Abstract
Electroceuticals provide promising opportunities for peripheral nerve regeneration, in terms of modulating the extensive endogenous tissue repair mechanisms between neural cell body, axons and target muscles. However, great challenges remain to deliver effective and controllable electroceuticals via bioelectronic implantable device. In this review, the modern fabrication methods of bioelectronic conduit for bridging critical nerve gaps after nerve injury are summarized, with regard to conductive materials and core manufacturing process. In addition, to deliver versatile electrical stimulation, the integration of implantable bioelectronic device is discussed, including wireless energy harvesters, actuators and sensors. Moreover, a comprehensive insight of beneficial mechanisms is presented, including up-to-date in vitro, in vivo and clinical evidence. By integrating conductive biomaterials, 3D engineering manufacturing process and bioelectronic platform to deliver versatile electroceuticals, the modern biofabrication enables comprehensive biomimetic therapies for neural tissue engineering and regeneration in the new era.
Keywords: peripheral nerve regeneration, electroceuticals, electrical stimulation, nerve conduit, conductive conduit
1. Introduction
Peripheral nerve injuries (PNIs) constitute 2%–5% of trauma cases, leading to significant economical and psychosocial burden to the individuals and society [1-3]. For severe injuries, PNIs adversely affect behavior, mobility, sensations, perception of skin, muscle and joints resulting in long-term disability. Unlike central nervous system (CNS) injuries, which commonly yield failure of injured axons to regenerate, PNIs are followed by robust regeneration with higher possibility of recovery of sensory and motor functions [4]. The frequency of axon regeneration is dependent on various factors including alterations within the cell body, stability of growth cone, and the hindrance of damaged tissue between neuron and its end organ. In humans, axonal regeneration is typically shown as 1–2 mm d−1 without additional treatments that can effectively accelerate the process [5]. This regenerative response is associated with widespread transcriptional and epigenetic changes in injured neurons [6, 7]. If the regeneration fails, however, the end organ such as motor unit would undergo irreversible degeneration 12–18 months after denervation [8]. Treatments of PNIs consist of surgical and non-surgical approaches and numerous modalities have been developed since the unsatisfactory outcome of severe PNIs remains a noteworthy clinical issue [9, 10] (figure 1). Among non-surgical modalities, numerous works have been studied including medication and phytochemicals [1, 11, 12]. Surgical therapeutic approaches for peripheral nerve recovery comprise a variety of techniques including direct repair [13, 14], nerve grafting (autografts, allografts) [15, 16], nerve transfer [17, 18], fibrin glue [1, 14, 19], nerve conduits [14, 20-30] and cell-based therapy [12, 31-33]. Advantages and disadvantages of each technique have been widely discussed [1, 10]. Currently, novel treatments that have been reported include phytochemicals, optogenetics, fat grafting, tissue-engineered nerve grafting and electrical nerve stimulation [6, 34-37].
Figure 1.
Current treatment options for promoting PNR. The state-of-art of multi-modality approaches to repair or reconstruct PNI include surgical, non-surgical and physical stimulation. Surgical intervention includes all kinds of microsurgical repair, nerve graft, nerve/muscle transfer. Non-surgical approach includes pharmaceuticals, various synthetic growth factors and cell-based therapies. Physical stimulation consists of optogenetics, ultrasound, microwave, radiofrequency and electroceuticals. Biomaterial approaches include synthetic NGCs, hydrogel and controlled release drug-containing vehicles.
For PNIs with a nerve defect or a gap needed to be bridged, autologous nerve graft remains the gold standard treatment. However, autografts have the drawback of donor site morbidity and limited supply. Other alternate methods are thus extensively explored. Nerve guidance conduits (NGCs) with the ease of various design such as combination of cell-based therapy or adjustment of the microenvironment with growth factors, gene therapy or tissue-engineered graft are increasingly being considered as an alternative to nerve autografts [22, 29, 31, 38].
Electroceuticals deliver electrical impulses targeting the neural circuits that regulate the body’s organs and functions, via either wearable or implantable electronic device [39, 40]. As a new category of novel therapeutic approach, electroceuticals have applied and demonstrated therapeutic potential for ischemic stroke [41], Alzheimer’s disease [42], type I diabetes [43], wound healing [44], cardiovascular regulation [45], gastrointestinal tract disorder [46] and even developmental disorders [47]. In the field of peripheral nerve regeneration (PNR), electrical stimulation (ES) as a therapeutic intervention for PNIs has been studied for decades. Percutaneous ES has long been clinically applied as prevention of muscular atrophy [48]. As for ES focused on injured nerve after repair, the positive effects of brief, low-frequency ES on PNR was established in various animal experiments [12, 35, 49]. This review will provide comprehensive information on (a) current animal and human evidence of ES therapy; (b) fabrication of conductive materials and electronic devices; and (c) integration of these two fundamental components of electroceutical approaches.
2. Electroceuticals as novel approaches for PNR
The main pathologies of PNIs include a cascade of changes in both the local injured axon and the associated neurons. Wallerian degeneration, which initiates within 24–48 h following nerve injury, consists of active axon degeneration and myelin degradation at nerve distal to the injury site [50]. This process leads to the degradation of neurofilaments, the rearrangement of nerve’s cytoskeleton with detachment of axon terminals from the target. In the meantime, the neurons proximal to the injured sites undergo a form of polarized growth in order to reinnervate towards their targets [51, 52]. However, the injured neuron might undergo programmed cell death activated within 6 h in unfavorable situations regulated by various extrinsic and intrinsic factors [53].
This regenerative response is associated with wide-spread transcriptional and epigenetic regulations in the injured neurons, axons and target organs. Substantial advances have been established in terms of the coordinated actions of transcription factors, epigenetic modifiers and microRNAs, which are widely investigated in the peripheral nervous system in recent studies [4, 6, 7, 54]. To facilitate the extensive regenerative process after PNIs, a comprehensive strategy should be considered for systemwide neuromodulations from proximal neurons, local injured axons to distal target organs.
2.1. Beneficial mechanisms of ES for PNR
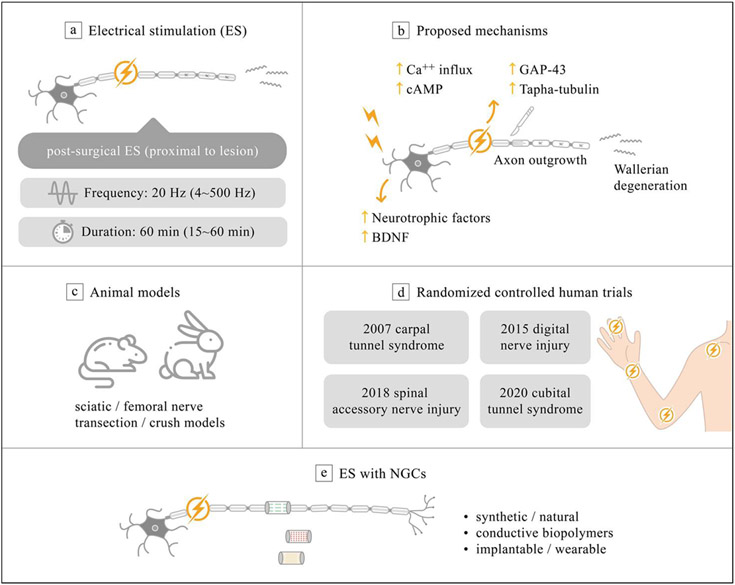
ES as an advanced therapeutic approach for PNIs, has been approved for its promising role in promoting PNR with specific target effect. Percutaneous ES has long been clinically applied for the prevention of muscular atrophy [48]. With direct stimulation on the proximal site to the injured nerve, brief low-frequency (20 Hz) ES has revealed beneficial effects in various animal experiments [12, 35, 49]. Gordon presented the first randomized controlled trial, demonstrating the beneficial therapeutic outcomes of proximal ES on patients with severe carpal tunnel syndrome after surgical decompression. The clinical evidence of direct nerve ES enables the additional proximal benefits on neuronal cell, in terms of promoting PNR during nerve surgery on injury site [35]. In contrast to low-frequency (20 Hz) ES, kilohertz stimulation lead to reversible nerve block under the same stimulation amplitude, which was usually being applied to alleviate neuropathic pain (see full review in [55, 56]).
ES has been widely demonstrated to offer benefits in the regeneration of bone, cartilage, skin, spinal nerves, and peripheral nerves [57]. The current evidence indicates postsurgical or intraoperative single proximal ES (SP-ES) as a promising therapeutic strategy to promote PNR after a variety of injuries [35, 49, 58-62]. SP-ES in which the electrical stimulus is applied directly on the nerve stump proximal to the injured site, is validated in various animal studies. A single dose of brief (1 h), low frequency (20 Hz) ES, has been proved to amplify axon regeneration after nerve transection and microsurgical repair (figure 2(a)) [35, 60, 63, 64]. Moreover, a recent research reveals that brief (10 min) SP-ES can provide identical therapeutic benefits to the abovementioned 60 min protocol in an acute sciatic nerve transection/repair rat model and thus imply the translational potential for future clinical application [65]. The current established mechanism of SP-ES contributing to the therapeutic effect of PNR involves increase of neurotrophic factors and upregulation of their receptors on neuronal cells, including brain derived neurotrophic factor (BDNF) [63, 66]. Subsequent elevation of intracellular cyclic adenosine monophosphate (cAMP) level and related cAMP response element binding protein pathway [67-69] further enhance expression of regeneration-associated genes, such as Talpha-1 tubulin and growth associated protein 43 (GAP-43), resulting in axon regeneration [70-72]. One in vitro study has demonstrated that ES increases production of nerve growth factor (NGF) from Schwann cell (SC), leading to efficient axon remyelination [73]. In addition, a recent in vitro study discovers that ES triggering the p38 mitogen-activated protein kinase pathway in PC12 mutant cells, which plays an important role in promoting neurite outgrowth (figure 2(b)) [74].
Figure 2.
Current evidence of electroceuticals for PNR. (a) Therapeutic stimulation parameters and application of brief ES. (b) Proposed mechanism of peripheral nerve ES. (c) Current established in vivo evidence for transection/crush injuries at sciatic/femoral nerves of rodent and rabbits. (d) Current human evidence among median nerve [64], digital nerve [34], spinal accessory nerve [75] and ulnar nerve [76]. (e) Choice of NGC with electroceuticals.
2.2. Current evidence of SP-ES after nerve injury
Numerous animal studies and human trials present the therapeutic benefits of PNR from SP-ES (figure 2(c)) [6, 35, 59]. In 1983, Nix and Hopf first described the positive effect from direct ES on injured nerves and the affected muscles. Better twitch force, tetanic tension, and muscle action potential were observed 2 weeks after ES (4 Hz, daily) treatment in a rabbit nerve transection model [77]. Further animal studies showed that postsurgical 1 h 20 Hz ES directly on injured nerve yield earlier recovery of motor and sensory functions in acute crush injury [78], transected femoral nerve [79, 80] and sciatic nerve in rats models [81, 82]. Moreover, in delayed nerve repair model of rats, a single session of 1 h ES at 20 Hz immediately after delayed nerve repair significantly increased the numbers of motoneurons reinnervating toward chronically denervated muscle [83]. Moreover, a recent research proposes that repetitive distal ES on nerve gap injury model, demonstrating the therapeutic potential on preservation of neuromuscular junction and improvement of motor function [84]. The current animal researches demonstrated the therapeutic evidence of SP-ES, whether for the injury mechanism (crush or transection), nerve type (femoral or sciatic nerve), treatment timing (acute or chronic) and stimulation site (proximal or distal to injured nerve).
For human therapeutic evidence, to our knowledge, there are currently four randomized controlled trials regarding the therapeutic benefits of SP-ES for PNR (figure 2(d)). Two randomized controlled trial had conducted to examined the effect of SP-ES immediately after carpal tunnel [64] and cubital tunnel syndrome [76] release, demonstrating motor unit number estimates increased significantly by postoperative 1 and 3 years as compared to the control unstimulated group, respectively. Wong et al further revealed immediate effect of SP-ES after digital nerve repair with improved digit sensation and nearly full functional recoveries [34]. For patients undergoing oncologic neck dissection, intraoperative SP-ES (continuous 60 min, 20 Hz) to spinal accessory nerve contributed to significant improvement in electrophysiologic outcome and overall shoulder function at 12 months after surgery [75]. Despite all the above-mentioned beneficial evidences of SP-ES, several translational challenges remain in terms of optimization of stimulation dosage, feasibility in critical nerve gap injury, and the potential benefit of preconditioning ES [85]. In addition, an innovative bioelectronic platform that combines such bi-directional ES is also crucial in order to achieve PNR in the future.
2.3. ES with conductive materials
Despite the abovementioned convincing evidence of proximal ES on PNR, the therapeutic evidence of ES on critical nerve gap remains unexplored due to limitations of biomaterials. NGC provides tissue engineered biomimetic tubular structures to bridge critical nerve gap when encountered and has drawn significant attention in terms of nerve tissue repair and regeneration (figure 2(e)). They can be made of natural or synthetic biopolymers such as chitosan, gelatin, collagen, polylactide, poly(lactic-co-glycolic acid) (PLGA), or polycaprolactone (PCL), which are designed to offer supportive mechanical and/or biochemical cues [29, 86, 87]. Either conductive or non-conductive biomaterials can be used as the scaffold of NGC. However, several issues should be still considered such as non-biodegradability, possible long-term in vivo toxicity, and non-homogeneous distribution of the conductive particles in neural tissue [21, 25, 29, 57, 88]. Recent development on implantable conductive materials and structure can offer the opportunity of delivering wide-spread ES from the implantation site, with the additional advantage of minimal stimulus needed as compared to transcutaneous approach. The efficacy of the combination of ES with NGC has been explored in literature [20, 57, 62, 86, 89, 90]. Choices of different types of conductive biomaterials with minimum toxicity and development of an implantable or wearable electronic device with numerous designs of interface are both hot topics in the field of neural tissue engineering [57, 87, 88, 91].
To engineer a novel NGCs for electroceuticals, the electrical conductivity of the fabricated biomaterials provides a compelling solution to the current clinical difficulties [92]. To achieve this goal, the optimal dosage of ES and protocol are both important determining factors for the therapeutic effects of neuromodulation. It is reported that SP-ES effectively accelerates the regenerating motoneurons with a low frequency of 20 Hz for an hour a day [79, 83, 93]. However, a recent study applying single or two sessions of proximal ES on critical nerve gaps, observed that such ES can only promote sensorimotor recovery at the first session of ES when delivered at the time of reconstruction, with no benefit of a second delayed session of ES 4 weeks after the initial reconstruction [94]. Therefore, the exploration of the application of repetitive ES and the integration of conductive conduit with ES would be of great value. Various ES conditions such as electrical conductivity of the NGCs, charged voltage, current, and duration have been summarized in table 1. From this perspective, the standard protocol has not yet been established. Although not as efficient as autograft, ES plus conductive conduit indeed demonstrates significant therapeutic benefits. The following section will introduce both the in vitro and in vivo influence of the PNR from the combination of ES and functional electrically conductive conduit.
Table 1.
Summarized parameters of current studies with engineering conductive conduit and ES for PNR.
| Materials | Conduit fabrication | Electrical properties | ES condition | Cell | Animal | References |
|---|---|---|---|---|---|---|
| PPy/chitosan | Injection molding technique | (1.5 ± 0.2) × 10−2 S cm−1 | 3 V of 20 Hz, 0.1 ms for 1 h | No data | Male SD rat sciatic 200–220 g, 15 mm defect | [90] |
| PPy/PLCL | Electrospun | 6.72–2.41 × 10−5 S cm−1 | 100 mV for 1 h/1, 3, 5 d | PC12, BDNF, GDNF, NT-3 mRNA | SD rat sciatic 15 mm defect | [95] |
| PPy/PLGA | Electrospun | (7.4 ± 3.2) × 103–(9.0 ± 6.0) × 104 Ω sq−1 | 10, 100 m V cm−1 for 2 h | PC12 | No data | [96] |
| PPy/PCLF | Injection molding technique | 2–25 kΩ | 10 μA of 20 Hz 1 h d−1 | PC12 | No data | [97] |
| PPy/SF | Electrospun | 114.46–3.98 × 10−3 ms mm−1 | 3 V of 20 Hz, 0.1 ms for 1 h | SCs | SD rat sciatic 180–220 g, 10 mm defect | [98] |
| PPy/PDLLA | Roll-up sheet, emulsion technique | 5.65–15.56 ms cm−1 | 100 mV for 2 h | PC12 | SD rat sciatic 150–200 g, 10 mm defect | [99] |
| PPy/BC/N-CNTs | Sheet with elecrospun conduit | 1.51 ± 0.05 S cm−1 | Self-powered stim. Similar to 50 mV mm−1 | DRGs | Female SD rat sciatic 220 g, 15 mm defect | [100] |
| PPy/PECA, PPy/PCL | Airbrushing, pressing, roll-up | 19–32 S cm−1 103–104 Ω sq−1 | 100 μA for 2 h | PC12 | Male SD rat sciatic 350 g, 10 mm defect | [101] |
| PPy/SA/CMCS | Nerve conduit filling material | 2.41 × 10−5–8.03 × 10−3 S cm−1 | 100 mV for 2 h d−1 | PC12, RSC96, BMMSC | SD rat sciatic 150 g, 10 mm defect | [102] |
| PANI/PCL/gelatin | Electrospun | 0.02 × 10−6 S | 1.5 V for 15, 30, 60 min during 1, 3, 5 d | NSC | No data | [103] |
| PANI/HEC/SPI | Injection molding technique | 1.7 S m−1 20.6 kΩ sq−1 | 3 V for 1 h 7 times during 2 d | SCs, BDNF, MBPs | SD rat sciatic, 180–220 g, 10 mm defect | [89] |
| CNT/Sericin | Injection molding technique | (3.73 ± 0.51) × 10−5–(3.90 ± 0.26) × 10−4 S cm−1 | 3 V of 20 Hz, 0.1 ms for 1 h | RSC96 | Male SD rat sciatic, 200–300 g, 10 mm defect | [104] |
| SWCNT/SF | Roll-up sheet manufactured by electrospun | Approx. 2.1 × 10−3 S m−1 | 1 V of 2 Hz, 0.2 ms | U373-MG | Male SD rat sciatic, 200–250 g, 10 mm defect | [105] |
| rGO/GelMA | Injection molding technique | 1.1 ± 0.1–8.7 ± 1.6 mS cm−1 10 ± 1–193 ± 13 kΩ | Stim. X Electromyogram (EMG) signal 1.5 V |
PC12 | Male SD rat sciatic, 180–200 g, 10 mm defect | [106] |
| rGO/ApF/PLCL | Electrospun | 1.96 × 10−3–4.05 × 10−2 S m−1 | DC 100 mV cm−1 for 1 h d−1 | SCs PC12 | Male SD rat sciatic, 200–250 g, 10 mm defect | [107] |
| CNT/PPDO | Electrospun | 1.73 × 10−10, 1.44–3.52 × 10−6 S cm−1 | 50 mV mm−1 for 1 h d−1 | hADMSCs | No data | [108] |
| MXene/PCL | Electrospun | 2.91 × 10−2 S cm−1 | Self-originated ES | RSCs | Male SD rat sciatic, 150–200 g, 15 mm defect | [109] |
| rGO/GelMA/BDNF/Morpho menelaus | Roll-up sheet | Approx. 0.5–1.0 S cm−1 | Stim. X. | PC12, NSC | Male SD rat sciatic, 180–250 g, 10 mm defect | [110] |
| ZnO/PCL | Electrospun | 40–120 mV | Piezoelectric | RSCs | 8 weeks old SD rat sciatic, 10 mm defect | [111] |
PPy; polypyrrole, PDLLA; poly(d, l-lactic acid), PCLF; polycaprolactone fumarate, SF; silk fibroin, PLCL; poly(l-lactic acid-co-ε-caprolactone) BDNF; brain-derived neurotrophic factor, GDNF; glial cell derived neurotrophic factor, NT-3; neurotrophin-3, PLGA; poly(lactic-co-glycolic acid), BC; bacteria cellulose, DRGs; dosal root ganglion cell, N-CNTs; nitrogen-doped CNTs, SWCNT; single wall CNTs, PECA; poly(ethyl cyanoacrylate), PCL; poly(ε-caprolactone), SA; sodium alginate, CMCS; carboxymethyl chitosan, PANI; polyaniline, HEC; hydroxyethyl cellulose, SPI; soy protein isolate, rGO; reduced graphene oxide, GelMA; gelatin-methacrylate, ApF; Antheraea pernyl silk fibroin, PC12; rat phaeochromocytoma cell line, SCs; Schwann cell, RSC96; Schwann cell, BMMSC; bone marrow mesenchymal stem cell, MBPs; myelin basic protein, NSC; nerve stem cell, U373-MG; human glioblastoma-astrocytoma, SD rat; Sprague–Dawley rat, PPDO; poly(p-dioxanone), hADMSCs; human adipose-derived mesenchymal stem cells, MXene; Ti3C2TX, RSCs; Rat Schwann cells.
2.3.1. Beneficial evidence of neural cell response on conductive materials in vitro
Conductive NGCs aims to reconnect nerve defects physically and communicate biophysical signals for facilitating neural tissue outgrowth. Although conductive substrates support cellular activity with or without ES [106, 113], it has been found that they can enhance axon outgrowth when applied in conjunction with ES [90, 99]. Recently, it has been widely studied the effect of cell stimulation and behavior on electrically conductive materials. When electrical stimuli are applied to the injured nerves, the neuronal cells are activated, resulting in cellular responses such as proliferation, migration, differentiation, neurite outgrowth, and remyelination, which extensively influence the peripheral nervous system [114].
SCs are a representative neuroglia cell to myelinate the axons in peripheral nervous system (PNS). It resides in peripheral nerve tissues, with important roles in chaperoning axon sprouting. More specifically, SCs activate proliferation and migration in the regeneration process by supporting axon growth and subsequent myelinization, resulting in nerve regeneration through the secretion of neurotrophic factors [115, 116]. Zhao et al evaluated the in vitro effect of ES on SCs, demonstrating enhanced viability, proliferation and migration, along with upregulated expression of neurotrophic factors (BDNF, NT-4/5, NGF, GDNF). Moreover, the constructed polypyrrole (PPy)/silk conductive NGC accompanying ES could effectively promote in vivo axonal regeneration and remyelination [98]. Accordingly, when NGCs is fabricated with electrical conductivity, it enables control of cell adhesion, migration and interaction of neural cells under an electric field. The in vitro cell responses in figure 3(a) showed that in the group adopting ES to the conductive matrix, SC growth, proliferation, and migration were significantly promoted than in the absence of ES. In addition, the remyelination by SCs was also facilitated by ES [107]. Figure 3(b) shows various histological benefits of conductive conduit with ES for sciatic nerve regeneration. It was observed that motor performance could be improved during rehabilitation by sciatic function index (SFI) analysis. This synergistic effect was more clearly demonstrated in the observation of immunostained images to evaluate regeneration of axonal growths in the conduits. With the electric field stimulation, the differentiation and elongation of neurons were enhanced on the conductive cross-linked poly(3,4-ethylene dioxythiophene) (PEDOT) substrate [117]. In addition, applied ES to the unidirectional aligned nanofiber matrix shows a synergetic effect for higher cell viability [98].
Figure 3.
The in vitro and in vivo beneficial effects of neural regeneration with combined conductive conduit and ES. (a) Neural cell response on the electrical field; in vitro. Illustration of various cell activity by ES on the conductive substrate. Reprinted from [107], Copyright (2019) with permission from Elsevier. (b) Electroceuticals to accelerate nerve repair; in vivo. Illustration of phenomena by ES in sciatic nerve repair. From [112] Reprinted with permission from AAAS. Reprinted from [107], Copyright (2019) with permission from Elsevier.
2.3.2. Electroceuticals to accelerate the nerve repair in vivo
As summarized in table 1, many groups have demonstrated the effect of this strategy using the transected sciatic nerve model in Sprague–Dawley (SD) rats. Researchers have investigated the accelerated regeneration of the sciatic nerve using over 10 mm nerve gap models in 150–250 g adult rats. Conductive NGCs had shown considerable improvement compared to non-conductive NGC groups. ES used in non-conductive materials can only apply directly to cells and cannot provide a large area. Furthermore, the combinations of conductive NGCs with ES contributed to higher regenerative ability than the solely conductive NGCs group [89, 90, 95, 98, 107].
Song et al had demonstrated the beneficial effects of ES on 15 mm nerve gap injury bridged by conductive NGC (PPy/PLCL) [95]. It revealed remarkable regeneration capability, and there was identical outcomes in sciatic function index, nerve conduction velocity, distal compound motor action potential, and recovery rate of triceps muscle weight between the PPy/PLCL with ES group and autograft group. In addition, Huang et al explain that that localized ES enhances the migration ability of SCs that migrating into the conductive conduit (PPY/chitosan) paves the way for axon regeneration [90]. It has been shown that these ES therapies allow a synergistic effect not only through the electrical properties of the neural conduit but also through the longer and faster neural filaments with highly aligned micropatterns [98]. So, the remarkable potential of this integrated strategy as electroceuticals suggests a promising future direction in PNS regeneration.
In summary, the integrated strategy of conductive NGC with ES shows substantial regeneration capacity comparable to autograft transplantation. Electroceuticals with conductive materials are promising therapeutic options in the future, providing both material and electrophysiological cues to support consequential PNR.
3. Fabrication of bioelectronic conduit
Providing well-developed conductive materials into the injured site is a key biotechnology to have synergic efficacy of regeneration without side effects and secondary damages. Conductive conduit implanted between the transected nerves can serve to use as an electrically conductive pathway for bilateral stimulation in the proximal and distal. Therefore, a comprehensive understanding of conductive materials, optimal structure and fabrication will help us develop more effective electrotherapy. In this section, we discuss these issues for a promising therapeutic strategy and optimization.
3.1. Candidate raw materials for conductive nerve conduit
Nerve regeneration requires intrinsic ionized electric signals and is wrapped in a myelin sheath (nonconductor) to prevent leakage of ions from the axon to transmit biological signals. However, in severe nerve injuries, transplantation of synthetic scaffolds is required and axons are guided in specific directions. But so far, artificial structures have not yet been as conductivity natural ions and tissues. Therefore, for bioinspired-mimic properties, implantable conduits are recommended alternative candidate materials with electrical conductivity.
Conductive polymers are called π-conjugated polymers, resulting in a conductive biostructure due to mostly carbon bonding structures, forming a valence band and involving electrons moving easily [118]. These π-conjugated polymers, such as PPy, polyaniline (PANI), and PEDOT, exhibit conductivity when dopants are added through a redox reaction [119]. The electrical stimulus on the conductive polymers drives the dopant to move through the structure, creating polarons and allowing the charge to flow through [20]. However, these conductive polymers are not biodegradable and have poor solubility in most solvents. Currently, these conductive polymers are usually not used independently but used a combination with natural or synthetic polymers [89, 90, 95, 97, 98, 103, 120-124]. The biodegradable natural and/or synthetic polymers are partially absorbed, the non-degradable conductive polymers still retain the debris and circulate through the in vivo environment. Although the effect of short-term ES after surgery provides acceleration of nerve growth, it has limitation that is difficult to track non-degradable conductive polymer fragment after recovery [29]. For this reason, it could not be a complete alternative material despite the potential application of electrical signal transmission.
Carbon nanomaterial has high electrical conductivity and excellent mechanical properties. The nanosized structure and large surface area could serve as a promising strategy to enhance neuro-regeneration. Carbon nanotube (CNT) provide electrical conductivity in state of dispersing on nanofiber that lead to improving cell proliferation of PC12 and SCs [125, 126]. However, the biocompatibility of CNTs still remains argumentation due to poor clear evidence in safety. In several studies, authors do not agree that non-functionalized CNTs have biocompatibility to support neuronal growth and regeneration [113, 127, 128]. The studies of degradation rate, discharge, and safety of CNTs are remained works to overcome the current limitations in future applications. Likewise, graphene (GO) also improves the biological cue between the biocompatible scaffold and the cell membrane because GO has strong π-bonding and a large surface area, resulting in high electrical conductivity and promoting signal transduction and metabolic activity. Therefore, it significantly improves neuronal expression both in vitro and in vivo. Many studies support the remarkable regeneration effects by dispersing graphene oxide (GO) or reduced GO (rGO) in a scaffold matrix [106, 129, 130]. GO, when inserted into the body, tends to accumulate in organs such as the lungs, liver, and spleen, and exposure to GO can cause severe cytotoxicity and disease [131]. On the other hand, as the result that GO could be biodegraded in vivo by macrophages is still being presented [132], the debate about the application of carbon nanomaterials as biomaterials in the future is expected to continue.
Metal particles can be used as conductive biomaterials including nanoparticles of gold (Au), silver (Ag), and copper (Cu) [133, 134]. Composite materials, mixed with nano metal materials and hydrogel, improve mechanical strength and electrical conductivity, and the level of cell adhesion and proliferation are able to control by the concentration of metal material [135]. Furthermore, metals can completely and harmlessly dissolve, reabsorb, or degrade at the molecular level, known as transient electronics. Such transient metals include magnesium (Mg), zinc (Zn), tungsten (W), iron (Fe), molybdenum (Mo) [136]. These metals are considered good bioresorbable materials, but their corrosion mechanisms are completely different. There are concerns that consumption of oxygen and byproducts in the corrosion process may cause necrosis of the surrounding tissues, so a lot of care should be taken when employing metal materials [137]. Besides, the allowance of each metal is different in the organs, sex, and ages; therefore, quantitative and systematic studies should be followed.
Ionically conductive materials called hydrogels, inogels, or polyionic elastomers have been introduced as a new type of conductors that use charged ions rather than electrons to allow electrical signals [138]. In general, ion conductors have excellent stretchability, transparency, and biocompatibility [139]. For example, polyvinyl alcohol/hydroxypropyl cellulose/fiber hydrogel is fabricated with artificial nerves to deliver stable AC and tunable DC electrical signals in robot finger movements for complete recovery [32]. Conductive hydrogels are used as 3D printing materials and have the advantage of constructing complex shapes. The ability to efficiently collect strain and vibration signals has been proven through the fabrication of sensors with a complex structure [138]. Most of the research results are used as wearable sensors under in vitro conditions. So far, ionically conductive materials with excellent electrical conductivity and physical properties and at the same time excellent biocompatibility have not been introduced.
For electroceuticals, exploring conductive materials is a key strategy, but above all, the nerve conduit must be biocompatible, biodegradable, and biostable. Also, conductive materials and/or composites must be applicable to the manufacturing process of conduit shapes, surface treatment, and internal micropatterns for guiding axon sprout.
3.2. Fabrication of a 3D conduit structure
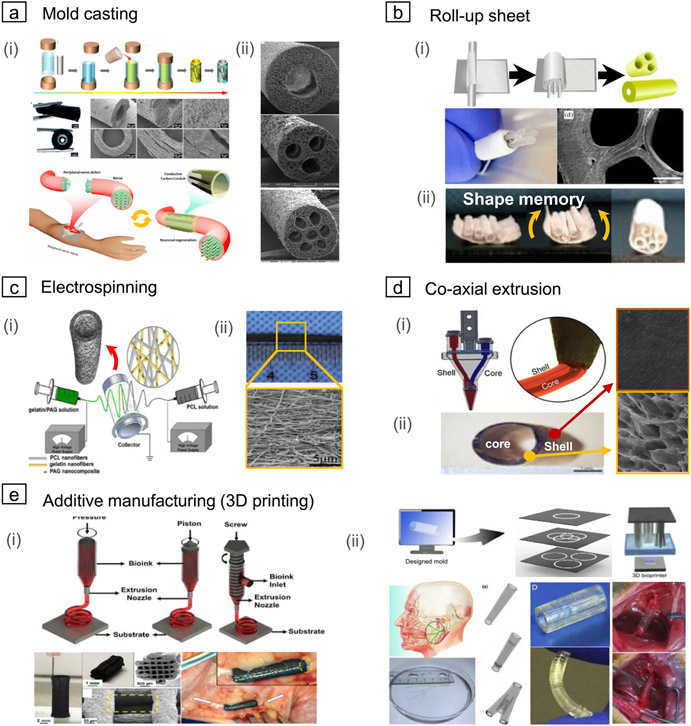
Fabrication methods of fine-scaled cylindrical shapes with conductive materials were a critical obstacle to facilitating the functionalized conduit in a clinical application. Mold casting can provide the simplest and easiest way to fabricate the hollow tube structure. More specifically, rods are placed in the core of the mold to create inner cavities. Then, injection of the prepared composite solutions into the rest of the casting mold serves hollow conduit structure, followed by demolding after complete solidification (figure 4(a) (i)). In addition, mold casting of a biodegradable polymer with carbon material can improve nerve conduction instead of non-conductive materials [106, 140]. The number of internal rods allows multi-channeled conduits as well as the single lumen, as shown in figure 4(a) (ii). The freeze-dried sponge-based conduit can even improve permeability by forming small pores on the cylinder wall [141]. The inner surface-to-volume ratio increased with more channels resulting in larger axon diameter and myelination thickness in the in vivo study.
Figure 4.
Various methods of constructing a nerve guidance conduit (NGC) with a cylindrical structure comprising single or multi-channel. (a) Mold casting; (i) representative illustration of the injection molding process and microstructure image of the manufactured conduit. Reprinted with permission from [140]. Copyright (2017) American Chemical Society. (ii) NGCs tube manufactured in various shapes according to the size and number of cores and location. Reprinted with permission from [141]. Copyright (2017) American Chemical Society. (b) Roll-up sheet; (i) schematic diagram of the rolled-up process using sheet and rod spacer (above) and manufactured conduit (below). [142] John Wiley & Sons. [original copyright notice]. (ii) Approach using electrospun shape memory nanofibers. The sheet keeps temporarily plane and then triggered by a physical temperature at 37 ° C to form a cylindrical conduit. Reprinted with permission from [143]. Copyright (2020) American Chemical Society. (c) Electrospinning; (i) illustration of dual electrospinning method. [142] John Wiley & Sons. [Original copyright notice]. Nano network conduits can be produced by jetting a polymer solution through a capillary nozzle with high voltage and depositing nanofibers in the collector. (ii) Photograph and microstructure of the electrospun NGCs. Reprinted from [144], Copyright (2019), with permission from Elsevier. (d) Co-axial extrusion; (i) schematic diagram of co-axial extrusion using a dual nozzle composed of inner and outer nozzles. Reproduced from [145], with permission from Springer Nature. (ii) The tube structure is produced by removing the core part of the extruded cylindrical filament. [146] Taylor & Francis Ltd. http://tandfonline.com. (e) Additive manufacturing (3D printing); (i) nozzle extrusion-based 3D plotting technique according to designed toolpath. [147] John Wiley & Sons. [Original copyright notice]. Reprinted with permission from [148]. Copyright (2015) American Chemical Society. (ii) Schematic diagram of the stereolithography based additive manufacturing using photocurable solution. Reprinted from [149], Copyright (2019), with permission from Elsevier. Stereolithography can provide customized therapy options for complex-shaped nerves defect. [150] John Wiley & Sons. [Original copyright notice]. Optical image of 3D printed NGCs, which has high flexibility. Reprinted from [151], Copyright (2018), with permission from Elsevier.
However, the restriction of internal rods in various shapes and homogeneous channels should be overcome. It is also challenging to fabricate a microchannel because there is a risk of shape deformation or collapse during the demolding process via dissolving or pulling out. Therefore, optimization of the size, number, and position of internal rods will be a key feature to improve axon guidance as to future works.
A hollow structure can be created by rolling the sheet onto cylindrical rods. This strategy enables forming a conduit shape by pulling out the rod after wrapping it with a rectangular electrical conductive sheet. Figure 4(b)(i) shows that multi-channel conduits can be manufactured according to the size and number of rods for channel formation [142]. There is a limit to downsize to the required regenerated nerve. This handwork means atypical and may vary the guiding ability of axon sprouting depending on skill level. Here, there is an interesting study of the automatically roll-up method using shape-memory materials. When triggered by a core body temperature of 37 °C, it is restored to a tubular shape of a multichannel conduit (figure 4(b)(ii)). Angiogenesis and blood vessel formation could be promoted by forming a microchannel of the prepared sheet with nanofiber which provides more space for cell proliferation, migration, and neural tissue regeneration [143]. Although it is still difficult to construct a uniform structure with more fined channels, this has infinite potential with prepared nanopatterning for morphology cues.
Electrospinning is one of the most useful methods for producing nanofiber network sheets improving permeability during neuro-regeneration. The electrostatic field deforms the polymer droplets on the nozzle tip into a cone shape, and if the electrostatic force exceeds the surface tension, a charged jet is ejected. At this time, when a high voltage is applied to both the capillary nozzle tip and the substrate, the polymer nanofibers forward the ground plate of the counter collector, and a thin fiber network is deposited (figure 4(c)(i)). It generally has collected randomly nanofiber. Randomly nanopores only provide permeability, but not topographical cues. For this reason, various attempts have been trying to manufacture nanofibers with aligned directionality (figure 4(c)(ii)). Highly aligned vertical and horizontal, and randomly oriented nanofibers on the single matrix induce the directionality and proliferation of neural cells. Randomly oriented nanofibers allow increasing mechanical properties [152]. This method is an effective strategy that can provide directional guidance at the nanoscale. It can be applied in various ways, such as directly jetting using a composite solution containing conductive materials [126] or coating the collected sheet after electrospinning [95, 153]. Either way, well-fabricated nanofibrous substrates could offer superior potential on PNR by neuronal expansion and topographical guide cues.
Simultaneous extrusion of the two materials using a dual nozzle allows a hollow shell structure by selective removing the core part (figure 4(d)(i)). This cost-effective and rapid construction method is a useful technique to control the inner channel by controlling the materials’ feed rate. However, since a thin nozzle is placed inside the outer nozzle, if the outer shell’s space becomes narrow, the fluid may not flow due to the rheological limitation. As a result, it may be challenging to manufacture fine-scaled conduits. Figure 4(d) (ii) shows that a conduit has a diameter of about 2.5 mm, and the shell part is composed of a pore structure for permeability. The electrical conductivity, swelling, degradation rate, mechanical properties, and cell proliferation ability can be controlled by the composition of the shell material [146]. In general, co-axial extrusion has the advantage of easily controlling the thickness of the shell according to the extrusion feed rate of the core and shell, but it is difficult to construct multiple channels. Therefore, it is suitable for manufacturing artificial blood vessels as well as NGC that requires a single channel. It will be exponential effect doubled when collaborating with electrospinning or 3D printing techniques.
Previous descriptions of traditional methods for manufacturing conduits are suitable for producing standardized simple lumen shapes. Therefore, it is challenging to build the freeform shapes of conduits or handle a complicated structure. Additive manufacturing allows us to construct tailored complex geometry for individual nerve injuries.
Recently, for the nozzle-based 3D plotting method, the extruded filament is deposited through the nozzle to consolidate while moving along the preprogrammed tool path (figure 4(e)(i)). This technique makes it possible to print cell-laden filaments so that more bioactive structures can be constructed. The extrusion-based 3D printing method has a low resolution because the depends on the thickness of the extruded filament through the nozzle. Attempts have been fabricated to develop the resolution by increasing the extrudability with high temperatures to facilitate extrusion [154]. However, there are still challenges to mimicking nerves.
Alternatively, the studies related to the manufacture of scaffolds using a stereolithography-based 3D printing technique have been started. Stereolithography-based printing can fabricate a fine multi-lumen architecture because of its higher resolution than extrusion-based 3D printing. This advanced approach aims at customized treatment by accurately scanning and rapidly fabricating damaged nerve areas. It suggests that it can be built as human nerve-sized with complex structures (figure 4(e)(ii) above) [155]. There are several biodegradable, 3D printable composite solutions for artificial nerve fabrication. The synthetics of photocurable copolymers and water-soluble hydrogel composite have opened a new process paradigm for constructing microchannels in artificial nerves. This presents the possibility of constructing a physical space of under 450 μm required for axon regeneration [156] unable to traditional fabrication methods. The conduit fabricated as shown in figure 4(e)(ii) below has sufficient flexibility and sufficient strength to resist compression modulus or sutures. A photo-initiator was added to produce a printable composite and reported unprecedented speed based on establishing a rapid continuous 3D direct light Pprocessing (DLP) printing platform [141]. Clinical application of 3D printed conduit upgrades fabrication technique and expands the scope of application of in vitro and in vivo platforms for personalized medical care for patients.
It has been reported to improve CNS regeneration via precisional medicine of biomimetic scaffolds such as the construction of 3D micro-architecture [157]. However, to our knowledge, there is no reports for manufacturing conductive conduits by stereolithography-based 3D printing by far. Therefore, this advanced manufacturing technique needs further study toward functional conduits for ES coupling, in terms of higher resolution and suitable properties for nerve bridges.
4. Integration of bioelectronic ES platform at the device level
Currently, it is limited to temporary and unsustainable stimulation through a wire-to-percutaneous electrode connection from an external power device. These percutaneous electrodes cannot be fully implanted, and the wires exposed to the outside of the skin may cause secondary infection after surgery, leading to a poor prognosis. Therefore, the needs of the fully fixation method are required for successful electroceuticals, which demands highly wireless power transfer. Here, we will introduce several wireless platforms, including energy harvesters from external power, actuators transmitting energy to tissue, and sensors for monitoring biosignals.
4.1. Energy harvester with wireless control
Recent implantable bioelectronic device research has focused on miniaturized and wireless energy transportation to operate devices battery-free because of the limited capacity of it. To facilitate operating battery-free devices, there is an in vivo energy harvester (IVEHS) that accumulates the energy generated in vivo such as piezoelectric, triboelectricity, automatic wristwatch, biofuel, endocochlear potential, optical energy [167-171]. However, these have poor output, conversion efficiency, and poor durability [172]. Therefore, we need transmission from a stable external power supply to an efficient wireless platform of in vivo devices, and there are several strategies; ultrasonic (figure 5(a)(i)), induced current by radiofrequency (RF) (figure 5(a)(ii)), and optogenetics (figure 5(a) (iii)).
Figure 5.
Integration between bioelectronics conduit and wireless platforms to efficient energy transmittance for improving neural regeneration. (a) Energy harvester with wireless control; (i) ultrasound-based wireless platform. © 2018 IEEE. Reprinted, with permission, from [158]. (ii) The induced current by radiofrequency (RF) signal from an external coil. Reproduced from [159], with permission from Springer Nature. (iii) Optogenetic wirelessly powered device by near-infrared (NIR) light. Reprinted from [160], Copyright (2021), with permission from Elsevier. (b) Actuator; (i) ultrasound-driven piezoelectric thin film nanogenerator. Reprinted from [161], Copyright (2021), with permission from Elsevier. (ii) Stretchable, bioresorbable electronic stimulator worked by induced current. Reproduced from [162], with permission from Springer Nature. (iii) Compact optical nerve cuff electrode for neural stimulation and monitoring. Reproduced from [163], with permission from Springer Nature. (c) Sensor; (i) nervous recording system with ultrasonic neural dust. Reprinted from [164], Copyright (2016), with permission from Elsevier. (ii) Long-term nerve impedance monitoring microsystem. © 2013 IEEE. Reprinted, with permission, from [165]. (iii) Multi-sites long-term recording electrodes. Reproduced from [166]. © IOP Publishing Ltd. All rights reserved.
Since the wireless stimulation devices are fully implanted nearby the sciatic nerve, they must be fabricated minimalize and flexible. Figure 5(a) presents illustrations of each energy harvester. They have an external power source external to the body and wirelessly harvest power from a receiver placed inside the body. Through optimal matching between the transmitter and receiver, the triggered energy is transmitted via the receiver to the actuator contacted with the sciatic nerve. All types of harvesters turned out to be feasible to harvest enough power to activate nerve stimulation. There is a miniature wireless peripheral nerve stimulator (6.5 mm3) called stim dust operated by ultrasonic. They succeeded in converting harvested ultrasonic waves with 82% peak chip efficiency, indicating that they can be operated with low power [158]. In particular, in the case of figure 4(a)(ii), all substances are composed of bioabsorbable materials and absorbed from the body, and released out of the body after full recovery with nerve stimulation [159]. In addition, near-infrared (NIR) light is a highly efficient energy source that is optically driven and controlled since it has high transmittance in biological tissues (655–900 nm). The flexible system Integration (SI) product validation (PV) arrays that build up the device generate power when illuminated by NIR light. At this time, the stimulation is activated by transmitting a signal to the connected optogenetic stimulator. They show that remote control can be efficiently delivered to optogenetic devices wirelessly through skin tissue [160].
Compared to IVEHS, these devices need to generate external power, but they are stable wireless communication systems with high efficiency electrically and functionally. Although it has the challenge of further extending the working distance in the future, these are innovative technologies that can allow long stimulation periods enabling the complete implantation of devices.
4.2. Actuator
The harvested energy could be delivered to the in vivo tissue in various forms. Research on implantable electroceuticals related to signal transmission at the nervous system and nerve regeneration through electrical signals is actively studied. Figure 5(b)(i) presents the piezoelectric thin film nanogenerator connected to the cuff for sciatic nerve stimulation. In general, the potential for stimulus is quite weak with harvested energy from the movement of the body or organs. Here, a programmable ultrasonic-driven stimulator combined with a battery-free thin-film nanogenerator for peripheral nerves was introduced to increase electric power. The piezoelectric thin film nanogenerator using ultrasound as an external energy source successfully achieved direct ES of the sciatic nerve in mice [161].
In the Choi et al study, a flexible expansion electrode and a targeted peripheral nerve target device were constructed by connecting RF power harvester that receives electricity from an external induction coil (figure 5(b)(ii)). It is encapsulated with bioabsorbable dynamic covalent polyurethane, which has excellent mechanical elasticity for in-vivo movement and provides minimal expansion, enabling powerful operation without limiting its working lifetime. As for the electrical interface, the exposed Mo electrodes surrounding the nerve were connected to the PLGA tubular conduit to perform nerve stimulation efficiently. Energy harvester, electrode, and conduit are all absorbent materials, and after the initial ES treatment, they completely decomposed in the body. Unlike the previously known concept of proximal nerve stimulation for regeneration, this bio-device prove a beneficial effect applied to the distal part of the damaged nerve to improve muscle strength and function and increase peripheral blood to recover nerve damage [162].
Optogenetic stimulation of the peripheral nervous system is a novel approach to motor control, sensory transmission, and pain block. However, it needs to be careful about the temperature change during optical stimulation. When the temperature increases, partial heat causes block of nerve signals, which can lead to irreversible nerve damage [173]. Figure 5(b)(iii) shows an optical nerve device for both optogenetic stimulation and synchronous monitoring of peripheral nerve neural activity using a single cuff electrode. The role of optogenetics is to stimulate sensitive genes with specific wavelengths of light optically. The increasing temperature during optical stimulation led to fatalities, but this actuator was found not to affect nerve tissue damage during signal recording with changes only in the range of 24 °C–26 °C. The device was suitable for monitoring neural activity and optical stimulation in transgenic mice [163].
4.3. Sensor
A wireless system that integrates with external power supply and internal energy harvester is evolving into in situ sensors to monitor vital signs beyond the stage of tissue stimulation. This is to observe how to interpret and control the electrophysiological activity of the body to prove the therapeutic effect in the target tissue. In future work, it is expected to develop into a patient-customized medical device capable of nerve stimulation and sensing simultaneously.
Seo et al show the design of a miniature receiver device working by ultrasound system (figure 5(c) (i)). This neural sensor recorded the electromyogram (EMG) response elicited from the gastrocnemius muscle in rats. The data were recorded for 20 ms around the stimulation window through stimulation with 200 μs duration and 6 s pulse. Monitoring could be continued indefinitely on the anesthesia, and there was no deterioration in the quality of the recordings even after continuous measurements for 30 min. Also, the difference between wireless and wired data was within ±0.4 mV, and the minimum signal detected by the sensor was about 0.25 mV. They proved that ultrasound effectively delivered energy to mm-scale devices through high-fidelity electromy-gram and electroneurogram signals [164].
Here, the Li group manufactured a neural impedance sensor for long-term monitoring of regeneration status for 42 d (figure 5(c)(ii)). Specifically, an implantable microsystem was inserted to observe the time-lapse variation of nerve impedance after wrapping the cuff electrode in an 8 mm injured sciatic nerve rat model. This system resulted in increasing myelin fiber density by offering long-term stimulation to accelerate nerve fiber growth as well as impedance evaluation to understanding as time goes by nerve regeneration. Continuous muscle stimulation can lead to better functional neural connections to reduce muscle atrophy and improve functional recovery [165].
The Vasudevan group further improved long-term monitoring by acquiring area mapping records with 16 sites records (figure 5(c) (iii)). They evaluated the changes in neural function through weekly in vivo impedance measurements and recordings. Although it was possible to achieve low-yield neural recordings of action potentials by floating microelectrode arrays in PNS, long-term recording performance was limited due to lead wire failure. After implantation, structural problems in which significant damage to the electrode and insulator, limiting continued reliability, but it is still expected to broadly expand the scope of application in the future by enabling evaluation and monitoring of the performance characteristics of neural arrays [166].
4.4. Optimize the therapeutic parameter
Despite the excellent neuro-regenerative effects of non-pharmacological electroceuticals, we still have not found a comprehensive and clinical therapeutic option. In the regeneration of damaged nerves, the degree of regenerative ability differs from the size and type of defect, location, age, and sex. Therefore, it is difficult to explain the set as ‘standard’ as the parameter of 1 h, 20 Hz, 0.1 ms for various conditions [85]. So far, evaluation is being performed under numerous healthy SD rats rather than clinical considerations. We need systematically strict investigation with more detailed conditions through multiple simple models. We may need the help of biocomputational tools for optimizing ES parameters and device design using artificial intelligence and machine learning in the future. But, this is a challenging issue because it needs numerous training samples are required for the utilization of deep learning applications. One of the approaches is to collect training data from computer simulation experiments. Another one might be meta-learning which learns to quickly adapt to a new environment with just a few samples [174]. Ultimately, optimizing parameters is a ‘key factor’ for boosting the therapeutic effect and applying clinical application.
5. Challenges and future works
Despite the remarkable potential of conductive NGCs to deliver ES for nerve regeneration, there is few optimal guideline of electroceuticals. As shown in table 1, the electrical properties (e.g. conductivity, impedance, resistance) employed in each research vary extensively. The biological reaction at nerveconduit interface might also increase the conductive variation, such as impedance and biofluid acumination. In addition, the evaluation of the electroreaction to the disrupted nerve being connected to the NGCs is not standardized. Furthermore, it should be recognized that the surface of NGCs is exposed to the bio-fluid directly, which does not fully deliver charges by causing electrical leakage in in vivo environment. Actual axons are encapsulated by an insulator called the myelin sheath, which increase neural activity of axon and prevent the leakage of electric signal. However, most conductive conduits are designed without this precise structure, which may cause a decrease in the efficiency of ES. In addition, the technical limitations of fabricating inner channels in a micro-scale allows only single-lumen type structures as nerve bridges, rather than multichannel structure. Researches on optimal pore size and channel structure through material combination and fabrication process are also important factors to increase mass transportation of nutrients and oxygen which are essentials for nerve regeneration. In a long-term implantation to mimic severe nerve injuries, the large nerve gap (>10 mm) requires sustainable mechanical and electrical properties during the designed degradation lifetime. The conductive and bioresorbable materials, including conductive hydrogels within the hollow NGCs should be carefully evaluated to prove the complete biosafety and biocompatibility, according to well-established protocol.
From the clinical perspective, the bioelectronic platform should be biocompatible, bioresorbable, miniaturized, and with ease of implantation under minimal invasive procedures. Different from other bioelectronic device designed for brain or spine, strong mechanical sustainability with stretchable property is crucial for peripheral nerve system, owning to its nature of high degree of mobility and pressure loading. Considering about the possibility of incomplete nerve regeneration or the formation of neuroma-in-continuity at the critical nerve gap, the detection of nerve action potential via implantable bioelectronic device can bring decisive information for the progress of nerve regeneration. In order to further investigate the underlying mechanisms of ES on PNR, several advanced technology can be expect to explore the full spectrum of peripheral nerve system, such as (a) single cell RNA sequencing for better understanding of cell responses, cell-cell interactions with unprecedented spatial and temporal resolutions; (b) biocomputational tools such as artificial intelligence and machine learning to help optimize the therapeutic parameters and device design. Furthermore, the versatile adjustment of the dosage of electroceuticals according to real-time nerve sensing will open a new era of precisional medicine as theranostic bioelectronic platform for electroceuticals in the future.
6. Conclusions
Both bio-engineered nerve conduit or electroceuticals has shown beneficial evidences in PNR for 20 years. Recent advances on biomaterials empower the modern bioresorbable and conductive nerve conduit, which enables to deliver versatile therapeutic electroceuticals. The biofabrication to integrate conductive nerve conduits with ES platform can offer versatile stimulation dosage, depending on the injury type, timing and progress of nerve regeneration. The modern bioelectronic platform for electroceuticals integrates the cutting-edge technology of tissue engineering and biofabrication to develop the future theranostic bioelectronic devices for regenerative precision medicine.
Acknowledgments
The authors are grateful to Professor Wentai Liu for the knowledge background regarding electrode design and electrical stimulation delivery, under the support and collaboration of Higher Education Sprout Project, Ministry of Education to the Headquarters of University Advancement at National Cheng Kung University. The authors would like to thank the financial support provided by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2020R1A6A3A01100464 to W Y M; NRF-2021R1A2C2094799 to J K). S L is supported by a grant from the National Institute of Health (USA) (R01NS126918). Y Y H is supported by National Science and Technology Council (NSTC) of Taiwan (NSTC-110-2628-B-006-036; NSTC-111-2311-B-006-001; NSTC-111-2923-B-006-001-MY2).
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
References
- [1].Hussain G et al. 2020. Current status of therapeutic approaches against peripheral nerve injuries: a detailed story from injury to recovery Int. J. Biol. Sci 16 116–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Noble J, Munro CA, Prasad VS and Midha R 1998. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries J. Trauma 45 116–22 [DOI] [PubMed] [Google Scholar]
- [3].Bray GM and Huggett DL 2016. Neurological diseases, disorders and injuries in Canada: highlights of a national study Can. J. Neurol. Sci 43 5–14 [DOI] [PubMed] [Google Scholar]
- [4].Mahar M and Cavalli V 2018. Intrinsic mechanisms of neuronal axon regeneration Nat. Rev. Neurosci 19 323–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pfister BJ, Gordon T, Loverde JR, Kochar AS, Mackinnon SE and Cullen DK 2011. Biomedical engineering strategies for peripheral nerve repair: surgical applications, state of the art, and future challenges Crit. Rev. Biomed. Eng 39 81–124 [DOI] [PubMed] [Google Scholar]
- [6].Gordon T 2016. Nerve regeneration in the peripheral and central nervous systems J. Physiol 594 3517–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Martin SL, Reid AJ, Verkhratsky A, Magnaghi V and Faroni A 2019. Gene expression changes in dorsal root ganglia following peripheral nerve injury: roles in inflammation, cell death and nociception Neural Regen. Res 14 939–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lee SK and Wolfe SW 2000. Peripheral nerve injury and repair J. Am. Acad. Orthop. Surg 8 243–52 [DOI] [PubMed] [Google Scholar]
- [9].Ruijs AC, Jaquet JB, Kalmijn S, Giele H and Hovius SE 2005. Median and ulnar nerve injuries: a meta-analysis of predictors of motor and sensory recovery after modern microsurgical nerve repair Plast. Reconstr. Surg 116 484–94 (discussion 495-6) [DOI] [PubMed] [Google Scholar]
- [10].Dahlin LB 2013. The role of timing in nerve reconstruction Int. Rev. Neurobiol 109 151–64 [DOI] [PubMed] [Google Scholar]
- [11].Grinsell D and Keating CP 2014. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies Biomed. Res. Int 2014 698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kubiak CA, Kung TA, Brown DL, Cederna PS and Kemp SWP 2018. State-of-the-art techniques in treating peripheral nerve injury Plast. Reconstr. Surg 141 702–10 [DOI] [PubMed] [Google Scholar]
- [13].Ramachandran S and Midha R 2019. Recent advances in nerve repair Neurol India 67 S106–14 [DOI] [PubMed] [Google Scholar]
- [14].Beris A, Gkiatas I, Gelalis I, Papadopoulos D and Kostas-Agnantis I 2019. Current concepts in peripheral nerve surgery Eur. J. Orthop. Surg. Traumatol 29 263–9 [DOI] [PubMed] [Google Scholar]
- [15].Trehan SK, Model Z and Lee SK 2016. Nerve repair and nerve grafting Hand Clin. 32 119–25 [DOI] [PubMed] [Google Scholar]
- [16].Li R, Liu Z, Pan Y, Chen L, Zhang Z and Lu L 2014. Peripheral nerve injuries treatment: a systematic review Cell Biochem. Biophys 68 449–54 [DOI] [PubMed] [Google Scholar]
- [17].Giuffre JL, Bishop AT, Spinner RJ and Shin AY 2015. The best of tendon and nerve transfers in the upper extremity Plast. Reconstr. Surg 135 617e–30e [DOI] [PubMed] [Google Scholar]
- [18].Rinker B 2015. Nerve transfers in the upper extremity: a practical user’s guide Ann. Plast. Surg 74 S222–8 [DOI] [PubMed] [Google Scholar]
- [19].Biscola NP, Cartarozzi LP, Ulian-Benitez S, Barbizan R, Castro MV, Spejo AB, Ferreira RS Jr, Barraviera B and Oliveira ALR 2017. Multiple uses of fibrin sealant for nervous system treatment following injury and disease J. Venom. Anim. Toxins Incl. Trop. Dis 23 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Anderson M, Shelke NB, Manoukian OS, Yu X, McCullough LD and Kumbar SG 2015. Peripheral nerve regeneration strategies: electrically stimulating polymer based nerve growth conduits Crit. Rev. Biomed. Eng 43 131–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Carvalho CR, Oliveira JM and Reis RL 2019. Modern trends for peripheral nerve repair and regeneration: beyond the hollow nerve guidance conduit Front. Bioeng. Biotechnol 7 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fornasari BE, Carta G, Gambarotta G and Raimondo S 2020. Natural-based biomaterials for peripheral nerve injury repair Front. Bioeng. Biotechnol 8 554257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Huang ML, Zhai Z, Chen ZX, Yang XN and Qi ZL 2020. Platelet-rich fibrin membrane nerve guidance conduit: a potentially promising method for peripheral nerve injuries Chin. Med. J 133 999–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jiang H, Qian Y, Fan C and Ouyang Y 2020. Polymeric guide conduits for peripheral nerve tissue engineering Front. Bioeng. Biotechnol 8 582646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sarker MD, Naghieh S, McInnes AD, Schreyer DJ and Chen X 2018. Regeneration of peripheral nerves by nerve guidance conduits: influence of design, biopolymers, cells, growth factors, and physical stimuli Prog. Neurobiol 171 125–50 [DOI] [PubMed] [Google Scholar]
- [26].Soman SS and Vijayavenkataraman S 2020. Perspectives on 3D bioprinting of peripheral nerve conduits J. Mol. Sci. (Int. Ed.) 21 5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Song S, Wang X, Wang T, Yu Q, Hou Z, Zhu Z and Li R 2020. Additive manufacturing of nerve guidance conduits for regeneration of injured peripheral nerves Front. Bioeng. Biotechnol 8 590596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Stewart CE, Kan CFK, Stewart BR, Sanicola HW 3rd, Jung JP, Sulaiman OAR and Wang D 2020. Machine intelligence for nerve conduit design and production J. Biol. Eng 14 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Vijayavenkataraman S 2020. Nerve guide conduits for peripheral nerve injury repair: a review on design, materials and fabrication methods Acta Biomater. 106 54–69 [DOI] [PubMed] [Google Scholar]
- [30].Zhu Y, Wang A, Patel S, Kurpinski K, Diao E, Bao X, Kwong G, Young WL and Li S 2011. Engineering bi-layer nanofibrous conduits for peripheral nerve regeneration Tissue Eng. C 17 705–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li R, Li DH, Zhang HY, Wang J, Li XK and Xiao J 2020. Growth factors-based therapeutic strategies and their underlying signaling mechanisms for peripheral nerve regeneration Acta Pharmacol. Sin 41 1289–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sayad Fathi S and Zaminy A 2017. Stem cell therapy for nerve injury World J. Stem Cells 9 144–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wang A, Tang Z, Park IH, Zhu Y, Patel S, Daley GQ and Li S 2011. Induced pluripotent stem cells for neural tissue engineering Biomaterials 32 5023–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wong JN, Olson JL, Morhart MJ and Chan KM 2015. Electrical stimulation enhances sensory recovery: a randomized controlled trial Ann. Neurol 77 996–1006 [DOI] [PubMed] [Google Scholar]
- [35].Gordon T 2016. Electrical stimulation to enhance axon regeneration after peripheral nerve injuries in animal models and humans Neurotherapeutics 13 295–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fang Z, Ge X, Chen X, Xu Y, Yuan WE and Ouyang Y 2020. Enhancement of sciatic nerve regeneration with dual delivery of vascular endothelial growth factor and nerve growth factor genes J. Nanobiotechnol 18 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].ElAbd R, Alabdulkarim A, AlSabah S, Hazan J, Alhalabi B and Thibaudeau S 2022. Role of electrical stimulation in peripheral nerve regeneration: a systematic review Plast. Reconstr. Surg. Glob. Open 10 e4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Xia B and Lv Y 2018. Dual-delivery of VEGF and NGF by emulsion electrospun nanofibrous scaffold for peripheral nerve regeneration Mater. Sci. Eng. C 82 253–64 [DOI] [PubMed] [Google Scholar]
- [39].Famm K, Litt B, Tracey KJ, Boyden ES and Slaoui M 2013. Drug discovery: a jump-start for electroceuticals Nature 496 159–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Long Y, Li J, Yang F, Wang J and Wang X 2021. Wearable and implantable electroceuticals for therapeutic electrostimulations Adv. Sci 8 2004023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Serruya MD and Rosenwasser RH 2021. An artificial nervous system to treat chronic stroke Artif. Organs 45 804–12 [DOI] [PubMed] [Google Scholar]
- [42].Slater C and Wang Q 2021. Alzheimer’s disease: an evolving understanding of noradrenergic involvement and the promising future of electroceutical therapies Clin. Transl. Med 11 e397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dirr EW, Urdaneta ME, Patel Y, Johnson RD, Campbell-Thompson M and Otto KJ 2020. Designing a bioelectronic treatment for type 1 diabetes: targeted parasympathetic modulation of insulin secretion Bioelectron. Med 3 17–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bhar B, Chouhan D, Pai N and Mandal BB 2021. Harnessing multifaceted next-generation technologies for improved skin wound healing ACS Appl. Bio. Mater 4 7738–63 [DOI] [PubMed] [Google Scholar]
- [45].Mishra S 2017. Electroceuticals in medicine—the brave new future Indian Heart J. 69 685–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ramadi KB, Srinivasan SS and Traverso G 2020. Electroceuticals in the gastrointestinal tract Trends Pharmacol. Sci 41 960–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Levin M 2021. Bioelectric signaling: reprogrammable circuits underlying embryogenesis, regeneration, and cancer Cell 184 1971–89 [DOI] [PubMed] [Google Scholar]
- [48].Willand MP 2015. Electrical stimulation enhances reinnervation after nerve injury Eur. J. Transl. Myol 25 243–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chan KM, Curran MW and Gordon T 2016. The use of brief post-surgical low frequency electrical stimulation to enhance nerve regeneration in clinical practice J. Physiol 594 3553–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Carroll SL and Worley SH 2017. Wallerian Degeneration Reference Module in Neuroscience and Biobehavioral Psychology (Amsterdam: Elsevier; ) 485–91 [Google Scholar]
- [51].Conforti L, Gilley J and Coleman MP 2014. Wallerian degeneration: an emerging axon death pathway linking injury and disease Nat. Rev. Neurosci 15 394–409 [DOI] [PubMed] [Google Scholar]
- [52].Cattin AL and Lloyd AC 2016. The multicellular complexity of peripheral nerve regeneration Curr. Opin. Neurobiol 39 38–46 [DOI] [PubMed] [Google Scholar]
- [53].Navarro X, Vivó M and Valero-Cabré A 2007. Neural plasticity after peripheral nerve injury and regeneration Prog. Neurobiol 82 163–201 [DOI] [PubMed] [Google Scholar]
- [54].Zhang J, Liu Y and Lu L 2018. Emerging role of microRNAs in peripheral nerve system Life Set. 207 227–33 [DOI] [PubMed] [Google Scholar]
- [55].Kilgore KL and Bhadra N 2014. Reversible nerve conduction block using kilohertz frequency alternating current Neuromodulation 17 242–54 (discussion 254-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Bhadra N, Vrabec TL, Bhadra N and Kilgore KL 2018. Reversible conduction block in peripheral nerve using electrical waveforms Bioelectron. Med 1 39–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ferrigno B, Bordett R, Duraisamy N, Moskow J, Arul MR, Rudraiah S, Nukavarapu SP, Vella AT and Kumbar SG 2020. Bioactive polymeric materials and electrical stimulation strategies for musculoskeletal tissue repair and regeneration Bioact. Mater 5 468–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Gordon T. Peripheral nerve regeneration and muscle reinnervation. J. Mol. Sci. (Int. Ed.) 2020;21:8652. doi: 10.3390/ijms21228652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ransom SC, Shahrestani S, Lien BV, Tafreshi AR, Brown NJ, Hanst B, Lehrich BM, Ransom RC and Sahyouni R 2020. Translational approaches to electrical stimulation for peripheral nerve regeneration Neurorehabil. Neural Repair 34 979–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gordon T and English AW 2016. Strategies to promote peripheral nerve regeneration: electrical stimulation and/or exercise Eur. J. Neurosci 43 336–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Willand MP, Nguyen MA, Borschel GH and Gordon T 2016. Electrical stimulation to promote peripheral nerve regeneration Neurorehabil. Neural Repair 30 490–6 [DOI] [PubMed] [Google Scholar]
- [62].Modrak M, Talukder MAH, Gurgenashvili K, Noble M and Elfar JC 2020. Peripheral nerve injury and myelination: potential therapeutic strategies J. Neurosci. Res 98 780–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Al-Majed AA, Brushart TM and Gordon T 2000. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons Eur. J. Neurosci 12 4381–90 [PubMed] [Google Scholar]
- [64].Gordon T, Amirjani N, Edwards DC and Chan KM 2010. Brief post-surgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel syndrome patients Exp. Neurol 223 192–202 [DOI] [PubMed] [Google Scholar]
- [65].Roh J, Schellhardt L, Keane GC, Hunter DA, Moore AM, Snyder-Warwick AK, Mackinnon SE and Wood MD 2022. Short-duration, pulsatile, electrical stimulation therapy accelerates axon regeneration and recovery following tibial nerve injury and repair in rats Plast. Reconstr. Surg 149 681e–90e [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Haastert-Talini K, Schmitte R, Korte N, Klode D, Ratzka A and Grothe C 2011. Electrical stimulation accelerates axonal and functional peripheral nerve regeneration across long gaps J. Neurotrauma 28 661–74 [DOI] [PubMed] [Google Scholar]
- [67].Udina E, Ladak A, Furey M, Brushart T, Tyreman N and Gordon T 2010. Rolipram-induced elevation of cAMP or chondroitinase ABC breakdown of inhibitory proteoglycans in the extracellular matrix promotes peripheral nerve regeneration Exp. Neurol 223 143–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].McGregor CE and English AW 2018. The role of BDNF in peripheral nerve regeneration: activity-dependent treatments and Val66Met Front. Cell Neurosci 12 522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Chu XL, Song XZ, Li Q, Li YR, He F, Gu XS and Ming D 2022. Basic mechanisms of peripheral nerve injury and treatment via electrical stimulation Neural Regen. Res 17 2185–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].English AW, Schwartz G, Meador W, Sabatier MJ and Mulligan A 2007. Electrical stimulation promotes peripheral axon regeneration by enhanced neuronal neurotrophin signaling Dev. Neurobiol 67 158–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Al-Majed AA, Tam SL and Gordon T 2004. Electrical stimulation accelerates and enhances expression of regeneration-associated genes in regenerating rat femoral motoneurons Cell. Mol. Neurobiol 24 379–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Geremia NM, Gordon T, Brushart TM, Al-Majed AA and Verge VM 2007. Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression Exp. Neurol 205 347–59 [DOI] [PubMed] [Google Scholar]
- [73].Huang J, Ye Z, Hu X, Lu L and Luo Z 2010. Electrical stimulation induces calcium-dependent release of NGF from cultured Schwann cells Glia 58 622–31 [DOI] [PubMed] [Google Scholar]
- [74].Kawamura K and Kano Y 2019. Electrical stimulation induces neurite outgrowth in PC12m3 cells via the p38 mitogen-activated protein kinase pathway Neurosci. Lett 698 81–84 [DOI] [PubMed] [Google Scholar]
- [75].Barber B. et al. Intraoperative brief electrical stimulation of the spinal accessory nerve (BEST SPIN) for prevention of shoulder dysfunction after oncologic neck dissection: a double-blinded, randomized controlled trial. J. Otolaryngol. Head Neck Surg. 2018;47:7. doi: 10.1186/s40463-017-0244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Power HA, Morhart MJ, Olson JL and Chan KM 2020. Postsurgical electrical stimulation enhances recovery following surgery for severe cubital tunnel syndrome: a double-blind randomized controlled trial Neurosurgery 86 769–77 [DOI] [PubMed] [Google Scholar]
- [77].Nix WA and Hopf HC 1983. Electrical stimulation of regenerating nerve and its effect on motor recovery Brain Res. 272 21–25 [DOI] [PubMed] [Google Scholar]
- [78].Pockett S and Gavin RM 1985. Acceleration of peripheral nerve regeneration after crush injury in rat Neurosci. Lett 59 221–4 [DOI] [PubMed] [Google Scholar]
- [79].Al-Majed AA, Neumann CM, Brushart TM and Gordon T 2000. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration J. Neurosci 20 2602–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Brushart TM, Hoffman PN, Royall RM, Murinson BB, Witzel C and Gordon T 2002. Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron J. Neurosci 22 6631–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Singh B, Xu QG, Franz CK, Zhang R, Dalton C, Gordon T, Verge VM, Midha R and Zochodne DW 2012. Accelerated axon outgrowth, guidance, and target reinnervation across nerve transection gaps following a brief electrical stimulation paradigm J. Neurosurg 116 498–512 [DOI] [PubMed] [Google Scholar]
- [82].Ahlborn P, Schachner M and Irintchev A 2007. One hour electrical stimulation accelerates functional recovery after femoral nerve repair Exp. Neurol 208 137–44 [DOI] [PubMed] [Google Scholar]
- [83].Elzinga K, Tyreman N, Ladak A, Savaryn B, Olson J and Gordon T 2015. Brief electrical stimulation improves nerve regeneration after delayed repair in Sprague Dawley rats Exp. Neurol 269 142–53 [DOI] [PubMed] [Google Scholar]
- [84].Choi YS. et al. Stretchable, dynamic covalent polymers for soft, long-lived bioresorbable electronic stimulators designed to facilitate neuromuscular regeneration. Nat. Commun. 2020;11:5990. doi: 10.1038/s41467-020-19660-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Zuo KJ, Gordon T, Chan KM and Borschel GH 2020. Electrical stimulation to enhance peripheral nerve regeneration: update in molecular investigations and clinical translation Exp. Neurol 332 113397. [DOI] [PubMed] [Google Scholar]
- [86].Nezakati T, Seifalian A, Tan A and Seifalian AM 2018. Conductive polymers: opportunities and challenges in biomedical applications Chem. Rev 118 6766–843 [DOI] [PubMed] [Google Scholar]
- [87].Someya T, Bao Z and Malliaras GG 2016. The rise of plastic bioelectronics Nature 540 379–85 [DOI] [PubMed] [Google Scholar]
- [88].Koo J et al. 2018. Wireless bioresorbable electronic system enables sustained nonpharmacological neuroregenerative therapy Nat. Med. 24 1830–6 [DOI] [PubMed] [Google Scholar]
- [89].Wu P, Zhao Y, Chen F, Xiao A, Du Q, Dong Q, Ke M, Liang X, Zhou Q and Chen Y 2020. Conductive hydroxyethyl cellulose/soy protein isolate/polyaniline conduits for enhancing peripheral nerve regeneration via electrical stimulation Front. Bioeng. Biotechnol 8 709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Huang J, Lu L, Zhang J, Hu X, Zhang Y, Liang W, Wu S and Luo Z 2012. Electrical stimulation to conductive scaffold promotes axonal regeneration and remyelination in a rat model of large nerve defect PLoS One 7 e39526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Larson CE and Meng E 2020. A review for the peripheral nerve interface designer J. Neurosci. Methods 332 108523. [DOI] [PubMed] [Google Scholar]
- [92].Burnstine-Townley A, Eshel Y and Amdursky N 2020. Conductive scaffolds for cardiac and neuronal tissue engineering: governing factors and mechanisms Adv. Funct. Mater 30 1901369 [Google Scholar]
- [93].Gordon T, Sulaiman OA and Ladak A 2009. Electrical stimulation for improving nerve regeneration: where do we stand? Int. Rev. Neurobiol 87 433–44 [DOI] [PubMed] [Google Scholar]
- [94].Zuo KJ, Shafa G, Antonyshyn K, Chan K, Gordon T and Borschel GH 2020. A single session of brief electrical stimulation enhances axon regeneration through nerve autografts Exp. Neurol 323 113074. [DOI] [PubMed] [Google Scholar]
- [95].Song J, Sun B, Liu S, Chen W, Zhang Y, Wang C, Mo X, Che J, Ouyang Y and Yuan W 2016. Polymerizing pyrrole coated poly (l-lactic acid-co-ε-caprolactone)(PLCL) conductive nanofibrous conduit combined with electric stimulation for long-range peripheral nerve regeneration Front. Mol. Neurosci 9 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Lee JY, Bashur CA, Goldstein AS and Schmidt CE 2009. Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications Biomaterials 30 4325–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Moroder P, Runge MB, Wang H, Ruesink T, Lu L, Spinner RJ, Windebank AJ and Yaszemski MJ 2011. Material properties and electrical stimulation regimens of polycaprolactone fumarate–polypyrrole scaffolds as potential conductive nerve conduits Acta Biomater. 7 944–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Zhao Y, Liang Y, Ding S, Zhang K, Mao H-Q and Yang Y 2020. Application of conductive PPy/SF composite scaffold and electrical stimulation for neural tissue engineering Biomaterials 255 120164. [DOI] [PubMed] [Google Scholar]
- [99].Xu H, Holzwarth JM, Yan Y, Xu P, Zheng H, Yin Y, Li S and Ma PX 2014. Conductive PPY/PDLLA conduit for peripheral nerve regeneration Biomaterials 35 225–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Sun Y, Quan Q, Meng H, Zheng Y, Peng J, Hu Y, Feng Z, Sang X, Qiao K and He W 2019. Enhanced neurite outgrowth on a multiblock conductive nerve scaffold with self-powered electrical stimulation Adv. Healthcare Mater 8 1900127. [DOI] [PubMed] [Google Scholar]
- [101].Durgam H, Sapp S, Deister C, Khaing Z, Chang E, Luebben S and Schmidt CE 2010. Novel degradable co-polymers of polypyrrole support cell proliferation and enhance neurite out-growth with electrical stimulation J. Biomater. Sci. Polym. Ed 21 1265–82 [DOI] [PubMed] [Google Scholar]
- [102].Bu Y, Xu H-X, Li X, Xu W-J, Yin Y-X, Dai H-L, Wang X-B, Huang Z-J and Xu P-H 2018. A conductive sodium alginate and carboxymethyl chitosan hydrogel doped with polypyrrole for peripheral nerve regeneration RSC Adv. 8 10806–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH and Ramakrishna S 2009. Electrical stimulation of nerve cells using conductive nanofibrous scaffolds for nerve tissue engineering Tissue Eng. A 15 3605–19 [DOI] [PubMed] [Google Scholar]
- [104].Li X, Yang W, Xie H, Wang J, Zhang L, Wang Z and Wang L 2020. A CNTs/sericin conductive nerve guidance conduit promotes functional recovery of transected peripheral nerve injury in a rat model ACS Appl. Mater. Interfaces 12 36860–72 [DOI] [PubMed] [Google Scholar]
- [105].Mottaghitalab F, Farokhi M, Zaminy A, Kokabi M, Soleimani M, Mirahmadi F, Shokrgozar MA and Sadeghizadeh M 2013. A biosynthetic nerve guide conduit based on silk/SWNT/fibronectin nanocomposite for peripheral nerve regeneration PLoS One 8 e74417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Park J, Jeon J, Kim B, Lee MS, Park S, Lim J, Yi J, Lee H, Yang HS and Lee JY 2020. Electrically conductive hydrogel nerve guidance conduits for peripheral nerve regeneration Adv. Funct. Mater 30 2003759 [Google Scholar]
- [107].Wang J, Cheng Y, Chen L, Zhu T, Ye K, Jia C, Wang H, Zhu M, Fan C and Mo X 2019. In vitro and in vivo studies of electroactive reduced graphene oxide-modified nanofiber scaffolds for peripheral nerve regeneration Acta Biomater. 84 98–113 [DOI] [PubMed] [Google Scholar]
- [108].Wu S, Qi Y, Shi W, Kuss M, Chen S and Duan B 2020. Electrospun conductive nanofiber yarns for accelerating mesenchymal stem cells differentiation and maturation into Schwann cell-like cells under a combination of electrical stimulation and chemical induction Acta Biomater. 139 91–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Nan L-P, Lin Z, Wang F, Jin X-H, Fang J-Q, Xu B, Liu S-H, Zhang F, Wu Z and Zhou Z-F 2022. Ti3C2Tx MXene-coated electrospun PCL conduits for enhancing neurite regeneration and angiogenesis Front. Bioeng. Biotechnol 10 850650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Hu Y, Chen Z, Wang H, Guo J, Cai J, Chen X, Wei H, Qi J, Wang Q and Liu H 2022. Conductive nerve guidance conduits based on morpho butterfly wings for peripheral nerve repair ACS Nano 16 1868–79 [DOI] [PubMed] [Google Scholar]
- [111].Mao R, Yu B, Cui J, Wang Z, Huang X, Yu H, Lin K and Shen SG 2022. Piezoelectric stimulation from electrospun composite nanofibers for rapid peripheral nerve regeneration Nano Energy 98 107322 [Google Scholar]
- [112].Wang L, Lu C, Yang S, Sun P, Wang Y, Guan Y, Liu S, Cheng D, Meng H and Wang Q 2020. A fully biodegradable and self-electrified device for neuroregenerative medicine Sci. Adv 6 eabc6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Zarrintaj P, Zangene E, Manouchehri S, Amirabad LM, Baheiraei N, Hadjighasem MR, Farokhi M, Ganjali MR, Walker BW and Saeb MR 2020. Conductive biomaterials as nerve conduits: recent advances and future challenges Appl. Mater. Today 20 100784 [Google Scholar]
- [114].Zhu R, Sun Z, Li C, Ramakrishna S, Chiu K and He L 2019. Electrical stimulation affects neural stem cell fate and function in vitro Exp. Neurol 319 112963. [DOI] [PubMed] [Google Scholar]
- [115].Stoll G and Müller HW 1999. Nerve injury, axonal degeneration and neural regeneration: basic insights Brain Pathol. 9 313–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Bhatheja K and Field J 2006. Schwann cells: origins and role in axonal maintenance and regeneration Int. J. Biochem. Cell Biol 38 1995–9 [DOI] [PubMed] [Google Scholar]
- [117].Pires F, Ferreira Q, Rodrigues CA, Morgado J and Ferreira FC 2015. Neural stem cell differentiation by electrical stimulation using a cross-linked PEDOT substrate: expanding the use of biocompatible conjugated conductive polymers for neural tissue engineering Biochim. Biophys. Acta 1850 1158–68 [DOI] [PubMed] [Google Scholar]
- [118].Balint R, Cassidy NJ and Cartmell SH 2014. Conductive polymers: towards a smart biomaterial for tissue engineering Acta Biomater. 10 2341–53 [DOI] [PubMed] [Google Scholar]
- [119].Kaur G, Adhikari R, Cass P, Bown M and Gunatillake P 2015. Electrically conductive polymers and composites for biomedical applications RSC Adv. 5 37553–67 [Google Scholar]
- [120].Huang J, Hu X, Lu L, Ye Z, Zhang Q and Luo Z 2010. Electrical regulation of Schwann cells using conductive polypyrrole/chitosan polymers J. Biomed. Mater. Res. A 93 164–74 [DOI] [PubMed] [Google Scholar]
- [121].Runge MB, Dadsetan M, Baltrusaitis J, Knight AM, Ruesink T, Lazcano EA, Lu L, Windebank AJ and Yaszemski MJ 2010. The development of electrically conductive polycaprolactone fumarate–polypyrrole composite materials for nerve regeneration Biomaterials 31 5916–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Wang G, Wu W, Yang H, Zhang P and Wang JY 2020. Intact polyaniline coating as a conductive guidance is beneficial to repairing sciatic nerve injury J. Biomed. Mater. Res. B 108 128–42 [DOI] [PubMed] [Google Scholar]
- [123].Prabhakaran MP, Ghasemi-Mobarakeh L, Jin G and Ramakrishna S 2011. Electrospun conducting polymer nanofibers and electrical stimulation of nerve stem cells J. Biosci. Bioeng 112 501–7 [DOI] [PubMed] [Google Scholar]
- [124].Shahini A, Yazdimamaghani M, Walker KJ, Eastman MA, Hatami-Marbini H, Smith BJ, Ricci JL, Madihally SV, Vashaee D and Tayebi L 2014. 3D conductive nanocomposite scaffold for bone tissue engineering Int. J. Nanomed 9 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Tosun Z and McFetridge P 2010. A composite SWNT–collagen matrix: characterization and preliminary assessment as a conductive peripheral nerve regeneration matrix J. Neural. Eng 7 066002. [DOI] [PubMed] [Google Scholar]
- [126].Hu X, Wang X, Xu Y, Li L, Liu J, He Y, Zou Y, Yu L, Qiu X and Guo J 2020. Electric conductivity on aligned nanofibers facilitates the transdifferentiation of mesenchymal stem cells into Schwann cells and regeneration of injured peripheral nerve Adv. Healthcare Mater 9 1901570. [DOI] [PubMed] [Google Scholar]
- [127].Bosi S, Fabbro A, Ballerini L and Prato M 2013. Carbon nanotubes: a promise for nerve tissue engineering? Nanotechnol. Rev 2 47–57 [Google Scholar]
- [128].Saito N, Haniu H, Usui Y, Aoki K, Hara K, Takanashi S, Shimizu M, Narita N, Okamoto M and Kobayashi S 2014. Safe clinical use of carbon nanotubes as innovative biomaterials Chem. Rev 114 6040–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Vijayavenkataraman S, Thaharah S, Zhang S, Lu WF and Fuh JYH 2019. 3D-printed PCL/rGO conductive scaffolds for peripheral nerve injury repair Artif. Organs 43 515–23 [DOI] [PubMed] [Google Scholar]
- [130].Qian Y, Zhao X, Han Q, Chen W, Li H and Yuan W 2018. An integrated multi-layer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration Nat. Commun 9 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Wang K, Ruan J, Song H, Zhang J, Wo Y, Guo S and Cui D 2011. Biocompatibility of graphene oxide Nanoscale Res. Lett 6 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Girish CM, Sasidharan A, Gowd GS, Nair S and Koyakutty M 2013. Confocal Raman imaging study showing macrophage mediated biodegradation of graphene in vivo Adv. Healthcare Mater 2 1489–500 [DOI] [PubMed] [Google Scholar]
- [133].Dong R, Ma PX and Guo B 2020. Conductive biomaterials for muscle tissue engineering Biomaterials 229 119584. [DOI] [PubMed] [Google Scholar]
- [134].Yin L, Cheng H, Mao S, Haasch R, Liu Y, Xie X, Hwang SW, Jain H, Kang SK and Su Y 2014. Dissolvable metals for transient electronics Adv. Funct. Mater 24 645–58 [Google Scholar]
- [135].Ahadian S et al. 2015. Hydrogels containing metallic glass sub-micron wires for regulating skeletal muscle cell behaviour Biomater. Sci 3 1449–58 [DOI] [PubMed] [Google Scholar]
- [136].Choi Y, Koo J and Rogers JA 2020. Inorganic materials for transient electronics in biomedical applications MRS Bull. 45 103–12 [Google Scholar]
- [137].Mei D, Lamaka SV, Lu X and Zheludkevich ML 2020. Selecting medium for corrosion testing of bioabsorbable magnesium and other metals—a critical review Corros. Sci 171 108722 [Google Scholar]
- [138].Yin X-Y, Zhang Y, Cai X, Guo Q, Yang J and Wang ZL 2019. 3D printing of ionic conductors for high-sensitivity wearable sensors Mater. Horiz 6 767–80 [Google Scholar]
- [139].Lee KY and Mooney DJ 2001. Hydrogels for tissue engineering Chem. Rev 101 1869–80 [DOI] [PubMed] [Google Scholar]
- [140].Liu X, Miller AL, Park S, Waletzki BE, Zhou Z, Terzic A and Lu L 2017. Functionalized carbon nanotube and graphene oxide embedded electrically conductive hydrogel synergistically stimulates nerve cell differentiation ACS Appl. Mater. Interfaces 9 14677–90 [DOI] [PubMed] [Google Scholar]
- [141].Zhao Y et al. 2017. Enhanced peripheral nerve regeneration by a high surface area to volume ratio of nerve conduits fabricated from hydroxyethyl cellulose/soy protein composite sponges ACS Omega 2 7471–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Mohammadi M, Ramazani Saadatabadi A, Mashayekhan S and Sanaei R 2020. Conductive multichannel PCL/gelatin conduit with tunable mechanical and structural properties for peripheral nerve regeneration J. Appl. Polym. Sci 137 49219 [Google Scholar]
- [143].Wang J, Xiong H, Zhu T, Liu Y, Pan H, Fan C, Zhao X and Lu WW 2020. Bioinspired multichannel nerve guidance conduit based on shape memory nanofibers for potential application in peripheral nerve repair ACS Nano 14 12579–95 [DOI] [PubMed] [Google Scholar]
- [144].Sun B, Zhou Z, Li D, Wu T, Zheng H, Liu J, Wang G, Yu Y and Mo X 2019. Polypyrrole-coated poly(l-lactic acid-co-ε-caprolactone)/silk fibroin nanofibrous nerve guidance conduit induced nerve regeneration in rat Mater. Sci. Eng. C 94 190–9 [DOI] [PubMed] [Google Scholar]
- [145].Duchi S, Onofrillo C, O’Connell CD, Blanchard R, Augustine C, Quigley AF, Kapsa RM, Pivonka P, Wallace G and di Bella C 2017. Handheld co-axial bioprinting: application to in situ surgical cartilage repair Sci. Rep 7 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Bayat A and Ramazani SA 2020. Biocompatible conductive alginate/polyaniline-graphene neural conduits fabricated using a facile solution extrusion technique Int. J. Polym. Mater. Polym. Biomater 70 486–95 [Google Scholar]
- [147].Heinrich MA. et al. 3D bioprinting: from benches to translational applications. Small. 2019;15:1805510. doi: 10.1002/smll.201805510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Jakus AE, Secor EB, Rutz AL, Jordan SW, Hersam MC and Shah RN 2015. Three-dimensional printing of high-content graphene scaffolds for electronic and biomedical applications ACS Nano 9 4636–48 [DOI] [PubMed] [Google Scholar]
- [149].Xu X et al. 2019. 3D printing of nerve conduits with nanoparticle-encapsulated RGFP966 Appl. Mater. Today 16 247–56 [Google Scholar]
- [150].Zhang X, Qu W, Li D, Shi K, Li R, Han Y, Jin E, Ding J and Chen X 2020. Functional polymer-based nerve guide conduits to promote peripheral nerve regeneration Adv. Mater. Interfaces 7 2000225 [Google Scholar]
- [151].Singh D, Harding AJ, Albadawi E, Boissonade FM, Haycock JW and Claeyssens F 2018. Additive manufactured biodegradable poly (glycerol sebacate methacrylate) nerve guidance conduits Acta Biomater. 78 48–63 [DOI] [PubMed] [Google Scholar]
- [152].Kim JI, Hwang TI, Aguilar LE, Park CH and Kim CS 2016. A controlled design of aligned and random nanofibers for 3D bi-functionalized nerve conduits fabricated via a novel electrospinning set-up Sci. Rep 6 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Jing W, Ao Q, Wang L, Huang Z, Cai Q, Chen G, Yang X and Zhong W 2018. Constructing conductive conduit with conductive fibrous infilling for peripheral nerve regeneration Chem. Eng. J 345 566–77 [Google Scholar]
- [154].Campos DFD, Blaeser A, Weber M, Jäkel J, Neuss S, Jahnen-Dechent W and Fischer H 2012. Three-dimensional printing of stem cell-laden hydrogels submerged in a hydrophobic high-density fluid Biofabrication 5 015003. [DOI] [PubMed] [Google Scholar]
- [155].Zhu W et al. 2018. Rapid continuous 3D printing of customizable peripheral nerve guidance conduits Mater. Today 21 951–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Joung D, Lavoie NS, Guo SZ, Park SH, Parr AM and McAlpine MC 2020. 3D printed neural regeneration devices Adv. Funct. Mater 30 1906237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Koffler J et al. 2019. Biomimetic 3D-printed scaffolds for spinal cord injury repair Nat. Med 25 263–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Johnson BC, Shen K, Piech D, Ghanbari MM, Li KY, Neely R, Carmena JM, Maharbiz MM and Muller R 2018. StimDust: a 6.5 mm3, wireless ultrasonic peripheral nerve stimulator with 82% peak chip efficiency 2018 IEEE Custom Integrated Circuits Conf. (CICC) (San Diego, CA, USA, 8–11 April 2018) (IEEE; ) pp 1–4 [Google Scholar]
- [159].Koo J et al. 2018. Wireless bioresorbable electronic system enables sustained nonpharmacological neuroregenerative therapy Nat. Med 24 1830–6 [DOI] [PubMed] [Google Scholar]
- [160].Jeong J, Jung J, Jung D, Kim J, Ju H, Kim T and Lee J 2021. An implantable optogenetic stimulator wirelessly powered by flexible photovoltaics with near-infrared (NIR) light Biosens. Bioelectron 180 113139. [DOI] [PubMed] [Google Scholar]
- [161].Chen P, Wu P, Wan X, Wang Q, Xu C, Yang M, Feng J, Hu B and Luo Z 2021. Ultrasound-driven electrical stimulation of peripheral nerves based on implantable piezoelectric thin film nanogenerators Nano Energy 86 106123 [Google Scholar]
- [162].Choi YS et al. 2020. Stretchable, dynamic covalent polymers for soft, long-lived bioresorbable electronic stimulators designed to facilitate neuromuscular regeneration Nat. Commun 11 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Song K-I, Park SE, Lee S, Kim H, Lee SH and Youn I 2018. Compact optical nerve cuff electrode for simultaneous neural activity monitoring and optogenetic stimulation of peripheral nerves Sci. Rep 8 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Seo D, Neely RM, Shen K, Singhal U, Alon E, Rabaey JM, Carmena JM and Maharbiz MM 2016. Wireless recording in the peripheral nervous system with ultrasonic neural dust Neuron 91 529–39 [DOI] [PubMed] [Google Scholar]
- [165].Li Y-T, Peng C-W, Chen L-T, Lin W-S, Chu C-H and Chen J-J-J 2012. Application of implantable wireless biomicrosystem for monitoring nerve impedance of rat after sciatic nerve injury IEEE Trans. Neural. Syst. Rehabil. Eng 21 121–8 [DOI] [PubMed] [Google Scholar]
- [166].Vasudevan S, Patel K and Welle C 2016. Rodent model for assessing the long term safety and performance of peripheral nerve recording electrodes 4 J. Neural. Eng 14 016008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Li Z, Zhu G, Yang R, Wang AC and Wang ZL 2010. Muscle-driven in vivo nanogenerator Adv. Mater 22 2534–7 [DOI] [PubMed] [Google Scholar]
- [168].Zheng Q, Shi B, Fan F, Wang X, Yan L, Yuan W, Wang S, Liu H, Li Z and Wang ZL 2014. In vivo powering of pacemaker by breathing-driven implanted triboelectric nanogenerator Adv. Mater 26 5851–6 [DOI] [PubMed] [Google Scholar]
- [169].Zurbuchen A, Haeberlin A, Pfenniger A, Bereuter L, Schaerer J, Jutzi F, Huber C, Fuhrer J and Vogel R 2016. Towards batteryless cardiac implantable electronic devices—the Swiss way IEEE Trans. Biomed. Circuits Syst 11 78–86 [DOI] [PubMed] [Google Scholar]
- [170].Nadeau P et al. 2017. Prolonged energy harvesting for ingestible devices Nat. Biomed. Eng 1 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Song K, Han JH, Lim T, Kim N, Shin S, Kim J, Choo H, Jeong S, Kim YC and Wang ZL 2016. Subdermal flexible solar cell arrays for powering medical electronic implants Adv. Healthcare Mater 5 1572–80 [DOI] [PubMed] [Google Scholar]
- [172].Shi B, Li Z and Fan Y 2018. Implantable energy-harvesting devices Adv. Mater 30 1801511. [DOI] [PubMed] [Google Scholar]
- [173].Xu D and Pollock M 1994. Experimental nerve thermal injury Brain 117 375–84 [DOI] [PubMed] [Google Scholar]
- [174].Ng WL, Chan A, Ong YS and Chua CK 2020. Deep learning for fabrication and maturation of 3D bioprinted tissues and organs Virtual Phys. Prototyp 15 340–58 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that support the findings of this study are included within the article (and any supplementary files).