Abstract
Background:
In cN1 patients rendered cN0 with neoadjuvant chemotherapy, the false-negative rate of sentinel lymph node biopsy (SLNB)is <10% when ≥3 sentinel lymph nodes (SLNs) are removed. The added value of nodal clipping in this scenario is unknown. Here we determine how often the clipped node is a sentinel node when ≥3 SLNs are retrieved.
Methods:
We identified cT1-3N1 patients treated between 02/2018-10/2021 with a clipped lymph node at presentation. SLNB was performed with a standardized approach of dual-tracer mapping and retrieval of ≥3 SLNs. Clipped nodes were not localized; SLNs were x-rayed intraoperatively to determine clip location. Axillary lymph node dissection (ALND) was performed for any residual disease or retrieval of<3 SLNs.
Results:
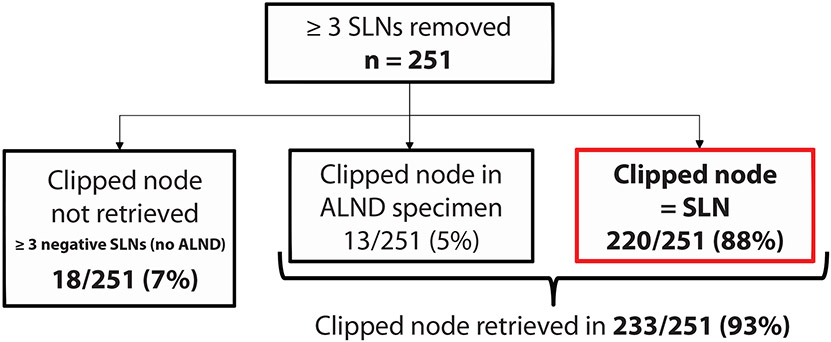
Of 269 patients, 251 (93%) had ≥3 SLNs. Median age was 51 years; the majority (92%) had ductal histology; 46% were HR+/HER2-. The median number of SLNs removed was 4(IQR 3,5). The clipped node was an SLN in 88% (220/251) of cases. Of the 31 where the clipped node was not, 13 had a positive SLN mandating ALND, and the clip was identified in the ALND specimen. In the remaining 18, where ≥3 negative SLNs were retrieved and an ALND was not performed, the clip was not retrieved, with no axillary failures in this group (median follow-up: 55 months).
Conclusion:
When the SLNB procedure is optimized with dual tracer and retrieval of ≥3 SLNs, the clipped node is an SLN in the majority of cases, suggesting that failure to retrieve the clipped node should not be an indication for ALND.
INTRODUCTION
In node-positive breast cancer patients who undergo neoadjuvant chemotherapy (NAC), improvements in systemic therapy regimens have resulted in increasing rates of pathological complete response1-4, allowing for de-escalation of axillary surgery in a significant proportion of patients.5-7 The accuracy of sentinel lymph biopsy (SLNB) in clinically node-positive (N+) patients who convert to clinically node negative with NAC has been evaluated in 4 multi-institutional prospective studies.8-11 In these trials, the overall false-negative rate (FNR) exceeded the 10% threshold considered clinically acceptable. However, with modification of the SLNB technique, including the use of dual tracer mapping and removal of ≥ 3 sentinel lymph nodes (SLNs), the FNR uniformly fell below 10%. In a meta-analysis of nearly 2000 biopsy-proven N+ patients, the pooled FNR was 4% with removal of ≥ 3 SLNs.12
Another method to decrease the false-negative rate of SLNB post-NAC is to retrieve the clipped biopsy-proven metastatic node at the time of SLNB, which results in an FNR of 2-4%.13-15 Studies evaluating the combination of localization of the clipped node with the SLNB procedure, also known as targeted axillary dissection (TAD)15,16, have shown that the clipped node is not a sentinel node in 20-35% of cases, which has resulted in the broad adoption of TAD, given its perceived accuracy.14-20 However, most of these studies were limited by a small sample size, suboptimal lymphatic mapping technique, and retrieval of < 3 SLNs.
In this study, we sought to determine how often the clipped node is an SLN when dual mapping is used and ≥ 3 SLNs are retrieved, and to assess the clinicopathological factors associated with the clipped node not being an SLN.
METHODS
At our institution, patients with clinical T1-3 biopsy-proven N1 breast cancer who receive NAC and convert to cN0 by physical exam are eligible for SLNB. Axillary ultrasound post-NAC was not routinely used to determine eligibility for SLNB. Patients with locally advanced breast cancer (cT4 and/or cN2/N3) were not considered eligible for downstaging to SLNB, irrespective of their response to NAC.
SLNB was performed using a pre-defined approach of dual tracer mapping with technetium-99m sulfur colloid and lymphazurin blue dye. The isotope was injected intradermally at a single site directly over the tumor, or just above the biopsy site. If the patient was given the isotope injection the day before surgery, a dose of 0.5 mCi was injected; if the isotope was given the morning of surgery, a dose of 0.1 mCi was injected. Lymphoscintigraphy was not routinely performed. All patients had 5 cc of isosulfan blue dye injected at the beginning of the operation, given either intraparenchymally near the tumor or near the biopsy site, or via subareolar injection, followed by a 5 minute breast massage. Nodes that were hot, blue, or palpably abnormal were considered SLNs, and surgeons were not asked to specify the exact method of SLN identification. ALND was performed for any residual nodal disease (including isolated tumor cells and micrometastases) or if 3 SLNs were not retrieved, irrespective of nodal response. Frozen section of the SLN was performed intraoperatively, followed by routine histological processing, and hematoxylin and eosin staining for permanent diagnosis. Immunohistochemistry was not routinely performed.
Placement of clips in metastatic nodes was not routine, and localization of the clipped node in patients presenting with clips placed elsewhere was not performed. Beginning in 2018, X-rays of the SLN specimen were routinely taken intraoperatively for all patients presenting with a clipped node in order to determine the clip location. Failure to retrieve the clipped node did not mandate an ALND in patients who otherwise had ≥ 3 negative SLNs. In cases where the clip was not seen on X-ray and ALND was performed for residual disease, the presence of the clip was documented in the pathology report.
Following receipt of institutional review board approval, consecutive patients with cT1-3N1 invasive breast cancer presenting with a clipped node who were treated with NAC and subsequent surgery between February 2018 and October 2021 were identified.
Statistical Analysis
The primary outcome was the frequency with which the clipped node was an SLN. The secondary aims were to identify factors associated with the clipped node not being an SLN and to determine how often the clipped node was the only positive lymph node. Rates were calculated and continuous variables were reported as a median (interquartile range [IQR]), and categorical variables were reported as n (%).
Clinicopathological characteristics were compared between patients for whom the clipped node was an SLN and those where the clipped node was not an SLN. The Wilcoxon rank sum test or t-test was used for continuous variables, and the Chi-square or Fisher’s exact test was used for categorical variables. All statistical analyses were conducted using R 3.5.3 (R Core Development Team, Vienna, Austria).
RESULTS
Between February 2018 and October 2021 there were 269 biopsy-proven T1-3N1 patients with a documented clipped node at presentation. All patients underwent SLNB with dual tracer mapping; the 251 patients (93%) who had ≥ 3 SLNs removed represent our study cohort. The median age of the cohort was 51 years and the majority of patients had T2 tumors and palpable adenopathy at presentation. The majority (92%) of tumors were of ductal histology and 46% were HR+/HER2−. The median number of SLNs removed was 4 (IQR 3,5) (Table 1).
TABLE 1.
Clinicopathological characteristics stratified by retrieval of the clipped node as an SLN (yes versus no)
| Overall cohort (n = 251) |
Clip in the sentinel node (n = 220) |
Clip not in the sentinel node (n = 31) |
p | |
|---|---|---|---|---|
| Age, years, median (IQR) | 51 (41, 58) | 50 (41, 59) | 53 (42, 57) | 0.7 |
| BMI, kg/m2, median (IQR) | 27 (23, 31) | 27 (23, 31) | 26 (21, 30) | 0.3 |
| Clinical T stage at presentation | 0.8 | |||
| 1 | 54 (22) | 49 (22) | 5 (16) | |
| 2 | 135 (54) | 118 (54) | 17 (55) | |
| 3 | 55 (22) | 47 (21) | 8 (26) | |
| X | 7 (2.8) | 6 (2.7) | 1 (3.2) | |
| Palpable node at presentation | 0.3 | |||
| Yes | 191 (76) | 170 (77) | 21 (68) | |
| Borderline | 6 (2.4) | 6 (2.7) | 0 (0) | |
| No | 54 (22) | 44 (20) | 10 (32) | |
| Number of abnormal nodes on ultrasound at presentation | 0.5 | |||
| 1 | 168 (67) | 146 (66) | 22 (71) | |
| 2 | 49 (20) | 43 (20) | 6 (19) | |
| 3 | 24 (9.6) | 23 (10) | 1 (3.2) | |
| >4 | 10 (4.0) | 8 (3.6) | 2 (6.5) | |
| Type of surgery | 0.2 | |||
| Mastectomy | 133 (53) | 113 (51) | 20 (65) | |
| BCT | 111(44) | 101 (46) | 10 (32) | |
| No breast surgery* | 7 (2.8) | 6 (2.7) | 1 (3.2) | |
| Number of sentinel nodes removed, median (IQR) | 4 (3, 5) | 4 (3, 5) | 4 (3, 5) | 0.8 |
| ypN stage | 0.3 | |||
| 0 | 117 (47) | 99 (45) | 18 (58) | |
| 1 | 91 (36) | 84 (38) | 7 (23) | |
| 2 | 32 (13) | 28 (13) | 4 (13) | |
| 3 | 11 (4.4) | 9 (4.1) | 2 (6.5) | |
| Breast pCR † | 0.8 | |||
| Yes | 81 (32) | 72 (33) | 9 (30) | |
| LVI †† | 0.2 | |||
| Yes | 82 (33) | 68 (31) | 14 (45) | |
| Histology | 0.6 | |||
| Ductal | 231 (92) | 201 (91) | 30 (97) | |
| Lobular or mixed | 11 (4.4) | 11 (5) | 0 (0) | |
| Other | 9 (3.6) | 8 (3.6) | 1 (3.2) | |
| Differentiation | 0.9 | |||
| Well | 1 (0.4) | 1 (0.5) | 0 (0) | |
| Moderately | 77 (31) | 67 (31) | 10 (33) | |
| Poorly | 168 (68) | 148 (69) | 20 (67) | |
| Unknown | 5 | 4 | 1 | |
| Subtype | 0.8 | |||
| HR+/HER2− | 116 (46) | 100 (45) | 16 (52) | |
| HER2+ | 83 (33) | 74 (34) | 9 (29) | |
| HR−/HER2− | 52 (21) | 46 (21) | 6 (19) |
Frequency (row percent) reported for categorical variables, and median (IQR) reported for continuous variables.
Occult primary carcinoma
Applies to non-occult cases only
LVI was present on core biopsy or final pathology
BMI body mass index, pCR pathological complete response, LVI lymphovascular invasion, HR hormone receptor, HER2 human epidermal growth factor receptor 2
There were no mapping failures. Inadequate mapping, defined as identification of < 3 SLNs, occurred in 18 cases (6.7%) with a median number of 2 SLNs removed (IQR 1,2). Clinicopathological features stratified by adequate versus inadequate mapping are shown in Supplementary Table 1.
How often is the clipped node a sentinel node when ≥ 3 SLNs are removed, and what are the associated clinicopathological factors?
The clipped node was an SLN in 88% (220/251) of cases. Of the 31 cases where the clipped node was not an SLN, 13 patients had a positive SLN mandating ALND and the clip was identified in the ALND specimen, representing an additional 5% of the cohort. In the remaining 18 cases where ≥ 3 negative SLNs were retrieved, ALND was not performed and the clipped node was not identified (Fig. 1). Of these, 12/18 (66%) had residual breast tumor and 6/18 (33%) had a complete pathological response (ypT0/is ypN0). At a median follow-up of 55 months, none of these 18 patients developed an axillary recurrence. There were no clinicopathologic features associated with failure to retrieve the clipped node during the SLN procedure (Table 1).
Fig. 1. Study Flowchart.
SLN sentinel lymph nodes, ALND axillary lymph node dissection
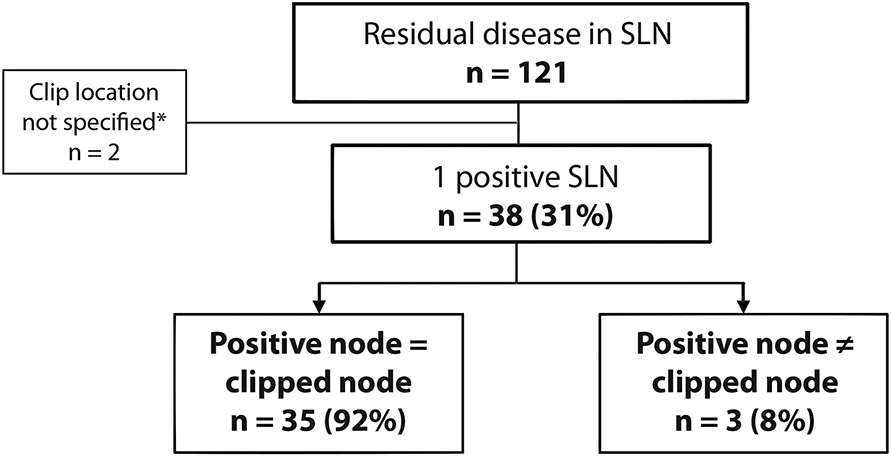
When there is only 1 residual positive node, how often is it the clipped node?
Residual nodal disease was found in 121/220 patients (55%) with a clipped SLN. Only 1 positive sentinel lymph node was present in 40 patients (33%); in 35 (87.5%), the clipped SLN was the positive node. Three patients (7.5%) had a positive SLN that was not the clipped node, and in 2 cases (5%) the pathology report did not specify whether the positive SLN was the clipped node (Fig. 2).
Fig. 2. How Often Is the Clipped Node the Only Metastatic Node?
*The pathology report did not specify whether the positive SLN was the clipped node
SLN sentinel lymph node
DISCUSSION
There is great variety across institutions and countries in the techniques used to reduce the FNR of SLNB in clinically N+ patients who downstage to clinically node negative with NAC.5,13,15,21-23
After publication of the multi-institutional trials8-11 demonstrating FNRs < 10% with retrieval of ≥ 3 SLNs in patients who downstage from clinically node positive to clinically node negative with NAC, it became standard at our institution to perform SLNB after NAC with dual tracer mapping and retrieval of ≥ 3 SLNs. By using this pre-defined approach in the present study, ≥ 3 SLNs were retrieved in 93% of cases. This is in line with prior studies from our institution showing that dual tracer mapping achieves adequate SLN retrieval in almost all cases.5,6
Data on nodal recurrence after SLNB alone in patients downstaged after NAC are emerging.24-27 In our experience of 234 consecutive patients for which ALND was omitted after retrieval of ≥ 3 negative SLNs, without nodal clipping, the rate of axillary recurrences was very low. At a median follow-up of 40 months, only 1 patient experienced an axillary failure synchronous with an in-breast recurrence after refusing radiation therapy.28 Similar excellent outcomes were reported by the group from the European Institute of Oncology, where the nodal recurrence rate without clipping was 1.6% in 123 patients at 10 years of follow-up.24 Although longer follow-up is needed, these results support the oncological safety of omission of ALND without nodal clipping.
Other techniques to lower the FNR include marking the metastatic biopsied node with either nodal clipping, nodal tattooing with carbon suspensions, or the use of magnetic seeds, with its subsequent localization at the time of surgery. Retrieval of the clipped node in combination with an SLNB (targeted axillary dissection [TAD]) results in an FNR of 2-4%.15,16 However, localization of the clipped node is not always possible, as the clip migrates into the perinodal fat or is not visible on intraoperative ultrasound in 2-30% of cases.16,29-31 In the German multicentric prospective study SenTa that evaluated the feasibility of TAD in a real-world cohort of cN+ patients, the identification rate of the clipped node was 77.8% for the clipped node alone and 86.9% when TAD was performed.18 This rate is similar to the one found in the present study without localization.
Studies that have evaluated the accuracy of TAD have found that the clipped node is not an SLN in 20-35% of cases.14-20 In the SenTa study, where only 8% of patients had dual tracer mapping and the median number of SLNs removed was 2, the clipped node was not an SLN in 35% of cases.18 Similarly, in the initial University of Texas MD Anderson Cancer Center experience, where only 55% of patients had dual mapping and a mean number of 2.7 SLNs was retrieved, the clipped node was not retrieved during the SLN procedure in 23% of cases.15 Given these results, TAD has become widely adopted. However, in the present study, where all patients had dual tracer mapping and the median number of SLNs removed was 4, the clipped node was not an SLN in only 12% of cases, demonstrating that optimization of the SLNB technique alone results in retrieval of the clipped node in most cases.
Identifying clinicopathological factors associated with the clipped node not being an SLN could help in patient selection for nodal clipping and localization. In the study from Caudle and colleagues, having ≥ 4 suspicious lymph nodes on ultrasound at presentation was associated with increased odds of not retrieving the clipped node during SLNB (odds ratio 3.45, 95% confidence interval 1.48-8.06).15 However, in the present study, we did not identify any clinicopathological factors associated with the clipped node not being an SLN.
Detecting residual disease post-NAC is important to tailor adjuvant systemic therapy, which results in improved outcomes.32,33 However, whether the additional reduction in the FNR obtained with nodal clipping translates to any clinical benefit remains unclear. Weiss and colleagues evaluated how often retrieval of the clipped node changed adjuvant therapy recommendations among 98 consecutive cN1 patients who converted to cN0 with NAC. In that study, dual tracer was used in 97% of cases, a median number of 4 SLNs were retrieved, and the clipped node was not an SLN in 19% of cases. Of the 58 patients with residual nodal disease, 3 (5.2%) had discordant pathology between the clipped node and the SLN. Of these, only 1 had a clipped non SLN that contained cancer when all other SLNs were negative; the tumor was HR+/HER2−, and systemic therapy recommendations were not affected by this finding.34 In a smaller study conducted by Hartmann and colleagues, discordance between the SLN and clipped node was present in 5/63 cases (7.9%) and the authors reported a change in systemic treatment recommendations for 1 patient who had a triple negative tumor. However, that patient had failed mapping, which is an indication for ALND. Therefore, residual nodal disease would have not been missed without nodal clipping.35
In our study, the clipped node was not retrieved in 18 cases;12/18 of these patients had residual disease in the breast and 6/18 had pCR. The 6 patients with a pCR had stage II disease at presentation, 5 had TNBC, and 1 had an HR−/HER2+ tumor. Given that nodal pCR is more common than breast pCR, and considering the tumor biology of these patients, the likelihood of undetected residual nodal disease is very low.36-38 Of the 12 patients with residual disease in the breast, 2 had TNBC and 3 had HER2+ tumors. Given the residual disease in the breast, these patients received adjuvant capecitabine and trastuzumab emtasine, respectively. Importantly, at a median follow-up of 55 months, none of these 18 patients in whom the clipped node was not retrieved developed an axillary recurrence. Multicenter studies23,39,40 comparing different staging techniques following NAC are ongoing and will provide robust data on whether nodal clipping translates to an appreciable clinical benefit. Until results of these studies are available, it should be appreciated that nodal clipping adds expense and may result in unnecessary axillary dissection if the clipped node cannot be retrieved.
Strength of our study include its large sample size and standardization of the SLNB procedure. Limitations are related to its retrospective monocentric design and to its tertiary cancer center setting, as results may not be generalizable. In particular, our rate of identification of 3 or more SLNs is higher than that reported by others, and we do not distinguish between hot, blue, or palpable sentinel lymph nodes. However, although it has been anecdotally reported that nodal clips may make lymph nodes palpable, which could have influenced our results, the fact that approximately 15% of clipped nodes cannot be retrieved16,29-31 suggests that not all clipped nodes are palpable irrespective of the type of clip used.18 Another limitation is that the median follow-up of the present cohort was too short to provide meaningful outcome data.
In conclusion, given the high frequency with which the clipped node is retrieved when the SLNB procedure is optimized, the very low rate of axillary recurrences without nodal clipping, and the lack of impact on systemic therapy recommendations, the role of routine localization of the clipped node post-NAC should be questioned. Failure to retrieve the clipped node in patients with ≥ 3 negative SLNs should not be an indication for ALND. Future studies should compare oncological outcomes and lymphedema incidence after different axillary staging procedures post-NAC and determine their cost-effectiveness.
Supplementary Material
Synopsis.
Here we determine how often the clipped node is an SLN when ≥3 SLNs are retrieved. We find that when SLNB is optimized with dual tracer/retrieval of ≥3 SLNs, the clipped node is an SLN in 88% of cases.
ACKNOWLEDGEMENTS
This study was a podium/oral presentation at the 23rd Annual Meeting of the American Society of Breast Surgeons, April 6-10, 2022, Las Vegas, NV. The preparation of this study was supported in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748 to Memorial Sloan Kettering Cancer Center. Dr. Monica Morrow has received honoraria from Exact Sciences and Roche. All other authors have no conflict of interest disclosures to report.
REFERENCES
- 1.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32. [DOI] [PubMed] [Google Scholar]
- 2.Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med. 2020;382(9):810–821. [DOI] [PubMed] [Google Scholar]
- 3.Mittendorf EA, Zhang H, Barrios CH, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet. 2020;396(10257):1090–1100. [DOI] [PubMed] [Google Scholar]
- 4.Samiei S, Simons JM, Engelen SME, et al. Axillary Pathologic Complete Response After Neoadjuvant Systemic Therapy by Breast Cancer Subtype in Patients With Initially Clinically Node-Positive Disease: A Systematic Review and Meta-analysis. JAMA Surg. 2021;156(6):e210891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mamtani A, Barrio AV, King TA, et al. How Often Does Neoadjuvant Chemotherapy Avoid Axillary Dissection in Patients With Histologically Confirmed Nodal Metastases? Results of a Prospective Study. Ann Surg Oncol. 2016;23(11):3467–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montagna G, Mamtani A, Knezevic A, Brogi E, Barrio AV, Morrow M. Selecting Node-Positive Patients for Axillary Downstaging with Neoadjuvant Chemotherapy. Ann Surg Oncol. 2020;27(11):4515–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simons JM, Koppert LB, Luiten EJT, et al. De-escalation of axillary surgery in breast cancer patients treated in the neoadjuvant setting: a Dutch population-based study. Breast Cancer Res Treat. 2020;180(3):725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310(14):1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609–618. [DOI] [PubMed] [Google Scholar]
- 10.Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33(3):258–264. [DOI] [PubMed] [Google Scholar]
- 11.Classe JM, Loaec C, Gimbergues P, et al. Sentinel lymph node biopsy without axillary lymphadenectomy after neoadjuvant chemotherapy is accurate and safe for selected patients: the GANEA 2 study. Breast Cancer Res Treat. 2019;173(2):343–352. [DOI] [PubMed] [Google Scholar]
- 12.Tee SR, Devane LA, Evoy D, et al. Meta-analysis of sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with initial biopsy-proven node-positive breast cancer. Br J Surg. 2018;105(12):1541–1552. [DOI] [PubMed] [Google Scholar]
- 13.Donker M, Straver ME, Wesseling J, et al. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg. 2015;261(2):378–382. [DOI] [PubMed] [Google Scholar]
- 14.Boughey JC, Ballman KV, Le-Petross HT, et al. Identification and Resection of Clipped Node Decreases the False-negative Rate of Sentinel Lymph Node Surgery in Patients Presenting With Node-positive Breast Cancer (T0-T4, N1-N2) Who Receive Neoadjuvant Chemotherapy: Results From ACOSOG Z1071 (Alliance). Ann Surg. 2016;263(4):802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caudle AS, Yang WT, Krishnamurthy S, et al. Improved Axillary Evaluation Following Neoadjuvant Therapy for Patients With Node-Positive Breast Cancer Using Selective Evaluation of Clipped Nodes: Implementation of Targeted Axillary Dissection. J Clin Oncol. 2016;34(10):1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siso C, de Torres J, Esgueva-Colmenarejo A, et al. Intraoperative Ultrasound-Guided Excision of Axillary Clip in Patients with Node-Positive Breast Cancer Treated with Neoadjuvant Therapy (ILINA Trial) : A New Tool to Guide the Excision of the Clipped Node After Neoadjuvant Treatment. Ann Surg Oncol. 2018;25(3):784–791. [DOI] [PubMed] [Google Scholar]
- 17.Diego EJ, McAuliffe PF, Soran A, et al. Axillary Staging After Neoadjuvant Chemotherapy for Breast Cancer: A Pilot Study Combining Sentinel Lymph Node Biopsy with Radioactive Seed Localization of Pre-treatment Positive Axillary Lymph Nodes. Ann Surg Oncol. 2016;23(5):1549–1553. [DOI] [PubMed] [Google Scholar]
- 18.Kuemmel S, Heil J, Rueland A, et al. A Prospective, Multicenter Registry Study to Evaluate the Clinical Feasibility of Targeted Axillary Dissection (TAD) in Node-Positive Breast Cancer Patients. Ann Surg. 2020. [DOI] [PubMed] [Google Scholar]
- 19.Cabioglu N, Karanlik H, Kangal D, et al. Improved False-Negative Rates with Intraoperative Identification of Clipped Nodes in Patients Undergoing Sentinel Lymph Node Biopsy After Neoadjuvant Chemotherapy. Ann Surg Oncol. 2018;25(10):3030–3036. [DOI] [PubMed] [Google Scholar]
- 20.Simons JM, v Nijnatten TJA, Koppert LB, et al. Abstract No. GS1-10. Radioactive Iodine Seed placement in the Axilla with the Sentinel lymoh node biopsy after neoadjuvant chemotherapy in breast cancer: Results of the prospective multicenter RISAS trial. Cancer Res. 2021;81(4_Supplement):GS1–10. [Google Scholar]
- 21.Simons JM, Maaskant-Braat AJG, Luiten EJT, et al. Patterns of axillary staging and management in clinically node positive breast cancer patients treated with neoadjuvant systemic therapy: Results of a survey amongst breast cancer specialists. Eur J Surg Oncol. 2020;46(1):53–58. [DOI] [PubMed] [Google Scholar]
- 22.Caudle AS, Bedrosian I, Milton DR, et al. Use of Sentinel Lymph Node Dissection After Neoadjuvant Chemotherapy in Patients with Node-Positive Breast Cancer at Diagnosis: Practice Patterns of American Society of Breast Surgeons Members. Ann Surg Oncol. 2017;24(10):2925–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banys-Paluchowski M, Gasparri ML, de Boniface J, et al. Surgical Management of the Axilla in Clinically Node-Positive Breast Cancer Patients Converting to Clinical Node Negativity through Neoadjuvant Chemotherapy: Current Status, Knowledge Gaps, and Rationale for the EUBREAST-03 AXSANA Study. Cancers (Basel). 2021;13(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahler-Ribeiro-Fontana S, Pagan E, Magnoni F, et al. Long-term standard sentinel node biopsy after neoadjuvant treatment in breast cancer: a single institution ten-year follow-up. Eur J Surg Oncol. 2021;47(4):804–812. [DOI] [PubMed] [Google Scholar]
- 25.Wong SM, Basik M, Florianova L, et al. Oncologic Safety of Sentinel Lymph Node Biopsy Alone After Neoadjuvant Chemotherapy for Breast Cancer. Ann Surg Oncol. 2021;28(5):2621–2629. [DOI] [PubMed] [Google Scholar]
- 26.Martelli G, Barretta F, Miceli R, et al. Sentinel Node Biopsy alone or with Axillary Dissection in Breast Cancer Patients after Primary Chemotherapy: Long-term Results of a Prospective Interventional Study. Ann Surg. 2020. [DOI] [PubMed] [Google Scholar]
- 27.Piltin MA, Hoskin TL, Day CN, Davis J Jr., Boughey JC. Oncologic Outcomes of Sentinel Lymph Node Surgery After Neoadjuvant Chemotherapy for Node-Positive Breast Cancer. Ann Surg Oncol. 2020;27(12):4795–4801. [DOI] [PubMed] [Google Scholar]
- 28.Barrio AV, Montagna G, Mamtani A, et al. Nodal Recurrence in Patients With Node-Positive Breast Cancer Treated With Sentinel Node Biopsy Alone After Neoadjuvant Chemotherapy-A Rare Event. JAMA Oncol. 2021;7(12):1851–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartmann S, Reimer T, Gerber B, Stubert J, Stengel B, Stachs A. Wire localization of clip-marked axillary lymph nodes in breast cancer patients treated with primary systemic therapy. Eur J Surg Oncol. 2018;44(9):1307–1311. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen TT, Hieken TJ, Glazebrook KN, Boughey JC. Localizing the Clipped Node in Patients with Node-Positive Breast Cancer Treated with Neoadjuvant Chemotherapy: Early Learning Experience and Challenges. Ann Surg Oncol. 2017;24(10):3011–3016. [DOI] [PubMed] [Google Scholar]
- 31.Wu S, Wang Y, Zhang N, et al. Intraoperative Touch Imprint Cytology in Targeted Axillary Dissection After Neoadjuvant Chemotherapy for Breast Cancer Patients with Initial Axillary Metastasis. Ann Surg Oncol. 2018;25(11):3150–3157. [DOI] [PubMed] [Google Scholar]
- 32.Masuda N, Lee SJ, Ohtani S, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med. 2017;376(22):2147–2159. [DOI] [PubMed] [Google Scholar]
- 33.von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med. 2019;380(7):617–628. [DOI] [PubMed] [Google Scholar]
- 34.Weiss A, King C, Grossmith S, et al. How Often Does Retrieval of a Clipped Lymph Node Change Adjuvant Therapy Recommendations? A Prospective, Consecutive, Patient Cohort Study. Ann Surg Oncol. 2022. [DOI] [PubMed] [Google Scholar]
- 35.Hartmann S, Stachs A, Schultek G, Gerber B, Reimer T. The Clinical Relevance of Target Lymph Node Biopsy after Primary Systemic Therapy in Initially Node-Positive Breast Cancer Patients. Cancers (Basel). 2021;13(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boughey JC, Ballman KV, McCall LM, et al. Tumor Biology and Response to Chemotherapy Impact Breast Cancer-specific Survival in Node-positive Breast Cancer Patients Treated With Neoadjuvant Chemotherapy: Long-term Follow-up From ACOSOG Z1071 (Alliance). Ann Surg. 2017;266(4):667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tadros AB, Yang WT, Krishnamurthy S, et al. Identification of Patients With Documented Pathologic Complete Response in the Breast After Neoadjuvant Chemotherapy for Omission of Axillary Surgery. JAMA Surg. 2017;152(7):665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Noordaa MEM, van Duijnhoven FH, Cuijpers FNE, et al. Toward omitting sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with clinically node-negative breast cancer. Br J Surg. 2021;108(6):667–674. [DOI] [PubMed] [Google Scholar]
- 39.de Wild SR, Simons JM, Vrancken Peeters M, Smidt ML, Koppert LB, Group M. MINImal vs. MAXimal Invasive Axillary Staging and Treatment After Neoadjuvant Systemic Therapy in Node Positive Breast Cancer: Protocol of a Dutch Multicenter Registry Study (MINIMAX). Clin Breast Cancer. 2022;22(1):e59–e64. [DOI] [PubMed] [Google Scholar]
- 40.Oncoplastic Breast Consortium (OPBC). Nodal recurrence following axillary downstaging with neoadjuvant chemotherapy and omission of axillary lymph node dissection (OPBC-04/EUBREAST-06). https://oncoplasticbc.org/documents/research/synopsis_opbc04_eubreast06_20211208.pdf (Accessed March 31, 2022). 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.