Abstract
Pediatric hydrocephalus, the leading reason for brain surgery in children, is characterized by enlargement of the cerebral ventricles classically attributed to cerebrospinal fluid (CSF) overaccumulation. Neurosurgical shunting to reduce CSF volume is the default treatment that intends to reinstate normal CSF homeostasis, yet neurodevelopmental disability often persists in hydrocephalic children despite optimal surgical management. Here, we discuss recent human genetic and animal model studies that are shifting the view of pediatric hydrocephalus from an impaired fluid plumbing model to a new paradigm of dysregulated neural stem cell (NSC) fate. NSCs are neuroprogenitor cells that comprise the germinal neuroepithelium lining the prenatal brain ventricles. We propose that heterogenous defects in the development of these cells converge to disrupt cerebrocortical morphogenesis, leading to abnormal brain–CSF biomechanical interactions that facilitate passive pooling of CSF and secondary ventricular distention. A significant subset of pediatric hydrocephalus may thus in fact be due to a developmental brain malformation leading to secondary enlargement of the ventricles rather than a primary defect of CSF circulation. If hydrocephalus is indeed a neuroradiographic presentation of an inborn brain defect, it suggests the need to focus on optimizing neurodevelopment, rather than CSF diversion, as the primary treatment strategy for these children.
Keywords: hydrocephalus, neural stem cell, neuroprogenitor, cerebrospinal fluid, neurodevelopmental disorders
The standard model of cerebrospinal fluid circulation and pediatric hydrocephalus
First described by Hippocrates, hydrocephalus (Gr., “water on the brain”) is one of the oldest known neurological disorders with archeological records suggesting its recognition as far back as ancient Egypt (Aschoff et al. 1999). Hydrocephalus is characterized by enlargement of the cerebrospinal fluid (CSF)-filled ventricles (ventriculomegaly) and clinical symptoms of elevated intracranial pressure. Although hydrocephalus can present at any age, pediatric hydrocephalus affects 1/1,000 live births and is the most common reason for children to undergo brain surgery (Tully and Dobyns 2014). Secondary or acquired hydrocephalus occurs as a complication from an underlying pathology, typically hemorrhage, infection, or obstructive mass. Intraventricular hemorrhage due to prematurity is the most common etiology of acquired hydrocephalus in developed countries, compared with pathogenic infections in developing nations. Pediatric hydrocephalus without a clear inciting event or etiology is classified as primary or congenital.
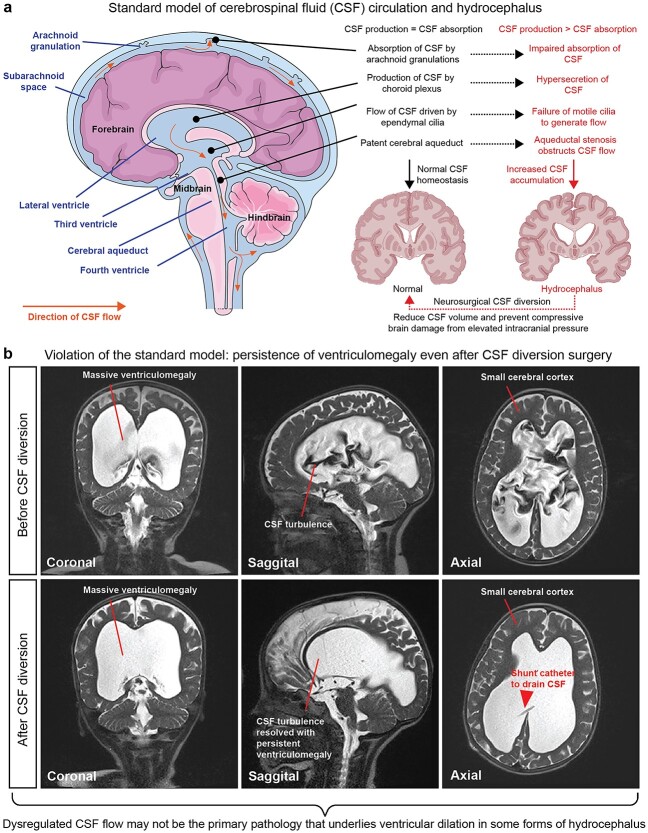
Embedded in the canonical definition of hydrocephalus is the belief that it is a “plumbing” disorder of failed CSF circulation (Fig. 1a). Pioneered by Walter Dandy more than a century ago, the “bulk flow” model posits that the choroid plexus secretes CSF into the ventricles, creating pressure gradients that drive CSF flow into the subarachnoid space, where it is reabsorbed into the systemic circulation via arachnoid granulations and villi (Symss and Oi 2013). Other proposed sites of CSF drainage include the para-vascular glymphatic system and lymphatic vessels (Iliff et al. 2012; Ma et al. 2017; Louveau et al. 2018). Ependymal cells lining the ventricles are also thought to contribute to CSF circulation, as they possess motile cilia that beat in an oar-like manner to produce near-wall flow (Worthington and Cathcart 1963; Ohata et al. 2014). Impaired absorption or obstruction of CSF flow is thought to cause hydrocephalus, and it can occur in the absence of anatomical obstruction (communicating or nonobstructive hydrocephalus), or with complete/partial obstruction (noncommunicating or obstructive hydrocephalus) most often due to aqueductal stenosis (Kahle et al. 2016). According to the standard model, an abnormality in any of the steps in CSF homeostasis (imbalance in production versus absorption of CSF, impaired cilia-driven fluid flow currents, or anatomical obstruction) is thought to initiate pathologic overaccumulation of CSF in the ventricular system (Fig. 1a). Fluid accumulation may result in elevated intracranial pressure and compression of the surrounding cerebral parenchyma (Fig. 1a). If left untreated, hydrocephalus may lead to neurologic compromise, coma, and death from fatal brain herniation.
Fig. 1.
Standard model of CSF circulation and hydrocephalus pathology. a) Standard model of CSF circulation in the adult human brain. Hydrocephalus is characterized by enlargement of the cerebral ventricles classically attributed to dysregulated CSF flow, leading to overaccumulation of CSF that distends the ventricles and raises the intracranial pressure. Neurosurgical CSF diversion that aims to reduce CSF volume and intracranial pressure is the primary treatment strategy for hydrocephalus. Image modified from Servier medical art (https://smart.servier.com) permitted by the creative commons attribution license (https://creativecommons.org/licenses/by/3.0/). b) Clinical case that demonstrates an example wherein the standard model of CSF circulation does not sufficiently explain the pathogenesis of hydrocephalus. Patient is a 3-year-old girl who presented with progressive macrocephaly. Neuroimaging prior to surgery (top panels) demonstrated extreme communicating ventriculomegaly with strikingly turbulent CSF flow and small cerebral cortex. A ventriculoperitoneal shunt was placed to divert CSF from the ventricles into the peritoneal cavity. Postoperative imaging showed complete resolution of CSF turbulence with persistence of ventriculomegaly. Although the patient’s progressive macrocephaly was arrested, she continued to exhibit mild neurocognitive impairments. Thus, reinstatement of CSF homeostasis may address some consequences of disease and prevent further deterioration but does not necessarily target the underlying developmental pathology. Images from patient were modified with permission from (Duy and Kahle 2021).
Persistence of neurodevelopmental disability despite CSF diversion in hydrocephalus
Based on the classical view that CSF overaccumulation begets hydrocephalus, lifelong neurosurgical shunting to reduce CSF volume has been the standard of care for this pathology since the 1950s. While neurosurgical shunting can be lifesaving for some patients by preventing further clinical deterioration, these surgical procedures are associated with complications and morbidity, including shunt malfunctions, infections, and repeat surgeries (Kahle et al. 2016). Furthermore, in many shunted hydrocephalic children, symptoms of intracranial hypertension may be relieved, yet neurodevelopmental outcomes remain poor and ventriculomegaly often persists (Maixner et al. 1990; Casey et al. 1997; Hoppe-Hirsch et al. 1998; Lindquist et al. 2005; Chance and Sandberg 2015; Rodríguez and Guerra 2017; Duy and Kahle 2021; Schiff et al. 2021) (see Fig. 1b for example of hydrocephalic patient who continues to exhibit enlarged ventricles and mild neurocognitive impairments after neurosurgical shunting). These observations underlie the fundamental challenge of pediatric hydrocephalus therapy: surgical CSF diversion can address some consequences of disease but does not necessarily target the underlying pathophysiological mechanisms of hydrocephalus. This raises the question of whether ventricular expansion in hydrocephalus is a primary active process or rather a consequence of an impaired developmental process that impacts multiple aspects of anatomic and functional brain maturation (Rodríguez and Guerra 2017; Duy et al. 2019; Vasung et al. 2022; Duy, Rakic, et al. 2022). The lack of satisfactory treatments highlights the urgency to better understand the mechanisms of pediatric hydrocephalus beyond the explanations provided by the standard model of CSF circulation.
A subset of pediatric hydrocephalus is increasingly being recognized not only as a disorder of aberrant CSF homeostasis but fundamentally as a brain parenchymal disorder secondary to impaired neurodevelopment (Miyan et al. 2001; Guerra and Guerra 2014; Guerra et al. 2017; Furey et al. 2018; Jin et al. 2020; Duy, Rakic, et al. 2022; Duy et al. 2022). Pediatric hydrocephalus often presents with a constellation of other structural brain defects, such as a markedly thin cerebral cortex (Furey et al. 2018; Jin et al. 2020), open lip schizencephaly (Furey et al. 2018; Allocco et al. 2019; Jin et al. 2020), agenesis of major axons such as in the corpus callosum and pyramidal tract (Adle-Biassette et al. 2013), and neural tube defects (Elgamal 2012; Chance and Sandberg 2015). Patients with primary and secondary forms of pediatric hydrocephalus may exhibit long-term neurodevelopmental deficits, including intellectual disability, motor disturbance, epilepsy, and cerebral palsy (Futagi et al. 2002; Persson et al. 2006; Gilard et al. 2018). Although brain dysfunction in the setting of hydrocephalus is commonly attributed to compression secondary to increased intracranial pressure (Del Bigio 1993; Khan et al. 2006; Del Bigio 2010), intact neurological functions or surprisingly mild impairments have been observed in rodents and human patients with ventriculomegaly and signs of mechanical compression (Lewin 1980; Canu et al. 2005; Feuillet et al. 2007; McMullen et al. 2012; Alvin and Miller 2016; Alders et al. 2018; Ferris et al. 2019). Furthermore, experimental blockade of CSF flow by mechanical obstruction often does not result in neurological symptoms despite leading to ventricular dilation (Campos-Ordonez and Gonzalez-Perez 2021; McAllister et al. 2021). Thus, brain damage due to mechanical compression or CSF hydrodynamic effects may not suffice to explain the persistence of neurodevelopmental disability in patients with hydrocephalus despite technically successful CSF diversion. The “paradoxes” of clinical hydrocephalus underscore the need to recognize pediatric hydrocephalus as a potential secondary sequela resulting from primary inborn brain defects and that ventriculomegaly is one cardinal feature accompanied by impairments in brain morphogenesis and neurobehavioral features.
Neural stem cells (NSCs) are the progenitor cells of all neurons and macroglia of the cerebral cortex. Normal brain morphogenesis depends on the ability of NSCs to expand and generate diverse neuronal and glial cell types at the appropriate brain regions and developmental time. As we describe below, genetic mutations, infections, and hemorrhage leading to pediatric hydrocephalus have all been identified as pathological processes that may disrupt prenatal NSC development. We posit that altered NSC development is a unifying cellular pathology that underlies diverse forms of pediatric hydrocephalus. We discuss the multitude of genetic and secondary triggers that cause pediatric hydrocephalus by impairing prenatal NSC development and provide a reappraisal of current therapeutic paradigms.
Neuroembryology and hydrocephalus: neuroprogenitors at the brain–CSF interface during development
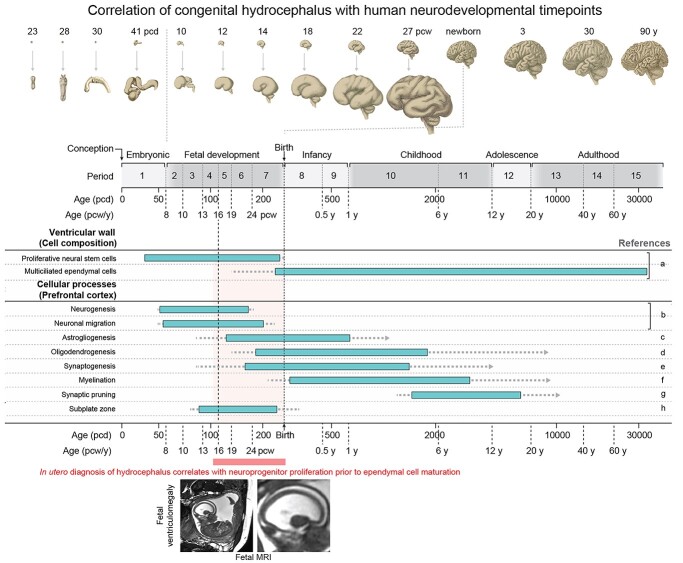
Figure 2 summarizes several major developmental events across time of human brain development (see Silbereis et al. 2016 for more detailed review). The average length of gestational development (defined as the time from conception to birth) is ~38 weeks and can be divided into 3 equal trimesters: first (postconception week, PCW, 0–11), second (PCW 12–25), and third trimester (PCW 26–38). Neural development begins early in the first trimester with the formation of the neural tube from the ectoderm at PCW4. The hollow fluid-filled lumen of the tube forms the ventricular system, whereas the wall of the neural tube is formed entirely of proliferative NSCs called neuroepithelial cells that later transition into radial glia NSCs. The first trimester is characterized by rapid NSC proliferative expansion at the ventricular wall, thereby amplifying the progenitor pool before the onset of neurogenesis by ~PCW7. The bulk of cortical neurogenesis (excitatory neurons) is completed during the second trimester (~PCW27) with certain other interneuron populations generated afterward with potential extension into postnatal life. Gliogenesis follows neurogenesis and generally peaks around birth. For most of gestational brain development, the cerebral ventricles are lined by a germinal neuroepithelium (also termed the ventricular zone) consisting of self-renewing NSCs capable of subsequent neurogenic or gliogenic divisions (Fig. 3). Neuronal progenies of NSCs are born at the ventricular neuroepithelium and migrate to their final positions in the overlying cortical plate, which is the prospective cerebral cortex. After birth, NSCs of the ventricular wall are replaced by a monolayer of multiciliated ependymal cells, progenies of terminally differentiated NSCs. Although immature ependymal cells begin to appear in the ventricular wall during the second trimester, mature multicilated ependymal cells do not cover the majority of the ventricular wall surface until after birth in both human and mouse (Coletti et al. 2018). Furthermore, the presence of ependymal cells on histology does not imply functionality, as optical coherence tomography imaging of ventricular explants has shown that ependymal cilia are not functionally mature until 1 week after birth in the mouse (Duy et al. 2022).
Fig. 2.
Timeline of key events in human cerebral cortex development and correlation to in utero diagnosis of hydrocephalus. Figure modified from Silbereis et al., Neuron, 2016 (Silbereis et al. 2016). Top and bottom panels provide a timeline of human cerebrocortical development (Kang et al. 2011) with age in postconceptional days (pcd), postconceptional weeks (pcw), and postnatal years (y). The schematic provides approximate timing and sequence of cellular processes in the ventricular wall and prefrontal cortex. Bars indicate the peak developmental period in which each feature is acquired; dotted lines indicate that feature acquisition occurs to lesser degrees at these ages, and arrows indicate that the feature is present thereafter throughout life. A human MRI showing in utero diagnosis of hydrocephalus is shown, corresponding to a period during which proliferative NSCs make up the ventricular wall well before appearance of multiciliated ependymal cells. Right column lists relevant references: a) Coletti et al. (2018); b) Bystron et al. (2006), Meyer (2007), Workman et al. (2013); c) Choi and Lapham (1978), deAzevedo et al. (2003), Kang et al. (2011); d) Kang et al. (2011), Yeung et al. (2014); e) Huttenlocher (1979), Kwan et al. (2012), Molliver et al. (1973), Petanjek et al. (2011); f) Miller et al. (2012), Yakovlev and Lecours (1967); g) Huttenlocher (1979), Petanjek et al. (2011); h) Kostovic and Rakic (1990).
Fig. 3.
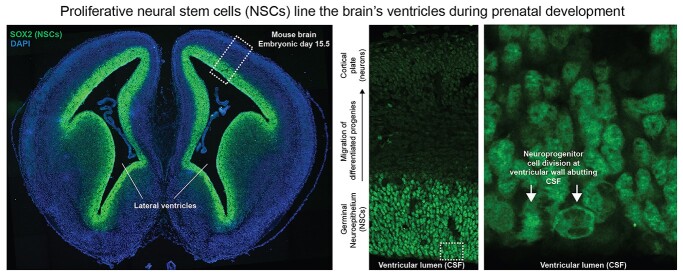
NSCs line the cerebral ventricles before birth. Shown are fluorescent images of a coronal section from an embryonic day 15.5 wild-type mouse brain that was stained with DAPI and SOX2 (a marker of NSCs). SOX2+ NSCs line the entire cerebral ventricular system. Zoomed in images show neuroprogenitor cell division at the apical surface of the ventricular wall directly adjacent to CSF. NSCs are therefore the predominant cell types at the brain–CSF interface before birth. Images were modified from (Allocco et al. 2019) permitted by the creative commons attribution license (https://creativecommons.org/licenses/by/4.0/).
Given that pediatric hydrocephalus can be diagnosed in utero by routine ultrasonography as early as PCW15 during early second trimester (Pretorius et al. 1985), hydrocephalus onset in some cases thus correlates more with periods of active NSC proliferation in the ventricular wall and neurogenesis rather than ependymogenesis (Fig. 2, example of a noninvasive MRI showing fetal hydrocephalus is shown). The manifestation of hydrocephalus prior to the functional emergence of mature ependymal cells and presumably cilia-driven CSF flow suggests that loss of CSF flow due to motile cilia failure is unlike to be the developmental mechanism that initiates ventriculomegaly in pediatric hydrocephalus diagnosed in utero. Other classic CSF circulation components, such as CSF absorption by arachnoid granulations and CSF secretion by the choroid plexus, also mature after birth (Radoš et al. 2021; Jones and Sellars 1982; Xu et al. 2021). Thus, the anatomical and cellular elements involved in driving active CSF circulation, at least according to the standard model, have yet to mature in the brain at time points when hydrocephalus can already be detected during early cortical development by routine pregnancy imaging. This challenges the dogma that dysfunction of CSF circulation components (such as “clogging” of the arachnoid granulations leading to reduced CSF drainage or defective cilia-generated CSF flow) is crucial for the pathogenesis of pediatric hydrocephalus (Kousi and Katsanis 2016). Rather, the detection of hydrocephalus in utero suggests that the primary pathogenesis in some cases may instead arise from abnormal development of ventricular wall NSCs and altered cortical neurogenesis.
Disruption of the ventricular neuroepithelium in human pediatric hydrocephalus
Neuropathological examinations of postmortem brain tissues from human patients with primary and posthemorrhagic forms of pediatric hydrocephalus have revealed abnormalities in the germinal neuroepithelium (Xue et al. 2003; Domínguez-Pinos et al. 2005; de Wit et al. 2008; Del Bigio 2011; Sival et al. 2011; Guerra et al. 2015; McAllister et al. 2017; Dohare et al. 2019). The major neuropathological findings in the human hydrocephalic brain tissues include: (i) abnormal expression of cell junction molecules in the neuroepithelium; (ii) formation of periventricular heterotopias (nodules of misplaced neurons), and (iii) disruption and denudation of the neuroepithelium, leading to NSC loss and release from the epithelial surface into CSF (Krueger et al. 2006). Intraventricular hemorrhage is also associated with a profound decrease in cell division and neural proliferation in the neuroepithelium (Xue et al. 2003; Del Bigio 2011; Dohare et al. 2019). Similar disruptions in the ventricular neuroepithelium have also been described in genetic rodent models of hydrocephalus, such as the hyh mutant mouse and the H-Tx rat (Jiménez et al. 2001; Chae et al. 2004; Guerra et al. 2015). These neuropathological events can precede the manifestation of hydrocephalus, appearing as early as the 16th gestational week in human pediatric hydrocephalus patients (Domínguez-Pinos et al. 2005), corresponding to critical developmental timepoints of proliferative NSC expansion and cortical neurogenesis.
It is also important to clarify that while previous reports have often used the term “ependyma” to refer to the ventricular lining (Jiménez et al. 2001), mature ependymal cells do not cover the majority of the lateral ventricular lining until 10 days after birth in humans (Coletti et al. 2018). Rather, the ventricles are lined primarily by NSCs (neuroepithelial cells and radial glia cells) throughout most of prenatal brain development, at least during the first and second trimesters of gestation. Thus, the major finding from neuropathological examinations of human fetuses with pediatric hydrocephalus is disruption of the NSC-enriched neuroepithelial lining, not of the multiciliated ependyma. These findings directly correlate the pathogenesis of human pediatric hydrocephalus with perturbations of the proliferative NSC compartments lining the cerebral ventricles.
Genetically encoded dysregulation of prenatal NSCs causes human congenital hydrocephalus
Perhaps, the most unbiased and clinically relevant approach to understand disease pathogenesis is through the discovery of causative gene mutations in human patients (Chong et al. 2015). Studies of familial congenital hydrocephalus (primarily of consanguineous unions) have identified several genes that cause disease with X-linked (L1CAM and AP1S2) or autosomal recessive (MPDZ and CCDC88C) inheritance (Rosenthal et al. 1992; Tarpey et al. 2006; Ekici et al. 2010; Al-Dosari et al. 2013; Shaheen et al. 2017). More recent studies using whole exome sequencing of numerous case-parent trios have found that damaging de novo gene mutations account for ~20% of patients with sporadic (nonfamilial) congenital hydrocephalus, with TRIM71, PTEN, SMARCC1, FOXJ1, and PIK3CA exhibiting exome-wide significant enrichment of de novo mutations (Furey et al. 2018; Jin et al. 2020; Duy et al. 2022). Over 100 other genes have been described to be mutated in syndromic hydrocephalus (Kousi and Katsanis 2016). Although hydrocephalus-associated genes have diverse molecular functions and are involved in numerous pathways, integrative genomic analyses have revealed the convergence of these genes in cortical neurogenesis elements (Jin et al. 2020; Hale et al. 2021; Duy et al. 2022), contrary to the dogma that hydrocephalus arises from abnormal fluid hydrodynamics. Below, we highlight several cellular and molecular pathways that illustrate the convergence of diverse genetic cause of congenital hydrocephalus on the regulation of prenatal NSC progression in the ventricular neuroepithelium, with particular focus on genes discovered in human hydrocephalus patients.
Ciliopathies
Dysfunction of motile cilia protruding from ependymal cells has been a popular explanation for the pathogenesis of hydrocephalus. It is believed that abnormal ciliary beating by the multiciliated ependyma reduces CSF circulation, contributing to pathologic overaccumulation of CSF responsible for ventricular dilation. This explanation is likely an oversimplification for several reasons.
First, much of the evidence linking cilia dysfunction to hydrocephalus has come from mouse studies in which knockout of cilia-related genes results in ventriculomegaly. However, human motile ciliopathies are rare causes of hydrocephalus (Jin et al. 2020). When hydrocephalus does occur in motile ciliopathies, it is often associated with systemic findings such as lung disease or altered body symmetry, reflecting the presence of motile cilia in peripheral organs (Wallmeier et al. 2019). The predominant phenotype of human ciliopathies is a syndrome characterized by symptoms referable to altered extracerebral ciliary function rather than a pathology that selectively impacts cilia in the cerebral ventricles. Thus, ciliopathies do not explain the pathogenesis of human hydrocephalus cases that lack clinical manifestations referable to altered motile cilia function outside of the brain.
Second, human and murine ventriculomegaly can occur independently of motile cilia function, as several genetic mouse models of hydrocephalus exhibit ventriculomegaly without defects in the multiciliated ependyma (Shimada et al. 2019; Ito et al. 2021; Duy et al. 2022). Similarly, knocking out S100A10 in the mouse disrupts ependymal cilia orientation associated with reduced CSF circulation, yet ventricular dilation does not occur (Seo et al. 2021). Despite that motile cilia are absent in the ventricular wall during most of gestational brain development (Coletti et al. 2018), hydrocephalus in humans can already be diagnosed as early as PCW15 by noninvasive fetal imaging (Pretorius et al. 1985). Similar to human observations, numerous mouse models hydrocephalus, including a ciliopathy model, exhibit ventriculomegaly at birth prior to the maturation of motile cilia at the ventricular wall (Carter et al. 2012; Banizs et al. 2005; Duy et al. 2022). The manifestation of hydrocephalus prior to maturation of motile cilia suggests that abnormal ependymal ciliary function is not causally related to the phenotype of ventricular dilation.
Third, the contribution of motile cilia to intraventricular CSF transport remains a topic of debate. A study of ventricular cilia in the zebrafish suggests that motile ciliary beating actually prevents mixing of CSF between ventricular compartments, while net CSF flow across compartments is driven by cardiac pulsations and body movement (Olstad et al. 2019). In vivo endoscopic video motion analyses of human ventricles demonstrate that ventricle wall motion and CSF pressure variations correspond to cardiac frequencies, pointing to pulsations driven by the heart rather than ependymal cilia as the main driver of intraventricular CSF flow (Butler et al. 2017). The causal links between abnormalities of ependymal ciliary function, presumably altered CSF transport, and ventriculomegaly remain to be established. At the very least, impaired cilia-driven CSF flow does not appear to be an absolute requirement for the development of the hydrocephalus phenotype (Shimada et al. 2019; Ito et al. 2021; Seo et al. 2021).
Finally, a CSF hydrodynamic-focused perspective of ciliary function neglects the developmental functions of nonmotile primary cilia that may also contribute to aberrant neurodevelopment in hydrocephalus (Guemez-Gamboa et al. 2014). Apical domains of ventricular zone NSCs contain primary cilia that are in direct contact with CSF, making them well-positioned to detect growth factors and morphogens that influence patterning, cell fate determination, and migration, among other aspects of NSC development (Guemez-Gamboa et al. 2014; Hasenpusch-Theil et al. 2020). Thus, dysfunction of the ventricular zone nonmotile primary cilia could impair prenatal NSC progression that directly contributes to ventriculomegaly and cortical thinning in hydrocephalus in the absence of impaired motile ciliary function. Multiple mouse studies have shown that genetic ablation of primary cilia restricted to prenatal NSCs results in hydrocephalus associated with altered neural proliferation and decreased numbers of cortical neurons, which is unlikely explained in full by altered CSF circulation (Tong et al. 2014; Foerster et al. 2017; Schock et al. 2017).
Neuroepithelial architecture and morphology
The cerebroventricular wall comprises proliferative NSCs forming a pseudostratified neuroepithelium during most of human gestation. Like other epithelial tissues, the neuroepithelium is notable for its polarized morphology, at the level of individual NSC (apical-basal polarity) and at the level of NSC populations within the same plane (planar cell polarity). Neuroepithelial integrity depends on the presence of junctional molecules that anchor NSCs to each other and to the apical surface of the ventricular wall. Multiple known congenital hydrocephalus genes are involved in adhesion/junction (MPDZ and L1CAM) (Weller and Gärtner 2001; Feldner et al. 2017), apical-basal polarity (CRB2) (Slavotinek et al. 2015), and planar cell polarity (reviewed extensively in (Kousi and Katsanis 2016). Disruptions of these genes could contribute to altered integrity and architecture of the neuroepithelium lining the cerebral ventricles, leading to a weakened wall more susceptible to deformation and ventricular dilation. Structural abnormalities in the neuroepithelium may also affect cortical neurogenesis and lead to hypoplasia of the cortical mantle in congenital hydrocephalus.
Embryonic patterning of NSCs lining the cerebral ventricles
The mammalian cerebral cortex is partitioned into discrete yet interconnected regions that process particular aspects of physiology and behavior, including sensation, movement, and cognition (Sur and Rubenstein 2005; Rakic 2009). The complex and intricate arealization of the cortex necessitates mechanisms that pattern proliferative NSCs lining the cerebral ventricles to generate the correct progeny at the appropriate time and place. Dysregulation of neural patterning is thus associated with severe malformations of the cortex (classically holoprosencephaly) and ventricular system. Several human congenital hydrocephalus genes are classically involved in patterning of the nervous system, including ventral-dorsal patterning (SHH and its receptor PTCH1) (Furey et al. 2018; Jin et al. 2020) and anterior–posterior patterning (CCDC88C encoding DAPLE, which is associated with the Wnt pathway) (Ekici et al. 2010). Experimental manipulation of these pathways in the mouse suggests that impaired morphology of the ventricular neuroepithelium is the primary developmental pathology that generates ventriculomegaly. De-repression of SHH signaling by removal of either Gpr161 or Ptch1 results in severe prenatal ventriculomegaly associated with increased NSC proliferation and irregular folding of the ventricular neuroepithelium, without apparent defects in ependymal cells or the cerebral aqueduct (Dave et al. 2011; Shimada et al. 2019). As efficient CSF transport depends on the shape of the ventricular system (Chiang et al. 2009; Longatti et al. 2019), abnormal neuroepithelial patterning leading to ventricular dysmorphology may directly decrease efficiency of CSF flow even in the absence of obvious physical obstruction (e.g. aqueductal stenosis) or motile ciliary dysfunction.
PI3K signaling
Phosphoinositide 3-kinase (PI3K) signaling is a major signal transduction pathway involved in cell proliferation and growth (Liu et al. 2009). PIK3CA encodes the PI3-kinase P110 alpha subunit that phosphorylates phosphatidylinositol-4,5-diphosphate (PIP2) to produce phosphatidylinositol (3,4,5) triphosphate (PIP3). PIP3 binds and stimulates AKT kinase, leading to mTOR activation. PTEN encodes the PIP3-phosphatase that reverses this reaction. Although these PI3K pathway genes are most often studied in the context of oncogenesis, they also influence proliferation, differentiation, and migration of prenatal NSCs in the brain (Groszer et al. 2001; Endo et al. 2009; Roy et al. 2019; Andrews et al. 2020). Germline and somatic mutations in components of the PI3K pathway have been identified in human patients with brain overgrowth disorders (hemimegaloencephaly) and autism (Varga et al. 2009; Lee et al. 2012). Whole-exome sequencing has recently identified de novo mutations in PIK3CA, PTEN, and MTOR in human patients with congenital hydrocephalus (Jin et al. 2020; DeSpenza et al. 2021). Both overactivation of Pik3ca (Roy et al. 2019) and deletion of Pten (Ohtoshi 2008) in the mouse result in severe hydrocephalus associated with excessive proliferation of prenatal ventricular zone NSCs, which provides a mechanistic explanation for cortical overgrowth in PI3K-related hemimegaloencephaly. Intriguingly, the Pik3ca-mutant mouse model also exhibits abnormal expression of cell junction proteins at the apical surface of the embryonic ventricular zone, suggesting that impaired integrity of the prenatal ventricular wall (consisting of NSCs) may contribute as a physiological cause of nonobstructive ventriculomegaly observed in the model (Roy et al. 2019).
SWI/SNF complex in hydrocephalus
The SWI/SNF complex is an ATP-dependent chromatin remodeling complex that is essential for gene expression regulation in the development of multiple organs, including the brain. Loss-of-function mutations in SMARCC1, encoding a core subunit of the SWI/SNF complex, have now been identified in human congenital hydrocephalus (Furey et al. 2018; Jin et al. 2020). The physiological cause of hydrocephalus in SMARCC1-mutant patients is presumed to be a CSF circulation defect since all patients presented with aqueductal stenosis (Jin et al. 2020). However, aqueductal stenosis may itself arise from a prenatal NSC defect, given that excessive proliferation of NSCs may initiate obliteration of the aqueduct (Lin et al. 2013; Zega et al. 2017). Indeed, a mouse model harboring a missense mutation in Smarcc1 exhibits failed neural tube closure associated with dysregulated NSC proliferation and survival (Harmacek et al. 2014). Conditional knockout of Smarcc1 in dorsal telencephalic NSCs results in detachment of NSCs from the apical surface due to abnormal establishment of adherens junctions at the ventricular lining (Narayanan et al. 2018), but without hydrocephalus. These NSC abnormalities may certainly contribute to impaired aqueduct development to cause hydrocephalus in the setting of SMARCC1 dysfunction. However, altered NSC development likely affects the development of the overall ventricular system and cerebral cortex rather than producing an isolated defect confined to the aqueduct, since SMARCC1 is widely expressed in all ventricular zone NSCs of the telencephalon (Narayanan et al. 2018). In fact, aqueductal stenosis is neither required or sufficient to initiate ventriculomegaly, as hydrocephalus often occurs with a patent aqueduct, and experimental blockade of the cerebral aqueduct in animal models may fail to cause ventricular dilation or even raise intracranial pressure (Klarica et al. 2009; Liu et al. 2020).
TRIM71 implicates an ancient heterochronic stem cell pathway in human congenital hydrocephalus
TRIM71 harbors the most de novo mutations in human sporadic congenital hydrocephalus (Furey et al. 2018; Jin et al. 2020; Duy et al. 2022). TRIM71 encodes an RNA-binding protein that is the primary target of the let-7 microRNA. The TRIM71/let-7 axis is conserved across diverse animal species, and it is believed that this regulatory axis constitutes a universal timekeeping mechanism to ensure that stem cell division and differentiation occur at the appropriate time in development (Ecsedi and Grosshans 2013; Duy et al. 2019). The best characterized molecular function of TRIM71 is its role in the posttranscriptional silencing RNA targets that influence stem cell proliferation and differentiation (Mitschka et al. 2015; Rand et al. 2018; Torres-Fernández et al. 2019; Welte et al. 2019; Liu et al. 2021). In the mouse, TRIM71 is highly expressed in the embryonic neuroepithelium, and knocking out TRIM71 results in embryonic lethality and failed neurulation associated with excessive neural differentiation at the expense of NSC proliferation (Maller Schulman et al. 2008; Chen et al. 2012; Mitschka et al. 2015). These findings suggest that TRIM71 functions to promote NSC self-renewal while suppressing differentiation, ensuring generation of a sufficient NSC pool to support subsequent neuro-gliogenesis. Remarkably, all human hydrocephalus-associated TRIM71 mutations clustered in its RNA-binding NHL domain (Furey et al. 2018; Jin et al. 2020), resulting in a severe loss of binding to RNA targets (Welte et al. 2019; Duy et al. 2022). We initially hypothesized that the resulting dysregulation of TRIM71 RNA targets depletes the NSC pool by causing premature differentiation at the expense of NSC proliferative expansion, ultimately leading to reduced neurogenesis that underlies the cortical thinning observed in congenital hydrocephalus patients with TRIM71 mutations (Duy et al. 2019). Consistent with this hypothesis, a humanized mouse model harboring a human hydrocephalus-associated missense mutation in Trim71 exhibits fetal-onset hydrocephalus associated with reduced cortical neurogenesis stemming from premature differentiation of embryonic neuroprogenitors (Duy et al. 2022). This reduced neurogenesis renders a thin and biomechanically unstable cerebral cortex that facilitates secondary ventricular enlargement in hydrocephalic Trim71 mutant mice without primary defects in CSF circulation (Duy et al. 2022). Thus, a genetically encoded dysregulation in brain morphogenesis is sufficient to cause hydrocephalus primarily by disrupting the mechanical stability of the cerebral parenchyma.
Convergence of hydrocephalus risk genes in neurogenesis-related elements of the developing human brain
Advances in genomics have enabled the identification an ever increasing number of new genes involved in susceptibility for congenital hydrocephalus, providing hope for mechanistic understanding and therapeutic development. However, these findings also pose new challenges in understanding biology given the extreme genetic heterogeneity and a large number of biological pathways potentially involved in disease. To that end, an integrative systems biology approach offers the ability to search for points of disease convergence in specific biological pathways and spatiotemporal loci of human brain development in a hypothesis-free manner (Parikshak et al. 2013; Willsey et al. 2013; Li et al. 2018). Combining human genetics with transcriptomic maps of gene expression during normal human brain development, pioneering studies have identified early human cortical development and multiple neuronal cell types in the cerebral cortex as spatiotemporal loci of disease convergence implicated by autism risk genes (Parikshak et al. 2013; Willsey et al. 2013). Applying a similar integrative genomics approach to congenital hydrocephalus, we have found that hydrocephalus risk genes converged in transcriptional networks that are also implicated in other neurodevelopmental disorders such as autism and microcephaly (Jin et al. 2020; Duy et al. 2022), a cortical malformation characterized by the development of a small cerebral cortex due to defective NSC proliferation and neurogenesis. Consistent with this, we also found that congenital hydrocephalus risk genes converged in discrete cell types involved in human cortical neurogenesis, including embryonic neuroepithelial NSCs and excitatory neurons (Jin et al. 2020; Duy et al. 2022).
The implication of these findings is 2-fold. First, in contrast to the dogma that hydrocephalus is a disorder of impaired fluid circulation, functional genomic analyses suggests that the etiology of congenital hydrocephalus is likely more related to impaired prenatal brain development and defective neurogenesis. Second, the convergence of hydrocephalus and autism risk genes in the same transcriptional module suggests shared disease mechanisms between hydrocephalus and other disorders of cortical maldevelopment. Thus, understanding the molecular genetic mechanisms of congenital hydrocephalus may facilitate the discovery of convergent developmental pathologies involved in related neurodevelopmental disorders that affect the growth and assembly of the cerebral cortex. Indeed, mutations in several congenital hydrocephalus-associated genes, PTEN (Jin et al. 2020) and WDR81 (Shaheen et al. 2017), can also cause autism (Satterstrom et al. 2020; DeSpenza et al. 2021) or microcephaly (Cavallin et al. 2017), respectively.
Environmental insults perturb ventricular NSC development, leading to acquired hydrocephalus
Although human genetics has provided valuable insights into the biology of congenital hydrocephalus, the most common causes of pediatric hydrocephalus worldwide are due to environmental perturbations. We focus here on 2 common causes of acquired pediatric hydrocephalus: intrauterine infections and intraventricular hemorrhage. The common theme between these 2 etiologies of acquired hydrocephalus is that in both situations, prenatal NSCs are exposed to external factors normally not present in their microenvironment, leading to dysregulation in NSC survival, self-renewal, and differentiation. These impairments culminate in an overall loss of NSCs and their differentiated progeny that populate the brain parenchyma. Understanding the impact of infections and hemorrhage on brain development provides yet another window into how NSC defects lead to pediatric hydrocephalus.
NSCs are the cellular target of teratogenic infectious agents
Congenital infections are an important cause of newborn mortality and morbidity. These infections occur when a mother transmits the pathogen to her child either during gestation (via the placenta) or delivery (via the vaginal canal). The common pathogens that cause in utero infections can be summarized by the TORCH acronym (Toxoplasmosis, Other agents including syphilis, varicella, and Zika, Rubella, Cytomegalovirus, and Herpes Simplex) (Coyne and Lazear 2016). TORCH infections that occur during prenatal development via the placenta have a devastating impact on the brain, often leading to microcephaly, hydrocephalus, and neurological sequelae such as intellectual disability, epilepsy, and sensorineural hearing loss. Although microcephaly and hydrocephalus are typically considered separate conditions of different underlying pathogenic mechanisms, detailed neuroradiographic studies of patients with congenital Zika syndrome showed that both malformations can appear together (Jucá et al. 2018; van der Linden et al. 2019; Niemeyer et al. 2020). Zika-infected infants initially presented with a classic picture of microcephaly (low head circumference and low brain volume) that then progressed to show symptoms of increased intracranial pressure and hydrocephalus, including ventricular dilation, worsening neurological symptoms, vomiting, and sudden increases in head circumference (van der Linden et al. 2019). These striking findings in congenital Zika syndrome suggest that clinical hydrocephalus that require neurosurgical CSF diversion can arise as a secondary complication of cerebrocortical hypoplasia.
The primary cellular pathology of congenital TORCH infections in the brain is unequivocally a detrimental impact on prenatal NSC development. In vivo studies of animal models and in vitro studies of human stem cells have demonstrated that TORCH pathogens preferentially infect NSCs by binding to specific receptors, while differentiated progeny cells are relatively spared (Onorati et al. 2016). Infected NSCs exhibit defects in pluripotency (decreased self-renewal and impaired neural differentiation) and undergo cell death (Luo et al. 2010; Dang et al. 2016; El Ghouzzi et al. 2016; Onorati et al. 2016). TORCH infections also induce a cascade of maternal inflammatory responses contributing to deficient NSC growth, in line with the vulnerability of NSCs to inflammatory insults (Dang et al. 2016; Vasistha et al. 2020). Indeed, even just the cytokines that are induced by the mother in the setting of infections may alter the ventricular integrity of the fetus, as transient changes in neuroprogenitor proliferation have been observed in a model of maternal inflammation induced by administration of lipopolysaccharide (Stolp et al. 2011). The overall outcome of TORCH infections is thus a depletion in the ventricular NSC pool, leading to deficient neuro-gliogenesis that manifests at the organ level as cortical hypoplasia, microcephaly, and hydrocephalus.
Hemorrhage exposes NSCs to foreign factors that disturb brain development
Intraventricular hemorrhage is the most common cause of acquired hydrocephalus in developed countries because of the resources available for sophisticated and expensive neonatal intensive care. Posthemorrhagic hydrocephalus commonly occurs due to premature birth, a condition in which the highly vascularized and fragile germinal matrix (an area of periventricular brain parenchyma) is prone to bleed under stress, allowing entry of blood into the adjacent ventricular system. A CSF hydrodynamic mechanism is classically invoked to explain the pathogenesis of posthemorrhagic hydrocephalus: obstructive ventriculomegaly occurs due to physical obstruction of intraventricular CSF flow by blood microthrombi with subsequent inflammation and fibrosis of the arachnoid granulations leading to reduced CSF clearance from the ventricular system (Strahle et al. 2012). Denudation of the multiciliated ependyma following intraventricular hemorrhage also contributes to pathologic CSF accumulation secondary to reduced cilia-driven CSF circulation (McAllister et al. 2017; Lummis et al. 2019). However, these explanations are an oversimplification, given that arachnoid granulations are not mature until after the 39th gestational week in humans (Gómez et al. 1982), whereas posthemorrhagic hydrocephalus occurs at much earlier timepoints. The classical explanations also do not account for observations of poor neurodevelopmental outcomes and reduced cortical gray matter volume in premature infants with intraventricular hemorrhage (Vasileiadis et al. 2004).
Emerging evidence demonstrates the deleterious impact of intraventricular hemorrhage on prenatal NSCs and neuro-gliogenesis. Studies of brain tissues from preterm infants with intraventricular hemorrhage show disorganization of the neurogenic ventricular zone and suppression of NSC proliferation (Xue et al. 2003; McAllister et al. 2017; Dohare et al. 2019). Investigations of animal models have begun to define the cellular and molecular mechanisms underlying the deleterious effects of hemorrhage on prenatal NSC and cortical neurogenesis. Using a fetal mouse model of posthemorrhagic hydrocephlaus, Yung et al. reported that a blood-borne lipid (lysophosphatidic acid) causes aberrant activation of Rho/Rac signaling, leading to disruption of NSC attachment at the ventricular zone and initiation of fetal ventriculomegaly (Yung et al. 2011). More recent investigations combining both examinations of fetal human brain tissues and rabbit models of premature birth have shown that intraventricular hemorrhage downregulates Wnt signaling, resulting in apoptosis of NSCs and suppression of cortical neurogenesis (Dohare et al. 2018, 2019). These findings highlight derangements in NSC signaling as disease mechanisms that initiate ventriculomegaly and cortical underdevelopment in posthemorrhagic hydrocephalus. Strikingly, pharmacological manipulations aimed at targeting the identified molecular alterations restored neurogenesis and prevented development of hydrocephalus in the studied models (Yung et al. 2011; Dohare et al. 2019).
Disruption of brain-CSF biomechanical interactions: linking NSC perturbations and impaired neurodevelopment to ventricular dilation in hydrocephalus
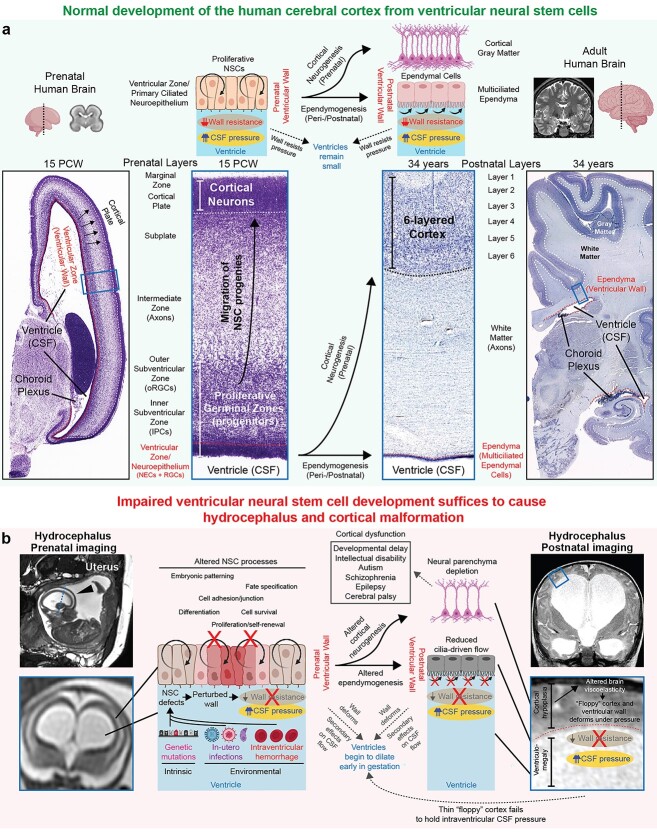
We propose a novel NSC model of pediatric hydrocephalus pathogenesis wherein intrinsic and environmental insults converge to perturb development of NSCs at the ventricular neuroepithelium, directly leading to ventricular and cortical malformation in primary and acquired hydrocephalus (Fig. 4). A particularly unique aspect of our model is that we emphasize the “vessel” holding the fluid (the neural tissue comprising the germinal neuroepithelium or brain parenchymal compartment) as the anatomical site of disease pathogenesis rather than the ventricular CSF compartment as classically believed. Thus, our model provides a pathophysiologic explanation of how ventriculomegaly occurs even in the absence of primary initiating defects in CSF circulation such as ependymal cilia failure or anatomical obstruction to CSF flow. This is in contrast to previous discussions of NSC involvement in hydrocephalus by other authors (Rodríguez et al. 2012; Rodríguez and Guerra 2017), who still endorsed a fluid hydrodynamic-view of hydrocephalus by proposing that the loss of NSCs initiates ventricular dilation by causing obliteration of the cerebral aqueduct or impaired ependymogeneis with attendant loss of cilia-driven CSF flow.
Fig. 4.
A NSC paradigm of human pediatric hydrocephalus. a) Normal development of the human ventricular system and cerebral cortex. During gestation, the ventricular wall is composed of a germinal neuroepithelium that has 2 crucial functions. First, as the epithelial barrier between fluid and brain parenchymal component, the germinal neuroepithelium has an important function in resisting the pressure generated by CSF in the ventricular lumen. Second, the germinal neuroepithelium contains proliferative NSCs that produce all neurons and macroglia of the cerebral cortex. After birth, this neuroepithelium is replaced by multiciliated ependymal cells that may contribute to local CSF flow driven by motile cilia, though in humans, the roles of ependymal ciliary motion in generating CSF circulation remain debated. MR image of control prenatal human brain from Gholipour et al. Scientific Reports, 2017 (Gholipour et al. 2017); MR image of control adult human brain from OpenNeuro dataset ds000221; images of histological sections from control prenatal and adult human brains from the BrainSpan atlas. NECs, neuroepithelial cells; RGCs, radial glia cells; IPCs, intermediate progenitor cells; oRGCs, outer radial glia cells. b) Pathophysiologic explanation of how altered ventricular NSCs during gestation lead to hydrocephalus and cortical maldevelopment. The developmental consequences of perturbed NSC fate are 2-fold. First, the germinal neuroepithelium of the developing brain ventricles weakens and thus fails to resist intraventricular CSF pressure, initiating ventricular dilation early in gestation. Second, abnormal development of the germinal neuroepithelium leads to abnormal cortical neurogenesis, leading to a thin and “floppy” cerebral cortex wall that also fails to hold the CSF pressure, leading to progression of ventriculomegaly. Impaired NSC fate can theoretically also result in abnormal ependymogenesis and failure of motile cilia-driven CSF flow, leading to secondary effects on CSF circulation. However, altered development of the ependyma is neither sufficient or required to cause hydrocephalus as discussed in the main text, thus the contribution of disrupted ependyma to the primary developmental pathogenesis of pediatric hydrocephalus remains questionable and should only be considered as a secondary consequence that may potentially worsen the phenotype but not absolutely required for initiation of the disease phenotype.
In fact, neither aqueductal obliteration nor loss of ependymal cells is sufficient or necessary to cause cortical malformation and ventriculomegaly in hydrocephalus for several reasons already discussed in prior sections but summarized again briefly here. First, experimental blockade of CSF flow does not always lead to ventricular dilation or raised intracranial pressure in animal models (Klarica et al. 2009; Liu et al. 2020), and even when ventriculomegaly does occur, there are no associated neurological sequelae similar to those observed in human patients with pediatric hydrocephalus (Campos-Ordonez and Gonzalez-Perez 2021; McAllister et al. 2021). Several case reports have also documented the presence of isolated aqueductal stenosis leading to complete obstruction of CSF flow in human individuals who otherwise are neurologically normal and do not exhibit hydrocephalus (Radoš et al. 2014; Radoš, Orešković, et al. 2021). Second, with regards to ependymal cilia-generated flow, pediatric hydrocephalus is often detected in utero prior to the emergence of multiciliated ependymal cells (Coletti et al. 2018). Furthermore, despite that knockout of motile cilia-related genes often causes hydrocephalus in the mouse, in humans, primary ciliary dyskinesia rarely results in hydrocephalus (Jin et al. 2020). These paradoxical observations challenge the current dogma that a gross defect in CSF compartment, stemming from aqueductal stenosis or loss of ependymal cells, is responsible for ventricular dilation and neurological symptoms observed in clinical hydrocephalus. In fact, the most important clinical observation challenging this dogma is that reduction of CSF volume by neurosurgical treatment still fails to improve neurodevelopment and restore brain growth in children with hydrocephalus (Duy and Kahle 2021; Schiff et al. 2021), suggesting that the relevant pathology arises from defective development of neural parenchyma instead of the fluid compartment.
Rather than requiring an obvious defect in active CSF circulation (cilia dysmotility or anatomical obstruction) to cause hydrocephalus, we propose that NSC defects leading to the depletion of the neural parenchyma are sufficient to initiate ventriculomegaly. Understanding this proposed model requires the recognition that a positive pressure always exists inside the lumen of the ventricular system, because otherwise the overlying neural tube and brain parenchymal compartment (the “vessels” holding the fluid) would collapse (Lowery and Sive 2009; Duy, Rakic, et al. 2022). This pressure generated by CSF in the ventricular lumen must also be balanced by forces exerted by neural tissue surrounding the fluid. The absence or reduction of such counteracting forces due to the depletion of the overlying neural parenchyma thus favors secondary ventricular dilation even when the intraventricular pressure is not abnormally high, as predicted by computational models (Peña et al. 2002). Indeed, impaired biomechanical properties of the brain parenchyma due to innate reduction/atrophy of brain tissue leading to decreased resistance to CSF pressure have been proposed to be the pathophysiologic explanation for ventricular dilation despite low intracranial pressure in some human hydrocephalus patients (Pang and Altschuler 1994).
During the earliest stages of neural development, the only neural tissue surrounding intraventricular CSF is proliferative NSCs, since the entire wall of the neural tube is a pseudostratified neuroepithelium comprised of neuroepithelial stem cells. This pseudostratified neuroepithelium of proliferative NSCs continues to cover the ventricular wall directly adjacent to CSF for the bulk of prenatal development. As the major and only epithelial barrier between fluid and brain parenchymal compartment during gestation, the germinal neuroepithelium (and therefore NSCs) has important functions in resisting intraventricular CSF pressure (Fig. 4a). Consequently, dysregulated NSC fate (due to mutations in hydrocephalus-associated genes or damage from exposure to environmental insults) may compromise the structural integrity of the germinal neuroepithelium, leading to a weakening of the developing ventricular wall and a failure to resist the intraventricular CSF pressure (Fig. 4b). In support of the hypothesis that dysregulated NSC fate initiates mechanical instability of the ventricular wall, histological studies have found abnormal expression of cell junction proteins in the developing ventricles of hydrocephalic mouse models and human patients (Chae et al. 2004; Rodríguez et al. 2012; McAllister et al. 2017; Roy et al. 2019). Ventricular dilation may thus initiate in utero due to a mechanically unstable ventricular neuroepithelium even in the absence of anatomically obstructed CSF flow.
Impaired development of NSCs in the germinal neuroepithelium also results in defective cortical neurogenesis, leading to decreased cortical cell mass that renders a weakened cortical mantle that is more susceptible to mechanical deformations (Fig. 4b). Additional downstream processes involved in the growth and assembly of the cerebral cortex may also be affected, such as neuropil formation, white matter growth, synaptogenesis, and gliogenesis. Depletion of the neural parenchyma components, such as neuronal density, likely results in reduced brain tissue stiffness (Schregel et al. 2012; Freimann et al. 2013; Duy et al. 2022). In the setting of reduced brain stiffness, continued production of CSF from the choroid plexus would expand the already large ventricular compartment, pushing the thin, low-resistance cortical ribbon to the dural-bone interface even at low-pressure gradients that are normally incapable of deforming the overlying brain parenchyma (Peña et al. 2002; Nagra et al. 2009; Wilkie et al. 2011, 2012). Thus, perturbations in cerebrocortical morphogenesis and brain biomechanics arising from NSC defects may contribute to the progression of ventricular dilation. Indeed, we have recently utilized atomic force microscopy to show that reduced neurogenesis in a genetic mouse model of hydrocephalus is associated with reduced stiffness of the hydrocephalic cortical mantle (Duy et al. 2022). In vivo measurements of brain biomechanics by magnetic resonance elastography have also revealed reduced brain parenchymal stiffness in human hydrocephalus patients (Olivero et al. 2016; Wagshul et al. 2021). Although altered NSC development can also theoretically perturb the development of multiciliated ependymal cells after birth (Fig. 4b), a defect in ependymal cells is still unlikely to explain subsets of fetal-onset hydrocephalus that manifests before the functional maturation of ependymal cilia (Coletti et al. 2018; Duy et al. 2022) (Fig. 2). Altogether, we hypothesize that a developmental pathology in cortical NSCs initiates a cascade of events that culminate in cerebrocortical dysgenesis and aberrant brain-fluid biomechanical interactions that directly facilitate ventricular expansion. This NSC paradigm of hydrocephalus frames the brain parenchyma as the anatomical site of pathogenesis rather than the CSF compartment in some or potentially many forms of pediatric hydrocephalus.
Clinical ramifications: optimizing neurodevelopment in pediatric hydrocephalus
The NSC paradigm provides explanatory power and translational value for the clinical care of patients with hydrocephalus, the mainstay treatments for which almost exclusively focus on surgical CSF drainage and reduction of ventricle size. Namely, the paradigm of hydrocephalus as a primary disorder of cerebrocortical development may in part explain the co-occurrence of hydrocephalus with other “brain parenchymal” phenotypes such as intellectual disability, epilepsy, and autism, as well as with other structural brain defects including cortical thinning and schizencephaly (Lindquist et al. 2005; Persson et al. 2006; Schoner et al. 2013; Allocco et al. 2019; Oegema et al. 2019; Munch et al. 2020; DeSpenza et al. 2021). Moreover, these findings may shed light on why some children with hydrocephalus fail to show significant neurodevelopmental improvement even after normal CSF circulation is presumably reinstated by CSF diversion surgeries (Gupta et al. 2007; Kulkarni et al. 2013; Riva-Cambrin et al. 2021; Schiff et al. 2021). While neurosurgical shunting may still be indicated in selected cases to prevent clinical deterioration and neurologic compromise, CSF diversion is largely palliative and does not address the underlying developmental pathology of pediatric hydrocephalus. This warrants the need to expand the therapeutic options beyond draining fluid to potentially include pharmacological and/or behavioral interventions directed toward abnormal cognitive and neurobehavioral function akin to those typically reserved for children with developmental neuropsychiatric disorders such as autism. Genetic testing by exome-wide or even genome-wide approaches may be a useful diagnostic adjunct in this regard (Sullivan et al. 2021; Duy, Timberlake, et al. 2022).
In the long-term, the definitive prevention and cure of pediatric hydrocephalus will require new interventions that target the underlying cellular and molecular perturbations that may arise before birth. Advances in prenatal genetic testing (Zhang et al. 2019), fetal surgical interventions (Farmer et al. 2003), and gene therapy successes in other human neurodevelopmental disorders (Mendell et al. 2017) suggest the therapeutic feasibility of early in utero interventions to correct molecular dysregulation of brain development and neuroprogenitor fate in hydrocephalus. Proof-of-concept studies in animal models of hydrocephalus have also demonstrated the ability of pharmacological interventions to prevent ventriculomegaly and improve neurological function (Foerster et al. 2017; Roy et al. 2019; Iwasawa et al. 2022). For instance, Foerster et al. (2017) showed in the mouse that the conditional depletion of primary cilia in NSCs results in ventriculomegaly and upregulation of the mTOR pathway, both of which can be corrected by prenatal administration of the commonly used mTOR inhibitor, rapamycin. In a more recent study, Iwasawa et al. (2022) showed that the administration of the anti-inflammatory agent Bindarit improves neurological function and cortical myelination in a genetic mouse model of hydrocephalus even though ventriculomegaly is only mildly reduced by therapeutic treatment. Although much work remains before clinical translation to humans, these promising studies show that precise targeting cellular and molecular defects by tailored pharmacological interventions is a viable strategy to optimize neurodevelopment in pediatric hydrocephalus without the need for invasive surgery. Thus, understanding NSC regulation and its alterations due to genetic mutations and environmental perturbations could shift our view of pediatric hydrocephalus from a lifelong neurosurgical condition to that of a preventable neurodevelopmental disorder. This shift to viewing pediatric hydrocephalus as a pharmacologically treatable neurodevelopmental disorder also suggests that the lessons learned in hydrocephalus may also be applied toward the prognostication, treatment stratification, and prevention of other common developmental neurologic and neuropsychiatric disorders (Duy, Rakic, et al. 2022; Duy, Timberlake, et al. 2022).
Authors’ contributions
PQD, PR, SLA, SMR, AJK, WEB, CAW, NS, DHG, SCJ, and KTK reviewed the literature and contributed to manuscript writing.
Funding
This work was supported by the NIH Medical Scientist Training Program Training Grant T32GM136651 (to PQD), F30HD106694 (to PQD), R01 NS111029-01A1 (to KTK), R01 NS109358 (to KTK), K12 228168 (to KTK); the Rudi Schulte Research Institute (to KTK); and National Institute on Drug Abuse grant DA023999 (to PR). SCJ is supported by a K99/R00 Pathway to Independence Award (K99HL143036 and R00HL143036-02). Cartoons and diagrams were created using BioRender.com.
Conflict of interest statement: PR and CAW are on the editorial board of Cerebral Cortex.
Contributor Information
Phan Q Duy, Department of Neuroscience, Yale University School of Medicine, New Haven, CT 06510, USA; Medical Scientist Training Program, Yale University School of Medicine, New Haven, CT 06510, USA; Department of Neurosurgery, Yale University School of Medicine, New Haven, CT 06510, USA.
Pasko Rakic, Department of Neuroscience, Yale University School of Medicine, New Haven, CT 06510, USA.
Seth L Alper, Division of Nephrology and Vascular Biology Research Center, Beth Israel Deaconess Medical Center and Department of Medicine, Harvard Medical School, Boston, MA 02215, USA.
Stephanie M Robert, Department of Neurosurgery, Yale University School of Medicine, New Haven, CT 06510, USA.
Adam J Kundishora, Department of Neurosurgery, Yale University School of Medicine, New Haven, CT 06510, USA.
William E Butler, Department of Neurosurgery, Massachusetts General Hospital, Boston, MA 02114, USA.
Christopher A Walsh, Division of Genetics and Genomics, Manton Center for Orphan Disease Research, Department of Pediatrics, and Howard Hughes Medical Institute, Boston Children’s Hospital, Boston, MA 02115, USA; Departments of Pediatrics and Neurology, Harvard Medical School, Boston, MA 02115, USA; Broad Institute of MIT and Harvard, Cambridge, MA 02142, USA.
Nenad Sestan, Department of Neuroscience, Yale University School of Medicine, New Haven, CT 06510, USA.
Daniel H Geschwind, Department of Human Genetics, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA 90095, USA.
Sheng Chih Jin, Department of Genetics, Washington University School of Medicine, St. Louis, MO 63110, USA.
Kristopher T Kahle, Department of Neurosurgery, Massachusetts General Hospital, Boston, MA 02114, USA; Broad Institute of MIT and Harvard, Cambridge, MA 02142, USA; Harvard Center for Hydrocephalus and Neurodevelopmental Disorders, Massachusetts General Hospital, Boston, MA 02114, USA.
References
- Adle-Biassette H, Saugier-Veber P, Fallet-Bianco C, Delezoide A-LL, Razavi F, Drouot N, Bazin A, Beaufrère A-M, Bessières B, Blesson S, et al. Neuropathological review of 138 cases genetically tested for X-linked hydrocephalus: evidence for closely related clinical entities of unknown molecular bases. Acta Neuropathol. 2013:126(3):427–442. 10.1007/s00401-013-1146-1. [DOI] [PubMed] [Google Scholar]
- Alders GL, Minuzzi L, Sarin S, Frey BN, Hall GB, Samaan Z. Volumetric MRI analysis of a case of severe Ventriculomegaly. Front Hum Neurosci. 2018:12:495. 10.3389/fnhum.2018.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Dosari MS, Al-Owain M, Tulbah M, Kurdi W, Adly N, Al-Hemidan A, Masoodi TA, Albash B, Alkuraya FS. Mutation in MPDZ causes severe congenital hydrocephalus. J Med Genet. 2013:50(1):54–58. 10.1136/jmedgenet-2012-101294. [DOI] [PubMed] [Google Scholar]
- Allocco AA, Jin SC, Duy PQ, Furey CG, Zeng X, Dong W, Nelson-Williams C, Karimy JK, DeSpenza T, Hao LT, et al. Recessive inheritance of congenital hydrocephalus with other structural brain abnormalities caused by compound heterozygous mutations in ATP1A3. Front Cell Neurosci. 2019:13:425. 10.3389/fncel.2019.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvin MD, Miller PE. Compensated hydrocephalus. Lancet (London, England). 2016:387(10036):2422. 10.1016/S0140-6736(16)00089-1. [DOI] [PubMed] [Google Scholar]
- Andrews MG, Subramanian L, Kriegstein AR. MTOR Signaling regulates the morphology and migration of outer radial glia in developing human cortex. elife. 2020:9(September). 10.7554/eLife.58737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschoff A, Kremer P, Hashemi B, Kunze S. The scientific history of hydrocephalus and its treatment. Neurosurg Rev. 1999:22(2–3):67–93discussion 94-5. 10.1007/s101430050035. [DOI] [PubMed] [Google Scholar]
- Banizs B, Pike MM, Millican CL, Ferguson WB, Komlosi P, Sheetz J, Bell PD, Schwiebert EM, Yoder BK. Dysfunctional cilia lead to altered Ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development. 2005:132(23):5329–5339. 10.1242/dev.02153. [DOI] [PubMed] [Google Scholar]
- Butler WE, Agarwalla PK, Codd P. CSF in the ventricles of the brain behaves as a relay medium for arteriovenous pulse wave phase coupling. PLoS One. 2017:12(11):e0181025. 10.1371/journal.pone.0181025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystron I, Rakic P, Molnár Z, Blakemore C. The first neurons of the human cerebral cortex. Nat Neurosci. 2006:9(7):880–886. 10.1038/nn1726. [DOI] [PubMed] [Google Scholar]
- Campos-Ordonez T, Gonzalez-Perez O. Characterization of a mouse model of chronic hydrocephalus induced by partial occlusion of the aqueduct of Sylvius in the adult brain. J Neurosci Methods. 2021:362(October):109294. 10.1016/J.JNEUMETH.2021.109294. [DOI] [PubMed] [Google Scholar]
- Canu EDG, Magnano I, Paulus KS, Piras MR, Conti M, Costantino S, Nuvoli S, Aiello I. Neuropsychophysiological findings in a case of long-standing overt Ventriculomegaly (LOVA). Neurosci Lett. 2005:385(1):24–29. 10.1016/j.neulet.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Carter CS, Vogel TW, Zhang Q, Seo S, Swiderski RE, Moninger TO, Cassell MD, Thedens DR, Keppler-Noreuil KM, Nopoulos P, et al. Abnormal development of NG2+ PDGFR-Α+ neural progenitor cells leads to neonatal hydrocephalus in a ciliopathy mouse model. Nat Med. 2012:18(12):1797–1804. 10.1038/nm.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey ATH, Kimmings EJ, Kleinlugtebeld AD, Taylor WAS, Harkness WF, Hayward RD. The long-term outlook for hydrocephalus in childhood. Pediatr Neurosurg. 1997:27(2):63–70. [DOI] [PubMed] [Google Scholar]
- Cavallin M, Rujano MA, Bednarek N, Medina-Cano D, Bernabe Gelot A, Drunat S, Maillard C, Garfa-Traore M, Bole C, Nitschké P, et al. WDR81 mutations cause extreme microcephaly and impair mitotic progression in human fibroblasts and drosophila neural stem cells. Brain J Neurol. 2017:140(10):2597–2609. 10.1093/brain/awx218. [DOI] [PubMed] [Google Scholar]
- Chae TH, Kim S, Marz KE, Hanson PI, Walsh CA. The Hyh mutation uncovers roles for ΑSnap in apical protein localization and control of neural cell fate. Nat Genet. 2004:36(3):264–270. 10.1038/ng1302. [DOI] [PubMed] [Google Scholar]
- Chance A, Sandberg DI. Hydrocephalus in patients with closed neural tube defects. Childs Nerv Syst. 2015:31(2):329–332. 10.1007/s00381-014-2492-6. [DOI] [PubMed] [Google Scholar]
- Chen J, Lai F, Niswander L. The ubiquitin ligase MLin41 temporally promotes neural progenitor cell maintenance through FGF Signaling. Genes Dev. 2012:26(8):803–815. 10.1101/gad.187641.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang WW, Takoudis CG, Lee SH, Weis-McNulty A, Glick R, Alperin N. Relationship between ventricular morphology and Aqueductal cerebrospinal fluid flow in healthy and communicating hydrocephalus. Investig Radiol. 2009:44(4):192–199. 10.1097/RLI.0b013e31819a640b. [DOI] [PubMed] [Google Scholar]
- Choi BH, Lapham LW. Radial glia in the human Fetal cerebrum: a combined Golgi, immunofluorescent and electron microscopic study. Brain Res. 1978:148(2):295–311. 10.1016/0006-8993(78)90721-7. [DOI] [PubMed] [Google Scholar]
- Chong JX, Buckingham KJ, Jhangiani SN, Boehm C, Sobreira N, Smith JD, Harrell TM, McMillin MJ, Wiszniewski W, Gambin T, et al. The genetic basis of Mendelian phenotypes: discoveries, challenges, and opportunities. Am J Hum Genet. 2015:97(2):199–215. 10.1016/j.ajhg.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coletti AM, Singh D, Kumar S, Shafin TN, Briody PJ, Babbitt BF, Pan D, Norton ES, Brown EC, Kahle KT, et al. Characterization of the ventricular-subventricular stem cell niche during human brain development. Development. 2018:145(20). 10.1242/dev.170100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne CB, Lazear HM. Zika virus-reigniting the TORCH. Nat Rev Microbiol. 2016:14(11):707–715. 10.1038/NRMICRO.2016.125. [DOI] [PubMed] [Google Scholar]
- Dang J, Tiwari SK, Lichinchi G, Qin Y, Patil VS, Eroshkin AM, Rana TM. Zika virus depletes neural progenitors in human cerebral organoids through activation of the innate immune receptor TLR3. Cell Stem Cell. 2016:19(2):258–265. 10.1016/J.STEM.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave RK, Ellis T, Toumpas MC, Robson JP, Julian E, Adolphe C, Bartlett PF, Cooper HM, Reynolds BA, Wainwright BJ. Sonic hedgehog and notch Signaling can cooperate to regulate neurogenic divisions of neocortical progenitors. PLoS One. 2011:6(2):e14680. 10.1371/journal.pone.0014680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deAzevedo LC, Fallet C, Moura-Neto V, Daumas-Duport C, Hedin-Pereira C, Lent R. Cortical radial glial cells in human Fetuses: depth-correlated transformation into astrocytes. J Neurobiol. 2003:55(3):288–298. 10.1002/neu.10205. [DOI] [PubMed] [Google Scholar]
- Wit OA, Dunnen WF, Sollie KM, Muñoz RI, Meiners LC, Brouwer OF, Rodríguez EM, Sival DA. Pathogenesis of cerebral malformations in human Fetuses with Meningomyelocele. Cerebrospinal Fluid Res. 2008:5(1):4. 10.1186/1743-8454-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bigio MR. Neuropathological changes caused by hydrocephalus. Acta Neuropathol. 1993:85(6):573–585. 10.1007/BF00334666. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR. Neuropathology and structural changes in hydrocephalus. Dev Disabil Res Rev. 2010:16(1):16–22. 10.1002/ddrr.94. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR. Cell proliferation in human ganglionic eminence and suppression after prematurity-associated haemorrhage. Brain J Neurol. 2011:134(Pt 5):1344–1361. 10.1093/BRAIN/AWR052. [DOI] [PubMed] [Google Scholar]
- DeSpenza T, Carlson M, Panchagnula S, Robert S, Duy PQ, Mermin-Bunnell N, Reeves BC, Kundishora A, Elsamadicy AA, Smith H, et al. PTEN mutations in autism Spectrum disorder and congenital hydrocephalus: developmental pleiotropy and therapeutic targets. Trends Neurosci. 2021:44(12):961–976. 10.1016/J.TINS.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohare P, Cheng B, Ahmed E, Yadala V, Singla P, Thomas S, Kayton R, Ungvari Z, Ballabh P. Glycogen synthase kinase-3β inhibition enhances myelination in preterm Newborns with intraventricular Hemorrhage, but not recombinant Wnt3A. Neurobiol Dis. 2018:118:22–39. 10.1016/j.nbd.2018.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohare P, Kidwai A, Kaur J, Singla P, Krishna S, Klebe D, Zhang X, Hevner R, Ballabh P. GSK3β inhibition restores impaired neurogenesis in preterm neonates with intraventricular Hemorrhage. Cerebral Cortex (New York, NY: 1991). 2019:29(8):3482–3495. 10.1093/cercor/bhy217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez-Pinos MD, Páez P, Jiménez A-J, Weil B, Arráez M-A, Pérez-Fígares J-M, Rodríguez E-M. Ependymal denudation and alterations of the subventricular zone occur in human Fetuses with a moderate communicating hydrocephalus. J Neuropathol Exp Neurol. 2005:64(7):595–604. 10.1097/01.jnen.0000171648.86718.bb. [DOI] [PubMed] [Google Scholar]
- Duy PQ, Kahle KT. Intraventricular CSF turbulence in Pediatric communicating hydrocephalus. Neurology. 2021:97(5):246–247. 10.1212/WNL.0000000000012237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duy PQ, Furey CG, Kahle KT. Trim71/Lin-41 links an ancient MiRNA pathway to human congenital hydrocephalus. Trends Mol Med. 2019:25(6):467–469. 10.1016/j.molmed.2019.03.004. [DOI] [PubMed] [Google Scholar]
- Duy PQ, Weise SC, Marini C, Li X-J, Liang D, Dahl PJ, Ma S, Spajic A, Dong W, Juusola J, et al. Impaired neurogenesis alters brain biomechanics in a Neuroprogenitor-based genetic subtype of congenital hydrocephalus. Nat Neurosci. 2022:25(4):458–473. 10.1038/s41593-022-01043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duy PQ, Rakic P, Alper SL, Butler WE, Walsh CA, Sestan N, Geschwind DH, Jin SC, Kahle KT. Brain ventricles as windows into brain development and disease. Neuron. 2022:110(1):12–15. 10.1016/j.neuron.2021.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duy PQ, Timberlake AT, Lifton RP, Kahle KT. Molecular genetics of human developmental neurocranial anomalies: towards ‘precision surgery’. Cerebral Cortex (New York, NY: 1991) June. 2022. 10.1093/cercor/bhac249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecsedi M, Grosshans H. LIN-41/TRIM71: emancipation of a MiRNA target. Genes Dev. 2013:27(6):581–589. 10.1101/gad.207266.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekici AB, Hilfinger D, Jatzwauk M, Thiel CT, Wenzel D, Lorenz I, Boltshauser E, Goecke TW, Staatz G, Morris-Rosendahl DJ, et al. Disturbed Wnt signalling due to a mutation in CCDC88C causes an autosomal recessive non-syndromic hydrocephalus with medial diverticulum. Mol Syndromol. 2010:1(3):99–112. 10.1159/000319859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Ghouzzi V, Bianchi FT, Molineris I, Mounce BC, Berto GE, Rak M, Lebon S, Aubry L, Tocco C, Gai M, et al. ZIKA virus elicits P53 activation and genotoxic stress in human neural progenitors similar to mutations involved in severe forms of genetic microcephaly and P53. Cell Death Dis. 2016:7(10):e2440–e2440. 10.1038/cddis.2016.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgamal EA. Natural history of hydrocephalus in children with spinal open neural tube defect. Surg Neurol Int. 2012:3(1):112. 10.4103/2152-7806.101801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M, Antonyak MA, Cerione RA. Cdc42-MTOR Signaling pathway controls Hes5 and Pax6 expression in retinoic acid-dependent neural differentiation. J Biol Chem. 2009:284(8):5107–5118. 10.1074/jbc.M807745200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer DL, Von Koch CS, Peacock WJ, Danielpour M, Gupta N, Lee H, Harrison MR. In utero repair of myelomeningocele. Arch Surg. 2003:138(8):872. 10.1001/archsurg.138.8.872. [DOI] [PubMed] [Google Scholar]
- Feldner A, Adam MG, Tetzlaff F, Moll I, Komljenovic D, Sahm F, Bäuerle T, Ishikawa H, Schroten H, Korff T, et al. Loss of Mpdz impairs ependymal cell integrity leading to perinatal-onset hydrocephalus in mice. EMBO Mol Med. 2017:9(7):890–905. 10.15252/emmm.201606430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Cai X, Qiao J, Switzer B, Baun J, Morrison T, Iriah S, Madularu D, Sinkevicius KW, Kulkarni P. Life without a brain: neuroradiological and Behavioral evidence of neuroplasticity necessary to sustain brain function in the face of severe hydrocephalus. Sci Rep. 2019:9(1):16479. 10.1038/s41598-019-53042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuillet L, Dufour H, Pelletier J. Brain of a white-collar worker. Lancet (London, England). 2007:370(9583):262. 10.1016/S0140-6736(07)61127-1. [DOI] [PubMed] [Google Scholar]
- Foerster P, Daclin M, Asm S, Faucourt M, Boletta A, Genovesio A, Spassky N. MTORC1 Signaling and primary cilia are required for brain ventricle morphogenesis. Development. 2017:144(2):201–210. 10.1242/dev.138271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freimann FB, Müller S, Streitberger KJ, Guo J, Rot S, Ghori A, Vajkoczy P, Reiter R, Sack I, Braun J. MR Elastography in a murine stroke model reveals correlation of macroscopic viscoelastic properties of the brain with neuronal density. NMR Biomed. 2013:26(11):1534–1539. 10.1002/NBM.2987. [DOI] [PubMed] [Google Scholar]
- Furey CG, Choi J, Jin SCC, Zeng X, Timberlake AT, Nelson-Williams C, Mansuri MSS, Lu Q, Duran D, Panchagnula S, et al. De novo mutation in genes regulating neural stem cell fate in human congenital hydrocephalus. Neuron. 2018:99(2):302–314.e4. 10.1016/j.neuron.2018.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futagi Y, Suzuki Y, Toribe Y, Morimoto K. Neurodevelopmental outcome in children with Fetal hydrocephalus. Pediatr Neurol. 2002:27(2):111–116. [DOI] [PubMed] [Google Scholar]
- Gholipour A, Rollins CK, Velasco-Annis C, Ouaalam A, Akhondi-Asl A, Afacan O, Ortinau CM, Clancy S, Limperopoulos C, Yang E, et al. A normative spatiotemporal MRI atlas of the Fetal brain for automatic segmentation and analysis of early brain growth. Sci Rep. 2017:7(1):476. 10.1038/s41598-017-00525-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilard V, Chadie A, Ferracci F-X, Brasseur-Daudruy M, Proust F, Marret S, Curey S. Post Hemorrhagic hydrocephalus and neurodevelopmental outcomes in a context of neonatal intraventricular Hemorrhage: an institutional experience in 122 preterm children. BMC Pediatr. 2018:18(1):288. 10.1186/s12887-018-1249-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez DG, DiBenedetto AT, Pavese AM, Firpo A, Hershan DB, Potts DG. Development of arachnoid villi and granulations in man. Acta Anat. 1982:111(3):247–258. 10.1159/000145473. [DOI] [PubMed] [Google Scholar]
- Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, Kornblum HI, Liu X, Wu H. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science (New York, NY). 2001:294(5549):2186–2189. 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- Guemez-Gamboa A, Coufal NG, Gleeson JG. Primary cilia in the developing and mature brain. Neuron. 2014:82(3):511–521. 10.1016/j.neuron.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra M, Guerra M. Neural stem cells: are they the hope of a better life for patients with Fetal-onset hydrocephalus? Fluids Barriers CNS. 2014:11(1):7. 10.1186/2045-8118-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra MM, Henzi R, Ortloff A, Lichtin N, Vío K, Jiménez AJ, Dominguez-Pinos MD, González C, Jara MC, Hinostroza F, et al. Cell junction pathology of neural stem cells is associated with ventricular zone disruption, hydrocephalus, and abnormal neurogenesis. J Neuropathol Exp Neurol. 2015:74(7):653–671. 10.1097/NEN.0000000000000203. [DOI] [PubMed] [Google Scholar]
- Guerra M, Blázquez JL, Rodríguez EM. Blood-brain barrier and foetal-onset hydrocephalus, with a view on potential novel treatments beyond managing CSF flow. Fluids Barriers CNS. 2017:14(1):19. 10.1186/s12987-017-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Park J, Solomon C, Kranz DA, Wrensch M, Yvonne WW. Long-term outcomes in patients with treated childhood hydrocephalus. J Neurosurg Pediatr. 2007:106(5):334–339. 10.3171/ped.2007.106.5.334. [DOI] [PubMed] [Google Scholar]
- Hale AT, Bastarache L, Morales DM, Wellons JC, Limbrick DD, Gamazon ER. Multi-Omic analysis elucidates the genetic basis of hydrocephalus. Cell Rep. 2021:35(5):109085. 10.1016/j.celrep.2021.109085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmacek L, Watkins-Chow DE, Chen J, Jones KL, Pavan WJ, Salbaum JM, Niswander L. A unique missense allele of BAF155, a Core BAF chromatin Remodeling complex protein, causes neural tube closure defects in mice. Dev Neurobiol. 2014:74(5):483–497. 10.1002/dneu.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenpusch-Theil K, Laclef C, Colligan M, Fitzgerald E, Howe K, Carroll E, Abrams SR, Reiter JF, Schneider-Maunoury S, Theil T. A transient role of the ciliary gene Inpp5e in controlling direct versus indirect neurogenesis in cortical development. elife. 2020:9(August). 10.7554/eLife.58162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe-Hirsch E, Laroussinie F, Brunet L, Sainte-Rose C, Renier D, Cinalli G, Zerah M, Pierre-Kahn A. Late outcome of the surgical treatment of hydrocephalus. Childs Nerv Syst. 1998:14(3):97–99. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979:163(2):195–205. 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, et al. A Paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012:4(147):147ra111. 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito N, Riyadh MA, Ahmad SAI, Hattori S, Kanemura Y, Kiyonari H, Abe T, Furuta Y, Shinmyo Y, Kaneko N, et al. Dysfunction of the proteoglycan Tsukushi causes hydrocephalus through altered neurogenesis in the subventricular zone in mice. Sci Transl Med. 2021:13(587). 10.1126/scitranslmed.aay7896. [DOI] [PubMed] [Google Scholar]
- Iwasawa E, Brown FN, Shula C, Kahn F, Lee SH, Berta T, Ladle DR, Campbell K, Mangano FT, Goto J. The anti-inflammatory agent Bindarit attenuates the impairment of neural development through suppression of microglial activation in a neonatal hydrocephalus mouse model. J Neurosci. 2022:42(9):1820–1844. 10.1523/JNEUROSCI.1160-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez AJ, Tomé M, Páez P, Wagner C, Rodríguez S, Fernández-Llebrez P, Rodríguez EM, Pérez-Fígares JM. A programmed ependymal denudation precedes congenital hydrocephalus in the Hyh mutant mouse. J Neuropathol Exp Neurol. 2001:60(11):1105–1119. 10.1093/jnen/60.11.1105. [DOI] [PubMed] [Google Scholar]
- Jin SC, Dong W, Kundishora AJ, Panchagnula S, Moreno-de-Luca A, Furey CG, Allocco AA, Walker RL, Nelson-Williams C, Smith H, et al. Exome sequencing implicates genetic disruption of prenatal neuro-Gliogenesis in sporadic congenital hydrocephalus. Nat Med. 2020:26(11):1754–1765. 10.1038/s41591-020-1090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H, Sellars R. The movement of fluid out of the cerebral ventricles in Fetal and neonatal rats. Eur J Pediatr Surg. 1982:37(12):130–133. 10.1055/s-2008-1059831. [DOI] [Google Scholar]
- Jucá E, Pessoa A, Ribeiro E, Menezes R, Kerbage S, Lopes T, Cavalcanti LP. Hydrocephalus associated to congenital Zika syndrome: does shunting improve clinical features? Childs Nerv Syst. 2018:34(1):101–106. 10.1007/s00381-017-3636-2. [DOI] [PubMed] [Google Scholar]
- Kahle KT, Kulkarni AV, Limbrick DD Jr, Warf BC, Limbrick DD Jr, Warf BC. Hydrocephalus in children. Lancet. 2016:387(10020):788–799. 10.1016/s0140-6736(15)60694-8. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Kawasawa YI, Cheng F, Zhu Y, Xu X, Li M, Sousa AMM, Pletikos M, Meyer KA, Sedmak G, et al. Spatio-temporal transcriptome of the human brain. Nature. 2011:478(7370):483–489. 10.1038/nature10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan OH, Enno TL, Del Bigio MR. Brain damage in neonatal rats following kaolin induction of hydrocephalus. Exp Neurol. 2006:200(2):311–320. 10.1016/j.expneurol.2006.02.113. [DOI] [PubMed] [Google Scholar]
- Klarica M, Oresković D, Bozić B, Vukić M, Butković V, Bulat M. New experimental model of acute Aqueductal blockage in cats: effects on cerebrospinal fluid pressure and the size of brain ventricles. Neuroscience. 2009:158(4):1397–1405. 10.1016/j.neuroscience.2008.11.041. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 1990:297(3):441–470. 10.1002/cne.902970309. [DOI] [PubMed] [Google Scholar]
- Kousi M, Katsanis N. The genetic basis of hydrocephalus. Annu Rev Neurosci. 2016:39(1):409–435. [DOI] [PubMed] [Google Scholar]
- Krueger RC, Hao W, Zandian M, Danielpour M, Kabos P, Yu JS, Sun YE. Neural progenitors populate the cerebrospinal fluid of preterm patients with hydrocephalus. J Pediatr. 2006:148(3):337–340. 10.1016/j.jpeds.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Kulkarni AV, Riva-Cambrin J, Butler J, Browd SR, Drake JM, Holubkov R, Kestle JRW, Limbrick DD, Simon TD, Tamber MS, et al. Outcomes of CSF shunting in children: comparison of hydrocephalus clinical research network cohort with historical controls. J Neurosurg Pediatr. 2013:12(4):334–338. 10.3171/2013.7.PEDS12637. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Lam MMS, Johnson MB, Dube U, Shim S, Rašin M-R, Sousa AMM, Fertuzinhos S, Chen J-G, Arellano JI, et al. Species-dependent posttranscriptional regulation of NOS1 by FMRP in the developing cerebral cortex. Cell. 2012:149(4):899–911. 10.1016/j.cell.2012.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Huynh M, Silhavy JL, Kim S, Dixon-Salazar T, Heiberg A, Scott E, Bafna V, Hill KJ, Collazo A, et al. De novo somatic mutations in components of the PI3K-AKT3-MTOR pathway cause Hemimegalencephaly. Nat Genet. 2012:44(8):941–945. 10.1038/ng.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin R. Is your brain really necessary? Science (New York, NY). 1980:210(4475):1232–1234. 10.1126/science.7434023. [DOI] [PubMed] [Google Scholar]
- Li M, Santpere G, Imamura Kawasawa Y, Evgrafov OV, Gulden FO, Pochareddy S, Sunkin SM, Li Z, Shin Y, Zhu Y, et al. Integrative functional genomic analysis of human brain development and neuropsychiatric risks. Science (New York, NY). 2018:362(6420). 10.1126/science.aat7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Liu B, Yang X, Yue X, Diao L, Wang J, Chang J. Genetic deletion of Rnd3 results in Aqueductal stenosis leading to hydrocephalus through up-regulation of notch Signaling. Proc Natl Acad Sci U S A. 2013:110(20):8236–8241. 10.1073/pnas.1219995110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden V, Lima Petribu NC, Pessoa A, Faquini I, Paciorkowski AR, Linden H, Silveira-Moriyama L, Cordeiro MT, Hazin AN, Barkovich AJ, et al. Association of severe hydrocephalus with congenital Zika syndrome. JAMA Neurol. 2019:76(2):203–210. 10.1001/jamaneurol.2018.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist B, Carlsson G, Persson E-K, Uvebrant P. Learning disabilities in a population-based Group of Children with hydrocephalus. Acta Paediatr. 2005:94(7):878–883. [DOI] [PubMed] [Google Scholar]
- Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009:8(8):627–644. 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Mestre H, Sweeney AM, Sun Q, Weikop P, Ting D, Nedergaard M. Direct measurement of cerebrospinal fluid production in mice. Cell Rep. 2020:33(12):108524. 10.1016/j.celrep.2020.108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Chen X, Novak MK, Zhang S, Hu W. Repressing Ago2 MRNA translation by Trim71 maintains pluripotency through inhibiting Let-7 MicroRNAs. elife. 2021:10. 10.7554/eLife.66288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longatti P, Fiorindi A, Peruzzo P, Basaldella L, Susin FM. Form follows function: estimation of CSF flow in the third ventricle-aqueduct-fourth ventricle complex Modeled as a diffuser/nozzle pump. J Neurosurg. 2019:(3):1–8. 10.3171/2019.5.JNS19276. [DOI] [PubMed] [Google Scholar]
- Louveau A, Herz J, Alme MN, Salvador AF, Dong MQ, Viar KE, Herod SG, Knopp J, Setliff JC, Lupi AL, et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci. 2018:21(10):1380–1391. 10.1038/s41593-018-0227-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery LA, Sive H. Totally tubular: the mystery behind function and origin of the brain ventricular system. BioEssays. 2009:31(4):446–458. 10.1002/bies.200800207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lummis NC, Sánchez-Pavón P, Kennedy G, Frantz AJ, Kihara Y, Blaho VA, Chun J. LPA 1/3 Overactivation induces neonatal posthemorrhagic hydrocephalus through ependymal loss and ciliary dysfunction. Sci Adv. 2019:5(10):eaax2011. 10.1126/sciadv.aax2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo MH, Hannemann H, Kulkarni AS, Schwartz PH, O'Dowd JM, Fortunato EA. Human cytomegalovirus infection causes premature and abnormal differentiation of human neural progenitor cells. J Virol. 2010:84(7):3528–3541. 10.1128/JVI.02161-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Ineichen BV, Detmar M, Proulx ST. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat Commun. 2017:8(1):1434. 10.1038/s41467-017-01484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maixner WJ, Morgan MK, Besser M, Johnston IH. Ventricular volume in infantile hydrocephalus and its relationship to intracranial pressure and cerebrospinal fluid clearance before and after treatment. A preliminary study. Pediatr Neurosurg. 1990:16(4–5):191–196. 10.1159/000120525. [DOI] [PubMed] [Google Scholar]
- Maller Schulman BR, Liang X, Stahlhut C, DelConte C, Stefani G, Slack FJ. The Let-7 MicroRNA target gene, Mlin41/Trim71 is required for mouse embryonic survival and neural tube closure. Cell Cycle. 2008:7(24):3935–3942. 10.4161/cc.7.24.7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister JP, Guerra MM, Ruiz LC, Jimenez AJ, Dominguez-Pinos D, Sival D, Dunnen W, Morales DM, Schmidt RE, Rodriguez EM, et al. Ventricular zone disruption in human neonates with intraventricular Hemorrhage. J Neuropathol Exp Neurol. 2017:76(5):358–375. 10.1093/jnen/nlx017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister JP, Talcott MR, Isaacs AM, Zwick SH, Garcia-Bonilla M, Castaneyra-Ruiz L, Hartman AL, Dilger RN, Fleming SA, Golden RK, et al. A novel model of acquired hydrocephalus for evaluation of neurosurgical treatments. Fluids Barriers CNS. 2021:18(1):49. 10.1186/s12987-021-00281-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen AB, Baidwan GS, McCarthy KD. Morphological and Behavioral changes in the pathogenesis of a novel mouse model of communicating hydrocephalus. PLoS One. 2012:7(1):e30159. 10.1371/journal.pone.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell JR, al-Zaidy S, Shell R, Arnold D, Rodino-Klapac LR, Prior TW, Lowes L, Alfano L, Berry K, Church K, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017:377(18):1713–1722. 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- Meyer G. Genetic control of neuronal migrations in human cortical development. Berlin/Heidelberg, Germany; 2007. [PubMed] [Google Scholar]
- Miller DJ, Duka T, Stimpson CD, Schapiro SJ, Baze WB, McArthur MJ, Fobbs AJ, Sousa AMM, Šestan N, Wildman DE, et al. Prolonged myelination in human neocortical evolution. Proc Natl Acad Sci U S A. 2012:109(41):16480–16485. 10.1073/pnas.1117943109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitschka S, Ulas T, Goller T, Schneider K, Egert A, Mertens J, Brüstle O, Schorle H, Beyer M, Klee K, et al. Co-existence of intact Stemness and priming of neural differentiation programs in MES cells lacking Trim71. Sci Rep. 2015:5(1):11126. 10.1038/srep11126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyan J, Sobkowiak C, Draper C. Humanity lost: the cost of cortical Maldevelopment. Is there light ahead? Eur J Pediatr Surg. 2001:11(Suppl 1):S4–S9. 10.1055/S-2001-19740. [DOI] [PubMed] [Google Scholar]
- Molliver ME, Kostović I, Loos H. The development of synapses in cerebral cortex of the human Fetus. Brain Res. 1973:50(2):403–407. 10.1016/0006-8993(73)90741-5. [DOI] [PubMed] [Google Scholar]
- Munch TN, Hedley P, Hagen CM, Bækvad-Hansen M, Bybjerg-Grauholm J, Grove J, Nordentoft M, Børglum AD, Mortensen PB, Werge TM, et al. Co-occurring hydrocephalus and polygenic risk in autism spectrum disorder: a Danish population-based cohort study. J Neurodev Disord 2020:PMID33910498. 10.21203/RS.3.RS-41560/V1. [DOI] [PMC free article] [PubMed]
- Nagra G, Koh L, Aubert I, Kim M, Johnston M. Intraventricular injection of antibodies to Beta1-Integrins generates pressure gradients in the brain Favoring hydrocephalus development in rats. Am J Physiol Regul Integr Comp Physiol. 2009:297(5):R1312–R1321. 10.1152/AJPREGU.00307.2009. [DOI] [PubMed] [Google Scholar]
- Narayanan R, Pham L, Kerimoglu C, Watanabe T, Castro Hernandez R, Sokpor G, Ulmke PA, Kiszka KA, Tonchev AB, Rosenbusch J, et al. Chromatin Remodeling BAF155 subunit regulates the genesis of basal progenitors in developing cortex. IScience. 2018:4(June):109–126. 10.1016/j.isci.2018.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer B, Hollanda R, Muniz B, Marchiori E. What we can find beyond the classic neuroimaging findings of congenital Zika virus syndrome? Eur Neurol. 2020:83(1):17–24. 10.1159/000505834. [DOI] [PubMed] [Google Scholar]
- Oegema R, McGillivray G, Leventer R, le Moing A-G, Bahi-Buisson N, Barnicoat A, Mandelstam S, Francis D, Francis F, Mancini GMS, et al. EML1- associated brain overgrowth syndrome with ribbon-like heterotopia. Am J Med Genet C Semin Med Genet. 2019:181(4):627–637. 10.1002/ajmg.c.31751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohata S, Nakatani J, Herranz-Perez V, Cheng J, Belinson H, Inubushi T, Snider WD, Garcia-Verdugo JM, Wynshaw-Boris A, Alvarez-Buylla A. Loss of Dishevelleds disrupts planar polarity in ependymal motile cilia and results in hydrocephalus. Neuron. 2014:83(3):558–571. 10.1016/j.neuron.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtoshi A. Hydrocephalus caused by conditional ablation of the Pten or Beta-catenin gene. Cerebrospinal Fluid Res. 2008:5(1):16. 10.1186/1743-8454-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivero WC, Wszalek T, Wang H, Farahvar A, Rieth SM, Johnson CL. Magnetic resonance elastography demonstrating low brain stiffness in a patient with low-pressure hydrocephalus: case report. Pediatr Neurosurg. 2016:51(5):257–262. 10.1159/000445900. [DOI] [PubMed] [Google Scholar]
- Olstad EW, Ringers C, Hansen JN, Wens A, Brandt C, Wachten D, Yaksi E, Jurisch-Yaksi N. Ciliary beating compartmentalizes cerebrospinal fluid flow in the brain and regulates ventricular development. Curr Biol. 2019:29(2):229–241.e6. 10.1016/j.cub.2018.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onorati M, Li Z, Liu F, Sousa AMM, Nakagawa N, Li M, Dell’Anno MT, Gulden FO, Pochareddy S, Tebbenkamp ATN, et al. Zika virus disrupts Phospho-TBK1 localization and mitosis in human Neuroepithelial stem cells and radial glia. Cell Rep. 2016:16(10):2576–2592. 10.1016/j.celrep.2016.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang D, Altschuler E. Low-pressure hydrocephalic state and viscoelastic alterations in the brain. Neurosurgery. 1994:35(4):643–655discussion 655-6. 10.1227/00006123-199410000-00010. [DOI] [PubMed] [Google Scholar]
- Parikshak NN, Luo R, Zhang A, Won H, Lowe JK, Chandran V, Horvath S, Geschwind DH. Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell. 2013:155(5):1008–1021. 10.1016/j.cell.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña A, Harris NG, Bolton MD, Czosnyka M, Pickard JD. Communicating hydrocephalus: the biomechanics of progressive ventricular enlargement revisited. Acta Neurochir Suppl. 2002:81:59–63. 10.1007/978-3-7091-6738-0_15. [DOI] [PubMed] [Google Scholar]
- Persson EK, Hagberg G, Uvebrant P. Disabilities in children with hydrocephalus--a population-based study of children aged between four and twelve years. Neuropediatrics. 2006:37(6):330–336. 10.1055/s-2007-964868. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judaš M, Šimic G, Rasin MR, Uylings HBM, Rakic P, Kostovic I. Extraordinary Neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci U S A. 2011:108(32):13281–13286. 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretorius DH, Davis K, Manco-Johnson ML, Manchester D, Meier PR, Clewell WH. Clinical course of Fetal hydrocephalus: 40 cases. AJR Am J Roentgenol. 1985:144(4):827–831. 10.2214/ajr.144.4.827. [DOI] [PubMed] [Google Scholar]
- Radoš M, Orešković D, Radoš M, Jurjević I, Klarica M. Long lasting near-obstruction stenosis of mesencephalic aqueduct without development of hydrocephalus--case report. Croat Med J. 2014:55(4):394–398. 10.3325/cmj.2014.55.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radoš M, Orešković D, Klarica M. The role of mesencephalic aqueduct obstruction in hydrocephalus development: a case report. Croat Med J. 2021:62(4):411–419. http://www.ncbi.nlm.nih.gov/pubmed/34472744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radoš M, Živko M, Periša A, Orešković D, Klarica M. No arachnoid granulations-no problems: number, size, and distribution of arachnoid granulations from birth to 80 years of age. Front Aging Neurosci. 2021:13:698865. 10.3389/fnagi.2021.698865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci. 2009:10(10):724–735. 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand TA, Sutou K, Tanabe K, Jeong D, Nomura M, Kitaoka F, Tomoda E, Narita M, Nakamura M, Nakamura M, et al. MYC releases early reprogrammed human cells from proliferation pause via retinoblastoma protein inhibition. Cell Rep. 2018:23(2):361–375. 10.1016/j.celrep.2018.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva-Cambrin J, Kulkarni AV, Burr R, Rozzelle CJ, Oakes WJ, Drake JM, Alvey JS, Reeder RW, Holubkov R, Browd SR, et al. Impact of ventricle size on neuropsychological outcomes in treated Pediatric hydrocephalus: an HCRN prospective cohort study. J Neurosurg Pediatr. 2021:(3):1–12. 10.3171/2021.8.PEDS21146. [DOI] [PubMed] [Google Scholar]
- Rodríguez EM, Guerra MM. Neural stem cells and Fetal-onset hydrocephalus. Pediatr Neurosurg. 2017:52(6):446–461. 10.1159/000453074. [DOI] [PubMed] [Google Scholar]
- Rodríguez EM, Guerra MM, Vío K, González C, Ortloff A, Bátiz LF, Rodríguez S, Jara MC, Muñoz RI, Ortega E, et al. A cell junction pathology of neural stem cells leads to abnormal neurogenesis and hydrocephalus. Biol Res. 2012:45(3):231–241. 10.4067/S0716-97602012000300005. [DOI] [PubMed] [Google Scholar]
- Rosenthal A, Jouet M, Kenwrick S. Aberrant splicing of neural cell adhesion molecule L1 MRNA in a family with X–linked hydrocephalus. Nat Genet. 1992:2(2):107. 10.1038/ng1092-107. [DOI] [PubMed] [Google Scholar]
- Roy A, Murphy RM, Deng M, MacDonald JW, Bammler TK, Aldinger KA, Glass IA, Millen KJ. PI3K-yap activity drives cortical Gyrification and hydrocephalus in mice. elife. 2019:8(May):e45961. 10.7554/eLife.45961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff SJ, Kulkarni AV, Mbabazi-Kabachelor E, Mugamba J, Ssenyonga P, Donnelly R, Levenbach J, Monga V, Peterson M, Cherukuri V, et al. Brain growth after surgical treatment for infant Postinfectious hydrocephalus in sub-Saharan Africa: 2-year results of a randomized trial. J Neurosurg Pediatr. 2021:28(3):1–9. 10.3171/2021.2.peds20949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schock EN, Struve JN, Chang C-F, Williams TJ, Snedeker J, Attia AC, Stottmann RW, Brugmann SA. A tissue-specific role for Intraflagellar transport genes during craniofacial development. PLoS One. 2017:12(3):e0174206. 10.1371/journal.pone.0174206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoner K, Kohlhase J, Müller AM, Schramm T, Plassmann M, Schmitz R, Neesen J, Wieacker P, Rehder H. Hydrocephalus, agenesis of the corpus callosum, and cleft lip/palate represent frequent associations in Fetuses with Peters’ plus syndrome and B3GALTL mutations. - Fetal PPS phenotypes, expanded by Dandy Walker cyst and Encephalocele. Prenat Diagn. 2013:33(1):75–80. 10.1002/pd.4012. [DOI] [PubMed] [Google Scholar]
- Schregel K, Wuerfel née Tysiak E, Garteiser P, Gemeinhardt I, Prozorovski T, Aktas O, Merz H, Petersen D, Wuerfel J, Sinkus R. Demyelination reduces brain parenchymal stiffness quantified in vivo by magnetic resonance Elastography. Proc Natl Acad Sci U S A. 2012:109(17):6650–6655. 10.1073/PNAS.1200151109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterstrom FK, Kosmicki JA, Wang J, Breen MS, de Rubeis S, An J-Y, Peng M, Collins R, Grove J, Klei L, et al. Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell. 2020:180(3):568–584.e23. 10.1016/j.cell.2019.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J-S, Mantas I, Svenningsson P, Greengard P. Ependymal cells-CSF flow regulates stress-induced depression. Mol Psychiatry. 2021:26(12):7308–7315. 10.1038/s41380-021-01202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen R, Sebai MA, Patel N, Ewida N, Kurdi W, Altweijri I, Sogaty S, Almardawi E, Seidahmed MZ, Alnemri A, et al. The genetic landscape of familial congenital hydrocephalus. Ann Neurol. 2017:81(6):890–897. 10.1002/ana.24964. [DOI] [PubMed] [Google Scholar]
- Shimada IS, Somatilaka BN, Hwang S-H, Anderson AG, Shelton JM, Rajaram V, Konopka G, Mukhopadhyay S. Derepression of sonic hedgehog Signaling upon Gpr161 deletion unravels forebrain and ventricular abnormalities. Dev Biol. 2019:450(1):47–62. 10.1016/J.YDBIO.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbereis JC, Pochareddy S, Zhu Y, Li M, Sestan N. The cellular and molecular landscapes of the developing human central nervous system. Neuron. 2016:89(2):248–268. 10.1016/J.NEURON.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sival DA, Guerra M, Dunnen WFA, Bátiz LF, Alvial G, Castañeyra-Perdomo A, Rodríguez EM. Neuroependymal denudation is in progress in full-term human foetal spina bifida Aperta. Brain Pathol. 2011:21(2):163–179. 10.1111/j.1750-3639.2010.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavotinek A, Kaylor J, Pierce H, Cahr M, DeWard SJ, Schneidman-Duhovny D, Alsadah A, Salem F, Schmajuk G, Mehta L. CRB2 mutations produce a phenotype resembling congenital nephrosis, Finnish type, with cerebral ventriculomegaly and raised alpha-fetoprotein. Am J Hum Genet. 2015:96(1):162–169. 10.1016/j.ajhg.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolp HB, Turnquist C, Dziegielewska KM, Saunders NR, Anthony DC, Molnar Z. Reduced ventricular proliferation in the foetal cortex following maternal inflammation in the mouse. Brain. 2011:134(11):3236–3248. 10.1093/brain/awr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahle J, Garton HJL, Maher CO, Muraszko KM, Keep RF, Xi G. Mechanisms of hydrocephalus after neonatal and adult intraventricular Hemorrhage. Transl Stroke Res. 2012:3(S1):25–38. 10.1007/s12975-012-0182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan W, Reeves BC, Duy PQ, Nelson-Williams C, Dong W, Jin SC, Kahle KT. Exome sequencing as a potential diagnostic adjunct in sporadic congenital hydrocephalus. JAMA Pediatr. 2021:175(3):310. 10.1001/jamapediatrics.2020.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur M, Rubenstein JLR. Patterning and plasticity of the cerebral cortex. Science (New York, NY). 2005:310(5749):805–810. 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- Symss NP, Oi S. Theories of cerebrospinal fluid dynamics and hydrocephalus: historical trend. J Neurosurg Pediatr. 2013:11(2):170–177. 10.3171/2012.3.PEDS0934. [DOI] [PubMed] [Google Scholar]
- Tarpey PS, Stevens C, Teague J, Edkins S, O’Meara S, Avis T, Barthorpe S, Buck G, Butler A, Cole J, et al. Mutations in the gene encoding the sigma 2 subunit of the adaptor protein 1 complex, AP1S2, cause X-linked mental retardation. Am J Hum Genet. 2006:79(6):1119–1124. 10.1086/510137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong CK, Han YG, Shah JK, Obernier K, Guinto CD, Alvarez-Buylla A. Primary cilia are required in a unique subpopulation of neural progenitors. Proc Natl Acad Sci U S A. 2014:111(34):12438–12443. 10.1073/pnas.1321425111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Fernández LA, Jux B, Bille M, Port Y, Schneider K, Geyer M, Mayer G, Kolanus W. The MRNA repressor TRIM71 cooperates with nonsense-mediated decay factors to destabilize the MRNA of CDKN1A/P21. Nucleic Acids Res. 2019:47(22):11861–11879. 10.1093/nar/gkz1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully HM, Dobyns WB. Infantile hydrocephalus: a review of epidemiology, classification and causes. Eur J Med Genet. 2014:57(8):359–368. 10.1016/j.ejmg.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga EA, Pastore M, Prior T, Herman GE, McBride KL. The prevalence of PTEN mutations in a clinical Pediatric cohort with autism Spectrum disorders, developmental delay, and macrocephaly. Genet Med. 2009:11(2):111–117. 10.1097/GIM.0b013e31818fd762. [DOI] [PubMed] [Google Scholar]
- Vasileiadis GT, Gelman N, Han VKM, Williams L-A, Mann R, Bureau Y, Thompson RT. Uncomplicated intraventricular hemorrhage is followed by reduced cortical volume at near-term age. Pediatrics. 2004:114(3):e367–e372. 10.1542/peds.2004-0500. [DOI] [PubMed] [Google Scholar]
- Vasistha NA, Pardo-Navarro M, Gasthaus J, Weijers D, Müller MK, García-González D, Malwade S, Korshunova I, Pfisterer U, von Engelhardt J, et al. Maternal inflammation has a profound effect on cortical interneuron development in a stage and subtype-specific manner. Mol Psychiatry. 2020:25(10):2313–2329. 10.1038/s41380-019-0539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasung L, Rollins CK, Zhang J, Velasco-Annis C, Yang E, Lin P-Y, Sutin J, Warfield SK, Soul J, Estroff J, et al. Abnormal development of transient Fetal zones in mild isolated fetal ventriculomegaly. Cereb Cortex. 2022:March. 10.1093/cercor/bhac125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagshul ME, McAllister JP, Limbrick DD, Yang S, Mowrey W, Goodrich JT, Meiri A, Morales DM, Kobets A, Abbott R. MR Elastography demonstrates reduced white matter shear stiffness in early-onset hydrocephalus. Neuroimage Clin. 2021:30:102579. 10.1016/j.nicl.2021.102579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallmeier J, Frank D, Shoemark A, Nöthe-Menchen T, Cindric S, Olbrich H, Loges NT, Aprea I, Dougherty GW, Pennekamp P, et al. De novo mutations in FOXJ1 result in a motile ciliopathy with hydrocephalus and randomization of left/right body asymmetry. Am J Hum Genet. 2019:105(5):1030–1039. 10.1016/j.ajhg.2019.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S, Gärtner J. Genetic and clinical aspects of X-linked hydrocephalus (L1 disease): mutations in the L1CAM gene. Hum Mutat. 2001:18(1):1–12. 10.1002/humu.1144. [DOI] [PubMed] [Google Scholar]
- Welte T, Tuck AC, Papasaikas P, Carl SH, Flemr M, Knuckles P, Rankova A, Buhler M, Grosshans H. The RNA hairpin binder TRIM71 modulates alternative splicing by repressing MBNL1. Genes Dev. 2019:33(17–18):1221–1235. 10.1101/gad.328492.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie KP, Drapaca CS, Sivaloganathan S. A nonlinear viscoelastic fractional derivative model of infant hydrocephalus. Appl Math Comput. 2011:217(21):8693–8704. 10.1016/J.AMC.2011.03.115. [DOI] [Google Scholar]
- Wilkie KP, Nagra G, Johnston M. A mathematical analysis of physiological and molecular mechanisms that modulate pressure gradients and facilitate ventricular expansion in hydrocephalus. Int J Numer Anal Model B. 2012:316:65–81. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4322945/. [PMC free article] [PubMed] [Google Scholar]
- Willsey AJ, Sanders SJ, Li M, Dong S, Tebbenkamp AT, Muhle RA, Reilly SK, Lin L, Fertuzinhos S, Miller JA, et al. Coexpression networks implicate human Midfetal deep cortical projection neurons in the pathogenesis of autism. Cell. 2013:155(5):997–1007. 10.1016/j.cell.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL. Modeling transformations of neurodevelopmental sequences across mammalian species. J Neurosci. 2013:33(17):7368–7383. 10.1523/JNEUROSCI.5746-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington WC, Cathcart RS. Ependymal cilia: distribution and activity in the adult human brain. Science (New York, NY). 1963:139(3551):221–222. 10.1126/science.139.3551.221. [DOI] [PubMed] [Google Scholar]
- Xu H, Fame RM, Sadegh C, Sutin J, Naranjo C, Syau Della, Cui J, Shipley FB, Vernon A, Gao F, et al. Choroid plexus NKCC1 mediates cerebrospinal fluid clearance during mouse early postnatal development. Nat Commun. 2021:12(1):447. 10.1038/s41467-020-20666-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Balasubramaniam J, Buist RJ, Peeling J, del Bigio MR. Periventricular/intraventricular hemorrhage in neonatal mouse cerebrum. J Neuropathol Exp Neurol. 2003:62(11):1154–1165. 10.1093/jnen/62.11.1154. [DOI] [PubMed] [Google Scholar]
- Yakovlev P, Lecours A. The Myelogenetic cycles of regional maturation of the brain. Undefined. 1967https://www.semanticscholar.org/paper/The-myelogenetic-cycles-of-regional-maturation-of-Yakovlev-Lecours/ae1b5cb44581d479695c9263f37aa04ea570c322.
- Yeung MSY, Zdunek S, Bergmann O, Bernard S, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Brundin L, et al. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell. 2014:159(4):766–774. 10.1016/j.cell.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Yung YC, Mutoh T, Lin M-E, Noguchi K, Rivera RR, Choi JW, Kingsbury MA, Chun J. Lysophosphatidic acid Signaling may initiate Fetal hydrocephalus. Sci Transl Med. 2011:3(99):99ra87. 10.1126/scitranslmed.3002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zega K, Jovanovic VM, Vitic Z, Niedzielska M, Knaapi L, Jukic MM, Partanen J, Friedel RH, Lang R, Brodski C. Dusp16 deficiency causes congenital obstructive hydrocephalus and brain overgrowth by expansion of the neural progenitor pool. Front Mol Neurosci. 2017:10:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li J, Saucier JB, Feng Y, Jiang Y, Sinson J, McCombs AK, Schmitt ES, Peacock S, Chen S, et al. Non-invasive prenatal sequencing for multiple Mendelian monogenic disorders using circulating cell-free Fetal DNA. Nat Med. 2019:25(3):439–447. 10.1038/s41591-018-0334-x. [DOI] [PubMed] [Google Scholar]