Abstract
The treatment of peripheral vascular disease is one of the most rapidly expanding fields of medicine today. At one time, patients who had peripheral vascular disease had few medical or surgical options. Now, however, options abound. The number of peripheral interventions increased from 90,000 in 1994 to more than 200,000 in 1997, and endovascular techniques may soon replace up to 50% of traditional vascular operations.
Cardiologists, interventional radiologists, and vascular surgeons bring various types of expertise to endovascular intervention; nonetheless, they seem to share similar levels of enthusiasm about this treatment option. The many advantages to the patient that such intervention offers over traditional surgery, such as the avoidance of anesthesia and other surgical risks, the rapid recovery time, and the relatively low treatment costs, provide encouragement to these specialists.
Endovascular intervention requires dedication on the part of practitioners, because it demands such complete knowledge of vascular disease and of the anatomic changes experienced by the patient. The challenge is intensified by the continual introduction of new products and methods. We hope, herein, to offer pertinent information about recent advances in interventional techniques and devices, and to provide a framework for future education.
Key words: Angioplasty, transluminal percutaneous; aortic aneurysm, abdominal; arterial occlusive diseases; blood vessel prosthesis; carotid stenosis; catheterization/methods; collagen/therapeutic use; embolism; peripheral vascular diseases/radiotherapy; gene transfer; hemostatic techniques; prosthesis design; thrombosis/drug therapy/radiotherapy
Endovascular intervention is the fastest growing area of vascular medicine. Peripheral vascular interventions have been developed with these aims: to avoid the risk of general or epidural anesthesia and the risk of conventional surgical procedures, to reduce the patient's discomfort and recovery time, and to lower the cost of treatment.
Endovascular intervention has generated great enthusiasm among specialists in cardiology, interventional radiology, and vascular surgery. The number of radiologic and angiographic heart procedures has doubled since 1994. 1 Approximately 2.5 million radiologic diagnostic procedures and 3.2 million angiographic heart procedures are performed annually in the United States. Since 1994, the number of peripheral interventional procedures has increased from 90,000 to more than 200,000 in 1997 2 —this far exceeds the increase in coronary interventional procedures.
Cardiovascular interventional techniques require specialized skills and training in diagnostic angiography and interventional techniques. To gain expertise in peripheral interventions, knowledge must also be acquired with regard to the natural history of peripheral vascular disease and the anatomic changes that occur in patients who have this disease. Familiarity with various therapeutic alternatives is necessary, as well. 3
Since the original description of percutaneous balloon angioplasty more than 25 years ago by Andreas Gruentzig, 4 endovascular interventionists have been able to treat patients with coronary and peripheral arterial disease using a variety of interventional techniques. Such treatment has undergone dramatic expansion during the last few decades (Table I).
TABLE I. Endovascular Treatment Methods for Peripheral Vascular Disease

Percutaneous Transluminal Balloon Angioplasty
Percutaneous transluminal balloon angioplasty (PTA) has been used successfully for treating coronary, renal, iliac, femoral, tibio-peroneal, subclavian, carotid, and other arterial stenoses. The best results of PTA are achieved in stenotic lesions that are short, concentric, and noncalcific. Despite substantial improvements in balloon and catheter technology, PTAstill produces unacceptable restenosis rates in complex lesions and often requires reintervention. This is particularly common when the complex lesions are in the carotid, renal, femoropopliteal, and tibio-peroneal arteries. 5 Several mechanisms can contribute to this recurrence, including elastic recoil, vascular remodeling, and intimal hyperplasia.
Through the technical innovation of stents, the problems of elastic recoil and vascular remodeling have for the most part been solved, and a large percentage of vessels remain free of restenosis. However, intimal hyperplasia, including smooth-muscle-cell proliferation, requires further research to find a solution.
Carotid Angioplasty
For many years, PTA was considered unsuitable as a treatment for atherosclerotic carotid stenosis, because atherosclerotic plaque is not removed by this method. Although PTA was 1st performed by Kerber's group in 1980, 6 the procedure is still considered controversial for extracranial carotid artery stenosis. The European trial CAVATAS, 7 which compared carotid endarterectomy to PTA of the extracranial carotid artery in a prospective randomized study, showed no essential difference between the results of the 2 methods over a period of more than 4 years.
Interventionists have been reluctant to use this technique due to the risk of dislodging atherosclerotic débris, causing cerebral embolism and stroke. Simple balloon angioplasty can lead to embolism and suboptimal long-term results for various reasons, including heterogeneous composition of atherosclerotic plaque at the carotid bifurcation, residual stenosis after PTA (frequently present), and intimal disruption and dissection leading to thrombus formation.
Stent-Supported Carotid Angioplasty
Technologic advances in the endovascular treatment of peripheral vascular disease, along with the introduction of stents, have been the impetus for various investigational trials concerned with treating extracranial carotid artery occlusive disease. The technique of stent-supported carotid angioplasty (SSCA) has expanded the indications and reduced the risk of neurologic complications that frequently occur with PTA for extracranial carotid artery stenosis. Stent-supported carotid angioplasty, however, is not an approved procedure in the United States. At this time, the consensus among experienced interventionists is that carotid angioplasty should not be performed without the use of a stent (even though no stent has been approved for this purpose). The preliminary results of SSCA 8–11 are encouraging; however, no randomized trial comparing PTA and stenting to carotid endarterectomy has been completed to validate this procedure. The preliminary results of SSCA that are currently available 8–11 are based on single-center, non-randomized trials that have used different study designs and techniques.
Roubin and colleagues 8 reported on a series of 238 SSCA procedures in which a 6.3% incidence of neurologic complications was observed. Diethrich's group, 9 in a series of 110 patients treated with stent placement (117 carotid arteries), reported 7 cerebrovascular accidents (6.4%) and 5 transient ischemic attacks (4.5%). In 1997, Wholey and co-authors 10 described 114 procedures, with successful Palmaz stent placement in 108 carotid arteries. Complications included 4 cerebrovascular accidents (2 major and 2 minor) and 5 transient ischemic attacks, all of which occurred only in the 61 symptomatic patients (8.2%). More recently, Wholey's group 11 reported the results of their international survey on SSCA, which included 2,591 procedures at 24 centers. The overall technical success rate was 98.8%. The complication rates of carotid stenting were 3.08% for minor strokes, 1.32% for major strokes, and 1.37% for periprocedural death. The combined periprocedural stroke and death rate was 5.77% and ranged from zero to 10% among the centers. The restenosis rate was 4.80% at 6 months, as determined by clinical and diagnostic studies. This survey also revealed that interventional cardiologists performed 63% of the procedures; radiologists, 25%; and vascular surgeons, 12%. At the present time, interventionists at 10 to 15 centers in the United States perform more than 50% of all carotid interventional procedures.
Stent-Supported Extracranial Carotid Artery Angioplasty Technique
Several factors can positively influence the results of SSCA:
Preprocedural performance of detailed clinical, noninvasive, and invasive cerebrovascular evaluation
Appropriate choice of arterial access site
Appropriate choice of guiding catheters, guide-wires, and PTA balloons
Appropriate choice of stents (balloon-expandable or self-expandable)
Use of cerebral protection devices when indicated
Use of essential pharmacologic therapy
Adequate knowledge of or support for intracranial vascular rescue
Postprocedural performance of neurologic, invasive, and noninvasive evaluation
The Choice of Stent
When a stent is placed across the carotid bifurcation, it must adapt to arteries of different diameters. The stent should be in close contact with the arterial wall in order to allow neointimal growth. Self-expandable stents, such as the Wallstent® Endoprosthesis (Boston Scientific Corp.; Natick, Mass) (Fig. 1) and the S.M.A.R.T.™ Stent (Cordis Corporation, a Johnson & Johnson company; Warren, NJ) (Fig. 2), have varied radial expansion capabilities, flexibility, and compressibility. Their narrow meshwork is beneficial in preventing embolism during balloon dilation. The disadvantages of the Wallstent are less accurate deployment than that of balloon-expandable stents and sharp strut ends. The S.M.A.R.T. Stent (the acronym S.M.A.R.T. refers to Shape Memory Alloy Recoverable Technology) is a self-expanding stent made of nitinol (as opposed to cobalt alloy like the Wallstent), and may have less shortening than Wallstent without the sharp strut ends. Some other self-expandable stents that have been used for SSCA are the Memotherm (CR Bard; Covington, Georgia) and the Integra stent (Boston Scientific). Currently available stent and balloon designs for SSCA are suboptimal, because the large profile of the 7-F stent delivery device can cause problems, especially in subtotal occlusions with tortuosity at the site of the lesion. The ability to track the stent may be limited by the high-profile delivery system. Nitinol technology is progressing rapidly, and a nondeformable super-elastic memory alloy may become the optimal stent material. In addition, a 5-F delivery system will soon be the subject of feasibility studies, and cerebral protection devices integrated with the stents will be available. Both the S.M.A.R.T. Stent and the Wallstent are now available with smaller outer diameters of 5.5 to 6 F that allow them to be used with smaller sheaths or guides.
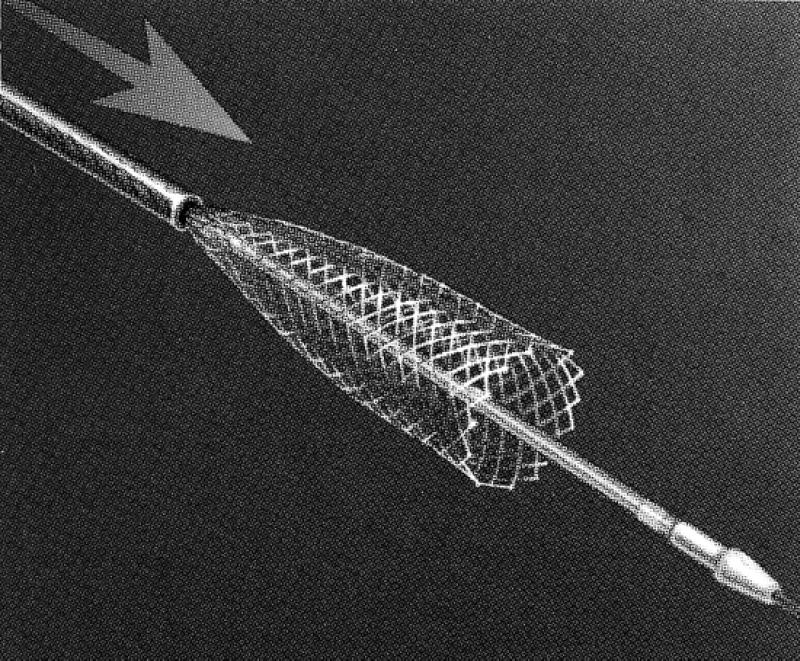
Fig. 1 The Wallstent® Endoprosthesis (Boston Scientific Corp.; Natick, Mass)
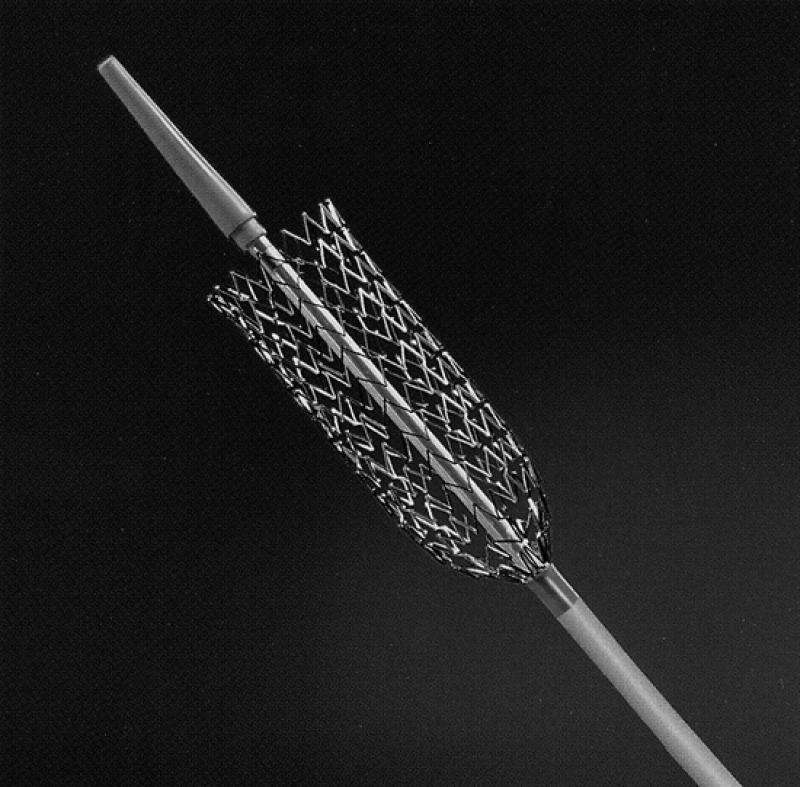
Fig. 2 The S.M.A.R.T.® Stent (Cordis Corp.; Warren, NJ)
Some of the balloon-expandable stents such as the Palmaz® Stent (Cordis) offer more precise location capabilities, provide more radial strength, and contain less metal. The disadvantages of balloon-expandable stents are the risk of deformity and their tendency to collapse with external compression or trauma. These complications occur at the rate of 4% to 15%. 8–12 For this reason, most of the interventionists in current trials are using only self-expandable stents.
Several types of stents have been used successfully for SSCA; however, no ideal stent is available yet. Several manufacturers are investigating covered stents, which could inhibit thrombus formation and myointimal proliferation. These coated stents are available outside the United States. However, the stents require several refinements in diameter and design to be of benefit for this application. It is possible that the covered stents will decrease the risk of cerebral embolism, but occlusion of the external carotid artery is a potential problem. Most investigators agree that SSCA would be of the greatest benefit to patients who are at high risk for surgery, which includes those with
High cervical carotid segmental lesions that are surgically inaccessible
Tandem lesions with proximal and distal lesions of the internal carotid artery
Postoperative recurrent stenosis of the carotid artery
Nonatherosclerotic cause of carotid artery stenosis (for example, fibromuscular dysplasia, Takayasu arteritis)
Ipsilateral stenosis due to prior radiation therapy to the neck
Stenosis due to prior radical neck surgery
Lesions of the common carotid artery with associated internal carotid artery lesions
Increased operative risk due to concomitant illnesses such as coronary artery disease requiring coronary artery bypass surgery
Contralateral occlusion and high-grade ipsilateral stenosis
Methods of Reducing the Incidence of Cerebral Emboli
Cerebral embolization can be caused by the manipulation of guidewires, balloons, and stents across complex atherosclerotic carotid artery lesions. Theron's group 13 analyzed the aspirated blood after patients had undergone angioplasty under cerebral protection, with the inflated balloon in the internal carotid artery. They found, in 17 of 21 cases, cholesterol crystals ranging from 600 to 1300 μm in length. Mathur and coworkers 14 reported that neurologic complications are related to patient selection. Advanced age (>80 years), severe stenosis, and long and multiple stenoses are independent predictors of procedural cerebrovascular accidents. Mathur's group did not find any correlation between neurologic complications and preprocedural symptoms, plaque ulceration, sex of the patient, presence of diabetes mellitus, coronary artery disease, hypercholesterolemia, prior carotid endarterectomy, history of smoking, contralateral carotid occlusion, or the type of stent. Several cerebral protection devices have been developed and are currently being investigated in an effort to reduce the incidence of cerebral embolism:
Theron's technique (cerebral protection with occlusion balloon) 13
Kachel's reversing flow technique 15
The PercuSurge® Guardwire™ temporary occlusion and aspiration system (PercuSurge, Inc.; Sunnyvale, Calif) (Fig. 3)
AngioGuard™ guidewire filter device (Cordis) (Fig. 4)
Medicorp, Henry-Amor-Frid-Rüfenacht (H.A.F.R.) device (Medicorp S.A.; Villers les Nancy, France)
MedNova NeuroShield Cerebral Protection System (MedNova USA; Topsfield, Mass) (Fig. 5)
EPI Filter Wire™ (Embolic Protection Inc.; San Carlos Calif) (Fig. 6)
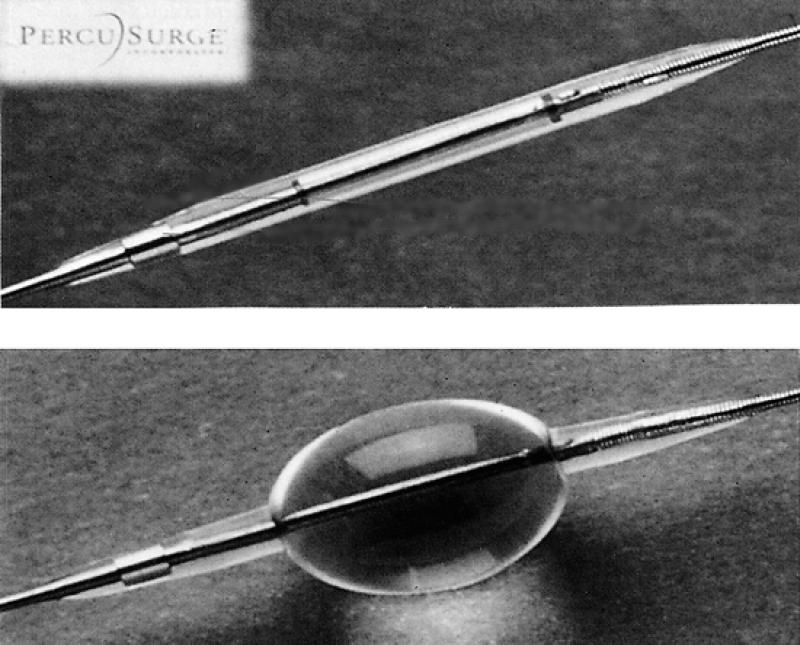
Fig. 3 The PercuSurge® Guardwire™ temporary occlusion and aspiration system (PercuSurge, Inc.; Sunnyvale, Calif)
Fig. 4 The AngioGuard™ guidewire filter device (Cordis)
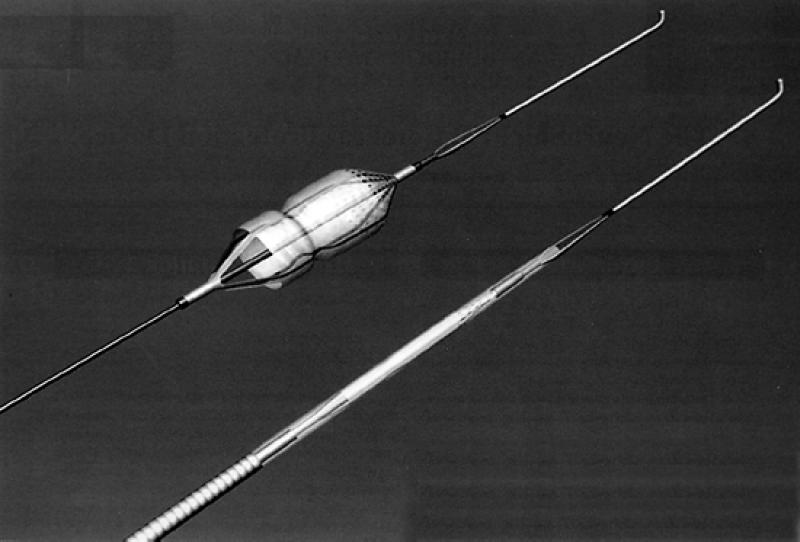
Fig. 5 The MedNova NeuroShield Cerebral Protection System (MedNova USA; Topsfield, Mass)
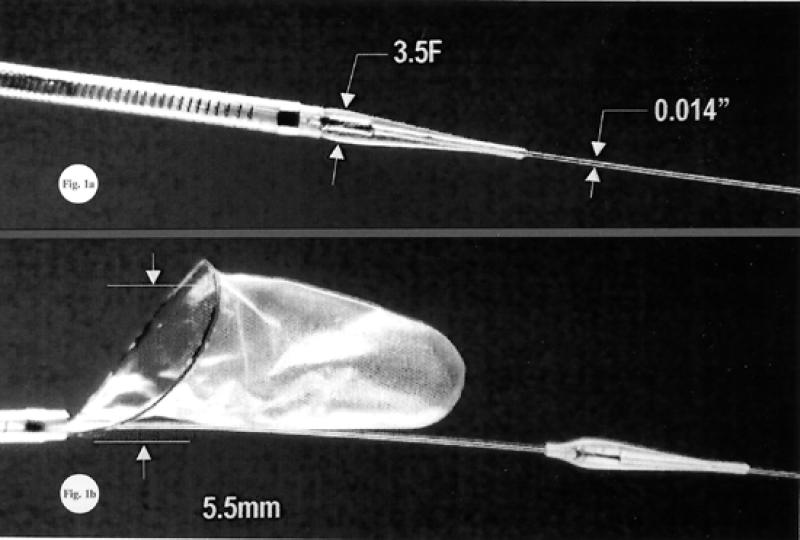
Fig. 6 The EPI filter wire™ (Embolic Protection Inc.; San Carlos, Calif)
Theron and associates 13 originally described their technique in 1990, in which they use a triple coaxial catheter that occludes the internal carotid artery beyond the stenosis with the use of a latex balloon. Angioplasty and stent placement are then performed with the patient under cerebral protection, thus avoiding distal embolism. Any débris from the procedure can be aspirated or flushed through the guiding catheter toward the external carotid artery. The limitations of Theron's technique include the absence of a guidewire in the shaft of the protection balloon and poor steerability of the catheter. No large study has yet evaluated this cerebral protection technique.
Kachel 15 developed a cerebral protection technique that consists of occluding the upper part of the common carotid artery with a balloon attached to the distal end of the guiding catheter. The occlusion created by the balloon allows the reversal of flow toward the external carotid artery. Angioplasty and stenting can be done through the guiding catheter. This technique seems easy to use; however, it does not offer sufficient safety against the risk of embolism. Kachel's series yielded a complication rate of 4.6%, which is not significantly different from complications reported in other studies that did not use cerebral protection.
The PercuSurge Guardwire (Fig. 3) is a device that consists of a 0.014- or 0.018-inch angioplasty guidewire constructed of a hollow nitinol hypotube. Incorporated into the distal wire segment is an inflatable balloon capable of occluding vessel flow. The proximal end of the wire incorporates a Microseal™ that allows inflation and deflation of the distal occlusion balloon. When the Microseal adapter is detached, the occlusion balloon remains inflated, at which time angioplasty and stenting are performed. An aspiration catheter can be advanced over the wire into the vessel, and manual suction is applied to retrieve particulate débris. This device was studied experimentally in animals by Osterle 16 in coronary vessels and then in human aortocoronary saphenous vein grafts. 17 These studies showed the PercuSurge Guardwire to be capable of capturing and retrieving atherosclerotic and thrombotic débris, which may aid in the prevention of distal embolism in a vessel. 16
The Medicorp device consists of a protection balloon and a dilation balloon that can be used over a 0.014-inch coronary guidewire. Although Henry's group has reported encouraging preliminary data with use of this device, larger numbers of cases are needed to determine the benefit of this cerebral protection device. 18 A technique of combined occlusion of the internal carotid and common carotid arteries could be considered as a reasonable alternative. Occlusion of the internal carotid artery above the lesion and the common carotid artery below the lesion would create a dilation zone without a flow, which could be aspirated and cleared of atherosclerotic débris easily.
Filtering devices are in the early experimental stages for cerebral protection. Afilter could stop detached embolic particles without interrupting blood flow to the brain. This technique might benefit patients with contralateral carotid occlusion or an incomplete circle of Willis who would have no tolerance for prolonged interruption of ipsilateral carotid flow. The Angio-Guard guidewire filter device is currently being studied (Fig. 4). Other cerebral protection devices are undergoing evaluation worldwide to determine their ability to prevent cerebral embolization during SSCA (Figs. 5 and 6).
Future Implications for SSCA
The preliminary results of SSCA from several non-randomized trials have been encouraging. However, randomized clinical trials are necessary to determine the benefits and the indications for SSCA. Two randomized clinical trials comparing SSCAwith carotid endarterectomy will soon begin, with the goal of determining which patients would benefit most from each procedure. The SAPPHIRE (Stenting and Angioplasty with Protection in Patients at High Risk for Carotid Endarterectomy) trial 19 and the CREST (Carotid Revascularization Endarterectomy versus Stent Trial) 20 will randomize high- and low-risk patients with carotid artery stenosis to stenting or surgery groups. Both trials will use cerebral protection devices in the stent arm of the study.
Endoluminal Treatment of Abdominal Aortic Aneurysms
Epidemiology of Abdominal Aortic Aneurysms
Abdominal aortic aneurysm (AAA) is characterized by permanent dilatation of the abdominal aorta with a diameter at least 50% larger than normal. This serious vascular disorder predominantly affects men who are 60 years of age or older. Men are affected 5 times as often as women are. More than 90% of AAAs are secondary to atherosclerosis and the majority (89%) are located in the infrarenal aorta. Previous studies have shown that 25% of patients with AAA who did not undergo corrective surgery died of ruptured aneurysm. 21,22 There is a 90% mortality rate associated with an out-of-hospital AAA rupture, but the mortality rate decreases to 50% for those who undergo emergency surgery. 21–23 As a preventive measure, over 40,000 surgical repairs of AAA are performed in the United States annually. 21 The generally accepted AAA diameter at which repair is indicated is 5 cm. 21–23 The standard treatment is replacement of the diseased aorta with a prosthetic graft. The surgical mortality rate in younger (<60 years), asymptomatic patients undergoing elective resection is 3% to 5%. 21,22 In patients who have undergone previous abdominal surgery or who have severe pulmonary, cardiovascular, or renal disease, the risk of perioperative death ranges from 20% to 60%. Such patients are often denied surgery, because the risks of surgery exceed the benefits. 21–24
Endovascular Treatment of AAAs
The 1st endoluminal treatment of AAAs in a clinical setting was reported in 1991 by Parodi and colleagues. 23 Since then, endovascular exclusion of AAAs has attracted many specialists: among them, vascular surgeons, interventional radiologists, and interventional cardiologists. Although vascular surgeons used to be the main practitioners of aortic grafting, more nonsurgical specialists are now getting involved, primarily due to the development of new transcatheter devices for delivery of vascular prostheses.
At first, the use of endoluminal devices was reserved for patients who had concomitant illnesses or other conditions that increased the risk of conventional surgery. 23 More recently, endoluminal grafts have been proposed for use in patients without additional illnesses. 25–30 The 1st-generation endovascular endoluminal grafts were tubular grafts, and later, aorto-uni-iliac grafts were developed. The early prostheses were relatively inflexible and required an introducing femoral sheath with a 24-F internal diameter. 23 The devices are now available as tube grafts or bifurcated grafts, are more flexible, and are available in smaller diameters. Their structures are either completely stent-supported or stented only at the level of attachment. Some of these devices consist of fabric grafts that are supported throughout their length by self-expanding metal stents to minimize kinking and migration. Stainless steel and nitinol (the latter of which has thermal memory characteristics) are the most common materials used for stents. 26–30
Some investigators have reported that fully supported grafts offer a higher degree of immediate and late success. 28–30 A stent may be placed on the outside of the graft material (exoskeleton) 28 or on the inside (endoskeleton). 30 The prosthetic wall can be made of a polyethylene terephthalate textile in a woven or knitted form, 28–30 of urethane polycarbonate, or of an expanded polytetrafluoroethylene (ePTFE) material. The stent grafts are either self-expandable 28,29 or balloon-expandable. 23 The stent graft is affixed by the radial force 28–30 of the stent or by a specific attachment system that uses barbs or hooks. 27 The bifurcated prostheses are available in either a 1-piece design 27 or a modular design. 28–30 The modular design consists of a bifurcated prosthesis, which is introduced through the femoral access as the 1st step, followed by insertion of the contralateral limb through the contralateral femoral access as the 2nd step. Which of these materials and designs will ultimately produce superior long-term results should be revealed when ongoing clinical studies 26–32 are completed. In 1999, well over 4,000 endoluminal abdominal aortic aneurysm repairs were performed with various devices world-wide (Table II). 5–12
TABLE II. Stent Grafts for Repair of Abdominal Aortic Aneurysms

Two endoluminal AAA exclusion stent graft systems have received FDA approval: the Ancure™ Endograft System (Guidant/EVT; Menlo Park, Calif) and the AneuRx™ device (Medtronic AVE; Santa Rosa, Calif). Both are over-the-wire systems that require bilateral femoral artery access.
The Ancure stent graft (Fig. 7) is an unsupported, single piece of woven Dacron fabric. The graft is bifurcated and thus has no intragraft junctions. The main device is delivered through a 24-F introducer sheath; a 12-F sheath is required to facilitate the deployment of the contralateral iliac limb. The graft is attached via a series of hooks that are located at the proximal aortic end and at both iliac ends. The hooks are seated transmurally in the aorta and the iliac arteries, initially by minimal radial force, and then affixed by low-pressure balloon dilation. Radiopaque markers are located on the body of the graft for correct alignment and positioning.
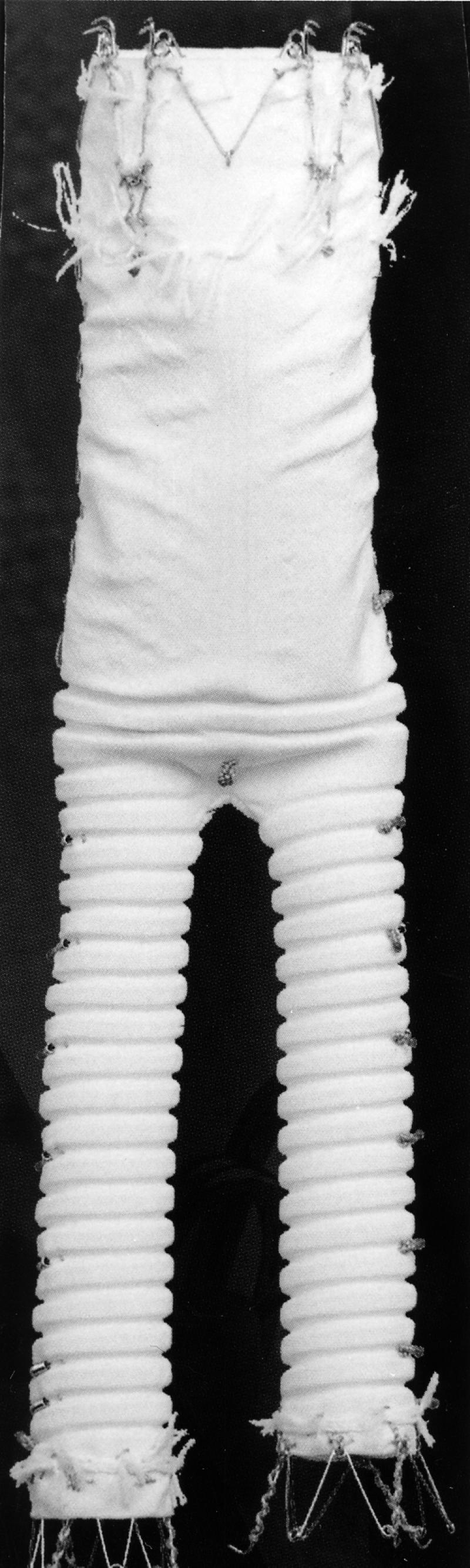
Fig. 7 The Ancure™ Bifurcated Endograft System (Guidant/EVT; Menlo Park, Calif)
The AneuRx device (Fig. 8) is a modular 2-piece system composed of a main bifurcation segment and a contralateral iliac limb. The graft is made of thin-walled woven polyester that is fully supported by a self-expanding nitinol exoskeleton. Attachment is accomplished by radial force at the attachment sites, which causes a frictional seal. The main bifurcated body is delivered through a 21-F sheath, and the contralateral limb requires a 16-F sheath. The body of the graft has radiopaque markers that facilitate correct alignment and positioning.
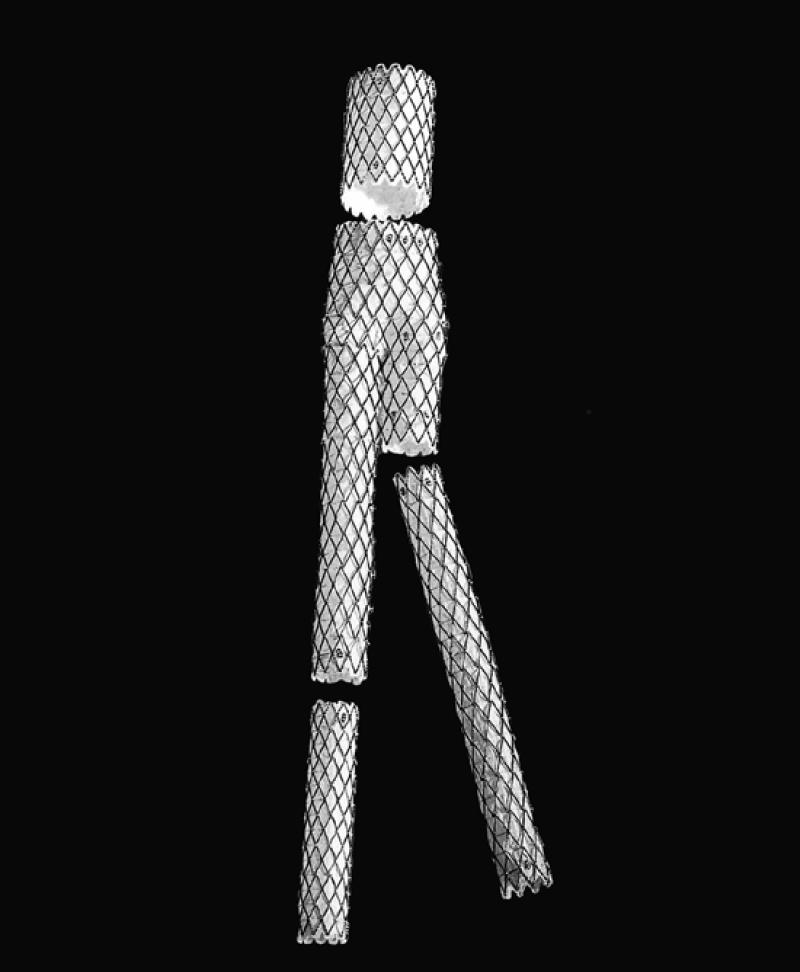
Fig. 8 The AneuRx™ device (Medtronic AVE; Santa Rosa, Calif)
Endoluminal AAA exclusion has been 90% successful with the devices currently being used. The need for surgical intervention due to a failed device is less than 8%. 27–30 The incidence of endoleaks after 1 month has been less than 10% for most devices, with the incidence at 1 and 2 years ranging from 15% to 20%. The procedural and early mortality rate was between 1% and 4% in a recently reported multicenter trial. 31 Rupture due to AAA after endovascular repair is rare: during phases II and III of that same clinical trial, no ruptures were reported with use of the EVT device in 597 cases. Nine ruptures were reported with use of the AneuRx device in 1,046 cases during phases I, II, and III. 32
Although substantial improvements have been made in stent grafts since the original procedure by Parodi and coworkers, 23 further follow-up in current trials is needed to determine the exact usefulness of this procedure for the treatment of AAAs. Some of the devices listed in Table I are currently undergoing clinical evaluation in the United States, and several have already been released for clinical use in other countries.
Thrombolytic Therapy for Arterial Occlusions
A principal goal of treatment for acute limb ischemia is rapid restoration of blood flow to the ischemic region before the occurrence of irreversible changes. Surgical treatment of acute limb ischemia, because of accompanying illnesses, has a 30-day mortality rate of 15% to 25%. 33,34
Intravenous infusion of exogenous plasminogen activators—specifically, streptokinase—was attempted nearly 40 years ago for the treatment of peripheral arterial occlusion. 35 Since then, several studies have shown that thrombolysis can be an effective initial treatment for many patients who have acute arterial occlusions. 33–36 One of the advantages of thrombolysis over surgical intervention is that after thrombolysis, angiographic evaluation can uncover hidden causes of the thrombus formation. 33–36 Then underlying lesions can be identified and treated by transluminal balloon angioplasty or stenting, or by elective surgical revascularization. 33,34
Reasons for using thrombolytic therapy for arterial thrombotic disease are listed below:
To remove the thrombus and establish blood flow to the ischemic limb
To identify hemodynamic causes of arterial or graft occlusion
To convert emergent surgery to elective surgery
To remove thrombus from the collateral circulation
To avoid the mechanical trauma of surgery in the tibio-peroneal vessels
Thrombolytic agents include streptokinase, acylated plasminogen streptokinase complex, urokinase (no longer available), pro-urokinase, and recombinant tissue plasminogen activator (rt-PA-alteplase and r-PA-reteplase). All of these agents induce a systemic fibrinolytic state. In comparative studies on the treatment of arterial thrombosis, 33–41 streptokinase, urokinase, rt-PA, and pro-urokinase have been shown to be more effective than heparin alone in lysing the thrombus. A retrospective study from the Cleveland Clinic 37 found that the clinical success rate was 60% for streptokinase, 95% for urokinase, and 91% for rt-PA. A recent report by McNamara 42 suggests that r-PA may have a clinical efficacy similar to that of rt-PA, but with less bleeding.
Early studies concerning the use of thrombolytic agents revealed that lysis is more likely to be successful if the thrombosis is recent and involves proximal vessels. 33–35 Studies of peripheral arterial occlusions have shown that urokinase has a higher success rate with fewer complications than does streptokinase. 35,37,39,40 McNamara and Fischer 38 found that the mean duration of infusion is also significantly shorter for urokinase than for streptokinase. Comparative studies of streptokinase, urokinase, and rt-PA have shown that rt-PA provides equal success in thrombolysis, but with a higher rate of major bleeding. 36,37,39 The use of streptokinase is limited when antibodies are being produced due to previous streptokinase use or when the patient has had a recent streptococcal infection. Urokinase, which had been the most frequently used thrombolytic agent, was recently removed from the market because of concerns about possible hepatitis contamination.
Methods of Administration of Thrombolytic Agents
Thrombolytic agents have been infused both systemically and locally. The systemic use of thrombolytic agents has been associated with severe bleeding complications. 35,39 On the other hand, some studies 33,34,38,39 have indicated that the local route (catheter-directed thrombolysis) increases the concentration of the thrombolytic agent in the treatment area, which increases the chance of interaction with the thrombus and decreases the incidence of hemorrhagic side effects. Several investigators have shown the usefulness of a guidewire traversal test to assess the outcome of thrombolysis. 34–40 McNamara and Fischer 38 have found that if a guidewire can easily be advanced through the thrombus before the initiation of thrombolysis, the thrombus is likely to respond; however, if the guidewire cannot be passed, thrombolysis is less likely to be successful. A variety of multi-sidehole catheters and infusion wires are available for local administration of thrombolytic agents. 39 A coaxial system of 2 catheters or a catheter and an infusion wire are often used to deliver thrombolytic agents throughout the length of a thrombotic occlusion. 38 This technique shortens the infusion time and requires less frequent angiographic monitoring, because lysing is achieved throughout the thrombus and because catheter repositioning is usually unnecessary. Some of the administration techniques that have been tried include bolus lacing (an initial bolus of the agent is given over a short period of time throughout the length of the thrombus), 34,38 pulsed-spray (a lytic agent is injected through a multi side-hole catheter using high-pressure intermittent pulses), 39 and continuous infusion of a thrombolytic agent over a longer period of time (hours to days). 34–40
Doses of Infusion of Thrombolytic Agents
The dosage and duration of infusion of thrombolytic agents depend on the indication; the agent used; the route of administration; the amount, age, and surface area of the thrombus; and the degree of ischemia. In general, the fresher the thrombus, the more effective the thrombolysis will be. 35,37–39 In addition, the greater the amount of thrombus (thrombus burden), the longer it will take for the completion of lysis. 38,39 The higher the concentration of the thrombolytic agent in the area of thrombosis, the more rapid the lysis will be. 37–39 Several investigators 35,37–39 have recommended the following dosage regimens for systemic infusion of thrombolytic agents in the treatment of deep venous thrombosis and pulmonary emboli:
Streptokinase: Administer a 250,000 IU intravenous bolus (loading dose) over a period of 30 minutes, followed by 100,000 IU/hr for 24 to 72 hours.
rt-PA-alteplase: Administer 100 mg as a continuous intravenous infusion given over 2 hours for pulmonary embolism and 0.06 mg/kg per hour for deep venous thrombosis.
r-PA-reteplase: The dosage recommendations for local infusion of r-PA for deep venous thrombosis and arterial occlusions is 0.5 to 1.0 U/hr intravenously for 5 to 24 hours, with or without a bolus of 2 to 5 U.
Low-dose intravenous heparin (500 U/hr) should be used with rt-PA-alteplase and r-PA-reteplase.
The more severe the degree of ischemia, the more important it is to achieve rapid lysis. Rapidity of thrombolysis is increased by high-dose regimens; however, the complication rates may also increase. 34,37–39 The duration of therapy usually depends on the response, as determined by clinical or angiographic results. Several investigators 37–40 have shown the benefit of concomitant anticoagulation and thrombolysis. Concomitant anticoagulation with heparin reduces thrombus formation around the catheter and retards thrombus propagation and reocclusion of the treated vessel segment, particularly in a proximal vessel that has low blood-flow above the occlusion. However, the addition of heparin can increase the severity of a bleeding complication.
The likelihood of success of thrombolysis depends on the factors listed in Table III. The end points of thrombolysis are as follows: restoration of antegrade flow, complete lysis of the thrombus, failure to lyse residual thrombus, extension of the thrombosis, and complications of therapy.
TABLE III. Factors Predicting the Success of Thrombolysis

Patient Selection for Thrombolysis
The selection of patients for thrombolysis depends on the presenting symptoms, medical history, physical findings, and objective laboratory test results. After the diagnosis of thrombosis has been established, it is essential to evaluate the indications, contraindications, risk factors, and likelihood of success. If thrombolysis is deemed a reasonable choice for therapy, the site of vascular access can be carefully selected and angiography performed. After the angiographic findings have been evaluated and the likelihood of success has been determined, the type of equipment and the dosage and type of thrombolytic agent can be selected.
Before the initiation of treatment with thrombolytic agents, possible hypercoagulable conditions should be considered:
Antithrombin III deficiency
Protein C and protein S deficiency
Factor V Leiden level
Anticardiolipin antibodies
Antiphospholipid antibodies
Malignancy
The presence of any of the above conditions is a contraindication to the use of thrombolytic therapy.
Extensive experience over the past decade has led to increased acceptance of selective intra-arterial thrombolytic therapy for peripheral arterial occlusions as an adjunct to definitive revascularization procedures. Although newer infusion techniques have substantially decreased treatment times, they remain at around 24 hours for lower-extremity occlusions. Work continues on the optimization of infusion methods and on the development of new drugs and dosages in order to shorten treatment times.
Mechanical Devices for Thrombus Removal
A number of mechanical devices have been developed to disrupt and remove freshly formed thrombus from the circulation (Table IV). Only one of these devices, the AngioJet® Rheolytic™ Thrombectomy System (Possis Medical, Inc.; Minneapolis, Minn), is currently approved in the United States for use in the arterial circulation. It appears that these devices are of most value when used to remove thrombi of recent onset. A brief description of some of the more promising devices follows.
TABLE IV. Commercially Available Thrombectomy Devices

The AngioJet (Fig. 9) is an over-the-wire percutaneous device that removes thrombus; the tip has a vacuum that operates on the Bernoulli principle. Several studies 43–47 have shown this device to be effective in treating thrombus-containing lesions in the peripheral and coronary circulation. It has been used successfully in native arteries, veins, saphenous vein grafts, prosthetic grafts, and renal dialysis shunts. The AngioJet is currently approved for use in vessels larger than 2.0 mm prior to balloon angioplasty or stent placement in patients who have symptomatic coronary artery or saphenous vein graft lesions. It can be used for thrombus removal and for breaking apart and removing unorganized thrombus from arteriovenous access.
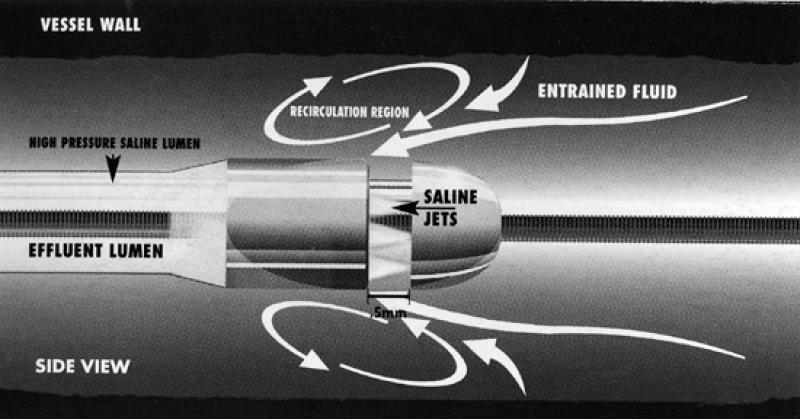
Fig. 9 The AngioJet® Rheolytic™ Thrombectomy System (Possis Medical, Inc.; Minneapolis, Minn)
The Hydrolyser™ Thrombectomy Catheter (Cordis Europa NV; Roden, The Netherlands) is an over-the-wire hydrodynamic thrombectomy catheter that uses the Venturi principle for aspiration and removal of intravascular thrombus. Negative pressure pulls the thrombus into the heparinized saline stream, resulting in microfragments that are discharged through the outflow lumen into the collection bag. Early reports from European trials 48,49 suggest a possible use for this device in thrombus-containing lesions and degenerated vein grafts. Currently, the device is investigational.
The Oasis™ Thrombectomy System (Boston Scientific) is another an over-the-wire hydrodynamic thrombectomy catheter that uses the Venturi principle for aspiration and removal of intravascular thrombus. This device is approved in the United States for use in obstructed renal dialysis grafts.
Vascular Radiation Therapy
Despite improvements in long-term outcomes after PTA and stenting of the peripheral vessels, restenosis remains a significant problem—particularly in long lesions, small-diameter vessels, and restenotic lesions. 50 Therapeutic approaches have focused on mechanical devices, atherectomy, stents, stent grafts, and pharmaceutical agents. None of these approaches has yet been successful in solving this problem. 50,51
Vascular radiation for the prevention of restenosis after PTA and stenting is a new frontier in the field of peripheral interventions. The 1st experience with in vivo endovascular radiation therapy was reported in 1964 by Friedman and colleagues 52 when they attempted to prevent the development of atherosclerosis.
Various types of radiation therapy have been tried to prevent restenosis after angioplasty, stenting, or both (Table V). One consideration is that large-diameter peripheral vessels require higher energy sources than the coronary vessels do. Nori and coworkers 53 have used external beam radiation in their pilot study, using 8 to 12 GΓ with encouraging preliminary results. To date, no randomized trial with long-term follow-up after external beam radiation has been performed to determine the long-term results and the consequences of the radiation to the adjacent tissues.
TABLE V. Radiation Therapy Methods Used Experimentally to Prevent Restenosis

Intravascular radiation therapy with various beta and gamma sources has been studied more extensively than has external beam radiation. A large number of animal investigations 54,55 and a few clinical trials 56,57 have established the ability of ionizing radiation to inhibit vascular smooth-muscle-cell proliferation associated with restenosis. Recently, several studies 58–60 have shown that localized irradiation of the angioplasty site by intraluminal delivery of low-dose beta-particle irradiation as well as gamma irradiation inhibits smooth-muscle-cell migration and proliferation in vitro and in vivo. 61
A number of isotopes have been tested and several others are being considered for future studies (Table VI). 62 Such tests have generally involved the use of high-activity gamma emitters. Two of the most controversial issues surrounding the delivery of intravascular radiation involve the preference of beta- or gamma-emitting radioisotope sources and the importance of source-centering in the arteries. Improper centering of the catheter-based solid source (off by as little as 0.5 mm) can lead to a dosing error as high as 5-fold. The consequences of these errors are considerably worse with beta emitters than with gamma emitters. However, because beta emitters deposit a large portion of their energy locally, these isotopes have substantial safety advantages over the gamma emitters for both the operator and the patient. Efforts to make use of beta radioisotopes in solution await the development of an appropriate compound with an adequate biodilution profile to safely handle the potential intravascular release of radioisotope-containing liquid. 63
TABLE VI. Isotopes Being Tested or Considered for Endovascular Brachytherapy

Clinical Trials of Endovascular Radiotherapy
The 1st clinical trial involving endovascular radiotherapy was started in 1990 by Liermann and co-workers 61 in an effort to reduce the restenosis rate following PTA in peripheral vessels. Their 6-year experience (May 1990 to June 1996) was described by Schopohl and co-authors (Frankfurt trial). 62 The study included 28 patients with in-stent restenosis in the femoropopliteal arteries who were treated with a repeat PTA procedure or with directional atherectomy; all 28 then underwent endovascular radiation with an Ir-192-HDR source. The radiation was well tolerated and the investigators reported a 5-year patency rate of 82% based on Doppler ultrasound results. Restenosis occurred in 11% of patients, and 7% of the patients developed thrombotic occlusion of the treated vessel. More recently, in a randomized trial comparing PTA and brachytherapy for superficial femoral artery lesions, Pokrajac's group 63 reported a restenosis rate of 51.7% for PTA alone versus 25% for PTA and brachytherapy combined.
The PARIS (Peripheral Arteries Radiation Investigational Study) trial 64 is currently evaluating the safety, feasibility, and efficacy of endovascular brachytherapy to prevent restenosis in the superficial femoropopliteal arteries immediately after PTA without stenting. Endovascular brachytherapy is administered through a balloon-centering catheter system using an Ir-192-HDR source delivered to the target site by a remote afterloader. Twenty-seven patients completed Phase II (the 6-month angiographic follow-up). Their restenosis rate was 11%. 64
Brachytherapy for treatment of peripheral arterial disease to prevent restenosis after an interventional procedure is in the early developmental stages. Various isotopes are being tested in an effort to minimize the radiation exposure to patients and personnel and to reduce the dose delivery in the near field. 61–64 There are now centering balloons that can center the catheter-based isotope within the lumen of the vessel, in spite of eccentric plaque. This improves the depth of dose delivery, especially for large vessels.
New techniques, such as radioactive liquid- or gas-filled balloons that improve dose delivery, are being investigated. Potential sites for brachytherapy include the superficial femoral arteries, popliteal arteries, tibio-peroneal arteries, hepatic vascular system-TIPS (transjugular intrahepatic portosystemic shunt), arteriovenous dialysis grafts, renal arteries, and carotid arteries.
Percutaneous Hemostatic Puncture Closure Devices
Vascular complications after endovascular treatment can cause morbidity and even death, and can increase the total cost of the procedure by prolonging the patient's hospital stay. Angiographic and angioplasty procedures involving femoral artery punctures lead to access site complications in 1% to 9% of cases. 65 These complications range from simple hematomas to arterial thrombosis, pseudoaneurysm, embolization, arteriovenous fistula, arterial hemorrhage requiring transfusion, and extended hospital stays including possible surgical repair. Krause and Klein 66 estimated a mean cost of $8,000 per vascular complication (assuming a 2-day hospital stay plus surgical repair in two-thirds of patients with complications). Prevention of vascular complications is therefore essential to optimize the outcome of interventional procedures. A variety of devices are available for arterial compression after sheath removal, including mechanical clamps and an inflatable pressure device, the FemoStop™ (CR Bard). These devices are commonly used for larger sheath sizes (8 to 16 F). The choice of technique is affected by patient size, the availability of a specific device, and the expertise of the individual using the device. Arterial compression is time consuming and labor intensive. The patient is often immobilized for an extended period of time; consequently, back pain and urinary retention may occur. Movement during compression can induce a local hematoma. In addition, anticoagulation therapy must be interrupted for this method of obtaining hemostasis.
Lately, there has been considerable interest in new methods to assist with hemostasis at the time of arterial catheter removal. This interest stems from an increased emphasis on patient mobilization and discharge on the day of the procedure. Recently introduced vascular hemostatic devices, deployable without compression and anticoagulation reversal, offer an alternative approach. The role of catheter techniques for arterial entry closure is evolving. Multiple devices are available, including collagen plugs, bioabsorbable pledgets, and vessel suturing devices, all of which can be introduced through specially designed catheters. VasoSeal™ VHD (Datascope Corporation; Montvale, NJ), the first of such devices, consists of an absorbable beef collagen cartridge delivered in the supra-arterial space by a preloaded, syringe-like system. The collagen, unaffected by antiplatelet or anticoagulant agents, attracts and activates platelets, rapidly forming a glue-like plug at the arterial puncture site and obliterating the subcutaneous tunnel. Ernst and associates 67 have shown that with the use of collagen plugs, hemostasis can be achieved in 87% of patients after a mean compression time of 4.8 minutes. Schrader's group 68 recently reported that a percutaneously applied collagen plug shortened manual compression time by 90%. This reduction in time to hemostasis was independent of the heparin load. Sanborn and colleagues, 69 in a multicenter randomized trial, found that major complications occurred in 1.4% of patients after angioplasty when collagen hemostatic devices were applied.
The Angio-Seal™ Hemostatic Puncture Closure Device (St. Jude Medical, Inc.; St. Paul, Minn) is a specially engineered bioabsorbable anchor (collagen sponge) that is deployed through a sheath, which is then drawn tightly against the arteriotomy. The device consists of 3 completely bioabsorbable components: 1) a flat rectangular anchor (2 × 10 mm) made from a copolymer of polylactic and polyglycolic acids; 2) a 27-mg bovine collagen plug; and 3) a positioning suture of polyglycolic acid that loops through the collagen plug and the anchor, exiting through the proximal end of the device.
The combination of the anchor with the collagen sponge retained by the suture forms a mechanical “sandwich” around the arteriotomy. When deployment of the anchor has been confirmed, the carrier tube and the insertion sheath are withdrawn and the tamper tube appears. This device is used to ensure proper positioning of the collagen. Atension spring is then applied over the suture, the suture is cut, and the carrier tube and the insertion sheath are removed. All the components are fully absorbed by the body in 60 to 90 days. This device is available in sizes from 6 to 10 F and is indicated for both diagnostic and interventional procedures. Blengino's group 70 achieved hemostasis with this device in 90% of patients, with a mean time to hemostasis of 2 ± 6 minutes. More recently, in 435 patients, the U.S. phase II clinical trial 71 showed that both the time to hemostasis and the compression time were 3.2 ± 10.5 minutes in the Angio-Seal device group, compared with a time to hemostasis of 16.0 ± 12.2 minutes and a compression time of 19.5 ± 11.9 minutes in the manual compression group (p < 0.0001). The overall complication rate was significantly lower in the device group than in the manual compression group (12% vs 18%, respectively; p = 0.08), as were bleeding complications (7% vs 15%; p = 0.007) and hematomas (2% vs 6%; p = 0.08%). The incidence of pseudoaneurysms and arteriovenous fistulae was the same in both groups. 71
The worst complication associated with the Angio-Seal device is anchor failure with subsequent distal embolization. Thus far, this complication has occurred once in the U.S. multicenter study and twice in the European study. 71 The Angio-Seal clinical trials did not specifically examine the use of this device in high-risk patients, such as those who are morbidly obese or who have severe peripheral vascular disease. Insertion of this device may be limited in patients who are obese, because the relatively short length of the tamper tube may make collagen compression difficult. A longer tamper rod is being designed to correct this problem. As currently designed, the device cannot be used during procedures that require catheters larger than 8 F. Larger sizes are being developed to extend device applicability to procedures that require larger sheaths. In addition, repuncture of the artery after device placement has not been studied in human beings. At this time, the manufacturer does not recommend reentry into an artery sealed with this device until 90 days have passed, in order to allow full collagen absorption.
In comparison with the Prostar® XL Percutaneous Vascular Surgery device (Perclose, Inc.; Redwood City, Calif), the Angio-Seal yielded a slightly lower rate of immediate hemostasis. Complication rates were similar for both devices. 72
The Prostar device is now being used in an unusual, off-label fashion that allows safe percutaneous access and closure of access sites up to 16 F. This method, described by Haas and colleagues 73 and by Krajcer and colleagues, 74,75 calls for placement of the Prostar device sutures before sheath placement. The sutures are left untied and the arterial access site is dilated with sheaths up to 16 F. The artery expands within the confines of the sutures, which are then closed at the end of the procedure. Howell reported that this technique, used in more than 54 patients, had a 94% success rate and no lower-extremity complications at the 1- and 6-month follow-up. 74,75
Gene Therapy
The prospect of growing new arteries, both in the coronary and in the peripheral circulation, generates much excitement. Preliminary results 76 with the use of vascular endothelial growth factor (VEGF) to induce new blood-vessel formation in animals and human beings have been encouraging. Both Baumgartner's and Isner's groups 77,78 have reported that intramuscular injection of naked plasmid DNA encoding VEGF induces therapeutic angiogenesis in patients with critical limb ischemia. 77,78 This treatment is still in the experimental phase.
Covered Stents
Covered stents are a recent development in peripheral vascular therapy. Originally, it was hoped that the prosthetic covering of the stents would decrease the restenosis rate, thus providing longer vessel patency. Early results, however, have shown no benefit over bare metal stents in the treatment of peripheral stenotic lesions. 79–83 Nevertheless, studies 84,85 have shown that covered stents may be very useful in providing an airtight seal for the treatment of such vascular lesions as arterial ruptures, dissections, aneurysms, pseudoaneurysms, and arteriovenous fistulae. A few of the more promising devices are described below.
The Wallgraft™ Endoprosthesis (Boston Scientific) (Fig. 10) is a self-expanding cobalt super alloy stent covered with polyethylene terephthalate graft material. The Hemobahn™ Endoprosthesis (W.L. Gore & Associates, Inc.; Flagstaff, Ariz) (Fig. 11) is a self-expanding nitinol stent covered with an ultra-thin PTFE graft. The Jostent® Peripheral Stent Graft (Jomed® USA; Conroe, Tex) (Fig. 12) is a single-piece self-expanding nitinol stent covered with an ultra-thin PTFE graft.

Fig. 10 The Wallgraft™ Endoprosthesis (Boston Scientific)

Fig. 11 Hemobahn™ Endoprosthesis (W.L. Gore & Associates, Inc.; Flagstaff, Ariz)

Fig. 12 The Jostent® Peripheral Stent Graft (Jomed® USA; Conroe, Tex)
Old Devices, New Uses
A number of devices that have been around for a while and used primarily in coronary artery interventions are being tried in peripheral interventions. Techniques such as intravascular therapeutic ultra-sound and laser angioplasty have not had a marked effect on peripheral interventions. 86–88 One exception is the excimer laser guidewire. Due to its success in crossing coronary arteries with chronic total occlusion, pilot studies are currently being carried out to evaluate its effectiveness in totally occluded peripheral arteries. 89
Footnotes
Address for reprints: Zvonimir Krajcer, MD, Suite 2780, 6624 Fannin Street, Houston, TX 77030
References
- 1.Bernstein EF, Dilley RB, Thomas WS, Randolph HF, Knowles HJ, Saeed M. Changing practice patterns in peripheral arterial disease. Ann Vasc Surg 1994;8:186–94. [DOI] [PubMed]
- 2.Hertzer NR, Beven EG, Young JR, O'Hara PJ, Ruschhaupt WF 3d, Graor RA, et al. Coronary artery disease in peripheral vascular patients. A classification of 1000 coronary angiograms and results of surgical management. Ann Surg 1984;199:223–33. [DOI] [PMC free article] [PubMed]
- 3.Levin DC, Becker GJ, Dorros G, Goldstone J, King SB 3d, Seeger JM, et al. Training standards for physicians performing peripheral angioplasty and other percutaneous peripheral vascular interventions. A statement for health professionals from the Special Writing Group of the Councils on Cardiovascular Radiology, Cardio-Thoracic and Vascular Surgery, and Clinical Cardiology of the American Heart Association. Circulation 1992;86:1348–50. [DOI] [PubMed]
- 4.Gruntzig A. Transluminal dilatation of coronary-artery stenosis [letter]. Lancet 1978;1(8058):263. [DOI] [PubMed]
- 5.Pentecost MJ, Criqui MH, Dorros G, Goldstone J, Johnston KW, Martin EC, et al. Guidelines for peripheral percutaneous transluminal angioplasty of the abdominal aorta and lower extremity vessels. A statement for health professionals from a special writing group of the Councils on Cardiovascular Radiology, Arteriosclerosis, Cardio-Thoracic and Vascular Surgery, Clinical Cardiology and Epidemiology and Prevention, the American Heart Association. Circulation 1994;89:511–31. [DOI] [PubMed]
- 6.Kerber CW, Cromwell LD, Loehden OL. Catheter dilatation of proximal carotid stenosis during distal bifurcation endarterectomy. AJNR Am J Neuroradiol 1980;1:348–9. [PMC free article] [PubMed]
- 7.Naylor AR, Bolia A, Abbott RJ, Pye IF, Smith J, Lennard N, et al. Randomized study of carotid angioplasty and stenting versus carotid endarterectomy: a stopped trial. J Vasc Surg 1998;28:326–34. [DOI] [PubMed]
- 8.Roubin GS, Yadav S, Iyer SS, Vitek J. Carotid stent-supported angioplasty: a neurovascular intervention to prevent stroke. Am J Cardiol 1996;78(3A):8–12. [DOI] [PubMed]
- 9.Diethrich EB, Ndiaye M, Reid DB. Stenting in the carotid artery: initial experience in 110 patients. J Endovasc Surg 1996;3:42–62. [DOI] [PubMed]
- 10.Wholey MH, Wholey MH, Jarmolowski CR, Eles G, Levy D, Buecthel J. Endovascular stents for carotid artery occlusive disease. J Endovasc Surg 1997;4:326–38. [DOI] [PubMed]
- 11.Wholey MH, Wholey M, Bergeron P, Diethrich EB, Henry M, Laborde JC, et al. Current global status of carotid artery stent placement. Cathet Cardiovasc Diagn 1998;44:1–6. [DOI] [PubMed]
- 12.Mathur A, Dorros G, Iyer SS, Vitek JJ, Yadav SS, Roubin GS. Palmaz stent compression in patients following carotid artery stenting. Cathet Cardiovasc Diagn 1997;41:137–40. [DOI] [PubMed]
- 13.Theron JG, Payelle GG, Coskun O, Huet HF, Guimaraens L. Carotid artery stenosis: treatment with protected balloon angioplasty and stent placement. Radiology 1996; 201:627–36. [DOI] [PubMed]
- 14.Mathur A, Roubin GS, Iyer SS, Piamsonboon C, Liu MW, Gomez CR, et al. Predictors of stroke complicating carotid artery stenting. Circulation 1998;97:1239–45. [DOI] [PubMed]
- 15.Kachel R. Results of balloon angioplasty in the carotid arteries. J Endovasc Surg 1996;3:22–30. [DOI] [PubMed]
- 16.Oesterle SN, Hayase M, Baim DS, Teirstein PS, Ramee SR, Whitlow PL, et al. An embolization containment device. Catheter Cardiovasc Interv 1999;47:243–50. [DOI] [PubMed]
- 17.Webb JG, Carere RG, Virmani R, Baim D, Teirstein PS, Whitlow P, et al. Retrieval and analysis of particulate debris after saphenous vein graft intervention. J Am Coll Cardiol 1999;34:468–75. [DOI] [PubMed]
- 18.Henry M, Amor M, Masson I, Henry I, Tzvetanov K, Chati Z, et al. Angioplasty and stenting of the extracranial carotid arteries. J Endovasc Surg 1998;5:293–304. [DOI] [PubMed]
- 19.Yadav JS. SAPPHIRE trial: concept and outline. Endocardiovasc Multimedia Mag 2000;4:77–79
- 20.Hobson RW 2nd, Brott T, Ferguson R, Roubin G, Moore W, Kuntz R, et al. CREST: carotid revascularization endarterectomy versus stent trial. Cardiovasc Surg 1997;5: 457–8. [DOI] [PubMed]
- 21.Johnston KW. Multicenter prospective study of nonruptured abdominal aortic aneurysm. Part II. Variables predicting morbidity and mortality. J Vasc Surg 1989;9: 437–47. [DOI] [PubMed]
- 22.Chen JC, Hildebrand HD, Salvian AJ, Taylor DC, Strandberg S, Myckatyn TM, et al. Predictors of death in nonruptured and ruptured abdominal aortic aneurysms. J Vasc Surg 1996;24:614–23. [DOI] [PubMed]
- 23.Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg 1991;5:491–9. [DOI] [PubMed]
- 24.Zarins CK, Harris EJ Jr. Operative repair for aortic aneurysms: the gold standard. J Endovasc Surg 1997;4:232–41. [DOI] [PubMed]
- 25.Balm R, Eikelboom BC, van Leeuwen MS, Noordzij J. Spiral CT-angiography of the aorta. Eur J Vasc Surg 1994;8: 544–51. [DOI] [PubMed]
- 26.May J, White GH, Yu W, Waugh R, Stephen M, Harris JP. Concurrent comparison of endoluminal repair vs. no treatment for small abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 1997;13:472–6. [DOI] [PubMed]
- 27.Moore WS. The EVT tube and bifurcated endograft systems: technical considerations and clinical summary. EVT Investigators. J Endovasc Surg 1997;4:182–94. [DOI] [PubMed]
- 28.Biasi GM, Piglionica MR, Meregaglia D, Ferrari SA, Cao PG, Barzi F, et al. European multicentre experience with modular device (Medtronic Aneuryx) for the endoluminal repair of infrarenal abdominal aortic aneurysms. J Mal Vasc 1998;23:374–80. [PubMed]
- 29.Taheri SA, Leonhardt HJ, Greenan T. The TALENT™ Endoluminal Graft Placement System. In: Yao JST, Pearce WH, editors. Techniques in vascular and endovascular surgery. Stamford, CT: Appleton & Lange, 1998. p. 433–45.
- 30.Beebe HG. Endovascular surgery: issues and opportunities as we approach the new millennium. J Endovasc Surg 1998;5:1–5. [DOI] [PubMed]
- 31.Zarins CK, White RA, Schwarten D, Kinney E, Diethrich EB, Hodgson KJ, et al. AneuRx stent graft versus open surgical repair of abdominal aortic aneurysms: multicenter prospective clinical trial. J Vasc Surg 1999;29:292–308. [DOI] [PubMed]
- 32.Zarins CK, White RA, Fogarty TJ. Aneurysm rupture after endovascular repair using the AneuRx stent graft. J Vasc Surg 2000;31:960–70. [DOI] [PubMed]
- 33.Yeager RA, Moneta GL, Taylor LM Jr, Hamre DW, McConnell DB, Porter JM. Surgical management of severe acute lower extremity ischemia. J Vasc Surg 1992;15:385–93. [DOI] [PubMed]
- 34.Ouriel K, Veith FJ, Sasahara AA. A comparison of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs. Thrombolysis or Peripheral Arterial Surgery (TOPAS) Investigators. N Engl J Med 1998;338:1105–11. [DOI] [PubMed]
- 35.McNicol GP, Reid W, Bain WH, Douglas AS. Treatment of peripheral arterial occlusion by streptokinase perfusion. Br Med J 1963 Jun 8;5344:1508–12. [DOI] [PMC free article] [PubMed]
- 36.Goldhabert SZ, Kessler CM, Heit JA, Elliott CG, Friedenberg WR, Heiselman DE, et al. Recombinant tissue-type plasminogen activator versus a novel dosing regimen of urokinase in acute pulmonary embolism: a randomized controlled multicenter trial. J Am Coll Cardiol 1992;20: 24–30. [DOI] [PubMed]
- 37.Groar RA, Olin J, Bartholomew JR, Ruschhaupt WF, Young JR. Efficacy and safety of intra-arterial local infusion of streptokinase, urokinase, or tissue plasminogen activator for peripheral arterial occlusion: a retrospective review. J Vasc Med Biol 1990;2:310–15.
- 38.McNamara TO, Fischer JR. Thrombolysis of peripheral arterial and graft occlusions: improved results using high-dose urokinase. AJR Am J Roentgenol 1985;144:769–75. [DOI] [PubMed]
- 39.Marder VJ, Sherry S. Thrombolytic therapy: current status parts 1 and 2. N Engl J Med 1988;318:1512–20 and 1585–95. [DOI] [PubMed]
- 40.van Breda A, Katzen BT, Deutsch AS. Urokinase versus streptokinase in local thrombolysis. Radiology 1987;165:109–11. [DOI] [PubMed]
- 41.Dale WA. Differential management of acute peripheral arterial ischemia. J Vasc Surg 1984;1:269–78. [PubMed]
- 42.McNamara TO, Chen JL, Temmins CJ, Quinn B. Bleeding associated with intrathrombus infusions of r-tPA for peripheral arterial and venous occlusions abstract. Am J Cardiol 1999; 84(Suppl 6A):37P.10404848
- 43.Ramee SR, Baim DS, Popma JJ, Ho KKL, Cutlip DE, Lanoue AS, et al. A randomized, prospective, multi-center study comparing intracoronary urokinase to rheolytic thrombectomy with the POSSIS AngioJet catheter for intracoronary thrombus: final results of the VeGAS 2 trial abstract. Circulation 1998;98(Suppl I):I-86.
- 44.Ramee SR, Schatz RA, Carrozza JP, Leon MB, Ho KK, Baim DS, et al. Results of the VeGAS I pilot study of the Possis Coronary AngioJet Thrombectomy Catheter. Circulation 1996;94(Suppl I):I-619.
- 45.Silva JA, Ramee SR, Collins TJ, Jenkins JS, Lansky AJ, Ansel GM, et al. Rheolytic thrombectomy in the treatment of acute limb-threatening ischemia: immediate results and six-month follow-up of the multicenter AngioJet registry. Possis Peripheral AngioJet Study AngioJet Investigators. Cathet Cardiovasc Diagn 1998;45:386–93. [DOI] [PubMed]
- 46.Vesely TM, Williams D, Weiss M, Hicks M, Stainken B, Matalon T, et al. Comparison of the AngioJet rheolytic catheter to surgical thrombectomy for the treatment of thrombosed hemodialysis grafts. Peripheral AngioJet Clinical Trial. J Vasc Interv Radiol 1999;10:1195–205. [DOI] [PubMed]
- 47.Voigtlander T, Rupprecht HJ, Nowak B, Post F, Mayer E, Stahr P, et al. Clinical application of a new rheolytic thrombectomy catheter system for massive pulmonary embolism. Catheter Cardiovasc Interv 1999;47:91–6. [DOI] [PubMed]
- 48.Henry M, Amor M, Henry I, Tricoche O, Allaoui M. The Hydrolyser thrombectomy catheter: a single-center experience. J Endovasc Surg 1998;5:24–31. [DOI] [PubMed]
- 49.van Ommen VG, van den Bos AA, Pieper M, den Heyer P, Thomas MR, Ozbeck S, et al. Removal of thrombus from aortocoronary bypass grafts and coronary arteries using the 6Fr Hydrolyser. Am J Cardiol 1997;79:1012–6. [DOI] [PubMed]
- 50.Johnston KW. Femoral and popliteal arteries: reanalysis of results of balloon angioplasty. Radiology 1992;183:767–71. [DOI] [PubMed]
- 51.Martin EC, Katzen BT, Benenati JF, Diethrich EB, Dorros G, Graor RA, et al. Multicenter trial of the Wallstent in the iliac and femoral arteries. J Vasc Interv Radiol 1995;6:843–9. [DOI] [PubMed]
- 52.Friedman M, Felton L, Byers S. The antiatherogenic effect of iridium-192 upon the cholesterol-fed rabbit. J Clin Invest 1964;43:186–92. [DOI] [PMC free article] [PubMed]
- 53.Nori D, Parikh S, Moni J. Management of peripheral vascular disease: innovative approaches using radiation therapy. Int J Radiat Oncol Biol Phys 1996;36:847–56. [DOI] [PubMed]
- 54.Ali NM, Kaluza G, Khan M, et al. The effect of intracoronary B-radiation on neointimal formation and vascular remodeling in balloon injured porcine coronary arteries: effect of dose rate. J Intervent Cardiol 1999;12:271–282.
- 55.Verin V, Popowski Y, Urban P, Belenger J, Redard M, Costa M, et al. Intraarterial beta irradiation prevents neointimal hyperplasia in a hypercholesterolemic rabbit restenosis model. Circulation 1995;92:2284–90. [DOI] [PubMed]
- 56.Raizner AE, Calfee RV, Ali MN. Clinical experience with a spiral balloon centering catheter for delivery of intracoronary radiation therapy. Cardiovascular Radiat Med 2000;3: 214–9. [DOI] [PubMed]
- 57.Kuntz RE, Baim DS. Prevention of coronary restenosis: the evolving evidence base for radiation therapy. Circulation 2000;101:2130–3. [DOI] [PubMed]
- 58.Raizner AE, Oesterle SN, Waksman R, Serruys PW, Colombo A, Lim YL, et al. Inhibition of restenosis with beta-emitting radiotherapy: report of the proliferation reduction with vascular energy trial (PREVENT). Circulation 2000;102:951–8. [DOI] [PubMed]
- 59.Waksman R, Bhargava B, White L, Chan RC, Mehran R, Lansky AJ, et al. Intracoronary ß-radiation therapy inhibits recurrence of in-stent restenosis. Circulation 2000;101: 1895–8. [DOI] [PubMed]
- 60.Teirstein PS, Massullo V, Jani S, Popma JJ, Mintz GS, Russo RJ, et al. Catheter-based radiotherapy to inhibit restenosis after coronary stenting. N Engl J Med 1997;336: 1697–703. [DOI] [PubMed]
- 61.Liermann D, Bottcher HD, Kollath J, Schopohl B, Strassman G, Strecker EP, et al. Prophylactic endovascular radiotherapy to prevent intimal hyperplasia after stent implantation in femoropopliteal arteries. Cardiovasc Intervent Radiol 1994;17:12–6. [PubMed]
- 62.Schopohl B, Leirmann D, Pohlit LJ, Heyd R, Strassmann G, Bauersachs R, et al. 192IR endovascular brachytherapy for avoidance of intimal hyperplasia after percutaneous transluminal angioplasty and stent implantation in peripheral vessels: 6 years of experience. Int J Radiat Oncol Biol Phys 1996;36:835–40. [DOI] [PubMed]
- 63.Pokrajac B, Potter R, Maca T, Fellner C, Mittlbock M, Ahmadi R, et al. Intraarterial (192)Ir high-dose-rate brachy-therapy for prophylaxis of restenosis after femoropopliteal percutaneous transluminal angioplasty: the prospective randomized Vienna-2-trial radiotherapy parameters and risk factors analysis. Int J Radiat Oncol Biol Phys 2000;48: 923–31. [DOI] [PubMed]
- 64.Waksman R, Laird JR, Benenati J, Kmowles H, Tripuraneni P, Ramee SR, et al. Intravascular radiation for prevention of restenosis after angioplasty of narrowed femoral-popliteal arteries: preliminary six month results of a feasibility study abstract. Circulation 1998;98(Suppl I):I-66.
- 65.Muller DW, Shamir KJ, Ellis SG, Topol EJ. Peripheral vascular complications after conventional and complex percutaneous coronary interventional procedures. Am J Cardiol 1992;69:63–8. [DOI] [PubMed]
- 66.Krause PB, Klein LW. Utility of a percutaneous collagen hemostasis device: to plug or not to plug? J Am Coll Cardiol 1993;22:1280–2. [DOI] [PubMed]
- 67.Ernst SM, Tjonjoegin RM, Schrader R, Kaltenbach M, Sigwart U, Sanborn TA, et al. Immediate sealing of arterial puncture sites after cardiac catheterization and coronary angioplasty using a biodegradable collagen plug: results of an international registry. J Am Coll Cardiol 1993;21:851–5. [DOI] [PubMed]
- 68.Schrader R, Steinbacher S, Burger W, Kadel C, Vallbracht C, Kaltenbach M. Collagen application for sealing of arterial puncture sites in comparison to pressure dressing: a randomized trial. Cathet Cardiovasc Diagn 1992;27:298–302. [DOI] [PubMed]
- 69.Sanborn TA, Gibbs HH, Brinker JA, Knopf WD, Kosinksi EJ, Roubin GS. A multicenter randomized trial comparing a percutaneous collagen hemostasis device with conventional manual compression after diagnostic angiography and angioplasty. J Invasive Cardiol 1999;11 Suppl B:6B–13B. [PubMed]
- 70.Blengino S, Hann B, Maiello L, Nakamura S, Hall P, Biagi G, et al. A randomized study of the 8 French Hemostatic Puncture Closure Device vs manual compression after coronary interventions abstract. J Am Coll Cardiol 1995; 25(1A):262A.
- 71.Fry SM. Review of the Angio-Seal™ Hemostatic Puncture Closure Device. J Invasive Cardiol 1998;10:111–20. [PubMed]
- 72.Baim DS, Pinkerton CA, Schatz RA, Vetter JW, FitzPatrick M, Ho KKL. Acute results of the STAND-II percutaneous vascular surgical device trial abstract. Circulation 1997; 96(Suppl I):I-442.
- 73.Haas PC, Krajcer Z, Diethrich EB. Closure of large percutaneous access sites using the Prostar XL Percutaneous Vascular Surgery device. J Endovasc Surg 1999;6:168–70. [DOI] [PubMed]
- 74.Krajcer Z, Howell M. A novel technique using the percutaneous vascular surgery device to close the 22 French femoral artery entry site used for percutaneous abdominal aortic aneurysm exclusion. Catheter Cardiovasc Interv 2000;50:356–60. [DOI] [PubMed]
- 75.Howell M, Villareal R, Krajcer Z. Percutaneous access and closure of femoral artery access sites associated with endoluminal repair of abdominal aortic aneurysms. J Endovasc Surg. In press. [DOI] [PubMed]
- 76.Tsurumi Y, Takeshita S, Chen D, Kearney M, Rossow ST, Passeri J, et al. Direct intramuscular gene transfer of naked DNA encoding vascular endothelial growth factor augments collateral development and tissue perfusion. Circulation 1996;94:3281–90. [DOI] [PubMed]
- 77.Baumgartner I, Pieczek A, Manor O, Blair R, Kearney M, Walsh K, et al. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation 1998;97:1114–23. [DOI] [PubMed]
- 78.Isner JM, Pieczek A, Schainfeld R, Blair R, Haley L, Asahara T, et al. Clinical evidence of angiogenesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. Lancet 1996;348:370–4. [DOI] [PubMed]
- 79.Krajcer Z, Sioco G, Reynolds T. Comparison of Wallgraft and Wallstent for treatment of complex iliac artery stenosis and occlusion. Preliminary results of a prospective randomized study. Tex Heart Inst J 1997;24:193–9. [PMC free article] [PubMed]
- 80.Satler LF, Muntz G. The promise of the covered stent [editorial]. Catheter Cardiovasc Interv 1999;46:466. [DOI] [PubMed]
- 81.Heuser RR, Woodfield S, Lopez A. Obliteration of a coronary artery aneurysm with a PTFE-covered stent: endoluminal graft for coronary disease revisited. Catheter Cardiovasc Interv 1999;46:113–6. [DOI] [PubMed]
- 82.Allen BT, Hovsepian DM, Reilly JM, Rubin BG, Malden E, Keller CA, et al. Endovascular stent grafts for aneurysmal and occlusive vascular disease. Am J Surg 1998;176: 574–80. [DOI] [PubMed]
- 83.Burger T, Meyer F, Tautenhahn J, Halloul Z, Fahlke J. Initial experiences with percutaneous endovascular repair of popliteal artery lesions using a new PTFE stent-graft. J Endovasc Surg 1998;5:365–72. [DOI] [PubMed]
- 84.Hernandez E, Krajcer Z, Strickman N, Mortazavi A, Perin E, Diethrich E, et al. Wallgraft™ endoprosthesis for endoluminal treatment of traumatic and aneurysmal peripheral vascular disease abstract. Am J Cardiol 1999; 84(Suppl 6A)37P.
- 85.Parodi JC, Schonholz C, Ferreira LM, Bergan J. Endovascular stent-graft treatment of traumatic arterial lesions. Ann Vasc Surg 1999;13:121–9. [DOI] [PubMed]
- 86.Siegel RJ, Gaines P, Crew JR, Cumberland DC. Clinical trial of percutaneous peripheral ultrasound angioplasty. J Am Coll Cardiol 1993;22:480–8. [DOI] [PubMed]
- 87.Conti CR, Barbeau GR, Seeger JM, Abela GS. Laser thermo-optical angioplasty in totally occluded peripheral arteries: immediate and short-term results. Trans Am Clin Climatol Assoc 1989;101:83–90. [PMC free article] [PubMed]
- 88.Lammer J. Laser angioplasty of peripheral arteries: an epilogue? Cardiovasc Intervent Radiol 1995;18:1–8. [DOI] [PubMed]
- 89.Oesterle SN, Bittl JA, Leon MB, Hamburger J, Tcheng JE, Litvack F, et al. Laser wire for crossing chronic total occlusions: “learning phase” results from the U.S. TOTAL trial. Total Occlusion Trial With Angioplasty by Using a Laser Wire. Cathet Cardiovasc Diagn 1998;44:235–43. [DOI] [PubMed]


