Abstract
Objectives
Treatment of respiratory infections with non-typeable Haemophilus influenzae (NTHi) in COPD patients is complicated by biofilm formation, protecting the bacteria against the hosts’ immune response and antibiotics. We investigated the antibiofilm and antibacterial effects of the alginate polymer OligoG, alone or combined with ampicillin or ciprofloxacin, on mature NTHi biofilms.
Materials and methods
Two unrelated COPD strains with PBP3-mediated β-lactam resistance, with additional TEM-1 β-lactamase (Hi-022) or quinolone resistance due to altered GyrA and ParC (Hi-072) were used. Antibiofilm and antibacterial effects were assessed macroscopically, by measurement of biofilm biomass (OD), and by viable cell counts, with determination of minimum biofilm inhibitory concentration (MBIC) and the novel parameter ‘minimum concentration for 2 log10 drop in viable cells in biofilm’ (MB2LDC). Drug interactions between OligoG and antibiotics were assessed by comparing expected and observed inhibitory effects (percent inhibition of no-treatment control) of combined treatment.
Results
OligoG had dose-dependent biofilm disruptive abilities and a weak inhibitory effect on viable cells. Combination with OligoG (64 g/L) significantly lowered MBIC for ampicillin (both strains) and MB2LDC for ciprofloxacin (Hi-022). For Hi-022, there was significant synergism between OligoG and both antibiotics. For Hi-072, interactions were subtle, but a tendency in direction of antagonism was significant at two concentrations of ciprofloxacin.
Conclusions
OligoG shows promise as a potential adjuvant to antibiotics in NTHi infections, but strain-specific factors appear to affect drug interactions and may lead to antagonism. More research is needed to clarify the mechanisms of action of OligoG and interactions with antibiotics.
Introduction
COPD was the third leading cause of death in humans worldwide in 2020.1,2 Non-typeable Haemophilus influenzae (NTHi), a major Gram-negative pathogen massively contributing to global morbidity, mortality and antibiotic usage, is the most frequent bacterial cause of lower respiratory tract infections in COPD patients and contributes to almost half of infectious exacerbations.3–5 The worldwide spread of transferable β-lactamases5,6 forced a switch from ampicillin to β-lactamase-stable β-lactams such as cephalosporins as recommended empirical therapy in severe NTHi infections.7 Since the year 2000, NTHi strains resistant to cefotaxime and other extended-spectrum cephalosporins have emerged in several geographical regions and constitute approximately half of the isolates in Japan,5,6 and dissemination of MDR strains with co-resistance to clinically important non-β-lactams such as quinolones, trimethoprim/sulfamethoxazole, tetracyclines and/or macrolides restrict alternative therapeutic options.6,8,9
The ability of NTHi to rapidly form biofilm is a central part of pathogenesis in COPD and complicated respiratory tract infections.2,10–12 The extracellular matrix (ECM) of NTHi biofilm contains large amounts of double-stranded DNA (dsDNA),13 as well as virulence determinants that facilitate adherence, tissue invasion, and evasion of the host’s immune system, such as lipooligosaccharides (LOSs), high molecular weight (HMW) adhesin protein, Hap protein and IgA proteases.2,13–17 It has been suggested that intra- and paracellular communities of NTHi may serve as seeds for recurrent and chronic infections, which may explain reports of antimicrobial therapy failure despite in vitro susceptibility.2,15,18 Notably, NTHi biofilm formation is promoted by subinhibitory concentrations of β-lactam antibiotics.19,20 Whereas biofilm protects NTHi against β-lactams and other antibiotics,18,20–22 ECM destabilizers (e.g. EDTA and DNase) can restore the efficacy of antibiotics against NTHi in biofilm in vitro.23 Biofilm destabilizers and inhibitors may therefore represent a novel approach to prevention and treatment of respiratory NTHi infections in COPD patients and other vulnerable patient groups.12
Alginate oligosaccharides (AOSs) are water-soluble, biologically active substances with demonstrated anti-inflammatory and antimicrobial effects.12,24–26 One of the most promising AOSs against biofilm infections is ‘OligoG’ (OligoG CF5/20), a low-toxic chelator with antibacterial, antibiofilm and antibiotic-potentiating effects against MDR strains of several Gram-negative species within the family Enterobacteriaceae and the genera Pseudomonas, Acinetobacter and Burkholderia.12,25 The exact mechanisms of action of OligoG on bacterial biofilm is not known, but current understanding was recently summarized by Hills et al.27 OligoG has orphan drug designation from the EMA and the FDA and is documented safe for human clinical use.28,29 Several Phase II clinical trials evaluating use of the substance in cystic fibrosis are completed or ongoing (www.ClinicalTrials.gov), and a recent study concluded that inhalation therapy with OligoG may have some effect in reducing Burkholderia spp. infection in cystic fibrosis.28
We are not aware of previous studies on the effects of OligoG or other AOSs on H. influenzae, and AOSs have to our knowledge not been evaluated for clinical use in COPD patients. This study aimed to investigate the in vitro antibiofilm and antibacterial effects of OligoG on mature NTHi biofilm, alone and in the presence of antibiotics (ampicillin or ciprofloxacin), with particular emphasis on drug interactions (synergism or antagonism).
Materials and methods
OligoG
OligoG (OligoG CF-5/20) was obtained from the manufacturer (AlgiPharma AS, Sandvika, Norway). The active ingredient, with the formula (NaC6H7O6)n is derived from the marine brown alga Laminaria hyperborea and has an average polymeric length of 18, a molecular weight of 3200 g/mol, an alpha-L-guluronic acid content of 85%, and a β-d-mannuronic acid content of 15%.24–26,30 For use in this study, spray dry powder was dissolved in sterile brain heart infusion (BHI) broth using magnetic rotation to obtain a homogenic 5% stock solution, which was diluted in sterile BHI to 2%, 1% and 0.5% (corresponding to 64, 32 and 16 g/L, respectively) for use in the experiments.
Bacterial strains
Strains Hi-022 and Hi-072 were obtained from a collection of well-characterized, anonymized clinical isolates of H. influenzae from a nationwide molecular epidemiologic study (D. Skaare, unpublished data). The two strains were selected based on clinical data (sampled from COPD patients), different phylogenetic lineages and phenotypically expressed resistance mechanisms towards quinolones31 and/or β-lactams (Table 1). Upon inclusion, the strains were characterized by antimicrobial susceptibility testing, including determination of MIC according to recommendations from EUCAST, and by WGS (Ion S5 XL, Thermo Fisher Scientific) with subsequent bioinformatic analyses (detailed in Table 1).
Table 1.
Characteristics of the H. influenzae strains used in the experiments
| Resistance mechanismsc | MIC (mg/L) and susceptibility categoryf | |||||||
|---|---|---|---|---|---|---|---|---|
| Strain | Source | Serotypea | STb | β-Lactams | Quinolones | Ampicillin | Ampicillin/sulbactam | Ciprofloxacin |
| Hi-022 | Sputum from patient with COPD | Non-typeable | ST836 | TEM-1 β-lactamase Group III(+) high-rPBP3d |
None | >16 (R) | 8 (R) | ≤0.06 (S) |
| Hi-072 | Sputum from patient with COPD | Non-typeable | ST1 | Group III(+) high-rPBP3d | Altered GyrA and ParCe | 2 (R) | 2 (R) | 1 (R) |
Using hicap v.1.0.3 (https://github.com/scwatts/hicap).
Transferable genes were detected using ResFinder v.4.1 (https://cge.food.dtu.dk/services/ResFinder); alterations in chromosomal genes were detected by multiple sequence alignment of translated genes, with H. influenzae Rd KW20 (GCA_000027305) as reference.
Characterized by the S385T, L389F and N526K substitutions in the transpeptidase region of PBP3.6
Substitutions in the QRDRs of GyrA (S84L) and ParC (S84I) present.31
MICs were determined by broth microdilution according to recommendations from EUCAST (https://www.eucast.org/ast_of_bacteria), using custom MIC panels (Sensititre NONAG7, Thermo Fisher Scientific), and interpreted (S, susceptible; R, resistant) according to the most recent version of the EUCAST breakpoint table (version 13.0).
Biofilm formation and antimicrobial challenge assay
A biofilm assay suitable for assessment of antibacterial and antibiofilm effects was established based on methods described in previous studies of NTHi biofilms, with some modifications.10,23,32–36 Frozen cultures (one pellet) were used to inoculate 5 mL of preheated BHI broth (Oxoid, Thermo Fisher Scientific) supplemented with 2 mg/L NAD (BioNor Laboratories AS) and 10 mg/L haemin (Sigma–Aldrich) (sBHI) and incubated at 35°C in 5% CO2 for 24 h.19,23,32,33,36 Cultures were diluted 1:10 in 5 mL of fresh sBHI to 1 McFarland (approximately 3 × 108 cfu/mL) and incubated statically for approximately 2 h to reach an OD at 600 nm (OD600) of 0.3 by spectrophotometry (GeneQuant 1300, GE Healthcare). For biofilm formation, 125 µL of culture and 100 µL of preheated sBHI were added to each well of a round-bottom, polystyrene, 96-well microtitre plate (Greiner Bio-One GmbH). The plate was incubated statically for 40 h at 35°C in 5% CO2, with careful replacement of broth with fresh preheated sBHI broth after 16–18 h.32,35 Finally, the biofilms were gently washed twice in sterile water to remove planktonic cells.23,37
Mature biofilms were treated (24 h) with OligoG at concentrations of 16, 32 and 64 g/L, and with ampicillin or ciprofloxacin at concentrations corresponding to three to four 2-fold dilutions centred around the respective MICs. In addition, mature biofilms were challenged with OligoG (fixed concentration of 64 g/L) in combination with ampicillin or ciprofloxacin, using the same antibiotic concentrations as described above. The OligoG concentrations were chosen based on an in vivo animal study showing a kill effect on Pseudomonas aeruginosa in mouse lungs of 1–2 log10 at concentrations 0.5%–3%.38
After treatment, biomasses of each biofilm were determined spectrophotometrically (OD600) by thoroughly scraping and pipetting, followed by vigorous resuspension in 5 mL of sBHI, as described by Dawe et al.32 (with modifications). For quantification of viable cells, the suspensions were diluted to 10−8 and seeded quantitatively on Colombia agar plates (BBL Colombia agar, Becton Dickinson) with 15 mg/L NAD and 15 mg/L haemin.8 The agar plates were incubated at 35°C in 5% CO2 for 48 h, with subsequent determination of cfu/mL by colony counts.
In each challenge experiment (OligoG, ampicillin, ciprofloxacin, OligoG/ampicillin and OligoG/ciprofloxacin), two sets of biofilm triplicates were included as pretreatment control and no-treatment control, with quantification of biofilm biomass and viable cells at 40 h (before treatment) and 64 h, respectively.32,39
The five challenge experiments were performed using different biological replicates (on separate days), each with three technical replicates. Fifteen replicates of control biofilms (five biological and three technical) were used to assess the relative contribution of biological and technical factors to variability in viable cell counts.
Assessment of antibiofilm and antibacterial effects of OligoG and/or antibiotics on mature biofilm
Antibiofilm effects were assessed by inspection for macroscopically visible changes in appearance and changes in biofilm biomass by measurement of OD600 after exposure to OligoG and/or antibiotics, compared with no treatment.
Antibacterial effects were assessed by determination of minimum biofilm inhibitory concentration (MBIC), the novel parameter ‘minimum concentration for 2 log10 drop in viable cells in biofilm’ (designated ‘MB2LDC’) and biofilm bactericidal concentration (BBC). MBIC was defined as the concentration of tested substance resulting in no increase in viable biofilm cells, while MB2LDC (this study) and BBC were defined as the concentrations resulting in ≥2 log10 and ≥3 log10 drop in viable biofilm cells, respectively, compared with no treatment.40 The rationale for the novel parameter MB2LDC is that a 2 log10 drop is a widely used pharmacodynamic parameter in time–kill studies and also corresponds to the clinical effect of amoxicillin and ciprofloxacin.41–43 For combined treatment with OligoG (64 g/L) and antibiotics, MBIC, MB2LDC and BBC values were expressed as the antibiotic concentration. For comparison of antibacterial effects of individual antibiotic treatment and combined antibiotic/OligoG treatment, we calculated ratios of MBIC, MB2LDC and BBC by antibiotic treatment to the same parameters by combined treatment. Ratios of ≥8 were considered significant, corresponding to the definition of synergy by determination of MIC:MIC ratio (3 or more 2-fold dilutions).44
For a more sophisticated assessment of interactions between OligoG and antibiotic (synergism or antagonism), we compared expected and observed inhibitory effects of combined treatment on viable cells, expressed as percent change in viable cells (log10 cfu/mL) compared with no-treatment control. The Bliss independence approach was used to calculate expected inhibitory effects at each antibiotic concentration, using the formula I1,2 = I1 + I2 − I1I2 where I1 and I2 are the inhibitory effects by individual treatments with drug 1 (OligoG) and 2 (antibiotic), respectively, I1,2 represents the expected inhibitory effect of combined treatment, and I1I2 represents the product of I1 and I2.45,46 Mean inhibitory effects by individual treatments (I1 and I2) (three technical replicates) were used as input in the formula to calculate the expected inhibitory effect of combined treatment (I1,2). The expected effect was compared with the observed inhibitory effect of combined treatment, calculated separately for each of the three technical replicates, with subsequent calculation of means and determination of 95% CI. Significant synergism was defined as ‘observed effect (lower 95% CI) > expected effect’ while significant antagonism was defined as ‘observed effect (upper 95% CI) < expected effect’.46
Statistical analysis
Excel (Microsoft Corporation, Redmond, WA, USA) was used to organize, analyse and visualize the data. Assessment of antibiofilm and antibacterial effects was based on calculated means (OD600 and mean log10 cfu/mL, respectively) from triplicate testing. The relative contribution of biological and technical factors to variability in viable cell counts (log10 cfu/mL) in the biofilm assay was assessed by comparing mean coefficients of variation (CV) for technical replicates with CV across five biological replicates of each strain. Normal distribution was assessed using Q-Q Plot in SPSS Statistics 28 (IBM, Armonk, NY, USA). Assessing significance of drug interaction was done by comparing mean expected inhibitory effect of combined treatment with mean observed inhibitory effect and 95% CI (described in detail above). CIs were determined assuming Student’s t-distribution to account for the low sample size (triplicates) and the lack of biological replicates in the challenge experiments.
Ethics
All personal data used in this study were anonymized. The study strains were recruited from a study (D. Skaare, unpublished data) approved by the Regional Committees for Medical and Health Research Ethics in Norway (reference number 2018/1558) and the Norwegian Data Protection Services (reference number 232381).
Data availability
Genomic sequences for strains Hi-022 and Hi-072 are deposited at https://www.ebi.ac.uk/ena under BioProject PRJEB49398, accession numbers GCA_923256855 and GCA_923258785, respectively.
Results
Biofilm assay variability
For both strains, viable cell counts (log10 cfu/mL) from the 15 replicates (5 biological and 3 technical) of untreated biofilms (no-treatment control) were normally distributed (data not shown). Variability between technical replicates (mean CV 3.6% for both strains) was comparable to the variability between biological replicates (CV 4.4% and 3.0% for Hi-022 and Hi-072, respectively).
Antibiofilm effects of individual treatment of biofilms with OligoG, ampicillin or ciprofloxacin
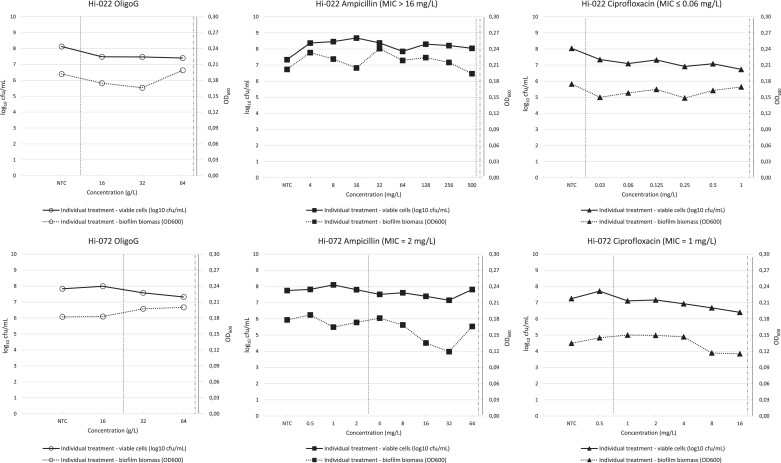
OligoG was found to have a concentration-dependent antibiofilm effect on mature biofilm from both strains, assessed by macroscopic inspection (not shown). Compared with the no-treatment control, treated biofilms were more dispersed and granulated, and less sheetlike. In contrast, there were no noticeable macroscopic differences between no treatment and treatment with ampicillin or ciprofloxacin. There were no clear dose-dependent associations between exposure to OligoG or antibiotics and changes in biofilm biomass at the tested concentrations (Figure 1, dotted lines).
Figure 1.
Antibacterial and antibiofilm effects of individual treatment of mature biofilms with OligoG, ampicillin or ciprofloxacin. Antibacterial effect was measured as change in viable cells (log10 cfu/mL, left axis), while antibiofilm effect was measured as change in biofilm biomass (OD600, right axis). All values are means of three technical replicates. NTC, no-treatment control. Vertical lines indicate MBIC (dashed), MB2LDC (dotted/dashed) and BBC by individual treatment (summarized in Table 2; see text for definitions). Vertical axis scales are harmonized between plots for easier comparison.
Antibacterial effects of individual treatment of biofilms with OligoG, ampicillin or ciprofloxacin
Antibacterial effects of individual treatments of mature biofilms with OligoG or antibiotics (expressed as changes in log10 cfu/mL) are shown in Figure 1. The corresponding MBIC, MB2LDC and BBC values (indicated by vertical lines) are summarized in Table 2. There was a weak concentration-dependent antibacterial effect of OligoG on both strains, with MBIC values of ≤16 g/L (Hi-022) and 32 g/L (Hi-072). At the concentration used for combined treatment (64 g/L), the inhibitory effects of OligoG on the two strains (calculated as percent inhibition of viable cells compared with no-treatment control) were 8.9% and 6.5%, respectively (not shown). The tested concentration ranges did not allow determination of exact antibiotic MBICs for strain Hi-022. For strain Hi-072, the ampicillin MBIC was one 2-fold dilution above MIC, whereas the ciprofloxacin MBIC was identical to the corresponding MIC value (Figure 1). All MB2LDC and BBC values exceeded the tested concentration ranges, i.e. a 2 log10 drop in cfu/mL compared with the no-treatment control was not achieved for any of the tested strain/drug combinations.
Table 2.
Antibacterial activity of individual or combined treatment with OligoG, ampicillin and/or ciprofloxacin on H. influenzae in mature biofilms
| Ampicillin (mg/L) alone or combined with OligoG (64 g/L) |
Ciprofloxacin (mg/L) alone or combined with OligoG (64 g/L) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Strain | Parameter | OligoG (g/L) | Individual treatment | Combined treatment | Ratiob | Individual treatment | Combined treatment | Ratiob |
| Hi-022 | MBIC | ≤16 | >500a | ≤4 | ≥125* | ≤0.03 | ≤0.03 | Indeterminate |
| MB2LDC | >64 | >500a | >500 | Indeterminate | >1 | ≤0.03 | ≥32* | |
| BBC | >64 | >500a | >500 | Indeterminate | >1 | 0.5 | ≥2 | |
| Hi-072 | MBIC | 32 | 4 | ≤0.5 | ≥8* | 1 | ≤0.5 | ≥1 |
| MB2LDC | >64 | >64 | >64 | Indeterminate | >16 | >16 | Indeterminate | |
| BBC | >64 | >64 | >64 | Indeterminate | >16 | >16 | Indeterminate | |
Asterisk indicates significant decrease (ratio ≥8).44
For technical reasons, the highest concentration tested was 500 mg/L (i.e. deviating from the standardized 2-fold scale)
Ratio of MBIC, MB2LDC or BBC by individual treatment to the same parameter by combined treatment.
Antibiofilm effects of combined treatment of biofilms with OligoG and ampicillin or ciprofloxacin
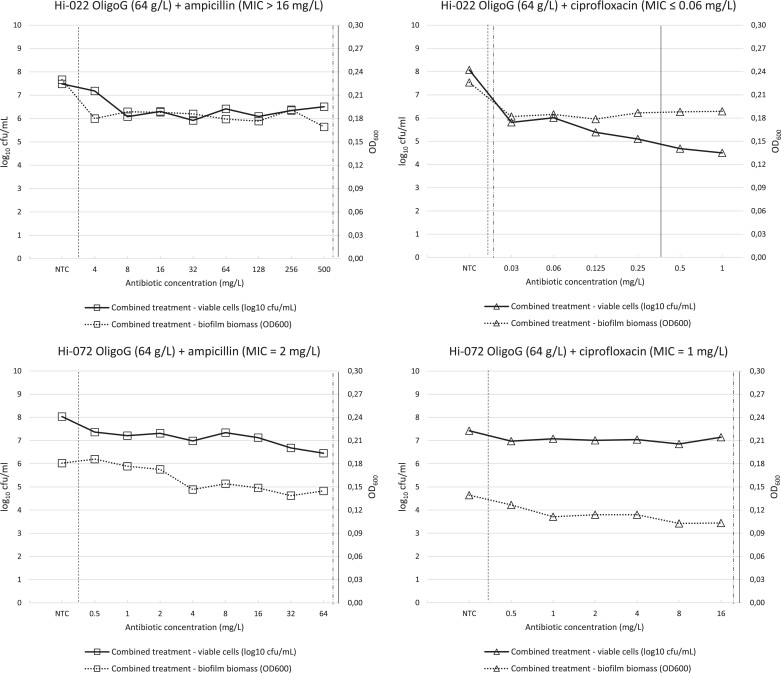
Biofilms treated with combinations of OligoG and antibiotics showed macroscopic changes similar to those observed after individual treatment with OligoG alone (not shown). Compared with no-treatment control, combined treatment resulted in consistently decreased biofilm biomass (as measured by OD600) for all strain/drug combinations (Figure 2, dotted lines).
Figure 2.
Antibacterial and antibiofilm effects of combined treatment of mature biofilms with antibiotic (ampicillin or ciprofloxacin) in combination with 64 g/L OligoG. Antibacterial effect was measured as change in viable cells (log10 cfu/mL, left axis), while antibiofilm effect was measured as change in biofilm biomass (OD600, right axis). All values are means of three technical replicates. NTC, no-treatment control. Vertical lines indicate MBIC (dashed), MB2LDC (dotted/dashed) and BBC by combined treatment (summarized in Table 2; see text for definitions). Vertical axis scales are harmonized between plots for easier comparison.
Antibacterial effects of combined treatment of biofilms with OligoG and ampicillin or ciprofloxacin
Antibacterial effects of combined treatment of mature biofilms with OligoG and antibiotics (expressed as changes in log10 cfu/mL) are shown in Figure 2. The corresponding MBIC, MB2LDC and BBC values (indicated by vertical lines) are summarized in Table 2. For both strains, all MBICs were equal to or lower than the lowest antibiotic concentration tested. Conversely, all MB2LDC and BBC values exceeded the tested concentration ranges, except for strain Hi-022 and OligoG/ciprofloxacin, where a 2 log10 drop (MB2LDC) was obtained with the lowest tested concentration of ciprofloxacin (0.03 mg/L), and a 3 log10 drop (BBC) was obtained with 0.5 mg/L antibiotic. For the β-lactamase-positive strain Hi-022, there was a marked drop in viable cells at the ampicillin concentration corresponding to MIC for ampicillin/sulbactam (8 mg/L) (Figure 2). Compared with individual antibiotic treatment, combined treatment with antibiotic and OligoG gave significantly lower MBIC for ampicillin (both strains) and MB2LDC for ciprofloxacin (Hi-022) (Table 2). For the β-lactamase-positive strain Hi-022, there was a marked drop in viable cells at the ampicillin concentration corresponding to MIC for ampicillin/sulbactam (8 mg/L) (Figure 2).
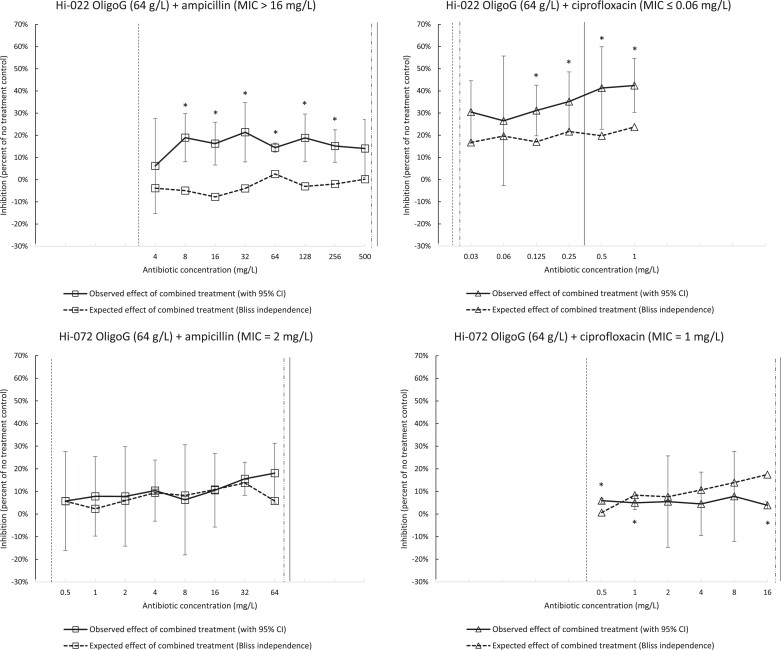
Drug interactions between OligoG and antibiotics
Expected and observed inhibitory effects of combined treatment of mature biofilms with OligoG and antibiotics (expressed as percent inhibition of no-treatment control) are shown in Figure 3. For strain Hi-022, we found significant synergistic effect between OligoG and ampicillin at ampicillin concentrations in the range 8–256 mg/L, and between OligoG and ciprofloxacin at ciprofloxacin concentrations in the range 0.125–1 mg/L. In contrast, there was generally little difference between the expected and observed inhibitory effects of combined treatment for strain Hi-072. For this strain, the observed effect of OligoG combined with ciprofloxacin tended to be weaker than the expected effect (i.e. in the direction of antagonism) at ciprofloxacin concentrations in the range 1–16 mg/L; however, the difference was significant only at two concentrations (1 and 16 mg/L).
Figure 3.
Expected and observed inhibitory effects of combined treatment of mature biofilms with antibiotic (ampicillin or ciprofloxacin) and 64 g/L OligoG. Inhibitory effects are expressed as percent change in viable cells (log10 cfu/mL) compared with no-treatment control. Expected effects (dashed lines) were calculated using the Bliss independence approach (see text for formula) with mean inhibitory effects by individual treatments (three technical replicates) as input. Observed inhibitory effects (full lines) were calculated separately for each of the three technical replicates, with subsequent calculation of means and determination of 95% CI (assuming Student’s t-distribution to account for low sample size). Significant synergism was defined as ‘observed effect (lower 95% CI) > expected effect’ while significant antagonism was defined as ‘observed effect (upper 95% CI) < expected effect’. Significant synergism or antagonism is indicated by asterisks above or below 95% CI, respectively. Vertical lines indicate MBIC (dashed), MB2LDC (dotted/dashed) and BBC by combined treatment (Table 2). Axis scales are harmonized for easier comparison.
Discussion
In this in vitro study, we investigated the potential of the AOS OligoG as a biofilm inhibitor for clinical use in combination with antibiotics in NTHi infections. OligoG has shown promise as adjuvant to several antibiotics for treatment of respiratory biofilm infections caused by a wide range of Gram-negative bacteria.12,30 To the best of our knowledge, the present study is the first investigation of the effects of OligoG on H. influenzae, the first study exploring drug interactions between OligoG and ampicillin, and only the second study performed independently of the manufacturer (the first being Hills et al.27). Using mature NTHi biofilms from two unrelated COPD strains, we found that OligoG inhibited growth of bacteria in biofilm at concentrations ≤16 g/L and 32 g/L (MBIC). We are not aware of previous studies with determination of OligoG MBICs, but Khan et al.30 reported that 64 g/L (2%) OligoG had no inhibitory effect on an Escherichia coli strain in liquid culture, and variable inhibitory effect on Klebsiella pneumoniae strains. Although we did not investigate the activity of OligoG against planktonic bacteria in the present study, these observations suggest that the intrinsic antibacterial activity of OligoG, similar to other antimicrobial agents, may vary between strains and species.
The basis for our selection of antibiotics was that ampicillin is the preferred drug for parenteral treatment of infections with susceptible strains of H. influenzae, while ciprofloxacin is an attractive option for oral treatment of infections caused by ampicillin-resistant strains. Moreover, some studies have suggested that quinolones are more effective than aminopenicillins against NTHi in mature biofilm.19–21 In an in vitro study on NTHi in biofilm, elimination rates of ciprofloxacin and amoxicillin/clavulanic acid were 68% and 3.6%, respectively.21 In contrast, another study found no significant difference between the abilities of ampicillin and ciprofloxacin to kill NTHi in biofilm in vitro.23
A recent report illustrated that susceptibility to ciprofloxacin as determined by conventional antimicrobial susceptibility testing is no guarantee for therapeutic success in complicated respiratory NTHi infections.9 Susceptibility categorization of bacteria based on planktonic bacteria (e.g. MIC) can not be used to predict therapeutic outcome in biofilm infections, and a standardized methodology for susceptibility testing of bacteria in biofilm that correlates to therapeutic outcome is lacking.40,47 MBIC is the biofilm-specific pharmacodynamic parameter that corresponds best to MIC. Although MBIC and MIC are based on different methodologies and therefore not directly comparable, it is generally assumed that the antibiotic concentration representing MBIC is 4- to 1000-fold higher than the corresponding MIC, depending on bug/drug combination.40,48 Interestingly, for strain Hi-072, antibiotic MBICs were similar to (ciprofloxacin) or only one dilution higher (ampicillin) than the corresponding MICs. Our results are in line with previous investigations of the activities of ampicillin and quinolones against NTHi in biofilm,19,49 suggesting that NTHi biofilms may have different properties in terms of antibiotic penetration compared with other species.
MBIC and BBC are often used to express antibiotic effect on bacteria in biofilm.40 Here, the additional novel parameter MB2LDC is introduced as a biofilm-specific parallel to the antibacterial effect measure (2 log10 drop) corresponding to clinical effect in time–kill studies.39–43 We found that MB2LDC for ciprofloxacin/OligoG and strain Hi-022 was significantly lower than MB2LDC for ciprofloxacin alone, and lower than the clinical MIC breakpoint recommended by EUCAST (susceptible ≤ 0.06 mg/L). Similarly, ampicillin/OligoG MBICs were significantly lower than ampicillin MBICs for both strains, and in the case of strain Hi-072 lower than the clinical MIC breakpoint (susceptible ≤ 1 mg/L). These observations suggest that combination with OligoG could increase the probability of therapeutic effect of ampicillin and ciprofloxacin in NTHi biofilm infections.
Current and emerging pharmaceutical strategies for combating respiratory biofilm infections, including combination of antibiotics and non-antibiotic adjuvants was recently reviewed by Zhang et al.12 The authors underlined that the exact mechanism of the antibiofilm effects of OligoG is unclear, but classified OligoG among extracellular matrix-interfering agents with the ability to potentiate a wide range of antibiotics.24–26,30 While studies have shown that OligoG has intrinsic antimicrobial activity24 and/or that antibiotics are more effective combined with OligoG than alone,24–26,30 we have not been able to identify studies describing interactions between OligoG and antibiotics in terms of assessing true synergy, i.e. whether the combined inhibitory effect exceeds the added effect of the two drugs. In the present study, we used the approach described by Prichard et al., based on comparison of expected and observed combined inhibitory effects, with calculation of expected effect based on the Bliss independence approach.46 Unlike the more widely used FIC index (FICI), this approach allows assessment of drug interactions without exact MBIC values for OligoG and all antibiotic/strain combinations.50 Our results suggest that OligoG may act synergistically with both ampicillin and ciprofloxacin against NTHi, but that the effect varies between strains and in some cases may even be antagonistic. Interestingly, for the β-lactamase-producing strain Hi-022, ampicillin/OligoG MBIC was lower than MIC for ampicillin/sulbactam. Further research should clarify whether this reflects a possible interaction between OligoG and β-lactamase activity. Furthermore, synergy was most evident for the strain with the lowest OligoG MBIC, suggesting that lack of synergy may be linked to unidentified, strain-specific OligoG resistance mechanisms. Nevertheless, the synergism observed for strain Hi-022 is to our knowledge the first report of demonstrated synergy between a biofilm inhibitor and antibiotics in H. influenzae, or between OligoG and antibiotics overall. In contrast, a previous investigation (using the FICI approach) found only additive effects between EDTA and ampicillin or ciprofloxacin against NTHi in biofilm.23
This study has several limitations. First, the five challenge experiments were performed with different, single biological replicates. Consequently, whether biological variation between different versions of the strains may have affected the results remains largely unexplored, although assessment of variability among untreated control biofilms suggested that technical factors had a larger impact than biological factors. Second, due to the small sample size in each experiment (three technical replicates), it was not possible to assess whether data were normally distributed. To compensate for these uncertainties, we chose a conservative statistical approach by assuming Student’s t-distribution in our calculations of 95% CI. This may, on the other hand, have led to underreporting of significant drug interactions. Nevertheless, the experimental design does not allow firm conclusions and the results should be interpreted with caution. Furthermore, the number of strains and drug concentration ranges were limited, and broader investigations (additional strains and antibiotics, combined with variable OligoG concentrations) should be undertaken to further explore clinically relevant drug interactions. In conclusion, OligoG shows promise as a potential adjuvant to ampicillin and ciprofloxacin in NTHi infections, but the usefulness in COPD patients must be validated in clinical studies. Importantly, strain-specific factors appear to affect drug interactions and may lead to significant antagonism. More research is therefore needed to clarify the mechanisms of action of OligoG and molecular mechanisms affecting interactions with antibiotics.
Acknowledgements
We thank Hedda Rath, Gunnhild Sommernes, Helene Johannessen Wefring, Mette Lundstrøm Dahl, Gløer Gløersen and Maren Nordsveen Davis for technical assistance, and the Department of Microbiology, Vestfold Hospital Trust, for access to the necessary infrastructure to perform the experimental procedures. We are also grateful to AlgiPharma AS for selling us OligoG CF-5/20 for use in the study.
Contributor Information
Kaja Marienborg, Department of Microbiology, Vestfold Hospital Trust, Tønsberg, Norway; Department of Life Sciences and Health, OsloMet—Oslo Metropolitan University, Oslo, Norway.
Ole Herman Ambur, Department of Life Sciences and Health, OsloMet—Oslo Metropolitan University, Oslo, Norway.
Ole Andreas Løchen Økstad, Section for Pharmacology and Pharmaceutical Biosciences, Department of Pharmacy, University of Oslo, Oslo, Norway.
Dagfinn Skaare, Department of Microbiology, Vestfold Hospital Trust, Tønsberg, Norway.
Funding
This work was supported by grants from the Helse Sør-Øst RHF, Norway (grant number 2020107), the regional research network ‘Turning the Tide of Antibiotic Resistance’ (des-2020), the Norwegian Surveillance System for Antimicrobial Drug Resistance (NORM) (19_07), Sykehuset i Vestfold (04.02.2021) and Storbyuniversitetet (OsloMet) (Forskerlinjen-2021).
Transparency declarations
All authors has stated they have none to declare. And AlgiPharma AS was not involved in funding, designing, or performing the study, nor in the analysis, interpretation, or publication of data.
References
- 1. WHO . Chronic obstructive pulmonary disease (COPD). https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd).
- 2. Weeks JR, Staples KJ, Spalluto CMet al. . The role of non-typeable Haemophilus influenzae biofilms in chronic obstructive pulmonary disease. Front Cell Infect Microbiol 2021; 11: 720742. 10.3389/fcimb.2021.720742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Su YC, Jalalvand F, Thegerström Jet al. . The interplay between immune response and bacterial infection in COPD: focus upon non-typeable Haemophilus influenzae. Front Immunol 2018; 9: 2530. 10.3389/fimmu.2018.02530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahearn CP, Gallo MC, Murphy TF. Insights on persistent airway infection by non-typeable Haemophilus influenzae in chronic obstructive pulmonary disease. Pathog Dis 2017; 75: ftx042. 10.1093/femspd/ftx042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van Eldere J, Slack MP, Ladhani Set al. . Non-typeable Haemophilus influenzae, an under-recognised pathogen. Lancet Infect Dis 2014; 14: 1281–92. 10.1016/S1473-3099(14)70734-0 [DOI] [PubMed] [Google Scholar]
- 6. Skaare D, Anthonisen IL, Kahlmeter Get al. . Emergence of clonally related multidrug resistant Haemophilus influenzae with penicillin-binding protein 3-mediated resistance to extended-spectrum cephalosporins, Norway, 2006 to 2013. Euro Surveill 2014; 19: 20986. 10.2807/1560-7917.ES2014.19.49.20986 [DOI] [PubMed] [Google Scholar]
- 7. Tristram S, Jacobs MR, Appelbaum PC. Antimicrobial resistance in Haemophilus influenzae. Clin Microbiol Rev 2007; 20: 368–89. 10.1128/CMR.00040-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johannessen H, Anthonisen IL, Zecic Net al. . Characterization and fitness cost of Tn7100, a novel integrative and conjugative element conferring multidrug resistance in Haemophilus influenzae. Front Microbiol 2022; 13: 945411. 10.3389/fmicb.2022.945411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lindemann PC, Mylvaganam H, Oppegaard Oet al. . Case report: whole-genome sequencing of serially collected Haemophilus influenzae from a patient with common variable immunodeficiency reveals within-host evolution of resistance to trimethoprim-sulfamethoxazole and azithromycin after prolonged treatment with these antibiotics. Front Cell Infect Microbiol 2022; 12: 896823. 10.3389/fcimb.2022.896823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baddal B. Characterization of biofilm formation and induction of apoptotic DNA fragmentation by nontypeable Haemophilus influenzae on polarized human airway epithelial cells. Microb Pathog 2020; 141: 103985. 10.1016/j.micpath.2020.103985 [DOI] [PubMed] [Google Scholar]
- 11. Swords WE. Nontypeable Haemophilus influenzae biofilms: role in chronic airway infections. Front Cell Infect Microbiol 2012; 2: 97. 10.3389/fcimb.2012.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang L, Bera H, Wang Het al. . Combination and nanotechnology based pharmaceutical strategies for combating respiratory bacterial biofilm infections. Int J Pharm 2022; 616: 121507. 10.1016/j.ijpharm.2022.121507 [DOI] [PubMed] [Google Scholar]
- 13. Jurcisek JA, Bakaletz LO. Biofilms formed by nontypeable Haemophilus influenzae in vivo contain both double-stranded DNA and type IV pilin protein. J Bacteriol 2007; 189: 3868–75. 10.1128/JB.01935-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Webster P, Wu S, Gomez G 3rdet al. . Distribution of bacterial proteins in biofilms formed by non-typeable Haemophilus influenzae. J Histochem Cytochem 2006; 54: 829–42. 10.1369/jhc.6A6922.2006 [DOI] [PubMed] [Google Scholar]
- 15. Sriram KB, Cox AJ, Clancy RLet al. . Nontypeable Haemophilus influenzae and chronic obstructive pulmonary disease: a review for clinicians. Crit Rev Microbiol 2018; 44: 125–42. 10.1080/1040841X.2017.1329274 [DOI] [PubMed] [Google Scholar]
- 16. Atack JM, Day CJ, Poole Jet al. . The HMW2 adhesin of non-typeable Haemophilus influenzae is a human-adapted lectin that mediates high-affinity binding to 2-6 linked N-acetylneuraminic acid glycans. Biochem Biophys Res Commun 2018; 503: 1103–7. 10.1016/j.bbrc.2018.06.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu S, Baum MM, Kerwin Jet al. . Biofilm-specific extracellular matrix proteins of nontypeable Haemophilus influenzae. Pathog Dis 2014; 72: 143–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Schilfgaarde M, Eijk P, Regelink Aet al. . Haemophilus influenzae localized in epithelial cell layers is shielded from antibiotics and antibody-mediated bactericidal activity. Microb Pathog 1999; 26: 249–62. 10.1006/mpat.1998.0269 [DOI] [PubMed] [Google Scholar]
- 19. Kaji C, Watanabe K, Apicella MAet al. . Antimicrobial effect of fluoroquinolones for the eradication of nontypeable Haemophilus influenzae isolates within biofilms. Tohoku J Exp Med 2008; 214: 121–8. 10.1620/tjem.214.121 [DOI] [PubMed] [Google Scholar]
- 20. Umar NK, Kono M, Sakatani Het al. . Respiratory quinolones can eradicate amoxicillin-induced mature biofilms and nontypeable Haemophilus influenzae in biofilms. J Infect Chemother 2022; 28: 1595–604. 10.1016/j.jiac.2022.07.019 [DOI] [PubMed] [Google Scholar]
- 21. Slinger R, Chan F, Ferris Wet al. . Multiple combination antibiotic susceptibility testing of nontypeable Haemophilus influenzae biofilms. Diagn Microbiol Infect Dis 2006; 56: 247–53. 10.1016/j.diagmicrobio.2006.04.012 [DOI] [PubMed] [Google Scholar]
- 22. Starner TD, Zhang N, Kim Get al. . Haemophilus influenzae forms biofilms on airway epithelia: implications in cystic fibrosis. Am J Respir Crit Care Med 2006; 174: 213–20. 10.1164/rccm.200509-1459OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cavaliere R, Ball JL, Turnbull Let al. . The biofilm matrix destabilizers, EDTA and DNaseI, enhance the susceptibility of nontypeable Hemophilus influenzae biofilms to treatment with ampicillin and ciprofloxacin. Microbiologyopen 2014; 3: 557–67. 10.1002/mbo3.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pritchard MF, Powell LC, Jack AAet al. . A low-molecular-weight alginate oligosaccharide disrupts pseudomonal microcolony formation and enhances antibiotic effectiveness. Antimicrob Agents Chemother 2017; 61: e00762-17. 10.1128/AAC.00762-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hengzhuang W, Song Z, Ciofu Oet al. . OligoG CF-5/20 disruption of mucoid Pseudomonas aeruginosa biofilm in a murine lung infection model. Antimicrob Agents Chemother 2016; 60: 2620–6. 10.1128/AAC.01721-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Powell LC, Pritchard MF, Ferguson ELet al. . Targeted disruption of the extracellular polymeric network of Pseudomonas aeruginosa biofilms by alginate oligosaccharides. NPJ Biofilms Microbiomes 2018; 4: 13. 10.1038/s41522-018-0056-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hills OJ, Yong CW, Scott AJet al. . Polyguluronate simulations shed light onto the therapeutic action of OligoG CF-5/20. Bioorg Med Chem 2022; 72: 116945. 10.1016/j.bmc.2022.116945 [DOI] [PubMed] [Google Scholar]
- 28. Fischer R, Schwarz C, Weiser Ret al. . Evaluating the alginate oligosaccharide (OligoG) as a therapy for Burkholderia cepacia complex cystic fibrosis lung infection. J Cyst Fibros 2022; 21: 821–9. 10.1016/j.jcf.2022.01.003 [DOI] [PubMed] [Google Scholar]
- 29. van Koningsbruggen-Rietschel S, Davies JC, Pressler Tet al. . Inhaled dry powder alginate oligosaccharide in cystic fibrosis: a randomised, double-blind, placebo-controlled, crossover phase 2b study. ERJ Open Res 2020; 6: 00132-2020. 10.1183/23120541.00132-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Khan S, Tondervik A, Sletta Het al. . Overcoming drug resistance with alginate oligosaccharides able to potentiate the action of selected antibiotics. Antimicrob Agents Chemother 2012; 56: 5134–41. 10.1128/AAC.00525-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Georgiou M, Muñoz R, Román Fet al. . Ciprofloxacin-resistant Haemophilus influenzae strains possess mutations in analogous positions of GyrA and ParC. Antimicrob Agents Chemother 1996; 40: 1741–4. 10.1128/AAC.40.7.1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dawe H, Berger E, Sihlbom Cet al. . D-methionine interferes with non-typeable Haemophilus influenzae peptidoglycan synthesis during growth and biofilm formation. Microbiology (Reading) 2017; 163: 1093–104. 10.1099/mic.0.000491 [DOI] [PubMed] [Google Scholar]
- 33. Marti S, Puig C, Merlos Aet al. . Bacterial lysis through interference with peptidoglycan synthesis increases biofilm formation by nontypeable Haemophilus influenzae. mSphere 2017; 2: e00329-16. 10.1128/mSphere.00329-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harrison A, Hardison RL, Wallace RMet al. . Reprioritization of biofilm metabolism is associated with nutrient adaptation and long-term survival of Haemophilus influenzae. NPJ Biofilms Microbiomes 2019; 5: 33. 10.1038/s41522-019-0105-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brockman KL, Azzari PN, Branstool MTet al. . Epigenetic regulation alters biofilm architecture and composition in multiple clinical isolates of nontypeable Haemophilus influenzae. mBio 2018; 9: e01682-18. 10.1128/mBio.01682-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Devaraj A, Buzzo J, Rocco CJet al. . The DNABII family of proteins is comprised of the only nucleoid associated proteins required for nontypeable Haemophilus influenzae biofilm structure. Microbiologyopen 2018; 7: e00563. 10.1002/mbo3.563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vermee Q, Cohen R, Hays Cet al. . Biofilm production by Haemophilus influenzae and Streptococcus pneumoniae isolated from the nasopharynx of children with acute otitis media. BMC Infect Dis 2019; 19: 44. 10.1186/s12879-018-3657-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brown JR, Jurcisek J, Lakhani Vet al. . In silico modeling of biofilm formation by nontypeable Haemophilus influenzae in vivo. mSphere 2019; 4: e00254-19. 10.1128/mSphere.00254-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thieme L, Hartung A, Tramm Ket al. . MBEC versus MBIC: the lack of differentiation between biofilm reducing and inhibitory effects as a current problem in biofilm methodology. Biol Proced Online 2019; 21: 18. 10.1186/s12575-019-0106-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Macia MD, Rojo-Molinero E, Oliver A. Antimicrobial susceptibility testing in biofilm-growing bacteria. Clin Microbiol Infect 2014; 20: 981–90. 10.1111/1469-0691.12651 [DOI] [PubMed] [Google Scholar]
- 41. EUCAST . Amoxicillin: Rationale for the clinical breakpoints, version 1.0. 2010. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Rationale_documents/Amoxicillin_rationale_Nov2010_v_1.0.pdf.
- 42. EUCAST . Ciprofloxacin: Rationale for the clinical breakpoints, version 1.9. 2007. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Rationale_documents/Ciprofloxacin_Rationale_Document_2.0_20210101.pdf.
- 43. EMA . Guideline on the use of pharmacokinetics and pharmacodynamics in the development of antimicrobial medicinal products. 2016. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-use-pharmacokinetics-pharmacodynamics-development-antimicrobial-medicinal-products_en.pdf.
- 44. Doern CD. When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J Clin Microbiol 2014; 52: 4124–8. 10.1128/JCM.01121-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Greco WR, Bravo G, Parsons JC. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev 1995; 47: 331–85. [PubMed] [Google Scholar]
- 46. Prichard MN, Shipman C Jr. A three-dimensional model to analyze drug-drug interactions. Antiviral Res 1990; 14: 181–205. 10.1016/0166-3542(90)90001-N [DOI] [PubMed] [Google Scholar]
- 47. Hoiby N, Bjarnsholt T, Moser Cet al. . ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect 2015; 21Suppl 1: S1–25. 10.1016/j.cmi.2014.10.024 [DOI] [PubMed] [Google Scholar]
- 48. Ciofu O, Moser C, Jensen POet al. . Tolerance and resistance of microbial biofilms. Nat Rev Microbiol 2022; 20: 621–35. 10.1038/s41579-022-00682-4 [DOI] [PubMed] [Google Scholar]
- 49. Kosikowska U, Andrzejczuk S, Plech Tet al. . Inhibitory effect of 1,2,4-triazole-ciprofloxacin hybrids on Haemophilus parainfluenzae and Haemophilus influenzae biofilm formation in vitro under stationary conditions. Res Microbiol 2016; 167: 647–54. 10.1016/j.resmic.2016.05.009 [DOI] [PubMed] [Google Scholar]
- 50. Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 2003; 52: 1. 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Genomic sequences for strains Hi-022 and Hi-072 are deposited at https://www.ebi.ac.uk/ena under BioProject PRJEB49398, accession numbers GCA_923256855 and GCA_923258785, respectively.