Abstract
Background
Increasing evidence suggests that chronic stress increases pain sensitivity and exacerbates existing pain. However, whether and how chronic unpredictable stress (CUS) affects surgical pain is unclear.
Methods
A postsurgical pain model was performed by longitudinal incision from 0.3 cm of the proximal edge of the heel toward the toes. The skin was sutured, and the wound site was covered. Sham surgery groups underwent the same procedure without an incision. The short-term CUS procedure was conducted by exposure of mice to 2 different stressors each day for 7 days. The behavior tests were conducted between 9:00 am and 4:00 pm. Mice were killed on day 19, and the mouse bilateral L4/5 dorsal root ganglia, spinal cord, anterior cingulate and insular cortex, and amygdala were collected for immunoblot analyses.
Results
Presurgical exposure of mice to CUS every day for 1–7 days showed significant depression-like behavior as evidenced by reduced sucrose preference in the sucrose consumption test and prolonged immobility time in the forced swimming task. This short-term CUS procedure did not affect the basal nociceptive response to mechanical and cold stimuli in the Von Frey and acetone-induced allodynia tests, but it delayed pain recovery after surgery, as indicated by the prolonged hypersensitivity in mechanical and cold stimuli by 12 days. The subsequent studies demonstrated that this CUS caused an increase in adrenal gland index. The abnormalities in pain recovery and adrenal gland index after surgery were reversed by a glucocorticoid receptor (GR) antagonist RU38486. Moreover, the prolonged pain recovery after surgery induced by CUS seemed to involve an increase in GR expression and decreases in cyclic adenosine monophosphate, phosphorylated cAMP response element binding protein, and brain-derived neurotrophic factor levels in emotion-related brain regions, such as anterior cingulate and insular cortex, amygdala, dorsal horn, and dorsal root ganglion.
Conclusions
This finding indicates that stress-induced GR change may result in dysfunction of GR-related neuroprotective pathway.
Keywords: Chronic unpredictable stress, pain recovery, glucocorticoid receptor, cAMP, pCREB, BDNF
Significance Statement.
Short-term stress could affect the recovery of pain after surgery, but the underlying neurobiological mechanism between this stress and pain recovery has not been clarified. The presurgery stress animal model that we established successfully simulates the clinical situation, where most patients undergoing incision surgery have mild depression before surgery, while the basal pain threshold has not changed. The present study was highly significant because it (1) identified the glucocorticoid receptor as a new regulator for control of prolonged surgical pain conditions; (2) suggested that short-term stress-mediated pain abnormality could be ameliorated by inhibition of glucocorticoid receptor and activation of downstream neuroprotective pathway; and (3) provided evidence that the prevention of perioperative stress may facilitate recovery of postoperative pain.
INTRODUCTION
Chronic pain is one of the most common and disabling mental disorders and affects approximately one-fifth of the population in the United States and Europe (Sheng et al., 2017). Clinical studies suggest that 85% of patients suffering from both chronic pain and depression (Bair et al., 2003; Williams et al., 2003). Chronic pain and depression are closely correlated in terms of occurrence and can promote each other’s deterioration. Recently therapeutics for relieving chronic pain include antidepressants as well as anxiolytics, underscoring the mutual correlation implicated in the pathology of depression and pain development.
Stress and pain share a common behavioral model of failure to extinguish negative memories. Previous studies suggested that chronic stress-related depression reduced basal pain threshold and prolonged recovery of pain (Raymond et al., 2001; Roehrs et al., 2006). Increasing evidence suggests that short-term stress or subacute stress delays surgical pain recovery (Caumo et al., 2002; Jackson et al., 2016) but does not affect the basal pain threshold. This phenomenon is consistent with patients who have a normal pain threshold although they suffer from short-term stress during hospital stays of 2–4 days for preoperative preparation (Wright, 2009; Theunissen et al., 2012; Hernández et al., 2015; Dekker et al., 2016; Sobol-Kwapinska et al., 2016; Su et al., 2017; Weinrib et al, 2017). Further studies demonstrate that this short-term stress could affect recovery of pain after surgery (Cremeans-Smith et al., 2006; Theunissen et al., 2012; Weinrib et al., 2017). However, the underlying neurobiological mechanism between short-term stress and pain recovery is less discernable. Indeed, mood disorders share the same pathway with the sensory occurrence, development, and maintenance of chronic pain from the central nervous system to the peripheral, such as the insular cortex, anterior cingulate cortex, amygdala, dorsal root ganglion (DRG), and dorsal horn (DH), which form a histological structural foundation for the coexistence of depression, anxiety, and pain (Meerwijk et al., 2013). Indeed, stress-induced glucocorticoid (GS) release and its receptor (glucocorticoid receptor [GR]) increase may play a crucial role in the process of pain because inhibition of this stress by treatment of a selective GR antagonist, RU38486 (RU), reverses the prolongation of pain hypersensitivity to mechanical and cold stimuli (Cao et al., 2015; Wang et al., 2015). However, whether this stress-induced increase in GR expression would prevent the neuroprotective effects by negatively regulating the expression of brain-derived neurotrophic factor (BDNF) and upstream transcription factors such as cAMP and cAMP response element-binding protein (CREB) is unknown.
In the present study, a CUS procedure was used to determine the time point at which short-term stress did not change basal pain threshold but prolonged surgical pain induced by a hind paw surgery. The depression-like behavior was also examined to analyze whether the prolonged pain response and depression-like behavior are relevant. The subsequent study determined depression and comorbid pain are related to the expression of GR, cAMP, the ratio of CREB phosphorylation (pCREB) to CREB, and BDNF in the brain regions related to emotional changes and the spinal cord and DRG, the main areas that regulate pain response.
MATERIALS AND METHODS
Animals
Adult male ICR mice (22–25 g) were obtained from the Animal Center of the Chinese Academy of Sciences (Shanghai, China). Mice were housed 4 per cage at controlled ambient temperature (24°C ± 1°C), humidity (50% ± 10%) with a light/dark cycle (12:12 hours) and had free access to ad libitum water and food. Mice were allowed an acclimation period of 5 days before the experiments. All efforts were made to minimize animal suffering and reduce the number of animals. Experiment procedures were conducted following the National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH publication No. 86-23, revised 1996) and approved by the Wenzhou Medical University Committee on Animal Care and Use.
Drug Administration
Sodium carboxymethyl cellulose was provided by the Beijing Institute of Pharmacology and Toxicology (China). Mifepristone (RU, Sigma-Aldrich, MO, USA) dissolved in 0.5% carboxymethyl cellulose suspended in saline was injected daily at 20 mg/kg 1 hour before CUS (Georén et al., 2005). They were i.p. administered in a constant volume of 10 mL/kg body weight.
Short-Term CUS
The procedure of short-term CUS was performed as previously described (Ortiz et al., 1996; Xu et al., 2018), with minor modifications. This stress procedure was administered twice per day over 1–7 days. Mice were exposed to 2 different stressors each day, such as food deprivation, restraint stress, tilted cage, cold swimming, and overnight illumination, etc. The sequence of stress was arranged in Table 1. The behavior tests were conducted between 9:00 am and 4:00 pm. Mice were killed on day 19, and the mouse bilateral L4/5 dorsal root ganglia, spinal cord, anterior cingulate and insular cortex, and amygdala were collected for immunoblot analyses. Control mice were housed in their usual cages under normal conditions.
Table 1.
Sequence of Chronic Unpredictable Stress
| Day | Stress |
|---|---|
| 1 | Food deprivation (12 h), overnight illumination (12 h) |
| 2 | Cold swim (4°C, 4 min), cage tilting (4 h) |
| 3 | Tail pinch (2 min), water deprivation (12 h) |
| 4 | Restraint (1 h), damp bedding (5 h) |
| 5 | Cold swim (4°C, 5 min), cage tilting (5 h) |
| 6 | Restraint (1.5 h), overnight illumination (12 h) |
| 7 | Tail pinch (3 min), food and water deprivation (24 h) |
Postsurgical Pain Model
The incisional surgery was carried out with minor modifications as previously described (Banik et al., 2006; Atianjoh et al., 2010). The surgical procedures were performed under isoflurane anesthesia (induction 4%, maintenance about 1.4%). The plantar aspect of the right hind paw was sterilely prepared (10% povidone-iodine solution) and longitudinally incised (8 mm; no. 11 blade through the skin and fascia) from 0.3 cm of the proximal edge of the heel toward the toes. The skin was sutured with 6-0 nylon, and the wound site was covered with triple antibiotic ointment. The mice were returned to cages for recovery after surgery, and the wounds healed well within about 6 days. Sham surgery groups underwent the same procedure without an incision.
Sucrose Preference Test (SPT)
The SPT was carried out as described in previous reports with slight modification (Grønli et al., 2005;Adebesin et al., 2017). All mice were adapted to a 2-bottle choice for 2 days (water or 1% sucrose in 1 bottle). After adaptation, mice were deprived of water and food for 24 hours, before SPT. Mice were then housed in individual cages and had free access to 2 bottles containing 100 mL of sucrose solution (1% w/v) and 100 mL of water, respectively. The consumption of water and sucrose during a 1-hour period for each mouse was determined using a standard weight scale. The sucrose preference was calculated as the ratio of the consumed sucrose solution to the total amount of liquid consumed.
Forced Swimming Test (FST)
The FST was carried out after the CUS procedure as previously described with minor modifications (Bourin et al., 2004; Xu et al., 2005). Each mouse was subjected to a preswim session (15 minutes) in a cylindrical and transparent container (height: 25 cm, diameter: 10 cm, depth: 10 cm) of water at 24°C ± 1°C. Mice were dropped in the container again for 6 minutes (test) 24 hours later. The period during which the mice were kept immobile was recorded during the last 4 minutes of the testing duration. A mouse was deemed to be immobile when it stopped struggling and kept floating motionless in the water and making only a few movements necessary to keep its head above water. The mice were dried after the behavioral test and returned to the home cages.
Mechanical Allodynia Test
Paw withdrawal responses to mechanical stimuli were determined using calibrated Von Frey filaments (0.07, 0.16, 0.40, 0.60, 1.0, 1.4, and 2.0 g), starting with 0.07 g and ending with 2.0 g in ascending order, as previously reported (Li et al., 2014; Xu et al., 2014). Mice were placed on an elevated wire-mesh floor (5 × 5 × 8 cm) and were covered with a clear Plexiglas chamber. After acclimation for 20 minutes, each monofilament was applied 5 times to the plantar side of the hind paw for approximately 1 second with a 10-second interval. A stimulus-related withdrawal was considered a positive response. The paw withdrawal threshold (PWT) was calculated as the force at which the positive response occurred in 3 of 5 stimuli. The PWT was regarded as the force at which withdrawal occurred (PWF ≥ 60%); 4 g was recorded as the PWT if PWF was ≤60% to all filaments.
Cold Allodynia Test
Cold allodynia of the hind paw was assessed using the acetone test. Cold allodynia was studied in the same plastic cages as those described above for the Von Frey test. After acclimation of at least 15 minutes, acetone (10 µL) was applied to the plantar skin. Acetone was alternately applied 5 times to each hind paw at 5-minute intervals, and the responses were recorded over a 1-minute period after application as follows according to the scale previously described (Bravo et al., 2012; Mizoguchi et al., 2016): 0, no response; 1, quick withdrawal, flick or stamp of the paw; 2, prolonged withdrawal or repeated flicking of the paw; 3, repeated flicking of the paw with persistent licking directed at the ventral side of the paw. The cumulative scores were obtained by summing the scores for each mouse and dividing by 5, the number of assays.
Adrenal Gland Index
The adrenal glands were quickly removed, and the weight was measured on day 19 after the mice were killed. The adrenal glands index was calculated as the ratio of the weight of the adrenal gland to the body weight of mice (Rubin et al., 1996; Pang et al., 2015).
Western-Blot Analysis
Mice were decapitated after the behavioral tests. The DRG, DH, anterior cingulated cortex, insular cortex, and amygdala were dissected and stored at −80°C until analysis after the mice were killed. All tissues were homogenized in Radio Immunoprecipitation Assay (RIPA) lysis buffer containing protease and phosphatase inhibitors and centrifuged at 14 000 rpm for 20 minutes at 4ºC. The supernatant was taken to assess GR, pCREB, CREB, and BDNF protein expression. As the internal reference protein, Actin expression was detected. Samples (45 µg protein) were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) before transferring to nitrocellulose membranes. Nonspecific bindings were blocked with a blocking buffer for 2 hours at room temperature. The membranes were incubated with the primary antibodies, that is, rabbit anti-GR (1:50000; Abcam), anti-protein kinase A (PKA; 1:50000; Abcam), anti-phosphorylated CREB (1:1000; Millipore), anti-CREB (1:500; Millipore), anti-BDNF (1:2000; Abcam), and anti-β-Actin (1: 1000; Abcam), overnight at 4°C. The membranes were incubated with the secondary antibodies, that is, goat anti-rabbit IgG-HRP (1:10 000; bio-sharp) for 60 minutes. The band signal was visualized by the electrochemiluminescence kit and was detected by the fluorescence scanner. The quantification of specific bands was analyzed by “Image-Lab” software (Zhang et al. 2013).
Statistical Analysis
Data shown are expressed as means ± SEM. The data were analyzed using 2-way ANOVA. When ANOVA showed a significant difference, pairwise comparisons between means were tested by the post hoc Tukey method (SigmaStat, San Jose, CA, USA). For two groups comparisons, data were statistically analyzed using Student’s t test. P < .05 was significant.
RESULTS
Time-Dependent Changes in Basal Pain Perception After Short-Term CUS
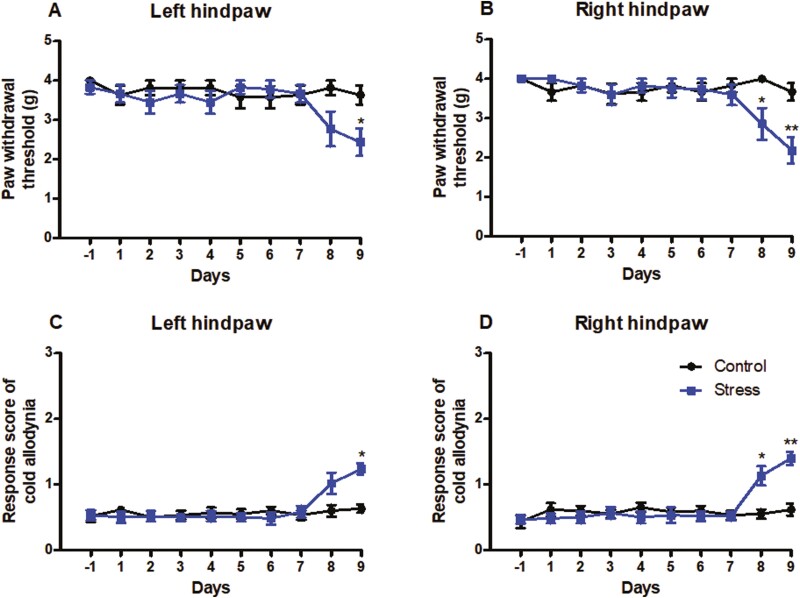
In the present study, we first examined the appropriate time of short-term CUS, which did not affect basal paw withdrawal responses in an established animal model of CUS. Mice were subjected to short-term CUS twice daily for 9 consecutive days. Paw withdrawal responses to mechanical and cold stimuli were examined 1 day before short-term CUS and 2 hours after CUS daily for 9 days. Marked reductions were found in PWTs in response to mechanical stimulation on day 9 post CUS on the left hind paws (Figure 1A; P < .05) and on day 8 and day 9 post CUS on right hind paws (Figure 1B; P < .05, P < .01) compared with the corresponding control group, which were indications of mechanical pain hypersensitivities. Similarly, significant increases in response scores on day 9 post CUS on the left hind paws (Figure 1C; P < .05) and on day 8 and 9 post CUS on right hind paws (Figure 1D; P < .05, P < .01) compared with the corresponding control group were behavioral indications of cold pain hypersensitivities. These results demonstrate that basal paw withdrawal responses did not significantly change during 7 consecutive days when mice were exposed to short-term CUS. Thus, short-term CUS for 7 consecutive days was defined as a short-term CUS procedure and was used in the following experiments.
Figure 1.
The time-dependent changes in basal paw withdrawal responses in mice to mechanical and cold stimuli after daily chronic unpredictable stress. (A, B) Mechanical allodynia test. (C, D) Cold allodynia test. (A, C) Responses of left hind paws. (B, D) Responses of right hind paws. Results are expressed as mean ± SEM. *P < .05, **P < .01 vs the corresponding time points in the control group (n = 10/group).
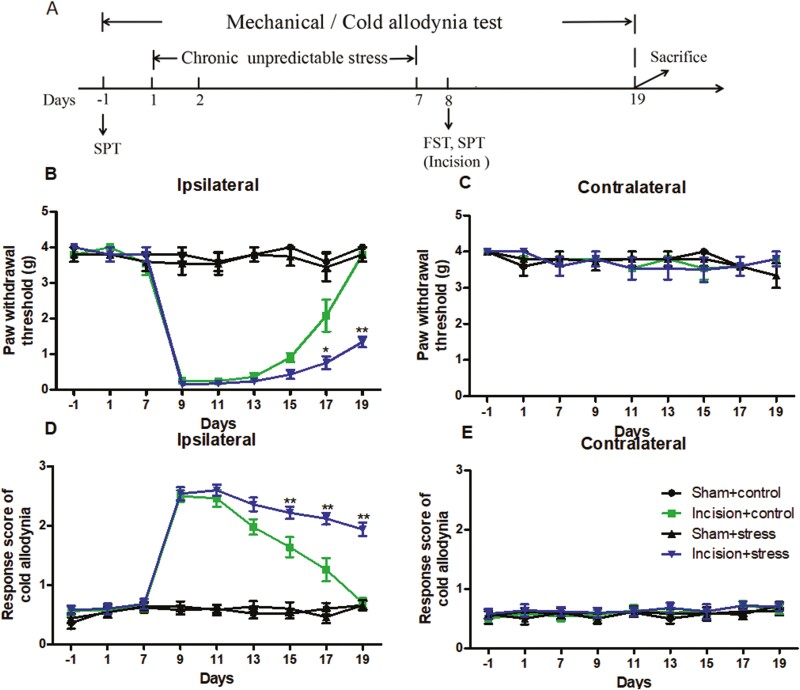
Pain Response Change in Mouse Exposure to Short-Term CUS Before Surgery
The experimental procedure of short-term CUS and the order of behavioral tests were shown in Figure 2A. To examine whether short-term CUS before surgery affected the magnitude or duration of surgery-induced hypersensitivity, we performed a unilateral plantar incision on the right hind paw in mice after they were exposed to short-term CUS for 7 consecutive days. The incision surgery induced persistent mechanical and cold nociceptive response on the ipsilateral (but not contralateral) side in the incision plus control group. For surgery group, pain hypersensitivity reached a peak 1 day after surgery (day 9) and completely disappeared 11 days after surgery (day 19) (Figure 2B and D). As expected, the preexposure to short-term CUS alone did not alter basal PWT to mechanical or cold stimuli on either side of stressed mice during our observation. However, the preexposure to short-term CUS delayed the recovery of surgical pain on the ipsilateral side in the incision plus stress group (Figure 2B and D). Two-way ANOVA revealed that short-term CUS and incision surgery significantly differed during the PWTs (short-term CUS: F(1,36) = 38.58, P < .001; incision surgery: F(1,36) = 4.807, P < .05 for day 17; short-term CUS: F(1,36) = 69.19, P < .001; incision surgery: F(1,36) = 49.94, P < .001 for day 19). The incision plus stress group had significantly lower PWTs to mechanical stimulation from day 17 to day 19 (Figure 2B; P < .05 or P < .01). Similar results were observed in the cold allodynia test: the incision plus stress group had significantly increased response scores of cold allodynia from day 15 to day 19 compared with the corresponding incision only group (P < .05 or P < .01) (Figure 2D).
Figure 2.
The experimental procedure (A) and postsurgical pain to preexposure to short-term chronic unpredictable stress in mice (B–E). (B, C) Mechanical allodynia test. (D, E) Cold allodynia test. (B, D) Responses of ipsilateral paws. (C, E) Responses of contralateral paws. Results are expressed as mean ± SEM. *P < .05, **P < .01 vs the corresponding time points in the incision plus control group (n = 10/group).
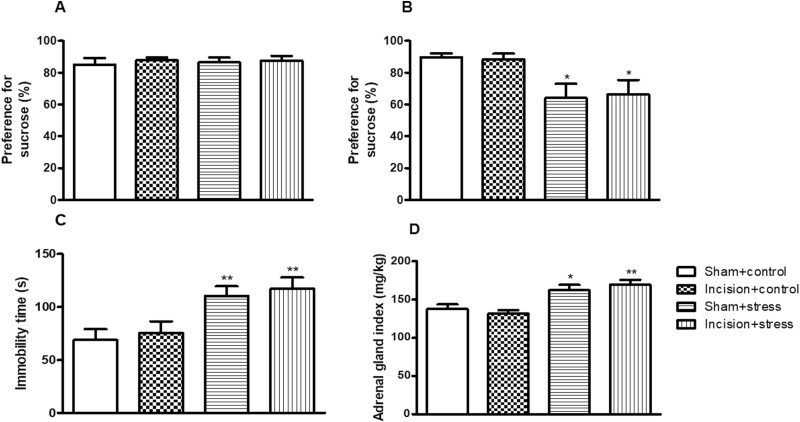
Depression-Like Behavior and Adrenal Gland Index in Mouse Exposure to Short-Term CUS Before Surgery
There were no significant differences in the baseline of sucrose preference in each group compared with the sham plus control group (Figure 3A). After receiving short-term CUS for 7 days, short-term CUS decrease the sucrose consumption (short-term CUS: F(1,36) = 11.28, P < .01; incision surgery: F(1,36) = 0.5, P > .05; Figure 3B), FST (short-term CUS: F(1,36) = 16.53, P < .001; incision surgery: F(1,36) = 0.4389, P > .05; Figure 3C), and adrenal gland index (short-term CUS: F(1,36) = 28.80, P < .001; incision surgery: F(1,36) = 1.401, P > .05; Figure 3D). The mice in the sham plus stress group and the incision plus stress group showed significantly less sucrose consumption than those in the sham plus control group (P < .05; P < .05; Figure 3B). No obvious difference in sucrose consumption was observed between the incision plus control group and the sham plus control group (Figure 3B). We further performed the FST, in which mice under stress displayed a longer duration of immobility. The mice in the sham plus stress group and the incision plus stress group showed more immobility time than those in the sham plus control group (P < .01; P < .01; Figure 3C). The incision plus control group and the sham plus control group did not show significant difference in immobility time (Figure 3C).
Figure 3.
The depression-like effects of short-term chronic unpredictable stress on the sucrose preference test (SPT; A, B) and forced swimming test (FST; C) in mice. Sucrose consumption before stress (baseline) (A) and day 8 after stress (B) as well as immobility time (C) were determined. The adrenal glands of mice were dissected after mice were killed on day 19 (D). Results are expressed as mean ± SEM. *P < .05, **P < .01 vs the corresponding sham plus control group (n = 10/group).
The subsequent study showed that the mice in the sham plus stress group and the incision plus stress group significantly increased adrenal gland index compared with those in the sham plus control group (P < .05; P < .01; Figure 3D). No obvious difference in adrenal gland index was observed between the incision plus control group and the sham plus control group (Figure 3D).
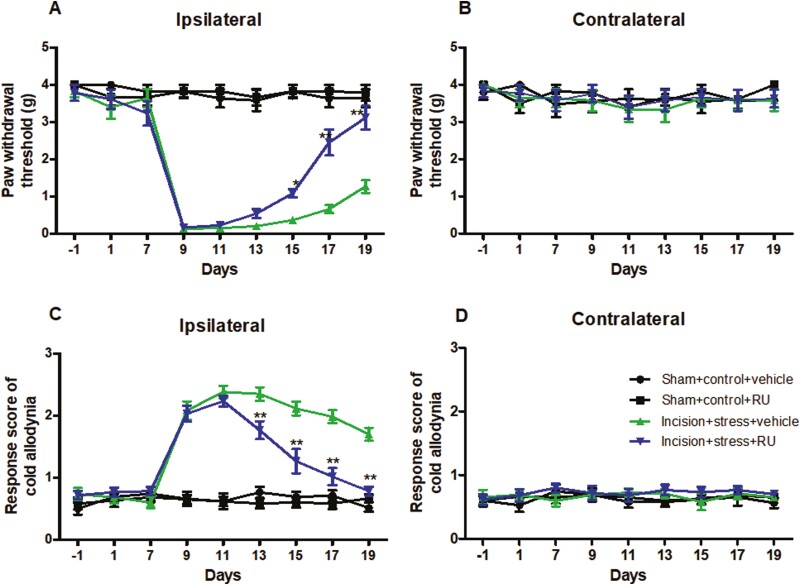
RU Reversed Short-Term CUS-Induced Postsurgical Pain Prolongation in Mice
In the incision plus stress group, pretreatment of the vehicle did not affect significant decreases in PWT to mechanical and cold stimuli on the ipsilateral side. However injection of RU completely abolished the decreases in PWT to mechanical stimulation in the incision plus stress group on days 15 (P < .05), 17 (P < .01), and 19 (P < .01) (Figure 4A). Similarly, compared with the corresponding incision plus stress plus vehicle group, treatment with RU to the incision plus stress group significantly decreased the response score to cold allodynia on days 13 (P < .01), 15 (P < .01), 17 (P < .01), and 19 (P < .01) (Figure 4C), suggesting that RU reversed pain recovery prolongation in the incision plus stress group. RU at the dose used alone did not alter basal paw withdrawal responses to mechanical and cold stimuli on the contralateral side of the incision plus stress group (Figure 4B and D) or on either ipsilateral or contralateral side of the remaining treated groups.
Figure 4.
RU38486 reversed short-term chronic unpredictable stress–induced postsurgical pain. Mechanical allodynia test (A, B) and cold allodynia test (C, D) were determined in ipsilateral paws (A, C) and contralateral paws (B, D). Results are expressed as mean ± SEM. *P < .05, **P < .01 vs the corresponding time points in the incision plus stress group with vehicle (n = 10/group).
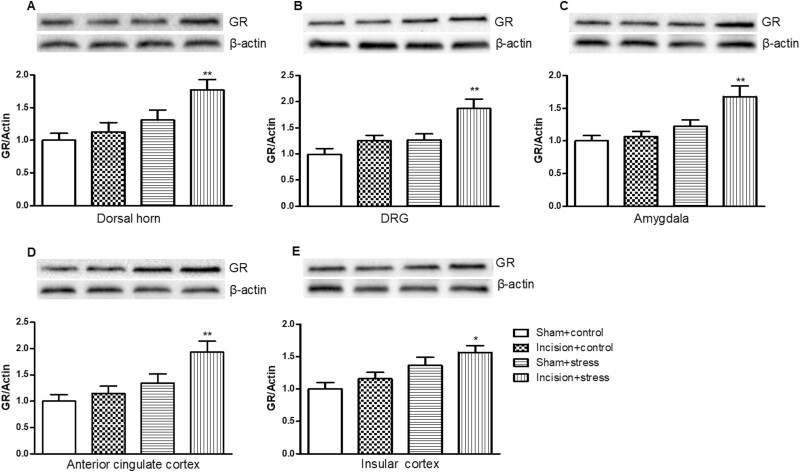
GR Expression in Depression Pain–Related Regions in Mouse Presurgical Exposure to Short-Term CUS
Two-way ANOVA revealed that short-term CUS significantly differed the expression of GR in the L4/5 DH (short-term CUS: F(1,36) = 4.45, P < .05; incision surgery: F(1,36) = 3.78, P > .05; Figure 5A), L4/5 DRG (short-term CUS: F(1,36) = 5.63, P < .05; incision surgery: F(1,36) = 1.94, P > .05; Figure 5B), amygdala (short-term CUS: F(1,36) = 9.517, P < .05; incision surgery: F(1,36) = 2.724, P > .05; Figure 5C), anterior cingulate cortex (short-term CUS: F(1,36) = 10.04, P < .01; incision surgery: F(1,36) = 3.08, P > .05; Figure 5D), and insular cortex (short-term CUS: F(1,36) = 11.7, P < .01; incision surgery: F(1,36) = 2.47, P > .05; Figure 5E). Presurgical exposure to short-term CUS significantly increased the expression of GR in the L4/5 DH (P < .01; Figure 5A), L4/5 DRG (P < .01; Figure 5B), amygdala (P < .01; Figure 5C), anterior cingulate cortex (P < .01; Figure 5D), and insular cortex (P < .05; Figure 5E) on the ipsilateral side 9 days after surgery in the incision plus short-term CUS group compared with the sham plus control group. Neither surgery alone nor short-term CUS caused significant alterations in the expression of GR in DH, DRG, amygdala, anterior cingulate cortex, and insular cortex in the incision plus control or the sham plus short-term CUS group.
Figure 5.
Presurgical exposure to chronic unpredictable stress–induced glucocorticoid receptor (GR) expression in the L4/5 dorsal horn (A), L4/5 dorsal root ganglion (DRG) (B), amygdala (C), anterior cingulate cortex (D), and insular cortex (E) on day 11 after surgery. Results are expressed as mean ± SEM. *P < .05, **P < .01 vs the corresponding incision plus control group (n = 10/group).
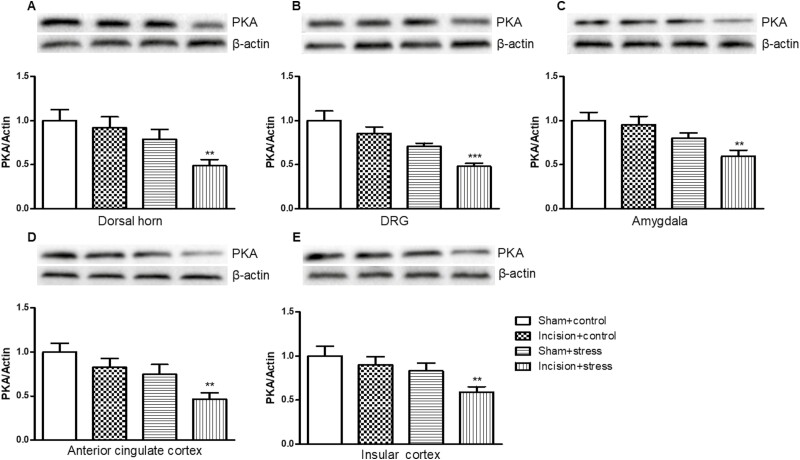
PKA Expression in Depression Pain–Related Regions in Mouse Presurgical Exposure to Short-Term CUS
To determine whether presurgical exposure affected PKA, we measured PKA expression in the L4/5 DH, L4/5 DRG, amygdala, anterior cingulate cortex, and insular cortex. Short-term CUS significantly decreased the expression of PKA in the L4/5 DH (short-term CUS: F(1,36) = 10.04, P < .05; incision surgery: F(1,36) = 3.08, P > .05; Figure 6A), amygdala (short-term CUS: F(1,36) = 5.56, P < .05; incision surgery: F(1,36) = 2.12, P > .05; Figure 6C), and insular cortex (short-term CUS: F(1,36) = 11.7, P < .01; incision surgery: F(1,36) = 2.47, P > .05; Figure 6E).
Figure 6.
Presurgical exposure to chronic unpredictable stress reduced protein kinase A (PKA) expression in the L4/5 dorsal horn (A), L4/5 dorsal root ganglion (DRG) (B), amygdala (C), anterior cingulate cortex (D), and insular cortex (E) on day 11 after surgery. Results are expressed as mean ± SEM. **P < .01, ***P < .001 vs the corresponding incision plus control group (n = 10/group).
Short-term CUS and incision surgery significantly decreased the expression of PKA in L4/5 DRG (short-term CUS: F(1,36) = 17.70, P < .001; incision surgery: F(1,36) = 6.96, P < .05; Figure 6B) and anterior cingulate cortex (short-term CUS: F(1,36) = 12.44, P < .01; incision surgery: F(1,36) = 6.54, P < .05; Figure 6D).
PKA expression in the incision plus stress group expression was decreased in the DH (P < .01; Figure 6A), DRG (P < .001; Figure 6B), amygdala (P < .01; Figure 6C), anterior cingulate cortex (P < .01, Figure 6D), and insular cortex (P < .01; Figure 6E) compared with the incision plus control group. Neither incision alone nor short-term CUS led to significant changes in the PKA expression in these detected areas in the incision plus control or the sham plus short-term CUS group.
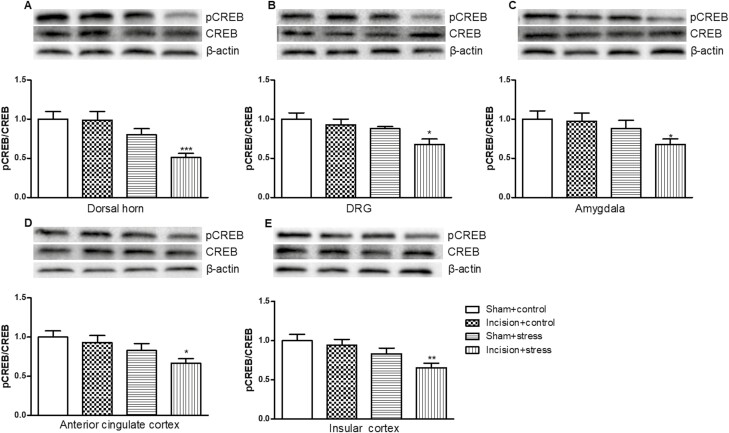
Ratio of pCREB to CREB and BDNF Expression in Depression Pain–Related Regions in Mouse Presurgical Exposure to Short-Term CUS
Both short-term CUS and incision surgery significantly differed the expression of pCREB/CREB in L4/5 DH (short-term CUS: F(1,36) = 19.67, P < .001; incision surgery: F(1,36) = 6.58, P < .05; Figure 7A). Only short-term CUS significantly differed the expression of pCREB/CREB in the L4/5 DRG (short-term CUS: F(1,36) = 6.67, P < .05; incision surgery: F(1,36) = 3.25, P > .05; Figure 7B), amygdala (short-term CUS: F(1,36) = 4.322, P < .05; incision surgery: F(1,36) = 0.43, P > .05; Figure 7C), anterior cingulate cortex (short-term CUS: F(1,36) = 8.13, P < .01; incision surgery: F(1,36) = 3.00, P > .05; Figure 7D), and insular cortex (short-term CUS: F(1,36) = 8.19, P < .01; incision surgery: F(1,36) = 3.57, P > .05; Figure 7E). Preexposure to short-term CUS in mice significantly decreased the ratio of pCREB/CREB in the L4/5 DH (P < .001; Figure 7A), L4/5 DRG (P < .05; Figure 7B), amygdala (P < .05; Figure 7C), anterior cingulate cortex (P < .05; Figure 7D), and insular cortex (P < .01; Figure 7E) compared with the incision plus control group.
Figure 7.
Presurgical exposure to chronic unpredictable stress reduced the ratio of cAMP response element-binding protein phosphorylation (pCREB)/CREB in the L4/5 dorsal horn (A), L4/5 dorsal root ganglion (DRG) (B), amygdala (C), anterior cingulate cortex (D), and insular cortex (E) on day 11 after surgery. Results are expressed as mean ± SEM. *P < .05, **P < .01, ***P < .001 vs the corresponding incision plus control group (n = 10/group).
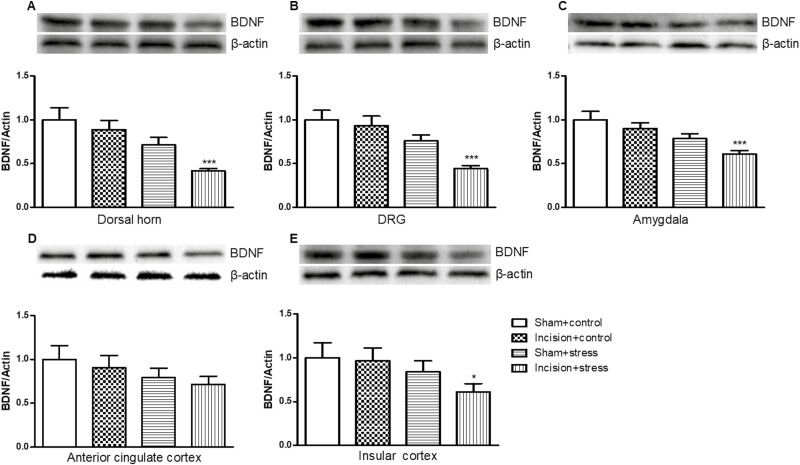
We also found that short-term CUS significantly differed the expression of BDNF in the L4/5 DH (short-term CUS: F(1,36) = 14.36, P < .001; incision surgery: F(1,36) = 3.992, P > .05; Figure 8A), DRG (short-term CUS: F(1,36) = 20.58, P < .001; incision surgery: F(1,36) = 2.15, P > .05; Figure 8B), anterior cingulate cortex (short-term CUS: F(1,36) = 3.57, P < .05,; incision surgery: F(1,36) = 0.39, P > .05; Figure 8D), and insular cortex (short-term CUS: F(1,36) = 4.12, P < .05; incision surgery: F(1,36) = 1.11, P > .05; Figure 8E). Short-term CUS and incision surgery significantly differed the expression of BDNF in amygdala (short-term CUS: F(1,36) = 8.98, P < .01; incision surgery: F(1,36) = 7.30, P < .05; Figure 8C).
Figure 8.
Presurgical exposure to chronic unpredictable stress reduced brain-derived neurotrophic factor (BDNF) proteins in the L4/5 dorsal horn (A), L4/5 dorsal root ganglion (DRG) (B), amygdala (C), anterior cingulate cortex (D), and insular cortex (E) on day 11 after surgery. Results are expressed as mean ± SEM. *P < .05, ***P < .001 vs the corresponding incision plus control group (n = 10/group).
BDNF expression was significant decreased in DH (P < .001; Figure 8A), DRG (P < .001; Figure 8B), amygdala (P < .001; Figure 8C), and insular cortex (P < .01; Figure 8E). In contrast, neither incision alone nor short-term CUS led to significant changes of pCREB/CREB and BDNF expression in these detected areas in other groups.
DISCUSSION
The present study suggested that exposure to short-term CUS in mice induces depressive-like symptoms but does not affect the basal pain threshold. In the SPT and FST, stress-reduced sucrose preferences and prolonged immobile duration indicated that short-term CUS caused a certain depressive state. In addition, short-term CUS reduced the pain thresholds to mechanical and cold stimuli in the Von Frey and acetone-induced allodynia tests. The subsequent studies demonstrated that chronic stress increased the adrenal gland index and that the GR antagonist RU can reverse the delayed recovery of postoperative pain induced by short-term CUS. This effect of short-term CUS delaying postsurgical pain recovery seemed to involve the regulation of the hypothalamus-pituitary-adrenal gland (HPA) axis and cerebrospinal system, specifically, the increase in GR and decreases of PKA, pCREB, and BDNF protein levels in the DH, DRG, and emotion-related brain regions such as the amygdala, anterior cingulate cortex, and insular cortex.
Acute postoperative pain is followed by persistent pain in 10%–50% of individuals after common operations (Kehlet et al., 2006). It differs from other gradual (such as fibromyalgia) or sudden unpredictable damage (such as trauma-induced nerve damage) (Kundermann et al., 2004; Huang et al., 2014; Vanini et al., 2016), where the surgical patients know in advance the precise time of the physical damage and subsequent pain (Roehrs et al., 2006). These conditions increase preoperative anxiety and depression and affect preoperative sleep, thereby causing stress (Wang et al., 2012). Identifying the risk factors that affect postoperative pain during the perioperative period can help us predict which patients may experience prolonged recovery and provide an opportunity to effectively manage postoperative pain. Chronic stress increases the basal pain threshold and exacerbates existing pathological pain (Raymond et al., 2001; Huang et al., 2014). However, most surgical patients have normal physical and mental health and normal pain threshold before the operation, although they have a certain degree of stress before and after the operation (Wang et al., 2015). Whether this short-term chronic stress affects postoperative pain recovery in surgical patients is still elusive.
This study established an animal model of mild depression. In this model, after the mice were subjected to CUS twice a day for 7 consecutive days, the freezing time of the mice in the FST was significantly increased, the preference for sucrose rate decreased in the SPT, and the adrenal gland index level was significantly increased, but the basal response to mechanical and cold stimuli hardly changed. The presurgery stress animal model that we established successfully simulates the clinical situation where most patients undergoing incision surgery have mild depression before surgery, but the basal pain threshold has not changed. More importantly, we found that the short-term CUS leads to mild depression and postpones pain recovery after surgery, as evidenced by the reduction of the hind PWT in response to noxious mechanical and cold stimuli, which indicates the exacerbation of pain. It is possible that the specific molecular biological changes in both the central nervous and peripheral system initiate negative regulation of downstream postreceptor signal cascade and neuroprotective function during chronic stress, leading to persistent pain after surgery.
Stress hormones such as GSs play an important role in a series of physiological processes such as inflammatory and immune responses in the brain and peripheral system (Neeck et al., 2002). Endogenous GS are mainly produced by the adrenal gland; cortisol is mainly produced in humans, and corticosterone is mainly produced in rodents (Wang et al., 2015). Stress-induced anxiety and depression activate the HPA axis and promote the release of GS. During the stress process, GS in the plasma increase significantly, thereby gradually activating the GR (Abrahám et al., 2000). Previous studies suggest that GS can exacerbate pain-like behaviors and participate in the development of depressive stress; it therefore can be speculated that GS plays an important role in mild depression-induced persistent postoperative pain (Blackburn-Munro et al., 2003; Cao et al., 2015). Our research showed that the short-term CUS transiently activated the HPA axis as reflected by the increased adrenal gland index and GR expression. These results were consistent with previous studies that suggested short-term CUS induced corticosterone secretion in mice and subsequent GR activation (Willner et al., 1992; Xu et al., 2006, 2013; Ding et al., 2014). Indeed, different brain regions, particularly in the limbic system, act in concert to mediate stress-related depression and/or anxiety (Ding et al., 2014). It is possible that compensatory adaptation was triggered in these brain regions manifested as activation or inhibition of GRs (Ding et al., 2014; Xu et al., 2019). The present study showed GR activation after stress plus incision, as evidenced by increased GR expression in different brain regions that were related to pain response. Further results suggested that the GR antagonist RU reversed the delayed recovery of postoperative pain induced by short-term CUS, indicating that the GR antagonist blocked the activation of GRs to eliminate the postsurgical pain recovery prolongation in stressed mice. These findings are consistent with previous studies that demonstrate that GR is highly expressed in emotion- and pain-related areas (Lerch et al., 2017; Luo et al., 2022). In addition to high expression in the brain regions such as hippocampus, amygdala, anterior cingulate cortex, and insular cortex (Myers et al., 2010; Baliki et al., 2015; Barthas et al., 2015), GR is broadly expressed in neurons of the DH and DRG (Condon et al., 1998; Villagrá et al., 2004;Wang et al., 2005; Gu et al., 2011). Our research showed that the increased adrenal gland index and GR levels in the DRG, DH, and brain regions related to emotional changes exacerbate persistent surgical pain, which indicates that the dysfunction of the HPA axis participates in CUS-induced pain prolongation. GR is negatively relevant to cyclic adenosine monophosphate (cAMP) activation in the brain. cAMP plays a key role in the intracellular signal cascade amplification and regulation of cell physiological activities and material metabolism. cAMP signaling is related to chronic pain and major depressive disorder (Zhu et al., 2018) and has been considered a potential target for the treatment of depression-pain disorders. G-protein coupled receptors (Gi or Gs) first transmit extracellular signal molecules to adenylate cyclase, thereby activating the second messenger cAMP in the cell, activating PKA and further triggering the downstream transcription factor pCREB to synthesize the corresponding functional protein (Lonze et al., 2002). pCREB combines with the classic downstream target gene of CREB, BDNF by CREB (Ge et al., 2015), and regulates early and late gene transcription. Previous studies found that dysregulation of the HPA axis decreased pCREB and corresponding functional protein expression, such as BDNF, thus inducing neurological damage (Bayatti et al., 2005; Li et al., 2018). Studies have also shown that depression is comorbid with chronic pain, and, consistent with our findings, chronicization of acute pain inhibits the expression of pCREB/BDNF-related proteins (Zhu et al., 2018). In the present study, the decreased expression of PKA, pCREB, and BDNF in the pain-/emotion-related areas of the brain may contribute to short-term CUS-induced persistent postsurgical pain. Further experiments are needed to verify the causal relationship between activation of GR and inhibition of its downstream PKA-CREB-BDNF signaling pathway in short-term stress–related pain recovery.
In summary, the present study demonstrated short-term CUS-induced postsurgical pain prolongation. The increased expression of GR in the neurons of the DH, DRG, amygdala, anterior cingulate cortex, and insular cortex in stress plus surgery mice triggered a series of downstream signaling changes, such as the decreased expression of PKA and decreased ratio of pCREB/CREB and BDNF, indicating that persistent postsurgical pain is related to dysfunction in the HPA axis–dependent cAMP/PKA/pCREB/BDNF signaling pathway.
Acknowledgments
This study was supported by the Shanghai Jinshan District Medical Backup Key Specialty Construction Project (no. JSZK2019H03) to Junfeng Zhu, the Natural Science Foundation of Zhejiang Province (no. LY21H310004) to Jianchun Pan, Medicine and Health Science Plan Project of Zhejiang Province (no. 2023RC217) to Yan Jiang, and the Zhejiang Provincial Public Welfare Research Project (no. 2018C37082) and Special Scientific Research Fund Project for Hospital Pharmacy of Zhejiang Pharmaceutical Association (no. 2021ZYY05) to Ling Chen.
Contributor Information
Jing Sun, Department of Anesthesiology, Jinshan Branch of the Sixth People’s Hospital of Shanghai, Shanghai Jiao Tong University, Shanghai, China.
Wenhua Xu, Department of orthopedics, Yichun People’s Hospital, The Affiliated Hospital of Yichun University, Yichun, China.
Han Ye, Department of Clinical Pharmacy, The First Affiliated Hospital, School of Medicine, Zhejiang University, China.
Dingzhong Tang, Department of Medicine, Jinshan Branch of the Sixth People’s Hospital of Shanghai, Shanghai Jiao Tong University, Shanghai, China.
Yan Jiang, Department of Traditional Chinese Medicine Pharmacy, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, China.
Yuqing Kang, Department of Anesthesiology, Jinshan Branch of the Sixth People’s Hospital of Shanghai, Shanghai Jiao Tong University, Shanghai, China.
Jianchun Pan, Brain Institute, School of Pharmacy, Wenzhou Medical University, Wenzhou, Zhejiang Province, China.
Junfeng Zhu, Department of Anesthesiology, Jinshan Branch of the Sixth People’s Hospital of Shanghai, Shanghai Jiao Tong University, Shanghai, China.
Min Zhou, Department of Medicine, Jinshan Branch of the Sixth People’s Hospital of Shanghai, Shanghai Jiao Tong University, Shanghai, China.
Ling Chen, Department of Clinical Pharmacy, Key Laboratory of Clinical Cancer Pharmacology and Toxicology Research of Zhejiang Province, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, China.
Author contributions
Jing Sun: investigation and original draft. Wenhua Xu and Han Ye: investigation and formal analysis. Dingzhong Tang: data curation. Yan Jiang: funding acquisition. Yuqing Kang: investigation and resources. Jianchun Pan: funding acquisition. Junfeng Zhu: conceptualization and funding acquisition. Min Zhou: conceptualization and project administration. Ling Chen: project administration and funding acquisition. All authors contributed to the article and approved the submitted version.
Interest Statement
The authors declare no conflicts of interest included in this manuscript, as applicable to each author, including specific financial interests, relationships, and/or affiliations relevant to the subject matter or materials.
Data Availability
The data that support the findings of this study are available on request from the corresponding author upon reasonable request.
References
- Abrahám I, Harkany T, Horvath KM, Veenema AH, Penke B, Nyakas C, Luiten PG (2000) Chronic corticosterone administration dose-dependently modulates Aβ((1-42))- and NMDA-induced neurodegeneration in rat magnocellular nucleus basalis. J Neuroendocrinol 12:486–494. [DOI] [PubMed] [Google Scholar]
- Adebesin A, Adeoluwa OA, Eduviere AT, Umukoro S (2017) Methyl jasmonate attenuated lipopolysaccharide-induced depressive-like behavior in mice. J Psychiatr Res 94:29–35. [DOI] [PubMed] [Google Scholar]
- Atianjoh FE, Yaster M, Zhao X, Takamiya K, Xia J, Gauda EB, Huganir RL, Tao YX (2010) Spinal cord protein interacting with C kinase 1 is required for the maintenance of complete Freund’s adjuvant-induced inflammatory pain but not for incision-induced post-operative pain. Pain 151:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bair MJ, Robinson RL, Katon WKK (2003) Depression and pain comorbidity: a literature review. Arch Intern Med 163:2433–2245. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Apkarian AV (2015) Nociception, pain, negative moods, and behavior selection. Neuron 87:474–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banik RK, Woo YC, Park SS, Brennan TJ (2006) Strain and sex influence pain sensitivity after plantar incision in the mouse. Anesthesiology 105:1246–1253. [DOI] [PubMed] [Google Scholar]
- Barthas F, Sellmeijer J, Hugel S, Waltisperger E, Barrot M, Yalcin I (2015) The anterior cingulate cortex is a critical hub for pain-induced depression. Biol Psychiatry 77:236–245. [DOI] [PubMed] [Google Scholar]
- Bayatti N, Hermann H, Lutz B, Behl C (2005) Corticotropin-releasing hormone-mediated induction of intracellular signaling pathways and brain-derived neurotrophic factor expression is inhibited by the activation of the endocannabinoid system. Endocrinology 146:1205–1213. [DOI] [PubMed] [Google Scholar]
- Blackburn-Munro G, Blackburn-Munro R (2003) Pain in the brain: are hormones to blame? Trends Endocrinol Metab 14:20–27. [DOI] [PubMed] [Google Scholar]
- Bourin M, Mocaër E, Porsolt R (2004) Antidepressant-like activity of S 20098 (agomelatine) in the forced swimming test in rodents: involvement of melatonin and serotonin receptors. J Psychiatry Neurosci 29:126–133. [PMC free article] [PubMed] [Google Scholar]
- Bravo L, Mico JA, Rey-Brea R, Pérez-Nievas B, Leza JC, Berrocoso E (2012) Depressive-like states heighten the aversion to painful stimuli in a rat model of comorbid chronic pain and depression. Anesthesiology 117:613–625. [DOI] [PubMed] [Google Scholar]
- Cao J, Wang PK, Tiwari V, Liang L, Lutz BM, Shieh KR, Zang WD, Kaufman AG, Bekker A, Gao XQ, Tao YX (2015) Short-term pre-and post-operative stress prolongs incision-induced pain hypersensitivity without changing basal pain perception. Mol Pain 11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caumo W, Schmidt AP, Schneider CN, Bergmann J, Iwamoto CW, Adamatti LC, Bandeira D, Ferreira MBC (2002) Preoperative predictors of moderate to intense acute postoperative pain in patients undergoing abdominal surgery. Acta Anaesthesiol Scand 46:1265–1271. [DOI] [PubMed] [Google Scholar]
- Condon J, Gosden C, Gardener D, Nickson P, Hewison M, Howie AJ, Stewart PM (1998) Expression of type 2 11β-hydroxysteroid dehydrogenase and corticosteroid hormone receptors in early human fetal life. J Clin Endocrinol Metab 83:4490–4497. [DOI] [PubMed] [Google Scholar]
- Cremeans-Smith JK, Millington K, Sledjeski E, Greene K, Delahanty DL (2006) Sleep disruptions mediate the relationship between early postoperative pain and later functioning following total knee replacement surgery. J Behav Med 29:215–222. [DOI] [PubMed] [Google Scholar]
- Dekker AP, Salar O, Karuppiah SV, Bayley E, Kurian J (2016) Anxiety and depression predict poor outcomes in arthroscopic subacromial decompression. J Shoulder Elb Surg 25:873–880. [DOI] [PubMed] [Google Scholar]
- Ding LS, Zhang C, Masood A, Li JX, Sun J, Nadeem A, Zhang HT, O’ Donnell JM, Xu Y (2014) Protective effects of phosphodiesterase 2 inhibitor on depression-and anxiety-like behaviors: involvement of antioxidant and anti-apoptotic mechanisms. Behav Brain Res 268:150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge L, Liu L, Liu H, Liu S, Xue H, Wang X, Yuan L, Wang Z, Liu D (2015) Resveratrol abrogates lipopolysaccharide-induced depressive-like behavior, neuroinflammatory response, and CREB/BDNF signaling in mice. Eur J Pharmacol 768:49–57. [DOI] [PubMed] [Google Scholar]
- Georén S, Åhnblad P, Stjärne P, Wikström AC, Stierna P (2005) Significance of endogenous glucocorticoid sensitivity for airway eosinophilia in a murine model of allergy. Acta Otolaryngol 125:378–385. [DOI] [PubMed] [Google Scholar]
- Grønli J, Murison R, Fiske E, Bjorvatn B, Sørensen E, Portas CM, Ursin R(2005) Effects of chronic mild stress on sexual behavior, locomotor activity and consumption of sucrose and saccharine solutions. Physiol Behav 84:571–577. [DOI] [PubMed] [Google Scholar]
- Gu XP, Peng L, Yang D, Ma Q, Zheng Y, Liu C, Zhu B, Song L, Sun X, Ma Z (2011) The respective and interaction effects of spinal GRs and MRs on radicular pain induced by chronic compression of the dorsal root ganglion in the rat. Brain Res 1396:88–95. [DOI] [PubMed] [Google Scholar]
- Hernández C, Díaz-Heredia J, Berraquero ML, Crespo P, Loza E, Ruiz Ibán MÁ (2015) Pre-operative predictive factors of post-operative pain in patients with hip or knee arthroplasty: a systematic review. Reumatol Clín 11:361–380. [DOI] [PubMed] [Google Scholar]
- Huang CT, Chiang RPY, Chen CL, Tsai YJ (2014) Sleep deprivation aggravates median nerve injury-induced neuropathic pain and enhances microglial activation by suppressing melatonin secretion. Sleep 37:1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson T, Tian P, Wang Y, Iezzi T, Xie W (2016) Toward identifying moderators of associations between presurgery emotional distress and postoperative pain outcomes: a meta-analysis of longitudinal studies. J Pain 17:874–888. [DOI] [PubMed] [Google Scholar]
- Kehlet H, Jensen TS, Woolf CJ (2006) Persistent postsurgical pain: risk factors and prevention. Lancet 367:1618–1625. [DOI] [PubMed] [Google Scholar]
- Kundermann B, Spernal J, Huber MT, Krieg JC, Lautenbacher S (2004) Sleep deprivation affects thermal pain thresholds but not somatosensory thresholds in healthy volunteers. Psychosom Med 66:932–937. [DOI] [PubMed] [Google Scholar]
- Lerch JK, Alexander JK, Madalena KM, Motti D, Quach T, Dhamija A, Zha A, Gensel JC, Webster Marketon J, Lemmon VP, Bixby JL, Popovich PG (2017) Stress increases peripheral axon growth and regeneration through glucocorticoid receptor-dependent transcriptional programs. eNeuro 4:ENEURO.0246-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Yang Y, Liu S, Fang H, Zhang Y, Furmanski O, Skinner J, Xing Y, Johns RA, Huganir RL, Tao F (2014) Stress induces pain transition by potentiation of AMPA receptor phosphorylation. J Neurosci 34:13737–13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Wang G, Shi J, Xie X, Fei N, Chen L, Liu N, Yang M, Pan J, Huang W, Xu Y (2018) Trans-resveratrol ameliorates anxiety-like behaviors and fear memory deficits in a rat model of post-traumatic stress disorder. Neuropharmacology 133:181–188. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD (2002) Function and regulation of CREB family transcription factors in the nervous system. Neuron 35:605–623. [DOI] [PubMed] [Google Scholar]
- Luo HY, Zhang YD, Zhang JJ, Shao JP, Ren XH, Zang WD, Cao J, Xu B (2022) Glucocorticoid receptor contributes to electroacupuncture-induced analgesia by inhibiting Nav1.7 expression in rats with inflammatory pain induced by complete Freund’s adjuvant. Neuromodulation 25:1393–1402. [DOI] [PubMed] [Google Scholar]
- Meerwijk EL, Ford JM, Weiss SJ (2013) Brain regions associated with psychological pain: implications for a neural network and its relationship to physical pain. Brain Imaging Behav 7:1–14. [DOI] [PubMed] [Google Scholar]
- Mizoguchi S, Andoh T, Yakura T, Kuraishi Y (2016) Involvement of c-Myc-mediated transient receptor potential melastatin 8 expressions in oxaliplatin-induced cold allodynia in mice. Pharmacol Rep 68:645–648. [DOI] [PubMed] [Google Scholar]
- Myers B, Greenwood-Van Meerveld B (2010) Divergent effects of amygdala glucocorticoid and mineralocorticoid receptors in the regulation of visceral and somatic pain. Am J Physiol - Gastrointest Liver Physiol 298:G295–303. [DOI] [PubMed] [Google Scholar]
- Neeck G, Renkawitz R, Eggert M (2002) Molecular aspects of glucocorticoid hormone action in rheumatoid arthritis. Cytokines Cell Mol Ther 7:61–69. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Fitzgerald LW, Lane S, Terwilliger R, Nestler EJ (1996) Biochemical adaptations in the mesolimbic dopamine system in response to repeated stress. Neuropsychopharmacology 14:443–452. [DOI] [PubMed] [Google Scholar]
- Pang C, Cao L, Wu F, Wang L, Wang G, Yu Y, Zhang M, Chen L, Wang W, Lv W, Chen L, Zhu J, Pan J, Zhang H, Xu Y, Ding L (2015) The effect of trans-resveratrol on post-stroke depression via regulation of hypothalamus–pituitary-adrenal axis. Neuropharmacology 97:447–456. [DOI] [PubMed] [Google Scholar]
- Raymond I, Nielsen TA, Lavigne G, Manzini C, Choinière M (2001) Quality of sleep and its daily relationship to pain intensity in hospitalized adult burn patients. Pain 92:381–388. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Hyde M, Blaisdell B, Greenwald M, Roth T (2006) Sleep loss and REM sleep loss are hyperalgesic. Sleep 29:145–151. [DOI] [PubMed] [Google Scholar]
- Rubin RT, Phillips JJ, McCracken JT, Sadow TF (1996) Adrenal gland volume in major depression: relationship to basal and stimulated pituitary-adrenal cortical axis function. Biol Psychiatry 40:89–97. [DOI] [PubMed] [Google Scholar]
- Sheng J, Liu S, Wang Y, Cui R, Zhang X (2017) The link between depression and chronic pain:neural mechanisms in the brain. Neural Plast 2017:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobol-Kwapinska M, Bąbel P, Plotek W, Stelcer B (2016) Psychological correlates of acute postsurgical pain: a systematic review and meta-analysis. Eur J Pain 20:1573–1586. [DOI] [PubMed] [Google Scholar]
- Su W, Lu F, Zhang X, Li G, Chen W, Ma T, Gao SL, Lou JY, Bai XL, Liang TB (2017) A hospital-to-home evaluation of an enhanced recovery protocol for elective pancreaticoduodenectomy in China. Medicine 96:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen M, Peters ML, Bruce J, Gramke HF, Marcus MA (2012) Preoperative anxiety and catastrophizing: a systematic review and meta-analysis of the association with chronic postsurgical pain. Clin J Pain 28:819–841. [DOI] [PubMed] [Google Scholar]
- Vanini G (2016) Sleep deprivation and recovery sleep prior to a noxious inflammatory insult influence characteristics and duration of pain. Sleep 39:133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villagrá NT, Berciano J, Altable M, Navascués J, Casafont I, Lafarga M, Berciano MT (2004) PML bodies in reactive sensory ganglion neurons of the Guillain- Barré syndrome. Neurobiol Dis 16:158–168. [DOI] [PubMed] [Google Scholar]
- Wang PK, Cao J, Wang H, Liang L, Zhang J, Lutz BM, Shieh KR, Bekker A, Tao YX (2015) Short-term sleep disturbance-induced stress does not affect basal pain perception but does delay postsurgical pain recovery. J Pain 16:1186–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Lim G, Zeng Q, Sung B, Yang L, Mao J (2005) Central glucocorticoid receptors modulate the expression and function of spinal NMDA receptors after peripheral nerve injury. J Neurosci 25:488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Tian Y, Song L, Lim G, Tan Y, You Z, Chen L, Mao J (2012) Exacerbated mechanical hyperalgesia in rats with genetically predisposed depressive behavior: role of melatonin and NMDA receptors. Pain 153:2448–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinrib AZ, Azam MA, Birnie KA, Burns LC, Clarke H, Katz J (2017) The psychology of chronic post-surgical pain: new frontiers in risk factor identification, prevention, and management. Br J Pain 11:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LS, Jones WJ, Shen J, Robinson RL, Weinberger MKK, Kroenke K (2003) Prevalence and impact of depression and pain in neurology outpatients. J Neurol Neurosurg Psychiatry 74:1587–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Muscat R, Papp M (1992) Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev 16:525–534. [DOI] [PubMed] [Google Scholar]
- Wright CE, Bovbjerg DH, Montgomery GH, Weltz C, Goldfarb A, Pace B, Silverstein JH (2009). Disrupted sleep the night before breast surgery is associated with increased postoperative pain. J Pain Symptom Manag 37:352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Guan XH, Yu JX, Lv J, Zhang HX, Fu QC, Xiang HB, Bu HL, Shi D, Shu B, Qin LS, Anne Manyande A, Tian YK (2014) Activation of spinal phosphatidylinositol 3-kinase/protein kinase B mediates pain behavior induced by plantar incision in mice. Exp Neurol 255:71–82. [DOI] [PubMed] [Google Scholar]
- Xu JJ, Wang R, Liu Y, Wang W, Liu DX, Jiang H, Pan F (2019) Short- and long-term alterations of FKBP5-GR and specific microRNAs in the prefrontal cortex and hippocampus of male rats induced by adolescent stress contribute to depression susceptibility. Psychoneuroendocrinology 101:204–215. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ku BS, Yao HY, Lin YH, Ma X, Zhang YH, Li XJ (2005) The effects of curcumin on depressive-like behaviors in mice. Eur J Pharmacol 518:40–46. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ku BS, Tie L, Yao HY, Jiang WG, Ma X, Li XJ (2006) Curcumin reverses the effects of chronic stress on behavior, the HPA axis, BDNF expression and phosphorylation of CREB. Brain Res 122:56–64. [DOI] [PubMed] [Google Scholar]
- Xu Y, Pan JC, Chen L, Zhang C, Sun J, Li J, Nguyen L, Nair N, Zhang H, O’Donnell JM (2013) Phosphodiesterase-2 inhibitor reverses corticosterone-induced neurotoxicity and related behavioural changes via cGMP/PKG dependent pathway. Int J Neuropsychopharmacol 16:835–847. [DOI] [PubMed] [Google Scholar]
- Xu Y, Cui SY, Ma Q, Ma Q, Shi J, Yu Y, Li JX, Zheng L, Zhang Y, Si JM, Yu YC (2018) Trans-resveratrol ameliorates stress-induced irritable bowel syndrome-like behaviors by regulation of brain-gut axis. Front Pharmacol 9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Guo J, Zhao X, Chen Z, Wang G, Liu A, Wang Q, Zhou W, Xu Y, Wang C (2013) Phosphodiesterase-5 inhibitor sildenafil prevents neuroinflammation, lowers beta-amyloid levels, and improves cognitive performance in APP/PS1 transgenic mice. Behav Brain Res 250:230–237. [DOI] [PubMed] [Google Scholar]
- Zhu C, Xu J, Lin Y, Ju P, Duan D, Luo Y, Ding WH, Huang SN, Jinghong Chen JH, Cui DH (2018) Loss of microglia and impaired brain-neurotrophic factor signaling pathway in a comorbid model of chronic pain and depression. Front Psychiatry 9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author upon reasonable request.