Abstract
Objective
Use of tacrolimus in mild to moderate myasthenia gravis (MG) is generally limited to glucocorticoid‐refractory cases; the advantage of mono‐tacrolimus over mono‐glucocorticoids is unknown.
Methods
We included mild to moderate MG patients treated with mono‐tacrolimus (mono‐TAC) or mono‐glucocorticoids (mono‐GC). The correlation between the immunotherapy options and the treatment efficacy and side effects were examined in 1:1 propensity‐score matching. The main outcome was time to minimal manifestations status or better (MMS or better). Secondary outcomes include time to relapse, the mean changes in Myasthenia Gravis‐specific Activities of Daily Living (MG‐ADL) scores and the rate of adverse events.
Results
Baseline characteristics showed no difference between matched groups (49 matched pairs). There were no differences in median time to MMS or better between the mono‐TAC group and mono‐GC group (5.1 vs. 2.8 months: unadjusted hazard ratio [HR], 0.73; 95% CI, 0.46–1.16; p = 0.180), as well as in median time to relapse (data unavailable for the mono‐TAC group since 44 of 49 [89.8%] participants remained in MMS or better; 39.7 months in mono‐GC group: unadjusted HR, 0.67; 95% CI, 0.23–1.97; p = 0.464). Changes in MG‐ADL scores between the two groups were similar (mean differences, 0.3; 95% CI, −0.4 to 1.0; p = 0.462). The rate of adverse events was lower in the mono‐TAC group compared to the mono‐GC group (24.5% vs. 55.1%, p = 0.002).
Interpretation
Mono‐tacrolimus performs superior tolerability with non‐inferior efficacy compared to mono‐glucocorticoids in mild to moderate myasthenia gravis patients who refuse or have a contraindication to glucocorticoids.
Introduction
Myasthenia gravis (MG) is an autoimmune disease with significant public and personal health burden that requires long‐term treatment and is prone to recurrence. 1 Glucocorticoids are commonly used as first‐line immunosuppressant therapy in MG patients displaying residual symptoms after treatment with cholinesterase inhibitor. It is highly effective for the majority of patients. However, the use of glucocorticoids is limited due to its poor response in the remaining 20%–30% of patients, causing initial worsening of symptoms, drug tapering‐related exacerbation and adverse events‐related drug withdrawal. 2 The exacerbation of existing comorbidities on metabolism, osteoporosis, or cardiovascular disease is an important concern under the increasing prevalence of elderly patients. 3 , 4 Moreover, appreciable quantity of young patients refuse to use glucocorticoids because of fear of its side effects in clinic. Therefore, optimal therapeutic regimens to alleviate symptoms, improve treatment tolerance and match complex conditions are required for different MG patients.
Tacrolimus is successfully used as a steroid‐sparing agent in conjunction with glucocorticoids to reduce the dosage and side effects of glucocorticoids in MG patients. 5 It has also been used as monotherapy for induced remission of various symptoms with fast onset in MG patients. 6 , 7 More recently, a retrospective, noncontrolled study showed the favorable effects of mono‐tacrolimus initial immunotherapy on MG. 8 However, no study has compared the effectiveness and safety between mono‐tacrolimus and mono‐glucocorticoids regimens in MG.
The aim of this study was to compare clinical outcomes between mono‐tacrolimus and mono‐glucocorticoid treatment in mild to moderate MG patients, and to seek for potential factors that potentially predict clinical effectiveness with mono‐tacrolimus as initial immunotherapy.
Materials and Methods
Participants and study population
We retrospectively identified participants registered from 1 July 2017 to 30 June 2021 in the Myasthenia Gravis Trial Database in Xuanwu Hospital, Capital Medical University. The Ethics Committee of Xuanwu Hospital approved this study (No. 2017084) and each participant provided written informed consent for participation. This cohort study followed the reporting guidelines of Strengthening the Reporting of Observational Studies in Epidemiology (STROBE). MG was diagnosed based on a fluctuating muscle weakness together with at least one test positive result among following tests: (a) anti‐AChR, anti‐MuSK, or anti‐LRP4 antibodies; (b) repetitive nerve stimulation or single‐fiber electromyography; and (c) response to acetylcholinesterase therapy. Seronegative MG patients suspected to have congenital myasthenic syndromes were excluded by gene sequencing.
Of the 825 MG patients in the database, participants were included and excluded based on the criteria as shown in Fig. 1. The baseline was defined as the time of first administration of immunotherapy. Patients who received mono‐tacrolimus (mono‐TAC group) or mono‐glucocorticoids (mono‐GC group) as initial treatment were included. Patients were excluded in three categories: (1) Unavailable data: lost to follow‐up, no baseline Myasthenia Gravis‐specific Activities of Daily Living (MG‐ADL) scores, no baseline Quantitative Myasthenia Gravis (QMG) scores, poor treatment compliance, or death with unclear report; (2) Undesired study population: patients with MGFA class IV or V, age younger than 18 years, or baseline MG‐ADL less than 2; (3) Interference in effectiveness evaluation: received intravenous immunoglobulin or plasma exchange within 4 weeks prior to baseline, underwent thymectomy within 24 weeks prior to baseline, or treated for shorter than 6 months from baseline.
Figure 1.
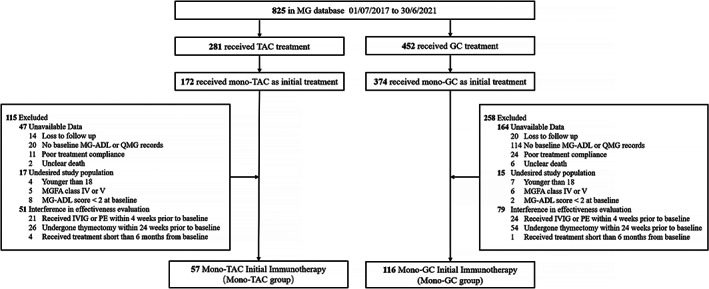
Flowchart of the participants included in the current study. Based on the inclusion and exclusion criteria, mono‐TAC group and mono‐GC group were identified for testing the association between immunotherapy choice and clinical outcomes. The factors associated with treatment response to tacrolimus were assessed in mono‐TAC group. MG, myasthenia gravis; TAC, tacrolimus; GC, glucocorticoids; MG‐ADL, Myasthenia Gravis‐specific Activities of Daily Living scale; QMG, quantitative MG score; IVIG, intravenous immunoglobulin; PE, plasma exchange; MGFA class, Myasthenia Gravis Foundation of America clinical classification.
Therapeutic regimens
The regimens of glucocorticoids or tacrolimus were formulated based on the international consensus guidance for management of myasthenia gravis in 2016. 5 In patients with glucocorticoids therapy, oral prednisone or intravenous methylprednisolone were given based on disease severity. The initial dose of oral prednisone in 110 patients was 15 or 20 mg, and was increased by 10 mg every one week up to 0.5–1.0 mg/kg of body weight per day for maintenance treatment. In 6 patients, methylprednisolone was given intravenously with an initial daily dose of 500 mg and decreased by half every 3 days to 120 mg for 3 days before switching to prednisone 60 mg per day, and then gradually decreased to the maintenance dose based on the clinical efficacy. None of the six patients had obviously transient deterioration of myasthenic symptoms during the pulse therapy. Patients were treated with the maintenance dose until clinical remission was confirmed or intolerable side effects occurred. Then the dosage was reduced further by 5 mg every 2 weeks until 20 mg with subsequent tapering of 5 mg every 1–3 months. A continued 5 mg per day of prednisone for at least 1 year helped to maintain the clinical remission status. A nonsteroidal immunosuppressive was added to glucocorticoids in case of contraindications, intolerability, or insufficient clinical disease control. 5
All patients with tacrolimus therapy were treated with an initial daily dose of 2 mg. Adjustment of tacrolimus regimen was based on clinical efficacy, side effects and tacrolimus trough concentration. According to physician preference, patients were permitted to take more tacrolimus or add Wuzhi tablets to achieve adequate concentration at a range of 4.8–10 ng/mL. 9 Wuzhi is a herbal medicine prepared from wuweizi (Schisandra sphenanthera). It increases the oral bioavailability of tacrolimus by inhibiting P‐gp‐mediated efflux and CYP3A‐mediated metabolism of tacrolimus. 10 Tacrolimus concentrations at steady state were monitored after 1 month of treatment by microparticle enzyme immunoassay. The tapering of tacrolimus was considered when clinical remission was maintained for at least 6 months. The dosage was reduced by no more than 1 mg per day every 6 months.
Data collection and outcome measurement
Demographic and clinical variables were retrieved from the MG database, include sex, age, age at onset, disease course, stage of disease at beginning of immunotherapy, therapeutic regimen, drug dose, tacrolimus concentration, thymectomy, thymoma, serum antibodies, MGFA classification, MG‐ADL scores, QMG scores, and MGFA post‐intervention status (MGFA‐PIS). Onset age at 50 year old was set as a cutoff point between early‐onset (<50 years) and late‐onset (≥50 years). 11 Disease stage at the beginning of immunotherapy was divided into early‐stage group (within 12 months) and late‐stage group (longer than 12 months).
The primary endpoint for effectiveness was the time to MMS or better. 5 The main secondary endpoint was the time to relapse, which was defined as recurrence of MG symptoms or a substantial increase in MG medications after the patient achieved MMS or better. 12 Other secondary endpoints include the changes in MG‐ADL scores from baseline to the end of the third month treatment (ΔMG‐ADL = [MG‐ADL scores at the third month] – [baseline MG‐ADL scores]), incidence of potential adverse events, and drug withdrawal. The MG‐ADL questionnaire was administered by telephone or face‐to‐face during clinic visits. Clinical assessment was performed at a fixed interval from the last administration of cholinesterase inhibitor to avoid modification by pyridostigmine. Gastrointestinal symptoms include nausea, diarrhea, abdominal pain and constipation. Elevation above the normal upper limit of blood urea nitrogen (BUN)/serum creatinine (sCr) and liver enzymes were recorded as renal and liver injury.
Statistical analysis
Differences in demographic and clinical features at baseline were evaluated between groups with Wilcoxon rank‐sum test for continuous variables and with the chi‐square test for categorical variables. Categorical variables were reported as portion of patients (%). Continuous variables were reported as mean with standard deviation [SD] or median with interquartile range [IQR]. To improve the balance of baseline characteristics, propensity score matching of age, sex, MGFA classification, disease course, stage of disease at beginning of immunotherapy and MG‐ADL score at baseline were used to create a 1 to 1 matched data set. Density plots of the distribution of propensity scores before and after matching for each group are shown in eFig. 1. Standardized mean differences between groups before and after propensity score matching were calculated (eFig. 2). Group differences in time‐to‐events were estimated by hazard ratios (HRs) and 95% confidence intervals (CIs) through Kaplan–Meier survival curve and unadjusted Cox proportional hazard regression model. Patients who had not yet achieved events were right‐censored at the last available follow‐up. An adjusted Cox proportional hazard regression model was used to investigate the association between achievement of events and multiple variables. Paired t‐tests were performed for within‐group comparisons in the ΔMG‐ADL. Between‐group differences in the ΔMG‐ADL were tested with analysis of covariance to adjust for baseline MG‐ADL scores. If MG‐ADL scores at the end of third month treatment were missing, the last record of MG‐ADL within the first 3 months was used to estimate MG‐ADL scores. The optimal cut‐off value was evaluated with receiver‐operating characteristics (ROC) curves by defining a point with Youden's index (sensitivity + specificity‐1) at maximum. Analysis and figures regarding the propensity score matching model were performed with R statistical software (version 3.6.0); in particular, the Nonrandom and the MatchIt packages with specified seed set at 1234 were used to ensure the consistency of repeated calculations. Other statistical analyses and figures were performed using SPSS (version 22.0) and Prism (version 8.3.0). p value < .05 was considered statistically significant.
Results
Baseline characteristics
We identified 57 and 116 patients in the mono‐TAC group and mono‐GC group, respectively. Patients in the mono‐TAC group were prescribed with tacrolimus monotherapy because glucocorticoids or other immunosuppressants were contraindicated in 38 patients or refused in 19 patients. Baseline characteristics were similar between the unmatched mono‐TAC group and mono‐GC group, which include sex, MGFA classification, serum antibodies, disease severity at baseline as reflected by MG‐ADL and QMG scores, time from disease onset to initial immunotherapy, follow‐up time after immunotherapy initiation, thymectomy, and thymoma. Compared with the mono‐GC group, the mono‐TAC group has a lower proportion of patients with early‐onset (21.1% vs. 45.7%, p = 0.002) and immunotherapy initiation at early‐stage (59.6% vs. 76.7%, p = 0.020). Patients in the mono‐TAC group were significantly older than those in the mono‐GC group (59.4 yr vs. 50.1 yr, p < .001) (Table 1).
Table 1.
Baseline characteristics of study participants in mono‐TAC group and mono‐GC group.
| Characteristics | Unmatched (n = 173) | Matched (n = 98) | ||||
|---|---|---|---|---|---|---|
| Mono‐TAC group (n = 57) | Mono‐GC group (n = 116) | p value | Mono‐TAC group (n = 49) | Mono‐GC group (n = 49) | p value | |
| Age, mean (SD), y | 59.4 (13.7) | 50.1 (15.7) | <0.001 | 57.5 (13.8) | 57.4 (12.0) | 0.504 |
| Age at onset, mean (SD), y | 58.0 (15.3) | 49.1 (16.1) | <0.001 | 56.3 (15.7) | 55.9 (13.4) | 0.440 |
| Early‐onset MG a , No. (%) | 12 (21.1) | 53 (45.7) | 0.002 | 12 (24.5) | 12 (24.5) | >0.99 |
| Female, No. (%) | 25 (43.9) | 43 (37.1) | 0.390 | 19 (38.8) | 19 (38.8) | >0.99 |
| MGFA classification, No. (%) | 0.372 | 0.838 | ||||
| I | 21 (36.8) | 51 (44.0) | 21 (42.9) | 20 (40.8) | ||
| II‐III | 36 (63.2) | 65 (56.0) | 28 (57.1) | 29 (59.2) | ||
| AChR‐Ab positive, No. (%) | 51 (89.5) | 95 (81.9) | 0.197 | 43 (87.8) | 42 (85.7) | 0.766 |
| MG‐ADL at baseline, median (IQR) | 6.0 (3.0–7.0) | 5.0 (4.0–6.0) | 0.359 | 6.0 (3.0–7.0) | 5.0 (4.0–7.0) | 0.911 |
| ΔMG‐ADL b | −3.0 ([−1.0]–[−5.0]) | −3.0 ([−2.0]–[−5.0]) | 0.201 | −3.0 ([−1.0]–[−5.0]) | −3.0 ([−2.0]–[−5.0]) | 0.167 |
| QMG at baseline, median (IQR) | 7.0 (5.0–10.0) | 6.5 (5.0–10.0) | 0.594 | 7.0 (5.0–10.0) | 7.0 (5.0–10.0) | 0.617 |
| Time from MG onset to initial IS treatment, median (IQR), mo | 6.0 (2.5–20.2) | 5.4 (2.2–10.9) | 0.301 | 4.4 (2.2–19.5) | 6.9 (3.1–29.5) | 0.197 |
| Initial IS at early‐stage c , No. (%) | 34 (59.6) | 89 (76.7) | 0.020 | 33 (67.3) | 33 (67.3) | >0.99 |
| Follow‐up time after initial IS, mean (SD), mo | 16.9 (10.9) | 20.9 (14.2) | 0.194 | 17.7 (10.8) | 23.0 (14.0) | 0.077 |
| Thymectomy, No. (%) | 6 (10.5) | 9 (7.8) | 0.572 | 5 (10.2) | 3 (6.1) | 0.715 |
| Thymoma, No. (%) | 2 (3.5) | 8 (6.9) | 0.500 | 1 (2.0) | 3 (6.1) | 0.617 |
AChR‐Ab, acetylcholine receptor antibodies; GC, glucocorticoids; IQR, interquartile range; IS, immunosuppression agents; MG, myasthenia gravis; MGFA classification, Myasthenia Gravis Foundation of America clinical classification; MG‐ADL, Myasthenia Gravis‐specific Activities of Daily Living scale; n, number of participants; QMG, quantitative MG score; SD, standard deviation; TAC, tacrolimus.
Onset age was younger than 50 year old.
Changes of MG‐ADL scores at the third month.
Patients started tacrolimus within 12 month from disease onset.
Immunotherapy treatment response
We compared the treatment response between mono‐tacrolimus and mono‐glucocorticoids regimens. To address possible hidden risk factors represented in the demographic differences in age, proportion of early‐onset and proportion of initial immunotherapy treatment at early‐stage, we conducted a propensity score‐matched cohort analysis. The matched cohort analysis showed no difference in baseline characteristics between the mono‐TAC group and mono‐GC group with 49 patients in each (Table 1). Kaplan–Meier curves showed no difference in median time to MMS or better between two groups (5.1 months in mono‐TAC group vs. 2.8 months in mono‐GC group: unadjusted HR, 0.73; 95% CI, 0.46–1.16; p = 0.180) (Fig. 2A), as well as in median time to relapse (data unavailable for the mono‐TAC group since 44 of 49 [89.8%] participants remained in MMS or better; 39.7 months in the mono‐GC group: unadjusted HR, 0.67; 95% CI, 0.23–1.97; p = 0.464) (Fig. 2B). Changes in MG‐ADL scores at month 3 (ΔMG‐ADL) were not significantly different between the groups by a mean differences of 0.3 (95% CI, −0.4 to 1.0; p = 0.462), with a change (mean ± SD) of −2.9 ± 2.1 in the mono‐TAC group vs. −3.1 ± 1.9 in the mono‐GC group (Fig. 2C). Furthermore, subgroup analysis in generalized MG patients showed no difference in median time to MMS or better between two groups, as well as in median time to relapse (eFig. 3). There was no ocular patient with mono‐TAC therapy relapsing during follow‐up period.
Figure 2.
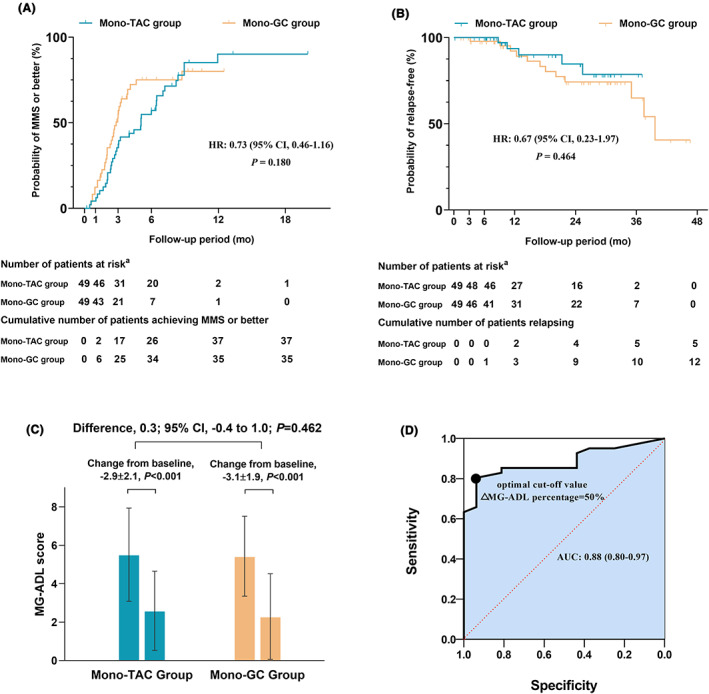
(A) Kaplan–Meier estimates of time to MMS or better in matched mono‐TAC group and mono‐GC group, plotted for subgroups by site of immunotherapy choice. There were 72 events recorded in 98 participants. Patient numbers at each follow‐up period are listed. (B) Kaplan–Meier estimate of relapse in matched mono‐TAC group and mono‐GC group, plotted for subgroups by site of immunotherapy choice. There were 17 events recorded in 98 participants. Patient numbers at each follow‐up period are listed. (C) Change in MG‐ADL scores from baseline to the end of the third month treatment in matched mono‐TAC and mono‐GC groups. Significant changes in MG‐ADL scores from baseline to the end of the third month treatment were observed in matched mono‐TAC and mono‐GC groups. There was no significant between‐group difference in favor of immunotherapy choices for MG‐ADL scores. (D) The receiver‐operating characteristics (ROC) curve represents the area under the curve (AUC) and 95% confidence interval (CI) are 0.88 and 0.80–0.97, respectively. The optimal cut‐off value of ΔMG‐ADL percentage was 50% (sensitivity, 80.5%; specificity, 93.8%).
A better tolerability was observed in the unmatched mono‐TAC group than in the unmatched mono‐GC group (24.6% vs. 41.4%, p = 0.03), and the advantage became more obvious in matched groups (24.5% vs. 55.1%, p = 0.002) (Table 2). A different profile of adverse events was observed, although the drug discontinuation rate due to adverse events were similar between the matched mono‐TAC group and matched mono‐GC group (6.1% vs. 8.2%, p > .99). Compared with the matched mono‐GC group, the matched mono‐TAC group had fewer patients experiencing blood glucose increases (1/49 vs. 9/49, p = 0.016), and there was no incidence of weight gain (0/49 vs. 12/49, p < .001) and osteoporosis (0/49 vs. 6/49, p = 0.027). All adverse events were resolved after dose reduction or drug withdrawal. No death occurred in either group.
Table 2.
Summary of potential drug‐related events reported in mono‐TAC group and mono‐GC group.
| Unmatched (n = 173) | Matched (n = 98) | |||||
|---|---|---|---|---|---|---|
| Mono‐TAC group, n (% of 57) | Mono‐GC group, n (% of 116) | p value | Mono‐TAC Group, n (% of 49) | Mono‐GC Group, n (% of 49) | p value | |
| Patients with any adverse events | 14 (24.6) | 48 (41.4) | 0.03 | 12 (24.5) | 27 (55.1) | 0.002 |
| Discontinuation due to adverse events | 4 (7.0) | 11 (9.5) | 0.776 | 3 (6.1) | 4 (8.2) | >0.99 |
| Total number of adverse events | 16 | 62 | 13 | 44 | ||
| BUN/sCr elevation | 3 a | 2 | 0.333 | 3 a | 2 | >0.99 |
| Liver enzyme elevation | 1 a | 0 | 0.329 | 0 | 0 | >0.99 |
| Tremor | 2 | 0 | 0.107 | 2 | 0 | 0.495 |
| Gastrointestinal Symptoms | 4 a | 2 a | 0.093 | 2 a | 2 a | >0.99 |
| Joint pain | 1 a | 0 | 0.329 | 1 a | 0 | >0.99 |
| Dizziness | 1 | 2 | >0.99 | 1 | 1 | >0.99 |
| Osteoporosis | 0 | 7 b | 0.097 | 0 | 6 b | 0.027 |
| Weight gain | 0 | 18 a | 0.002 | 0 | 12 | <0.001 |
| Leg cramps | 0 | 5 | 0.173 | 0 | 4 | 0.117 |
| Moon face | 0 | 4 | 0.304 | 0 | 2 | 0.495 |
| Insomnia | 0 | 4 | 0.304 | 0 | 3 | 0.242 |
| High blood pressure | 1 | 2 | >0.99 | 1 | 1 | >0.99 |
| Blood glucose increased | 1 | 13 | 0.037 | 1 | 9 | 0.016 |
| Blood uric acid increased | 1 | 0 | 0.329 | 1 | 0 | >0.99 |
| Blood lipid increased | 1 | 3 | >0.99 | 1 | 2 | >0.99 |
BUN, blood urea nitrogen; GC, glucocorticoids; n, number of patients; sCr, serum creatinine; TAC, tacrolimus.
One patient discontinued therapy due to this adverse event.
Three patients discontinued therapy due to this adverse event.
Factors associated with treatment response to tacrolimus
We assessed the factors associated with time to MMS or better or relapse to explore outcome predictors for mono‐tacrolimus treatment by a Cox proportional hazard model. As showed in Table 3, in the unadjusted model, the time to MMS or better is associated with disease stage at the beginning of immunotherapy (4.4 months in early‐stage group vs. 7.1 months in late‐stage group: unadjusted HR, 2.22; 95% CI, 1.12–4.39; p = 0.022) and with changes of MG‐ADL at month 3 (ΔMG‐ADL) (unadjusted HR, 0.85; 95% CI, 0.76–0.95; p = 0.005). In the adjusted model, besides association with disease stage (adjusted HR, 2.97; 95% CI, 1.43–6.15; p = 0.003) and ΔMG‐ADL (adjusted HR, 0.75; 95% CI, 0.65–0.86; p < .001), the time to MMS or better is also associated with MGFA class I with reference of other classes (adjusted HR, 2.80; 95% CI, 1.34–5.84; p = 0.006). However, the time to MMS or better is not associated with age, age of onset, sex, AChR‐Ab, QMG scores at baseline, tacrolimus trough concentration or thymectomy (p > .05). No factors affecting relapse time were found (eTable 1).
Table 3.
Variables associated with time to MMS or better in mono‐tacrolimus treatment predicted by Cox proportional hazard model.
| Variables | Unadjusted HR with 95% CI | p value | Adjusted HR with 95% CI | p value |
|---|---|---|---|---|
| Age | 0.99 (0.97–1.02) | 0.542 | NA | NA |
| Early‐stage group a (Ref., Late‐stage group) | 2.22 (1.12–4.39) | 0.022 | 2.97 (1.43–6.15) | 0.003 |
| Early‐onset MG b (Ref., Late‐onset MG) | 0.73 (0.32–1.66) | 0.452 | NA | NA |
| Female (Ref., Male) | 0.62 (0.33–1.17) | 0.136 | NA | 0.500 |
| MGFA class I (Ref., class II‐III) | 1.68 (0.88–3.21) | 0.115 | 2.80 (1.34–5.84) | 0.006 |
| AChR‐Ab positive (Ref., negative) | 0.75 (0.26–2.12) | 0.585 | NA | NA |
| ΔMG‐ADL c | 0.85 (0.76–0.95) | 0.005 | 0.75 (0.65–0.86) | <0.001 |
| QMG at baseline | 0.96 (0.87–1.06) | 0.392 | NA | NA |
| Tacrolimus trough concentration | 1.04 (0.86–1.24) | 0.712 | NA | NA |
| Thymectomy (Ref., No thymectomy) | 1.03 (0.32–3.35) | 0.965 | NA | NA |
AChR‐Ab, acetylcholine receptor antibodies; MG, myasthenia gravis; MGFA classification, Myasthenia Gravis Foundation of America clinical classification; MG‐ADL, Myasthenia Gravis‐specific Activities of Daily Living scale; QMG, quantitative MG score.
Patients started tacrolimus within 12 month from disease onset.
Onset age was younger than 50 year old.
Changes of MG‐ADL scores at the third month.
Furthermore, we converted the change of MG‐ADL scores at the third month into ΔMG‐ADL percentage defined as ‐[ΔMG‐ADL]/[baseline MG‐ADL scores] for Receiver‐Operating Characteristics (ROC) analyses. That revealed the achievement of MMS or better is associated with ΔMG‐ADL percentage (area under the receiver operating characteristic curve, 0.88; 95% CI, 0.80–0.97, p < .001). The optimal cut‐off value was 50% (sensitivity, 80.5%; specificity, 93.8%) (Fig. 2D).
Discussion
We found mono‐tacrolimus had equivalent effectiveness in treating mild to moderate myasthenia gravis compared to mono‐glucocorticoids but with lower incidence of adverse events. Thus, mono‐tacrolimus is a promising option in mild to moderate MG patients, particularly benefitting those who refused steroids treatment or when other immunosuppressants were contraindicated. Furthermore, our analysis also demonstrated that mono‐tacrolimus as initial immunotherapy could be more effective in patients with MGFA class I (vs. MGFA class II to III), within 12 months from MG onset (vs. longer than 12 months), or improving MG‐ADL no less than 50% at month three (vs. less than 50%).
Several non‐steroidal immunosuppressants, including azathioprine, mycophenolate mofetil, and cyclosporin A, could be effective as a singular immunotherapy for MG patients, for whom steroids were contraindicated or refused. 13 , 14 , 15 However, the application of these regimens could be limited due to their slow onset of action and adverse events. 2 , 13 Previous studies suggest treatment of tacrolimus in MG may circumvent these limitations. 6 , 7 , 8 , 16 Mono‐tacrolimus has been applied in membranous nephropathy, refractory ulcerative colitis and heart transplantation. 17 , 18 , 19 , 20 , 21 Our results demonstrated that mono‐tacrolimus therapy has fast action onset with minimum adverse events in MG. Tacrolimus exerts its effect by inhibiting the calcineurin activity and downregulating the transcription of inflammatory cytokines, which results in the suppression of T‐cells activity and antibody production in B cells. 22 Tacrolimus also improves ryanodine receptor function at an early phase resulting in the enhancement of skeletal strength, which explains its fast‐action in treating MG. 23 However, to our best knowledge, no study has been reported to compare the efficacy and side effects between mono‐tacrolimus therapy and mono‐glucocorticoid therapy in these diseases. Our results also showed that mono‐tacrolimus had noninferior effectiveness and fewer adverse events compared to mono‐glucocorticoids in MG. The most frequent adverse events of tacrolimus were BUN/sCr elevation, tremor and gastrointestinal symptoms. All of the adverse events were mild and reversible. The favorable safety profile was consistent with our previous study. 6 This is encouraging. Because complex comorbidities in elder MG patients limits the application of steroids, our findings suggest extensive application of mono‐tacrolimus in elder MG patients would be beneficial. The cost‐effectiveness of drug should be an important concern in the treatment of myasthenia gravis. As we observed in our clinic, nearly all of the patients could afford the treatment with tacrolimus and very few cases withdrew the drug for the economic reason. Unfortunately, there is no data about cost‐effectiveness of mono‐tacrolimus therapy in published studies or in our current study, a dedicated cost analysis comparing tacrolimus to glucocorticoids would be of benefit.
Although the rate of achieving MMS or better gradually increased to 75% in mono‐TAC group, the remaining 25% of patients suffered a prolonged and insufficient treatment. Accurate prediction of final outcomes and early modification of mono‐TAC regimen are of utmost importance. In this study, we found that ocular MG, an earlier initiation of tacrolimus treatment or a sufficient response within the first 3 months is important for a desirable outcome in MG patients with mono‐tacrolimus. The greater effectiveness for ocular MG in the present study was in accordance with our previous findings. 6 Furthermore, there were no statistically differences between mono‐tacrolimus and mono‐glucocorticoids in therapeutic effect of inducing remission and reducing relapsing for generalized MG. Mono‐TAC therapy is a considerable regimen to benefit both ocular and generalized MG. Structural changes in long‐term damage of the neuromuscular junction, such as loss of synaptic folds, widened clefts, and relocation of the nerve terminal, may block the effects of therapy, which may explains the particular effectiveness of early intervention of immunotherapy. 24 The advantage of early intervention with tacrolimus in MG patients were reported and recommended in clinical guidelines for MG in Japan. 24 , 25 , 26 Our results demonstrated improved prognosis with initiation of mono‐tacrolimus within 12 months from MG onset. Favorable response at month 3 has been reported as a predictor for effectiveness in thymectomized MG patients with oral prednisone. 27 However, neither the role nor a cut‐off point of favorable response in the early phase is clear in MG patients with tacrolimus therapy. Our study demonstrated that no less than 50% improvement of MG‐ADL at month 3 could be a predictor for desirable outcomes. As such, mono‐tacrolimus therapy is more suitable than mono‐glucocorticoids in mild to moderate MG patients for whom ocular symptoms are predominant or immunotherapy is able to be initiated within 12 months from onset. Moreover, mono‐tacrolimus therapy could be adjusted or switch to other immunosuppressants according to the therapeutic effect within the first 3 months.
Robust evidence showed that tacrolimus has a dose‐dependent or concentration‐dependent effect in organ transplantation and other autoimmune diseases. 28 , 29 However, the correlation between tacrolimus concentration and therapeutic effect in MG is undetermined. Kanai et al. found a significantly higher rate of achievement of MMS or better in adequate concentration group (92.6%) than in low concentration group (54.2%). 9 Nagane et al. observed no significant differences of tacrolimus concentration in between good responders (5.95 ± 1.16 ng/mL) and poor responders (5.75 ± 1.14 ng/mL). 24 Our results also did not show the correlation. However, the optimal target range of tacrolimus concentration in MG still plays an important role in therapeutic drug monitoring. Further evaluation on the relationship between tacrolimus dosage and its effect in large MG population is necessary.
Limitations
This study has some limitations. It is an observational study and is limited by selection bias attributed to the retrospective nature of participation selection as well as small sample size. Review of the baseline characteristics indicated that patients in mono‐TAC group tended to be an older, lower proportion of early‐onset MG and lower proportion of immunotherapy initiation at early‐stage from disease onset. To mitigate confounding effects of baseline characteristics, we used propensity score‐based matching in combination with covariate adjustment in the statistical models. As shown by the standardized mean differences before and after matching, the differences between the groups were smaller after propensity score matching. It was reassuring that similar effects of treatment were observed in the propensity score–matched comparison. Furthermore, the effect of mono‐tacrolimus therapy might be inadequate to improve symptoms in MG severe cases. The use of mono‐tacrolimus as initial immunotherapy should be limited for mild to moderate MG, but not probably for severe cases. Further prospective studies or high‐quality randomized controlled trials are needed to clarify the comparative safety and effective outcomes of tacrolimus monotherapy in a large population.
Conclusion
Mono‐tacrolimus as initial immunotherapy is an effective and safe regimen for mild to moderate MG patients who refuse glucocorticoids due to potential adverse events or have contraindication to glucocorticoids. Ocular MG, initiation of tacrolimus within 12 months from MG onset, or improvement no less than 50% of MG‐ADL scores at the third month predict a desirable clinical outcome of mono‐tacrolimus treatment.
Author Contributions
ZF conceived the idea, designed the study, collect and analyze the data, wrote and revised the manuscript, and provided figures. LL, SS, SZ, NX and LL collect the data. YL, LD, MW and MX interpretated the data and revised the manuscript. XS and YD conceived the idea, designed the study, analyze the data and revised the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
Not applicable.
Funding Information
This work was supported by National Natural Science Foundation of China (No. 62171299), Clinical Cohort Study of Myasthenia Gravis, National Key R&D Program of China, Precision Medicine Project (No. 2017YFC0907700) and NIH R01 NS109491.
Conflict of Interest Statement
The authors declare no financial or other conflicts of interest.
Supporting information
Appendix S1.
Funding Statement
This work was funded by Clinical Cohort Study of Myasthenia Gravis, National Key R&D Program of China, Precision Medicine Project grant 2017YFC0907700; National Natural Science Foundation of China grant 62171299; NIH R01 grant NS109491.
Contributor Information
Xin‐Ming Shen, Email: shen.xinming@mayo.edu.
Yuwei Da, Email: dayuwei100@hotmail.com.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Fang W, Li Y, Mo R, et al. Hospital and healthcare insurance system record‐based epidemiological study of myasthenia gravis in southern and northern China. Neurol Sci. 2020;41(5):1211‐1223. [DOI] [PubMed] [Google Scholar]
- 2. Gotterer L, Li Y. Maintenance immunosuppression in myasthenia gravis. J Neurol Sci. 2016;369:294‐302. [DOI] [PubMed] [Google Scholar]
- 3. Fardet L, Flahault A, Kettaneh A, et al. Corticosteroid‐induced clinical adverse events: frequency, risk factors and patient's opinion. Br J Dermatol. 2007;157(1):142‐148. [DOI] [PubMed] [Google Scholar]
- 4. Cortes‐Vicente E, Alvarez‐Velasco R, Segovia S, et al. Clinical and therapeutic features of myasthenia gravis in adults based on age at onset. Neurology. 2020;94(11):e1171‐e1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sanders DB, Wolfe GI, Benatar M, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology. 2016;87(4):419‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fan Z, Li Z, Shen F, et al. Favorable effects of tacrolimus monotherapy on myasthenia gravis patients. Front Neurol. 2020;11:594152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yagi Y, Sanjo N, Yokota T, Mizusawa H. Tacrolimus monotherapy: a promising option for ocular myasthenia gravis. Eur Neurol. 2013;69(6):344‐345. [DOI] [PubMed] [Google Scholar]
- 8. Duan W, Peng Y, Jin W, Ouyang S, Yang H. Tacrolimus as single‐agent immunotherapy and minimal manifestation status in nonthymoma myasthenia gravis. J Immunol Res. 2021;2021:9138548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kanai T, Uzawa A, Kawaguchi N, et al. Adequate tacrolimus concentration for myasthenia gravis treatment. Eur J Neurol. 2017;24(2):270‐275. [DOI] [PubMed] [Google Scholar]
- 10. Qin XL, Bi HC, Wang XD, et al. Mechanistic understanding of the different effects of Wuzhi tablet (Schisandra sphenanthera extract) on the absorption and first‐pass intestinal and hepatic metabolism of tacrolimus (FK506). Int J Pharm. 2010;389(1–2):114‐121. [DOI] [PubMed] [Google Scholar]
- 11. Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. 2015;14(10):1023‐1036. [DOI] [PubMed] [Google Scholar]
- 12. Jaretzki AR, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Task force of the medical scientific advisory Board of the Myasthenia Gravis Foundation of America. Neurology. 2000;55(1):16‐23. [DOI] [PubMed] [Google Scholar]
- 13. Mantegazza R, Antozzi C, Peluchetti D, Sghirlanzoni A, Cornelio F. Azathioprine as a single drug or in combination with steroids in the treatment of myasthenia gravis. J Neurol. 1988;235(8):449‐453. [DOI] [PubMed] [Google Scholar]
- 14. Tindall RS, Rollins JA, Phillips JT, et al. Preliminary results of a double‐blind, randomized, placebo‐controlled trial of cyclosporine in myasthenia gravis. N Engl J Med. 1987;316(12):719‐724. [DOI] [PubMed] [Google Scholar]
- 15. Hehir MK, Burns TM, Alpers J, Conaway MR, Sawa M, Sanders DB. Mycophenolate mofetil in AChR‐antibody‐positive myasthenia gravis: outcomes in 102 patients. Muscle Nerve. 2010;41(5):593‐598. [DOI] [PubMed] [Google Scholar]
- 16. Itani K, Nakamura M, Wate R, et al. Efficacy and safety of tacrolimus as long‐term monotherapy for myasthenia gravis. Neuromuscul Disord. 2021;31:512‐518. [DOI] [PubMed] [Google Scholar]
- 17. Praga M, Barrio V, Juarez GF, Luno J. Tacrolimus monotherapy in membranous nephropathy: a randomized controlled trial. Kidney Int. 2007;71(9):924‐930. [DOI] [PubMed] [Google Scholar]
- 18. Liang Q, Li H, Xie X, Qu F, Li X, Chen J. The efficacy and safety of tacrolimus monotherapy in adult‐onset nephrotic syndrome caused by idiopathic membranous nephropathy. Ren Fail. 2017;39(1):512‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boschetti G, Nancey S, Moussata D, et al. Tacrolimus induction followed by maintenance monotherapy is useful in selected patients with moderate‐to‐severe ulcerative colitis refractory to prior treatment. Dig Liver Dis. 2014;46(10):875‐880. [DOI] [PubMed] [Google Scholar]
- 20. Baran DA, Zucker MJ, Arroyo LH, et al. Randomized trial of tacrolimus monotherapy: tacrolimus in combination, tacrolimus alone compared (the TICTAC trial). J Heart Lung Transplant. 2007;26(10):992‐997. [DOI] [PubMed] [Google Scholar]
- 21. Medjeral‐Thomas NR, Lawrence C, Condon M, et al. Randomized, controlled trial of tacrolimus and prednisolone monotherapy for adults with De novo minimal change disease: a multicenter, randomized, controlled trial. Clin J Am Soc Nephrol. 2020;15(2):209‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Siekierka JJ, Sigal NH. FK‐506 and cyclosporin a: immunosuppressive mechanism of action and beyond. Curr Opin Immunol. 1992;4(5):548‐552. [DOI] [PubMed] [Google Scholar]
- 23. Imai T, Tsuda E, Hozuki T, et al. Early effect of tacrolimus in improving excitation‐contraction coupling in myasthenia gravis. Clin Neurophysiol. 2012;123(9):1886‐1890. [DOI] [PubMed] [Google Scholar]
- 24. Nagane Y, Suzuki S, Suzuki N, Utsugisawa K. Factors associated with response to calcineurin inhibitors in myasthenia gravis. Muscle Nerve. 2010;41(2):212‐218. [DOI] [PubMed] [Google Scholar]
- 25. Wang L, Xi J, Zhang S, et al. Effectiveness and safety of tacrolimus therapy for myasthenia gravis: a single arm meta‐analysis. J Clin Neurosci. 2019;63:160‐167. [DOI] [PubMed] [Google Scholar]
- 26. Murai H, Utsugisawa K, Nagane Y, Suzuki S, Imai T, Motomura M. Rationale for the clinical guidelines for myasthenia gravis in Japan. Ann N Y Acad Sci. 2018;1413(1):35‐40. [DOI] [PubMed] [Google Scholar]
- 27. Lee I, Kuo HC, Aban IB, et al. Minimal manifestation status and prednisone withdrawal in the MGTX trial. Neurology. 2020;95(6):e755‐e766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43(10):623‐653. [DOI] [PubMed] [Google Scholar]
- 29. Wu B, Tong J, Ran Z. Tacrolimus therapy in steroid‐refractory ulcerative colitis: a review. Inflamm Bowel Dis. 2019;26:24‐32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


