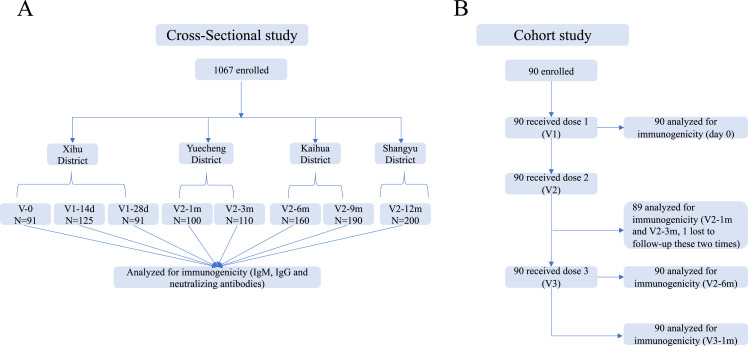
Figure 1. Schedule of sample collection.
(A) Cross-sectional survey: a total of 1067 participants aged 18–59 were enrolled in five counties in Zhejiang, China. The participants had no previous vaccination or were vaccinated with one or two doses of CoronaVac. Venous blood (3–5 ml) was collected on day 0 (V-0, no vaccination), day 14±2 (V1-14d), and day 28±3 (V1-28d) after the first dose, and day 30±3 (V2-1m), day 90±7 (V2-3m), day 180±14 (V2-6m), day 270±14 (V2-9m), and day 365±30 (V2-12m) after the second dose. (B) Prospective cohort study: 90 healthy adults aged 18–80 years in Jiaxing city were recruited and administered 4 µg/0.5 mL of CoronaVac following a 3-shot vaccine schedule 28 days and 6 months apart. Following that, venous blood was collected from recipients at five timepoints: day 0 (Pre-V, before vaccination), day 30±3 (V2-1m), day 90±7 (V2-3m), and day 180±14 (V2-6m) after the second dose, and day 30±3 (V3-1m) after the third dose.