Abstract
A fronto-temporal brain network has long been implicated in language comprehension. However, this network’s role in language production remains debated. In particular, it remains unclear whether all or only some language regions contribute to production, and which aspects of production these regions support. Across 3 functional magnetic resonance imaging experiments that rely on robust individual-subject analyses, we characterize the language network’s response to high-level production demands. We report 3 novel results. First, sentence production, spoken or typed, elicits a strong response throughout the language network. Second, the language network responds to both phrase-structure building and lexical access demands, although the response to phrase-structure building is stronger and more spatially extensive, present in every language region. Finally, contra some proposals, we find no evidence of brain regions—within or outside the language network—that selectively support phrase-structure building in production relative to comprehension. Instead, all language regions respond more strongly during production than comprehension, suggesting that production incurs a greater cost for the language network. Together, these results align with the idea that language comprehension and production draw on the same knowledge representations, which are stored in a distributed manner within the language-selective network and are used to both interpret and generate linguistic utterances.
Keywords: language production, language comprehension, language network, syntax, fMRI
Introduction
Although the scientific enterprise of language neuroscience began with a report about an aspect of language production (articulatory abilities; Broca 1861), the field has been largely dominated by investigations of language comprehension. As a result, many questions remain about the cognitive and neural mechanisms of language production and their relationship to language comprehension mechanisms.
Language production encompasses multiple cognitive processes, from formulating a thought to selecting the right words and constructions (lexical access), to putting them together into a well-formed string (phrase-structure building), to retrieving the phonological forms associated with each word, to finally planning and executing a series of motor movements (e.g. articulatory movements for spoken language) (e.g. Levelt 1989; Goldrick et al. 2014). The latter, lower-level aspects of language production draw on a relatively well-characterized network of superior temporal and frontal areas, including an area in posterior left inferior frontal gyrus (IFG), in line with Broca’s original report (e.g. Hillis et al. 2004; Bohland and Guenther 2006; Bouchard et al. 2013; Flinker et al. 2015; Fridriksson et al. 2016; Guenther 2016; Long et al. 2016; Basilakos et al. 2018). This “articulation network” appears to be distinct from what we refer to here as the “language network”—the network of (more inferior) temporal and (more anterior) frontal areas that have been implicated in higher-level linguistic processing (cf. Miozzo et al. 2015; Riès et al. 2017; Strijkers et al. 2017 for claims that some articulation areas may contribute to higher-level language production).
The language network can be defined in individual participants using a) functional localizer contrasts (Saxe et al. 2006), like reading sentences vs. nonwords, or listening to speech vs. acoustically degraded speech (e.g. Fedorenko et al. 2010; Scott et al. 2017; Lipkin et al. 2022) across diverse languages (Malik-Moraleda et al. 2022); or b) patterns of inter-voxel functional correlations during naturalistic cognition paradigms (e.g. Braga et al. 2020; see also Blank et al. 2014). These language-responsive brain areas are highly selective for language relative to diverse non-linguistic cognitive processes (e.g. Fedorenko et al. 2011; Monti et al. 2012; Ivanova et al. 2020; see Fedorenko and Varley 2016 and Fedorenko and Blank 2020 for reviews) and support both lexical semantic (i.e. relating to the processing of word meanings) and combinatorial processes during language comprehension (e.g. Fedorenko et al. 2010, 2020; Bautista and Wilson 2016; see e.g. Hagoort and Indefrey 2014 for a meta-analysis).
What role does the language-selective network play in language production? Of course, comprehension and production are intimately linked. Most agree that they draw on the same set of linguistic knowledge representations (e.g. Chomsky 1965; Pickering and Garrod 2004). Some further argue that certain computations may be analogous between comprehension and production. For example, Chater et al. 2016 have suggested that both rely on chunking—compressing detailed representations in order to pass them down (in production) or up (in comprehension) to the next processing stage. Others argue that the production system is always active during comprehension and serves as a vehicle for linguistic prediction (e.g. Federmeier 2007; Pickering and Garrod 2013; Dell and Chang 2014), and that the comprehension system is always active during production, serving as a monitoring mechanism (e.g. Levelt 1989; cf. Nozari et al. 2011).
This tight relationship between comprehension and production finds empirical support. For example, syntactic structures that are experienced through listening or reading are more likely to be subsequently produced (e.g. Branigan et al. 2000), and the magnitude of this priming effect is not diminished relative to production-to-production priming (e.g. Tooley and Bock 2014). Comprehension difficulties tend to arise in structures that are dispreferred during production planning (e.g. Hsiao and MacDonald 2016). And the same (lexico-semantic and phonological) word knowledge is available at the same early latencies during word production and comprehension (Fairs et al. 2021).
Given this theoretical and empirical backdrop, it seems reasonable to expect that the same mechanisms would support comprehension and production. Indeed, a number of past brain imaging studies have directly compared neural responses during comprehension and production tasks and reported overlap within the language network (e.g. Awad et al. 2007; Menenti et al. 2011; Segaert et al. 2012; Silbert et al. 2014; Giglio et al. 2022; see Indefrey 2018 and Walenski et al. 2019 for meta-analyses; see Strijkers and Costa 2016 and Gambi and Pickering 2017 for theoretical proposals of shared circuitry; cf. Indefrey and Levelt 2004 and Indefrey 2011 for arguments for distinct circuits supporting word-level comprehension vs. production). However, these studies and meta-analyses have a) often reported overlap in only some of the language regions, and these regions differ across studies (e.g. Awad et al. 2007 report overlap within anterior temporal cortex and temporo-parietal junction, but Segaert et al. 2012 in posterior temporal and inferior frontal areas); and/or b) have not controlled for lower-level perceptual/motor demands, making the nature of the overlap difficult to interpret (e.g. Silbert et al. 2014). Furthermore, all past studies (cf. Matchin and Wood 2020; see Discussion) have relied on the traditional random-effects analytic approach where activations are averaged voxel-wise across individuals and inferences are drawn based on the resulting group-level maps. Because of the well-established inter-individual variability in the precise locations of functional areas (e.g. Frost and Goebel 2012; Tahmasebi et al. 2012)—including language areas (e.g. Fedorenko et al. 2010; Mahowald and Fedorenko 2016; Braga et al. 2020; Lipkin et al. 2022)—and the high functional heterogeneity of the association cortex, where distinct areas often lay adjacent to each other (e.g. Scholz et al. 2009; Fedorenko, Duncan, and Kanwisher 2012; Deen et al. 2015; Braga and Buckner 2017; Braga et al. 2020; Deen and Freiwald 2022), this approach can both miss activations and overestimate activation overlap between nearby areas (e.g. Saxe et al. 2006; Nieto-Castañón and Fedorenko 2012; Fedorenko and Shain 2021).
Another unresolved question about the architecture of language production concerns the relationship between the mechanisms that support lexical access (word retrieval) vs. those that support phrase-structure building (combining words together to form phrases and sentences). Some past studies that examined neural responses to language production have conflated these 2 kinds of demands (e.g. Awad et al. 2007; Silbert et al. 2014). Other studies have attempted to separate them and argued for different parts of the language network supporting lexical and combinatorial aspects of language production (e.g. Menenti et al. 2011). However, if the same representations subserve comprehension and production, and given that lexico-semantic and syntactic processing do not appear to be spatially segregated for comprehension (e.g. Fedorenko et al. 2020), a dissociation between lexical access and phrase-structure building in production would seem surprising.
Of high relevance to the relationships a) between production and comprehension, and b) between lexical access and combinatorial processing in production is a hypothesis about the existence of mechanisms that selectively support phrase-structure building in production (e.g. Bock 1982, 1995; Matchin and Hickok 2019). This hypothesis is motivated by a putative asymmetry between production and comprehension with respect to combinatorial processing. In particular, whereas linearization (putting words in a particular order) and morpho-syntactic agreement processes are necessary aspects of language production (cf. Swets et al. 2013; Goldberg and Ferreira 2022), comprehension is possible even when word order and other morpho-syntactic cues are degraded or absent (e.g. Ferreira et al. 2002; Ferreira 2003; Levy 2008; Levy et al. 2009; Gibson et al. 2013; Ferreira and Lowder 2016; Mollica et al. 2020; Mahowald et al. 2022; cf. Shain et al. 2022 for evidence that comprehenders compute fine-grained syntactic representations even during passive listening in naturalistic paradigms). Matchin and Hickok (2019), based largely on data from aphasia, recently proposed that such a mechanism may be housed in the inferior frontal component of the language network (in contrast with its posterior temporal component, which they hypothesized supports morpho-syntactic demands in both comprehension and production). However, this proposal has not been thoroughly evaluated to date (cf. the 2 recent studies that are summarized in section Discussion).
To illuminate the contribution of the language-selective network to language production, we use functional magnetic resonance imaging (fMRI) to examine the responses of the language areas—defined in individual participants by an extensively validated comprehension-based language localizer (Fedorenko et al. 2010)—during production tasks. To examine both phrase-structure building and lexical access using this precision fMRI approach, we adapt a paradigm that has proven fruitful in probing combinatorial and lexico-semantic processes in comprehension (e.g. Friederici et al. 2000; Humphries et al. 2007; Fedorenko et al. 2010; Pallier et al. 2011; Fedorenko, Nieto-Castañon, and Kanwisher 2012; Shain et al. 2021). In particular, we examine neural responses during spoken (Experiments 1–2) and typed (Experiment 3) production of sentences and lists of words (as well as control nonword sequences in Experiments 1 and 3). Brain areas that support phrase-structure building should work harder (i.e. exhibit stronger blood oxygenation level-dependent [BOLD] responses) when words must be combined (sentence production) compared to retrieval of unrelated words (word-list production), given that sentence production requires additional operations, including semantic composition, ordering the words, and implementing the relevant morpho-syntactic agreement processes. And brain areas that support lexical retrieval should work harder when words must be accessed (word-list production) compared to a simple articulatory task (nonword production), given the additional computations required for word retrieval. (We, of course, acknowledge that both phrase-structure building and lexical access may themselves consist of multiple distinct sub-processes [e.g. some have distinguished between syntactic vs. semantic combinatorial processing; e.g. Hagoort and Indefrey 2014; Pylkkänen 2019]; here, we probe these aspects of language production using relatively broad contrasts given that this is the first attempt to examine language production using precision fMRI.) To evaluate the hypothesis about brain mechanisms that are selective for phrase-structure building in production relative to comprehension, we additionally included sentence and word-list comprehension conditions.
Materials and methods
Participants
Forty-one individuals (age 18–31, mean 23.3 years; 28 (68.3%) females) from the Cambridge/Boston, MA community participated for payment across 3 fMRI experiments (n = 29 in Experiment 1; n = 12 in Experiment 2; and n = 14 in Experiment 3; participants in Experiment 3 were a proper subset of the participants in Experiment 1). All were native speakers of English. Of the 32 participants for whom handedness data were available, 28 participants (87.5%) were right-handed, as determined by the Edinburgh Handedness Inventory (Oldfield 1971) or self-report, 2 (6.25%) were left-handed, and 2 (6.25%) were ambidextrous (see Willems et al. 2014, for arguments for including non-right-handers in cognitive neuroscience experiments). Handedness data were not recorded for the remaining 9 participants. All but 1 participant showed typical left-lateralized language activations in the language localizer task; 1 (right-handed) participant in Experiment 2 showed right-lateralized language activations; we chose to include this participant to err on the conservative side. For Experiment 3, we recruited participants who could type without seeing the written output or the keyboard itself. All participants gave informed written consent in accordance with the requirements of MIT’s Committee on the Use of Humans as Experimental Subjects (COUHES).
Design, materials, and procedure
Each participant completed a comprehension-based localizer task for the language network (Fedorenko et al. 2010) and a critical language production experiment. All but one participant (in Experiment 1) additionally completed a localizer task for the domain-general multiple demand (MD) network (Duncan 2010, 2013). Because the MD network has been shown to be generally sensitive to task difficulty across domains (e.g. Duncan and Owen 2000; Fedorenko et al. 2013; Hugdahl et al. 2015; Shashidhara et al. 2019; Assem et al. 2020), activity levels therein can be used to determine the relative difficulty levels of the different production conditions, to aid the interpretation of the results. Some participants also completed one or more tasks for unrelated studies. The scanning sessions lasted approximately 2 h.
Language network localizer
The regions of the language network were localized using a task described in detail in Fedorenko et al. (2010) and subsequent studies from the Fedorenko lab (the task is available for download from https://evlab.mit.edu/funcloc/). Briefly, participants silently read sentences and lists of unconnected, pronounceable nonwords in a blocked design. The sentences > nonwords contrast targets brain regions that that support high-level language comprehension. This contrast generalizes across tasks (e.g. Fedorenko et al. 2010; Scott et al. 2017; Ivanova et al. 2020) and presentation modalities (reading vs. listening; e.g. Fedorenko et al. 2010; Scott et al. 2017; Chen et al. 2021; Malik-Moraleda et al. 2022). All the regions identified by this contrast show sensitivity to lexico-semantic processing (e.g. stronger responses to real words than nonwords) and combinatorial semantic and syntactic processing (e.g. stronger responses to sentences and Jabberwocky sentences than to unstructured word and nonword lists) (e.g. Fedorenko et al. 2010, 2016, 2020; Fedorenko, Nieto-Castañon, and Kanwisher 2012; Blank et al. 2016; Shain et al. 2021). More recent work further shows that these regions are also sensitive to sub-lexical regularities (Regev et al. 2021), in line with the idea that this system stores our linguistic knowledge, which encompasses regularities across representational grains, from phonological and morphological schemas to words and constructions (e.g. Jackendoff and Audring 2019; Jackendoff 2002).
Stimuli were presented one word/nonword at a time at the rate of 350–450 ms (differing slightly between variants of the localizer; Supplementary Table SI-1) per word/nonword. Participants read the materials passively and performed either a simple button-press or a memory probe task at the end of each trial, which were included in order to help participants remain alert. The memory probe required the participant to indicate whether a given word was from the sentence/list of nonwords they had just read. Each participant completed 2 ~6 min runs. In Experiments 1 and 3, all participants completed the language localizer in the same session as the production experiment. In Experiment 2, 8 participants completed the language localizer in the same session as the production experiment and the remaining 4 participants completed the language localizer in an earlier scanning session (see Mahowald and Fedorenko 2016 and Braga et al. 2020 for evidence of high across-session reliability of the activation patterns).
Multiple demand network localizer
The regions of the MD network (Duncan 2010; Duncan et al. 2020) were localized using a spatial working memory task contrasting a harder condition with an easier condition (e.g. Fedorenko et al. 2011, 2013; Blank et al. 2014). The hard > easy contrast targets brain regions engaged in cognitively demanding tasks. Fedorenko et al. (2013) have established that the regions activated by this task are also activated by a wide range of other demanding tasks (see also Duncan and Owen 2000; Hugdahl et al. 2015; Shashidhara et al. 2019; Assem et al. 2020).
On each trial (8 s), participants saw a fixation cross for 500 ms, followed by a 3 x 4 grid within which randomly generated locations were sequentially flashed (1 s per flash) 2 at a time for a total of 8 locations (hard condition) or 1 at a time for a total of 4 locations (easy condition). Then, participants indicated their memory for these locations in a 2-alternative, forced-choice paradigm via a button press (the choices were presented for 1,000 ms, and participants had up to 3 s to respond). Feedback, in the form of a green checkmark (correct responses) or a red cross (incorrect responses), was provided for 250 ms, with fixation presented for the remainder of the trial. Hard and easy conditions were presented in a standard blocked design (4 trials in a 32 s block, 6 blocks per condition per run) with a counterbalanced order across runs. Each run included 4 blocks of fixation (16 s each) and lasted a total of 448 s. Each participant completed 2 runs, except for 1 participant in Experiment 1, who completed 1 run. In Experiment 1, of the 28 participants who completed the MD localizer, 25 did so in the same session as the production experiment (this included 11 of the 14 participants who also participated in Experiment 3) and the remaining 3 participants completed the MD localizer in an earlier scanning session. In Experiment 2, 9 participants completed the MD localizer in the same session as the production experiment and the remaining 3 participants completed the MD localizer in an earlier session.
Like the language localizer, the MD localizer has been extensively validated, and a network that closely corresponds to the one activated by the MD localizer emerges from task-free (resting state) data (e.g. Assem et al. 2020; Braga et al. 2020; also Blank et al. 2014; Malik-Moraleda et al. 2022).
General approach for the language production tasks
Tapping mental computations related to high-level language production—including both lexical access and combining words into phrases and sentences—is notoriously challenging because linguistic productions originate from internal conceptual representations (e.g. Levelt 1989; Bock 1996; Goldrick et al. 2014). These representations are difficult to probe and manipulate without sacrificing ecological validity. However, given that many open questions remain about how language production is implemented in the mind and brain, and the need for careful comparisons (critical for interpretability; e.g. Mook 1983), we opted for a controlled experimental approach. In particular, building on a strong foundation of behavioral work on language production, we used pictorial stimuli to elicit object labels and event-level linguistic descriptions.
Experiment 1 (spoken production)
Design. Participants were presented with a variety of visual stimuli across 6 conditions. In the 2 critical language production conditions—sentence production and word-list production—participants were instructed to speak out loud, but to move their heads as little as possible. The sentence production (SProd) condition is the closest to reflecting the language production demands of everyday life, where we often communicate event-level descriptions using phrases and sentences. In this condition, participants viewed photographs of common events involving humans, animals, and inanimate objects (Fig. 1a-i and b-i) and were asked to produce a description of the event (e.g. “The girl is smelling a flower”). This condition targets sentence-level production planning and execution, which includes a) retrieving the words for the entities/objects and actions, and b) combining them into an utterance, including semantic composition, ordering the words, and implementing the relevant syntactic agreement processes. Of course, participants are not guaranteed to produce complete sentences. In fact, when asked to describe event pictures, participants commonly revert to “headlinese” (the register used in newspaper headlines) and produce descriptions like “girl smelling a flower” or “girl smells flower” (Supplementary Fig. SI-1). Importantly, such elliptical productions still require cognitive operations above and beyond lexical retrieval, including semantic composition but also morpho-syntactic planning and execution given that they obey the syntactic constraints of this particular register, including word order and agreement constraints (see e.g. Halliday 1967; Mårdh 1980; van Dijk 1988 for discussions of linguistic features of headlinese).
Fig. 1.
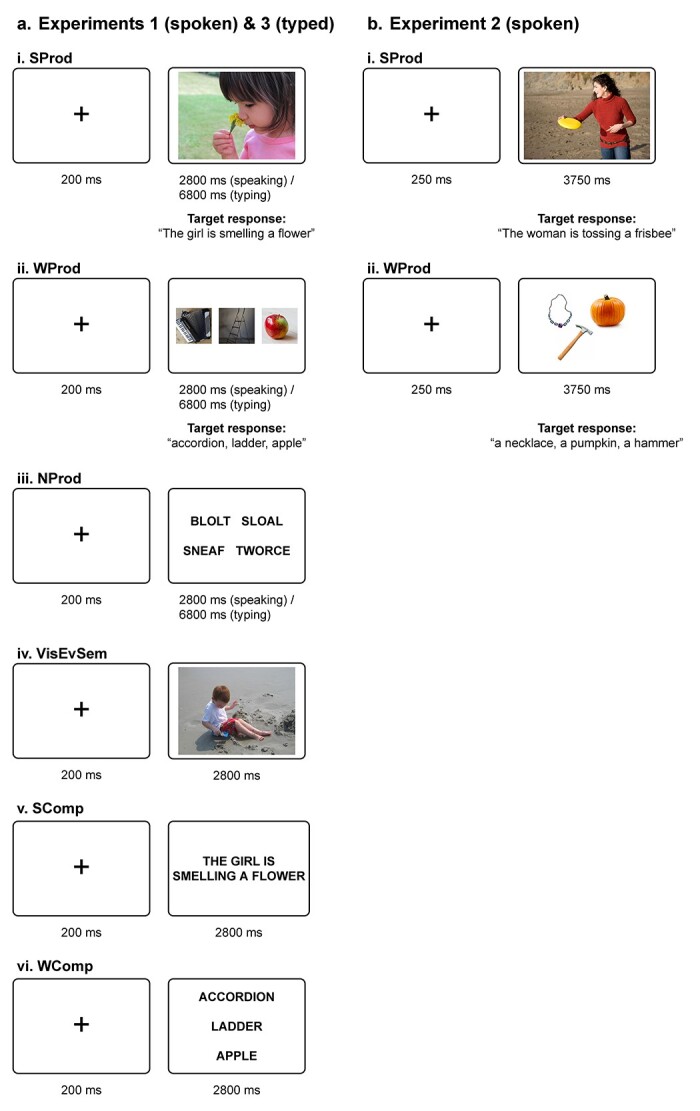
Sample trials for each condition of Experiments 1–3. a) In Experiments 1 and 3, participants performed 2 production tasks: producing descriptions of events depicted in naturalistic photographs (SProd), and producing names of unrelated, isolated objects depicted in separate photographs (WProd). In 2 control conditions, participants spoke or typed monosyllabic nonwords (NProd), and indicated whether events depicted in photographs took place indoors or outdoors (VisEvSem). Finally, participants performed 2 comprehension tasks: silently reading sentences (SComp) and word lists (WComp), mirroring the structure and content of target responses from the production trials (see Methods for details). For the production conditions, trials lasted 2,800 ms in Experiment 1 (spoken production) and were increased to 6,800 ms in Experiment 3 (typed production). b) In Experiment 2, participants performed the sentence and word production conditions (SProd, WProd) with a different set of materials.
In the word-list production (WProd) condition, participants viewed groups of 2, 3, or 4 photographs of inanimate objects (Fig. 1a-ii and b-ii) and were asked to name each object in the set (e.g. “accordion, ladder, apple”). The number of objects in each group (2–4) matched the number of content words in the target productions in the SProd condition. This condition targets word-level production planning and execution. To isolate the mental processes related to single-word production, photographs were manually grouped in a way that minimized semantic associations between the objects, to prevent participants from unintentionally forming phrases/clauses with the retrieved words.
The experiment also included 2 control conditions: low-level (nonword-list) production and semantic judgments about visual events. In the nonword-list production (NProd) condition, participants viewed lists of 4 monosyllabic nonwords (Fig. 1a-iii) and were asked to say them out loud (e.g. “blolt, sloal, sneaf, tworce”). The nonwords obeyed the phonotactic constraints of English and were selected to be sufficiently distant from phonologically neighboring words. This condition targets low-level articulatory planning and execution and was included as a more stringent baseline (in addition to the low-level fixation baseline) to which sentence and word production conditions could be compared (see e.g. Bohland and Guenther 2006; Okada and Hickok 2006; Flinker et al. 2015 for evidence that this kind of condition robustly activates articulation areas). We acknowledge the difference between this condition and the other production conditions in that this condition involves visually presented linguistic strings (cf. pictures in the SProd and WProd conditions); it is difficult to create minimally different conditions one of which does and the other does not require lexical access while having similar lower-level (e.g. articulation) demands. And in the visual event semantics (VisEvSem) condition, participants viewed photographs of events (as in SProd) and were asked to indicate whether the depicted event takes place indoors or outdoors (a relatively high-level judgment that requires visual event perception and also draws on world knowledge) via a 2-choice button box (Fig. 1a-iv). This condition targets visual and conceptual processing of events and was included to ensure that responses to the SProd condition, which uses these pictorial stimuli, were not due to these cognitive processes (see e.g. Ivanova et al. 2021 and Sueoka et al. 2022 for some evidence of engagement of the language areas in visual event semantics).
Finally, the experiment included 2 reading comprehension conditions: sentence comprehension and word-list comprehension. In both conditions, participants were instructed to read the stimuli silently (as in the language localizer). In the sentence comprehension (SComp) condition, participants viewed short sentences describing common events (e.g. “The girl is smelling a flower”) and were asked to read them. These sentences described the same events that were depicted in the photographs used in the SProd condition (Fig. 1a-v); however, as is common in sentence-level experiments, any given participant experienced an event once—either as a sentence (SComp condition) or as a photograph (SProd condition) (elaborated in Procedure below). The SComp condition targets sentence-level comprehension processes, including lexico-semantic and combinatorial (semantic and syntactic) processes. In the word-list comprehension (WComp) condition, participants viewed lists of 2, 3, or 4 object names (Fig. 1a-vi) and were asked to read them (e.g. “accordion, ladder, apple”). This condition targets word-level comprehension. As in the WProd condition, object names in the WComp condition were grouped in a way that minimized semantic associations. These conditions were included to enable comparisons of responses to content-matched sentences and word lists across production and comprehension as relevant to the question of production-selective mechanisms.
Materials. To obtain the event photographs for the SProd and VisEvSem conditions, we first manually selected 400 images clearly depicting everyday events from the Flickr30k dataset (Young et al. 2014). We then ran a norming study on Amazon.com’s Mechanical Turk to identify the stimuli that would elicit the most consistent linguistic descriptions across participants. On each trial, participants viewed a single photograph and were given the instructions “Please provide a one-sentence description of what is happening in the photo.” They were able to type freely in a textbox below the image and could only proceed to the next trial after submitting a non-empty response. We recruited n = 30 participants for each of the 400 images, and each participant produced descriptions for 100 images.
To analyze the resulting 12,000 responses, we used the Python spaCy natural language processing library (Honnibal et al. 2020) to parse each production into the subject noun phrase (NP), verb phrase (VP), subject NP head, and VP head. After manually cleaning the parses for consistency, we computed 3 metrics for each photograph: (1) the number of unique responses in each of the parsed categories, (2) the number of unique lemmas for the single-word parsed categories (subject NP head and VP head), and (3) the standard deviation of the number of tokens per production. We then obtained a “linguistic variability” score by summing these 3 values for each image and chose the 200 photographs with the lowest scores. Finally, we hand-selected 128 from these 200 to maximally cover a range of objects and actions. These photographs were used in the SProd and VisEvSem conditions, and the associated sentence descriptions (the most frequently used description for each photograph) were used in the SComp condition.
For the WProd and WComp conditions, we wanted to use materials that would be semantically (and lexically) similar to the ones used in the SProd and SComp conditions. As a result, to obtain the object photographs for the WProd condition, we first identified between 2 and 4 words in each of the 128 sentence descriptions that referred to inanimate objects (we avoided animate entities like “a man” or “a woman” because in the setup that we used, with multiple objects presented at once, we wanted to avoid the possibility of participants constructing event-level representations). For example, from the description “A man is playing saxophone in a park” we selected “saxophone,” and from the description “A man is sitting on a bench reading the newspaper” we selected “bench” and “newspaper.” This resulted in a total of 120 words. Next, we selected images of each object from the THINGS database (Hebart et al. 2019) as well as a repository of license-free stock photographs. In those images, each object is presented on a neutral but naturalistic background, which isolates the object from possibly associated events or concepts. We generated all possible groups of 2-, 3-, and 4-object images, and then took a random sample of 40 2-object, 80 3-object, and 40 4-object groups, as there was an average of 3 content words in our target sentence productions. We then manually selected the final 128 object groups by discarding groups with semantically related objects and ensuring that each object appeared 1–3 times. The associated words (grouped in the same way) were used in the WComp condition. The order of objects and words was randomized within each group during presentation.
Finally, the nonwords for the NProd condition were selected from the ARC Nonword Database (Rastle et al. 2002). We began by selecting all the monomorphemic syllables involving orthographically existing onsets, bodies, and legal bigrams. We then obtained the final set of 256 nonwords by filtering for low numbers of onset and phonological neighbors in order to minimize the likelihood of these nonwords priming real words. These 256 nonwords were then randomly distributed into 64 groups of 4.
All the materials for this experiment and Experiment 2 are available on GitHub (https://github.com/jennhu/LanguageProduction); the sentences, word lists, and nonword lists are also provided in the SI for convenience (Supplementary Material, Appendix A).
Procedure. Following the general practice in the field of sentence processing, the same event or object group did not appear in both a production condition and its corresponding comprehension condition for any given participant (in order to avoid influences on the processing of sentence 1 in condition A from the earlier processing of sentence 1 in condition B). To achieve this, we distributed the materials in the SProd, WProd, SComp, and WComp conditions (i.e. 128 event images, 128 corresponding target sentences, 128 object group images, and 128 corresponding target word lists) into 2 experimental lists. We assigned a unique number 1–128 to each event and object group, such that event image x corresponds to sentence x, and object group x corresponds to word list x. These numbers were assigned such that sets 1–64 and 65–128 were each semantically diverse (e.g. 2 images of a person playing a musical instrument were assigned to different sets). Furthermore, the 2-, 3-, and 4-object groups were evenly distributed across the 2 sets (1–64 and 65–128). Finally, this numbering was used to create 2 lists. In List 1, event images 1–64 in SProd appeared with sentences 65–128 in SComp, and similarly object group images 1–64 in WProd appeared with word lists 65–128 in WComp. And in List 2, event/object group images 65–128 in SProd/WProd appeared with sentences/word lists 1–64 in SComp/WComp. The materials for the NProd condition were identical across lists, and the materials for the VisEvSem condition were the same as the SProd materials in that list.
The materials in each condition (and each list, where relevant) were grouped into 16 blocks of 4 trials each; this was done separately for each participant. (Note that although we had enough materials to yield 16 blocks per condition, we ended up presenting 12 blocks per condition for any given participant because—based on pilot participants—this number of blocks per condition gave us sufficient power to elicit clear between-condition differences.) Each block was preceded by instructions, which told the participants what they would be doing in the trials to come: “Describe the event out loud” for SProd, “Name the objects out loud” for WProd, “Say the nonwords out loud” for NProd, “Inside (=1) or outside (=2)?” for VisEvSem, “Read the sentence silently” for SComp, and “Read the words silently” for WComp. The instructions remained on the screen (in small font in the bottom left corner of the screen) throughout the block to minimize the demands associated with holding onto the instructions and to help participants in case they missed the block-initial instructions screen. Each trial lasted 3 s and consisted of an initial fixation cross (0.2 s) and stimulus presentation (2.8 s). In the SProd and VisEvSem conditions, the stimulus was a single event picture; in the WProd condition, the stimulus was a set of 2–4 object pictures (presented all at once); in the NProd condition, the stimulus was a set of 4 nonwords (presented all at once); in the SComp condition, the stimulus was a sentence (presented all at once); and in the WComp, the stimulus was a set of 2–4 words (presented all at once) (see Fig. 1a). The block-initial instructions were presented for 2 s. Thus, each block lasted 14 s (2 s instructions and 4 trials 3 s each).
The total of 72 experimental blocks (12 blocks * 6 conditions) were distributed into 6 sets, corresponding to runs, of 12 blocks each (2 blocks per condition). Each run additionally included 3 fixation blocks of 12 s each: 1 at the beginning of the run, 1 after the first 6 experimental blocks, and 1 at the end. Thus, each run consisted of 12 experimental blocks of 14 s each and 3 fixation blocks of 12 s each, lasting a total of 204 s (3 min 24 s). Each participant completed 4–6 runs (for a total of 8–12 blocks per condition). The order of conditions was palindromic within each run and varied across runs and participants.
Prior to entering the scanner, participants were provided with printed instructions and were guided through sample items that mimicked the experimental stimuli. The experimental script with all the materials is available at GitHub: https://github.com/jennhu/LanguageProduction.
Experiment 2 (spoken production; conceptual replication of Experiment 1)
Design. Experiment 2 was designed and conducted prior to Experiment 1 and constituted an early attempt (~2012) to develop a word and sentence production paradigm for fMRI. For this reason, it does not include all the critical control conditions that are included in Experiment 1. Nevertheless, it serves as a conceptual replication of the findings from the critical production conditions (SProd and WProd) in Experiment 1 while generalizing the results to a new set of materials and an independent group of participants. The design was identical except that in the word-list production (WProd) condition, participants always viewed groups of 3 object photographs (cf. 2, 3, or 4 object photographs in Experiment 1), and they were asked to name each object in the set with an indefinite article (e.g. “a necklace, a pumpkin, a hammer”), which includes some basic phrase-level combinatorial processing in addition to lexical retrieval. The experiment also included 2 other conditions that are not directly relevant to the current investigation and are therefore not discussed.
Materials. The materials were selected from the publicly available images in the Google Images database and consisted of 96 event images for the SProd condition (Fig. 1b-i), and 288 object images for the WProd (Fig. 1b-ii) condition. The event photographs were similar in style to those used in Experiments 1, but were more semantically diverse, including not only humans interacting with inanimate objects (as most events in Experiments 1), but also humans interacting with other humans, and humans interacting with animals. The object photographs were also similar in style to those used in Experiment 1, but did not include any background, and were also more semantically diverse, including not only inanimate objects, but also humans (where the occupation of the person is clear: e.g. a chef, a juggler, a ballerina, etc.) and animals. As in Experiment 1, the object photographs were grouped in a way that minimized semantic associations between the objects.
Procedure. The materials in each condition were grouped into 24 blocks of 4 trials each; this was done separately for each participant. (The materials were further divided into 2 experimental lists of 12 blocks per condition.) Each block was preceded by instructions, which told the participants what they would be doing in the trials to come: “Describe the events” for SProd, and “Name the objects” for WProd. Each trial lasted 4 s and consisted of an initial fixation cross (0.25 s) and stimulus presentation (3.75 s). In the SProd condition, a trial consisted of a single event picture, and in the WProd condition, a trial consisted of 3 object pictures (presented all at once in a triangular configuration) (see Fig. 1b). Participants were instructed to describe the events with complete sentences (e.g. “The woman is tossing a frisbee”) and to name the objects with indefinite determiners (e.g. “a necklace, a pumpkin, a hammer”). The block-initial instructions were presented for 2 s. Thus, each block lasted 18 s (2 s instructions and 4 trials 4 s each).
The total of 48 experimental blocks in each list (12 blocks * 4 conditions, 2 of which are of interest to the current study) were distributed into 4 sets, corresponding to runs, of 12 blocks each (3 blocks per condition). Each run additionally included 4 fixation blocks of 18 s each: one at the beginning of the run, and one after each set of 4 experimental blocks. Thus, each run consisted of 12 experimental blocks of 18 s each and 4 fixation blocks of 18 s each, lasting a total of 288 s (4 min 48 s). Eleven participants completed 4 runs (for a total of 12 blocks per condition) and 1 participant completed 2 runs (for a total of 6 blocks per condition). The order of conditions was palindromic within each run and varied across runs and participants.
Prior to entering the scanner, participants were provided with printed instructions and were guided through sample items that mimicked the experimental stimuli. The experimental script with all the materials is available at GitHub: https://github.com/jennhu/LanguageProduction.
Experiment 3 (typed production; extension to another output modality)
Design and materials. Experiment 3 served to generalize the results from Experiment 1 to another output modality and was performed by 14 of the 29 participants in Experiment 1. If the data patterns observed for spoken production reflect cognitive processes related to high-level aspects of language production (i.e. lexical access and phrase-structure building), then they should be similar regardless of whether the utterances are spoken or written. This logic is similar to the logic in past studies of language comprehension that have compared neural responses to spoken and written (or signed for sign languages) linguistic input. Such studies have found that the high-level frontal and temporal language areas are sensitive to lexico-semantic and combinatorial processing during comprehension, regardless of the input modality (e.g. Fedorenko et al. 2010, 2016; Vagharchakian et al. 2012; Regev et al. 2013; Scott et al. 2017; Deniz et al. 2019). The design of Experiment 3 was identical to that of Experiment 1, except that in the production conditions (the 2 critical conditions—SProd and WProd—and the NProd control condition), participants were asked to type their responses on a scanner-safe keyboard (described below) instead of saying them out loud. For the control VisEvSem condition, participants were asked to type their answers (1 or 2) on the keyboard instead of the button box. The 2 critical production conditions target the same cognitive processes as in Experiment 1, and the control NProd condition targets low-level hand motor planning and execution. Given that the participants in this experiment also participated in Experiment 1, for any given participant, a different experimental list was used than the list used for Experiment 1 (see Experiment 1 for details of how the lists were constructed).
Procedure. The procedure for Experiment 3 only differed from that of Experiment 1 in the trial timing for the 3 production conditions (SProd, WProd, and NProd). In particular, for these conditions, trial duration was increased from 3 to 7 s (0.2 s fixation and 6.8 stimulus presentation) given that typing takes longer than speaking (especially when typing in an unusual position, as described below). Each run therefore consisted of 12 experimental blocks (6 were 14 s each, as in Experiment 1, and 6 were 23 s each) and 3 fixation blocks of 12 s each, lasting a total of 300 s (5 min). The on-screen instructions for the production conditions were also adjusted to reflect the difference in output modality: “Type a description of the event” for SProd, “Type the names of the objects” for WProd, and “Copy the nonwords (typing)” for NProd. Each participant completed 6 runs (for a total of 12 blocks per condition). The order of experiments (Experiment 1 and Experiment 3) was counterbalanced across participants.
To collect the typed responses, we built a custom MR-safe wireless keyboard. We purchased an off-the shelf wireless keyboard (Inland model ic210) and removed all the ferrous mechanical parts, such as the case screws and the steel wires used to stabilize the wide keys (shift, return, and space keys). We then replaced the highly ferrous alkaline AA battery and pulse width modulated step-up voltage regulator with a lithium ion polymer (LiPo) battery and a linear low-drop out voltage regulator. The keyboard uses silicon dome switches and flexible conductive traces that were not found to be ferrous. The wireless USB receiver was plugged into the MRI suite’s penetration panel through a USB to DB9 filter to prevent the introduction of radio frequency (RF) interference into the MR images. The absence of RF interference introduced by the keyboard was confirmed by collecting time series of BOLD scans with and without the presence of the keyboard and keys being pressed during these scans and calculating the pixel-by-pixel temporal signal-to-noise ratio (tSNR) on a static quality assurance phantom.
During the experiment, the keyboard was placed directly on the participant’s abdomen or on a small non-ferrous platform placed on their abdomen, so they could quite comfortably type while lying in the scanner (akin to working on one’s laptop in bed); however, they were unable to see the output of their typing or their own keystrokes. Participants were given a chance to practice typing prior to the experiment to get accustomed to the setup and the keyboard layout. We collected and monitored the participants’ productions on a computer outside the scanning room.
The experimental script with all the materials is available at GitHub: https://github.com/jennhu/LanguageProduction.
fMRI data acquisition, preprocessing, and first-level modeling
Data acquisition
Whole-brain structural and functional data were collected on a whole-body 3 Tesla Siemens Trio scanner with a 32-channel head coil at the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research at MIT. T1-weighted structural images were collected in 176 axial slices with 1 mm isotropic voxels (repetition time (TR) = 2,530 ms; echo time (TE) = 3.48 ms). Functional, BOLD data were acquired using an EPI sequence with a 90o flip angle and using GRAPPA with an acceleration factor of 2; the following parameters were used: 31 4.4 mm thick near-axial slices acquired in an interleaved order (with 10% distance factor), with an in-plane resolution of 2.1 × 2.1 mm, FoV in the phase encoding (A > > P) direction 200 mm and matrix size 96 × 96 voxels, TR = 2,000 ms and TE = 30 ms. The first 10 s of each run were excluded to allow for steady state magnetization.
Preprocessing
fMRI data were analyzed using SPM12 (release 7487), CONN EvLab module (release 19b), and other custom MATLAB scripts. Each participant’s functional and structural data were converted from DICOM to NIFTI format. All functional scans were coregistered and resampled using B-spline interpolation to the first scan of the first session (Friston et al. 1995). Potential outlier scans were identified from the resulting subject-motion estimates as well as from BOLD signal indicators using default thresholds in CONN preprocessing pipeline (5 standard deviations above the mean in global BOLD signal change, or framewise displacement values above 0.9 mm; Nieto-Castañón 2020). Functional and structural data were independently normalized into a common space (the Montreal Neurological Institute [MNI] template; IXI549Space) using SPM12 unified segmentation and normalization procedure (Ashburner and Friston 2005) with a reference functional image computed as the mean functional data after realignment across all timepoints omitting outlier scans. The output data were resampled to a common bounding box between MNI-space coordinates (−90, −126, −72) and (90, 90, 108), using 2 mm isotropic voxels and 4th order spline interpolation for the functional data, and 1 mm isotropic voxels and trilinear interpolation for the structural data. Last, the functional data were smoothed spatially using spatial convolution with a 4 mm FWHM Gaussian kernel.
First-level modeling
For both the language localizer task and the critical experiments, effects were estimated using a general linear model (GLM) in which each experimental condition was modeled with a boxcar function convolved with the canonical hemodynamic response function (HRF) (fixation was modeled implicitly, such that all timepoints that did not correspond to one of the conditions were assumed to correspond to a fixation period). Temporal autocorrelations in the BOLD signal timeseries were accounted for by a combination of high-pass filtering with a 128 s cutoff, and whitening using an AR(0.2) model (first-order autoregressive model linearized around the coefficient a = 0.2) to approximate the observed covariance of the functional data in the context of restricted maximum likelihood estimation. In addition to experimental condition effects, the GLM design included first-order temporal derivatives for each condition (included to model variability in the HRF delays), as well as nuisance regressors to control for the effect of slow linear drifts, subject-motion parameters, and potential outlier scans on the BOLD signal.
Definition of the language network functional regions of interest
For each participant, we defined a set of language functional regions of interest (fROIs) using group-constrained, subject-specific localization (Fedorenko et al. 2010). In particular, each individual map for the sentences > nonwords contrast from the language localizer was intersected with a set of 6 binary masks. These masks (Fig. 2a; available at https://evlab.mit.edu/funcloc/) were derived from a probabilistic activation overlap map for the same contrast in a large set of participants (n = 220) using watershed parcellation, as described in Fedorenko et al. (2010) for a smaller set of participants. These masks covered the fronto-temporal language network in the left hemisphere. Within each mask, a participant-specific language fROI was defined as the top 10% of voxels with the highest t-values for the localizer contrast. (Note that we here included the language fROI within the angular gyrus (AngG) even though this fROI consistently patterns differently from the rest of the language fROIs in its functional response profile and patterns of functional correlations.) In other recent papers, we have started excluding the AngG language fROI to focus on the core set of 5 language fROIs. However, we chose to include it here given the importance of the “dorsal stream”—white matter tracts of the arcuate and/or superior longitudinal fasciculus that connect posterior-most temporal/parietal language areas and inferior frontal language areas—in language production (e.g. Hickok and Poeppel 2004; Fridriksson et al. 2016).
Fig. 2.
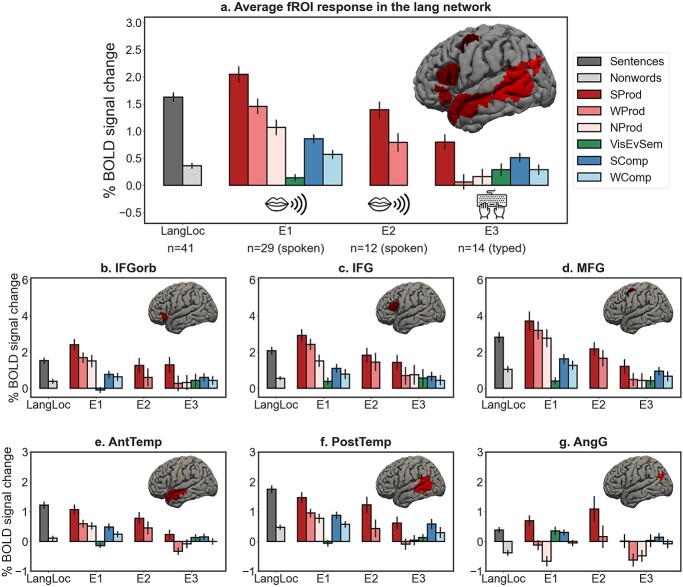
Responses in the language network. Responses in the language network to the language localizer conditions (dark gray = sentences, light gray = nonwords; the responses are pooled across all participants) and the conditions of the critical experiments (red shades = production conditions (from darker to lighter: sentence production (SProd), word-list production (WProd), and nonword-list production (NProd)); green = visual event semantics condition (VisEvSem); blue shades = comprehension conditions (dark = sentence comprehension (SComp), light = word-list comprehension (WComp))) in Experiments 1–3. The top panel shows the responses averaging across the 6 regions of the language network. On the brain inset, we show the parcels that were used to define the individual language functional ROIs (any individual fROI is 10% of the parcel, as described in Methods). The bottom panels show the responses for each of the 6 regions of the language network. Error bars represent standard errors of the mean over participants.
Definition of the MD network fROIs
For each participant (except for one participant in Experiment 1 who did not perform the relevant localizer), we defined a set of MD fROIs using group-constrained, subject-specific localization (Fedorenko et al. 2010). Each individual map for the hard > easy spatial working memory contrast from the MD localizer was intersected with a set of twenty binary masks (10 in each hemisphere). These masks (Fig. 3a; available at https://evlab.mit.edu/funcloc/) were derived from a probabilistic activation overlap map for the same contrast in a large set of participants (n = 197) using watershed parcellation. The masks covered the frontal and parietal components of the MD network (Duncan 2010, 2013) bilaterally. Within each mask, a participant-specific MD fROI was defined as the top 10% of voxels with the highest t-values for the localizer contrast.
Fig. 3.
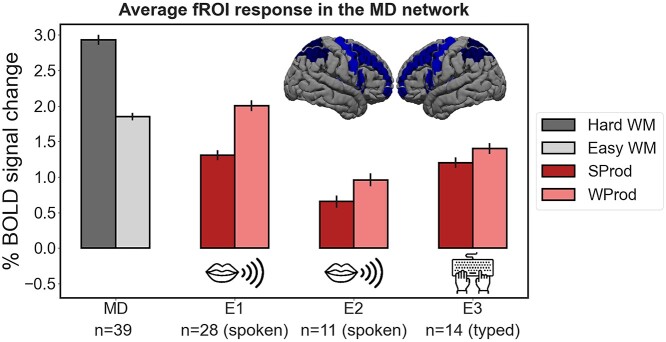
Responses in the MD network. Responses in the bilateral MD network to the MD localizer conditions (dark gray = hard spatial working memory (hard WM), light gray = easy spatial working memory (easy WM); the responses are pooled across all participants) and the production conditions of the critical experiments (dark red = sentence production (SProd), light red = word-list production (WProd)) in Experiments 1–3. Responses are averaged across the 20 regions of the MD network. On the brain inset, we show the parcels that were used to define the individual MD functional ROIs (any individual fROI is 10% of the parcel, as described in Methods). Error bars represent standard errors of the mean over participants.
Analyses
All analyses were performed with linear mixed-effects models using the “lme4” package in R (version 1.1.26; Bates et al. 2015) with P-value approximation performed by the “lmerTest” package (version 3.1.3; Kuznetsova et al. 2017) and effect sizes (Cohen’s d) estimated by the “EMAtools” package (version 0.1.3; Kleiman 2017).
Validation of the language and MD fROIs
To ensure that the language and MD fROIs behave as expected (i.e. show a reliable localizer contrast effect), we used an across-runs cross-validation procedure (e.g. Nieto-Castañón and Fedorenko 2012). In these analyses, the first run of the localizer was used to define the fROIs, and the second run to estimate the responses (in percent BOLD signal change, PSC) to the localizer conditions, ensuring independence (e.g. Kriegeskorte et al. 2009); then the second run was used to define the fROIs, and the first run to estimate the responses; finally, the extracted magnitudes were averaged across the 2 runs to derive a single response magnitude for each of the localizer conditions. Statistical analyses were performed on these extracted PSC values. As expected, the language fROIs showed a robust sentences > nonwords effect (Ps < 10−8, |d|s > 2.05; P-values corrected for the number of fROIs using the false discovery rate (FDR) correction; Benjamini and Yekutieli 2001), and the MD fROIs showed a robust hard > easy spatial working memory effect (Ps < 10−6, |d|s > 1.84). For participants with a single run of the MD localizer, the activation maps were visually inspected to ensure they look as expected.
Critical analyses
To estimate the responses in the language fROIs (and MD fROIs for one analysis) to the conditions of the critical experiments, the data from all the runs of the language (or MD) localizer were used to define the fROIs, and the responses to each condition were then estimated in these regions by averaging the effects across the voxels that comprise each individual fROI. Statistical analyses were performed on these PSC values.
For each relevant contrast (as described in detail below, in 1–3), we used 2 types of linear mixed-effect regression models: i) the network-wise model, which examined the language network as a whole; and ii) the fROI-wise models, which examined each language fROI separately, to paint a more detailed picture. As discussed in section Introduction, treating the language network as an integrated system is reasonable given that the regions of this network a) show similar functional profiles, both with respect to their selectivity for language (e.g. Fedorenko et al. 2011; Fedorenko and Blank 2020) and their role in lexico-semantic and combinatorial processing during language comprehension (e.g. Fedorenko et al. 2010, 2016, 2020; Fedorenko, Nieto-Castañon, and Kanwisher 2012; Bautista and Wilson 2016; Blank et al. 2016); and b) exhibit strong inter-region correlations in their activity during naturalistic cognition paradigms (e.g. Blank et al. 2014; Paunov et al. 2019; Braga et al. 2020) and in key functional markers, like the strength of response or the extent of activation in response to language stimuli (e.g. Mahowald and Fedorenko 2016; Mineroff et al. 2018). However, to examine potential differences among the language regions with respect to their role in language production, we supplement the network-wise analyses with the analyses of the 6 language fROIs separately. For the analyses of the MD network, we only use network-wise models given that we use the MD network for control purposes and do not have any hypotheses about differences among the MD regions with respect to the current experiments.
For the network-wise analyses, we fit a linear mixed-effect regression model, predicting the level of BOLD response in the language (or MD) fROIs in the contrasted conditions (as detailed in 1–3 below). The model included a fixed effect for condition and random intercepts for fROI and participant.
Effect size ~ condition + (1 | fROI) + (1 | participant)
For the fROI-wise analyses, we fit a linear mixed-effect regression model, predicting the level of BOLD response in the target fROI in the contrasted conditions. The model included a fixed effect for condition and a random intercept of participant. The results were FDR-corrected for the number of fROIs.
Effect size ~ condition + (1 | participant)
To characterize the responses in the language network to language production, we asked the following 3 questions.
Does the language network respond to sentence production?
First, we asked whether sentence production elicits a response in the language network. As discussed in section Introduction, overlap between the language comprehension system (targeted by our language localizer; Fedorenko et al. 2010) and the language production system is expected based on a) current theorizing about their relationship, b) evidence from psycholinguistic and neurophysiological studies, and c) previous PET/fMRI reports of overlap in some of the language regions in studies that have relied on the group-averaging approach (e.g. Awad et al. 2007; Menenti et al. 2011; Segaert et al. 2012; Silbert et al. 2014; Giglio et al. 2022). We also asked whether this response is ubiquitous across the language network or restricted to some of its regions, as some past studies and meta-analyses have suggested (e.g. Awad et al. 2007; Segaert et al. 2012; Walenski et al. 2019; Giglio et al. 2022), and whether it generalizes across output modalities.
To evaluate this question, we examined 4 contrasts. First, we compared the responses to the sentence production condition (SProd in each experiment) against the fixation baseline. Although fixation is a liberal baseline, it is important to note that many perceptual and cognitive conditions do not elicit a response in the language network above the fixation baseline, including executive tasks (Fedorenko and Blank 2020) and music perception (Chen et al. 2021). Second, we compared the responses to the sentence production condition against the response to the nonword strings condition from the language localizer—an unstructured and meaningless linguistic stimulus. Third and fourth, in Experiments 1 and 3, we compared the responses to the sentence production condition against the response to the low-level production condition (NProd) and the visual event semantics condition (VisEvSem). A brain region that supports sentence production should exhibit a response during the SProd condition (across output modalities) that falls above both the fixation baseline, the nonword strings condition from the language localizer, and the low-level production condition. If that brain region responds to language production demands rather than the visual and/or semantic processing associated with the processing of event pictures (cf. Ivanova et al. 2021; Ivanova 2022), it should also respond more strongly during sentence production than during a semantic task on the same pictures.
Does the language network contribute to phrase-structure building, lexical access, or both?
Focusing primarily on the well-powered Experiment 1, we probed the responses in the language network to 2 core aspects of high-level language production. As discussed in section Introduction, if the knowledge representations are shared between comprehension and production, and given that for comprehension, all regions of the language network respond to both lexico-semantic and combinatorial linguistic demands (e.g. Fedorenko et al. 2020), we might expect a similar picture to hold for production. To test the responses of the language regions to phrase-structure building demands above and beyond those associated with single word retrieval, we compared the responses to the sentence production condition (SProd) against the word-list production condition (WProd). To test the responses to lexical access demands above and beyond lower-level articulation demands, we compared the responses to the word-list production condition (WProd) against the nonword-list production condition (NProd). A brain region that contributes to phrase-structure building should show a SProd > WProd effect, and a brain region that contributes to lexical access should show a WProd > NProd effect.
In a control analysis, we asked whether the SProd condition might elicit stronger responses than the WProd condition because it is more cognitively demanding. To test this possibility, we examined the responses to these conditions in a set of brain regions that have been previously established to be robustly sensitive to general cognitive effort across domains: the regions of the fronto-parietal MD network (Duncan 2010, 2013; Fedorenko et al. 2013; Hugdahl et al. 2015; Shashidhara et al. 2019; Assem et al. 2020). This network is functionally distinct from the language network (see Fedorenko and Blank 2020 for review), and appears to respond during linguistic tasks only in the presence of external task demands, at least for language comprehension (Diachek et al. 2020; Fedorenko and Shain 2021).
Do any brain regions selectively support phrase-structure building during language production relative to comprehension?
Finally, we evaluated the idea from the literature about an asymmetry between production and comprehension: namely, that morpho-syntactic aspects of phrase-structure building are relatively more important for production than comprehension (e.g. Bock 1982, 1995; Matchin and Hickok 2019). For example, Bock (1982) writes, “many of the aspects of a sentence’s surface form appear to play a relatively minor role in comprehension”; sentence production, on the other hand, “requires the paraphernalia of the correct morphology, constituent structure, and order”. This hypothesis postulates the existence of some production-selective mechanisms. Matchin and Hickok (2019) advocate a version of this idea where the inferior frontal component of the language network selectively/preferentially supports morpho-syntactic demands in production (what they call “morpho-syntactic linearization,” or transforming “nonsequential conceptual information” into “sequences of morphemes”), in contrast to the posterior temporal component, which is hypothesized to support morpho-syntactic demands (“hierarchical structuring”) in both comprehension and production. Although the SProd > WProd contrast in the current study does not isolate morpho-syntactic aspects of combinatorial linguistic processing (i.e. it also includes semantic compositional aspects), it certainly includes those aspects. To evaluate the prediction of Matchin & Hickok’s proposal, we examined the responses of the IFG and PostTemp language fROIs (and other language fROIs, for completeness) to the SProd, WProd, SComp, and WComp conditions. The sizes of the SComp > WComp and SProd > WProd effects are predicted to be similar for the PostTemp fROI, and the SProd > WProd effect is predicted to be larger than the SComp > WComp effect in the IFG fROI, as would be evidenced by an interaction between task (production vs. comprehension) and stimulus (sentences vs. word lists). (If such an interaction were to occur in the IFG fROI, a claim about a difference in the profiles of the IFG and the PostTemp fROIs would further require a three-way interaction between task, stimulus, and fROI.)
In addition, we evaluated the production/comprehension asymmetry idea more broadly by asking whether any brain regions—including outside the core left-hemisphere language network—show selective responses to computations related to phrase-structure building during production relative to comprehension. To do so, we searched across the brain for regions that respond more strongly during the SProd condition than each of the WProd and SComp conditions. The SProd > WProd contrast targets voxels that are sensitive to phrase-structure building demands during language production—the aspect of production that is hypothesized to engage selective mechanisms, and the SProd > SComp contrast targets voxels that respond more strongly during sentence production than during sentence comprehension. For this search, we used a whole-brain group-constrained, subject-specific approach (Fedorenko et al. 2010), which is akin to the traditional whole-brain random-effects analysis (Holmes and Friston 1998), but is more statistically powerful and robust (Blank et al. 2022) given that it a) takes into account inter-individual variability in the precise locations of functional areas, and b) has built into it an across-runs cross-validation procedure to ensure that the regions that emerge show replicable responses over time. Using data from Experiment 1, we created for each participant a whole-brain map that represented a conjunction of contrasts (SProd > WProd and SProd > SComp; for each contrast, we selected the top 10% of most responsive voxels across the brain; the results were similar when selecting voxels based on fixed significance thresholds). Each participant’s map was binarized, with ones corresponding to voxels that fall in the top 10% of voxels for both contrasts above, and zeros otherwise. These individual maps were then overlaid, and watershed parcellation was performed, as described in Fedorenko et al. (2010); see also Julian et al. (2012), to search for areas that that show spatially consistent responses in the majority of participants. The resulting regions were then used as masks to define the individual fROIs using the same 2 contrasts, selecting the top 10% of voxels based on the t-values for each contrast in each parcel and taking the intersection of those voxel sets (the n% approach allows for the definition of the fROIs in each individual). Finally, across-runs cross-validation was used to estimate the responses to the critical conditions (SProd, WProd, and SComp) in these individually defined fROIs and to test for the replicability of the SProd > WProd and SProd > SComp contrasts. The responses of the fROIs with replicable response profiles were then examined with respect to the full range of experimental conditions (including the localizer conditions) to evaluate whether any region(s) is/are indeed selective for phrase-structure building during language production.
Results
Every region of the language network responds robustly to sentence production (across output modalities)
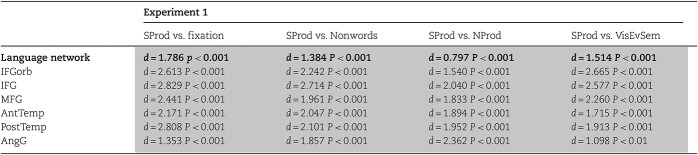
In line with past studies (e.g. Awad et al. 2007; Menenti et al. 2011; Segaert et al. 2012; Silbert et al. 2014; Giglio et al. 2022), spoken sentence production elicited a robust response in the language network across the 2 spoken production experiments. This response was stronger than i) the fixation baseline (Experiment 1: d = 1.786, P < 0.001; Experiment 2: d = 1.731, P < 0.001), ii) the nonword reading control condition from the language localizer (Experiment 1: d = 1.384, P < 0.001; Experiment 2: d = 1.587, P < 0.001), iii) the low-level production condition (Experiment 1: d = 0.797, P < 0.001), and iv) the visual event semantic processing condition (Experiment 1: d = 1.514, P < 0.001) (Fig. 2a, Table 1, Supplementary Table SI-2). (These effects held in all individual language fROIs [Fig. 2b-g, Table 1, Supplementary Table SI-2].)
Table 1.
Responses in the language network to spoken sentence production. Effect sizes (Cohen’s d) and estimated P-values for the effect of the spoken SProd condition (relative to 4 baselines) in linear mixed-effects regression models in experiment 1 (see Analyses, Q1). Models were fit to perform pairwise comparisons of sentence production (SProd) vs. each of the following conditions: Fixation, nonword comprehension (nonwords; from the language localizer), nonword production (NProd), and visual event semantics processing (VisEvSem). The results are shown averaged across the language network (top row), as well as at the level of individual functional ROIs in the language network (bottom 6 rows; FDR corrected). Gray cells highlight significance at P<0.05 in the predicted direction.
Moreover, the effects generalized to the typed output modality. Typed sentence production (Experiment 3) also elicited a stronger response in the language network than i) fixation (d = 1.027, P < 0.001), ii) nonword reading (d = 0.498, P = 0.003), iii) low-level production (d = 0.705, P < 0.001), and iv) visual event semantic processing (d = 0.576, P < 0.001) (Fig. 2a, Supplementary Table SI-2).
The language network contributes to both phrase-structure building and lexical access, but phrase-structure building elicits a stronger and more spatially extensive response
The language network responded to both phrase-structure building and lexical access. At the network level, sentence production (SProd) elicited a stronger response than word-list production (WProd) (d = 0.499, P < 0.001; Fig. 2, Table 2). This effect replicated in Experiment 2 (d = 0.609, P < 0.001) and Experiment 3 (d = 0.830, P < 0.001) (Fig. 2, Supplementary Table SI-3). Further, in Experiment 1, this effect was reliable in each of the 6 fROIs (Fig. 2, Table 2). Similarly, at the network level, word-list production (WProd) elicited a stronger response than nonword production (NProd) (d = 0.325, P = 0.004) (Fig. 2, Table 2; see Supplementary Fig. SI-2 for a control analysis that shows that the NProd condition elicits the expected strong responses in the premotor and motor cortex), but unlike the SProd > WProd effect, the WProd > NProd effect was only reliable in 3 of the 6 fROIs. Further, at the network level and in 5 of the 6 fROIs (the IFG language fROI being the exception), the SProd > WProd effect was numerically larger than the WProd > NProd effect, which suggests that the response to sentence production is more strongly driven by phrase-structure building demands. Interestingly, the region where lexical access demands elicited a larger effect than phrase-structure building demands was the IFG language fROI—a region that has been associated with combinatorial, not lexical, processing in much prior literature (e.g. Friederici 2002, 2011; Hagoort 2005).
Table 2.
Responses in the language network to phrase-structure building and lexical access. Effect sizes (Cohen’s d) and estimated P-values for the effects associated with phrase-structure building and lexical access in linear mixed-effects regression models in experiment 1 (see Analyses, Q2). Models were fit to perform pairwise comparisons of sentence production (SProd) vs. word-list production (WProd), and word-list production vs. nonword production (NProd). The results are shown averaged across the language network (top row), as well as at the level of individual functional ROIs in the language network (bottom 6 rows; FDR corrected).
| fROI | Experiment 1 | |
|---|---|---|
| SProd vs. WProd | WProd vs. NProd | |
| Language network | d = 0.499 p < 0.001 | d = 0.325 p < 0.01 |
| IFGorb | d = 1.852 P < 0.001 | d = 0.381 P = 0.387 |
| IFG | d = 1.029 P = 0.011 | d = 1.533 P < 0.01 |
| MFG | d = 1.068 P = 0.010 | d = 0.900 P = 0.049 |
| AntTemp | d = 1.756 P < 0.001 | d = 0.304 P = 0.428 |
| PostTemp | d = 1.994 P < 0.001 | d = 0.712 P = 0.105 |
| AngG | d = 2.519 P < 0.001 | d = 1.197 P = 0.011 |
In contrast to the language network, the MD network responded more strongly to the WProd condition than the SProd condition (d = 0.692, P < 0.001), providing evidence that word-list production is, in fact, more cognitively demanding than sentence production, and ruling out general cognitive difficulty as the explanation of the SProd > WProd effect in the language network (Fig. 3). This effect replicated in Experiment 2 (d = 0.353, P < 0.001) and Experiment 3 (d = 0.225, P = 0.01). It is worth noting that strong responses in the MD network during single-word production underscore the contributions of domain-general executive mechanisms to performance in confrontation naming tasks—one of the most commonly used clinical language assessment tools (e.g. Kaplan et al. 1983).
No evidence of brain regions that selectively support phrase-structure building during language production relative to comprehension
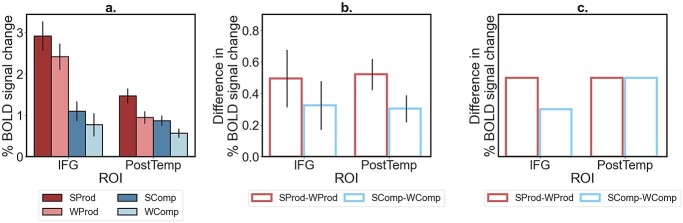
We do not find support for Matchin and Hickok’s (2019) proposal about the IFG language fROI selectively supporting phrase-structure building during production relative to comprehension. As shown in Figure 4a and b, contra this proposal’s prediction (illustrated in Fig. 4c; see also Matchin and Wood 2020 for a similar predictions figure), the effect of phrase-structure sensitivity in the IFG language fROI does not statistically differ between production and comprehension (P = 0.66; Supplementary Table SI-4), and the magnitude of the SProd > WProd effect (as well as the SComp > WComp effect) is strikingly similar between the IFG and PostTemp language fROIs (Fig. 4b). Instead, in both the IFG language fROI and the PostTemp language fROI, the language production conditions elicit a stronger response than the language comprehension conditions (Ps < 0.02). This effect also holds for the language network as a whole (P < 0.001) and is present in all language fROIs, except for the AngG fROI (Supplementary Table SI-4).
Fig. 4.
Responses of the IFG and PostTemp language fROIs during sentence and word-list production and comprehension. a) Responses of the IFG and PostTemp language fROIs to the language production and comprehension conditions: sentence production (SProd), word-list production (WProd), sentence comprehension (SComp), and word-list comprehension (WComp). Error bars represent standard errors of the mean over participants. b) Observed difference in response magnitudes for the SProd–WProd effect (red bars) and the SComp–WComp effect (blue bars). c) A pattern of differences in response magnitudes for the SProd–WProd and SComp–WComp effects in the 2 ROIs, as predicted by Matchin and Hickok’s (2019) proposal.
Our whole-brain search for brain regions that support phrase-structure building during production (as evidenced by the SProd > WProd effect) and also respond more strongly during sentence production than sentence comprehension (as evidenced by the SProd > SComp effect) revealed 9 regions (Supplementary Fig. SI-3). However, based on the full response profiles of these fROIs, none of them appear to be selective for phrase-structure building during production. In particular, 2 kinds of profiles characterize these regions. Four fROIs (marked in teal in Supplementary Fig. SI-3) in the posterior inferior temporal, occipital, and cerebellar cortex respond strongly to the visual event semantic condition, suggesting that their strong and selective response to the sentence production condition (relative to the word-list production and sentence comprehension conditions) is driven by the visual and/or semantic demands associated with the processing of event pictures (see also Ivanova 2022). And the remaining 5 fROIs (marked in yellow in Supplementary Fig. SI-3) in the left lateral and medial frontal cortex appear to overlap with the extended language-selective network, as they all show a robust response to the language localizer contrast (and no response to the MD localizer). The extended language network includes a number of cortical, as well as subcortical and cerebellar, areas outside of the left frontal and temporal cortex (e.g. Fedorenko et al. 2011; Lipkin et al. 2022), including the medial frontal areas that emerge in this analysis. It is not clear why these fROIs show relatively weak responses to the sentence comprehension (SComp) condition, not strongly differing from the response to the word-list comprehension (WComp) condition (cf. the response to the language localizer contrast). One possibility is that the sentences in the SComp condition are relatively short and simple, and semantically and structurally similar to one another (Appendix A; cf. the sentences in the language localizer, which are longer and more semantically and syntactically diverse; https://evlab.mit.edu/funcloc/). In any case, the strong response to the comprehension-based localizer contrast shows that these regions are not strongly selective for phrase-structure building in language production and also support high-level linguistic comprehension.
Discussion
We examined neural responses to cognitive demands associated with high-level language production—lexical access and phrase-structure building—in the fronto-temporal language-selective network (Fedorenko et al. 2011) using a robust precision fMRI approach. Across 3 experiments that employed a picture naming/description paradigm to elicit word- and sentence-level productions, we found that i) sentence production, spoken or typed, elicits a strong response across the entire language-selective network as defined by a comprehension-based localizer (Fedorenko et al. 2010); ii) the language network is sensitive to both phrase-structure building and lexical access, but phrase-structure building demands elicit a stronger and more spatially extensive effect, reliably manifesting in every language fROI; and iii) no region within the language network, or in the rest of the brain, appears to selectively support phrase-structure building in production relative to comprehension. Below, we contextualize these results in the current theoretical and empirical landscape of the field and discuss their implications.
Ubiquitous sensitivity to sentence production across the language network
Sentence production appears to recruit the entire fronto-temporal language-selective network. In each of the 6 language fROIs, we observed a large and reliable response during the sentence production condition (spoken or typed) in all 3 fMRI experiments. Because we focused on the fROIs that have been unambiguously and selectively linked to high-level language processing, including lexico-semantic and combinatorial processes (e.g. Fedorenko et al. 2010, 2020; Bautista and Wilson 2016), we can conclude that high-level comprehension and production draw on the same network, ruling out the possibility that the overlap is due to shared perceptual and/or motor demands. This finding aligns with earlier findings from neuroimaging studies that have relied on the group-averaging approach and reported overlap in parts of the language network (e.g. Awad et al. 2007; Menenti et al. 2011; Segaert et al. 2012; Silbert et al. 2014; Giglio et al. 2022), but goes beyond them in 2 ways. First, the individual-subject analytic approach ensures that the overlap between comprehension and production is not due to the artifacts of blurring nearby distinct regions, which is inherent in traditional group analyses (e.g. Nieto-Castañón and Fedorenko 2012).
Second, this is the first study that demonstrates that responses to sentence production are robust to the output modality (speaking vs. typing). In particular, a key signature of the language network is input-modality independence during comprehension, as evidenced by similar responses across listening and reading (e.g. Fedorenko et al. 2010; Vagharchakian et al. 2012; Regev et al. 2013; Scott et al. 2017; Chen et al. 2021), as well as during visual processing in sign language comprehension (e.g. Mac Sweeney et al. 2008). Here, we show that the language network also exhibits output-modality-independent responses during production, across speaking and typing. This generalization across modalities demonstrates that the observed effects concern higher-level aspects of production (access of linguistic representations and utterance planning) rather than lower-level implementation parts of the production pipeline. As discussed in section Introduction, the network of areas that support articulation (e.g. Bohland and Guenther 2006; Fedorenko and Thompson-Schill 2014; Basilakos et al. 2018; see Supplementary Fig. SI-2a for a profile of regions in this network) is distinct from the higher-level language network examined here. The hand motor control areas associated with writing or typing linguistic utterances have been less extensively investigated, but are also distinct from the language network (e.g. Roux et al. 2009; Longcamp et al. 2014; Willett et al. 2021; Supplementary Fig. SI-2b).
The fact that every region of the language network responds during both interpretation and generation of linguistic utterances suggests that this network plausibly stores our language knowledge—mappings between forms and meanings—that are, of course, necessary for both comprehension (by evaluating the input relative to these stored representations) and production (by searching these representations for the right words/constructions). These results align well with past theoretical proposals (e.g. Strijkers and Costa 2016; Gambi and Pickering 2017). The fact that responses to sentence production (as well as to sentence comprehension) are distributed across the language network aligns with growing evidence that this network constitutes a “natural kind” in the mind and brain, working as an integrated system to solve comprehension and production (e.g. Mesulam 1990; Blank et al. 2014; Fedorenko and Thompson-Schill 2014; Braga et al. 2020).
The language network supports both lexical access and phrase-structure building
As discussed in Section Introduction, high-level production includes selecting the right words and constructions (lexical access) and putting them together into well-formed strings, including ordering the words and selecting the right form of each word according to the intended meaning and the structure being built (e.g. selecting the plural form of a noun, or selecting the right tense for a verb) (phrase-structure building). We found that the language network is sensitive to cognitive demands associated with both of these components of high-level production, although the response to phrase-structure building demands is generally stronger.
We used a paradigm adapted from comprehension (e.g. Friederici et al. 2000; Humphries et al. 2007; Fedorenko et al. 2010) in an effort to separate lexical access and phrase-structure building demands. In comprehension, the question of whether different brain regions in the language network support the understanding of individual word meanings vs. combinatorial (syntactic/semantic) processing has long been controversial. Based on the critical review of the literature and several additional studies, Fedorenko et al. (2020) argued that no brain region within the language network is selective for combinatorial processing over the processing of single words (see also Chee et al. 1999; Keller et al. 2001; Röder et al. 2002; Bautista and Wilson 2016; Blank et al. 2016; see Toneva and Wehbe 2019; Schrimpf et al. 2021; Caucheteux et al. 2021 for converging evidence from relating human neural representations to those from artificial neural network models; and see Dick et al. 2001 for earlier arguments and evidence against syntax selectivity).
Here, we asked this question for language production. The response to phrase-structure building demands (evidenced by a stronger response during sentence production than word-list production) was reliable in every language fROI. This distributed nature of phrase-structure building a) parallels the distributed effects of syntactic demands during language comprehension (e.g. Blank et al. 2016; Shain et al. 2020; Shain et al. 2022), and b) aligns with evidence from aphasia, where damage to both frontal and temporal language areas and the white matter tracts connecting them can result in syntactic deficits (e.g. Kempler et al. 1991; Caplan et al. 1996; Dick et al. 2001; Wilson and Saygın 2004; Mesulam et al. 2015; Wilson et al. 2022; see de Bleser 1987 for a discussion of earlier evidence), thus adding to the growing evidence against focal implementation of combinatorial linguistic processing. Importantly, we showed that the sentence > word-list effect in production cannot be explained by general cognitive demands. In particular, the domain-general MD network (Duncan 2010, 2013)—which is sensitive to effort across domains (e.g. Duncan and Owen 2000; Fedorenko et al. 2013; Hugdahl et al. 2015; Shashidhara et al. 2019; Assem et al. 2020)—responded more strongly during word-list production than sentence production, in line with a similar effect that had been reported for comprehension (e.g. Diachek et al. 2020).
The response to lexical access demands was positive in all 6 language fROIs, but generally lower than the response to phrase-structure building and only statistically reliable in 3 of the 6 fROIs. This picture differs somewhat from what has been reported for language comprehension, where lexical access demands manifest reliably in every region of the language network (e.g. Fedorenko et al. 2010; Shain et al. 2021). Why the responses to lexical access demands in production in the current study were not as robust as those previously reported for comprehension is not clear. One possibility is that the objects that we used all had relatively high-frequency names, and some of these words may have already been activated in other conditions (e.g. sentence production or sentence/word-list comprehension) due to the semantic and lexical overlap in the materials. We leave to future work to investigate responses to lexical access demands in greater detail and across a wider range of materials and paradigms. We also acknowledge that some areas outside the boundaries of the language network, as defined here, may selectively contribute to lexical access in production, as has been suggested in some patient studies (e.g. Bi et al. 2011; Mesulam et al. 2013).
Evidence from aphasia deserves a brief mention given that some patients with aphasia have been argued to exhibit a selective grammatical deficit (“agrammatism”; see de Bleser 1987 for a historical overview). We would argue that no single study has compellingly established the existence of syntax-selective machinery (see also e.g. Badecker and Caramazza 1985; Berndt et al. 1996; for recent claims about the existence of such machinery, see e.g. Matchin et al. 2020). Such a demonstration would require establishing that the syntactic deficit a) is present in both production and comprehension (i.e. affects the underlying syntactic representations), b) generalizes across spoken and written modalities, diverse linguistic materials, and experimental paradigms, c) cannot be explained by low-level perceptual or motor difficulties, or non-linguistic factors (e.g. executive limitations), and d) is selective relative to other aspects of language (like lexical access). In fact, some evidence exists against a syntax-selective deficit in aphasia. First, anomia (lexical retrieval difficulties) is ubiquitous in aphasia, including for patients with agrammatic production and/or comprehension (e.g. Goodglass and Geschwind 1976; Blumstein 1988; see Lu et al. 2021 for related evidence from cortical stimulation), suggesting that brain areas whose damage leads to syntactic deficits also contribute to lexical access (see also Bates and Goodman 1997). And second, syntactic comprehension deficits and the kinds of errors that are observed in patients with expressive agrammatism have been shown to be inducible in neurotypical adults (e.g. Butterworth and Howard 1987; Miyake et al. 1994; Blackwell and Bates 1995), arguing against a representation-level explanation of such behaviors.
No evidence of brain regions that are selective for phrase-structure building during language production
We do not find evidence for the existence of brain mechanisms that selectively support phrase-structure building during production relative to comprehension. Such mechanisms have been hypothesized to exist given that morpho-syntactic processes play a critical role in sentence encoding during production (cf. Swets et al. 2013; Goldberg and Ferreira 2022) but are not strictly necessary during sentence comprehension, which is possible even when such cues are degraded or absent (e.g. Ferreira et al. 2002; Ferreira 2003; Levy 2008; Levy et al. 2009; Gibson et al. 2013; Ferreira and Lowder 2016; Mahowald et al. 2022). We evaluated a particular proposal due to Matchin and Hickok (2019), whereby the inferior frontal component of the language network is hypothesized to selectively support morpho-syntactic planning for language production (relative to comprehension). We found that effects associated with phrase-structure building demands were similar for production and comprehension in the inferior frontal (IFG) language fROI, and this pattern was similar to that in the posterior temporal (PostTemp) and other language fROIs. Instead, language regions responded overall more strongly to the production than the comprehension conditions, perhaps unsurprisingly given that production trails comprehension in development and is more challenging for adult language learners (e.g. Jakobson 1941). We also searched across the brain for regions with the hypothesized profile (a selective response to phrase-structure building during production) and did not find such regions.
Taken at face value, the lack of the asymmetry in our data between the inferior frontal and posterior temporal language regions is at odds with 2 recent published fMRI findings. In particular, Matchin and Wood (2020) and Giglio et al. (2022) both claim to find support for Matchin and Hickok’s (2019) hypothesis. However, both studies have limitations that makes their results difficult to interpret. Matchin and Wood (2020) report a study where participants i) read, ii) read and subvocally repeated, or iii) listened to Jabberwocky phrases (e.g. “these clopes this pand”), real words (e.g. “hermit dogma”), or nonwords (e.g. “ninyo pobset”). One problematic aspect of the design is that the production condition (reading and subvocally repeating strings) does not require high-level planning: participants simply repeat the already constructed stimuli (presented to them on the screen). More importantly, however, the authors do not report the magnitudes of response to their conditions, as would be needed for evaluating the hypothesis in question. In particular, the main results figure in their paper (Fig. 4) shows the t-statistics for the relevant contrasts, not beta weights or percent BOLD signal change values. Because t-values are scale-free (the ratio of the effect size to the standard error), they do not tell us about the size of the change in the BOLD signal. Instead, they provide a measure of confidence in the deflection from zero, with bigger t-values reflecting greater confidence in a positive effect, not a larger effect. As a result, as reported, the findings do not speak to Matchin and Hickok’s (2019) hypothesis.
Giglio et al. (2022) report a study that adapted the design from Pallier et al. (2011), who had compared neural responses to linguistic strings composed of the same number of words but varying in the syntactic structure so as to allow for shorter vs. longer composite phrases. Pallier et al. (2011) found that the neural response in the language regions increases as a function of the length of composite phrases (e.g. a string like “a girl with pigtails” elicits a stronger response than a string like “a girl a bag”). Giglio et al. (2022) developed a paradigm where on each trial, participants saw pictures of 2 possible agents (e.g. a girl and a woman) and were provided with the verbs (e.g. “to clap,” “to sleep”). They were then asked to either view the pictures and listen to the accompanying linguistic strings (comprehension conditions) or to generate linguistic strings (production conditions). Critically, the way the pictures were structured on the screen indicated the target syntactic structure. For example (as shown in Fig. 1 in Giglio et al’.s paper), when each agent had a box around it and a verb in a box above each, participants heard—or were asked to generate—strings like “a girl claps, a woman sleeps” (two clauses, each 2 content words long), but when the agents and the verbs were all within the same box, participants heard, or were asked to generate, strings like “a girl hears that a woman claps” (a clause that is 4 content words long). The authors conceptually replicated Pallier et al.’s (2011) results of stronger neural responses to longer phrases in their comprehension conditions (see also Shain et al. 2021). They also found a similar pattern in their production conditions. Of most relevance to the current investigation, they report similar slopes for these structural effects for comprehension and production in their middle temporal gyrus (MTG) ROI, and a higher slope for production in their IFG ROI. They take this pattern as evidence for a greater role of the IFG ROI in structure building during language production, compared to comprehension. In the Giglio et al.’s study, the interpretation difficulty results from how the ROIs are defined. In particular, the authors use large anatomical masks (Fig. 4d in their paper): one encompasses the entire middle temporal gyrus, and the other encompasses the IFG. This is problematic because in the inferior frontal cortex, language-selective areas lay adjacent to the areas of the MD network, which is functionally distinct from the language network (Fedorenko, Duncan, and Kanwisher 2012; see Fedorenko and Blank 2020 for review). Because MD areas are robustly sensitive to task difficulty across domains (e.g. Duncan and Owen 2000; Fedorenko et al. 2013; Shashidhara et al. 2019; Assem et al. 2020) and because the structurally more complex production conditions are more difficult (as evidenced by the behavioral data in Figs 2 and 3a in their paper), the inclusion of parts of the MD cortex in the region of interest effectively guarantees the pattern observed for the IFG ROI. (The MTG ROI also includes some non-language selective cortex given what we know about the topography of individual language areas (e.g. Fedorenko et al. 2010; Lipkin et al. 2022), but it does not include areas that are sensitive to general cognitive effort.)
We acknowledge the possibility that the lack of selectivity for morpho-syntactic encoding in production in our data may have to do with the fact that many participants relied on “headlinese” (Supplementary SI-1) at least some of the time (i.e. saying or typing “girl smelling a flower” instead of “A girl is smelling a flower”). Headlinese has its own set of syntactic constraints (e.g. Halliday 1967; Mårdh 1980; van Dijk 1988), and whether morpho-syntactic demands that are associated with the production of headlinese-style utterances are lower compared to the production of complete well-formed sentences is an empirical question that, to the best of our knowledge, has not been tackled before. But even if producing headlinese is easier with respect to morpho-syntactic encoding, it seems valuable to investigate sentence production under as natural conditions as possible. During everyday communication, we rarely speak in complete sentences, and if many/most participants produce headlinese-style responses when asked to describe pictures of events, then it seems worthwhile to understand the cognitive and neural infrastructure supporting this behavior.
The data pattern we observe here, along with the findings reported in past studies of language comprehension (e.g. Fedorenko et al. 2010, 2020; Fedorenko, Nieto-Castañon, and Kanwisher 2012; Pallier et al. 2011; Blank et al. 2016; Shain et al. 2020)—whereby phrase-structure building demands elicit strong responses across the language network during both comprehension and production—aligns with recent evidence from Shain et al. (2022). Shain and colleagues report a large-scale fMRI study that suggests that language comprehension involves computationally demanding word-by-word structure building operations even when participants passively listen to naturalistic stories. Thus, although comprehension is possible in many cases where morpho-syntactic cues are degraded or absent (e.g. Ferreira et al. 2002; Ferreira 2003; Levy 2008; Levy et al. 2009; Gibson et al. 2013; Ferreira and Lowder 2016; Mollica et al. 2020; Mahowald et al. 2022), it appears that rich syntactic structures are nevertheless always computed, contrary to arguments that human language processing is mostly approximate and shallow (e.g. Frank and Bod 2011).
In conclusion, we have shown that the language-selective network, which supports comprehension across modalities, also supports sentence production during speaking and typing. Similar to the strong integration between the processing of word meanings and combinatorial processing that has been observed for language comprehension (e.g. Fedorenko et al. 2020), we found that the language network is sensitive to both the demands associated with phrase-structure building and those associated with lexical access during language production, although the effects of phrase-structure building are more pronounced and reliably present in every language region. Finally, contra prior hypotheses, we did not find evidence of brain areas that are selective for phrase-structure building during production relative to comprehension; instead, sentence production appears to pose a higher cost to the language network than sentence comprehension. These results support the idea that the language network stores integrated linguistic knowledge, from phonotactic regularities, to morphological schemas, to words, to constructions, and this knowledge is accessed during both decoding and encoding of linguistic messages.
Supplementary Material
Acknowledgements
We would like to acknowledge the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research at MIT, and its support team. We thank former and current EvLab members (especially Josef Affourtit and Alvincé Pongos) for their help with data collection, Rachel Ryskin for extensive discussions of the relationship between comprehension and production, and the audience at the 2020 Neurobiology of Language conference and members of the Fedorenko and Gibson labs for helpful comments and discussions.
Contributor Information
Jennifer Hu, Department of Brain and Cognitive Sciences, MIT, Cambridge, MA 02139, United States.
Hannah Small, Department of Cognitive Science, Johns Hopkins University, Baltimore, MD 21218, United States.
Hope Kean, Department of Brain and Cognitive Sciences, MIT, Cambridge, MA 02139, United States; McGovern Institute for Brain Research, MIT, Cambridge, MA 02139, United States.
Atsushi Takahashi, McGovern Institute for Brain Research, MIT, Cambridge, MA 02139, United States.
Leo Zekelman, Program in Speech and Hearing Bioscience and Technology, Harvard University, Cambridge, MA 02138, United States.
Daniel Kleinman, Haskins Laboratories, New Haven, CT 06511, United States.
Elizabeth Ryan, St. George’s Medical School, St. George’s University, Grenada, West Indies.
Alfonso Nieto-Castañón, McGovern Institute for Brain Research, MIT, Cambridge, MA 02139, United States; Department of Speech, Language, and Hearing Sciences, Boston University, Boston, MA 02215, United States.
Victor Ferreira, Department of Psychology, UCSD, La Jolla, CA 92093, United States.
Evelina Fedorenko, Department of Brain and Cognitive Sciences, MIT, Cambridge, MA 02139, United States; McGovern Institute for Brain Research, MIT, Cambridge, MA 02139, United States; Program in Speech and Hearing Bioscience and Technology, Harvard University, Cambridge, MA 02138, United States.
Funding
This work was partially supported by the National Institutes of Health (awards R01-DC016607 and R01-DC016950 from NICHD to E.F.), the National Science Foundation (Graduate Research Fellowship #1745302 to J.H.), and research funds from the McGovern Institute for Brain Research, the Department of Brain and Cognitive Sciences, and the Simons Center for the Social Brain. E.F. was additionally supported by award U01-NS121471 from the National Institute of Neurological Disorders and Stroke.
Conflicts of interest statement: The authors declare no competing financial interests.
Authors’ contribution
| Role/Author | JH* | HS* | HK | AT | LZ | DK | ER | ANC | VF | EF |
|---|---|---|---|---|---|---|---|---|---|---|
| Conceptualization | ☑ | ☑ | ☑ | ☑ | ☑ | |||||
| Methodology | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ||
| Investigation (data collection) | ☑ | ☑ | ☑ | ☑ | ☑ | |||||
| Data curation | ☑ | ☑ | ||||||||
| Formal analysis | ☑ | ☑ | ||||||||
| Validation | ☑ | ☑ | ||||||||
| Visualization | ☑ | ☑ | ||||||||
| Software | ☑ | ☑ | ☑ | |||||||
| Resources | ☑ | |||||||||
| Writing—original draft | ☑ | ☑ | ☑ | |||||||
| Writing—review and editing | ☑ | ☑ | ☑ | ☑ | ||||||
| Project administration | ☑ | |||||||||
| Supervision | ☑ |
References
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005:26(3):839–851. [DOI] [PubMed] [Google Scholar]
- Assem M, Glasser MF, Van Essen DC, Duncan J. A domain-general cognitive core defined in multimodally parcellated human cortex. Cereb Cortex. 2020:30(8):4361–4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad M, Warren JE, Scott SK, Turkheimer FE, Wise RJS. A common system for the comprehension and production of narrative speech. J Neurosci. 2007:27(43):11455–11464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badecker W, Caramazza A. On considerations of method and theory governing the use of clinical categories in neurolinguistics and cognitive neuropsychology: the case against agrammatism. Cognition. 1985:20(2):97–125. [DOI] [PubMed] [Google Scholar]
- Basilakos A, Smith KG, Fillmore P, Fridriksson J, Fedorenko E. Functional characterization of the human speech articulation network. Cereb Cortex. 2018:28(5):1816–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E, Goodman JC. On the inseparability of grammar and the lexicon: evidence from acquisition, aphasia and real-time processing. Lang Cogn Process. 1997:12(5–6):507–584. [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw Articles. 2015:67(1):1–48. [Google Scholar]
- Bautista A, Wilson SM. Neural responses to grammatically and lexically degraded speech. Lang Cogn Neurosci. 2016:31(4):567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001:29(4):1165–1188. [Google Scholar]
- Berndt RS, Mitchum CC, Haendiges AN. Comprehension of reversible sentences in “agrammatism”: a meta-analysis. Cognition. 1996:58(3):289–308. [DOI] [PubMed] [Google Scholar]
- Bi Y, Wei T, Wu C, Han Z, Jiang T, Caramazza A. The role of the left anterior temporal lobe in language processing revisited: evidence from an individual with ATL resection. Cortex. 2011:47(5):575–587. [DOI] [PubMed] [Google Scholar]
- Blackwell A, Bates E. Inducing agrammatic profiles in normals: evidence for the selective vulnerability of morphology under cognitive resource limitation. J Cogn Neurosci. 1995:7(2):228–257. [DOI] [PubMed] [Google Scholar]
- Blank I, Kanwisher N, Fedorenko E. A functional dissociation between language and multiple-demand systems revealed in patterns of BOLD signal fluctuations. J Neurophysiol. 2014:112(5):1105–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank I, Balewski Z, Mahowald K, Fedorenko E. Syntactic processing is distributed across the language system. NeuroImage. 2016:127:307–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank A, Fedorenko E, Blank I. Group-constrained subject specific analysis as a more powerful alternative to the traditional random-effects fMRI analysis. 2022.
- Bleser R. From agrammatism to paragrammatism: German aphasiological traditions and grammatical disturbances. Cogn Neuropsychol. 1987:4(2):187–256. [Google Scholar]
- Blumstein SE. Neurolinguistics: an overview of language–brain relations in aphasia. In: Newmeyer FJ, editors. Linguistics: the Cambridge survey: volume 3: language: psychological and biological aspects. Vol. 3. Cambridge Core: Cambridge University Press; 1988. pp. 210–236. [Google Scholar]
- Bock K. Toward a cognitive psychology of syntax: information processing contributions to sentence formulation. Psychol Rev. 1982:89(1):1–47. [Google Scholar]
- Bock, K. (1995). Sentence production: from mind to mouth. In: Miller J. L. & Eimas P. D., editors, Speech, language, and communication (2nd ed., p. 181–216). Academic Press. https://www.sciencedirect.com/science/article/pii/B978012497770950008X. [Google Scholar]
- Bock K. Language production: methods and methodologies. Psychon Bull Rev. 1996:3(4):395–421. [DOI] [PubMed] [Google Scholar]
- Bohland JW, Guenther FH. An fMRI investigation of syllable sequence production. NeuroImage. 2006:32(2):821–841. [DOI] [PubMed] [Google Scholar]
- Bouchard KE, Mesgarani N, Johnson K, Chang EF. Functional organization of human sensorimotor cortex for speech articulation. Nature. 2013:495(7441):327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga RM, Buckner RL. Parallel interdigitated distributed networks within the individual estimated by intrinsic functional connectivity. Neuron. 2017:95(2):457–471.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga RM, DiNicola LM, Becker HC, Buckner RL. Situating the left-lateralized language network in the broader organization of multiple specialized large-scale distributed networks. J Neurophysiol. 2020:124(5):1415–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branigan HP, Pickering MJ, Cleland AA. Syntactic co-ordination in dialogue. Cognition. 2000:75(2):B13–B25. [DOI] [PubMed] [Google Scholar]
- Broca P. Remarks on the seat of the Faculty of Articulated Language, following an observation of Aphemia (loss of speech). Bull Soc Anat. 1861:6:330–357. [Google Scholar]
- Butterworth B, Howard D. Paragrammatisms. Cognition. 1987:26(1):1–37. [DOI] [PubMed] [Google Scholar]
- Caplan D, Hildebrandt N, Makris N. Location of lesions in stroke patients with deficits in syntactic processing in sentence comprehension. Brain. 1996:119(3):933–949. [DOI] [PubMed] [Google Scholar]
- Caucheteux, C., Gramfort, A., & King, J.-R. (2021). Disentangling syntax and semantics in the brain with deep networks. Proceedings of the 38th International Conference on Machine Learning. Proceedings of Machine Learning Research. http://proceedings.mlr.press/v139/caucheteux21a/caucheteux21a.pdf.
- Chater N, McCauley SM, Christiansen MH. Language as skill: intertwining comprehension and production. J Memory Lang. 2016:89:244–254. [Google Scholar]
- Chee MWL, Tan EWL, Thiel T. Mandarin and English single word processing studied with functional magnetic resonance imaging. J Neurosci. 1999:19(8):3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Affourtit J, Ryskin R, Regev TI, Norman-Haignere S, Jouravlev O, Malik-Moraleda S, Kean H, Varley R, Fedorenko E. The human language system does not support music processing. BioRxiv. 2021:2021(06):01.446439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomsky N. Aspects of the theory of syntax. MIT Press; 1965. [Google Scholar]
- Christiansen MH, Chater N. The now-or-never bottleneck: a fundamental constraint on language. Behav Brain Sci. Vol. 39. Cambridge Core; 2016. p. e62. [DOI] [PubMed] [Google Scholar]
- Deen B, Freiwald WA. Parallel systems for social and spatial reasoning within the cortical apex. BioRxiv. 2022. [Google Scholar]
- Deen B, Koldewyn K, Kanwisher N, Saxe R. Functional organization of social perception and cognition in the superior temporal sulcus. Cereb Cortex. 2015:25(11):4596–4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell GS, Chang F. The P-chain: relating sentence production and its disorders to comprehension and acquisition. Philos Trans R Soc B: Biol Sci. 2014:369(1634):20120394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deniz F, Nunez-Elizalde AO, Huth AG, Gallant JL. The representation of semantic information across human cerebral cortex during listening versus reading is invariant to stimulus modality. J Neurosci. 2019:39(39):7722–7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diachek E, Blank I, Siegelman M, Affourtit J, Fedorenko E. The domain-general multiple demand (MD) network does not support core aspects of language comprehension: a large-scale fMRI investigation. J Neurosci. 2020:40(23):4536–4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick F, Bates E, Wulfeck B, Utman JA, Dronkers N, Gernsbacher MA. Language deficits, localization, and grammar: evidence for a distributive model of language breakdown in aphasic patients and neurologically intact individuals. Psychol Rev. 2001:108(4):759–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk TA. News as discourse. Hillsdale, NJ: Lawrence Erlbaum Associates, Publishers; 1988. [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci. 2010:14(4):172–179. [DOI] [PubMed] [Google Scholar]
- Duncan J. The structure of cognition: attentional episodes in mind and brain. Neuron. 2013:80(1):35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000:23(10):475–483. [DOI] [PubMed] [Google Scholar]
- Duncan J, Assem M, Shashidhara S. Integrated intelligence from distributed brain activity. Trends Cogn Sci. 2020:24(10):838–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairs A, Michelas A, Dufour S, Strijkers K. The same ultra-rapid parallel brain dynamics underpin the production and perception of speech. Cereb Cortex Commun. 2021:2(3):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federmeier KD. Thinking ahead: the role and roots of prediction in language comprehension. Psychophysiology. 2007:44(4):491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Blank IA. Broca’s area is not a natural kind. Trends Cogn Sci. 2020:24(4):270–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Shain C. Similarity of computations across domains does not imply shared implementation: the case of language comprehension. Curr Dir Psychol Sci. 2021:30(6):526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Thompson-Schill SL. Reworking the language network. Trends Cogn Sci. 2014:18(3):120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Varley R. Language and thought are not the same thing: evidence from neuroimaging and neurological patients. Ann N Y Acad Sci. 2016:1369(1):132–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Hsieh P-J, Nieto-Castañón A, Whitfield-Gabrieli S, Kanwisher N. New method for fMRI investigations of language: defining ROIs functionally in individual subjects. J Neurophysiol. 2010:104(2):1177–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Behr MK, Kanwisher N. Functional specificity for high-level linguistic processing in the human brain. Proc Natl Acad Sci. 2011:108(39):16428–16433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Nieto-Castañon A, Kanwisher N. Lexical and syntactic representations in the brain: an fMRI investigation with multi-voxel pattern analyses. Neuropsychologia. 2012:50(4):499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, Kanwisher N. Language-selective and domain-general regions lie side by side within Broca’s area. Curr Biol. 2012:22(21):2059–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, Kanwisher N. Broad domain generality in focal regions of frontal and parietal cortex. Proc Natl Acad Sci. 2013:110(41):16616–16621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko, E., Scott, T. L., Brunner, P., Coon, W. G., Pritchett, B., Schalk, G., & Kanwisher, N. (2016). Neural correlate of the construction of sentence meaning. Proc Natl Acad Sci, 113 (41), E 6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Blank IA, Siegelman M, Mineroff Z. Lack of selectivity for syntax relative to word meanings throughout the language network. Cognition. 2020:203:104348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira VS. The persistence of optional complementizer production: why saying “that” is not saying “that” at all. J Mem Lang. 2003:48(2):379–398. [Google Scholar]
- Ferreira F, Lowder MW. Prediction, information structure, and good-enough language processing. In: Ross BH, editors. Psychology of learning and motivation. Vol. 65. Academic Press; 2016. pp. 217–247. [Google Scholar]
- Ferreira F, Bailey KGD, Ferraro V. Good-enough representations in language comprehension. Curr Dir Psychol Sci. 2002:11(1):11–15. [Google Scholar]
- Flinker A, Korzeniewska A, Shestyuk AY, Franaszczuk PJ, Dronkers NF, Knight RT, Crone NE. Redefining the role of Broca’s area in speech. Proc Natl Acad Sci. 2015:112(9):2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank SL, Bod R. Insensitivity of the human sentence-processing system to hierarchical structure. Psychol Sci. 2011:22(6):829–834. [DOI] [PubMed] [Google Scholar]
- Fridriksson J, Yourganov G, Bonilha L, Basilakos A, Den Ouden D-B, Rorden C. Revealing the dual streams of speech processing. Proc Natl Acad Sci. 2016:113(52):15108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD. Towards a neural basis of auditory sentence processing. Trends Cogn Sci. 2002:6(2):78–84. [DOI] [PubMed] [Google Scholar]
- Friederici AD. The brain basis of language processing: from structure to function. Physiol Rev. 2011:91(4):1357–1392. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Meyer M, Von Cramon DY. Auditory language comprehension: an event-related fMRI study on the processing of syntactic and lexical information. Brain Lang. 2000:74(2):289–300. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline J-B, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Hum Brain Mapp. 1995:3(3):165–189. [Google Scholar]
- Frost MA, Goebel R. Measuring structural–functional correspondence: spatial variability of specialized brain regions after macro-anatomical alignment. NeuroImage. 2012:59(2):1369–1381. [DOI] [PubMed] [Google Scholar]
- Gambi C, Pickering MJ. Models linking production and comprehension. In: The handbook of psycholinguistics. John Wiley & Sons, Ltd; 2017. pp. 157–181. [Google Scholar]
- Gibson E, Bergen L, Piantadosi ST. Rational integration of noisy evidence and prior semantic expectations in sentence interpretation. Proc Natl Acad Sci. 2013:110(20):8051–8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giglio L, Ostarek M, Weber K, Hagoort P. Commonalities and asymmetries in the neurobiological infrastructure for language production and comprehension. Cereb Cortex. 2022:32(7):1405–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AE, Ferreira F. Good-enough language production. Trends Cogn Sci. 2022:26(4):300–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldrick M, Ferreira V, Miozzo M, editors. The Oxford handbook of language production. Oxford University Press; 2014. [Google Scholar]
- Goodglass, H., & Geschwind, N. (1976). Language disturbance (aphasia). In: Carterette E. C. & Friedman M. P., editors, Handbook of perception.Vol. 7, p. 389–428). Academic Press. [Google Scholar]
- Guenther FH. Neural control of speech. MIT Press; 2016. [Google Scholar]
- Hagoort P. On Broca, brain, and binding: a new framework. Trends Cogn Sci. 2005:9(9):416–423. [DOI] [PubMed] [Google Scholar]
- Hagoort P, Indefrey P. The neurobiology of language beyond single words. Annu Rev Neurosci. 2014:37(1):347–362. [DOI] [PubMed] [Google Scholar]
- Halliday MAK. Grammar, society and the noun. London: H. K. Lewis for University College, London; 1967. [Google Scholar]
- Hebart MN, Dickter AH, Kidder A, Kwok WY, Corriveau A, Wicklin CV, Baker CI. THINGS: a database of 1, 854 object concepts and more than 26, 000 naturalistic object images. PLoS One. 2019:14(10):e0223792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004:92(1):67–99. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Work M, Barker PB, Jacobs MA, Breese EL, Maurer K. Re-examining the brain regions crucial for orchestrating speech articulation. Brain. 2004:127(7):1479–1487. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Friston KJ. Generalisability, random effects & population inference. NeuroImage. 1998:7(4, Part 2):S754. [Google Scholar]
- Honnibal M, Montani I, Van Landeghem S, Boyd A. Spacy: industrial-strength natural language processing in Python. Zenodo. 2020. [Google Scholar]
- Hsiao Y, Mac Donald MC. Production predicts comprehension: animacy effects in mandarin relative clause processing. J Mem Lang. 2016:89:87–109. [Google Scholar]
- Hugdahl K, Raichle ME, Mitra A, Specht K. On the existence of a generalized non-specific task-dependent network. Front Hum Neurosci. 2015:9:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries C, Binder JR, Medler DA, Liebenthal E. Time course of semantic processes during sentence comprehension: an fMRI study. NeuroImage. 2007:36(3):924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P. The spatial and temporal signatures of word production components: a critical update. Front Psychol. 2011:2:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P. The relationship between syntactic production and comprehension. In: Rueschemeyer S-A, Gaskell MG, editors. The Oxford handbook of psycholinguistics. 2nd ed. Oxford University Press; 2018. pp. 486–505. [Google Scholar]
- Indefrey P, Levelt WJM. The spatial and temporal signatures of word production components. Cognition. 2004:92(1):101–144. [DOI] [PubMed] [Google Scholar]
- Ivanova AA, Srikant S, Sueoka Y, Kean HH, Dhamala R, O’Reilly U-M, Bers MU, Fedorenko E. Comprehension of computer code relies primarily on domain-general executive brain regions. elife. 2020:9:e58906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova AA, Mineroff Z, Zimmerer V, Kanwisher N, Varley R, Fedorenko E. The language network is recruited but not required for nonverbal event semantics. Neurobiol Lang. 2021:2(2):176–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova AA. The role of language in broader human cognition: evidence from neuroscience. Doctoral dissertation, MIT. 2022.
- Jackendoff R. Foundations of language: brain, meaning, grammar, evolution. Oxford University Press; 2002. [DOI] [PubMed] [Google Scholar]
- Jackendoff R, Audring J. The texture of the lexicon: relational morphology and the parallel architecture. Oxford University Press; 2019. [Google Scholar]
- Jakobson R. Child language, aphasia, and phonological universals. Mouton; 1941. [Google Scholar]
- Julian JB, Fedorenko E, Webster J, Kanwisher N. An algorithmic method for functionally defining regions of interest in the ventral visual pathway. NeuroImage. 2012:60(4):2357–2364. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Bekkering H, Weintraub S, Goodglass H. The Boston naming test. Lea & Febiger; 1983. [Google Scholar]
- Keller TA, Carpenter PA, Just MA. The neural bases of sentence comprehension: a fMRI examination of syntactic and lexical processing. Cereb Cortex. 2001:11(3):223–237. [DOI] [PubMed] [Google Scholar]
- Kempler D, Curtiss S, Metter EJ, Jackson CA, Hanson WR. Grammatical comprehension, aphasic syndromes and neuroimaging. J Neurolinguistics. 1991:6(3):301–318. [Google Scholar]
- Kleiman E. EMAtools: data management tools for real-time monitoring/ecological momentary assessment data. R package version. 2017:0(1):3.https://CRAN.R-project.org/package=EMAtools. [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009:12(5):535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB. Lmer test package: tests in linear mixed effects models. J Stat Softw. 2017:82(13):1–26. [Google Scholar]
- Levelt WJM. Speaking: from intention to articulation. MIT Press; 1989. [Google Scholar]
- Levy, R. (2008). A noisy-channel model of human sentence comprehension under uncertain input. Proceedings of the 2008 Conference on Empirical Methods in Natural Language Processing, 234–243. Association for Computational Linguistics. https://aclanthology.org/D08-1025.
- Levy R, Bicknell K, Slattery T, Rayner K. Eye movement evidence that readers maintain and act on uncertainty about past linguistic input. Proc Natl Acad Sci. 2009:106(50):21086–21090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkin B, Small H, Affourtit J, Mineroff Z, Nieto-Castañón A, Fedorenko E. In defense of individual-level functional neural markers: evidence from large-scale fMRI datasets of functional ‘localizers’ for the language and the multiple demand networks. 2022.
- Long MA, Katlowitz KA, Svirsky MA, Clary RC, Byun TM, Majaj N, Oya H, Howard MA, Greenlee JDW. Functional segregation of cortical regions underlying speech timing and articulation. Neuron. 2016:89(6):1187–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longcamp M, Lagarrigue A, Nazarian B, Roth M, Anton J-L, Alario F-X, Velay J-L. Functional specificity in the motor system: evidence from coupled fMRI and kinematic recordings during letter and digit writing. Hum Brain Mapp. 2014:35(12):6077–6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Zhao Z, Zhang J, Wu B, Zhu Y, Chang EF, Wu J, Duffau H, Berger MS. Functional maps of direct electrical stimulation-induced speech arrest and anomia: a multicentre retrospective study. Brain. 2021:144(8):2541–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Sweeney M, Capek CM, Campbell R, Woll B. The signing brain: the neurobiology of sign language. Trends Cogn Sci. 2008:12(11):432–440. [DOI] [PubMed] [Google Scholar]
- Mahowald K, Fedorenko E. Reliable individual-level neural markers of high-level language processing: a necessary precursor for relating neural variability to behavioral and genetic variability. NeuroImage. 2016:139:74–93. [DOI] [PubMed] [Google Scholar]
- Mahowald K, Diachek E, Gibson E, Fedorenko E, Futrell R. Grammatical cues are largely, but not completely, redundant with word meanings in natural language. arXiv. 2022. [Google Scholar]
- Malik-Moraleda S, Ayyash D, Gallée J, Affourtit J, Hoffmann M, Mineroff Z, Jouravlev O, Fedorenko E. The universal language network: a cross-linguistic investigation spanning 45 languages and 12 language families. BioRxiv. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårdh I. Headlinese: on the grammar of English front page headlines. Liberläromedel/Gleerup; 1980. [Google Scholar]
- Matchin W, Hickok G. The cortical organization of syntax. Cereb Cortex. 2019:30(3):1481–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchin W, Wood E. Syntax-sensitive regions of the posterior inferior frontal gyrus and the posterior temporal lobe are differentially recruited by production and perception. Cereb Cortex Commun. 2020:1(1):tgaa 029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchin W, Basilakos A, Stark BC, Ouden D-B, Fridriksson J, Hickok G. Agrammatism and paragrammatism: a cortical double dissociation revealed by lesion-symptom mapping. Neurobiol Lang. 2020:1(2):208–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menenti L, Gierhan SME, Segaert K, Hagoort P. Shared language: overlap and segregation of the neuronal infrastructure for speaking and listening revealed by functional MRI. Psychol Sci. 2011:22(9):1173–1182. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990:28(5):597–613. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M, Wieneke C, Hurley R, Rademaker A, Thompson CK, Weintraub S, Rogalski EJ. Words and objects at the tip of the left temporal lobe in primary progressive aphasia. Brain J Neurol. 2013:136(Pt 2):601–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M, Thompson CK, Weintraub S, Rogalski EJ. The Wernicke conundrum and the anatomy of language comprehension in primary progressive aphasia. Brain. 2015:138(8):2423–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineroff Z, Blank IA, Mahowald K, Fedorenko E. A robust dissociation among the language, multiple demand, and default mode networks: evidence from inter-region correlations in effect size. Neuropsychologia. 2018:119:501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miozzo M, Pulvermüller F, Hauk O. Early parallel activation of semantics and phonology in picture naming: evidence from a multiple linear regression MEG study. Cereb Cortex. 2015:25(10):3343–3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Carpenter PA, Just MA. A capacity approach to syntactic comprehension disorders: making normal adults perform like aphasic patients. Cogn Neuropsychol. 1994:11(6):671–717. [Google Scholar]
- Mollica F, Siegelman M, Diachek E, Piantadosi ST, Mineroff Z, Futrell R, Kean H, Qian P, Fedorenko E. Composition is the core driver of the language-selective network. Neurobiol Lang. 2020:1(1):104–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti MM, Parsons LM, Osherson DN. Thought beyond language: neural dissociation of algebra and natural language. Psychol Sci. 2012:23(8):914–922. [DOI] [PubMed] [Google Scholar]
- Mook DG. In defense of external invalidity. Am Psychol. 1983:38(4):379–387. [Google Scholar]
- Nieto-Castañón A. Handbook of functional connectivity magnetic resonance imaging methods in CONN. Hilbert Press; 2020. [Google Scholar]
- Nieto-Castañón A, Fedorenko E. Subject-specific functional localizers increase sensitivity and functional resolution of multi-subject analyses. NeuroImage. 2012:63(3):1646–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozari N, Dell GS, Schwartz MF. Is comprehension necessary for error detection? A conflict-based account of monitoring in speech production. Cogn Psychol. 2011:63(1):1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Hickok G. Left posterior auditory-related cortices participate both in speech perception and speech production: neural overlap revealed by fMRI. Brain Lang. 2006:98(1):112–117. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971:9(1):97–113. [DOI] [PubMed] [Google Scholar]
- Pallier C, Devauchelle A-D, Dehaene S. Cortical representation of the constituent structure of sentences. Proc Natl Acad Sci. 2011:108(6):2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paunov AM, Blank IA, Fedorenko E. Functionally distinct language and theory of mind networks are synchronized at rest and during language comprehension. J Neurophysiol. 2019:121(4):1244–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering, M. J., & Garrod, S. (2004). Toward a mechanistic psychology of dialogue. Behav Brain Sci, 27(2), 169–190(Cambridge Core). [DOI] [PubMed] [Google Scholar]
- Pickering, M. J., & Garrod, S. (2013). An integrated theory of language production and comprehension. Behav Brain Sci, 36(4), 329–347(Cambridge Core). [DOI] [PubMed] [Google Scholar]
- Pylkkänen L. The neural basis of combinatory syntax and semantics. Science. 2019:366(6461):62–66. [DOI] [PubMed] [Google Scholar]
- Rastle K, Harrington J, Coltheart M. 358, 534 nonwords: the ARC nonword database. Q J Exp Psychol. 2002:55A:1339–1362. [DOI] [PubMed] [Google Scholar]
- Regev M, Honey CJ, Simony E, Hasson U. Selective and invariant neural responses to spoken and written narratives. J Neurosci. 2013:33(40):15978–15988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev TI, Affourtit J, Chen X, Schipper AE, Bergen L, Mahowald K, Fedorenko E. High-level language brain regions are sensitive to sub-lexical regularities. BioRxiv. 2021:2021(06):11.447786. [Google Scholar]
- Riès, SK, Dhillon, RK, Clarke, A, King-Stephens, D, Laxer, KD, Weber, PB, Kuperman, RA, Auguste, KI, Brunner, P, Schalk, G, et al. (2017). Spatiotemporal dynamics of word retrieval in speech production revealed by cortical high-frequency band activity. Proc Natl Acad Sci, 114 (23), E4530–E 4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röder B, Stock O, Neville H, Bien S, Rösler F. Brain activation modulated by the comprehension of normal and pseudo-word sentences of different processing demands: a functional magnetic resonance imaging study. NeuroImage. 2002:15(4):1003–1014. [DOI] [PubMed] [Google Scholar]
- Roux F-E, Dufor O, Giussani C, Wamain Y, Draper L, Longcamp M, Démonet J-F. The graphemic/motor frontal area Exner’s area revisited. Ann Neurol. 2009:66(4):537–545. [DOI] [PubMed] [Google Scholar]
- Saxe R, Brett M, Kanwisher N. Divide and conquer: a defense of functional localizers. NeuroImage. 2006:30(4):1088–1096. [DOI] [PubMed] [Google Scholar]
- Scholz J, Triantafyllou C, Whitfield-Gabrieli S, Brown EN, Saxe R. Distinct regions of right temporo-parietal junction are selective for theory of mind and exogenous attention. PLoS One. 2009:4(3):e 4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrimpf M, Blank I, Tuckute G, Kauf C, Hosseini EA, Kanwisher N, Tenenbaum J, Fedorenko E. The neural architecture of language: integrative modeling converges on predictive processing. Proc Natl Acad Sci. 2021:118(45):e2105646118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott TL, Gallée J, Fedorenko E. A new fun and robust version of an fMRI localizer for the frontotemporal language system. Cogn Neurosci. 2017:8(3):167–176. [DOI] [PubMed] [Google Scholar]
- Segaert K, Menenti L, Weber K, Petersson KM, Hagoort P. Shared syntax in language production and language comprehension—an fMRI study. Cereb Cortex. 2012:22(7):1662–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shain C, Blank IA, Van Schijndel M, Schuler W, Fedorenko E. FMRI reveals language-specific predictive coding during naturalistic sentence comprehension. Neuropsychologia. 2020:138:107307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shain C, Kean H, Lipkin B, Affourtit J, Siegelman M, Mollica F, Fedorenko E. ‘Constituent length’ effects in fMRI do not provide evidence for abstract syntactic processing. Bio Rxiv. 2021. [Google Scholar]
- Shain C, Blank IA, Fedorenko E, Gibson E, Schuler W. Robust effects of working memory demand during naturalistic language comprehension in language-selective cortex. J Neurosci. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashidhara S, Mitchell DJ, Erez Y, Duncan J. Progressive recruitment of the frontoparietal multiple-demand system with increased task complexity, time pressure, and reward. J Cogn Neurosci. 2019:31(11):1617–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert LJ, Honey CJ, Simony E, Poeppel D, Hasson U. Coupled neural systems underlie the production and comprehension of naturalistic narrative speech. Proc Natl Acad Sci. 2014:111(43):E 4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strijkers K, Costa A. The cortical dynamics of speaking: present shortcomings and future avenues. Lang Cogn Neurosci. 2016:31(4):484–503. [Google Scholar]
- Strijkers K, Costa A, Pulvermüller F. The cortical dynamics of speaking: lexical and phonological knowledge simultaneously recruit the frontal and temporal cortex within 200 ms. NeuroImage. 2017:163:206–219. [DOI] [PubMed] [Google Scholar]
- Sueoka Y, Paunov A, Ivanova A, Blank IA, Fedorenko E. The language network reliably ‘tracks’ naturalistic meaningful non-verbal stimuli. BioRxiv. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swets B, Jacovina ME, Gerrig RJ. Effects of conversational pressures on speech planning. Discourse Process. 2013:50(1):23–51. [Google Scholar]
- Tahmasebi AM, Davis MH, Wild CJ, Rodd JM, Hakyemez H, Abolmaesumi P, Johnsrude IS. Is the link between anatomical structure and function equally strong at all cognitive levels of processing? Cereb Cortex. 2012:22(7):1593–1603. [DOI] [PubMed] [Google Scholar]
- Toneva M, Wehbe L. Interpreting and improving natural-language processing (in machines) with natural language-processing (in the brain). Adv Neural Inf Proces Syst. 2019:14954–14964https://arxiv.org/abs/1905.11833. [Google Scholar]
- Tooley KM, Bock K. On the parity of structural persistence in language production and comprehension. Cognition. 2014:132(2):101–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagharchakian L, Dehaene-Lambertz G, Pallier C, Dehaene S. A temporal bottleneck in the language comprehension network. J Neurosci. 2012:32(26):9089–9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walenski M, Europa E, Caplan D, Thompson CK. Neural networks for sentence comprehension and production: an ALE-based meta-analysis of neuroimaging studies. Hum Brain Mapp. 2019:40(8):2275–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems RM, Haegen LV, Fisher SE, Francks C. On the other hand: including left-handers in cognitive neuroscience and neurogenetics. Nat Rev Neurosci. 2014:15(3):193–201. [DOI] [PubMed] [Google Scholar]
- Willett FR, Avansino DT, Hochberg LR, Henderson JM, Shenoy KV. High-performance brain-to-text communication via handwriting. Nature. 2021:593(7858):249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Saygın AP. Grammaticality judgment in aphasia: deficits are not specific to syntactic structures, aphasic syndromes, or lesion sites. J Cogn Neurosci. 2004:16(2):238–252. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Entrup JL, Schneck SM, Onuscheck CF, Levy DF, Rahman M, Willey E, Casilio M, Yen M, Brito A, et al. Recovery from aphasia in the first year after stroke. Brain. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P, Lai A, Hodosh M, Hockenmaier J. From image descriptions to visual denotations: new similarity metrics for semantic inference over event descriptions. Trans Assoc Comput Linguist. 2014:2:67–78. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.