Abstract
Serious manifestations of respiratory virus infections such as influenza and coronavirus disease 2019 (COVID-19) are associated with a dysregulated immune response and systemic inflammation. Treating the immunological/inflammatory dysfunction with glucocorticoids, Janus kinase inhibitors, and monoclonal antibodies against the interleukin-6 receptor has significantly reduced the risk of respiratory failure and death in hospitalized patients with severe COVID-19, but the proportion of those requiring invasive mechanical ventilation (IMV) and dying because of respiratory failure remains elevated. Treatment of severe influenza-associated pneumonia and acute respiratory distress syndrome (ARDS) with available immunomodulators and anti-inflammatory compounds is still not recommended. New therapies are therefore needed to reduce the use of IMV and the risk of death in hospitalized patients with rapidly increasing oxygen demand and systemic inflammation who do not respond to the current standard of care. This paper provides a critical assessment of the published clinical trials that have tested the investigational use of intravenously administered allogeneic mesenchymal stem/stromal cells (MSCs) and MSC-derived secretome with putative immunomodulatory/antiinflammatory/regenerative properties as add-on therapy to improve the outcome of these patients. Increased survival rates are reported in 5 of 12 placebo-controlled or open-label comparative trials involving patients with severe and critical COVID-19 and in the only study concerning patients with influenza-associated ARDS. Results are encouraging but inconclusive for the following reasons: small number of patients tested in each trial; differences in concomitant treatments and respiratory support; imbalances between study arms; differences in MSC source, MSC-derived product, dosing and starting time of the investigational therapy; insufficient/inappropriate reporting of clinical data. Solutions are proposed for improving the clinical development plan, with the aim of facilitating regulatory approval of the MSC-based investigational therapy for life-threatening respiratory virus infections in the future. Major issues are the absence of a biomarker predicting responsiveness to MSCs and MSC-derived secretome and the lack of pharmacoeconomic evaluations.
Keywords: Acute respiratory distress syndrome, Add-on therapy, Cell-based therapy, Clinical trial, COVID-19, Exosome, Extracellular vesicle, Influenza, Mesenchymal stem cell, Mesenchymal stromal cell
Key Summary Points
| New therapeutic options are needed to treat life-threatening manifestations of respiratory virus infections such as coronavirus disease-19 (COVID-19) and influenza that do not resolve despite appropriate respiratory support and management with the currently recommended antivirals, immunomodulators, and antiinflammatory agents. |
| The therapeutic potential of mesenchymal stem/stromal cells (MSCs) and MSC-derived products is under evaluation in a huge number of clinical trials, on the basis of the favorable results of preclinical studies. |
| Although it is unclear how this investigational therapy could be integrated into the approved clinical management protocol for serious respiratory virus infections in the future, its use as an adjunctive therapy would fulfill the currently unmet need. |
| This paper therefore focuses on the critical assessment of published clinical studies that have specifically tested MSCs and MSC-derived products as add-on therapy to reduce mortality and the requirement for invasive mechanical ventilation in hospitalized patients with severe and critical COVID-19 and influenza. |
| Results are promising but inconclusive and solutions are proposed for improving the clinical development plan for the currently most needed add-on-therapy indication, with adequate consideration of regulatory and pharmacoeconomic issues. |
Introduction
Acute respiratory tract infections are among the commonest infectious diseases [1, 2]. Until December 2019, the most serious and prolonged outbreaks of these diseases had been observed with infections caused by strains of the influenza viruses type A and type B and by coronaviruses such as the severe acute respiratory syndrome coronavirus and the Middle East respiratory syndrome coronavirus [1–3]. Nonetheless, respiratory mortality associated with seasonal influenza has remained elevated worldwide even outside periods of major outbreaks caused by new strains, with global influenza-associated respiratory deaths ranging between 291,243 and 654,832 annually (4.0–8.8 deaths per 100,000 individuals), according to the latest estimate published in March 2018 [4].
The first outbreak of a pneumonia associated with a new coronavirus, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was reported in China in December 2019 and was followed by a rapid spreading of the infection worldwide [1, 5]. At the time of this writing (22 September 2022) the illness caused by SARS-CoV-2 and termed coronavirus disease 2019 (COVID-19) has already killed more than 6.5 million individuals and the pandemic is not over yet [6]. The devastating impacts on healthcare systems, economies, and education and social relationships have resulted in a global crisis with no precedent since the Second World War [7–9]. Highly efficacious vaccines have been developed in less than 1 year from SARS-CoV-2 identification [10] and the speed at which many countries have rolled out the vaccination program is unparalleled [9–11]. High levels of immunity induced by the mass vaccination efforts and by natural infections have greatly changed the course of the COVID-19 pandemic, but the continued generation of highly transmissible and virulent SARS-CoV-2 genetic variants capable of evading the existing level of immunity and still causing multiple waves of infections is hampering transition of COVID-19 from the pandemic to an endemic phase [12].
Like influenza viruses, SARS-CoV-2 can cause severe pneumonitis and acute respiratory distress syndrome (ARDS) with high frequency in the elderly, in immunocompromised patients, and in those with comorbidities such as obesity, diabetes, chronic cardiovascular disorders, and kidney and liver diseases [1, 2, 4, 13–15]. Invasive mechanical ventilation (IMV) is commonly needed in critically ill patients admitted to intensive care units (ICUs) for seasonal influenza and COVID-19, but patients with COVID-19 require longer duration of IMV and are at greater risk of mortality during the hospitalization than patients with influenza, irrespective of age, sex, and comorbidities [15]. The survivors may not recover completely and may suffer from disabling symptoms for the rest of their lives.
Because the influenza viruses and SARS-CoV-2 can cause serious pneumonitis and ARDS in the same groups of individuals, even minor outbreaks of COVID-19 occurring with a simultaneous influenza wave in the Northern Hemisphere in late autumn and winter could lead to another surge in admissions to ICUs and deaths. In addition, the prevalence of coinfections, which are associated with increased odds of ICU admission and death in those individuals [16], may also escalate because of the easing of non-pharmaceutical measures that greatly reduced the circulation of SARS-CoV-2 as well as the circulation of influenza viruses in 2020–2021, the current absence of a systematic virologic surveillance [17–19], and the limited effectiveness of influenza vaccines in high-risk subjects [19].
Rationale for the Potential Use of MSCs and MSC-Derived Secretome as Add-On Therapy
The severe life-threatening manifestations of influenza and COVID-19 are associated with a dysregulated immune response and hyperproduction of proinflammatory cytokines and chemokines such as interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, interferon (IFN)-γ inducible protein-10, monocyte chemoattractant protein-1, and IL-8 [20–25]. The unchecked immunological/inflammatory alterations lead to further tissue damage [20–25], in addition to that caused by virus replication alone, and to increased risk of thrombosis [26] not responding to anticoagulation alone [27]. Targeting the immunological/inflammatory dysfunction with glucocorticoids, Janus kinase inhibitors, and humanized monoclonal antibodies against the IL-6 receptor (IL-6R) [28–30], in addition to providing maximal supportive therapy [31], significantly reduces the risk of respiratory failure and death in hospitalized patients with severe COVID-19-associated pneumonia, hypoxia, and evidence of systemic inflammation, but the residual numbers of individuals requiring IMV and dying because of respiratory failure remain elevated in the clinical trial setting [28–30] and in clinical practice [32]. The evaluation of the efficacy of immunomodulators and antiinflammatory compounds in severe influenza-associated pneumonia and ARDS has generated conflicting results and there is evidence of detrimental effects of glucocorticoids in influenza-related ARDS [31, 33, 34]. Effective therapeutic options are therefore needed to reduce the use of IMV and the risk of death in hospitalized patients with rapidly increasing oxygen demand and systemic inflammation who do not respond to the evidence-based therapeutic regimen currently recommended by international guidelines (Table 1) [19, 35].
Table 1.
Therapeutic management of hospitalized adult patients by disease severity.
Source: published international guidelines [19, 35], last accessed 12 February 2023
| Disease severity | Moderate disease | Severe disease | Critical disease | |
| Dyspnea, SpO2 ≥ 94% on room air at sea level, pulmonary infiltrate < 50% of the lung fields | Dyspnea, SpO2 < 94% on room air at sea level, PaO2/FiO2 < 300 mm Hg, RR > 30 breaths/min, or pulmonary infiltrates > 50% of the lung fields |
Hypoxemic respiratory failure, ARDS, shock, multiorgan dysfunction/failure |
||
| Not requiring supplemental oxygen | Requiring supplemental oxygen through mask or nasal prongs | Requiring oxygen through a high-flow device or NIV | Requiring IMV, IMV and vasopressors, or ECMO | |
| WHO CPS | 4 | 5 | 6 | 7–9 |
| COVID-19 |
Treatment with the antivirals nirmatrelvir (boosted with ritonavir) or remdesivir for patients at high risk of disease progression Prophylactic dose of heparin to reduce the risk of thromboembolic disease, unless contraindicated |
Treatment with remdesivir alone only for patients requiring minimal supplemental oxygen Add the glucocorticoid dexamethasone to the antiviral remdesivir For dexamethasone-treated patients who have rapidly increasing oxygen needs and systemic inflammation, add the Janus kinase inhibitor baricitinib or the IL-6 receptor antagonist tocilizumab Therapeutic dose of heparin for nonpregnant patients with increased D-dimer levels and without increased bleeding risk Prophylactic dose of heparin for the other patients, unless contraindicated |
Prompt treatment with the glucocorticoid dexamethasone plus the Janus kinase inhibitor baricitinib or with dexamethasone plus the IL-6 receptor antagonist tocilizumab Add remdesivir, if required in certain patients, including immunocompromised patients Prophylactic dose of heparin, unless contraindicated |
Prompt treatment with the glucocorticoid dexamethasone plus the Janus kinase inhibitor baricitinib or with dexamethasone plus the IL-6 receptor antagonist tocilizumab Prophylactic dose of heparin, unless contraindicated Critical care management similar to that recommended for other critically ill patients admitted to the ICU |
| Identification and treatment of concomitant or secondary infections | ||||
| Influenza |
Treatment with one antiviral of the class of neuraminidase inhibitors (oral oseltamivir, intravenous peramivir in intubated patients) Identification and treatment of concomitant or secondary infections Adjunctive therapy with glucocorticoids or immunomodulators not recommended |
|||
ARDS acute respiratory distress syndrome, ECMO extracorporeal membrane oxygenation, ICU intensive care unit, IL-6 interleukin-6, IMV invasive mechanical ventilation, min minute, NIV noninvasive ventilation, PaO2/FiO2 arterial oxygen partial pressure/fractional inspired oxygen, RR respiratory rate, SpO2 saturation of peripheral oxygen, WHO CPS World Health Organization Clinical Progression Scale
Because of this unmet need, the therapeutic potential of mesenchymal stem/stromal cells (MSCs) and MSC-derived products is under evaluation in a huge number of clinical trials [36–40] on the basis of the results of preclinical studies that have demonstrated the ability of intravenously injected MSCs to transiently accumulate in the pulmonary circulation and to exert multiple beneficial effects, including the modulation of immunological responses, the prevention of bacterial superinfections, the promotion of the repair of damaged alveolo-capillary barriers, and the alleviation of fibrosis in the injured lungs [40–46], mainly through paracrine signaling [42–45]. The MSCs under evaluation are a heterogeneous population of self-renewable multipotent cells that are most commonly harvested from the perinatal tissues (umbilical cord tissue, umbilical cord blood, or placenta), the menstrual blood, adult bone marrow or adult adipose tissue of one or more healthy unrelated donor(s) and are expanded in culture to large quantities for treating many patients [39, 42, 47, 48]. The investigational therapy is either the allogeneic population of MSCs expanded in culture or its secretome, which is composed of soluble factors and extracellular vesicles such as exosomes and microvesicles [38, 45].
It is widely recognized that the allogeneic MSCs under evaluation only acquire immunomodulatory properties in inflammatory conditions [49]. The induction of the expression of a predominant immunosuppressive phenotype is known as MSC licensing and has been reported to be elicited in the circulation and at the tissue sites by IFN-γ [50, 51], particularly in the concomitant presence of one of the proinflammatory cytokines TNF-α, IL-1α, and IL-1β [52]. The importance of the licensing activity of IFN-γ is supported by the results of studies in an animal model of graft versus host disease (GVHD), where the recipients of IFN-γ–/– T lymphocytes did not respond to treatment with bone-marrow-derived MSCs and died [53].
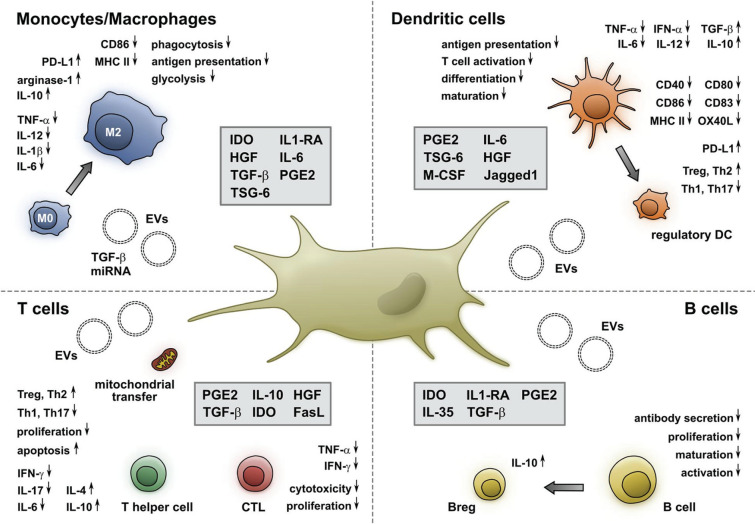
Licensed MSCs acquire the ability to generate powerful immunoregulatory effects by modulating the proliferation and function of diverse cells involved in the innate and adaptive immunity through the release of biologically active soluble molecules and extracellular vesicles, and the transfer of mitochondria via intercellular communication [49, 54] (Fig. 1). Soluble factors with immunomodulatory properties include indoleamine 2,3-dioxygenase (IDO), prostaglandin E2 (PGE2), transforming growth factor (TGF)-β, and IL-1R antagonist (IL-RA). Through the release of IDO and PGE2, MSCs can reduce the proliferation, cytotoxic activity, and cytokine production of effector T lymphocytes, and the proliferation of B lymphocytes. Importantly, MSCs can favor the differentiation and expansion of functional regulatory T lymphocytes (Treg) through IDO, PGE2, cyclooxygenase (COX)-2, and TGF-β [49, 54] (Fig. 1). Furthermore, they can promote the generation of IL-10-producing regulatory B cells (Breg) that inhibit the differentiation of effector T cells into T helper-17 (Th-17) lymphocytes [49, 54] (Fig. 1). MSCs can also block the activation of effector immune cells via cell-to-cell interaction through the association of the programmed death (PD)-1 and its ligand PD-L1 [49]. In presence of macrophage colony-stimulating factor (M-CSF), MSCs promote the differentiation of monocytes and type 1 macrophages with proinflammatory activity into M2 type macrophages with antiinflammatory and regenerative properties, which produce IL-10 and TGF-β. MSCs also inhibit the differentiation and maturation of dendritic cells and switch their profile toward a tolerogenic one by reducing their expression of the costimulatory molecules HLA-DR, CD1a, CD80, and CD83, downregulating their production of IL-12 and increasing their expression of PD-L1 [54] (Fig. 1).
Fig. 1.
Immunomodulatory properties of mesenchymal stem/stromal cells. Copyright 2021 Müller, Tunger, Wobus, von Bonin, Towers, Bornhäuser, Dazzi, Wehner, and Schmitz [54], Figure reproduced without changes under the terms of the Creative Commons Attribution License, https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors(s) and the copyright owner(s) are credited, the original publication is cited, the link to the license is given, and it is indicated if changes were made. CD cluster of differentiation, CTL cytotoxic T lymphocytes, EVs extracellular vesicles, FasL Fas ligand, HGF hepatocyte growth factor, IDO indoleamine 2,3-dioxygenase, IFN interferon, IL interleukin, IL1-RA interleukin 1 receptor antagonist, MHC major histocompatibility complex, M-CSF macrophage colony-stimulating factor, miRNA microRNA, OX40L OX40 ligand, PD-L1 programmed death-ligand 1, PGE2 prostaglandin 2, Th T helper lymphocyte, TGF transforming growth factor, TNF tumor necrosis factor, Treg T regulatory lymphocyte, TSG tumor necrosis factor-stimulated gene
In addition to the process of MSC licensing described above, another mechanism has been recently proposed to explain the immunomodulatory function of intravenously injected allogeneic MSCs [49, 55]. The infused cells would undergo apoptosis by interaction with the granules released by cytotoxic CD8 lymphocytes and natural killer (NK) cells of the host, and the apoptotic cells would be taken up by the circulating mononuclear phagocytes. This efferocytosis would induce a sort of reprogramming of the phagocytic cells of the MSC recipient, which would produce PGE2 and IDO themselves and in this manner mediate the immunosuppressive effects of MSCs. The two mechanisms may coexist because there is evidence that viable MSCs cannot be replaced with apoptotic or dead MSCs from a therapeutic perspective [56], and more studies are required to clarify this issue. In addition, it would be important to understand if the reprogramming of phagocytic cells depends at least in part on the biological activity of the extracellular vesicles of the apoptotic MSCs and whether it can also occur with the infusion of MSC-derived extracellular vesicles, which are in large part taken up by the phagocytes of the reticuloendothelial system because of their size.
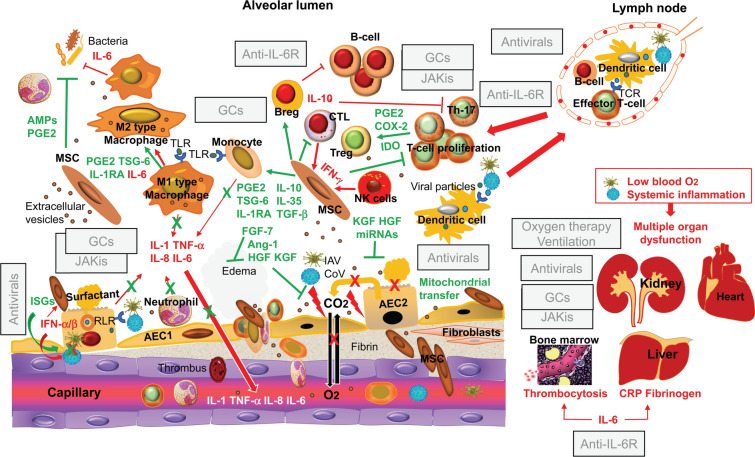
MSC tracking experiments in an animal model of infectious pneumonitis [57], using real-time, intravital imaging of the kinetics of MSCs in lung vessels, demonstrated the immediate influx of MSCs following their intravascular injection and their persistence in the alveolar capillaries for more than 24 h. Moreover, MSC administration was associated with improvements in the gas-exchange function of the alveolar-capillary barrier, resulting in increased arterial oxygen levels [57]. Figure 2 schematically shows the possible mechanisms through which intravenously injected allogeneic MSCs, or MSC-derived extracellular vesicles, can restore the impaired gas exchange and counteract the effects of the dysregulated immune response and persisting inflammation in severe and critical COVID-19 and influenza. The indicated effects of MSCs and MSC-derived extracellular vesicles are based on the immunomodulatory properties of MSCs discussed above and the results of preclinical studies in animal models of lung injury associated with influenza virus infection [58–61] or in experimentally-induced ARDS [62]. The figure also shows a possible mechanism through which MSCs become or remain not permissive to viral growth in the inflamed alveoli. This is related to their ability to express INF-stimulated genes in response to INFs such as the type I IFNs (IFN-α/β) produced by infected alveolar epithelial cells and the IFN-γ present in the inflammatory infiltrate [63].
Fig. 2.
Mechanistic rationale for investigating the clinical use of mesenchymal stem/stromal cells and their products as adjunctive therapy for the management of severe and critical coronavirus disease 2019 and influenza. The pathological mechanisms leading to alveolar damage, hypoxemia, and systemic inflammation are highlighted in red, and the counteracting effects of intravenously injected allogeneic mesenchymal stem/stromal cells that have been induced to express an antiinflammatory/immunosuppressive phenotype systemically and in the inflamed alveoli are highlighted in green. In severe and critical coronavirus disease 2019, virus replication, the proliferation of effector T lymphocytes, the release of proinflammatory cytokines, and the recruitment of leukocytes from the peripheral blood are inhibited by the recommended treatment with antivirals and the glucocorticoid dexamethasone in combination with a Janus kinase inhibitor of the JAK1/JAK2 subtype, such as baricitinib, or with the humanized antibody against the interleukin-6 receptor tocilizumab. This combination also reduces the systemic effects of viral replication and excessive inflammation, but in abolishing the acute-phase response and the IL-6 mediated enhancement of bacterial phagocytosis, the combination of antiinflammatory agents concurs to render the host more vulnerable to pulmonary and systemic infections. Key adjunctive effects of mesenchymal stem/stromal cells are the following: reestablishment of the regulatory function of subpopulations of T and B lymphocytes (Treg and Breg cells) that normally suppress excessive and deleterious immunological/inflammatory responses; activation of the mechanisms involved in the repair of the alveolar-capillary barrier via the release of soluble factors (Ang-1, HGF, and KGF) and extracellular vesicles delivering microRNAs; enhancement of the viability of alveolar epithelial cells through the transfer of healthy mitochondria by intercellular communication; prevention of the development of secondary bacterial infections by producing antimicrobial peptides and by enhancing the phagocytic activity of neutrophils and macrophages through the release of prostaglandin E2. In severe and critical influenza, where a combination of antivirals and antiinflammatory or immunoregulators is not allowed, most of the biological effects of mesenchymal stem/stromal cells highlighted in this figure would be desirable. Generated using in part ScienceSlides graphics from VisiScience Corp., licensed use. AEC alveolar epithelial cell, AMPs antimicrobial peptides, Ang angiopoietin, Breg B regulatory lymphocytes, CRP C-reactive protein, CoV coronavirus, COX cyclooxygenase, CTL cytotoxic T lymphocytes, EVs extracellular vesicles, FGF fibroblast growth factor, GCs glucocorticoids, HGF hepatocyte growth factor, IAV influenza virus, IDO indoleamine 2,3-dioxygenase, IFN interferon, IL interleukin, IL1-RA interleukin 1 receptor antagonist, IL-6R interleukin-6 receptor, ISGs interferon-stimulated genes, JAKis Janus kinase inhibitors, KGF keratinocyte growth factor, miRNA microRNA, NK natural killer, PGE2 prostaglandin 2, RLR retinoic acid-inducible gene-1-like receptor, TCR T cell receptor, TGF transforming growth factor, Th T helper lymphocyte, TLR toll-like receptor, TNF tumor necrosis factor, Treg T regulatory lymphocyte, TSG tumor necrosis factor-stimulated gene
The currently approved pharmacological treatment for severe and critical COVID-19 includes the glucocorticoid dexamethasone as standard of care, the humanized monoclonal antibody of the IgG1 class against the IL-6R tocilizumab, and the Janus kinase inhibitor of the JAK/1/JAK2 subtype baricitinib as adjunctive therapies (Table 1) [19, 35]. Dexamethasone is known to have a broad antiinflammatory activity, and the transcriptomic data on pulmonary and circulating immune cells from patients with severe COVID-19 has suggested that the therapeutic effect of this glucocorticoid in this disease may be specifically related to TNF-α, IL-1a, IL-1b, IFN-α, and IFN-γ signaling but does not involve the IL-6 pathway [64]. Tocilizumab targets IL-6-mediated signal transduction by binding to both the transmembrane and the soluble receptors, and in so doing it irreversibly blocks the proinflammatory and prothrombotic effects of IL-6 for 2–3 weeks, as well as its still desirable effects on the development of an acute-phase response against infections and on the enhancement of bacterial phagocytosis [65]. Baricitinib predominantly blocks IL-6 and INF-γ signaling and IL-10 and IFN-α signaling to a lesser extent [66], and similarly to dexamethasone, has a short half-life. Both dexamethasone and baricitinib inhibit the function of type I IFNs involved in viral clearance and must be administered in combination with antivirals in immunocompromised patients. Baricitinib also reduces, albeit to a lesser extent than other Janus kinase inhibitors [66], the desirable regulatory activity of IL-10, which is related to its ability to promote the emergence of Tregs while suppressing the development of Th-17 lymphocytes. None of these therapeutic agents has the direct effects mediated by whole MSCs and by their extracellular vesicles on the viability of alveolar epithelial cells, the repair of the alveolar-capillary barrier, and the prevention of the development of secondary bacterial infections (Fig. 2). In severe and critical influenza, the potential biological effects of whole MSCs or of their extracellular vesicles are unrivaled, because a combination of antivirals and antiinflammatory or immunoregulators is not allowed (Table 1) [19, 35].
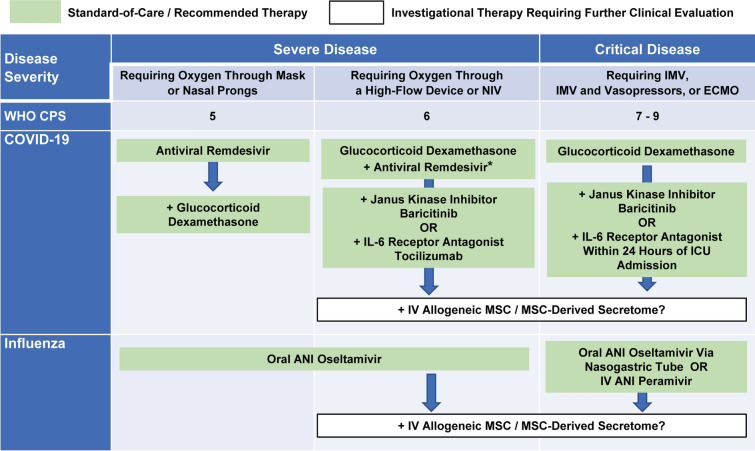
A mechanistic rationale therefore emerges for the use of MSC-based therapy as an adjunctive therapy in patients with severe and critical COVID-19 who do not respond to dexamethasone, and in patients with severe and critical influenza who show increasing oxygen demand and systemic inflammation on treatment with antivirals alone. The combination of an MSC-based therapy and glucocorticoids has been already used for the treatment of GVHD and excellent results have been reported in terms of safety and efficacy [67]. Moreover, glucocorticoids at high doses are used as standard of care in studies testing the potential additional benefits of MSC-based therapies in GVHD [68]. The potential future integration of investigational MSC-based therapy into the currently recommended therapeutic management of hospitalized adult patients with severe and critical COVID-19 or influenza is illustrated in Fig. 3.
Fig. 3.
Potential future integration of investigational therapy into the recommended therapeutic management of hospitalized adult patients with severe and critical COVID-19 or influenza, which is based on published international guidelines [19, 35, 90]. The arrows indicate potential sequential treatment strategies, where allogeneic mesenchymal stem/stromal cells or their products are administered intravenously as an adjunctive or alternative add-on therapy to prevent further disease progression and death in patients not responding to the recommended first-line treatment with dexamethasone, while receiving adequate respiratory support and the required critical care management. The asterisk indicates the allowed addition of the antiviral remdesivir in immunocompromised patients with severe coronavirus disease 2019 who require oxygen through a high-flow device or noninvasive ventilation and are receiving dexamethasone. ANI antiviral neuraminidase inhibitor, COVID-19 coronavirus disease 2019, ECMO extracorporeal membrane oxygenation, ICU intensive care unit, IL-6 interleukin-6, IMV invasive mechanical ventilation, IV intravenous, MSC mesenchymal stem/stromal cells, NIV noninvasive ventilation, WHO CPS World Health Organization Clinical Progression Scale
Aims and Methodological Approach of this Review
The focus of this review was on the status of the clinical investigations testing the potential use of MSCs and MSC-derived secretome to improve the outcome of patients with severe and critical diseases already managed according to the evidence-based therapeutic approach outlined in Table 1. The main objectives were the following: to identify progresses in the assessment of the potential added value of the investigational therapy in clinical trials, to highlight unresolved issues, and to discuss how to address them.
An extensive literature search was conducted to retrieve all articles reporting on the clinical use of MSCs and MSC-derived products as investigational therapy for lung conditions related to COVID-19 and influenza as described in Table 2. To assess if investigational therapy significantly accelerated the recovery and decreased the mortality of patients with severe or critical diseases in comparison with the recommended/standard therapeutic regimen, controlled prospective clinical trials on the use of the investigational therapy as add-on therapy and single-arm uncontrolled clinical trials on the use of the investigational therapy when the recommended/standard treatments have failed were taken into consideration. Clinical studies in patients in stable conditions on the recommended/standard treatment, clinical studies where the recommended/standard treatment was not described, clinical studies where the recommended/standard treatment did not include antivirals or glucocorticoids or immunomodulators, case series, and case reports were excluded and are reviewed elsewhere [40], together with registered but still unpublished studies.
Table 2.
Criteria of the literature search
| Electronic databases | |
| PubMed/MEDLINE, Scopus, Cochrane Collaboration, Web of Science | |
| Publications | |
| Language | English |
| Period of time | 1 January 1970–31 July 2022 |
| Subject | Human clinical studies |
| Setting | Hospital wards and intensive care units |
| Included study type | Randomized double-blind placebo-controlled clinical trials on the use of investigational therapy in addition to recommended/standard therapy (add-on therapy) |
| Randomized open-label placebo-controlled clinical trial on the use of investigational therapy in addition to recommended/standard therapy (add-on therapy) | |
| Nonrandomized open-label parallel-group clinical trial on the use of investigational therapy in addition to recommended/standard therapy (add-on therapy) | |
| Prospective single-arm study on the use of investigational therapy when recommended/standard therapy has failed | |
| Excluded publications | |
| Any prospective study on the use of investigational therapy in stable patients on recommended/standard therapy | |
| Any study on the use of investigational therapy as add-on therapy if recommended/standard therapy was not described | |
| Any study on the use of investigational therapy as add-on therapy, or when recommended/standard therapy has failed, if recommended/standard therapy did not include antivirals, glucocorticoids, or immunomodulators, singly or in combination | |
| Retrospective studies | |
| Case series | |
| Case report | |
| Articles posted on pre-print servers | |
| Abstracts | |
| Conference proceeding | |
| Opinion article | |
| Review articles | |
| Editorials | |
| Theses | |
| Book chapters | |
| Keywords | |
| Mesenchymal stem cells | |
| Mesenchymal stromal cells | |
| Exosomes | |
| Extracellular vesicles | |
| Pneumonia | |
| Acute respiratory distress syndrome | |
| Cytokine storm | |
| Systemic inflammation | |
| Coronavirus | |
| COVID-19 | |
| Influenza | |
COVID-19 coronavirus disease 2019, IT investigational therapy
This review was based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Overview and Critical Assessment of Published Clinical Trial Results
As of 31 July 2022, 21 published reports were found [69–89], including 6 randomized, double-blind, placebo-controlled trials [71, 74, 75, 81, 82, 84], 3 randomized, open-label parallel-group studies [76, 77, 85], 6 nonrandomized prospective studies with control groups [69, 70, 72, 78, 86, 89], and 6 prospective, uncontrolled single-arm studies [73, 79, 80, 83, 87, 88] conducted in diverse countries worldwide (Table 3). A total of 20 reports [69–88] concerned treatment of patients with severe and or critical illness caused by laboratory-confirmed SARS-CoV-2 infection, but 6 of the reported studies also included patients with mild [85] or moderate [69, 70, 78, 79, 86] disease, and 1 study [87] was conducted in patients with a condition defined as moderate pneumonia by the investigators, although the reported clinical and laboratory data at baseline (Table 3) reflected a more severe stage according to international guidelines [35, 90]. The total number of patients treated with MSCs or MSC-derived products in these studies was 343 (Table 3). One report [89] concerned treatment of patients with ARDS caused by laboratory-confirmed H7N9 influenza virus infection: 17 out of 61 individuals received the investigational therapy, in addition to standard care including antivirals and glucocorticoids, while the others served as control (Table 3). The characteristics of all studies and their main findings are summarized in Tables 3, 4, 5, and 6.
Table 3.
Study type and patient characteristics
| Author and reference no. | Country | Study type | Total, n IT; Ctrl | Age (y) IT; Ctrl |
Comorbidities IT; Ctrl |
Disease severity IT; Ctrl |
Pharmacological treatment IT; Ctrl |
Follow-up |
|---|---|---|---|---|---|---|---|---|
| Disease: COVID-19/IT: MSCs | ||||||||
| Shi L, et al. [71, 72] | China | Randomized, double-blind, placebo-controlled, phase 2 clinical trial, with up to 1-y open-label follow-up period in moderate and severe disease |
100 65; 35 |
IT: mean 60.72, SD 9.14; Ctrl: mean 59.94, SD 7.79 |
IT: HT 17 (26.15%), DM 12 (18.46%), CB 2 (3.08%) COPD 2 (3.08%); Ctrl: HT 10 (28.6%), DM 5 (14.3%), CB 3 (8.6%), COPD 0 |
IT: 14 (21.5%) pts not requiring supplemental oxygen, 50 (76.9%) pts requiring supplemental oxygen, 1 (1.5%) pt on NIV or high-flow oxygen; Ctrl: 10 (28.6%) pts not requiring supplemental oxygen, 25 (71.4%) pts requiring supplemental oxygen, 0 pts on NIV or high-flow oxygen Comparable proportions of total lung lesion volume and solid component lesion volume by high-resolution chest CT and image analysis No differences for time from symptom onset to study entry, laboratory data, levels of CRP, IL-6, and D-dimer |
Comparable proportions of pts on treatment with antivirals, antibiotics, and glucocorticoids |
28 D Open-label follow-up at 3, 6, 9, and 12 mo, when 56/65 pts in IT group and 30/35 pts in ctrl group were finally assessed |
| Lanzoni G, et al. [74] | USA | Randomized, double-blind, placebo-controlled, phase 1/2a clinical trial in ARDS, with randomization and stratification by ARDS severity |
24 12; 12 |
IT: mean 58.58, SD 15.93; Ctrl: mean 58.83, SD 11.61 |
IT: DM 5 (41.7%), HT 7 (58.3%), obesity 11 (91.7%), cancer 0, heart disease 1 (8.3%); Ctrl: DM 6 (50%,) HT 9 (75%), obesity 5 (41.7%), cancer 1 (8.3%), heart disease 3(25%) Significantly higher BMI in the IT group |
Bilateral infiltrates on frontal chest radiograph or bilateral ground glass opacities on a chest CT scan 3 pts (25%) in each group with mild-to-moderate ARDS: PaO2/FiO2 > 150 mm Hg 9 pts (75%) in each group with moderate-to-severe ARDS: PaO2/FiO2 < 150 mm Hg IMV: 4 pts in the IT group and 7 pts in the ctrl group High-flow oxygen therapy: 8 pts in the IT group and 5 in the ctrl group No significant differences in median viral load between groups at D 0 Significantly higher levels of IL-6 in the peripheral blood of pts in the ctrl group. Comparable levels of GM-CSF, IFNγ, IL-2, IL-5, IL-7, TNFα TNFβ, PDGF-BB, and RANTES |
No significant differences in concomitant treatments with heparin, remdesivir, convalescent plasma, glucocorticoids, tocilizumab, hydroxychloroquine. Alteplase treatment only in 2 pts in the ctrl group | 28 D from last infusion |
| Dilogo IH, et al. [75] | Indonesia | Randomized, double-blind, placebo-controlled, multicenter clinical trial in critical disease |
40 20; 20 |
IT: < 40 4, 40–60 8, > 60 8; Ctrl: < 40 3, 40–60 7, > 60 10 |
IT: DM 8, HT 6, CHKD 2, CAD 2, congestive heart failure 1, tuberculosis 1, others 10; Ctrl: DM 12, HT 10, CHKD 5, CAD 3, congestive heart failure 1, tuberculosis 1, others 6 |
All pts intubated in the ICU | Concomitant medications in all pts: azithromycin 500 mg and oseltamivir 75 mg (according to local guidelines) | Period of stay in ICU until death or recovery |
| Monsel A, et al. [81] | France | Randomized, double-blind, placebo-controlled, multicenter clinical trial in ARDS |
45 21; 24 |
IT: mean 64, SD 10.4; Ctrl: mean 63.2, SD 11.4 |
IT: obesity 33.3%, COPD 0, AF 13.3%, HT 73.3%, CAD 13.3%, stroke 13.3%; Ctrl: obesity 25%, COPD 6.7%, AF 0, HT 66.7%, CAD 13.3%, stroke 6.7%; |
IT: (NIV and/or HFNO) 10 (47.6%), IMV 11 (52.4%), SpO2 94.6% (3.4%), PaO2/FiO2 156.2 (68.2) mmHg, LIS 3.0 (0.7), mean SOFA score 5.5 (SD 2.7); Ctrl: (NIV and/or HFNO) 4 (16.7%), IMV 20 (83.6%), SpO2 96.0% (3.0%), PaO2/FiO2 171.2 (72.9) mmHg, LIS 2.8 (0.5), mean SOFA score 5.9 (SD 2.7) |
IT: 5 pts (23.8%) on vasopressor, 6 pts (28.6%) on neuromuscular blockade, 15 pts (71.4%) using glucocorticoids for 7 D; Ctrl: 14 pts (58.3%) on vasopressor, 16 pts (66.7%) on neuromuscular blockade, 19 pts (79.2%) using glucocorticoids for 7 D |
28 D |
| Rebelatto CLK, et al. [82] | Brazil | Randomized, double-blind, placebo-controlled, single-center clinical trial in ARDS |
17 11; 6 |
IT: mean 53, SD 15.3; Ctrl: mean 61.7, SD 9.7 |
IT: obesity 54.5%, HT 54.5%, DM 36.4%, CHKD 9.1%, COPD 0%, schizophrenia 9.1%; Ctrl: obesity 50%, HT 50%, DM 50%, CHKD 0%, COPD 16.7%, schizophrenia 0% |
IT: IMV in the ICU 100%, 200 < PaO2/FiO2 ≤ 300 mmHg 36.4%, 100 < PaO2/FiO2 ≤ 200 mmHg 54.5%, PaO2/FiO2 ≤ 100 mmHg 9.1%, time from symptom onset to first infusion: 10.7 ± 3.9 D; Ctrl: IMV in the ICU 100%, 200 < PaO2/FiO2 ≤ 300 mmHg 83.3%, 100 < PaO2/FiO2 ≤ 200 mmHg 0%, PaO2/FiO2 ≤ 100 mmHg 16.6%, time from symptom onset to first infusion: 12.1 ± 2.2 D Total lymphocyte count and proportion of CD3 and CD4 T lymphocytes significantly lower in the IT group. No differences between groups for D-dimer, CRP, ferritin troponin, and creatinine |
Concomitant treatment with anticoagulant and glucocorticoids in 100% of pts in both groups, antiviral drugs in 2 pts in the IT group, antibiotics when needed | 4 mo |
| Zhu R, et al. [85] | China | Randomized, single-blind, placebo-controlled, 2-center clinical trial in common/mild, severe, and critical COVID-19 |
58 29; 29 |
IT: median 64, IQR 54.5–68; Ctrl: median 66, IQR 59.5–69.5 |
IT: CAD 10.3%, DM 13.8%, cerebrovascular disease 10.3%, HT 41.4%, chronic respiratory disease 3.4%, history of liver and kidney disease 6.9%; Ctrl: CAD 10.3%, DM 13.8%, cerebrovascular disease 6.9%, HT 37.9%, chronic respiratory disease 0%, history of liver and kidney disease 10.3% |
IT: pts with common/mild disease 51.7%, severe disease 37.9%, critical disease 10.3%, requiring oxygen therapy 93.1%, on NIMV 10.3%, on IMV 0%; Ctrl: pts with common/mild disease 55.2%, severe disease 34.5%, critical disease 10.3%, requiring oxygen therapy 88.9%, on NIMV 6.9%, on IMV 0% No significant between-group differences in laboratory data Median D (IQR) from symptom onset to starting treatment 13 D (9.5–15.5) in the IT group and 11 D (8–14.5) in the ctrl group |
Before enrollment IT: glucocorticoids in 70% of pts (median D 4, QR 3–6, median dose 40 mg/D, IQR 40–73.3), antibiotics in 62.1% of pts, antivirals (IFN alpha, ribavirin, or ganciclovir) in 44.8% of pts; Before enrollment ctrl: glucocorticoids in 65.5% of pts (median D 4, IQR 2–7, median dose 40 mg/D, IQR 40–80), antibiotics in 65.5% of pts, antivirals (IFN alpha, ribavirin, or ganciclovir) in 44.8% of pts Concomitant treatment IT: glucocorticoids in 55.2% of pts (median D 4, IQR 1–9, median dose 24.4 mg/D, IQR 3–41.7), antibiotics in 55.2% of pts, antivirals (IFN-alpha or ribavirin) in 41.4% of pts; Concomitant treatment ctrl: glucocorticoids in 58.6% of pts (median D 7, IQR 5–14, median dose 28.6 mg/D, IQR 13.3–46.4), antibiotics in 62.1% of pts, antivirals (IFN alpha or ribavirin) in 51.7% of pts |
28 D |
| Shu L, et al. [76] | China | Randomized, open-label parallel-group, phase 1 clinical trial in severe disease, not responding to standard therapy for 7–10 D |
41 12; 29 |
IT: mean 61.00, SD 17.87; Ctrl: mean 57.86, SD 15.79 |
IT: DM 3 (25%), HT 3 (33.33%); Ctrl: DM 5 (17.24%), HT 6 (20.69%) |
IT: no supplemental oxygen 1 (8.33%), supplemental oxygen 7 (58.33%), HFNC or NIV 4 (33.33%), HMO or IMV 0; Ctrl: no supplemental oxygen 2 (6.90%), supplemental oxygen 21 (72.41%), HFNC or NIV 6 (20.69%), HMO or IMV 0 IT: median CT score 18.50 (IQR 16.25, 20.75), median no. lobes involved 4 (IQR 4, 5) Ctrl: median CT score 16.00 (IQR 15.00, 20.00), median no. of lobes involved 4 (IQR 3.5, 5) |
In all pts concomitant standard treatment with antiviral agents (abidor/oseltamivir) and glucocorticoids (1–2 mg/Kg) Antibiotic agents in 10 pts in the IT group (83.33%) and in 26 pts in the ctrl group (89.65%) |
28 D |
| Adas G, et al. [77] | Turkey | Randomized, open-label parallel-group phase 1 clinical trial in critically ill pts, with 10 pts with moderate disease evaluated as additional control group |
30 10; 10 + 10 |
Mean 56 NR; NR + NR |
NR |
Critically ill pts in the IT and in the ctrl group were all intubated and followed up in the ICU Additional ctrl group of hospitalized pts with moderate COVID-19: no signs of severe pneumonia and no need for supplemental oxygen Inflammatory markers significantly increased in the two groups of critically ill pts versus group of patients with moderate disease. No significant between-group difference in the critically ill pts |
IT as an add-on therapy to conventional therapy including antibiotics, antivirals, dexamethasone, hydroxychloroquine, and enoxaparin Additional ctrl group treated and followed up in the infectious disease clinic |
Markers of systemic inflammation and cytokine storm evaluated on D 0, 1, 4, and 7. Clinical outcome monitored during the entire hospital stay |
| Leng Z, et al. [69] | China | Nonrandomized, open-label, parallel-group, phase I study in moderate, severe, and critical disease |
10 7; 3 |
IT: mean 57; Ctrl: mean 65 |
IT: HT (1); Ctrl: NR |
IT: 2 pts with moderate disease, 4 pts with severe disease, 1 pt with critical disease, mean SpO2 92% (SD 0.02), mean SOB 2.29 (scale 1–3, SD 0.95); Ctrl: all pts with severe disease, mean SpO2 92% (SD 0.01), mean SOB 2 (scale 1–3; SD 1) |
IT initiated when worsening on antiviral drugs (lopinavir-ritonavir, with/without antipyretics) and supportive therapy | 14 D |
| Meng F, et al. [70] | China | Nonrandomized, open-label, parallel group, phase I study in moderate and severe disease |
18 9; 9 |
IT: mean 45.1; Ctrl: mean 49.6 |
IT: HT (2) DM (1) Liver disease (1); Ctrl: HT (1) Asthma (1) |
Five pts in each group with moderate disease: fever, respiratory symptoms, confirmed pneumonia on CT imaging or x-ray Four pts in each group with severe disease: SOB or dyspnea after activity, and/or RR ≥ 30/min, and/or oxygen saturation ≤ 93% at rest and/or PaO2/FiO2 < 300 |
Glucocorticoids in all pts in the IT group and in 6/9 pts in the ctrl group Lopinavir-ritonavir in 8/9 pts in the IT group and all pts in the ctrl group |
28 D |
| Xu X, et al. [78] | China | Nonrandomized, multicenter, open-label, parallel-group, exploratory clinical trial in severe and critical disease |
44 26; 18 |
IT: mean 58.31, SD 12.49; Ctrl: mean 61.11, SD 11.03 |
NR |
IT: 16 (61.5%) pts with severe disease and 10 (38.5%) critically ill pts; Ctrl: 10 (55.6%) pts with severe disease and 8 (44.4%) critically ill pts |
No significant between-group differences in concomitant medications: symptomatic treatment, antiviral therapy, antibacterial treatment, glucocorticoids, gut microflora modulator, traditional Chinese medicine. In both groups, significantly more critically ill pts than pts with severe disease received extracorporeal blood system purification | 30 D |
| Wei F, et al. [86] | China | Prospective, parallel-group two-center trial in moderate, severe, and critical COVID-19 |
25 12; 13 |
IT: median 67, IQR 56–70; Ctrl: median 68, IQR 65–78 |
IT: DM (1), hemorrhagic cerebral infarction (1); Ctrl: NR |
IT: 5 pts with moderate disease, 6 pts with severe disease, 1 pt with critical disease, median PaO2/FiO2 321, IQR 170–455; Ctrl: no. of pts with moderate or severe disease NR, 2 pts with critical disease, median PaO2/FiO2 and IQR NR Oxygen support: in the IT group 10 pts on LFNC, 1 pt on HFNC, 1 pt on IMV; in the ctrl group NR Median time (IQR) from disease onset to IT infusion: 42 D (29–46). Median time (IQR) from admission to IT infusion: 18 D (9–27) |
In both groups, pts treated with arbidol and lopinavir-ritonavir (12/12 in the IT group, NR for the ctrl group), plus methylprednisolone (4/12 pts in the IT group, NR for the ctrl group) | 60 D for pts in the IT group; NR for the ctrl group |
| Grégoire C, et al. [88] | Belgium | Prospective, single-arm study in severe ARDS with retrospectively selected ctrl group of matched pts |
32 8; 24 IT: males 7/8; Ctrl: males NR |
IT: median 50, IQR 43–58; Ctrl: median 54, IQR 49.5–63 |
IT: NR; Ctrl: NR |
IT: pts requiring HFNC (7) or IMV (1) within 24 h of ICU admission, median PaO2/FiO2 85.5 (IQR 77.9–93.4), WHO severity score of 6 (7 pts) and 8 (1 pt), median SOFA score 4 (IQR 3–5), elevated levels of CRP, ferritin, and D-dimer; Ctrl: pts requiring HFNC with 24 h of ICU admission and with comparable functional data, severity scores, and levels of CRP, ferritin, and D-dimer |
All pts received dexamethasone (6 mg/D for 10 D) and prophylactic doses of heparin unless a therapeutic dose was indicated | 60 D |
| Iglesias M, et al. [73] | Mexico | Prospective, single-arm study in severe ARDS not responding to standard medical management |
5 5; 0 |
Mean 52.6 | Obesity 3, Overweight 1, DM 2, HT 1, hypotiroidism 1, PAD 1, dyslipidemia 1, PF 1 |
Bilateral COVID-19 pneumonia by chest CT, complicated with severe ARDS, persistent PaO2/FiO2 < 100 (median 76, IQR 62–84), requiring IMV (4 pts) or BiPAP (1 pt, 15 L/min) Persistent fever, increase in D-dimer concentrations ≥ 50% from baseline and/or ferritin concentrations > 1000 ng/mL despite standard medical management in intensive care unit; SOFA score < 11 (mean 5.8 from 4 to 7) |
No clinical improvement after 48 h of standard pharmacological treatment with antibiotics and enoxaparin in all pts, glucocorticoids in 2 pts, and tocilizumab in 1 pt plus supportive therapy in the ICU | 21 D |
| Sánchez-Guijo F, et al. [79] | Spain | Prospective, single-arm, proof-of-concept study in patients requiring IMV despite treatment with antivirals and anti-inflammatory agents |
13 13; 0 (12/13 males) |
Mean 60.31 (median 60, IQR 11) |
None 3, Hepatitis B virus 1, hypertension 6, COPD 2, DM 1, hyperthyroidism 1, hypothyroidism 1, Behçet Syndrome 1 Ex-smokers 5 BMI range 24.49–35.16 kg/m2 |
All pts under IMV in the ICU. Median time from hospital admission to IMV 4 D (IQR 3 D). Median duration of IMV before first IT dose 7 D (IQR 12 D) Mean SOFA score 4.08, from 2 to 11 |
Previous treatment with glucocorticoids, antibiotics, and low molecular weight heparin in all patients, tocilizumab and hydroxychloroquine with/without azithromycin, and lopinavir–ritonavir in 85% of pts, anakinra in 15% of pts after tocilizumab with further administration of siltuximab in one pt Glucocorticoids administered concomitantly with IT, together with standard supportive therapy for IMV |
28 D |
| Guo Z, et al. [80] (2020) | China | Prospective, single-arm study in patients with severe or critical disease already receiving standard treatment |
31 31; 0 25/31 (80%) males |
Median (IQR): 70 (61–71) | HT 13 (41.9%), COPD 6 (19.4%), CAD 5 (16.1%), DM 5 (16.1%) | 23 pts (74.2%) with severe disease requiring oxygen inhalation (19 pts, 61.3%) or NIMV (4 pts, 12.9%); 8 pts (25.8%) with critical disease requiring IMV. Median (IQR) PaO2/FiO2 242 mm Hg (200–294 mm Hg). Persistent fever and increased levels of CRP, IL-6, and D-dimer before IT infusion. Mean D (SD) between symptom onset and IT infusion 50.7 (12.6), median D (IQR) between hospital admission and IT infusion 10.0 (6.0–22.0). ICU admission for 16 pts (51.6%) | Standard treatment with antivirals (83.9%), arbidol (64.5%) interferon alpha-2b (29.0%), antibiotics (74.2%), glucocorticoids (19.4%), oseltamivir (9.7%), chloroquine (9.7%), and/or IV immunoglobulin therapy (25.8%) | NR |
| Sharma A, et al. [87] | India | Prospective, single-arm, single-center study in patients with moderate pneumonia, first stage |
10 10 (8 males); 0 |
Mean 47.3, range 28–65 | DM 6, HT 4, vitiligo 1, history of tuberculosis 1, none 3 |
Shortness of breath in 100% of pts, RR > 24/min, SaO2 ≤ 93% on room air, PaO2/FiO2 200–300 mm Hg Pts receiving supplemental oxygen if/when SpO2 < 95% on room air, n = NR |
Concomitant standard treatment with one antiviral (lopinavir–ritonavir or favipiravir or remdesivir), methylprednisolone, low molecular weight heparin | 6 mo |
| Disease: COVID-19/IT: MSC-derived products | ||||||||
| Fathi-Kazerooni M, et al. [84] | Iran | Randomized, double-blind placebo-controlled clinical trial of MSC-derived secretome in severe disease |
30 15; 15 |
IT: mean 46.43, SD 11.91; Ctrl: mean 53.67, SD 10.30 |
IT: DM 3 (21.5%) HT 4 (28.5%); Ctrl: DM 4 (26.6%) HT 5 (33.3%) |
IT: RR > 30/min 15, resting SpO2 ≤ 90% 15 (< 80% 9), PaO2/FiO2 ≤ 300 mmHg 15, pulmonary infiltration > 50% in 24–48 h 15 (> 75% 7), O2 support: IMV 2, NIV 5, O2 Reserve Mask 7; Ctrl: RR > 30/min 15, resting SpO2 ≤ 90% 15 (< 80% 6), PaO2/FiO2 ≤ 300 mmHg 15, pulmonary infiltration > 50% in 24–48 h 15 (> 75% 6), O2 support: IMV 2, NIV 6, O2 Reserve Mask 7 |
Concomitant best standard of care as per institutional guidelines in both groups, with all pts already receiving remdesivir, glucocorticoids, and anticoagulants at study entry | 28 D |
| Sengupta V, et al. [83] | USA | Prospective, single-center cohort study of MSC-derived exosomes in severe disease not responding to the institutional standard treatment |
27 27; 0 |
Median 59 (range 29–84) | Pre-DM 3, DM 20, HT 12, hyperlipidemia 5, any condition 25 |
Mild ARDS (PaO2/FiO2 200 to < 300) 1, moderate ARDS (PaO2/FiO2 100 to ≤ 200) 11, severe ARDS (PaO2/FiO2 < 100) 13 O2 support: IMV 2, BiPAP 2, HFNC 5, NRBM 10, NC 4, room air 1 |
IT when fever and/or dyspnea for > 72 h and overall clinical deterioration as evidenced by down-trending PaO2/FiO2 on treatment with hydroxychloroquine and azithromycin | 14 D post-treatment |
| Disease: influenza/IT: MSCs | ||||||||
| Chen J, et al. [89] | China | Nonrandomized, open-label, parallel-group study in H7N9 influenza virus-induced ARDS |
61 17; 44 |
IT: mean 62.8, SD 14.4; Ctrl: mean 61.6, SD 11.8 |
IT: HT 58.8%, CAD 0%, COPD 0%, DM 29.4%, liver disease 5.9%, renal failure complication 9%, shock complication 70.6% (P = 0.03 versus ctrl); Ctrl: HT 52.3%, CAD 18.2%, COPD 2.3%, DM 15.9%, liver disease 2.3%, renal failure complication 22.7%, shock complication 36.4% |
All pts: PaO2/FiO2 < 200, requiring IMV and/or ECMO SOB and fatigue more frequently reported in the IT group than in the ctrl group IT: IMV 14 (82.4%), and/or ECMO 8 (47.1%); Ctrl: IMV 31 (70.5%), and/or ECMO 14 (31.8%) Inflammatory index PCT significantly more elevated in the ctrl group |
IT concomitant treatment (% of pts): antivirals 100%, glucocorticoids 52.9%, antibiotics 82.4%, vasoactive drugs 70.6%, ALSS 76.5%, CRRT 70.6% (P = 0.016 versus ctrl); Ctrl concomitant treatment (% of pts): antivirals 100%, glucocorticoids 54.5%, antibiotics 81.8%, vasoactive drugs 43.2%, ALSS 40.9%, CRRT 36.4% |
5 y Performed only in 4 survivors in the IT group |
AF atrial fibrillation, AKI acute kidney injury, ALSS artificial support liver system, AML acute myeloid leukemia, APACHE Acute Physiology and Chronic Health Evaluation, ARDS acute respiratory distress syndrome, BiPAP bilevel positive airway pressure, BM-MSCs bone marrow derived mesenchymal stem cells, CAD coronary artery disease, CHKD chronic kidney disease, CB chronic bronchitis, CLL chronic lymphocytic leukemia, CMP cardiomyopathy, COPD chronic obstructive pulmonary disease, COVID coronavirus disease, CRP C-reactive protein, CRRT continuous renal replacement therapy, CT computed tomography, Ctrl control, D day(s), DM diabetes mellitus, ECMO extracorporeal membrane oxygenation, GM-CSF granulocyte–macrophage colony-stimulating factor, hr hour, HT hypertension, IFN interferon, IL interleukin, IMV invasive mechanical ventilation, IQR interquartile range, HFNC high-flow nasal cannula oxygen therapy, IQR interquartile range, IT investigational therapy, ITP idiopathic thrombocytopenic purpura, LFNC low-flow nasal cannula oxygen therapy, LIS lung injury score, MAP mean airway pressure, min minute(s), mo month(s), MSCs mesenchymal stem/stroma cells, NA not applicable, NC nasal cannula, NIMV noninvasive mechanical ventilation, NIV noninvasive ventilation, NK natural killer, NR not reported, NRBM non-rebreather mask, PAD peripheral artery disease, PaO2/FiO2 arterial oxygen partial pressure/fractional inspired oxygen, PCT procalcitonin, PDGF platelet-derived growth factor, PF pulmonary fibrosis, Pt patient, RANTES regulated on activation, normal T-cell expressed and secreted, RR respiratory rate, SaO2 arterial oxygen saturation, SD standard deviation, SOB shortness of breath, SOFA sequential organ failure assessment, SpO2 peripheral oxygen saturation (by pulse oximeter), SpO2/FiO2 pulse oximetry oxygen saturation/fractional inspired oxygen, TNF tumor necrosis factor, wks weeks, y year(s)
Table 4.
MSC and MSC-derived product characterization, dosing and delivery
| Author and reference no. | IT source | Donor(s) n (sex) | IT markers and function | Cell passages | Culture media | Cell viability | IT dose | Frequency | Route of delivery | Ctrl/placebo |
|---|---|---|---|---|---|---|---|---|---|---|
| Disease: COVID-19/IT: MSCs | ||||||||||
| Shi L, et al. [71, 72] | Clinical-grade cells from umbilical cord tissue produced by VCGEC under cGMP | 1F |
> 95% of the cell population expressed CD105, CD73, and CD90, and < 2% positive for CD45, CD34, CD11b, CD19, and HLA-DR |
5 | Cell cultured in DMEM/F12 medium with 10% bovine serum and then in serum-free medium |
Mean 94.4% (SD 1.9%) after preparation, mean 88.48% (SD 4.8%) before infusion |
4 × 107 cells/kg | 3 (D 0, D 3, and D 6) | IV | Equal amount of cell medium without cells |
| Lanzoni G, et al. [74] |
Clinical-grade cells from umbilical cord tissue produced under cGMP and tested according to FDA, AABB, and FACT |
1 (F) |
Mean ± SD: 97.9% ± 2.6% CD90+, 98.1% ± 1.4% CD105+ and 2.2% ± 4.9% CD34/CD45+ |
NR: use of frozen cell samples of a master cell bank from a single umbilical cord |
Frozen cells thawed and diluted in Plasma-Lyte A with human serum albumin and heparin |
Mean ± SD: 96.2% ± 1.8% by trypan blue and 88.4% ± 7.6% by flow cytometry |
Mean ± SD: 100 ± 20 × 106 cells/kg in 50 ml of endotoxin-free and sterile vehicle solution containing human serum albumin and heparin |
2 (D 0 and D 3) |
IV over mean 10 (SD 5) minutes |
Vehicle solution alone |
| Dilogo IH, et al. [75] | Umbilical cord | NR | > 95% CD90+ and CD73+, < 2% CD34+ | 5 or 6 | NR | NR | 1 × 106 cells/kg in 100 ml of normal saline | 1 on D 8 (range 2–30 D) of treatment in ICU | IV | 100 ml of normal saline |
| Monsel A, et al. [81] | Umbilical cord Wharton’s jelly | NR (F) |
Mean ± SD: 99.2 ± 1.6% CD90+, 99.9 ± 0.1% CD73+, 97.0 ± 1.9% CD105+ CD45, CD34, D11b, CD19, and HLA-DR below positivity threshold of 2% (0.8 ± 0.7%) Confirmed immune-modulatory properties by mixed lymphocyte-reaction assay |
3–4 |
MSCs cultured in 5% human platelet lysate |
Mean ± SD: 78.4 ± 5.3% | Mean ± SD: 0.9 ± 0.1 × 106 cells/kg (range 0.6–1 × 106 cells/kg) in 150 ml 0.9% NaCl/0.5% albumin | 1 (81.0%), 2 (9.5%), or 3 (9.5%) doses, on D 1, D 3 ± 1 D, and D 5 ± 1 D | IV | 150 ml 0.9% NaCl/0.5% albumin |
| Rebelatto CLK, et al. [82] | Clinical-grade cells from umbilical cord |
NR Full-term newborns by cesarean section |
MSCs characterized according to the criteria of the International Society for Cellular Therapy | 3–5 |
IMDM with 20% FBS and antibiotics for initial expansion before cryopreservation |
Mean ± SD: 96.6% ± 0.01% infusion 1, 95.4% ± 0.03% infusion 2, 95.5% ± 0.02% infusion 3 |
5 × 105 cells/kg in 30 ml of saline solution with 5% citrate dextrose and 20% albumin | 3, one infusion every other D | IV | 30 ml of vehicle |
| Zhu R, et al. [85] | Clinical-grade cells from umbilical cord tissue certified by the Chinese regulatory authority | NR | NR | NR | NR | NR | 1 × 106 cells/kg in 100 ml normal saline | 1 | IV over about 40 min (20–30 drops/min) | 100 ml of normal saline |
| Shu L, et al. [76] | Clinical-grade cells from umbilical cord tissue produced under cGMP conditions | NR (F) |
> 95% CD73+, CD90+, and CD105+, < 2% CD34+, CD45+, CD14+, or CD11b+, CD79α+ or CD19+, and HLA-DR+ |
3 or 5 | NR | NR | 2 × 106 cells/kg in 100 ml of normal saline | 1 | IV over 1 h, 35 drops /min | No placebo |
| Adas G, et al. [77] | Clinical-grade cells from umbilical cord Wharton’s jelly produced under cGMP | NR (F) | NR | 4 | NR | NR | 3 × 106 cells/kg in 150 ml 0.9% NaCl | 3 (D 0, D 3, and D 6) | IV over 1 h | No placebo |
| Leng Z, et al. [69] | Clinical-grade cells certified by the Chinese regulatory authority. Source: NR | NR |
> 99% of cells were positive for CD105, CD90, CD73, CD44, and CD29; ≤ 0.13% were CD45 and CD14 negative in the representative experiment shown. Tested for adipogenic, chondrogenic, and osteogenic differentiation Only rare cells expressed ACE2 and TMPRSS2 by RNA sequencing |
3 | Cultured in DMEM/F12 medium with 2% FBS, 2% GlutaMAXTM-I, 1% antibiotics, and 2 mM GlutaMAXTM-I at 37 °C with 5% CO2 | 91.6% at passage 3 in the representative experiment shown | 1 × 106 cells/kg in 100 ml of normal saline | 1 | IV over 40 min | Placebo use not described |
| Meng F, et al. [70] | Clinical-grade cells from umbilical cord tissue produced by VCGEC under cGMP | NR (F) | Cells were CD73+, CD90+, CD105+, and negative for CD19, CD34, CD11b, CD46, and HLA-DR in the representative experiment shown | 5 | MSC culture medium for plating and expansion and then serum-free medium | 82.12% at passage 5 in the representative experiment shown | 3 × 107 cells/kg | 3 (on D 0, D 3, and D 6) | IV | Equal amount of cell medium without cells |
| Xu X, et al. [78] | Menstrual blood | 3 (F) |
Cells were CD29+, CD73+, CD90+, and CD105+ and negative for CD34, CD45, CD117, and HLA-DR Cells did not express ACE2 mRNA |
5 |
Frozen MSCs thawed and diluted in Plasma-Lyte A 148 |
> 90% | 3 × 107 cells/kg in 500 ml saline solution | 3 (D 1, D 3, and D 5) |
IV over 2 h, at a speed of 30–40 drops/min for about 15 min, followed by a speed of 100–120 drops/ min |
No placebo |
| Wei F, et al. [86] | Clinical-grade cells from umbilical cord tissue certified by the Chinese regulatory authority | NR (F) |
Cells were CD44+, CD73+, CD90+, and CD105+ and negative for CD34 and CD45. Demonstrated multilineage differentiation ability |
NR | NR | NR | 1 × 106 cells/kg in 100 ml normal saline | 1 | IV over about 30 min (60 drops/min) | No placebo |
| Grégoire C, et al. [88] | Clinical-grade cells from bone marrow, produced under cGMP | NR |
Adherent cells of fibroblastic morphology CD90+ > 95% CD105+ > 95% CD73+ > 95% CD29+ > 90% CD44+ > 90% CD166+ > 90% CD14+ < 2% CD34+ < 2% CD45+ < 2% CD3+ < 1% with immunosuppressive properties confirmed after thawing by mixed lymphocyte reaction assay |
2 | 90% DMEM-LGGLX medium and 10% γ-irradiated FBS by | Pre-freezing ≥ 80%; post-thawing viability ranging from 56% to 93% (median 76%) |
1.5–3 × 106/kg infused after treatment with anti-coagulants, cetirizine, and paracetamol |
3 at 3 (± 1) D intervals, starting within 2 D of ICU admission |
IV infused within 1 h of thawing |
No ctrl |
| Iglesias M, et al. [73] | Clinical-grade cells from umbilical cord tissue produced by CBCBT under cGMP | NR (F) | 99.99% CD105+, 99.96% CD90+, 99.98% CD73+, 99.29% CD44+, 0.03% CD45+, and CD34+, 0.1% HLA-DR+ | NR | NR | 99.95% | 1 × 106 cells/kg | 1 | IV over 15 min | No ctrl |
| Sánchez-Guijo F, et al. [79] | Adipose tissue | 5 (NR) | NR | Less than 2 |
Frozen MSCs thawed and suspended in medium containing AB serum and 10% dimethyl sulfoxide In some cases, thawed cells re-cultured for less than 72 h and suspended in Ringer’s lactate with 1% albumin |
NR |
1 × 106 cells/kg Steroids and dexchlorpheni-ramine administered prior to cell infusion |
1 infusion on D 1 and repeated 1 or 2 infusions at a distance of 48 h or 96 h if deemed necessary on the basis of the response to the first infusion | IV | No ctrl |
| Guo Z, et al. [80] | Umbilical cord | NR (F) | NR | NR | NR | NR | 1 × 106 cells/kg in 100 ml of normal saline | 1–3 as deemed necessary. Interval between infusions: NR | IV | No ctrl |
| Sharma A, et al. [87] | Mix of cells from human umbilical cord blood and placenta, produced at a GMP-compliant facility with cord blood banking license | NR (F) | NR | NR | StemProTM MSC SFM XenoFree for cell cultures | NR | 100 × 106 cells/kg in 100 ml of normal saline | 2 (D 1 and D 4) | IV over 40 min (40 drops /min) | No ctrl |
| Disease: COVID-19 / IT: MSC-derived products | ||||||||||
| Fathi-Kazerooni M, et al. [84] | Menstrual blood MSCs | > 5 (F) | Culture-expanded cells from established and previously characterized master cell bank | NR | DMEM-F12 medium containing platelet lysate, followed by phenol-red-free DMEM-F12 for 48–72 h | NR | 5 ml of cell supernatant following sterile filtration and centrifugation at 2000 rpm for 5 min at room temperature. Infused after dilution in 100 ml normal saline | 5, on fifth consecutive D | IV over 60 min | 100 ml of normal saline |
| Sengupta V, et al. [83, 118] | Bone marrow MSCs | NR | NR | NR | NR | NR | ExoFlow from Direct Biologics, FDA-approved for investigational use: 15 ml of a solution containing about 40 × 106 nanoparticles/ml in 100 ml normal saline | 1 | IV over 60 min | No ctrl |
| Disease: influenza/IT: MSCs | ||||||||||
| Chen J, et al. [89] | Menstrual blood | 1 (F) | NR | NR | NR | 90–95% | 1 × 106 cells/kg in 100 ml of Plasma-Lyte A |
3 (3 pts in the acute phase of ARDS and 3 pts at a later stage) 4 (8 pts at a late stage of ARDS) |
IV | No placebo |
All MSCs were allogeneic and culture expanded
AABB American Association of Blood Banks, ACE2 angiotensin-converting enzyme 2, ARDS acute respiratory distress syndrome, CBCBT CBCells BioTechnology, cGMP current good manufacturing practice, COVID coronavirus disease, Ctrl control, D day(s), DMEM Dulbecco’s modified Eagle’s medium, F female, FACT Foundation for the Accreditation for Cellular Therapy, FBS fetal bovine serum, FDA Food and Drug Administration of the United States of America, hr hour, IMDM Iscove’s Modified Dulbecco’s Medium, IT investigational therapy, IV intravenous, min minute(s), MSCs mesenchymal stem/stromal cells, NA not applicable, NR not reported, Pt patient, PBMC peripheral blood mononuclear cells, SD standard deviation, TMPRSS2 transmembrane serine protease type 2, VCGEC Vcanbio Cell & Gene Engineering Corp., Ltd
Table 5.
Adverse events and serious adverse events
| Author and reference no. | Treatment-related AEs | Nontreatment-related AEs | Treatment-related SAEs/deaths | Nontreatment-related SAEs/deaths |
|---|---|---|---|---|
| Disease: COVID-19/IT: MSCs | ||||
| Shi L, et al. [71, 72] | None |
Incidence of AEs: 55.38% in the IT group and 60% in the ctrl group by D 28 Incidence of most common AEs (all grade 1–2): LDH increase 13.85% in the IT group and 20% in the ctrl group; elevated ALT, 10.77% in the IT group and 11.43% in the ctrl group; hypokalemia, 9.23% in the IT group and 2.86% in the ctrl group; AST increase 7.69% in the IT group and 11.43% in the ctrl group; hyperuricemia, 7.69% in the IT group and 8.75% in the ctrl group Only one grade 3–4 AE: pneumothorax in 1 pt in the IT group during the first 28 days of the trial, recovered under conservative treatment Total incidence of AEs over 1 year 83.1% in the IT group and 74.3% in the ctrl group |
None | One death from cancer at 3 mo in the ctrl group |
| Lanzoni G, et al. [74] | 1 pt experienced worsening of bradycardia requiring transient vasopressor treatment within 6 h after IT infusion; 1 pt experienced new cardiac arrhythmia 2 h after vehicle infusion | 31 in the IT group; 45 in the ctrl group | None |
2 (2 pts) in the IT group; 16 (8 pts) in the ctrl group (P = 0.04) 2 deaths in the IT group: 1 due to failed endotracheal intubation, 1 due to acute respiratory failure; 7 deaths in the ctrl group: 1 due to acute respiratory failure, 6 due to multiorgan dysfunction |
| Dilogo IH, et al. [75] | None | NR | None | 4 deaths |
| Monsel A, et al. [81] |
Possible IT-related diarrhea (1); Adverse hemodynamic event within 6 h of vehicle infusion |
49 (18 pts, 85.7%) in the IT group; 48 (18 pts, 75.0%) in the ctrl group | None |
10 (6 pts, 28.6%), 5 deaths in the IT group; 6 (6 pts, 25.0%), 4 deaths in the ctrl group |
| Rebelatto CLK, et al. [82] |
Transient hypotension after the first infusion in 1 pt, uncertain relation to treatment; Tachycardia immediately after the first infusion in 1 pt, uncertain relation to treatment |
None |
IT group: 1 death due to multiorgan dysfunction syndrome at D 8 after the first infusion, 4 deaths due to bacterial septic shock at D 8, D 17, D 20, and D 23 after the first infusion; Ctrl group: 1 death due to bacterial septic shock at D 38 after the first infusion |
|
| Zhu R, et al. [85] | No significant differences between groups for changes in vital signs during the first 24 h after IT or placebo infusion |
More pts in the placebo group (13, 44.8%) than in the IT group (3, 10.3%) experienced AEs. Disturbance of consciousness, urinary tract infection, headache, fever, diarrhea/bloating, and inappetence were only reported in the placebo group No significant differences between groups for the levels of ALT, TBIL, and sCr within 3 D after IT or placebo infusion |
NR | 2 deaths in the placebo group |
| Shu L, et al. [76] | No rash, allergic reaction, or febrile reaction after the infusion in the IT group | NR | None | NR |
| Adas G, et al. [77] | None | NR | None | 6 deaths in the IT group |
| Leng Z, et al. [69] | None: no acute infusion-related events or allergic reactions within 2 h after IT administration, no delayed hypersensitivity or secondary infections | None | None | None |
| Meng F, et al. [70] |
IT group: 1 pt with moderate disease, facial flushing within 4 h after first infusion; ctrl group: 1 pt with moderate disease, transient fever within 2 h after third infusion |
None | None | IT group, 1 pt with severe disease, severe hypoxemia within 12 h after first infusion, recovered after HFNC oxygen therapy |
| Xu X, et al. [78] | None | Frequency of high blood pressure significantly higher in the ctrl group. No significant differences between groups for number of AEs and severity grade | None | 2 deaths in the IT group |
| Wei F, et al. [86] |
No allergic reactions in the IT group on the D of IT infusion Follow-up for at least 60 D for the IT group only AEs: NR SAEs: the pt with critical disease on IMV died 17 D after IT infusion from respiratory failure, circulatory failure, and secondary infections; the SAE was not considered treatment related |
|||
| Grégoire C, et al. [88] | None | NR | None |
1 pt had multifocal ischemic cerebral lesions after the second IT dose and did not receive the third dose. An aortic endocarditis involving a bicuspid valve was considered the embolic source responsible for the SAE |
| Iglesias M, et al. [73] |
AEs during the first hr post-infusion: Pt no. 4, muscle spasms in the extremities and chest, respiratory efforts, hypoxemia, and arterial hypertension lasting 5 min on treatment with propofol and increased FiO2; Pt. no. 5, hypotension lasting 60 min on treatment with vasopressin; Pt. no. 3, muscle spasms in the extremities lasting 15 min without treatment |
|||
| Sánchez-Guijo F, et al. [79] | None | Concurrent bacteria pneumonia in 1 pt, fungal infection in 1 pt. Both patients recovered under appropriate antibacterial and antifungal therapy and were extubated | None |
1 death due to massive gastrointestinal bleeding because of nasogastric-tube-related gastric ulcer, 1 death due to secondary fungal pneumonia |
| Guo Z, et al. [80] | None | NR | None | 72 deaths |
| Sharma A, et al. [87] | None | NR | None | 1 death caused by cardiac arrest after 3.5 mo after last IT infusion |
| Disease: COVID-19/IT: MSC-derived product | ||||
| Fathi-Kazerooni M, et al. [84] |
None observed in the IT group but 1 pt discontinued after first dose for unknown causes; no data reported for the ctrl group |
IT group: NR Ctrl group: NR |
None in the IT group; no data reported for the ctrl group |
IT group: aggravated hypoxic respiratory failure requiring intubation (n = 7), pulmonary embolism (n = 3), myocardial infarction (n = 1), sepsis (n = 1), death (n = 6); ctrl group: aggravated hypoxic respiratory failure requiring intubation (n = 12), death (n = 12) |
| Sengupta V, et al. [83] | None observed within 72 h of ExoFlo administration | NR | None observed within 72 h of ExoFlo administration | Pulmonary embolism (n = 1), acute renal failure (n = 3), worsening of hypoxic respiratory failure requiring intubation (n = 4), and 4 deaths at post-treatment D 4–13 |
| Disease: influenza/IT: MSCs | ||||
| Chen J, et al. [89] | None | NR | None | 3 deaths in the IT group; 24 deaths in the ctrl group |
AE adverse events, ALB albumin, ALT alanine aminotransferase, AST aspartate aminotransferases, COVID coronavirus disease, CRP C-reactive protein, Ctrl control, D day, DBIL direct bilirubin, FiO2 fractional inspired oxygen, HFNC high-flow nasal cannula, hr hour(s), IMV invasive mechanical ventilation, IT investigational therapy, LDH lactate dehydrogenase, min minute(s), mo month(s), MSC mesenchymal stem/stromal cells, NR not reported, PCT procalcitonin, Pt patient, SAE serious adverse events, sCr serum creatinine, TBIL total bilirubin
Table 6.
Clinical, laboratory, and imaging findings
| Author | Mortality in IT group (death/n) | Mortality in ctrl group (death/n) | Pulmonary function outcome | Systemic outcome | Inflammatory/coagulation markers | Imaging outcome |
|---|---|---|---|---|---|---|
| COVID-19—MSCs | ||||||
| Shi L, et al. [71, 72] | 0/65 (0%) | 1/35 (2.86%) |
Pts in the IT group tended to improve more than pts in the placebo group but no statistically significant differences between groups for 6-MWD, status of oxygen therapy, VCmax, DLCO, and mMRCds by D 28 and at any time during the 1-year follow-up. No significant differences in the inhibition rate of neutralizing antibodies by mo 12. No significant differences in tumor markers between groups at mo 12 |
By D 28, no significant differences in the subsets of peripheral lymphocyte counts (CD4+ T cells, CD8+ T cells, B cells, NK cells) and inflammatory markers (including also PCR and IL-6) between the two groups. No significant differences in the subsets of peripheral lymphocyte counts by mo 12 |
Significant improvement in whole lung lesion volume from baseline to D 10 compared with placebo (primary endpoint); significant reduction in the proportion of lung solid component lesion volume from baseline to mo 1 and to mo 9; 10/56 pts in the IT group had normal CT images at mo 12 and none in the placebo group | |
| Lanzoni G, et al. [74] | 2/12 (16.67%) | 7/12 (58.33%) | Time to recovery significantly shorter in the IT group (P = 0.0307) |
Survival by D 28 after the last infusion significantly improved in the IT group (P = 0.015) SAE-free survival significantly improved in the IT group (P = 0.0081) |
Significant decrease in the plasma concentrations of all the tested inflammatory markers from D 0 to D 6 in the IT group; no significant change in the vehicle group No significant differences in median viral load between groups at D 0 and D 6 |
NR |
| Dilogo IH, et al. [75] |
10/20 (50.0%) 7/11 with ≥ 2 comorbidities |
16/20 (80.0%) 12/13 with ≥ 2 comorbidities |
No significant differences between groups in terms of length of stay in the ICU and ventilator usage |
Overall, survival rate 2.5 times higher in the IT group (71.4%) than in the ctrl group (28.6%) In pts with ≥ 2 comorbidities, survival rate 4.5 times higher in the IT group than in the ctrl group |
Significant decrease in the levels of circulating IL-6 from D 0 to D 7 post-infusion only in the recovered patients in the IT group (n = 10, P = 0.023) No significant changes in the levels of other inflammatory markers |
NR |
| Monsel A, et al. [81] | 5/21 (23.81%) | 4/24 (16.66%) |
No significant difference between groups for PaO2/FiO2-ratio change from D 0 to D 7 (primary endpoint) No significant between-group differences for SOFA scores, compliance, driving pressure change between D 0 and D 7 or D 14, organ-failure-free days, ventilation-free days, duration of ventilation, and time to reach PaO2/FiO2 > 200 or > 300 |
No significant between-group differences for time to ICU discharge and mortality to D 28 |
Significant decrease of plasma inflammatory markers (i.e., IP-10, MCP-2, IL-1b RAGE) in the IT group at D 14 versus D 0 in comparison with pts in the placebo group | NR |
| Rebelatto CLK, et al. [82] | 5/11(45.45%) | 1/6 (16.66%) | No significant between-groups difference in time to recovery and time to hospital discharge |
No significant between-groups differences in the reduction of viral load over time Significantly higher levels of troponin I and creatinine in the MSC group than in the placebo group between D 4 and D 14 Significant increase in the creatinine level from baseline at 2 mo and 4 mo only in the placebo group |
Significant decrease from baseline of the levels of ferritin, IL-6, MCP-1, and CCL2 at D 14 in the IT group. In the same group, significant decrease of the levels of CRP and neutrophil counts, with a concomitant increase in the numbers of CD4, CD8, and NK lymphocytes at 2 mo Significant reduction of the levels of D-dimer in the IT group in comparison with the placebo group at 2 mo after first cell infusion |
Significantly higher degree of clearance of pulmonary opacities in the IT group than in the placebo group at 4 mo in comparison with baseline and 14 D by chest CT scan |
| Zhu R, et al. [85] | 0/29 (0%) | 2/29 (6.9%) |
Median time to symptom remission significantly shorter in the IT group than in the placebo group Pts with severe and critical disease in the IT group achieved significantly better symptom outcome than similar pts in the placebo group on D 14 and D 21. No significant differences in patients with common/mild disease Three pts in the placebo group showed disease progression, requiring NIMV (1 pt) or IMV (2 pts) |
Median time of hospital stay (primary endpoint) significantly shorter in the IT group than in the placebo group (11 D, IQR 8–14, versus 15 D, IQR 11–19) |
For the pts with severe and critical disease: more rapid and more marked decrease in the levels of CRP in the IT group than in the placebo group, with statistically significant differences at D 3 and D 5; significantly higher reduction of the levels of IL-27, IL-5, IL-17E/IL-25, IL-18, and growth-regulated protein alpha from baseline to D 28 in the IT group than in the placebo group, but no significant difference for the levels of IL-6, IL-1 alpha, IFN-gamma, TNF-alpha, and IL-12 Circulating markers indicative of immune thrombosis, NET, significantly reduced in the IT group Plasma levels of the antibodies against SARS-CoV-2 significantly higher in the IT group than in the ctrl group on D 28 |
Significantly higher degree of clearance of pulmonary opacities in the severe and critically ill pts in the IT group than in those in the placebo group at D 7 and D 21 by chest CT scan |
| Shu L, et al. [76] | 0/12 (0%) | 3/29 (10.34%) |
Significant improvement of PaO2/FiO2 in the IT group versus the ctrl group from D 7 post-infusion Significant improvement of symptoms in the IT group versus the ctrl group by D 7 post-infusion |
All patients in the IT group recovered and were discharged 4 pts in the ctrl group progressed from severe to critical illness, requiring invasive ventilation, and 3 died |
Significantly higher and faster reduction of circulating levels of CRP and IL-6 in the IT group than in the ctrl group Significantly shorter time to normalization of the total lymphocyte count in the IT group that in the ctrl group |
Significant improvement of the CT score in the IT group versus the ctrl group by D 14, particularly for the numbers of lobes involved and consolidation score |
| Adas G, et al. [77] | 3/10 (30.0%) |
6/10 (60.0%) in critically ill ctrl group 0/10 (0%) in moderately ill ctrl group |
Mortality rate and length of stay in ICU significantly lower in the IT group versus the ctrl group of critically ill pts |
Significant decrease in the levels of ferritin, CRP, IL-6, IFN-γ, IL-2, IL-12, and IL-17A and significant increase of IL-10, IL-13, and IL-1ra in the IT group versus the ctrl group of critically ill pts Significant decrease in the levels of fibrinogen and D-dimer in the IT group versus the ctrl group of critically ill pts |
NR | |
| Leng Z, et al. [69] | 0/7 (0%) | 1/3 (33.33%) | 2–4 D after IT infusion: oxygen saturation rose to ≥ 95% at rest, with or without oxygen administration (5 L/min) | 2–4 D after IT infusion: all symptoms disappeared in all patients |
IT group: decrease in TNFα, increase in IL-10 (all groups) In pts with most critical conditions: CRP decreased; cytokine-secreting immune cells CXCR3+ CD4+ T cells, CXCR3+ CD8 + T cells, and CXCR3+ NK cells decreased; IP-10, VEGF, lymphocytes, CD14+ CD11c+ CD11bmid regulatory DCs dramatically increased |
In pts with most critical conditions: signs of bilateral pneumonia on CT improved on D 9 after IT infusion, with only residual ground-glass opacity detected on D 15 |
| Meng F, et al. [70] | 0/9 (0%) | 0/9 (0%) |
IMV in 1/9 pts in the IT group and in 4/9 pts in the ctrl group High-flow oxygen support in 3/9 pts in the IT group and in 5/9 pts in the ctrl group Low-flow oxygen support in 7/9 pts in both groups In pts with most severe disease, PaO2/FiO2 ratio stably improved only in the IT group |
Time to discharge: 20 D in the IT group and 23 D in the ctrl group No significant differences in duration of symptoms between groups |
IL-6 decrease within 3 D after the first IT infusion in 2 pts with moderate disease and 2 pts with severe disease, all having high levels of IL-6 at baseline. Similar changes not observed in treated pts with lower levels of IL-6 at baseline and in the ctrl group | Chest CT scan results shown for 2 pts with severe disease: better outcome in the treated pts than in the ctrl pts, with improvement detectable on D 6 |
| Xu X, et al. [78] | 2/26 (7.69%) | 6/18 (33.33%) |
Significant improvement of cough on D 1 and in expiratory dyspnea on D 1, D 3, and D 5 in the IT group versus the ctrl group Significant improvement in SpO2 and PaO2 following treatment in the IT group Overall time to symptom improvement significantly shortened in the IT group (difference of 5.8 D) |
Significantly higher survival rate in the IT group versus ctrl group. The increase in survival rate more pronounced in the critically ill pts than in the pts with severe disease after adjustment for sex and age No significant differences between groups for use of respiratory support, number of days in ICU and length of hospital stay No difference between groups for occurrence of shock or multiple organ failure |
No significant differences in CRP and IL-6 levels before and after treatment in the IT group |
Significantly higher improvement rate of CT imaging results in the IT group versus the ctrl group At the end of the follow-up period, 17 (85%) pts in the IT group showed improvement while 6 (50%) patients in the ctrl group showed no significant changes |
| Wei F, et al. [86] | 1/12 | NR | Significant increase in SpO2/FiO2 at D 12 and D 16 post-IT infusion in pts with severe disease. Missing information about SpO2/FiO2 changes in the ctrl group | In the IT group, 9/10 pts on LFNC no longer needed oxygen support 2 wks after IT infusion. Missing information about the ctrl group |
No significant changes in laboratory data in the IT group and in the ctrl group, including total lymphocyte count and levels of CRP, IL-6, and TNFα Antibodies against SARS-CoV-2: significant decrease in the levels of IgM in the IT group only, and no changes in the levels of IgG in the IT group and in the ctrl group |
Significant decrease in the area of pulmonary inflammation over 2 wks post-IT infusion in the survived pts of the IT group by CT scan. Missing data for the ctrl group |
| Grégoire C, et al. [88] |
0/8 at D 28; 0/8 at D 60 |
Matched ctrl group: 5/24 (20.8%) at D 28; 7/24 (29.2%) at D 60 Whole ctrl group: 65/247 (26.3%) at D 28; 79/247 (32.0%) at D 60 |
Transient progression of disease severity in 2/7 pts on HFNO at inclusion in the IT group: requiring IMV, followed by ECMO in one case, after IT infusions The other pts in the IT group rapidly improved |
Survival rate significantly higher in the IT group than in the matched ctrl group at D 28 (100% versus 79.2%) and at D 60 (100% versus 70.8%) No significant differences between groups for ICU-free D, need for IMV, IMV-free D, HFNO support-free D, O2 support-free D, and need for noradrenaline |
The levels of D 7 D-dimer were much lower in the IT group than in the matched ctrl group: median 821.0 μg/L (IQR 362.0–1305.0) versus median 3553.0 mg/L (IQR 1155.0–6433.5), P = 0.0085 No significant differences in the levels of D 7 CRP between groups |
NR |
| Iglesias M, et al. [73] | 2/5 (40.0%) Pts. nos. 4 and 5 | 0/0 | Statistically significant increase in PaO2/FiO2 over 7 D post-infusion |
3 pts survived and were extubated at 9 D post-infusion Pts. nos. 4 and 5 had severe systemic complications of COVID-19 with thrombosis, bleeding, and liver or kidney failure and died at 13 D and 15 D post-infusion |
Increased D-dimer concentrations after the first 48 h post-infusion in all patients CRP concentrations remained normal in the 3 pts who survived. It increased during the first 72 h post-infusion and then remained elevated in the patients who died Total lymphocytes were slightly elevated at 7 D post-infusion. Only one of the survived pts had a decrease in total lymphocytes from 1570/ml at baseline to 984/ml at 7 D |
CT scan at 2–3 wks post-infusion: reduction of ground glass opacities and consolidations in the lungs with decrease in the estimated % of damaged lung parenchyma in the survived pts |
| Sánchez-Guijo F, et al. [79] | 2/13 (15.38%) | 0/0 |
10 pts received 2 doses, with the second dose administered a median of 3 D (IQR 1 D) after the first one. 2 pts received a single dose and another pt received 3 doses. Median number of cells per dose was 0.98 × 106 (IQR 0.50 × 106) /kg 9 pts (70%) improved clinically and 7 (53%) were extubated within a median time of 16 D (IQR 9 D) from D 1. 2 of the extubated pts were discharged Extubated pts had received the first IT infusion earlier than the other pts, with a median difference of 5 D 2 pts required ECMO despite 2 IT infusions and then remained stable |
In the pts who improved clinically: decrease in inflammatory parameters at D 5 after infusion, with a decrease in CRP in 8 pts (88%), LDH in 9 (100%), and D-dimer and ferritin in 5 of 8 evaluable Pts (63%); increased number of total lymphocytes in 5 of 6 pts tested by flow cytometry (86%), with increased frequency of CD4+ and CD8+ T cells |
Radiological improvement was confirmed after first IT administration in 40% of 10 evaluable patients | |
| Guo Z, et al. [80] | 4/31 (12.9%) | 0/0 |
IT infusions: 1 in 35.5% of pts, 2 in 29.0% of pts, 3 in 35.5% of pts. After first IT infusion, viral clearance in 96.8% of pts after a mean time of 10.7 D (SD, 4.2 D). Pts discharged: 87.1% Significant increase in PaO2/FiO2 from median (IQR) 242 (200–294) to 332 (288–364) mm Hg, P < 0.001 |
Significant increase of total lymphocyte count (P < 0.001) and significant decrease of the levels of CRP, procalcitonin, IL-6, and D-dimer (P ≤ 0.010) | NR | |
| Sharma A, et al. [87] | 1/10 | 0/0 |
Resolution of symptoms in all pts within 10 D, with none of the pts still requiring oxygen support on D 9 9/10 pts discharged within 9 D of their admission Improvement of median SpO2/FiO2 from 259.43 mmHg on D 1 to 458.09 mmHg on D 8. Improvement of median PaO2/FiO2 from 230.1 mmHg on D 1 to 402.14 mmHg on D 8 |
Normalization of the median levels of CRP by D 4. Decrease of the median neutrophil/ lymphocyte ratio and normalization of the levels of ferritin, D-dimer, and IL-6 by D 14, after abnormal increases over baseline between D 2 and D 6 |
Average CT scan scores decreased from 14 on D 1 (n = 10) to 8 on D 28 (n = 8) No evidence of pulmonary fibrosis at D 28 and at 6 mo by chest x-ray. No evidence of new abnormalities at 6 mo |
|
| COVID-19—MSC-derived products | ||||||
| Fathi-Kazerooni M, et al. [84] | 6/14 (42.86%) | 12/15 (80.0%) | Significant improvement of SpO2 within 5 D of starting infusions in 64% of pts in the IT group. Significantly lower percentage of pts requiring intubation after study start in the IT group (50%) than in the ctrl group (80%) |
Survival rate significantly higher in the IT group (57%) than in the ctrl group (20%) All non-intubated pts in the IT group (7/14, 50%) recovered and were discharged from hospital after 12.3 ± 3.68 D after the first IT dose |
Significant reduction in mean levels of CRP (77%), ferritin (43%), and D-dimer (42%) in the IT group Significant improvement of lymphopenia and increase in mean CD4+ (20%) and CD8+ (15%) lymphocyte counts in the IT group |
CT scan: significant reduction in the mean percentage of lung involvement from baseline (72.57%) to discharge (28.67%) in the survivors of the IT group |
| Sengupta V, et al. [83] | 4/24 (16%) | 0/0 | Significant increase of PaO2/FiO2 from baseline to D 14 or last D of hospitalization (mean 191%, P < 0.001), with an improvement within 3 D of treatment in 80% of pts |
Overall survival rate: 83%. Overall recovery rate: 71% Recovery rate in the cohort of 20 pts not requiring IMV at baseline: 75%. The remaining 25% progressed to IMV. In the cohort of 3 intubated pts with severe ARDS at baseline, all pts remained critically ill, requiring IMV |
Significant reduction in absolute neutrophil count and increase in CD3+, CD4+, and CD8+ lymphocyte counts. Mean CRP, ferritin, and D-dimer reduction of 77% (P < 0.001), 43% (P < 0.001), and 42% (P < 0.05), respectively |
NR |
| Influenza—MSCs | ||||||
| Chen J, et al. [89] | 3/17 (17.6%) | 24/44 (54.5%) |
No information about changes in PaO2/FiO2, duration of IMV, length of stay in the ICU and time to discharge in the survivors 4 pts in the IT group followed up for 5 y. No long-term significant changes in FEV1, FVC, FEV1/FVC, and FEF50% |
Significantly higher survival rate in the IT group than in the ctrl group CK and sCr levels significantly lower in the IT group than in the ctrl group at discharge No significant changes in the quality of life scores during the follow up of pts in the IT group |
CRP, PCT, PT, and D-dimer significantly lower in the IT group than in the ctrl group at discharge | At 24 wks and 1 y after IT, all pts showed improvement on chest CT |
6-MWT 6-min walk test, 6-MWD 6-min walking distance, ALB albumin, ALT alanine aminotransferase, APACHE Acute Physiology and Chronic Health Evaluation, ARDS acute respiratory distress syndrome, AST aspartate aminotransferase, BAL bronchoalveolar lavage, BM bone marrow, CCT cardiac computerized tomography, CK creatine kinase, CK-MB creatine kinase-MB, CLL chronic lymphocytic leukemia, CMP cardiomyopathy, Cr creatinine, COVID coronavirus disease, CRP C-reactive protein, CT computed tomography, cTnT cardiac troponin T, Ctrl control, CXCR C-X-C motif chemokine receptor, CXR chest X-ray, D day(s), DBIL direct bilirubin, DC(s) dendritic cell(s), DLCO diffusion lung capacity for carbon monoxide, ECMO extracorporeal membrane oxygenation, FEF50% forced expiratory flow at 50% of vital capacity, FEV1 forced expiratory volume in one second, FVC forced vital capacity, HFNC high-flow nasal cannula, hr hour, IL interleukin, IMV invasive mechanical ventilation, NIV noninvasive ventilation, IP-10 interferon-gamma-induced protein-10, IQR interquartile range, IT investigational therapy, ICU intensive care unit, KGF keratinocyte growth factor, LAC lactate, LDH lactate dehydrogenase, LFNC low-flow nasal cannula, LIS lung injury score, MCP monocyte chemoattractant protein, mMRCds modified Medical Research Council dyspnea scale, mo month(s), MSC mesenchymal stem/stromal cell, NA not applicable, NET neutrophil extracellular traps, NR not reported, PaO2/FiO2 arterial oxygen partial pressure/fractional inspired oxygen, PCT procalcitonin, Pt patient, PT prothrombin time, RAGE receptor for advanced glycation end products, SaO2 arterial oxygen saturation, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, sCr serum creatinine, SD standard deviation, SpO2 peripheral oxygen saturation (by pulse oximeter), SpO2/FiO2 pulse oximetry oxygen saturation/fractional inspired oxygen, SF-36 36-Item Short Form Survey for Quality of Life, SOFA sequential organ failure assessment score, TBIL total bilirubin, TNF tumor necrosis factor, VCmax maximum forced vital capacity, WBC white blood cells, WHO World Health Organization, wk week(s), y year(s)
The most frequent source of MSCs was the umbilical cord tissue [70–76, 80–83, 85–87], followed by the menstrual blood [78, 84, 89] and bone marrow [83, 88] (Table 4). In one study the MSC source was adipose tissue [79] (Table 4). In the report of another study [69] the MSC origin was not mentioned (Table 4). Seven reports [77, 79, 80, 83, 85, 87, 89] lacked the required description of the surface markers and multilineage differentiation ability of MSCs [91], and the only report of a study using extracellular vesicles of the exosome subtype as investigational therapy [83] did not contain the required minimal information about the isolation, analysis, and quantification of the MSC-derived product [92]. The report of the study using the secretome of menstrual-blood-derived MSCs as investigational treatment [84] lacked information about the composition of the MSC-conditioned medium, which likely contained a mix of extracellular vesicles and soluble factors [93]. The putative immunomodulatory /immunosuppressive properties of the administered MSCs were confirmed by mixed lymphocyte reaction assay only in two studies [81, 88], employing umbilical-cord- and bone-marrow-derived MSCs, respectively (Table 4). The susceptibility to virus infection of the MSCs to be injected into the circulation of an infected host was tested in a minority of the studies, although infected MSCs may have reduced survival and function as a virus reservoir in the body of the treated patients. The expression of the main cell entry receptor for SARS-CoV-2, the angiotensin-converting enzyme 2 (ACE2), by the infused MSCs was only tested in 2 out of 20 studies concerning COVID-19 [69, 78], but the results were reassuring because cells from menstrual blood [78] and those from an unreported origin [69] were marginally ACE2-positive [69] or ACE2-negative [78] (Table 4). The MSCs from an unreported origin [69] also did not express a protease involved in viral cell entry, the transmembrane serine protease type 2 (TMPRSS2). These results would be in keeping with the in vitro observations that human MSCs from fetal and adult tissues are indeed ACE2- and TMPRSS2-negative and may not be permissive to SARS-CoV-2 [94], but diverse studies have uncovered new mechanisms of viral entry into human host cells [95–99], and these data should be taken into account in clinical trials testing the therapeutic potential of MSCs in COVID-19. The previously demonstrated susceptibility of human MSCs of bone marrow and cord blood origin to infection with avian influenza A H5N1 virus [100] was not excluded in the only study evaluating the potential therapeutic effectiveness of MSCs in H7N9-influenza-induced ARDS [89]. Thus, the possibility that the various MSC populations infused in the studies reviewed here were infected by the hosted viruses, once injected, cannot be excluded. Nonetheless, the viral load over time was not significantly affected by MSC treatment in the randomized, double-blind, placebo-controlled trials that properly evaluated this outcome in COVID-19-associated ARDS [74, 82].
The studies testing MSCs in COVID-19 differed greatly in terms of patient selection, MSC dosage, and infusion schedule (Tables 3 and 4). The outcome measures and follow-up periods also varied greatly (Tables 3, 6), precluding the possibility to perform meaningful meta-analyses, as previously recognized by the authors of recent systematic reviews not specifically focusing on the assessment of MSCs and MSC-derived products as add-on therapy to the currently recommended standard of care [101–104]. Considering the safety outcomes, most reports did not contain complete and convincing information about the incidence of serious and non-serious adverse events and their relation to treatment (Table 5). For example, in the prospective single-arm study on severe ARDS not responding to standard treatment [73] and in the prospective single-arm study on moderate pneumonitis [87], there was a transient increase in the circulating levels of the fibrin degradation product D-dimer after MSC infusions (Table 6), which was not reported as a treatment-related adverse event although it may reflect the effects of the procoagulant activity of tissue factor (TF)/CD142-expressing MSCs from umbilical cord and placenta [105] in patients who already have an increased risk of thrombosis because of the infection. In one of these studies [87], the observed transient increase in the levels of the proinflammatory chemokine IL-6 following MSC infusion (Table 6) was also not reported as an adverse event. It is, however, reassuring to know that in the controlled clinical trial with an open-label follow-up period of up to 1 year [71, 72], patients with less severe disease who had received three doses of viable 4 × 107 umbilical-cord-derived MSCs/kg of body weight (Table 4) did not show significant reductions in the inhibition rate of neutralizing antibodies against SARS-CoV-2, changes in pulmonary function, alterations of laboratory parameters, or evidence of tumor developments in comparison with the controls (Table 6).
In terms of concomitant treatment, some investigators included drugs that were empirically used during the initial phases of the COVID-19 pandemic and that are no longer recommended by current guidelines [35, 90], such as hydroxychloroquine and various antivirals used for other infections (Table 3). Nonetheless, in most studies patients received glucocorticoids (Table 3), the current standard of care in severe and critical COVID-19 [35, 90], albeit with substantial differences in terms of glucocorticoid type, dosage, and duration of treatment. Three of these studies were randomized, double-blind, placebo-controlled trials of umbilical-cord-derived MSCs in ARDS [74, 81, 82], and only one [74] demonstrated significantly higher survival rate by day 28 and shorter time to recovery in the group of patients who received MSCs as investigational add-on therapy than in the control group (Table 6). Possible explanations for the discrepancy were MSC dosing (Table 4) and imbalance of the patients’ condition at baseline (Table 3). In the study by Lanzoni and colleagues [74], the mean number of infused cells at each infusion and in total was much higher than in the other two trials, and the cell viability was comparable to that detected in the negative study by Rebellato and colleagues [82], despite the use of thawed cells from frozen cell samples (Table 4). In the same study by Lanzoni and colleagues [74], only 4 of 12 patients (33.33%) in the MSC-treated group versus 7 of 12 patients (58.33%) in the placebo group received IMV, and the baseline levels of IL-6 were significantly higher in the control group (Table 3), suggesting that control patients were at higher risk of death than MSC-treated patients. In the study by Rebelatto and colleagues [82], in which all MSC-treated and placebo-treated patients required IMV in the ICU and received glucocorticoids and anticoagulants as concomitant therapy, the mortality rate was even higher in the group of MSC-treated patients (45.45%) than in the group of patients who received placebo (16.66%), and the MSC-treated patients also showed increased levels of the cardiac troponin I and of creatinine, suggesting further cardiac damage and renal insufficiency between day 4 and day 14 post-treatment (three infusions of 5 × 105 cells/kg of body weight every other day, starting 10.7 days, on average, after symptom onset). In the study by Monsel and colleagues [81], low level of alloimmunization developed in 3 of 21 patients (14.3%) on day 14 post-infusion but an additional 6 of the 21 MSC recipients (28.6%) already had pre-formed antibodies against the human leukocyte antigen of the infused cells before treatment. In this study, antibody-mediated loss of functional cells may contribute to explain the lack of significant effects of the investigational therapy on most efficacy outcome measures, except for a significant decrease of plasma inflammatory markers at day 14 post-infusion (Table 6), but the relative impact cannot be estimated because none of the other studies reviewed here reported data concerning possible alloimmunization.
In another randomized, double-blind, placebo-controlled trial of umbilical-cord-derived MSCs in critical COVID-19 [75], there was a significantly higher survival rate in patients treated with MSCs than in the controls (2.5 times higher overall and 4.5 times higher in patients with more than two comorbidities known to worsen disease outcome), which was associated with a significant decrease in the circulating levels of IL-6 (Table 6), but the concomitant treatment only included oseltamivir and azithromycin (Table 3), drugs not considered as effective in severe or critical COVID-19 [35, 90], and there were no significant differences between groups in terms of length of stay in the ICU and ventilator usage (Table 6). Thus, MSC treatment significantly improved survival in critical COVID-19, but this effect is known to be achievable in similar patients with the very less expensive and easier to use glucocorticoids [35, 90].
Taking into account the putative immunomodulatory/immunosuppressive properties of MSCs, it is worth noting that in most placebo-controlled studies involving severely and critically ill patients with COVID-19, investigational therapy was found to significantly reduce the levels of inflammatory markers in comparison with placebo (Table 6). This was not the case for studies involving patients with less severe COVID-19, such as the controlled clinical trial with 1-year follow-up mentioned above [71, 72], in which the administration of MSCs as add-on therapy resulted in significant radiological improvements not accompanied by significant functional or laboratory changes by day 28 (Table 6).
Considering the other comparative trials testing MSCs as investigational add-on therapy in COVID-19 [69, 70, 76–78, 85, 86, 88], significantly higher survival rates in the treated groups than in the control groups were reported in three of the eight studies [77, 78, 88], and the increase in survival rates was more pronounced in critically ill patients than in patients with severe disease (Table 6). In one of these studies [77], which was a randomized open-label parallel-group trial, the length of stay in ICU was also significantly lower in the MSC-treated group than in the control group of critically ill patients. In another randomized, single-blind, placebo-controlled study involving patients with mild, severe, and critical disease [85], the duration of hospital stays (primary endpoint) was significantly shorter in the MSC-treated group than in the placebo group (Table 6). For the patients with severe and critical COVID-19 enrolled in the comparative trials cited above, MSC treatment was associated with significant decreases in the levels of circulating inflammatory markers and/or coagulation markers, and with significantly shorter time to normalization of the total lymphocyte counts in MSC-treated patients than in the controls, although these improvements in laboratory parameters did not consistently translate into reduced mortality and reduced use of IMV (Table 6), possibly because of the low number of enrolled patients. Promising preliminary results were provided by Grégoire and colleagues [88], with the initial analysis of data from the only still ongoing study testing bone-marrow-derived MSCs as add-on investigational therapy in severe ARDS (Tables 3, 4). The concomitant treatment with glucocorticoids and anticoagulants was in keeping with current guidelines [35, 90] and efficacy outcome measures were in accordance with those proposed by the World Health Organization (WHO) for COVID-19 clinical research, including the use of the WHO Clinical Progression Scale to evaluate patient trajectory over the course of disease [106]. MSC-treated patients (n = 8) required high-flow oxygen therapy (n = 7) or IMV (n = 1) within 24 h of ICU admission and received three infusions of 1.5–3 × 106 clinical-grade cells/kg body weight at an average interval of 3 days starting within 2 days of ICU admission. Matched controls (n = 24) were only retrospectively selected among the patients admitted to the ICU in the same hospital, the major limitation of this study. Although a progression of disease severity was initially observed in two of the seven patients after MSC infusion, survival rate was significantly higher in the MSC-treated group than in the matched control group at day 28 (100% versus 79.2%) and at day 60 (100% versus 70.8%) (Table 6). The risk of thrombosis was also significantly reduced by the investigational treatment, as indicated by the levels of the fibrin degradation product D-dimer, which were much lower in this group than in the matched control group by day 7 (Table 6).
The two studies testing MSC-derived products in severe COVID-19 [83, 84] differed greatly in terms of study design, source of MSCs, type of MSC-derived products, and concomitant treatments. In the randomized, double-blind placebo-controlled trial testing the conditioned medium from menstrual-blood-derived MSCs as investigational therapy in severe disease [84], concomitant treatment agreed with current guidelines [35, 90] and the investigational therapy was an effective add-on as it significantly reduced mortality at day 28 and the need for intubation in the treated group in comparison with the control group (Table 6). Treatment was not apparently associated with the occurrence of adverse events, but the adverse event reporting was incomplete, and one patient discontinued after first dose for unknown causes (Table 5). The second study [83] was a prospective uncontrolled cohort study testing a single intravenous infusion of exosomes from bone-marrow-derived MSCs in patients with mild, moderate, or severe COVID-19-associated ARDS, who showed clinical deterioration for more than 72 h on treatment with the institutional standard treatment, consisting in oxygen support and administration of hydroxychloroquine and azithromycin, two drugs not recommended for the management of similar patients by current guidelines [35, 90]. During the follow-up period of 14 days, the overall survival rate was 83%. The recovery rate in the cohort of 20 patients not requiring IMV at baseline was 75%, but the other patients worsened to the point of requiring IMV. In the cohort of patients with severe ARDS at baseline, all three patients remained critically ill, still requiring IMV (Table 6). Overall, a single exosome infusion was associated with a significant improvement of laboratory data, including the markers of inflammation and markers of thrombosis, such as D-dimer (Table 6). The occurrence of treatment-related adverse events was specifically evaluated over a period of 72 h after exosome administration and no adverse events were reported. The cases of pulmonary embolism, acute renal failure, worsening of hypoxic respiratory failure requiring intubation, and four deaths, all occurring at post-treatment days 4–13, were not reported as related to the exosome infusion (Table 5). Thus, investigational therapy was apparently safe and effective at improving the outcome of patients with mild or moderate COVID-19-associated ARDS in comparison with the outcome reported in literature for similar patients [107], even though a large fraction of intravenously injected exosomes is immediately taken up by the mononuclear phagocytes of the reticuloendothelial system in the liver and spleen [108]. However, better outcomes have been reported in clinical trials, for all levels of ARDS severity, with the use of glucocorticoids [107], the current standard of care in severe and critical COVID-19 [35, 90], and the addition of Janus kinase inhibitors and monoclonal antibodies against IL-6R, if required [35, 90].
Concerning the nonrandomized, open-label, parallel-group study that tested the therapeutic potential of MSCs from menstrual blood when added to standard care in H7N9-influenza-virus-induced ARDS [89] (Table 3), investigational therapy consisted in the intravenous administration of 1 × 106 90–95% viable MSCs 3–4 times (Table 4), depending on patients’ consent. The group of MSC-treated patients had a significant increase in survival rate and more marked improvement of the inflammatory parameters and D-dimer at discharge than the control group that received standard care alone (Table 6). In addition to the supportive therapy for critically ill patients with multiorgan dysfunction, the concomitant treatment in the MSC-treated group and in the control group included the antivirals recommended by international guidelines (oseltamivir or peramivir) [19] in all patients. Over 50% of them in both study arms also received glucocorticoids, which are not recommended for the treatment of severe influenza because of possible detrimental effects on the outcome [19]. However, the mortality rate in the control group (54.5%) was only slightly higher than that reported in literature [107] for similar patients with severe ARDS [107]. The more than threefold lower mortality rate (17.6%) in the MSC-treated group is impressive but may be explained at least in part by the lower proportion of critically ill patients with severe renal injury at study entry in this group in comparison with the control group (Tables 3 and 6). There was no report of adverse events related to the MSC infusions (Table 5). A 5-year follow-up period was limited to four survivors in the MSC-treated arm and no harmful effects of the MSC transplantation were observed in these subjects (Table 6).
Conclusions
The results of published clinical studies on the therapeutic potential of MSCs and MSC-derived products in COVID-19 and influenza suggest that MSCs and MSC-derived products may significantly increase the survival of hospitalized patients with severe and critical disease and that this beneficial effect may be related to the putative immunomodulatory/immunosuppressive properties of MSCs and their secretome. However, in COVID-19-associated ARDS, similar or better outcomes have been reported in clinical trials with glucocorticoids alone [107], the current standard of care in severe and critical COVID-19 [35, 90], with further improvement attainable with the addition of Janus kinase inhibitors and monoclonal antibodies against IL-6R if required to block disease progression according to current guidelines [90, 107]. The studies reviewed here have not consistently demonstrated that adding MSCs or MSC-derived products to this currently recommended therapeutic regimen can reduce the use of IMV and the risk of death in patients still showing rapidly increasing oxygen demand and systemic inflammation despite appropriate therapeutic management. Overall, increased survival rates were observed in 5 of 12 prospective comparative trials that tested MSCs or MSC-derived secretome as add-on therapy in severe and critical COVID-19. Four of these trials were randomized, double-blind, and placebo controlled. The standard of care included glucocorticoids and anticoagulants in all these trials and remdesivir, glucocorticoids, monoclonal antibodies against IL-6R, and anticoagulants in some recent studies, but the proportion of patients receiving these therapeutics in each study varied greatly across studies, and most reports do not contain information about dosing and duration of treatment. The results of the only available study about the use of MSCs as add-on therapy for influenza-associated ARDS are promising, particularly because no immunomodulator/antiinflammatory treatment is currently recommended for severe and critical disease, but the study is a small open-label trial with imbalances between the arms, which may contribute to explaining the superior efficacy outcome attributed to the MSC infusion. The positive results reported in this study and in 5 of the 12 comparative studies that evaluated the therapeutic added values of MSCs and MSC-derived products in severe and critical COVID-19 must be confirmed in controlled clinical trials conducted in compliance with the current Good Manufacturing Practice and Good Clinical Practice guidelines. Compliance with these guidelines is a condition for the generation of data that can be submitted to the regulatory authorities when seeking the mandatory authorization for the use of a therapeutic candidate outside the setting of a clinical investigation [109].
Implications for Future Research
To comply with the current international guidelines for the clinical development of cell and cell-based therapies, several issues need to be addressed in the design and conduction of future clinical trials and in the reporting of the clinical data. Possible solutions for improving the clinical development plan are proposed in tabular form (Table 7). The key issue is how to choose the starting time of the MSC-based add-on therapy. This challenge is difficult to overcome because the studies reviewed here suggest that blood biomarkers of ongoing inflammation recognized thus far are not consistently predictive of a response to MSCs and MSC-derived secretome when the recommended therapeutic regimen is insufficient to block disease progression. It may be useful to evaluate one of the most recently identified specific biomarkers of prolonged inflammation and dysregulated immune activation, the elevated level of plasma soluble urokinase plasminogen activator receptor, which has been found to predict disease progression in hospitalized patients with COVID-19 and other viral pneumonia more accurately than other inflammatory parameters [109–111]. Alternatively, or concomitantly, a composite score such as the COVID-19-associated Hyperinflammation Syndrome score [112, 113] may be introduced to evaluate the risk of further disease progression in critically ill patients already receiving the recommended immunomodulatory/antiinflammatory regimen (Table 7), although this score system still needs to be fully validated.
Table 7.
Proposed solutions for the improvement of a development plan aiming for regulatory approval
| Challenge | Solution |
|---|---|
| MSC and MSC-derived secretome |
MSC obtained and characterized according to the criteria of the International Society of Cell & Gene Therapy [91]. Tissue factor and hemocompatibility assessment [105] to be included for the evaluation of the product suitability for intravenous use MSC-derived extracellular vesicles isolated according to the guidelines of the International Society for Extracellular Vesicles [92] MSC-derived conditioned medium characterized for the presence of extracellular vesicles and for the contents of soluble biologically active factors [92, 93]. Optimization of formulation and manufacturing [120] Scalable production according to the current Good Clinical Manufacturing guidelines Evaluation of the potency of the product (e.g., immunomodulatory/immunosuppressive properties assessed by mixed lymphocyte reaction) Dose estimated on the basis of preclinical studies or previous pilot clinical studies |
| Characteristic of the clinical trials |
Prospective, randomized, double-blind, or open-label placebo-controlled two-arm studies in hospitalized patients with severe and critical COVID-19 and influenza Concomitant therapies with antivirals, glucocorticoids, and other immunomodulators and antiinflammatory agents according to current guidelines Sample size calculated to detect significant differences for the selected primary efficacy outcome |
| Functional and biochemical parameters indicating the need for add-on therapy |
Patients showing rapidly increasing oxygen needs and systemic inflammation despite treatment with antivirals, immunomodulators, and antiinflammatory agents according to current guidelines Use of the Hyperinflammation Syndrome score at enrollment [113, 114] Recording and monitoring of C-reactive protein, D-dimers, interleukin-6, serum ferritin concentrations, soluble urokinase plasminogen activator receptor, and leukocyte counts [106, 110–112] |
| Main outcome measures |
All-cause mortality at day 28 and at hospital discharge Clinical progression assessed daily by using the WHO Clinical Progression Scale [106] |
| Secondary outcomes |
Length of stay in ICU Need for intubation Length of stay in the hospital Changes in biochemical parameters (C-reactive protein, D-dimers, interleukin-6, serum ferritin concentrations, soluble urokinase plasminogen activator receptor, leukocyte counts) Organ dysfunction score Pulmonary function at 1, 6, 12 months Radiological findings Tolerability and adverse events Viral burden assessed by quantitative real-time polymerase chain reaction |
| Clinical data recording and reporting | According to the Good Clinical practice guidelines |
| Data for economic analysis | Recording of costs and resource use |
COVID-19 coronavirus disease 2019, ICU intensive care unit, MSC mesenchymal stem/stromal cell, WHO World Health Organization
Finally, it should be considered that the high costs of MSC-based therapy would still represent an obstacle to its clinical acceptance [114], even if its effectiveness at reducing the healthcare expenditures associated with the prolonged hospitalizations of critically ill patients were conclusively demonstrated. The costs of obtaining clinical-grade allogeneic MSCs varies depending on the MSC source [114], and the use of cell-free MSC-derived products, such as exosomes and other extracellular vesicles, can greatly increase these costs. The use of properly isolated MSC-derived exosomes would render the costs of the MSC-based therapy particularly high because of the expensive isolation procedure and the necessity to produce a huge number of clinical-grade vesicles to overcome problems with the biodistribution of these nanoparticles after intravenous infusion [108, 115]. One of the possible solutions may be to avoid their systemic administration [116], but the first published small single-arm study on the investigational use of repeated doses of aerosolized MSC-derived extracellular vesicles in severe COVID-19 [117] has not provided favorable results in terms of efficacy outcome, and the total dosage of extracellular vesicles (2 × 108 nanoparticles per day for 5 consecutive days) is higher than that used for intravenous administration (6 × 108) [83, 118].
Another possible solution would be the systemic administration of exosomes that have been engineered to escape phagocytosis and/or to specifically target the lungs [119], but these manipulations would likely affect some of their desired biological activities against viral respiratory tract infections and would require a lot of preclinical research work before first investigational use in humans, without reducing or even increasing the costs of the final product for the indication discussed here. Thus, the decision to go ahead with a rigorous clinical development of an MSC-based therapy as add-on therapy for the treatment of severe and critical COVID-19, influenza, and other severe viral respiratory infections should also be based on pharmacoeconomic considerations, and studies with an adequate evaluation of the cost-effectiveness of these investigational therapies are highly demanded (Table 7).
Acknowledgements
Funding
No external funding or sponsorship was received for this review or publication of this article. The journal’s Rapid Service fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given approval for this version to be published.
Author Contributions
Sabrina Mattoli conceptualized the review paper. Matthias Schmidt performed the literature search. Both authors independently assessed the retrieved articles and agreed on the final evaluation. Sabrina Mattoli wrote the original draft. Matthias Schmidt critically revised the work. Both authors read and approved the final manuscript.
Disclosures
Sabrina Mattoli is consultant to the European Commission and to the Eureka Association and is shareholder in Novartis Pharma AG and Pfizer Inc. Matthias Schmidt is named as co-inventor on a submitted patent application concerning the generation of engineered exosomes for targeted delivery of biologics to the lungs.
Compliance with Ethics Guidelines
This review was based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article.
Contributor Information
Sabrina Mattoli, Email: smattoli@avail-bri.com, Email: smattoli@inavail.org.
Matthias Schmidt, Email: mschmidt@avail-bri.com, Email: mschmidt@avail-research.com.
References
- 1.Clementi N, Ghosh S, De Santis M, et al. Viral respiratory pathogens and lung injury. Clin Microbiol Rev. 2021;34(3):e00103–e120. doi: 10.1128/CMR.00103-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan M, Adil SF, Alkhathlan HZ, et al. COVID-19: a global challenge with old history, epidemiology and progress so far. Molecules. 2021;26(1):39. doi: 10.3390/molecules26010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.V’kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19(3):155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iuliano AD, Roguski KM, Chang HH, Global Seasonal Influenza-associated Mortality Collaborator Network et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391(10127):1285–1300. doi: 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.To KK, Sridhar S, Chiu KH, et al. Lessons learned 1 year after SARS-Cov-2 emergence leading to COVID-19 pandemic. Emerg Microbes Infect. 2021;10(1):507–535. doi: 10.1080/22221751.2021.1898291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johns Hopkins University and Medicine. Johns Hopkins Coronavirus Resource Center. Updated 22 September. https://coronavirus.jhu.edu/map.html. Accessed 22 Sept 2022.
- 7.Han E, Tan MMJ, Turk E, et al. Lessons learnt from easing COVID-19 restrictions: an analysis of countries and regions in Asia Pacific and Europe. Lancet. 2020;396(10261):1525–1534. doi: 10.1016/S0140-6736(20)32007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicola M, Alsafi Z, Sohrabi C, et al. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg. 2020;78:185–193. doi: 10.1016/j.ijsu.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattoli S. Filling the gap until full vaccine deployment in the war on coronavirus disease-19. Infect Dis Ther. 2021;10(1):27–34. doi: 10.1007/s40121-020-00394-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fauci AS. The story behind COVID-19 vaccines. Science. 2021;372(6538):109. doi: 10.1126/science.abi8397. [DOI] [PubMed] [Google Scholar]
- 11.Ledford H. Six months of COVID vaccines: what 1.7 billion doses have taught scientists. Nature. 2021;594:164–167. doi: 10.1038/d41586-021-01505-x. [DOI] [PubMed] [Google Scholar]
- 12.Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, evaluation, and treatment of coronavirus (COVID-19). 2022 Jun 30. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. [PubMed]
- 13.Short KR, Kroeze EJBV, Fouchier RAM, Kuiken T. Pathogenesis of influenza-induced acute respiratory distress syndrome. Lancet Infect Dis. 2014;14(1):57–69. doi: 10.1016/S1473-3099(13)70286-X. [DOI] [PubMed] [Google Scholar]
- 14.Tzotzos SJ, Fischer B, Fischer H, Zeitlinger M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey. Crit Care. 2020;24(1):516. doi: 10.1186/s13054-020-03240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cobb NL, Sathe NA, Duan KI, et al. Comparison of clinical features and outcomes in critically ill patients hospitalized with COVID-19 versus influenza. Ann Am Thorac Soc. 2021;18(4):632–640. doi: 10.1513/AnnalsATS.202007-805OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swets MC, Russell CD, Harrison EM, et al. SARS-CoV-2 co-infection with influenza viruses, respiratory syncytial virus, or adenoviruses. Lancet. 2022;399(10334):1463–1464. doi: 10.1016/S0140-6736(22)00383-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh DY, Buda S, Biere B, et al. Trends in respiratory virus circulation following COVID-19-targeted nonpharmaceutical interventions in Germany, January–September 2020: analysis of national surveillance data. Lancet Reg Health Eur. 2021;6:100112. doi: 10.1016/j.lanepe.2021.100112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsen SJ, Winn AK, Budd AP, et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic—United States, 2020–2021. MMWR Morb Mortal Wkly Rep. 2021;70(29):1013–1019. doi: 10.15585/mmwr.mm7029a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis. 2019;68(6):e1–47. 10.1093/cid/ciy866 [Erratum in: Clin Infect Dis. 2019 May 2;68(10):1790. 10.1093/cid/ciz044]. [DOI] [PMC free article] [PubMed]
- 20.Darwish I, Mubareka S, Liles WC. Immunomodulatory therapy for severe influenza. Expert Rev Anti Infect Ther. 2011;9(7):807–822. doi: 10.1586/eri.11.56. [DOI] [PubMed] [Google Scholar]
- 21.Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. 2020;383:2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Del Valle DM, Kim-Schulze S, Huang HH, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laing AG, Lorenc A, Del Molino Del Barrio I, et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med. 2020;26(10):1623–35. 10.1038/s41591-020-1038-6 [Erratum in: Nat Med. 2020 Oct; 26(10):1663. 10.1038/s41591-020-1079-x. Erratum in: Nat Med. 2020 Dec;26(12):1951. 10.1038/s41591-020-01186-5]. [DOI] [PubMed]
- 24.Lucas C, Wong P, Klein J, et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584(7821):463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reynolds D, Vazquez Guillamet C, Day A, et al. Comprehensive immunologic evaluation of bronchoalveolar lavage samples from human patients with moderate and severe seasonal influenza and severe COVID-19. J Immunol. 2021;4(207):1229–1238. doi: 10.4049/jimmunol.2100294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stals MAM, Grootenboers MJJH, van Guldener C, Dutch COVID & Thrombosis Coalition (DCTC) et al. Risk of thrombotic complications in influenza versus COVID-19 hospitalized patients. Res Pract Thromb Haemost. 2021;5(3):412–420. doi: 10.1002/rth2.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chowdary P. COVID-19 coagulopathy—what should we treat? Exp Physiol. 2022;107(7):749–758. doi: 10.1113/EP089404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization (WHO) Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA. 2021;326(6):499–518. 10.1001/jama.2021.11330. [DOI] [PMC free article] [PubMed]
- 30.Ely EW, Ramanan AV, Kartman CE, et al., COV-BARRIER Study Group. Efficacy and safety of baricitinib plus standard of care for the treatment of critically ill hospitalised adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: an exploratory, randomised, placebo-controlled trial. Lancet Respir Med. 2022;10(4):327–36. 10.1016/S2213-2600(22)00006-6 [Erratum in: Lancet Respir Med. 2022 Feb 11. 10.1016/S2213-2600(22)00057-1]. [DOI] [PMC free article] [PubMed]
- 31.Meyer NJ, Gattinoni L, Calfee CS. Acute respiratory distress syndrome. Lancet. 2021;398:622–637. doi: 10.1016/S0140-6736(21)00439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lambermont B, Rousseau AF, Seidel L, et al. Outcome improvement between the first two waves of the coronavirus disease 2019 pandemic in a single tertiary-care hospital in Belgium. Crit Care Explor. 2021;3(5):e0438. doi: 10.1097/CCE.0000000000000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Q, Zhou YH, Yang ZQ. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell Mol Immunol. 2016;13(1):3–10. doi: 10.1038/cmi.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hui DS, Lee N, Chan PK, Beigel JH. The role of adjuvant immunomodulatory agents for treatment of severe influenza. Antiviral Res. 2018;150:202–216. doi: 10.1016/j.antiviral.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.COVID-19 treatment guidelines panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/. Accessed 12 Feb 2023. [PubMed]
- 36.Khoury M, Cuenca J, Cruz FF, Figueroa FE, Rocco PRM, Weiss DJ. Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. Eur Respir J. 2020;55(6):2000858. doi: 10.1183/13993003.00858-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qu W, Wang Z, Hare JM, et al. Cell-based therapy to reduce mortality from COVID-19: systematic review and meta-analysis of human studies on acute respiratory distress syndrome. Stem Cells Transl Med. 2020;9(9):1007–1022. doi: 10.1002/sctm.20-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorman E, Millar J, McAuley D, O'Kane C. Mesenchymal stromal cells for acute respiratory distress syndrome (ARDS), sepsis, and COVID-19 infection: optimizing the therapeutic potential. Expert Rev Respir Med. 2021;15(3):301–324. doi: 10.1080/17476348.2021.1848555. [DOI] [PubMed] [Google Scholar]
- 39.Spinetti G, Avolio E, Madeddu P. Treatment of COVID-19 by stage: any space left for mesenchymal stem cell therapy? Regen Med. 2021;16(5):477–494. doi: 10.2217/rme-2020-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao W, Shi L, Zhang Y, Dong H, Zhang Y. Mesenchymal stem/stromal cell therapy for COVID-19 pneumonia: potential mechanisms, current clinical evidence, and future perspectives. Stem Cell Res Ther. 2022;13(1):124. doi: 10.1186/s13287-022-02810-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gotts JE, Matthay MA. Mesenchymal stem cells and acute lung injury. Crit Care Clin. 2011;27(3):719–733. doi: 10.1016/j.ccc.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barczyk M, Schmidt M, Mattoli S. Stem cell-based therapy in idiopathic pulmonary fibrosis. Stem Cell Rev Rep. 2015;11(4):598–620. doi: 10.1007/s12015-015-9587-7. [DOI] [PubMed] [Google Scholar]
- 43.Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;2(4):22. doi: 10.1038/s41536-019-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chow L, Johnson V, Impastato R, Coy J, Strumpf A, Dow S. Antibacterial activity of human mesenchymal stem cells mediated directly by constitutively secreted factors and indirectly by activation of innate immune effector cells. Stem Cells Transl Med. 2020;9(2):235–249. doi: 10.1002/sctm.19-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiong J, Chen L, Zhang L, Bao L, Shi Y. Mesenchymal stromal cell-based therapy: a promising approach for severe COVID-19. Cell Transplant. 2021;30:963689721995455. doi: 10.1177/0963689721995455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samarelli AV, Tonelli R, Heijink I, et al. Dissecting the role of mesenchymal stem cells in idiopathic pulmonary fibrosis: cause or solution. Front Pharmacol. 2021;12:692551. doi: 10.3389/fphar.2021.692551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samsonraj RM, Raghunath M, Nurcombe V, Hui JH, van Wijnen AJ, Cool SM. Concise review: multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl Med. 2017;6(12):2173–2185. doi: 10.1002/sctm.17-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fitzsimmons REB, Mazurek MS, Soos A, Simmons CA. Mesenchymal stromal/stem cells in regenerative medicine and tissue engineering. Stem Cells Int. 2018;2018:8031718. doi: 10.1155/2018/8031718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheung TS, Bertolino GM, Giacomini C, Bornhäuser M, Dazzi F, Galleu A. Mesenchymal stromal cells for graft versus host disease: mechanism-based biomarkers. Front Immunol. 2020;25(11):1338. doi: 10.3389/fimmu.2020.01338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ryan JM, Barry F, Murphy JM, Mahon BP. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. 2007;149(2):353–363. doi: 10.1111/j.1365-2249.2007.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krampera M, Cosmi L, Angeli R, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24(2):386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 52.Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2(2):141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 53.Polchert D, Sobinsky J, Douglas G, et al. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol. 2008;38(6):1745–1755. doi: 10.1002/eji.200738129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Müller L, Tunger A, Wobus M, et al. Immunomodulatory properties of mesenchymal stromal cells: an update. Front Cell Dev Biol. 2021;9:637725. doi: 10.3389/fcell.2021.637725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pang SHM, D'Rozario J, Mendonca S, et al. Mesenchymal stromal cell apoptosis is required for their therapeutic function. Nat Commun. 2021;12(1):6495. doi: 10.1038/s41467-021-26834-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weiss DJ, English K, Krasnodembskaya A, Isaza-Correa JM, Hawthorne IJ, Mahon BP. The neurobiology of mesenchymal stromal cells affects therapeutic efficacy. Front Immunol. 2019;4(10):1228. doi: 10.3389/fimmu.2019.01228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Masterson CH, Tabuchi A, Hogan G, et al. Intra-vital imaging of mesenchymal stromal cell kinetics in the pulmonary vasculature during infection. Sci Rep. 2021;11(1):5265. doi: 10.1038/s41598-021-83894-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Y, Xu J, Shi W, et al. Mesenchymal stromal cell treatment prevents H9N2 avian influenza virus-induced acute lung injury in mice. Stem Cell Res Ther. 2016;7:159. doi: 10.1186/s13287-016-0395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chan MC, Kuok DI, Leung CY, et al. Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc Natl Acad Sci USA. 2016;113:3621–3626. doi: 10.1073/pnas.1601911113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khatri M, Richardson LA, Meulia T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res Ther. 2018;9:17. doi: 10.1186/s13287-018-0774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Loy H, Kuok DIT, Hui KPY, et al. Therapeutic implications of human umbilical cord mesenchymal stromal cells in attenuating influenza A (H5N1) virus-associated acute lung injury. J Infect Dis. 2019;219:186–196. doi: 10.1093/infdis/jiy478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dutra Silva J, Su Y, Calfee CS, et al. Mesenchymal stromal cell extracellular vesicles rescue mitochondrial dysfunction and improve barrier integrity in clinically relevant models of ARDS. Eur Respir J. 2021;58(1):2002978. doi: 10.1183/13993003.02978-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu X, Dao Thi VL, Huang Y, et al. Intrinsic immunity shapes viral resistance of stem cells. Cell. 2018;172(3):423–438.e25. doi: 10.1183/13993003.02978-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sharma A. Inferring molecular mechanisms of dexamethasone therapy in severe COVID-19 from existing transcriptomic data. Gene. 2021;788:145665. doi: 10.1016/j.gene.2021.145665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McInnes IB, Byers NL, Higgs RE, et al. Comparison of baricitinib, upadacitinib, and tofacitinib mediated regulation of cytokine signaling in human leukocyte subpopulations. Arthritis Res Ther. 2019;21(1):183. doi: 10.1186/s13075-019-1964-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kebriaei P, Isola L, Bahceci E, et al. Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15(7):804–811. doi: 10.1016/j.bbmt.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 68.Kurtzberg J, Prockop S, Teira P, et al. Allogeneic human mesenchymal stem cell therapy (remestemcel-L, Prochymal) as a rescue agent for severe refractory acute graft-versus-host disease in pediatric patients. Biol Blood Marrow Transplant. 2014;20(2):229–235. doi: 10.1016/j.bbmt.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 69.Leng Z, Zhu R, Hou W, et al. Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meng F, Xu R, Wang S, et al. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduct Target Ther. 2020;5(1):172. doi: 10.1038/s41392-020-00286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shi L, Huang H, Lu X, et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct Target Ther. 2021;6(1):58. doi: 10.1038/s41392-021-00488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shi L, Yuan X, Yao W, et al Human mesenchymal stem cells treatment for severe COVID-19: 1-year follow-up results of a randomized, double-blind, placebo-controlled trial. EBioMedicine. 2022;75:103789. 10.1016/j.ebiom.2021.103789. [DOI] [PMC free article] [PubMed]
- 73.Iglesias M, Butrón P, Torre-Villalvazo I, et al. Mesenchymal stem cells for the compassionate treatment of severe acute respiratory distress syndrome due to COVID 19. Aging Dis. 2021;12(2):360–370. doi: 10.14336/AD.2020.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lanzoni G, Linetsky E, Correa D, et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med. 2021;10(5):660–673. doi: 10.1002/sctm.20-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dilogo IH, Aditianingsih D, Sugiarto A, et al. Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: a randomized controlled trial. Stem Cells Transl Med. 2021;10:1279–1287. doi: 10.1002/sctm.21-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shu L, Niu C, Li R, et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11(1):361. doi: 10.1186/s13287-020-01875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Adas G, Cukurova Z, Yasar KK, et al. The systematic effect of mesenchymal stem cell therapy in critical covid-19 patients: a prospective double controlled trial. Cell Transplant. 2021;30:9636897211024942. doi: 10.1177/09636897211024942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu X, Jiang W, Chen L, et al. Evaluation of the safety and efficacy of using human menstrual blood-derived mesenchymal stromal cells in treating severe and critically ill COVID-19 patients: an exploratory clinical trial. Clin Transl Med. 2021;11(2):e297. doi: 10.1002/ctm2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sánchez-Guijo F, García-Arranz M, López-Parra M, et al. Adipose-derived mesenchymal stromal cells for the treatment of patients with severe SARS-CoV-2 pneumonia requiring mechanical ventilation. A proof of concept study. EClinicalMedicine. 2020;25:100454. doi: 10.1016/j.eclinm.2020.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo Z, Chen Y, Luo X, He X, Zhang Y, Wang J. Administration of umbilical cord mesenchymal stem cells in patients with severe COVID-19 pneumonia. Crit Care. 2020;24(1):420. doi: 10.1186/s13054-020-03142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Monsel A, Hauw-Berlemont C, Mebarki M, APHP STROMA–CoV-2 Collaborative Research Group et al. Treatment of COVID-19-associated ARDS with mesenchymal stromal cells: a multicenter randomized double-blind trial. Crit Care. 2022;26(1):48. doi: 10.1186/s13054-022-03930-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rebelatto CLK, Senegaglia AC, Franck CL, et al. Safety and long-term improvement of mesenchymal stromal cell infusion in critically COVID-19 patients: a randomized clinical trial. Stem Cell Res Ther. 2022;13(1):122. doi: 10.1186/s13287-022-02796-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020;29(12):747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fathi-Kazerooni M, Fattah-Ghazi S, Darzi M, et al. Safety and efficacy study of allogeneic human menstrual blood stromal cells secretome to treat severe COVID-19 patients: clinical trial phase I and II. Stem Cell Res Ther. 2022;13(1):96. doi: 10.1186/s13287-022-02771-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu R, Yan T, Feng Y, et al. Mesenchymal stem cell treatment improves outcome of COVID-19 patients via multiple immunomodulatory mechanisms. Cell Res. 2021;31(12):1244–1262. doi: 10.1038/s41422-021-00573-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wei F, Kong D, Li T, et al. Efficacy and safety of umbilical cord mesenchymal stem cells for the treatment of patients with COVID-19. Clinics (São Paulo) 2021;76:e2604. doi: 10.6061/clinics/2021/e2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sharma A, Kulkarni R, Sane H, et al. Phase 1 clinical trial for intravenous administration of mesenchymal stem cells derived from umbilical cord and placenta in patients with moderate COVID-19 virus pneumonia: results of stage 1 of the study. Am J Stem Cells. 2022;11(3):37–55. [PMC free article] [PubMed] [Google Scholar]
- 88.Grégoire C, Layios N, Lambermont B, et al. Bone marrow-derived mesenchymal stromal cell therapy in severe COVID-19: preliminary results of a phase I/II clinical trial. Front Immunol. 2022;13:932360. doi: 10.3389/fimmu.2022.932360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen J, Hu C, Chen L, et al. Clinical study of mesenchymal stem cell treatment for acute respiratory distress syndrome induced by epidemic influenza A (H7N9) infection: a hint for COVID-19 treatment. Engineering (Beijing) 2020;6(10):1153–1161. doi: 10.1016/j.eng.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Agarwal A, Rochwerg B, Lamontagne F, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379. doi: 10.1136/bmj.m3379. [DOI] [PubMed] [Google Scholar]
- 91.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 92.Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bozorgmehr M, Gurung S, Darzi S, et al. Endometrial and menstrual blood mesenchymal stem/stromal cells: biological properties and clinical application. Front Cell Dev Biol. 2020;9(8):497. doi: 10.3389/fcell.2020.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Avanzini MA, Mura M, Percivalle E, et al. Human mesenchymal stromal cells do not express ACE2 and TMPRSS2 and are not permissive to SARS-CoV-2 infection. Stem Cells Transl Med. 2021;10(4):636–642. doi: 10.1002/sctm.20-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cantuti-Castelvetri L, Ojha R, Pedro LD, et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370(6518):856–860. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Daly JL, Simonetti B, Klein K, et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;370(6518):861–865. doi: 10.1126/science.abd3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang K, Chen W, Zhang Z, et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther. 2020;5(1):283. doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang S, Qiu Z, Hou Y, et al. AXL is a candidate receptor for SARS-CoV-2 that promotes infection of pulmonary and bronchial epithelial cells. Cell Res. 2021;31(2):126–140. doi: 10.1038/s41422-020-00460-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gu Y, Cao J, Zhang X, et al. Receptome profiling identifies KREMEN1 and ASGR1 as alternative functional receptors of SARS-CoV-2. Cell Res. 2022;32(1):24–37. 10.1038/s41422-021-00595-6 [Erratum in: Cell Res. 2022 Jun;32(6):600. 10.1038/s41422-022-00654-6]. [DOI] [PMC free article] [PubMed]
- 100.Thanunchai M, Kanrai P, Wiboon-Ut S, Puthavathana P, Hongeng S, Thitithanyanont A. Tropism of avian influenza A (H5N1) virus to mesenchymal stem cells and CD34+ hematopoietic stem cells. PLoS ONE. 2013;8(12):e81805. doi: 10.1371/journal.pone.0081805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang M, Yan X, Shi M, et al. Safety and efficiency of stem cell therapy for COVID-19: a systematic review and meta-analysis. Glob Health Res Policy. 2022;7(1):19. doi: 10.1186/s41256-022-00251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen CY, Chen WC, Hsu CK, Chao CM, Lai CC. The clinical efficacy and safety of mesenchymal stromal cells for patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials. J Infect Public Health. 2022;15(8):896–901. doi: 10.1016/j.jiph.2022.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yao W, Dong H, Qi J, Zhang Y, Shi L. Safety and efficacy of mesenchymal stem cells in severe/critical patients with COVID-19: a systematic review and meta-analysis. EClinicalMedicine. 2022;51:101545. doi: 10.1016/j.eclinm.2022.101545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kirkham AM, Bailey AJM, Monaghan M, et al. Updated living systematic review and meta-analysis of controlled trials of mesenchymal stromal cells to treat COVID-19: a framework for accelerated synthesis of trial evidence for rapid approval-FASTER approval. Stem Cells Transl Med. 2022;11(7):675–687. doi: 10.1093/stcltm/szac038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moll G, Ankrum JA, Olson SD, Nolta JA. Improved MSC minimal criteria to maximize patient safety: a call to embrace tissue factor and hemocompatibility assessment of MSC products. Stem Cells Transl Med. 2022;11(1):2–13. doi: 10.1093/stcltm/szab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20(8):e192–7. 10.1016/S1473-3099(20)30483-7 [Erratum in: Lancet Infect Dis. 2020 Oct;20(10):e250. 10.1016/S1473-3099(20)30637-X]. [DOI] [PMC free article] [PubMed]
- 107.Gorman EA, O'Kane CM, McAuley DF. Acute respiratory distress syndrome in adults: diagnosis, outcomes, long-term sequelae, and management. Lancet. 2022;400(10358):1157–1170. doi: 10.1016/S0140-6736(22)01439-8. [DOI] [PubMed] [Google Scholar]
- 108.Wiklander OP, Nordin JZ, O'Loughlin A, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;20(4):26316. doi: 10.3402/jev.v4.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Levy O, Kuai R, Siren EMJ, et al. Shattering barriers toward clinically meaningful MSC therapies. Sci Adv. 2020;6(30):eaba6884. doi: 10.1126/sciadv.aba6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Velissaris D, Dimopoulos G, Parissis J, et al. Prognostic role of soluble urokinase plasminogen activator receptor at the emergency department: a position paper by the Hellenic Sepsis Study Group. Infect Dis Ther. 2020;9(3):407–416. doi: 10.1007/s40121-020-00301-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Arnold DT, Attwood M, Barratt S, et al. Predicting outcomes of COVID-19 from admission biomarkers: a prospective UK cohort study. Emerg Med J. 2021;38(7):543–548. doi: 10.1136/emermed-2020-210380. [DOI] [PubMed] [Google Scholar]
- 112.Velissaris D, Lagadinou M, Paraskevas T, et al. Evaluation of plasma soluble urokinase plasminogen activator receptor levels in patients with COVID-19 and non-COVID-19 pneumonia: an observational cohort study. J Clin Med Res. 2021;13(9):474–478. doi: 10.14740/jocmr4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Webb BJ, Peltan ID, Jensen P, et al. Clinical criteria for COVID-19-associated hyperinflammatory syndrome: a cohort study. Lancet Rheumatol. 2020;2(12):e754–e763. doi: 10.1016/S2665-9913(20)30343-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yildirim M, Halacli B, Yuce D, Gunegul Y, Ersoy EO, Topeli A. Assessment of admission COVID-19 associated hyperinflammation syndrome score in critically-ill COVID-19 patients. J Intensive Care Med. 2023;38(1):70–77. doi: 10.1177/08850666221131265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Golchin A. Cell-based therapy for severe COVID-19 patients: clinical trials and cost-utility. Stem Cell Rev Rep. 2021;17(1):56–62. doi: 10.1007/s12015-020-10046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Patel S, Schmidt KF, Farhoud M, et al. In vivo tracking of [89Zr]Zr-labeled engineered extracellular vesicles by PET reveals organ-specific biodistribution based upon the route of administration. Nucl Med Biol. 2022;112–113:20–30. doi: 10.1016/j.nucmedbio.2022.06.004. [DOI] [PubMed] [Google Scholar]
- 117.Zhu YG, Shi MM, Monsel A, et al. Nebulized exosomes derived from allogenic adipose tissue mesenchymal stromal cells in patients with severe COVID-19: a pilot study. Stem Cell Res Ther. 2022;13(1):220. doi: 10.1186/s13287-022-02900-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sengupta V, Sengupta S, Lazo A, Jr, Hicok KC, Moseley T. Response to Lim et al. re: "Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19". Stem Cells Dev. 2020;29(14):879–881. doi: 10.1089/scd.2020.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.He J, Ren W, Wang W, et al. Exosomal targeting and its potential clinical application. Drug Deliv Transl Res. 2022;12(10):2385–2402. doi: 10.1007/s13346-021-01087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mocchi M, Bari E, Marrubini G, et al. Freeze-dried mesenchymal stem cell-secretome pharmaceuticalization: optimization of formulation and manufacturing process robustness. Pharmaceutics. 2021;13(8):1129. doi: 10.3390/pharmaceutics13081129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.