Abstract
Background
Autism spectrum disorders (ASD) comprise many complex and clinically distinct neurodevelopmental conditions, with increasing evidence linking them to parkinsonism.
Methods
We searched Medline and Embase from inception to 21 March 2022 and reviewed the bibliographies of relevant articles. Studies were screened and reviewed comprehensively by two independent authors.
Results
Of 863 references from our search, we included eight clinical studies, nine genetic studies, and five case reports. Regardless of age group, Parkinson's disease (PD) and parkinsonian syndromes were more frequently observed in patients with ASD, though the evidence for increased rates of parkinsonism is less clear for children and adolescents. Parkinsonian features and hypokinetic behavior were common in Rett syndrome, with prevalence estimates ranging from 40% to 80%. Frequently observed parkinsonian features include bradykinesia, rigidity, hypomimia, and gait freezing. PD gene PARK2 copy number variations appear more frequently in ASD cases than controls. Evidence suggests that RIT2 and CD157/BST1 are implicated in ASD and PD, while the evidence for other PD‐related genes (DRD2, GPCR37, the SLC gene family, and SMPD1) is less clear. Rare mutations, such as ATP13A2, CLN3, and WDR45, could result in autistic behavior and concomitant parkinsonism.
Conclusion
The prevalence of parkinsonism in ASD is substantially greater than in the general population or matched controls. Various PD‐associated gene loci, especially PARK2, could confer susceptibility to ASD as well. Important future directions include conducting prospective cohort studies to understand how parkinsonian symptoms may progress, genetic studies to reveal relevant gene loci, and pathophysiologic studies to identify potential therapeutic targets.
Introduction
Autism spectrum disorders (ASDs) comprise a multitude of complex and clinically distinct neurodevelopmental conditions. These conditions are typically diagnosed in childhood and persist for life in most patients. They are characterized by difficulties with communication and social interactions, and patients also frequently demonstrate repetitive movements and behaviors. ASDs are increasingly common, and recent estimates in 2018 in the United States suggest one in 44 children to be affected by an ASD. 1
With the steadily rising prevalence estimates of ASD, the number of adults with ASD will increase concordantly. Recent literature has already indicated the increased vulnerability, relative to the general population, to medical and mental conditions experienced by adults with ASD. 2 , 3 With more patients on the autistic spectrum progressing in adulthood and even older adulthood (i.e., greater than 65 years of age), the associated risk of ASD patients getting age‐dependent neurodegenerative disorders has attracted increasing attention.
A recent study suggested that middle‐aged and older autistic adults with no intellectual disabilities reported a higher prevalence of parkinsonism (bradykinesia, poor balance, etc.) compared with the general population, 3 supporting other reports of parkinsonism in patients with ASD. 4 , 5 , 6 The rates of parkinsonism remain unexpectedly high among ASD patients, even after excluding those (both currently and previously) on atypical antipsychotics. 6
There is also suggestion of genetic overlap between Parkinson's disease (PD) and ASD, where certain genes associated with PD were found to be implicated in ASD. 7 It would be interesting to determine if pathogenic gene mutations responsible for PD are also found in patients with ASD. There is also biological plausibility as both conditions may share similar pathophysiologic mechanisms. Both animal and human studies have demonstrated dopaminergic dysregulation in ASD, which is central to the pathogenesis of PD, 8 , 9 , 10 Moreover, these studies have identified dopamine receptors to be potential drug targets, though more research must be done to confirm this hypothesis. 8
Despite reports linking ASD with parkinsonian‐like features, there has not been a systematic review of the clinical, genetic, and pathophysiologic data on the association between the two conditions. To address current gaps in knowledge, we conducted a systematic review to comprehensively consider the current evidence linking parkinsonism with ASD. In this article, the term “PD” refers the specific diagnosis of PD, while “parkinsonism” and “parkinsonian” features refer to the extrapyramidal signs that may be present in hypokinetic (such as in PD) and hyperkinetic (such as in Huntington's disease) disorders.
Methods
Search strategy
In accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines, 11 this systematic review summarizes the current evidence on the associations between ASD and parkinsonism. We registered this study on PROSPERO at CRD42022320765 and searched Medline and Embase on 21 March 2022 for relevant articles. In Medline, we utilized the following search strategy: (Parkinson*.ti,ab. OR exp “Parkinsonian Disorders”/) AND (Autis*.ti,ab. OR exp “Autism Spectrum Disorder”/). Furthermore, the bibliographies of related reviews and meta‐analyses were screened to ensure a comprehensive search.
Study selection
Two reviewers (ASM and DWJW) screened the title and abstract of each reference in an independent manner, before retrieving the full texts for further review. Any disputes were referred to a third author for resolution (FST). We included primary research articles reporting parkinsonian features in ASD and genetic associations, as well as case studies highlighting patients presenting with ASD and parkinsonism. Reviews, meta‐analyses, editorials, commentaries, conference abstracts, non‐human studies (animal and in vitro studies), and articles not published in English were excluded.
Data extraction
Two blinded reviewers (ASM and DWJW) extracted the following data items for clinical studies: first author and year of publication, study design, population studied, sample size, method of ASD diagnosis and parkinsonism assessment (based on the cardinal features of rigidity, bradykinesia, resting tremor, and postural instability), as well as baseline patient demographics (e.g., age and sex). For gene studies, we further extracted the nationality and ancestry of included patients, method of gene sequencing and analysis, as well as the genes studied. For case studies, we retrieved information for the following fields: patient demographics (age, gender, and ethnicity), final diagnosis, family history, associated genetic mutations, features of ASD as well as parkinsonism, other reported problems, and attempted treatments. Unless otherwise stated, the data reported were presented as mean ± standard deviation for continuous outcomes, and percentage (events/sample size).
Quality assessment
For clinical studies, we employed the Joanna Briggs Institute (JBI)'s Critical Appraisal Tools for quality assessment. The cross‐sectional studies were evaluated using the Checklist for Prevalence Studies (maximum score of 9), case–control studies using the Checklist for Case–Control Studies (maximum score of 10), case series using the Checklist for Case Series (maximum score of 10), and lastly case reports using the Checklist for Case Reports (maximum score of 8). The quality ratings were reported as scores out of the maximum score attainable for each study design. Two reviewers (ASM and DWJW) assessed the quality of each study independently, and any unresolved conflicts were directed to a third reviewer (FST). All appropriate studies were included, irrespective of quality ratings, as this is an under‐researched topic.
Results
Summary of included articles
Following the search, 863 references with 29 duplicates were exported to Zotero. We removed the duplicates and screened the remaining 834 references. The full texts of 31 articles were retrieved, but we were unable to obtain the full texts of another eight references, as they were either conference abstracts or were not published in English. After a review of the full texts, 22 articles were included in the final review. The PRISMA flow diagram can be found in Supplementary Figure S1. Of the included studies, eight were clinical studies involving 6894 patients, 2 , 3 , 4 , 5 , 6 , 12 , 13 , 14 nine were genetic studies of 6975 subjects, 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 and five were case reports of patients presenting with both ASD and parkinsonism features. 24 , 25 , 26 , 27 , 28 A summary of the key ideas presented in this review can be found in Fig. 1.
Figure 1.
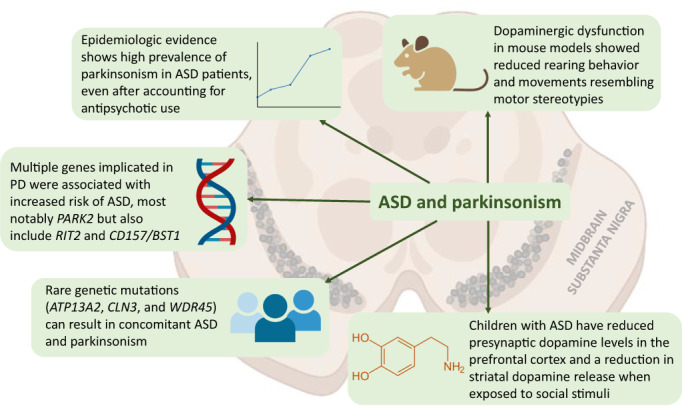
Central illustration.
Of the clinical studies, five were descriptive cross‐sectional studies 4 , 6 , 12 , 13 , 14 and three were case–control studies. 2 , 3 , 5 Three studies scored the maximum score for the quality assessment, 2 , 3 , 4 while four cross‐sectional studies scored 8 out of 9 points due to their small sample sizes (<100 participants), 6 , 12 , 13 , 14 and one case–control study scored 8 out of 10 points as confounding factors were not identified and controlled for. 5
Among the genetic studies, five studied single genes, 16 , 17 , 18 , 19 , 21 two studied the whole genome, 22 , 23 one studied the whole exome, one used a mix of whole‐exome sequencing (WES), next‐generation sequencing (NGS) panel, and targeted single‐gene testing. 20 Five studies employed a case–control study design, 16 , 18 , 21 , 22 , 23 while the remaining employed either a cross‐sectional 17 , 19 or a case series study design. 15 , 20
Five genetic studies involved only Asian populations, 15 , 18 , 20 , 21 , 22 except for three which included only Caucasian populations 16 , 17 , 23 and one study which recruited a mix of Asian and Caucasian patients. 19 Most studies scored the maximum number of points, except for three studies which had a small sample size, 19 lack of case–control matching, 16 , 22 or identification and adjustments for confounders. 16 All case reports scored the maximum number of points for quality assessment.
Clinical Studies
Autism and related conditions
This systematic review included clinical studies characterizing features of parkinsonism in ASD, as well as potential risk factors. We present the study characteristics, as well as their quality ratings, in Table 1 and their key findings in Table 2. Parkinsonism is more prevalent in adult patients with ASD when compared with non‐ASD controls, irrespective of age group. 2 , 3 Among younger adults (mean age 29.0 ± 12.2 years), PD were significantly more common at 0.93% (14/1507), compared with 0.03% (5/15,070) in controls, with an odds ratio (OR) of 32.73 (95% CI 7.76–137.96, P < 0.001). 2 Similarly, older adults (ages ≥65 years) with ASD were also more prone to developing PD (adjusted OR [aOR] 6.1, 95% CI 5.3–7.0). 3 Females, however, had higher odds of PD (aOR 8.2, 95% CI 6.2–10.7) relative to males (aOR 5.4, 95% CI 4.6–6.4). 3
Table 1.
Clinical studies on parkinsonism in autism spectrum disorders.
| Study | Design | Population | Sample size | Criteria for ASD diagnosis | Method of parkinsonism assessment | Age, years | Female, N (%) | Quality a |
|---|---|---|---|---|---|---|---|---|
| Croen et al, 2015 b | Case–control | Adults with ASD | 1507/15,070 | ICD‐9 codes in the electronic medical record system of Kaiser Permanente in Northern California | 29 + 12.2/29.4 + 12.1 | 405 (26.9%)/4050 (26.9%) | 10/10 | |
| FitzGerald et al, 1990 | Cross‐sectional | Rett syndrome | 32 | Motor‐behavioral assessment scale and assessment of ≥2 videotapes | Assessment of ≥1 videotape over 2‐year follow‐up | Range 30 months to 28 years | 32 (100%) | 8/9 |
| Geurts et al, 2022 | Cross‐sectional | ASD adults without suspected intellectual disability |
Netherlands sample: 296 US sample: 209 |
DSM‐4 or DSM‐5 | Parkinsonism Screening Questionnaire (parkinsonism defined as ≥7) |
Netherlands sample: 58.4 + 5.9 US sample: 59.35 + 7.15 |
Netherlands sample: 113 (38%) US sample: 109 (52%) |
9/9 |
| Hand et al, 2020 b | Case–control | Older adults (ages ≥65 years) with ASD | 4685/46,850 | ICD‐10 codes from Medicare Standard Analytic Files |
65–69 years 2,442 (52.1%)/24,420 (52.1%) 70–74 years 1190 (25.4%)/11,900 (25.4%) ≥75 years 1053 (22.5%)/10,530 (22.5%) |
1510 (32.2%)/15,100 (32.2%) | 10/10 | |
| Humphreys and Barrowman, 2016 | Cross‐sectional | Rett syndrome | 51 | Standardized historical questionnaire and neurologic examination | Rett Syndrome Rigidity Distribution score c | Range 2 years 5 months to 54 years | 51 (100%) | 8/9 |
| Mostert‐Kerckhoffs et al, 2020 b | Case–control | Children and adolescents with ASD |
6–12 years: 22/22 13–26 years: 23/26 |
ADI‐R and ADOS | UPDRS and mechanical assessment |
6–12 years: 10.4 + 1.7/10.3 + 1.8 13–26 years: 18.7 + 4.6/20.2 + 4.1 |
6–12 years: 5 (22.7%)/5 (22.7%) 13–26 years: 6 (26.1%)/6 (23.1%) |
8/10 |
| Starkstein et al, 2015 d | Cross‐sectional | Adults with autism |
Study 1: 19 Study 2: 37 |
DSM‐5, ADI‐R, and ADOS | UPDRS and MDS‐UPDRS |
Study 1: 57 + 6.7 Study 2: 51.2 + 8.5 |
Study 1: 0 Study 2: 5 (13.5%) |
8/9 |
| Young et al, 2020 | Cross‐sectional | Rett syndrome | 14 | Revised diagnostic criteria by Neul et al | Videotapes assessed by ≥2 independent assessors | 9.2 + 5.4 | 14 (100%) | 8/9 |
ADI‐R, Autism Diagnostic Interview‐Revised; ADOS, Autism Diagnostic Observation Schedule; ASD, autism spectrum disorders; DSM, Diagnostic and Statistical Manual of Mental Disorders; ICD, International Classification of Diseases; MDS, Movement Disorder Society; UPDRS, Unified Parkinson's Disease Rating Scale; US, United States.
The quality of cross‐sectional studies was assessed using the Joanna Briggs Institute's Checklist for Prevalence Studies (rated out of 9 points), and case–control studies using the Checklist for Case–Control Studies (rated out of 10 points).
Relevant variables (i.e., sample size, age, and proportion of females) were reported as cases/controls for these case–control studies.
The Rett Syndrome Rigidity Distribution score is an investigator‐developed survey evaluating the extent of rigidity in Rett syndrome.
The authors first conducted a hypothesis‐generating pilot study of 19 adults with ASD, followed by a second study of 37 individuals with ASD to confirm their findings.
Table 2.
Key findings of clinical studies.
| Study | Key findings |
|---|---|
| Croen et al, 2015 |
|
| FitzGerald et al, 1990 |
|
| Geurts et al, 2022 |
|
| Hand et al, 2020 |
|
| Humphreys and Barrowman, 2016 |
|
| Mostert‐Kerckhoffs et al, 2020 |
|
| Starkstein et al, 2015 |
|
| Young et al, 2020 |
|
aOR, adjusted odds ratio; ASD, autism spectrum disorders; CI, confidence intervals; OR, odds ratio; RTT, Rett syndrome; RTTRD, Rett Syndrome Rigidity Distribution score; US, United States.
The Rett Syndrome Rigidity Distribution score is an investigator‐developed survey evaluating the extent of rigidity in Rett syndrome.
ASD patients of ages 6–12 years were considered children, while those of ages 13–26 years were considered adolescents.
Parkinsonian features in ASD adults were common. 4 , 6 Starkstein et al 6 similarly found a high frequency of parkinsonism in adults with autism (32% [12/37] of the whole sample), and after excluding those taking atypical antipsychotics, 20% (4/20) had significant parkinsonism. In another study of 505 ASD adults without intellectual disability, 23.6% (119/505) scored above the cutoff score (≥7 points, defined as the “screen‐positive group”) for the Parkinsonism Screening Questionnaire (PSQ), which is suggestive of clinically significant parkinsonism. 4 In the United States sample, the proportion of females in the screen‐positive group was significantly higher than that in the screen‐negative group (65.2% [45/69] vs. 45.7% [64/140], P = 0.008). 4 In the Netherlands sample, screen‐positive patients also reported lower rates of cognitive failures than screen‐negative patients (70.4 ± 15.7 vs. 83.8 ± 14.5, P < 0.001), but more medical (87.8% [43/49] vs. 63.1% [147/233], P = 0.002) and mental health diagnoses (62.2% [28/45] vs. 38.2% [87/228], P = 0.003). Importantly, antipsychotic use did not differ between the screen‐positive and screen‐negative groups for both samples (P = 0.98 for the Netherlands sample; P = 0.05 for the US sample). 4
In children and adolescents, though, the evidence for increased rates of parkinsonism is less clear. Compared with matched controls, bradykinesia was significantly more common in both children and adolescents with ASD (children [ages 6–12 years]—50.0% [11/22] vs. 4.5% [1/22], P < 0.001; adolescents [ages 13–26 years]—65.2% [15/23] vs. 7.7% [2/26], P < 0.001). 5 However, rigidity was significantly more frequent in adolescents with ASD (30.4% [7/23] vs. 0, P < 0.001) but not in children (P = 0.325). In addition, the prevalence of tremors was not significantly increased in both age groups (P = 0.144 for children; P = 0.439 for adolescents). 5
Rett syndrome (RTT)
Despite being classified within the autism spectrum, RTT is clinically distinct and is associated with movement disorders of greater severity. Indeed, parkinsonism features were very prevalent in patients with RTT, with many patients reporting rigidity (43.8–84.3%), hypomimia (62.5%), bradykinesia (40.6%), and freezing (85.7%). 12 , 13 , 14 A cross‐sectional study by FitzGerald et al 12 observed hypomimia in 62.5% (20/32), rigidity in 43.8% (14/32), and bradykinesia in 40.6% (13/32).
Furthermore, Humphreys and Barrowman 13 reported rigidity in 84.3% (43/51) of patients, which was evaluated by an investigator‐developed survey, the RTT rigidity distribution (RTTRD) score (higher scores indicate greater severity). The rigidity was more severe in older age groups (3.00 ± 2.16 for ages 6–10 years; 6.70 ± 3.47 for ages 11–19 years; and 7.67 ± 3.80 for ages >20 years) and higher RTTRD scores were correlated with lower levels of homovanillic acid in the cerebrospinal fluid (R ‐0.83, P = 0.005). 13
Freezing was observed in 85.7% of RTT patients, with gait shuffling observed in 21.4% and initiation freeze in 35.7%. 14 In addition, RTT patients often experience hyperkinetic movement disorders as well (such as bruxism in 100% [32/32], oculogyric crises in 62.5% [20/32], and dystonia in 59.4% [19/32]), combined with the hypokinetic parkinsonian features described above. 12
Genetic studies
The genetic studies included in this review examined a variety of genes, such as PARK2, RIT2, CD157/BST1, DRD2, SLC, GPCR37, and SMPD1. We present a detailed summary of these articles in Table 3. PARK2 was investigated in three studies, and associated mutations were more commonly observed in ASD patients. In a study of 342 Portuguese individuals with ASD, the prevalence of PARK2 deletions was 1.5% (5/342), which was relatively high for a single gene variant in ASD. 17 In addition, 53 patients had copy number variations (CNVs) in PARK2 between introns 4 and 6, of which 24 also had CNVs spanning intron 9. 17 Another study of 335 Han Chinese ASD patients found six patients who had CNVs in the PARK2 exonic regions, ranging over exons 2 to 7. 22 Lastly, another genetic study of patients of European ancestry (with comparison to healthy controls) found PARK2 CNVs to be exclusive to ASD cases. 23
Table 3.
Genetic studies on genes potentially involved in parkinsonism and autism spectrum disorders.
| Study | Study design | Genetic testing employed | Population | Sample size | Key genes studied | Age, years | Female, N (%) | Findings | Quality a |
|---|---|---|---|---|---|---|---|---|---|
| Chang et al, 2019 | Case series | Whole‐exome sequencing | Taiwanese patients with ASD | 5 | SMPD1 | Range 6–21 years | 0 | Pathogenic mutation in SMPD1 in a patient with autism and epilepsy | 10/10 |
| Comings et al, 1991 b | Case–control | Single gene testing | Non‐Hispanic white ASD and separately PD | 50 (33 ASD and 17 PD)/314 | DRD2 | Not reported | Not reported | Prevalence of A1 allele significantly higher in autism‐PDD patients than controls (54.5% vs. 24.5%, P = 0.0005) but not significant in PD (17.6% vs. 24.5%, P = 0.42) | 7/10 |
| Conceicao et al, 2017 | Cross‐sectional | Single gene testing | Portuguese ASD patients | 342 | PARK2 | Range 3–9 years | 0 | Five patients have PARK2 deletions, 53 patients with neurodevelopmental disorders have PARK2 CNVs in region between introns 4 and 6, and 24 spanned intron 9 | 9/9 |
| Emamalizadeh et al, 2017 b | Case–control | Single gene testing | Unrelated Iranian subjects with ASD and separately PD |
PD: 520/520 ASD: 470/470 |
RIT2 |
PD: 59.5 + 12.5/58.12 + 12.22 ASD: 7.9 + 2.7/8.2 + 2.5 |
PD:242 (46.5%)/252 (48.5%) ASD:184 (39.1%)/191 (40.6%) |
Genotype and allele frequencies of rs12456492 differed significantly for PD (P = 0.001 and P = 0.007, respectively), as well as for ASD with borderline significance (P = 0.05 and P = 0.06, respectively) | 10/10 |
| Fujita‐Jimbo et al, 2012 | Cross‐sectional | Single gene testing | Unrelated Japanese and Caucasian ASD patients |
Japanese: 72 Caucasian: 200 |
GPCR37 |
Japanese: Range 2–32 Caucasian: Not reported |
Japanese:15 (20.8%) Caucasian:28 (14%) |
Mutations in GPCR37 found in one Japanese patient and in one Caucasian patient | 8/9 |
| Mir et al, 2022 | Case series | A mix of whole‐exome sequencing, NGS panel, or single gene testing | Pediatric Saudi Arabian patients | 25 | SLC | Range 1.5–14 | 10 (40%) | SLC9A9 mutations found in a patient with autism, and SLC6A3 mutations found in another with infantile parkinsonism‐dystonia 1 | 10/10 |
| Yokoyama et al, 2015 b | Case–control | Single gene testing | Japanese ASD patients | 147/150 | CD157/BST1 | 15.6 + 0.6/ 23.8 + 0.3 | 34 (23.1%)/35 (23.3%) | rs4301112 (OR 6.4, 95% CI 1.9–22, P = 0.0007), rs28532698 (OR 6.2, 95% CI 1.8–21, P = 0.0012), and rs10001565 (OR 5.5, 95% CI 1.6–19, P = 0.0038) demonstrated significantly higher allele frequencies in ASD cases than unaffected controls | 10/10 |
| Yin et al, 2016 b | Case–control | Whole‐genome sequencing | Han Chinese ASD patients | 335/1093 | PARK2 | 9.39 + 4.04/68.07 + 10.12 | 36 (10.7%)/568 (52%) | Six patients had PARK2 CNVs over exons 2–7 | 9/10 |
| Glessner et al, 2009 b | Case–control | Whole‐genome sequencing | ASD patients of European ancestry | 859/2519 | PARK2 | Range 2–21 years (mean or median not reported)/8.7 + 5.46 | 156 (18.2%)/1197 (47.5%) | PARK2 CNVs were exclusive to ASD cases | 10/10 |
ASD, autism spectrum disorders; CI, confidence interval; CNV, copy number variation; OR, odds ratio; PD, Parkinson's disease.
The quality of case–control studies was assessed using the Checklist for Case–Control Studies (rated out of 10 points), cross‐sectional studies using the Joanna Briggs Institute's Checklist for Prevalence Studies (rated out of 9 points), and case series using the Checklist for Case Series (rated out of 10 points).
Relevant variables (i.e., sample size, age, and proportion of females) were reported as cases/controls for these case–control studies.
Single‐nucleotide polymorphisms (SNPs) in RIT2 appear to confer susceptibility to both PD and ASD. 18 When compared to matched controls, the genotype and allele frequencies of rs12456492 differed significantly for PD (P = 0.001 and P = 0.007, respectively), as well as for ASD with borderline significance (P = 0.05 and P = 0.06, respectively). 18 Polymorphisms in CD157/BST1, another gene associated with PD, were associated with ASD as well. Three SNPs—rs4301112 (OR 6.4, 95% CI 1.9–22, P = 0.0007), rs28532698 (OR 6.2, 95% CI 1.8–21, P = 0.0012), and rs10001565 (OR 5.5, 95% CI 1.6–19, P = 0.0038)—demonstrated significantly higher allele frequencies in ASD cases than unaffected controls. 21
The evidence supporting the involvement of other genes in ASD and PD, however, is less convincing. While the prevalence of the Taq I allele of DRD2 was significantly higher in ASD than in controls (54.5% vs. 24.5%, P = 0.0005), there were no significant differences for PD (17.6% vs. 24.5%, P = 0.42). 16 Fujita‐Jimbo et al 19 reported two male patients—one Japanese and one Caucasian—with mutations in GPCR37, which is a PD‐associated gene. SLC has also been implicated in ASD and PD. Mir et al 20 reported SLC9A9 mutations in a patient with autism, and SLC6A3 mutations in another with infantile parkinsonism‐dystonia 1. Lastly, a pathogenic mutation in SMPD1—variants of which were recently associated with PD—was found in a patient presenting with autism and epilepsy. 15 Further large‐scale studies will be needed to understand the role of these genes in ASD and PD.
Multiple case reports also presented various rare genetic mutations that have resulted in parkinsonism and autistic behavior. This review included five such case reports, two of which presented patients with RTT (caused by mutations in MECP2). The two patients described here demonstrated bradykinesia with rigidity and were unresponsive to levodopa therapy. The remaining three cases each had mutations in ATP13A2, CLN3, and WDR45, respectively. Levodopa therapy appeared to have variable efficacy in these individuals. Further details are presented in Table 4.
Table 4.
Case reports of patients with features of parkinsonism and autism spectrum disorders.
| Study | Age, gender, and ethnicity | Diagnosis | Family history | Associated genetic mutations | Features of ASD | Features of Parkinsonism | Treatments attempted |
|---|---|---|---|---|---|---|---|
| Balint et al, 2020 | 18‐year‐old Pakistani male | Pallido‐pyramidal syndrome | Consanguineous couple | ATP13A2 (NM_022089.4: c.2218C>T mutation) | Difficulty with social interaction, as well as stereotypies, gaze avoidance, and reclusive behavior | Young‐onset dystonia‐parkinsonism with slow finger tapping | Levodopa with good response but development of dyskinesia |
| Roze et al, 2007 | 49‐year‐old French female | Rett syndrome | None | MECP2 (heterozygous frameshift mutation c.1163del35 in exon 4) | Stereotypies and was unable to speak, but could make good eye contact with her family | Generalized bradykinesia and rigidity with dystonic posturing of the distal limbs, unsteady broad‐based gait, and difficulties with gait initiation | Levodopa failed to improve dystonia and parkinsonism, and trihexyphenidyl discontinued due to daytime sedation |
| Valadares et al, 2011 | 12‐year‐old Brazilian female | Juvenile neuronal ceroid lipofuscinosis | Consanguineous couple | CLN3 (1.02 kb deletion involving exons 7 and 8) | Autistic behavior with tics but understands and cooperates | Parkinsonism, ataxia, and can only walk with support since 10 years | None |
| Venkateswaran et al, 2014 | 15‐year‐old European female | Rett syndrome | None | MECP2 (missense mutation: c.419C>T; p.Ala140Val) | Socialized parallel to peer group and thrived with routine | Bradykinesia and rigidity with hypomimia | Levodopa/carbidopa did not improve symptoms; quetiapine partially controlled psychiatric symptoms |
| Verhoeven et al, 2014 | 42‐year‐old female (unknown ethnicity) | Beta‐propeller protein‐associated neurodegeneration | Father died from Parkinsonian dementia at 73 years | WDR45 (c.1030del leading to frameshift) | Poor language and social communication skills considered to fall on the autism spectrum | Bradykinesia and broad‐based gait with freezing tendency | Levodopa/carbidopa with partial response |
Discussion
In this systematic review, we evaluate the clinical and genetic association between ASD and parkinsonian features. We found that persons with ASD have a higher prevalence of parkinsonism, including PD for older adults. We also highlighted various genes, most notably PARK2, that are associated with ASD and PD, including individuals carrying certain rare genetic variants presenting with autistic behavior and parkinsonism.
While the motor disturbances in ASD remain to be better characterized, most reports suggest that motor deficits are common in patients with ASD. A meta‐analysis led by Fournier et al 29 indicated a presence of impairments across various motor domains, such as in motor planning, upper extremity function, as well as gait and balance. Some reports also likened the gait observed in patients with ASD to the gait in patients with other movement disorders, such as PD 30 , 31 and cerebellar ataxia. 32
A series of recent studies corroborated these findings. They found that a significant proportion of individuals with ASD demonstrated clinically apparent motor difficulties, but only a small portion of them received a specific diagnosis. 33 , 34 , 35 About 80–90% of pediatric patients with ASD presented with motor impairment as assessed by a well‐established parent report measure. However, there is gross under‐recognition of these symptoms and only a fraction (ranging from 1.5% to 15%) received a motor‐specific diagnosis.
The link between ASD and parkinsonism has garnered substantial interest, particularly because of the presence of motor abnormalities in individuals with autism. Gait abnormalities in ASD have been previously studied, both qualitatively and quantitatively, but whether the pattern is more suggestive of cerebellar dysfunction, or striatal dysfunction, remains to be clarified. 10 It is also unclear if motor dysfunction in ASD is a risk factor or predisposes to the development of parkinsonism.
Sex differences may play an important role in the presentation of parkinsonism in ASD patients. The study by Geurts et al 4 found a substantial difference in the proportion of subjects with ASD screening positive for parkinsonism between the United States and the Netherlands samples. Of note, the US sample included more females and found parkinsonism to be more prevalent in females than males. This suggestion is in line with the findings of a previous study by Rydzewska et al, 36 which concluded female ASD patients were at greater risk of developing health conditions compared with their male counterparts. Rydzewska et al 36 did not evaluate parkinsonism in their study, which will limit the applicability of their results to support sex differences in the parkinsonism rates among ASD patients.
Age could be another important factor affecting the presentation of parkinsonism in ASD patients. The study by Mostert‐Kerckhoffs et al 5 demonstrated that, relative to healthy age‐matched controls, rigidity was significantly more prevalent in adolescents but not in children with ASD. This may be attributed to thalamocortical dysconnectivity, which was more pronounced in adolescents than in children and adults. 37 The hypothesis that there are age‐specific effects on the abnormalities in brain connectivity is supported by other studies as well. 38 , 39
Among the various genes evaluated in this systematic review, the association of PARK2—mutations in which is the common cause of recessive forms of PD 40 —with ASD is of particular interest. PARK2 encodes the Parkin protein, which is a cytosolic ubiquitin E3 ligase, and plays an important role (along with PINK1) to regulate mitophagy. Defects in this pathway are hypothesized to result in greater vulnerability of the dopaminergic neurons to neurotoxins, resulting in their degeneration and hence PD. 41 The relationship between PARK2 mutations and autistic symptoms still needs to be evaluated, though abnormalities in this gene were thought to cause developmental anomalies 42 , 43 and even deficits in facial recognition. 44
Dopaminergic pathways could be implicated in the pathogenesis of ASD since dopamine is among the main neurotransmitters responsible for social behavior as well as movement control. Mutations in the dopamine transporter affecting dopaminergic transmission within the brain have been shown to result in autism‐like behavioral patterns. 45 , 46 In addition, drug‐induced alterations in the nigrostriatal circuit also resulted in stereotypical ASD‐like behavior in mouse models. 47 Abnormalities with dopamine metabolism in the brain could contribute to the behavioral pattern observed in patients with ASD.
Dopaminergic projections from the midbrain extend into multiple brain regions such as the basal ganglia, cortex, and amygdala. The dopaminergic dysfunction in these pathways would likely contribute to the pathogenesis of ASD. In children with ASD, there were reduced presynaptic dopamine levels in the prefrontal cortex 48 and a reduction in phasic striatal dopamine release when exposed to social stimuli. 49 , 50 Brain imaging studies have consistently demonstrated abnormalities in dopaminergic structures and their connectivity. Firstly, enlargement of the caudate nucleus (a major target of the dopaminergic system) was found in individuals with ASD, 51 even in those who were medication‐naïve. 52 Secondly, an MRI study found substantial and localized reductions in the gray matter of the frontostriatal networks, which implies abnormal connectivity between dopaminergic and cortical structures. 53
Mouse models of ASD have also been employed to study the derangements in dopamine metabolism. A mouse model carrying the Val559 mutation in SLC6A3 demonstrated an increase in the basal striatal dopamine levels with reduced rearing behavior. 54 A separate model with the T356M mutation in DAT demonstrated a decrease in the clearance and synthesis of dopamine with an increase in striatal dopamine metabolism. 45 These mice were more active and exhibited behavior that resembled motor stereotypies. 45 Evidently, ASD‐relevant changes can arise from alterations in dopaminergic neurotransmission.
There are inherent challenges to evaluating parkinsonism in ASD subjects. First, atypical antipsychotics—which are commonly associated with extrapyramidal side effects—are frequently used in the management of ASD and were previously thought to explain the parkinsonism observed in persons with ASD. However, current evidence suggests that the prevalence of parkinsonism was higher than expected, even when accounting for antipsychotic use. Starkstein et al 6 found a substantial portion of ASD subjects to demonstrate clinical parkinsonism despite excluding patients on atypical antipsychotics. In addition, Geurts et al 4 failed to find a significant difference in the use of atypical antipsychotics between patients with and without parkinsonism. The frequent use of atypical antipsychotics nonetheless presents a great challenge in the evaluation of parkinsonism in persons with ASD.
Another challenge is with the ascertainment of parkinsonism in these individuals, especially those with hyperkinetic disorders. Signs of parkinsonism may be missed in individuals with ASD, either due to lack of awareness or mischaracterization as stereotypies related to the ASD itself. This is also because most individuals with ASD (except those with profound movement disorders) do not follow up with movement disorders specialists, making an accurate assessment of parkinsonism even more difficult.
Most ASD patients also have communication problems, which will complicate the history‐taking process, especially in nonverbal subjects. As such, the progression of parkinsonian signs is also difficult to ascertain. The medical care providers may also change with time, leading to discontinuity of care and thereby difficulties with constructing an accurate timeline of parkinsonian symptoms. 55 History taking, therefore, should involve caregivers and family members, as well as primary care providers, and be complemented by a comprehensive review of past medical records. 55 Severity of parkinsonian symptoms should also be quantified using validated scales, to better detect symptomatic progression.
Future prospective studies with a longer follow‐up of patients with and without ASD will be useful, as this allows for a better assessment of the progression of parkinsonism and the patients' response to levodopa and pharmacotherapeutic options. This would address the current limitation of inadequate follow‐up. Functional imaging studies (such as diffusion tensor imaging, positron emission tomography, and dopamine transporter scan) to evaluate the dopaminergic function would provide quantification of the dopaminergic reserve and its changes over time.
The identification of suitable biomarkers to diagnose, monitor, and prognosticate idiopathic PD in ASD is another area of great importance. α‐synuclein is currently the best‐studied biomarker for PD, and its concentrations in the cerebrospinal fluid (CSF) or blood have been used in the investigative workup for and diagnosis of PD. 56 Other promising biomarkers include lysosomal enzymes, neurofilament light chain (NfL), and even the classic biomarkers for Alzheimer's disease. 56
CSF NfL levels can differentiate idiopathic PD from atypical parkinsonian syndromes. 57 , 58 , 59 The identification of a reliable biomarker would greatly facilitate the monitoring of the severity of parkinsonism in ASD patients, which is currently limited to the histories taken from the patients and their caregivers.
Conclusion
The prevalence of ASD has been steadily rising over the past decades, especially among those who have progressed to adulthood. Our systematic review highlights evidence to suggest that parkinsonian symptoms appeared to be more prevalent in ASD cases as compared to matched controls regardless of age group. Variants in PD gene PARK2 may also confer susceptibility to ASD, in addition to other PD‐related gene loci such as RIT2, CD157/BST1, GPCR37, and the SLC gene family. Rare genetic mutations (such as ATP13A2, CLN3, and WDR45) could also result in autistic behavior and concomitant parkinsonism. Further prospective cohort studies will be useful to evaluate the progression of parkinsonian features in ASD patients. Genetic screening and clinical genetic correlations in ASD families with parkinsonism will also provide additional clues. Pathophysiologic studies in transgenic animal models and human organoid models derived from ASD patients with and without parkinsonism can identify novel clues that may uncover potential therapeutic targets. The additional risk of parkinsonism observed in ASD patients, as well as genetic associations and common pathogenetic mechanisms underlying the two conditions, are key areas to be further investigated.
Conflicts of Interest
The authors do not have any competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Author Contributions
AS Mai, DWJ Wan, FS Tseng, QXJ Foo, DQ Wang, and Prof E‐K Tan contributed to (1) the conception and design of this project; (2) acquisition, analysis, and interpretation of data; and (3) drafting and revising it critically for important intellectual content. All authors gave their final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Consent for Publication
All authors consent to the publication of this article and related materials.
Ethics Approval and Consent to Participate
Not applicable.
Supporting information
Figure S1. PRISMA flow diagram.
Acknowledgements
None.
Funding Information:
Prof E‐K Tan is supported by the National Medical Research Council (grant numbers: OF PD LCG 000207 and STaR 0030/2018).
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1. Maenner MJ. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2018. MMWR Surveill Summ. 2021;70:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Croen LA, Zerbo O, Qian Y, et al. The health status of adults on the autism spectrum. Autism Int J Res Pract. 2015;19(7):814‐823. [DOI] [PubMed] [Google Scholar]
- 3. Hand BN, Angell AM, Harris L, Carpenter LA. Prevalence of physical and mental health conditions in Medicare‐enrolled, autistic older adults. Autism Int J Res Pract. 2020;24(3):755‐764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Geurts HM, McQuaid GA, Begeer S, Wallace GL. Self‐reported parkinsonism features in older autistic adults: a descriptive study. Autism. 2022;26(1):217‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mostert‐Kerckhoffs MAL, Willems AE, Tenback DE, Koning JP, Van Harten P, Staal WG. Motor disturbance in ASD: a pilot study showing hypokinetic behavior? J Autism Dev Disord. 2020;50(2):415‐428. [DOI] [PubMed] [Google Scholar]
- 6. Starkstein S, Gellar S, Parlier M, Payne L, Piven J. High rates of parkinsonism in adults with autism. J Neurodev Disord. 2015;7(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morato Torres CA, Wassouf Z, Zafar F, Sastre D, Outeiro TF, Schule B. The role of alpha‐synuclein and other Parkinson's genes in neurodevelopmental and neurodegenerative disorders. Int J Mol Sci. 2020;21(16):5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kosillo P, Bateup HS. Dopaminergic dysregulation in syndromic autism Spectrum disorders: insights from genetic mouse models. Front Neural Circuits. 2021;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mandic‐Maravic V, Grujicic R, Milutinovic L, Munjiza‐Jovanovic A, Pejovic‐Milovancevic M. Dopamine in autism Spectrum disorders—focus on D2/D3 partial agonists and their possible use in treatment. Front Psychiatry. 2022;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nayate A, Bradshaw JL, Rinehart NJ. Autism and Asperger's disorder: are they movement disorders involving the cerebellum and/or basal ganglia? Brain Res Bull. 2005. Oct 30;67(4):327‐334. [DOI] [PubMed] [Google Scholar]
- 11. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. FitzGerald PM, Jankovic J, Percy AK. Rett syndrome and associated movement disorders. Mov Disord. 1990;5(3):195‐202. [DOI] [PubMed] [Google Scholar]
- 13. Humphreys P, Barrowman N. The incidence and evolution of parkinsonian rigidity in Rett syndrome: a pilot study. Can J Neurol Sci. 2016;43(4):567‐573. [DOI] [PubMed] [Google Scholar]
- 14. Young DR, Suter B, Levine JT, Glaze DG, Layne CS. Characteristic behaviors associated with gait of individuals with Rett syndrome. Disabil Rehabil. 2020. Sep;15:1‐8. [DOI] [PubMed] [Google Scholar]
- 15. Chang YS, Lin CY, Huang HY, Chang JG, Kuo HT. Chromosomal microarray and whole‐exome sequence analysis in Taiwanese patients with autism spectrum disorder. Mol Genet Genomic Med. 2019;7(12):e996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Comings DE, Comings BG, Muhleman D, et al. The dopamine D2 receptor locus as a modifying gene in neuropsychiatric disorders. Jama. 1991;266(13):1793‐1800. [PubMed] [Google Scholar]
- 17. Conceicao IC, Rama MM, Oliveira B, et al. Definition of a putative pathological region in PARK2 associated with autism spectrum disorder through in silico analysis of its functional structure. Psychiatr Genet. 2017;27(2):54‐61. [DOI] [PubMed] [Google Scholar]
- 18. Emamalizadeh B, Jamshidi J, Movafagh A, et al. RIT2 polymorphisms: is there a differential association? Mol Neurobiol. 2017;54(3):2234‐2240. [DOI] [PubMed] [Google Scholar]
- 19. Fujita‐Jimbo E, Yu ZL, Li H, et al. Mutation in Parkinson disease‐associated, G‐protein‐coupled receptor 37 (GPR37/PaelR) is related to autism spectrum disorder. PLoS One. 2012;7(12):e51155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mir A, Almudhry M, Alghamdi F, et al. SLC gene mutations and pediatric neurological disorders: diverse clinical phenotypes in a Saudi Arabian population. Hum Genet. 2022;141(1):81‐99. [DOI] [PubMed] [Google Scholar]
- 21. Yokoyama S, Al Mahmuda N, Munesue T, et al. Association study between the CD157/BST1 gene and autism spectrum disorders in a Japanese population. Brain Sci. 2015;5(2):188‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yin CL, Chen HI, Li LH, et al. Genome‐wide analysis of copy number variations identifies PARK2 as a candidate gene for autism spectrum disorder. Mol Autism. 2016;7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Glessner JT, Wang K, Cai G, et al. Autism genome‐wide copy number variation reveals ubiquitin and neuronal genes. Nature. 2009;459(7246):569‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Balint B, Damasio J, Magrinelli F, Guerreiro R, Bras J, Bhatia KP. Psychiatric manifestations of ATP13A2 mutations. Mov Disord Clin Pract. 2020;7(7):838‐841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roze E, Cochen V, Sangla S, et al. Rett syndrome: an overlooked diagnosis in women with stereotypic hand movements, psychomotor retardation, parkinsonism, and dystonia? Mov Disord. 2007;22(3):387‐389. [DOI] [PubMed] [Google Scholar]
- 26. Valadares ER, Pizarro MX, Oliveira LR, et al. Juvenile neuronal ceroid‐lipofuscinosis: clinical and molecular investigation in a large family in Brazil. Arq Neuropsiquiatr. 2011;69(1):13‐18. [DOI] [PubMed] [Google Scholar]
- 27. Venkateswaran S, McMillan HJ, Doja A, Humphreys P. Adolescent onset cognitive regression and neuropsychiatric symptoms associated with the A140V MECP2 mutation. Dev Med Child Neurol. 2014;56(1):91‐94. [DOI] [PubMed] [Google Scholar]
- 28. Verhoeven WMA, Egger JIM, Koolen DA, et al. Beta‐propeller protein‐associated neurodegeneration (BPAN), a rare form of NBIA: novel mutations and neuropsychiatric phenotype in three adult patients. Parkinsonism Relat Disord. 2014;20(3):332‐336. [DOI] [PubMed] [Google Scholar]
- 29. Fournier KA, Hass CJ, Naik SK, Lodha N, Cauraugh JH. Motor coordination in autism spectrum disorders: a synthesis and meta‐analysis. J Autism Dev Disord. 2010. Oct;40(10):1227‐1240. [DOI] [PubMed] [Google Scholar]
- 30. Vernazza‐Martin S, Martin N, Vernazza A, et al. Goal directed locomotion and balance control in autistic children. J Autism Dev Disord. 2005. Feb;35(1):91‐102. [DOI] [PubMed] [Google Scholar]
- 31. Hollander E, Wang AT, Braun A, Marsh L. Neurological considerations: autism and Parkinson's disease. Psychiatry Res. 2009;170(1):43‐51. [DOI] [PubMed] [Google Scholar]
- 32. Esposito G, Venuti P. Analysis of toddlers' gait after six months of independent walking to identify autism: a preliminary study. Percept Mot Skills. 2008;106(1):259‐269. [DOI] [PubMed] [Google Scholar]
- 33. Licari MK, Alvares GA, Varcin K, et al. Prevalence of motor difficulties in autism Spectrum disorder: analysis of a population‐based cohort. Autism Res off J Int Soc Autism Res. 2020;13(2):298‐306. [DOI] [PubMed] [Google Scholar]
- 34. Bhat AN. Is motor impairment in autism spectrum disorder distinct from developmental coordination disorder? A report from the SPARK study. Phys Ther. 2020;100(4):633‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ketcheson LR, Pitchford EA, Wentz CF. The relationship between developmental coordination disorder and concurrent deficits in social communication and repetitive behaviors among children with autism Spectrum disorder. Autism Res. 2021;14(4):804‐816. [DOI] [PubMed] [Google Scholar]
- 36. Rydzewska E, Hughes‐McCormack LA, Gillberg C, et al. Prevalence of long‐term health conditions in adults with autism: observational study of a whole country population. BMJ Open. 2018. Sep 1;8(8):e023945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Woodward ND, Giraldo‐Chica M, Rogers B, Cascio CJ. Thalamocortical dysconnectivity in autism spectrum disorder: an analysis of the autism brain imaging data exchange. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2(1):76‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Uddin LQ, Supekar K, Menon V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front Hum Neurosci. 2013. Aug;7(7):458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nomi JS, Uddin LQ. Developmental changes in large‐scale network connectivity in autism. NeuroImage Clin. 2015;7:732‐741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lunati A, Lesage S, Brice A. The genetic landscape of Parkinson's disease. Rev Neurol (Paris). 2018;174(9):628‐643. [DOI] [PubMed] [Google Scholar]
- 41. Lim KL, Ng XH, Grace LGY, Yao TP. Mitochondrial dynamics and Parkinson's disease: focus on parkin. Antioxid Redox Signal. 2012;16(9):935‐949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mariani M, Crosti F, Redaelli S, et al. Partial duplication of the PARK2 gene in a child with developmental delay and her normal mother: a second report. Am J Med Genet Part B Neuropsychiatr Genet. 2013;162B(5):485‐486. [DOI] [PubMed] [Google Scholar]
- 43. Scheuerle A, Wilson K. PARK2 copy number aberrations in two children presenting with autism spectrum disorder: further support of an association and possible evidence for a new microdeletion/microduplication syndrome. Am J Med Genet Part B Neuropsychiatr Genet. 2011;156B(4):413‐420. [DOI] [PubMed] [Google Scholar]
- 44. Anders S, Sack B, Pohl A, et al. Compensatory premotor activity during affective face processing in subclinical carriers of a single mutant Parkin allele. Brain J Neurol. 2012;135(Pt 4):1128‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. DiCarlo GE, Aguilar JI, Matthies HJ, et al. Autism‐linked dopamine transporter mutation alters striatal dopamine neurotransmission and dopamine‐dependent behaviors. J Clin Invest. 2019;129(8):3407‐3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Herborg F, Andreassen TF, Berlin F, Loland CJ, Gether U. Neuropsychiatric disease‐associated genetic variants of the dopamine transporter display heterogeneous molecular phenotypes. J Biol Chem. 2018;293(19):7250‐7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lewis MH, Tanimura Y, Lee LW, Bodfish JW. Animal models of restricted repetitive behavior in autism. Behav Brain Res. 2007;176(1):66‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ernst M, Zametkin AJ, Matochik JA, Pascualvaca D, Cohen RM. Low medial prefrontal dopaminergic activity in autistic children. Lancet. 1997;350(9078):638. [DOI] [PubMed] [Google Scholar]
- 49. Scott‐Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer SY. Reward processing in autism. Autism Res. 2010;3(2):53‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zürcher NR, Walsh EC, Phillips RD, et al. A simultaneous [11C]raclopride positron emission tomography and functional magnetic resonance imaging investigation of striatal dopamine binding in autism. Transl Psychiatry. 2021;11(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sears LL, Vest C, Mohamed S, Bailey J, Ranson BJ, Piven J. An MRI study of the basal ganglia in autism. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23(4):613‐624. [DOI] [PubMed] [Google Scholar]
- 52. Langen M, Durston S, Staal WG, Palmen SJMC, van Engeland H. Caudate nucleus is enlarged in high‐functioning medication‐naive subjects with autism. Biol Psychiatry. 2007;62(3):262‐266. [DOI] [PubMed] [Google Scholar]
- 53. McAlonan GM, Cheung V, Cheung C, et al. Mapping the brain in autism. A voxel‐based MRI study of volumetric differences and intercorrelations in autism. Brain. J Neurol. 2005;128(Pt 2):268‐276. [DOI] [PubMed] [Google Scholar]
- 54. Mergy MA, Gowrishankar R, Gresch PJ, et al. The rare DAT coding variant Val559 perturbs DA neuron function, changes behavior, and alters in vivo responses to psychostimulants. Proc Natl Acad Sci U S A. 2014;111(44):E4779‐E4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Malik‐Soni N, Shaker A, Luck H, et al. Tackling healthcare access barriers for individuals with autism from diagnosis to adulthood. Pediatr Res. 2021;25:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Parnetti L, Gaetani L, Eusebi P, et al. CSF and blood biomarkers for Parkinson's disease. Lancet Neurol. 2019;18(6):573‐586. [DOI] [PubMed] [Google Scholar]
- 57. Magdalinou NK, Paterson RW, Schott JM, et al. A panel of nine cerebrospinal fluid biomarkers may identify patients with atypical parkinsonian syndromes. J Neurol Neurosurg Psychiatry. 2015;86(11):1240‐1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hansson O, Janelidze S, Hall S, et al. Blood‐based NfL: a biomarker for differential diagnosis of parkinsonian disorder. Neurology. 2017;88(10):930‐937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Herbert MK, Aerts MB, Beenes M, et al. CSF neurofilament light chain but not FLT3 ligand discriminates parkinsonian disorders. Front Neurol. 2015;6:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. PRISMA flow diagram.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


