Introduction
Invasive mucormycosis is a life-threatening fungal infection that most frequently occurs in patients with underlying comorbidities impacting immune system function.1–4 Rhino-orbital-cerebral involvement is most frequently seen in those with poorly-controlled diabetes mellitus, while immunocompromised patients (including those with hematological malignancies and transplant recipients) frequently present with pulmonary involvement and disseminated infection.3,5,6.
Even with advances in microbiologic tools and antifungal therapies, many challenges remain in both the diagnosis and treatment of mucormycosis. A multi-faceted approach including the elimination of predisposing factors, aggressive surgical debridement, and effective antifungal therapy is critical to improve patient survival. However, despite these interventions, the outcome of invasive mucormycosis remains ominous.2,3.
Microbiology
Mucormycosis refers to infections caused by members of the order Mucorales. While most human infections are caused by Rhizopus, Mucor and Rhizomucor, other clinically relevant organisms within the order Mucorales include: Actinomucor, Apophysomyces, Cunninghamella, Lichtheimia (previously named Absidia), Saksenaea, and Syncephalastrum.7
Fungi of the order Mucorales have unique features that distinguish them from other clinically relevant fungi such as Aspergillus spp. First, Mucorales do not form true conidia (Figure 1). Instead, Mucorales produce unicelled asexual spores (sporangiospores) endogenously without the involvement of pre-existing cell walls.7 Additionally, Mucorales hyphae are broad, ribbon-like, multinucleated cells with none to rare septation (coenocytic hyphae). These hyphae typically develop from the germinal tube by apical extension, and during tissue invasion may occasionally septate to delimit reproductive structures or swollen areas.8 Occasional irregular branching may occur, representing a departure from apical growth related to nutrient resources in the cell wall (Figure 2). In contrast, Aspergillus forms true conidia and hyphae growth occurs in an isotropic fashion during germination. During this process, as the hyphae continue to grow apically, additional polarity axes enable septa formation (using the internal cellular wall) and lateral branching at regular intervals (Figure 2).8,9
Figure 1:
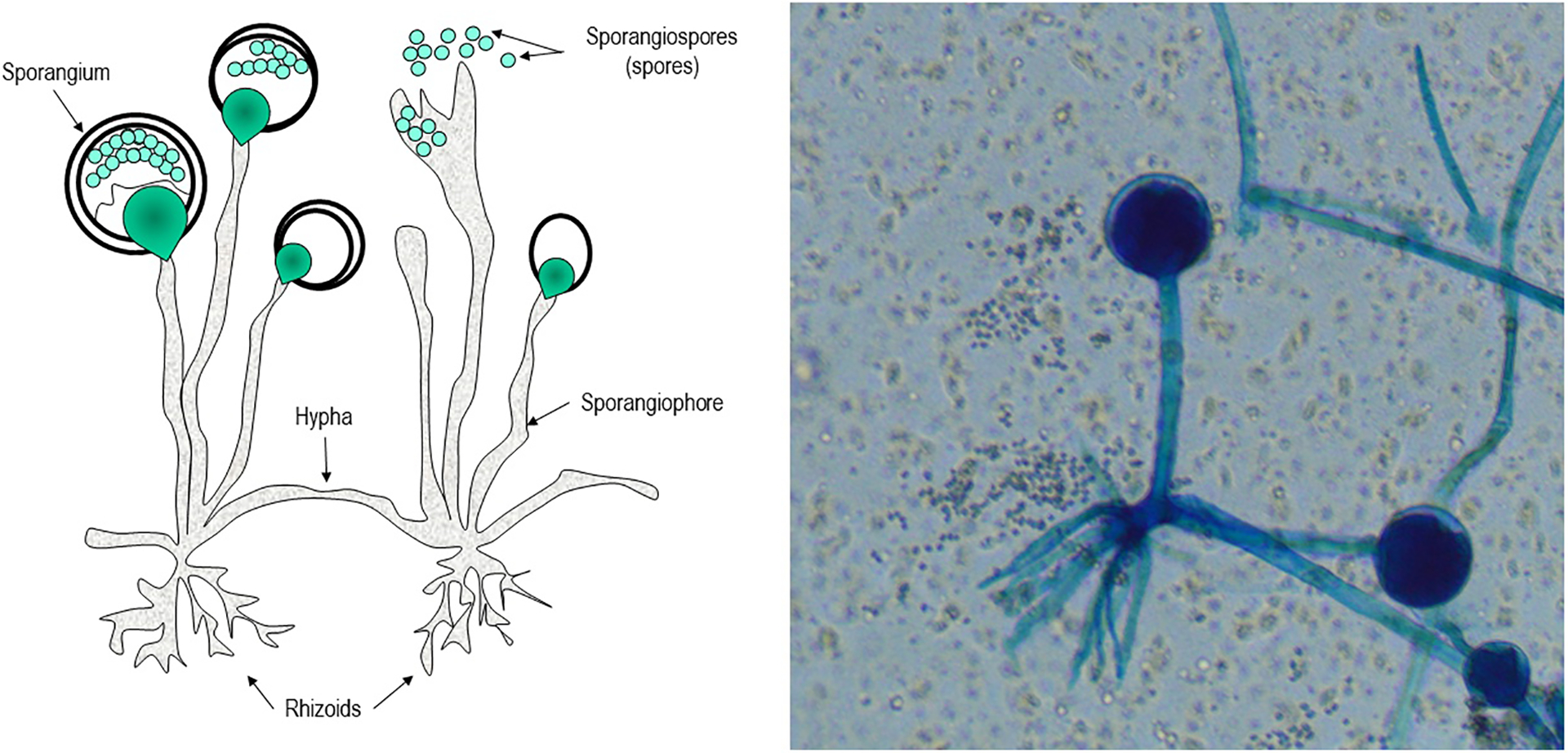
Structure of Rhizopus
Figure 2:
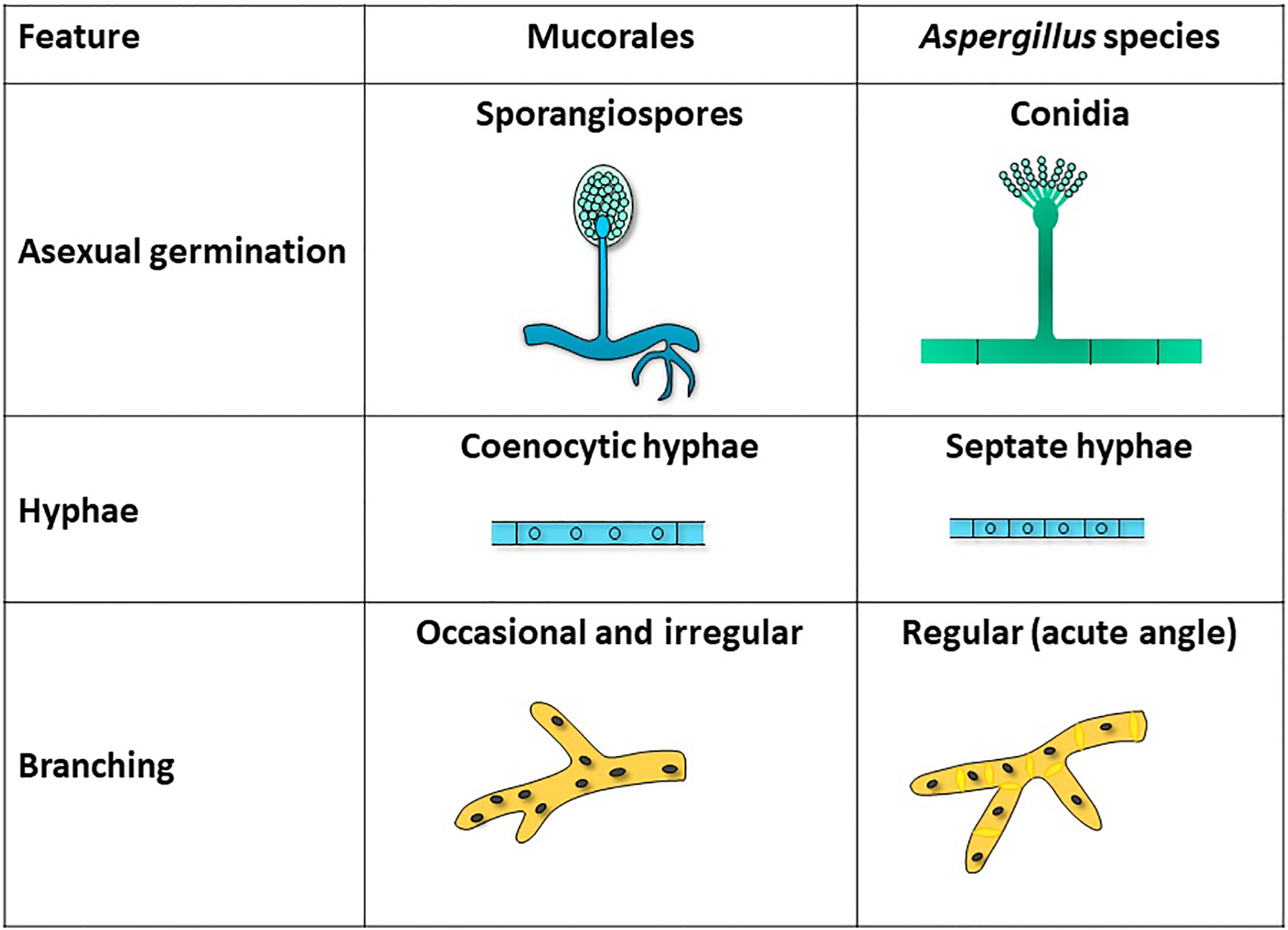
Distinctive features of Mucorales vs. Aspergillus species
Epidemiology and host factors
Mucorales are ubiquitous fungi usually found in soil, decaying organic matter, compost and contaminated foods. Mucormycosis is considered a rare infection – diabetes remains the most prominent underlying medical comorbidity in infected patients, and was identified as an independent risk factor for rhino-orbital-cerebral mucormycosis in a meta-analysis of 851 cases.3,6,10 However, over the last 2 decades, the number of cases reported in vulnerable patients with underlying immunosuppression (either innate or acquired) has increased.11,12 The reason behind this increase remains unclear, but it is likely multifactorial, related to the increased use of immunosuppressant drugs, improvement in fungal diagnostics, and selection by the widespread use of voriconazole prophylaxis.3,10,13 Patients with hematologic malignancies (particularly those with prolonged neutropenia) and hematopoietic stem cell transplant recipients appear to become infected at higher rates than solid organ transplant recipients, particularly among those receiving treatment for graft-versus-host disease.10,11,14–20 Cases of mucormycosis have also been linked to direct inoculation in the setting of trauma (primarily for cutaneous infection), iron overload, intravenous drug use, and malnourishment, even in the absence of diabetes and immunosuppression.3,21–23 Health-care associated outbreaks of mucormycosis (from infected laundry, bandages, hospital construction) or infection in the setting of natural disasters have also been described.24–28
Pathogenesis
Fungal spores enter the respiratory tract through inhalation, the skin through direct inoculation to areas of trauma, or are ingested through the gastrointestinal tract.29 Following initial entry, spores germinate into hyphae, resulting in angioinvasion with the potential for hematogenous dissemination and multiorgan involvement. Key virulence factors specific to the pathogenesis of Mucorales include the high-affinity iron permease (FTR1), which allows pathogen survival in iron-poor environments.30,31 The spore coat (CotH) protein is present on the spore surface of Mucorales and impairs host immune defenses,32 while the ADP-ribosylation factor (Arf) appears to have a role in Mucorales growth and morphology.33 Further research is needed to fully detail the effects of these and additional Mucorales virulence factors – including alkaline Rhizopus protease enzyme (Arp), calcineurin (CaN), and serine and aspartate proteases (SAPs) – to allow fungi to survive and invade the host.
Underlying host factors that impair immune system function can also contribute to the aggressive nature of mucormycosis infection. For instance, glucocorticoids are known to impair macrophage function, leading to infection progression and invasive disease.34 Hyperglycemia, acidosis, and iron overload have also been found to play an important role in the pathogenesis of Mucorales.2,35
In addition to factors attributable to the fungi and the host, external influences can also play a significant role in infection. It has been suggested that some of the toxins responsible for endothelial disruption may not be directly produced by the fungus itself, but rather as a result of bacterial endosymbiosis enhancing fungal virulence.36,37 Voriconazole exposure has also been shown to augment the growth and virulence of Mucorales beyond selective pressure, but the exact mechanisms remain unknown.38,39 Further studies are necessary to better understand the intricate mechanisms involved in the fascinating pathogenesis of Mucorales.
Clinical Presentation
Individuals with mucormycosis can have diverse clinical manifestations contingent on the immune status of the host, the extent of the infection, and the involved organs. The most common and distinct presentation is rhino-orbital-cerebral infection, which typically occurs when fungal spores are inhaled into the sinuses. From there, the infection can remain localized, with symptoms consistent with acute sinusitis along with fever, headache, sinus pain, and nasal congestion. In vulnerable hosts however, progression of the infection with invasion of the orbit and palate and further extension to the brain may occur. This can result in a number of significant clinical abnormalities including vision loss, cranial nerve palsies, and changes in mental status.40 Rhino-orbital-cerebral mucormycosis is the most frequent presentation among patient with diabetes mellitus and hyperglycemia, particularly with ketoacidosis, and has also been reported in about one-third of solid organ transplant recipients with mucormycosis.3–6,10,41,42 Rarely, this syndrome may occur in the absence of clear immunocompromising risk factors.43 Clinical progression and invasive infection typically occurs rapidly over days without appropriate treatment, although more protracted courses over weeks to months have been reported.44 Rhino-orbital-cerebral mucormycosis has been reported to have a 25–62% mortality, without significant improvement in survival over the past 20 years, despite earlier and more aggressive medical and surgical therapy.3–5,45,46 Of note, central nervous system infection can also occur without sinus involvement and direct extension. This is thought to be secondary to hematogenous seeding, and more frequently seen in patients with a history of intravenous drug use or acquired immunodeficiency syndrome.47,48
Mucormycosis can also present with pulmonary infection after spore inhalation. This is more common among patients with neutropenia due to hematologic malignancies or recipients of hematopoietic stem cell or solid organ transplants.3,16,42,49 Fever, chest pain, dyspnea, and hemoptysis (potentially massive and fatal) are often seen, due to hyphal invasion of blood vessels and subsequent hemorrhage. Contiguous spread of this aggressive infection can lead to involvement of surrounding tissues, including bronchi, cardiac involvement, and mediastinitis.50,51 The mortality rate of pulmonary mucormycosis has been reported between 48–87%.3,5,15,16,19
Cutaneous mucormycosis can be seen in both immunocompetent and immunocompromised patients but is the form of infection least likely to be associated with an underlying illness. As many as 50% of cases do not have overt immunosuppression but have undergone major antecedent trauma.24–26,52 Infection can remain localized or extend to deeper structures, including surrounding bones, muscles, and tendons. It is less frequently seen as a component of disseminated infection.53 Lesions start with painful erythema and induration, and progressively become necrotic as they evolve over several days (Figure 3), often with progression to necrotizing fasciitis (Figure 4). Mortality is lower (~25%) than that noted in other forms of mucormycosis.5
Figure 3:
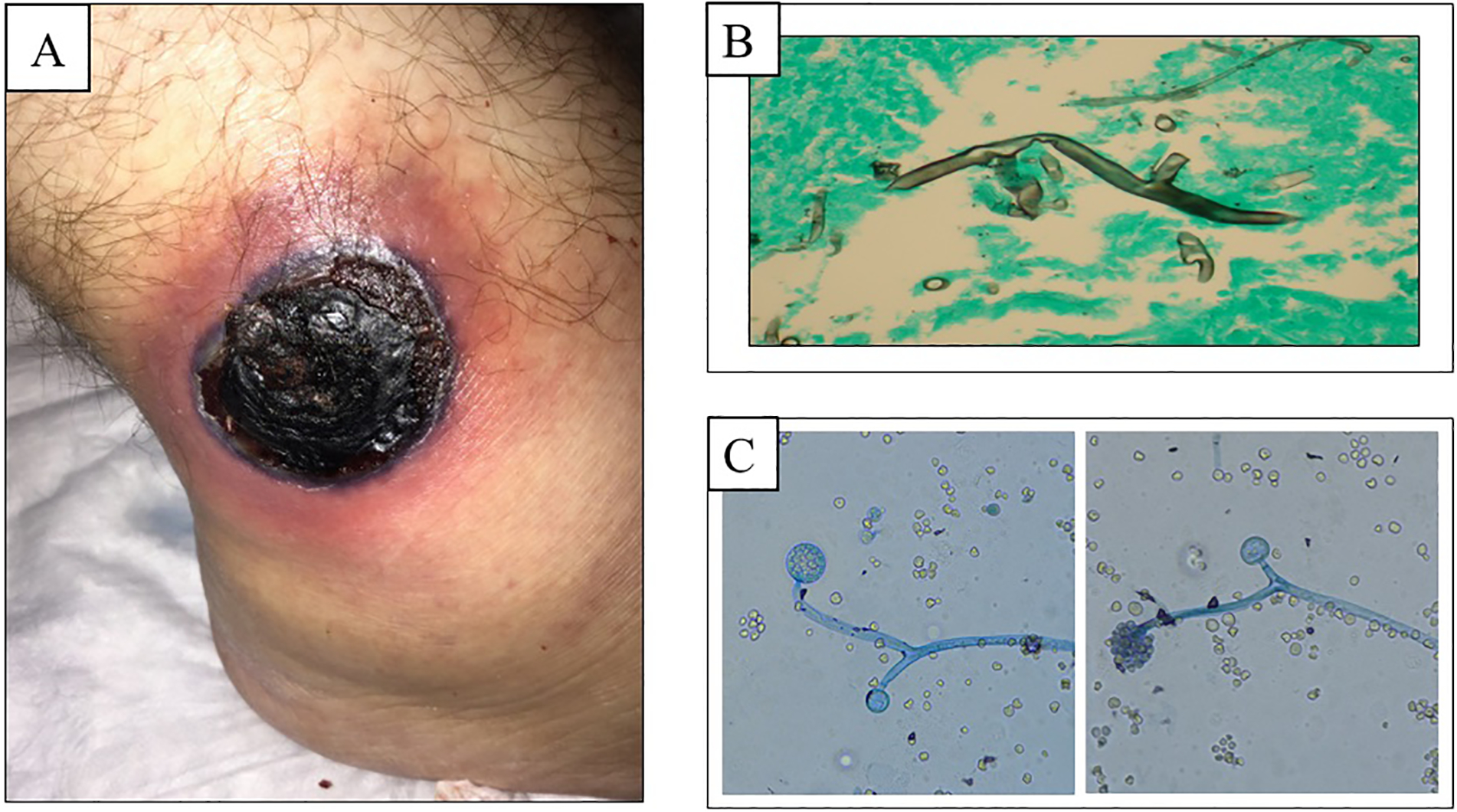
Skin lesion secondary to trauma in patient with cutaneous mucormycosis
Figure 4:
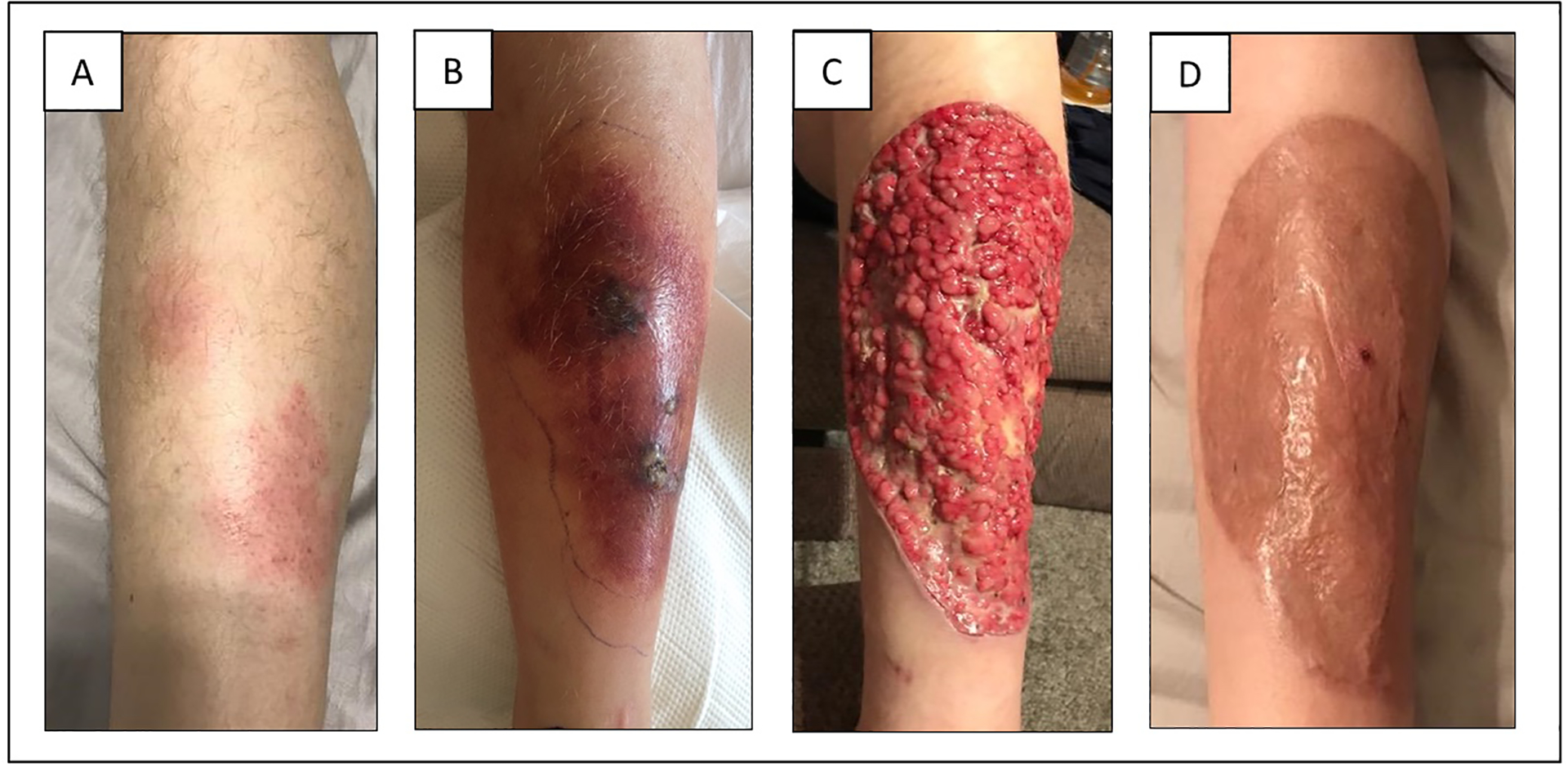
Clinical course of localized cutaneous mucormycosis in patient with non-Hodgkin lymphoma
There are also more rare forms of disease. Gastrointestinal mucormycosis has been reported, thought to be secondary to spore ingestion. This can involve multiple components of the gastrointestinal tract, including gastric ulceration of the stomach and intestinal involvement including bowel perforation.3,54–56 Many patients initially present with gastrointestinal bleeding. Renal mucormycosis has also been reported in increased frequency in patients with a history of intravenous drug use and acquired immunodeficiency syndrome.57,58 It is thought to stem from hematogenous seeding during bloodstream fungal infection, similar to central nervous system infection.
Disseminated mucormycosis is seen in patients with significant immunocompromise. In one systematic review of 67 cases of mucormycosis in patients with human immunodeficiency virus, the most common presentation was disseminated infection, at 20%.59 This presentation has the highest reported mortality at 96% despite appropriate treatment.3
Diagnosis
Early diagnosis of mucormycosis is key to rapid and appropriate treatment and improved outcomes. The diagnosis of mucormycosis requires demonstration of characteristic wide, ribbon-like, non-septate hyphae invading tissues on histopathology, accompanied with culture growth from specimens of involved sites.60,61 Pathogen identification and antifungal susceptibilities are critical to determine appropriate antifungal therapy. However, it is not unusual that specimens are not sent for culture or that organisms do not grow. In such instances, diagnosis is made from histopathology alone, leading to significant limitations in the management of this disease.61
Radiographic findings alone are non-specific and are usually insufficient for complete and accurate diagnosis of mucormycosis. Pulmonary infection has a spectrum of nonspecific radiographic appearances, similar to other fungal pneumonias, particularly aspergillosis.61 Several computed tomography (CT) findings – namely pleural effusion and multiple pulmonary nodules – along with clinical evidence of sinusitis, point toward mucormycosis as opposed to other fungi, particularly in the presence of an immunocompromised host.62 The ‘reverse halo’ sign has also been frequently reported, more commonly in pulmonary mucormycosis than in aspergillosis.63,64 This presents as a central ground glass opacification surrounded by a consolidative ring, reflective of central lung infarction surrounded by dense peripheral hemorrhage (Figure 5).
Figure 5:
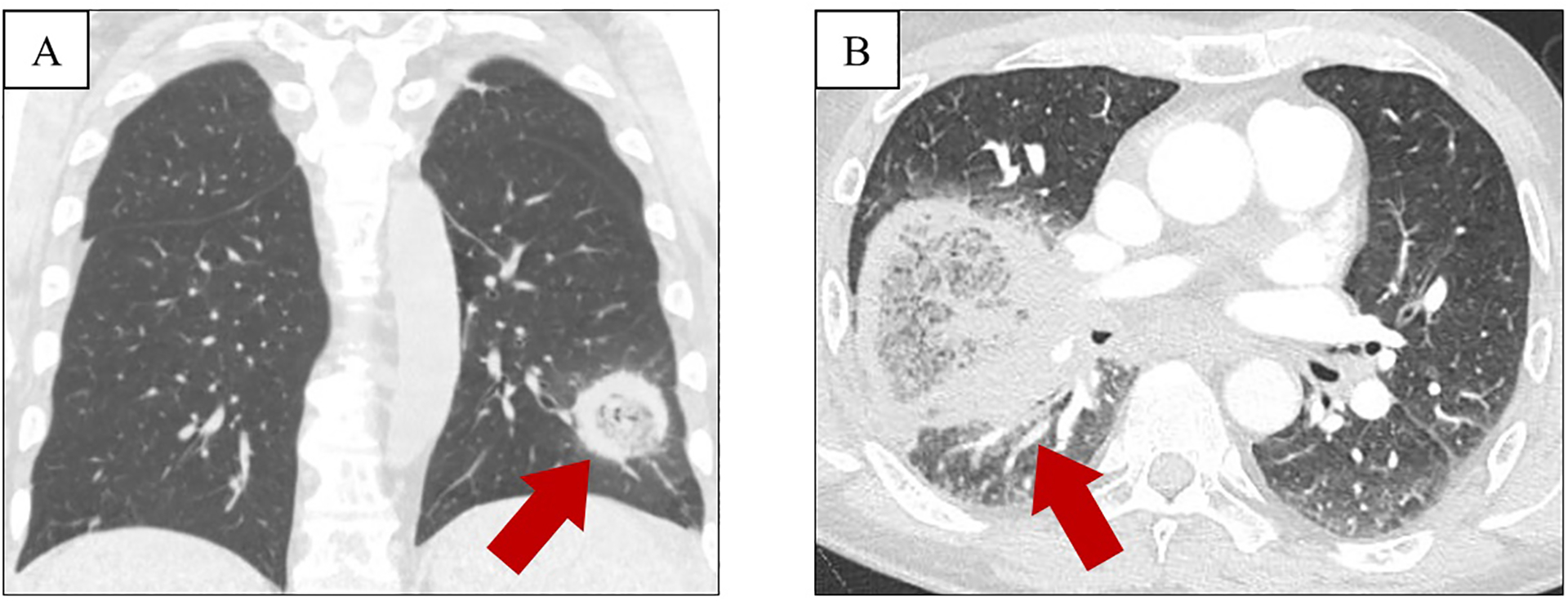
Reverse halo sign in patients with pulmonary mucormycosis
Non-culture-based serologic tests for the diagnosis of invasive fungal infections are currently available. However, such serum markers, including 1,3-beta-D-glucan (BDG) and Aspergillus galactomannan, are derived from fungal cell wall components not present in Mucorales.65,66 Thus, while a positive BDG or galactomannan can be suggestive of fungal infection with alternative pathogens to mucormycosis (i.e., to ‘rule out’ mucormycosis), these tests will not be able to identify a specific pathogen. Currently, there are no serum assays specific to mucormycosis.
Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry can be used for better identification of culture specimens, but further development of available databases is necessary for more widespread use.67 Molecular methods, including polymerase chain reaction (PCR)-based approaches, are increasingly used due to their ability to improve detection in tissues, and often aid in identification to the level of the species, through targets such as the internal transcribed spacer (ITS) or 18s ribosomal RNA.60,67–71 Additional non-invasive approaches of fungal identification continue to be investigated as well, including gene expression profiling, next-generation sequencing, and breath-based metabolomics.72–74
Management
Early clinical recognition and prompt diagnosis are key in the management of mucormycosis. While the clinical presentation and radiological features may be suggestive, urgent tissue diagnosis (pathology and culture) should be pursued whenever possible. Early initiation of systemic antifungals has a direct impact on the outcomes for mucormycosis and does not appear to alter the yield of tissue diagnosis or cultures.75 Eliminating the predisposing factor should also be attempted – for example, achieving control of blood sugar in diabetes has shown to be an important component of treatment.46 When eradication of the predisposing factor is not possible, such as in patients with hematological malignancies or transplant recipients, immunosuppression should be decreased as much as possible. Persistent immunosuppression (e.g., persistent neutropenia) makes management of this infection extremely challenging (Figure 6).
Figure 6:
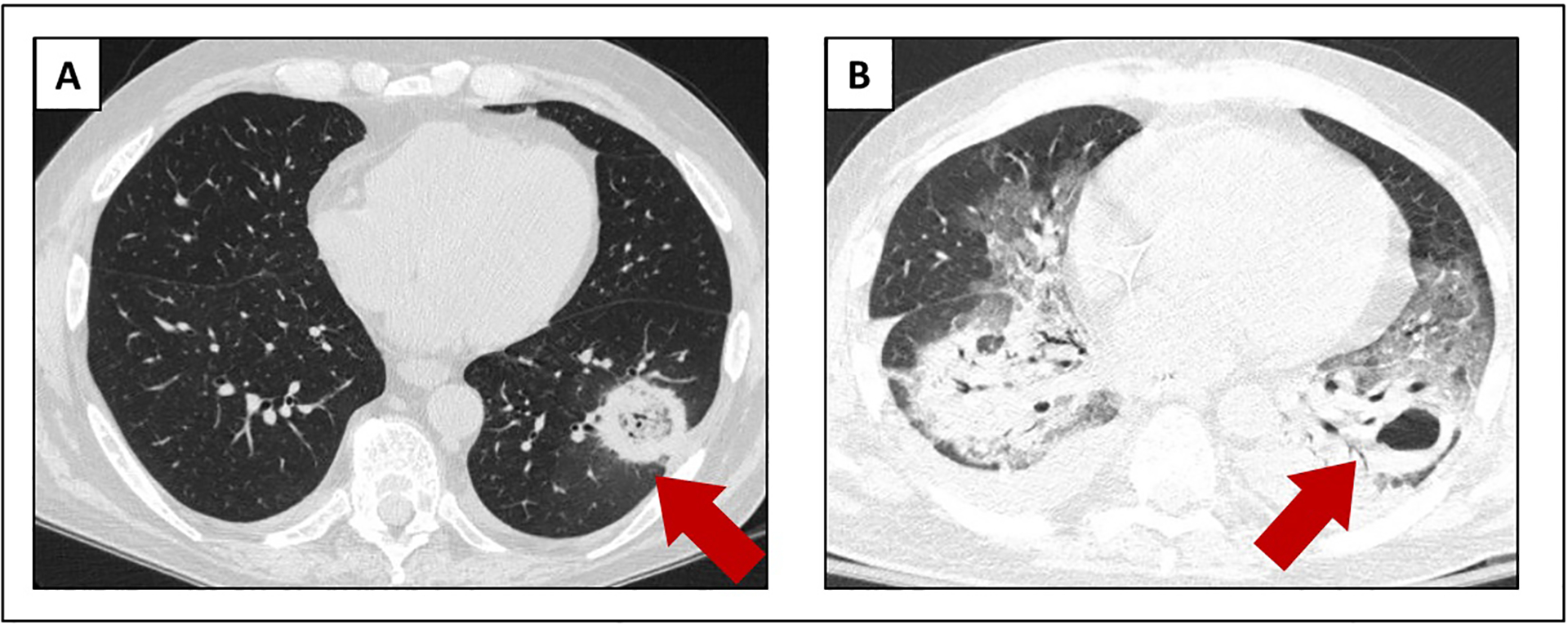
Radiological progression of pulmonary mucormycosis in a patient with relapsed acute leukemia and persistent neutropenia
Amphotericin B (AmB) is the most active drug in vitro against Mucorales and is considered the drug of choice for initial therapy (Table 1).76–79 For decades, AmB deoxycholate (AmB-D) was the sole available agent available for the treatment of mucormycosis. However, the use of AmB-D was limited by toxicity, especially infusion-related reactions and nephrotoxicity. Lipid formulations of AmB with an improved safety profile were developed to obviate these frequent side effects. The AmB lipid complex (ABLC) is composed of large ribbon-like complexes of phospholipids, amphotericin B colloidal dispersion (ABCD) contains disk-like structures of cholesteryl sulfate, and liposomal AmB (L-AmB) encompasses AmB within spherical liposomes.80 The efficacy of lipid formulations of AmB appears to be comparable and perhaps even superior to that of AmB-D.3,5,81–84 Of the lipid formulations, L-AmB and ABLC are most frequently used; the role of ABCD is limited and will not be discussed further. The use of lipid formulation AmB allows for prolonged therapy and higher daily doses with less toxicity, however the optimal daily dosage of lipid formulations of AmB for mucormycosis has not been established. The usual dose for mucormycosis is 5 mg/kg/day, but higher doses (7–10 mg/kg/d) are used in severe cases.80 Data suggest that high dose L-AmB (10 mg/kg/d) does not improve outcome and increases toxicity.85
Table 1:
Antifungal drugs used for the treatment of Mucormycosis
| Antifungal drug | Dose and route | Common Side Effects |
|---|---|---|
| Initial therapy | ||
| AmB -deoxycholate | 1 to 1.5 mg/kg/day IV | Infusion reactions Phlebitis Acute kidney injury Hypokalemia and hypomagnesemia Anemia |
| Liposomal AmB | 5 to 10 mg/kg/day IV | |
| ABLC | 5 to 10 mg/kg/day IV | |
| Step-down or salvage therapy | ||
| Posaconazole | IV formulation: 300 mg twice daily on day 1, followed by 300 mg daily | Nausea, vomiting, diarrhea, and headache QTc prolongation Hepatotoxicity |
| Oral suspension: 200 mg four times daily, followed by 400 mg twice daily after stabilization of disease.2 | ||
| Delayed-release tablets: 300 mg twice daily on day 1, followed by 300 mg daily3 | ||
| Isavuconazole | IV formulation: 372 mg every 8 h for 6 doses, followed by 372 mg once daily | Nausea, vomiting, diarrhea, headache, and rash Edema, hypokalemia Hepatotoxicity Shortened QTc interval Infusion reactions |
| Oral tablets: 372 mg (2 capsules) every 8 h for 6 doses, followed by 372 mg (2 capsules) once daily | ||
Infusion reactions (i.e.: fever, rigors, nausea, and vomiting) are most frequent with AmB deoxycholate.
Posaconazole oral suspension is taken with a full meal, liquid nutritional supplement, or acidic carbonated beverage to improve gastric absorption.
Delayed release tablet Posaconazole is taken regardless of food intake, H2- receptor antagonists or proton pump inhibitors.
Among the azole class of antifungals, posaconazole and isavuconazole are the most active agents against Mucorales and are used for stepdown therapy after response has been achieved with AmB. Posaconazole and isavuconazole are also used as salvage therapy in patients that cannot be treated with AmB.86,87 Mucorales are intrinsically resistant to other azoles (fluconazole, itraconazole, and voriconazole), echinocandins and flucytosine.88–90
Posaconazole is active against Mucorales, and comes in multiple formulations.91 Two studies using oral suspension posaconazole as salvage therapy in patients with invasive mucormycosis have been reported.92,93 Greenberg, et al reported on 24 patients enrolled in an open label salvage trial. In this study, 19 patients had infection refractory to AmB therapy.92 A favorable outcome (partial or complete response) was reported in 79% of patients, who were followed for 8 to 1004 days (median 182 days). The variable endpoint used to assess response to therapy makes these results difficult to interpret, rather than the standard 6-week or 12-week endpoint used more frequently in antifungal trials. The second study is a retrospective review of 91 patients with refractory mucormycosis (48 with hematologic malignancies, 37 were transplant recipients, and 30 with diabetes).93 In this study, the 12-week success rate was 60%. Both of these trials used the oral suspension of posaconazole, which has problematic absorption and has to be administered multiple times daily with fatty food or a nutritional supplement. Breakthrough mucormycosis has been reported in patients receiving oral suspension posaconazole prophylaxis.94 This could be a consequence of resistance of some Mucorales species to posaconazole or could be related to the poor absorption of the oral suspension resulting in suboptimal serum levels. New formulations of posaconazole, a delayed-release tablet and an intravenous formulation, are now available.95,96 Extended-release tablet posaconazole offers a more reliable absorption independent of gastric acidity with fewer drug-drug interactions and side effects.95,97 However, clinical data suggest that sub-therapeutic levels can still occur and are linked to obesity, diarrhea, and the use of proton pump inhibitors.98–100 Thus, therapeutic drug monitoring (TDM) is recommended both for treatment and prophylaxis.101 Serum trough levels are recommended within 5–7 days after the first dose. The suggested serum level is >0.7 μg/mL for prophylaxis, but levels ≥2 μg/mL are preferred for treatment of mucormycosis.
Isavuconazole is a newer triazole agent that is active against Mucorales, including Rhizomucor spp., Rhizopus spp., and Mucor spp.89,102,103 Isavuconazole was granted FDA approval for the treatment of mucormycosis based on the results of a phase 3, open-label, non- comparative, multi-center study of invasive fungal infections that included 37 patients with invasive mucormycosis.104 Of the 37 patients, 32 (86%) had proven invasive mucormycosis and 5 (14%) had probable invasive mucormycosis. In this study, 16 patients (43%) received isavuconazole as salvage therapy after failing or being intolerant of standard therapy, while 21 patients (57%) received only isavuconazole. The overall success at day 42 was 32%, and mortality was 38%. Mortality was highest among patients with refractory disease (46%). In a separate matched case-control study, the outcomes of 21 patients treated with isavuconazole were compared with 33 patients treated with AmB. In this study, mortality rate was 33% in those receiving isavuconazole and 41% in those receiving AmB.102 Additionally, case reports have noted success with isavuconazole as salvage therapy for disseminated and sino-orbital mucormycosis when other therapies failed.105,106
Available data supports the use of isavuconazole as an alternative to posaconazole for step-down therapy following initial therapy with L-AmB. However, some species are resistant and breakthrough cases of mucormycosis have been described in patients receiving either agent.88,89,102,107,108 Recent literature has shown that Mucorales species exhibit varying degrees of sensitivity to isavuconazole leading to clinical failure.107,109 Therefore, species identification and MIC testing should be obtained prior to initiating therapy with this agent. The role of isavuconazole TDM is uncertain and routine monitoring is not recommended.110 The use of isavuconazole in clinical practice will better define its future role in the treatment of mucormycosis.
Combination antifungal therapy is typically used by many physicians in an attempt to maximize treatment of this devastating disease, especially in patients with profound immunosuppression that cannot be reverted. The combination of lipid formulation AmB with oral posaconazole is based on case reports demonstrating efficacy.111–113 However, in the absence of a clinical trial, it is not clear that the outcomes of combined therapy are significantly improved over those noted with lipid formulation AmB alone. Despite a paucity of data, many experts support the use of combination therapy with L-AmB and posaconazole, given the potential clinical benefit and the lack of evidence for antagonism between the drugs.
Echinocandins have also been used in combination with AmB despite their lack of activity against Mucorales.114–117 A mouse model of mucormycosis infected with Rhizopus oryzae showed a modest improvement in survival when treated with caspofungin and AmB.114 The clinical experience using combination therapy with echinocandins and AmB is limited to small retrospective series and case reports.116,117 While echinocandins have a very low side effect profile, it is not clear that combination therapy has superior antifungal activity compared to monotherapy with liposomal AmB.115,116 In rare cases, an echinocandin has been successfully combined with posaconazole in patients with anaphylaxis to AmB.118 Currently, there is not enough data to support the routine use of an echinocandin combined with another antifungal agent for the treatment of mucormycosis.
It should be noted that the duration of antifungal therapy for mucormycosis is unknown, but typically ranges from months to years depending on organ involvement and the persistence of underlying risk factors (e.g., ongoing immunosuppression or persistent neutropenia). Sequential clinical and radiological assessments are necessary to determine response to antifungal therapy, and management of these patients is typically personalized to their unique clinical circumstances.67
Adjunctive management
Surgical debridement is instrumental in the treatment of this disease and has been shown to improve survival.1–5,119 Thus, aggressive debridement of all necrotic tissue should be carried out expeditiously (Figure 4). Surgery is especially important in rhino-orbital mucormycosis.3,6,120 Frequently, repeated debridements are required to effectively remove all necrotic tissue to a clean and viable surgical margin, increasing the effectiveness of antifungal therapy.121,122 Particular sites of infection that are more difficult to access, such as the lungs, throat, or genitals, make surgical resection more challenging. Similarly, surgical debridement may be precluded in patients with hematological malignancy or hematopoietic stem cell transplant due to severe thrombocytopenia.
Strategies to augment the number and function of neutrophils using granulocyte colony stimulating factor (G-CSF) or granulocyte-macrophage colony stimulating factor (GM-CSF) have shown benefit in animal studies.115,121 However, human clinical data is limited by small patient numbers.3,123–125 The use of interferon-gamma (IFN-γ) has also been used as an immunological booster in patients receiving antifungal therapy for mucormycosis.123 Case reports using IFN-γ have shown anecdotal success, however, there is not enough experience to recommend its routine use.
Rhizopus and other Mucorales require iron as a growth factor and utilize siderophores to capture iron from the host. Experimental animal studies have shown that the iron chelator deferasirox does not act as a siderophore, and denies iron to Rhizopus, inhibiting its growth.115 In a mouse model of mucormycosis, deferasirox efficacy was equal to that of AmB.126 A few case reports and a small open-label salvage study appeared to show benefit of deferasirox added to AmB or posaconazole therapy.127,128 However, a double-blind, randomized study of L-AmB with either deferasirox or placebo showed no survival advantage.129 In fact, 90-day mortality was significantly higher in patients who received the iron chelator. Based on these data, the use of deferasirox is not recommended as an adjuvant in the treatment of mucormycosis.
Hyperbaric oxygen has been used for adjunctive therapy in mucormycosis for many years.130–132 However, its antifungal mechanism has not yet been fully determined. It is possible that the increased partial pressure of oxygen leads to an increase of free oxygen radicals that exert a fungicidal effect, increasing neutrophil phagocytosis and killing, as well as improving angiogenesis.131 The use of a hyperbaric oxygen adjunct to surgical and antifungal therapy may have a role in diabetic patients with sinusitis or those with cutaneous mucormycosis.130,132 However, overall there is not enough experience using hyperbaric oxygen therapy to recommend its routine use in the treatment of mucormycosis.
Conclusion
Invasive mucormycosis is a rare but aggressive fungal infection with high morbidity and mortality, particularly in patients with underlying medical comorbidities or immunosuppression. Clinical and radiographical presentations can vary between patients based on the immune status of the host and mode of infection. However, it is important to keep a high level of suspicion of infection, as early diagnosis and rapid initiation of surgical and antifungal therapy are key to improve survival.
Key Points:
Mucormycosis is a rare but aggressive fungal disease that mainly affects patients with poorly controlled diabetes mellitus and those who are severely immunocompromised – including patients with hematological malignancies and solid organ transplant recipients.
Early recognition of infection is critical for treatment success, followed by prompt initiation of antifungal therapy with lipid formulation amphotericin B. Posaconazole and isavuconazole should be used for stepdown and salvage therapy.
Surgical debridement is key, both for tissue diagnosis and for treatment, and should be pursued without delay whenever possible.
In addition to surgery and antifungal therapy, reverting the underlying risk factor for infection is important for treatment response.
Disclosures:
J.M.S. has patents pending for gene expression-based classifiers of fungal infection and is funded by NIH NIAID T32 - AI100851.
M.H.M. has received research support and consulting honoraria from Astellas US, Scynexis, Inc. and Mayne Pharma.
References
- 1.Gomes MZ, Lewis RE, Kontoyiannis DP. Mucormycosis caused by unusual mucormycetes, non-Rhizopus, -Mucor, and -Lichtheimia species. Clin Microbiol Rev. 2011;24(2):411–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13(2):236–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–653. [DOI] [PubMed] [Google Scholar]
- 4.Prabhu RM, Patel R. Mucormycosis and entomophthoramycosis: a review of the clinical manifestations, diagnosis and treatment. Clin Microbiol Infect. 2004;10 Suppl 1:31–47. [DOI] [PubMed] [Google Scholar]
- 5.Lanternier F, Dannaoui E, Morizot G, et al. A global analysis of mucormycosis in France: the RetroZygo Study (2005–2007). Clin Infect Dis. 2012;54 Suppl 1:S35–43. [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarti A, Das A, Mandal J, et al. The rising trend of invasive zygomycosis in patients with uncontrolled diabetes mellitus. Med Mycol. 2006;44(4):335–342. [DOI] [PubMed] [Google Scholar]
- 7.Walther G, Wagner L, Kurzai O. Updates on the Taxonomy of Mucorales with an Emphasis on Clinically Important Taxa. J Fungi (Basel). 2019;5(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orlowski M Mucor dimorphism. Microbiol Rev. 1991;55(2):234–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinberg G, Peñalva MA, Riquelme M, Wösten HA, Harris SD. Cell Biology of Hyphal Growth. Microbiol Spectr. 2017;5(2). [DOI] [PubMed] [Google Scholar]
- 10.Jeong W, Keighley C, Wolfe R, et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019;25(1):26–34. [DOI] [PubMed] [Google Scholar]
- 11.Park BJ, Pappas PG, Wannemuehler KA, et al. Invasive non-Aspergillus mold infections in transplant recipients, United States, 2001–2006. Emerg Infect Dis. 2011;17(10):1855–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azie N, Neofytos D, Pfaller M, Meier-Kriesche HU, Quan SP, Horn D. The PATH (Prospective Antifungal Therapy) Alliance® registry and invasive fungal infections: update 2012. Diagn Microbiol Infect Dis. 2012;73(4):293–300. [DOI] [PubMed] [Google Scholar]
- 13.Kontoyiannis DP, Yang H, Song J, et al. Prevalence, clinical and economic burden of mucormycosis-related hospitalizations in the United States: a retrospective study. BMC Infect Dis. 2016;16(1):730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baddley JW, Stroud TP, Salzman D, Pappas PG. Invasive mold infections in allogeneic bone marrow transplant recipients. Clin Infect Dis. 2001;32(9):1319–1324. [DOI] [PubMed] [Google Scholar]
- 15.Kontoyiannis DP, Wessel VC, Bodey GP, Rolston KV. Zygomycosis in the 1990s in a tertiary-care cancer center. Clin Infect Dis. 2000;30(6):851–856. [DOI] [PubMed] [Google Scholar]
- 16.Almyroudis NG, Sutton DA, Linden P, Rinaldi MG, Fung J, Kusne S. Zygomycosis in solid organ transplant recipients in a tertiary transplant center and review of the literature. Am J Transplant. 2006;6(10):2365–2374. [DOI] [PubMed] [Google Scholar]
- 17.Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis. 2010;50(8):1101–1111. [DOI] [PubMed] [Google Scholar]
- 18.Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50(8):1091–1100. [DOI] [PubMed] [Google Scholar]
- 19.Kontoyiannis DP, Lionakis MS, Lewis RE, et al. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: a case-control observational study of 27 recent cases. J Infect Dis. 2005;191(8):1350–1360. [DOI] [PubMed] [Google Scholar]
- 20.Lanternier F, Sun H-Y, Ribaud P, Singh N, Kontoyiannis DP, Lortholary O. Mucormycosis in Organ and Stem Cell Transplant Recipients. Clin Infect Dis. 2012;54(11):1–8. [DOI] [PubMed] [Google Scholar]
- 21.Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54 Suppl 1:S23–34. [DOI] [PubMed] [Google Scholar]
- 22.Reid G, Lynch JP 3rd, Fishbein MC, Clark NM. Mucormycosis. Semin Respir Crit Care Med. 2020;41(1):99–114. [DOI] [PubMed] [Google Scholar]
- 23.Trifilio SM, Bennett CL, Yarnold PR, et al. Breakthrough zygomycosis after voriconazole administration among patients with hematologic malignancies who receive hematopoietic stem-cell transplants or intensive chemotherapy. Bone Marrow Transplant. 2007;39(7):425–429. [DOI] [PubMed] [Google Scholar]
- 24.Andresen D, Donaldson A, Choo L, et al. Multifocal cutaneous mucormycosis complicating polymicrobial wound infections in a tsunami survivor from Sri Lanka. Lancet. 2005;365(9462):876–878. [DOI] [PubMed] [Google Scholar]
- 25.Neblett Fanfair R, Benedict K, Bos J, et al. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med. 2012;367(23):2214–2225. [DOI] [PubMed] [Google Scholar]
- 26.Warkentien T, Rodriguez C, Lloyd B, et al. Invasive mold infections following combat-related injuries. Clin Infect Dis. 2012;55(11):1441–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rammaert B, Lanternier F, Zahar JR, et al. Healthcare-associated mucormycosis. Clin Infect Dis. 2012;54 Suppl 1:S44–54. [DOI] [PubMed] [Google Scholar]
- 28.Hartnett KP, Jackson BR, Perkins KM, et al. A Guide to Investigating Suspected Outbreaks of Mucormycosis in Healthcare. J Fungi (Basel). 2019;5(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrikkos G, Tsioutis C. Recent Advances in the Pathogenesis of Mucormycoses. Clin Ther. 2018;40(6):894–902. [DOI] [PubMed] [Google Scholar]
- 30.Hassan MIA, Voigt K. Pathogenicity patterns of mucormycosis: epidemiology, interaction with immune cells and virulence factors. Med Mycol. 2019;57(Supplement_2):S245–S256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu Y, Lee H, Collins M, et al. Cloning and functional characterization of the Rhizopus oryzae high affinity iron permease (rFTR1) gene. FEMS Microbiol Lett. 2004;235(1):169–176. [DOI] [PubMed] [Google Scholar]
- 32.Gebremariam T, Liu M, Luo G, et al. CotH3 mediates fungal invasion of host cells during mucormycosis. J Clin Invest. 2014;124(1):237–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patiño-Medina JA, Maldonado-Herrera G, Pérez-Arques C, et al. Control of morphology and virulence by ADP-ribosylation factors (Arf) in Mucor circinelloides. Curr Genet. 2018;64(4):853–869. [DOI] [PubMed] [Google Scholar]
- 34.Lionakis MS, Kontoyiannis DP. Glucocorticoids and invasive fungal infections. Lancet. 2003;362(9398):1828–1838. [DOI] [PubMed] [Google Scholar]
- 35.Binder U, Maurer E, Lass-Flörl C. Mucormycosis--from the pathogens to the disease. Clin Microbiol Infect. 2014;20 Suppl 6:60–66. [DOI] [PubMed] [Google Scholar]
- 36.Lackner G, Partida-Martinez LP, Hertweck C. Endofungal bacteria as producers of mycotoxins. Trends Microbiol. 2009;17(12):570–576. [DOI] [PubMed] [Google Scholar]
- 37.Partida-Martinez LP, Hertweck C. Pathogenic fungus harbours endosymbiotic bacteria for toxin production. Nature. 2005;437(7060):884–888. [DOI] [PubMed] [Google Scholar]
- 38.Lamaris GA, Ben-Ami R, Lewis RE, Chamilos G, Samonis G, Kontoyiannis DP. Increased virulence of Zygomycetes organisms following exposure to voriconazole: a study involving fly and murine models of zygomycosis. J Infect Dis. 2009;199(9):1399–1406. [DOI] [PubMed] [Google Scholar]
- 39.Lewis RE, Liao G, Wang W, Prince RA, Kontoyiannis DP. Voriconazole pre-exposure selects for breakthrough mucormycosis in a mixed model of Aspergillus fumigatus-Rhizopus oryzae pulmonary infection. Virulence. 2011;2(4):348–355. [DOI] [PubMed] [Google Scholar]
- 40.Mattingly JK, Ramakrishnan VR. Rhinocerebral Mucormycosis of the Optic Nerve. Otolaryngol Head Neck Surg. 2016;155(5):888–889. [DOI] [PubMed] [Google Scholar]
- 41.McNulty JS. Rhinocerebral mucormycosis: predisposing factors. Laryngoscope. 1982;92(10 Pt 1):1140–1143. [PubMed] [Google Scholar]
- 42.Sun HY, Forrest G, Gupta KL, et al. Rhino-orbital-cerebral zygomycosis in solid organ transplant recipients. Transplantation. 2010;90(1):85–92. [DOI] [PubMed] [Google Scholar]
- 43.Elinav H, Zimhony O, Cohen MJ, Marcovich AL, Benenson S. Rhinocerebral mucormycosis in patients without predisposing medical conditions: a review of the literature. Clin Microbiol Infect. 2009;15(7):693–697. [DOI] [PubMed] [Google Scholar]
- 44.Harrill WC, Stewart MG, Lee AG, Cernoch P. Chronic rhinocerebral mucormycosis. Laryngoscope. 1996;106(10):1292–1297. [DOI] [PubMed] [Google Scholar]
- 45.Vaughan C, Bartolo A, Vallabh N, Leong SC. A meta-analysis of survival factors in rhino-orbital-cerebral mucormycosis-has anything changed in the past 20 years? Clin Otolaryngol. 2018;43(6):1454–1464. [DOI] [PubMed] [Google Scholar]
- 46.Yohai RA, Bullock JD, Aziz AA, Markert RJ. Survival factors in rhino-orbital-cerebral mucormycosis. Surv Ophthalmol. 1994;39(1):3–22. [DOI] [PubMed] [Google Scholar]
- 47.Siddiqi SU, Freedman JD. Isolated central nervous system mucormycosis. South Med J. 1994;87(10):997–1000. [DOI] [PubMed] [Google Scholar]
- 48.Nagy-Agren SE, Chu P, Smith GJ, Waskin HA, Altice FL. Zygomycosis (mucormycosis) and HIV infection: report of three cases and review. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10(4):441–449. [DOI] [PubMed] [Google Scholar]
- 49.Feng J, Sun X. Characteristics of pulmonary mucormycosis and predictive risk factors for the outcome. Infection. 2018;46(4):503–512. [DOI] [PubMed] [Google Scholar]
- 50.Connor BA, Anderson RJ, Smith JW. Mucor mediastinitis. Chest. 1979;75(4):525–526. [DOI] [PubMed] [Google Scholar]
- 51.Hamilos G, Samonis G, Kontoyiannis DP. Pulmonary mucormycosis. Semin Respir Crit Care Med. 2011;32(6):693–702. [DOI] [PubMed] [Google Scholar]
- 52.Prakash H, Chakrabarti A. Global Epidemiology of Mucormycosis. J Fungi (Basel). 2019;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skiada A, Petrikkos G. Cutaneous zygomycosis. Clin Microbiol Infect. 2009;15 Suppl 5:41–45. [DOI] [PubMed] [Google Scholar]
- 54.Cheng VC, Chan JF, Ngan AH, et al. Outbreak of intestinal infection due to Rhizopus microsporus. J Clin Microbiol. 2009;47(9):2834–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corey KE, Gupta NK, Agarwal S, Xiao HD. Case records of the Massachusetts General Hospital. Case 32–2013. A 55-year-old woman with autoimmune hepatitis, cirrhosis, anorexia, and abdominal pain. N Engl J Med. 2013;369(16):1545–1553. [DOI] [PubMed] [Google Scholar]
- 56.Martinez EJ, Cancio MR, Sinnott JTt, Vincent AL, Brantley SG. Nonfatal gastric mucormycosis in a renal transplant recipient. South Med J. 1997;90(3):341–344. [DOI] [PubMed] [Google Scholar]
- 57.Levy E, Bia MJ. Isolated renal mucormycosis: case report and review. J Am Soc Nephrol. 1995;5(12):2014–2019. [DOI] [PubMed] [Google Scholar]
- 58.Weng DE, Wilson WH, Little R, Walsh TJ. Successful medical management of isolated renal zygomycosis: case report and review. Clin Infect Dis. 1998;26(3):601–605. [DOI] [PubMed] [Google Scholar]
- 59.Moreira J, Varon A, Galhardo MC, et al. The burden of mucormycosis in HIV-infected patients: A systematic review. J Infect. 2016;73(3):181–188. [DOI] [PubMed] [Google Scholar]
- 60.Walsh TJ, Gamaletsou MN, McGinnis MR, Hayden RT, Kontoyiannis DP. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis). Clin Infect Dis. 2012;54 Suppl 1:S55–60. [DOI] [PubMed] [Google Scholar]
- 61.Donnelly JP, Chen SC, Kauffman CA, et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020;71(6):1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chamilos G, Marom EM, Lewis RE, Lionakis MS, Kontoyiannis DP. Predictors of pulmonary zygomycosis versus invasive pulmonary aspergillosis in patients with cancer. Clin Infect Dis. 2005;41(1):60–66. [DOI] [PubMed] [Google Scholar]
- 63.Jung J, Kim MY, Lee HJ, et al. Comparison of computed tomographic findings in pulmonary mucormycosis and invasive pulmonary aspergillosis. Clin Microbiol Infect. 2015;21(7):684.e611–688. [DOI] [PubMed] [Google Scholar]
- 64.Hammer MM, Madan R, Hatabu H. Pulmonary Mucormycosis: Radiologic Features at Presentation and Over Time. AJR Am J Roentgenol. 2018;210(4):742–747. [DOI] [PubMed] [Google Scholar]
- 65.Ostrosky-Zeichner L, Alexander BD, Kett DH, et al. Multicenter clinical evaluation of the (1-->3) beta-D-glucan assay as an aid to diagnosis of fungal infections in humans. Clin Infect Dis. 2005;41(5):654–659. [DOI] [PubMed] [Google Scholar]
- 66.Miceli MH, Maertens J. Role of Non-Culture-Based Tests, with an Emphasis on Galactomannan Testing for the Diagnosis of Invasive Aspergillosis. Semin Respir Crit Care Med. 2015;36(5):650–661. [DOI] [PubMed] [Google Scholar]
- 67.Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19(12):e405–e421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bialek R, Konrad F, Kern J, et al. PCR based identification and discrimination of agents of mucormycosis and aspergillosis in paraffin wax embedded tissue. J Clin Pathol. 2005;58(11):1180–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hammond SP, Bialek R, Milner DA, Petschnigg EM, Baden LR, Marty FM. Molecular methods to improve diagnosis and identification of mucormycosis. J Clin Microbiol. 2011;49(6):2151–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soare AY, Watkins TN, Bruno VM. Understanding Mucormycoses in the Age of “omics”. Front Genet. 2020;11:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Skiada A, Pavleas I, Drogari-Apiranthitou M. Epidemiology and Diagnosis of Mucormycosis: An Update. J Fungi (Basel). 2020;6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steinbrink JM, Zaas AK, Betancourt M, Modliszewski JL, Corcoran DL, McClain MT. A transcriptional signature accurately identifies Aspergillus Infection across healthy and immunosuppressed states. Transl Res. 2020;219:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steinbrink JM, Hong DK, Bergin SP, Al-Rohil RN, Perfect JR, Maziarz EK. The robust and rapid role of molecular testing in precision fungal diagnostics: A case report. Med Mycol Case Rep. 2020;27:77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koshy S, Ismail N, Astudillo CL, et al. Breath-Based Diagnosis of Invasive Mucormycosis (IM). Open Forum Infect Dis. 2017;4(Suppl 1):S53–S54. [Google Scholar]
- 75.Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47(4):503–509. [DOI] [PubMed] [Google Scholar]
- 76.Alastruey-Izquierdo A, Castelli MV, Cuesta I, et al. In vitro activity of antifungals against Zygomycetes. Clin Microbiol Infect. 2009;15:71–76. [DOI] [PubMed] [Google Scholar]
- 77.Dannaoui E, Meletiadis J, Mouton JW, Meis JF, Verweij PE. In vitro susceptibilities of zygomycetes to conventional and new antifungals. J Antimicrob Chemother. 2003;51(1):45–52. [DOI] [PubMed] [Google Scholar]
- 78.McCarthy M, Rosengart A, Schuetz AN, Kontoyiannis DP, Walsh TJ. Mold infections of the central nervous system. N Engl J Med. 2014;371(2):150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vitale RG, de Hoog GS, Schwarz P, et al. Antifungal susceptibility and phylogeny of opportunistic members of the order mucorales. J Clin Microbiol. 2012;50(1):66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miceli MH, Chandrasekar P. Safety and efficacy of liposomal amphotericin B for the empirical therapy of invasive fungal infections in immunocompromised patients. Infect Drug Resist. 2012;5:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Skiada A, Pagano L, Groll A, et al. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect. 2011;17(12):1859–1867. [DOI] [PubMed] [Google Scholar]
- 82.Rüping MJ, Heinz WJ, Kindo AJ, et al. Forty-one recent cases of invasive zygomycosis from a global clinical registry. J Antimicrob Chemother. 2010;65(2):296–302. [DOI] [PubMed] [Google Scholar]
- 83.Petrikkos GL. Lipid formulations of amphotericin B as first-line treatment of zygomycosis. Clin Microbiol Infect. 2009;15 Suppl 5:87–92. [DOI] [PubMed] [Google Scholar]
- 84.Forrest GN, Mankes K. Outcomes of invasive zygomycosis infections in renal transplant recipients. Transpl Infect Dis. 2007;9(2):161–164. [DOI] [PubMed] [Google Scholar]
- 85.Lanternier F, Poiree S, Elie C, et al. Prospective pilot study of high-dose (10 mg/kg/day) liposomal amphotericin B (L-AMB) for the initial treatment of mucormycosis. J Antimicrob Chemother. 2015;70(11):3116–3123. [DOI] [PubMed] [Google Scholar]
- 86.Espinel-Ingroff A, Chakrabarti A, Chowdhary A, et al. Multicenter evaluation of MIC distributions for epidemiologic cutoff value definition to detect amphotericin B, posaconazole, and itraconazole resistance among the most clinically relevant species of Mucorales. Antimicrob Agents Chemother. 2015;59(3):1745–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Biswas D, Kotwal A, Kakati B, Ahmad S. Amphotericin B Resistant Apophysomyces elegans Causing Rhino-oculo-Cerebral Mucormycosis in an Immunocompetent Host. Journal of clinical and diagnostic research : JCDR. 2015;9(8):DD01–DD02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guinea J, Peláez T, Recio S, Torres-Narbona M, Bouza E. In vitro antifungal activities of isavuconazole (BAL4815), voriconazole, and fluconazole against 1,007 isolates of zygomycete, Candida, Aspergillus, Fusarium, and Scedosporium species. Antimicrob Agents Chemother. 2008;52(4):1396–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Verweij PE, González GM, Wiedrhold NP, et al. In vitro antifungal activity of isavuconazole against 345 mucorales isolates collected at study centers in eight countries. J Chemother. 2009;21(3):272–281. [DOI] [PubMed] [Google Scholar]
- 90.Torres-Narbona M, Guinea J, Martínez-Alarcón J, Peláez T, Bouza E. In vitro activities of amphotericin B, caspofungin, itraconazole, posaconazole, and voriconazole against 45 clinical isolates of zygomycetes: comparison of CLSI M38-A, Sensititre YeastOne, and the Etest. Antimicrob Agents Chemother. 2007;51(3):1126–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Torres HA, Hachem RY, Chemaly RF, Kontoyiannis DP, Raad II. Posaconazole: a broad-spectrum triazole antifungal. Lancet Infect Dis. 2005;5(12):775–785. [DOI] [PubMed] [Google Scholar]
- 92.Greenberg RN, Mullane K, van Burik JA, et al. Posaconazole as salvage therapy for zygomycosis. Antimicrob Agents Chemother. 2006;50(1):126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006;42(7):e61–65. [DOI] [PubMed] [Google Scholar]
- 94.Dolton MJ, Ray JE, Chen SC, Ng K, Pont L, McLachlan AJ. Multicenter study of posaconazole therapeutic drug monitoring: exposure-response relationship and factors affecting concentration. Antimicrob Agents Chemother. 2012;56(11):5503–5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krishna G, Ma L, Martinho M, Preston RA, O’Mara E. A new solid oral tablet formulation of posaconazole: a randomized clinical trial to investigate rising single- and multiple-dose pharmacokinetics and safety in healthy volunteers. J Antimicrob Chemother. 2012;67(11):2725–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Duarte RF, López-Jiménez J, Cornely OA, et al. Phase 1b study of new posaconazole tablet for prevention of invasive fungal infections in high-risk patients with neutropenia. Antimicrob Agents Chemother. 2014;58(10):5758–5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pettit NN, Miceli MH, Rivera CG, et al. Multicentre study of posaconazole delayed-release tablet serum level and association with hepatotoxicity and QTc prolongation. J Antimicrob Chemother. 2017;72(8):2355–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Farmakiotis D, Kontoyiannis DP. Emerging issues with diagnosis and management of fungal infections in solid organ transplant recipients. Am J Transplant. 2015;15(5):1141–1147. [DOI] [PubMed] [Google Scholar]
- 99.Miceli MH, Perissinotti AJ, Kauffman CA, Couriel DR. Serum posaconazole levels among haematological cancer patients taking extended release tablets is affected by body weight and diarrhoea: single centre retrospective analysis. Mycoses. 2015;58(7):432–436. [DOI] [PubMed] [Google Scholar]
- 100.Tang LA, Marini BL, Benitez L, et al. Risk factors for subtherapeutic levels of posaconazole tablet. J Antimicrob Chemother. 2017;72(10):2902–2905. [DOI] [PubMed] [Google Scholar]
- 101.Tverdek FP, Heo ST, Aitken SL, Granwehr B, Kontoyiannis DP. Real-Life Assessment of the Safety and Effectiveness of the New Tablet and Intravenous Formulations of Posaconazole in the Prophylaxis of Invasive Fungal Infections via Analysis of 343 Courses. Antimicrob Agents Chemother. 2017;61(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miceli MH, Kauffman CA. Isavuconazole: A New Broad-Spectrum Triazole Antifungal Agent. Clin Infect Dis. 2015;61(10):1558–1565. [DOI] [PubMed] [Google Scholar]
- 103.Marty FM, Perfect JR, Cornely OA, et al. 824: An Open-Label Phase 3 Study of Isavuconazole (VITAL): Focus on Mucormycosis. Open Forum Infect Dis. 2014;1(Suppl 1):S235–S236. [Google Scholar]
- 104.Marty FM, Ostrosky-Zeichner L, Cornely OA, et al. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis. 2016;16(7):828–837. [DOI] [PubMed] [Google Scholar]
- 105.Peixoto D, Gagne LS, Hammond SP, et al. Isavuconazole treatment of a patient with disseminated mucormycosis. J Clin Microbiol. 2014;52(3):1016–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ervens J, Ghannoum M, Graf B, Schwartz S. Successful isavuconazole salvage therapy in a patient with invasive mucormycosis. Infection. 2014;42(2):429–432. [DOI] [PubMed] [Google Scholar]
- 107.Bellanger AP, Berceanu A, Scherer E, et al. Invasive Fungal Disease, Isavuconazole Treatment Failure, and Death in Acute Myeloid Leukemia Patients. Emerg Infect Dis. 2019;25(9):1778–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dannaoui E Antifungal resistance in mucorales. Int J Antimicrob Agents. 2017;50(5):617–621. [DOI] [PubMed] [Google Scholar]
- 109.Denis J, Ledoux MP, Nivoix Y, Herbrecht R. Isavuconazole: A new broad-spectrum azole. Part 1: In vitro activity. J Mycol Med. 2018;28(1):8–14. [DOI] [PubMed] [Google Scholar]
- 110.Furfaro E, Signori A, Di Grazia C, et al. Serial monitoring of isavuconazole blood levels during prolonged antifungal therapy. J Antimicrob Chemother. 2019;74(8):2341–2346. [DOI] [PubMed] [Google Scholar]
- 111.Vehreschild JJ, Birtel A, Vehreschild MJ, et al. Mucormycosis treated with posaconazole: review of 96 case reports. Crit Rev Microbiol. 2013;39(3):310–324. [DOI] [PubMed] [Google Scholar]
- 112.Cornely OA, Vehreschild JJ, Rüping MJ. Current experience in treating invasive zygomycosis with posaconazole. Clin Microbiol Infect. 2009;15 Suppl 5:77–81. [DOI] [PubMed] [Google Scholar]
- 113.Pagano L, Cornely OA, Busca A, et al. Combined antifungal approach for the treatment of invasive mucormycosis in patients with hematologic diseases: a report from the SEIFEM and FUNGISCOPE registries. Haematologica. 2013;98(10):e127–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Spellberg B, Fu Y, Edwards JE Jr., Ibrahim AS. Combination therapy with amphotericin B lipid complex and caspofungin acetate of disseminated zygomycosis in diabetic ketoacidotic mice. Antimicrob Agents Chemother. 2005;49(2):830–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Spellberg B, Ibrahim A, Roilides E, et al. Combination therapy for mucormycosis: why, what, and how? Clin Infect Dis. 2012;54 Suppl 1(Suppl 1):S73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Reed C, Bryant R, Ibrahim AS, et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008;47(3):364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Voitl P, Scheibenpflug C, Weber T, Janata O, Rokitansky AM. Combined antifungal treatment of visceral mucormycosis with caspofungin and liposomal amphotericin B. Eur J Clin Microbiol Infect Dis. 2002;21(8):632–634. [DOI] [PubMed] [Google Scholar]
- 118.Sheybani F, Naderi HR, Sarvghad M, Ghabouli M, Arian M. How should we manage a patient with invasive mucoromycosis who develops life-threatening reaction to amphotericin B? Report of two cases and literature review. Med Mycol Case Rep. 2015;8:29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cornely OA, Arikan-Akdagli S, Dannaoui E, et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin Microbiol Infect. 2014;20 Suppl 3:5–26. [DOI] [PubMed] [Google Scholar]
- 120.Vironneau P, Kania R, Morizot G, et al. Local control of rhino-orbito-cerebral mucormycosis dramatically impacts survival. Clin Microbiol Infect. 2014;20(5):O336–339. [DOI] [PubMed] [Google Scholar]
- 121.Spellberg B, Edwards J Jr., Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rapidis AD. Orbitomaxillary mucormycosis (zygomycosis) and the surgical approach to treatment: perspectives from a maxillofacial surgeon. Clin Microbiol Infect. 2009;15 Suppl 5:98–102. [DOI] [PubMed] [Google Scholar]
- 123.Garcia-Diaz JB, Palau L, Pankey GA. Resolution of rhinocerebral zygomycosis associated with adjuvant administration of granulocyte-macrophage colony-stimulating factor. Clin Infect Dis. 2001;32(12):e145–150. [DOI] [PubMed] [Google Scholar]
- 124.Abzug MJ, Walsh TJ. Interferon-gamma and colony-stimulating factors as adjuvant therapy for refractory fungal infections in children. Pediatr Infect Dis J. 2004;23(8):769–773. [DOI] [PubMed] [Google Scholar]
- 125.Ma B, Seymour JF, Januszewicz H, Slavin MA. Cure of pulmonary Rhizomucor pusillus infection in a patient with hairy-cell leukemia: role of liposomal amphotericin B and GM-CSF. Leuk Lymphoma. 2001;42(6):1393–1399. [DOI] [PubMed] [Google Scholar]
- 126.Ibrahim AS, Gebermariam T, Fu Y, et al. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. J Clin Invest. 2007;117(9):2649–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Reed C, Ibrahim A, Edwards JE Jr., Walot I, Spellberg B. Deferasirox, an iron-chelating agent, as salvage therapy for rhinocerebral mucormycosis. Antimicrob Agents Chemother. 2006;50(11):3968–3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Spellberg B, Andes D, Perez M, et al. Safety and outcomes of open-label deferasirox iron chelation therapy for mucormycosis. Antimicrob Agents Chemother. 2009;53(7):3122–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Spellberg B, Ibrahim AS, Chin-Hong PV, et al. The Deferasirox-AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J Antimicrob Chemother. 2012;67(3):715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.John BV, Chamilos G, Kontoyiannis DP. Hyperbaric oxygen as an adjunctive treatment for zygomycosis. Clin Microbiol Infect. 2005;11(7):515–517. [DOI] [PubMed] [Google Scholar]
- 131.Tragiannidis A, Groll AH. Hyperbaric oxygen therapy and other adjunctive treatments for zygomycosis. Clin Microbiol Infect. 2009;15 Suppl 5:82–86. [DOI] [PubMed] [Google Scholar]
- 132.Segal E, Menhusen MJ, Shawn S. Hyperbaric oxygen in the treatment of invasive fungal infections: a single-center experience. Isr Med Assoc J. 2007;9(5):355–357. [PubMed] [Google Scholar]


