Abstract
When there is an echocardiographic diagnosis of severe mobile atherosclerotic plaque in the aortic arch or descending aorta, perfusion toward the aortic arch during cardiopulmonary bypass may create a high risk of embolic neurologic injury. Other perfusion methods, such as cannulation of the femoral or axillary arteries, are not always possible, due to atherosclerosis. The ascending aorta may be an alternative site for perfusion, since it is less frequently diseased. We assessed a new technique of perfusion toward the aortic valve using a new cannula designed for this purpose (Dispersion aortic cannula).
Our study included 100 consecutive patients, 72 men and 28 women, with an average age of 68 ± 1.0 years (range, 39–89 years). There were no complications related to insertion of the cannula or perfusion. The ascending aorta could be cross-clamped and side-clamped without perfusion problems. Three deaths occurred; none was related to the cannulation technique. No intra-operative stroke occurred. Two patients suffered neurologic events, one on day 1 and the other on day 6; both had been fully alert after surgery. Perfusion toward the aortic valve appears to be safe and hemodynamically effective. This cannulation technique appears to be an acceptable alternative to present methods. Comparative studies will be needed to determine whether this alternative technique is effective in patients with severe aortic arch disease.
Key words: Aorta, thoracic/surgery; cardiac surgical procedures; cardiopulmonary bypass/methods; cerebrovascular accident/prevention and control; embolism, cholesterol/prevention and control; surgical instruments
Neurologic injury associated with myocardial revascularization is an uncommon complication that has been increasingly reported 1 as a significant cause of disability and death. Intra-operative hypotension, 2 postoperative cardiac arrest, 3 and air embolism 4 are established causes of brain injury. Age 5–7 and prolonged cardiopulmonary bypass (CPB), 3,5,8 as well as carotid disease and peripheral vascular disease, 7 are also strongly associated risk factors. Atherosclerosis of the ascending aorta 5,9,10 has been reported as a cause of perioperative embolic stroke. However, we have observed that cerebral embolization often occurs when the ascending aorta appears normal. While the known risk factors may provide some degree of predictability for perioperative stroke risk, friable aortic arch atherosclerosis has been implicated in cerebral injury, and it has been found to be much more common than atherosclerotic disease of the ascending aorta. 11,12 Is this the significant information that cardiac surgeons could use to reduce perioperative stroke rate in the high-risk patient?
For some time, we have suspected that friable atheromatous plaque in or near the aortic arch may be a frequent source of emboli. Our suspicions have arisen from autopsy studies performed on our patients who died of unexplained massive perioperative stroke, and on a previous case in which the ascending aorta appeared normal but deep hypothermia was required to perform an endarterectomy of mobile débris from the aortic arch. 13 As a consequence of these experiences, we have been led to question the routine practice of directing the perfusion cannula and its “jet” into the aortic arch.
In this report, we describe an alternative technique—perfusion toward the aortic valve—which eliminates the “sandblast” effect of the perfusion cannula into the aortic arch and may thereby reduce the rate of embolic stroke. This technique is made possible with a new cannula that has a soft, fan-shaped exit flow. It is angled away from the orifice, which enables the cannula tip to maintain adequate distance when the aorta is cross-clamped.
We report our experience with this technique in our first 100 patients.
Patients and Methods
The average age of the 100 consecutive patients on whom coronary artery bypass surgery only was performed was 68 ± 1.0 years (range, 39 to 89). Twenty patients were 59 years old or less, 26 patients were 60 to 69 years of age, 41 patients were 70 to 79 years of age, and 13 patients were 80 years or older. Seventy-two were male, and 28 were female. Ten patients had emergency operations. Table I lists other clinical characteristics, including aortic insufficiency that was found either by angiography or by preoperative echocardiography and determined to be non-surgical.
TABLE I. Clinical Characteristics of Patients
From June to September 1998, 100 consecutive patients underwent CPB with perfusion directed only toward the aortic valve. Study of this technique was limited to patients undergoing coronary artery bypass grafting, in whom it was possible to perform aortic cannulation in the sub-innominate artery position on the distal ascending aorta. We did not use this technique if calcification of the ascending aorta or atherosclerosis involving the distal ascending aorta made cannulation impossible, or if cardiopulmonary resuscitation was required prior to the surgical procedure.
Age, sex, perfusion time, and clinical features such as diabetes mellitus, hypertension, obesity, smoking history, number of bypass grafts, previous stroke history, and carotid artery disease were recorded for all patients. Routine echocardiography of the aorta was not performed preoperatively or intraoperatively. Patients were examined preoperatively and postoperatively by the cardiologist and the cardiovascular surgeon for neurologic deficits and for evidence of distal embolization. Patients in whom neurologic changes were suspected were examined by a neurologist, and computerized axial tomography of the brain was performed to confirm neurologic injury.
At surgery, all 100 patients underwent a median sternotomy, and, if needed, the left internal mammary artery was harvested. The pericardium was opened, and the ascending aorta was carefully palpated for plaque or calcification. A double purse-string suture was then placed at a “clean” site next to the origin of the innominate artery or proximal to any disease of the ascending aorta, provided that it left enough room for cannulation, cross-clamping, and side-clamping for proximal anastomosis. A single purse-string suture was used for right atrial cannulation by means of a double-staged cannula. Beef heparin was given to keep the activated clotting time in excess of 500 seconds. Each patient was cannulated with the Dispersion aortic cannula, size 24, (Edwards Life Science Research Medical; Midvale, Utah), with the lip and orifice of the cannula directed toward the ascending aorta (Fig. 1).
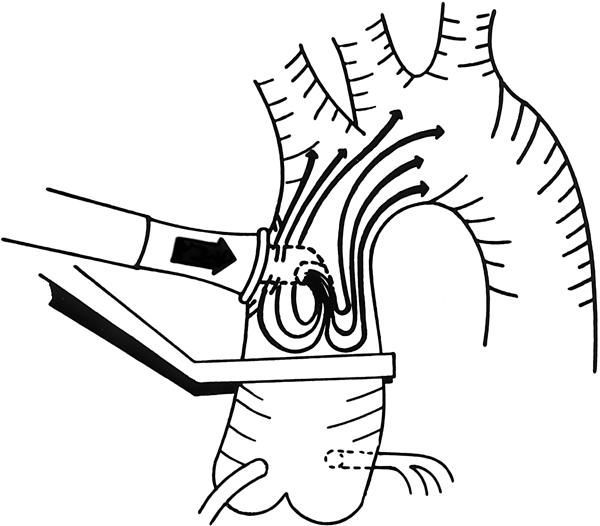
Fig. 1 Perfusion into the ascending aorta using the Dispersion cannula. The distance between the cannula's orifice and the aortic cross-clamp is usually 1-2 cm. Side clamping can be performed without problems.
Cardiopulmonary bypass on all patients was conducted with a Carmeda coated hollow-fiber membrane oxygenator (Medtronic Bio-Medicus; Eden Prairie, Minn) using a centrifugal pump with a 40-micron blood filter (Pall Biomedical; East Hills, NY) incorporated into the arterial perfusion line. Flow rates were maintained at a cardiac index of 2.4 L/min/kg, with mild systemic hypothermia of 31 to 33 °C and rewarming to a maximum of 37 °C. After the ascending aorta was cross-clamped, usually 1 cm proximal to the insertion point of the cannula, cold blood cardioplegic solution (6 to 8 °C) was given antegrade via the ascending aorta to cool the myocardium to 15 °C or lower. This was repeated antegrade or retrograde between each grafting or when the myocardial temperature approached 20 °C.
We recorded any problems with cannulation or decannulation and aortic cross-clamping or side-clamping, and we noted elevations in the perfusion line pressure. The heart was carefully observed for evidence of aortic insufficiency and ventricular distention during the entire pump run. Clinical outcomes of death, major morbidity, and postoperative length of stay were collected by the cardiac coordinating nurse. For continuous data, mean values ± 1 SEM are reported. The study of this cannulation technique was approved by the Institutional Review Board at Iowa Methodist Medical Center, January 1998.
Results
The number of grafts constructed in each patient is listed in Table I. Eighty-two patients received 1 or more internal mammary anastomoses. Five patients had reoperative bypass procedures. In 99 patients, aortic cannulation was easy. In 1 patient, the surgeon considered insertion of the aortic cannula to be of moderate difficulty; this was during a redo operation. No complications were encountered with insertion or removal of the cannula. The ascending aorta was cross-clamped in all patients. Problems such as cardiac distention, persistent hypotension (< 50 mmHg), or high perfusion-line pressure (>240 mmHg) were not encountered either before or after the ascending aorta was cross-clamped. In 96 patients, the side-clamp was applied in the usual manner. Across-clamp alone was used in 2 patients who received a single internal mammary graft and in 2 patients who underwent redo surgery. Other significant perfusion data are listed in Table II. Most often, the lowest perfusion pressure was recorded at the onset of perfusion or at the release of the aortic cross-clamp.
TABLE II. Perfusion Data
Major morbidity and mortality occurred in 9 patients (Table III). Two postoperative strokes occurred: 1 on day 1, and the other on day 6. Both patients had been neurologically normal after recovering from anesthesia. The patient who had a stroke on postoperative day 6 died on day 15. One intra-operative death occurred when the patient could not be weaned from CPB. Another patient died of cardiac arrest the evening before he was scheduled for discharge. No intra-operative strokes, defined as a neurologic deficit discovered upon anesthetic clearance, were diagnosed in the 99 patients who recovered from the anesthetic.
TABLE III. Major Morbidity and Mortality
Discussion
During the past 3 decades, cannulation of the ascending aorta with perfusion directed into the aortic arch has remained the standard technique for conducting CPB. This method is simple and readily achievable, and it has a lower rate of aortic dissection than does femoral cannulation. 14 Nevertheless, even with this technique and perfusion improvements such as inline arterial filters, membrane oxygenators, hemodilution technique, hypothermia, and management of intra-cardiac air, neurologic injury is still most often of embolic origin. 11,15,16 What is the primary source of the emboli? Perioperative stroke associated with CABG has been correlated with advanced age, 5–7 prolonged CPB, 3,5,8 carotid disease, 7 atherosclerotic ascending aorta, 15 and even peripheral vascular disease, 7 but none of these associations completely accounts for the primary source of the embolic phenomenon, particularly when the ascending aorta is normal.
Recently, echocardiographic studies 11,12 have demonstrated that friable or mobile atherosclerotic plaque of the aortic arch and descending aorta is common and likely to be an embolic cause of stroke. Especially significant are the findings of Barbut and associates 11 demonstrating that high grade, mobile plaque (Grade V) is found more frequently in the aortic arch (18%) and descending aorta (34.5%) than in the ascending aorta (5.3%). This finding is important because much of the blame for atherosclerotic emboli has previously been attributed to the ascending aorta, 15 and the aortic arch has not been implicated. Only recently has the “sandblasting” effect of the high-velocity jet of blood that exits the aortic cannula and strikes the friable atherosclerosis within the ascending aorta been recognized as a frequent source of perioperative stroke. 17 The “Soft Flow” cannula (Sarns, Inc.; Ann Arbor, Mich) was designed with the intention of preventing embolization from within the aorta.
We previously reported a case in which angiography diagnosed severe aortic arch atherosclerosis that required deep hypothermia to perform an endarterectomy of the arch simultaneously with carotid endarterectomy and quadruple bypass surgery. 13 This, along with autopsy findings on our patients who died of unexplained perioperative stroke, has led us to suspect for some time that atherosclerotic plaque in or near the aortic arch may be the primary source of emboli. In all of these patients, the ascending aorta was free of atherosclerotic disease. The increased frequency of aortic arch disease can be explained by Bernoulli's theorem. 18 Accelerated atherosclerotic buildup at a curve or bifurcation may be caused by “lift” within the vascular wall similar to the “lift” produced by the air flow over the greater curvature of an airplane wing. As the patient ages, the patches of atherosclerosis in the arch enlarge, then degenerate into soft friable or potentially mobile lesions. The continuous kinetic energy and turbulence produced by the jet of blood from the standard perfusion cannula produces a sandblast effect that can dislodge débris, which can then be carried by the blood flow to the brain or other organs and cause embolic events. Our observations, as well as the published echocardiographic studies, 11,16 have led us to speculate that perfusion into the aortic arch may be hazardous, while perfusion toward the aortic valve may reduce intraoperative stroke by decreasing the likelihood of plaque disruption in the arch.
We are still very cautious about considering this technique if a large portion (greater than 1/3rd) of the ascending aorta is diseased. In such a case, we would use the “no touch” technique described by Dietl and associates. 19 We found no such patients in our study. In the 5 patients who had disease of the ascending aorta, we were able to cannulate proximal to the disease (on the aortic valve side) and still perform this technique successfully and easily.
Our initial study of perfusion toward the aortic valve in 100 consecutive patients demonstrates the feasibility and safety of this technique. The most encouraging outcome is that none of our 100 patients suffered intraoperative stroke. The Dispersion aortic cannula, with its particular angulation and lip at the orifice for reduction of exit force and velocity, seems ideally designed for this technique and enables easy cross-clamping and side-clamping of the ascending aorta without occluding the cannula. The perfusion data confirm that no hemodynamic difficulties occurred, but more studies are needed to determine how effective this alternative technique might be in patients with severe aortic arch disease.
Acknowledgment
We gratefully acknowledge Dixie Van Syoc for her secretarial assistance and Cynthia Bik, RN, for data collection. Norman Paradise, PhD, and James Hopkins, MD, are thanked for their data review and editorial assistance. We also wish to thank Terry Plummer for the illustration.
Footnotes
Address for reprints: Ronald K. Grooters, MD, FACS, The Iowa Clinic–Heart and Vascular Care, Cardio-Thoracic Surgery Division, 1440 Pleasant Street, Suite 150, Des Moines, IA 50309
The Dispersion aortic cannula is manufactured under a patent license agreement between Edwards Life Sciences Research Medical, Midvale, Utah, and Ronald K. Grooters, MD.
References
- 1.Cosgrove DM, Loop FD, Lytle BW, Baillot R, Gill CC, Golding LA, et al. Primary myocardial revascularization. Trends in surgical mortality. J Thorac Cardiovasc Surg 1984;88:673–84. [PubMed]
- 2.Henriksen L. Evidence suggestive of diffuse brain damage following cardiac operations. Lancet 1984;1(8381):816–20. [DOI] [PubMed]
- 3.Sotaniemi KA. Brain damage and neurological outcome after open-heart surgery. J Neurol Neurosurg Psychiatry 1980;43:127–35. [DOI] [PMC free article] [PubMed]
- 4.Mills NL, Ochsner JL. Massive air embolism during cardiopulmonary bypass. Causes, prevention, and management. J Thorac Cardiovasc Surg 1980;80:708–17. [PubMed]
- 5.Gardner TJ, Horneffer PJ, Manolio TA, Hoff SJ, Pearson TA. Major stroke after coronary artery bypass surgery: changing magnitude of the problem. J Vasc Surg 1986;3:684–7. [PubMed]
- 6.Slogoff S, Girgis KZ, Keats AS. Etiological factors in neuropsychiatric complications associated with cardiopulmonary bypass. Anesth Analg 1982;61:903–11. [PubMed]
- 7.Horneffer PJ, Gardner TJ, Maniolio TA, Hoff SJ, Rykiel MF, Pearson TA. The effects of age on outcome after coronary bypass surgery. Circulation 1987;76(5 Pt 2):V6–12. [PubMed]
- 8.Tufo HM, Ostfeld AM, Shekelle R. Central nervous system dysfunction following open-heart surgery. JAMA 1970; 212:1333–40. [PubMed]
- 9.Culliford AT, Colvin SB, Rohrer K, Baumann FG, Spencer FC. The atherosclerotic ascending aorta and transverse arch: a new technique to prevent cerebral injury during bypass: experience with 13 patients. Ann Thorac Surg 1986; 41:27–35. [DOI] [PubMed]
- 10.Landymore RW, Kinley CE, Murphy DA, Sullivan JA. Prevention of neurological injury during myocardial revascularization in patients with calcific degenerative aortic disease. Ann Thorac Surg 1986;41:293–96. [DOI] [PubMed]
- 11.Barbut D, Lo YW, Hartman GS, Yao FS, Trifiletti RR, Hager DN, et al. Aortic atheroma is related to outcome but not numbers of emboli during coronary bypass. Ann Thorac Surg 1997;64:454–9. [DOI] [PubMed]
- 12.Konstadt SN, Reich DL, Kahn R, Viggiani RF. Transesophageal echocardiography can be used to screen for ascending aortic atherosclerosis. Anesth Analg 1995;81:225–8. [DOI] [PubMed]
- 13.Nishida H, Grooters RK, Yeager AA, Soltanzadeh H, Thieman KC, Schneider RF. Carotid and aortic arch endarterectomy using hypothermic arrest with coronary bypass. Ann Thoracic Surg 1989;48:865–66. [DOI] [PubMed]
- 14.Salerno TA, Lince DP, White DN, Lynn RB, Charrette EJ. Arch versus femoral artery perfusion during cardiopulmonary bypass. J Thorac Cardiovasc Surg 1978;76:681–4. [PubMed]
- 15.Blauth CI, Cosgrove DM, Webb BW, Ratliff NB, Boylan M, Piedmonte MR, et al. Atheroembolism from the ascending aorta. An emerging problem in cardiac cardiac surgery. J Thorac Cardiovasc Surg 1992;103:1104–12. [PubMed]
- 16.Hartman GS, Yao FS, Bruefach M 3rd, Barbut D, Peterson JC, Purcell MH, et al. Severity of aortic atheromatous disease diagnosed by transesophageal echocardiography predicts stroke and other outcomes associated with coronary artery surgery: a prospective study. Anesth Analg 1996;83:701–08. [DOI] [PubMed]
- 17.Muehrcke DD, Cornhill JF, Thomas JD, Cosgrove DM. Flow characteristics of aortic cannulae. J Card Surg 1995; 10(4 Suppl):514–9. [DOI] [PubMed]
- 18.Moses C. Pathogenesis of atherosclerosis. In: Moses C. Atherosclerosis. Philadelphia: Lea & Febiger, 1963:32–6.
- 19.Dietl CA, Madigan NP, Laubach CA, Chapman JH, Bering JP, Holcomb PH, et al. Myocardial revascularization using the “no-touch” technique, with mild systemic hypothermia, in patients with a calcified ascending aorta. J Cardiovasc Surg 1995;36:39–44. [PubMed]


