Abstract
Background
This phase 2b part of a randomized phase 2/3 study assessed the efficacy and safety of ensitrelvir for mild-to-moderate coronavirus disease 2019 (COVID-19) during the Omicron epidemic.
Methods
Patients were randomized (1:1:1) to orally receive ensitrelvir fumaric acid 125 mg (375 mg on day 1) or 250 mg (750 mg on day 1) or placebo once daily for 5 days. The co-primary endpoints were the change from baseline in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) titer on day 4 and time-weighted average change from baseline up to 120 hours in the total score of predefined 12 COVID-19 symptoms. Safety was assessed through adverse events.
Results
A total of 341 patients (ensitrelvir 125-mg group: 114; ensitrelvir 250-mg group: 116; and placebo group: 111; male: 53.5–64.9%; mean age: 35.3–37.3 years) were included in the efficacy analyses. The change from baseline in SARS-CoV-2 titer on day 4 was significantly greater with both ensitrelvir doses than with placebo (differences from placebo: −0.41 log10 50% tissue-culture infectious dose/mL; P < .0001 for both). The total score of the 12 COVID-19 symptoms did not show a significant difference between the ensitrelvir groups and placebo group. The time-weighted average change from baseline up to 120 hours was significantly greater with ensitrelvir versus placebo in several subtotal scores, including acute symptoms and respiratory symptoms. Most adverse events were mild in severity.
Conclusions
Ensitrelvir treatment demonstrated a favorable antiviral efficacy and potential clinical benefit with an acceptable safety profile.
Clinical Trials Registration
Japan Registry of Clinical Trials: jRCT2031210350 (https://jrct.niph.go.jp/en-latest-detail/jRCT2031210350)
Keywords: COVID-19, ensitrelvir, S-217622, SARS-CoV-2 3C-like protease inhibitor, viral titer
Treatment with 5-day, once-daily, oral ensitrelvir demonstrated a reduction in viral titer and viral RNA against the SARS-CoV-2 Omicron variant, potential clinical benefit, and an acceptable safety profile in patients with mild-to-moderate COVID-19 in a phase 2b study setting.
As of 20 July 2022, more than 560 million confirmed cases of coronavirus disease 2019 (COVID-19) and over 6 million COVID-19–associated deaths have been reported worldwide [1]. Several vaccines for COVID-19 have been approved for clinical use [2–5], and more than 12 billion vaccine doses have been administered to the worldwide population [1]. However, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) neutralizing antibody titers decrease substantially with time after vaccination [6], potentially leading to postvaccination infections [7]. Therefore, effective antiviral agents are needed for COVID-19 treatment.
The SARS-CoV-2 Omicron (B.1.1.529) variant, one of the variants of concern, was first detected in South Africa in November 2021 and quickly spread worldwide [8]. The disease associated with the Omicron variant is less severe than that caused by previous variants [9–11], but the high transmissibility and infectivity of this variant, resulting from a significant number of mutations in the SARS-CoV-2 receptor-binding domain [12, 13], may pose an additional threat to global health security. Several antiviral treatment options have demonstrated efficacy in patients with COVID-19 at risk of severe disease [14–17]. However, in view of the public burden of the infectious virus transmission and to draw maximum benefits from early pharmacological treatment initiation [18], additional oral antiviral agents that can be administered regardless of the risk of severe disease are needed.
Ensitrelvir fumaric acid (S-217622; hereafter, ensitrelvir), a novel oral SARS-CoV-2 3C-like protease inhibitor, was discovered through joint research by Hokkaido University and Shionogi & Co, Ltd [19]. Ensitrelvir showed antiviral efficacy against different SARS-CoV-2 variants, including the Omicron variant, in preclinical studies [19–21]. In a phase 1 study of ensitrelvir, the once-daily oral dose was well tolerated and demonstrated a favorable pharmacokinetic profile [22]. A multicenter, randomized, double-blind, placebo-controlled, phase 2/3 study is also underway to assess the efficacy, safety, and pharmacokinetics of 5-day oral ensitrelvir treatment. In its phase 2a part, ensitrelvir treatment led to a reduction in the SARS-CoV-2 viral titer and viral RNA level compared with placebo, with an acceptable safety profile [23]. Herein, we present the antiviral and clinical efficacy and safety of ensitrelvir derived from the phase 2b part of this phase 2/3 study.
METHODS
Study Design
This phase 2b, dose-finding part of the study (Japan Registry of Clinical Trials identifier: jRCT2031210350) was conducted from 2 January to 9 February 2022 at 87 institutions in Japan and South Korea (Supplementary Table 1). Patients with mild-to-moderate COVID-19 were randomized (1:1:1) to orally receive ensitrelvir fumaric acid (375 mg on day 1, followed by 125 mg on days 2 through 5, or 750 mg on day 1, followed by 250 mg on days 2 through 5) or matching placebo once daily without dose modification and followed up until day 28.
This study was conducted in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice guidelines, and other applicable laws and regulations. The study was reviewed and approved by the institutional review boards of all participating institutions listed in Supplementary Table 1. All patients or their legally acceptable representatives provided written informed consent.
Patients, Randomization, Blinding, and Treatment
Patients aged 12–69 years who tested positive for SARS-CoV-2 (assessed by SARS-CoV-2 antigen or nucleic acid detection testing) within 120 hours prior to randomization and who had a symptom duration of 120 hours or less were eligible for study enrollment. Patients with mild-to-moderate COVID-19—that is, those having at least 1 moderate or severe symptom or worsening of an existing symptom among the 12 COVID-19 symptoms (Supplementary Table 2) based on the US Food and Drug Administration guidance [24]—were included. Patients with an awake oxygen saturation of 93% or less (room air) or those requiring oxygen administration were excluded (Supplementary Methods).
Patient randomization was performed through an interactive response technology system using time from the onset of COVID-19 to randomization (<72 hours/≥72 hours) and the first COVID-19 vaccination (yes/no) as stratification factors. Patients received allocated study drugs orally (ensitrelvir fumaric acid 125 mg, 250 mg, or placebo tablets). Treatment was discontinued when liver function abnormalities, pregnancy, COVID-19 exacerbation, or serious or intolerable adverse events (AEs) were observed (Supplementary Methods).
Outcomes and Assessments
The primary virologic outcome was change from baseline (day 1, before drug administration) in the SARS-CoV-2 viral titer on day 4 of treatment. The primary clinical outcome was time-weighted average change from baseline up to 120 hours in the total score of 12 COVID-19 symptoms. Based on the results of the phase 2a part [23], which showed a statistically significant reduction in the SARS-CoV-2 viral titer and an improving trend in the total score of the 12 COVID-19 symptoms after ensitrelvir treatment, these outcomes were used as the co-primary endpoints. The secondary outcomes included the SARS-CoV-2 viral titer and viral RNA level (absolute values and change from baseline) up to day 21, time to first negative SARS-CoV-2 viral titer (infectious viral clearance), proportion of patients with positive viral titers, time to first improvement in COVID-19 symptoms (Supplementary Table 2), and subtotal scores of the 12 COVID-19 symptoms (acute symptoms, main clinical symptoms, respiratory symptoms, systemic symptoms, and digestive symptoms) (Supplementary Table 2) self-recorded on an electronic diary. Additionally, post hoc analyses were performed to assess a composite subtotal score (respiratory symptoms and feverishness) for the 12 COVID-19 symptoms. Details of the virologic and clinical assessments are provided in the Supplementary Methods.
Safety was assessed by the occurrence of treatment-emergent AEs (TEAEs). All safety data were evaluated by an independent data and safety monitoring board (Supplementary Methods).
Statistical Analyses
Based on the interim evaluation of the phase 2a part of this study (Shionogi & Co, Ltd, unpublished data), the difference in time-weighted average change in the total score of COVID-19 symptoms from baseline up to 120 hours between the ensitrelvir and placebo groups was assumed to be −1, with a standard deviation of 2.6. Considering the mean baseline score observed in the phase 2a part (7.3 to 10.2) [23], a 1-point improvement in the total score of 12 COVID-19 symptoms by day 6 was deemed meaningful to patients. A total of 108 patients per group (324 in total) were required to detect this difference with 80% power using a 2-sample t-test at a 1-sided significance level of.025. Considering a dropout rate of 25% (proportion of patients with a negative SARS-CoV-2 viral titer at baseline), the final sample size was set at 145 patients per group (435 in total) (Supplementary Methods).
All randomized patients with a positive SARS-CoV-2 viral titer (≥1.1 log10 50% tissue-culture infectious dose [TCID50]/mL) at baseline were included in the intention-to-treat (ITT) population and subjected to all efficacy analyses. All randomized patients who received at least 1 dose of the study drug were included in the safety analysis population. All analyses were performed in the planned treatment groups. Statistical significance was assessed at a 1-sided significance level of.025 (for the primary virologic and clinical outcomes) or a 2-sided significance level of.05 (Supplementary Methods).
No imputation was performed for missing data. All analyses were performed using SAS version 9.4 (SAS Institute, Inc, Cary, NC, USA).
RESULTS
Patient Disposition
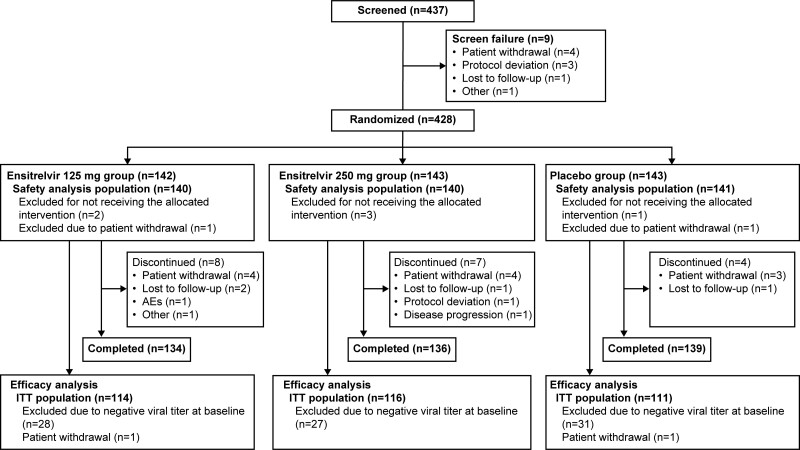
Of the 437 patients who provided informed consent, 9 were excluded prior to randomization due to screening failure. Among the 428 patients in total, 142, 143, and 143 were randomized to the ensitrelvir 125 mg, ensitrelvir 250 mg, and placebo groups, respectively; 140, 140, and 141 patients, respectively, were included in the safety analysis population. After the exclusion of patients with undetectable SARS-CoV-2 titer at baseline or those who provided incomplete informed consent, 341 patients (ensitrelvir 125 mg, n = 114; ensitrelvir 250 mg, n = 116; and placebo, n = 111) were included in the ITT population (Figure 1).
Figure 1.
Patient disposition. Some patients were excluded from the analysis populations due to more than 1 reason. Abbreviations: AE, adverse event; ITT, intention-to-treat.
Demographics and Clinical Characteristics
No notable difference was observed in the baseline characteristics across the treatment groups; the mean age was 35.3–37.3 years, and 4 patients were aged more than 65 years. Men accounted for 53.5–64.9% of the patients in each group. Nearly half of the patients were randomized within 72 hours of COVID-19 symptom onset, and more than 80% had received at least 1 dose of mRNA SARS-CoV-2 vaccine. Among the predefined 12 COVID-19 symptoms, sore throat and cough were most frequently observed in all treatment groups. Most patients were infected with the Omicron variant BA.1 (Table 1).
Table 1.
Baseline Demographics and Clinical Characteristics (ITT Population) and Treatment Compliance Rate (Safety Analysis Population)
| Variables | Ensitrelvir 125 mg (N = 114) |
Ensitrelvir 250 mg (N = 116) |
Placebo (N = 111) |
|---|---|---|---|
| Male sex, n (%) | 61 (53.5) | 66 (56.9) | 72 (64.9) |
| Age, mean (SD), years | 35.6 (13.5) | 35.3 (13.1) | 37.3 (12.6) |
| Age, n (%) | |||
| ȃ≥12 to <18 years | 4 (3.5) | 2 (1.7) | 2 (1.8) |
| ȃ≥18 to <65 years | 109 (95.6) | 112 (96.6) | 108 (97.3) |
| ȃ≥65 to <70 years | 1 (0.9) | 2 (1.7) | 1 (0.9) |
| Patient conditions,a n (%) | |||
| ȃHospitalized | 29 (25.4) | 44 (37.9) | 33 (29.7) |
| ȃOutpatient | 49 (43.0) | 40 (34.5) | 48 (43.2) |
| ȃRecuperation at home | 2 (1.8) | 1 (0.9) | 0 (0.0) |
| ȃRecuperation at hotels | 34 (29.8) | 31 (26.7) | 30 (27.0) |
| Time from onset to randomization, n (%) | |||
| ȃ<24 hours | 11 (9.6) | 2 (1.7) | 5 (4.5) |
| ȃ≥24 to <48 hours | 21 (18.4) | 21 (18.1) | 24 (21.6) |
| ȃ≥48 to <72 hours | 23 (20.2) | 30 (25.9) | 25 (22.5) |
| ȃ≥72 to <96 hours | 37 (32.5) | 38 (32.8) | 33 (29.7) |
| ȃ≥96 to ≤120 hours | 22 (19.3) | 23 (19.8) | 24 (21.6) |
| ȃ>120 hours | 0 (0.0) | 2 (1.7) | 0 (0.0) |
| COVID-19 vaccination history, n (%) | |||
| ȃ≥1 vaccination | 97 (85.1) | 97 (83.6) | 97 (87.4) |
| ȃ≥2 vaccinations | 94 (82.5) | 96 (82.8) | 95 (85.6) |
| ȃ≥3 vaccinations | 3 (2.6) | 1 (0.9) | 2 (1.8) |
| Patients with COVID-19 symptoms, n (%) | |||
| ȃRespiratory symptoms | |||
| ȃȃStuffy or runny nose | 29 (25.4) | 34 (29.3) | 26 (23.4) |
| ȃȃSore throat | 65 (57.0) | 63 (54.3) | 54 (48.6) |
| ȃȃShortness of breath (difficulty breathing) | 8 (7.0) | 8 (6.9) | 1 (0.9) |
| ȃȃCough | 48 (42.1) | 46 (39.7) | 49 (44.1) |
| ȃSystemic symptoms | |||
| ȃȃLow energy or tiredness | 37 (32.5) | 42 (36.2) | 27 (24.3) |
| ȃȃMuscle or body aches | 26 (22.8) | 19 (16.4) | 23 (20.7) |
| ȃȃHeadache | 28 (24.6) | 30 (25.9) | 24 (21.6) |
| ȃȃChills or shivering | 31 (27.2) | 20 (17.2) | 17 (15.3) |
| ȃȃFeeling hot or feverish | 43 (37.7) | 41 (35.3) | 36 (32.4) |
| ȃDigestive symptoms | |||
| ȃȃNausea | 4 (3.5) | 5 (4.3) | 2 (1.8) |
| ȃȃVomiting | 3 (2.6) | 3 (2.6) | 2 (1.8) |
| ȃȃDiarrhea | 6 (5.3) | 6 (5.2) | 8 (7.2) |
| ȃSensation disturbance | |||
| ȃȃAnosmia | 16 (14.0) | 10 (8.6) | 10 (9.0) |
| ȃȃDysgeusia | 19 (16.7) | 7 (6.0) | 9 (8.1) |
| Total score of 12 COVID-19 symptoms,b mean (SD) | 9.9 (5.0) | 9.3 (4.5) | 8.6 (3.8) |
| Patients with fever (body temperature ≥37.0°C), n (%) | 45 (39.5) | 39 (33.6) | 30 (27.0) |
| Treatment compliance rate in the safety population,c mean (SD) | 97.9 (10.3) | 99.3 (6.0) | 99.3 (5.8) |
| SARS-CoV-2 variant, n (%) | |||
| ȃ21I (Delta) | 0 (0.0) | 1 (0.9) | 0 (0.0) |
| ȃ21J (Delta) | 0 (0.0) | 1 (0.9) | 0 (0.0) |
| ȃ21K (Omicron BA.1 lineage) | 114 (100.0) | 112 (96.6) | 110 (99.1) |
| ȃ21L (Omicron BA.2 lineage) | 0 (0.0) | 1 (0.9) | 1 (0.9) |
| ȃUnknown | 0 (0.0) | 1 (0.9) | 0 (0.0) |
Abbreviations: COVID-19, coronavirus disease 2019; ITT, intention-to-treat; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
In Japan, patients were eligible for enrollment irrespective of treatment settings (inpatient, outpatient, recuperation at home, or recuperation at designated hotels) because some of them required hospitalization or recuperation for the purpose of isolation or clinical trial participation, regardless of disease severity, at the time of this research.
n = 110, n = 113, and n = 110 for the ensitrelvir 125-mg, ensitrelvir 250-mg, and placebo groups, respectively.
n = 140, n = 140, and n = 141 for the ensitrelvir 125-mg, ensitrelvir 250-mg, and placebo groups, respectively.
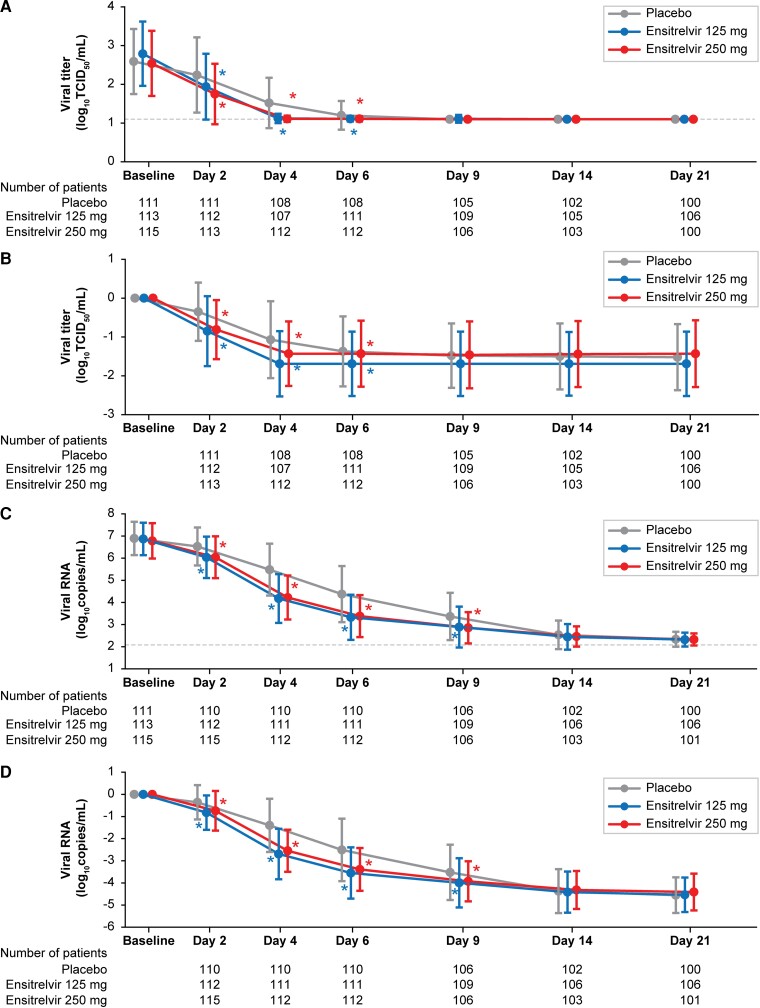
Co-Primary Efficacy Endpoint: SARS-CoV-2 Viral Titer
The mean SARS-CoV-2 viral titer was comparable across groups at baseline (2.5–2.8 log10 TCID50/mL). In all treatment groups, the SARS-CoV-2 viral titer decreased with time up to day 4 (after the third drug administration) and remained stable at the lower limit of detection levels (1.1 log10 TCID50/mL) until day 21 (Figure 2A and 2B ). The change from baseline in the SARS-CoV-2 viral titer (log10 TCID50/mL) on day 4, assessed using analysis of covariance (ANCOVA), was significantly greater with ensitrelvir 125 mg and 250 mg (least-squares [LS] mean [standard error (SE)]: −1.49 [0.04]; difference from placebo: −0.41 [95% confidence interval (CI): −.51 to −.31]; P < .0001) versus placebo (LS mean [SE]: −1.08 [0.04]). The change from baseline in the SARS-CoV-2 viral titer on day 4 was significantly greater with ensitrelvir 125 mg and 250 mg versus placebo irrespective of COVID-19 vaccination history or time from COVID-19 onset to randomization (Supplementary Table 3).
Figure 2.
Absolute values (A) and change from baseline (B) in SARS-CoV-2 viral titer. Absolute values (C) and change from baseline (D) in SARS-CoV-2 RNA level (ITT population). Data are presented as means ± SDs. Differences in viral titer and viral RNA from placebo were assessed using ANCOVA. Dotted lines indicate the lower limit of detection for SARS-CoV-2 viral titer (1.1 log10 TCID50/mL) and lower limit of quantification for SARS-CoV-2 RNA level (2.08 log10 copies/mL). Abbreviations: ANCOVA, analysis of covariance; ITT, intention-to-treat; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation; TCID50, 50% tissue-culture infectious dose. *P < .05 versus placebo.
Co-Primary Efficacy Endpoint: Total Scores for 12 COVID-19 Symptoms
The mean total scores of the predefined 12 COVID-19 symptoms are depicted in Supplementary Figure 1. There was no significant difference in the time-weighted average change from baseline up to 120 hours in the total score of the 12 COVID-19 symptoms between the ensitrelvir 125-mg or 250-mg groups and the placebo group (Table 2), irrespective of COVID-19 vaccination history (Supplementary Table 4).
Table 2.
Time-Weighted Average Change From Baseline Up to 120 Hours in the Total Score of 12 COVID-19 Symptoms (ITT Population)
| Statistics | Ensitrelvir 125 mg (N = 114) |
Ensitrelvir 250 mg (N = 116) |
Placebo (N = 111) |
|---|---|---|---|
| No. | 109 | 113 | 110 |
| Mean (SD) change from baseline | −5.95 (4.02) | −5.42 (3.70) | −4.92 (3.25) |
| LS mean (SE) change from baseline assessed by ANCOVA | −5.37 (0.24) | −5.17 (0.23) | −5.12 (0.24) |
| LS mean (SE) difference in change from baseline versus placebo | −0.24 (0.30) | −0.04 (0.29) | … |
| ȃ95% CI | −.83 to .34 | −.62 to .53 | … |
| ȃP | .4171 | .8806 | … |
Abbreviations: ANCOVA, analysis of covariance; CI, confidence interval; COVID-19, coronavirus disease 2019; ITT, intention-to-treat; LS, least squares; SD, standard deviation; SE, standard error.
SARS-CoV-2 Viral RNA Level
The SARS-CoV-2 RNA level decreased with time in all groups (Figure 2C and 2D ). The change from baseline in SARS-CoV-2 RNA level (log10 copies/mL) on day 4, assessed using ANCOVA, was significantly greater with ensitrelvir 125 mg (LS mean [SE]: −2.58 [0.11]; difference from placebo: −1.30; 95% CI: −1.57 to −1.03; P < .0001) and 250 mg (LS mean [SE]: −2.49 [0.11]; difference from placebo: −1.21; 95% CI, −1.48 to −.94; P < .0001) versus placebo (LS mean [SE]: −1.28 [0.11]). Similarly, the change from baseline in SARS-CoV-2 RNA level was significantly greater in the ensitrelvir groups versus the placebo group on days 2, 6, and 9.
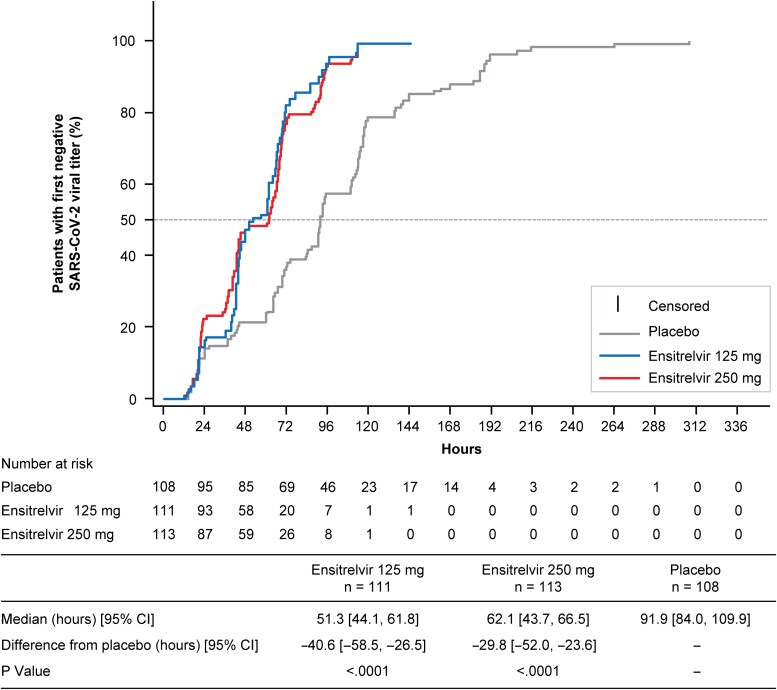
Time to First Negative SARS-CoV-2 Viral Titer
The median time to first negative SARS-CoV-2 viral titer was significantly shorter with ensitrelvir 125 mg and 250 mg versus placebo (P < .0001 for both) (Figure 3), irrespective of COVID-19 vaccination history (Supplementary Table 5).
Figure 3.
Time to first negative SARS-CoV-2 viral titer (viral clearance; ITT population). Abbreviations: CI, confidence interval; ITT, intention-to-treat; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Proportion of Patients With a Positive SARS-CoV-2 Viral Titer
The proportion of patients with a positive SARS-CoV-2 viral titer on day 4 was significantly lower with ensitrelvir 125 mg and 250 mg versus placebo (P < .0001 for both). Similar results were derived on day 6 (Supplementary Figure 2).
Time to First Improvement in COVID-19 Symptoms
No significant difference was observed in the time to first improvement in COVID-19 symptoms between the ensitrelvir groups and placebo group (median [95% CI] hours: 28.0 [21.5–36.6], 27.8 [24.6–40.0], and 36.6 [28.0–40.8] for ensitrelvir 125 mg, ensitrelvir 250 mg, and placebo, respectively).
Subtotal Scores for the 12 COVID-19 Symptoms
The mean changes from baseline in the subtotal scores of the 12 COVID-19 symptoms are depicted in Supplementary Figure 3. The time-weighted average change from baseline up to 120 hours was significantly greater with ensitrelvir versus placebo in the subtotal scores for acute symptoms (250-mg group; P = .0070), main clinical symptoms (250-mg group; P = .0149), respiratory symptoms (125-mg and 250-mg groups; P = .0153 and.0033, respectively), and the composite of respiratory symptoms and feverishness (125-mg and 250-mg groups; P = .0164 and.0039, respectively). No significant difference was observed between the ensitrelvir and placebo groups in the time-weighted average change for systemic symptoms and digestive symptoms (Supplementary Table 6). The anosmia and dysgeusia scores generally showed a transient increase from baseline, suggesting a delayed onset of these symptoms in patients with COVID-19 (Supplementary Figure 4).
Safety
Overall, 48 (34.3%), 60 (42.9%), and 44 (31.2%) patients in the ensitrelvir 125 mg, ensitrelvir 250 mg, and placebo groups, respectively, reported TEAEs, most of which were mild in severity. Treatment-related AEs were observed in 19 (13.6%), 31 (22.1%), and 7 (5.0%) patients in the ensitrelvir 125 mg, ensitrelvir 250 mg, and placebo groups, respectively, a majority of which resolved without sequelae. A decrease in high-density lipoprotein (HDL) levels was most frequently reported as a TEAE across groups and was the most common treatment-related AE in both ensitrelvir groups. No TEAEs leading to death were reported during the study (Table 3).
Table 3.
Summary of Treatment-Emergent Adverse Events (Safety Analysis Population)
| Ensitrelvir 125 mg (N = 140) |
Ensitrelvir 250 mg (N = 140) |
Placebo (N = 141) | |
|---|---|---|---|
| Patients with events, n (%) | |||
| ȃPatients with any TEAE | 48 (34.3) | 60 (42.9) | 44 (31.2) |
| ȃPatients with any treatment-related AE | 19 (13.6) | 31 (22.1) | 7 (5.0) |
| ȃPatients with any serious TEAE | 0 (0.0) | 0 (0.0) | 2 (1.4) |
| ȃPatients with TEAEs leading to death | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| ȃPatients with TEAEs leading to treatment discontinuation | 2 (1.4) | 0 (0.0) | 0 (0.0) |
| TEAEs occurring in ≥2% of patients in either group, n (%) | |||
| ȃDyslipidemia | 0 (0.0) | 3 (2.1) | 0 (0.0) |
| ȃHeadache | 3 (2.1) | 3 (2.1) | 0 (0.0) |
| ȃDiarrhea | 2 (1.4) | 3 (2.1) | 1 (0.7) |
| ȃUpper abdominal pain | 0 (0.0) | 0 (0.0) | 4 (2.8) |
| ȃRash | 2 (1.4) | 1 (0.7) | 3 (2.1) |
| ȃBack pain | 1 (0.7) | 3 (2.1) | 1 (0.7) |
| ȃHDL decrease | 31 (22.1) | 40 (28.6) | 5 (3.5) |
| ȃBlood triglycerides increase | 1 (0.7) | 9 (6.4) | 1 (0.7) |
| ȃBlood creatine phosphokinase increase | 0 (0.0) | 0 (0.0) | 4 (2.8) |
| Treatment-related AEs occurring in ≥5% of patients in either group, n (%) | |||
| ȃHDL decrease | 13 (9.3) | 22 (15.7) | 0 (0.0) |
Abbreviations: AE, adverse event; HDL, high-density lipoprotein; TEAE, treatment-emergent adverse event.
Two patients in the placebo group had serious TEAEs (thoracic vertebral fracture [recovered without sequelae] and facial paralysis [recovering]), both of which were determined as not being treatment related. All 3 TEAEs in the ensitrelvir 125-mg group leading to treatment discontinuation (mild eczema on day 2 in 1 patient and moderate nausea and mild headache on day 2 in 1 patient) were judged as being treatment related by the investigator, and both patients recovered without sequelae after drug discontinuation.
A dose-dependent, transient change in the HDL-cholesterol, triglyceride, total bilirubin, and iron levels was observed on day 6 in the ensitrelvir groups (Supplementary Figure 5). These changes were asymptomatic and resolved without additional treatment by day 14 for triglyceride and total bilirubin and by day 28 for HDL cholesterol. No notable difference was observed with ensitrelvir treatment versus placebo in haptoglobin, reticulocyte, or low-density lipoprotein levels, and no laboratory or clinical signs of hemolysis were observed. None of the patients showed an increase in serum bilirubin occurring concurrently with liver enzyme elevation.
DISCUSSION
This phase 2b part of a phase 2/3 trial of ensitrelvir in patients with mild-to-moderate COVID-19 was conducted in early 2022 during the SARS-CoV-2 Omicron epidemic. Most of the patients enrolled were vaccinated, whereas currently available COVID-19 treatments were evaluated in unvaccinated patients at risk of severe disease [14, 15]. On day 4, ensitrelvir treatment significantly reduced the SARS-CoV-2 viral titer versus placebo. There was no significant difference from placebo in the time-weighted average change from baseline up to 120 hours in the total score of the 12 COVID-19 symptoms.
The rapid and significant reduction in the SARS-CoV-2 viral titer and viral RNA level is consistent with the findings of the previous phase 2a part, which was conducted during the SARS-CoV-2 Delta epidemic [23]. However, the level of viral titer reduction from baseline to day 4 was lower in the current phase 2b part (−1.49 log10 TCID50/mL) than in the phase 2a part (−2.81 log10 TCID50/mL with ensitrelvir 250 mg) [23]. These differences in findings may be attributed to the lower baseline titer recorded in the phase 2b part than in the phase 2a part (2.5–2.8 vs 3.3–3.7 log10 TCID50/mL) and other unidentified differences in viral characteristics between the Omicron and Delta variants, such as infectivity or peak viral titer measured in Vero-E6 cells [25] and peak viral load and viral clearance [26]. The difference from placebo in the reduction in SARS-CoV-2 RNA level observed from baseline to day 4 in the current phase 2b part (−1.30 and −1.21 log10 copies/mL for ensitrelvir 125 mg and 250 mg, respectively) appeared greater than that observed with molnupiravir 800 mg (day 5, −0.547 log10 copies/mL) [27], nirmatrelvir 300 mg in combination with ritonavir 100 mg (day 5, −0.868 log10 copies/mL) [15], and casirivimab/imdevimab 2400 mg (day 7, −0.86 log10 copies/mL) [17].
Patients with COVID-19 present with diverse symptoms [28] and symptoms may differ depending on the epidemic variants. Among the 12 symptoms assessed, upper respiratory symptoms and feverishness were recorded in many patients enrolled in the phase 2b part, whereas systemic and digestive symptoms were less frequent at baseline. These findings are consistent with an epidemiology report in Japan, where the most commonly reported COVID-19 symptoms associated with the Omicron variant were cough (46.0%), sore throat (33.8%), runny nose (18.0%), and fever (30.9%) [29]. The absence of a significant difference in the total score of the 12 COVID-19 symptoms can be attributed to the low baseline subtotal scores recorded for some patients (eg, digestive symptoms). Interestingly, ensitrelvir treatment improved respiratory symptoms and feverishness, which are among the most common symptoms associated with the Omicron variant. The early cessation of infectious virus shedding could be associated with the upper respiratory symptom improvement with ensitrelvir treatment. The efficacy of ensitrelvir in suppressing long-term complications after COVID-19, such as loss of taste and/or smell, fatigue, and headache [30, 31], will be assessed in an optional exploratory period (days 28–337) of the current phase 2/3 study.
No clear dose-response was observed in the current phase 2b part, which is consistent with the findings of the phase 2a part [23]. Pharmacokinetic data for 5-day ensitrelvir 125-mg treatment (375 mg on day 1) derived from the phase 1 study (maximum plasma concentration: 30.4 µg/mL; area under the curve: 597.4 µg · h/mL) [22] suggest that this regimen is sufficient to achieve a reduction in SARS-CoV-2 in humans while minimizing drug exposure.
Similar to the findings of previous studies [22, 23], a transient change in HDL-cholesterol and triglyceride levels was observed following ensitrelvir treatment, as well as transient increases in total bilirubin and iron levels. The underlying causes of these laboratory findings and their potential relationship with ensitrelvir warrant further investigation.
This study has some limitations, including the limited number of elderly patients. As the risk of severe COVID-19 increases with age [32], the safety and efficacy of ensitrelvir in a wide range of patients with COVID-19 will be further assessed in the phase 3, multinational, randomized, placebo-controlled study SCORPIO-HR (ClinicalTrials.gov identifier: NCT05305547). Moreover, differences in the predominant SARS-CoV-2 variants, virologic characteristics, and clinical disease course between this phase 2b part and previous COVID-19 clinical trials may have affected the interpretation of the virologic and clinical efficacy.
In conclusion, 5-day, once-daily, oral ensitrelvir treatment demonstrated rapid and favorable antiviral efficacy with an acceptable safety profile in patients with mild-to-moderate COVID-19, a majority of whom had been vaccinated.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Hiroshi Mukae, Department of Respiratory Medicine, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan.
Hiroshi Yotsuyanagi, The Institute of Medical Science, The University of Tokyo, Tokyo, Japan.
Norio Ohmagari, Disease Control and Prevention Center, National Center for Global Health and Medicine, Tokyo, Japan.
Yohei Doi, Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA; Departments of Microbiology and Infectious Diseases, Fujita Health University School of Medicine, Toyoake, Japan.
Hiroki Sakaguchi, Drug Development and Regulatory Science Division, Shionogi & Co, Ltd, Osaka, Japan.
Takuhiro Sonoyama, Drug Development and Regulatory Science Division, Shionogi & Co, Ltd, Osaka, Japan.
Genki Ichihashi, Drug Development and Regulatory Science Division, Shionogi & Co, Ltd, Osaka, Japan.
Takao Sanaki, Pharmaceutical Research Division, Shionogi & Co, Ltd, Toyonaka, Japan.
Keiko Baba, Pharmaceutical Research Division, Shionogi & Co, Ltd, Toyonaka, Japan.
Yuko Tsuge, Drug Development and Regulatory Science Division, Shionogi & Co, Ltd, Osaka, Japan.
Takeki Uehara, Drug Development and Regulatory Science Division, Shionogi & Co, Ltd, Osaka, Japan.
Notes
Acknowledgments. The authors and research team thank all the patients involved in this study and Masahiro Kinoshita and Satoshi Kojima (Shionogi & Co, Ltd) for preparing technical support documents and an earlier version of the manuscript draft. They acknowledge Ryu Yoshida (Shionogi & Co, Ltd) and Merime Oota and Takashi Hashimoto (Shionogi Techno Advance Research Co, Ltd) for their excellent technical assistance. Support for study monitoring and data management was provided by EPS Corporation and funded by Shionogi & Co, Ltd. Medical writing and editorial assistance was provided by Mami Hirano, MS, of Cactus Life Sciences (part of Cactus Communications) and funded by Shionogi & Co, Ltd. All authors retained full ownership of the manuscript content and approved the final draft for submission.
Author Contributions. H. M., H. Y., N. O., Y. D., T. Sonoyama, Y. T., and T. U. conceived and designed the experiments and wrote the paper. H. S. conceived and designed the experiments, analyzed the data, contributed materials/analysis tools, and wrote the paper. G. I. conceived and designed the experiments and contributed materials/analysis tools. T. Sanaki conceived and designed the experiments, performed the experiments, analyzed the data, contributed materials/analysis tools, and wrote the paper. K. B. performed the experiments, analyzed the data, contributed materials/analysis tools, and wrote the paper.
Data availability. Shionogi & Co, Ltd, is committed to disclosing the synopses and results of its clinical trials and sharing the clinical trial data with researchers on reasonable request. For further details, please refer to the websites of Shionogi & Co, Ltd (https://www.shionogi.com/shionogi/global/en/company/policies/shionogi-group-clinical-trial-data-transparency-policy.html) and Vivli (https://vivli.org/).
Financial support. This work was sponsored by Shionogi & Co, Ltd, and financially supported by the Organization of the Ministry of Health, Labour, and Welfare. Employees of Shionogi & Co, Ltd, participated in and approved the design and conduct of the study; wrote the protocol; and were involved in the collection, management, analysis, and interpretation of data. Institutional authors reviewed and approved the protocol and collected and interpreted the data. H. M. has received funding relevant to the submitted work from Shionogi Co, Ltd.
References
- 1. World Health Organization . WHO coronavirus (COVID-19) dashboard. Available at: https://covid19.who.int/. Accessed 21 July 2022.
- 2. Baden LR, El Sahly HM, Essink B, et al. . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Polack FP, Thomas SJ, Kitchin N, et al. . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Voysey M, Clemens SAC, Madhi SA, et al. . Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet 2021; 397:881–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heath PT, Galiza EP, Baxter DN, et al. . Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med 2021; 385:1172–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levin EG, Lustig Y, Cohen C, et al. . Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med 2021; 385:e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bergwerk M, Gonen T, Lustig Y, et al. . Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med 2021; 385:1474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization . Classification of Omicron (B.1.1.529): SARS-CoV-2 variant of concern. Available at: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern. Accessed 21 July 2022.
- 9. Wolter N, Jassat W, Walaza S, et al. . Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet 2022; 399:437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Auvigne V, Vaux S, Strat YL, et al. . Severe hospital events following symptomatic infection with Sars-CoV-2 omicron and Delta variants in France, December 2021–January 2022: a retrospective, population-based, matched cohort study. EClinicalMedicine 2022; 48:101455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nyberg T, Ferguson NM, Nash SG, et al. . Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet 2022; 399:1303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ao D, Lan T, He X, et al. . SARS-CoV-2 omicron variant: immune escape and vaccine development. MedComm (2020) 2022; 3:e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumar S, Thambiraja TS, Karuppanan K, Subramaniam G, et al. . Omicron and delta variant of SARS-CoV-2: a comparative computational study of spike protein. J Med Virol 2022; 94:1641–9. [DOI] [PubMed] [Google Scholar]
- 14. Jayk Bernal A, da Silva MMG, Musungaie DB, et al. . Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med 2022; 386:509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hammond J, Leister-Tebbe H, Gardner A, et al. . Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med 2022; 386:1397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gupta A, Gonzalez-Rojas Y, Juarez E, et al. . Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med 2021; 385:1941–50. [DOI] [PubMed] [Google Scholar]
- 17. Weinreich DM, Sivapalasingam S, Norton T, et al. . REGEN-COV antibody combination and outcomes in outpatients with Covid-19. N Engl J Med 2021; 385:e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Japanese Association for Infectious Diseases . Considerations towards pharmacological treatment for COVID-19, version 13.1, 18 February 2022 (website in Japanese). Available at: https://www.kansensho.or.jp/uploads/files/topics/2019ncov/covid19_drug_220218.pdf. Accessed 21 July 2022.
- 19. Unoh Y, Uehara S, Nakahara K, et al. . Discovery of S-217622, a non-covalent oral SARS-CoV-2 3CL protease inhibitor clinical candidate for treating COVID-19. J Med Chem 2022; 65:6499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uraki R, Kiso M, Iida S, et al. . Characterization and antiviral susceptibility of SARS-CoV-2 Omicron/BA.2. Nature 2022; 607:119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sasaki M, Tabata K, Kishimoto M, et al. . Oral administration of S-217622, a SARS-CoV-2 main protease inhibitor, decreases viral load and accelerates recovery from clinical aspects of COVID-19. bioRxiv 2022.02.14.480338 [Preprint]. 15 February 2022 [cited 21 July 2022]. Available at: https://www.science.org/doi/10.1126/scitranslmed.abq4064. [Google Scholar]
- 22. Shimizu R, Sonoyama T, Fukuhara T, et al. . Safety, tolerability, and pharmacokinetics of the novel antiviral agent ensitrelvir fumaric acid, a SARS-CoV-2 3CL protease inhibitor, in healthy adults. Antimicrob Agents Chemother 2022; 66:e0063222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mukae H, Yotsuyanagi H, Ohmagari N, et al. . A randomized phase 2/3 study of ensitrelvir, a novel oral SARS-CoV-2 3C-like protease inhibitor, in Japanese patients with mild-to-moderate COVID-19 or asymptomatic SARS-CoV-2 infection: results of the phase 2a part. Antimicrob Agents Chemother 2022; 66:e0069722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. US Food and Drug Administration . Assessing COVID-19-related symptoms in outpatient adult and adolescent subjects in clinical trials of drugs and biological products for COVID-19 prevention or treatment. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/assessing-covid-19-related-symptoms-outpatient-adult-and-adolescent-subjects-clinical-trials-drugs. Accessed 21 July 2022.
- 25. Mautner L, Hoyos M, Dangel A, Berger C, Ehrhardt A, Baiker A. Replication kinetics and infectivity of SARS-CoV-2 variants of concern in common cell culture models. Virol J 2022; 19:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hay JA, Kissler SM, Fauver JR, et al. . Viral dynamics and duration of PCR positivity of the SARS-CoV-2 omicron variant. medRxiv 2022.01.13.22269257 [Preprint]. 14 January 2022 [cited 21 July 2022]. Available at: https://www.medrxiv.org/content/10.1101/2022.01.13.22269257v1. [Google Scholar]
- 27. Fischer WA II, Eron JJ Jr, Holman W, et al. . A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Sci Transl Med 2022; 14:eabl7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rodebaugh TL, Frumkin MR, Reiersen AM, et al. . Acute symptoms of mild to moderate COVID-19 are highly heterogeneous across individuals and over time. Open Forum Infect Dis 2021; 8:ofab090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. National Institute of Infectious Diseases . Active surveillance of COVID-19 and SARS-CoV-2 B.1.1.529 (Omicron) variant (5th report): epidemiological and clinical characteristics (website in Japanese). Available at: https://www.niid.go.jp/niid/ja/2019-ncov/2484-idsc/10969-covid19-72.html. Accessed 21 July 2022.
- 30. Blomberg B, Mohn KGI, Brokstad KA, et al. . Long COVID in a prospective cohort of home-isolated patients. Nat Med 2021; 27:1607–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. . More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep 2021; 11:16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pennington AF, Kompaniyets L, Summers AD, et al. . Risk of clinical severity by age and race/ethnicity among adults hospitalized for COVID-19-United States, March–September 2020. Open Forum Infect Dis 2020; 8:ofaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.