Abstract
This retrospective, observational, single-center study analyzed the results of a “stent-when-feasible” policy in a real-world setting. The study began in the “pre-stent” period (1993) and ended after the beginning of the “routine stent” period (1997). When the 1993 and 1997 global data were compared, the early and 6-month results included significant improvements in the rates of angiographic success (89.3% vs 97.1%), emergency surgical revascularization (1.0% vs 0.3%), freedom from in-hospital major events (91.2% vs 95.9%), and freedom from 6-month major events (77.2% vs 85.1%). The 6-month redo revascularization rate was reduced by almost half for “any catheter intervention” (19.6% vs 10.7%) and was lowest after stent use (7.7% in 1997).
Key words: Atherectomy, balloon angioplasty, coronary catheter intervention, restenosis, stent angioplasty
Since the 1979 clinical introduction in the United States of coronary catheter interventions (CCI),* the use of CCI for coronary artery disease has increased progressively, with and without balloons. Recently, the advent of coronary stenting 1,2 has further promoted the use of CCI. Although stenting was originally devised to treat failed balloon angioplasty, its uses were soon extended to improve the late results of initially successful balloon-CCI. In 1994, the Stent Restenosis Study (STRESS) 3 and the Belgian Netherlands Stent trial (BENESTENT) 4 showed that, for selected coronary conditions (clin-ical and angiographic subsets), stent-CCI generally yields better early and mid-term (6-month) results, specifically with respect to mid-term restenosis, than does balloon-CCI alone. This claim was soon widely accepted and empirically interpreted by the medical community to mean that, when possible, stent treatment is always better than balloon angioplasty alone. The resulting generalized policy of “stent when feasible,” as currently practiced at most interventional cardiology centers, has not yet been validated by large, randomized protocols or by observational studies of large populations.
In the present study, we analyzed the early and late results of progressive implementation of the “stent-when-feasible” policy at a single large cardiovascular center. The study period began in 1993, immediately before stenting became clinically available, and was completed in 1997, after the stent-when-feasible policy was adopted.
Patients and Methods
We examined the records of all consecutive patients with coronary artery disease who underwent any type of coronary revascularization procedure from 1 January 1993 through 31 December 1997. Data for this retrospective analysis were derived from an integrated database established at the Texas Heart Institute specifically to document early and late outcomes of cardiovascular interventional procedures, involving surgery or catheter devices, at a single institution (St. Luke's Episcopal Hospital). Every patient who undergoes catheter or surgical revascularization is entered into the database, which documents approximately 1,000 clinically relevant parameters for each case. As each patient is followed up, additional data are entered to reflect new hospital admissions, the results of routine written questionnaires (completed at 12 months), and mortality records from the Texas State Bureau of Vital Statistics. In this particular study, postoperative follow-up observation was terminated at 6 months. During the study period, the pattern of referrals and follow-up compliance did not change significantly with time.
Operators
As a tertiary referral hospital, St. Luke's Episcopal Hospital has a large staff of cardiologists (about 90 who have admitting privileges), most of whom (approximately 60) practice interventional cardiology. The hospital's 11 catheterization laboratories have an open-staff policy by which each qualified cardiologist independently performs his or her own interventional procedures. The training and experience of individual operators varies, although fairly strict supervision is enforced, in accordance with agreed-upon rules and regulations and subject to counseling by a peer-review committee. Intravascular ultrasound (IVUS) imaging was used sparingly (in 3% of all cases), by some operators.
Study Period
The study period started on 1 January 1993 when balloon-CCI was the dominant CCI and coronary stenting had not yet been introduced into clinical practice. Enrollment of new patients ended on 31 December 1997, and follow-up data were collected through 30 June 1998. By 1997, a progressive “stent-when-feasible” plan had been adopted by all of the involved cardiologists. According to established directives, stents were used only in arteries or vein grafts with a luminal diameter of at least 3.0 mm but less than 4.5 mm.
Catheter Devices
For balloon-CCI, we used many different types of commercially available balloon catheters. Table I lists the types of stents implanted. The Palmaz-Schatz stent (Johnson & Johnson; New Brunswick, NJ) was initially implanted according to the rules of an experimental multicenter protocol (the STRESS study). This device was approved by the United States Food and Drug Administration in mid-1994. It became the most widely used model until mid-1997, when it was largely replaced by the Multilink stent (Guidant; Redwood City, Calif). The Gianturco-Roubin stent (Cook Cardiology; Bloomington, Ind) was used primarily to treat balloon-CCI early failure and/or coronary dissection from 1993 to 1995. We implanted several stents while they were undergoing preclinical-release assessment; these devices included the Multilink, the NIR (Scimed; Maple Grove, Minn), the Wall-Stent (Schneider; Minneapolis, Minn), and the Wiktor (Medtronic; Minneapolis, Minn). During the study period, only the Gianturco-Roubin, Multilink, Palmaz-Schatz, and Wiktor devices were used in great number (>100).
TABLE I. Stent Models Implanted (n = 4,616), 1993–1997
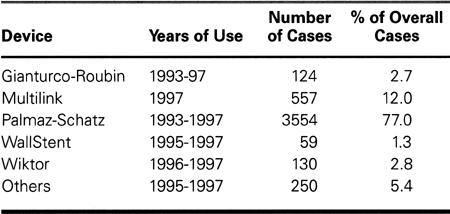
Whereas atherectomy instruments consisted essentially of directional devices (DVI Simpson Coronary AtheroCath; Guidant) and rotational devices (Rota-blator; Boston Scientific; Natick, Mass), the transluminal extraction catheter (TEC; Interventional Technologies, Inc.; San Diego, Calif) was rarely used.
Anticoagulation
During the period covered by the study, our techniques and procedures conformed to the prevailing national trends. These trends included the use of anticoagulants during and early after stent implantation (specifically, dextran, aspirin, dipyridamole, and heparin administered for 12 to 48 hours as an intravenous infusion, and warfarin). After the introduction of routine high-pressure (>12 atm) stent expansion in mid-1995, this anticoagulation regimen was replaced by aspirin (325 mg/day indefinitely) and ticlopidine (250 mg twice a day for 1 month), without heparin infusion. Newer-generation platelet inhibitors (platelet glycoprotein IIb/IIIa receptor antagonists) were administered at a progressively higher rate as the study period progressed (Fig. 1).
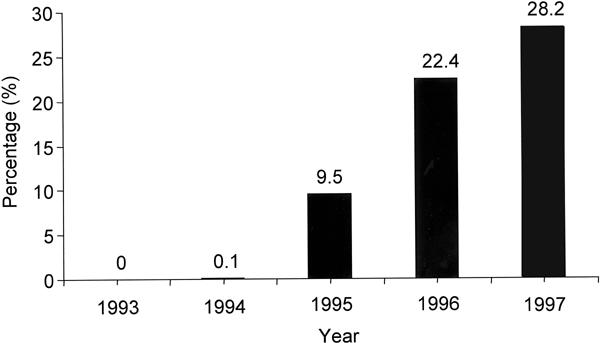
Fig. 1 Yearly percentage of patients who received intravenous abciximab. The drug was administered as a bolus dose at the start of coronary catheter intervention and then as a contin-uous infusion for the next 12 hours.
End-Points
The end-points of the study were 1) “angiographic success,” defined as an angiographically assessed early outcome in which the residual luminal stenosis was less than 50% at the site of at least 1 treated lesion that originally had an angiographically assessed stenosis of greater than 70%; 2) “clinical success,” defined as a successful clinical outcome at hospital discharge (early angiographic success in the absence of the following major in-hospital events: acute myocardial infarction,* death, and redo coronary angioplasty or surgical revascularization); and 3) event-free survival at the 6-month follow-up evaluation, in the absence of the same major events between hospital discharge and follow-up.
Statistical Analysis
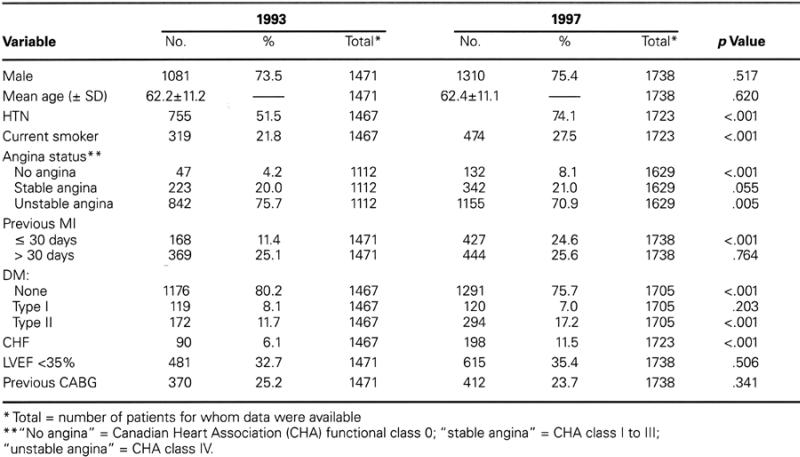
Statistical comparisons of categorical variables were performed with χ2 analysis, and the results were compared by means of t-tests. Differences were considered significant when the p value was less than 0.05. Unless otherwise indicated, comparisons are between data obtained during the 1st year (1993) and during the final year (1997) of the study (i.e., the pre-stent sample versus the routine-stent sample). The effects on the clinical outcomes of variations between the 1993 and 1997 populations in their baseline characteristics (Table IIA) were evaluated by means of multivariate logistic regression analysis. The “6-month event rate” includes the hospitalization period following the initial procedure (the “in-hospital” event rate).
TABLE IIA. Demographic and Clinical Characteristics
Results
From 1 January 1993 through 31 December 1997, our hospital staff performed 17,956 coronary revas-cularization procedures in 15,770 patients. Of these procedures, 9,857 were CCI, and 8,099 were aortocoronary bypass operations (Fig. 2). The 9,857 CCI were performed to treat 15,474 lesions in 7,671 patients (2,146 patients had 2 or more CCI). The CCI were categorized according to the most sophisticated device used. In increasing order of sophistication, the categories were: balloon-CCI (5,449 procedures); atherectomy-CCI using any type of atherectomy device (723 procedures); and stent-CCI (3,685 procedures involving 4,616 stents) (Figs. 2 and 3). In 253 of the cases, an atherectomy preceded stent use.
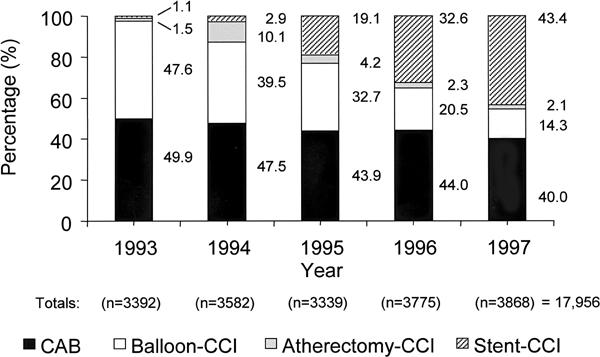
Fig. 2 Yearly coronary interventions at St. Luke's/Texas Heart Institute, broken down by the type of intervention, as a percentage of each annual total.
CAB = coronary artery bypass; CCI = coronary catheter intervention
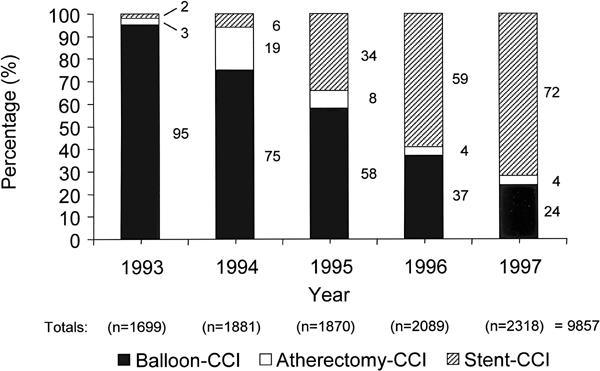
Fig. 3 Yearly percutaneous interventions at St. Luke's/Texas Heart Institute, broken down by the type of intervention, as a percentage of each annual total.
CCI = coronary catheter intervention
Demographic, Clinical, and Angiographic Characteristics
Table II (A,B) shows the demographic, clinical, and angiographic characteristics for the 1993 and 1997 cohorts. Throughout the study, the mean age remained about 62 years, and the male-to-female ratio remained basically constant (percentage of males: 73.5% in 1993 in comparison with 75.4% in 1997; p = 0.517). Each year, the percentage of patients treated for saphenous vein graft lesions remained stable, at around 10%; similarly, the percentage of patients who had undergone coronary artery bypass surgery remained stable, at about 24% per year.
TABLE IIB. Angiographic Characteristics
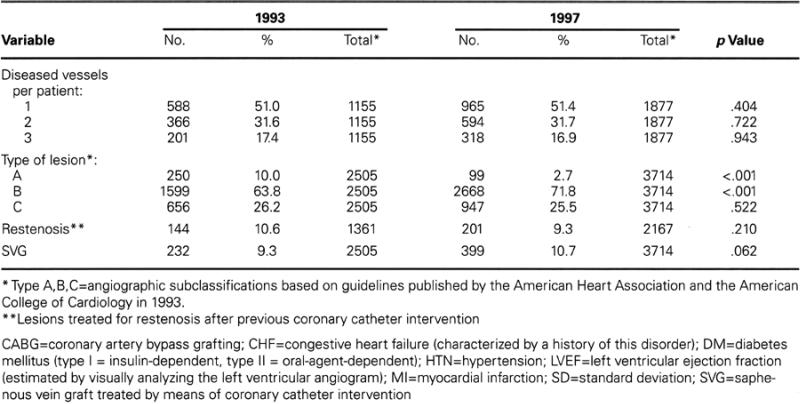
From 1993 to 1997, the incidence of the following factors increased significantly: hypertension (51.5% to 74.1%); diabetes mellitus (19.8% to 24.2%); myocardial infarction (MI) within 30 days before the CCI (11.4% to 24.6%); and a history of congestive heart failure (6.1% to 11.5%). Substantially unchanged were the number of lesions treated per patient (1.3 to 1.5) and the incidence of angiographically difficult lesions (22.5% to 26.2%), which were identified as type C in accordance with the American College of Cardiology/American Heart Association classification system.
Procedures and Outcomes
Over the study period, the yearly percentage of patients who underwent surgical revascularization decreased from 49.9% to 40.0% (Fig. 2). Stent use increased from 1.1% to 43.4% of the total number of coronary revascularization procedures. The percentage of CCI that involved the use of stents increased from 2.0% to 72.5% (Fig. 3). The incidence of atherectomy peaked in 1994, when the popularity of the DVI directional device was at its highest level; after 1995, the incidence decreased to 4% of the total CCI done, and the Rotablator became essentially the only atherectomy device used.
From 1993 to 1997, the CCI angiographic success rate increased significantly (from 89.3% to 97.1%; p <0.001), as did the clinical success rate (from 82.2% to 92.1%; p <0.001) for all procedures combined (Figs. 4 and 5). The clinical success rate also improved for balloon-CCI considered separately, although it remained inferior to that for stent-CCI. The need for urgent or emergency surgery (performed after any CCI, on the same day) decreased significantly (1.0% to 0.3%; p <0.001). The total incidence of in-hospital redo revascularizations (in which the treated lesions underwent an additional procedure before hospital discharge) decreased from 7.3% to 2.0% (p <0.001) (Fig. 6). The mean length of stay in the hospital decreased significantly from 4.9 to 3.6 days (p <0.0001).
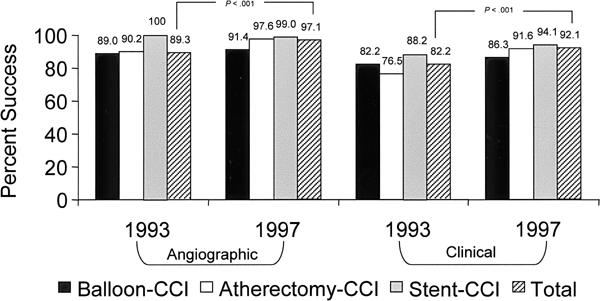
Fig. 4 Comparative angiographic and clinical success rates (per procedure) in 1993 and 1997. (See text for the definitions of angiographic and clinical success.)
CCI = coronary catheter intervention
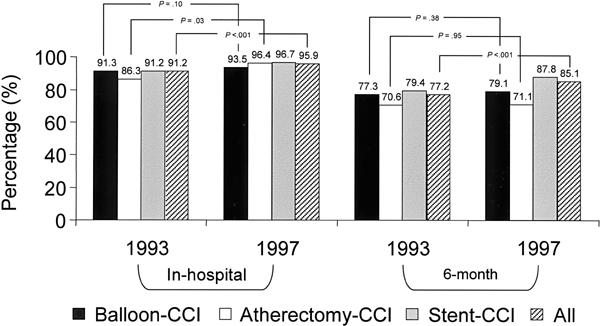
Fig. 5 Comparison of freedom from major adverse events (death, myocardial infarction, redo balloon-CCI, or aortocoronary bypass), excluding angiographic failure, in hospital and at 6 months, after CCI performed in 1993 and in 1997. Figures are provided for each type of CCI and also for all CCI.
CCI = coronary catheter intervention
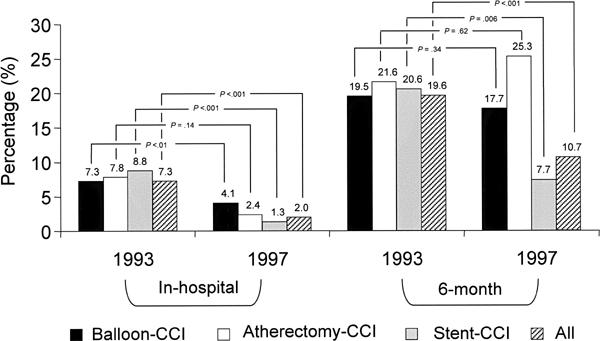
Fig. 6 Reintervention rates (1993 compared with 1997)
CCI = coronary catheter intervention
Figures 5 through 8 show the rates of freedom from major cardiac events (death, MI, redo revascularization) before hospital discharge and at 6 months for the 1993 and 1997 cohorts. Over the study period, freedom from any of these major events during the first 6 postoperative months increased significantly, both for each individual catheter technique and for the group as a whole (77.2% to 85.1%; p <0.001) (Fig. 5).
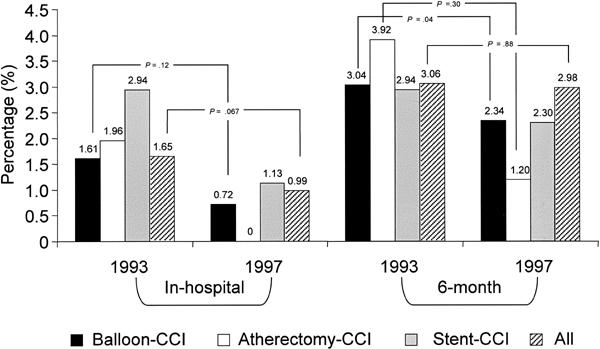
Fig. 7 Mortality rates (1993 compared with 1997)
CCI = coronary catheter intervention
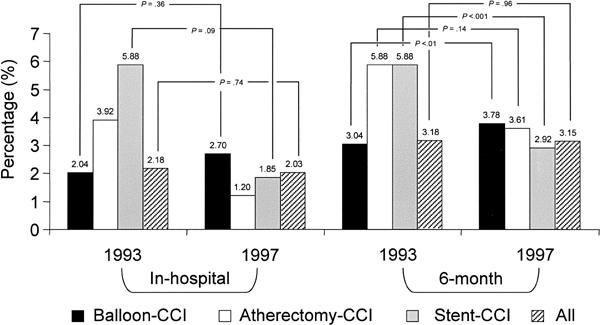
Fig. 8 Myocardial infarction rates (1993 compared with 1997)
CCI = coronary catheter intervention
There was a clear trend towards a decreased mortality rate for each type of procedure. In particular, hospital deaths associated with any CCI decreased from 1.65% to 0.99% (p = 0.067) (Fig. 7). This advantage was not maintained at 6 months (3.06% vs 2.98%; p = 0.88), because the postdischarge mortality tended to increase (1.39% vs 1.99%) from the 1993 to the 1997 cohorts.
Figure 8 shows the yearly MI rates. Over the study period, there was no significant change in the in-hospital MI rate (2.18% in 1993 in comparison with 2.03% in 1997; p = 0.74) or in the MI rate during the first 6 postoperative months (3.18% in comparison with 3.15%; p = 0.96).
The need for redo revascularization (CCI or coronary artery bypass) at 6 months decreased significantly for the combined CCI but not for balloon-CCI or atherectomy-CCI (Fig. 6). Most importantly, the incidence of redo revascularization was substantially lower after stent-CCI (7.7%) than after balloon-CCI (17.7%).
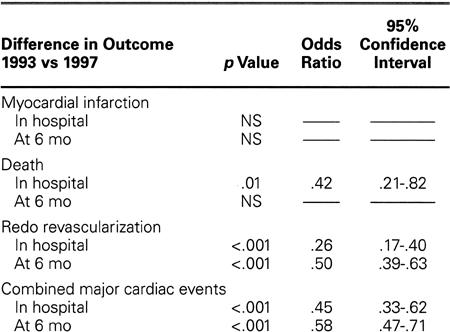
Table III shows the results of a multivariate logistic regression analysis performed to adjust for baseline clinical characteristics that were significantly different in 1993 than in 1997. After this adjustment, the incidence of MI in the 2 populations did not change, either at hospital discharge or at 6 months. After the adjustment, mortality and need for redo revascularization during hospitalization appeared to decrease significantly (by 58% and 74%, respectively). The 6-month mortality rate was not significantly changed, but the need for revascularization at 6 months decreased by 50%.
TABLE III. Risk-Adjusted Difference in Outcomes (In-Hospital and 6-Month Results): 1993 versus 1997
Discussion
When the STRESS 3 and BENESTENT 4 trials were presented in the literature as evidence that stent-CCI is generically better than balloon-CCI in coronary arteries with a diameter of at least 3 mm, several interventional cardiologists felt that this statement might have been radical and unwarranted. Indeed, it was conceivable that subsets of lesions could be found for which stand-alone balloon-CCI would still be easier, simpler, and more cost-effective than stent-CCI. However, it soon became apparent that cardiologists were unlikely to be able to identify variables that would allow “proper” selection of patients for stand-alone balloon-CCI. 5 The reliable, consistently “beautiful” results of coronary stenting (as documented by angiography and IVUS) empirically and forcefully tilted the balance in favor of the broad stent-when-feasible policy. This policy subsequently became established in clinical practice, even without proper validation, except possibly in cases involving total occlusion, aortocoronary ostial obstruction, acute MI, or vein graft lesions. 6–9 In our own experience from 1993 to 1997, more than 40% of the patients treated with stent-CCI did not meet the inclusion criteria of the STRESS and BENESTENT trials, which also differed from our experience because they did not involve a continuous series. We investigated the practice of routine stent use by analyzing our extensive experience with a continuous series of patients undergoing CCI at a large center that practices a relatively aggressive policy of coronary revascularization. The most striking results of our study can be summarized as follows.
Summary of Our Results
The indications for CCI were extended, mainly because of the advent of coronary stents. The incidence of CCI increased from 50.2% to 59.8% of the total number of revascularization procedures performed at our hospital during the study period (Fig. 2). At the same time, the incidence of surgical revascularization decreased from 49.9% to 40.0% (in absolute terms, from 1,692 to 1,540 procedures).
The availability of coronary stents (whether or not they were actually used) enabled CCI to be performed in a much safer and more controlled fashion, even in patients at relatively high risk. The increased risk in the 1997 population (compared with 1993) is evidenced by the higher incidence of factors associated with early and late adverse events (diabetes, recent MI, and congestive heart failure).
The efficiency (success rate) of CCI improved globally. The early angiographic success rate increased from 89.3% to 97.1% (Fig. 4).
After the introduction of coronary stents, the stability of the early results of CCI greatly improved, as evidenced by decreased in-hospital morbidity and mortality and by an increased clinical success rate (from 82.2% to 92.1%) (Fig. 4).
The need for emergent or urgent (same-day) surgical revascularization for failed angioplasty decreased by 70% (from 1.0% to 0.3%).
The in-hospital mortality rate after CCI decreased by 40% (from 1.65% to 0.99%) (Fig. 7). After adjustment for risk stratification, in-hospital mortality was found to have decreased by 50% (Table III).
The in-hospital and 6-month MI rates did not change significantly, but the global rate of freedom from major events during the first 6 months clearly increased, from 77.2% to 85.1% (Fig. 5). When these results were adjusted for risk, the combined major cardiac event rate was found to have decreased by 55% in the hospital and 42% at 6 months (Table III).
In particular, the incidence of redo revascularization decreased significantly during the first 6 postoperative months, from 19.6% to 10.7% for the overall group of CCI. After stent-CCI in the 1997 population, the incidence was 7.7% (lower than for any other type of CCI). When adjusted for risk, the 6-month rate of redo revascularization decreased by 42% (Table III).
Previous Reports
Using data from the large Palmaz-Schatz stent registry, Ellis and coworkers 10 reported on restenosis rates, but that early report (1992) concerned indications and technical circumstances that were quite different from ours. More recently, the literature has presented studies similar to ours, concerning “real-world” repercussions of the introduction of stenting into clinical practice. Schunkert, 8 McGrath, 9 Altmann, 11 Farshid, 12 and their associates have confirmed the same impressive decrease in redo revascularization after the progressive enactment of a policy of general stent use.
The report that most resembles ours appears to be the multicenter British Columbia survey published by Rankin and coworkers, 13 which analyzes their experience from April 1994 through 1997 and includes 1-year follow-up results. Their rate of stent use increased (from 14.2% to 58.7%) in a manner similar to ours, but the improvements in the early morbidity (MI, emergency surgery) and mortality rates were more dramatic in our population. In Rankin's survey, the 1-year mortality rate did not change significantly over the study period; likewise, our 6-month mortality rate remained stable, at about 3%.
Clinical Implications
The provisional or conditional stent-usage option (“stent only if certain operative criteria cannot be achieved by balloon-CCI alone”) has been pursued on the basis of parameters obtained in prospective studies involving coronary angiography, 5,14–16 IVUS, 17,18 pressure wires, 19 or Doppler measurement of flow velocity. 20–22 Despite their small size, these studies suggest that such a strategy is unlikely to be cost-efficient: when compared with routine stent-CCI, a policy of provisional stenting results in minor early savings that are outweighed by late increased costs for treating a heightened rate of restenosis. 15
Indeed, in our study, routine stent-CCI had significant advantages in the treatment of a large, mixed-risk population of patients at a single institution. Since 1994, when initial investigations were reported concerning coronary stent usage in limited and well-defined subsets of patients, 3,4 the indications have been widely extended in clinical practice. Today, it seems reasonable to reiterate that CCI in vessels measuring at least 3 mm in diameter should generally include the use of stents. The search for specific indications for stand-alone balloon-CCI seems futile and, at best, worth pursuing only for limited subgroups of lesions (for example, those treatable with the newer antiplatelet regimens as adjuncts to balloon-CCI). 7,23,24
Our experience confirms that coronary stents do not prevent the generation of restenotic tissue per se. Rather, they prevent elastic recoil, which is a major cause of restenosis after balloon-CCI. 25 By providing a larger, more stable, initial luminal opening, stents decrease the incidence of early complications and of redo revascularization at 6 months.
Limitations of Our Study
Our study is based on an observational, retrospective analysis of prospectively collected data. Therefore, it lacks the advantages of the strict experimental conditions seen in prospective randomized trials (clearly set indications and contraindications, uniform procedural protocols, and operator performance criteria). Also, the study is weakened by the lack of complete follow-up information and of objective, precise (angiographic or IVUS) evaluation at 6 months. With respect to in-hospital events, the completeness of parameter entry into our database was 100%. With respect to 6-month follow-up, the completeness was 80% for the written questionnaire but was probably higher for “events requiring hospitalization.” All of the events that required hospitalization at St. Luke's Episcopal Hospital were accounted for, but some patients may have been referred to other centers. Nevertheless, our study includes a wide variety of clinical conditions and anatomic/functional subgroups, and it involves a real-world analysis of a large, relatively stable population, which has been under the care of the same group of cardiologists for many years at a single institution. We believe that these advantages may compensate for the aforementioned methodologic inadequacies and that our data may complement the findings from smaller, controlled, prospective studies.
For a control group, we used our own balloon-CCI patients instead of those in the National Heart, Lung, and Blood Institute registry, 26 the most frequently used historical control group for the assessment of new devices. We believe that a cohort of patients who underwent “almost contemporary” CCI during the prestent period, at the same institution and by the same physicians, was a better control group for our study.
We were unable to clearly define the stent-when-feasible concept. As of 1997, it was probably still evolving. The main technical limitation of stent use is coronary luminal size: the 3.0-mm limit is based on preliminary experience 2 and the fact that only 3.0- and 3.5-mm stents were initially available. As experience has grown over the years, operators' aggressiveness has increased, and the early and mid-term outcomes have been encouraging. In our study, the only measure of our operators' aggressiveness in 1997 is the fact that stent-CCI accounted for 72.5% of the total number of CCI. In 1999, because of improved stent design and the advent of 2.25- and 2.50-mm stents, some of our operators used stents in 85% of their cases.
Our clinical-outcome analysis implies that stent use alone was responsible for beneficial changes observed during the study period. It is possible (but not amenable to proof except by an ad hoc prospective study) that other factors such as operator experience or newer medication protocols 23,24 could also have significantly affected the in-hospital outcomes, but these were unlikely to have affected our 6-month results.*
Cost issues were not addressed by our study, but they would surely be relevant to a final judgment concerning the merits of any CCI device. Earlier studies have suggested that the cost of stents is definitely justified by the lower restenosis and complication rates associated with these devices. 27 Treatment of in-stent restenosis may be more problematic than treatment of post-balloon-CCI restenosis. 28,29 So far, only local brachytherapy seems to promise consistently favorable short-term results for the treatment of in-stent obstruction, 30 but the late results of radiation may limit the early optimism. The possibility of late unfavorable consequences many years after the introduction of a stent into a coronary artery cannot be discounted on the basis of the limited currently available information. 31,32For these reasons, caution is still necessary in extending the indications for stent utilization.
Conclusions
The introduction of a “stent-when-feasible” policy at our institution has resulted in a dramatic decrease in adverse early and late events after CCI: both early success rates and 6-month adverse event rates support routine stenting of obstructed coronary arteries that have a diameter of at least 3 mm. This observation in a large, “real-world” population also may have important implications regarding the need for routine surgical standby during CCI. 33
Footnotes
*In the absence of a generic, widely accepted term for “angioplasty carried out by catheter,” we prefer to avoid the original term “percutaneous transluminal coronary angioplasty (PTCA),” which is often inconsistently used as a synonym for balloon catheter angioplasty. We also prefer to avoid “percutaneous coronary intervention (PCI),” because percutaneous entry is also involved in surgery. (According to Merriam-Webster's Dictionary, 1995 edition, percutaneous means “administered, removed, or absorbed by way of the skin.”) We favor “coronary catheter intervention (CCI),” which clearly indicates any procedure performed in the coronary arteries with a catheter.
*As diagnosed by the admitting physician on the basis of a clinical event and as documented by enzyme-level elevation (creatine phosphokinase levels higher than twice the maximal normal value, with isoenzymes positive for myocardial infarction) or by electrocardiographic changes (Q and non-Q waves), or by both.
*Although abciximab reportedly decreases the incidence of major adverse coronary events during the first 30 days after coronary angioplasty, our retrospective study was unable to confirm or deny this finding. Analysis of the 2,538 stent-CCI cases performed in 1996–97 revealed that abciximab was used during the procedure in 700 cases (36.3%) for “high-risk” conditions that could not be analyzed retrospectively. The rate of major adverse coronary events before hospital discharge was 6.1% with abciximab and 3.4% without abciximab (p = 0.002). At 6 months, the same rates were 12.9% and 10.9%, respectively (p = 0.16), including in-hospital events.
Address for reprints: Paolo Angelini, MD, P.O. Box 20206, Houston, TX 77030
References
- 1.Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med 1987;316:701–6. [DOI] [PubMed]
- 2.Schatz RA, Baim DS, Leon M, Ellis SG, Goldberg S, Hirshfeld JW, et al. Clinical experience with the Palmaz-Schatz coronary stent. Initial results of a multicenter study. Circulation 1991;83:148–61. [DOI] [PubMed]
- 3.Fischman DL, Leon MB, Baim DS, Schatz RA, Savage MP, Penn I, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med 1994;331:496–501. [DOI] [PubMed]
- 4.Serruys PW, de Jaegere P, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med 1994;331:489–95. [DOI] [PubMed]
- 5.Foley DP, Serruys PW. Provisional stenting—stent-like balloon angioplasty: evidence to define the continuing role of balloon angioplasty for percutaneous coronary revascularization. Semin Interv Cardiol 1996;1:269–73. [PubMed]
- 6.Mahdi NA, Lopez J, Leon M, Pathan A, Harrell L, Jang IK, et al. Comparison of primary coronary stenting to primary balloon angioplasty with stent bailout for the treatment of patients with acute myocardial infarction. Am J Cardiol 1998;81:957–63. [DOI] [PubMed]
- 7.Randomised placebo-controlled and balloon-angioplasty-controlled trial to assess safety of coronary stenting with use of platelet glycoprotein-IIb/IIIa blockade. The EPI-STENT Investigators. Evaluation of Platelet IIb/IIIa Inhibitor for Stenting. Lancet 1998;352:87–92. [DOI] [PubMed]
- 8.Schunkert H, Harrel L, Palacios IF. Implications of small reference vessel diameter in patients undergoing percutaneous coronary revascularization. J Am Coll Cardiol 1999;34:40–8. [DOI] [PubMed]
- 9.McGrath PD, Malenka DJ, Wennberg DE, Shubrooks SJ Jr, Bradley WA, Robb JF, et al. Changing outcomes in percutaneous coronary interventions: a study of 34,752 procedures in northern New England, 1990 to 1997. Northern New England Cardiovascular Disease Study Group. J Am Coll Cardiol 1999;34:674–80. [DOI] [PubMed]
- 10.Ellis SG, Savage M, Fischman D, Baim DS, Leon M, Goldberg S, et al. Restenosis after placement of Palmaz-Schatz stents in native coronary arteries. Initial results of a multicenter experience. Circulation 1992;86:1836–44. [DOI] [PubMed]
- 11.Altmann DB, Racz M, Battleman DS, Bergman G, Spo-kohny A, et al. Reduction in angioplasty complications after the introduction of coronary stents: results from a consecutive series of 2242 patients. Am Heart J 1996;132:503–7. [DOI] [PubMed]
- 12.Farshid A, Allan RM, Giles RW, McCredie RM, Pitney MR, Walsh WF. Impact of an aggressive coronary stenting strategy on the incidence of target lesion revascularization. Am J Cardiol 1998;82:1441–4. [DOI] [PubMed]
- 13.Rankin JM, Spinelli JJ, Carere RG, Ricci DR, Penn IM, Hilton JD, et al. Improved clinical outcome after widespread use of coronary-artery stenting in Canada. N Engl J Med 1999;341:1957–65. [DOI] [PubMed]
- 14.Rodriguez AE, Santaera O, Larribau M, Fernandez M, Sar-miento R, Perez Balino, et al. Coronary stenting decreases restenosis in lesions with early loss in luminal diameter 24 hours after successful PTCA. Circulation 1995;91:1397–402. [DOI] [PubMed]
- 15.Weaver WD. Optimal angioplasty versus primary stenting (OPUS) [abstract: Late-breaking trials in clinical cardiology] J Am Coll Cardiol 1999;34:1.
- 16.Rodriguez AE, Grinfeld L, Balino NP, et al. Argentine randomized study optimal coronary balloon angioplasty and stenting vs coronary bypass surgery in multiple vessel disease (ERACI II): in hospital and 30-day results [abstract]. J Am Coll Cardiol 1999;33(Suppl A):33A.9935005
- 17.Haase KK, Athanasiadis A, Mahrholdt H, Treusch A, Wullen B, Jarmillo C, et al. Acute and one year follow-up results after vessel size adapted PTCA using intracoronary ultrasound. Eur Heart J 1998;19:263–72. [DOI] [PubMed]
- 18.de Jaegere P, Mudra H, Figulla H, Almagor Y, Doucet S, Penn I, et al. Intravascular ultrasound-guided optimized stent deployment. Immediate and 6 months clinical and angiographic results from the Multicenter Ultrasound Stenting in Coronaries Study. Eur Heart J 1998;19:1214–23. [DOI] [PubMed]
- 19.Kern MJ, de Bruyne B, Pijls NH. From research to clinical practice: current role of intracoronary physiologically based decision making in the cardiac catheterization laboratory. J Am Coll Cardiol 1997;30:613–20. [DOI] [PubMed]
- 20.Di Mario C. The DESTINI-CFR Study Group. Doppler and QCA guided aggressive PTCA has the same target lesion revascularization of stent implantation: 6-month-results of the DESTINI study [abstract]. J Am Coll Cardiol 1999;33(Suppl A):47A.
- 21.Serruys PW, di Mario C, Piek J, Schroeder E, Vrints C, Probst P, et al. Prognostic value of intracoronary flow velocity and diameter stenosis in assessing the short- and long-term outcomes of coronary balloon angioplasty: the DEBATE Study (Doppler Endpoints Balloon Angioplasty Trial Europe). Circulation 1997;96:3369–77. [DOI] [PubMed]
- 22.Bech GJ, Pijls NH, De Bruyne B, Peels KH, Michels HR, Bonnier HJ, et al. Usefulness of fractional flow reserve to predict clinical outcome after balloon angioplasty. Circulation 1999;99:883–8. [DOI] [PubMed]
- 23.Kereiakes DJ. Preferential benefit of platelet glycoprotein IIb/IIIa receptor blockade: specific considerations by device and disease state. Am J Cardiol 1998;81(7A):49E–54E. [DOI] [PubMed]
- 24.Lincoff AM, Califf RM, Moliterno DJ, Ellis SG, Ducas J, Kramer JH, et al. Complementary clinical benefits of coronary-artery stenting and blockade of platelet glycoprotein IIb/IIIa receptors. Evaluation of Platelet Iib/IIIa Inhibition in Stenting Investigators. N Engl J Med 1999;341:319–27. [DOI] [PubMed]
- 25.Topol EJ, Serruys PW. Frontiers in interventional cardiology. Circulation 1998;98:1802–20. [DOI] [PubMed]
- 26.Detre K, Holubkov R, Kelsey S, Cowley M, Kent K, Williams D, et al. Percutaneous transluminal coronary angioplasty in 1985–1986 and 1977–1981. The National Heart, Lung, and Blood Institute Registry. N Engl J Med 1988;318:265–70. [DOI] [PubMed]
- 27.Cohen DJ, Krumholz HM, Sukin CA, Ho KK, Siegrist RB, Cleman M, et al. In-hospital and one-year economic outcomes after coronary stenting or balloon angioplasty. Results from a randomized clinical trial. Stent Restenosis Study Investigators. Circulation 1995;92:2480–7 [DOI] [PubMed]
- 28.Mintz GS, Hoffmann R, Mehran R, Pichard AD, Kent KM, Satler LF, et al. In-stent restenosis: the Washington Hospital Center experience. Am J Cardiol 1998;81(7A):7E–13E. [DOI] [PubMed]
- 29.Farb A, Sangiorgi G, Carter AJ, Walley VM, Edwards WD, Schwartz RS, et al. Pathology of acute and chronic coronary stenting in humans. Circulation 1999;99:44–52. [DOI] [PubMed]
- 30.Teirstein PS, Massullo V, Jani S, Russo RJ, Cloutier DA, Schatz RA, et al. Two-year follow-up after catheter-based radiotherapy to inhibit coronary restenosis. Circulation 1999;99:243–7. [DOI] [PubMed]
- 31.Laham RJ, Carrozza JP, Berger C, Cohen DJ, Kuntz RE, Baim DS. Long-term (4- to 6-year) outcome of Palmaz-Schatz stenting: paucity of late clinical stent-related problems. J Am Coll Cardiol 1996;28:820–6. [DOI] [PubMed]
- 32.Kimura T, Yokoi H, Nakagawa Y, Tamura T, Kaburagi S, Sawada Y, et al. Three-year follow-up after implantation of metallic coronary-artery stents. N Engl J Med 1996;334:561–6. [DOI] [PubMed]
- 33.Angelini P. Guidelines for surgical standby for coronary angioplasty: should they be changed? J Am Coll Cardiol 1999;33:1266–8. [DOI] [PubMed]


