or apoptosis, exudation, pneumocyte desquamation, and formation of hyaline membranes that are typical for diffuse alveolar damage. The interstitial edema and inflammatory infiltrates of mononuclear and multinucleated syncytial cells also contribute to alveolar dysfunction. Thus, a severe impairment of alveolar gas exchange and oxygenation is a typical pathophysiological peculiarity of COVID-19 pneumonia/ARDS. This process accompanies long COVID-19 with a prolonged course.
When studying the blood of patients with SARS-CoV-2 infection and ARDS during the beginning of mechanical ventilation compared with healthy individuals, a significant increase in circulating histone–DNA complexes was detected. At the same time, increased thro mbin formation was observed in plasma samples using calibrated automatic thrombography in patients with COVID-19. Despite increased endogenous thrombin potential, the plasma samples from patients in the presence of a tissue factor demonstrated a delay in clot formation and an increase in the time to peak thrombin. Strikingly different results were observed when using endothelial cells instead of tissue factor: thrombin was not formed after 60 min in control plasma samples of healthy individuals; on the contrary, thrombin appeared in the plasma samples of patients with COVID-19 (the average delay time corresponded to 20 min). The observations for thrombin formation demonstrate that the clots in the blood of patients with COVID-19 show a dense fibrin network, thin fibers, and low fibrin solubility. An increased content of histones, aberrant formation of fibrin, and increased endothelial-dependent thrombin production can contribute to coagulopathy in COVID-19. SARS-CoV-2 not only impairs alveolar gas diffusion, but also causes inflammation of the respiratory tract, reducing the ventilation function of the respiratory tract. The widespread association of bronchopneumonia with pneumonia caused by COVID-19 is direct evidence that SARS-CoV-2 affects the function of ventilation of the respiratory tract.
CONCLUSIONS
At present, there is increasing evidence that long COVID-19 is a complex and multifactorial syndrome, which includes virus-specific pathophysiological variations that affect many mechanisms, but especially oxidative stress, immune function, and inflammation. The term long COVID-19 refers to the traits and symptoms that continue or develop after the acute phase of COVID-19. There is an opinion that severe symptoms or abnormal clinical parameters that persist for more than two weeks after the beginning of COVID-19 disease and do not return to the initial healthy level can be considered as long-term consequences of the pathological process (López Castro, 2020; Crook et al., 2021). However, most researchers tend to believe that manifestations of the disease lasting more than 28 days can be considered as long COVID-19.
The most frequent symptoms of long COVID-19 include fatigue, dyspnea, cough, headache, brain fog, anosmia and dysgeusia, hair loss, joint pain, heartbeat disorders, weight loss, ear pain, eye problems, sneezing, cold nose, and a burning sensation in the trachea. Debilitating symptoms that affect the skin, respiratory, cardiovascular, digestive, urogenital, and central nervous systems often occur with long COVID-19.
With CNS damage, exacerbations of a psychoneurological nature or the appearance of new mental disorders and cognitive symptoms (memory and attention deficit, dementia), as well as psychosocial distress (loneliness, anxiety, depression, and sleep disturbances), can occur. At the same time, the brain size reduces, and damage to the cells in the hippocampus, cingulate gyrus, and cerebral cortex (directly related to cognitive activity) occurs. These changes can be both organic and functional in nature, which determines their duration and the possibility of recovery.
No less severe disorders in long COVID-19 are associated with the activity of the cardiovascular system. Myocarditis, myocardial infarction, stroke, and thrombosis of small and large vessels can develop.
Diseases of the respiratory system in long COVID-19 are manifested by severe damage to the lung parenchyma, in which the pathological process and respiratory dysfunction persist for many weeks and months, often requiring continuous respiratory support.
Thus, significant functional changes in vital systems with long COVID-19 can last not only for weeks, months, but possibly even years, which makes the need for the rehabilitation of such patients a global medical and social problem.
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement of the welfare of humans or animals. The article does not contain any studies involving humans or animals in experiments performed by any of the authors.
Footnotes
Translated by A. Barkhash
REFERENCES
- 1.Adeloye D., Elneima O., Daines L. The long-term sequelae of COVID-19: An international consensus on research priorities for patients with pre-existing and new-onset airways disease. Lancet Respir. Med. 2021;9:1467–1478. doi: 10.1016/S2213-2600(21)00286-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agyeman A.A., Chin K.L., Landersdorfer C.B. Smell and taste dysfunction in patients with COVID-19: A systematic review and meta-analysis. Mayo Clin. Proc. 2020;95:1621–1631. doi: 10.1016/j.mayocp.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alberti P., Beretta S., Piatti M. Guillain–Barre syndrome related to COVID-19 infection. Neurol. Neuroimmunol. Neuroinflammation. 2020;7:e741. doi: 10.1212/NXI.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohmwald K., Gálvez N.M.S., Ríos M., Kalergis A.M. Neurologic alterations due to respiratory virus infections. Front. Cell. Neurosci. 2018;12:386. doi: 10.3389/fncel.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bornstein S.R., Voit-Bak K., Donate T. Chronic post-COVID-19 syndrome and chronic fatigue syndrome: Is there a role for extracorporeal apheresis. Mol. Psychiatry. 2021;27:34–37. doi: 10.1038/s41380-021-01148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calabrese F., Pezzuto F., Fortarezza F. Pulmonary pathology and COVID-19: Lessons from autopsy. The experience of European pulmonary pathologists. Virchows Arch. 2020;477:359–372. doi: 10.1007/s00428-020-02886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callard F., Perego E. How and why patients made long COVID. Soc. Sci. Med. 2021;268:113426. doi: 10.1016/j.socscimed.2020.113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carvalho-Schneider C., Laurent E., Lemaignen A. Follow-up of adults with noncritical COVID-19 two months after symptoms onset. Clin. Microbiol. Infect. 2021;27:258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C., Amelia A., Ashdown G.W. Risk surveillance and mitigation: Autoantibodies as triggers and inhibitors of severe reactions to SARS-CoV-2 infection. Mol. Med. 2021;27:160–167. doi: 10.1186/s10020-021-00422-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chippa, V., Aleem, A., and Anjum, F., Post Acute Coronavirus (COVID- 19) Syndrome, Treasure Island: StatPearls, 2022. [PubMed]
- 11.Crook H., Raza S., Nowell J. Long covid-mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 12.Dani M., Dirksen A., Taraborrelli P. Autonomic dysfunction in “Long COVID”: Rationale, physiology and management strategies. Clin. Med. 2021;21:e63–e67. doi: 10.7861/clinmed.2020-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DosSantos M.F., Devalle S., Aran V. Neuromechanisms of SARS-CoV-2: A review. Front. Neuroanat. 2020;14:37–42. doi: 10.3389/fnana.2020.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Espinosa Rodríguez P., Martínez Aguilar A., Ripoll Muñoz M.P. Long COVID: Is it really myalgic encephalomyelitis? Bibliographic review and considerations. Semergen. 2022;48:63–69. doi: 10.1016/j.semerg.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estiri H., Strasser Z.H., Brat G.A. Evolving phenotypes of non-hospitalized patients that indicate long COVID. BMC Med. 2021;19:249. doi: 10.1186/s12916-021-02115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frija-Masson J., Debray M.P., Gilbert M. Functional characteristics of patients with SARS-CoV-2 pneumonia at 30 days post-infection. Eur. Respir. J. 2020;56:2001754. doi: 10.1183/13993003.01754-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garg M., Maralakunte M., Garg S. The conundrum of “Long–COVID-19”: A narrative review. Int. J. Gen. Med. 2021;14:2491–2506. doi: 10.2147/IJGM.S316708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garg S., Garg M., Prabhakar N. Unraveling the mystery of COVID-19 cytokine storm: From skin to organ systems. Dermatol. Ther. 2020;33:1385–1389. doi: 10.1111/dth.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goh K.J., Choong M.C., Cheong E.H. Rapid progression to acute respiratory distress syndrome: Review of current understanding of critical illness from COVID-19 infection. Ann. Acad. Med. Singapore. 2020;49:108–118. doi: 10.47102/annals-acadmedsg.202057. [DOI] [PubMed] [Google Scholar]
- 20.Graham E.L., Clark J.R., Orban Z.S. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized COVID-19 “long haulers”. Ann. Clin. Transl. Neurol. 2021;8:1073–1085. doi: 10.1002/acn3.51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hadjadj J., Yatim N., Barnabei L. Impaired type I interferon activity and exacerbated inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haidar, M.A., Jourdi, H., Haj Hassan, Z., et al., Neurological and neuropsychological changes associated with SARS-CoV-2 infection: New observations new mechanisms, Neuroscientist, 2021, vol. 2021, p. 1073858420984106. [DOI] [PubMed]
- 23.Haidar M.A., Shakkour Z., Reslan M.A. SARS-CoV-2 involvement in central nervous system tissue damage. Neural Regener. Res. 2022;17:1228–1239. doi: 10.4103/1673-5374.327323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang N., Pérez P., Kato T. SARS-COV-2 infection of the oral cavity and saliva. Nat. Med. 2021;27:892–903. doi: 10.1038/s41591-021-01296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ilonzo N., Kumar S., Borazan N. Endotheliitis in coronavirus disease-19-positive patients after extremity amputation for acute thrombotic events. Ann. Vasc. Surg. 2021;72:209–215. doi: 10.1016/j.avsg.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kananeh M.F., Thomas T., Sharma K. Arterial and venous strokes in the setting of COVID-19. J. Clin. Neurosci. 2020;79:60–66. doi: 10.1016/j.jocn.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanberg N., Ashton N.J., Andersson L.M. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology. 2020;95:e1754–e1759. doi: 10.1212/WNL.0000000000010111. [DOI] [PubMed] [Google Scholar]
- 28.Khavinson V., Linkova N., Dyatlova A. Peptides: Prospects for use in the treatment of COVID-19. Molecules. 2020;25:4389–4393. doi: 10.3390/molecules25194389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khavinson V.Kh., Kuznik B.I., Trofimova S.V. Results and prospects of using activator of hematopoietic stem cell differentiation in complex therapy for patients with COVID-19. Stem Cell Rev. Rep. 2021;17:285–290. doi: 10.1007/s12015-020-10087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komaroff A.L., Lipkin W.I. Insights from myalgic encephalomyelitis/chronic fatigue syndrome may help unravel the pathogenesis of postacute COVID-19 syndrom. Trends Mol. Med. 2021;27:895–906. doi: 10.1016/j.molmed.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuznik B.I., Khavinson V.Kh., Lin’kova N.S. COVID-19: Impact on immunity, hemostasis and possible methods of correction. Usp. Fiziol. Nauk. 2020;51:51–63. [Google Scholar]
- 32.Kuznik B.I., Khavinson V.Kh., Luk’yanov S.A. Effect of tocilizumab and thymalin on systemic inflammation in patients with COVID-19. Vrach. 2020;31:87–96. doi: 10.29296/25877305-2020-11-17. [DOI] [Google Scholar]
- 33.Lingel H., Meltendorf S., Billing U. Unique autoantibody prevalence in long-term recovered SARS-CoV-2-infected individuals. J. Autoimmun. 2021;122:102682. doi: 10.1016/j.jaut.2021.102682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Logue J.K., Franko N.M., McCulloch D.J. Sequelae in adults at 6 months after COVID-19 infection. JAMA Network Open. 2021;4:e210830. doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.López Castro J. Post-COVID-19 syndrome (PC19S): Chronic reactive endotheliitis and disseminated vascular disease. Acta Med. Port. 2020;33:859–864. doi: 10.20344/amp.14612. [DOI] [PubMed] [Google Scholar]
- 36.Lu J.Q., Lu J.Y., Wang W. Clinical predictors of acute cardiac injury and normalization of troponin after hospital discharge from COVID-19. EBioMedicine. 2022;76:103821. doi: 10.1016/j.ebiom.2022.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lucas C., Wong P., Klein J. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyubavina N.A., Sal’tsev S.G., Men’kov N.V. Immunological approaches to the treatment of novel coronavirus infection. Sovrem. Tekhnol. Med. 2021;13:81–101. doi: 10.17691/stm2021.13.3.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahase E. COVID-19: What do we know about “long covid”? BMJ. 2020;370:m2815. doi: 10.1136/bmj.m2815. [DOI] [PubMed] [Google Scholar]
- 40.Mahdi A., Collado A., Tengbom J. Erythrocytes induce vascular dysfunction in COVID-19. JACC: Basic Transl. Sci. 2022;7:193–204. doi: 10.1016/j.jacbts.2021.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mantovani E., Mariotto S., Gabbiani D. Chronic fatigue syndrome: An emerging sequela in COVID-19 survivors? J. Neurovirol. 2021;27:631–637. doi: 10.1007/s13365-021-01002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mao L., Jin H., Wang M. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maracaja L., Khanna A.K., Royster R. Selective lobe ventilation and a novel platform for pulmonary drug delivery. J. Cardiothorac. Vasc. Anesth. 2021;35:3416–3422. doi: 10.1053/j.jvca.2021.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meier, I.B., Vieira Ligo Teixeira, C., Tarnanas, I., et al., Neurological and mental health consequences of COVID-19: Potential implications for well-being and labour force, Brain Commun., 2021, vol. 3, no. 1, p. fcab012. [DOI] [PMC free article] [PubMed]
- 45.Meinhardt J., Radke J., Dittmayer C. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021;24:168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 46.Moriguchi T., Harii N., Goto J. A first case of meningitis/encephalitis associated with SARS–Coronavirus-2. Int. J. Infect. Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morris G., Bortolasci C.C., Puri B.K. The cytokine storms of COVID-19, H1N1 influenza, CRS and MAS compared. Can one sized treatment fit all? Cytokine. 2021;144:155593. doi: 10.1016/j.cyto.2021.155593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nassau D.E., Best J.C., Kresch E. Impact of the SARS-CoV-2 virus on male reproductive health. BJU Int. 2022;129:143–150. doi: 10.1111/bju.15573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nguyen N.N., Hoang V.T., Lagier J.C. Long-term persistence of olfactory and gustatory disorders in COVID-19 patients. Clin. Microbiol. Infect. 2021;27:931–932. doi: 10.1016/j.cmi.2020.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okada Y., Yoshimura K., Toya S., Tsuchimochi M. Pathogenesis of taste impairment and salivary dysfunction in COVID-19 patients. Jpn. Dent. Sci. Rev. 2021;57:111–122. doi: 10.1016/j.jdsr.2021.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pazuhina E., Angreeva M., Spiridonova E. Prevalence and risk factors of post-COVID-19 condition in adults and children at 6 and 12 months after hospital discharge: A prospective. BMC Med. 2022;20:244. doi: 10.1186/s12916-022-02448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pezzini A., Padovani A. Lifting the mask on neurological manifestations of COVID-19. Nat. Rev. Neurol. 2020;16:636–644. doi: 10.1038/s41582-020-0398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pierce J.D., Shen Q., Cintron S.A. Post-COVID-19 syndrome. Nurs. Res. 2022;71:164–174. doi: 10.1097/NNR.0000000000000565. [DOI] [PubMed] [Google Scholar]
- 54.Premraj L., Kannapadi N.V., Briggs J. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis. J. Neurol. Sci. 2022;434:120162. doi: 10.1016/j.jns.2022.120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rubin R. As their numbers grow, COVID-19 “long haulers” stump experts. JAMA. 2020;324:1381–1383. doi: 10.1001/jama.2020.17709. [DOI] [PubMed] [Google Scholar]
- 56.Scordo K.A., Richmond M.M., Munro N. Post-COVID-19 syndrome: Theoretical basis, identification, and management. AACN Adv. Crit. Care. 2021;32:188–194. doi: 10.4037/aacnacc2021492. [DOI] [PubMed] [Google Scholar]
- 57.Shah W., Hillman T., Playford E.D., Hishmeh L. Managing the long term effects of COVID-19: Summary of NICE, SIGN, and RCGP rapid guideline. BMJ. 2021;372:n136. doi: 10.1136/bmj.n136. [DOI] [PubMed] [Google Scholar]
- 58.Shi H., Zuo Y., Navaz S. Endothelial cell-activating antibodies in COVID-19. Arthritis Rheumatol. 2022;74:1132–1138. doi: 10.1002/art.42094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silva Andrade B., Siqueira S., Soares W.R.A. Long-COVID and post-COVID health complications: An up-to-date review on clinical conditions and their possible molecular mechanisms. Viruses. 2021;13:700–710. doi: 10.3390/v13040700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simarova, I.B., Perekhodov, S.N., and Bulanov, A.Yu., Hemorrhagic complications of a novel coronavirus infection: Actual clinical problem, Tromboz, Gemostaz Reol., 2021, no. 3, pp. 12–15.
- 61.Singer-Cornelius T., Cornelius J., Oberle M. Objective gustatory and olfactory dysfunction in COVID-19 patients: A prospective cross-sectional study. Eur. Arch. Otorhinolaryngol. 2021;278:3325–3332. doi: 10.1007/s00405-020-06590-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Slukhanchuk, E.V., Bitsadze, V.O., Khizroeva, Yu.K., et al., COVID-19 and thrombotic microangiopathy, Akush., Ginekol., Reprod., 2021, vol. 15, no. 6, pp. 639–657.
- 63.Strauss S.B., Lantos J.E., Heier L.A. Olfactory bulb signal abnormality in patients with COVID-19 who present with neurologic symptoms. Am. J. Neuroradiol. 2020;41:1882–1887. doi: 10.3174/ajnr.A6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Su Y., Yuan D., Chen D.G. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185:881–895. doi: 10.1016/j.cell.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takahashi T., Ota M., Numata Y. Relationships between the fear of COVID-19 scale and regional brain atrophy in mild cognitive impairment. Acta Neuropsychiatrica. 2022;34:153–162. doi: 10.1017/neu.2022.7. [DOI] [PubMed] [Google Scholar]
- 66.Tian T., Wu J., Chen T. Long-term follow-up of dynamic brain changes in patients recovered from COVID-19 without neurological manifestations. JCI Insight. 2022;7:e155827. doi: 10.1172/jci.insight.155827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Torres-Castro R., Vasconcello-Castillo L., Alsina-Restoy X. Respiratory function in patients post-infection by COVID-19: A systematic review and meta-analysis. Pulmonology. 2021;27:328–337. doi: 10.1016/j.pulmoe.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tran V.-T., Riveros C., Clepier B. Development and validation of the Long coronavirus disease (COVID) symptom and impact tools, a set of patient-reported instruments constructed from patients’ lived experience. Clin. Infect. Dis. 2022;74:278–287. doi: 10.1093/cid/ciab352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tremblay M.-E., Madore C., Bordeleau M. Neuropathobiology of COVID-19: The role for glia. Front. Cell. Neurosci. 2020;14:592214. doi: 10.3389/fncel.2020.592214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verity R., Okell L.C., Dorigatti I. Estimates of the severity of coronavirus disease 2019: A model-based analysis. Lancet Infect. Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wallukat G., Hohberger B., Wenzel K. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J. Transl. Autoimmun. 2021;4:100100. doi: 10.1016/j.jtauto.2021.100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang F., Kream R.M., Stefano G.B. Long-term respiratory and neurological sequelae of COVID-19. Med. Sci. Monit. 2020;26:e928996. doi: 10.12659/MSM.928996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang F., Nie J., Wang H. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J. Infect. Dis. 2020;221:1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wong A.W., Shah A.S., Johnston J.C. Patient-reported outcome measures after COVID-19: A prospective cohort study. Eur. Respir. J. 2020;56:2003276. doi: 10.1183/13993003.03276-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xie Y., Xu E., Bowe B., Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiong Q., Xu M., Li J. Clinical sequelae of COVID-19 survivors in Wuhan, China: A single-centre longitudinal study. Clin. Microbiol. Infect. 2021;27:89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yap J.K.Y., Moriyama M., Iwasaki A. Inflammasomes and pyroptosis as therapeutic targets for COVID-19. J. Immunol. 2020;205:307–312. doi: 10.4049/jimmunol.2000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ye G., Pan Z., Pan Y. Clinical characteristics of severe acute respiratory syndrome coronavirus 2 reactivation. J. Infect. 2020;80:e14–e17. doi: 10.1016/j.jinf.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yelin D., Margalit I., Yahav D. Long COVID-19 – it’s not over until? Clin. Microbiol. Infect. 2021;27:506–508. doi: 10.1016/j.cmi.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou H., Lu S., Chen J. The landscape of cognitive function in recovered COVID-19 patients. J. Psychiatr. Res. 2020;129:98–102. doi: 10.1016/j.jpsychires.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zuo, Y., Estes, S.K., Ali, R.A., et al., Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19, Sci. Transl. Med., 2020, vol. 12, no. 570, p. eabd3876. [DOI] [PMC free article] [PubMed]