ABSTRACT
Chikungunya virus (CHIKV), a mosquito-borne alphavirus, is an emerging global threat identified in more than 60 countries across continents. The risk of CHIKV transmission is rising due to increased global interactions, year-round presence of mosquito vectors, and the ability of CHIKV to produce high host viral loads and undergo mutation. Although CHIKV disease is rarely fatal, it can progress to a chronic stage, during which patients experience severe debilitating arthritis that can last from several weeks to months or years. At present, there are no licensed vaccines or antiviral drugs for CHIKV disease, and treatment is primarily symptomatic. This Review provides an overview of CHIKV pathogenesis and explores the available therapeutic options and the most recent advances in novel therapeutic strategies against CHIKV infections.
Keywords: Chikungunya virus, Chikungunya pathogenesis, Antiviral compounds, Vaccines, Monoclonal antibodies, Immunomodulatory drugs
Summary: Although chikungunya virus (CHIKV) cases are rising globally, therapeutic options remain extremely limited. Here, we provide an overview of CHIKV pathogenesis and explore recent advances in strategies to prevent and treat infections.
Introduction
Chikungunya virus (CHIKV) disease causes severe, incapacitating joint pain, which may persist for several months or years, resulting in serious economic and social impact for affected communities (Mohan et al., 2010). CHIKV is an arthropod-borne virus (see Glossary, Box 1) that belongs to the Togaviridae family (genus Alphavirus) (Box 1, Box 2) and is transmitted by two species of mosquitoes, Aedes albopictus and Aedes aegypti (Dubrulle et al., 2009; Nuckols et al., 2013; Díaz-González et al., 2015). CHIKV was first recognised as a human pathogen in 1952, when it was isolated from human serum during an epidemic in Tanzania (Robinson, 1955). The origin of the name chikungunya comes from a Makonde word that translates as “that which bends up”, a reference to the contorted posture exhibited in infected patients with severe joint pain characteristic of the disease (Robinson, 1955).
Box 1. Glossary.
Arthralgia: pain in a joint.
Arthropod-borne virus: also known as an arbovirus, a general term used to describe a group of viruses that spread to humans by the bite of arthropods (insects), such as mosquitoes or ticks. Chikungunya virus (CHIKV) is transmitted to humans by the bite of infected mosquitoes.
Autochthonous transmission: spread of a disease from one individual to another individual in the same location.
Myalgia: muscle pain.
Neutrophil extracellular trap (NET): a network of extracellular fibres, mainly composed of DNA associated with host defence peptides, which can engulf and kill pathogens.
Osteoclast: large, multinucleated cell responsible for bone resorption (bone destruction and release of minerals into blood). Osteoclasts are derived from precursors in the myeloid/monocyte lineage that circulate in the blood.
Polyarthralgia: pain in several joints.
Structure–activity relationship: the relationship between the chemical structure of a compound and its biological effect.
Tendonitis: inflammation of a tendon.
Tenosynovitis: inflammation of the synovial fluid that surrounds a tendon; associated with tendonitis.
Togaviridae family: a family of enveloped viruses with single-stranded positive-sense RNA genomes of 10-12 kb. Alphavirus is a genus within the Togaviridae family and includes a large number of viruses transmitted by arthropods, typically mosquitoes. Well-studied members of this genus include CHIKV, Sindbis virus, Semliki Forest virus, Venezuelan equine encephalitis virus and Ross River virus.
Vector control: limiting the transmission of a virus by reducing or eliminating human contact with mosquitos, through the use of chemical and non-chemical based tools (reviewed in Wilson et al., 2020).
Box 2. CHIKV structure.
CHIKV is an enveloped positive-sense RNA alphavirus of ∼60-70 nm in diameter. The viral genome consists of single-stranded, linear RNA that is 11.8 kb in size, encompassing two open reading frames (ORFs), encoding two polyproteins. ORF1 encodes for non-structural proteins (nsPs; nsP1, helicase nsP2, nsP3, polymerase nsP4) and ORF2 encodes for structural proteins [capsid (C) protein, envelope proteins (E1, E2, E3), 6K] (Voss et al., 2010; Ahola and Merits, 2016).
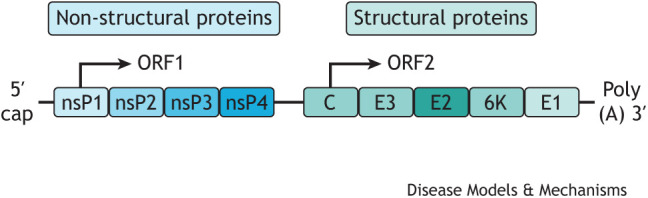
The nsPs are important for viral replication; however, none of these proteins are packaged in the final virion (Powers, 2017). The structural surface glycoproteins E1 and E2 assemble into spikes on the virion surface – each spike consisting of a trimer of E2-E1 heterodimers – and are the major viral epitopes responsible for the attachment and entry into the host cell.
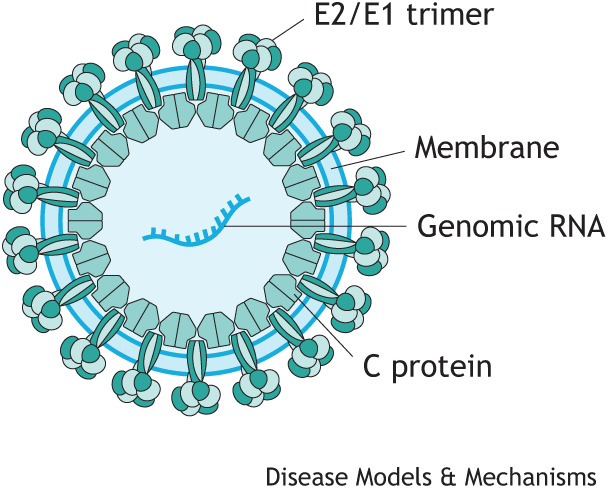
The E1 glycoprotein is necessary for membrane fusion and the E2 protein is responsible for receptor binding (Voss et al., 2010). The E3 glycoprotein serves as a signal sequence for the translocation of E3-E2-6K-E1 polyprotein into the endoplasmic reticulum, where it undergoes complete cleavage into individual proteins important for virus maturation and spike assembly (Snyder and Mukhopadhyay, 2012). The C protein associates with the genomic RNA to form a nucleocapsid that is coated with surface proteins E1 and E2.
CHIKV disease in humans is typically marked by two phases, an acute phase and a chronic phase. Symptoms of CHIKV infection start abruptly, normally presenting with a high fever (>38.9°C) that can last from several days to up to 2 weeks. The majority of infected patients develop polyarthralgia (Box 1) after the onset of fever, but other common symptoms can include rash, myalgia (Box 1) and headaches (Burt et al., 2017). After the acute phase of the illness, some patients develop long-term symptoms, known as the chronic phase, that can last from several weeks (Chopra et al., 2012) to months or years (Chang et al., 2018; Paixão et al., 2018). Studies vary widely in terms of the percentage of patients that experience the chronic disease and the disease longevity. Further research is warranted to better assess the burden of chronic disease upon inflicted populations with CHIKV epidemics.
The long-term sequelae include arthralgia (Box 1), joint stiffness, swelling and tendonitis/tenosynovitis (Box 1), as well as alopecia and depression (Hawman et al., 2013; van Aalst et al., 2017). CHIKV-induced arthritis resembles rheumatoid arthritis (RA), but, unlike RA, there is no evidence that CHIKV-associated arthropathies are caused by autoimmunity. Although the case fatality rate for CHIKV disease has been estimated to be 0.009% [Pan American Health Organization/World Health Organization Annual Arbovirus Bulletin, 2021], a meta-analysis study by Rodríguez-Morales et al. (2016) predicted that 25% of CHIKV infections would lead to chronic inflammatory rheumatism and 14% of patients would develop chronic arthritis. However, the estimates of the rate of progression to chronic arthritis post-CHIKV infection vary widely due to the criteria used and the populations examined. Therefore, a more recent systematic review suggests that, on average, 42.5% of patients experience chronic arthritis post-CHIKV infection (Puntasecca et al., 2021). The population risk groups identified as being more likely to develop severe disease include infants, the elderly and immunocompromised individuals (Sebastian et al., 2009; Lang et al., 2017).
In the past 20 years, CHIKV has re-emerged as a major threat to public health with worldwide distribution and has been associated with frequent regional outbreaks (Box 3). In the most recent outbreak in 2013 starting in the Caribbean island of Saint Martin, CHIKV spread to 22 countries in the Caribbean and Central and South America, resulting in hundreds of thousands of infections (Van Bortel et al., 2014; Kautz et al., 2015). The rapid spread of CHIKV worldwide is a clear indicator that vector control (Box 1) strategies, which are currently the only method available to protect populations from CHIKV and other vector-borne viruses, are insufficient to contain arboviral diseases. To date, there are no licensed vaccines or preventative approaches for the targeted treatment of CHIKV. Although several vaccine strategies are being pursued, with some in various stages of clinical trials, these vaccines are still several years away from being licensed and available to the public. Currently, the best protection against CHIKV disease is preventing infection by avoiding mosquito bites. Furthermore, with no approved antiviral therapy, the existing treatment for patients with CHIKV disease is based on supportive care and consists of the administration of analgesics, antipyretics and non-steroid anti-inflammatory drugs (NSAIDs). Effective therapeutic and preventative approaches for CHIKV infection are urgently required.
Box 3. Evolution and spread of CHIKV.
Phylogenetic analysis of CHIKV has identified four distinct lineages with different genotypes corresponding to their geographic region, including Asian (sub-lineage Asian/American), East/Central/South African (ECSA), West African and Indian Ocean lineage (IOL) genotypes (Wahid et al., 2017). In 2004, CHIKV re-emerged in coastal Kenya and spread throughout the western Indian Ocean islands (Chretien et al., 2007). This led to the most recent major epidemic of CHIKV that occurred between 2005 and 2006 and affected several countries located in the Indian Ocean. On La Réunion island, one-third of the population was infected with CHIKV (Vazeille et al., 2007; Staples et al., 2009). The CHIKV isolated during this epidemic represented a novel ECSA genotype with a mutation in the E1 envelope glycoprotein (E1-A226V), which was subsequently described as the IOL (Sahadeo et al., 2015). As CHIKV glycoproteins play an important role in viral transmission, emergence and spread, the E1-A226V mutation increased infectivity and transmission by Aedes albopictus mosquitoes, with less preferential transmission by Aedes aegypti (Tsetsarkin et al., 2007). Other mutations within the IOL have been identified on CHIKV envelope glycoprotein E2 (e.g. L210Q, K252Q), which further increase viral fitness in A. albopictus (Tsetsarkin et al., 2014). The mosquito A. albopictus is more abundant and widely distributed than A. aegypti in southern Europe and America, and several CHIKV outbreaks and autochthonous transmission (Box 1) have been reported recently in these regions, including Italy (Rezza et al., 2007), France (Roiz et al., 2015), Spain, Caribbean islands (Van Bortel et al., 2014), Argentina, Mexico (Kautz et al., 2015) and USA (Madariaga et al., 2016). CHIKV isolates originating from the 2013 Saint Martin outbreak, which spread throughout the Caribbean and into Central and South America, also form a novel American sublineage within the Asian lineage (Asian/American) (Van Bortel et al., 2014; Kautz et al., 2015). The continuous evolution of CHIKV glycoproteins increases the virus’ fitness in a more widely distributed vector, which expands its geographical impact. Owing to globalisation and the year-round presence of mosquito vectors, especially in highly populated urban areas (Kraemer et al., 2015), there is a high risk that the virus will become endemic in several different regions of the world.
In this Review, we provide a current perspective on CHIKV pathogenesis, as well as novel therapeutic strategies used to prevent, treat and manage CHIKV disease, including vaccines, monoclonal antibodies, antiviral compounds and immunomodulatory drugs.
CHIKV infection and host immune responses
CHIKV and other arboviruses infect the mosquito midgut, following the ingestion of viraemic blood, then replicate and disseminate to the salivary glands, being transmitted via saliva when the mosquito bites. Following the mosquito bite, CHIKV replicates at the site of the inoculation (Fig. 1). The dermal fibroblasts have been shown to constitute the main site of viral amplification (Ekchariyawat et al., 2015; Wichit et al., 2017). The spread of the virus from the skin to other peripheral organs is thought to be via the circulatory system (Fig. 2), as CHIKV was shown to infect human peripheral blood mononuclear cells (PBMCs) in vitro, with monocytes primarily infected and B cells and myeloid dendritic cells to a lesser extent infected (Her et al., 2010; Ruiz Silva et al., 2016). CHIKV antigens were also detected in vivo in the monocytes of acutely infected patients (Her et al., 2010). In contrast, a previous study by Sourisseau et al. (2007) showed that PBMCs – including monocytes, B cells, T cells and monocyte-derived dendritic cells – were not susceptible to CHIKV infection. These contradictory results indicate that more research is required.
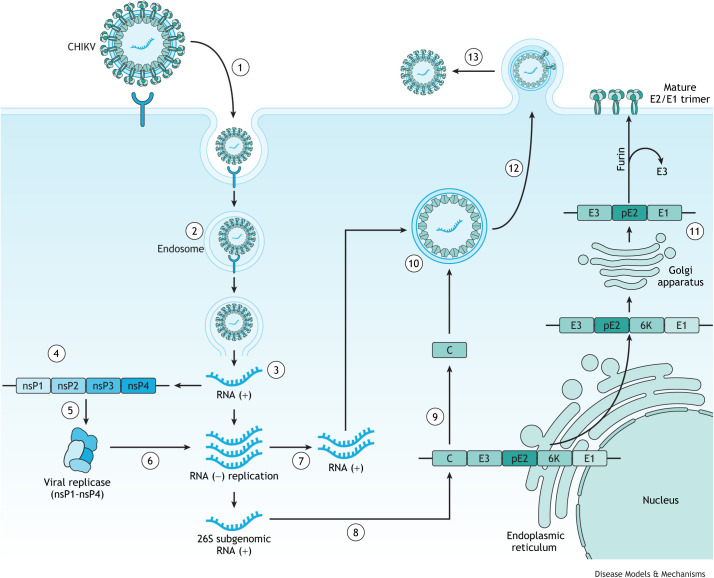
Fig. 1.
CHIKV life cycle. (1) CHIKV enters the host cells through receptor-mediated endocytosis. The viral protein E2 interacts with specific host surface receptors. A number of receptors [for example, prohibitin (Wintachai et al., 2012), MXRA8 (Zhang et al., 2018), PS receptors (Moller-Tank et al., 2013) and GAG (Silva et al., 2014)] have been implicated in this process. (2) Once in the endosome, the acidic pH triggers conformational changes in the viral envelope, exposing the E1 peptide and leading to its fusion with the endosomal membrane (Voss et al., 2010). (3) This allows for the cytoplasmic delivery of the nucleocapsid and the release of the viral RNA genome. (4) The viral genome is translated to the non-structural polyprotein nsP1- nsP2-nsP3-nsP4. (5) The viral protease nsP2 then cleaves the polyprotein into the individual nsPs – nsP1, nsP2, nsP3 and nsP4 – that then form the viral replicase complex. (6,7) The viral replicase is responsible for the synthesis of the negative-strand RNA that will be a template for both new positive-strand RNA and the sub-genomic RNA (26S RNA) (van der Heijden and Bol, 2002). (8) The 26S RNA drives the expression of the structural polyprotein C-pE2-6K-E1 in the endoplasmic reticulum. (9,10) The capsid protein (C) dissociates from the polyprotein by self-cleavage activity and binds to the newly synthesised viral RNA, forming the nucleocapsid core in the cytoplasm. (11) In the meantime, E2 and E1 associate in the Golgi apparatus and are exported to the cell membrane, where pE2 is cleaved by the host protease furin into E2 and E3. E3, which stabilises the E2/E1 trimer, then dissociates from the trimer when it reaches the cell membrane. (12) The already-formed nucleocapsid migrates to the host cell membrane region rich in E2/E1 trimers that bind the virion membrane. (13) The mature virions are released by the budding process from the infected cells (Yap et al., 2017). CHIKV, chikungunya virus; GAG, glycosaminoglycan; MXRA8, matrix remodelling-associated protein 8; nsP, non-structural protein; PS receptor, phosphatidylserine-mediated entry-enhancing receptor.
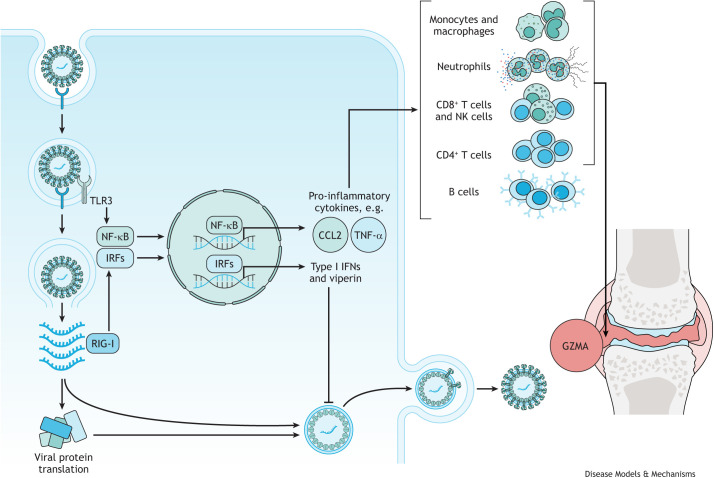
Fig. 2.
CHIKV infection activates host immune responses, leading to joint/muscle inflammation. CHIKV is detected by TLR3, TLR7 and TLR8, as well as by RIG-I-like receptors, which activate transcription factors, NF-κB and IRFs. IRFs stimulate a strong antiviral type I IFN response through the transcription of IFN-stimulated genes that encode antiviral proteins, such as viperin, IFN-α/β and OAS (Teng et al., 2012; Priya et al., 2014; Onomoto et al., 2021). NF-κB activates a pro-inflammatory response by stimulating the transcription of many pro-inflammatory cytokines and chemokines, including TNF-α and CCL2 (Teng et al., 2015; Onomoto et al., 2021). The chemokine CCL2 is responsible for the recruitment of monocytes/macrophages to the site of infection, while TNF-α has been linked to the recruitment of cytolytic lymphocytes, including NK cells and CD8+ T cells that secrete the pro-inflammatory granule GZMA, which has demonstrated a prominent role in driving arthritic inflammation. Studies have also shown that antibody-producing B cells are involved in CHIKV clearance and control. CD4+ T cells were shown to be activated during the chronic phase of CHIKV infection and play a role in pathogenesis of CHIKV-induced joint inflammation. CD8+ T cells, neutrophils and monocytes/macrophages can also accumulate in the joints, with monocytes and macrophages even differentiating into osteoclasts that can lead to damage in the joint. CCL2, chemokine ligand 2; CHIKV, chikungunya virus; IFN, interferon; IRF, interferon regulatory factor; GZMA, granzyme A; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NF-κB, nuclear factor kappa B; NK, natural killer; OAS, 2'-5'-oligoadenylate synthetase 1; RIG-I, retinoic acid-inducible gene I; TLR, Toll-like receptor; TNF-α, tumour necrosis factor alpha.
Myalgia and arthralgia are two main symptoms associated with CHIKV reaching the muscles and joints, predominantly attributed to infection of myoblasts, skeletal muscle fibroblasts and synovial fibroblasts, as well as to joint macrophages (Dubrulle et al., 2009; Hoarau et al., 2010; Phuklia et al., 2013; Lohachanakul et al., 2015; Guerrero-Arguero et al., 2020). The ability of CHIKV to affect multiple systems/organs might be due to the widespread expression of receptors, which permits infection of a wide range of different cell types. In mammalian cells, suggested receptors for CHIKV include prohibitin (Wintachai et al., 2012), matrix remodelling-associated protein 8 (MXRA8) (Zhang et al., 2018), phosphatidylserine-mediated entry-enhancing receptors (PS receptors) (Moller-Tank et al., 2013) and glycosaminoglycans (GAGs) (Silva et al., 2014); however, their precise roles are not well elucidated.
Acute phase of CHIKV disease
The acute phase of CHIKV disease lasts typically <21 days after the onset of infection. It is divided into two different stages, the viraemic phase (5-10 days), marked by high fever and polyarthralgia/arthritis, myalgia, headaches and skin rashes; and the post-viraemic phase (6-21 days), characterised by a lack of fever, polyarthralgia/arthritis and, to a lesser extent, myalgia, fatigue and anorexia (Thiberville et al., 2013).
During the acute phase of infection, CHIKV RNA is detected by pattern recognition receptors, including Toll-like receptor (TLR)3, TLR7 and TLR8, as well as retinoic acid-inducible gene I (RIG-I)-like receptors, which trigger the production of type I interferons (IFNs) through the activation of the transcription factors IFN regulatory factors (IRFs) and nuclear factor kappa B (NF-κB). Both IRFs and NF-κB induce the transcription of IFN-stimulated genes (ISGs) and pro-inflammatory cytokine genes (Onomoto et al., 2021). Type I ISGs represented 50% of all upregulated genes in an RNA-sequencing (RNA-seq) analysis (Wilson et al., 2017), with IFN-α and IFN-γ, in particular, upregulated in all acutely infected patients during the first week of symptoms (Wauquier et al., 2011). IFNs, and specifically type I IFNs, appear to play essential roles in controlling the severity of CHIKV infection. Of note, results from CHIKV infection of mice deficient in IFN-α/β receptors have suggested that rapid early induction of type I IFNs is required to control virus replication and protect against disease severity (Couderc et al., 2008; Gardner et al., 2012; Rudd et al., 2012). Furthermore, in vivo studies have identified fibroblasts as the predominant target cell of CHIKV and the main source of type I IFNs (specifically IFN-β). Type I IFN produced by and acting on fibroblasts appears to be essential for the control and early clearance of CHIKV in vivo (Couderc et al., 2008; Schilte et al., 2010).
Many ISGs upregulated during the initial CHIKV infection encode proteins involved in host defence (Teng et al., 2012; Priya et al., 2014). Viperin, an inducible antiviral protein, was elevated in monocytes from CHIKV-infected patients (Teng et al., 2012). Furthermore, in viperin-deficient mice, there was a direct correlation between the lack of viperin expression and higher CHKV replication and joint inflammation (Teng et al., 2012). Hence, the upregulation of viperin could be a potential host strategy to control CHIKV infection.
In addition to a strong antiviral type I response, the acute phase of CHIKV disease is also associated with a potent inflammatory response (Chow et al., 2011; Wauquier et al., 2011; Teng et al., 2015; Wilson et al., 2017). A systematic meta-analysis characterised the circulatory immune mediators in plasma or serum samples from different patient cohorts with acute CHIKV infection and identified upregulation of pro-inflammatory cytokines, including IL-6, IL-8, CCL2, RANTES (also known as CCL5), TNF-α (also known as TNF) and IL-1α, as well as the anti-inflammatory cytokines IL-10 and IL-13, growth factors GM-CSF (also known as CSF2), G-CSF (also known as CSF3) and VEGF, and other mediators IL-4, IL-17, CXCL9, IFN-α and IFN-γ (Teng et al., 2015). The production of pro-inflammatory cytokines and chemokines at the primary sites of infection, mainly joints and muscles, mediates the infiltration of monocytes/macrophages, neutrophils, natural killer (NK) cells and T cells to these infection sites, resulting in tissue inflammation and damage that manifests as arthralgia in patients (Zare et al., 2004; Gardner et al., 2010).
The monocytes/macrophages that are recruited to the sites of inflammation during CHIKV infection have been shown to have antiviral activity against CHIKV (Haist et al., 2017; Fox et al., 2019), and to contribute to the resolution of inflammation and tissue repair (Ikeda et al., 2018). However, the musculoskeletal tissues of CHIKV-infected patients were shown to be greatly infiltrated with monocytes and macrophages (Hoarau et al., 2010), which strongly implicates these cells in CHIKV arthritic immunopathology. Studies in mice in which monocytes/macrophages were depleted (Gardner et al., 2010) or migration was inhibited (Rulli et al., 2011; Chen et al., 2015) suggested that these cells can be pathogenic effectors during CHIKV infection. However, CHIKV infection of mice deficient in CCR2, the receptor for CCL2, a potent monocyte-attracting chemokine, resulted in exacerbation of the arthritic disease, in which the monocytes/macrophages infiltrate was replaced by a severe neutrophil and eosinophil infiltrate (Poo et al., 2014a). Thus, the role of monocytes/macrophages in CHIKV infection is complex, and although their persistent recruitment may contribute to chronic inflammation, initial macrophage recruitment can also prevent excessive pathology.
As mentioned above, neutrophils are also recruited to the site of inflammation during CHIKV infection, where they can produce reactive oxygen species (ROS) and other inflammatory mediators important in fighting infection. Similar to that of macrophages, the role of neutrophils during CHIKV infection is poorly understood. A study using zebrafish demonstrated that CHIKV infection induced a strong type I IFN response and identified neutrophils as the main source of type I IFNs. The depletion of neutrophils in this model led to an increase in disease severity and mortality, which was associated with high viral load, demonstrating the crucial role neutrophils have in fighting CHIKV infection (Palha et al., 2013). Of note, a study by Hiroki et al. (2019) identified neutrophil extracellular traps (NETs; Box 1) as a protective mechanism during CHIKV infection. NETs were shown to directly inhibit CHIKV replication in vitro. Furthermore, in IFN receptor-deficient (Ifnar−/−) mice, the inhibition of NETs by DNAse treatment resulted in increased susceptibility to CHIKV infection and viral load (Hiroki et al., 2019).
The acute phase is also mediated by CD8+ T-cell responses in the early stages of infection, whereas CD4+ T-cell responses are present in the later stages of infection (Wauquier et al., 2011). During acute CHIKV infection, activated CD8+ T cells were significantly elevated in the peripheral blood of patients, with high expression of cytolytic markers granzyme B and perforin, and the degranulation marker CD107A (also known as LAMP1) (Hoarau et al., 2010; Dias et al., 2018). This suggests that CD8+ T cells mediate cytolytic activity against CHIKV-infected cells by releasing the cytolytic granules, perforin and granzyme B. Furthermore, in mice infected with CHIKV, CD4+ T cells and CD8+ T cells infiltrated inflamed joints (Morrison et al., 2011). Despite this, mice deficient in CD8+ T cells still developed joint inflammation (Teo et al., 2013). Interestingly, immunisation with a vaccine that induces virus-specific effector CD8+ T cells prior to infection increased the clearance of CHIKV infection in the spleen but not in joint-associated tissues (Davenport et al., 2020). This was also observed in Ross River virus (RRV) infection in mice, in which CD8+ T cells contributed to the control of RRV infection in certain tissues but failed to mediate an antiviral response in the joints (Burrack et al., 2015). This suggests that CHIKV establishes and maintains a persistent infection in joint-associated tissues partly by evading CD8+ T-cell immunity.
Granzyme A (GZMA), a serine protease granule secreted by cytotoxic lymphocytes, including CD8+ T cells, NK cells and natural killer T (NKT) cells, was elevated in the serum of CHIKV-infected patients and correlated with viral loads and disease severity. In a CHIKV mouse model, serum GZMA levels were also elevated and NK cells were attributed as the main source (Schanoski et al., 2019). Previous studies also showed that Gzma−/− mice had a pronounced reduction in foot swelling and arthritis compared to wild-type C57BL/6J mice, suggesting a pro-inflammatory role of GZMA in CHIKV infection (Wilson et al., 2017). However, Gzma−/− mice have a mixed C57BL/6J and C57BL/6N genetic background and retain the full-length nicotinamide nucleotide transhydrogenase (Nnt) gene, whereas in the wild-type C57BL/6J mice the Nnt gene is truncated. Hence, the genetic background and the presence of the full-length Nnt gene, rather than the loss of GZMA expression, were shown to be responsible for the ameliorated CHIKV arthritis phenotype of Gzma−/− mice (Rawle et al., 2022). Nevertheless, this cannot be directly extrapolated to humans, and further studies are warranted to determine whether GZMA could be a potential target for therapy in CHIKV-mediated inflammation.
During the acute phase of infection, CHIKV-specific IgM can be detected by day 4 and CHIKV-specific IgG antibodies can be detected by day 10 (Chua et al., 2017). Numerous studies have established an association between antibody response and viral titres, cytokine levels and disease progression during the acute and chronic phases of infection (Kam et al., 2012; Jain et al., 2017). Patients with high viremia were shown to develop high levels of neutralising IgG3 antibodies. Although these patients underwent a more severe disease progression during the acute viraemic phase, they were able to clear the virus faster and did not develop persistent arthralgia. Conversely, patients that presented low viremia produced IgG3 at a later stage and were shown to develop persistent arthralgia. Therefore, determining the levels of early CHIKV-specific IgG3 could serve as a marker for the identification of patients at risk of developing the severe chronic disease (Kam et al., 2012). In support of this, animal studies have provided evidence of the role of antibodies in CHIKV infection. Lum et al. (2013) showed that CHIKV infection in B-cell knockout mice resulted in viremia that persisted for more than 1 year, with a more severe disease than that of wild-type mice, indicating that antibody-producing cells are directly involved in viral clearance and control (Lum et al., 2013).
Overall, the acute phase of CHIKV infection is characterised by a type I IFN response alongside inflammatory responses that recruit innate and adaptive immune cells. This orchestrated recruitment of leukocytes is important for controlling and resolving infection, but if the fine-tuned balance is disrupted, more severe and/or prolonged symptoms can arise.
Chronic phase of CHIKV disease
It is estimated that, on average, 42.5% of CHIKV-infected patients will develop arthritis/arthralgia that can persist for several months and even years (Puntasecca et al., 2021). This is known as the chronic phase of CHIKV disease.
The main symptom in chronic CHIKV disease is arthralgia; however, the underlying inflammatory stimuli responsible for chronic CHIKV arthropathy are far from understood. An RNA-seq analysis of CHIKV infection in wild-type mice suggested that chronic arthritic disease represents a prolongation of the acute inflammatory response (Wilson et al., 2017), rather than the activation of new immunopathological inflammatory responses (Suhrbier and Mahalingam, 2009), which continues until the virus material is cleared (Poo et al., 2014b).
During the chronic stage, CHIKV particles have predominantly been cleared from the blood; however, a substantial body of evidence reports that CHIKV RNA and CHIKV-specific proteins can persist in tissues. Hoarau et al. (2010) reported that CHIKV RNA and proteins were detected in synovial macrophages of one patient 1.5 years after infection (Hoarau et al., 2010). CHIKV antigens were also detected in human muscle satellite cells 3 months after acute infection (Ozden et al., 2007). These results are consistent with animal studies that found that CHIKV RNA persisted in muscle fibroblasts in mice (Young et al., 2019) and in macrophages in non-human primates (NHPs) (Labadie et al., 2010). As previously noted, CHIKV-induced arthritis draws several parallels with autoimmune arthritis, although there is very little evidence that viral arthritis leads to autoimmune disease. Rather, it is thought that the persistence of viral antigens could be a contributing factor to the development of chronic CHIKV-induced arthritis. One hypothesis is that double-stranded RNA (dsRNA) intermediates found in infected tissues act as pro-inflammatory pattern-associated molecular patterns that trigger an arthritogenic response (Zare et al., 2004; Hawman et al., 2013; McCarthy and Morrison, 2017).
The chronic phase has consistently been associated with increased levels of circulating IL-6 (Ng et al., 2009; Chow et al., 2011; Noret et al., 2012), as well as GM-CSF, IL-12, IL-17, IL-27, receptor activator of NF-κB ligand (RANKL; also known as TNFSF11) and IL-8 (Hoarau et al., 2010; Chaaitanya et al., 2011; Kelvin et al., 2011; Noret et al., 2012; Gualberto Cavalcanti et al., 2019). The plasma levels of IL-6 in patients with persistent arthralgia were higher than those in convalescent-phase patients (Chow et al., 2011), and this observation was further supported by the findings of Hoarau et al. (2010), showing that IL-6 was specifically expressed in the affected joint during chronic disease. The bone-forming osteoblasts (OBs) express RANKL and osteoprotegerin (OPG; also known as TNFRSF11B), and a high ratio of RANKL/OPG is associated with the formation of osteoclasts (Box 1) from monocytic precursors. CHIKV has been shown to infect OBs, inducing the expression of IL-6 and RANKL and repressing OPG expression (Noret et al., 2012), which would presumably lead to increased osteoclast formation. In addition, CHIKV is known to infect fibroblast-like synoviocytes, which induces the secretion of RANKL, IL-6, IL-8 and CCL2, and leads to the migration and differentiation of monocytes into osteoclasts (Phuklia et al., 2013). The presence of osteoclasts in the joints can lead to damage of the joint structure and contribute to the arthritic-like syndrome, which has been described in RA (Schett, 2007).
T cells play an important role in the development of chronic CHIKV infection. CD8+ T cells in patients with chronic disease showed a reduction in activation marker CD69 and in cytolytic activity, demonstrated by the decreased expression of granzyme B, perforin and CD107A, compared to that in patients in the acute phase of the infection (Dias et al., 2018). In the acute phase of the infection, the continuous presentation of CHIKV epitopes by antigen-presenting cells to CD8+ T cells leads to the sustained activation of T-cell receptors (Mueller and Ahmed, 2009; Dias et al., 2018). This could cause CD8+ T-cell exhaustion, leading to cells shutting down and rendering them unable to eliminate infected cells, which may lead to chronic CHIKV infection with lower levels of functioning CD8+ T cells (Mueller and Ahmed, 2009; Dias et al., 2018; Hashimoto et al., 2018). By contrast, CD4+ T cells were shown to be activated during the chronic phase of CHIKV infection, playing a primary role in the pathogenesis of CHIKV-induced joint inflammation (Hoarau et al., 2010; Teo et al., 2013). An elegant study by Teo et al. (2017) illustrated the essential role of CD4+ T cells in the CHIKV-induced joint inflammation, as transferring splenic CD4+ T cells from CHIKV-infected wild-type mouse into CHIKV-infected T cell receptor-deficient (Tcr−/−) mice recapitulated severe joint disease (Teo et al., 2017).
The complex interactions between CHIKV and the host can determine the outcome of the viral infection. Although co-evolution of the virus with the host has led to the development of specialised immune responses to control viral replication, CHIKV, like many other pathogens, has evolved to evade the immune response of the host by hijacking antiviral pathways. For the development of therapeutic approaches aimed at enhancing early viral clearance and limiting the development of chronic disease, a greater understanding is required of both the acute and chronic phases of CHIKV disease and the role of host defence in the pathobiology of the disease.
Antiviral strategies targeting the virus replication cycle
Viruses are obligatory intracellular microbes that require the host cell to replicate; therefore, it is difficult to define virus-specific functions as suitable targets for antiviral therapy. Nevertheless, extensive research has identified several molecules with anti-CHIKV potential, targeting the virus and/or the host. This section will summarise the antiviral strategies directed at the pathogen itself, as well as host cell pathways or molecules important for viral replication (Fig. 3). Currently, no approved antiviral treatment is available for CHIKV infection.
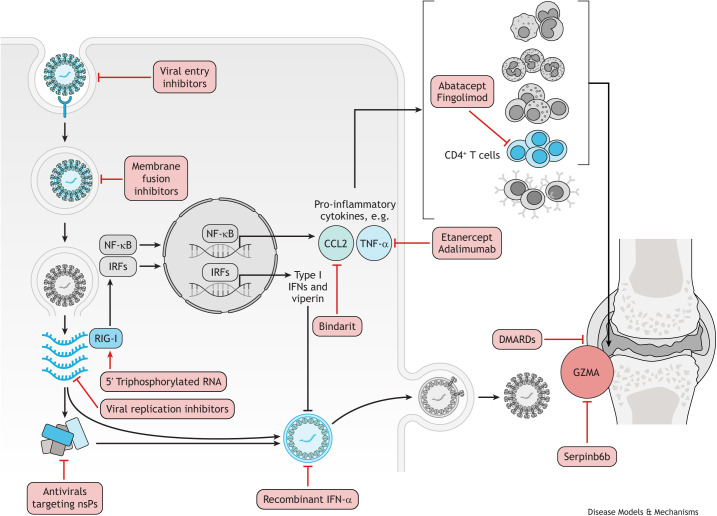
Fig. 3.
CHIKV treatments targeting the viral life cycle, host defence mechanisms and host immunopathology. Antivirals that block viral entry into the host cell include the natural compounds epigallocatechin gallate (Weber et al., 2015), flavaglines (Wintachai et al., 2015) and curcumin (Mounce et al., 2017). In vitro, chloroquine was shown to increase the endosomal pH, which then prevents the fusion of CHIKV E1 glycoprotein with the endosomal membrane (Khan et al., 2010). Broad-spectrum antivirals, including ribavirin and 6-azauridine, inhibit viral replication by introducing mutations in the viral genome, whereas favipravir targets RNA polymerase and prevents viral replication (Briolant et al., 2004). The viral nsPs are also a potential target for the development of antiviral molecules, and several compounds have been identified to target different nsPs, including harringtonine (Bassetto et al., 2013; Das et al., 2016). In response to CHIKV infection, the host elicits a strong antiviral type I IFN; therefore, recombinant IFN-α has shown the ability to inhibit CHIKV replication in vitro (Briolant et al., 2004). Furthermore, activating the RIG-I receptor with 5′ triphosphorylated RNA can augment this host response (Olagnier et al., 2014). A prolonged pro-inflammatory host response to CHIKV infection can lead to the chronic disease; therefore, some treatments are aimed at dampening this response. Inhibiting the chemokine CCL2 with bindarit ameliorated the inflammation in joints and skeletal muscles in mice (Rulli et al., 2011). TNF-α inhibitors, such as etanercept and adamimumab, were shown to reduce arthritic symptoms in patients (Blettery et al., 2016). CD4+ T cells also have a primary role in the pathogenesis of CHIKV-induced joint inflammation (Teo et al., 2017). Fingolimod efficiently suppressed CHIKV-induced joint pathology in virus-infected mice by blocking T-cell migration from the lymph nodes to the joints (Teo et al., 2017), and abatacept, which inhibits CD4+ T-cell priming in combination with neutralising anti-CHIKV monoclonal antibody, reduced T-cell accumulation in the joints (Miner et al., 2017). This resulted in ameliorated joint inflammation and decrease in proinflammatory cytokine secretion (Miner et al., 2017). The pro-inflammatory granule GZMA is released by natural killer cells and CD8+ T cells in response to CHIKV infection, and the GZMA inhibitor, Serpinb6b, was shown to reduce joint inflammation (Wilson et al., 2017). DMARDs, including hydroxychloroquine, sulfasalazine and methotrexate, have been trialled for chronic CHIKV arthritis (Ganu and Ganu, 2011; Martí-Carvajal et al., 2017; Ravindran and Alias, 2017). However, owing to contradictory results, it remains unclear whether the use of specific DMARDs is effective in treating chronic CHIKV arthritis. CHIKV, chikungunya virus; DMARD, disease-modifying antirheumatic drug; GZMA, granzyme A; IFN, interferon; IRF, interferon regulatory factor; NF-κB, nuclear factor kappa B; nsP, non-structural protein; RIG-I, retinoic acid-inducible gene I; TNF-α, tumour necrosis factor alpha.
Antiviral strategies targeting the virus
One approach for identifying novel anti-CHIKV therapies is to assess compounds that have shown antiviral potential against other viruses. Known compounds tested against CHIKV that have shown the potential to block viral entry in vitro include chloroquine, arbidol and derivatives (Di Mola et al., 2014), epigallocatechin gallate (EGCG; Weber et al., 2015), flavaglines (Wintachai et al., 2015), curcumin (Mounce et al., 2017) and phenothiazines (Pohjala et al., 2011). Chloroquine is an anti-malarial drug with anti-inflammatory properties, which is also used to treat a variety of inflammatory diseases, including RA, osteoarthritis and lupus. In in vitro studies, chloroquine showed direct antiviral activity against flaviviruses, retroviruses and coronaviruses (Savarino et al., 2003). Chloroquine treatment of CHIKV-infected Vero cells strongly inhibited viral replication, and the underlying mechanism is thought to be associated with an increase in endosomal pH that prevents the fusion of E1 glycoprotein with the endosomal membrane (Khan et al., 2010). However, in a clinical trial in Réunion Island, oral chloroquine had no significant effect on viremia or duration of symptoms compared to placebo treatment (De Lamballerie et al., 2008). A comparable study performed in India showed that chloroquine improved patient symptoms but failed to offer any advantage over treatment with the NSAID meloxicam (Chopra et al., 2014). Furthermore, another clinical trial and an NHP study suggested that chloroquine had a negative impact on the immunological response, causing a possible delay in viral clearance (Roques et al., 2018). The events leading to the revocation of chloroquine for COVID-19 treatment, owing to its inefficacy in hospitalised patients, further highlight the importance of translating in vitro studies to carefully planned animal studies and/or clinical trials.
Natural compounds mostly derived from plants, roots and spices have also been investigated for their anti-CHIKV activity. Among the first compounds to be tested in vitro against CHIKV was EGCG, the major constituent of green tea. EGCG was shown to prevent CHIKV entry into HEK293T cells, inhibiting its replication (Weber et al., 2015). Other natural compounds that have shown the ability to block CHIKV entry in vitro include flavaglines (Wintachai et al., 2012) and curcumin (von Rhein et al., 2016). Flavaglines are known ligands of prohibitin, and, recently, prohibitin-1 has been identified as a potential cellular receptor used by CHIKV to enter mammalian cells (Wintachai et al., 2012). Flavaglines were shown to inhibit CHIKV replication in HEK293T cells only when compounds were added prior to infection, which indicates that flavaglines interfere with CHIKV entry. This observation was further confirmed by the significant reduction in the colocalisation of CHIKV E2 protein with prohibitin-1 when incubated in the presence of flavaglines (Wintachai et al., 2015).
Other antiviral compounds have been shown to inhibit CHIKV replication, including well-known and characterised broad-spectrum antiviral agents, such as favipiravir (Delang et al., 2014), ribavirin, 6-azauridine, and glycyrrhizin. A study by Briolant et al. (2004) evaluated the anti-CHIKV activity and cytotoxicity of ribavirin, 6-azauridine and glycyrrhizin in Vero cells. 6-Azauridine was shown to reduce virus-induced cytopathogenicity and was more potent than ribavirin, inhibiting CHIKV replication at very-low concentrations (S et al., 2004). Glycyrrhizin inhibited CHIKV in Vero cells; however, this was only achieved at high subtoxic concentrations (Briolant et al., 2004). Ribavirin showed potent antiviral activity against CHIKV and exhibited synergistic effects with doxycycline (Rothan et al., 2015) and with recombinant IFN-α2 in vitro (Briolant et al., 2004). Despite the potent antiviral properties of ribavirin in vitro, this did not translate in vivo. A small non-randomised clinical trial (n=20) showed no evidence of greater efficacy of ribavirin compared to placebo (Ravichandran and Manian, 2008).
The replication cycle of CHIKV can also be utilised to identify novel targets for the development of antiviral compounds. The four non-structural proteins (nsPs) represent interesting targets for the identification of selective CHIKV inhibitors, as these proteins possess enzymatic activities that are essential for virus replication. An in silico study by Das et al. (2016) designed compounds predicted to interact with the CHIKV active centre of nsP2 proteases. Several of these identified compounds were then shown to inhibit nsP2 functionality, as well as suppress CHIKV replication in BHK-21 cells (Das et al., 2016). Bassetto et al. (2013) performed a virtual screening simulation of more than 5 million compounds on the CHIKV nsP2 protease. A series of 26 compounds was identified after performing analysis of the structure–activity relationship (Box 1). From those, only nine compounds were tested in vitro and were shown to inhibit CHIKV replication in Vero cells at non-cytotoxic concentrations (Bassetto et al., 2013).
In recent years, the in-depth characterisation of CHIKV nsP3 functional domains has identified nsP3 as a potential drug target. The N-terminal macrodomain of nsP3 is highly conserved amongst alphaviruses and other positive-strand RNA viruses (Eckei et al., 2017). Therefore, the nsP3 macrodomain represents an ideal site for the development of antivirals with broad antiviral activity. In silico screenings have helped identify putative CHIKV nsP3 inhibitors that target specific binding sites in the macrodomain. In vitro analysis demonstrated that two of the selected molecules inhibited CHIKV replication in Huh-7 cells (Shimizu et al., 2020). This study highlights the importance of validating in vitro the antiviral properties and mode of action of inhibitors discovered using in silico methods.
Other CHIKV proteins have also been identified as potential targets for the development of inhibitors. The benzimidazole-related compound, designated Compound A, was found to inhibit several CHIKV strains at very-low concentrations. Compound A was identified as an inhibitor of CHIKV RNA polymerase by it targeting a residue in the nsP4 protein and therefore inhibiting the replication of CHIKV in Vero cells (Wada et al., 2017). Kaur et al. (2013), used an immunofluorescence-based screening platform to identify 44 compounds that resulted in >70% inhibition of CHIKV replication. Among these, harringtonine, hypocrellin A, rottlerin and daunorubicin inhibited CHIKV replication in BHK21 cells in a dose-dependent manner, and harringtonine was identified as the most potent antiviral compound. Harringtonine treatment of CHIKV-infected cells resulted in a decrease in CHIKV RNA products and synthesis of nsP3 and E2 proteins, which suggested that harringtonine interfered with viral protein translation (Kaur et al., 2013).
Several promising antiviral compounds have been developed; however, most of them still have to be validated in vivo and in clinical trials. The use of re-purposed antiviral compounds, such as chloroquine, ribavirin and favipiravir, for the treatment of CHIKV has its advantages as these have already been intensively evaluated in patients, potentially fast-tracking clinical trials for them against CHIKV. So far, however, these have failed to maintain their efficacy in vivo and in clinical trials (e.g. chloroquine and ribavirin). Although targeting the virus provides a specific and direct inhibitory effect, there is the risk of the virus mutating and developing resistance to antiviral therapy (Delang et al., 2014). An alternative antiviral approach, with the potential to lower the likelihood of drug resistance, is the identification of host pathways as possible targets for CHIKV inhibitors.
Antiviral strategies targeting host defence mechanisms
In addition to targeting the pathogen itself, novel antiviral approaches target host cell pathways or molecules to interfere with CHIKV replication, an approach that has shown considerable efficacy with other viral pathogens. A genome loss-of-function screen in which HEK293 cells were transfected with a library of short interfering RNAs (siRNAs) targeting host cell genes prior to CHIKV infection identified a diverse range of novel host factors and pathways, including ATPases, calmodulin signalling and fatty acid synthesis, which were essential for CHIKV replication (pro-viral) or limited its replication (antiviral) (Karlas et al., 2016). Two compounds, the calmodulin inhibitor pimozide and the fatty acid synthesis inhibitor TOFA, demonstrated potent antiviral effects in HeLa and HEK-293T cells and C57BL/6 mice. These effects were enhanced when the drugs were used in combination, suggesting that the use of drugs targeting different cellular pathways may enhance antiviral effects and reduce the chance of CHIKV developing resistance (Karlas et al., 2016). Another approach includes targeting host cell receptors, such as intracellular RIG-I receptors that recognise dsRNA and activate multiple antiviral factors blocking viral replication. Experimentally, an optimised RIG-I agonist has been shown to trigger RIG-I and protect lung fibroblast MRC-5 cells from CHIKV infection (Olagnier et al., 2014), demonstrating a promising approach for therapeutic development.
Targeting host factors can offer several advantages over just targeting the virus itself. For instance, modulating host pathways may allow the inhibition of all viruses that depend on that particular function. Additionally, combining these compounds with specific direct antiviral compounds may lead to synergistic effects with the potential to develop more comprehensive treatment plans that target multiple pathways. Chatterjee et al. (2022) recently identified checkpoint kinase proteins Chk1 and Chk2 (also known as CHEK1 and CHEK2), which are commonly known as DNA damage response proteins, as host factors that are involved in CHIKV replication. Similarly, De Caluwé et al. (2021) characterised the role of the CD147 protein complex in CHIKV entry into host cells. With inhibitors for both of these host targets currently being used to treat other disorders, establishing whether these compounds suppress viral replication merits experimental testing. The number of novel therapeutic compounds aimed at inhibiting host molecules involved in CHIKV replication is steadily growing. However, as these approaches target host factors, potential serious adverse or off-target effects may occur.
Thus far, several classes of compounds have shown the potential to inhibit CHIKV either by directly targeting the virus or by targeting host cell factors; however, many of these inhibitors have only been tested in vitro. Currently, there is no standardised protocol to determine the efficacy and toxicity of antiviral drugs in vitro, which makes comparison of the different hits extremely challenging. The efficacy and toxicity values vary widely depending on the cell line, assay method and viral strain (Battisti et al., 2021). A more comprehensive validation of potential antiviral drugs in vitro could help make translational research more robust and efficient (Emmerich et al., 2021). The use of animal models to further examine the potential of these antivirals is clearly needed, which will help to determine their effect in different stages of CHIKV disease and enable clinical translation.
Therapeutic targets to limit the development of CHIKV-associated immunopathology
The recent outbreaks of chikungunya have allowed for a better understanding of the innate and adaptive immune responses induced by CHIKV infection (reviewed in the ‘CHIKV infection and host immune responses’ section). Many responses that contribute to CHIKV immunopathology are also required for protection against viral infections. Therefore, an important consideration for the development of new therapeutic interventions is to target excessive inflammation without compromising antiviral immunity. In this section, we will discuss new potential therapeutic avenues aimed at enhancing early viral clearance and limiting the development of chronic disease (Fig. 3).
IFNs play an essential role in the control of CHIKV infection and provide a promising avenue for the development of antivirals against this virus. One of the most well characterised and effective clinical applications for IFNs is the use of recombinant IFN-α against chronic hepatitis C virus (Hoofnagle and Seeff, 2006). The use of exogenous IFNs was also investigated for CHIKV infection, as Briolant et al. (2004) demonstrated that treatment with recombinant IFN-α inhibited CHIKV replication in Vero cells. Furthermore, it has been shown that a combination approach of IFN-α paired with the antiviral drug ribavirin resulted in a synergistic inhibitory effect on CHIKV replication in vitro (Gallegos et al., 2016). Conversely, in addition to inhibiting viral replication, type I IFNs can promote arthritis. For instance, injection of polyinosinic acid–polycytidylic acid (poly I:C), which structurally resembles dsRNA and is a potent stimulator of type I IFN, into the feet of mice can induce arthritis (Prow et al., 2017). This indicates that further translational studies are required to determine the efficacy of this regimen, in order to find balance between antiviral activity and the inflammatory response.
The identification of appropriate pro-inflammatory mediators that can be targeted without compromising the protective antiviral responses has been a major objective in the development of novel therapeutics for CHIKV disease (Long and Heise, 2015). TNF-α is induced during CHIKV infection (Teng et al., 2015; Thanapati et al., 2017; Wilson et al., 2017), suggesting that TNF-α inhibitors could treat viral arthritis. In fact, treatment of patients with CHIKV with TNF-α antagonists, etanercept and adalimumab, showed promise with good tolerance without the reappearance of the viral infections manifestations (Blettery et al., 2016; Brunier et al., 2016). However, TNF-α also plays an important antiviral role (Rahman and McFadden, 2006). For example, treatment with etanercept resulted in a dramatic disease exacerbation and 100% mortality of mice infected with RRV, which closely resembles CHIKV (Zaid et al., 2011). The protective role of TNF-α in viral infections raises concerns about TNF antagonists for the treatment of patients with CHIKV during the acute phase. However, for patients with chronic manifestations of CHIKV, treatment with TNF-α antagonists has not been associated with obvious disease worsening (Guaraldo et al., 2018), and TNF-α antagonist treatment is included in guidelines to treat CHIKV chronic disease (Simon et al., 2015; Marques et al., 2017). A critical observation is the likelihood of compromising antiviral immunity in locations in which other arboviruses circulate. Ideally, therapies that target CHIKV immunopathology do not compromise the patient's ability to generate immunity in subsequent viral infections.
Another potential anti-inflammatory intervention is supressing CCL2 expression, which is strongly induced during CHIKV infection (Teng et al., 2015; Michlmayr et al., 2018), using bindarit. The treatment of CHIKV-infected mice with bindarit resulted in reduced monocyte recruitment, joint and skeletal muscle tissue swelling, and bone loss (Rulli et al., 2011). This suggests that bindarit, and other CCL2 inhibitors, could potentially be used as a treatment for CHIKV-induced arthritis in humans. However, in the absence of monocytes and macrophages, neutrophils and eosinophils are instead recruited into the joints of mice deficient for the CCL2 receptor, CCR2, promoting joint destruction (Poo et al., 2014a). This raises concerns in using anti-inflammatory interventions due to the potential risk of inadvertently promoting immunopathology.
GZMA was also shown to be elevated in the serum of patients with CHIKV, and its role in driving arthritic inflammation was demonstrated in infected mice (Schanoski et al., 2019). CHIKV-infected wild-type mice were treated with Serpinb6b, a murine GZMA inhibitor, which reduced arthritic inflammation, without compromising the antiviral activity against CHIKV (Wilson et al., 2017). Targeting and inhibiting the activity of GZMA may, therefore, be a potential therapeutic approach for CHIKV-induced arthritis. However, there are distinct differences between murine GZMA and human GZMA (Kaiserman et al., 2006; 2014), indicating the need for more refined translational studies.
Targeting pathogenic CD4+ T cells has also shown some promising results in animal models (Teo et al., 2017). For example, the sphingosine 1-phosphate receptor agonist, fingolimod, efficiently suppressed CHIKV-induced joint pathology in virus-infected mice by blocking CD4+ T-cell migration from lymphoid organs into the joints (Teo et al., 2017). Fingolimod is a clinically approved treatment for multiple sclerosis, although currently there are no clinical trials to assess efficacy in chronic CHIKV disease. Another approach has involved the use of biological disease-modifying antirheumatic drugs (DMARDs) such as abacept, which blocks CD4+ T cell co-stimulation (CTLA4-Ig), in combination with neutralising anti-CHIKV human monoclonal antibodies (mAbs). In CHIKV-infected mice, the combination therapy successfully reduced CD4+ T-cell accumulation in the joints and decreased proinflammatory cytokines, which ameliorated joint inflammation (Miner et al., 2017).
Owing to the fact that chronic CHIKV-induced arthritis resembles RA, the use of DMARDs, including hydroxychloroquine (HCQ), sulfasalazine and methotrexate (MTX) have been trialled for chronic CHIKV arthritis (Ganu and Ganu, 2011; Martí-Carvajal et al., 2017; Ravindran and Alias, 2017). A randomised controlled trial in patients with chronic CHIKV-induced arthritis compared the efficacy of combining MTX, sulfasalazine and HCQ versus HCQ monotherapy and showed that the combination of DMARDs was more effective than HCQ monotherapy (Ravindran and Alias, 2017). However, a longitudinal study showed that most patients displayed progression of disease even with continued treatment with these DMARDs (Bouquillard and Combe, 2009). Owing to conflicting results and the lack of statistical power in trials, it remains unclear whether the use of specific DMARDs is effective in treating chronic CHIKV arthritis.
Although the drugs discussed herein are an exciting avenue for targeting specific immunoinflammatory pathways, the high costs of these drugs might prevent their widespread use, especially in resource-poor settings. In addition, human data for the use of these therapies are very limited, inconclusive and, in most cases, non-existent.
Therapeutic antibodies
Passive antibody therapy that involves the administration of convalescent serum to susceptible individuals to prevent or treat viral infections, such as measles (Park and Freeman, 1926) and influenza (Luke et al., 2010), has a long history and has recently been investigated for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Montelongo-Jauregui et al., 2020). Similarly, antibody therapy has been considered for treatment of alphaviruses, in which immune sera protected mice against pathology associated with Sindbis (Wust et al., 1989), Semliki Forest (Boere et al., 1983) and Venezuelan equine encephalitis viruses (Rabinowitz and Adler, 1973). These studies encouraged the exploration of antibody therapy to combat CHIKV infection.
An elegant study by Couderc et al. (2008) showed that the passive transfer of anti-CHIKV human polyclonal antibodies from donors in the convalescent phase of CHIKV disease prevented and treated CHIKV in adult and neonate mice, providing the rationale that isolation and administration of CHIKV immunoglobulins may be a safe and effective strategy for CHIKV prophylaxis and/or treatment. Building upon this, studies have investigated the use of mAbs to target specific CHIKV antigens, such as E1 and E2 glycoproteins that block viral attachment and entry into host cells (Bréhin et al., 2008; Selvarajah et al., 2013; Masrinoul et al., 2014; Smith et al., 2015; Weger-Lucarelli et al., 2015; Clayton, 2016). Human mAbs isolated from recovered patients have effectively inhibited CHIKV infection in vitro and in vivo (Selvarajah et al., 2013; Smith et al., 2015). For instance, the human mAb C9, which recognises the acid-sensitive region in CHIKV E2 that is critical for viral fusion and entry into host cells, provided complete protection against CHIKV viremia and CHIKV-induced arthritis when administered prophylactically to CHIKV-infected mice. In addition, when mAb C9 was administered therapeutically to mice at 8 h or 18 h after a lethal dose of CHIKV, all mice were protected against lethality (Selvarajah et al., 2013). This was also demonstrated in immunocompromised Ifnar−/− mice, which were protected from a lethal CHIKV challenge even 60 h post-infection when treated with human mAbs that target the A domain of E2, known to be required for viral fusion (Smith et al., 2015). A study by Pal et al. (2013) screened 230 mouse anti-CHIKV mAbs and identified four mAbs that provided complete protection when administered prophylactically to Ifnar−/− mice (Pal et al., 2013). Interestingly, in humans, it has been demonstrated that anti-CHIKV antibodies have wide-ranging neutralising activity against viruses belonging to different CHIKV genotypes (Smith et al., 2015), and antibodies arising from infection with a closely related non-CHIKV alphavirus could have cross-neutralising activity against CHIKV (Kam et al., 2015).
Despite the therapeutic potential of anti-CHIKV antibodies, it is important to consider that viruses can develop resistance to neutralising antibodies by undergoing genetic mutations to inhibit antigen specificity and antigen–antibody binding (Lee et al., 2011), or through indirect immune evasion strategies, including cell-to-cell transmission, where the virus can directly be transmitted between cells, bypassing the extracellular space (Hahon and Zimmerman, 1970; Lee et al., 2011; reviewed in Jin and Simmons, 2019). A number of neutralisation-escape mutants have been identified for CHIKV, and in vitro studies reported that these can be generated under the selective pressure of mAbs (Lee et al., 2011). Combining neutralising mAbs that target distinct viral epitopes can prevent virus immune escape. Pal et al. (2013) demonstrated that the combination of pairs of mAbs protected mice from lethal doses of CHIKV and extended the treatment window. The most promising pair of mAbs targeted distinct epitopes and was shown to limit the generation of viral resistance, possibly by blocking multiple stages of viral entry. The efficacy of this combination of mAbs was further evaluated in an NHP model of CHIKV infection. The treatment of rhesus macaques with these mAbs reduced viral spread and infection; however, viral RNA persisted in the presence of mAb therapy, which could reflect populations of cells that actively replicate viral RNA (Pal et al., 2014). Therefore, the use of mAbs against CHIKV infection can provide immediate immune-mediated viral suppression, although high viral loads and viral RNA persistence observed in CHIKV disease complicate the use of mAb therapy.
The practical use of antibody-based therapies for viral infections poses several limitations. For example, antibody therapy works best if administered early during the acute phase of the infection when viral loads are high. The administration of exogenous antibodies once an infection is established has little benefit. In addition, the development of antibody-based therapies is expensive. As infectious disease incidences are higher in low-income countries, the adoption of these complex and costly antibody-based therapies would be challenging.
Vaccine development
Many tropical diseases are often neglected as they disproportionally affect low-income populations and do not always, therefore, provide the investment potential that companies developing vaccine candidates demand (Rezza, 2015; Rezza and Weaver, 2019). To date, no vaccine has been licensed to control CHIKV infections. However, CHIKV vaccine development started in the 1960s, soon after the virus was first isolated, and CHIKV vaccine candidates that balance both immunogenicity and safety have been developed and tested in clinical trials. CHIKV has limited antigenic diversity, and, after infection, the production of anti-CHIKV neutralising antibodies is typically enough to protect against re-infection (Kam et al., 2012); therefore, vaccine development continues to be an attractive approach to control urban CHIKV outbreaks.
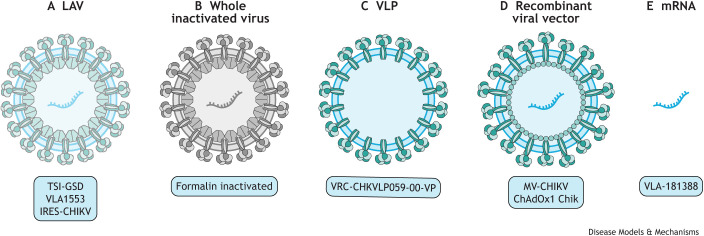
Various strategies have been adopted to develop vaccines and these can be classified as live-attenuated virus (LAV) vaccines, whole inactivated viral vaccines, subunit or virus-like particle (VLP) vaccines, chimeric or recombinant viral-vectored vaccines, and mRNA vaccines. This Review focuses on the recent progress made in vaccine development, in particular, vaccines that have advanced into clinical trials (Table 1 and Fig. 4). Of note, there are currently no whole inactivated vaccines that are being tested in clinical trials for CHIKV, despite early work on a formalin-inactivated CHIKV vaccine that displayed good safety, tolerability and immunogenicity profiles in a phase I clinical trial (Harrison et al., 1971).
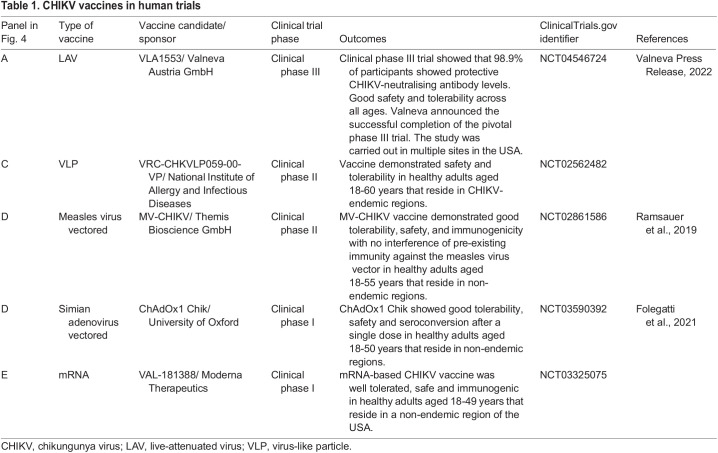
Table 1.
CHIKV vaccines in human trials
Fig. 4.
Several platforms used for the development of CHIKV vaccines that have entered human clinical trial testing. (A) LAV vaccines are derived from CHIKV, with structural changes introduced to reduce its virulence but retain its immunogenicity. The CHIKV LAV vaccines that have reached human clinical trials include TSI-GSD (The Salk Institute-Government Services Division), which showed promising results in phase I and II trials, but, owing to limited funding and uncertainty about safety, the development of this vaccine was terminated (Edelman et al., 2000); the Δ5nsP3 vaccine (VLA1553), which recently entered phase III clinical trial (NCT04546724); and IRES-CHIKV, which is in its late preclinical stages, in which immunogenicity and efficacy have been tested in mice and non-human primates (Plante et al., 2011; 2015). (B) The use of whole inactivated virus via chemical treatment, with formalin, was one of the first strategies for the development of a vaccine for CHIKV. The formalin-inactivated CHIKV vaccine entered clinical phase I (Harrison et al., 1971); however, despite the good safety, tolerability and immunogenicity profiles, the vaccine programme was discontinued. (C) VLP vaccines closely resemble the wild-type virus by containing self-assembled structural proteins but no viral genetic material. A CHIKV VLP vaccine candidate, VRC-CHKVLP059-00-VP, has now advanced into clinical phase II evaluation (NCT02562482) (Akahata et al., 2010). (D) Chimeric or recombinant viral-vectored vaccines are obtained by incorporating genetic elements of CHIKV into a vector virus genome. The recombinant viral-vectored vaccine MV-CHIKV, which uses attenuated measles virus as a vector, is currently in clinical phase II (NCT02861586), and ChAdOx1 Chik vaccine, known as the chimpanzee adenovirus Oxford 1 as it uses the Simian adenovirus as a vector, is in phase I of a clinical trial (NCT03590392). (E) mRNA vaccines are the newest strategy for the development of CHIKV vaccine candidates, based on the delivery of engineered mRNAs that can instruct host cells to express viral antigens, mimicking a viral infection that could elicit an immune response and lead to the generation of antibodies. There is currently one CHIKV mRNA vaccine, VLA-181388, in phase I clinical trial (NCT03325075) (Goyal et al., 2018). CHIKV, chikungunya virus; LAV, live-attenuated virus; VLP, virus-like particle.
LAVs are derived from CHIKV, with structural changes introduced to reduce its virulence but retain its immunogenicity. LAV vaccine candidates contain very-specific mutations or alterations of the original virus genome that increase specificity, improve safety and offer high immunogenicity, providing protection with one single dose of the vaccine. Early immune responses to CHIKV are activated by viral nsPs; therefore, several LAV vaccines have been designed to target nsPs. A recent paper on the LAV vaccine Δ5nsP3, in which the replicase region of nsP3 was deleted, showed that this vaccine generated a strong, long-lasting and protective antibody response upon a single immunisation in Cynomolgus macaques. This vaccine also generated significant IFN-γ T-cell responses and activation of specific CD4+ T cells, which are required for B-cell maturation, and long-lived B cells that correlate with a long-lasting protective effects (Roques et al., 2017). Of note, the Δ5nsP3 vaccine candidate named VLA1553 has recently advanced to clinical phase III trials, having previously shown good immunogenicity and safety profiles in healthy adults (NCT04546724). Several other strategies to attenuate CHIKV have been exploited for vaccine development, such as complete capsid deletion (Zhang et al., 2019), the introduction of point mutations in nsPs (Chan et al., 2019), and the replacement of the subgenomic promoter in a cDNA CHIKV clone by the internal ribosome entry site (IRES) from encephalomyocarditis virus (Plante et al., 2011). Owing to its high immunogenic profile, LAV is the platform with one of the best prospects.
Another successful vaccine platform is the VLP vaccines that closely resemble the wild-type virus by containing self-assembled structural proteins but no viral genetic material. VLPs are highly immunogenic, typically elicit high-titre neutralising antibodies, and are considered to be safe as they are non-replicative, non-infectious viral constructs (Akahata et al., 2010; DeZure et al., 2016). A CHIKV VLP candidate vaccine, VRC-CHKVLP059-00-VP, has now advanced into clinical phase II evaluation (NCT02562482) following reassuring preclinical and early clinical evaluation (Akahata et al., 2010). This vaccine is produced in mammalian cells (HEK293 cells) by transfecting expression vectors encoding CHIKV structural proteins (C-E3-E2-6K-E1) from a West African CHIKV strain. This results in the production of VLPs that mimic wild-type virus with E1/E2 glycoproteins. Immunisation of NHPs with VLPs resulted in high neutralising antibody titres, which protected the adult rhesus macaques against viremia after high-dose challenge (Akahata et al., 2010). A potential drawback of this vaccine platform is that VLPs require multiple dosages to induce maximal immunogenicity, which in turn can increase the cost of manufacturing. Additionally, phase I and II clinical trials are carried out in adults between the ages of 18 and 60 years of age, which introduces a knowledge gap regarding the CHIKV VLP vaccine efficacy in the elderly. A study by Arévalo et al. (2019) combined CHIKV VLP vaccine with different adjuvants to enhance immunogenicity and protection in adult and aged mice. Results showed that VLP vaccine alone was able to protect adult mice against CHIKV disease; however, the disease was exacerbated in aged mice when vaccinated with VLP alone or with an adjuvant. This study suggests the need for an improved or alternative vaccine approach to protect the elderly against CHIKV infections (Arévalo et al., 2019).
Chimeric or recombinant viral-vectored vaccines are obtained by incorporating genetic elements of CHIKV into a vector virus genome. Several different virus vectors have been used for CHIKV vaccine development and include measles virus, alphavirus, vaccinia virus, adenovirus and vesiculovirus. Recombinant viral-vectored vaccines are live replicating viruses with avirulent vectors, which make these vaccines safe and highly immunogenic. The most developed of these vaccines for CHIKV is based on the measles virus vector (MV). The MV-CHIKV vaccine uses attenuated measles virus, which contains a large structural coding gene (C-E3-E2-6K-E1) derived from the East/Central/South African (ECSA) CHIKV strain. MV-CHIKV vaccine has performed well in clinical phases I and II, demonstrating good tolerability, safety and immunogenicity with no interference of pre-existing immunity against the MV (Ramsauer et al., 2015; 2019). NHP models can further demonstrate vaccine efficacy before proceeding into pivotal phase III clinical trials, as they closely mimic human CHIKV infection responses. Studies have revealed that cynomolgus macaques vaccinated with MV-CHIKV demonstrated a robust immune response and protection from any clinical symptoms of the disease and viremia after challenge with wild-type CHIKV (Rossi et al., 2019). However, important aspects, such as the contribution of T cells and the length of the immune responses, were not addressed in this study and are critical for the progression of this vaccine. Nevertheless, MV-CHIKV is a promising vaccine candidate for CHIKV.
The newest category of vaccine to advance into clinical trials is mRNA vaccine, currently used for SARS-CoV-2 (Jackson et al., 2020). One approach for using this platform is based on the delivery of engineered mRNAs that can instruct host cells to express viral antigens, mimicking a viral infection that could elicit an immune response and lead to the generation of antibodies. Moderna Therapeutics have developed an mRNA vaccine candidate for CHIKV, VAL-181388, that is currently in a phase I clinical trial (NCT03325075). No preclinical data have been published, but it has been reported that a single dose of CHIKV mRNA vaccine protected mice from infection and generated a robust antibody response in NHPs (Goyal et al., 2018). The recent development of COVID-19 vaccines may, hopefully, instigate a new era for the development and approval of vaccines for CHIKV and other neglected infectious diseases that disproportionally affect low-income regions.
Knowledge of the lineage-specific variations (Box 3) in virulence and cross-protection among CHIKV strains is an important consideration when developing disease prevention therapies, such as vaccines, and other treatments. A study by Langsjoen et al. (2018) showed that in type I IFN receptor knockout mice, distinct CHIKV lineages differ in their virulence. West African (WA) strains produced higher levels of viremia than ECSA, Indian Ocean lineage (IOL) and Asian/American strains, whereas the Asian/American strains, in general, appeared to be more attenuated than WA, ECSA and Asian strains. In addition, the IOL-derived, LAV strain, IRES-CHIKV, provided protection in mice and NHPs against the Asian/American strain and WA strain, suggesting that the IOL-based vaccine offers cross-protection against strains from other CHIKV lineages (Langsjoen et al., 2018). This is in accordance with the general opinion that CHIKV has a single serotype (Volk et al., 2010; Chua et al., 2016; Langsjoen et al., 2018). Nevertheless, further research is necessary to correlate the observed variation of CHIKV virulence with pathogenicity and vaccine efficacy.
Vaccine efficacy is traditionally demonstrated in randomised, controlled vaccine phase III trials in an outbreak area (Marshall and Baylor, 2011). However, it is extremely difficult to predict when and where CHIKV outbreaks will happen due to the sporadic epidemiology of this disease (Rezza and Weaver, 2019). The current vaccine candidate in clinical trial III, VLA1553, relied on neutralising antibody titres as the endpoints (NCT04546724); however, this surrogate of protection is still controversial (Yang et al., 2017). Completion of phase III vaccine efficacy trials depends not just on a CHIKV outbreak happening somewhere, but in a specific population that can be enrolled and followed during a longitudinal trial. The ongoing outbreaks of CHIKV worldwide (Bettis et al., 2022) provide some opportunity for carrying out these efficacy trials; however, thus far, vaccine candidates have not been able to leverage these outbreaks for phase III efficacy trials that are required for vaccines to be licensed.
Future perspectives
The spread of CHIKV across the globe confirms that the virus is expanding at a significant rate and has the potential to migrate to new geographic areas. Millions of people in the tropics and subtropics have already been affected or are at high risk of infection, and countries with mild climates may experience severe outbreaks due to the year-round presence of the mosquito vector A. albopictus. Rapid global warming is thought to have long-term implications for the prevention and control of vector-borne diseases, as vectors thrive in warmer conditions (Brady et al., 2013). Climate change is predicted to get worse in the near future, and mathematical models predict a geographical expansion of climates that are suitable for a number of vector-borne diseases, including CHIKV (Fischer et al., 2013; Tjaden et al., 2017; Carlson et al., 2022). In a warming world, there is also increased potential for pathogens and vectors to evolve due to higher replication rates (Fay et al., 2021). Such evolution could play an important part in vector-borne disease emergence, re-emergence and spread. The origin and the extent of a future CHIKV outbreak are difficult to foresee, which underlines the importance of developing effective countermeasures. Currently, no specific antiviral treatments or vaccines are available to prevent CHIKV infection, and the containment measures for future outbreaks mainly rely on the interruption of the transmission chain by controlling vector density and distribution (Rezza and Weaver, 2019).
Effective therapeutic and preventative approaches for CHIKV infection are urgently required. In the area of antiviral therapy, there are several compounds that have been shown to be effective at inhibiting CHIKV replication. However, owing to limited knowledge on CHIKV pathogenesis, viral mutation dynamics and a lack of studies performed in animal models and humans, this has proven insufficient for licencing an antiviral therapy for CHIKV disease.
Neutralising mAbs have proven to be effective against CHIKV infection, but additional research is required to understand the kinetics of CHIKV infection. This will in turn inform medical practitioners of optimum treatment timings, doses and whether mAbs can prevent and/or treat chronic CHIKV-induced arthritis.
Notably, there has been promising progress in the development of vaccine candidates for CHIKV disease. For example, the LAV, VLA1553, has now entered phase III clinical trial. However, licensing a vaccine for CHIKV is challenging due to the amount of funding required to bring a new vaccine to the market.
Despite this, the ongoing COVID-19 pandemic has challenged our paradigm of what is possible in vaccine development, proving that fast vaccine development can be achieved when there is a global effort and sufficient resources. However, what does this signify for the development of CHIKV and other vaccines? For a start, it may establish the use of mRNA vaccines, which until now had not been approved for general use in people. Currently, the CHIKV mRNA vaccine candidate, VAL-181388, is in phase I clinical trial (NCT03325075). This may facilitate efficacy and safety testing in humans, pushing vaccine development forward to the next phase of the clinical trials. However, for a vaccine to be developed at a comparable speed, the infection levels need to be high to enable rapid and large clinical trials, with enormous amounts of funding.
The development of vaccines for CHIKV and other neglected tropical viral diseases is not considered highly profitable by industry. For example, the global market for CHIKV vaccine has been estimated to be ∼US$500 million annually (Valneva Press Release, 2022), whereas the global influenza vaccine market value is estimated to reach US$7.547 billion by 2024 (Global Influenza Vaccine Market Report, 2018-2024). Strategies have been implemented to overcome this problem, including the creation of public and private partnerships, in particular the Coalition for Epidemic Preparedness Innovations (CEPI) (see CEPI ‘Priority diseases’ information), which are essential to incentivise and accelerate vaccine development against emerging infectious diseases (Rezza and Weaver, 2019). Currently, CEPI is providing funding worth US$14.1 million for the development of an inactivated whole virion vaccine against CHIKV (BioPharma Reporter, 2020).
Although important advances have been made in developing novel therapeutics for CHIKV disease, further characterisation of the molecular mechanisms employed by the virus, host–pathogen interactions and inflammatory responses that lead to disease progression are required in order to design better and more targeted approaches.
Footnotes
Funding
The authors acknowledge the funding of the Medical Research Council (MR/S019790), RISTEKDIKTI and the Newton Fund.
References
- Ahola, T. and Merits, A. (2016). Functions of chikungunya virus nonstructural proteins. Chikungunya Virus 75-98. 10.1007/978-3-319-42958-8_6 [DOI] [Google Scholar]
- Akahata, W., Yang, Z.-Y., Andersen, H., Sun, S., Holdaway, H. A., Kong, W.-P., Lewis, M. G., Higgs, S., Rossmann, M. G., Rao, S.et al. (2010). A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat. Med. 16, 334-338. 10.1038/nm.2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arévalo, M. T., Huang, Y., Jones, C. A. and Ross, T. M. (2019). Vaccination with a chikungunya virus-like particle vaccine exacerbates disease in aged mice. PLoS Negl. Trop. Dis. 13, e0007316. 10.1371/journal.pntd.0007316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassetto, M., De Burghgraeve, T., Delang, L., Massarotti, A., Coluccia, A., Zonta, N., Gatti, V., Colombano, G., Sorba, G., Silvestri, R.et al. (2013). Computer-aided identification, design and synthesis of a novel series of compounds with selective antiviral activity against chikungunya virus. Antivir. Res. 98, 12-18. 10.1016/j.antiviral.2013.01.002 [DOI] [PubMed] [Google Scholar]
- Battisti, V., Urban, E. and Langer, T. (2021). Antivirals against the Chikungunya Virus. Viruses 13, 1307. 10.3390/v13071307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettis, A. A., L'Azou Jackson, M., Yoon, I.-K., Breugelmans, J. G., Goios, A., Gubler, D. J. and Powers, A. M. (2022). The global epidemiology of chikungunya from 1999 to 2020: A systematic literature review to inform the development and introduction of vaccines. PLoS Negl. Trop. Dis. 16, e0010069. 10.1371/journal.pntd.0010069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blettery, M., Brunier, L., Polomat, K., Moinet, F., Deligny, C., Arfi, S., Jean-Baptiste, G. and De Bandt, M. (2016). Brief report: management of chronic post-chikungunya rheumatic disease: the martinican experience. Arthritis Rheumatol. 68, 2817-2824. 10.1002/art.39775 [DOI] [PubMed] [Google Scholar]
- Boere, W. A., Benaissa-Trouw, B. J., Harmsen, M., Kraaijeveld, C. A. and Snippe, H. (1983). Neutralizing and non-neutralizing monoclonal antibodies to the E2 glycoprotein of Semliki Forest virus can protect mice from lethal encephalitis. J. Gen. Virol. 64, 1405-1408. 10.1099/0022-1317-64-6-1405 [DOI] [PubMed] [Google Scholar]
- Bouquillard, E. and Combe, B. (2009). A report of 21 cases of rheumatoid arthritis following Chikungunya fever. A mean follow-up of two years. Joint Bone Spine 76, 654-657. 10.1016/j.jbspin.2009.08.005 [DOI] [PubMed] [Google Scholar]
- Brady, O. J., Johansson, M. A., Guerra, C. A., Bhatt, S., Golding, N., Pigott, D. M., Delatte, H., Grech, M. G., Leisnham, P. T., Maciel-De-Freitas, R.et al. (2013). Modelling adult Aedes aegypti and Aedes albopictus survival at different temperatures in laboratory and field settings. Parasit. Vectors 6, 351. 10.1186/1756-3305-6-351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bréhin, A.-C., Rubrecht, L., Navarro-Sanchez, M. E., Maréchal, V., Frenkiel, M.-P., Lapalud, P., Laune, D., Sall, A. A. and Desprã¨S, P. (2008). Production and characterization of mouse monoclonal antibodies reactive to Chikungunya envelope E2 glycoprotein. Virology 371, 185-195. 10.1016/j.virol.2007.09.028 [DOI] [PubMed] [Google Scholar]
- Briolant, S., Garin, D., Scaramozzino, N., Jouan, A. and Crance, J. M. (2004). In vitro inhibition of Chikungunya and Semliki Forest viruses replication by antiviral compounds: synergistic effect of interferon-alpha and ribavirin combination. Antivir. Res. 61, 111-117. 10.1016/j.antiviral.2003.09.005 [DOI] [PubMed] [Google Scholar]
- Brunier, L., Polomat, K., Deligny, C., Dehlinger, V., Numéric, P., Jeanbaptiste, G., Arfi, S. and De Bandt, M. (2016). Chikungunya virus infection in patients on biotherapies. Joint Bone Spine 83, 245-246. 10.1016/j.jbspin.2015.08.004 [DOI] [PubMed] [Google Scholar]
- Burrack, K. S., Montgomery, S. A., Homann, D. and Morrison, T. E. (2015). CD8+ T cells control Ross River virus infection in musculoskeletal tissues of infected mice. J. Immunol. 194, 678-689. 10.4049/jimmunol.1401833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt, F. J., Chen, W., Miner, J. J., Lenschow, D. J., Merits, A., Schnettler, E., Kohl, A., Rudd, P. A., Taylor, A., Herrero, L. J.et al. (2017). Chikungunya virus: an update on the biology and pathogenesis of this emerging pathogen. Lancet Infect. Dis. 17, e107-e117. 10.1016/S1473-3099(16)30385-1 [DOI] [PubMed] [Google Scholar]
- Carlson, C. J., Albery, G. F., Merow, C., Trisos, C. H., Zipfel, C. M., Eskew, E. A., Olival, K. J., Ross, N. and Bansal, S. (2022). Climate change increases cross-species viral transmission risk. Nature 607, 555-562. 10.1038/s41586-022-04788-w [DOI] [PubMed] [Google Scholar]
- Chaaitanya, I. K., Muruganandam, N., Sundaram, S. G., Kawalekar, O., Sugunan, A. P., Manimunda, S. P., Ghosal, S. R., Muthumani, K. and Vijayachari, P. (2011). Role of proinflammatory cytokines and chemokines in chronic arthropathy in CHIKV infection. Viral Immunol. 24, 265-271. 10.1089/vim.2010.0123 [DOI] [PubMed] [Google Scholar]
- Chan, Y.-H., Teo, T. H., Utt, A., Tan, J. J. L., Amrun, S. N., Abu Bakar, F., Yee, W. X., Becht, E., Lee, C. Y. P., Lee, B.et al. (2019). Mutating chikungunya virus non-structural protein produces potent live-attenuated vaccine candidate. EMBO Mo. Med 11, e10092. 10.15252/emmm.201810092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, A. Y., Encinales, L., Porras, A., Pacheco, N., Reid, S. P., Martins, K. A. O., Pacheco, S., Bravo, E., Navarno, M., Rico Mendoza, A.et al. (2018). Frequency of chronic joint pain following chikungunya virus infection: a colombian cohort study. Arthritis Rheumatol. 70, 578-584. 10.1002/art.40384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee, S., Kumar, S., Mamidi, P., Datey, A., Sengupta, S., Mahish, C., Laha, E., De, S., Keshry, S. S., Nayak, T. K.et al. (2022). DNA damage response signaling is crucial for effective chikungunya virus replication. J. Virol. 96, e0133422. 10.1128/jvi.01334-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W., Foo, S.-S., Taylor, A., Lulla, A., Merits, A., Hueston, L., Forwood, M. R., Walsh, N. C., Sims, N. A., Herrero, L. J.et al. (2015). Bindarit, an inhibitor of monocyte chemotactic protein synthesis, protects against bone loss induced by chikungunya virus infection. J. Virol. 89, 581-593. 10.1128/JVI.02034-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra, A., Anuradha, V., Ghorpade, R. and Saluja, M. (2012). Acute Chikungunya and persistent musculoskeletal pain following the 2006 Indian epidemic: a 2-year prospective rural community study. Epidemiol. Infect. 140, 842-850. 10.1017/S0950268811001300 [DOI] [PubMed] [Google Scholar]
- Chopra, A., Saluja, M. and Venugopalan, A. (2014). Effectiveness of chloroquine and inflammatory cytokine response in patients with early persistent musculoskeletal pain and arthritis following chikungunya virus infection. Arthritis Rheumatol. 66, 319-326. 10.1002/art.38221 [DOI] [PubMed] [Google Scholar]
- Chow, A., Her, Z., Ong, E. K. S., Chen, J.-, Dimatatac, F., Kwek, D. J. C., Barkham, T., Yang, H., Rénia, L., Leo, Y.-S.et al. (2011). Persistent arthralgia induced by chikungunya virus infection is associated with interleukin-6 and granulocyte macrophage colony-stimulating factor. J. Infect. Dis. 203, 149-157. 10.1093/infdis/jiq042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chretien, J.-P., Anyamba, A., Bedno, S. A., Breiman, R. F., Sang, R., Sergon, K., Powers, A. M., Onyango, C. O., Small, J., Tucker, C. J.et al. (2007). Drought-associated chikungunya emergence along coastal East Africa. Am. J. Trop. Med. Hyg. 76, 405-407. 10.4269/ajtmh.2007.76.405 [DOI] [PubMed] [Google Scholar]
- Chua, C.-L., Sam, I.-C., Merits, A. and Chan, Y.-F. (2016). Antigenic variation of East/Central/South African and asian chikungunya virus genotypes in neutralization by immune sera. PLoS Negl. Trop. Dis. 10, e0004960. 10.1371/journal.pntd.0004960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua, C.-L., Sam, I.-C., Chiam, C.-W. and Chan, Y.-F. (2017). The neutralizing role of IgM during early Chikungunya virus infection. PLoS ONE 12, e0171989. 10.1371/journal.pone.0171989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton, A. M. (2016). Monoclonal antibodies as prophylactic and therapeutic agents against chikungunya virus. J. Infect. Dis. 214 Suppl. 5, S506-S509. 10.1093/infdis/jiw324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couderc, T., Chrétien, F., Schilte, C., Disson, O., Brigitte, M., Guivel-Benhassine, F., Touret, Y., Barau, G., Cayet, N., Schuffenecker, I.et al. (2008). A mouse model for chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog. 4, e29. 10.1371/journal.ppat.0040029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, P. K., Puusepp, L., Varghese, F. S., Utt, A., Ahola, T., Kananovich, D. G., Lopp, M., Merits, A. and Karelson, M. (2016). Design and validation of novel chikungunya virus protease inhibitors. Antimicrob. Agents Chemother. 60, 7382-7395. 10.1128/AAC.01421-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport, B. J., Bullock, C., Mccarthy, M. K., Hawman, D. W., Murphy, K. M., Kedl, R. M., Diamond, M. S. and Morrison, T. E. (2020). Chikungunya virus evades antiviral CD8+ T cell responses to establish persistent infection in joint-associated tissues. J. Virol. 94, e02019-e02036. 10.1128/JVI.02036-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Caluwé, L., Coppens, S., Vereecken, K., Daled, S., Dhaenens, M., Van Ostade, X., Deforce, D., Ariën, K. K. and Bartholomeeusen, K. (2021). The CD147 protein complex is involved in entry of chikungunya virus and related alphaviruses in human cells. Front. Microbiol. 12, 615165. 10.3389/fmicb.2021.615165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lamballerie, X., Boisson, V., Reynier, J.-C., Enault, S., Charrel, R. N., Flahault, A., Roques, P., Grand, R. L., et al. (2008). On chikungunya acute infection and chloroquine treatment. Vector Borne Zoonotic Dis. 8, 837-839. [DOI] [PubMed] [Google Scholar]
- Delang, L., Segura Guerrero, N., Tas, A., Quérat, G., Pastorino, B., Froeyen, M., Dallmeier, K., Jochmans, D., Herdewijn, P., Bello, F.et al. (2014). Mutations in the chikungunya virus non-structural proteins cause resistance to favipiravir (T-705), a broad-spectrum antiviral. J. Antimicrob. Chemother. 69, 2770-2784. 10.1093/jac/dku209 [DOI] [PubMed] [Google Scholar]
- Dezure, A. D., Berkowitz, N. M., Graham, B. S. and Ledgerwood, J. E. (2016). Whole-inactivated and virus-like particle vaccine strategies for chikungunya virus. J. Infect. Dis. 214 Suppl. 5, S497-S499. 10.1093/infdis/jiw352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mola, A., Peduto, A., La Gatta, A., Delang, L., Pastorino, B., Neyts, J., Leyssen, P., De Rosa, M. and Filosa, R. (2014). Structure-activity relationship study of arbidol derivatives as inhibitors of chikungunya virus replication. Bioorg. Med. Chem. 22, 6014-6025. 10.1016/j.bmc.2014.09.013 [DOI] [PubMed] [Google Scholar]
- Dias, C. N. d. S., Gois, B. M., Lima, V. S., Guerra-Gomes, I. C., Araújo, J. M. G., Gomes, J. A. S., Araújo, D. A. M., Medeiros, I. A., Azevedo, F. d. L. A. A. d., Veras, R. C.et al. (2018). Human CD8 T-cell activation in acute and chronic chikungunya infection. Immunology 155, 499-504. 10.1111/imm.12992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-González, E. E., Kautz, , T. F., Dorantes-Delgado, A., Malo-García, I. R., Laguna-Aguilar, M., Langsjoen, R. M., Chen, R., Auguste, D. I., Sánchez-Casas, R. M., Danis-Lozano, R.et al. (2015). First report of Aedes aegypti transmission of chikungunya virus in the Americas. Am. J. Trop. Med. Hyg. 93, 1325-1329. 10.4269/ajtmh.15-0450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrulle, M., Mousson, L., Moutailler, S., Vazeille, M. and Failloux, A.-B. (2009). Chikungunya virus and aedes mosquitoes: saliva is infectious as soon as two days after oral infection. PLOS ONE 4, e5895. 10.1371/journal.pone.0005895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckei, L., Krieg, S., Bütepage, M., Lehmann, A., Gross, A., Lippok, B., Grimm, A. R., Kümmerer, B. M., Rossetti, G., Lüscher, B.et al. (2017). The conserved macrodomains of the non-structural proteins of Chikungunya virus and other pathogenic positive strand RNA viruses function as mono-ADP-ribosylhydrolases. Sci. Rep. 7, 41746. 10.1038/srep41746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman, R., Edelman, R., Tacket, C. O., Wasserman, S. S., Bodison, S. A. and Mangiafico, J. A. (2000). Phase II safety and immunogenicity study of live chikungunya virus vaccine TSI-GSD-218. Am. J. Trop. Med. Hyg. 62, 681-685. 10.4269/ajtmh.2000.62.681 [DOI] [PubMed] [Google Scholar]
- Ekchariyawat, P., Hamel, R., Bernard, E., Wichit, S., Surasombatpattana, P., Talignani, L., Thomas, F., Choumet, V., Yssel, H., Desprès, P.et al. (2015). Inflammasome signaling pathways exert antiviral effect against Chikungunya virus in human dermal fibroblasts. Infect. Genet. Evol. 32, 401-408. 10.1016/j.meegid.2015.03.025 [DOI] [PubMed] [Google Scholar]
- Emmerich, C. H., Gamboa, L. M., Hofmann, M. C. J., Bonin-Andresen, M., Arbach, O., Schendel, P., Gerlach, B., Hempel, K., Bespalov, A., Dirnagl, U.et al. (2021). Improving target assessment in biomedical research: the GOT-IT recommendations. Nat. Rev. Drug Discov. 20, 64-81. 10.1038/s41573-020-0087-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay, R. L., Ngo, K. A., Kuo, L., Willsey, G. G., Kramer, L. D. and Ciota, A. T. (2021). Experimental evolution of West Nile virus at higher temperatures facilitates broad adaptation and increased genetic diversity. Viruses 13, 1889. 10.3390/v13101889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, D., Thomas, S. M., Suk, J. E., Sudre, B., Hess, A., Tjaden, N. B., Beierkuhnlein, C. and Semenza, J. C. (2013). Climate change effects on Chikungunya transmission in Europe: geospatial analysis of vector's climatic suitability and virus’ temperature requirements. Int. J. Health Geogr. 12, 51. 10.1186/1476-072X-12-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folegatti, P. M., Harrison, K., Preciado-Llanes, L., Lopez, F. R., Bittaye, M., Kim, Y. C., Flaxman, A., Bellamy, D., Makinson, R., Sheridan, J.et al. (2021). A single dose of ChAdOx1 Chik vaccine induces neutralizing antibodies against four chikungunya virus lineages in a phase 1 clinical trial. Nat. Commun. 12, 4636. 10.1038/s41467-021-24906-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, J. M., Roy, V., Gunn, B. M., Huang, L., Edeling, M. A., Mack, M., Fremont, D. H., Doranz, B. J., Johnson, S., Alter, G.et al. (2019). Optimal therapeutic activity of monoclonal antibodies against chikungunya virus requires Fc-FcγR interaction on monocytes. Sci. Immunol. 4, eaav5062. 10.1126/sciimmunol.aav5062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos, K. M., Drusano, G. L., Argenio, D. Z. D. and Brown, A. N. (2016). Chikungunya virus: in vitro response to combination therapy with ribavirin and interferon alfa 2a. J. Infect. Dis. 214, 1192-1197. 10.1093/infdis/jiw358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganu, M. A. and Ganu, A. S. (2011). Post-chikungunya chronic arthritis--our experience with DMARDs over two year follow up. J. Assoc. Physicians India 59, 83-86. [PubMed] [Google Scholar]
- Gardner, J., Anraku, I., Le, T. T., Larcher, T., Major, L., Roques, P., Schroder, W. A., Higgs, S. and Suhrbier, A. (2010). Chikungunya virus arthritis in adult wild-type mice. J. Virol. 84, 8021-8032. 10.1128/JVI.02603-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner, C. L., Burke, C. W., Higgs, S. T., Klimstra, W. B. and Ryman, K. D. (2012). Interferon-alpha/beta deficiency greatly exacerbates arthritogenic disease in mice infected with wild-type chikungunya virus but not with the cell culture-adapted live-attenuated 181/25 vaccine candidate. Virology 425, 103-112. 10.1016/j.virol.2011.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal, M., Chauhan, A., Goyal, V., Jaiswal, N., Singh, S. and Singh, M. (2018). Recent development in the strategies projected for chikungunya vaccine in humans. Drug Des. Devel. Ther. 12, 4195-4206. 10.2147/DDDT.S181574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualberto Cavalcanti, N., Melovilar, K., Branco Pinto Duarte, A. L., Jesus Barreto de Melo Rêgo, M., Cristiny Pereira, M., da Rocha Pitta, I., Diniz Lopes Marques, C. and Galdino da Rocha Pitta, M. (2019). IL-27 in patients with Chikungunya fever: A possible chronicity biomarker? Acta Trop. 196, 48-51. 10.1016/j.actatropica.2019.05.005 [DOI] [PubMed] [Google Scholar]
- Guaraldo, L., Wakimoto, M. D., Ferreira, H., Bressan, C., Calvet, G. A., Pinheiro, G. C., Siqueira, A. M. and Brasil, P. (2018). Treatment of chikungunya musculoskeletal disorders: a systematic review. Expert Rev. Anti Infect. Ther. 16, 333-344. 10.1080/14787210.2018.1450629 [DOI] [PubMed] [Google Scholar]
- Guerrero-Arguero, I., Høj, T. R., Tass, E. S., Berges, B. K. and Robison, R. A. (2020). A comparison of Chikungunya virus infection, progression, and cytokine profiles in human PMA-differentiated U937 and murine RAW264.7 monocyte derived macrophages. PLOS ONE 15, e0230328. 10.1371/journal.pone.0230328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahon, N. and Zimmerman, W. D. (1970). Chikungunya virus infection of cell monolayers by cell-to-cell and extracellular transmission. Appl. Microbiol. 19, 389-391. 10.1128/am.19.2.389-391.1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haist, K. C., Burrack, K. S., Davenport, B. J. and Morrison, T. E. (2017). Inflammatory monocytes mediate control of acute alphavirus infection in mice. PLoS Pathog. 13, e1006748. 10.1371/journal.ppat.1006748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, V. R., Eckels, K. H., Bartelloni, P. J. and Hampton, C. (1971). Production and evaluation of a formalin-killed Chikungunya vaccine. J. Immunol. 107, 643-647. 10.4049/jimmunol.107.3.643 [DOI] [PubMed] [Google Scholar]
- Hashimoto, M., Kamphorst, A. O., Im, S. J., Kissick, H. T., Pillai, R. N., Ramalingam, S. S., Araki, K. and Ahmed, R. (2018). CD8 T cell exhaustion in chronic infection and cancer: opportunities for interventions. Annu. Rev. Med. 69, 301-318. 10.1146/annurev-med-012017-043208 [DOI] [PubMed] [Google Scholar]
- Hawman, D. W., Stoermer, K. A., Montgomery, S. A., Pal, P., Oko, L., Diamond, M. S. and Morrison, T. E. (2013). Chronic joint disease caused by persistent chikungunya virus infection is controlled by the adaptive immune response. J. Virol. 87, 13878-13888. 10.1128/JVI.02666-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Her, Z., Malleret, B., Chan, M., Ong, E. K. S., Wong, S.-C., Kwek, D. J. C., Tolou, H., Lin, R. T. P., Tambyah, P. A., Rénia, L.et al. (2010). Active infection of human blood monocytes by chikungunya virus triggers an innate immune response. J. Immunol. 184, 5903-5913. 10.4049/jimmunol.0904181 [DOI] [PubMed] [Google Scholar]
- Hiroki, C. H., Toller-Kawahisa, J. E., Fumagalli, M. J., Colon, D. F., Figueiredo, L. T. M., Fonseca, B. A. L. D., Franca, R. F. O. and Cunha, F. Q. (2019). Neutrophil extracellular traps effectively control acute chikungunya virus infection. Front. Immunol. 10, 3108. 10.3389/fimmu.2019.03108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoarau, J.-J., Jaffar Bandjee, M.-C., Krejbich Trotot, P., Das, T., Li-Pat-Yuen, G., Dassa, B., Denizot, M., Guichard, E., Ribera, A., Henni, T.et al. (2010). Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J. Immunol. 184, 5914-5927. 10.4049/jimmunol.0900255 [DOI] [PubMed] [Google Scholar]
- Hoofnagle, J. H. and Seeff, L. B. (2006). Peginterferon and ribavirin for chronic hepatitis C. N. Engl. J. Med. 355, 2444-2451. 10.1056/NEJMct061675 [DOI] [PubMed] [Google Scholar]
- Ikeda, N., Asano, K., Kikuchi, K., Uchida, Y., Ikegami, H., Takagi, R., Yotsumoto, S., Shibuya, T., Makino-Okamura, C., Fukuyama, H.et al. (2018). Emergence of immunoregulatory Ym1+Ly6Chi monocytes during recovery phase of tissue injury. Sci. Immunol. 3, eaat0207. 10.1126/sciimmunol.aat0207 [DOI] [PubMed] [Google Scholar]
- Jackson, L. A., Anderson, E. J., Rouphael, N. G., Roberts, P. C., Makhene, M., Coler, R. N., Mccullough, M. P., Chappell, J. D., Denison, M. R., Stevens, L. J.et al. (2020). An mRNA vaccine against SARS-CoV-2 - preliminary report. N Engl. J. Med. 383, 1920-1931. 10.1056/NEJMoa2022483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain, J., Nayak, K., Tanwar, N., Gaind, R., Gupta, B., Shastri, J. S., Bhatnagar, R. K., Kaja, M. K., Chandele, A. and Sunil, S. (2017). Clinical, serological, and virological analysis of 572 chikungunya patients from 2010 to 2013 in India. Clin. Infect. Dis. 65, 133-140. 10.1093/cid/cix283 [DOI] [PubMed] [Google Scholar]
- Jin, J. and Simmons, G. (2019). Antiviral functions of monoclonal antibodies against chikungunya virus. Viruses 11, 305. 10.3390/v11040305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiserman, D., Bird, C. H., Sun, J., Matthews, A., Ung, K., Whisstock, J. C., Thompson, P. E., Trapani, J. A. and Bird, P. I. (2006). The major human and mouse granzymes are structurally and functionally divergent. J. Cell Biol. 175, 619-630. 10.1083/jcb.200606073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiserman, D., Stewart, S. E., Plasman, K., Gevaert, K., Van Damme, P. and Bird, P. I. (2014). Identification of Serpinb6b as a species-specific mouse granzyme A inhibitor suggests functional divergence between human and mouse granzyme A. J. Biol. Chem. 289, 9408-9417. 10.1074/jbc.M113.525808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam, Y.-W., Simarmata, D., Chow, A., Her, Z., Teng, T.-S., Ong, E. K. S., Rénia, L., Leo, Y.-S. and Ng, L. F. P. (2012). Early appearance of neutralizing immunoglobulin G3 antibodies is associated with chikungunya virus clearance and long-term clinical protection. J. Infect. Dis. 205, 1147-1154. 10.1093/infdis/jis033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam, Y.-W., Pok, K.-Y., Eng, K. E., Tan, L.-K., Kaur, S., Lee, W. W. L., Leo, Y.-S., Ng, L.-C. and Ng, L. F. P. (2015). Sero-prevalence and cross-reactivity of chikungunya virus specific anti-E2EP3 antibodies in arbovirus-infected patients. PLoS Negl. Trop. Dis. 9, e3445. 10.1371/journal.pntd.0003445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlas, A., Berre, S., Couderc, T., Varjak, M., Braun, P., Meyer, M., Gangneux, N., Karo-Astover, L., Weege, F., Raftery, M.et al. (2016). A human genome-wide loss-of-function screen identifies effective chikungunya antiviral drugs. Nat. Commun. 7, 11320. 10.1038/ncomms11320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur, P., Thiruchelvan, M., Lee, R. C. H., Chen, H., Chen, K. C., Ng, M. L. and Chu, J. J. H. (2013). Inhibition of chikungunya virus replication by harringtonine, a novel antiviral that suppresses viral protein expression. Antimicrob. Agents Chemother. 57, 155-167. 10.1128/AAC.01467-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautz, T. F., Díaz-González, E. E., Erasmus, J. H., Malo-García, I. R., Langsjoen, R. M., Patterson, E. I., Auguste, D. I., Forrester, N. L., Sanchez-Casas, R. M., Hernández-Ávila, M.et al. (2015). Chikungunya virus as cause of febrile illness outbreak, chiapas, Mexico, 2014. Emerg. Infect. Dis. 21, 2070-2073. 10.3201/eid2111.150546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelvin, A. A., Banner, D., Silvi, G., Moro, M. L., Spataro, N., Gaibani, P., Cavrini, F., Pierro, A., Rossini, G., Cameron, M. J.et al. (2011). Inflammatory cytokine expression is associated with chikungunya virus resolution and symptom severity. PLoS Negl. Trop. Dis. 5, e1279. 10.1371/journal.pntd.0001279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, M., Santhosh, S. R., Tiwari, M., Lakshmana Rao, P. V. and Parida, M. (2010). Assessment of in vitro prophylactic and therapeutic efficacy of chloroquine against Chikungunya virus in vero cells. J. Med. Virol. 82, 817-824. 10.1002/jmv.21663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer, M. U. G., Sinka, M. E., Duda, K. A., Mylne, A. Q. N., Shearer, F. M., Barker, C. M., Moore, C. G., Carvalho, R. G., Coelho, G. E., Van Bortel, W.et al. (2015). The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife 4, e08347. 10.7554/eLife.08347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labadie, K., Larcher, T., Joubert, C., Mannioui, A., Delache, B., Brochard, P., Guigand, L., Dubreil, L., Lebon, P., Verrier, B.et al. (2010). Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J. Clin. Invest. 120, 894-906. 10.1172/JCI40104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, P. O., Loulergue, P. and Aspinall, R. (2017). Chikungunya virus infection: why should U.S. geriatricians be aware of it? J. Am. Geriatr. Soc. 65, 2529-2534. 10.1111/jgs.15104 [DOI] [PubMed] [Google Scholar]
- Langsjoen, R. M., Haller, S. L., Roy, C. J., Vinet-Oliphant, H., Bergren, N. A., Erasmus, J. H., Livengood, J. A., Powell, T. D., Weaver, S. C. and Mahalingam, S. (2018). Chikungunya virus strains show lineage-specific variations in virulence and cross-protective ability in murine and nonhuman primate models. mBio 9, e02449-17. 10.1128/mBio.02449-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C. Y., Kam, Y.-W., Fric, J., Malleret, B., Koh, E. G. L., Prakash, C., Huang, W., Lee, W. W. L., Lin, C., Lin, R. T. P.et al. (2011). Chikungunya virus neutralization antigens and direct cell-to-cell transmission are revealed by human antibody-escape mutants. PLoS Pathog. 7, e1002390. 10.1371/journal.ppat.1002390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohachanakul, J., Phuklia, W., Thannagith, M., Thongsakulprasert, T., Smith, D. R. and Ubol, S. (2015). Differences in response of primary human myoblasts to infection with recent epidemic strains of Chikungunya virus isolated from patients with and without myalgia. J. Med. Virol. 87, 733-739. 10.1002/jmv.24081 [DOI] [PubMed] [Google Scholar]
- Long, K. M. and Heise, M. T. (2015). Protective and pathogenic responses to chikungunya virus infection. Curr. Trop. Med. Rep. 2, 13-21. 10.1007/s40475-015-0037-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke, T. C., Casadevall, A., Watowich, S. J., Hoffman, S. L., Beigel, J. H. and Burgess, T. H. (2010). Hark back: passive immunotherapy for influenza and other serious infections. Crit. Care Med. 38 Suppl. 4, e66-e73. 10.1097/CCM.0b013e3181d44c1e [DOI] [PubMed] [Google Scholar]
- Lum, F.-M., Teo, T.-H., Lee, W. W. L., Kam, Y.-W., Rénia, L. and Ng, L. F. P. (2013). An essential role of antibodies in the control of chikungunya virus infection. J. Immunol. 190, 6295-6302. 10.4049/jimmunol.1300304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madariaga, M., Ticona, E. and Resurrecion, C. (2016). Chikungunya: bending over the Americas and the rest of the world. Braz. J. Infect. Dis. 20, 91-98. 10.1016/j.bjid.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques, C. D. L., Duarte, A. L. B. P., Ranzolin, A., Dantas, A. T., Cavalcanti, N. G., Gonçalves, R. S. G., Junior, L. F. R., Valadares, L. D. A., Melo, A. K. G., Freire, E. A. M.et al. (2017). Recommendations of the Brazilian Society of Rheumatology for the diagnosis and treatment of chikungunya fever. Part 2 – Treatment. Rev. Bras. Reumatol. 57, 438-451. 10.1016/j.rbre.2017.06.004 [DOI] [PubMed] [Google Scholar]
- Marshall, V. and Baylor, N. W. (2011). Food and Drug Administration regulation and evaluation of vaccines. Pediatrics 127 Suppl. 1, S23-S30. 10.1542/peds.2010-1722E [DOI] [PubMed] [Google Scholar]
- Martí-Carvajal, A., Ramon-Pardo, P., Javelle, E., Simon, F., Aldighieri, S., Horvath, H., Rodriguez-Abreu, J. and Reveiz, L. (2017). Interventions for treating patients with chikungunya virus infection-related rheumatic and musculoskeletal disorders: A systematic review. PLoS One 12, e0179028. 10.1371/journal.pone.0179028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masrinoul, P., Puiprom, O., Tanaka, A., Kuwahara, M., Chaichana, P., Ikuta, K., Ramasoota, P. and Okabayashi, T. (2014). Monoclonal antibody targeting chikungunya virus envelope 1 protein inhibits virus release. Virology 464-465, 111-117. 10.1016/j.virol.2014.05.038 [DOI] [PubMed] [Google Scholar]
- Mccarthy, M. K. and Morrison, T. E. (2017). Persistent RNA virus infections: do PAMPS drive chronic disease? Curr. Opin. Virol. 23, 8-15. 10.1016/j.coviro.2017.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michlmayr, D., Pak, T. R., Rahman, A. H., Amir, E. €. A. D., Kim, E. Y., Kim-Schulze, S., Suprun, M., Stewart, M. G., Thomas, G. P., Balmaseda, A.et al. (2018). Comprehensive innate immune profiling of chikungunya virus infection in pediatric cases. Mol. Syst. Biol. 14, e7862. 10.15252/msb.20177862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner, J. J., Cook, L. E., Hong, J. P., Smith, A. M., Richner, J. M., Shimak, R. M., Young, A. R., Monte, K., Poddar, S., Crowe, J. E.et al. (2017). Therapy with CTLA4-Ig and an antiviral monoclonal antibody controls chikungunya virus arthritis. Sci. Transl. Med. 9, 375. 10.1126/scitranslmed.aah3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan, A., Kiran, D. H. N., Manohar, C. I. and Kumar, P. D. (2010). Epidemiology, clinical manifestations, and diagnosis of chikungunya fever: lessons learned from the re-emerging epidemic. Indian J. Dermatol. 55, 54-63. 10.4103/0019-5154.60355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller-Tank, S., Kondratowicz, A. S., Davey, R. A., Rennert, P. D. and Maury, W. (2013). Role of the phosphatidylserine receptor TIM-1 in enveloped-virus entry. J. Virol. 87, 8327-8341. 10.1128/JVI.01025-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montelongo-Jauregui, D., Vila, T., Sultan, A. S. and Jabra-Rizk, M. A. (2020). Convalescent serum therapy for COVID-19: A 19th century remedy for a 21st century disease. PLoS Pathog. 16, 8. 10.1371/journal.ppat.1008735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison, T. E., Oko, L., Montgomery, S. A., Whitmore, A. C., Lotstein, A. R., Gunn, B. M., Elmore, S. A. and Heise, M. T. (2011). A mouse model of chikungunya virus–induced musculoskeletal inflammatory disease: evidence of arthritis, tenosynovitis, myositis, and persistence. Am. J. Pathol. 178, 32-40. 10.1016/j.ajpath.2010.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounce, B. C., Cesaro, T., Carrau, L., Vallet, T. and Vignuzzi, M. (2017). Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding. Antivir. Res. 142, 148-157. 10.1016/j.antiviral.2017.03.014 [DOI] [PubMed] [Google Scholar]
- Mueller, S. N. and Ahmed, R. (2009). High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. U.S.A. 106, 8623-8628. 10.1073/pnas.0809818106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, L. F. P., Chow, A., Sun, Y.-J., Kwek, D. J. C., Lim, P.-L., Dimatatac, F., Ng, L.-C., Ooi, E.-E., Choo, K.-H., Her, Z.et al. (2009). IL-1β, IL-6, and RANTES as biomarkers of chikungunya severity. PLoS ONE 4, e4261. 10.1371/journal.pone.0004261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noret, M., Herrero, L., Rulli, N., Rolph, M., Smith, P. N., Li, R. W., Roques, P., Gras, G. and Mahalingam, S. (2012). Interleukin 6, RANKL, and osteoprotegerin expression by chikungunya virus-infected human osteoblasts. J. Infect. Dis. 206, 455-457. 10.1093/infdis/jis368 [DOI] [PubMed] [Google Scholar]
- Nuckols, J. T., Ziegler, S. A., Huang, Y.-J. S., Mcauley, A. J., Vanlandingham, D. L., Klowden, M. J., Spratt, H., Davey, R. A. and Higgs, S. (2013). Infection of Aedes albopictus with chikungunya virus rectally administered by enema. Vector Borne Zoonotic Dis. 13, 103-110. 10.1089/vbz.2012.1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olagnier, D., Scholte, F. E. M., Chiang, C., Albulescu, I. C., Nichols, C., He, Z., Lin, R., Snijder, E. J., Van Hemert, M. J. and Hiscott, J. (2014). Inhibition of dengue and chikungunya virus infections by RIG-I-mediated type I interferon-independent stimulation of the innate antiviral response. J. Virol. 88, 4180-4194. 10.1128/JVI.03114-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onomoto, K., Onoguchi, K. and Yoneyama, M. (2021). Regulation of RIG-I-like receptor-mediated signaling: interaction between host and viral factors. Cell. Mol. Immunol. 18, 539-555. 10.1038/s41423-020-00602-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozden, S., Huerre, M., Riviere, J.-P., Coffey, L. L., Afonso, P. V., Mouly, V., De Monredon, J., Roger, J.-C., El Amrani, M., Yvin, J.-L.et al. (2007). Human muscle satellite cells as targets of Chikungunya virus infection. PloS One 2, e527. 10.1371/journal.pone.0000527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paixão, E. S., Rodrigues, L. C., Costa, M. C. N., Itaparica, M., Barreto, F., Gérardin, P. and Teixeira, M. G. (2018). Chikungunya chronic disease: a systematic review and meta-analysis. Trans. R. Soc. Trop. Med. Hyg. 112, 301-316. 10.1093/trstmh/try063 [DOI] [PubMed] [Google Scholar]
- Pal, P., Dowd, K. A., Brien, J. D., Edeling, M. A., Gorlatov, S., Johnson, S., Lee, I., Akahata, W., Nabel, G. J., Richter, M. K. S.et al. (2013). Development of a highly protective combination monoclonal antibody therapy against chikungunya virus. PLoS Pathog. 9, e1003312. 10.1371/journal.ppat.1003312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal, P., Fox, J. M., Hawman, D. W., Huang, Y.-J. S., Messaoudi, I., Kreklywich, C., Denton, M., Legasse, A. W., Smith, P. P., Johnson, S.et al. (2014). Chikungunya viruses that escape monoclonal antibody therapy are clinically attenuated, stable, and not purified in mosquitoes. J. Virol. 88, 8213-8226. 10.1128/JVI.01032-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palha, N., Guivel-Benhassine, F., Briolat, V., Lutfalla, G., Sourisseau, M., Ellett, F., Wang, C.-H., Lieschke, G. J., Herbomel, P., Schwartz, O.et al. (2013). Real-time whole-body visualization of chikungunya virus infection and host interferon response in zebrafish. PLoS Pathog. 9, e1003619. 10.1371/journal.ppat.1003619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, W. H. and Freeman, R. G. (1926). The prophylactic use of measles convalescent serum. J. Am. Med. Assoc. 87, 556-558. 10.1001/jama.1926.02680080022009 [DOI] [Google Scholar]
- Phuklia, W., Kasisith, J., Modhiran, N., Rodpai, E., Thannagith, M., Thongsakulprasert, T., Smith, D. R. and Ubol, S. (2013). Osteoclastogenesis induced by CHIKV-infected fibroblast-like synoviocytes: a possible interplay between synoviocytes and monocytes/macrophages in CHIKV-induced arthralgia/arthritis. Virus Res. 177, 179-188. 10.1016/j.virusres.2013.08.011 [DOI] [PubMed] [Google Scholar]
- Plante, K., Wang, E., Partidos, C. D., Weger, J., Gorchakov, R., Tsetsarkin, K., Borland, E. M., Powers, A. M., Seymour, R., Stinchcomb, D. T.et al. (2011). Novel chikungunya vaccine candidate with an IRES-based attenuation and host range alteration mechanism. PLoS Pathog. 7, e1002142. 10.1371/journal.ppat.1002142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plante, K. S., Rossi, S. L., Bergren, N. A., Seymour, R. L. and Weaver, S. C. (2015). Extended preclinical safety, efficacy and stability testing of a live-attenuated chikungunya vaccine candidate. PLoS Negl. Trop. Dis. 9, e0004007. 10.1371/journal.pntd.0004007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohjala, L., Utt, A., Varjak, M., Lulla, A., Merits, A., Ahola, T. and Tammela, P. (2011). Inhibitors of alphavirus entry and replication identified with a stable chikungunya replicon cell line and virus-based assays. PLOS ONE 6, e28923. 10.1371/journal.pone.0028923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo, Y. S., Nakaya, H., Gardner, J., Larcher, T., Schroder, W. A., Le, T. T., Major, L. D. and Suhrbier, A. (2014a). CCR2 deficiency promotes exacerbated chronic erosive neutrophil-dominated chikungunya virus arthritis. J. Virol. 88, 6862-6872. 10.1128/JVI.03364-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo, Y. S., Rudd, P. A., Gardner, J., Wilson, J. A. C., Larcher, T., Colle, M.-A., Le, T. T., Nakaya, H. I., Warrilow, D., Allcock, R.et al. (2014b). Multiple immune factors are involved in controlling acute and chronic chikungunya virus infection. PLoS Negl. Trop. Dis. 8, e3354. 10.1371/journal.pntd.0003354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers, A. M. (2017). Vaccine and therapeutic options to control chikungunya virus. Clin. Microbiol. Rev. 31, e00104-16. 10.1128/CMR.00104-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priya, R., Patro, I. K. and Parida, M. M. (2014). TLR3 mediated innate immune response in mice brain following infection with Chikungunya virus. Virus Res. 189, 194-205. 10.1016/j.virusres.2014.05.010 [DOI] [PubMed] [Google Scholar]
- Prow, N. A., Tang, B., Gardner, J., Le, T. T., Taylor, A., Poo, Y. S., Nakayama, E., Hirata, T. D. C., Nakaya, H. I., Slonchak, A.et al. (2017). Lower temperatures reduce type I interferon activity and promote alphaviral arthritis. PLoS Pathog. 13, e1006788. 10.1371/journal.ppat.1006788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puntasecca, C. J., King, C. H. and Labeaud, A. D. (2021). Measuring the global burden of chikungunya and Zika viruses: a systematic review. PLoS Negl. Trop. Dis. 15, e0009055. 10.1371/journal.pntd.0009055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz, S. G. and Adler, W. H. (1973). Host defenses during primary Venezuelan equine encephalomyelitis virus infection in mice. I. Passive transfer of protection with immune serum and immune cells. J. Immunol. 110, 1345-1353. 10.4049/jimmunol.110.5.1345 [DOI] [PubMed] [Google Scholar]
- Rahman, M. M. and Mcfadden, G. (2006). Modulation of tumor necrosis factor by microbial pathogens. PLoS Pathog. 2, e4. 10.1371/journal.ppat.0020004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsauer, K., Schwameis, M., Firbas, C., Müllner, M., Putnak, R. J., Thomas, S. J., Desprès, P., Tauber, E., Jilma, B. and Tangy, F. (2015). Immunogenicity, safety, and tolerability of a recombinant measles-virus-based chikungunya vaccine: a randomised, double-blind, placebo-controlled, active-comparator, first-in-man trial. Lancet. Infect. Dis. 15, 519-527. 10.1016/S1473-3099(15)70043-5 [DOI] [PubMed] [Google Scholar]
- Ramsauer, K., Reisinger, E., Firbas, C., Wiedermann-Schmidt, U., Beubler, E., Pfeiffer, A., Müllner, M., Aberle, J. and Tauber, E. (2019). Phase 2 clinical results: Chikungunya vaccine based on measles vector (MV-CHIK) induces humoral and cellular responses in the presence of pre-existing anti measles immunity. Int. J. Infect. Dis. 79, 118. 10.1016/j.ijid.2018.11.291 [DOI] [Google Scholar]
- Ravichandran, R. and Manian, M. (2008). Ribavirin therapy for Chikungunya arthritis. J. Infecti. Dev. Countries 2, 140-142. 10.3855/T2.2.140 [DOI] [PubMed] [Google Scholar]
- Ravindran, V. and Alias, G. (2017). Efficacy of combination DMARD therapy vs. hydroxychloroquine monotherapy in chronic persistent chikungunya arthritis: a 24-week randomized controlled open label study. Clin. Rheumatol. 36, 1335-1340. 10.1007/s10067-016-3429-0 [DOI] [PubMed] [Google Scholar]
- Rawle, D. J., Le, T. T., Dumenil, T., Bishop, C., Yan, K., Nakayama, E., Bird, P. I. and Suhrbier, A. (2022). Widespread discrepancy in Nnt genotypes and genetic backgrounds complicates granzyme A and other knockout mouse studies. eLife 11, e70207. 10.7554/eLife.70207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezza, G. (2015). Do we need a vaccine against chikungunya? Pathog. Glob. Health 109, 170-173. 10.1179/2047773215Y.0000000017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezza, G. and Weaver, S. C. (2019). Chikungunya as a paradigm for emerging viral diseases: Evaluating disease impact and hurdles to vaccine development. PLoS Negl. Trop. Dis. 13, e0006919. 10.1371/journal.pntd.0006919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezza, G., Nicoletti, L., Angelini, R., Romi, R., Finarelli, A. C., Panning, M., Cordioli, P., Fortuna, C., Boros, S., Magurano, F.et al. (2007). Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet 370, 1840-1846. 10.1016/S0140-6736(07)61779-6 [DOI] [PubMed] [Google Scholar]
- Robinson, M. C. (1955). An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952–53. I. Clinical features. Trans. R. Soc. Trop. Med. Hyg. 49, 28-32. 10.1016/0035-9203(55)90080-8 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Morales, A. J., Cardona-Ospina, J. A., Fernanda Urbano-Garzón, S. and Sebastian Hurtado-Zapata, J. (2016). Prevalence of post-chikungunya infection chronic inflammatory arthritis: a systematic review and meta-analysis. Arthritis Care Res. 68, 1849-1858. 10.1002/acr.22900 [DOI] [PubMed] [Google Scholar]
- Roiz, D., Boussès, P., Simard, F., Paupy, C. and Fontenille, D. (2015). Autochthonous chikungunya transmission and extreme climate events in southern france. PLoS Negl. Trop. Dis. 9, 6. 10.1371/journal.pntd.0003854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roques, P., Ljungberg, K., Kümmerer, B. M., Gosse, L., Dereuddre-Bosquet, N., Tchitchek, N., Hallengärd, D., García-Arriaza, J., Meinke, A., Esteban, M.et al. (2017). Attenuated and vectored vaccines protect nonhuman primates against Chikungunya virus. JCI Insight 2, e83527. 10.1172/jci.insight.83527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roques, P., Thiberville, S.-D., Dupuis-Maguiraga, L., Lum, F.-M., Labadie, K., Martinon, F., Gras, G., Lebon, P., Ng, L., De Lamballerie, X.et al. (2018). Paradoxical effect of chloroquine treatment in enhancing chikungunya virus infection. Viruses 10, 268. 10.3390/v10050268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi, S. L., Comer, J. E., Wang, E., Azar, S. R., Lawrence, W. S., Plante, J. A., Ramsauer, K., Schrauf, S. and Weaver, S. C. (2019). Immunogenicity and efficacy of a measles virus-vectored chikungunya vaccine in nonhuman primates. J. Infect. Dis. 220, 735-742. 10.1093/infdis/jiz202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan, H. A., bahrani, H., Mohamed, Z., Teoh, T. C., Shankar, E. M., Rahman, N. A. and Yusof, R. (2015). A combination of doxycycline and ribavirin alleviated chikungunya infection. PloS One 10, e0126360. 10.1371/journal.pone.0126360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd, P. A., Wilson, J., Gardner, J., Larcher, T., Babarit, C., Le, T. T., Anraku, I., Kumagai, Y., Loo, Y.-M., Gale, M.et al. (2012). Interferon response factors 3 and 7 protect against Chikungunya virus hemorrhagic fever and shock. J. Virol. 86, 9888-9898. 10.1128/JVI.00956-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz Silva, M., Van Der Ende-Metselaar, H., Mulder, H. L., Smit, J. M. and Rodenhuis-Zybert, I. A. (2016). Mechanism and role of MCP-1 upregulation upon chikungunya virus infection in human peripheral blood mononuclear cells. Sci. Rep. 6, 32288. 10.1038/srep32288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulli, N. E., Rolph, M. S., Srikiatkhachorn, A., Anantapreecha, S., Guglielmotti, A. and Mahalingam, S. (2011). Protection from arthritis and myositis in a mouse model of acute chikungunya virus disease by bindarit, an inhibitor of monocyte chemotactic protein-1 synthesis. J. Infect. Dis. 204, 1026-1030. 10.1093/infdis/jir470 [DOI] [PubMed] [Google Scholar]
- S, B., Garin, D., Scaramozzino, N., Jouan, A. and Crance, J. M. (2004). In vitro inhibition of chikungunya and semliki forest viruses replication by antiviral compounds: synergistic effect of interferon-alpha and ribavirin combination, antiviral research. Antiviral Res. 61, 111-117. 10.1016/j.antiviral.2003.09.005 [DOI] [PubMed] [Google Scholar]
- Sahadeo, N., Mohammed, H., Allicock, O. M., Auguste, A. J., Widen, S. G., Badal, K., Pulchan, K., Foster, J. E., Weaver, S. C. and Carrington, C. V. F. (2015). Molecular characterisation of chikungunya virus infections in trinidad and comparison of clinical and laboratory features with dengue and other acute febrile cases. PLoS Negl. Trop. Dis. 9, e0004199. 10.1371/journal.pntd.0004199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarino, A., Boelaert, J. R., Cassone, A., Majori, G. and Cauda, R. (2003). Effects of chloroquine on viral infections: an old drug against today's diseases. Lancet Infect. Dis. 3, 722-727. 10.1016/S1473-3099(03)00806-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanoski, A. S., Le, T. T., Kaiserman, D., Rowe, C., Prow, N. A., Barboza, D. D., Santos, C. A., Zanotto, P. M. A., Magalhães, K. G., Aurelio, L.et al. (2019). Granzyme A in chikungunya and other arboviral infections. Front. Immunol. 10, 3083. 10.3389/fimmu.2019.03083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schett, G. (2007). Cells of the synovium in rheumatoid arthritis. Osteoclasts. Arthritis Res. Ther. 9, 203. 10.1186/ar2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilte, C., Couderc, T., Chretien, F., Sourisseau, M., Gangneux, N., Guivel-Benhassine, F., Kraxner, A., Tschopp, J., Higgs, S., Michault, A.et al. (2010). Type I IFN controls chikungunya virus via its action on nonhematopoietic cells. J. Exp. Med. 207, 429-442. 10.1084/jem.20090851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian, M. R., Lodha, R. and Kabra, S. K. (2009). Chikungunya infection in children. Indian J. Pediatr. 76, 185-189. 10.1007/s12098-009-0049-6 [DOI] [PubMed] [Google Scholar]
- Selvarajah, S., Sexton, N. R., Kahle, K. M., Fong, R. H., Mattia, K.-A., Gardner, J., Lu, K., Liss, N. M., Salvador, B., Tucker, D. F.et al. (2013). A neutralizing monoclonal antibody targeting the acid-sensitive region in chikungunya virus E2 protects from disease. PLoS Negl. Trop. Dis. 7, e2423. 10.1371/journal.pntd.0002423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu, J. F., Martins, D. O. S., Mcphillie, M. J., Roberts, G. C., Zothner, C., Merits, A., Harris, M. and Jardim, A. C. G. (2020). Is the ADP ribose site of the Chikungunya virus NSP3 Macro domain a target for antiviral approaches? Acta Trop. 207, 105490. 10.1016/j.actatropica.2020.105490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, L. A., Khomandiak, S., Ashbrook, A. W., Weller, R., Heise, M. T., Morrison, T. E. and Dermody, T. S. (2014). A single-amino-acid polymorphism in Chikungunya virus E2 glycoprotein influences glycosaminoglycan utilization. J. Virol. 88, 2385-2397. 10.1128/JVI.03116-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, F., Javelle, E., Cabie, A., Bouquillard, E., Troisgros, O., Gentile, G., Leparc-Goffart, I., Hoen, B., Gandjbakhch, F., Rene-Corail, P.et al. (2015). French guidelines for the management of chikungunya (acute and persistent presentations). November 2014. Med. Mal. Infect. 45, 243-263. 10.1016/j.medmal.2015.05.007 [DOI] [PubMed] [Google Scholar]
- Smith, S. A., Silva, L. A., Fox, J. M., Flyak, A. I., Kose, N., Sapparapu, G., Khomandiak, S., Ashbrook, A. W., Kahle, K. M., Fong, R. H.et al. (2015). Isolation and characterization of broad and ultrapotent human monoclonal antibodies with therapeutic activity against chikungunya virus. Cell Host Microbe 18, 86-95. 10.1016/j.chom.2015.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder, A. J. and Mukhopadhyay, S. (2012). The alphavirus E3 glycoprotein functions in a clade-specific manner. J. Virol. 86, 13609-13620. 10.1128/JVI.01805-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourisseau, M., Schilte, C., Casartelli, N., Trouillet, C., Guivel-Benhassine, F., Rudnicka, D., Sol-Foulon, N., Roux, K. L., Prevost, M.-C., Fsihi, H.et al. (2007). Characterization of reemerging chikungunya virus. PLoS Pathog. 3, e89. 10.1371/journal.ppat.0030089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples, J. E., Breiman, R. F. and Powers, A. M. (2009). Chikungunya fever: an epidemiological review of a re-emerging infectious disease. Clin. Infect. Dis. 49, 942-948. 10.1086/605496 [DOI] [PubMed] [Google Scholar]
- Suhrbier, A. and Mahalingam, S. (2009). The immunobiology of viral arthritides. Pharmacol. Ther. 124, 301-308. 10.1016/j.pharmthera.2009.09.005 [DOI] [PubMed] [Google Scholar]
- Teng, T.-S., Foo, S.-S., Simamarta, D., Lum, F.-M., Teo, T.-H., Lulla, A., Yeo, N. K. W., Koh, E. G. L., Chow, A., Leo, Y.-S.et al. (2012). Viperin restricts chikungunya virus replication and pathology. J. Clin. Invest. 122, 4447-4460. 10.1172/JCI63120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng, T.-S., Kam, Y.-W., Lee, B., Hapuarachchi, H. C., Wimal, A., Ng, L.-C. and Ng, L. F. P. (2015). A systematic meta-analysis of immune signatures in patients with acute chikungunya virus infection. J. Infect. Dis. 211, 1925-1935. 10.1093/infdis/jiv049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo, T.-H., lum, F.-M., Claser, C., Lulla, V., Lulla, A., Merits, A., Rénia, L. and Ng, L. F. P. (2013). A pathogenic role for CD4+ T cells during chikungunya virus infection in mice. J. Immunol. 190, 259-269. 10.4049/jimmunol.1202177 [DOI] [PubMed] [Google Scholar]
- Teo, T.-H., Chan, Y.-H., Lee, W. W. L., Lum, F.-M., Amrun, S. N., Her, Z., Rajarethinam, R., Merits, A., Rötzschke, O., Rénia, L.et al. (2017). Fingolimod treatment abrogates chikungunya virus-induced arthralgia. Sci. Transl. Med. 9, 375. 10.1126/scitranslmed.aal1333 [DOI] [PubMed] [Google Scholar]
- Thanapati, S., Ganu, M., Giri, P., Kulkarni, S., Sharma, M., Babar, P., Ganu, A. and Tripathy, A. S. (2017). Impaired NK cell functionality and increased TNF-α production as biomarkers of chronic chikungunya arthritis and rheumatoid arthritis. Hum. Immunol. 78, 370-374. 10.1016/j.humimm.2017.02.006 [DOI] [PubMed] [Google Scholar]
- Thiberville, S.-D., Moyen, N., Dupuis-Maguiraga, L., Nougairede, A., Gould, E. A., Roques, P. and De Lamballerie, X. (2013). Chikungunya fever: Epidemiology, clinical syndrome, pathogenesis and therapy. Antivir. Res. 99, 345-370. 10.1016/j.antiviral.2013.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjaden, N. B., Suk, J. E., Fischer, D., Thomas, S. M., Beierkuhnlein, C. and Semenza, J. C. (2017). Modelling the effects of global climate change on Chikungunya transmission in the 21st century. Sci. Rep. 7, 3813. 10.1038/s41598-017-03566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsetsarkin, K. A., Vanlandingham, D. L., Mcgee, C. E. and Higgs, S. (2007). A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 3, 12. 10.1371/journal.ppat.0030201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsetsarkin, K. A., Chen, R., Yun, R., Rossi, S. L., Plante, K. S., Guerbois, M., Forrester, N., Perng, G. C., Sreekumar, E., Leal, G.et al. (2014). Multi-peaked adaptive landscape for chikungunya virus evolution predicts continued fitness optimization in Aedes albopictu s mosquitoes. Nat. Commun. 5, 4084. 10.1038/ncomms5084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aalst, M., Nelen, C. M., Goorhuis, A., Stijnis, C. and Grobusch, M. P. (2017). Long-term sequelae of chikungunya virus disease: a systematic review. Travel Med. Infect. Dis. 15, 8-22. 10.1016/j.tmaid.2017.01.004 [DOI] [PubMed] [Google Scholar]
- Van Bortel, W., Dorleans, F., Rosine, J., Blateau, A., Rousset, D., Matheus, S., Leparc-Goffart, I., Flusin, O., Prat, C. M., Cesaire, R.et al. (2014). Chikungunya outbreak in the Caribbean region, December 2013 to March 2014, and the significance for Europe. Euro Surveill. 19, 20759. 10.2807/1560-7917.ES2014.19.13.20759 [DOI] [PubMed] [Google Scholar]
- Van Der Heijden, M. W. and Bol, J. F. (2002). Composition of alphavirus-like replication complexes: involvement of virus and host encoded proteins. Arch. Virol. 147, 875-898. 10.1007/s00705-001-0773-3 [DOI] [PubMed] [Google Scholar]
- Vazeille, M., Moutailler, S., Coudrier, D., Rousseaux, C., Khun, H., Huerre, M., Thiria, J., Dehecq, J.-S., Fontenille, D., Schuffenecker, I.et al. (2007). Two Chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito, Aedes albopictus. PloS One 2, e1168. 10.1371/journal.pone.0001168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk, S. M., Chen, R., Tsetsarkin, K. A., Adams, A. P., Garcia, T. I., Sall, A. A., Nasar, F., Schuh, A. J., Holmes, E. C., Higgs, S.et al. (2010). Genome-scale phylogenetic analyses of chikungunya virus reveal independent emergences of recent epidemics and various evolutionary rates. J. Virol. 84, 6497-6504. 10.1128/JVI.01603-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Rhein, C., Weidner, T., Henãÿ, L., Martin, J., Weber, C., Sliva, K. and Schnierle, B. S. (2016). Curcumin and Boswellia serrata gum resin extract inhibit chikungunya and vesicular stomatitis virus infections in vitro. Antivir. Res. 125, 51-57. 10.1016/j.antiviral.2015.11.007 [DOI] [PubMed] [Google Scholar]
- Voss, J. E., Vaney, M.-C., Duquerroy, S., Vonrhein, C., Girard-Blanc, C., Crublet, E., Thompson, A., Bricogne, G. and Rey, F. A. (2010). Glycoprotein organization of Chikungunya virus particles revealed by X-ray crystallography. Nature 468, 709-712. 10.1038/nature09555 [DOI] [PubMed] [Google Scholar]
- Wada, Y., orba, Y., Sasaki, M., Kobayashi, S., Carr, M. J., Nobori, H., Sato, A., Hall, W. W. and Sawa, H. (2017). Discovery of a novel antiviral agent targeting the nonstructural protein 4 (nsP4) of chikungunya virus. Virology 505, 102-112. 10.1016/j.virol.2017.02.014 [DOI] [PubMed] [Google Scholar]
- Wahid, B., Ali, A., Rafique, S. and Idrees, M. (2017). Global expansion of chikungunya virus: mapping the 64-year history. Int. J. Infect. Dis. 58, 69-76. 10.1016/j.ijid.2017.03.006 [DOI] [PubMed] [Google Scholar]
- Wauquier, N., Becquart, P., Nkoghe, D., Padilla, C., Ndjoyi-Mbiguino, A. and Leroy, E. M. (2011). The acute phase of chikungunya virus infection in humans is associated with strong innate immunity and T CD8 cell activation. J. Infect. Dis. 204, 115-123. 10.1093/infdis/jiq006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, C., Sliva, K., Von Rhein, C., Kümmerer, B. M. and Schnierle, B. S. (2015). The green tea catechin, epigallocatechin gallate inhibits chikungunya virus infection. Antivir. Res. 113, 1-3. 10.1016/j.antiviral.2014.11.001 [DOI] [PubMed] [Google Scholar]
- Weger-Lucarelli, J., Aliota, M. T., Kamlangdee, A. and Osorio, J. E. (2015). Identifying the role of E2 domains on alphavirus neutralization and protective immune responses. PLoS Negl. Trop. Dis. 9, e0004163. 10.1371/journal.pntd.0004163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichit, S., Diop, F., Hamel, R., Talignani, L., Ferraris, P., Cornelie, S., Liegeois, F., Thomas, F., Yssel, H. and Missé, D. (2017). Aedes Aegypti saliva enhances chikungunya virus replication in human skin fibroblasts via inhibition of the type I interferon signaling pathway. Infect. Genet. Evol. 55, 68-70. 10.1016/j.meegid.2017.08.032 [DOI] [PubMed] [Google Scholar]
- Wilson, A. L., Courtenay, O., Kelly-Hope, L. A., Scott, T. W., Takken, W., Torr, S. J. and Lindsay, S. W. (2020). The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl. Trop. Dis. 14, e0007831. 10.1371/journal.pntd.0007831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, J. A. C., Prow, N. A., Schroder, W. A., Ellis, J. J., Cumming, H. E., Gearing, L. J., Poo, Y. S., Taylor, A., Hertzog, P. J., Di Giallonardo, F.et al. (2017). RNA-Seq analysis of chikungunya virus infection and identification of granzyme A as a major promoter of arthritic inflammation. PLoS Pathog. 13, 2. 10.1371/journal.ppat.1006155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintachai, P., Wikan, N., Kuadkitkan, A., Jaimipuk, T., Ubol, S., Pulmanausahakul, R., Auewarakul, P., Kasinrerk, W., Weng, W.-Y., Panyasrivanit, M.et al. (2012). Identification of prohibitin as a Chikungunya virus receptor protein. J. Med. Virol. 84, 1757-1770. 10.1002/jmv.23403 [DOI] [PubMed] [Google Scholar]
- Wintachai, P., Thuaud, F., Basmadjian, C., Roytrakul, S., Ubol, S., Désaubry, L. and Smith, D. R. (2015). Assessment of flavaglines as potential chikungunya virus entry inhibitors. Microbiol. Immunol. 59, 129-141. 10.1111/1348-0421.12230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wust, C. J., Nicholas, J. A. W., Fredin, D., Dodd, D. C., Brideau, R. J., Levely, M. E. and Brown, A. (1989). Monoclonal antibodies that cross-react with the E1 glycoprotein of different alphavirus serogroups: characterization including passive protection in vivo. Virus Res. 13, 101-112. 10.1016/0168-1702(89)90009-9 [DOI] [PubMed] [Google Scholar]
- Yang, S., Fink, D., Hulse, A. and Pratt, R. D. (2017). Regulatory considerations in development of vaccines to prevent disease caused by Chikungunya virus. Vaccine 35, 4851-4858. 10.1016/j.vaccine.2017.07.065 [DOI] [PubMed] [Google Scholar]
- Yap, M. L., Klose, T., Urakami, A., Hasan, S. S., Akahata, W. and Rossmann, M. G. (2017). Structural studies of Chikungunya virus maturation. Proc. Natl. Acad. Sci. U.S.A. 114, 13703-13707. 10.1073/pnas.1713166114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, A. R., Locke, M. C., Cook, L. E., Hiller, B. E., Zhang, R., Hedberg, M. L., Monte, K. J., Veis, D. J., Diamond, M. S. and Lenschow, D. J. (2019). Dermal and muscle fibroblasts and skeletal myofibers survive chikungunya virus infection and harbor persistent RNA. PLoS Pathog. 15, e1007993. 10.1371/journal.ppat.1007993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaid, A., Rulli, N. E., Rolph, M. S., Suhrbier, A. and Mahalingam, S. (2011). Disease exacerbation by etanercept in a mouse model of alphaviral arthritis and myositis. Arthritis. Rheum. 63, 488-491. 10.1002/art.30112 [DOI] [PubMed] [Google Scholar]
- Zare, F., Bokarewa, M., Nenonen, N., Bergström, T., Alexopoulou, L., Flavell, R. A. and Tarkowski, A. (2004). Arthritogenic properties of double-stranded (Viral) RNA. J. Immunol. 172, 5656-5663. 10.4049/jimmunol.172.9.5656 [DOI] [PubMed] [Google Scholar]
- Zhang, R., Kim, A. S., Fox, J. M., Nair, S., Basore, K., Klimstra, W. B., Rimkunas, R., Fong, R. H., Lin, H., Poddar, S.et al. (2018). Mxra8 is a receptor for multiple arthritogenic alphaviruses. Nature 557, 570-574. 10.1038/s41586-018-0121-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y.-N., Deng, C.-L., Li, J.-Q., Li, N., Zhang, Q.-Y., Ye, H.-Q., Yuan, Z.-M. and Zhang, B. (2019). Infectious chikungunya virus (CHIKV) with a complete capsid deletion: a new approach for a CHIKV vaccine. J. Virol. 93, e00504-19. 10.1128/JVI.00504-19 [DOI] [PMC free article] [PubMed] [Google Scholar]