Abstract
Objective
To evaluate the effect of overall peripheral inflammatory levels on cognitive function, we explored the relationship between established biomarkers of peripheral inflammation (circulating C‐reactive protein [CRP], interleukin‐6 [IL‐6], and tumor necrosis factor‐α [TNF‐α]) and cognitive decline by performing a review of observational studies and creating an updated summary.
Methods
We included literatures exploring the relationship between peripheral levels of CRP, IL‐6, and TNF‐α and subsequent cognitive decline, published until July 2022, by searching the following databases: PubMed, Embase, Web of Science, the Cochrane Library, ClinicalTrials, CNKI, and VIP databases. We used random‐effects models to pool the odds ratios (ORs) for the risks of subsequent cognitive decline in older adults with high levels of peripheral inflammation. We initially screened out 501 literatures, of which only 17 were ultimately eligible. Overall, there were 19,516 older individuals included in our meta‐analysis, and 2134 of them experienced subsequent cognitive change.
Results
Individuals with high levels of peripheral inflammation may have 14% more chance to develop subsequent cognitive decline than those with low levels (OR = 1.14, 95% CI: 1.03–1.27; p < 0.00001). In the subgroup analysis, the incidence of cognitive decline was higher in individuals with high levels of IL‐6. This study further demonstrates the link between systemic inflammation and cognitive status.
Interpretation
Detecting CRP, IL‐6, and TNF‐α in peripheral blood is necessary, as they may become effective indicators for forthcoming cognitive performance.
Introduction
Cognitive functions decline inevitably. 1 Age‐related cognitive decline manifests as the deterioration of memory and response sensitivity. 2 Previous studies on older adults reported that the proportion of cognitive decline can reach 28% 3 ; it not only affects the quality of life but also places heavy economic and social burdens on the family and society. 4 Currently, cognitive decline is generally diagnosed using neuropsychological assessment scales; however, it is time‐consuming, limited by poor patient compliance, and susceptible to the influence of educational levels. 5 Increasing evidence indicates that chronic low‐grade peripheral inflammation can significantly accelerate the development of cognitive deterioration 6 and can modulate neuroinflammation and the progression of neurodegeneration. 7
Peripheral inflammation increases with age and is known as “inflammatory aging”. 8 Elevated C‐reactive protein (CRP), interleukin‐6 (IL‐6), and tumor necrosis factor‐α (TNF‐α) levels were strongly correlated with cognitive disorders. 9 , 10 , 11 CRP is an acute‐phase reactive protein 12 and a peripheral inflammatory biomarker. 13 , 14 Increased CRP levels are related to a higher incidence of subsequent cognitive deterioration. 11 Among the inflammatory cytokines involved in inflammatory aging, IL‐6 plays an essential role. It can disrupt the integrity and function of the blood–brain barrier and result in age‐related neurodegeneration. 15 Elevated IL‐6 levels are also connected with cognitive degeneration and can promote the conversion from normal cognition to mild cognitive impairment or Alzheimer's disease. 16 , 17 TNF‐α is abundantly expressed around β‐amyloid (Aβ) plaques in the brain tissues of patients with Alzheimer's disease, 18 initiating the subsequent inflammatory cascade amplification reaction. 19 Increasing studies indicate that cognitive decline is connected with overactivation of the immune system. 20 Elevated peripheral inflammatory biomarkers can lead to the decline of global cognitive 21 and specific domain functions. 22 , 23 , 24 Although many studies have explored the connection between peripheral inflammation and cognitive decline, the conclusions are controversial owing to different backgrounds, methods, and eligibility criteria. Some studies reveal a close connection, 25 , 26 while others show no associations. 27 , 28 Therefore, further analysis is necessary.
We carried out this meta‐analysis of observational studies on the relevance of peripheral inflammatory cytokines for future cognitive decline in older individuals without dementia by expanding the sample size and enhancing the efficiency of statistical tests. Therefore, we could determine whether elevated peripheral inflammatory biomarkers indicate a higher incidence of cognitive decline, providing an evidence‐based medicine basis for diagnosis and prevention of cognitive decline.
Materials and Methods
Protocol and guidance
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA). The protocol of our meta‐analysis was registered with PROSPERO (CRD4202230429).
Search strategy
One team member (YHW) conducted the literature retrieval from databases: PubMed, Embase, Web of Science, Cochrane Library, ClinicalTrials, CNKI, and VIP, from inception to July 2022. The search strategy was as follows: (“Interleukin‐6” OR “IL‐6” OR “C‐reactive protein” OR “CRP” OR “Tumor necrosis factor‐α” OR “TNF‐α”) AND (“cognitive decline” OR “cognition” OR “cognitive function” OR “cognitive impairment” OR “cognitive loss” OR “memory”) AND (“aging” OR “aging”) AND (“prospective” OR “longitudinal” OR “cross‐sectional”). We also manually retrieved related literatures.
Eligibility criteria
The literature eligibility criteria were as follows: (1) Participants were nondemented older people at baseline (≥60 years); (2) had a cross‐sectional or longitudinal cohort design; (3) the literature reporting CRP, IL‐6, and TNF‐α levels was in English; (4) cognitive performance was evaluated at baseline and follow‐up; and (5) odds ratios (ORs) or hazard ratios (HRs) were provided for the association between inflammatory cytokines and future cognitive decline. The exclusion criteria were as follows: (1) participants with cognitive impairment or dementia previously; (2) the connection between peripheral inflammatory cytokines and cognitive decline was not reported by ORs or HRs; (3) generally accepted cognitive scales or methods were not used to assess overall cognition; (4) published repeatedly; and (5) a review or meta‐analysis.
Literature screening, data extraction, and quality assessment
We searched the databases for both published and unpublished data. Titles and abstracts were reviewed to exclude irrelevant literature, eliminate repetitive ones, and screen out qualified literature. Studies deemed potentially relevant were retrieved in full‐text form and reviewed by at least two co‐authors. Any discrepancy concerning inclusion was resolved by a senior author. The data were extracted and recorded using Microsoft Excel. In some cases, the corresponding author was contacted for supplementary data. Two researchers independently evaluated the studies included using the Newcastle‐Ottawa Scale (NOS). The risk of bias in individual studies was assessed by two reviewers using the Quality in Prognosis Studies tool (QUIPS). 29 This tool assesses six domains: study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, and statistical analysis and reporting.
Outcomes
The primary outcome was the incidence of cognitive decline. The secondary outcome was changes in global or specific cognitive domain functions. This study evaluated cognitive performance using the Mini‐Mental State Examination (MMSE), Modified Mini‐Mental State Examination (3MS), and a battery of cognitive tests that are commonly used for cognitive function. 28
Statistical analysis
We considered low levels of CRP/IL‐6/TNF‐α as reference values to evaluate the risk of cognitive decline with high peripheral inflammatory cytokine levels. We used random‐effect models and presented the pooled results as ORs, 95% confidence intervals (CIs), and p values 30 ; p < 0.05 indicated a significant difference. We performed the I 2 statistic and the Cochrane Q test to evaluate the statistical heterogeneity. 31 The subgroup analysis was performed according to the length of follow‐up (<3 or ≥3 years), year of publication (<2014 or ≥2014), age (<75 or ≥75 years), number of participants (<1500 or ≥1500), study design (cross‐sectional or longitudinal), and NOS score (<7 or ≥7). In addition, we also performed a sensitivity analysis, eliminating each study at a time to assess which study would make a significant impact on the combined results. Two statistical analysis models were transformed to evaluate the robustness of the combined results. Publication bias was intuitively determined by drawing funnel plots. All the statistical analyses were performed using the RevMan (version 5.4.1; The Cochrane Collaboration) software.
Results
Search results
Figure 1 shows the flow chart of the literature screening process, including the reasons for exclusion. The search strategy returned 492 studies. A reference search of relevant reviews and meta‐analyses revealed nine additional studies. After removing duplicates and irrelevant articles based on titles and abstracts, 60 articles were assessed for eligibility. Among them, 43 studies that were not in accord with the eligibility criteria were eliminated, leaving 17 literatures for our meta‐analysis.
Figure 1.
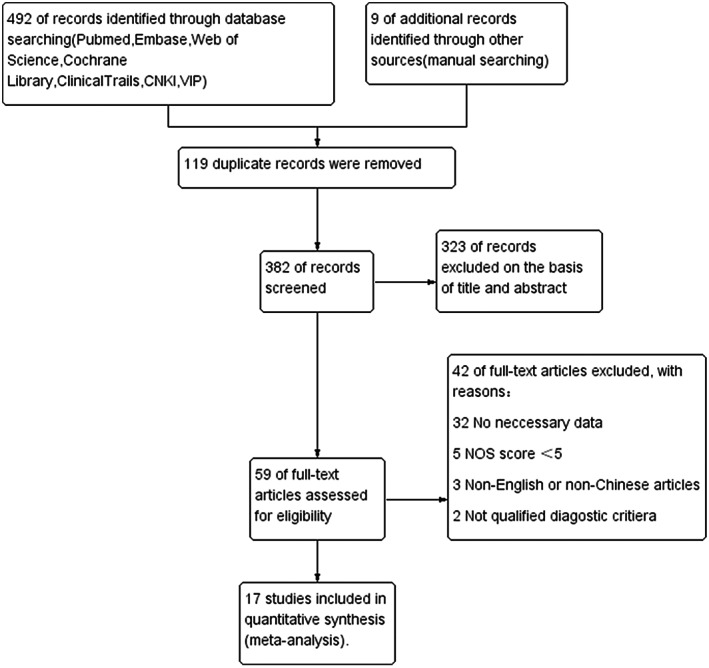
Flow chart summarizing the literature search for the meta‐analysis.
Characteristics of included studies
The basic characteristics of the 17 included studies were presented in tabular form (Table 1). Overall, our meta‐analysis comprised 19,516 participants, and 2134 of them developed age‐related cognitive decline. One study reported the total sample size but did not mention the number of primary outcome events. 32 Each study reported different levels of peripheral CRP, IL‐6, and TNF‐α. We summarized different global cognitive tests and definitions of cognitive decline (Table S1).
Table 1.
Summary characteristics of the included studies.
| Characteristics | No. of studies (no. of participants) |
|---|---|
| Eligible studies | |
| Total no. of trials (no. of participants) | 17 (19,516) |
| Follow‐up at least three years | 11 (12,761) |
| Total no. of outcome events | 2134 |
| Median (IQR) % female | 53 (49–59) |
| Median (IQR) age (years) | 74 (65–80) |
| Country | |
| American | 12 (17,041) |
| European | 4 (2335) |
| China | 1 (140) |
| Inflammatory markers | |
| CRP | 11 (14,324) |
| IL‐6 | 15 (18,710) |
| TNF‐α | 5 (7183) |
IQR, interquartile range.
Risk of bias within and across included studies
A total of seventeen articles were included in this meta‐analysis, of which 15 had NOS scores ≥7 and were high‐quality articles (Table S2). The risk of bias assessment in individual studies is shown in Table S3. The overall risk of bias in the studies was judged to be low and moderate for seven 16 , 27 , 33 , 34 , 35 , 36 , 37 and ten 14 , 25 , 26 , 32 , 38 , 39 , 40 , 41 , 42 , 43 studies, respectively. Study participation, prognostic factor measurement, and statistical analysis and reporting were typically associated with low or moderate risks of bias. The domains most commonly at high risk of bias were study attrition (n = 4), 32 , 34 , 38 , 41 outcome measurement (n = 5), 14 , 25 , 26 , 38 , 42 and study confounding (n = 4). 36 , 39 , 40 , 43 The funnel plot of cognitive decline‐related inflammatory cytokines (CRP, IL‐6, and TNF‐α) was symmetrical and within 95% CI, thus revealing an inverted funnel‐shaped distribution without obvious publication bias (Fig. 2).
Figure 2.
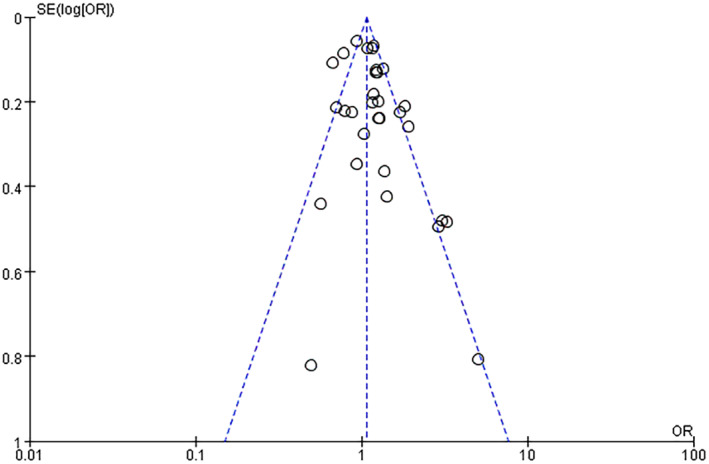
Funnel plot for the association between high peripheral levels of inflammatory biomarkers and future cognitive decline analysis. OR, odds ratio; SE, standard error.
Association of high peripheral inflammation with future cognitive decline: meta‐analysis
Individuals with high levels of baseline peripheral inflammation were 1.14 times more likely to experience cognitive decline at follow‐up (OR = 1.14, 95% CI: 1.03–1.27, I 2 = 68%, p < 0.00001; Fig. 3), suggesting that older adults with higher peripheral CRP, IL‐6, and TNF‐α levels were at increased risk of future cognitive decline by 14%. The funnel plot was symmetric upon visual inspection, and therefore publication bias was not evident (Fig. 2). We performed sensitivity analysis by excluding each study one by one to control for confounding variables among studies, and the combined results were robust. We employed the fixed and random‐effects models to estimate the pooled ORs and 95% CIs; the pooled results were similar. Subgroup analysis determined that the association was related to the publication year, and the incidence of cognitive decline in articles published in or after 2014 was significantly lower than that of those published before 2014. Further subgroup analysis of cytokine types revealed that cognitive decline was significantly correlated with an increase in IL‐6 level but not with CRP and TNF‐α. Details of the subgroup analysis are shown in Table S4 and Fig. 1.
Figure 3.
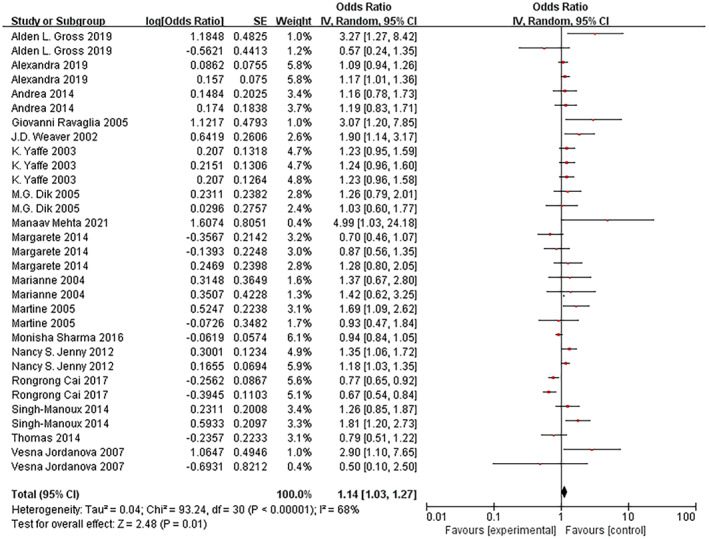
Forest plot for the association between high peripheral levels of inflammatory biomarkers and future cognitive decline analysis. CI, confidence interval.
Peripheral CRP level and future cognitive decline
All 11 studies classified peripheral CRP levels into different categories. Only two of them revealed a higher risk of future cognitive decline for higher levels of peripheral CRP after adjusting confounding factors 38 , 41 ; the remaining nine reported no associations. 14 , 25 , 26 , 35 , 36 , 37 , 40 , 42 , 43 Elevated peripheral CRP levels did not increase the risk of cognitive decline compared to lower peripheral CRP levels (OR = 1.05, 95% CI: 0.86–1.29; Fig. 4). Study heterogeneity (I 2 = 68%, p = 0.0006) was evident. Sensitivity analyses were performed by eliminating each study at a time to detect the potential sources of interstudy heterogeneity. The overall pooled OR estimate became statistically significant after Rongrong Cai's 42 (pooled OR = 1.15, 95% CI: 1.00–1.32) study was excluded and heterogeneity was significantly reduced from 68% to 18%.
Figure 4.
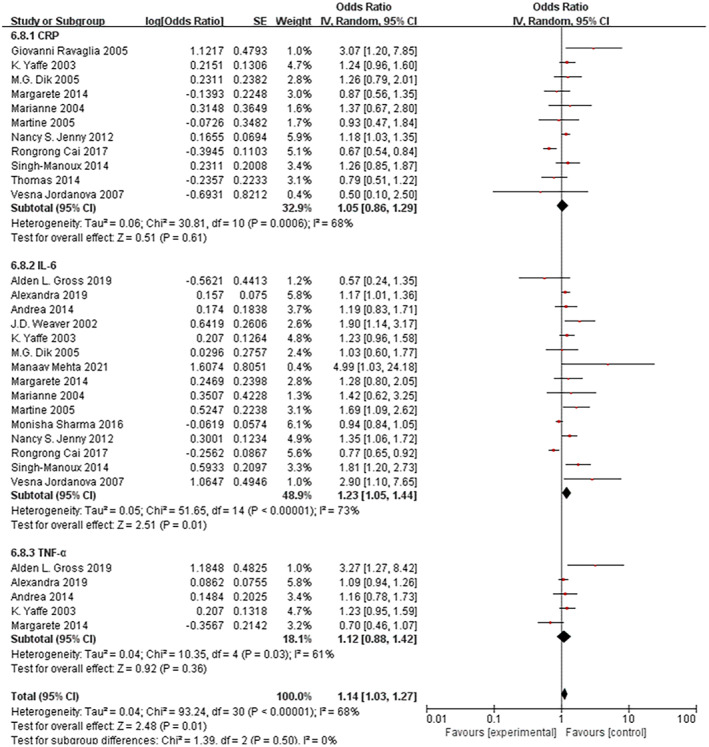
Subgroup analyses for the association between high peripheral inflammatory biomarkers and future cognitive decline analysis.
Peripheral IL‐6 level and future cognitive decline
Among the 15 studies reporting peripheral IL‐6 levels, seven reported a higher risk of future cognitive decline for elevated IL‐6, 14 , 16 , 27 , 36 , 37 , 39 , 41 and eight revealed no correlation. 25 , 26 , 32 , 33 , 34 , 35 , 40 , 42 Compared to those with lower peripheral IL‐6, older adults with higher peripheral IL‐6 had a 23% enhanced risk of future cognitive decline (OR = 1.23, 95% CI: 1.05–1.44, Fig. 4); study heterogeneity (I 2 = 73%, p = 0.00001) was still statistically significant. We performed a sensitivity analysis, and the pooled results were robust.
Peripheral TNF‐α level and future cognitive decline
A total of five studies examined the relevance of peripheral TNF‐α levels to future cognitive performance. Only one reported an enhanced risk of future cognitive decline with higher levels of peripheral TNF‐α 33 ; four reported no association. 25 , 27 , 34 , 35 Likewise, an increase in peripheral TNF‐α levels was not statistically significantly correlated with future cognitive decline (OR = 1.12, 95% CI: 0.88–1.42, Fig. 4). Study heterogeneity (I 2 = 68%, p = 0.03) remained evident.
Discussion
This collective analysis found that high levels of established peripheral inflammatory biomarkers such as CRP, IL‐6, and TNF‐α increased the likelihood of future cognitive decline by 1.14 times compared to their low levels. Further subgroup analysis revealed that cognitive decline was significantly correlated with an increase in peripheral IL‐6 but not with CRP or TNF‐α levels. In addition, the incidence of cognitive decline in articles published before 2014 was significantly higher, which may be related to fewer social activities, 44 a low level of education, 45 and a disadvantaged neighborhood environment. 46
The effect of CRP on cognitive function has been controversial. It has been known to directly damage cerebral brain microvascular endothelial cells (ECs) 47 and further increase the permeability of the blood–brain barrier (BBB), thus activating neuroinflammation and resulting in cognitive impairment. 48 A previous meta‐analysis revealed a weak but significant relevance of elevated peripheral CRP levels to subsequent cognitive decline. 49 However, our subgroup analysis demonstrated no significant association between elevated CRP levels and cognitive decline. Notably, there was significant heterogeneity across studies in our results (I 2 = 68%). A CRP study was the main source of this significant heterogeneity, 42 reporting that elevated CRP levels were not related to mild cognitive impairment (MCI) (OR = 0.674, 95% CI: 0.543–0.838). An explanation could be that this study explored the correlation between higher peripheral CRP levels and MCI rather than subsequent cognitive performance. The participants' cognitive statuses (normal cognition or MCI) were evaluated using the 2006 European Alzheimer's Disease Consortium criteria, 50 which led to fewer evaluations of cognitive decline. Coincidentally, the association was significant once Rongrong Cai's 42 study was excluded, and the interstudy heterogeneity was significantly reduced (I 2 = 18%). Therefore, we suggest that peripheral CRP levels may be considered an early warning biomarker of future cognitive decline in nondemented individuals.
In addition, we assessed the relevance of peripheral IL‐6 levels to cognitive decline. Elevated peripheral IL‐6 levels are significantly related to a higher incidence of future cognitive decline. A previous meta‐analysis demonstrated a significant association between higher IL‐6 and future cognitive decline, 8 and we reached a consensus. Peripheral IL‐6 has been demonstrated to directly enter the central nervous system through the BBB, even at very low levels in a mouse model. 51 Additionally, elevated peripheral IL‐6 levels, due to repeated infections or increased exogenous IL‐6, increase BBB permeability. 52 IL‐6 levels in the peripheral blood and cerebrospinal fluid were simultaneously elevated in patients with dementia 53 and in cognitively normal individuals. 54 Therefore, BBB dysfunction due to age‐related or underlying infections might have occurred in these individuals. Peripheral IL‐6 may enter the CNS and be further elevated during cognitive aging.
TNF‐α plays an essential role in AD pathogenesis and is abundantly expressed around amyloid plaques. 55 TNF‐α can significantly upregulate the expression of intercellular cell adhesion molecule‐1 (ICAM‐1) and vascular cell adhesion molecule (VCAM) in brain microvascular endothelial cells (BMECs), as well as increase BBB permeability. 56 Excessively expressed TNF‐α in the brain affects the clearance of Aβ by microglia, 57 causes synaptic dysfunction, 58 and aggravate AD and cognitive decline. However, some observational studies 25 , 27 , 34 , 35 did not report significant associations between peripheral TNF‐α and cognitive decline in nondemented individuals. To explore the underlying association between peripheral TNF‐α and cognitive decline, we first performed this meta‐analysis to reveal the relationship between these factors. In our subgroup analysis, there was no significant correlation between higher peripheral TNF‐α and future cognitive decline. Considering the limited studies included in our analysis, caution should be exercised while generalizing our findings on future cognitive decline in all nondemented older adults.
The limitations of this meta‐analysis should also be considered when interpreting the results. First, the included studies were all observational studies, which contributed to interstudy heterogeneity. Second, the impacts of other peripheral inflammatory biomarkers, for example interleukin 1‐β (IL‐1β), 59 interleukin‐10 (IL‐10), 60 S100β, 61 and prostaglandin‐endoperoxide synthase 2 (ptgs2) 62 on cognitive performance have been reported recently. Owing to the lack of data, these studies were not included in our meta‐analysis; this fact has an impact on the combined results. Moreover, general cognition was evaluated using different cognitive tests including MMSE, 3MS, and other cognitive test batteries. The MMSE has been recommended by guidelines to determine MCI. However, in clinical practice, some individuals with MCI have normal MMSE scores. Therefore, cognitive decline must be defined using standardized cognitive assessments.
In conclusion, this study revealed a weak but significant association between older individuals with higher peripheral inflammatory biomarkers and future cognitive decline. It may become reliable to identify high‐risk older adults by measuring levels of peripheral inflammatory biomarkers. Further exploration should put emphasis on the clinical application of peripheral inflammatory cytokines (CRP, IL‐6, and TNF‐α) as biomarkers for future cognitive health.
Authors' Contributions
Xiaoxia Duan involved in study design, literature search, statistical analysis, quality assessment, and reviewing of the manuscript. Lan Feng involved in literature search, quality assessment, data analyses, and writing of the manuscript. Yuhao Wang involved in interpretation and drafting of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest Statement
The authors do not have any competing financial or nonfinancial interests related to the manuscript.
Supporting information
Acknowledgements
This work was supported by the Science and Technology Department of Sichuan Province (grant number 2022NSFSC1360). The Department of Human Resources and Social Security of Sichuan Province (grant number 19058); the Science and Technology Strategic Cooperation Programs of Luzhou Municipal People's Government and Southwest Medical University (grant number 2019LZXNYDJ36); and the Doctoral Research Initiation Fund of Affiliated Hospital of Southwest Medical University (grant number 19023). We would also like to acknowledge the contribution of Editage (www.editage.cn) for English language editing.
Funding Statement
This work was funded by Department of Human Resources and Social Security of Sichuan Province grant 19058; Department of Science and Technology of Sichuan Province grant 2022NSFSC1360; The Doctoral Research Initiation Fund of Affiliated Hospital of Southwest Medical University grant 19023; The Science and Technology Strategic Cooperation Programs of Luzhou Municipal People's Government and Southwest Medical University grant 2019LZXNYDJ36.
References
- 1. Rabin LA, Smart CM, Crane PK, et al. Subjective cognitive decline in older adults: an overview of self‐report measures used across 19 international research studies. J Alzheimers Dis. 2015;48(Suppl 1):S63‐S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kennedy G, Hardman RJ, Macpherson H, Scholey AB, Pipingas A. How does exercise reduce the rate of age‐associated cognitive decline? A review of potential mechanisms. J Alzheimers Dis. 2017;55(1):1‐18. [DOI] [PubMed] [Google Scholar]
- 3. Scafato E, Gandin C, Galluzzo L, et al. Prevalence of aging‐associated cognitive decline in an Italian elderly population: results from cross‐sectional phase of Italian PRoject on epidemiology of Alzheimer's disease (IPREA). Aging Clin Exp Res. 2010;22(5–6):440‐449. [DOI] [PubMed] [Google Scholar]
- 4. Patnode CD, Perdue LA, Rossom RC, et al. Screening for cognitive impairment in older adults: updated evidence report and systematic review for the US preventive services task force. JAMA. 2020;323(8):764‐785. [DOI] [PubMed] [Google Scholar]
- 5. Chen YX, Liang N, Li XL, Yang SH, Wang YP, Shi NN. Diagnosis and treatment for mild cognitive impairment: a systematic review of clinical practice guidelines and consensus statements. Front Neurol. 2021;12:719849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krabbe KS, Mortensen EL, Avlund K, et al. Genetic priming of a proinflammatory profile predicts low IQ in octogenarians. Neurobiol Aging. 2009;30(5):769‐781. [DOI] [PubMed] [Google Scholar]
- 7. Wang RP, Ho YS, Leung WK, Goto T, Chang RC. Systemic inflammation linking chronic periodontitis to cognitive decline. Brain Behav Immun. 2019;81:63‐73. [DOI] [PubMed] [Google Scholar]
- 8. Bradburn S, Sarginson J, Murgatroyd CA. Association of Peripheral Interleukin‐6 with global cognitive decline in non‐demented adults: a meta‐analysis of prospective studies. Front Aging Neurosci. 2017;9:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25‐year follow‐up of the Honolulu‐Asia aging study. Ann Neurol. 2002;52(2):168‐174. [DOI] [PubMed] [Google Scholar]
- 10. Huang HH, Chang JC, Liu HC, et al. Handgrip strength, tumor necrosis factor‐alpha, interlukin‐6, and visfatin levels in oldest elderly patients with cognitive impairment. Exp Gerontol. 2020;142:111138. [DOI] [PubMed] [Google Scholar]
- 11. Salgado J, Cruz B, Campos S, et al. Elevated Il‐6 levels are associated with social cognitive impairment in stable patients with schizophrenia. Eur Psychiatry. 2015;30:383. [Google Scholar]
- 12. Pathak A, Agrawal A. Evolution of C‐reactive protein. Front Immunol. 2019;10:943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fard MT, Cribb L, Nolidin K, Savage K, Wesnes K, Stough C. Is there a relationship between low‐grade systemic inflammation and cognition in healthy people aged 60‐75 years? Behav Brain Res. 2020;383:112502. [DOI] [PubMed] [Google Scholar]
- 14. Jordanova V, Stewart R, Davies E, Sherwood R, Prince M. Markers of inflammation and cognitive decline in an African‐Caribbean population. Int J Geriatr Psychiatry. 2007;22(10):966‐973. [DOI] [PubMed] [Google Scholar]
- 15. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age‐associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S4‐S9. [DOI] [PubMed] [Google Scholar]
- 16. Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin‐6 and risk of cognitive decline MacArthur studies of successful aging. Neurology. 2002;59 (3):371‐378. [DOI] [PubMed] [Google Scholar]
- 17. Lyra ESNM, Goncalves RA, Pascoal TA, et al. Pro‐inflammatory interleukin‐6 signaling links cognitive impairments and peripheral metabolic alterations in Alzheimer's disease. Transl Psychiatry. 2021;11(1):251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paouri E, Tzara O, Kartalou GI, Zenelak S, Georgopoulos S. Peripheral tumor necrosis factor‐alpha (TNF‐alpha) modulates amyloid pathology by regulating blood‐derived immune cells and glial response in the brain of AD/TNF transgenic mice. J Neurosci. 2017;37(20):5155‐5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao YF, Yang HW, Yang TS, Xie W, Hu ZH. TNF‐alpha—Mediated peripheral and central inflammation are associated with increased incidence of PND in acute postoperative pain. BMC Anesthesiol. 2021;21(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin T, Liu GA, Perez E, et al. Systemic inflammation mediates age‐related cognitive deficits. Front Aging Neurosci. 2018;10:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fard MT, Savage KM, Stough CK. Peripheral inflammation marker relationships to cognition in healthy older adults—a systematic review. Psychoneuroendocrinology. 2022;144:105870. [DOI] [PubMed] [Google Scholar]
- 22. Teunissen CE, Van Boxtel MP, Bosma H, et al. Inflammation markers in relation to cognition in a healthy aging population. Journal of Neuroimmunology. 2003;134:142‐150. [DOI] [PubMed] [Google Scholar]
- 23. Bettcher BM, Watson CL, Walsh CM, et al. Interleukin‐6, age, and corpus callosum integrity. PLoS ONE. 2014;9(9):106521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heringa SM, van den Berg E, Reijmer YD, et al. Markers of low‐grade inflammation and endothelial dysfunction are related to reduced information processing speed and executive functioning in an older population—the Hoorn study. Psychoneuroendocrinology. 2014;40:108‐118. [DOI] [PubMed] [Google Scholar]
- 25. Yaffe K, Lindquist K, Penninx BW, et al. Inflammatory markers and cognition in well‐functioning African‐American and white elders. Neurology. 2003;61(1):76‐80. [DOI] [PubMed] [Google Scholar]
- 26. Dik MG, Jonker C, Hack CE, Smit JH, Comijs HC, Eikelenboom P. Serum inflammatory proteins and cognitive decline in older persons. Neurology. 2005;64(8):1371‐1377. [DOI] [PubMed] [Google Scholar]
- 27. Wennberg AMV, Hagen CE, Machulda MM, Knopman DS, Petersen RC, Mielke MM. The cross‐sectional and longitudinal associations between IL‐6, IL‐10, and TNFalpha and cognitive outcomes in the Mayo Clinic study of aging. J Gerontol A Biol Sci Med Sci. 2019;74(8):1289‐1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Crane PK, Narasimhalu LE, Gibbons LE, et al. Item response theory facilitated cocalibrating cognitive tests and reduced bias in estimated rates of decline. J Clin Epidemiol. 2008;61:1018‐1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280‐286. [DOI] [PubMed] [Google Scholar]
- 30. DerSimonian R, Kacker R. Random‐effects model for meta‐analysis of clinical trials: an update. Contemp Clin Trials. 2006;28:105‐114. [DOI] [PubMed] [Google Scholar]
- 31. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539‐1558. [DOI] [PubMed] [Google Scholar]
- 32. Sharma M, Fitzpatrick AL, Arnold AM, et al. Inflammatory biomarkers and cognitive decline: the ginkgo evaluation of memory study. J Am Geriatr Soc. 2016;64(6):1171‐1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gross AL, Anja Soldan ARM, Walker KA, Pettigrew C, Albert MS, Walston JD. Plasma markers of inflammation linked to clinical progression and decline during preclinical AD. Front Aging Neurosci. 2019;11:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Metti AL, Yaffe K, Boudreau RM, et al. Change in inflammatory markers and cognitive status in the oldest‐old women from the study of osteoporotic fractures. J Am Geriatr Soc. 2014;62(4):662‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wichmann MA, Cruickshanks KJ, Carlsson CM, et al. Long‐term systemic inflammation and cognitive impairment in a population‐based cohort. J Am Geriatr Soc. 2014;62(9):1683‐1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Puts MT, Visser M, Twisk JW, Deeg DJ, Lips P. Endocrine and inflammatory markers as predictors of frailty. Clin Endocrinol (Oxf). 2005;63(4):403‐411. [DOI] [PubMed] [Google Scholar]
- 37. Archana Singh‐Manoux AD, Brunner E, Kumari M, Shipley M, Elbaz A, Kivimaki M. Interleukin‐6 and C‐reactive protein as predictors of cognitive decline in late midlife. Neurology. 2014;83(6):486‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Giovanni Ravaglia PF, Maioli F, Brunetti N, et al. Serum CRP and cognitive function in healthy elderly Italian community dwellers. J Gerontol Series A. 2005;8:1017‐1021. [DOI] [PubMed] [Google Scholar]
- 39. Mehta M, Louissaint J, Parikh NS, Long MT, Tapper EB. Cognitive function, sarcopenia, and inflammation are strongly associated with frailty: a Framingham cohort study. Am J Med. 2021;134(12):1530‐1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Engelhart MJ, Geerlings MI, Meijer J. Inflammatory proteins in plasma and the risk of dementia. Arch Neurol. 2004;61(5):668‐672. [DOI] [PubMed] [Google Scholar]
- 41. Jenny NS, French B, Arnold AM, et al. Long‐term assessment of inflammation and healthy aging in late life: the cardiovascular health study all stars. J Gerontol A Biol Sci Med Sci. 2012;67(9):970‐976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cai R, Huang R, Han J, et al. Lipoprotein‐associated phospholipase A2 is associated with risk of mild cognitive impairment in Chinese patients with type 2 diabetes. Sci Rep. 2017;7(1):12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lima TA, Adler AL, Minett T, et al. C‐reactive protein, APOE genotype and longitudinal cognitive change in an older population. Age Ageing. 2014;43(2):289‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sommerlad A, Sabia S, Singh‐Manoux A, Lewis G, Livingston G. Association of social contact with dementia and cognition: 28‐year follow‐up of the Whitehall II cohort study. PLoS Med. 2019;16(8):e1002862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ramos J, Chowdhury AR, Caywood LJ, et al. Lower levels of education are associated with cognitive impairment in the old order Amish. J Alzheimers Dis. 2021;79(1):451‐458. [DOI] [PubMed] [Google Scholar]
- 46. Wang Y, Jiang Y, Wu W, et al. Education, neighborhood environment, and cognitive decline: findings from two prospective cohort studies of older adults in China. Alzheimer's & Dementia. 2022;18:12679. [DOI] [PubMed] [Google Scholar]
- 47. Zhang J, Rui Y‐C, Yang P‐Y, Lu L, Li T‐J. C‐reactive protein induced expression of adhesion molecules in cultured cerebral microvascular endothelial cells. Life Sci. 2006;78:2983‐2988. [DOI] [PubMed] [Google Scholar]
- 48. Kuhlmann CRW, Librizzi L, Closhen D, et al. Mechanisms of C‐reactive protein‐induced blood‐brain barrier disruption. Stroke. 2009;40:1458‐1466. [DOI] [PubMed] [Google Scholar]
- 49. Yang J, Fan C, Pan L, et al. C‐reactive protein plays a marginal role in cognitive decline: a systematic review and meta‐analysis. Int J Geriatr Psychiatry. 2015;30(2):156‐165. [DOI] [PubMed] [Google Scholar]
- 50. Portet F, Ousset PJ, Visser PJ, et al. Mild cognitive impairment (MCI) in medical practice: a critical review of the concept and new diagnostic procedure. Report of the MCI working Group of the European Consortium on Alzheimer's disease. J Neurol Neurosurg Psych. 2006;77:714‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Banks WA, Kastin AJ, Gutierrez EG. Penetration of interleukin‐6 across the murine blood‐brain barrier. Neurosci Lett. 1994;179:53‐56. [DOI] [PubMed] [Google Scholar]
- 52. Varatharaj A, Galea I. The blood‐brain barrier in systemic inflammation. Brain Behav Immun. 2017;10:1‐12. [DOI] [PubMed] [Google Scholar]
- 53. Sun YX, Minthon L, Wallmark A, Warkentin S, Blennow K, Janciauskiene S. Inflammatory markers in matched plasma and cerebrospinal fluid from patients with Alzheimer's disease. Dement Geriatr Cogn Disord. 2003;16:136‐144. [DOI] [PubMed] [Google Scholar]
- 54. Agorastos A, Hauger RL, Barkauskas DA, et al. Circadian rhythmicity, variability and correlation of interleukin‐6 levels in plasma and cerebrospinal fluid of healthy men. Psychoneuroendocrinology. 2014;44:71‐82. [DOI] [PubMed] [Google Scholar]
- 55. Perry RT, Collins JS, Wiener H, Acton R, Go RC. The role of TNF and its receptors in Alzheimer's disease. Neurobiol Aging. 2003;22:873‐883. [DOI] [PubMed] [Google Scholar]
- 56. Ge S, Jiang X, Paul D, Song L, Wang X, Pachter JS. Human ES‐derived MSCs correct TNF‐α‐mediated alterations in a blood–brain barrier model. Fluids Barriers CNS. 2019;16:1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Koenigsknecht‐Talboo J, Landreth GE. Microglial phagocytosis induced by fibrillar beta‐amyloid and IgGs are differentially regulated by proinflammatory cytokines. J Neurosci. 2005;25:8240‐8249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ralay Ranaivo H, Craft JM, Hu W, et al. Glia as a therapeutic target: selective suppression of human amyloid‐beta‐induced upregulation of brain proinflammatory cytokine production attenuates neurodegeneration. J Neurosci. 2006;26(2):662‐670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Samatra D, Pratiwi NMD, Widyadharma IPE. High Il‐1β serum as a predictor of decreased cognitive function in mild traumatic brain injury patients. Open Access Macedonian J Med Sci. 2018;6(9):1674‐1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tegeler C, O'Sullivan JL, Bucholtz N, et al. The inflammatory markers CRP, IL‐6, and IL‐10 are associated with cognitive function—data from the Berlin aging study II. Neurobiol Aging. 2016;38:112‐117. [DOI] [PubMed] [Google Scholar]
- 61. Wang F, Zou ZR, Yuan D, et al. Correlation between serum S100β protein levels and cognitive dysfunction in patients with cerebral small vessel disease: a case‐control study. Biosci Rep. 2017;37(2):BSR20160446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang YH, Chen YW, Xiao WL, et al. MiR‐214‐3p prevents the development of perioperative neurocognitive disorders in elderly rats. Curr Med Sci. 2022;42(4):871‐884. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


