Abstract
Persistence of symptoms beyond the initial acute phase of coronavirus disease-2019 (COVID-19) is termed postacute SARS-CoV-2 (PASC) and includes neurologic, autonomic, pulmonary, cardiac, psychiatric, gastrointestinal, and functional impairment. PASC autonomic dysfunction can present with dizziness, tachycardia, sweating, headache, syncope, labile blood pressure, exercise intolerance, and “brain fog.” A multidisciplinary team can help manage this complex syndrome with nonpharmacologic and pharmacologic interventions.
Keywords: PASC, Autonomic dysfunction, COVID-19, Brain fog, Tilt table test
Key points
-
•
Autonomic dysfunction related to postacute SARS-CoV-2 can present with dizziness, tachycardia, sweating, headache, syncope, labile blood pressure, exercise intolerance, and “brain fog.”
-
•
Nonpharmacologic management involves increased salt and water intake, compression garments, progressive aerobic exercises, and enhanced external counterpulsation.
-
•
Pharmacologic treatment can include β-blockers, fludrocortisone, midodrine, pyridostigmine, and ivabradine.
Introduction
The SARS-CoV-2 virus, a member of the coronavirus family, has been responsible for the coronavirus disease-2019 (COVID-19) pandemic with an acute phase causing pneumonia and pulmonary disorders, but it has been shown to result in extrapulmonary manifestations including cardiovascular and neurologic diseases. Moreover, residual symptoms have been reported to persist past the acute phase. In a cross-sectional study of SARS-CoV-2–positive patients, at 48 days postdischarge the most common persistent symptoms were fatigue, difficulty breathing, and psychological distress.1 In a cohort study of 1733 patients with COVID-19 from Wuhan, China, patients reported persistence of fatigue, muscle weakness, sleeping difficulties, palpitations, anxiety, or depression at 6 months after initial onset.2 Numerous other studies now indicate the presence of persistent symptoms following COVID-19 infection, with more than 200 symptoms reported. This syndrome has been coined as the postacute SARS-CoV-2 (PASC) syndrome and has been defined as the persistence of symptoms or development of new symptoms after the time of infection, which can include fatigue, brain fog, palpitations, and a plethora of other manifestations.
Background
Infection with SARS-CoV-2 in the acute phase can lead to extrapulmonary manifestations including fatigue, myalgias, gastrointestinal dysfunction, as well as cardiovascular complications.3, 4, 5, 6 It has also been reported that SARS-CoV-2 infection can have neurologic involvement in more than one-third of acute infections.7 In a meta-analysis of patients with acute COVID-19 of more than 1585 records, acute SARS-CoV-2 infection can lead to autonomic dysfunction during the acute stages in terms of cardiovascular, sudomotor, and pupillometric functions.8
However, there have been growing reports that patients continue to remain unwell beyond 3 weeks.9 In a systematic review of 57 studies, it was found that 250,351 survivors of acute COVID-19 had persistent symptoms after 6 months of initial diagnosis that could be divided into 4 major categories: neurologic symptoms, generalized symptoms, mental health disorders, and mobility impairment.10 The same study reported common symptoms including headaches, difficulty concentrating, cognitive impairment, fatigue, functional impairment, and mobility decline. Therefore, postacute COVID syndrome continues to remain an ongoing issue that requires more examination.
Currently, there have been multiple names and criteria for this postacute phase COVID-19 syndrome. The Mayo Clinic uses the term “long COVID” to refer to long-term sequelae that occurred on or after the initial positive SARS-CoV-2 test.11 Meanwhile, the United Kingdom National Institute for Health and Care Excellence (NICE) has defined various phases of COVID-19 including “post-COVID-19 syndrome” for symptoms that develop around the acute phase and persist for more than 12 weeks that are not explained by an alternative diagnosis and “long COVID” that describes symptomatic COVID-19 and post-COVID-19 syndrome based on the criteria.12 In addition, a common term used has been postacute COVID-19 syndrome (PACS) and PASC and defined as the persistence of symptoms for more than 3 weeks after the initial onset of COVID-19.13
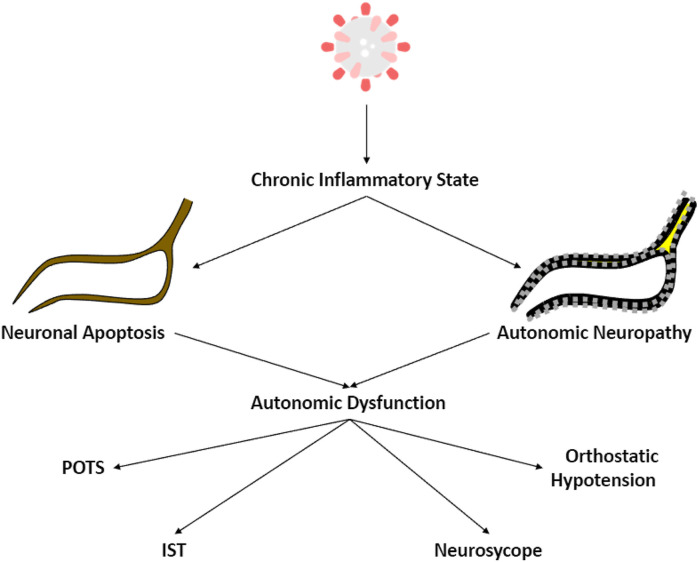
The mechanism of postacute COVID-19 autonomic dysfunction is thought to be multifactorial.14 One major proposed mechanism is through direct viral effects with multiple hypotheses. Persistent viremia can lead to a persistent highly inflammatory state with cellular injury.15, 16, 17, 18, 19 One of the proposed pathophysiology is cytokine- and hypoxia induced injury leading to neuronal apoptosis affecting the while matter fiber bundles causing impaired neurological function. Another hypothesis is that this inflammatory pathway could lead to autonomic and small fiber neuropathies, as previously seen in viral infections from herpes simplex and infectious mononucleosis.20 , 21
Postacute autonomic manifestations of coronavirus disease-2019
In a systematic review involving 54 articles involving 154 cases of COVID-19, the most common clinical presentation was orthostatic intolerance (including orthostatic lightheadedness, dizziness, tachycardia, sweating, headache, and “brain fog”) and syncope (including reflex and orthostatic hypotension-related syncope) (Fig. 1).22 Orthostatic intolerance has been defined as the inability to tolerate the upright position due to symptoms caused by cerebral hypoperfusion, sympathetic activation, or both and is relieved by recumbency.23 In a prospective study of 24 patients with PASC, 23 demonstrated orthostatic intolerance based on the head-up tilt table test (HUTT).24 In the same study, they found that these patients can present with postural orthostatic tachycardia syndrome (POTS), inappropriate sinus tachycardia, neurocardiogenic syncope (NCS), and orthostatic hypotension.
Fig. 1.
Mechanism of post-acute SARS-CoV-2 autonomic dysfunction.
POTS, characterized by partial dysautonomia and hyperadrenergic orthostatic intolerance, typically affects young high-achieving adults, particularly Caucasian women of childbearing age who are at the beginning of their working lives.25 POTS is heterogeneous in presentation and is associated with palpitations, dizziness, headache, fatigue, and blurry vision.26, 27, 28 Typically, POTS is diagnosed by a tilt table test or 10-minute stand test and is characterized by an increase in heart rate of at least 30 beats per minute (bpm) from supine to standing position in the absence of orthostatic hypotension.11 , 29 Before the arrival of COVID-19, approximately 50% of patients with POTS report a history of infection before symptom onset.11 , 30 Most reported infections associated with POTS included mycoplasma pneumonia,31 Epstein-Barr virus,32 , 33 Trypanosoma cruzi,34 and Borrelia burgdorferi.35 , 36 Now, since the inception of the SARS-CoV-2 virus, POTS seems to be a prevalent manifestation of PASC syndrome, with this viral infection being an inciting event.
Similarly, inappropriate sinus tachycardia (IST) has been characterized by unexpected fast sinus rates (greater than 100 beats bpm) at rest and/or with minimal physical activity and can be accompanied by symptoms including palpitations, dyspnea, or dizziness.37 Typically, IST can be diagnosed based on resting heart rate greater than 100 bpm with an average rate greater than 90 beats bpm on a 24-hour Holter monitor in symptomatic patients.37 The underlying pathophysiology is not completely understood, although it may be related to sinus node automaticity or autonomic imbalance. It has been seen in patients with Trypanosoma cruzi antibodies that cross-react with beta receptors and stimulate tachycardia.38 , 39 In a recent study of 200 patients with PASC, 20% were found to have inappropriate sinus tachycardia and can commonly affect younger women without previous comorbidities who have had a mild initial SARS-CoV-2 infection.40 It is suggested that IST in these patients may be related to autonomic dysfunction due to decreased heart rate variability based on 24-hour electrocardiogram (ECG) monitoring.
NCS has been reported as the most common cause of syncope, with a median of 3 episodes in a lifetime and a recurrence rate of 30% within 30 months.41 There have been case reports of NCS.42 , 43 There may be some overlap between neurogenic syncope with POTS, as NCS can occur in up to 38% of patients with POTS.35 , 44, 45, 46
Orthostatic hypotension, defined as a drop of systolic blood pressure by at least 20 mm Hg or drop of diastolic blood pressure by at least 10 minutes within 3 minutes of standing from a supine position, has been accompanied by similar symptoms of dizziness and syncope. It often involves excessive pooling of blood into the splanchnic and leg circulation, leading to decreased venous return with change to standing position and resulting in decreased cardiac output.47 Typically, the autonomic nervous system can compensate with changes in vascular tone, heart rate, and cardiac contractility. However, in neurogenic orthostatic hypotension, there may be a defective or delayed response.47 Similarly, there have been case reports of orthostatic hypotension as a presenting symptom of autonomic dysfunction in patients with PASC.22
Evaluation and management
The initial evaluation should begin with a detailed history and physical examination with particular attention to symptoms including dizziness, lightheadedness, fatigue, dyspnea, diarrhea or constipation, presyncope, anxiety, panic attacks, and brain fog.48 Vital signs evaluation should include a review of blood pressure, heart rate, breathing rate, oxygen saturation, and pain scale. Physical examination should include a full neurologic examination (involving cranial nerve evaluation, sensory and motor function, deep tendon reflexes, and coordination), pulmonary evaluation, cardiovascular examination, musculoskeletal tone and range of motion, skin exam for rashes, and psychiatric evaluation.
The Composite Autonomic Symptom Scale (COMPASS-31) questionnaire, originally developed as an 84-question scoring instrument for autonomic symptoms,49 has been growing in use for patients with PASC.50 The survey has been validated in patients with autonomic dysfunction and seems to be a quick and efficient method to have patients with PASC screened.
Initial laboratory evaluation should include a complete blood cell count, renal function panel, B-type natriuretic peptide, electrolytes, thyroid stimulating hormone, morning cortisol, and resting 12-lead ECG. Based on the history, physical examination, and clinical evaluation, further laboratory testing may be warranted. There has been a high prevalence of G-protein–coupled receptor antibodies and ganglionic neuronal nicotinic acetylcholine receptor antibodies in patients with PASC and POTS.51 POTS itself has also been reported to be associated with antinuclear, antithyroid, anti-NMDA glutamate receptor, antiphospholipid, and Sjogren antibodies and may be reasonably considered in patients with PASC with POTS if clinically appropriate.51
The 3-minute standing test, involving patients going from the supine position to the standing position, has been recommended by the Centers of Disease Control and Prevention and can be used as a quick and easy method for evaluating autonomic dysfunction in patients with PASC.52 In addition to the standing test, the HUTT uses a similar concept but requires patients to be on a table with restraining belts, and the table moves into supine and upright vertical positions that can leave patients feeling uncomfortable. Despite this, the HUTT has been the most used autonomic testing mechanism in patients with PASC with concerns for cardiac autonomic dysfunction.22
In addition, Holter ECG monitoring and 24-hour ambulatory blood pressure monitoring can also be used in patients with PASC to evaluate for autonomic dysfunction53; this would particularly allow close examination for heart rate variability (HRV) in sitting and standing positions. Before COVID-19, reduced HRV has been associated with chronic fatigue syndrome and myalgic encephalomyelitis54 and has been a tool used to evaluate autonomic nervous system functions.8
If further testing is needed, an examination can involve skin biopsy for intraepidermal nerve fiber density evaluating for small fiber (aδ and C fiber) neuropathy and quantitative sudomotor axon reflex test to evaluate postganglionic sympathetic cholinergic sudomotor function by evaluating sweat response time.55 Similarly, the thermoregulatory sweat test evaluates the central and peripheral sympathetic sudomotor pathways from central nervous system to the sweat glands.56 Electrochemical skin conductance (ESC) is a noninvasive test of sudomotor function. It is an indirect index of sympathetic nonmyelinated C-fiber activity and be estimated with ESC, due to the lack of parasympathetic innervation in the skin.57 An abnormal ESC result can also suggest autonomic small fiber neuropathy and autonomic dysfunction in patients with PASC.
Treatment
With autonomic dysfunction, initial treatment can include conservative and nonpharmacological measures including increased water consumption of up to 2 to 3 L per day, increased sodium consumption of up to 10 to 12 g per day, lower limb compression stockings, and progressive aerobic exercise training programs that start in supine or sitting position for physical reconditioning. It is also important to review the existing medication and supplementation list to remove agents that can worsen the symptoms of autonomic dysfunction (anticholinergics, antihypertensives, and so forth)
Multidisciplinary care can play an essential role in the treatment and management of these patients. Primary care, neurology, physical medicine and rehabilitation, cardiology, neuropsychologists, psychiatrists, occupational therapists, physical therapists, speech therapists, and dietitians may be part of the care team to assist with the complex syndrome of PASC autonomic dysfunction.10 , 58 , 59 Rehabilitation therapeutics include breathwork exercises, yoga/pranayama, autonomic reconditioning, symptom-titrated physical activity/movement, and functional restoration.
If symptoms persist despite the above measures, pharmacologic management may be considered. The Heart Rhythm Society recommends medical management with fludrocortisone, midodrine, pyridostigmine, β-blockers (specifically propranolol), or ivabradine.37 However, ivabradine is still being studied for safety and efficacy in patients with a specific subtype of POTS.60 These therapies can theoretically help with volume expansion (fludrocortisone), heart rate inhibition (propranolol, ivabradine, and pyridostigmine), and vasoconstriction (midodrine) that may help with autonomic dysfunction response (Table 1 ).
Table 1.
Options for pharmacologic treatment
| Drug | Mechanism of Action | Dosing | Side Effects |
|---|---|---|---|
| Propranolol | Reduces HR by beta-adrenergic blockade | 10–20 mg up to 4 times a day | Bradycardia, hypotension, fatigue, bronchospasm |
| Fludrocortisone | Volume expansion | 0.1–0.2 mg daily | Hypokalemia, edema, headache |
| Desmopressin (DDAVP) | Volume expansion | 0.1–0.2 mg daily | Hyponatremia, edema |
| Midodrine | Vasoconstriction | 2.5–15 mg 3 times a day | Headache, scalp tingling, hypertension |
| Pyridostigmine | Cholinergic, reduces HR | 30–60 mg 3 times a day | Nausea, abdominal cramps, diarrhea |
| Ivabradine | Reduces HR | 2.5–7.5 mg twice a day | Headache, palpitations, hypertension, visual disturbance |
Abbreviation: HR, heart rate.
There have been case reports where enhanced external counterpulsation (EECP), a noninvasive therapy that involves retrograde aortic flow to decrease the number of inflammatory cytokines, has been added as a suitable treatment method for patients with PASC.61 , 62 EECP has been approved by Food and Drug Administration and shown to improve morbidity and mortality for patients with chronic stable angina or ischemic heart failure. The benefit has been theorized to be due to vascular changes and improve endothelial function by affecting vasodilation and proinflammatory agents.63 Therefore, EECP may improve response to PASC autonomic dysfunction.
Blitshteyn and colleagues made additional multidisciplinary recommendations for evaluating and treating PASC-related autonomic dysfunction, and their consensus guidance was published in 2022 and is now accessible.64 In the authors’ preliminary study looking at 42 patients with PASC-related autonomic dysfunction, despite treatments, they continue to be symptomatic at 1 year since PASC onset with mild to moderate improvement of their symptoms. Long-term follow-up and studies are needed to bring the much-needed respite for these symptoms.
Clinics care points
-
•
There are several subtypes of autonomic dysfunction related to postacute SARS-CoV-2 including postural orthostatic tachycardia syndrome (POTS), inappropriate sinus tachycardia, neurocardiogenic syncope (NCS), and orthostatic hypotension.
-
•
Evaluation beyond a history and physical, should be inclusive of a Composite Autonomic Symptom Scale (COMPASS-31) questionnaire and a 3-minute standing test. The head-up tilt table test (HUTT) can be done when there is concern for cardiac autonomic dysfunction and stand test is nondiagnostic.
-
•
Treatment for autonomic dysfunction should include both conservative and nonpharmacological management to start. It is also imperative to consider pharmacologic management to help treat symptoms.
Disclosures
None related to this work.
References
- 1.Halpin S.J., McIvor C., Whyatt G., et al. Postdischarge symptoms and rehabilitation needs in survivors of Covid-19 infection: A cross-sectional evaluation. J Med Virol. 2020;93(2):1013–1022. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- 2.Huang C, Huang L, Wang Y, et al. 6-Month Consequences of COVID-19 in Patients Discharged from Hospital: A Cohort Study, Lancet, 397 (10270), 2021, 220–232. [DOI] [PMC free article] [PubMed]
- 3.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan J.F., Yuan S., Kok K.H., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang Y., Chen T., Mui D., et al. Cardiovascular manifestations and treatment considerations in COVID-19. Heart. 2020;106:1132–1141. doi: 10.1136/heartjnl-2020-317056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao L., Jin H., Wang M., et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scala I., Rizzo P.A., Bellavia S., et al. Autonomic Dysfunction during Acute SARS-CoV-2 Infection: A Systematic Review. J Clin Med. 2022;11(13):3883. doi: 10.3390/jcm11133883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kings College London. COVID symptom study. how long does COVID-19 last? 2020. Available: https://covid19. joinzoe. com/post/covid- long- term? fbclid= IwAR1RxIcmmdLEFjh_aI-.
- 10.Groff D., Sun A., Ssentongo A.E., et al. Short-term and Long-term Rates of Postacute Sequelae of SARS-CoV-2 Infection: A Systematic Review. JAMA Netw Open. 2021;4(10):e2128568. doi: 10.1001/jamanetworkopen.2021.28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thieben M.J., Sandroni P., Sletten D.M., et al. Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin Proc. 2007;82(3):308–313. doi: 10.4065/82.3.308. [DOI] [PubMed] [Google Scholar]
- 12.National Institute for Health and Care Excellence (NICE) (2021) COVID-19 rapid guideline: managing the long-term effects of COVID-19. https://www. nice. org. uk/guida nce/ng188. Accessed 30 Dec 2021.
- 13.Dixit N.M., Churchill A., Nsair A., et al. Post-Acute COVID-19 Syndrome and the cardiovascular system: What is known? Am Heart J. 2021;5:100025. doi: 10.1016/j.ahjo.2021.100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker R.C. Autonomic dysfunction in SARS-COV-2 infection acute and long-term implications COVID-19 editor’s page series. J Thromb Thrombolysis. 2021;52:692–707. doi: 10.1007/s11239-021-02549-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oronsky B, Larson C, Hammond TC, et al. A review of persistent post-COVID syndrome (PPCS). Clin Rev Allergy Immunol. 2021;1-9. [DOI] [PMC free article] [PubMed]
- 16.Lan L., Xu D., Ye G., et al. Positive RT-PCR test results in patients recovered from COVID-19. JAMA. 2020;323(15):1502–1503. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellul M.A., Benjamin L., Singh B., et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colafrancesco S., Alessandri C., Conti F., et al. COVID-19 gone bad: a new character in the spectrum of the hyperferritinemic syndrome? Autoimmun Rev. 2020;19(7):102573. doi: 10.1016/j.autrev.2020.102573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baig A.M., Khaleeq A., Ali U., et al. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 20.Neville B.G., Sladen G.E. Acute autonomic neuropathy following primary herpes simplex infection. J Neurol Neurosurg Psychiatry. 1984;47:648–650. doi: 10.1136/jnnp.47.6.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vassallo M., Camilleri M., Caron B.L., et al. Gastrointestinal motor dysfunction in acquired selective cholinergic dysautonomia associated with infectious mononucleosis. Gastroenterology. 1991;100:252–258. doi: 10.1016/0016-5085(91)90609-o. [DOI] [PubMed] [Google Scholar]
- 22.Reis Carneiro D., Rocha I., Habek M., et al. Clinical presentation and management strategies of cardiovascular autonomic dysfunction following a COVID-19 infection - A systematic review. Eur J Neurol. 2023 doi: 10.1111/ene.15714. Epub ahead of print. PMID: 36694382. [DOI] [PubMed] [Google Scholar]
- 23.Cutsfort-Gregory J.K. Postural tachycardia syndrome and neurally mediated syncope. Continuum. 2020;26:93–115. doi: 10.1212/CON.0000000000000818. [DOI] [PubMed] [Google Scholar]
- 24.Jamal S.M., Landers D.B., Hollenberg S.M., et al. Prospective Evaluation of Autonomic Dysfunction in Post-Acute Sequela of COVID-19. J Am Coll Cardiol. 2022;79(23):2325–2330. doi: 10.1016/j.jacc.2022.03.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw B.H., Stiles L.E., Bourne K., et al. The face of postural tachycardia syndrome - insights from a large cross-sectional online community-based survey. J Intern Med. 2019;286(4):438–448. doi: 10.1111/joim.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fedorowski A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J Intern Med. 2019;285:352–366. doi: 10.1111/joim.12852. [DOI] [PubMed] [Google Scholar]
- 27.Bryarly M., Phillips L.T., Fu Q., et al. Postural orthostatic tachycardia syndrome: JACC focus seminar. J Am Coll Cardiol. 2019;73:1207–1228. doi: 10.1016/j.jacc.2018.11.059. [DOI] [PubMed] [Google Scholar]
- 28.Schondorf R., Low P.A. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43:132–137. doi: 10.1212/wnl.43.1_part_1.132. [DOI] [PubMed] [Google Scholar]
- 29.Raj S.R., Guzman J.C., Harvey P., et al. Canadian Cardiovascular Society position statement on postural orthostatic tachycardia syndrome (POTS) and related disorders of chronic orthostatic intolerance. Can J Cardiol. 2020;36:357–372. doi: 10.1016/j.cjca.2019.12.024. [DOI] [PubMed] [Google Scholar]
- 30.Sandroni P., Opfer-Gehrking T.L., McPhee B.R., et al. Postural tachycardia syndrome: clinical features and follow-up study. Mayo Clin Proc. 1999;74:1106–1110. doi: 10.4065/74.11.1106. [DOI] [PubMed] [Google Scholar]
- 31.Kasmani R., Elkambergy H., Okoli K. Postural orthostatic tachycardia syndrome associated with mycoplasma pneumoniae. Infect Dis Clin Pract. 2009;17:342–343. doi: 10.1097/IPC.0b013e318191781b. [DOI] [Google Scholar]
- 32.Pohlgeers K.M., Stumbo J.R. Syncope in an athlete: a case of infectious mononucleosis induced postural tachycardia syndrome. Curr Sports Med Rep. 2016;15:41–45. doi: 10.1249/JSR.0000000000000227. [DOI] [PubMed] [Google Scholar]
- 33.Yaxley K.L. Infectious mononucleosis complicated by peritonsillar abscess and postural orthostatic tachycardia syndrome: a case report. SAGE Open Med Case Rep. 2020;8 doi: 10.1177/2050313X20915413. 2050313X20915413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmero H.A., Caeiro T.F., Josa D.J. Distinctive abnormal responses to tilting test in chronic Chagas’ disease. Klin Wochenschr. 1980;58:1307–1311. doi: 10.1007/BF01478139. [DOI] [PubMed] [Google Scholar]
- 35.Kanjwal K., Karabin B., Kanjwal Y., et al. Postural orthostatic tachycardia syndrome following Lyme disease. Cardiol J. 2011;18:63–66. doi: 10.1097/MJT.0b013e3181da0763. [DOI] [PubMed] [Google Scholar]
- 36.Noyes A.M., Kluger J. A tale of two syndromes: lyme disease preceding postural orthostatic tachycardia syndrome. Ann Noninvasive Electrocardiol. 2015;20:82–86. doi: 10.1111/anec.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheldon R.S., Grubb B.P., Olshansky B., et al. Heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. 2015;12(6):e41–e63. doi: 10.1016/j.hrthm.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiale P.A., Garro H.A., Schmidberg J., et al. Inappropriate sinus tachycardia may be related to an immunologic disorder involving cardiac β andrenergic receptors. Heart Rhythm. 2006;3(10):1182–1186. doi: 10.1016/j.hrthm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 39.Baruscotti M., Bianco E., Bucchi A., et al. Current understanding of the pathophysiological mechanisms responsible for inappropriate sinus tachycardia: Role of the if “funny” current. J. Interv. Card. Electrophysiol. 2016;46:19–28. doi: 10.1007/s10840-015-0097-y. [DOI] [PubMed] [Google Scholar]
- 40.Aranyó J., Bazan V., Lladós G., et al. Inappropriate sinus tachycardia in post-COVID-19 syndrome. Sci Rep. 2022;12(1):298. doi: 10.1038/s41598-021-03831-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baron-Esquivias G., Morillo C.A. Definitive pacing therapy in pateints with neuromediated syncope. Lessons from the SPAIN study. Rev Esp cardio. 2018;71:320–322. doi: 10.1016/j.rec.2017.10.037. [DOI] [PubMed] [Google Scholar]
- 42.Shouman K., Vanichkachorn G., Cheshire W.P., et al. Autonomic dysfunction following COVID-19 infection: an early experience. Clin Auton Res. 2021;31(3):385–394. doi: 10.1007/s10286-021-00803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blitshteyn S., Whitelaw S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: a case series of 20 patients. Immunol Res. 2021;69(2):205–211. doi: 10.1007/s12026-021-09185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ojha A., McNeeley K., Heller E., et al. Orthostatic syndromes differ in syncope frequency. Am J Med. 2010;123:245–249. doi: 10.1016/j.amjmed.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 45.Chouksey D., Rathi P., Sodani A., et al. Postural orthostatic tachycardia syndrome in patients of orthostatic intolerance symptoms: an ambispective study. AIMS Neurosci. 2020;8(1):74–85. doi: 10.3934/Neuroscience.2021004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raj S.R. The Postural Tachycardia Syndrome (POTS): pathophysiology, diagnosis & management. Indian Pacing Electrophysiol J. 2006;6(2):84–99. [PMC free article] [PubMed] [Google Scholar]
- 47.Freeman R., Wieling W., Axelrod F.B., et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69–72. doi: 10.1007/s10286-011-0119-5. [DOI] [PubMed] [Google Scholar]
- 48.Dani M., Dirksen A., Taraborrelli P., et al. Autonomic dysfunction in 'long COVID': rationale, physiology and management strategies. Clin Med. 2021;21 doi: 10.7861/clinmed.2020-0896. e63–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sletten D.M., Suarez G.A., Low P.A., et al. COMPASS 31: a refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin Proc. 2012;87(12):1196–1201. doi: 10.1016/j.mayocp.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buoite Stella A., Furlanis G., Frezza N.A., et al. Autonomic dysfunction in post-COVID patients with and witfhout neurological symptoms: A prospective multidomain observational study. J Neurol. 2021 doi: 10.1007/s00415-021-10735-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fedorowski A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J Intern Med. 2019;285:352–366. doi: 10.1111/joim.12852. [DOI] [PubMed] [Google Scholar]
- 52.Centers for Disease Control and Prevention: evaluating and caring for patients with post-COVID conditions: interim guidance. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-index.html (2021). Accessed March 21, 2023.
- 53.Davido B., Seang S., Tubiana R., et al. Post-COVID-19 chronic symptoms: a postinfectious entity? Clin Microbiol Infect. 2020;26:1448–1449. doi: 10.1016/j.cmi.2020.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Escorihuela R.M., Capdevila L., Castro J.R., et al. Reduced heart rate variability predicts fatigue severity in individuals with chronic fatigue syndrome/myalgic encephalomyelitis. J Transl Med. 2020;18:4. doi: 10.1186/s12967-019-02184-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Low P.A., Caskey P.E., Tuck R.R., et al. Quantitative sudomotor axon reflex test in normal and neuropathic subjects. Ann Neurol. 1983;14:573–580. doi: 10.1002/ana.410140513. [DOI] [PubMed] [Google Scholar]
- 56.Fealey R.D., Low P.A., Thomas J.E. Thermoregulatory sweating abnormalities in diabetes mellitus. Mayo Clin Proc. 1989;64:617–628. doi: 10.1016/s0025-6196(12)65338-5. [DOI] [PubMed] [Google Scholar]
- 57.Bellavia S., Scala I., Luigetti M., et al. Instrumental Evaluation of COVID-19 Related Dysautonomia in Non-Critically-Ill Patients: An Observational, Cross-Sectional Study. J Clin Med. 2021;10(24):5861. doi: 10.3390/jcm10245861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nasserie T., Hittle M., Goodman S.N. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open. 2021;4(5):e2111417. doi: 10.1001/jamanetworkopen.2021.11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sivan M., Taylor S. NICE guideline on long COVID. BMJ. 2020;371:m4938. doi: 10.1136/bmj.m4938. [DOI] [PubMed] [Google Scholar]
- 60.Taub P.R., Zadourian A., Lo H.C., et al. Randomized trial of ivabradine in patients with Hyperadrenergic postural orthostatic tachycardia syndrome. J Am Coll Cardiol. 2021;77:861–871. doi: 10.1016/j.jacc.2020.12.029. [DOI] [PubMed] [Google Scholar]
- 61.Dayrit J.K., Verduzco-Gutierrez M., Teal A., et al. Enhanced External Counterpulsation as a Novel Treatment for Post-acute COVID-19 Sequelae. Cureus. 2021;13(4):e14358. doi: 10.7759/cureus.14358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Varanasi S., Sathyamoorthy M., Chamakura S., et al. Management of Long-COVID Postural Orthostatic Tachycardia Syndrome With Enhanced External Counterpulsation. Cureus. 2021;13(9):e18398. doi: 10.7759/cureus.18398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raza A., Steinberg K., Tartaglia J., et al. Enhanced external counterpulsation therapy: past, present, and future. Cardiol Rev. 2017;25:59–67. doi: 10.1097/CRD.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 64.Blitshteyn S., Whiteson J.H., Abramoff B., et al. Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of autonomic dysfunction in patients with post-acute sequelae of SARS-CoV-2 infection (PASC) Pharm Manag PM R. 2022;14(10):1270–1291. doi: 10.1002/pmrj.12894. [DOI] [PMC free article] [PubMed] [Google Scholar]