Abstract
The rapid adoption of immune checkpoint blockade (ICB) therapies has led to a need to understand the mechanistic drivers of efficacy and the identification of novel biomarkers that enrich for patients who benefit from ICB therapy. Here, we provide a perspective on emerging biomarker candidates, their underlying biological mechanisms, and how they may fit into the current landscape of cancer biomarkers. We discuss new frameworks to identify and evaluate biomarker candidates and review the opportunities and challenges of utilizing biomarker-derived models to facilitate the development of new immunotherapies.
Keywords: immunotherapy, biomarker, immune checkpoint blockade, tumor immunology
Collecting pieces of the puzzle
With the rapid development of immunotherapy, particularly immune checkpoint blockade (ICB), more and more patients have achieved durable remission. Currently, it is estimated that about 40% of cancer patients were eligible for one or more types of ICB therapy in 2020 [1]. However, to date, the overall response rate of single agent ICB therapy still remains around 20–40% in many cancer types and much lower for other types (10%) [2]. Therefore, the need to identify biomarkers that can accurately predict which patients will benefit from ICB therapy is very important. As the interaction between tumor cells and the immune system is complex, it is a virtual certainty that the information provided from a single biomarker will not be sufficient for optimal prediction of response and resistance. This is perhaps aptly embodied by the ancient Indian parable of the blind men and the elephant. In this story, a group of blind men who have never encountered an elephant learn about what an elephant is like by touching a part of it. Each man feels a different part of the animal. The men then describe their limited experience and their descriptions differ from each other. They then become suspicious and think the other men are dishonest and come to blows. The moral of the story is that humans have a tendency to claim absolute truth based on their limited, subjective experience while discounting other people’s similarly limited experiences [3]. This lesson is very applicable to the modern field of cancer immunotherapy where warring factions spar over incomplete data in an environment where no one has achieved a complete understanding. Once we collect more pieces of the puzzle, the drivers of response underlying a complex biology will become increasingly apparent (Figure 1). Moreover, it is important to note that not all biological phenomena that can affect tumor immunity are suitable as clinical biomarkers. The oncology biomarker field is populated by thousands of candidate markers, etc. that will never see clinical utility. This is because adoption for clinical use requires technical feasibility, an easily standardized workflow, and cost-efficiency. Here, we share some perspectives on this ongoing process, propose inclusive approaches to examining emerging biomarkers of ICB therapy as well as new strategies and concerns for future development.
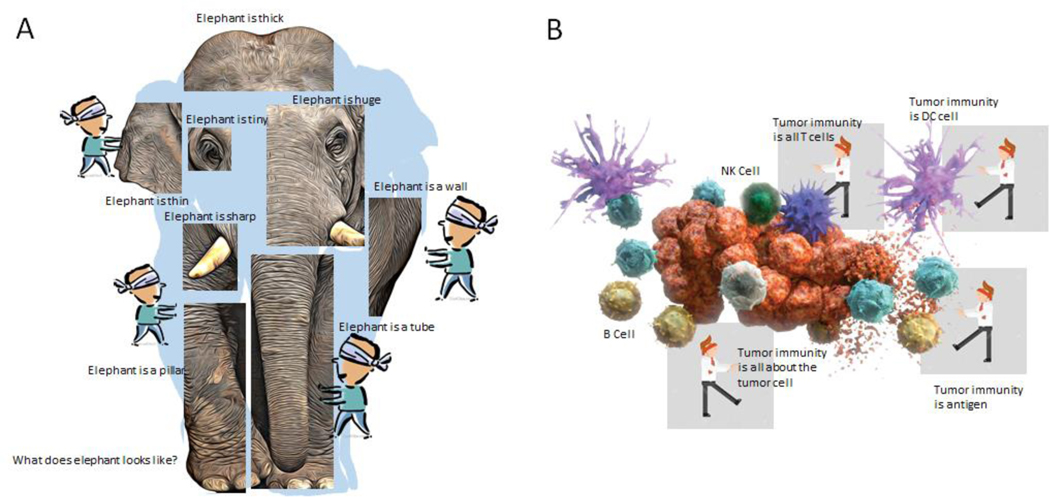
Figure 1. Individual investigation without broad vision leads to suboptimal understanding.
When there is insufficiently broad examination of an entity or a process, or a failure to integrate data that leads to a complete picture, flawed interpretations can result. (A) Blindfolded observers examining only single parts of an elephant can develop incomplete (and wrong) impressions of the nature of the elephant. (B) Blindfolded scientists examining the process of anti-tumor immunity may develop biomarkers that fail to capture the entirety of the process. Single biomarkers may not be sufficient for complex processes such as anti-tumor immunity. Instead, successful integration of information from diverse viewpoints can lead to a better interpretation and understanding of anti-tumor immunity. Abbreviations: DC, dendritic cell; NK, natural killer.
Fundamental factors that determine the efficacy of ICB therapy and current FDA-approved biomarkers
There are at least three fundamental factors that determine the efficacy of ICB therapy (also see Box 1) [4]: (i) the capability of the immune system to ‘recognize’ tumor cells, (ii) the ‘resilience’ of the effector immune cells to be re-activated by ICB therapy, and (iii) ‘reinforcement’ of the immune system at the systemic and microenvironmental level to support the function of the effector immune cells. These three factors interact with each other and cooperatively determine the success of anti-tumor immunity and ICB therapy. A common saying is that ‘a bucket’s capacity is determined by its shortest stave’[5], and similarly, dysregulation of any of these three factors will lead to suboptimal activity of ICB therapy. Importantly, many of the current or emerging biomarkers provide information on the status of one or more of these three factors.
Box 1. Three fundamental factors that contribute to the efficacy of ICB therapy.
The capability of T cells to ‘recognize’ tumor cells. This reflects the immunogenicity of the tumor, and the pre-existing immune reactivity against the tumor, which is related to the quality and quantity of antigens (including neoantigens and self-antigens), the ability of tumor cells and antigen presenting cells to present cancer antigens to effector immune cells, and the abundance of effector immune cells that are capable of recognizing and targeting them.
The ‘resilience’ of the effector immune cells. This measures the extent that effector immune cells can recover from exhaustion and the expression profile of relevant immune checkpoint molecules. These factors can help determine whether the therapy is sufficient to recall the activity of functional immune cells.
The ‘reinforcement’ of the immune system from systemic and microenvironmental factors to support the function of effector immune cells. This measures how good the overall microenvironment and other factors could support or suppress the durability of the effector cells to kill tumor cells.
Currently, the FDA have approved the use of three main biomarkers for ICB therapy. PD-L1 (see Glossary) quantitation by immunohistochemistry (IHC) was the first biomarker that was approved, first in non-small cell lung cancer (NSCLC) for anti-PD-1 therapy [6]. PD-L1 IHC is also used in various ad hoc settings in other cancer types [7,8]. While PD-L1 levels do enrich for responders in some cases, its applicability is not universal. Tumor mutation burden (TMB) and DNA damage repair mutations, such as mismatch repair gene mutation, are two other biomarkers for ICB that have been FDA approved. Our group first discovered the association of these features with ICB efficacy [9,10], which was subsequently validated in multiple prospective trials. TMB is one of the first tumor-type agnostic genomic biomarkers and is used to predict ICB response [11,12]. Microsatellite instability-high or mismatch repair deficiency (MSI-H/MMRd) is an approved biomarker that is used to identify tumors that are likely sensitive to ICB. MSI-H results in a high TMB, which is the ultimate target of anti-tumor immunity. The underlying biological mechanism, current status and challenges associated with these three approved biomarkers are summarized in Box 2.
Box 2. FDA-approved ICB biomarkers.
PD-L1 is the first biomarker that was approved for ICB. Indeed, as the major ligand of the PD-1 molecule, high expression of PD-L1 is associated with improved response rate in a number of clinical trials of anti-PD-1/PD-L1. However, in many cancer types, the expression of PD-L1 is not associated with response and PD-L1-low patients still benefit from treatment. There are different ways to quantify the expression of PD-L1 based on different antibodies and different patterns of distribution of PD-L1 on cells. Histological evaluation of PD-L1 expression is also affected by the time of sample collection and many other factors. It has also been shown that ICB therapy or other combination therapies could convert immune cold microenvironments (i.e.. PD-L1 low) into immune active microenvironments. Indeed, some therapeutic agents such as chemotherapy or radiation can induce expression of PD-L1 and hence render the baseline PD-L1 expression less relevant.
TMB is one of the most important biomarkers that can be useful across many cancer types [11]. The FDA has approved ICB therapy for all cancers with high TMB defined with the cutoff of 10 or more mutation/Mb. Although this cutoff is a start, evidence shows that each cancer type might has their own optimal TMB cutoff [111]. Similar to PD-L1, TMB can be measured in a variety of different ways. The sensitivity and specificity of specific cutoffs can vary between different next generation sequencing (NGS) assays. TMB calculated from different sequencing approaches can lead to different results. Indeed, results from different targeted NGS panels or various ctDNA assays measuring blood TMB may produce different TMB tallies. A recent study indicated that the TMB calculated from blood controls can potentially overestimate TMB when compared to assays using paired solid tissue-normal samples [112]. Nevertheless, substantial progress has been made to harmonize the TMB calculated from different sequencing assays [113].
MSI-H/MMRd is another approved biomarker that can evaluate the immunogenicity of a tumor. High mutational burden, particularly the increased number of insertion/deletion mutations in these tumors, - drives high response rates (as high as 50–100%) in the MMRd patients [11,104]. Moreover, recent evidence has shown that in addition to increased neoantigen load, MMRd could also directly activate intracellular innate immune signals that help generate proinflammatory signals. Several challenges exist with the current utilization of MSI-H/MMRd as a biomarker. First, MMRd itself does not always guarantee MSI-H status, and thus, could lead to overestimation of the estimated response rate to ICB. Second, the current approach to detecting MSI-H/MMRd also does not take tumor purity or intratumorally heterogeneity into account. Low tumor purity, which results from a large fraction of non-cancerous cells in the tumor, can lead to underestimation of MSI intensity. Moreover, sub-clonal MMRd events can also lead to acquired resistance to ICB therapy.
Emerging biological factors as biomarker candidates
Without a doubt, current approved biomarkers can be improved upon. The field is exceedingly active with efforts to decipher the mechanisms underlying anti-tumor immunity. What are some of the emerging factors that can identify ICB responders? During investigations to decipher the molecular and genetic bases of response and resistance to ICB therapy, many novel and unexpected biological factors that play important roles had been identified. Many of these biological factors unveil important information about the three fundamental factors that determine the efficacy of ICB therapy, and at the same time, emerge as biomarker candidates.
Germline factors
Germline genetic features have been shown to be very important for cancer development, as well as for the function of the immune system [13–16]. One of the most important germline features that affect the immune system is HLA genotype [17,18]. Indeed, HLA genotype determines the spectrum and repertoire size of the peptide epitopes that can be presented by the host antigen presentation machinery [19]. Moreover, HLA makeup is known to differ across the world and has been linked to outcomes from infectious diseases and differential risk of developing cancers [20,21]. Increased HLA type I allele divergence is associated with enhanced ability for the tumor to present broader types of neoantigens and can be correlated with improved response to ICB therapy [22–24]. While this association has been observed in some immunotherapy cohorts, the effect of HLA divergence was not observed in all studies [25]. This could be attributed to different methodologies, different cutoffs used, or other potential biological confounders that are unique to each individual cohort. By comparison, how divergence of HLA II impacts ICB remains unclear and is worthy of further investigation. Specific HLA alleles have also been shown to be associated with the outcome of ICB therapy. Melanoma patients with alleles belonging to the HLA-B44 superfamily are more likely to respond to ICB therapy due to the fact that HLA-B44 alleles have higher affinity for the neopeptides with negatively charged glutamic acid anchors, which is more common in melanoma [26,27]. Importantly, another group found that HLA B44 strongly affected response patterns in NSCLC, in a manner driven by how lung cancer mutation patterns influence neoepitope formation [27]. Patients with HLA alleles in the HLA-A3 family, however, are associated with unfavorable outcome with ICB therapy in multiple observation cohorts and clinical trials [28]. The underlying mechanisms are likely multifaceted and still being worked out.
Recent evidence shows that other germline features can affect tumor immunity, although their use as biomarkers remains unclear. The genetic ancestry of patients has been shown to be associated with varying tumor molecular features and immune characteristics and could potentially affect the outcome of immunotherapy [29]. Analyses of pan-cancer cohorts found that germline variants in immune modulatory genes and DNA damage repair genes can affect intra-tumor interferon (IFN) signaling as well as infiltration of T cell and natural killer (NK) cell subsets that function during ICB therapy [30–32]. Germline traits that are associated with risk of autoimmune disease could also potentially be connected to ICB response, and perhaps to immune-mediated adverse events [33].
Somatic genetic factors
Neoantigen load is the most direct measurement of the immunogenetic potential of a tumor, or how well the tumor can be recognized by the immune system [34]. TMB, MSI, and HLA divergence are all surrogates of the true immunogenic neoantigen load. It is still quite difficult to predict whether a putative neoantigen is functionally important without direct experimental evaluation. However, various approaches have demonstrated some success in predicting functionally important neoantigens. Typically, direct prediction of neoantigen load requires a combination of somatic mutation calling from tumor tissues or circulating tumor DNA (ctDNA), and transcriptome analysis to find which mutations are expressed in tumors. It is also important to determine HLA type and any potential HLA- loss of heterozygosity (LOH) events. Various algorithms can then be used to predict the HLA-binding affinity and immunogenicity of putative neoantigens [35]. While many approaches have been made to automate this process [36,37], the accuracy of prediction is not perfect, particularly for uncommon HLA types [38]. Larger scale analyses and developing better prediction models are needed to overcome this challenge and these efforts are ongoing.
Chromosomal instability is a hallmark of tumor development and can lead to somatic copy number alterations that have been shown to associate with signatures of immune evasion [39]. Somatic copy number alterations (SCNAs) are associated with inferior clinical response rates to ICB therapy [40,41]. Furthermore, recent evidence shows that loss of chromosome 9p, including 9p21.3 (where CDKN2A/B and the IFN gene cluster is located) or 9p24 (where the genes encoding JAK2 and PD-L1 are located), are strong predictors of unfavorable outcome to ICB therapy [42,43]. Interestingly, it has been observed that SCNAs can be associated with the presence of extrachromosomal DNA (ecDNA) in tumor cells [44]. Whether ecDNAs can affect tumor immunity is still controversial and it is too early to make definitive conclusions here.
Somatic alterations in key oncogenic or immune modulatory pathways have been linked to differential response to ICB [41,45]. For example, patients with EGFR, ALK alterations, PTEN, WNT pathway members, or STK11/KEAP1 with KRAS are unlikely to respond to ICB therapy [46,47]. Some of these alterations may be associated with lower TMB or suppressed immune microenvironments. Investigations on patients with high mutation load or MSI-H patients that did not respond or whose disease progressed on ICB therapy identified genomic alterations or transcriptional programs involved in key pathways related to immune activation and antigen presentation [48–50]. In contrast, alterations in pathways related to DNA damage repair or epigenetic regulation are more likely to be linked to better response [51–54]. DNA damage deficiencies cause increased TMB load and increased immunosurveillance [55], while defective epigenetic regulation can cause abnormally expressed cancer testis antigens, endogenous retroviruses or transposable elements. These all have been shown to boost tumor immunity [56]. With the exception of DNA damage repair genes, mutations in various pathways are quite diverse and difficult to adopt to current clinical decision making paradigms. As such, alterations in single signaling pathways are not currently used to make decisions on the appropriateness of ICB therapy for patients. It is possible that, in the future, alterations in various pathways that influence immunity may be used together to inform therapeutic decisions but this will require extensive validation.
Gene expression biomarkers
As stated earlier, the primary gene expression biomarker used today is quantitation of PD-L1. Here, it is actually protein and not mRNA levels that are quantified. Transcriptional analysis, applied to tumors in bulk or at the single cell level, have been used extensively to understand the nature of tumor microenvironment (TME) changes and can provide information about the composition and function of tumor infiltrating immune cells [57]. RNA expression of various genes such as CXCL9/CXCL13, single cell RNA-seq (sc-RNA-seq) signatures from certain tumor infiltrating lymphocyte subpopulations (i.e., tissue resident lymphocytes), and various transcriptional programs associate with TMEs that are amenable to ICB action [25,58,59] while other signatures such as the TGF-β-related and epithelial-mesenchymal-transition (EMT) signatures are associated with a T cell exclusion phenotype [60,61]. With the development of advanced deconvolution tools, it has become more and more practical to utilize the findings from single-cell and spatial transcriptome analyses to overcome the resolution limitation of bulk transcriptome profiling. As has been the case for decades, however, use of transcriptional signatures in the clinic has been hampered by feasibility, cost, and reproducibility. Therefore, as of now, transcriptional analysis has not been adopted in the clinical setting to guide immunotherapy use. This may change if more robust standard operating procedures are developed to implement these assays in the clinic, such has been done with OncoType Dx [62].
Etiology, pathology, histology, and treatment history
Specific etiological and pathological features of a tumor can impact the likelihood of response to ICB therapy. For example, hepatocellular carcinoma associated with non-alcoholic steatohepatitis (NASH) is less likely to respond because NASH can drive dysfunction of CD8+ T cells and dampen immune surveillance [63]. Interestingly, desmoplastic melanomas, a rare type of melanoma featuring high TMB, high PD-L1 expression, and high CD8+ T cell infiltration, have a particularly high response rate (70%) to ICB [64]. This tumor type is enriched for mutations in Serpin B3, which can influence immunological processes [65]. Evidence has also emerged that liver metastases can lessen the actions of antigen-specific T cells and restrain the efficacy of ICB [66].
Histology and imaging analysis can provide information on the overall immune characteristics of tumors as well as tumor-associated immune infiltrates [67,68]. Previous studies have classified tumors into several categories based on immune infiltration patterns and noted that T cell desert/excluded tumors are less likely to response to ICB [60]. Recently, specific lymphoid entities - tertiary lymphoid structures (TLS) - were found to associate with improved response [69]. Interestingly, recently developed pathological image-based deep learning models can predict MSI status [70], the presence of oncogenic mutations [71], spatial gene expression organization [72], and response to ICB [73,74]. Similarly, radiomics and immune positron emission tomography (PET) also show clear potential for tracking ongoing response during therapy, particularly when combined with advanced feature selection and machine learning modeling [75,76].
Similarly, blood-based tests and high dimensional flow cytometry have also been used to nominate biological factors that have potential for use as biomarkers for ICB. Elevation of pretreatment neutrophil/lymphocyte ratio (NLR), an indicator of systematic inflammation, has been demonstrated to be a negative predictive factor for response and survival to ICB [77]. High serum albumin level is also identified as a strong predictive factor for improved ICB response and survival [78]. The abundance and phenotype of certain circulating immune cells, ctDNA, circulating cytokines and chemokines, or extracellular vesicles have also been nominated and are worth further evaluation [79–81].
Prior treatment history is a factor that can impact the outcome of ICB therapy. Prior or concurrent chemotherapy had been shown to be a predictive factor for response to ICB [78]. Indeed, this is exactly what has been shown to be beneficial in a number of prospective clinical trials specifically testing the efficacy of giving chemotherapy with ICB [82–84]. Chemotherapy given with ICB can enhance antigen presentation and cooperate with ICB therapy [85]. Furthermore, emerging evidence shows that prior treatment with MAPK inhibitors could render tumors less responsive to ICB therapy [86]. Other clinical features, such as body-mass-index [87] and gender [88] have also been shown to affect the outcome of ICB therapy.
Metabolic biomarkers
Metabolic alterations can affect the function of tumor cells and immune cells. Key metabolic enzymes or metabolites act as checkpoints that influence the efficacy of ICB therapy [89]. Elevated lactate dehydrogenase (LDH) levels in the serum, a reflection of glucose deprivation, is associated with impaired response to ICB [90]. Serum kynurenine-to-tryptophan ratio and quinolinic acid concentration, indicators of IDO1/2 and TDO1 activity, are predictive factors associated with progression free survival (PFS) in NSCLC patients treated with anti-PD1 [91]. Soluble CD73, the ecto-5’-nucleotidase that converts AMP to the immune suppressive adenosine, was reported as a marker of response to anti-PD-1 therapy in melanoma patients [91].
Viruses, microbiota, and heterologous immunity
Viruses or pathogen infection can induce cancer development. Infection related cancers show distinct immunologic features [92]. Evidence of virus infection, in some circumstances, can render the tumors more likely to be recognized by the immune system and enhance inflammation in the TME, facilitating better response to ICB. Human papillomavirus (HPV) positive head and neck squamous cell carcinoma (HNSCC) [93] and Epstein–Barr virus (EBV) associated gastric cancers [94] show increased PD1 and PD-L1 expression and enhanced T cell infiltration as well as improved response to ICB therapy.
The microbiota plays an important role in the development of the immune system and affects the progression of cancer [95]. Emerging evidence shows that the composition of gut microbiota can affect the efficacy of ICB for cancer treatment [96,97]. The gut microbiota has also been found to associate with immune-related adverse events from ICB [97,98]. Microbiota can also reside in other tissues, such as the airway, the oral cavity, and the bile duct. The effect of these tissue resident microbiota on ICB outcome needs to be further studied [95]. Recently, several studies illustrated the importance of intratumoral microbiota on immune surveillance [99,100], indicating that they may also influence tumor immunity.
Pathogens and microbiota generate a diverse antigenic repertoire, which can influence adaptive immune cells and help shape peripheral tolerance [95,101]. Studies have shown that some peptides homologous to microbiota-derived peptides can activate the same T cells that are also reactive to tumor-associated antigens or neoantigen derived peptides with similar amino acid sequences [93–95]. These findings demonstrate that tumor:microbe heterologous immunity can exist, which our group and others have previously suggested [9]. Pre-existing heterologous neoantigen-reactive T cells could boost immune surveillance against the tumor and help effect response to ICB therapy [102–106].
Challenges and opportunities
We believe there are at least two key goals in immunotherapy biomarker development efforts: (i) to develop systems for decision-making that can guide the physician’s choice of treatment plan in order to maximize patient benefit, and (ii) to advance our understanding of tumor immunology by identifying biological factors and associated underlying mechanisms. Here we provide some of our thoughts on how to pursue these two goals.
Multivariable models, machine learning frameworks, and nomograms
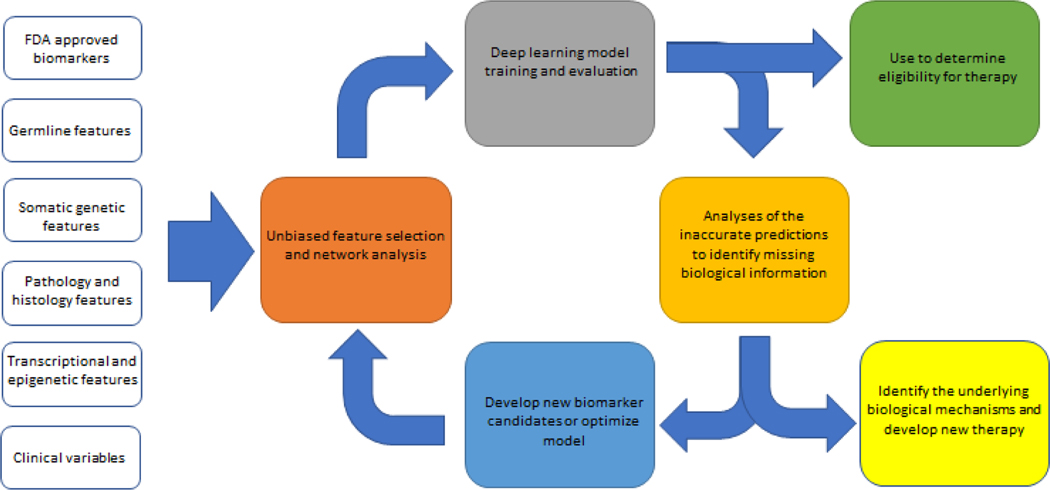
To measure complex biology such as immunotherapy response, more than one biomarker may be required. The aggregation of data from omics and clinical tests have nominated thousands of variables, only a fraction of which are clinically feasible biomarkers for prediction of efficacy from ICB therapy. The information provided by various features can also be overlapping. The major challenges we now face are identifying the most useful signals and understanding how to use the most important biomarkers in an integrated manner. Ideally, one can identify a minimal set of biomarkers with minimal redundancy and which, at the same time, provides the most comprehensive coverage of predictive variables. To better achieve this goal, a framework that includes unbiased feature selection followed by modeling can be used [25,78,107,108]. First, all features and established biomarkers can be assessed using multiple variable models to explore their contribution as well as their interactions. Then, advanced machine learning approaches could be used on informative features to generate a model that fully utilizes this information. Last, the inaccuracies from or exceptions to the model should be assessed to explore underlying biological causes, which in turn, can be used to improve the model. Figure 2 describes this iterative model.
Figure 2. Framework for biomarker selection, model building, feedback, and improvement.
Biomarkers are analyzed in a multivariable network model to determine which ones can provide non-redundant information. Then, the candidate factors are used to build deep learning models with additional optimization of the prediction power. Factors used need to be cost effective and clinically feasible. The result can be adapted for us as nomograms or weighted scores based on the most important variables.
Dynamic monitoring of ICB response and biomarker-based decision-making system
Currently, most biomarkers are assayed before the initiation of immunotherapy has begun. This includes measurements of TMB, MSI, and PD-L1 levels. Biomarkers derived from non-invasive measurements, such as ctDNA [79,109,110], make it possible to track on-therapy changes for monitoring of response to ICB. These assays can supplement pretreatment biomarkers to further optimize treatment plans.Furthermore, with the rapidly developing landscape of novel immunotherapy and combination therapies, more and more therapy options will be available for both naïve patients and the ICB-refractory patients due to primary or acquired resistance. It will be essential to determine how to utilize the existing and emerging biomarkers to build a decision-making system to generate personalized treatment plans to maximize patient benefit.
Importance of open data sharing to propel the development of integrated biomarker approaches
The identification and development of biomarkers relies on patient cohorts with complete molecular and clinical data. Biomarkers can be developed from post hoc analyses of clinical trials and retrospective patient cohorts but these need to be validated in prospective trials. It is essential for both corporations and academic investigators to share all published data. Data availability will enable harmonization and accelerate validation.
Concluding remarks
With more and more immunotherapy biomarkers being proposed, the major challenge facing us today is not just the limited numbers of biomarker candidates we have identified, or limited exploratory data we currently have access to. On the translational side, the priority is to integrate the best biomarkers in a rational manner for clinical use (see Outstanding questions). Individual biomarkers can only provide limited information – elucidating only part of the proverbial elephant. We believe that optimal use of some of the primary predictive variables already available today, in the context of a nomogram or weighted scoring model, can provide excellent performance as ICB biomarkers. Along these lines, it will be critical for investigators to avoid closed mindedness that can result from conflicts of interest and nihilism, something that the author of the parable we invoked above warned about centuries ago. On the basic research side, the focus should be shifted to understanding the underlying mechanism associated to the biomarkers, especially the mechanisms leading to false predictions, for development of better therapeutic strategies (see Outstanding questions). Many other questions remain, such as those related to eliminating disparate applicability regarding ethnicities, genders, diseases and economic conditions (see Outstanding questions). We hope our opinions and views can shed light on strategies to answer these questions and help solve the puzzle of what makes immunotherapies work.
Outstanding questions.
How do we select the most potent features that can be used as biomarkers in an unbiased way?
How do we decipher the correlations and associations between different biomarkers?
What is the biology underlying the observation that some biomarkers work in one cancer type but not in another?
What can we learn from inaccuracies in prediction? What information is missing?
What is the best way to stratify the outcome of patients for development of biomarkers?
How do we tailor existing biomarkers to prevent disparate applicability in rare cancer types and understudied ethnicities?
Is it possible to develop an efficient prediction model based on combination biomarkers with minimal cost and high clinical feasibility that could benefit third world countries with limited medical resources?
How do we utilize existing biomarkers optimally?
Highlights.
The fast-evolving landscape of immune checkpoint therapy requires development of effective biomarkers.
Many emerging biological factors affecting tumor immunity are also potentially useful biomarker candidates.
Because tumor immunity is a complex process, a framework using multiple factors, unbiased feature selection, and application of machine learning could maximize the potential power of biomarker sets.
Data sharing and feature integration will help fuel the development of biomarkers for immunotherapy that are feasible to use in the clinic.
Acknowledgements
We thank the Chan lab for helpful discussions. We acknowledge funding sources including NIH R01 CA205426 (T.A.C.) and NIH R35 CA232097 (T.A.C.).
Declaration of interests
T.A.C. is a co-founder of Gritstone Oncology and holds equity. T.A.C. holds equity in An2H. T.A.C. acknowledges grant funding from Bristol-Myers Squibb, AstraZeneca, Illumina, Pfizer, An2H, and Eisai. T.A.C. has served as an advisor for Bristol-Myers, MedImmune, Squibb, Illumina, Eisai, AstraZeneca, and An2H. T.A.C., is the inventor on intellectual property held by Memorial Sloan Kettering Cancer Center on using tumor mutation burden to predict immunotherapy response, with pending patent, which has been licensed to Personal Genome Diagnostics. X.M. has no interests to declare.
Glossary
- HLA genotype
human leukocyte antigen genes exhibit very high levels of polymorphism. Many of these polymorphisms are associated with the expression, stability, and substrate specificity of the HLA proteins. The HLA haplotype system has been generated to describe the unique polymorphisms of the HLA genes. HLA genotype is the sum of the haplotypes of all of the HLA genes of an individual
- HLA-LOH
HLA loss of heterozygosity. Tumors frequently lose the expression of one or more HLA alleles due to genomic alterations such as mutation or copy number loss, or silencing at epigenetic, transcriptional, or post-transcriptional levels. HLA-LOH events are strongly associated with presence of extensive immune pressure. Of note, clonal HLA-LOH events had been shown to be associated with worse response and prognosis after treatment with ICB while patients with sub-clonal HLA-LOH events can still benefit from the ICB
- MAPK inhibitors
inhibitors targeting and inhibiting the activation of mitogen-activated protein kinases
- Neoantigen
a new antigen resulting from a genomic alteration (such as nonsynonymous mutations or gene fusions), RNA transcriptional and splicing error (such as exon skipping or intron retention), or post-translational modifications (glycosylation or conformational changes) that is specific to tumor cells. Neoantigens must contain novel amino acid sequences or post-translational motifs that are distinct from their wild-type counterparts or other proteins in the normal proteome
- Neoepitope
neoantigen generated peptides that have been processed by the antigen presenting machinery and are able to bind to the HLA-I or II molecules and be recognized by T cells. Neoepitopes are neopeptides, while only a small portion of the neopeptides are neoepitopes, due to the substrate specificity of the antigen presenting machine and T cell receptor recognition
- Neopeptide
neoantigen derived peptides that contain unique novel amino acids or motifs. Each neoantigen can generate multiple neopeptides
- PD-L1
programmed death-ligand 1 (CD274, B7H1), is one of the major immune checkpoint molecules expressed by tumors cells or immune suppressive stromal cells. PD-L1 is the major ligand of PD-1. The PD-1/PD-L1 axis is the direct target of the anti-PD-1/anti-PD-L1 checkpoint therapies
- Tumor mutational burden (TMB)
the total number of somatic (nonsynonymous) mutations or alterations found in the genome of the tumor cells. TMB is usually adjusted by normalizing to the sizes of the genome or gene regions from which the absolute number of mutations were counted
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haslam A. and Prasad V. (2019) Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw Open 2, e192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yarchoan M. et al. (2017) Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N Engl J Med 377, 2500–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.E.B G. (2010) Encyclopedia of Perception (Vol. 1, SAGE Publications [Google Scholar]
- 4.Lee JS and Ruppin E. (2019) Multiomics Prediction of Response Rates to Therapies to Inhibit Programmed Cell Death 1 and Programmed Cell Death 1 Ligand 1. JAMA Oncol 5, 1614–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitson AR and Walster HL (1912) Soils and soil fertility Webb publishing co. [Google Scholar]
- 6.Garon EB et al. (2015) Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372, 2018–2028 [DOI] [PubMed] [Google Scholar]
- 7.Davis AA and Patel VG (2019) The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer 7, 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doroshow DB et al. (2021) PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol 18, 345–362 [DOI] [PubMed] [Google Scholar]
- 9.Snyder A. et al. (2014) Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 371, 2189–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rizvi NA et al. (2015) Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samstein RM et al. (2019) Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 51, 202–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marabelle A. et al. (2020) Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 21, 1353–1365 [DOI] [PubMed] [Google Scholar]
- 13.Garber JE and Offit K. (2005) Hereditary cancer predisposition syndromes. J Clin Oncol 23, 276–292 [DOI] [PubMed] [Google Scholar]
- 14.Rahman N. (2014) Realizing the promise of cancer predisposition genes. Nature 505, 302–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orru V. et al. (2013) Genetic variants regulating immune cell levels in health and disease. Cell 155, 242–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang JY and Sarwal MM (2017) Transplant genetics and genomics. Nat Rev Genet 18, 309–326 [DOI] [PubMed] [Google Scholar]
- 17.International HIVCS et al. (2010) The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 330, 1551–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rock KL et al. (2016) Present Yourself! By MHC Class I and MHC Class II Molecules. Trends Immunol 37, 724–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marty R. et al. (2017) MHC-I Genotype Restricts the Oncogenic Mutational Landscape. Cell 171, 1272–1283 e1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arora J. et al. (2020) HLA Heterozygote Advantage against HIV-1 Is Driven by Quantitative and Qualitative Differences in HLA Allele-Specific Peptide Presentation. Mol Biol Evol 37, 639–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferreiro-Iglesias A. et al. (2018) Fine mapping of MHC region in lung cancer highlights independent susceptibility loci by ethnicity. Nat Commun 9, 3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chowell D. et al. (2019) Evolutionary divergence of HLA class I genotype impacts efficacy of cancer immunotherapy. Nat Med 25, 1715–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu Z. et al. (2021) Germline HLA-B evolutionary divergence influences the efficacy of immune checkpoint blockade therapy in gastrointestinal cancer. Genome Med 13, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee CH et al. (2021) High Response Rate and Durability Driven by HLA Genetic Diversity in Patients with Kidney Cancer Treated with Lenvatinib and Pembrolizumab. Mol Cancer Res 19, 1510–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Litchfield K. et al. (2021) Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell 184, 596–614 e514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chowell D. et al. (2018) Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science 359, 582–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cummings AL et al. (2020) Mutational landscape influences immunotherapy outcomes among patients with non-small-cell lung cancer with human leukocyte antigen supertype B44. Nat Cancer 1, 1167–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naranbhai V. et al. (2022) HLA-A*03 and response to immune checkpoint blockade in cancer: an epidemiological biomarker study. Lancet Oncol 23, 172–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carrot-Zhang J. et al. (2020) Comprehensive Analysis of Genetic Ancestry and Its Molecular Correlates in Cancer. Cancer Cell 37, 639–654 e636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim YW et al. (2018) Germline genetic polymorphisms influence tumor gene expression and immune cell infiltration. Proc Natl Acad Sci U S A 115, E11701–E11710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sayaman RW et al. (2021) Germline genetic contribution to the immune landscape of cancer. Immunity 54, 367–386 e368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shahamatdar S. et al. (2020) Germline Features Associated with Immune Infiltration in Solid Tumors. Cell Rep 30, 2900–2908 e2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirchhoff T. and Ferguson R. (2020) Germline Genetics in Immuno-oncology: From Genome-Wide to Targeted Biomarker Strategies. Methods Mol Biol 2055, 93–117 [DOI] [PubMed] [Google Scholar]
- 34.Srivastava RM et al. (2020) Diverse Neoantigens and the Development of Cancer Therapies. Semin Radiat Oncol 30, 113–128 [DOI] [PubMed] [Google Scholar]
- 35.Blass E. and Ott PA (2021) Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat Rev Clin Oncol 18, 215–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fotakis G. et al. (2021) Computational cancer neoantigen prediction: current status and recent advances. Immuno-Oncology and Technology 12, 100052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borden ES et al. (2022) Cancer Neoantigens: Challenges and Future Directions for Prediction, Prioritization, and Validation. Front Oncol 12, 836821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wells DK et al. (2020) Key Parameters of Tumor Epitope Immunogenicity Revealed Through a Consortium Approach Improve Neoantigen Prediction. Cell 183, 818–834 e813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bakhoum SF and Cantley LC (2018) The Multifaceted Role of Chromosomal Instability in Cancer and Its Microenvironment. Cell 174, 1347–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davoli T. et al. (2017) Tumor aneuploidy correlates with markers of immune evasion and with reduced response to immunotherapy. Science 355, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Havel JJ et al. (2019) The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer 19, 133–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.William WN Jr. et al. (2021) Immune evasion in HPV(−) head and neck precancer-cancer transition is driven by an aneuploid switch involving chromosome 9p loss. Proc Natl Acad Sci U S A 118, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han G. et al. (2021) 9p21 loss confers a cold tumor immune microenvironment and primary resistance to immune checkpoint therapy. Nat Commun 12, 5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu S. et al. (2022) Extrachromosomal DNA: An Emerging Hallmark in Human Cancer. Annu Rev Pathol 17, 367–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aldea M. et al. (2021) Overcoming Resistance to Tumor-Targeted and Immune-Targeted Therapies. Cancer Discov 11, 874–899 [DOI] [PubMed] [Google Scholar]
- 46.Calles A. et al. (2020) Checkpoint Blockade in Lung Cancer With Driver Mutation: Choose the Road Wisely. Am Soc Clin Oncol Educ Book 40, 372–384 [DOI] [PubMed] [Google Scholar]
- 47.Ricciuti B. et al. (2022) Diminished Efficacy of Programmed Death-(Ligand)1 Inhibition in STK11- and KEAP1-Mutant Lung Adenocarcinoma Is Affected by KRAS Mutation Status. J Thorac Oncol 17, 399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zaretsky JM et al. (2016) Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med 375, 819–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gurjao C. et al. (2019) Intrinsic Resistance to Immune Checkpoint Blockade in a Mismatch Repair-Deficient Colorectal Cancer. Cancer Immunol Res 7, 1230–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dhatchinamoorthy K. et al. (2021) Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front Immunol 12, 636568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mouw KW et al. (2017) DNA Damage and Repair Biomarkers of Immunotherapy Response. Cancer Discov 7, 675–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samstein RM et al. (2020) Mutations in BRCA1 and BRCA2 differentially affect the tumor microenvironment and response to checkpoint blockade immunotherapy. Nature Cancer, [DOI] [PMC free article] [PubMed]
- 53.Li J. et al. (2020) Epigenetic driver mutations in ARID1A shape cancer immune phenotype and immunotherapy. J Clin Invest 130, 2712–2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Griffin GK et al. (2021) Epigenetic silencing by SETDB1 suppresses tumour intrinsic immunogenicity. Nature 595, 309–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang J. et al. (2020) Role of DNA repair defects in predicting immunotherapy response. Biomark Res 8, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones PA et al. (2019) Epigenetic therapy in immune-oncology. Nat Rev Cancer 19, 151–161 [DOI] [PubMed] [Google Scholar]
- 57.Binnewies M. et al. (2018) Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 24, 541–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yost KE et al. (2021) Recruiting T cells in cancer immunotherapy. Science 372, 130–131 [DOI] [PubMed] [Google Scholar]
- 59.Jerby-Arnon L. et al. (2018) A Cancer Cell Program Promotes T Cell Exclusion and Resistance to Checkpoint Blockade. Cell 175, 984–997 e924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mariathasan S. et al. (2018) TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Horn LA et al. (2020) Tumor Plasticity and Resistance to Immunotherapy. Trends Cancer 6, 432–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sparano JA and Paik S. (2008) Development of the 21-gene assay and its application in clinical practice and clinical trials. J Clin Oncol 26, 721–728 [DOI] [PubMed] [Google Scholar]
- 63.Pfister D. et al. (2021) NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 592, 450–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eroglu Z. et al. (2018) High response rate to PD-1 blockade in desmoplastic melanomas. Nature 553, 347–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Riaz N. et al. (2016) Recurrent SERPINB3 and SERPINB4 mutations in patients who respond to anti-CTLA4 immunotherapy. Nat Genet 48, 1327–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu J. et al. (2021) Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med 27, 152–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bera K. et al. (2019) Artificial intelligence in digital pathology - new tools for diagnosis and precision oncology. Nat Rev Clin Oncol 16, 703–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saltz J. et al. (2018) Spatial Organization and Molecular Correlation of Tumor-Infiltrating Lymphocytes Using Deep Learning on Pathology Images. Cell Rep 23, 181–193 e187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Helmink BA et al. (2020) B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kather JN et al. (2019) Deep learning can predict microsatellite instability directly from histology in gastrointestinal cancer. Nat Med 25, 1054–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coudray N. et al. (2018) Classification and mutation prediction from non-small cell lung cancer histopathology images using deep learning. Nat Med 24, 1559–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.He B. et al. (2020) Integrating spatial gene expression and breast tumour morphology via deep learning. Nat Biomed Eng 4, 827–834 [DOI] [PubMed] [Google Scholar]
- 73.Johannet P. et al. (2021) Using Machine Learning Algorithms to Predict Immunotherapy Response in Patients with Advanced Melanoma. Clin Cancer Res 27, 131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park S. et al. (2022) Artificial Intelligence-Powered Spatial Analysis of Tumor-Infiltrating Lymphocytes as Complementary Biomarker for Immune Checkpoint Inhibition in Non-Small-Cell Lung Cancer. J Clin Oncol, JCO2102010 [DOI] [PMC free article] [PubMed]
- 75.Colen RR et al. (2021) Radiomics analysis for predicting pembrolizumab response in patients with advanced rare cancers. J Immunother Cancer 9, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van de Donk PP et al. (2020) Molecular imaging biomarkers for immune checkpoint inhibitor therapy. Theranostics 10, 1708–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Valero C. et al. (2021) Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat Commun 12, 729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chowell D. et al. (2021) Improved prediction of immune checkpoint blockade efficacy across multiple cancer types. Nat Biotechnol, [DOI] [PMC free article] [PubMed]
- 79.Nabet BY et al. (2020) Noninvasive Early Identification of Therapeutic Benefit from Immune Checkpoint Inhibition. Cell 183, 363–376 e313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li S. et al. (2020) Emerging Blood-Based Biomarkers for Predicting Response to Checkpoint Immunotherapy in Non-Small-Cell Lung Cancer. Front Immunol 11, 603157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang M. et al. (2021) The Role of Cytokines in Predicting the Response and Adverse Events Related to Immune Checkpoint Inhibitors. Front Immunol 12, 670391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gandhi L. and Garassino MC (2018) Pembrolizumab plus Chemotherapy in Lung Cancer. N Engl J Med 379, e18. [DOI] [PubMed] [Google Scholar]
- 83.Wang X. et al. (2021) Comparative Efficacy and Safety of Immunotherapy Alone and in Combination With Chemotherapy for Advanced Non-small Cell Lung Cancer. Front Oncol 11, 611012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Akinboro O. et al. (2021) Outcomes of anti-PD-(L1) therapy in combination with chemotherapy versus immunotherapy (IO) alone for first-line (1L) treatment of advanced non-small cell lung cancer (NSCLC) with PD-L1 score 1–49%: FDA pooled analysis. Journal of Clinical Oncology 39, 9001–9001 [Google Scholar]
- 85.Salas-Benito D. et al. (2021) Paradigms on Immunotherapy Combinations with Chemotherapy. Cancer Discov 11, 1353–1367 [DOI] [PubMed] [Google Scholar]
- 86.Haas L. et al. (2021) Acquired resistance to anti-MAPK targeted therapy confers an immune-evasive tumor microenvironment and cross-resistance to immunotherapy in melanoma. Nat Cancer 2, 693–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yoo SK et al. (2022) Outcomes Among Patients With or Without Obesity and With Cancer Following Treatment With Immune Checkpoint Blockade. JAMA Netw Open 5, e220448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ye Y. et al. (2020) Sex-associated molecular differences for cancer immunotherapy. Nat Commun 11, 1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.DePeaux K. and Delgoffe GM (2021) Metabolic barriers to cancer immunotherapy. Nat Rev Immunol 21, 785–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Diem S. et al. (2016) Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br J Cancer 114, 256–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hu-Lieskovan S. et al. (2020) SITC cancer immunotherapy resource document: a compass in the land of biomarker discovery. J Immunother Cancer 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen S. et al. (2021) The viral expression and immune status in human cancers and insights into novel biomarkers of immunotherapy. BMC Cancer 21, 1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tray N. et al. (2018) Predictive Biomarkers for Checkpoint Immunotherapy: Current Status and Challenges for Clinical Application. Cancer Immunol Res 6, 1122–1128 [DOI] [PubMed] [Google Scholar]
- 94.Kim ST et al. (2018) Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med 24, 1449–1458 [DOI] [PubMed] [Google Scholar]
- 95.Zheng D. et al. (2020) Interaction between microbiota and immunity in health and disease. Cell Res 30, 492–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhou CB et al. (2021) Gut Microbiota in Cancer Immune Response and Immunotherapy. Trends Cancer 7, 647–660 [DOI] [PubMed] [Google Scholar]
- 97.McCulloch JA et al. (2022) Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti-PD-1. Nat Med 28, 545–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dubin K. et al. (2016) Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun 7, 10391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shi Y. et al. (2020) Intratumoral accumulation of gut microbiota facilitates CD47-based immunotherapy via STING signaling. J Exp Med 217, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cogdill AP et al. (2018) The Impact of Intratumoral and Gastrointestinal Microbiota on Systemic Cancer Therapy. Trends Immunol 39, 900–920 [DOI] [PubMed] [Google Scholar]
- 101.Boesch M. et al. (2021) Tumour neoantigen mimicry by microbial species in cancer immunotherapy. Br J Cancer 125, 313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Balachandran VP et al. (2017) Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature 551, 512–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sioud M. (2018) T-cell cross-reactivity may explain the large variation in how cancer patients respond to checkpoint inhibitors. Scand J Immunol 87, [DOI] [PubMed] [Google Scholar]
- 104.Leng Q. et al. (2020) Pre-existing heterologous T-cell immunity and neoantigen immunogenicity. Clin Transl Immunology 9, e01111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bessell CA et al. (2020) Commensal bacteria stimulate antitumor responses via T cell cross-reactivity. JCI Insight 5, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fluckiger A. et al. (2020) Cross-reactivity between tumor MHC class I-restricted antigens and an enterococcal bacteriophage. Science 369, 936–942 [DOI] [PubMed] [Google Scholar]
- 107.Topol EJ (2019) High-performance medicine: the convergence of human and artificial intelligence. Nat Med 25, 44–56 [DOI] [PubMed] [Google Scholar]
- 108.Leclercq M. et al. (2019) Large-Scale Automatic Feature Selection for Biomarker Discovery in High-Dimensional OMICs Data. Front Genet 10, 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cindy Yang SY et al. (2021) Pan-cancer analysis of longitudinal metastatic tumors reveals genomic alterations and immune landscape dynamics associated with pembrolizumab sensitivity. Nat Commun 12, 5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nisar S. et al. (2020) Non-invasive biomarkers for monitoring the immunotherapeutic response to cancer. J Transl Med 18, 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Valero C. et al. (2021) Response Rates to Anti-PD-1 Immunotherapy in Microsatellite-Stable Solid Tumors With 10 or More Mutations per Megabase. JAMA Oncol 7, 739–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sturgill EG et al. (2022) Discordance in Tumor Mutation Burden from Blood and Tissue Affects Association with Response to Immune Checkpoint Inhibition in Real-World Settings. Oncologist 27, 175–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vega DM et al. (2021) Aligning tumor mutational burden (TMB) quantification across diagnostic platforms: phase II of the Friends of Cancer Research TMB Harmonization Project. Ann Oncol 32, 1626–1636 [DOI] [PubMed] [Google Scholar]