Abstract
Background:
The continued increase in prevalence of methamphetamine use in the United States has resulted in a significant increase in the number of patients entering treatment for methamphetamine use. However, no robustly efficacious pharmacologic treatment for methamphetamine use or withdrawal has been identified to date after stopping methamphetamine use.
Aims:
Given the association between methamphetamine withdrawal and relapse during early treatment, this study tested a controlled d-amphetamine withdrawal paradigm among methamphetamine-using individuals.
Methods:
Treatment-seeking adults who used methamphetamine (N=34; 47% female; 100% white) were enrolled in a four-week, randomized, double-blind, placebo-controlled trial in a residential setting, in which all participants were maintained on d-amphetamine (30 mg BID) during week 1, then half were switched to placebo during weeks 2–3. All participants received placebo during week 4. Outcomes included vital signs, withdrawal, cravings for methamphetamine, mood, and cognition. Bivariate analyses tested treatment group differences on baseline demographic and outcome variables. Repeated measures models examined main and interaction effects of treatment over time.
Results/Outcomes:
Participants were successfully randomized and safely stabilized on d-amphetamine. Craving for methamphetamine increased during weeks 2–3 in the placebo group relative to those on d-amphetamine. Interactions with age and heart rate were noted.
Conclusions/Interpretation:
To our knowledge, this is the first double blind, placebo-controlled trial measuring pharmacologic effects of abruptly stopping controlled d-amphetamine administration in adults who use methamphetamine. Results support the potential of this withdrawal paradigm to further examine the efficacy of pharmacologic agents in ameliorating methamphetamine withdrawal symptoms.
Keywords: methamphetamine, pharmacologic treatment, methamphetamine withdrawal, methamphetamine withdrawal paradigm
Introduction
Methamphetamine use is increasingly prevalent in the United States (US; DHHS and SAMHSA, 2019) and is associated with profound medical (Gonzales et al., 2010; Kosten, 1990; Hong et al., 1991; Albertson et al., 1999; Sibanda et al., 2019), psychiatric (Kosten, 1990; Murray, 1998; Leshner and Koob, 1999), legal (NACO, 2005), and social (Gonzales et al., 2010; Murray, 1998) problems. In 2019, 2 million people aged 12 or older reported using methamphetamine (DHHS and SAMHSA, 2019), with a recent increase in patients entering treatment for methamphetamine use disorder. According to the Treatment Episode Data Set (TEDS) data, the rate of nationwide treatment admissions for methamphetamine misuse was 49 per 100,000 in 2015 (NIDA, 2019). During 2019, methamphetamine (58%) far outpaced cocaine (19%) as the greatest threat of state and local agencies in the US (NIDA, 2019).
Acute withdrawal from chronic amphetamine use in humans has been associated with drug craving, dysphoric mood, anhedonia, depression, fatigue, aggression, agitation, anxiety, hyperphagia, hypersomnia, and psychomotor retardation (American Psychiatric Association, 2013; Shoptaw et al., 2009; Gossop et al., 1982). The duration of amphetamine withdrawal tends to range from 5 days to more than 2 weeks (Shoptaw et al., 2009; McGregor et al., 2005), much longer than withdrawal from other stimulants, such as cocaine (Srisurapanont et al., 2005). Although 87%–97% of recently-abstinent amphetamine users are thought to experience withdrawal (Shoptaw et al., 2009; McGregor et al., 2005; Cantwell and McBride, 1998; Schuckit, 1999) and drug withdrawal is a predictor of drug relapse (Sinha, 2011), a dearth of studies has examined the impact of potential treatment agents on alleviating symptoms of amphetamine withdrawal among individuals with methamphetamine use disorder.
Although some medications have shown potential in counteracting the effects of methamphetamine, prolonging abstinence from use, and/or reducing use (e.g., bupropion [Elkashef et al., 2008; Shoptaw et al., 2008; Newton et al., 2006], bupropion plus injection naltrexone [Trivedi et al., 2021], and methylphenidate [Tiihonen et al., 2007; Gonzales et al., 2010]), no approved pharmacological treatments for methamphetamine use disorder exist to date (Montoya and Vocci, 2008; NIDA, 2019), despite decades of research. Previous placebo-controlled trials have failed to demonstrate any efficacy of medications in managing methamphetamine withdrawal (e.g., Cruickshank et al., 2009; Heinzerling et al., 2006; Johnson et al., 2007; Shoptaw et al., 2006; Tiihonen et al., 2007). Furthermore, although a few maintenance trials utilizing substitution treatment with dexamphetamine have demonstrated a decrease in methamphetamine use, they were not necessarily associated with any change in the acute amphetamine withdrawal syndrome (Longo et al., 2009; Shearer et al., 2001). Given this lack of understanding regarding the impact of potential pharmacologic treatment modalities on both outcome and management of the withdrawal syndrome, it is imperative to design a procedure to examine other potential substitution agents, preferably with low abuse liability, that alleviate any symptoms that might occur with the abrupt discontinuation of methamphetamine (e.g., craving, withdrawal severity, mood, cognition).
Although the Diagnostic and Statistical Manual-IV (DSM-IV; American Psychiatric Association, 2000) and DSM-5 (American Psychiatric Association, 2013) indicate criteria for an amphetamine withdrawal syndrome, amphetamine withdrawal, much less methamphetamine withdrawal, has been studied on a very limited basis in humans. A four-week study on the natural course of symptoms, mood, and cognitive performance among patients with methamphetamine dependence who were admitted to a residential facility (Mancino et al., 2011) found that self-reported measures of depression, anxiety, and methamphetamine withdrawal symptoms decreased significantly during the first two weeks of the residential stay with the most dramatic reduction occurring during the first week. Cognitive testing did not reveal significant changes during the course of the study, although performance did appear to decline during the second week of treatment. Additionally, participants in the study were older and had used methamphetamine longer, which might have contributed to the longer duration of withdrawal symptoms. These findings suggest that the time course of withdrawal is complex (highlighting the importance of obtaining a wide array of measures) and the duration of methamphetamine withdrawal-related symptoms may be longer than previously reported and highlight the need for a double-blind, placebo-controlled methamphetamine withdrawal paradigm in humans.
Given that a prior study demonstrated that daily sustained-release dexamphetamine produced no serious adverse events among individuals who use methamphetamine (Longo et al., 2009), the present study tested the utility of a human clinical model of d-amphetamine withdrawal, in which participants who used methamphetamine were admitted to a residential facility and initially placed on controlled d-amphetamine administration. Then, half were switched to placebo to determine whether amphetamine-associated withdrawal symptoms occurred. Specifically, this study addressed the following research questions: (1) Can individuals who use methamphetamine with varying lengths of use be safely stabilized on d-amphetamine (60 mg/day), successfully randomized to 2 ‘equivalent’ groups, and retained without reporting serious adverse events? (2) Are study outcomes (i.e., severity of methamphetamine use, methamphetamine withdrawal, craving for methamphetamines, depression, anxiety, cognition, and vital signs) impacted over the 1-week standardized exposure to d-amphetamine? (3) Does abrupt termination of d-amphetamine administration in oral d-amphetamine-stabilized methamphetamine users acutely impact (i.e., one week) study outcomes compared to those who continue to receive d-amphetamine? (4) What is the time course (up to two weeks) of any impacts abrupt termination of d-amphetamine administration on study outcomes among individuals stabilized on oral d-amphetamine-stabilized compared to those who continue to receive d-amphetamine?
Methods
Participants
Adults who met the following criteria were recruited from newspaper and radio advertisements, posters/flyers, and word-of-mouth advertising to participate in this study: 20–65 years of age; not currently enrolled in a treatment program; history of methamphetamine use with recent use confirmed by a positive urine toxicology screen for amphetamines during the month prior to study entry; self-reported methamphetamine use on at least 15 of the past 30 days; use of at least one-half gram of methamphetamine per week during the month prior to study entry; and negative pregnancy test for women of childbearing age; no current diagnosis of alcohol, opiate, or sedative physical dependence; no ill health (e.g., major cardiovascular, renal, endocrine, hepatic, pain, seizure disorder); no history of schizophrenia, or bipolar type I disorder; no present or recent use of over-the-counter or prescription psychoactive drug or drug(s) that would be expected to have major interaction with d-amphetamine; no use of monoamine oxidase inhibitor (MAOI) within 2 weeks of study entry; and no medical condition contraindicative to receiving study medications (e.g., previous adverse reaction to d-amphetamine). Participants received the following payments for assessment completion: for week one, $10 ($30 total), week two, $15 ($45 total), week three, $20 ($60 total), week four, $25 ($75 total). The Institutional Review Board at the University of Arkansas for Medical Sciences (UAMS) approved all study procedures.
Research Design and Procedures
Treatment-seeking adults who used methamphetamine were enrolled in a one-month, double-blind, placebo-controlled trial examining the impact of abruptly terminating controlled oral immediate-release d-amphetamine administration (stabilized on d-amphetamine for one week) on withdrawal, cravings for methamphetamine, mood, cognition, vital signs, and adverse events. Participants were admitted to a residential facility, inducted onto d-amphetamine during week 1 (to standardize exposure to stimulants prior to initiation of randomization), and randomized via urn randomization to receive d-amphetamine or placebo during weeks 2–3. All participants received placebo during week 4. At regular timepoints, assessments of amphetamine withdrawal, craving, blood pressure and cognitive measures were obtained. Participants received residential substance use treatment for the duration of their participation.
D-amphetamine or placebo (microcrystalline cellulose) was prepared in identical blue opaque capsules contained in blister packs. All study medications were kept locked in a secure area at the residential facility. At predetermined times, facility or research staff gave participants their respective blister pack of the study medication, who then ingested their dose under observation. No concomitant medications were allowed. Subjects were initiated on d-amphetamine at 10 mg twice daily with the first dose administered within 2 hours of awakening and the second dose 6 hours later. This dose was increased by 20 mg daily (10 mg per dose) until day 3, when a dose of 60 mg per day had been achieved. At the beginning of week 2, half the subjects received placebo and the other half continued on d-amphetamine. At the beginning of week 4, all subjects were placed on placebo to allow a week of washout and final assessments. At the end of the study, participants were discharged from the residential facility and referred for longer-term treatment, if desired.
Measures
Primary outcome measures included Methamphetamine Selective Severity Assessment (MSSA; McGregor et al., 2005), Methamphetamine Withdrawal Assessment (MAWA; Trevisan et al., 1998), and Visual Analog Scale of Methamphetamine Craving, in which craving in the past 24 hours was measured on a scale from 0 (no craving) to 20 (great deal of craving). Secondary outcome measures included Hamilton Depression (HAM-D; Hamilton 1960) and Hamilton Anxiety (HAM-A; Hamilton, 1959) Scales, the Stroop Color Word Test (Golden, 1978), and vital signs. Vital signs were obtained to evaluate for orthostatic hypotension with 3 minutes between seated and standing measures. Assessments were repeated at least thrice weekly, except for cognitive measures that were repeated weekly. Typically, measures were taken within 2–3 hours after d-amphetamine dosing. Participants also reported the number of days they used amphetamines per week, grams used per day, age of first use, and years of regular use and whether or not they used methamphetamine intravenously. They reported the use of other drugs (alcohol, benzodiazepines, cannabis, cocaine, opioids, and tobacco) in the past 30 days as well. Adverse effects were monitored and recorded to determine the relative safety of stabilizing participants on d-amphetamine before abruptly terminating oral d-amphetamine administration and throughout 1 month study timeframe. Urine samples to test for illicit substances were not collected due to confounds with the detection of study medication.
Data Analyses
In order to determine the utility, preliminary effectiveness, and safety of a human model of d-amphetamine withdrawal among adults who use methamphetamine, the following analyses were conducted to examine randomization and retention, effects of 1-week standardized exposure to d-amphetamine, effects of abrupt termination of d-amphetamine during week 2, and length of effects of amphetamine withdrawal over the course of study. Missing data were managed by an assumption of missing at random. Repeated measures modelling was done as mixed effects repeated measures using SAS PROC MIXED which retains observed values for participants even when some of a participant’s values may be missing, unlike SAS PROC GLM which would have eliminated all data from participants with any missing value.
Randomization and Retention:
In order to determine whether or not individuals who use methamphetamine could be successfully randomized to 2 equivalent groups, and retained as such, over the course of the study, bivariate analyses (t-test, ANOVA, chi-square) were conducted to examine group differences in methamphetamine withdrawal, craving, and severity, depression, anxiety, cognition, and vital signs at intake and one-week after standardized exposure to d-amphetamine. Kaplan-Meier Survival Analysis were conducted to determine whether or not retention differed between treatment and placebo groups over the course of the study, specifically in the first week of the study (during 1-week standardized exposure to d-amphetamine) and from week 2 forward (after assigned to treatment versus placebo groups). In order to ensure the reproducibility of study findings, analyses were completed to determine whether or not: participants were successfully and safely randomized to 2 equivalent groups (i.e., no statistically significant differences in demographics, methamphetamine withdrawal, craving, or severity, depression, anxiety, cognition, vital signs, adverse events, methamphetamine use/route histories, or other drug use) at intake; treatment groups remained equivalent after 1-week standardized exposure to d-amphetamine; and retention significantly differed by treatment group.
Effects of 1-Week Standardized Exposure to D-Amphetamine:
Repeated measures analysis of variance (ANOVA) models examined within and between group differences on continuous outcome measures obtained thrice-weekly over week 1 standardized exposure to d-amphetamine in order to identify more specific and possibly adverse changes in outcomes and vital signs that might have occurred throughout this time period as well as ensure no group baseline differences occurred during d-amphetamine administration.
Effects of Abrupt Termination of D-Amphetamine During Week 2:
Repeated measures ANOVA models examined the immediate (over 3 visits during week 2) impact of abruptly terminating d-amphetamine administration in methamphetamine-using individuals stabilized on d-amphetamine. Models including main effects of treatment and timepoint were fit as well as models including the interaction of those main effects. Residuals from both were examined for model assumptions and transformations were used where needed. After choosing transformations, a final model was chosen based on if the interaction term was significant or not. Several outcome variables needed transformations, after testing several options, rank transformation was used for them. All estimated model effect coefficients are reported. For all pairwise comparisons, only those with an unadjusted p-value<=0.05 are reported. Tukey adjusted p-values are also reported for pairwise comparisons.
Length of Effects of Amphetamine Withdrawal over Course of Study:
Thrice-weekly assessments collected at the third time point of data collection were used as the baseline measures included in analyses of week 1 visit 3 to week 4 visit 3 data. Repeated measures ANOVA models examined the length of effects of amphetamine withdrawal in methamphetamine users over the course of study. Repeated measures models were fit with main effects of treatment, time, and the interaction. Transformations were used to achieve normality and constant variance of residuals.
Results
Baseline Participant Characteristics:
Of 40 who were found to be eligible and signed informed consent, 34 participants (47% female, 100% White, 21% less than high school education, 21% employed, and 26% never married) were enrolled into the study proper. Despite randomization procedures, treatment groups differed, in that individuals in the placebo group were significantly younger than those in the d-amphetamine group (see Table 1). No other statistically significant differences in intake demographics, methamphetamine use/route, other drug use, or baseline measures were found between groups.
Table 1.
Comparison of Intake Sample Demographics and Methamphetamine/Other Drug Use (N=34).
| VARIABLE | D-AMP (N= 17) | PLACEBO (N= 17) | |
|---|---|---|---|
| N (%) or Mean (SD) | N (%) or Mean (SD) | p-value | |
| Gender | |||
| Male | 9 (53%) | 9 (53%) | 1.0000 |
| Female | 8 (47%) | 8 (47%) | |
| Age (20–65) | 40.25 (9.31) | 34.18 (7.33) | .0450 |
| Race/Ethnicity | |||
| White | 17 (100%) | 17 (100%) | N/A |
| Other | 0 | 0 | |
| Education | |||
| Less than High School Education | 2 (13%) | 5 (29%) | 0.3983 |
| High School Graduate or Higher | 14 (87%) | 12 (71%) | |
| Employment Status | |||
| Employed | 4 (25%) | 3 (18%) | 0.6880 |
| Unemployed | 12 (75%) | 14 (82%) | |
| Relationship Status | |||
| Married/Separated/Divorced | 14 (87%) | 10 (59%) | 0.1175 |
| Never Married | 2 (13%) | 7 (41%) | |
| Methamphetamine Use | |||
| Days Used Per Week | 5.50 (1.67) | 5.91 (1.35) | 0.5813 |
| Grams Used Per Day | 1.39 (0.97) | 1.17 (0.80) | 0.625 |
| Age First Used | 24.06 (8.96) | 20.03 (5.06) | 0.2204 |
| Years of Regular Use | 12.64 (6.89) | 11.03 (6.56) | 0.4847 |
| Use Intravenously | 9 (50%) | 9 (53%) | 0.8619 |
| Other Drug Use (Past 30 days) | |||
| Alcohol | 7 (39%) | 7 (41%) | 0.8902 |
| Benzodiazepines | 2 (11%) | 5 (29%) | 0.1761 |
| Cannabis | 10 (56%) | 8 (47%) | 0.6152 |
| Cocaine | 0 (0%) | 2 (12%) | 0.1340 |
| Opioids | 1 (6%) | 5 (29%) | 0.0613 |
| Tobacco | 16 (89%) | 15 (88%) | 0.9516 |
Retention and Adverse Events:
No significant differences were found in study retention by treatment group during week 1 (Log-Rank test chi-square=0.0184, df=1, p-value=0.8921); Figure 1) or from the start of week 2 through the end of the study (Log-Rank test chi-square=0.0658, df=1, p-value=0.7975). Eight participants in each group completed the entire study (47%). Reasons for drop out included voluntary withdrawal (n=11), residential treatment facility rule violations (n=2), family emergency (n=2), study medication refusal (n=1), sought medical care outside facility (n=1), and discharged by principal investigator for elevated heart rate (n=1). Most adverse events were reported during week 1 during d-amphetamine administration, regardless of group (Table 2). The most commonly reported adverse event was increased heart rate (6 in the placebo group and 4 in the d-amphetamine group). The one participant removed from the study by the principal investigator during week 1 due to clinically significant elevated heart rate was asymptomatic and this was a conservative clinical decision. All adverse events were rated as mild.
Figure 1. Percentage of participants retained during the trial by treatment group*.
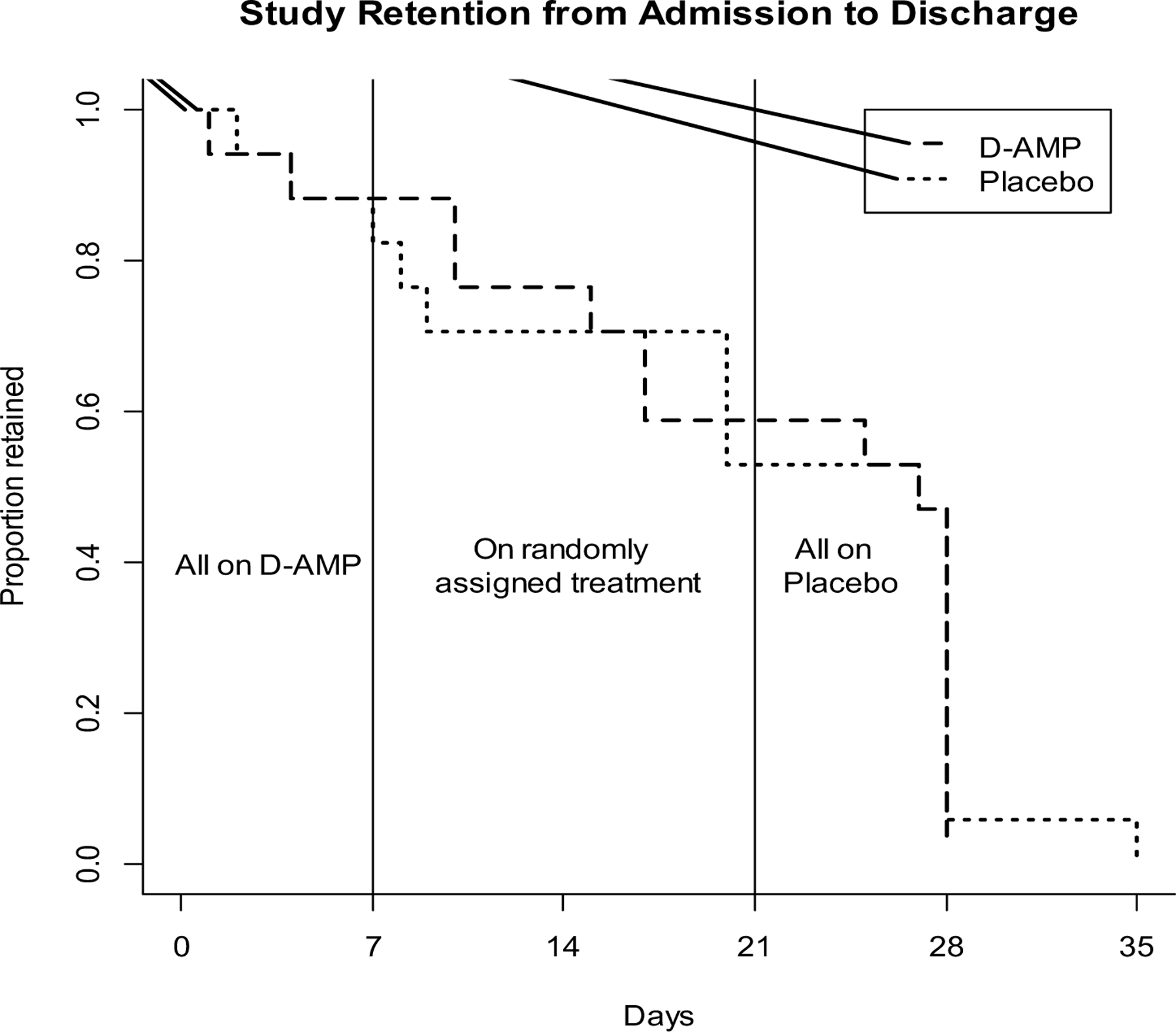
*Placebo (lighter dashed lines), D-Amphetamine (bold dashed lines).
Table 2.
The number of spontaneously-reported adverse events by week and treatment group *
| Adverse Event | Week 1 | Weeks 2–3 | Week 4 | |||
|---|---|---|---|---|---|---|
| Placebo | D-AMP ** | Placebo | D-AMP | Placebo | D-AMP | |
| Body Aches | 1 | --- | --- | --- | --- | --- |
| Coldness | 1 | --- | --- | --- | --- | --- |
| Constipation | --- | 1 | --- | --- | --- | --- |
| Coughing | 1 | --- | --- | --- | --- | --- |
| Decreased Appetite | 1 | --- | --- | --- | --- | --- |
| Fatigue/Low Energy | --- | 1 | 1 | --- | --- | --- |
| Flushed | --- | 1 | --- | --- | --- | --- |
| Headaches | 2 | 1 | 1 | 1 | 1 | --- |
| Heartburn | --- | 2 | --- | --- | --- | --- |
| Hot/Sweaty | --- | 1 | --- | 1 | --- | --- |
| Increased Blood Pressure | --- | 1 | --- | 1 | --- | --- |
| Increased Heart Rate | 6 | 4 | 1 | --- | 1 | --- |
| Insomnia | 1 | 2 | 1 | 1 | --- | --- |
| Itching | --- | 1 | --- | --- | --- | --- |
| Lightheaded/Dizzy | 2 | 1 | --- | 1 | --- | --- |
| Nasal Congestion | 1 | --- | --- | --- | --- | --- |
| Nausea/Vomiting | 1 | --- | --- | --- | 1 | --- |
| Paleness | 1 | --- | --- | --- | --- | --- |
| Restless Lower Extremities | 1 | --- | --- | --- | --- | --- |
| Shaking Hands | 1 | --- | --- | --- | --- | --- |
| Twitching | --- | --- | --- | 1 | --- | --- |
| Weakness | --- | 1 | --- | --- | --- | --- |
| Wheezing | 1 | --- | --- | --- | --- | --- |
| Total Number of Events | 21 | 17 | 4 | 6 | 3 | 0 |
Number of times adverse events were attributed as possible, probable or definite. Adverse events attributed as unrelated or unlikely were not reported. Week 1: all participants on d-amphetamine; Week2–3: participants randomized to d-amphetamine or placebo; Week 4: all participants on placebo.
D-AMP = D-Amphetamine.
Effects of Standardized Exposure to D-Amphetamine During Week 1:
Heart rate showed a main effect of treatment group, such that seated (p=0.0042, Cohen’s d effect size=0.68), standing (p=0.0028), d=0.72, and supine (p=0.0095, d=0.61) heart rates were elevated in the placebo relative to d-amphetamine group between during week 1, regardless of time. No measures showed any group x time interactions (p’s>0.05) while several measures (MSSA, MAWA, Methamphetamine Craving, Ham-D scores) showed decreases over time regardless of medication group.
After standardized exposure to d-amphetamine during week 1, no statistically significant group differences existed in methamphetamine withdrawal, craving, or severity, depression, anxiety, or cognition (see Table 3). Supine, seated, and standing heart rates were significantly higher in the placebo group; however, only the difference in standing heart rate was clinically significant.
Table 3.
Comparison of Intake Sample Cognition, Vital Signs, and Outcomes, Total and By Condition, to Baseline (after 1-week standardized exposure to d-amphetamine) Sample Cognition, Vital Signs, and Outcomes, Total and By Condition (N=24).
| INTAKE | BASELINE | |||||
|---|---|---|---|---|---|---|
| VARIABLE | D-AMP (N= 12) | PLACEBO (N= 12) | D-AMP (N= 12) | PLACEBO (N= 12) | ||
| N (%) or Mean (SD) | N (%) or Mean (SD) | p-value | N (%) or Mean (SD) | N (%) or Mean (SD) | p-value | |
| COGNITION | ||||||
| Stroop Color and Word Test Score | 49.86 (8.69) | 60.71 (22.70) | 0.4808 | 43.9 (16.1) | 63.1 (18.3) | 0.0509 |
| VITAL SIGNS | ||||||
| Supine Systolic Blood Pressure | 123.75 (17.49) | 120.83 (8.12) | 0.6077 | 119.8 (10.7) | 120.8 (12.2) | 0.8333 |
| Supine Diastolic Blood Pressure | 73.08 (9.29) | 75.42 (7.49) | 0.5052 | 72.7 (6.3) | 73.4 (7.1) | 0.7882 |
| Supine Heart Rate | 80.92 (18.63) | 77.33 (11.25) | 0.5742 | 79.3 (11.4) | 90.4 (7.8) | 0.0109 |
| Seated Systolic Blood Pressure | 127.42 (14.63) | 122.92 (11.14) | 0.4056 | 122.0 (12.9) | 121.5 (9.5) | 0.9147 |
| Seated Diastolic Blood Pressure | 81.83 (9.35) | 83.50 (6.08) | 0.6099 | 79.3 (7.6) | 76.8 (7.7) | 0.4463 |
| Seated Heart Rate | 85.25 (16.48) | 84.75 (11.15) | 0.7504 | 85.3 (8.2) | 96.8 (10.5) | 0.0065 |
| Standing Systolic Blood Pressure | 125.08 (14.68) | 123.33 (11.03) | 0.74444 | 120.3 (11.8) | 120.5 (11.9) | 0.9729 |
| Standing Diastolic Blood Pressure | 79.92 (9.06) | 84.50 (9.40) | 0.2367 | 82.8 (9.0) | 78.9 (11.9) | 0.3826 |
| Standing Heart Rate | 92.08 (18.45) | 95.92 (11.77) | 0.5501 | 93.4 (9.2) | 107.5 (12.5) | 0.0048 |
| PRIMARY OUTCOMES | ||||||
| MAWA Result Score | 16.92 (11.16) | 18.92 (10.33) | 0.6533 | 7.3 (8.3) | 7.4 (5.6) | 0.9544 |
| MSSA Total Score | 39.58 (23.92) | 41.42 (20.80) | 0.8431 | 17.3 (17.9) | 12.6 (6.8) | 0.3992 |
| Craving for Methamphetamine | 13.83 (5.18) | 13.75 (4.83) | 0.9679 | 4.4 (6.1) | 3.3 (4.2) | 0.5907 |
D-AMP = D-Amphetamine ; SD=standard deviation. MAWA: Methamphetamine Withdrawal Assessment; MSSA: Methamphetamine Selective Severity Assessment; Craving for methamphetamine: 0–20 Visual analog scale of craving.
Effects of Abrupt Termination of D-Amphetamine During Week 2:
Only Methamphetamine Craving showed a significant treatment group x time interaction (p=0.0354), such that methamphetamine craving was increased over time in the placebo group relative to d-amphetamine. Specifically, pairwise comparisons found methamphetamine craving was higher in the treatment compared to placebo group at week 2 visit 2 (p=0.0311, d=0.34) and week 2 visit 3 (p=0.0391, d=0.32).
Length of Effects of Amphetamine Withdrawal over Course of Study:
Methamphetamine Craving scores decreased over time within the d-amphetamine (week 4 visit 3 lower than week 1 visit 3; p=0.0008, d=0.49), but not placebo (p= 0.2167, d=0.17) group. Methamphetamine craving was lower in the d-amphetamine than placebo group at week 2 visit 3 (p=0.0251, d=0.32). MAWA scores decreased over time within the d-amphetamine (week 4 visit 3 lower than week 1 visit 3; p=0.0017, d=0.47), and placebo (week 4 visit 3 lower than week 1 visit 3; p=0.0003, d=0.56) groups. Methamphetamine withdrawal scores based on the MAWA were lower in the d-amphetamine than placebo group at week 2 visit 3 (p=0.0091, d=0.38). MSSA scores decreased over time within the d-amphetamine (week 4 visit 3 lower than week 1 visit 3; p<0.0001, d=0.62) and placebo (week 4 visit 3 lower than week 1 visit 3; p=0.0135, d=0.35) groups. No significant differences were found between groups at any time point (see Figure 2).
Figure 2. Withdrawal and Craving Total Scores*.
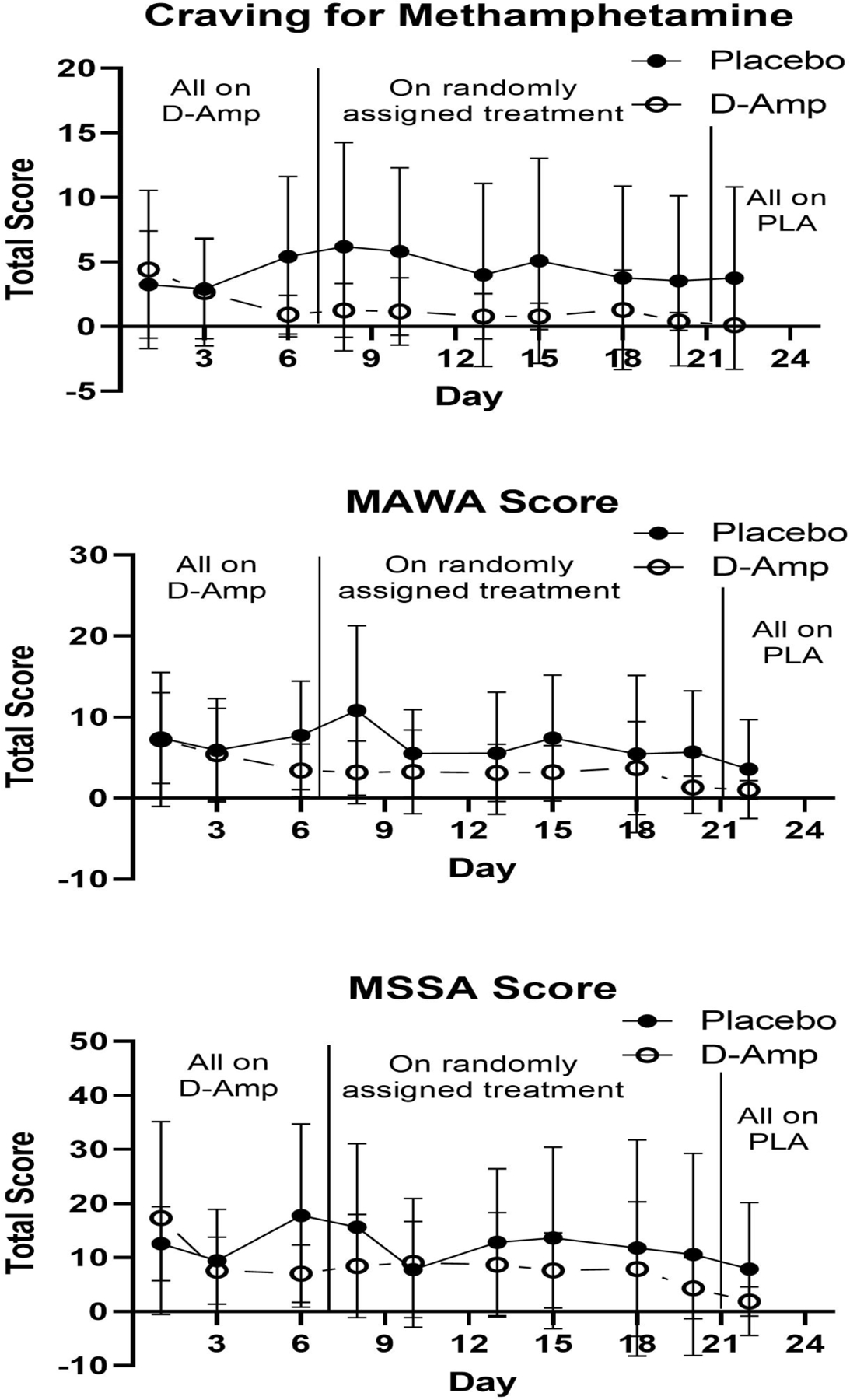
*Scores depicting the mean intensity of methamphetamine craving on the 20 point Visual Analog Scale for methamphetamine craving (top panel), and the Methamphetamine Withdrawal Assessment (MAWA) (middle panel). Mean intensity of craving on the Methamphetamine Selective Severity Assessment (MSSA) (bottom panel). X-axis represents each study day during the first 24 days of the study. The first vertical line demarcates the time when participants transitioned from all receiving d-amphetamine to being randomized to d-amphetamine or placebo. The second vertical line demarcates the transition from randomization to all being on placebo. Y-axis represents mean total score.
Ham-A (rank-transformed total score) decreased over time within both d-amphetamine (week 4 visit 3 lower than week 1 visit 3; p=0.0007, d=0.50) and placebo (week 4 visit 3 lower than week 1 visit 3; p=0.0132, d=0.35) groups. No significant treatment group differences were found. Ham-D (log-transformed) decreased over time within the d-amphetamine (week 4 visit 3 lower than week 1 visit 3; p=0.0132, d=0.36), but not placebo, group. No significant treatment group differences were found (see Figure 3). The Stroop Color and Word Test scores showed no significant effects.
Figure 3. HAM-A and HAM-D Total Scores*.
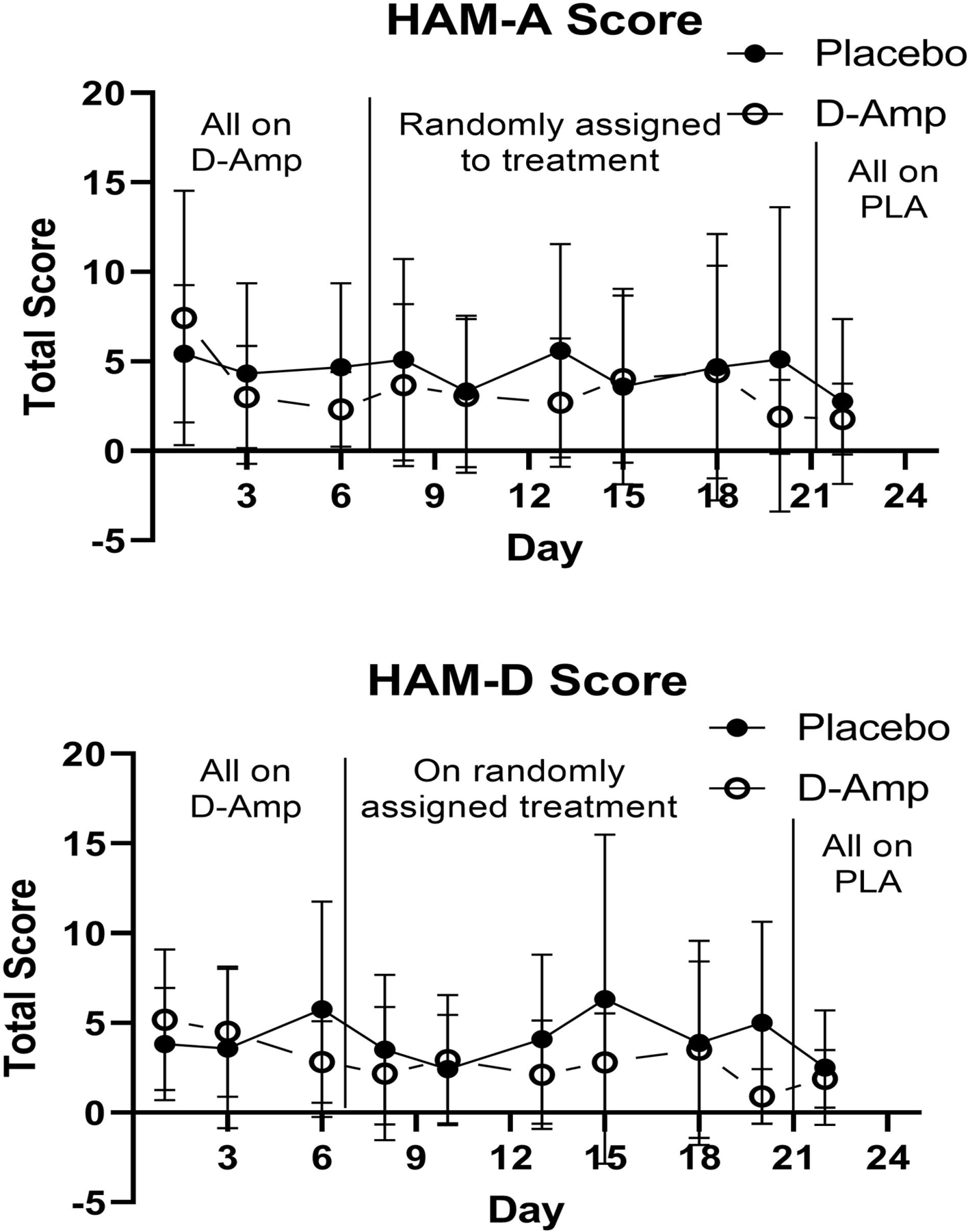
*Scores depicting the mean total HAM-A score at each study assessment (top panel), mean total HAM-D score at each study assessment (bottom panel). X-axis represents each study day during the first 24 days of the study. The first vertical line demarcates the time when participants transitioned from all receiving d-amphetamine to being randomized to d-amphetamine or placebo. The second vertical line demarcates the transition from randomization to all being on placebo. Y-axis represents mean total score.
None of Supine Systolic, Supine Diastolic, Seated Systolic, Seated Diastolic, or Standing Systolic Blood Pressure showed significant model coefficients, main effect differences, within-group differences, or treatment group x time interactions. However, Standing Diastolic Blood Pressure increased over time within the placebo group (week 4 visit 3 higher than week 1 visit 3; p<0.0483, d=0.31). Whether Supine, Seated, or Standing, Heart Rate was high for both treatment groups at the end of week 1, and was significantly higher in the placebo group than the d-amphetamine group (p=0.0128, d=0.39; p=0.0046, d=0.45; p=0.0021, d=0.49; respectively); however, heart rate decreased in both groups over time (week 4 visit 3 lower than week 1 visit 3; p<0.0001, d=0.72; data not shown for Standing. No other significant treatment differences were found. There was one participant in the control and one in the d-amphetamine group that met the criteria for orthostatic hypotension at week 2 visit 1 and one patient in the control and one in the d-amphetamine group at week 4 visit 1. None of these four participants were symptomatic.
Discussion
This study demonstrated that individuals who use methamphetamine with varying methamphetamine use histories can be relatively safely stabilized on d-amphetamine (60 mg/day) and successfully randomized for short-term medication trials. Moreover, d-amphetamine was shown to reduce several measures of methamphetamine withdrawal during early methamphetamine abstinence among patients who use methamphetamine, while abruptly terminating d-amphetamine administration resulted in a temporary increase in methamphetamine craving among those assigned to placebo. These findings suggest that a controlled amphetamine withdrawal paradigm is feasible and may have utility for examining treatment agents aimed at reducing methamphetamine craving.
Individuals who regularly used methamphetamine for a varying number of years did not experience significant adverse changes in outcomes and vital signs throughout the one-week d-amphetamine stabilization period other than heart rate (though higher in placebo condition during week 1). Statistically significant differences between groups by age and heart rate (supine, seated, standing) are noted. However, only the difference in standing heart rate was clinically significant between groups (placebo mean standing heart rate of 107.5 versus active treatment of 93.4). Thus, findings indicate that younger individuals might not tolerate d-amphetamine as well as older individuals who use methamphetamine. Additional analyses found that average number of days used methamphetamine per week, grams used per day, age of first use, and years of regular methamphetamine use, incidence of intravenous use, and use of other drugs (alcohol, benzodiazepines, cannabis, cocaine, opioids, and tobacco) did not significantly differ between placebo and d-amphetamine groups. Thus, elevated heart rate, specifically standing heart rate, was not necessarily a reflection of methamphetamine use history prior to entering treatment. Elevated heart rate was the most common adverse event in both treatment groups while receiving d-amphetamine and might be related to unmeasured variables (e.g., hydration status), not necessarily the d-amphetamine stabilizing dosage. Otherwise, from a cardiovascular standpoint, d-amphetamine was well tolerated given that heart rate (supine, seated, and standing) decreased over time in both groups and no orthostatic hypotension was observed in either group throughout the trial. Regardless, these findings suggest that precautions/regular monitoring should be taken when stabilizing younger individuals and those with elevated heart rates on d-amphetamine.
The finding that d-amphetamine might help reduce methamphetamine craving during early abstinence among participants who use methamphetamine is consistent with other randomized, placebo controlled trials of d-amphetamine for methamphetamine dependence (Longo et al., 2009; Shearer et al., 2001; Galloway et al., 2011). For instance, one trial examining sustained release d-amphetamine up to 110 mg daily for three months found it to be well tolerated, decrease severity of dependence and improve treatment retention compared to placebo (Longo et al., 2009). Another study demonstrated that d-amphetamine was well tolerated at a dose of 60 mg daily for up to 12 weeks and attendance at counseling sessions was superior in the intervention arm as well (Shearer et al., 2001). Additionally, a third trial found that 30 mg twice daily of sustained release d-amphetamine was well tolerated and associated with decreased cravings and fewer withdrawal symptoms over 8 weeks (Galloway et al., 2011). These findings suggest that even short-term d-amphetamine administration may alleviate initial symptoms of withdrawal and craving, which in turn may help to improve treatment outcomes. Our findings suggest that a time period shorter than 8 weeks may be efficacious in alleviating craving in methamphetamine-using individuals and more acceptable to those concerned about administering drugs of known abuse potential, such as amphetamines, to those with stimulant use disorder.
A higher dropout rate was expected in the placebo group, due to the increased risk of methamphetamine withdrawal after abrupt termination of d-amphetamine. This was not found to be the case and might be partially explained by initial d-amphetamine-induced reductions in methamphetamine craving as well as by the support services provided at the residential facility. At the same time, approximately half (53%) of participants dropped out at some point over the four-week study, although dropout/retention rates did not significantly differ between treatment and placebo groups. Although this is comparable to drop out rates (43–53%) of other randomized controlled outpatient trials of d-amphetamine for methamphetamine use (Shearer et al, 2001; Longo et al., 2010), another randomized placebo controlled trial of d-amphetamine reported only a 7% dropout rate over the course of 8 weeks (Galloway et.al., 2011). Future studies may want to consider higher monetary payments to enhance retention, given that subjects earned up to only $210 for the four-week residential stay/participation. Nevertheless, the present study showed statistically significant findings and minimal, mild adverse events were reported, suggesting this type of paradigm is feasible.
Study Strengths and Limitations
This study is strengthened by its rigorous research design (randomized, double blinded, placebo-controlled trial) and the fact that randomization resulted in two groups that did not differ significantly on baseline sociodemographics (other than age) and other key potential confounders of outcomes. Study findings are limited by a small sample size, particularly during week 4 (8 participants per group completed the study), which limited the ability to detect any group differences when the d-amphetamine groups was switched to placebo. This also limits reproducibility. Further, the sample might not be homogenous enough because it was not limited to those with methamphetamine use disorder based on DSM criteria (no standardized diagnostic assessment tool was administered). At the same time, d-amphetamine produced a significant decrease in methamphetamine craving during week one that was sustained through weeks 2–3 in those continuing to receive d-amphetamine, whereas craving increased during week 2 among those switched to placebo. These findings demonstrate that a paradigm like this to produce abstinence-induced methamphetamine craving under controlled conditions is feasible. The study timeline was short and follow-up did not occur with participants who voluntarily left the facility without notifying research staff to ascertain reasons for such. However, although about half of the participants dropped out of the study, retention rates did not significantly differ between treatment and placebo groups or impede the production of statistically significant findings. At the same time, a higher stabilization dose of d-amphetamine is likely necessary to enhance expression of withdrawal symptoms after abrupt termination and may be feasible given the overall favorable side effect profile of the 60 mg dose. A shorter stabilization period may also increase subject retention, enhancing feasibility and rigor.
Conclusions
To our knowledge, this is the first double blind, placebo-controlled trial measuring pharmacologic effects of abruptly stopping controlled d-amphetamine administration in adults who use methamphetamine. The preliminary utility of is d-amphetamine withdrawal paradigm is supported in terms of safe stabilization on d-amphetamine (60 mg/day), successful randomization, detection of withdrawal-associated symptoms, and short-term retention.
Additional research is needed on the role of d-amphetamine dose in relation to withdrawal symptoms during early abstinence among individuals who use methamphetamine. Results suggest the potential of the amphetamine withdrawal model for examining the efficacy of pharmacologic agents in alleviating early methamphetamine craving and other withdrawal symptoms.
Figure 4. Seated Vital Signs*.
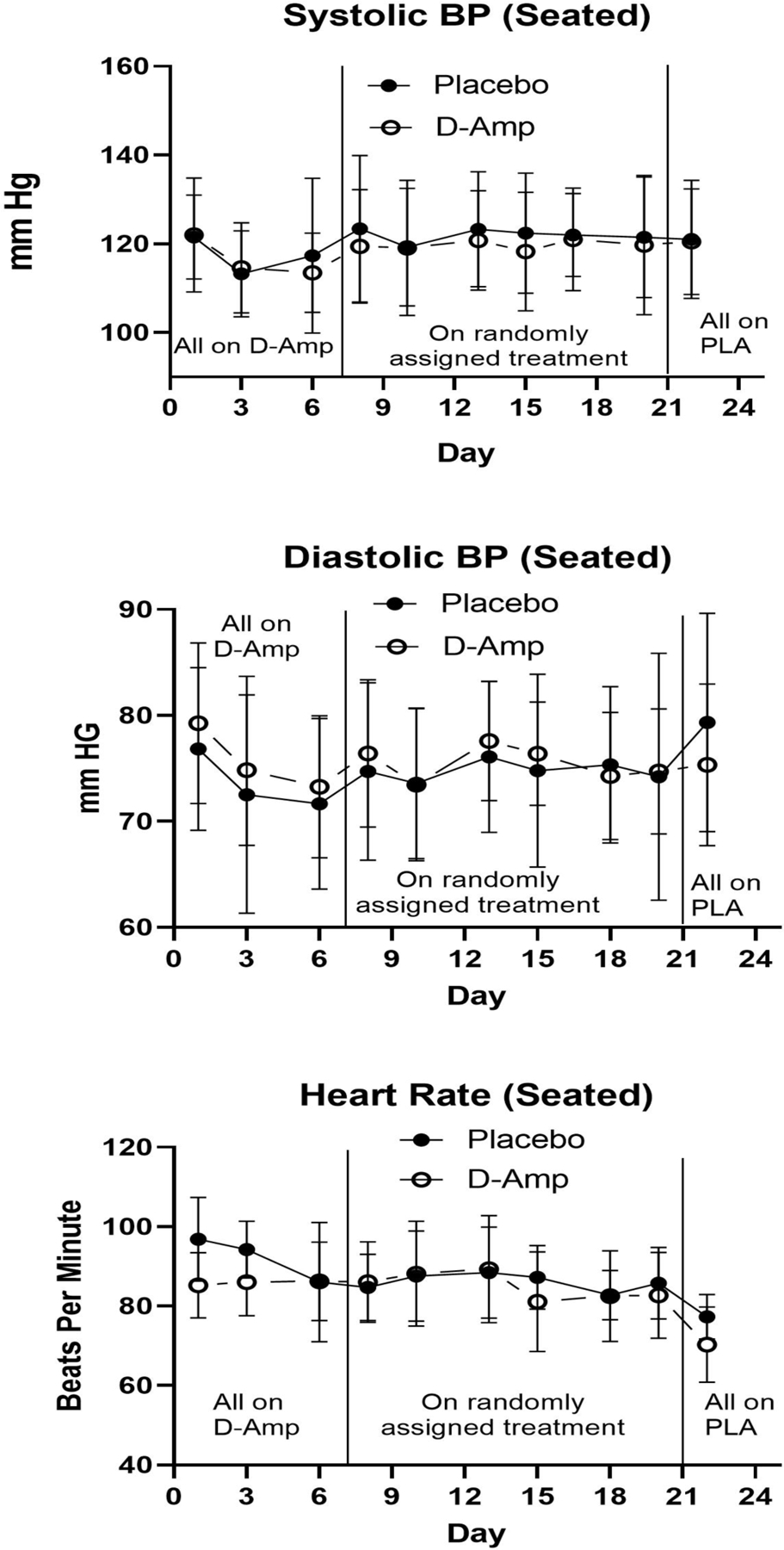
*Mean Seated vital sign measures at each study visit for treatment group versus placebo. Seated Systolic BP (Blood Pressure) (top panel); Seated Diastolic BP (Blood Pressure) middle panel); Heart Rate (bottom panel). X-axis represents each study day during the first 24 days of the study. The first vertical line demarcates the time when participants transitioned from all receiving d-amphetamine to being randomized to d-amphetamine or placebo. The second vertical line demarcates the transition from randomization to all being on placebo. Y-axis represents mean total score. Y-axis represents mean of each measure in mm Hg or beats per minute.
Declaration of Interest/Funding:
Research supported by NCRR-RR020146.
References
- Albertson TE, Derlet RW, Van Hoozen BE (1999) Methamphetamine and the expanding complications of amphetamines. West J Med 170(4): 214–219. [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2000) Diagnostic and Statistical Manual for Mental Disorders: DSM-IV 4th ed. Washington DC: American Psychiatric Association. [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders: DSM-5 5th ed. Washington DC: American Psychiatric Association. [Google Scholar]
- Cantwell B, McBride AJ (1998) Self-detoxification by amphetamine dependent patients: A pilot study. Drug Alcohol Dependence 49: 157–163. [DOI] [PubMed] [Google Scholar]
- Cruickshank CC, Dyer KR (2009) A review of the clinical pharmacology of methamphetamine. Addiction 104: 1085–1099. [DOI] [PubMed] [Google Scholar]
- DHHS and SAMHSA (2019) Results from the 2019 National Survey on Drug Use and Health: National Findings in: Department of Health and Human Services Substance Abuse and Mental Health Services Administration Office of Applied Studies: Rockville, MD. [Google Scholar]
- Elkashef AM, Rawson RA, Anderson AL, Li SH, Holmes T, Smith EV, Chiang N, Kahn R, Vocci F, Ling W, Pearce VJ, McCann M, Campbell J, Gorodetzky C, Haning W, Carlton B, Mawhinney J, Weis D (2008) Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology 33(5): 1162–1170. [DOI] [PubMed] [Google Scholar]
- Galloway GP, Buscemi R, Coyle JR, Flower K, Siegrist JD, Fiske LA, Baggott MJ, Li L, Polcin D, Che CYA, Mendelson J (2011) A randomized, placebo-controlled trial of sustained-release dextroamphetamine for treatment of methamphetamine addiction. Clin Pharmacol Ther 89(2):276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden CJ (1978) Stroop color and word test Stoelting Co: Wood Dale, IL. [Google Scholar]
- Gonzales R, Mooney L, Rawson R (2010) The methamphetamine problem in the United States. Annu Rev Public Health 31: 385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop MR, Bradley BP, Brewis RK (1982) Amphetamine withdrawal and sleep disturbances. Drug Alc Dep 10:177–183. [DOI] [PubMed] [Google Scholar]
- Hamilton M (1959) The assessment of anxiety states by rating. British J Medical Psychology 32: 50–55. [DOI] [PubMed] [Google Scholar]
- Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzerling KG, Shoptaw S, Peck JA, Yang X, Liu J, Roll J, Ling W (2006) Randomized, placebo-controlled trial of baclofen and gabapentin for the treatment of methamphetamine dependence. Drug Alc Dep 85(3): 177–184. [DOI] [PubMed] [Google Scholar]
- Hong R, Matsuyama E, Nur K (1991) Cardiomyopathy associated with the smoking of crystal methamphetamine. Jama 265(9): 1152–1154. [PubMed] [Google Scholar]
- Johnson BA, Roache JD, Ait-Daoud N, Wells LT, Wallace CL, Dawes MA, Liu L, Wang X-Q (2007) Effects of topiramate on methamphetamine-induced changes in attentional and perceptual-motor skills of cognition in recently abstinent methamphetamine-dependent individuals. Progress in Neuro-Psychopharmacology and Biological Psychiatry 31(1): 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR (1990) Neurobiology of abused drugs: Opioids and stimulants. J Nerv Ment Dis 178(4): 217–227. [DOI] [PubMed] [Google Scholar]
- Leshner AI, Koob GF (1999) Drugs of abuse and the brain. Proc Assoc Am Physicians 111(2): 99–108. [DOI] [PubMed] [Google Scholar]
- Longo M, Wickes W, Smout M, Harrison S, Cahill S, White JM (2009) Randomized controlled trial of dexamphetamine maintenance for the treatment of methamphetamine dependence. Addiction 105: 146–154. [DOI] [PubMed] [Google Scholar]
- Mancino MJ, Gentry BW, Feldman Z, Mendelson J, Oliveto A (2011) Characterizing methamphetamine withdrawal in recently abstinent methamphetamine users: A pilot field study. Am J Drug Alcohol Abuse 37(2): 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM (2005) The nature, time course and severity of methamphetamine withdrawal. Addiction 100: 1320–1329. [DOI] [PubMed] [Google Scholar]
- Montoya ID, Vocci F (2008) Novel medications to treat addictive disorders. Curr Psychiatry Rep 10(5): 392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JB (1998) Psychophysiological aspects of amphetamine-methamphetamine abuse. J Psychol 132(2): 227–237. [DOI] [PubMed] [Google Scholar]
- NACO (2005) The Meth Epidemic in America, in: The National Association of Counties
- Newton TF, Roache JD, De La Garza R 2nd, Fong T, Wallace CL, Li SH, Elkashef A, Chiang N, Kahn R (2006) Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacol 31(7): 1537–1544. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse (2019) Methamphetamine Research Report
- Schuckit M, Daeppen J-B, Danko GP, et al. (1999) Clinical implications for four drugs of the DSM-IV distinction between substance dependence with and without a physiological component. American Journal Psychiatry 156: 41–49. [DOI] [PubMed] [Google Scholar]
- Shaw K (1999) Human methamphetamine-related fatalities in Taiwan during 1991–1996. Journal Forensic Science 44(2): 7–31. [PubMed] [Google Scholar]
- Shearer J, Wodak A, Mattick RP, Van Beek I, Lewis J, Hall W, Dolan K (2001) Pilot randomized controlled study of dexamphetamine substitution for amphetamine dependence. Addiction 96: 1289–1296. [DOI] [PubMed] [Google Scholar]
- Shoptaw SJ, Kao U, Heinzerling K, Ling W (2009) Treatment for amphetamine withdrawal. Cochrane Database of Systematic Reviews [DOI] [PMC free article] [PubMed]
- Shoptaw S, Huber A, Peck JA, Yang X, Liu J, Dang J, Roll J, Shapiro B, Rotheram-Fuller E, Ling W (2006) Randomized, placebo-controlled trail of sertraline and contingency management to treatment of methamphetamine dependence. Drug and Alcohol Dependence 85: 12–18. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Heinzerling KG, Rotheram-Fuller E, Kao UH, Wang PC, Bholat MA, Ling W (2008) Bupropion hydrochloride versus placebo, in combination with cognitive behavioral therapy, for the treatment of cocaine abuse/dependence. J Addict Dis 27(1): 13–23. [DOI] [PubMed] [Google Scholar]
- Sibanda NC, Kornhaber R, Hunt GE, Morley K, Cleary M. Prevalence and Risk Factors of Emergency Department Presentations with Methamphetamine Intoxication or Dependence: A Systematic Review and Meta-analysis. Issues Ment Health Nurs Jul 2019;40(7):567–578. doi: 10.1080/01612840.2018.1553003 [DOI] [PubMed] [Google Scholar]
- Sinha R New Findings on Biological Factors Predicting Addiction Relapse Vulnerability. Current Psychiatry Reports 2011;13(5):398–405. doi: 10.1007/s11920-011-0224-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisurapanont M, Jarusuraisin N, Kittirattanapaiboon (2005) Treatment of amphetamine withdrawal. The Cochrane Library 2 [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Kuoppasalmi K, Föhr J, Tuomola P, Kuikanmäki O, Vorna H, Sokero P, Haukka J, Meririnne E (2007) A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am J Psychiatry 164: 160–162. [DOI] [PubMed] [Google Scholar]
- Trevisan LA, Boutros N, Petrakis IL, Krystal JH (1998) Complications of alcohol withdrawal: Pathophysiological insights. Alcohol Health Res World 22: 61–66. [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Walker R, Ling W, dela Cruz A, Sharma G, Carmody T, Ghitza U, Wahle A, Kim M, Shores-Wilson K, Sparenborg S, Coffin P, et al. (2021) Bupropion and naltrexone in methamphetamine use disorder. N Engl J Med 384: 140–153. [DOI] [PMC free article] [PubMed] [Google Scholar]


