Abstract
Background
In this study, we compared admission incidence risk and the risk of mortality in the Omicron BA.4/BA.5 wave to previous waves.
Methods
Data from South Africa's SARS-CoV-2 case linelist, national COVID-19 hospital surveillance system, and Electronic Vaccine Data System were linked and analyzed. Wave periods were defined when the country passed a weekly incidence of 30 cases/100 000 population. In-hospital case fatality ratios (CFRs) during the Delta, Omicron BA.1/BA.2, and Omicron BA.4/BA.5 waves were compared using post-imputation random effect multivariable logistic regression models.
Results
The CFR was 25.9% (N = 37 538 of 144 778), 10.9% (N = 6123 of 56 384), and 8.2% (N = 1212 of 14 879) in the Delta, Omicron BA.1/BA.2, and Omicron BA.4/BA.5 waves, respectively. After adjusting for age, sex, race, comorbidities, health sector, and province, compared with the Omicron BA.4/BA.5 wave, patients had higher risk of mortality in the Omicron BA.1/BA.2 wave (adjusted odds ratio [aOR], 1.3; 95% confidence interval [CI]: 1.2–1.4) and Delta wave (aOR, 3.0; 95% CI: 2.8–3.2). Being partially vaccinated (aOR, 0.9; 95% CI: .9–.9), fully vaccinated (aOR, 0.6; 95% CI: .6–.7), and boosted (aOR, 0.4; 95% CI: .4–.5) and having prior laboratory-confirmed infection (aOR, 0.4; 95% CI: .3–.4) were associated with reduced risks of mortality.
Conclusions
Overall, admission incidence risk and in-hospital mortality, which had increased progressively in South Africa's first 3 waves, decreased in the fourth Omicron BA.1/BA.2 wave and declined even further in the fifth Omicron BA.4/BA.5 wave. Mortality risk was lower in those with natural infection and vaccination, declining further as the number of vaccine doses increased.
Keywords: COVID-19, hospital admissions, mortality, Omicron BA.4, Omicron BA.5
Admission incidence risk and in-hospital mortality decreased in the Omicron BA.1/BA.2 wave, reducing even further in the Omicron BA.4/BA.5 wave. Mortality risk was lower in those with natural infection and vaccination, declining further as the number of vaccine doses increased.
Until the emergence of the Omicron variant in November 2021, each new variant of concern that spread in South Africa led to more infections, hospitalizations, and deaths. The Omicron variant of concern (VOC) marked a shift in the coronavirus disease 2019 (COVID-19) pandemic. The Omicron BA.1 subvariant, while more transmissible and associated with immune escape, was reported to be less virulent as it showed attenuated replication in mice [1] and more upper respiratory tract infections compared with the lower respiratory tract [2]. It emerged at a time when 73% of South Africans had developed hybrid immunity through vaccination and/or prior infection [3]. As a result of high levels of immunity and a less virulent variant, in the fourth Omicron BA.1–dominated wave in South Africa, lower levels of hospitalization, severity, and mortality were observed compared with previous waves dominated by D614G, Beta, and Delta variants [4, 5]. Reduced severity with Omicron BA.1 was also reported in other countries [6–9]. However, in a study in Hong Kong, similar mortality was reported among individuals infected with Omicron who did not have preexisting immunity compared with those infected with earlier variants [10].
After the Omicron BA.1/BA.2 wave, a number of additional Omicron subvariants (rather than a new variant of concern) caused resurgences in many parts of the world. In South Africa, BA.3 never became dominant and circulated at low levels [11]. Omicron BA.4 and BA.5 were detected in South Africa in February 2022 [12], and both jointly dominated the fifth wave from April 2022 to June 2022. Shortly after having experienced waves related to Omicron BA.1/BA.2, Omicron BA.4 and/or BA.5 became dominant globally, and cases increased in many countries [13].
Omicron subvariants are more transmissible than previous VOCs, with greater immune escape, with BA.4/BA.5 showing reduced neutralization from antibodies induced by BA.1 infection, more so in the unvaccinated [14, 15]. Early data showed that Omicron BA.4/BA.5 waves had reduced severity in South Africa [16] and the United States [17].
It remains important to understand the characteristics of severe disease caused by new VOCs or viral lineages in different geographic locations in order to guide public health policy and planning. Our purpose in this study was to investigate trends in the incidence of laboratory-confirmed COVID-19 hospitalizations by age group in each COVID-19 wave in South Africa and compare the risk of mortality in the most recent Omicron BA.4/BA.5 wave with the Omicron BA.1 and the Delta waves.
METHODS
Data on real-time reverse-transcription polymerase chain reaction and antigen-positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cases were collated daily from laboratory reports, while data on COVID-19 hospital admissions were collected through DATCOV, an active surveillance program established specifically for COVID-19. These case and hospital surveillance systems have been previously described [18].
Data for the period from the first case in South Africa on 5 March 2020 to 28 May 2022 were analyzed. Three data sources were linked for this analysis using South African identification numbers, first names, surnames, and dates of birth: the DATCOV national COVID-19 hospital surveillance; the national SARS-CoV-2 case linelist and the Notifiable Medical Conditions Surveillance System (NMC-SS), both established by the National Institute for Communicable Diseases; and the Electronic Vaccine Data System (EVDS), established by the National Department of Health.
Incidence risks were calculated using Statistics South Africa mid-year population figures for 2021 [19]. A weekly incidence of 30 cases/100 000 population was the threshold for the start and end of each wave, thereby defining the distinct periods of analysis, namely, D614G (first) wave from 7 June 2020 to 22 August 2020, Beta (second) wave from 15 November 2020 to 6 February 2021, Delta (third) wave from 9 May 2021 to 18 September 2021, Omicron BA.1/BA.2 (fourth) wave from 28 November 2021 to 5 February 2022, and Omicron BA.4/BA.5 (fifth) wave from 17 April 2022 to 28 May 2022.
Analysis of mortality was restricted to admissions that had already accumulated outcomes and all patients still in the hospital or transferred to other hospitals without final outcomes were excluded because they remained at risk of still developing severe outcomes including death. The proportion of patients with reported outcomes in each wave (including the most recent Omicron BA.4/BA.5 wave) was more than 98% (Supplementary Table 2). Descriptive statistics were used to describe the trends in numbers of cases, admissions, mortality, and case fatality ratios (CFRs) over the equivalent periods of the D614G, Beta, Delta, Omicron BA.1/BA.2, and Omicron BA.4/BA.5 waves.
Post-imputation random effect (on admission facility) multivariable logistic regression models were used to compare mortality among the Delta, Omicron BA.1/BA.2, and Omicron BA.4/BA.5 waves. To account for incomplete or missing data on selected variables, we used multivariate imputation by chained equation (MICE) and generated 10 complete imputed datasets that were used for subsequent analyses. Variables analyzed using MICE included race and comorbidities, where up to one-third of the data were missing. Complete or near complete variables included in the imputation process were age, sex, province, health sector (ie, public or private), in-hospital outcome (ie, discharged alive or died), and vaccination status (see Supplementary Table 1 for variable completeness).
Age, sex, race, presence of a comorbidity (which included hypertension, diabetes, chronic cardiac disease, chronic kidney disease, asthma/chronic pulmonary disease, malignancy, human immunodeficiency virus, and tuberculosis), type of health sector (private or public), province, vaccination status, and recorded laboratory-confirmed prior infection were included in the model to assess the relationship between each wave period and mortality in SARS-CoV-2–positive patients admitted to the hospital. These variables were selected a priori as they are known risk factors for COVID-19 mortality cited in the literature; all possible confounders were included; and the interaction terms that were used revealed no significant change to the model estimates. Prior infection was determined through linking to the NMC-SS and regarded as affirmative if an individual had a recorded positive SARS-CoV-2 test more than 90 days after a previous positive test. Vaccination status was determined at date of admission through EVDS linkage. Individuals were considered to be unvaccinated if they had not received any COVID-19 vaccine doses, partially vaccinated if they received 1 dose of BNT162b, fully vaccinated if they received 2 doses of BNT162b or 1 dose of Ad26.COV2.S with the most recent dose at least 14 days earlier, and boosted if they received at least 1 additional COVID-19 vaccine dose of any kind in addition to full vaccination. South Africa first rolled out COVID-19 vaccination among healthcare workers in February 2021, using Ad26.COV2.S under the Sisonke program. In May 2021, vaccination using BNT162b or Ad26.COV2.S was introduced for individuals aged >60 years, then expanding to those aged 35–50 years in July 2021, 18–35 years in August 2021, and 12–18 years in October 2021. In December 2021, booster doses were introduced.
The statistical analysis was implemented using Stata 15 (StataCorp, College Station, TX). Ethical approval was obtained from the University of the Witwatersrand Human Research Ethics Committee (medical) for the collection of SARS-CoV-2 case data and for the DATCOV hospital surveillance program.
RESULTS
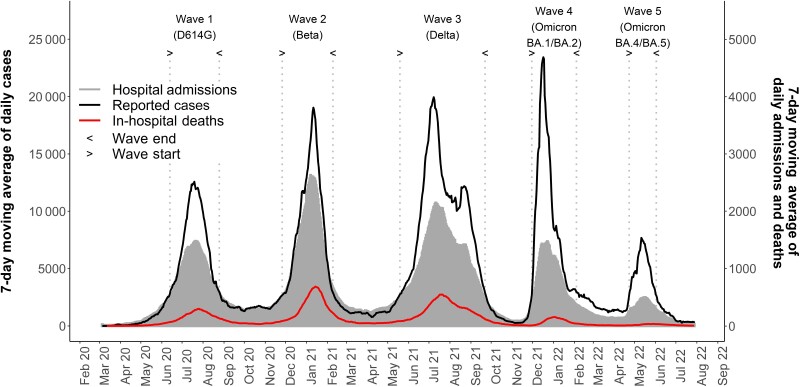
The 7-day moving average of daily SARS-CoV-2 cases had a peak that was higher in each successive wave in the first 4 waves but lower in the fifth wave (Figure 1). The numbers of hospital admissions in each of the 5 waves were 71 248 (D614G), 105 623 (Beta), 147 516 (Delta), 57 510 (Omicron BA.1/BA.2), and 15 185 (Omicron BA.4/BA.5). Unlike the pattern observed in the prior waves, the rise in cases during the Omicron BA.1/BA.2 and Omicron BA.4/BA.5 waves was not accompanied by a concomitant rise in hospital admissions and in-hospital deaths.
Figure 1.
The 7-day moving average of daily severe acute respiratory syndrome coronavirus 2 cases and admissions.
The incidence of COVID-19 admissions per 100 000 population was highest in those aged ≥60 years in each wave (Table 1, Supplementary Figure 1). In the fourth and fifth waves, incidence was highest in those aged ≥60 years followed by aged <1 years. In adults and children aged 5–18 years, admission incidence risk increased from the first to the third wave, then decreased in the fourth and fifth waves to the lowest incidence seen in any previous waves. In children aged ≤5 years, admission incidence risk increased from the first wave to the fourth wave, then decreased in the fifth wave to similar incidence as the second wave.
Table 1.
Coronavirus Disease 2019 Admissions Incidence Risk Per 100 000 Population by Age Group (in Years) and Wave Period, South Africa, 5 March 2020–28 May 2022 (N = 397 082)
| Age Group, y (Population Size) | Incidence Risk Per 100 000 Population (Number of Admissions) | ||||
|---|---|---|---|---|---|
| Wave 1 D614G | Wave 2 Beta | Wave 3 Delta | Wave 4 Omicron BA.1/BA.2 | Wave 5 Omicron BA.4/BA.5 | |
| <1 (1 175 632) | 37.7 (443) | 72.9 (857) | 151.7 (1783) | 172.4 (2027) | 80.0 (940) |
| 1–4 (4 533 324) | 8.3 (375) | 15.2 (689) | 34.6 (1570) | 44.0 (1993) | 19.1 (868) |
| 5–18 (15 299 104) | 8.8 (1346) | 11.0 (1683) | 31.1 (4751) | 26.2 (4003) | 8.1 (1242) |
| 19–39 (21 624 489) | 71.0 (15 343) | 86.6 (18 737) | 134.2 (29 026) | 89.0 (19 245) | 16.7 (3602) |
| 40–59 (12 005 082) | 245.8 (29 506) | 344.3 (41 339) | 455.1 (54 634) | 113.7 (13 653) | 27.9 (3359) |
| 60+ (5 505 347) | 440.2 (24 235) | 768.7 (42 318) | 1012.7 (55 752) | 301.3 (16 589) | 94.1 (5180) |
| Total (60 142 978) | 118.5 (71 248) | 175.6 (105 623) | 245.3 (147 516) | 95.6 (57 510) | 25.2 (15 185) |
The in-hospital CFR was 25.9% (37 538 of 144 778), 10.9% (6123 of 56 384), and 8.2% (1212 of 14 879) in the Delta, Omicron BA.1/BA.2, and Omicron BA.4/BA.5 periods, respectively (Table 2). The age-specific CFRs for each wave show that the CFR was higher in older age groups across all waves and was lowest in each age group in the Omicron BA.1/BA.2 and Omicron BA.4/BA.5 waves (Table 2).
Table 2.
Coronavirus Disease 2019 In-Hospital Case Fatality Ratio by Age Group (in Years) and Wave Period, South Africa, 5 March 2020–28 May 2022 (N = 390 023)
| Age Group, y | CFR % (Number of Deaths/Total Admissions With Outcomes) | ||||
|---|---|---|---|---|---|
| Wave 1 D614G | Wave 2 Beta | Wave 3 Delta | Wave 4 Omicron BA.1/BA.2 | Wave 5 Omicron BA.4/BA.5 | |
| <1 | 8.9 (38/429) | 5.1 (42/830) | 5.9 (104/1754) | 2.4 (47/1987) | 1.3 (12/917) |
| 1–4 | 0.8 (3/368) | 3.1 (21/676) | 1.2 (19/1549) | 1.3 (25/1968) | 0.3 (5/853) |
| 5–18 | 3.8 (50/1303) | 3.8 (62/1637) | 2.9 (131/4555) | 1.6 (61/3908) | 1.2 (15/1215) |
| 19–39 | 7.0 (1051/15 044) | 9.8 (1807/18 381) | 9.0 (2543/28 406) | 4.8 (899/18 804) | 4.3 (151/3530) |
| 40–59 | 17.2 (4998/29 080) | 22.8 (9283/40 740) | 22.9 (12 282/53 724) | 11.4 (1525/13 371) | 7.6 (250/3283) |
| 60+ | 37.8 (9028/23 876) | 43.6 (18 138/41 618) | 41.0 (22 459/54 790) | 21.9 (3566/16 310) | 15.3 (779/5081) |
| Total | 21.6 (15 168/70 100) | 28.3 (29 353/103 882) | 25.9 (37 538/144 778) | 10.9 (6123/56 384) | 8.2 (1212/14 879) |
Abbreviation: CFR, case fatality ratio.
Vaccination coverage among 144 778 patients admitted in the Delta wave was 12 774 (8.8%) partially vaccinated, 5501 (3.8%) fully vaccinated, and 2 (<0.01%) boosted, while 126 501 (87.4%) were unvaccinated. Among 56 384 patients admitted in the Omicron BA.1/BA.2 wave, 2075 (3.7%) were partially vaccinated, 13 494 (23.9%) were fully vaccinated, and 435 (0.8%) were boosted, while 40 380 (71.6%) were unvaccinated. Among 14 879 patients admitted in the Omicron BA.4/BA.5 wave, 446 (3.0%) were partially vaccinated, 3927 (26.4%) were fully vaccinated, and 1271 (8.5%) were boosted, while 9235 (62.1%) were unvaccinated.
On multivariable analysis (Table 3), after adjusting for age, sex, race, presence of a comorbidity, health sector (private/'public), and province, compared with the Omicron BA.4/BA.5 wave, patients had a higher risk of mortality in the Omicron BA.1 (adjusted odds ratio [aOR], 1.3; 95% confidence interval [CI]: 1.2–1.4) and Delta (aOR, 3.0; 95% CI: 2.8–3.2) waves. Being partially vaccinated (aOR, 0.9; 95% CI: .9–.9), fully vaccinated (aOR, 0.6; 95% CI: .6–.7), and boosted (aOR, 0.4; 95% CI: .4–.5) and having prior laboratory-confirmed infection (aOR, 0.4; 95% CI: .3–.4) were associated with reduced mortality (Table 3).
Table 3.
Factors Associated With Mortality Among Severe Acute Respiratory Syndrome Coronavirus 2–Positive Hospitalized Patients in the Delta (9 May–18 September 2021), Omicron BA.1/BA.2 (28 November 2021–5 February 2022), and Omicron BA.4/BA.5 Waves (17 April–28 May 2022), South Africa
| Characteristic | Unimputed Case Fatality Ratio, % (n/N) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | P Value |
|---|---|---|---|---|
| Wave period | ||||
| ȃOmicron BA.4/BA.5 | 8.2 (1212/14 879) | Reference | Reference | |
| ȃDelta | 25.9 (37 538/144 778) | 3.9 (3.7–4.1) | 3.0 (2.8–3.2) | <.001 |
| ȃOmicron BA.1/BA.2 | 10.9 (6123/56 384) | 1.3 (1.2–1.4) | 1.3 (1.2–1.4) | <.001 |
| Prior infection | ||||
| ȃNo | 21.4 (44 297/207 029) | Reference | Reference | |
| ȃYes | 6.4 (576/9012) | 0.3 (.3–.3) | 0.4 (.3–.4) | <.001 |
| Vaccination | ||||
| ȃNo | 21.4 (37 672/176 116) | Reference | Reference | |
| ȃPartial | 28.6 (4381/15 295) | 1.6 (1.5–1.6) | 0.9 (.9–.9) | <.001 |
| ȃFull | 11.8 (2712/22 922) | 0.6 (.5–.6) | 0.6 (.6–.7) | <.001 |
| ȃBoosted | 6.3 (108/1708) | 0.3 (.2–.4) | 0.4 (.4–.5) | <.001 |
| Age group, y | ||||
| ȃ<20 | 2.3 (450/19 968) | Reference | Reference | |
| ȃ20–39 | 7.2 (3562/49 514) | 3.2 (2.9–3.5) | 2.9 (2.6–3.2) | <.001 |
| ȃ40–59 | 20.0 (14 057/70 378) | 10.8 (9.8–11.9) | 7.8 (7.1–8.6) | <.001 |
| ȃ60+ | 35.2 (26 804/76 181) | 23.6 (21.5–26.0) | 18.2 (16.5–20.1) | <.001 |
| Sex | ||||
| ȃFemale | 19.1 (22 617/118 577) | Reference | Reference | |
| ȃMale | 22.8 (22 236/97 340) | 1.3 (1.3–1.3) | 1.3 (1.3–1.3) | <.001 |
| Race | ||||
| ȃWhite | 24.9 (4494/18 772) | Reference | Reference | |
| ȃMixed | 25.2 (2089/8285) | 0.8 (.8–.9) | 1.1 (1.1–1.2) | .0004 |
| ȃBlack | 21.3 (19 882/93 367) | 0.6 (.6–.6) | 1.1 (1.1–1.2) | <.001 |
| ȃIndian | 21.7 (1331/6141) | 1.0 (.9–1.0) | 1.2 (1.1–1.3) | <.001 |
| ȃOther | 21.3 (97/455) | 0.7 (.5–.8) | 0.9 (.7–1.2) | .6150 |
| Comorbid conditiona | ||||
| ȃNo | 15.3 (11 795/77 123) | Reference | Reference | |
| ȃYes | 26.0 (13 560/52 169) | 2.3 (2.3–2.4) | 1.5 (1.4–1.5) | <.001 |
| Health sector | ||||
| ȃPrivate | 17.4 (18 312/105 456) | Reference | Reference | |
| ȃPublic | 24.0 (26 561/110 585) | 1.6 (1.4–1.9) | 1.5 (1.4–1.5) | <.001 |
| Province | ||||
| ȃWestern Cape | 20.2 (8220/40 757) | Reference | Reference | |
| ȃEastern Cape | 24.8 (3642/14 702) | 1.3 (1.0–1.7) | 1.3 (1.1–1.6) | .0038 |
| ȃFree State | 20.6 (2688/13 064) | 1.1 (.8–1.5) | 1.0 (.8–1.3) | .6768 |
| ȃGauteng | 20.9 (15 663/75 037) | 1.0 (.8–1.2) | 1.2 (1.0–1.5) | .0211 |
| ȃKwaZulu-Natal | 18.4 (6025/32 789) | 0.9 (.7–1.1) | 1.1 (.9–1.4) | .1741 |
| ȃLimpopo | 25.0 (2466/9851) | 1.8 (1.3–2.4) | 1.5 (1.2–1.9) | .0002 |
| ȃMpumalanga | 21.7 (2219/10 206) | 1.6 (1.2–2.2) | 1.4 (1.1–1.8) | .0084 |
| ȃNorth West | 18.3 (2771/15 115) | 0.8 (.6–1.2) | 0.8 (.6–1.1) | .1133 |
| ȃNorthern Cape | 26.1 (1179/4520) | 1.8 (1.2–2.7) | 1.5 (1.1–2.0) | .0143 |
Univariate and multivariable analysis implemented on the imputed dataset (N = 216 041). Random effects multivariate logistic regression model controlling for clustering by facility.
Abbreviations: CI, confidence interval; OR, odds ratio.
Comorbid conditions included hypertension, diabetes, chronic cardiac disease, chronic kidney disease, asthma/chronic pulmonary disease, malignancy, human immunodeficiency virus, and active and past tuberculosis.
DISCUSSION
The fifth COVID-19 wave in South Africa, due predominantly to Omicron BA.4 and BA.5 subvariants, had a lower peak number of cases, risk of hospital admission, and risk of in-hospital mortality compared with all previous waves. Vaccination and prior infection were protective against COVID-19 mortality.
The reduced severity in the Omicron BA.1/BA.2 wave was thought to be related to multiple factors including a less virulent variant and widespread immunity due to vaccination and natural infection in South Africa [3–5]. A combination of natural infection and vaccination, referred to as hybrid immunity, has been shown to protect better against Omicron BA.1 symptomatic COVID-19 than infection-only immunity [20].
While some South African studies have suggested that Omicron BA.4 and BA.5 had disease severity that was similar to that of Omicron BA.1 and BA.2 [16, 21], our data indicate a lower risk of mortality in the Omicron BA.4/BA.5 wave compared with the Omicron BA.1/BA.2 wave. In our study, we analyzed data from a national dataset rather than a single province compared with Davies et al [21] and included all admissions by wave period rather than only a sample of those sequenced or with S-gene target failure, and our data were fully linked to the national vaccination database rather than relying on self-reported vaccine status compared with Wolter et al [16]. Immunological observations from South Africa point to considerable immune escape of Omicron BA.4 and BA.5 from Omicron BA.1 elicited immunity, but much less so in those with vaccination [15]. In other settings such as the United States, a higher risk of breakthrough infection with Omicron BA.4 or BA.5 was observed among previously vaccinated or infected individuals, indicating greater immune escape, but these breakthrough infections were associated with low severity [17]. Cell-mediated immunity likely contributed to the lower levels of hospitalization and mortality in the Omicron BA.4/BA.5 wave [22].
A South African study with prospective ascertainment of repeat infections demonstrated that prior infection, whether symptomatic or not, provided durable protection against reinfection beyond 1 year and resulted in a complex immune landscape [23]. In contrast, Hong Kong's Omicron BA.2.2 outbreak and high mortality among older individuals could be explained by low immunity (the incidence of COVID-19 was very low in Hong Kong as a result of pandemic control efforts, and protection for most individuals was likely elicited by vaccination rather than by infection) [24]. The fifth wave in Hong Kong showed mortality that was similar to mortality in previous waves in unvaccinated individuals and double risk of mortality among those unvaccinated compared with vaccinated [10]. In Australia, with low levels of cases in previous waves, mortality in the Omicron wave was the highest to date compared with the reduced mortality seen in South Africa, Israel, and a number of European countries [25].
The reduced mortality in the Omicron BA.4/BA.5 wave was more specifically presumed to be related to the high prevalence of humoral and cell-mediated immunity in South Africa. Studies of humoral immunity reported SARS-CoV-2 antibody seroprevalence estimates of 71% [26] and 73% [3] before the Omicron BA.1/BA.2 wave, while estimates after the Omicron BA.1/BA.2 wave but just before the Omicron BA.4/BA.5 wave were 97% [27] and 91% [28]. The latter serosurvey indicated that 61% of individuals had serological evidence of SARS-CoV-2 infection during the Omicron BA.1/BA.2 wave in South Africa [28]. In addition, 49% of adult South Africans had received at least 1 vaccine dose by April 2022, although the uptake of COVID-19 booster doses was low, with less than 5% of people having received a booster [29]. The South African vaccination program is described in the Methods section. The prevalence of cell-mediated immunity following natural infection or vaccination is likely to follow a similar trend in South Africa.
Some have expressed uncertainty about ascribing lower severity in the Omicron waves to an intrinsically less virulent variant [30]. The propensity of Omicron BA.1 to infect the upper airways rather than the lower airways suggests a mechanism for less severe disease [2], and mice studies have suggested less pathogenic disease [31]. However, an animal study suggested that Omicron BA.4/BA.5 in the absence of prior protection may be more pathogenic than Omicron BA.2 [32].
Admission incidence in the fifth wave was the lowest seen to date in individuals aged ≥5 years. However, in children aged <5 years, where vaccination is not yet provided in South Africa, admission incidence in the BA.4/BA.5 wave was similar to the Beta wave and higher than in the D614G wave. The U-shaped distribution of COVID-19 admissions (with higher admissions in children aged <5 years compared with older children and young adults) is similar to what is observed for other severe respiratory illnesses including influenza [33]. This pattern is likely due to the immunity gap with young children having lower infection rates than adults and not being eligible for vaccination. Modelers have suggested that characteristics of the shift toward endemicity include a transition in the age structure once the disease reaches seasonal endemism [34].
A strength of this analysis is its high-quality national database and linked data on COVID-19 vaccination and prior infection for the comparisons of Omicron BA.4/BA.5 severity with previous variants. This study has several limitations. Reported cases are dependent on case ascertainment that is influenced by uptake of testing. Testing strategies for determining cases have changed over time, and testing rates have been lowest during the Omicron BA.4/BA.5 wave compared with any previous waves. However, testing of hospitalized patients who present with suggestive symptoms has changed little and the criteria for hospital admission with COVID-19 has not changed much over time, except that lower admissions in the 2 Omicron waves resulted in more hospital bed capacity than in previous waves, so more patients could have been admitted with milder disease due to available bed capacity.
A further limitation is that the DATCOV database has less than 50% completeness of the reason for admission field, which makes it difficult to discern between those admitted for COVID-19 and those admitted to the hospital coincidentally with SARS-CoV-2 infection. However, this did not vary significantly between wave periods and among patients who did have admission reason completed; COVID-19 symptoms were the reason for admission in 75%, 78%, 76%, 70%, and 72% of admissions in the D614G, Beta, Delta, Omicron BA.1, and Omicron BA.4/BA.5 waves. The DATCOV dataset uses wave period as a proxy for variants and does not contain individual-level data on infecting lineage; however, each wave has correlated well with a particular VOC. While DATCOV is able to link with EVDS and contains full vaccination history of all COVID-19 admissions, the analysis did not take into account time since last vaccine dose to factor in the effect of waning immunity. Also, the South African vaccination program eligibility changed over time during this period, starting with healthcare workers and individuals aged >60 years, then expanding to other age groups and later introducing booster doses (detailed in the Methods section). However, there was no significant interaction between wave period and COVID-19 vaccination status, although it is possible that we did not have sufficient power to detect any interaction. In a small number of cases, when national identification numbers were not available in either dataset being linked, linkage required “fuzzy” matching using first name, surname, and date of birth. Therefore, this may be incomplete when correctly ascertaining prior infection and vaccination status, though this would have minimal impact as most matching is on identification numbers. Finally, the data on prior SARS-CoV-2 infections are likely to be incomplete as infection and reinfection are substantially underascertained due to the high proportion of asymptomatic infections and challenges in testing. We relied on the testing data available, and it was therefore impossible to determine the extent to which ascertainment of reinfection varied over time.
CONCLUSIONS
There were fewer SARS-CoV-2 cases, lower risk of hospitalization, and a substantially reduced risk of in-hospital mortality in the Omicron BA.4/BA.5 wave compared with all previous waves, including the Omicron BA.1/BA.2 wave. This trend is different in young children who are not yet included in South Africa's vaccination strategy. The overall trend toward lower severity, reduced hospitalizations, and fewer deaths is likely due to the combination of lower viral virulence and increasing immunity, especially hybrid immunity from increasing vaccination coverage against a backdrop of widespread natural infection.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Waasila Jassat, National Institute for Communicable Diseases, Division of the National Health Laboratory Service, Johannesburg, South Africa; Right to Care, Pretoria, South Africa.
Salim S Abdool Karim, Centre for the AIDS Programme of Research in South Africa, Durban, South Africa; Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, New York, USA.
Lovelyn Ozougwu, National Institute for Communicable Diseases, Division of the National Health Laboratory Service, Johannesburg, South Africa; Right to Care, Pretoria, South Africa.
Richard Welch, National Institute for Communicable Diseases, Division of the National Health Laboratory Service, Johannesburg, South Africa; Right to Care, Pretoria, South Africa.
Caroline Mudara, National Institute for Communicable Diseases, Division of the National Health Laboratory Service, Johannesburg, South Africa.
Maureen Masha, National Institute for Communicable Diseases, Division of the National Health Laboratory Service, Johannesburg, South Africa; Right to Care, Pretoria, South Africa.
Petro Rousseau, National Department of Health, Pretoria, South Africa.
Milani Wolmarans, National Department of Health, Pretoria, South Africa.
Anthony Selikow, Council for Scientific and Industrial Research, Pretoria, South Africa.
Nevashan Govender, National Institute for Communicable Diseases, Division of the National Health Laboratory Service, Johannesburg, South Africa.
Sibongile Walaza, National Institute for Communicable Diseases, Division of the National Health Laboratory Service, Johannesburg, South Africa; School of Public Health, Faculty of Health Sciences, University of Witwatersrand, Johannesburg, South Africa.
Anne von Gottberg, National Institute for Communicable Diseases, Division of the National Health Laboratory Service, Johannesburg, South Africa; School of Pathology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Nicole Wolter, National Institute for Communicable Diseases, Division of the National Health Laboratory Service, Johannesburg, South Africa; School of Pathology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Pedro Terrence Pisa, Right to Care, Pretoria, South Africa; Department of Human Nutrition and Dietetics, Faculty of Health Sciences, University of Pretoria, Pretoria, South Africa.
Ian Sanne, Right to Care, Pretoria, South Africa; Clinical HIV Research Unit, Department of Internal Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Sharlene Govender, Right to Care, Pretoria, South Africa.
Lucille Blumberg, National Institute for Communicable Diseases, Division of the National Health Laboratory Service, Johannesburg, South Africa; Right to Care, Pretoria, South Africa.
Cheryl Cohen, National Institute for Communicable Diseases, Division of the National Health Laboratory Service, Johannesburg, South Africa; School of Public Health, Faculty of Health Sciences, University of Witwatersrand, Johannesburg, South Africa.
Michelle J Groome, National Institute for Communicable Diseases, Division of the National Health Laboratory Service, Johannesburg, South Africa; School of Pathology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Notes
Author Contributions. W. J., L. O., and C. M. contributed to the literature search. W. J., L. B., C. C., M. M., L. O., and C. M. contributed to study design and refining methods of analysis. L. O., W. J., C. M., and R. W. contributed to data analysis and creation of tables and figures. W. J., M. G., C. C., R. W., and L. O. contributed to data interpretation and the initial draft. W. J., R.W., and L. O. drafted the initial manuscript, and all other co-authors contributed scientific input equally toward the interpretation of the findings and the final draft of the manuscript. W. J., L. O., C. M., and R. W. verified the underlying data.
Acknowledgments. We acknowledge the National Institute for Communicable Diseases (NICD) that was team responsible for reporting test, case, and hospitalization data. Thanks to the National Department of Health and the Council for Scientific and Industrial Research for implementation support and access to vaccination rates (in particular Melissa Burgess and Lauren Hankel), the NICD for support and oversight, and the Network for Genomics Surveillance in South Africa for sequence frequencies. Our gratitude to the laboratories, clinicians, and data teams in all public and private sector hospitals throughout the country who reported cases and hospitalization data. These team members are acknowledged and listed as the DATCOV author group (see https://www.nicd.ac.za/diseases-a-z-index/COVID-19/surveillance-reports/daily-hospital-surveillance-datcov-report/).
Disclaimer. The contents of this study are the sole responsibility of the National Institute for Communicable Diseases and National Department of Health and do not necessarily reflect the views of US Agency for International Development (USAID), US President's Emergency Plan for AIDS Relief (PEPFAR), or the US government. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Financial support. DATCOV is a national surveillance system that was initially funded by the National Institute for Communicable Diseases and the South African national government and subsequently by the support of the American people through the USAID via the mechanism awarded to Right to Care.
Data sharing agreement. The dataset analyzed for this article is available upon reasonable request. The data dictionary is available at request to the corresponding author: waasilaj@nicd.ac.za
References
- 1. Shuai H, Chan JF, Hu B, et al. . Attenuated replication and pathogenicity of SARS-CoV-2 B.1.1.529 Omicron. Nature 2022; 603:693–9. [DOI] [PubMed] [Google Scholar]
- 2. Hui KP, Ho JC, Cheung MC, et al. . SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature 2022; 603:715–20. [DOI] [PubMed] [Google Scholar]
- 3. Madhi SA, Kwatra G, Myers JE, et al. . Population immunity and Covid-19 severity with Omicron variant in South Africa. N Engl J Med 2022; 386:1314–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wolter N, Jassat W, Walaza S, et al. . Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet 2022; 399:437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jassat W, Karim SS, Mudara C, et al. . Clinical severity of COVID-19 in patients admitted to hospital during the Omicron wave in South Africa: a retrospective observational study. Lancet Glob Health 2022; 10:E961–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nyberg T, Ferguson NM, Nash SG, et al. . Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) variants in England: a cohort study. Lancet 2022; 399:1303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bager P, Wohlfahrt J, Bhatt S, et al. . Risk of hospitalisation associated with infection with SARS-CoV-2 Omicron variant versus Delta variant in Denmark: an observational cohort study. Lancet Infect Dis 2022; 22:967–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lewnard JA, Hong VX, Patel MM, Kahn R, Lipsitch M, Tartof SY. Clinical outcomes associated with SARS-CoV-2 Omicron (B.1.1.529) variant and BA.1/BA.1.1 or BA.2 subvariant infection in southern California. Nat Med 2022; 28:1933–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sievers C, Zacher B, Ullrich A, et al. . SARS-CoV-2 Omicron variants BA.1 and BA.2 both show similarly reduced disease severity of COVID-19 compared to Delta, Germany, 2021 to 2022. Eurosurveillance 2022; 27:2200396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mefsin YM, Chen D, Bond HS, et al. . Epidemiology of infections with SARS-CoV-2 Omicron BA.2 variant, Hong Kong, January–March 2022. Emerg Infect Dis 2022; 28:1856–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Network for Genomics Surveillance in South Africa . SARS-CoV-2 genomic surveillance update. Posted online3June2022. Available at: https://www.nicd.ac.za/diseases-a-z-index/disease-index-covid-19/sars-cov-2-genomic-surveillance-update/. Accessed 12 June 2022. [Google Scholar]
- 12. Tegally H, Moir M, Everatt J, et al. . Emergence of SARS-CoV-2 Omicron lineages BA.4 and BA.5 in South Africa. Nat Med 2022; 28:1785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. European Centre for Disease Prevention and Control . Epidemiological update: SARS-CoV-2 Omicron sub-lineages BA.4 and BA.5. Available at: https://www.ecdc.europa.eu/en/news-events/epidemiological-update-sars-cov-2-omicron-sub-lineages-ba4-and-ba5. Accessed 16 July 2022. [Google Scholar]
- 14. Tuekprakhon A, Nutalai R, Dijokaite-Guraliuc A, et al. . Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell 2022; 185:2422–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khan K, Karim F, Ganga Y, et al. . 4/BA.5 escape neutralizing immunity elicited by BA.1 infection. Nat Commun 2022; 13:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wolter N, Jassat W, Walaza S, et al. . Clinical severity of SARS-CoV-2 Omicron BA.4 and BA.5 lineages in South Africa. Preprint. Posted online28June2022. (Version 1). Available at Research Square. 10.21203/rs.3.rs-1792132/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lewnard JA, Hong V, Tartof SY. Association of SARS-CoV-2 BA.4/BA.5 Omicron lineages with immune escape and clinical outcome. medRxiv PPR527995 [Preprint]. August 2, 2022 [cited 2022 Nov 3]. Available from: 10.1101/2022.07.31.22278258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Silal SP, Groome MJ, Govender N, et al. . Leveraging epidemiology as a decision support tool during the COVID-19 epidemic in South Africa. S Afr Med J 2022; 112:361–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Statistics South Africa . Mid-year population estimates, 2021. Statistical release P0302, Pretoria; 2022. Available at: https://www.statssa.gov.za/publications/P0302/P03022021.pdf. Accessed 12 June 2022. [Google Scholar]
- 20. Suryawanshi R, Ott M. SARS-CoV-2 hybrid immunity: silver bullet or silver lining? Nat Rev Immunol 2022; 22:591–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Davies M-A, Morden E, Rosseau P, et al. . Outcomes of laboratory-confirmed SARS-CoV-2 infection during resurgence driven by Omicron lineages BA.4 and BA.5 compared with previous waves in the Western Cape Province, South Africa. Int J Infect Dis .2022. 10.1016/j.ijid.2022.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Keeton R, Tincho MB, Ngomti A, et al. . T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature 2022; 603:488–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun K, Tempia S, Kleynhans J, et al. SARS-CoV-2 transmission, persistence of immunity, and estimates of Omicron's impact in South African population cohorts. Sci Transl Med 2022; 14:eabo7081. 10.1126/scitranslmed.abo7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen LL, Abdullah SM, Chan WM, et al. . Contribution of low population immunity to the severe Omicron BA, 2, outbreak in Hong Kong. Nat Commun 2022; 13:3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arnaout R, Arnaout R. Visualizing omicron: COVID-19 deaths vs. cases over time. PLoS One 2022; 17:e0265233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cable R, Coleman C, Glatt T, et al. . Estimates of prevalence of anti-SARS-CoV-2 antibodies among blood donors in eight provinces of South Africa in November 2021. Res Sq 2022; 10.21203/rs.3.rs-1359658/v1. [DOI] [Google Scholar]
- 27. Bingham J, Cable R, Coleman C, et al. . Estimates of prevalence of anti-SARS-CoV-2 antibodies among blood donors in South Africa in March 2022. Res Sq 2022; 10.21203/rs.3.rs-1687679/v2. [DOI] [Google Scholar]
- 28. Madhi S, Kwatra G, Myers JE, et al. . SARS-CoV-2 infections during Omicron (BA.1) dominant wave and subsequent population immunity in Gauteng, South Africa. medRxiv [Preprint]. July 15, 2022. Available from: 10.1101/2022.07.13.22277575. [DOI] [Google Scholar]
- 29. National Department of Health South Africa . Latest Vaccine Statistics. SAcoronavirus website. Available at: https://sacoronavirus.co.za/latest-vaccine-statistics/. Accessed 12 June 2022. [Google Scholar]
- 30. Bhattacharyya RP, Hanage WP. Challenges in inferring intrinsic severity of the SARS-CoV-2 Omicron variant. N Engl J Med 2022; 386:e14. [DOI] [PubMed] [Google Scholar]
- 31. Halfmann PJ, Iida S, Iwatsuki-Horimoto K, et al. . SARS-CoV-2 Omicron virus causes attenuated disease in mice and hamsters. Nature 2022; 603:687–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kimura I, Yamasoba D, Tamura T, et al. Virological characteristics of the SARS-CoV-2 Omicron BA.2 subvariants, including BA.4 and BA.5. Cell 2022; 185(21):P3992–4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tempia S, Moyes J, Cohen AL, et al. . The national burden of influenza-like illness and severe respiratory illness overall and associated with nine respiratory viruses in South Africa, 2013–2015. Influenza Other Respir Viruses 2022; 16:438–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li R, Metcalf CJ, Stenseth NC, Bjørnstad ON. A general model for the demographic signatures of the transition from pandemic emergence to endemicity. Sci Adv 2021; 7:eabf9040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.