Abstract
Objective
The purpose was to validate the prognostic value of an early optimal morphological response on CT in patients treated with bevacizumab-containing chemotherapy for unresectable colorectal cancer liver metastases (CLM). It also evaluated the prognostic value of size-based criteria and the association of optimal morphological response with the receipt of bevacizumab.
Design
141 patients treated first using bevacizumab and 142 patients from a randomised study evaluating the addition of bevacizumab to oxaliplatin-based chemotherapy were retrospectively analysed. Radiologists evaluated pretreatment and restaging CT scans using morphological response criteria. Responses were also assessed with size-based criteria: Response Evaluation Criteria in Solid Tumors (RECIST), early tumour shrinkage (ETS) and deepness of response (DpR). The ability of each criterion to predict progression-free survival (PFS), overall survival (OS) and postprogression survival (PPS) was determined using a univariate Cox proportional hazards model.
Results
In both populations, median PFS was significantly longer for patients achieving an optimal morphological response (10.4 vs 6.8 months, p=0.03; and 8.3 vs 4.9 months, p<00001, respectively). Neither RECIST nor ETS responses were associated with a prolonged PFS. Median OS was longer for those with an optimal morphological response but only at second restaging in the first population (n=141, 20.8 vs 12.3 months, p=0.002). DpR but not optimal morphological response was associated with PPS. In the randomised study, an optimal morphological response was 6.2 times more likely among patients receiving bevacizumab (p<0.0001).
Conclusion
In patients with unresectable CLM, early morphological response may be a better predictor of PFS than size-based response. The addition of bevacizumab improves morphological response rate.
INTRODUCTION
Although half of patients with colorectal cancer (CRC) develop metastases during their disease course,1 overall survival (OS) has improved during the last 20 years owing to advances in surgical technique and medical management.2 One such advance is the development of bevacizumab, a monoclonal antibody against vascular endothelial growth factor A, which improves progression-free survival (PFS) and OS when combined with cytotoxic chemotherapy as first-line or second-line therapy for metastatic CRC.3–5
However, one issue encountered with bevacizumab is the lack of association between outcome and radiological response of metastatic CRCs based on Response Evaluation Criteria in Solid Tumors (RECIST).6 Alternative methods of categorising changes in tumour size on traditional images have been introduced but not sufficiently studied in patients receiving bevacizumab. Two such methods, early tumour shrinkage (ETS, ≥20% decrease in size from baseline to first restaging) and deepness of response (DpR, percentage of shrinkage observed at nadir of size), have been strongly associated with long-term outcomes in patients treated with cytotoxic chemotherapy alone7 or combined with cetuximab.8–10 Similar results for ETS have been reported with bevacizumab-based treatments,11 but only in one small study. Morphological changes, based on tumour attenuation and normal liver-tumour interface analysis, in CRC metastases to the liver, detected by CT, better predict surgical outcomes after bevacizumab-containing chemotherapy. Indeed, in three non-randomised cohorts of patients with resectable CRC liver metastase (CLM) treated with bevacizumab, an optimal morphological response predicted pathological response, disease or recurrence-free survival and OS better than RECIST did.12–14 Two of these studies also suggested that optimal morphological response was more frequently achieved in patient treated with bevacizumab.13 14 Furthermore another study found a reversal of the morphological pattern of response when progression occurred after an initial optimal morphological response.15 However, in patients with unresectable CLM, the prognostic value of morphological response has been confirmed only in a small, non-randomised cohort.16 Thus, the primary aim of our retrospective study was to establish the value of morphological response as an early indicator of survival outcomes in patients with unresectable CLM treated with bevacizumab-containing chemotherapy. Secondary goals were to compare the prognostic values of morphological, RECIST and alternative size-related methods of measuring response; to evaluate the prognostic value of a reversal of the morphological pattern of optimal response; and to determine whether adding bevacizumab to cytotoxic chemotherapy increases the rate of optimal morphological response in a randomised trial population.
METHODS
Study design
We performed a retrospective study based on CT images review of two distinct prospectively accrued cohorts of patients with unresectable CLM treated in first-line therapy.
Patient selection
Single-institution non-randomised population
From a prospectively accrued cohort of patients at MD Anderson Cancer Center, we identified 141 patients with unresectable CLM treated first using bevacizumab plus cytotoxic chemotherapy between March 2004 and September 2009 and with at least one liver metastasis larger than 2 cm (to optimise our ability to score the morphological response). The period was chosen so that mature survival data were available. All patients had undergone contrast-enhanced CT of the abdomen before starting chemotherapy and at 2-month intervals thereafter until discontinuation of treatment because of progression or unacceptable toxicity. Carcinoembryonic antigen (CEA) levels were also measured regularly.
NO16966 population
We selected patients enrolled in a multi-institutional phase III randomised trial that evaluated the efficacy and safety of first-line therapy with bevacizumab and oxaliplatin-based chemotherapy in patients with unresectable CLM.17 Patients received either bevacizumab or placebo with capecitabine with oxaliplatin (XELOX) or infusional fluorouracil and leucovorin with oxaliplatin (FOLFOX)-4 regimens until disease progression or for 48 weeks. Response was evaluated every 6 weeks using RECIST V.1.0. To be included in the present study, patients had to have at least one liver metastasis larger than 2 cm and a baseline CT evaluation and at least two subsequent sequential restaging CT studies of sufficient quality to allow scoring of morphological response.
Image analysis
In the non-randomised population, contrast-enhanced CT studies had been performed with a multidetector row 4-slice or 16-slice CT scanner (Light-Speed, GE Healthcare, Piscataway, New Jersey, USA) using 20 mm radiation beam width with 5 mm image thickness and 2.5 mm reconstruction interval. Contrast enhancement was performed with a triphasic liver protocol or single-phase technique, as previously described.12 Scanning parameters were chosen to achieve optimal spatial and contrast resolution using a noise index of 10–12.
For the NO16966 population, contrast-enhanced CT studies with single venous-phase contrast enhancement had been performed according to the practice at each centre using a cut thickness of 5 mm or 7.5 mm and 2.5 or 7.5 mm reconstruction interval.
For this study, CT images were reviewed by two radiologists with 4 and 20 years’ experience. CT scans before treatment and at first and second restaging were scored in both populations. Subsequent follow-up scans up to the time of morphological progression were also scored in the non-randomised population. Radiologists were blinded to clinical data and outcomes.
Liver metastases were assessed according to morphological criteria as previously defined.12 An optimal morphological response to chemotherapy was defined as the transformation from a pretreatment appearance characterised by heterogeneous attenuation and poorly defined tumour–normal liver interface to a homogeneous lesion of low attenuation with a thin, sharply defined tumour–normal liver interface mimicking a pseudocystic pattern. A suboptimal morphological response was defined as an increase in tumour homogeneity and sharpness of the tumour–normal liver interface but not to the same extent as in the optimal responders. A lack of morphological change regardless of change in size was considered to indicate no morphological response. In patients with multiple liver tumours, the reported morphological response was the response seen in the majority of tumours. Morphological progression after optimal response in the non-randomised patients was defined as a reversal of the response pattern previously reported for at least one metastasis.15
Response was also assessed using RECIST V.1.1 in the non-randomised patients and RECIST V.1.0 in the NO16966 patients (as measured by the independent response review committee). In addition, ETS and DpR were calculated. ETS was defined as the relative change in the sum of the longest diameters of RECIST target lesions from baseline to first restaging. As described by Piessevaux et al,9 a 20% decrease was adopted as the cut-off value to discriminate early responders from non-responders. DpR was defined as the relative change in the sum of the longest diameters of RECIST target lesions at their nadir size in the absence of new lesions or progression of non-target lesions compared with baseline. DpR was 0 for no change and negative if the tumour load increased.
Statistical analysis
Continuous variables were compared using the Mann-Whitney U test, and categorical variables were compared using Fisher’s exact test, where appropriate. PFS was calculated from the start of the bevacizumab-containing regimen (in the non-randomised population) or from randomisation (in the NO16966 population) to the first documentation of progression or death from any cause. OS was measured from the same start points to death from any cause. We employed a landmark analysis approach using the first and the second restaging time points to generate survival curves with the Kaplan-Meier method, and differences between curves were evaluated with the log-rank test. Postprogression survival (PPS) was the time between first documentation of progression and death from any cause. To assess the prognostic value of different radiological parameters in predicting survival, a univariate analysis was performed using a Cox proportional hazards model with the same landmark approach. Analyses were performed with IBM SPSS (V.22.0; SPSS) and PRISM GraphPad (V.6.0, La Jolla, California, USA) software. Significance was set at p<0.05. This retrospective study received institutional review board approval, with a waiver of the informed consent requirement.
RESULTS
Single-institution non-randomised population
Patients and responses
Demographic and clinical characteristics of the 141 patients are presented in table 1. The median follow-up was 22.6 months (range, 2.4–99 months). The most recent date of follow-up was March 2014. Restaging was performed every 8–9 weeks, with an average of 8 weeks. At first restaging, 28% of patients had an optimal morphological response. During the entire follow-up, an optimal morphological response was observed in 59 (42%), a suboptimal response in 46 (33%) and no morphological response in 36 (26%) patients. The median time to achieve an optimal or suboptimal morphological response was 2.5 months.
Table 1.
Baseline characteristics of non-randomised population (n=141)
| Characteristic | Value |
|---|---|
| Age, years | |
| Median | 55 |
| Range | 26–81 |
|
| |
| Sex, number of patients (%) | |
| Male | 84 (59.6) |
| Female | 57 (40.4) |
|
| |
| Primary tumour, number of patients (%) | |
| Colon | 111 (78.7) |
| Rectum | 29 (20.6) |
| Unknown | 1 (0.7) |
|
| |
| Extrahepatic disease, number of patients (%) | |
| Yes | 84 (59.6) |
| No | 57 (40.4) |
|
| |
| Chemotherapy regimen, number of patients (%) | |
| FOLFIRI+bevacizumab | 55 (39.0) |
| FOLFOX+bevacizumab | 79 (56.0) |
| XELOX+bevacizumab | 4(2.8) |
| Other 5-FU/capecitabine+bevacizumab regimen | 3(2.1) |
|
| |
| CEA level before treatment, ng/mL | |
| Mean | 690 |
| Range | 1–16 271 |
CEA, carcinoembryonic antigen; FOLFIRI, infusional fluorouracil and leucovorin with irinotecan; FOLFOX, infusional fluorouracil and leucovorin with oxaliplatin; FU, fluorouracil; LV5FU2, infusional FU and leucovorin; XELIRI, capecitabine with irinotecan; XELOX, capecitabine with oxaliplatin.
According to RECIST 1.1, 46 patients (33%) had a response at first restaging. The best RECIST response observed was a complete response or partial response (PR) in 73 patients (52%). The median time to achieve a RECIST response was 2.6 months. A response based on ETS (≥20% size decrease at first restaging) was reached in 78 patients (55%). The median DpR was 32.4% (IQR, 17.8%–44.8%).
At the first restaging, there was no association between morphological response and RECIST. Thirty-seven per cent of patients (17 of 46) with PR and 23% (22 of 95) with stable disease or progressive disease by RECIST were classified as having an optimal morphological response (p=0.1). Figure 1 shows examples of different patterns of response by RECIST and morphological criteria at first restaging.
Figure 1.
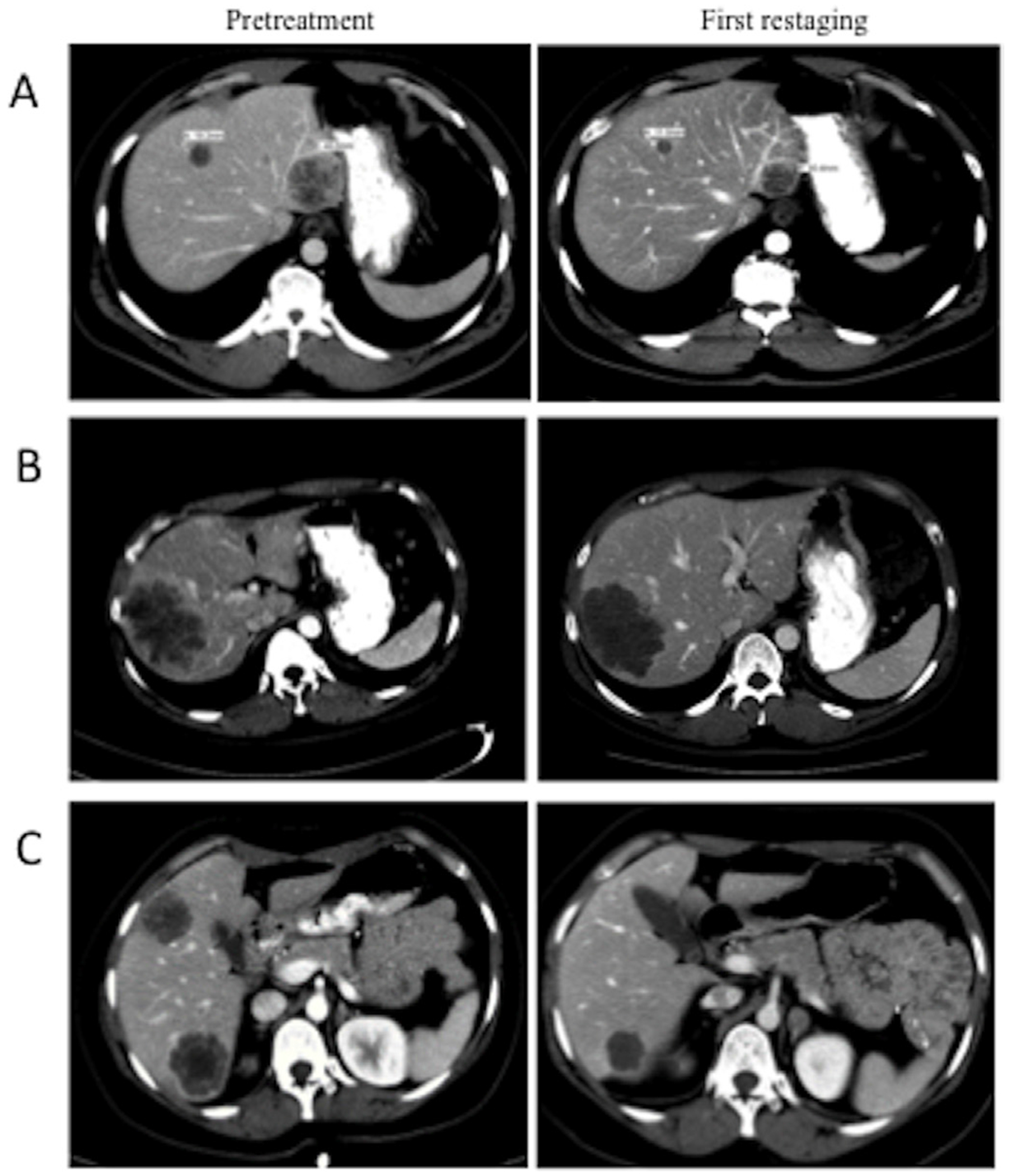
Contrast-enhanced CT scans from three patients in the non-randomised population at baseline (left) and first restaging (right) showing variations in the pattern of response by RECIST 1.1 and morphological criteria. (A) RECIST 1.1 response but no morphological response. (B) RECIST 1.1 stable disease but optimal morphological response. (C) RECIST 1.1 response and optimal morphological response. RECIST, Response Evaluation Criteria in Solid Tumors.
Associations between radiological parameters and outcomes
Patients with an optimal morphological response at first restaging had a significantly longer median PFS than patients with a suboptimal or no response (10.4 months and 6.8 months, respectively; p=0.028; HR=0.57; 95%CI 0.36 to 0.93) (figure 2A). There was no difference in PFS (figure 2B) or OS (figure 3A) according to RECIST 1.1 response at first restaging. The median OS was not significantly increased in patients with an optimal morphological response at first restaging but was significantly longer for those with an optimal morphological response at the second restaging (20.8 months vs 12.3 months, respectively; p=0.002, HR=0.56; 95%CI 0.38 to 0.8) (figure 3B).
Figure 2.
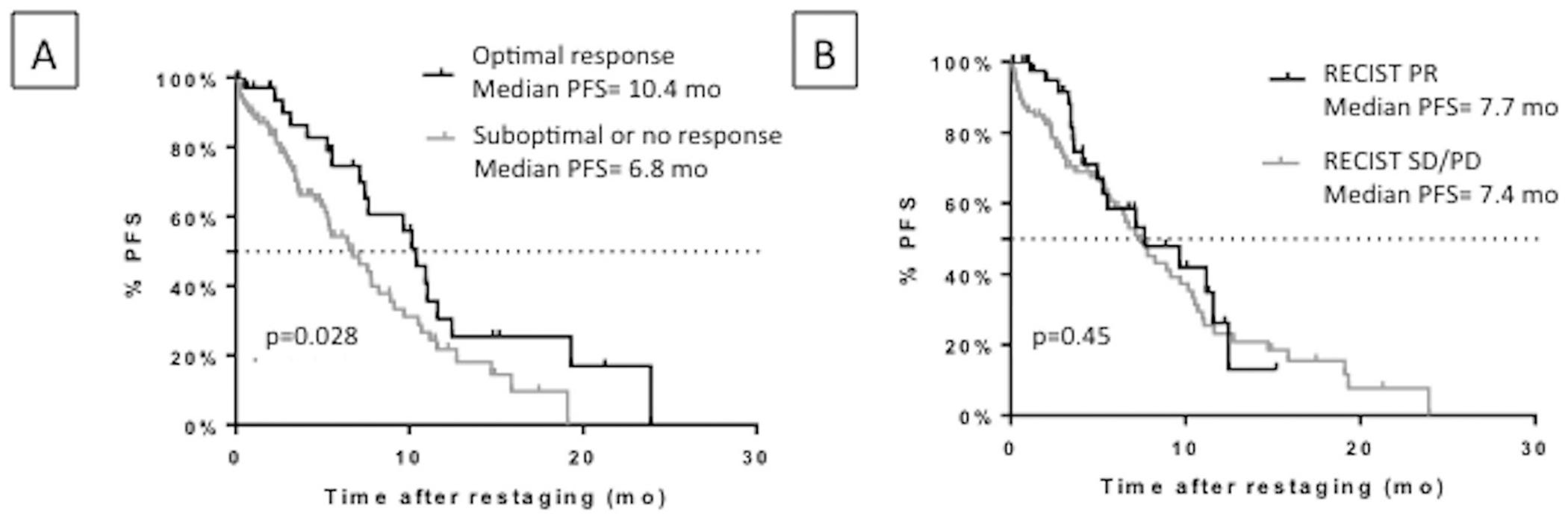
PFS in responders and non-responders by morphological criteria (A) and RECIST 1.1 (B) at first restaging (8 week) in the non-randomised population. PFS, progressive-free survival; PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease.
Figure 3.
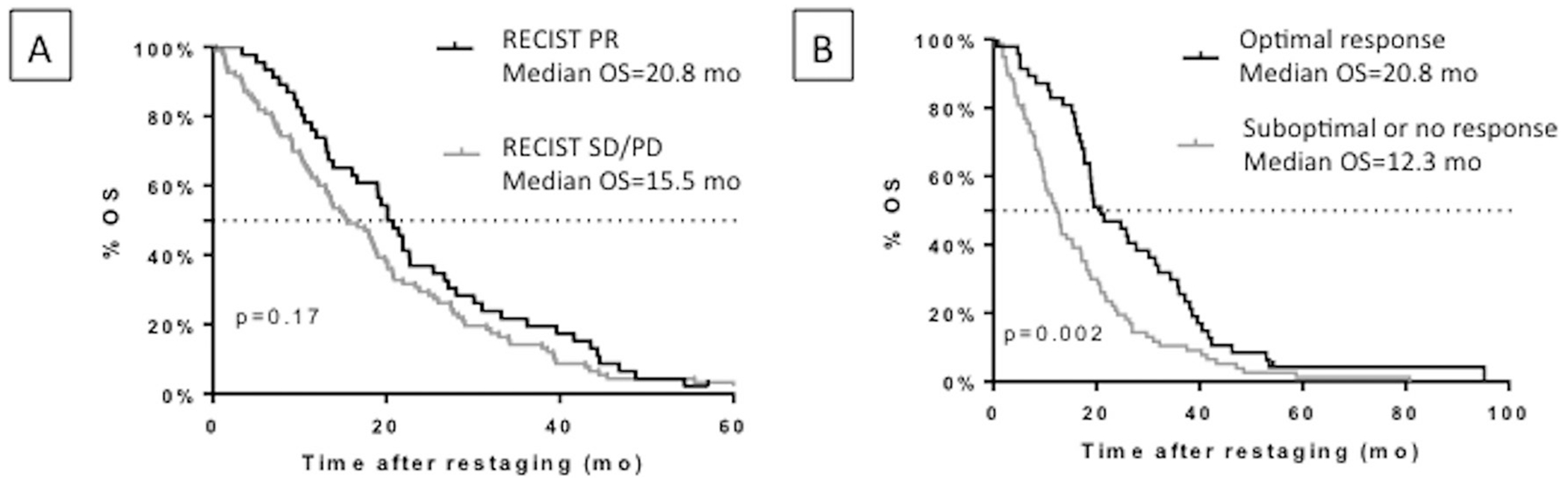
OS in the non-randomised population (A) in responders and non-responders by RECIST 1.1 at first (8 week) restaging and (B) in responders and non-responders by morphological criteria at second (16 week) restaging. OS, overall survival; PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease.
An optimal morphological response at any restaging was associated with a greater decline in CEA during treatment than was a suboptimal or no response (p=0.002) (figure 4).
Figure 4.
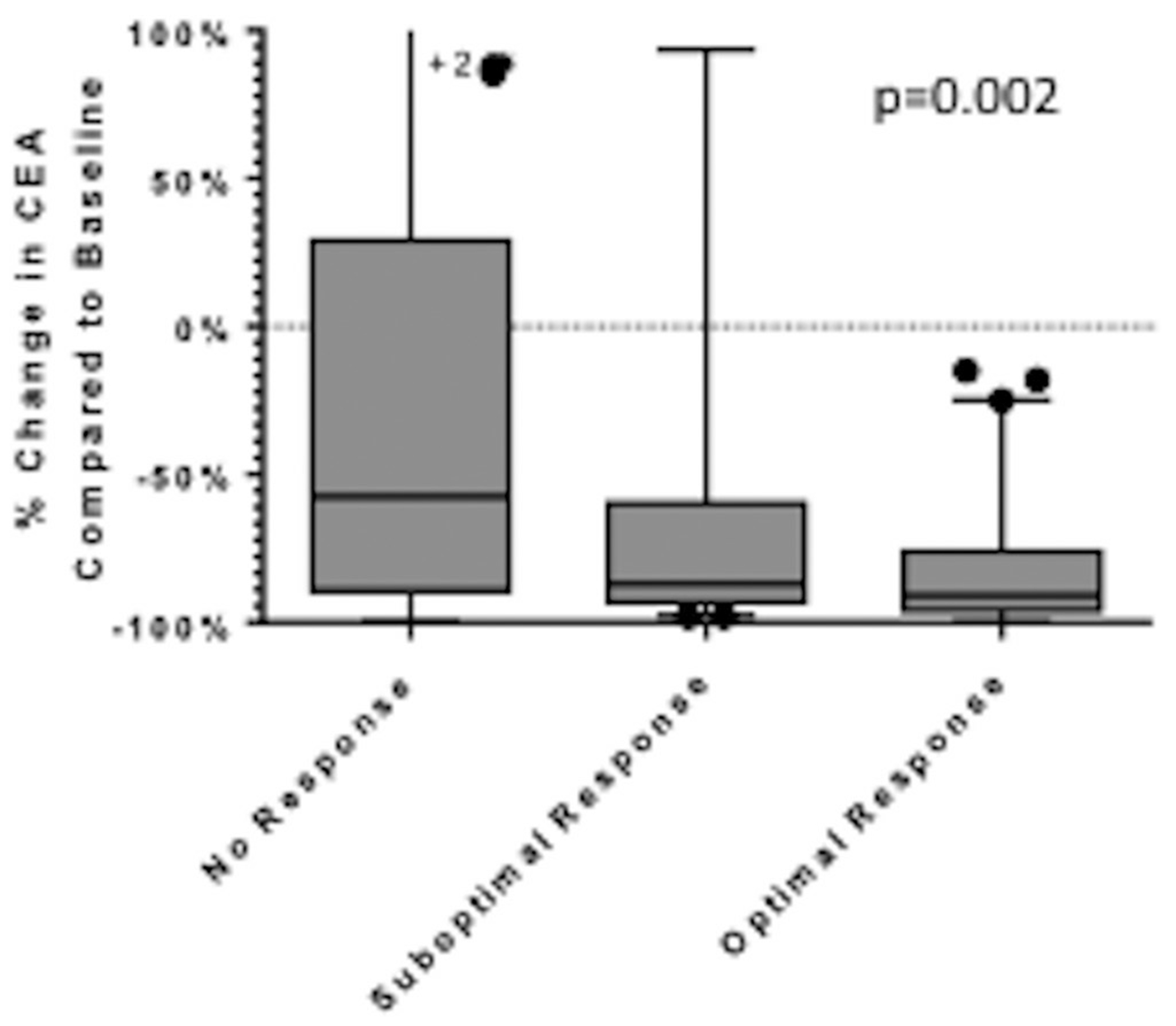
CEA level changes by best morphological response achieved in each patient in the non-randomised population. Lines inside boxes represent the median; end points of whiskers represent minimum and maximum values; lower and upper edges of boxes represent 25th and 75th percentiles. CEA, carcinoembryonic antigen.
A reversal of a previously detected optimal response without a change in tumour size was seen in 8% of the scans and increased the odds of disease progression (by RECIST) on a subsequent scan 9.4 times (95%CI 2.5 to 36.9; p<0.0001). Figure 5 shows an example of reversal of a morphological response.
Figure 5.
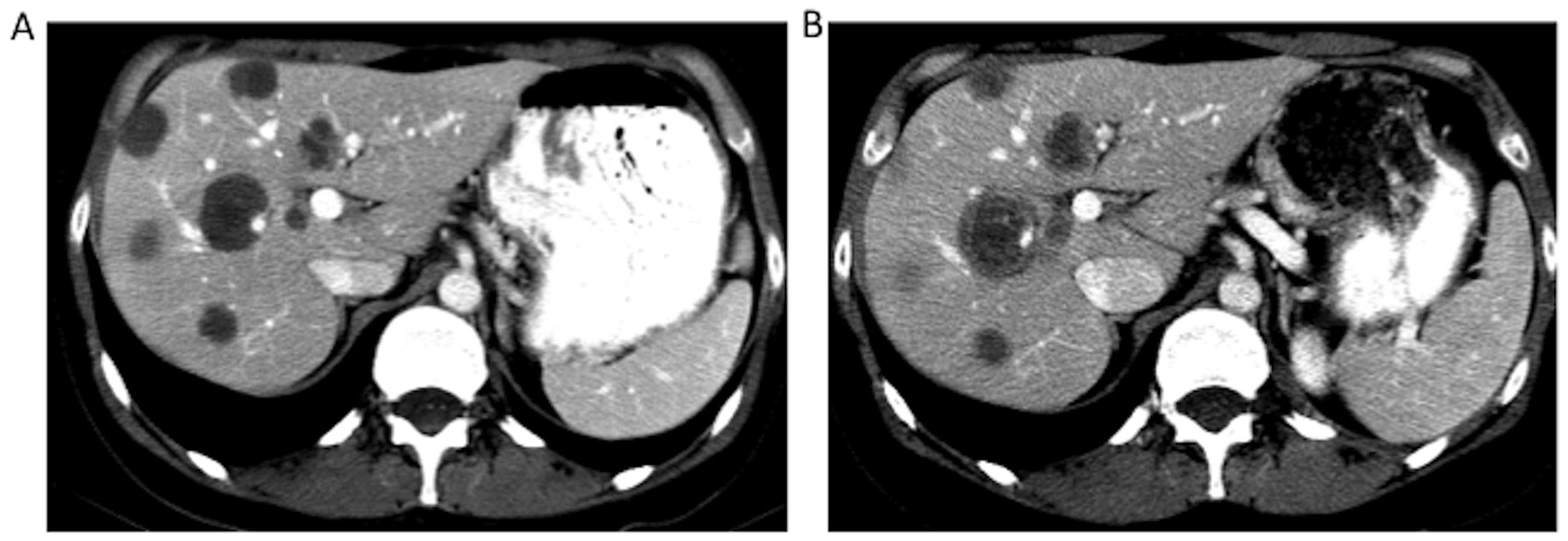
Contrast-enhanced CT scans from a patient in the non-randomised population shows reversal of a morphological response. (A) At first restaging, metastases are hypoattenuating and homogeneous. (B) Six months after treatment began, a marginal increase in the size of the metastases and increasing heterogeneity consistent with progression are evident.
NO16966 population
Patients and responses
To match the size of the non-randomised population, we selected 142 patients from the NO16966 trial with suitable image quality. To reach this number, we needed to analyse CT studies from 200 patients and exclude 29% of them because of image quality inadequacy for morphological assessment. They were treated with the following regimens: FOLFOX and bevacizumab (41 patients), XELOX and bevacizumab (35), FOLFOX and placebo (33) or XELOX and placebo (33). A majority of this population presented with widespread disease (82 patients had extrahepatic metastasis). Median PFS and OS in the 142 selected patients did not differ from the remaining NO16966 population.
At first and second restaging (specified at 6 and 12 weeks, respectively), an optimal morphological response was observed in 27 (19%) and 65 (46%) patients, respectively. An optimal morphological response at first and/or second restaging was 6.2 times more likely (95%CI 2.8 to 13.7) to occur in patients randomised to receive bevacizumab than in those who received placebo (70% vs 27%; p<0.0001).
As previously reported for the full study cohort, the addition of bevacizumab did not improve the rate of response by RECIST.17 ETS was noted in 79 (56%) patients and also was not influenced by bevacizumab (OR=0.86; 95%CI 0.41 to 1.77; p=0.7). The median DpR reached 45.8% (IQR, 33%–67.6%) in the bevacizumab group and 53.4% in the placebo group (IQR, 30.7%–69.5%; p=0.66).
Associations between radiological parameters and outcomes
Patients with an optimal morphological response at the first (6 weeks) restaging tended to have a lower risk of progression than patients with a suboptimal or no response (HR=0.70; 95% CI 0.49 to 1.05; p=0.1). An optimal morphological response at the second (12 weeks) restaging was associated with a significantly improved PFS compared with a suboptimal or no response (8.3 vs 4.9 months; p<0.0001; HR=0.52; 95%CI 0.35 to 0.7) (figure 6). However, there was no association with OS (figure 7). Response by RECIST at first or second restaging did not predict PFS.
Figure 6.

PFS in responders and non-responders by morphological criteria at second restaging (12 week) in NO16966 population. PFS, progression-free survival.
Figure 7.

OS in responders and non-responders by morphological criteria at second restaging (12 week) in the NO16966 population. OS, overall survival.
Prognostic factors for PFS, OS and PPS
Table 2 presents the results of a univariate Cox proportional hazards model analysis of the ability of morphological response, RECIST and ETS to predict PFS and OS. Optimal morphological response was the only predictor of longer PFS. This significant association was found for optimal morphological response at first and second restaging in the non-randomised population and at second restaging in the NO16966 population. An optimal morphological response at first restaging was not significantly associated with OS in either cohort, but an optimal morphological response at second restaging was significantly associated with OS in the non-randomised population. RECIST response at second restaging, but not first, was significantly associated with OS but not PFS in both cohorts. ETS had no prognostic value.
Table 2.
Univariate analysis of radiological predictors of PFS and OS in non-randomised and NO16966 populations
| Optimal morphological response | RECIST response | ETS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| Non-randomised | NO16966 | Non-randomised | NO16966 | Non-randomised | NO16966 | |||||||
|
| ||||||||||||
| Outcome and restaging study | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95%CI) | p |
| PFS postfirst restaging | 0.53 (0.31 to 0.93) | 0.03 | 0.69 (0.45 to 1.05) | 0.09 | 0.8 (0.47 to 1.37) | 0.41 | 0.86 (0.6 to 1.21) | 0.39 | 0.74 (0.47 to 1.18) | 0.21 | 0.73 (0.52 to 1.02) | 0.07 |
|
| ||||||||||||
| PFS postsecond restaging | 0.42 (0.24 to 0.75) | 0.003 | 0.5 (0.35 to 0.7) | <0.0001 | 0.82 (0.47 to 1.43) | 0.48 | 0.78 (0.55 to 1.1) | 0.16 | ||||
|
| ||||||||||||
| OS postfirst restaging | 0.79 (0.54 to 1.16) | 0.23 | 0.69 (0.43 to 1.12) | 0.13 | 0.78 (0.54 to 1.11) | 0.17 | 0.94 (0.64 to 1.37) | 0.73 | 0.78 (0.55 to 1.1) | 0.16 | 0.8 (0.55- 1.15) | 0.25 |
|
| ||||||||||||
| OS postsecond restaging | 0.56 (0.38 to 0.8) | 0.002 | 0.72 (0.5 to 1.05) | 0.09 | 0.60 (0.41 to 0.87) | 0.007 | 0.65 (0.45 to 0.95) | 0.03 | ||||
Each predictor was evaluated at first and second restaging except ETS, which, by definition, is determined at first restaging.
ETS, early tumour shrinkage; OS, overall survival; PFS, progression-free survival.
A significant association between DpR and PPS was noted. Patients with a DpR higher than the median value in each cohort had a significantly longer PPS than did those with a lower DpR (table 3). Optimal morphological response did not predict PPS regardless of restaging interval or cohort.
Table 3.
Univariate analysis of radiological predictors of pps in non-randomised and NO16966 populations
| DpR >median value | Optimal morphological response at any restaging | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Non-randomised | NO16966 | Non-randomised | NO16966 | |||||
|
| ||||||||
| Outcome | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p |
| PPS | 0.59 (0.36 to 0.92) | 0.02 | 0.67 (0.45 to 0.97) | 0.03 | 0.78 (0.50 to 1.23) | 0.28 | 1.00 (0.69 to 1.45) | 0.99 |
DpR, deepness of response; PPS, postprogression survival.
DISCUSSION
In this study, we show in a large sample of patients treated with bevacizumab for unresectable CLM that morphological radiological criteria provide useful prognostic information. Indeed, we found that an optimal morphological response as early as the first or second restaging CT was strongly predictive of prolonged PFS. Thus, our data may support the use of morphological response as an early marker of treatment efficacy.
The overall optimal morphological response rates in the two populations were consistent with those obtained in our previous studies of bevacizumab-containing therapy,12 13 even though the majority of patients studied here had more advanced disease. We also confirmed in a randomised trial population that an optimal morphological response occurs more frequently in patients treated with bevacizumab and may better reflect antiangiogenic activity.
We also found that the detection of progression based on morphological criteria increased the risk of disease progression by RECIST being detected on subsequent scans (approximately 2 months later) by an OR of 9.4. It would be interesting to explore whether this morphological worsening parallels the elevations of pro-angiogenic cytokines noted before RECIST evidence of disease progression in previous studies.18 19
This study confirms the inadequacy of RECIST used alone to predict outcome in patients with CLM receiving bevacizumab, first reported by Grothey et al,6 and expands the data comparing morphological criteria, RECIST and new size-based response parameters. Several recent retrospective studies demonstrated that an early size decrease of less than the RECIST cut-off value of 30% was associated with significantly longer PFS and OS in patients with metastatic CRC receiving cytotoxic chemotherapy alone7 20 21 or combined with cetuximab.8 9 In our study with bevacizumab-containing regimens, morphological response was a better predictor of PFS than size criteria, including ETS. Our findings are consistent with those of Chung et al,22 who found that an early tumour size response assessed by modified criteria (≥ 10% decrease in target lesion at 2 months) was not significantly associated with time to tumour progression in a small group who received bevacizumab-containing chemotherapy. However, our findings are in contrast with other studies showing that early response endpoints based on tumour size measurements (ETS and early objective tumour response) are significantly associated with improved PFS and OS in patients with metastatic CRC treated with bevacizumab and chemotherapy when cut-off values of 15%–20% are used.23–25 Despite these conflicting results, tumour shrinkage remains a key parameter in response evaluation even in patients treated with bevacizumab since we demonstrate, as did Mansmann et al,10 a significant association between DpR and PPS and between RECIST at second restaging and OS. Thus, given the inconsistent overlap between patterns of radiological response (size and morphology), both methods are needed for accurate assessment in each patient.
A limitation of morphological assessment is its subjective nature, but because the observations indicative of optimal response are quite evident (pseudo-cystic appearance versus solid heterogeneously enhancing tumour), the subjectivity does not impact the reproducibility or data quality. The learning curve is short and the interobserver agreement excellent across a wide range of experience. It was recently confirmed by an independent group from Japan.14 The only limitation to the scoring is the image quality. Scoring is easy and reliable only if the spatial and contrast resolution are optimised. Regarding objective measurements, the density alone is not sufficient to reproduce the morphological criteria but requires measures of tumour homogeneity and sharpness of margins, which can be very dependent on imaging techniques and equipment variability. As a result, the efforts to make this an objective (and automated) assessment have been difficult and will be the subject of future work. Chung et al22 has defined criteria combining attenuation measurement and size and showed that these were more accurate than RECIST alone. Research is under way to use change in texture as a tool to assess response but currently not ready for clinical use. One could argue that the subjectivity is a strength in the clinical setting as response can be judged immediately and reliably without the need to resort to measurement or special software. Objective measure, however, is preferable in the trial setting.
Another point supporting the robustness of the morphological criteria is its excellent correlation with pathological scores and data. They correlate with the percentage of residual tumour cells12 13 and with the thickness of the rim of residual tumour cells at the interface between the tumour and the normal liver.26 A recent publication shows a decreased in microscopic cancer spread surrounding resected CLM in patients with optimal morphological response.27 Moreover, a team at the Institute of Cancer Research in London has reported a link between the subjective radiographic analysis of response and the histological growth pattern of the metastases. The pushing growth pattern is associated with optimal response, while the replacement growth pattern is associated with the lack of response.28
While a strength of our study is the use of a prospective randomised trial cohort to validate our findings, the association of morphological response with outcome may be underestimated in the NO16966 population, as only 29% of bevacizumab recipients were treated until progression. This may partially explain our inability to confirm an association of morphological response with OS.
To conclude, our results confirm that morphological criteria provide substantial information on treatment efficacy and subsequent outcomes in patients with CLM treated with bevacizumab-containing chemotherapy. Both size and morphological criteria need to be applied simultaneously for a reliable response assessment. Further validation of these findings in prospective cohorts is under way, as is the development of morphological analysis software to improve external validity and adoption into clinical trials.
Significance of the study.
What is already known on this subject?
Bevacizumab is widely used in patients with hepatic metastases from colorectal cancer whether in first-line or second-line settings in association with cytotoxic chemotherapy.
One major issue encountered with the use of this antiangiogenic monoclonal antibody is the absence of relation between the improved clinical outcome and tumour shrinkage that hinders an objective measure of response by Response Evaluation Criteria in Solid Tumors (RECIST).
Morphological but non-size-based criteria have been developed at MD Anderson Cancer Center and have been shown to predict pathological response and survivals better than RECIST in patients with resectable colorectal liver metastases in three retrospective studies.
These criteria, however, have not been validated for patients with unresectable disease, and non-RECIST methods of categorising changes in tumour size (early tumour shrinkage, deepness of response) have not been tested in this setting.
What are the new findings?
Optimal morphological response is generally observed as early as the first or second restaging and predicts prolonged progression-free survival unlike RECIST on a large population of patients with unresectable metastatic colorectal
Non-size-related morphological changes can also be used as an indicator of early recurrence.
There is a strong correlation between bevacizumab and the occurrence of an optimal morphological response.
Alternative methods of categorising changes in tumour size have weaker prognostic performances than morphological criteria.
How might it impact on clinical practice in the foreseeable future?
In patients with unresectable metastatic colorectal cancer receiving bevacizumab, tumour size-based criteria do not thoroughly capture its impact on outcomes.
Morphological criteria provide substantial information on the treatment efficacy and should be systematically used in this setting in addition to the RECIST.
Acknowledgements
We thank all the investigators and the patients who contributed to this study. We would like to acknowledge the editorial assistance of Melissa G Burkett. We would also like to thank the funding support of Roche pharmaceuticals.
Funding
This study was sponsored by F Hoffmann-La Roche Ltd, Basel, Switzerland. No grant number is applicable.
Competing interests
TM disclosed research funding from ROCHE; honoraria from AMGEN and SANOFI; and travel, accommodations, expenses paid by AMGEN. SK declared research funding from ROCHE, AMGEN, GSK, SANOFI, SYSMEX, BIOCARTIS, GUARDANT HEALTH and AGENDIA and consulting or advisory role for AMGEN, ROCHE, GSK, JANSSEN, BMS, AGENDIA, MERRIMACK, SYSMEX, BAYER, TAIHO, SANOFI and ARRAY BIOPHARMA. CE declared honoraria from ROCHE, GENENTECH and BAYER; consulting or advisory role for BAYER and SIRTEX; participation in a speakers’ bureau for GENENTECH; and travel, accommodations and expenses paid by GENENTECH, SIRTEX and BAYER. HC disclosed honoraria from NOVARTIS; consulting or advisory role for NOVARTIS; and travel, accommodations and expenses paid by NOVARTIS. MJO declared consulting or advisory role for MERRIMACK and SIRTEX and research funding from ROCHE, AMGEN, BMS, CELGENE, MEDIMMUNE and MERCK. MY disclosed honoraria from ROCHE and consulting and advisory role for ROCHE. J-NV disclosed research funding from ROCHE. All remaining authors have declared no conflicts of interest.
Footnotes
Patient consent Detail has been removed from this case description/these case descriptions to ensure anonymity. The editors and reviewers have seen the detailed information available and are satisfied that the information backs up the case the authors are making.
Ethics approval Institutional Review Board.
REFERENCES
- 1.Van Cutsem E, Cervantes A, Nordlinger B, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25(Suppl 3):iii1–9. [DOI] [PubMed] [Google Scholar]
- 2.Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009;27:3677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuchs CS, Marshall J, Barrueco J. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: updated results from the BICC-C study J Clin Oncol 2008;26:689–90. [DOI] [PubMed] [Google Scholar]
- 4.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 2007;25:1539–44. [DOI] [PubMed] [Google Scholar]
- 5.Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 2013;14:29–37. [DOI] [PubMed] [Google Scholar]
- 6.Grothey A, Hedrick EE, Mass RD, et al. Response-independent survival benefit in metastatic colorectal cancer: a comparative analysis of N9741 and AVF2107. J Clin Oncol 2008;26:183–9. [DOI] [PubMed] [Google Scholar]
- 7.Giessen C, Laubender RP, Fischer von Weikersthal L, et al. Early tumor shrinkage in metastatic colorectal cancer: retrospective analysis from an irinotecan-based randomized first-line trial. Cancer Sci 2013;104:718–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Modest DP, Laubender RP, Stintzing S, et al. Early tumor shrinkage in patients with metastatic colorectal cancer receiving first-line treatment with cetuximab combined with either CAPIRI or CAPOX: an analysis of the German AIO KRK 0104 trial. Acta Oncol 2013;52:956–62. [DOI] [PubMed] [Google Scholar]
- 9.Piessevaux H, Buyse M, Schlichting M, et al. Use of early tumor shrinkage to predict long-term outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol 2013;31:3764–75. [DOI] [PubMed] [Google Scholar]
- 10.Mansmann UR, Sartorius U, Laubender RP, et al. Quantitative analysis of the impact of deepness of response on post-progression survival time following first-line treatment in patients with mCRC. ASCO Meet Abstr 2013;31:3630. [Google Scholar]
- 11.Ichante JL, Adenis A, Francois E, et al. 6094 POSTER impact of early tumour shrinkage on long-term outcome in metastatic colorectal cancer (mCRC) treated with 5FU+lrinotecan+Leucovorin (FOLFIRI) or Capecitabine+lrinotecan XELIRI plus Bevacizumab. Eur J Cancer 2011;47:S419. [Google Scholar]
- 12.Chun YS, Vauthey JN, Boonsirikamchai P, et al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA 2009;302:2338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shindoh J, Loyer EM, Kopetz S, et al. Optimal morphologic response to preoperative chemotherapy: an alternate outcome end point before resection of hepatic colorectal metastases. J Clin Oncol 2012;30:4566–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishioka Y, Shindoh J, Yoshioka R, et al. Radiological morphology of colorectal liver metastases after preoperative chemotherapy predicts tumor viability and postoperative outcomes. J Gastrointest Surg 2015;19:1653–61. [DOI] [PubMed] [Google Scholar]
- 15.Boonsirikamchai P, Asran MA, Maru DM, et al. CT findings of response and recurrence, independent of change in tumor size, in colorectal liver metastasis treated with bevacizumab. AJR Am J Roentgenol 2011;197:W1060–1066. [DOI] [PubMed] [Google Scholar]
- 16.Yoshita H, Hosokawa A, Ueda A, et al. Predictive value of optimal morphologic response to first-line chemotherapy in patients with colorectal liver metastases. Digestion 2014;89:43–8. [DOI] [PubMed] [Google Scholar]
- 17.Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013–9. [DOI] [PubMed] [Google Scholar]
- 18.Kopetz S, Hoff PM, Morris JS, et al. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol 2010;28:453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lieu CH, Tran H, Jiang ZQ, et al. The association of alternate VEGF ligands with resistance to anti-VEGF therapy in metastatic colorectal cancer. PLoS One 2013;8:3533:e77117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki C, Blomqvist L, Sundin A, et al. The initial change in tumor size predicts response and survival in patients with metastatic colorectal cancer treated with combination chemotherapy. Ann Oncol 2012;23:948–54. [DOI] [PubMed] [Google Scholar]
- 21.Sommeijer DW, Shi Q, Saad ED, et al. Early predictors of prolonged overall survival (OS) in patients (pts) on first-line chemotherapy (CT) for metastatic colorectal cancer (mCRC): an ARCAD study with individual patient data (IPD) on 10,962 pts. ASCO Meet Abstr 2014;32:3538. [Google Scholar]
- 22.Chung WS, Park MS, Shin SJ, et al. Response evaluation in patients with colorectal liver metastases: RECiST version 1.1 versus modified CT criteria. AJR Am J Roentgenol 2012;199:809–15. [DOI] [PubMed] [Google Scholar]
- 23.Cremolini C, Loupakis F, Antoniotti C, et al. Assessing tumor response beyond RECiST criteria: Early tumor shrinkage (ETS) and deepness of response (DpR) in phase III TRIBE trial by the GONO group. Eur J Cancer 2013;49:S491. [Google Scholar]
- 24.Mazard T, Assenat E, Ychou M, et al. 1402 POSTER DiSCUSSiON CT evaluation of the response of colorectal liver metastasis after bevacizumab treatment—a density quantitative analysis correlated with patient outcome. Eur J Cancer 2011;47:S169–170. [Google Scholar]
- 25.Saad ED, Coart E, Sommeijer DW, et al. Early predictors of improved long-term outcomes in first-line antiangiogenics plus chemotherapy (anti-ANG/CT) in metastatic colorectal cancer (mCRC): Analysis of individual patient (pt) data from the ARCAD database. ASCO Meet Abstr 2014;32:3578. [Google Scholar]
- 26.Maru DM, Kopetz S, Boonsirikamchai P, et al. Tumor thickness at the tumor-normal interface: a novel pathologic indicator of chemotherapy response in hepatic colorectal metastases. Am J Surg Pathol 2010;34:1287–94. [DOI] [PubMed] [Google Scholar]
- 27.Nishioka Y, Shindoh J, Yoshioka R, et al. Clinical impact of preoperative chemotherapy on microscopic cancer spread surrounding colorectal liver metastases. Ann Surg Oncol 2017;24:2326–33. [DOI] [PubMed] [Google Scholar]
- 28.Frentzas S, Simoneau E, Bridgeman VL, et al. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat Med 2016;22:1294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]


