Abstract
Objective
The aim of the study is to test the association of a functional variant each in DRD2 and COMT genes with schizophrenia and its endophenotypes.
Basic methods
Effect of two functional variants rs1076560 in DRD2 and rs4680 in COMT on (1) schizophrenia (502 cases, 448 controls) diagnosed by Diagnostic and Statistical Manual of Mental Disorders-IV criteria and in subsets with (2) tardive dyskinesia (80 positive, 103 negative), assessed by Abnormal Involuntary Movement Scale (AIMS), positive and negative symptoms assessed by Positive and Negative Syndrome Scale (PANSS) and (3) cognition (299 cases, 245 controls), estimated by Penn Computerized Neurocognitive Battery, were analysed either using analysis of variance (ANOVA) or regression analysis.
Main results
No association of two SNPs with schizophrenia, but association of rs4680 (P < 0.05) with tardive dyskinesia was observed. On ANOVA, main effect of smoking [F(2,148) = 16.3; P = 3.9 × 10−7]; rs4680 [F(2,148) = 3.3; P = 0.04] and interaction effect of tardive dyskinesia-status*Smoking [F(2,148) = 5.4, P = 0.006]; Smoking*rs1076560 [F(3,148) = 3.6; P = 0.01]; Smoking*rs4680 [F(4,148) = 5.3; P = 4.7 × 10−4] were significant with AIMS tardive dyskinesia score. The main effect of rs1076560 [F(2,148) = 4.5; P = 0.013] and rs4680 [F(2,148) = 4.0; P = 0.02] were significant with limb truncal tardive dyskinesia. Allelic/genotypic (P = 0.004/P = 0.01) association of rs1076560 with negative scale of PANSS in tardive dyskinesia-negative; diminished expression factor of PANSS in tardive dyskinesia-negative subcohort (allelic/genotypic P = 3.3 × 10−5/6.6 × 10−5) and tardive dyskinesia cohorts (P = 0.003/0.002); genotypic association (P = 0.05) with disorganised/concrete factor in tardive dyskinesia-positive subcohorts were observed by regression analysis using gPLINKv2.050. Further allelic/genotypic (P = 0.02) association of rs4680 with depressed factor of PANSS in tardive dyskinesia cohort was observed. Allelic/genotypic association of rs1076560 with abstraction and mental flexibilityaccuracy (P = 0.03/0.04), abstraction and mental flexibilityefficiency (P = 0.01/0.02); allelic association with spatial abilityprocessing speed (P = 0.03), emotionefficiency (P = 0.05); and with spatial abilityefficiency (genotypic, P = 0.05) in healthy controls and allelic association of rs4680 with emotionefficiency in cases with schizophrenia (P = 0.04) were notable.
Principal conclusion
Dopaminergic genes seem to contribute to tardive dyskinesia and cognition warranting replication. Psychiatr Genet 30: 125–135 Copyright © 2020 Wolters Kluwer Health, Inc. All rights reserved.
Keywords: catechol-O-methyltransferase, cognition, rs1076560, rs4680, dopamine receptor D2, schizophrenia, tardive dyskinesia
Introduction
Schizophrenia is a debilitating neuropsychiatric disorder characterised by hallucinations, delusions and lack of perception of reality. Genetic, environmental factors and their interactions are implicated in the etiology of this illness. Risk alleles of variants in genes of dopaminergic (Howes and Kapur, 2009), glutamatergic (Bitanihirwe et al., 2009; Coyle et al., 2012), serotoninergic (Aghajanian and Marek, 2000), adrenergic (Cubells et al., 2011) and GABAergic pathways (Blum and Mann, 2002; Nakazawa et al., 2012) are implicated in conferring risk for schizophrenia.
Catechol-O-methyltransferase (COMT; EC: 2.1.1.6) an enzyme that is encoded by the COMT gene (22q11.1-q11.2) (Grossman et al., 1992) is involved in the catabolism of catecholamines which includes neurotransmitters such as dopamine, epinephrine and norepinephrine (Axelrod, 1957). COMT exists in two forms soluble (S-COMT) and membrane-bound forms- (MB-COMT). As the levels of catecholamines are altered in many neuropsychiatric disorders (Craddock et al., 2006), variants in these genes encoding these enzymes involved in the metabolism of these neurotransmitters including COMT may alter disease susceptibility to these disorders (Weinshilboum et al., 1999). Hence, it is widely considered as a candidate gene in conditions such as Parkinson’s disease (PD) (Tai and Wu, 2002), schizophrenia (Glatt et al., 2003; Fan et al., 2005; Okochi et al., 2009), bipolar disorder (Zhang et al., 2009), depression (Porcelli et al., 2011), etc. A functional polymorphism (rs4680) in COMT gene (Val158Met in MB-COMT and Val108Met in S-COMT) involving a transition of guanine (G) to adenine (A) was reported (Lachman et al., 1996b; Lachman et al., 1996a). The proteoform of COMT with Met at position 158 was found unstable at 37°C and had one-fourth activity of the Val proteoform (Lotta et al., 1995). So subjects with the latter proteoforms may have lower levels of catecholamines than those with Met proteoforms. Subjects with A allele were shown to have higher mRNA expression in both brain and lymphoblasts (Zhu et al., 2004). Because Val/Val shows the highest self-reported schizotypy scores in healthy males, rs4680 or another variant in linkage disequilibrium with it was hypothesised to be involved in the schizophrenia pathophysiology (Avramopoulos et al., 2002). However, no significant association between rs4680 and clinical symptoms or cognitive function was observed in Han Chinese schizophrenia subjects at baseline before 8 weeks of antipsychotic treatment (Sun et al., 2018).
Dopamine receptor D2 (D2R) is a protein encoded by DRD2 in humans which is coupled to subtype of G protein-coupled receptor (Gi) that inhibits adenylate cyclase activity (Usiello et al., 2000). D2 receptors are well-known targets for neuroleptic drugs (Miyamoto et al., 2004) and D2 agonists were found to improve working memory performance (Mehta and Riedel, 2006). A single nucleotide polymorphism (SNP) in DRD2 (rs2514218) was among the 108 loci that were associated with schizophrenia in a genome-wide association study (Schizophrenia Working Group of the Psychiatric Genomics, 2014). Two distinct isoforms of D2 receptors (D2S and D2L) are generated through alternate splicing mechanisms in human body. Polymorphisms in DRD2 were shown to be associated with development of motor fluctuations in PD (Wang et al., 2001). Postsynaptic D2R were found to exhibit enhanced sensitivity in schizophrenia (Seeman et al., 2005; Seeman et al., 2007). Two intronic SNPs (rs2283265 and rs1076560) lead to a decrease in expression of presynaptic DRD2 short splice forms relative to postsynaptic DRD2 long forms (Zhang et al., 2007). An intronic SNP of DRD2 (rs1076560) affecting alternate splicing and memory processing was found to be associated with cocaine abuse (Moyer et al., 2011). The activity of prefrontal-striatal pathway was reduced in schizophrenia subjects as compared to controls (Bertolino et al., 2009). The T allele of this SNP (rs1076560) is associated with reduced expression of D2S in prefrontal cortex and impaired working memory (Bertolino et al., 2010). This SNP was also associated with emotional control, brain activity and connectivity during emotion processing (Blasi et al., 2009). Family-based association study of polymorphisms in DRD2 and COMT genes with schizophrenia was also carried out (Pawel et al., 2010).
Tardive dyskinesia is a movement disorder caused by chronic treatment with antipsychotics that block dopamine D2 and D3 receptors. Subjects who are undergoing prolonged treatment with typical antipsychotics have a greater chance of developing tardive dyskinesia. So atypical antipsychotics were introduced that reduce the incidence of tardive dyskinesia but irrespective of the class of antipsychotics, neuroleptics that block dopamine receptors may increase the risk for tardive dyskinesia incidence (O’Brien, 2016; Correll et al., 2004; Correll and Schenk, 2008). With the advent of atypical antipsychotics from typical antipsychotics, a decrease in prevalence of tardive dyskinesia from 31% to 10–12% was observed (Chouinard and Chouinard, 2008; Woods et al., 2010). Genetic variants in dopamine receptors (D2 and D3) and COMT genes are implicated in predisposition for tardive dyskinesia (Aquino and Lang, 2014). The prevalence and severity of tardive dyskinesia were found to be more among schizophrenia subjects who were smokers than non-smokers (Chong et al., 2003; Diehl et al., 2009). Identification of key genetic or environmental factors that affect tardive dyskinesia may help in development of tailored therapies that may help to reduce incidence of tardive dyskinesia leading to personalised medicine (Thelma et al., 2008).
The objective of our study was to check association of two functional SNPs namely rs1076560 and rs4680 of DRD2 and COMT, respectively, with schizophrenia and its endophenotypes namely tardive dyskinesia and cognition in a North Indian cohort.
Methods
Recruitment of subjects and diagnosis
The recruitment of samples for this study was done as described previously (Tiwari et al., 2005a; Tiwari et al., 2007; Kukshal et al., 2013). The Diagnostic and Statistical Manual of Mental Disorders-IV criteria were used to diagnose subjects with schizophrenia or schizoaffective disorder at Postgraduate Institute of Medical Education and Research – Dr. Ram Manohar Lohia Hospital, New Delhi. A Hindi version of Diagnostic Interview for Genetic Studies (Deshpande et al., 1998; Nurnberger et al., 1994) and Family Interview from Genetic Studies (Maxwell, 1992) were used for the assessment of all the participants. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Institutional ethical committee clearance from ethical committee at Dr. Ram Manohar Lohia Hospital, approval number 18–15/2002-RMLH(HA1)/3140 dated 5 March 2004; 18–62/06-RMLH(HA-1)/vol.II/63 dated 30 November 2008 and 18–9/2002-RMLH(HA-I)/1088 dated 15 January 2008 was obtained. Further, informed consent was obtained from all individual participants included in the study.
Assessment of tardive dyskinesia
In a subset of schizophrenia cases, Abnormal Involuntary Movement Scale (AIMS) (Guy, 1976) scores were determined to assess tardive dyskinesia for items 1–7 using the Schooler and Kane criterion (Schooler and Kane, 1982) as described elsewhere (Tiwari et al., 2005b). While a total of 80 schizophrenia subjects [male n = 46 (Age = 34 ± 11); female n = 34 (Age = 34 ± 14); AIMS score = 6.1 ± 3.6] constituted tardive dyskinesia positive cohort, 103 schizophrenia subjects [male n = 47 (Age = 31 ± 11); female n = 56 (Age = 31 ± 9.4); AIMS score = 0.6 ± 0.9) constituted tardive dyskinesia negative cohort. There were 140 nonsmokers, 33 subjects who were smokers at the time of the study and 10 subjects who smoked in the past in the tardive dyskinesia cohort. In the tardive dyskinesia positive cohort, 26 subjects received typical antipsychotics (illness duration = 12 ± 8.7 years; AIMS score = 6.5 ± 3.6), 23 received atypical antipsychotics (illness duration = 6.9 ± 5.1 years; AIMS score = 5.7 ± 3.2) and 42 were treated with both typical and atypical antipsychotics during different times of their illness (illness duration 10 ± 8.4 years; AIMS score = 6.2 ± 3.3). While the category of antipsychotic treatment for all the subjects is available, the dosage is not available (Tiwari et al., 2005a; Tiwari et al., 2005b). Two mild, one moderate or higher rating of symptoms were used to demarcate schizophrenia subjects into tardive dyskinesia positive and tardive dyskinesia negative. For association testing AIMS total score served as continuous variable and tardive dyskinesia status served as dichotomous variable. Assessment of Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987) was done in schizophrenia subjects of tardive dyskinesia cohort (tardive dyskinesia positive, PANSS total score = 63 ± 19; tardive dyskinesia negative, PANSS total score = 55 ± 20). Disorganized/concrete, excited and depressed factors constituted by P2 + N5 + G11, P4 + P7 + G8 + G14 and G2 + G3 + G6 were extracted from the PANSS scores as described earlier (Wallwork et al., 2012). Further amotivation (N2 + N4 + G16), diminished expression (N1 + N3 + N6 + G7) factors (Fervaha et al., 2014; Kaliuzhna et al., 2020) were extracted from PANSS scores. These extracted factors were used for association testing.
Neurocognitive assessment using Penn Computerized Neurocognitive Battery
Cognitive assessment was done using a Hindi version of Penn Computerized Neurocognitive Battery (CNB) (Bhatia et al., 2012; Kukshal et al., 2013) measuring performance functions namely accuracy, processing speed and efficiency of neurobehavioral functions of eight domains such as abstraction and flexibility, attention, working memory, face memory, spatial memory, spatial processing, sensorimotor dexterity and emotional processing (Gur et al., 2001b; Gur et al., 2001a; Gur et al., 2007) was administered on 245 adult controls comprising 147 male (age = 41 ± 17) and 98 female (age = 36 ± 11) and 299 schizophrenia cases comprising 218 male (age = 33 ± 9.4) and 81 female (age = 32 ± 8.9) at the time of recruitment. The study cohort details are given in Table 1. For testing association, the scores obtained from PennCNB was transformed to near normality. Positive symptoms, negative symptoms and disease severity were assessed in schizophrenia subjects of cognition cohort by Scale for Assessment of Positive Symptoms (SAPS, 13.7 ± 12), Scale for Assessment of Negative Symptoms (SANS, 28.6 ± 20) (Andreasen and Olsen, 1982)and Global Assessment Scale (GAS, current episode 22.4 ± 5.3; past month 36.8 ± 14.2) (Endicott et al., 1976).
Table 1.
Study cohort composition
| Case-control | Cognition samples | Tardive dyskinesia samples | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | Male | Female | Male | Female | Male | Female | Male | Female | ||||
| Case | Control | Case | Control | Case | Control | Case | Control | Tardive dyskinesia positive | Tardive Dyskinesia negative | Tardive dyskinesia positive positive |
Tardive Dyskinesia negative |
|
| Samples (N) | 322 | 268 | 180 | 180 | 218 | 147 | 81 | 98 | 46 | 47 | 34 | 56 |
| Age (mean ± SD) | 33 ± 9.9 | 39 ± 16 | 31 ± 10 | 36 ± 12 | 33 ± 9.4 | 41 ± 17 | 32 ± 8.9 | 36 ± 11 | 34 ± 11 | 31 ± 11 | 34 ± 14 | 31 ± 9.4 |
Demographics of the study cohort along with description of gender and mean age in different subclasses of the cohort used in this study for disease association, cognition and tardive dyskinesia.
Selection of single nucleotide polymorphisms and genotyping
Two variants namely rs1076560 and rs4680 in DRD2 and COMT respectively, were selected for genotyping. While primers for amplicon encompassing rs1076560 of DRD2 were designed online using Primer 3 (v.0.4.0) (http://bioinfo.ut.ee/primer3-0.4.0/), that for rs4680 of COMT was as previously described (Norton et al., 2002). The restriction fragment length polymorphism (RFLP) patterns corresponding to these markers were generated using WatCut (http://watcut.uwaterloo.ca/template.php?act=snp_new). The oligos used for amplification and the RFLP profile for these amplicons are given in Supplemental digital content 1, http://links.lww.com/PG/A241. PCR was done on a Veriti 96-Well Thermal Cycler (Thermo Fisher Scientific Waltham, Massachusetts, USA) using 3B DNA polymerase (Biotools B&M Labs, SA Madrid, Spain) and constituents of PCR reactions are given in Supplemental digital content 1, http://links.lww.com/PG/A241. The thermal cycling and restriction digestion conditions for these two amplicons are given in Supplemental digital content 2, http://links.lww.com/PG/A242. Sanger sequence confirmed DNA samples for each of the three genotypes were used in each 96-well plate as controls. Post restriction digestion, the samples were loaded on to a 3.0% Agarose gel in 1 mM lithium borate buffer (Brody et al., 2004) and electrophoresis was done at 150 and 100 V for rs1076560 and rs4680, respectively. The gels were visualised on Gel Doc-It Imaging system (Ultra-Violet Products Ltd Upland, California, USA) following electrophoresis and the alleles were called.
Statistical analysis
Deviation of the two variants rs4680 and rs1076560 in COMT and DRD2 from Hardy–Weinberg equilibrium was assessed using Plink 1.07 (Purcell et al., 2007). Associations of the two variants with dichotomous variables (schizophrenia or tardive dyskinesia) were evaluated using Chi square (χ2) tests with Plink 1.07. The power of the entire sample set to detect an association was determined with Quanto software (Gauderman and Morrison, 2006).
To assess the effect of tardive dyskinesia status, smoking, genotypes of rs4680 and rs1076560 on tardive dyskinesia, a full factorial analysis of variance (ANOVA) model was done with tardive dyskinesia status, smoking status and genotypes of these two markers as fixed factors and aligned rank transformed (Wobbrock et al., 2011) Abnormal Involuntary Movement Scale (AIMSart) score, orofacial (items 1–4 of AIMS) or limb-truncal (items 5–7 of AIMS) tardive dyskinesia scores separately as dependent variables using IBM SPSS Statistics Subscription for Windows (IBM Corp. Armonk, New York, USA). The main effects of tardive dyskinesia status, smoking status, genotypes of the two markers, interaction of genotypes of the two SNPs, smoking × genotypes and tardive dyskinesia status × genotypes of the two SNPs on AIMSart score were assessed in the model. If significant main effects or interactions were found in full factorial ANOVA model, Bonferroni multiple comparisons were done to identify significantly different groups. Association of the two SNPs with PANSS total score; positive scale; negative scale; general psychopathology scales; disorganised/concrete; excited; depressed; amotivation and diminished expression factors of PANSS were tested by linear regression using gPLINK v.2.050.
Cognition scores for healthy controls in cognition cohort were conditioned for the two SNP genotypes, age and gender, and made to conform to normality (Hawkins and Weisberg, 2017) using car package in R (Fox and Weisberg, 2011). Association of the two SNPs with cognitive scores in healthy controls and schizophrenia cases was assessed by linear regression with age and gender as covariates using gPLINK v.2.050. For multivariate analysis of covariance (MANCOVA), the cognition scores were transformed by skew power transformation and were made to conform to multivariate normality. The correlation structure of cognition data was visualised using corrplot package with hclust option for reordering variables to reveal highly correlated variables in R (Kuhn M, Johnson K 2013). MANCOVA was done with case-control status (dichotomous variable) and the genotypes of these two variants as fixed factors and transformed cognition scores (continuous variables) from PennCNB corresponding to accuracy, processing speed and efficiency for eight cognitive domains namely (a) abstraction and mental flexibility, (b) attention, (c) face memory, (d) spatial memory, (e) working memory, (f) spatial ability, (g) sensorimotor dexterity and (h) emotional processing as dependent variable, with gender and age as covariates using IBM SPSS Statistics Subscription for Windows. Association of the two SNPs with SANS, SAPS and GAS scores were done separately using gPLINK v.2.050 with age and gender as covariates for schizophrenia subjects in cognition cohort.
Results
Genotyping and association with schizophrenia and tardive dyskinesia
The RFLP patterns of the two SNPs analysed in this study are presented in supplemental digital content 3, http://links.lww.com/PG/A243. The total genotyping rate of the two SNPs in the cohort was 0.98. Though no allelic or genotypic association with schizophrenia was observed (Table 2), a significant association of rs4680 on tardive dyskinesia status was observed under the dominant model (P = 0.02). ANOVA with align rank transformed AIMS total score (AIMSart) as dependent variable and tardive dyskinesia status, smoking status, genotypes of two SNPs as fixed factors revealed that the main effects of smoking status [F(2,148) = 16.3, P = 3.9 × 10–7] and rs4680 [F(2,148) = 3.3, P = 0.04] have an effect on AIMSart total score (Table 3). The interactions of tardive dyskinesia status*smoking status [F(2,148) = 5.4, P = 0.01]; smoking status*rs1076560 [F(3,148) = 3.6, P = 0.02]; Smoking status*rs4680 [F(4,148)=5.3, P = 4.7 × 10–4] were found to have an effect on AIMSart total score of tardive dyskinesia (Table 3). The main effects and interactions that had a significant effect on AIMSart total score of tardive dyskinesia after Bonferroni multiple comparisons are presented in Fig. 1. With Orofacial tardive dyskinesia scores, smoking status [F(2,148) = 9.1; P = 1.9 × 10–4] and smoking status*rs4680 [F(4,148) = 7.3; P = 2.1 × 10–5] were found to have a significant effect. Smoking status [F(2,148) = 12.9; P = 7.0 × 10–6], rs1076560 [F(2,148) = 4.5; P = 0.013 (Fig. 2a)], rs4680 [F(2,148) = 4.0; P = 0.020] (Fig. 2b), tardive dyskinesia status*smoking status [F(2,148) = 5.4; P = 0.006], tardive dyskinesia status*rs1076560 [F(2,148) = 4.2; P = 0.017], smoking status*rs1076560 [F(3,148) = 5.2; P = 0.002], tardive dyskinesia status*rs4680 [F(2,148) = 3.9; P = 0.002] and smoking status*rs4680 [F(4,148) = 2.4; P = 0.05] were found to be significant with limb truncal tardive dyskinesia.
Table 2.
Lack of association of SNPs of DRD2 (rs1076560) and COMT (rs4680) with schizophrenia
| CHR | SNP | A1 | A2 | TEST | AFF | UNAFF | CHISQ | P |
|---|---|---|---|---|---|---|---|---|
| 11 | rs1076560 | A | C | GENO | 35/192/268 | 28/166/243 | 0.28 | 0.87 |
| 11 | rs1076560 | A | C | TREND | 262/728 | 222/652 | 0.27 | 0.60 |
| 11 | rs1076560 | A | C | ALLELIC | 262/728 | 222/652 | 0.27 | 0.60 |
| 11 | rs1076560 | A | C | DOM | 227/268 | 194/243 | 0.20 | 0.65 |
| 11 | rs1076560 | A | C | REC | 35/460 | 28/409 | 0.16 | 0.69 |
| 22 | rs4680 | A | G | GENO | 97/245/156 | 90/198/149 | 1.44 | 0.49 |
| 22 | rs4680 | A | G | TREND | 439/557 | 378/496 | 0.13 | 0.72 |
| 22 | rs4680 | A | G | ALLELIC | 439/557 | 378/496 | 0.13 | 0.72 |
| 22 | rs4680 | A | G | DOM | 342/156 | 288/149 | 0.81 | 0.37 |
| 22 | rs4680 | A | G | REC | 97/401 | 90/347 | 0.18 | 0.67 |
Absence of allelic and genotypic association of these two makers with the disease as modelled using Plink 1.07.
SNP, single nucleotide polymorphism.
Table 3.
Effect of tardive dyskinesia status, smoking status, the two SNPs and their interaction on Aligned Rank Transform of Abnormal Involuntary Movement Scale tardive dyskinesia score
| Effect | F | P value | Power (%) |
|---|---|---|---|
| Tardive dyskinesia status | F(1,148) = 75.39 | 6.5 × 10−15# | 100.0 |
| Smoking | F(2,148) = 16.34 | 3.9 × 10−7# | 100.0 |
| rs1076560 | F(2,148) = 2.33 | 0.10 | 46.6 |
| rs4680 | F(2,148) = 3.27 | 0.04# | 61.4 |
| Tardive dyskinesia status*Smoking | F(2,148) = 5.36 | 0.01# | 83.5 |
| Tardive dyskinesia status* rs1076560 | F(2,148) = 0.07 | 0.93 | 6.1 |
| Smoking* rs1076560 | F(3,148) = 3.57 | 0.02# | 78.0 |
| Tardive dyskinesia status* rs4680 | F(2,148) = 1.46 | 0.24 | 30.8 |
| Smoking* rs4680 | F(4,148) = 5.35 | 4.7 × 10−4# | 97.0 |
| rs1076560*rs4680 | F(4,148) = 7.53 | 1.5 × 10−5# | 99.6 |
| Tardive dyskinesia status*Smoking* rs4680 | F(2,148) = 0.38 | 0.68 | 11.0 |
| Tardive dyskinesia status*Smoking* rs1076560 | F(1,148) = 0.59 | 0.44 | 11.9 |
| Tardive dyskinesia status* rs1076560*rs4680 | F(3,148) = 2.72 | 0.05# | 65.1 |
| Smoking* rs1076560*rs4680 | F(2,148) = 15.29 | 9.2 × 10−7# | 99.9 |
| Tardive dyskinesia status*Smoking* rs1076560*rs4680 | F(1,139) = 0.60 | 0.44 | 12.0 |
The main effects of tardive dyskinesia status, Smoking and rs4680 were found to significantly affect AIMSart tardive dyskinesia score. The interactions of tardive dyskinesia status*Smoking, Smoking*rs1076560, Smoking*rs4680, tardive dyskinesia status* rs1076560*rs4680 and smoking* rs1076560*rs4680 withstood Bonferroni multiple comparisons post-ANOVA.
ANOVA, analysis of variance; SNP, single nucleotide polymorphism.
#P value < 0.05.
Fig. 1.
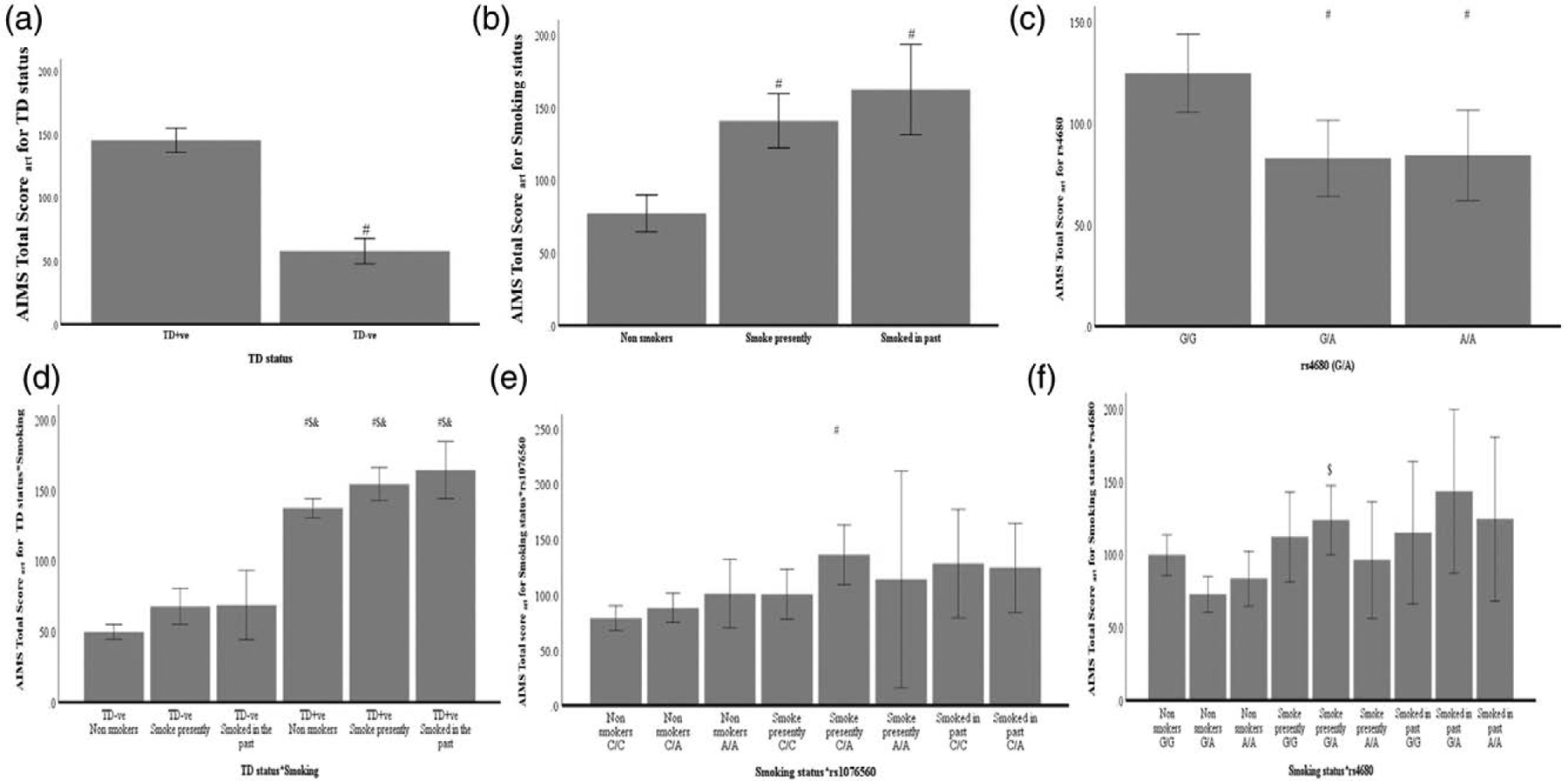
Effect of tardive dyskinesia status, smoking status, rs4680, rs1076560 and their interactions on Aligned Rank Transformed Abnormal Involuntary Movement Scale (AIMSart) score of tardive dyskinesia. A full factorial ANOVA model identified main effects of (a) tardive dyskinesia status, (b) smoking status and (c) rs4680 (COMT) to have an effect on AIMSart score of tardive dyskinesia. Further, interactions of (d) tardive dyskinesia status and smoking status, smoking status with (e) rs1076560 (DRD2) and (f) rs4680 were also found to have a significant effect on AIMSart score of tardive dyskinesia. #, $ and & represents significantly different from first, second and third bars post-Bonferroni multiple comparisons. Error bars indicate 95% CI. ANOVA, analysis of variance.
Fig. 2.
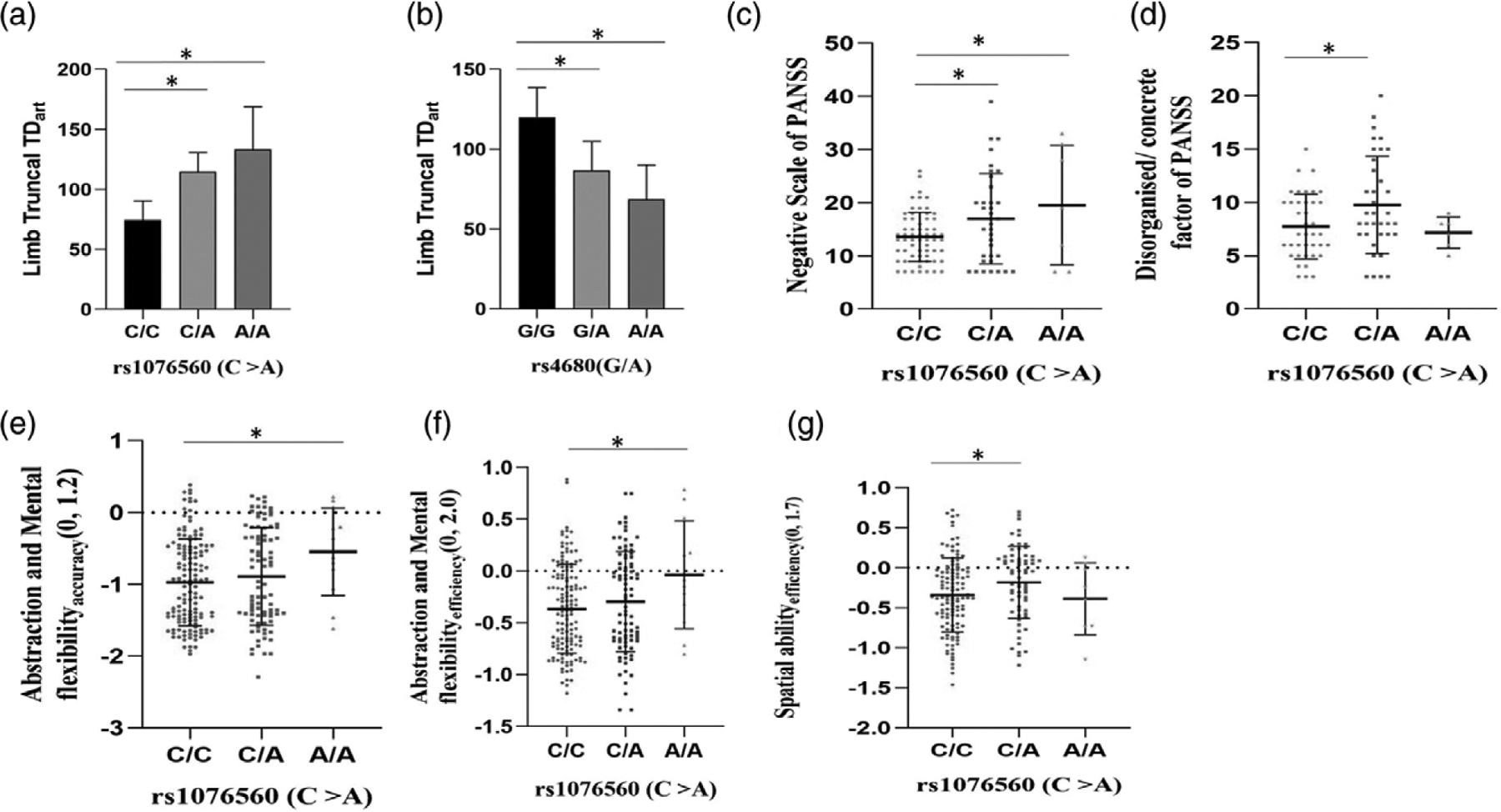
Effect of rs1076560(DRD2) and rs4680(COMT) on limb truncal tardive dyskinesia, PANSS subscales and on cognition in healthy controls. On ANOVA, schizophrenia subjects (a) with A/A and C/A genotypes at rs1076560 of DRD2 were found to have higher align rank transformed limb truncal tardive dyskinesia scores than those with C/C genotypes post-Bonferroni multiple comparisons. Schizophrenia subjects (b) with G/G genotype at rs4680 were found to have higher limb truncal tardive dyskinesia scores than those with G/A and A/A genotypes post-Bonferroni multiple comparisons. Tardive dyskinesia negative subjects (c) with C/C genotype had lower Negative scale scores of Positive and Negative Syndrome Scale (PANSS) than those with C/A and A/A genotypes post-Fisher’s Least Significant Difference (LSD) multiple comparisons. Tardive dyskinesia positive subjects (d) with C/A genotype were found to have higher disorganised/concrete factor score of PANSS than those with C/C genotypes. Among healthy controls, those with A/A genotype at rs1076560 were found to have higher (e) abstraction and mental flexibilityaccuracy and (f) abstraction and mental flexibilityefficiency than those with C/C genotypes post-Fisher’s LSD multiple comparisons. Healthy controls with C/A genotype at rs1076560 were found to have higher Spatial abilityefficiency than those with C/C genotypes post-Fisher’s LSD multiple comparisons. (a and b) error bars indicate 95%CI, (c–g) error bars indicate SD. * denotes P < 0.05. ANOVA, analysis of variance; LSD, least significant difference.
There was allelic association of DRD2 SNP (rs1076560) with negative scale of PANSS in tardive dyskinesia-negative subcohort (β = 3.1, P = 0.004). Further, there was genotypic association of rs1076560 with disorganised/concrete factor (P = 0.05) and negative scale (P = 0.01) of PANSS in tardive dyskinesia-positive and tardive dyskinesia negative subcohorts (Fig. 2c and d). In tardive dyskinesia-positive subcohort, subjects with C/A genotype of rs1076560 were significantly different (P = 0.02) in disorganised/ concrete factor of PANSS from those with C/C genotypes post-Fisher’s least significant difference (LSD) multiple comparisons. In tardive dyskinesia-negative subjects, those with C/A and A/A genotypes of rs1076560 had significantly different negative scale scores of PANSS than those with C/C genotypes post-Fisher’s LSD multiple comparisons. Allelic and genotypic association of DRD2 SNP (rs1076560) with (1) negative subscale (allelic β = 2.3, P = 0.008; genotypic P = 0.005) of PANSS in tardive dyskinesia cohort (Fig. 3a) (2) diminished expression factor (allelic β = 2.3, P = 3.3 × 10–5; genotypic P = 6.6 × 10–5) in tardive dyskinesia negative subcohort (Fig. 3b) and (3) in tardive dyskinesia cohort (allelic β = 1.4, P = 0.003; genotypic P = 0.002) was observed (Fig. 3c). Allelic and genotypic association of the COMT SNP (rs4680) with depressed factor of PANSS (allelic β = −0.64, P = 0.02; genotypic P = 0.02) was also observed (Fig. 3d).
Fig. 3.
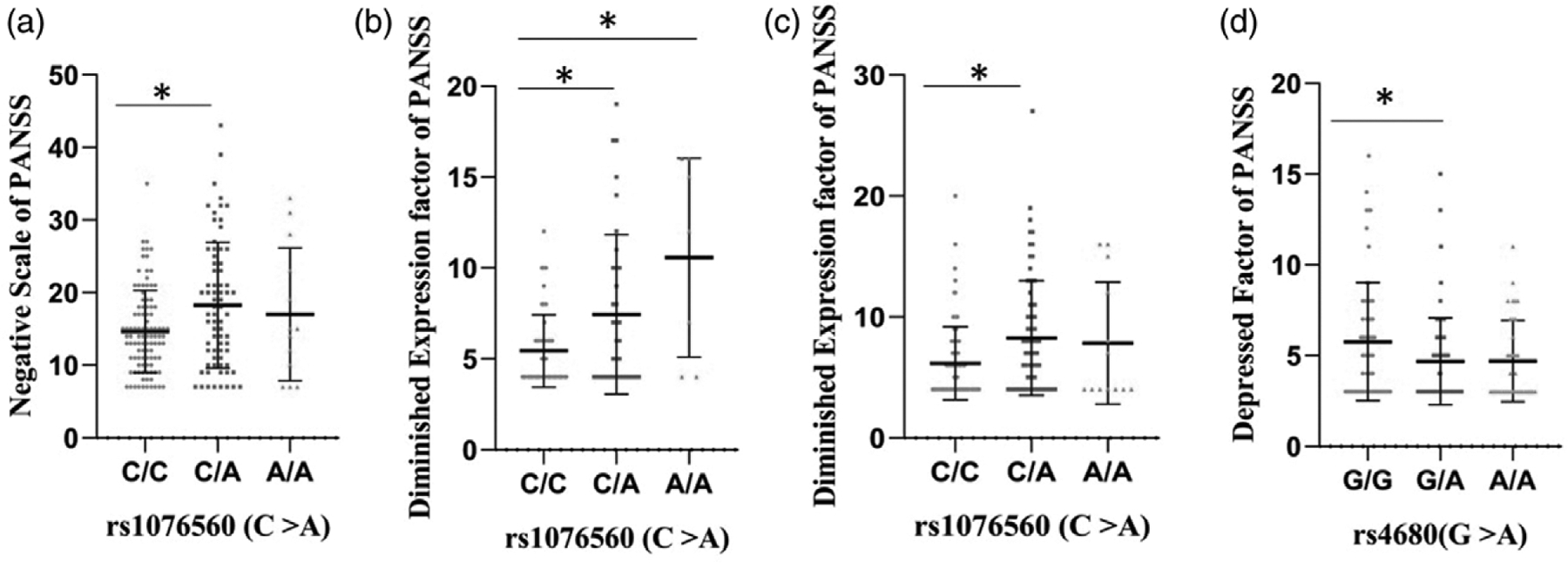
Effect of rs1076560(DRD2) and rs4680(COMT) on Negative scale, diminished expression factor and depressed factor of PANSS. Schizophrenia subjects in (a) tardive dyskinesia cohort who were heterozygous at rs1076560 (C/A) had significantly higher negative subscale scores of PANSS than those who were homozygous for the anscestral allele (C/C) (b) tardive dyskinesia negative subcohort with C/C genotype at rs1076560 were significantly different from those with C/A and A/A genotypes in diminished expression factor of PANSS (c) tardive dyskinesia cohort with C/A genotype at rs1076560 were significantly different from those with C/C genotype in diminished expression factor of PANSS (d) tardive dyskinesia cohort with G/A genotype at rs4680 were found to have lower scores of depressed factor of PANSS than those with G/G genotype in.* denotes significantly different post-Bonferroni’s multiple comparisons. Error bars denotes SD.
Association with cognition
The correlation matrix of cognition data visualised using corrplot package is depicted in Fig. 4. Allelic association of rs1076560 C > A with abstraction and mental flexibilityaccuracy (β = 0.15, P = 0.03), spatial abilityprocessing speed (β = 0.13, P = 0.03), abstraction and mental flexibility efficiency (β = 0.12, P = 0.01) and emotionefficiency (β = 0.10, P = 0.05) was observed in healthy controls. Further there was genotypic association of rs1076560 with abstraction and mental flexibilityaccuracy (P = 0.04), abstraction and mental flexibilityefficiency (P = 0.02) and spatial abilityefficiency (P = 0.05) (Fig. 2e to g). Healthy controls with A/A genotype at rs1076560 had significantly higher abstraction and mental flexibilityaccuracy (P = 0.03) and abstraction and mental flexibility efficiency (P = 0.02) scores than those with C/C genotype post-Fisher’s LSD multiple comparisons. Further, those with C/A genotype were found to have higher spatial abilityefficiency scores than those with C/C genotypes in healthy controls post-Fisher’s LSD multiple comparisons. Allelic association of the A allele of rs4680 of COMT with Emotionefficiency (β = 0.09, P = 0.04) in schizophrenia cases were also observed. Though there was no significant effect of interaction of health status* genotypes of two SNPs on cognition scores in the combined cohort using MANCOVA, a significant effect of health status and gender on cognitive scores was observed (Table 4).
Fig. 4.
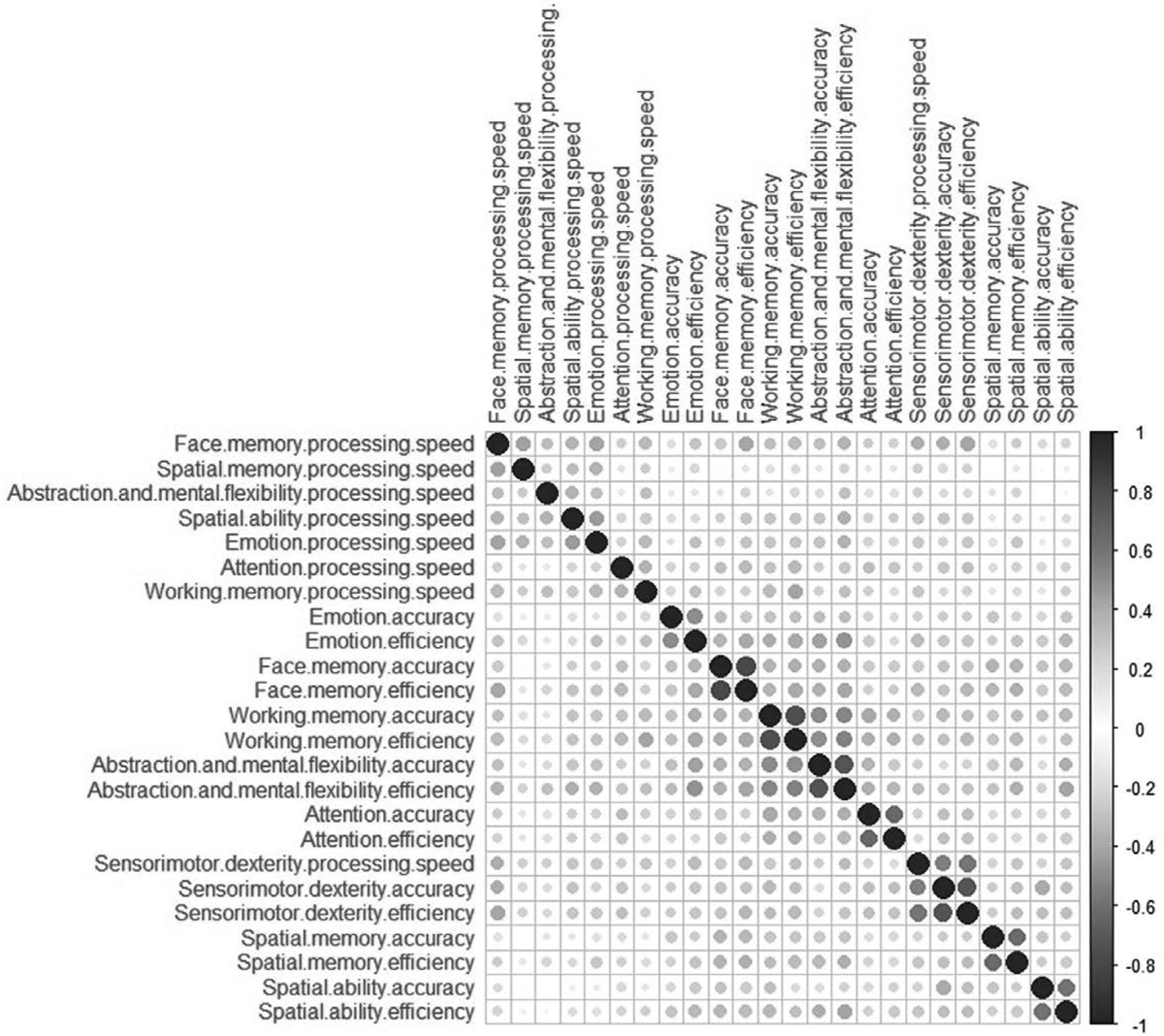
Correlation matrix of cognition data. Correlation between the cognitive scores of accuracy, processing speed and efficiency of eight domains of Penn Computerized neurocognitive battery (PennCNB). Cognitive scores that are clustered together have higher correlation. Blue circles denote positive correlation, negative correlation, if any, is depicted as red circles.
Table 4.
Multivariate testsa of health status and genotypes of the two SNPs on cognitive scores
| Effect | Value | F | Hypothesis df | Error df | Sig. | Partial η2 | Powerd |
|---|---|---|---|---|---|---|---|
| Intercept | |||||||
| Pillai’s Trace | 1.0 | 66680.16b | 24 | 415 | <1 × 10−30 | 1.0 | 1.0 |
| Wilks’ Lambda | 2.6 × 10−4 | 66680.16b | 24 | 415 | <1 × 10−30 | 1.0 | 1.0 |
| Hotelling’s Trace | 3856.20 | 66680.16b | 24 | 415 | <1 × 10−30 | 1.0 | 1.0 |
| Roy’s Largest Root | 3856.20 | 66680.16b | 24 | 415 | <1 × 10−30 | 1.0 | 1.0 |
| Gender | |||||||
| Pillai’s Trace | 0.13 | 2.68b | 24 | 415 | 4.3 × 10−5 | 0.13 | 1.0 |
| Wilks’ Lambda | 0.87 | 2.68b | 24 | 415 | 4.3 × 10−5 | 0.13 | 1.0 |
| Hotelling’s Trace | 0.16 | 2.68b | 24 | 415 | 4.3 × 10−5 | 0.13 | 1.0 |
| Roy’s Largest Root | 0.16 | 2.68b | 24 | 415 | 4.3 × 10−5 | 0.13 | 1.0 |
| Health status | |||||||
| Pillai’s Trace | 0.17 | 3.63b | 24 | 415 | 4 × 10−8 | 0.17 | 1.0 |
| Wilks’ Lambda | 0.83 | 3.63b | 24 | 415 | 4 × 10−8 | 0.17 | 1.0 |
| Hotelling’s Trace | 0.21 | 3.63b | 24 | 415 | 4 × 10−8 | 0.17 | 1.0 |
| Roy’s Largest Root | 0.21 | 3.63b | 24 | 415 | 4 × 10−8 | 0.17 | 1.0 |
| rs1076560 (C/A) | |||||||
| Pillai’s Trace | 0.10 | 0.95 | 48 | 832 | 0.57 | 0.05 | 0.96 |
| Wilks’ Lambda | 0.90 | 0.95b | 48 | 830 | 0.57 | 0.05 | 0.96 |
| Hotelling’s Trace | 0.11 | 0.95 | 48 | 828 | 0.57 | 0.05 | 0.96 |
| Roy’s Largest Root | 0.07 | 1.25c | 24 | 416 | 0.20 | 0.07 | 0.91 |
| rs4680 (G/A) | |||||||
| Pillai’s Trace | 0.08 | 0.73 | 48 | 832 | 0.91 | 0.04 | 0.87 |
| Wilks’ Lambda | 0.92 | 0.73b | 48 | 830 | 0.91 | 0.04 | 0.87 |
| Hotelling’s Trace | 0.09 | 0.73 | 48 | 828 | 0.91 | 0.04 | 0.87 |
| Roy’s Largest Root | 0.06 | 1.01c | 24 | 416 | 0.45 | 0.06 | 0.82 |
| Health status * rs1076560 (C/A) | |||||||
| Pillai’s Trace | 0.08 | 0.75 | 48 | 832 | 0.90 | 0.04 | 0.88 |
| Wilks’ Lambda | 0.92 | 0.75b | 48 | 830 | 0.90 | 0.04 | 0.88 |
| Hotelling’s Trace | 0.09 | 0.74 | 48 | 828 | 0.90 | 0.04 | 0.88 |
| Roy’s Largest Root | 0.05 | 0.81c | 24 | 416 | 0.72 | 0.05 | 0.70 |
| Health status * rs4680 (G/A) | |||||||
| Pillai’s Trace | 0.08 | 0.71 | 48 | 832 | 0.93 | 0.04 | 0.85 |
| Wilks’ Lambda | 0.92 | 0.71b | 48 | 830 | 0.93 | 0.04 | 0.85 |
| Hotelling’s Trace | 0.08 | 0.71 | 48 | 828 | 0.93 | 0.04 | 0.85 |
| Roy’s Largest Root | 0.05 | 0.87c | 24 | 416 | 0.65 | 0.05 | 0.73 |
| rs1076560 (C/A) * rs4680 (G/A) | |||||||
| Pillai’s Trace | 0.16 | 0.71 | 96 | 1672 | 0.99 | 0.04 | 0.98 |
| Wilks’ Lambda | 0.85 | 0.71 | 96 | 1646.5 | 0.99 | 0.04 | 0.98 |
| Hotelling’s Trace | 0.16 | 0.70 | 96 | 1654 | 0.99 | 0.04 | 0.98 |
| Roy’s Largest Root | 0.06 | 0.96c | 24 | 418 | 0.52 | 0.05 | 0.79 |
| Health status * rs1076560 (C/A) * rs4680 (G/A) | |||||||
| Pillai’s Trace | 0.13 | 0.82 | 72 | 1251 | 0.87 | 0.05 | 0.98 |
| Wilks’ Lambda | 0.87 | 0.81 | 72 | 1241.1 | 0.87 | 0.05 | 0.98 |
| Hotelling’s Trace | 0.14 | 0.81 | 72 | 1241 | 0.87 | 0.05 | 0.98 |
| Roy’s Largest Root | 0.06 | 1.10c | 24 | 417 | 0.34 | 0.06 | 0.86 |
Though an effect of gender and health status was observed on cognitive scores, no significant effect of these SNPs by itself or its interaction with health status was observed on cognitive scores in this study cohort.
Design: Intercept + gender + health + rs1076560 (C/A) + rs4680 (G/A) + health * rs1076560 (C/A) + health * rs4680 (G/A) + rs1076560 (C/A) * rs4680 (G/A) + health * rs1076560 (C/A) * rs4680 (G/A).
Exact statistic.
The statistic is an upper bound on F that yields a lower bound on the significance level.
Computed using alpha = 0.05.
SNP, single nucleotide polymorphism.
In schizophrenia subjects of cognition cohort, no allelic association of rs1076560 and rs4680 with SANS (β = 2.0, P = 0.30; β = −1.8, P = 0.30), SAPS (β = 0.13, P = 0.91; β = 1.2, P = 0.23), GAS during current episode (β = 0.10, P = 0.85; β = 0.55, P = 0.24) and past month GAS (β = −0.80, P = 0.6; β = −0.61, P = 0.6) was observed. Further, no genotypic association of rs1076560 and rs4680 with SANS (P = 0.56/0.50), SAPS (P = 0.41/0.44), current episode GAS (P = 0.52/0.22) and GAS during past month (P = 0.7/0.9) was observed in schizophrenia subjects of cognition cohort.
Discussion
Dopaminergic pathway has been long implicated in the pathophysiology of schizophrenia. However, the effect of variants in these genes to various endophenotypes of this disease is seldom documented. In this study, we have tried to assess the effect of two functional variants in dopaminergic genes namely DRD2 and COMT on schizophrenia and its endophenotypes namely tardive dyskinesia and cognition. The association of rs4680 with tardive dyskinesia under the dominant model should be viewed in light of an earlier study (Srivastava et al., 2006). In our study, we found that smoking was found to increase tardive dyskinesia scores (Fig. 1b). The Aligned ranked transformed Abnormal Involuntary Movement Scale (AIMSart) tardive dyskinesia scores of nonsmokers were significantly lower than those who were smokers at the time of study and those who smoked in the past (Fig. 1b). Further the tardive dyskinesia scores of subjects with G/A and A/A genotypes of rs4680 of COMT significantly different from those with G/G genotype post-Bonferroni comparisons (Fig. 1c). Nonsmokers, those who were smokers at the time of study and those who smoked in the past significantly differed between the tardive dyskinesia positive and tardive dyskinesia negative groups (Fig. 1d). There were previous reports on the effect of smoking on tardive dyskinesia (Chong et al., 2003; Diehl et al., 2009; Zhang et al., 2011). The tardive dyskinesia scores of subjects who were smokers at the time of study and with C/A genotype of rs1076560 differed significantly from those with C/C genotype who were nonsmokers (Fig. 1e). The AIMSart tardive dyskinesia scores of subjects who were smokers at the time of study and who had G/A genotype of rs4680 significantly differed from those who are non-smokers with the same genotype (Fig. 1f). However, the interaction of the two SNPs (rs1076560*rs4680) that was associated with AIMSart in full model did not exhibit association on recoding as a single variable with nine possible combinations [F(8, 174) = 1.66, P = 0.11]. Though there were a few studies on the effect of SNPs in these genes on tardive dyskinesia (Zai et al., 2010; Kang et al., 2008; Bakker et al., 2008), they were inconclusive and very few studies on the genetically distinct Indian population (Srivastava et al., 2006) were undertaken till date. The effect of rs1076560 on diminished expression factor of PANSS could be viewed in the light of earlier report that this SNP is associated with reduced expression of D2S in prefrontal cortex (Bertolino et al., 2010), emotional control, brain activity and connectivity during emotion processing (Blasi et al., 2009).
In healthy controls, rs1076560 was found to be an expression quantitative trait loci for abstraction and mental flexibilityaccuracy, spatial abilityprocessing speed, abstraction and mental flexibilityefficiency and emotionefficiency. Though there was an effect of gender and health on cognitive scores, there was no effect of interaction of health status and rs1076560 or rs4680 genotypes on the cognitive scores. Further, the interaction between rs1076560 and rs4680 with health status was also found not significant. Despite being a well-powered study (>80%), the lack of association or interaction rules out the likely effect of these SNPs in schizophrenia or its endophenotype of cognition in this study cohort. Though there was previous evidence from structural MRI (Di Giorgio et al., 2014) and functional MRI (Blasi et al., 2009), we were not able to identify an effect of the splice variant of DRD2 either on schizophrenia or its interaction with health status on cognition. This may partly due to the fact that the study cohort used in the fMRI data was not large enough to ascertain the effect of these variants. Further, we were not able to identify any effect of the COMT SNP rs4680 or its interaction with health status on schizophrenia or cognition. This is in line with the previous reports of absence of association of this SNP itself (Williams et al., 2005) or its epistatic interaction with variants in monoamine oxidase A (Norton et al., 2002; Matsumoto et al., 2004). However, schizophrenia being a genetically heterogeneous condition, the effect of these SNPs may not be uniform. Because we have only genotyped one SNP each in DRD2 and COMT, the effect of two or more SNPs/SNP haplotypes needs to be investigated which warrants additional genotyping in the study cohort.
The effect of smoking, rs4680 and the interaction of smoking with tardive dyskinesia status, rs1076560 and rs4680 imply that subjects could be grouped into different categories for the incidence of tardive dyskinesia. Association of the rs1076560 with negative scale of PANSS in tardive dyskinesia negative subcohort; diminished expression and limb truncal tardive dyskinesia has pharmacological significance and could lead to personalised medicine.
Supplementary Material
Acknowledgements
Skew power transformations using car package in R was done with help from Prof. Sanford Weisberg, School of Statistics, University of Minnesota. Prof. James J. Higgins of Department of Statistics, Kansas State University for Help with Aligned Rank transformation ARTool. This work was supported by Department of Biotechnology (DBT), Government of India, New Delhi (Grant number BKT, BT/PR/2425/MED/13/089/2001), (Grants numbers SND, BT/IC-2/Israel/Deshpande/2002 and BT/IC-2/00/smita/99 to SND); Department of Science and Technology - Science and Engineering Research Board (Grant number BKT, JC Bose National fellowship SR/S2/JCB-44/2011- phase II)
Footnotes
Conflicts of interest
There are no conflicts of interest.
Study was designed by B.K.T. and T.J.P.: experiments were conducted by T.J.P. and P.K.: Statistical analysis was done by T.B. and T.J.P.: samples were recruited by SND and her team at RML hospital.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.psychgenetics.com.
References
- Aghajanian GK, Marek GJ (2000). Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res Brain Res Rev 31:302–312. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Olsen S (1982). Negative v positive schizophrenia. Definition and validation. Arch Gen Psychiatry 39:789–794. [DOI] [PubMed] [Google Scholar]
- Aquino CC, Lang AE (2014). Tardive dyskinesia syndromes: current concepts. Parkinsonism Relat Disord 20 (Suppl 1):S113–S117. [DOI] [PubMed] [Google Scholar]
- Avramopoulos D, Stefanis NC, Hantoumi I, Smyrnis N, Evdokimidis I, Stefanis CN (2002). Higher scores of self reported schizotypy in healthy young males carrying the COMT high activity allele. Mol Psychiatry 7:706–711. [DOI] [PubMed] [Google Scholar]
- Axelrod J (1957). O-methylation of epinephrine and other catechols in vitro and in vivo. Science 126:400–401. [DOI] [PubMed] [Google Scholar]
- Bakker PR, Van Harten PN, Van Os J (2008). Antipsychotic-induced tardive dyskinesia and polymorphic variations in COMT, DRD2, CYP1A2 and MnSOD genes: a meta-analysis of pharmacogenetic interactions. Mol Psychiatry 13:544–556. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Fazio L, Caforio G, Blasi G, Rampino A, Romano R, et al. (2009). Functional variants of the dopamine receptor D2 gene modulate prefronto-striatal phenotypes in schizophrenia. Brain 132:417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Taurisano P, Pisciotta NM, Blasi G, Fazio L, Romano R, et al. (2010). Genetically determined measures of striatal D2 signaling predict prefrontal activity during working memory performance. PLoS One 5:e9348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia T, Agarwal A, Shah G, Wood J, Richard J, Gur RE, et al. (2012). Adjunctive cognitive remediation for schizophrenia using yoga: an open, non-randomized trial. Acta Neuropsychiatr 24:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitanihirwe BK, Lim MP, Kelley JF, Kaneko T, Woo TU (2009). Glutamatergic deficits and parvalbumin-containing inhibitory neurons in the prefrontal cortex in schizophrenia. BMC Psychiatry 9:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BlasI G, lo Bianco L, Taurisano P, Gelao B, Romano R, Fazio L, et al. (2009). Functional variation of the dopamine D2 receptor gene is associated with emotional control as well as brain activity and connectivity during emotion processing in humans. J Neurosci 29:14812–14819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum BP, Mann JJ (2002). The GABAergic system in schizophrenia. Int J Neuropsychopharmacol 5:159–179. [DOI] [PubMed] [Google Scholar]
- Brody JR, Calhoun ES, Gallmeier E, Creavalle TD, Kern SE (2004). Ultra-fast high-resolution agarose electrophoresis of DNA and RNA using low-molarity conductive media. Biotechniques 37:598, 600, 602. [DOI] [PubMed] [Google Scholar]
- Chong SA, Tan EC, Tan CH, Mythily (2003). Smoking and tardive dyskinesia: lack of involvement of the CYP1A2 gene. J Psychiatry Neurosci 28:185–189. [PMC free article] [PubMed] [Google Scholar]
- Chouinard G, Chouinard VA (2008). Atypical antipsychotics: CATIE study, drug-induced movement disorder and resulting iatrogenic psychiatric-like symptoms, supersensitivity rebound psychosis and withdrawal discontinuation syndromes. Psychother Psychosom 77:69–77. [DOI] [PubMed] [Google Scholar]
- Correll CU, Leucht S, Kane JM (2004). Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry 161:414–425. [DOI] [PubMed] [Google Scholar]
- Correll CU, Schenk EM (2008). Tardive dyskinesia and new antipsychotics. Curr Opin Psychiatry 21:151–156. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Basu A, Benneyworth M, Balu D, Konopaske G (2012). Glutamatergic synaptic dysregulation in schizophrenia: therapeutic implications. Handb Exp Pharmacol 213:267–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock N, Owen MJ, O’Donovan MC (2006). The catechol-O-methyl transferase (COMT) gene as a candidate for psychiatric phenotypes: evidence and lessons. Mol Psychiatry 11:446–458. [DOI] [PubMed] [Google Scholar]
- Cubells JF, Sun X, LI W, Bonsall RW, Mcgrath JA, Avramopoulos D, et al. (2011). Linkage analysis of plasma dopamine beta-hydroxylase activity in families of patients with schizophrenia. Hum Genet 130:635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande SN, Mathur MN, Das SK, Bhatia T, Sharma S, Nimgaonkar VL (1998). A Hindi version of the diagnostic interview for Genetic Studies. Schizophr Bull 24:489–493. [DOI] [PubMed] [Google Scholar]
- Di Giorgio A, Smith RM, Fazio L, D’Ambrosio E, Gelao B, Tomasicchio A, et al. (2014). DRD2/CHRNA5 interaction on prefrontal biology and physiology during working memory. PLoS One 9:e95997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl A, Reinhard I, Schmitt A, Mann K, Gattaz WF (2009). Does the degree of smoking effect the severity of tardive dyskinesia? A longitudinal clinical trial. Eur Psychiatry 24:33–40. [DOI] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL, Fleiss JL, Cohen J (1976). The Global Assessment Scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry 33:766–771. [DOI] [PubMed] [Google Scholar]
- Fan JB, Zhang CS, Gu NF, Li XW, Sun WW, Wang HY, et al. (2005). Catechol-O-methyltransferase gene Val/Met functional polymorphism and risk of schizophrenia: a large-scale association study plus meta-analysis. Biol Psychiatry 57:139–144. [DOI] [PubMed] [Google Scholar]
- Fervaha G, Foussias G, Agid O, Remington G (2014). Motivational and neurocognitive deficits are central to the prediction of longitudinal functional outcome in schizophrenia. Acta Psychiatr Scand 130:290–299. [DOI] [PubMed] [Google Scholar]
- Fox J, Weisberg S (2011). An R Companion to Applied Regression. Los Angeles, Calif: London SAGE Publications. [Google Scholar]
- Gauderman WJ, Morrison JM (2006). QUANTO 1.1: A computer program for power and sample size calculations for genetic-epidemiology studies [Online]. Available at: http://biostats.usc.edu/Quanto.html [Accessed 13 September 2019]. [Google Scholar]
- Glatt SJ, Faraone SV, Tsuang MT (2003). Association between a functional catechol O-methyltransferase gene polymorphism and schizophrenia: meta-analysis of case-control and family-based studies. Am J Psychiatry 160:469–476. [DOI] [PubMed] [Google Scholar]
- Grossman MH, Emanuel BS, Budarf ML (1992). Chromosomal mapping of the human catechol-O-methyltransferase gene to 22q11.1----q11.2. Genomics 12:822–825. [DOI] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, Bilker WB, Kohler C, Siegel SJ, et al. (2001a). Computerized neurocognitive scanning: II. The profile of schizophrenia. Neuropsychopharmacology 25:777–788. [DOI] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, Turner TH, Kohler C, et al. (2001b). Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology 25:766–776. [DOI] [PubMed] [Google Scholar]
- Gur RE, Nimgaonkar VL, Almasy L, Calkins ME, Ragland JD, Pogue-Geile MF, et al. (2007). Neurocognitive endophenotypes in a multiplex multigenerational family study of schizophrenia. Am J Psychiatry 164:813–819. [DOI] [PubMed] [Google Scholar]
- GUY W (1976). Early Clinical Drug Evaluation Unit (ECDEU) Assessment Manual for Psychopharmacology. 534–537. [Google Scholar]
- Hawkins DM, Weisberg S (2017). Combining the box-cox power and generalised log transformations to accommodate nonpositive responses in linear and mixed-effects linear models South African Statistics J 51:317–328. [Google Scholar]
- Howes OD, Kapur S (2009). The dopamine hypothesis of schizophrenia: version III – the final common pathway. Schizophr Bull 35:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliuzhna M, Kirschner M, Carruzzo F, Hartmann-Riemer MN, Bischof M, Seifritz E, et al. (2020). Clinical, behavioural and neural validation of the PANSS amotivation factor. Schizophr Res.pii: S0920–9964(20)30225–5. [DOI] [PubMed] [Google Scholar]
- Kang SG, Choi JE, Park YM, Lee HJ, Han C, Kim YK, et al. (2008). Val158Met polymorphism in the catechol-O-methyltransferase (COMT) gene is not associated with tardive dyskinesia in schizophrenia. Neuropsychobiology 57:22–25. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA (1987). The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 13: 261–276. [DOI] [PubMed] [Google Scholar]
- Kuhn M, Johnson K (2013). Applied predictive modeling 55–56. [Google Scholar]
- Kukshal P, Bhatia T, Bhagwat AM, Gur RE, Gur RC, Deshpande SN, et al. (2013). Association study of neuregulin-1 gene polymorphisms in a North Indian schizophrenia sample. Schizophr Res 144:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman HM, Morrow B, Shprintzen R, Veit S, Parsia SS, Faedda G.,et al. (1996a). Association of codon 108/158 catechol-O-methyltransferase gene polymorphism with the psychiatric manifestations of velo-cardio-facial syndrome. Am J Med Genet 67:468–472. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM (1996b). Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics 6:243–250. [DOI] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I, Taskinen J (1995). Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry 34:4202–4210. [DOI] [PubMed] [Google Scholar]
- Matsumoto C, Shinkai T, Hori H, Ohmori O, Nakamura J (2004). Polymorphisms of dopamine degradation enzyme (COMT and MAO) genes and tardive dyskinesia in patients with schizophrenia. Psychiatry Res 127:1–7. [DOI] [PubMed] [Google Scholar]
- Maxwell ME (1992). Family Interview for Genetic Studies (FIGS): a manual for FIGS. Bethesda, MD: Clinical Neurogenetics Branch, Intramural Research Program, National Institute of Mental Health. [Google Scholar]
- Mehta MA, Riedel WJ (2006). Dopaminergic enhancement of cognitive function. Curr Pharm Des 12:2487–2500. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Duncan GE, Marx CE, Lieberman JA (2004). Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Molecular Psychiatry 10:79. [DOI] [PubMed] [Google Scholar]
- Moyer RA, Wang D, Papp AC, Smith RM, Duque L, Mash DC, Sadee W. (2011). Intronic polymorphisms affecting alternative splicing of human dopamine D2 receptor are associated with cocaine abuse. Neuropsychopharmacology 36:753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, Belforte JE (2012). GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology 62:1574–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton N, Kirov G, Zammit S, Jones G, Jones S, Owen R, et al. (2002). Schizophrenia and functional polymorphisms in the MAOA and COMT genes: no evidence for association or epistasis. Am J Med Genet 114:491–496. [DOI] [PubMed] [Google Scholar]
- Nurnberger JIJr Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, et al. (1994). Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH genetics initiative. Arch Gen Psychiatry 51:849–859; discussion 863–4. [DOI] [PubMed] [Google Scholar]
- O’Brien A (2016). Comparing the risk of tardive dyskinesia in older adults with first-generation and second-generation antipsychotics: a systematic review and meta-analysis. Int J Geriatr Psychiatry 31:683–693. [DOI] [PubMed] [Google Scholar]
- Okochi T, Ikeda M, Kishi T, Kawashima K, Kinoshita Y, Kitajima T, et al. (2009). Meta-analysis of association between genetic variants in COMT and schizophrenia: an update. Schizophr Res 110:140–148. [DOI] [PubMed] [Google Scholar]
- Pawel K, Hauser J, Skibinska M, Szczepankiewicz A, Dmitrzak-Weglarz M, Gorzkowska K, et al. (2010). Family based association study of DRD1, DRD2, DRD3, DRD4, DAT, COMT gene polymorphism in schizophrenia. Psychiatr Pol 44:405–413. [PubMed] [Google Scholar]
- Porcelli S, Drago A, Fabbri C, Gibiino S, Calati R, Serretti A (2011). Pharmacogenetics of antidepressant response. J Psychiatry Neurosci 36:87–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. (2007). PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia working group of the psychiatric genomics consortium (2014). Biological insights from 108 schizophrenia-associated genetic loci. Nature 511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schooler NR, Kane JM (1982). Research diagnoses for tardive dyskinesia. Arch Gen Psychiatry 39:486–487. [DOI] [PubMed] [Google Scholar]
- Seeman P, Ko F, Jack E, Greenstein R, Dean B (2007). Consistent with dopamine supersensitivity, RGS9 expression is diminished in the amphetamine-treated animal model of schizophrenia and in postmortem schizophrenia brain. Synapse 61:303–309. [DOI] [PubMed] [Google Scholar]
- Seeman P, Weinshenker D, Quirion R, Srivastava LK, Bhardwaj SK, Grandy DK, et al. (2005). Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis. Proc Natl Acad Sci USA 102:3513–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava V, Varma PG, Prasad S, Semwal P, Nimgaonkar VL, Lerer B, et al. (2006). Genetic susceptibility to tardive dyskinesia among schizophrenia subjects: IV. Role of dopaminergic pathway gene polymorphisms. Pharmacogenet Genomics 16:111–117. [DOI] [PubMed] [Google Scholar]
- Sun Z, Zhang Z, Mao P, Ma Y, Li W, Li J, et al. (2018). Association between COMT gene polymorphisms, clinical symptoms, and cognitive functions in Han Chinese patients with schizophrenia. Psychiatr Genet 28:47–54. [DOI] [PubMed] [Google Scholar]
- Tai CH, Wu RM. (2002). Catechol-O-methyltransferase and Parkinson’s disease. Acta Med Okayama 56:1–6. [DOI] [PubMed] [Google Scholar]
- Thelma B, Srivastava V, Tiwari AK, (2008). Genetic underpinnings of tardive dyskinesia: passing the baton to pharmacogenetics. Pharmacogenomics 9:1285–1306. [DOI] [PubMed] [Google Scholar]
- Tiwari AK, Deshpande SN, Lerer B, Nimgaonkar VL, Thelma BK (2007). Genetic susceptibility to tardive dyskinesia in chronic schizophrenia subjects: V. Association of CYP1A2 1545 C>T polymorphism. Pharmacogenomics J 7:305–311. [DOI] [PubMed] [Google Scholar]
- Tiwari AK, Deshpande SN, Rao AR, Bhatia T, Lerer B, Nimgaonkar VL, Thelma BK (2005a). Genetic susceptibility to tardive dyskinesia in chronic schizophrenia subjects: III. Lack of association of CYP3A4 and CYP2D6 gene polymorphisms. Schizophr Res 75:21–26. [DOI] [PubMed] [Google Scholar]
- Tiwari AK, Deshpande SN, Rao AR, Bhatia T, Mukit SR, Shriharsh V, et al. (2005b). Genetic susceptibility to tardive dyskinesia in chronic schizophrenia subjects: I. Association of CYP1A2 gene polymorphism. Pharmacogenomics J 5:60–69. [DOI] [PubMed] [Google Scholar]
- Usiello A, Baik JH, Rouge-Pont F, Picetti R, Dierich A, Lemeur M, et al. (2000). Distinct functions of the two isoforms of dopamine D2 receptors. Nature 408:199–203. [DOI] [PubMed] [Google Scholar]
- Wallwork RS, Fortgang R, Hashimoto R, Weinberger DR, Dickinson D (2012). Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr Res 137:246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Liu ZL, Chen B (2001). Association study of dopamine D2, D3 receptor gene polymorphisms with motor fluctuations in PD. Neurology 56:1757–1759. [DOI] [PubMed] [Google Scholar]
- Weinshilboum RM, Otterness DM, Szumlanski CL (1999). Methylation pharmacogenetics: catechol O-methyltransferase, thiopurine methyltransferase, and histamine N-methyltransferase. Annu Rev Pharmacol Toxicol 39:19–52. [DOI] [PubMed] [Google Scholar]
- Williams HJ, Glaser B, Williams NM, Norton N, Zammit S, Macgregor S, et al. (2005). No association between schizophrenia and polymorphisms in COMT in two large samples. Am J Psychiatry 162:1736–1738. [DOI] [PubMed] [Google Scholar]
- Wobbrock JO, Findlater L, Gergle D, Higgins JJ (2011). The Aligned Rank Transform for nonparametric factorial analyses using only ANOVA procedures. Proceedings of the ACM Conference on Human Factors in Computing Systems (CHI ‘11); 7–12 May 2011; Vancouver, BC, Canada; 143–146. [Google Scholar]
- Woods SW, Morgenstern H, Saksa JR, Walsh BC, Sullivan MC, Money R, et al. (2010). Incidence of tardive dyskinesia with atypical versus conventional antipsychotic medications: a prospective cohort study. J Clin Psychiatry 71:463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zai CC, Tiwari AK, Muller DJ, De Luca V, Shinkai T, Shaikh S, et al. (2010). The catechol-O-methyl-transferase gene in tardive dyskinesia. World J Biol Psychiatry 11: 803–812. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Yu YQ, Sun S, Zhang X, Li W, Xiu MH, et al. (2011). Smoking and tardive dyskinesia in male patients with chronic schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 35:1765–1769. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bertolino A, Fazio L, Blasi G, Rampino A, Romano R, et al. (2007). Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc Natl Acad Sci USA 104:20552–20557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Lindpaintner K, Che R, He Z, Wang P, Yang P, et al. (2009). The Val/Met functional polymorphism in COMT confers susceptibility to bipolar disorder: evidence from an association study and a meta-analysis. J Neural Transm (Vienna) 116:1193–1200. [DOI] [PubMed] [Google Scholar]
- Zhu G, Lipsky RH, Xu K, Ali S, Hyde T, Kleinman J, et al. (2004). Differential expression of human COMT alleles in brain and lymphoblasts detected by RT-coupled 5′ nuclease assay. Psychopharmacology (Berl) 177:178–184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


