ABSTRACT
Background
Vascular calcification (VC) is a common comorbidity among patients with chronic kidney disease (CKD), indicating major cardiovascular events. This study aimed to evaluate the effects and safety of intravenous sodium thiosulphate (STS) for VC in CKD patients.
Methods
Electronic databases were searched for clinical trials that provided data comparing outcomes among patients treated with and without STS. The PRISMA guidelines were followed. Efficacy was assessed using calcification scores and arterial stiffness. Safety was examined by analyzing adverse symptoms, electrolytes and bone mineral density (BMD). Random-effects models were performed. Meta-regression and sensitivity analysis were done. The risk of bias was assessed using the Cochrane tools.
Results
Among the 5601 publications, 6 studies involving 305 participants (mean age: 56 years, male: 56.6%) with all participants on maintenance hemodialysis met eligibility criteria. For efficacy, the progression in Agatston scores in the coronary arteries [107 patients, mean difference (MD): −241.27, 95% confidence interval (95% CI): −421.50 to −61.03] and iliac arteries (55 patients, MD: −382.00, 95% CI: −751.07 to −12.93) was lower in the STS treated group compared with controls. The increase in pulse wave velocity was lower in the STS group (104 patients, MD: −1.29 m/s, 95% CI: −2.24 to −0.34 m/s). No association was found between the change in calcification scores and STS regimen. For safety, gastrointestinal symptoms (e.g. nausea) and increased anion gap acidosis were noted. No reduction in BMD by STS was observed.
Conclusions
Intravenous STS may attenuate the progression of VC and arterial stiffness in hemodialysis patients. Large and well-designed randomized controlled trials are warranted.
Keywords: arterial stiffness, bone mineral density, chronic kidney disease, sodium thiosulphate, vascular calcification
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What is already known about this subject?
Vascular calcification (VC) is widely prevalent in patients with chronic kidney disease and indicative of poor cardiovascular consequences and a shorter lifetime.
There is a dearth of treatment that can reverse or stabilize the progression of VC.
What this study adds?
This systemic review and meta-analysis found that intravenous sodium thiosulphate (STS) may reduce the progression of VC and help ameliorate arterial stiffness in hemodialysis patients.
Gastrointestinal symptoms (e.g. nausea) and increased anion gap acidosis were noted in the trials included, but no prolonged adverse effect was noted after the completion of STS therapy. No change in bone mineral density was found to be caused by STS.
A lack of large and well-conducted randomized control trials (RCTs) was noted.
What impact this may have on practice or policy?
Our findings may prompt the use of intravenous STS in treating VC among hemodialysis patients. Clinicians should be cautious in adverse effects monitoring due to the paucity of evidence in the area.
RCTs with larger populations and higher quality should be conducted in the future.
INTRODUCTION
Vascular calcification (VC) is a strong indicator of stroke, myocardial infarction and cardiovascular (CV) mortality [1, 2]. The incidence of VC in patients with chronic kidney disease (CKD) is 2- to 5-fold that of the age-matched non-CKD population [3]. Inflammation, calcium-phosphate disturbance and uremic toxins could catalyze transformation from vascular smooth muscle cells and adventitial cells to osteoblast-like cells, which eventually results in VC [4]. VC can affect all levels of arteries, valves and heart structures but mainly indicates calcification in macrovascular circulation, primarily consisting of large- and medium-sized arteries (e.g. aorta, coronary artery and iliac artery).
In recent years, several calcification scores [e.g. Kauppila index, Agatston score, calcium volume score (CVS), etc.] have been linked to CV risks. For instance, coronary artery calcification (CAC) score is independently associated with CV consequences (heart failure, sudden cardiac death, stroke, etc.) in the general population, while abdominal aortic calcification score (AACS) has comparable values in patients treated with hemodialysis (HD) [5, 6]. However, there is a dearth of studies examining the reversal or stabilization of VC for CKD patients.
Sodium thiosulphate (STS) presents a plausible option for CKD patients with VC [7]. It was suggested as a potential treatment for calciphylaxis (featured calcification in subcutaneous arterioles and small vessels) in 2004 in a case report and was shown to prevent macrovascular calcification in uremic rats in 2008 [8, 9]. Since 2010, it has been generalized to and tested in patients with VC [10, 11]. STS has been posited to chelate calcium deposits into soluble calcium thiosulphate complexes and has vasodilatory and antioxidant activities [12]. However, severe side effects related to STS treatment have been reported anecdotally in some studies, including severe metabolic acidosis and bone mineral density (BMD) reduction, given its potential role in inhibiting hydroxyapatite formation [13, 14].
We performed a systematic review and meta-analysis to evaluate the efficacy and safety of STS for VC in CKD patients.
MATERIALS AND METHODS
We followed the ‘‘Preferred reporting items for systematic reviews and meta-analyses (PRISMA)’’ guidelines and the recommendations of the Cochrane collaboration to conduct this systematic review and meta-analysis. The protocol was registered and published on PROSPERO (CRD42021235860).
Eligibility criteria
We searched for clinical trials that met the following criteria: (i) included adult patients (≥18 years old) diagnosed with CKD (defined as either kidney damage or a decreased glomerular filtration rate of <60 mL/min/1.73 m2 for at least 3 months [15]); (ii) having VC in macrovascular circulation (e.g. coronary artery, iliac artery and aorta) as the main complication studied and the primary indication for STS treatment; and (iii) included both the patients treated with and without intravenous STS to provide a comparison between intervention and control groups. Studies were excluded if (i) they reported outcomes only from non-intravenous administration of STS (e.g. oral, intra-peritoneal, intra-lesional, etc.) or (ii) the data among CKD patients could not be extracted from the study.
Data sources and search strategy
MEDLINE/PubMed, EMBASE, Cochrane Library, Web of Science and ClinicalTrials.gov were searched using relevant terms and synonyms including ‘‘sodium thiosulphate’’ and ‘‘calci*’’ without language restriction. The controlled vocabulary terms, synonyms and the complete search strategy are listed in Supplementary data, Tables S1 and S2. We contacted the authors of eligible articles to retrieve missing data. Our data search included studies published before the end of August 2021.
Study selection and data collection process
Two authors (W.W. and I.P.-C.) screened the records independently using Endnote X20 to identify eligible studies. From the eligible studies, data were independently extracted by two authors (W.W. and I.P.-C.) regarding characteristics of studies (e.g. trial design, randomization, blinding, etc.), participants (e.g. population, age, gender, sample size, etc.), intervention and outcome measures. Discrepancies among the reviewers were rechecked by a third author (S.U.N.) and discussed to obtain a consensus.
Risk-of-bias assessment
For the studies included, risk of bias was assessed using Cochrane tools. The risk of bias in the randomized control trials (RCTs) was evaluated using ‘‘Revised Cochrane risk-of-bias tool for randomized trials (RoB 2) [16]’’. The risk of bias in the non-RCTs was assessed using ‘‘Risk of bias in non-randomized studies of interventions (ROBINS-I) [17]’’. Two authors (W.W. and I.P.-C.) independently graded the risk of bias in the studies and consulted the third author (S.U.N.) when discrepancies arose.
Outcomes
Targeted outcomes regarding VC were collected and analyzed. For efficacy assessment, calcification scores (Agatston score, CVS and Kauppila index) and arterial stiffness measurements [pulse wave velocity (PWV) and cardio-ankle vascular index (CAVI)] were examined. For safety assessment, adverse symptoms, chronic kidney disease-mineral and bone disorder (CKD-MBD) parameters [calcium, phosphate, intact parathyroid hormone (iPTH) and 25-hydroxyvitamin D3 (25(OH)VitD3)], electrolytes and BMD were studied.
Data synthesis
The results were tabulated and synthesized quantitatively by performing the random-effects model. Mean difference (MD) and standard deviation (SD) of continuous variables (e.g. Agatston score, CVS, PWV, CAVI, electrolytes, etc.) were calculated and synthesized to compute a weighted MD [18]. Hedge's g as the standard mean difference was used when analyzing data from different measurements. All pooled estimates with their 95% confidence intervals (CIs) were displayed. Subgroup analyses regarding different locations of VC and BMD, as well as laboratory tests at different time points, were performed. In each subgroup analysis, difference among the group-specific overall effect size was examined using the Qb test. A sensitivity analysis was performed to test the influence of the statistical model, effect measurements and main outcomes in our study. Meta-regression was performed to examine the impact of dose and duration of STS administration and publication year on VC measurements. Egger's test was used to measure publication bias. Heterogeneity was assessed using the I2 test. An I2 index >50% indicates obvious to high heterogeneity. Stata IC 16 was used for statistical analyses.
RESULTS
Description of the included studies
In total, 5601 publications were retrieved from the targeted databases among which 514 full-texts were screened. Six studies [19–24] (five RCTs and one non-randomized trial) involving 305 participants (mean age: 56 years, male: 56.6%) met our eligibility criteria. The detailed flow diagram of literature search and screening is listed in Fig. 1.
FIGURE 1:
Flow chart of study inclusion.
Characteristics of the six clinical trials, including population, age, gender, dialysis vintage, complicated disease, lab results and medications, are summarized in Table 1 and Supplementary data, Table S3. The six trials [19–24] were all focused on patients treated with HD. None of the patients had complicated calciphylaxis. The STS dosage ranged from 5 to 25 g, during or after dialysis, and was administrated 2–3 times a week. Treatment duration ranged from 3 to 12 months. Medications were documented in five of the six studies. Notably, patients in the five trials only received active vitamin D and calcium-based phosphate binders for iPTH and phosphate control.
Table 1.
Characteristics of the six clinical trials for VC
| Study ID | Study design | Country | Population | No. of participants (T:C) | Age (T versus C) (mean ± SD, years) | Gender (T versus C) | Dialysis vintage (T versus C) | STS treatment | Control treatment | Duration | Outcome measurements |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adirekkiat et al. [19] | Non-randomized trial | Thailand | HD patients (CAC score ≥300) | 16:16 | 59.6 ± 14.4 versus 60.4 ± 11.5 | 11M, 5F versus 8M, 8F | 44.7 ± 33.7a mo versus 47.2 ± 28.5a mo | 12.5 g i.v. twice a week over 15–20 min after HD treatment was completed | Usual care | 4 mo | Agatston score |
| Messa et al. [20] | RCT | Italy | HD patients | 43:43 | NA | NA | NA | 5 g i.v. at the end of each dialysis session | Usual care | 12 mo | Kauppila index |
| Yu et al. [21] | RCT | China | HD patients (CAC score ≥50) | 17:10 | NA | NA | 7 (4–23)b years in the whole population | 0.18 g/kg dissolved in 100 mL saline i.v. 3 times a week for 30 min after every HD session | Usual care | 3 mo | Agatston score |
| Saengpanit et al. [22] | RCT | Thailand | ESRD on HD with CAVI ≥8 | 24:26 | 50.4 ± 9.5 versus 54.4 ± 10.7 | 12M, 12F versus 16M, 10F | 69 (38–110)c mo versus 55 (30–101)c mo | 12.5 g during the last hour of HD twice a week | Usual care | 6 mo | CVS; PWV; CAVI |
| Djuric et al. [23] | RCT | Serbia | ESRD on HD with AACS ≥100 | 30:30 | 63.8 ± 13.2 versus 64.1 ± 9.7 | 17M, 13F versus 21M, 9F | 104.4 ± 80.5a mo versus 103.7 ± 75.1a mo | 25 g/1.73 m2 dissolved in 100 mL saline i.v. during the last 15 min of every HD session | 100 ml of 0.9% saline | 6 mo | Agatston score; CVS; PWV |
| Bian et al. [24] | RCT | China | HD patients without severe infection, diabetes, hypercalcaemia, or iPTH <150 pg/mL | 25:25 | 52.1 ± 20.8 versus 52.5 ± 19.4 | 12M, 13F versus 12M, 13F | 29 (4–101)d mo versus 30 (3–107)d mo | 0.18 g/kg dissolved in 100 mL normal saline three times a week | Usual care | 6 mo | Agatston score; CVS; PWV |
ID: identity; T: treatment group; C: control group; ESRD: end-stage renal disease; M: male; F: female; i.v.: intravenous; mo: months; and NA: not available.
Mean ± SD.
Median (range).
Median (IQR1–IQR3).
Median (P2.5–P97.5).
Efficacy of intravenous STS among CKD patients
Calcification scores
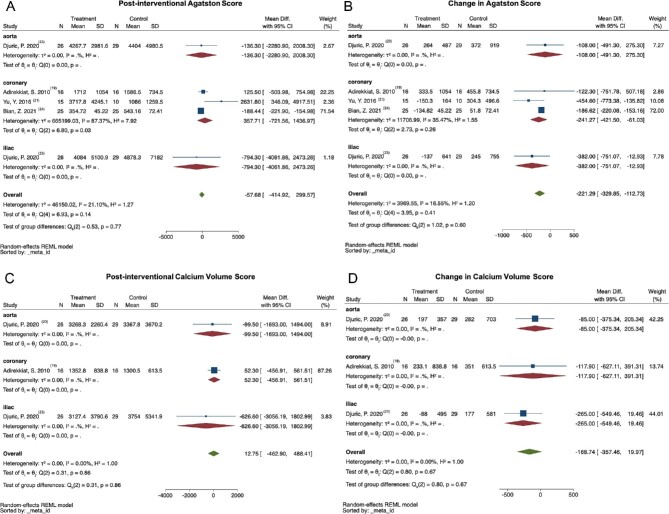
Comparisons of the calcification scores were categorized into subgroups according to the location of their involvement (aorta, coronary artery and iliac artery). Both the post-interventional level and the change of calcification scores from four trials [19, 21, 23, 24] were analyzed (Fig. 2). As displayed in Fig. 2B, the progression of Agatston score for coronary artery (107 patients, MD: −241.27, 95% CI: −421.50 to −61.03) and iliac artery (55 patients, MD: −382, 95% CI: −751.07 to −12.93) was lower in the STS group compared with the control group, whereas the change of Agatston score for aorta did not show a difference between the two groups (55 patients, MD: −108, 95% CI: −491.30 to 275.30). No difference between the two groups was found in each subgroup for the post-interventional Agatston score, the post-interventional CVS and the change of CVS (shown in Fig. 2A, C and D, respectively). Meanwhile, no group difference was noticed among the overall effect sizes in subgroup analysis for all the four endpoints (P > .05) (Fig. 2A–D).
FIGURE 2:
Meta-analysis of the effect of STS on Agatston score and CVS in patients with VC. Calcification scores in different locations were synthesized. No difference was found in both post-interventional Agatston score and CVS (A and C). The increase in Agatston score was lower in the STS group compared with the control group for coronary artery (107 patients, MD: –241.27, 95% CI: −421.50 to −61.03) and iliac artery (55 patients, MD: −382, 95% CI: −751.07 to −12.93) (B). The change in CVS was not different between the two groups (D).
After Messa et al. [20], which utilized the Kauppila index to evaluate aortic calcification, was added to the meta-analysis using Hedge's g function, no difference was noted between the STS group and the control group (Supplementary data, Fig. S1). In meta-regression analyses, no correlation was found between the assessed characteristics of STS therapy and change in calcification scores (Agatston score and CVS) (P > .05) (Supplementary data, Table S4).
Arterial stiffness
Two RCTs [22, 23] studied the effect of STS on arterial stiffness. As shown in Fig. 3, the increase of PWV was lower in the STS group compared with the control group (104 patients, MD: −1.29 m/s, 95% CI: −2.24 to −0.34 m/s). No difference between the two groups was found in the post-interventional level (49 patients, MD: −0.51, 95% CI: −1.03 to 0.01) or the change (49 patients, MD: −0.46, 95% CI: −0.98 to 0.06) of CAVI.
FIGURE 3:
Meta-analysis of the effect of STS on PWV and CAVI in patients with VC. No difference was found in the post-interventional PWV between the two groups (A). The increase in PWV was lower in the STS group compared with the control group (104 patients, MD: −1.29 m/s, 95% CI: −2.24 to −0.34 m/s) (B). No difference in the post-interventional level or the change of CAVI was found between the two groups (P > .05) (C and D).
Safety of intravenous STS among CKD patients
Adverse symptoms
In the four studies [19, 21–23], which reported adverse symptoms related to STS treatment, gastrointestinal (GI) symptoms (e.g. anorexia, poor appetite, nausea and vomiting) were the most commonly observed (25.9%), followed by hypotension (4.7%), sneezing (4.7%), flushing (2.3%), dizziness (2.3%) and excessive thirst (1.2%) (Table 2). The study [19] with the highest frequency of GI symptoms (75%) employed post-dialysis administration and short infusion times (15–20 min).
Table 2.
Adverse symptoms related to STS treatment among included studies
| Study ID | No. of participants | GI symptoms | Hypotension | Sneezing | Flushing | Dizziness | Thirsty |
|---|---|---|---|---|---|---|---|
| Adirekkiat et al. [19] | 20 | 15 (anorexia and poor appetite) | 2 | 3 | 1 | ||
| Yu et al. [21] | 15 | 3 (nausea and vomiting) | 1 | 1 | 1 | ||
| Saengpanit et al. [22] | 24 | 3 (anorexia and poor appetite) | 2 | 2 | |||
| Djuric et al. [23] | 26 | 1 (nausea) | |||||
| Total | 85 (100%) | 22 (25.9%) | 4 (4.7%) | 4 (4.7%) | 2 (2.3%) | 2 (2.3%) | 1 (1.2%) |
ID: identity; No.: number.
CKD-MBD parameters
As shown in Fig. 4, four RCTs [21–24] measured calcium, phosphate and iPTH levels, while two of them [21, 22] documented 25(OH)VitD3 levels as well. The laboratory values were measured before dialysis at baseline, while on STS treatment (during the trials) and at the completion of the trials (after the trials). The point values (during or after the trials) and the changes from the baseline were compared between the STS group and the control group, respectively. During the trials, calcium, phosphate and iPTH were comparable between the STS group and the control group, and there were no significant changes in these parameters. The mean level of 25(OH)VitD3 in the STS group is less than that of the controls (49 participants, MD: −5.80 ng/mL, 95% CI: −9.59 to −2.01 ng/mL) during the trials, but no difference was noticed in the changes from baseline between the two groups (49 participants, MD: −0.40 ng/mL, 95% CI: −4.19 to 3.39 ng/mL). After the trials, the post-interventional levels and the change of serum calcium, serum phosphate, blood iPTH and blood 25(OH)VitD3 in the STS group showed no difference between the two groups.
FIGURE 4:
Meta-analysis of the effect of STS on CKD-MBD parameters in patients with VC. Calcium, phosphate and iPTH were comparable between the STS group and the control group there were no significant changes in these parameters during and after the trials (A–F). During the trials, the mean level of 25(OH)VitD3 in the STS group was less than that of the controls (49 participants, MD: −5.80 ng/mL, 95% CI: −9.59 to −2.01 ng/mL), but no difference was noticed in the change during the trials between the two groups (G and H).
Electrolytes
In Adirekkiat et al. [19], serum sodium, chloride, bicarbonate and anion gap were found to be changed immediately after the infusion of STS. When comparing the electrolyte levels during the entire STS therapy with baseline levels, Adirekkiat et al. [19] found an elevation in pre-dialysis serum anion gap and sodium in 16 patients treated with HD undergoing a 4-month STS therapy. Serum electrolytes in the control group were also reported in three trials [21–23] during or after the trials. As displayed in Fig. 5, no significant difference was found between the STS group and the control group in serum sodium, potassium, chloride and bicarbonate during or after the trials. During the trial period, a higher anion gap (49 participants, MD: 3.00 mmol/L, 95% CI: 1.03 to 4.97 mmol/L) and a larger increase in the anion gap (49 participants, MD: 2.50 mmol/L, 95% CI: 0.53 to 4.47 mmol/L) were noted in the STS group compared with the control group. However, anion gaps showed no difference between the two groups after the trials.
FIGURE 5:
Meta-analysis of the effect of STS on electrolytes in patients with macrovascular calcification. Panels (A–J) show the point values (during or after the trials) and the changes from the baseline of serum sodium, potassium, chloride, bicarbonate and anion gap. During the trial period, higher anion gaps (49 participants, MD: 3.00 mmol/l, 95% CI: 1.03 to 4.97 mmol/L) and a larger increase in the anion gap (49 participants, MD: 2.50 mmol/l, 95% CI: 0.53 to 4.47 mmol/L) were noted in the STS group compared with the control group (I and J).
BMD
Two studies [19, 21] reported BMD in the STS and control groups. Yu et al. [21] showed that no significant change was observed in both groups, but the data were not retrievable. Adirekkiat et al. [19] reported a decline in the total hip BMD in the treatment group, but no comparison between the two groups was conducted. The lumbar and the total hip BMD in Adirekkiat et al. [19] were extracted and compared between the STS group and the control group. As presented in Fig. 6, the post-trial levels and the changes were not significantly different between the STS group and the control group for both the lumbar and total hip BMD values. In addition, no significant difference was found in the effect size between the two locations (lumbar and total hip).
FIGURE 6:
Analysis of the effect of STS on BMD in patients with VC. BMD in different locations was synthesized. Compared with controls, no difference was noticed in both post-interventional levels (A) and the change (B) of BMD in the STS group in both sites (lumbar and hip) (P > .05).
Sensitivity analysis
Analyses were repeated using a fixed-effect model in which little differences were noted in the overall effect sizes. Furthermore, we performed sensitivity analyses on the impact of single studies. The overall effect size and its 95% CIs when omitting the denoted study are presented in Supplementary data, Fig. S2. For the change in Agatston score using Hedge's g, omitting Messa et al. [20] resulted in lower progression in the STS group. No change in the conclusion or direction of other results was noticed.
Heterogeneity
Various sources of heterogeneity were noted among the studies included in this analysis, mainly from different countries of origin, diverse study designs, various dosages, timing and duration of STS administration and distinct outcome measures. Based on the I2 test, obvious or high heterogeneity was observed in data related to blood 25(OH)VitD3, serum calcium, serum bicarbonate and serum anion gap.
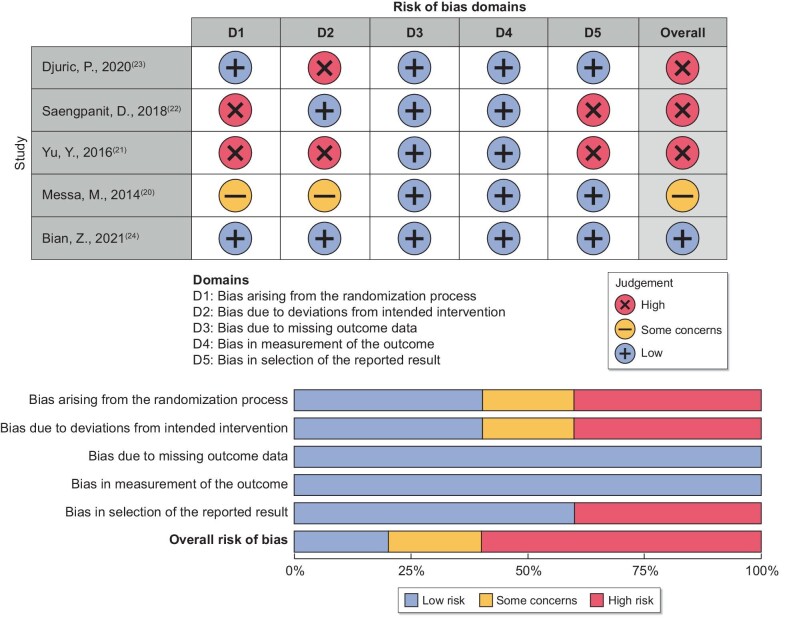
Risk-of-bias assessment
Risk-of-bias assessment for the five RCTs [20–24] using the RoB 2 tool is shown in Fig. 7. The study by Bian et al. [24] had a low risk of bias, while the others were with some concern [20] or high risk of bias [21–23]. Based on the ROBINS-I tool, the non-RCT [19] was evaluated as having a low risk of bias (Fig. 8).
FIGURE 7:
Risk-of-bias assessment of randomized control trials using RoB 2 tool.
FIGURE 8:
Risk-of-bias assessment of the non-randomized control trial using ROBINS-I tool.
Egger's tests were performed to evaluate publication bias. As presented in Supplementary data, Table S5, no small study effect was discovered in our study.
DISCUSSION
To date, no medication has been approved to treat VC. Most of the conventional therapies (e.g. phosphate binders, calcimimetics, vitamin D therapy, etc.) yielded conflicting or inconclusive results [25]. Magnesium supplementation showed attenuation on VC but needs further exploration [25]. SNF472, as a new agent that directly inhibits calcium phosphate crystal formation and aggregation, has completed a Phase 2 clinical trial showing attenuation of coronary artery and aortic valve calcification in HD patients [26]. In this systematic review and meta-analysis, we found that intravenous STS may attenuate the progression of macrovascular calcification and arterial stiffness in patients treated with HD.
In clinical trials lacking controls, a reduction or non-progression for VC was observed among patients receiving STS. Ghiandai et al. [10] reported a modest reduction of Kauppila's index was detected in 18 HD patients administrated with 6-month intravenous STS. Mathews et al. [11] treated 22 HD patients with CAC with intravenous STS for 5 months, and no progression in the mean annualized rate of change of VC was observed. In our meta-analysis, comparisons were made between patients treated with and without intravenous STS, and less progression of the Agatston score for coronary artery and iliac artery in the STS group compared with controls was revealed. However, this finding was not replicated in the aorta, suggesting that diverse mechanisms might be involved (e.g. aging, smoking, metabolic disorders [27]). Progression in CVS was also compared between the two groups, but no difference was noticed. This could partly be due to the conversion from median to mean scores in meta-analysis and the fewer number of studies reporting CVS. Thus, STS's role in CVS scores may need further investigation. Compared with CVS, more evidence lies in Agatston scores for predicting CV events [28]. In addition to calcification scores, STS could help ameliorate arterial stiffness and presented prolonged benefits at 48 weeks in an extended study of Saengpanit's trial [29]. Although no benefit has been shown by current cohort studies, an overall survival improvement in patients with calciphylaxis has been reported by Gaisne et al. with an effective therapeutic regimen of STS for not <2 weeks or with a cumulative STS dose of no <150 g [30, 31]. For studies examining the effect of STS on macrovascular calcification, however, a short follow-up duration and a lack of evidence on long-term survival have been noted.
Common adverse events in prior studies on STS treatment included electrolyte disorders, GI symptoms, decreased appetite/anorexia, skin disorders, metabolic acidosis and transient hypotension/hypertension [32]. These were all noted in our review. Our study indicates that GI symptoms were the most commonly seen and might be related to fast infusion or post-dialysis administration of STS in patients treated with dialysis. Serum electrolytes and anion gap could be dramatically altered after infusion and were likely to change in the middle of a certain period of STS therapy depending on STS dosage [33]. Our analysis suggests that these effects might not last after the completion of STS therapy. However, bicarbonate supplement via dialysates or other routes might be a key element not adjusted in the included trials. Whether STS administration casts an impact on calcium-phosphate metabolism has not been well-defined. For iPTH and 25(OH)VitD3, although stated in some of the included trials, are not expected to be directly affected by STS treatment. Elevated serum calcium or phosphate levels in STS treated patients have been reported in case studies [34]. In our meta-analysis, calcium and phosphate levels seemed not impacted by STS administration both during and after the completion. BMD seemed not to be impacted by STS use in our analysis, but a paucity of evidence should be noted. To avoid unnecessary risks and make the best use of the therapy, close observation and dynamic regimen adjustment are suggested.
Several limitations should be noted in the present study. First, the trials included in our analysis only provided per-protocol data in which the effect size may be exaggerated. Second, some of the subgroups (e.g. Agatston scores, BMD) were derived from duplicated participants. Here, we should concentrate on the subgroup effects and the between-group differences rather than the overall effects. Third, missing data, various sources of heterogeneity and a high risk of bias are notable. We reached out to the primary study authors via email to address the missing data. No reply, however, was obtained. Of note, the sample size was relatively small in current studies included given the paucity of randomized trials in this area. Furthermore, the absence of calcimimetics or non-calcium-based phosphate binder's use may make the participants included less representative of patients with easy access to those medications. Nonetheless, this meta-analysis is the first to systematically examine and report the current state of knowledge on the effects of STS for VC in CKD patients.
In conclusion, intravenous STS may attenuate the progression of VC and arterial stiffness in individuals on HD. Future large and well-designed randomized controlled trials are warranted to further establish the effect of STS.
Supplementary Material
ACKNOWLEDGEMENTS
We appreciate colleagues from Massachusetts General Hospital for their support in this study. V.C. is supported by R21-DK119740, 1R01HL132325.
Contributor Information
Wen Wen, Department of Nephrology, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, China; Division of Nephrology, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA.
Ignacio Portales-Castillo, Division of Nephrology, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA.
Rituvanthikaa Seethapathy, Division of Nephrology, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA.
Scott Krinsky, Division of Nephrology, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA.
Daniela Kroshinsky, Department of Dermatology, Massachusetts General Hospital, Boston, MA, USA.
Sahir Kalim, Division of Nephrology, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA.
Jeremy Goverman, Sumner Redstone Burn Center, Massachusetts General Hospital, Boston, MA, USA.
Rosalynn M Nazarian, Department of Pathology, Massachusetts General Hospital, Boston, MA, USA.
Vipul Chitalia, Renal Section, Department of Medicine, Boston University Medical Center, Boston, MA, USA.
Rajeev Malhotra, Cardiovascular Research Center and the Cardiology Division of the Department of Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Rafael Kramann, Division of Nephrology and Clinical Immunology, Medical Faculty RWTH Aachen University, Aachen, Germany; Institute of Experimental Medicine and Systems Biology, Medical Faculty RWTH Aachen University, Aachen, Germany; Department of Internal Medicine, Nephrology and Transplantation, Erasmus Medical Center, Rotterdam, The Netherlands.
Cindy K Malhotra, Department of Pharmacy, Massachusetts General Hospital, Boston, MA, USA.
Sagar U Nigwekar, Division of Nephrology, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA.
AUTHORS’ CONTRIBUTIONS
S.U.N. and W.W. conceived the study. W.W. and I.P.-C. were involved in data collection, statistical design and analysis. W.W., R.S., D.K., S.K., J.G., R.M.N., V.C., R.M., R.K., C.K.M. and S.U.N. were involved in the study design, analysis and editing of the manuscript. All authors contributed to the article and approved the submitted version.
CONFLICT OF INTEREST STATEMENT
S.U.N. received grant support from Hope Pharmaceuticals. R.K. received grants from Chugai, Travere Therapeutics and Galapagos, honoraria for lectures and advisory boards from Bayer Healthcare. The results presented in this paper have not been published previously in whole or in part.
DATA AVAILABILITY STATEMENT
The data used or analyzed in the study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Sin HK, Wong PN, Lo KYet al. An echocardiography-derived calcium score as a predictor of major adverse cardiovascular events in peritoneal dialysis patients––a prospective cohort study. Nephrology (Carlton) 2022; 27: 181–189 [DOI] [PubMed] [Google Scholar]
- 2. Chen L, Vavrenyuk A, Ren JHet al. Prognostic value of coronary artery calcification identified by the semi-quantitative weston method in the emergency room or other hospitalized patients. Front Cardiovasc Med 2021; 8: 684292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dusing P, Zietzer A, Goody PRet al. Vascular pathologies in chronic kidney disease: pathophysiological mechanisms and novel therapeutic approaches. J Mol Med (Berl) 2021; 99: 335–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kakani E, Elyamny M, Ayach T, El-Husseini A. Pathogenesis and management of vascular calcification in CKD and dialysis patients. Semin Dial 2019; 32: 553–561 [DOI] [PubMed] [Google Scholar]
- 5. Nelson AJ, Raggi P, Wolf Met al. Targeting vascular calcification in chronic kidney disease. JACC Basic Transl Sci 2020; 5: 398–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen HC, Wang WT, Hsi CNet al. Abdominal aortic calcification score can predict future coronary artery disease in hemodialysis patients: a 5-year prospective cohort study. BMC Nephrol 2018; 19: 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Piccoli GB, Torreggiani M, Gendrot Let al. Setting the clock back: new hope for dialysis patients. Sodium thiosulphate and the regression of vascular calcifications. J Nephrol 2021; 34: 23–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cicone JS, Petronis JB, Embert CDet al. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis 2004; 43: 1104–1108 [DOI] [PubMed] [Google Scholar]
- 9. Pasch A, Schaffner T, Huynh-Do Uet al. Sodium thiosulfate prevents vascular calcifications in uremic rats. Kidney Int 2008; 74: 1444–1453 [DOI] [PubMed] [Google Scholar]
- 10. Ghiandai G, Ralli C, Imperiali Pet al. [Is the Sodium Thiosulfate Therapy useful for vascular calcification in dialysis Pts?]. G Ital Nefrol 2015; 32: gin/32.3.6. [PubMed] [Google Scholar]
- 11. Mathews SJ, De Las Fuentes L, Podaralla Pet al. Effects of sodium thiosulfate on vascular calcification in end-stage renal disease: a pilot study of feasibility, safety and efficacy. Am J Nephrol 2011; 33: 131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen NC, Hsu CY, Chen CL. The strategy to prevent and regress the vascular calcification in dialysis patients. Biomed Res Int 2017; 2017: 9035193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Selk N, Rodby RA.. Unexpectedly severe metabolic acidosis associated with sodium thiosulfate therapy in a patient with calcific uremic arteriolopathy. Semin Dial 2011; 24: 85–88 [DOI] [PubMed] [Google Scholar]
- 14. Dobry AS, Ko LN, Kroshinsky D.. Fractures in calciphylaxis patients following intravenous sodium thiosulfate therapy. J Eur Acad Dermatol Venereol 2017; 31: e445–e446 [DOI] [PubMed] [Google Scholar]
- 15. Levey AS, Eckardt KU, Dorman NMet al. Nomenclature for kidney function and disease-executive summary and glossary from a Kidney Disease: Improving Global Outcomes (KDIGO) consensus conference. Eur Heart J 2020; 41: 4592–4598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang ZR, Sun F, Zhan SY. [Risk on bias assessment: (2) revised cochrane risk of bias tool for individually randomized, parallel group trials (RoB2.0)]. Zhonghua Liu Xing Bing Xue Za Zhi 2017; 38: 1285–1291 [DOI] [PubMed] [Google Scholar]
- 17. Sterne JA, Hernan MA, Reeves BCet al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355: i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wan X, Wang W, Liu Jet al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adirekkiat S, Sumethkul V, Ingsathit Aet al. Sodium thiosulfate delays the progression of coronary artery calcification in haemodialysis patients. Nephrol Dial Transplant 2010; 25: 1923–1929 [DOI] [PubMed] [Google Scholar]
- 20. Messa M, Tomei P, Motton Met al. Effect of sodium thiosulphate on aortic calcifications in hemodialysis patients. Nephrol Dial Transplant 2014; 29: iii48 [Google Scholar]
- 21. Yu Y, Bi ZM, Wang Yet al. [Effect of sodium thiosulfate on coronary artery calcification in maintenance hemodialysis patients]. Zhonghua Yi Xue Za Zhi 2016; 96: 3724–3728 [DOI] [PubMed] [Google Scholar]
- 22. Saengpanit D, Chattranukulchai P, Tumkosit Met al. Effect of sodium thiosulfate on arterial stiffness in end-stage renal disease patients undergoing chronic hemodialysis (Sodium Thiosulfate-Hemodialysis study): a randomized controlled trial. Nephron 2018; 139: 219–227 [DOI] [PubMed] [Google Scholar]
- 23. Djuric P, Dimkovic N, Schlieper Get al. Sodium thiosulphate and progression of vascular calcification in end-stage renal disease patients: a double-blind, randomized, placebo-controlled study. Nephrol Dial Transplant 2020; 35: 162–169 [DOI] [PubMed] [Google Scholar]
- 24. Bian Z, Zhang Q, Shen Let al. The effect of sodium thiosulfate on coronary artery calcification in hemodialysis patients. ASAIO J 2022; 68: 402–406 [DOI] [PubMed] [Google Scholar]
- 25. Xu C, Smith ER, Tiong MKet al. Interventions to attenuate vascular calcification progression in chronic kidney disease: a systematic review of clinical trials. J Am Soc Nephrol 2022; 33: 1011–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raggi P, Bellasi A, Bushinsky Det al. Slowing progression of cardiovascular calcification with SNF472 in patients on hemodialysis: results of a randomized phase 2b study. Circulation 2020; 141: 728–739 [DOI] [PubMed] [Google Scholar]
- 27. Singh A, Tandon S, Tandon C. An update on vascular calcification and potential therapeutics. Mol Biol Rep 2021; 48: 887–896 [DOI] [PubMed] [Google Scholar]
- 28. Blaha MJ, Mortensen MB, Kianoush Set al. Coronary artery calcium scoring: is it time for a change in methodology? JACC Cardiovasc Imaging 2017; 10: 923–937 [DOI] [PubMed] [Google Scholar]
- 29. Saengpanit D, Sitprija V, Praditpornsilpa Ket al. Effect of sodium thiosulfate on arterial stiffness in end-stage renal disease patients undergoing chronic hemodialysis: an extended follow-up of the sodium thiosulfate-hemodialysis study (a randomized controlled trial). Nephrol Dial Transplant 2019; 34: a254. [DOI] [PubMed] [Google Scholar]
- 30. Udomkarnjananun S, Kongnatthasate K, Praditpornsilpa Ket al. Treatment of calciphylaxis in CKD: a systematic review and meta-analysis. Kidney Int Rep 2019; 4: 231–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gaisne R, Péré M, Menoyo Vet al. Calciphylaxis epidemiology, risk factors, treatment and survival among French chronic kidney disease patients: a case-control study. BMC Nephrol 2020; 21: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nigwekar SU, Brunelli SM, Meade Det al. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol 2013; 8: 1162–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hundemer GL, Fenves AZ, Phillips KMet al. Sodium thiosulfate and the anion gap in patients treated by hemodialysis. Am J Kidney Dis 2016; 68: 499–500 [DOI] [PubMed] [Google Scholar]
- 34. Hlusicka J, Veisova E, Ullrych Met al. Serum calcium and phosphorus concentrations and the outcome of calciphylaxis treatment with sodium thiosulfate. Monatshefte für Chemie - Chem Mon 2017; 148: 435–440 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used or analyzed in the study are available from the corresponding author on reasonable request.