Abstract
Neutrophils are immune cells involved in several inflammatory and homeostatic processes. Their capacity to release cargo can be classified based on whether the cargo is released on its own, or in conjunction with plasma membrane structures. Examples of plasma membrane-free secretion modes are degranulation, neutrophil extracellular trap (NET) release, and cytokine release through inflammasome formation. The most studied membrane-covered neutrophil-derived structures are exosomes and ectosomes that are collectively called extracellular vesicles (EV). Apoptotic vesicles are another recognized EV subtype. Over the last decade, additional membrane-covered neutrophil-derived structures were characterized: migratory cytoplasts, migrasomes, and elongated neutrophil-derived structures (ENDS). All these structures are smaller than the neutrophils, cannot reproduce themselves, and thus meet the latest consensus definition of EVs. In this review, we focus on the less well-studied neutrophil EVs: apoptotic vesicles, cytoplasts, migrasomes, and ENDS.
Keywords: apoptotic body, cytoplast, ectosome, elongated neutrophil-derived particles, ENDS, Exosome, migrasome, mitosome, NET, neutrophil-derived extracellular trap
1 |. INTRODUCTION
Neutrophils are the most abundant immune cells in human blood and play an important role in host defense against bacterial and fungal infections. These cells are also involved in tissue remodeling and pathological conditions like sepsis, autoimmune diseases, allergies, chronic obstructive pulmonary diseases, atherosclerosis, and cancer.1–3
Neutrophils are professional phagocytes that are highly effective in killing pathogens and cleaning up tissue debris. Neutrophils also release a vast array of inflammatory and antimicrobial molecules. Many of these molecules are stored in granules within the neutrophils and are released when the granules fuse with the plasma membrane. This exocytotic process is also called degranulation.1,4 Degranulation already begins when circulating neutrophils get primed. This is suggested by the presence of CD66a-positive neutrophils and MPO in the circulation,5 both of which are intracellular molecules. Degranulation reaches its peak in the extravascular space where the fully activated neutrophils release their most toxic molecules like the serine proteases neutrophil elastase and cathepsin G.1 Neutrophils also release several molecules in conjunction with nuclear or mitochondrial DNA. This process is called neutrophil extracellular trap (NET) release. NET release can happen on the endothelial surface and in the extravascular space.6,7 NETs may be released by neutrophil plasma membrane lysis that is accompanied by the death of the neutrophil or by fusion of NET-containing intracellular vesicles with the neutrophil plasma membrane that leaves the neutrophil alive.8 NETs are devoid of plasma membrane.
Neutrophils have several mechanisms to release molecules enclosed by phospholipid bilayer structures. These structures are smaller than the neutrophils, do not contain a functional nucleus, and cannot replicate. These properties define them as extracellular vesicles (EV) according to the International Society of Extracellular Vesicles (ISEV).9 EVs are categorized into subsets based on their mechanism of formation and size. The best-studied subsets are exosomes and ectosomes. This review focuses on the understudied or recently discovered subsets: apoptotic EVs, cytoplasts, migrasomes, and elongated neutrophil-derived structures (ENDS).
2 |. EXOSOMES AND ECTOSOMES
Exosomes form intracellularly inside endosomes that become multivesicular bodies. The multivesicular bodies fuse with the plasma membrane, resulting in exosome release into the extracellular space. On the contrary, ectosomes (or microvesicles) bud off directly from the cell membrane into the extravascular space.10,11 The molecular mechanisms behind these processes were thoroughly reviewed.10,11 The diameter of exosomes is limited by the size of endosomes and varies between 50 and 150 nm, which is below but overlapping with the size range of ectosomes that range from 100 nm to 1 μm in diameter (Figure 1 and Table 1). Currently, this size difference is used to estimate the ratio of exosome and ectosome release.12 Based on such estimates, the majority of EVs released by neutrophils are ectosomes.13 However, the characterization of release, composition, and effect of exosomes and ectosomes remains a major challenge due to the difficulty of identifying reliable markers for differentiation of exosomes and ectosomes.9,14
FIGURE 1.
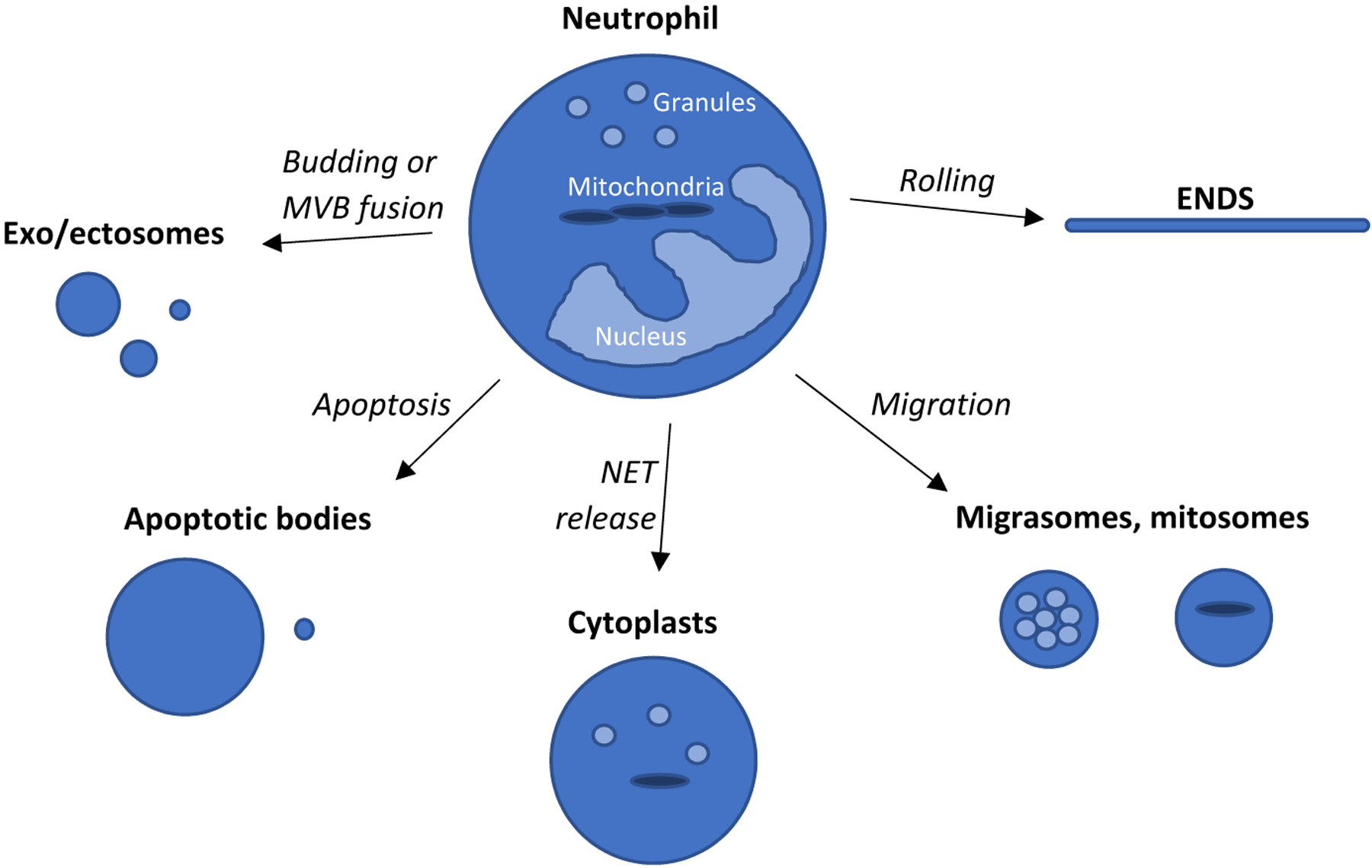
Graphical summary of neutrophil-derived EVs. The mechanism related to EV formation is indicated on the arrows. Presence of granules or mitochondria in the EVs is indicated with light-blue spheres or with dark-blue elongated objects. The size of different type of EVs is close to proportional. MVB—multivesicular body, NET—neutrophil extracellular trap, and ENDS—elongated neutrophil-derived structures
TABLE 1.
Comparison of the main highly sensitive EV analysis methods
| Method | Sample preparation | Counting | Information about shape | Marker-based phenotyping |
|---|---|---|---|---|
|
Electron Microscopy EVs are fixed to a substrate, labeled and scanned with electron microscopy |
Difficult | Semi-quantitative | Yes | Limited |
|
Nanoparticle Tracking Analysis Particle size is estimated based on the particle’s Brownian motion detected by laser scattering |
Easy, but requires the right sample concentration | Quantitative | No | Limited |
|
Dynamic Light Scattering Laser scattering from suspended particles is detected over time |
Requires monodisperse suspension | Quantitative | No | No |
|
Ultrasensitive Flow Cytometry Fluorescent signal is detected of EVs passing by the detector |
Easy, but sensitive to sample concentration | Semi-quantitative | No | Yes |
|
Imaging Cytometry (Amnis) Fluorescent image is captured of EVs passing by the detector |
Easy, least sensitive to sample concentration | Quantitative | Yes, limited by optical resolution | Yes |
Recently Kolonics and colleagues thoroughly reviewed the literature of exo/ectosome release by neutrophils.13 Since 1998, over 55 studies were published where neutrophil-derived exo/ectosomes with or without isolation by density centrifugation, dialysis, filtration, or precipitation were analyzed by electron microscopy, nanoparticle tracking analysis, dynamic light scattering, tunable resistive pulse sensing, ultrasensitive flow cytometry, or imaging cytometry (ImageStream – Amnis15,16). Some of the older studies preceded the publication of the latest ISEV consensus guidelines.9 Thus, results from older studies must be viewed with caution. Also, ultracentrifugation aggregates exosomes and ectosomes, and freezing followed by thawing compromises their membrane integrity.17,18
Due to technical difficulties, very few studies reported exact exosome or ectosome counts for body fluids. Imaging cytometry appears to be the ideal tool for quantitative analysis of such counts, because this technique requires minimal sample preparation, has high throughput and sensitivity, and enables neutrophil-derived exo/ectosome identification through neutrophil-specific surface markers like Ly6G in mice and CD66b in humans.19,20 During imaging cytometry, a multi-channel fluorescent image is captured of each particle that passes by the detector. The resulting dataset is analyzed with image masking-based analysis tools that allow to quantify the fluorescent signal and shape of the captured exo/ectosome. Imaging cytometry can exclude crowding events, defined as multiple objects captured in the detection area at the same time. Flow cytometry can be also used to enumerate exo/ectosome populations in suspensions; however, this requires a dedicated high-sensitivity flow cytometer,21 the analysis does not provide information about the shape of the exo/ectosome and it is vulnerable to the above described crowding events. The advantages and disadvantages of the most frequently used exo/ectosome analysis methods are shown in Table 1 and discussed in more detail in recent publications.22–25
Neutrophils release exo/ectosomes even without stimulation, which can be detected in supernatant of cultured neutrophils and also in healthy human blood. With imaging cytometry, Headland and colleagues counted about 100 neutrophil-derived exo/ectosomes per microliter of healthy human platelet-poor plasma.15 With the same technique but slightly different sample preparation protocol that included lower centrifugation forces, our group counted about 1000 neutrophil-derived exo/ectosomes per microliter of healthy human plasma.16 In vitro experiments showed that neutrophils increase their exo/ectosome release upon numerous types of stimulation including inflammatory mediators (TNF, IFN-γ, GM-CSF, C5a, PAF, IL-8), bacteria and bacterial molecules (S. aureus, M. tuberculosis, Meningococcus, fMLP, LPS); this was recently reviewed.13,26 Increased blood exo/ectosome counts were measured in numerous infectious, autoimmune and traumatic disease states; however, most of the applied detection methods do not give absolute counts. For those that do, the results cannot be compared directly due to the different exo/ectosome detection methods used.13,26,27 With imaging cytometry, we counted 120,000 exo/ectosomes / μl of septic patients’ blood, more than 100 times the number in healthy donors’ blood.16 Besides blood plasma, neutrophil-derived exo/ectosomes have also been detected in the bronchoalveolar and peritoneal lavage of septic patients28 and in the bronchoalveolar lavage of chronic obstructive pulmonary diseases (COPD) patients.29
The effect of exo/ectosome release on the neutrophil itself is unknown. However, the effect of the neutrophil-derived exo/ectosomes on other cells was extensively studied. While exo/ectosomes do not contain organelles, they carry various molecules including adhesion molecules (integrins, selectins), Fc and complement receptors, neutrophil granule-associated molecules (e.g., MPO, elastase, and CD63), neutrophil cytosolic proteins (S100A8), and micro RNAs through which exo/ectosomes can influence other cells, bacteria, blood coagulation, or the extracellular matrix.13,26 Numerous in vitro co-incubation experiments showed that neutrophil-derived exo/ectosomes can exert pro-or anti-inflammatory effects on various immune cells including monocytes, macrophages, and neutrophils.13,30 Neutrophil-derived exo/ectosomes were found to inhibit bacterial growth by aggregating bacteria,31 to activate platelets by delivering arachidonic acid,32 to mediate neutrophil swarming by prolonging the lifetime of leukotriene B4 (LTB4) released by neutrophils,33 and to contribute to COPD development by protecting the neutrophil elastase from α1-antitrypsin in the lungs.29 Recently, neutrophil-derived exo/ectosomes were shown to contribute to vascular inflammation at atherosclerosis-prone vessel sites with disturbed flow by delivering miR-155 into endothelial cells.34 Neutrophil exo/ectosomes also contribute to epithelial injury in inflammatory bowel diseases by delivering miR-26a and miR-155 into the epithelial cells.35
3 |. APOPTOTIC EXTRACELLULAR VESICLES
Apoptosis is a programmed cell death that results in regulated cell removal by efferocytosis.36 During apoptosis, several cell types are known to release apoptotic EVs which can be stratified by their size as small (diameter 50–1000 nm) and large (diameter 1–5 μm), the latter also called apoptotic bodies (Figure 1 and Table 1).37,38 While small apoptotic EVs may form similarly as exo/ectosomes,39 apoptotic bodies are released in a different way. Caspase-3 mediated activation of Rho-associated kinase 1 and other kinases orchestrate cell contraction that results in membrane blebbing due to the increased intracellular pressure.38 Sometimes, the blebs separate from the cell and become apoptotic bodies with up to 5 μm diameter.40 More often, under the regulation of the plasma membrane ATP channel pannexin-1 and transmembrane protein plexin-b2, filaments (apoptopodia) grow out from the apoptotic cell. Sometimes, these structures turn into beaded apoptopodia as 10–20 beads form along their length. Eventually, apoptopodia detach from the cell forming 1–4 μm large apoptotic bodies.38,41,42
Apoptotic EVs often express the surface markers of the source cell, like CD3 for T cells, CD11b for myeloid cells, CD31 for endothelial cells, and CD45 for leukocytes.43 While apoptotic bodies can be distinguished from cells by flow cytometry due to their smaller size and lower granularity,40,44,45 separation of apoptotic EVs from exo/ectosomes is challenging.
Apoptotic EVs carry the apoptotic marker phosphatidylserine (PS, detected with Annexin-5)40,44; however, the specificity of PS for apoptotic EVs is questionable46 because several groups have reported PS enrichment on exo/ectosomes too.47 As soon as 20 min after incubation, isolated human neutrophils release PS-positive exo/ectosomes smaller than 1 μm,31 but these neutrophils are most likely not apoptotic yet. While apoptotic EVs form from cell surfaces that already expose PS, on non-apoptotic exo/ectosomes PS exposure is suggested to happen due to absence of flippases from the exo/ectosomes.48 Poon and colleagues introduced TO-PRO-3 Iodide staining as a criterion to identify apoptotic bodies by flow cytometry.40 Uptake of TO-PRO-3 dye is indicative of the presence of the pannexin-1 ATP channel in the plasma membrane of the apoptotic body and, once internal, TO-PRO-3 binds to double-stranded DNA. However, some apoptotic bodies do not contain DNA and thus cannot be distinguished from exo/ectosomes using this method.46
The literature of neutrophil-derived apoptotic vesicles is scarce. Shi and colleagues showed by electron microscopy that apoptotic neutrophils and their apoptotic bodies are phagocytosed by Kupffer cells in rat liver sinusoids.49 In another study, Dalli and colleagues collected neutrophil-derived EVs from human neutrophils after 1 day of culture50 and found that >85% of EVs were Annexin-5 positive. Although the authors did not aim to study apoptotic vesicles, their sample most likely contained apoptotic vesicles, because neutrophils turn apoptotic after 5 h in culture.51 Dieker and colleagues studied EVs in the blood of systemic lupus erythematosus patients and found that 15% of the Annexin-5+ and apoptosis-modified histone-positive EVs were of neutrophil origin (CD31+CD45+CD66b+), suggesting the release in vivo of neutrophil-derived apoptotic EVs.52
Neutrophil apoptosis happens at steady state and has an important role in negatively regulating neutrophil production. In healthy humans, about 1 billion neutrophils are made per kg body weight a day. Within a few hours of production, most of the neutrophils in bone marrow, liver, and spleen become apoptotic and are phagocytosed by macrophages and dendritic cells.53 This tempers IL-23 release by macrophages and dendritic cells, which in turn reduces the production of IL-17A by γδ T cells and other T-cell populations leading to reduced GCSF levels and reduced neutrophil production.54
Since neutrophil apoptosis happens at steady state, neutrophil-derived apoptotic EVs must form very commonly as well. However, this probably happens mainly in the bone marrow, spleen, liver, and lungs.53 Due to the increase of eat-me signals (e.g., PS) and decrease of do not eat me signals (e.g., CD47), neutrophil-derived apoptotic EVs are probably rapidly cleared by local phagocytes.
Apoptopodia formation was documented in monocytes41; however, it is not known whether apoptopodia form in neutrophils, too. Some reports suggest that it might happen. The apoptosis-inducing compound staurosporine was found to induce long filament outgrowth from various cells.55,56 This was observed in neutrophils as well, and fragments of these filaments broke off from the neutrophils.57
4 |. CYTOPLASTS
In 1967, Carter noticed that the nuclei of adhered fibroblasts are extruded after prolonged cytochalasin B treatment.58 The extruded nuclei connected to the cell through an easily severable thin plasma membrane tether which sometimes broke spontaneously. Based on this phenomenon, Wigler and Weinstein combined cytochalasin B treatment with Ficoll density centrifugation to establish a scalable method for preparation of nucleus-free cells.59 In 1983, Roos and colleagues applied this technique to human neutrophils to generate vesicles without nuclei and granules, called neutrophil cytoplasts.60 Another method for neutrophil fragment production was discovered by Keller and Bessis; heating adhered neutrophils to 46°C induced the formation of blebs, which separated from the cell body as the neutrophils migrated away.61,62 These structures were named cytokineplasts.62
During the next two decades, several groups employed in vitro-made cytoplasts and cytokineplasts to study neutrophil functions. These nucleus- and granule-free neutrophil bodies were about half of the neutrophil’s size, had an intact plasma membrane,60 were able to migrate,63 react to fMLP,64,65 form LTB466 and were capable of phagocytosis and bacterial killing.60,67,68 Unlike neutrophils, cytoplasts and cytokineplasts were able to maintain their migratory and antibacterial functions even after thawing from cryopreservation, which raised hope that cytoplasts or cytokineplasts could become a therapeutic option.67
In 2004, Brinkman and colleagues discovered that neutrophils exposed to inflammatory mediators such as PMA, IL-8, or LPS in vitro can release their nuclear DNA and form NETs.69 Almost a decade later, Yipp and colleagues found that injection of S. pyogenes Gram-positive bacteria into the mouse skin induces NET release in vivo. The remaining neutrophils with diffuse nuclei resembled the in vitro-made cytoplasts in their shape and migration.70 The authors detected anuclear neutrophils in the fluid of S.pyogenes abscesses of patients, and they also observed anuclear neutrophil formation by human neutrophils injected into the mouse skin together with S.aureus. These findings showed for the first time that anuclear neutrophil (or cytoplast) formation can happen in vivo and is associated with NET release.
Subsequently, in vivo NET release was observed on the surface of large veins during deep vein thrombosis,71 in atherosclerotic plaque,72,73 during infection in the lungs,74 in the liver and the gastrointestinal system.75 Likely all these NET-forming events resulted in the formation of cytoplasts; however, in these studies, presence of cytoplasts was not investigated.
In mice stimulated with house dust mite extract and LPS, neutrophils undergo NET release and cytoplasts become detectable in the bronchoalveolar fluid and in the mediastinal lymph nodes.76 Peptidyl arginine deaminase 4 (PAD4) is one of the NET release regulators. In PAD4 knockout mice, LPS did not induce NET release and cytoplasm formation. The authors isolated cytoplasts from the lungs of these mice by FACS as CD45+CD11b+Ly6G+DNA- objects. They found that cytoplasts excluded trypan blue, showed chemokinesis (but no chemotaxis toward LTB4), phagocytosed and acidified E. coli particles, and killed S.pneumoniae. Cytoplasts also trigger dendritic cells, resulting in increased antigen-specific IL-17 and IL-13 production by CD4+ T cells. The authors also detected CD45+CD66b+CD16+DNA-cytoplasts in the BALF of severe asthmatic patients.
By appearance, cytoplasts resemble apoptotic bodies: both have diameters between 3 and 5 μm, little or no DNA content, and are decorated with neutrophil surface markers (Figure 1 and Table 1). So far, no study compared the two entities directly; however, based on published data, cytoplasts should be distinguishable from apoptotic bodies by lack of PS on their surface76 and by their higher side scatter signal in flow cytometry caused by their higher intracellular granularity.
5 |. MIGRASOMES
Migrating neutrophils form long and thin traction fibers from their uropod, which may detach from the neutrophil and form a trail of membrane-covered structures along the migrated path. This was first shown by Hyun and colleagues, who found that neutrophils extravasating from mesenteric venules in mice leave behind about 1 μm large spherical structures in the perivascular area.77 These structures are positive for β2 integrins, and their formation depends on the LFA-1 (αLβ2)-mediated arrest and VLA-3 (α3β1)-mediated transmigration of the neutrophils. The authors confirmed these findings in human neutrophils, which leave behind membrane-covered structures during transmigration across cultured human endothelial cells.77
Later, Lim and colleagues reported that in neutrophil-depleted mice, fewer T cells migrate to the flu-infected trachea than in control mice78 and hypothesized that migrating neutrophils leave behind trails that guide T cells to the inflamed tissue. The authors supported their hypothesis with in vitro experiments showing that migrating neutrophils leave behind membrane-covered structures that release CXCL12. Additionally, in in vitro chemotaxis assays, T cells migrated toward the zone where neutrophils had migrated earlier and in vivo, in the ear of mice, T cells migrated along the path demarcated by neutrophil-derived spherical membrane-covered structures. Both phenomena were attenuated by pharmacological inhibition of CXCR4, the receptor for CXCL12, suggesting that the trails released by migrating neutrophils guide T-cell migration through CXCL12 recognition. Trail formation by migrating neutrophils was also observed in the lungs79 and liver80,81 of mice; however, whether these trails guide other cells is not known.
In the same year, Ma and colleagues reported the discovery of migrasomes, which are 2–3 μm spherical vesicles that form at the tips and branching points of traction fibers that extend from the uropod of migrating cells82 and thus might be the same type of structures discussed above. Migrasomes are enriched for tetraspanin-4 and may contain up to 300 smaller vesicles inside each of them. Migrasomes were detected in intestinal capillaries and lung alveoli of mice and rats. A detailed protocol for migrasome detection was recently published.83 Activated α5β1 integrin was found to be enriched in the basal surface of retraction fibers and migrasomes of MGC803 cells, suggesting that integrins are necessary for the formation of these structures.84 Huang and colleagues identified tetraspanin-4 and cholesterol as key drivers of migrasome formation from a normal rat epithelial kidney cell line; both form clusters and increase the membrane stiffness that forces sphere formation from membrane tubules.85 Proteomic analysis revealed that relative to the cell bodies migrasomes are enriched in migration- and adhesion-related proteins and have a different composition that exosomes, as their proteomes overlap only by 27%.86
The above-discussed studies suggest that migrasome formation depends on integrin-mediated anchorage and tetraspanin-mediated membrane sphere formation. The proposed requirement for integrin anchorage fits well with trail and migrasome formation on the vessel wall, where neutrophil migration is integrin-dependent.87 In the extravascular space, even though swarming neutrophils do not require high-affinity integrin interaction for interstitial migration (only for compaction at the center of the injury88), migrasome formation was observed.78
Jiao and colleagues reported migrasome formation by mouse neutrophils in vivo and discovered that these migrasomes can contain mitochondria with impaired membrane potential, suggesting that migrasome release can serve as removal mechanism for damaged mitochondria.81 The authors designated mitochondriacontaining migrasomes as mitosomes, and their formation process as mitocytosis (Figure 1 and Table 1). Jiao and colleagues also found that neutrophils from tetraspanin-9 knockout mice produce fewer mitosomes and have more splenic neutrophils with impaired mitochondrial membrane potential. These findings suggest that mitocytosis is important for neutrophil health; however, its role in neutrophil aging and host defense requires further investigation.
6 |. ELONGATED NEUTROPHIL-DERIVED STRUCTURES (ENDS)
Cryo-EM investigation of EVs released by a human mast cell line in vitro showed that cells can produce EVs of different shapes,89 suggesting that EV shape should be considered during EV classification. Focusing on blood, Yuana and colleagues detected elongated particles in plasma of healthy human donors by cryo-EM.90 In their study, surface markers were not assessed; thus, the elongated particles’ cell of origin remained undefined. The subcellular structures were pelleted and resuspended for imaging, which might have caused artificial formation of elongated particles as indicated by a recent study.91 Arraud and colleagues reported elongated particles in healthy human donors’ plasma as well.92 The authors performed cryo-EM by freezing platelet-free plasma onto a perforated carbon film without pelleting and resuspension. About half of the observed subcellular objects had an elongated shape with an average length of 2.2 μm and maximal length over 6 μm and width between 50 and 400 nm. Most of the elongated particles did not present PS on their surface as indicated by lack of labeling with gold-conjugated Annexin-5. About 70% of the elongated particles were either CD235 or CD41 positive, indicating erythrocyte or platelet origin, respectively. Some of the remaining 30% may have been of leukocyte origin, but the samples were not stained for leukocyte markers. Tersteeg and colleagues confirmed the existence of platelet-derived elongated particles in vitro and in vivo on the atherosclerotic vessel wall of mice.93 The authors also identified a formation mechanism that entails platelet adhesion, activation-induced disassembly of the cytoskeleton and flow-induced membrane extraction from the platelet.
More recently, our group has shown that neutrophils release elongated neutrophil-derived structures (ENDS) into the circulation of septic patients and mice.16 ENDS form as their anchored microvilli are pulled out into long thin tethers and break off from the rolling cell. This process is dependent on the pulling force applied on the cell surface, as indicated by the correlation between the number of released ENDS and the magnitude of wall shear stress. ENDS stain for the same surface markers as the parent neutrophil (Ly6G, CD11a, and CD11b in mice; CD66b, CD16, CD11a, and CD11b in humans) and have functional adhesion molecules. ENDS are 115 nm thin and median 7 μm long membrane-covered structures that contain cytoplasm but no mitochondria, endoplasmic reticulum, or DNA (Figure 1 and Table 1). Proteomic analysis showed that ENDS are enriched for several proteins and are rich in MRP8 and MRP14 (also called S100A8 and S100A9). Initially, the ENDS membrane is intact as indicated by its ability to exclude calcium and retain intracellular dyes such as Fluo4 or CMRA. Over the course of a few hours, ENDS expose PS on their surface as detected by Annexin-5 binding. With degradation, ENDS release MRP8/14 complex,16 which is a damage-associated molecular pattern multimer that can bind TLR-4 and can activate neutrophils and monocytes, but may also desensitize macrophages.94 MRP8/14 is rapidly inactivated in the presence of calcium.95 Since ENDS can exclude calcium from their lumen for a limited period, ENDS might help disseminate MRP8/14.
ENDS formation most likely depends on so far unidentified characteristics of the cells. During intravital imaging of mice, only about 6% of neutrophils left behind ENDS when in the same vessel (and hence under the same wall shear stress) where 94% of neutrophils did not. It is not known which cellular properties might promote tether formation and rupture of the tether. It is not known what happens to neutrophils that released ENDS. In vitro and in vivo experiments revealed that ENDS formation is accompanied by unstable neutrophil rolling. How and where these neutrophils leave the circulation is not known. One possibility could be rolling-independent recruitment mechanisms such as in the lungs or liver.96,97
7 |. CONCLUSION
Neutrophils release various membrane-covered structures that occur in suspension, on vessel walls and in the interstitial space. These structures differ in mechanisms of release, size, shape, surface markers, and organelle content (Figure 1 and Table 2). All neutrophil EVs are membrane-covered, smaller than neutrophils, and cannot reproduce themselves. While cytoplasts retain several neutrophil functions, including migration, phagocytosis, and degranulation, exosomes, ectosomes, ENDS, and migrasomes are non-migrating structures. They likely extend the lifetime of their cargo by protecting it from the microenvironment.
TABLE 2.
Characteristics of neutrophil-derived extracellular vesicles (EV)
| Release | Shape | Size | In tissue | Organelle cargo | |
|---|---|---|---|---|---|
| Exosomes | Multivesicular body fusion with plasma membrane | Spherical | 50–150 nm | Blood | – |
| Ectosomes | Bud off from cell surface | Spherical | 100–1000 nm | Blood | – |
| Small apoptotic EVs | Similar to exo/ectosomes | Spherical | 50–150 nm | Blood | – |
| Large apoptotic EVs or apoptotic bodies | Bud off from cell surface, detachment of apoptopodia or beaded apoptopodia | Spherical | 1–5 μm | – | |
| Cytoplast | Neutrophil extracellular trap NET release | Spherical | 1–5 μm | Blood, bronchoalveolar fluid | Fragments of organelles |
| Elongated neutrophil-derived structures (ENDS) | Tether detachment from rolling neutrophils | Elongated, spherical | Median 7 μm long and 115 nm thick | Blood | – |
| Migrasomes | Vesicle formation at the tip or branching point | Spherical | 2–3 μm | Trachea, lung, liver, Blood | Smaller vesicles |
| Mitosomes | of traction fibers or migrating neutrophils | 2–3 μm | Blood, liver, spleen | Damaged mitochondria |
Funding information
This work was funded by National Institutes of Health R35 145241 and program project grant P01 HL151433 of Klaus Ley
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
This article is part of a series of reviews covering Extracellular vesicles appearing in Volume 312 of Immunological Reviews
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study
REFERENCES
- 1.Ley K, Hoffman HM, Kubes P, et al. Neutrophils: New insights and open questions. Sci Immunol. 2018;3(30):eaat4579. [DOI] [PubMed] [Google Scholar]
- 2.Ng LG, Ostuni R, Hidalgo A. Heterogeneity of neutrophils. Nat Rev Immunol. 2019;19(4):255–265. [DOI] [PubMed] [Google Scholar]
- 3.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175. [DOI] [PubMed] [Google Scholar]
- 4.Sheshachalam A, Srivastava N, Mitchell T, Lacy P, Eitzen G. Granule protein processing and regulated secretion in neutrophils. Front Immunol. 2014;5:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fine N, Barzilay O, Sun C, et al. Primed PMNs in healthy mouse and human circulation are first responders during acute inflammation. Blood Adv. 2019;3(10):1622–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18(2):134–147. [DOI] [PubMed] [Google Scholar]
- 7.Jorch SK, Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med. 2017;23(3):279–287. [DOI] [PubMed] [Google Scholar]
- 8.Castanheira FVS, Kubes P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood. 2019;133(20):2178–2185. [DOI] [PubMed] [Google Scholar]
- 9.Thery C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–228. [DOI] [PubMed] [Google Scholar]
- 11.Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. [DOI] [PubMed] [Google Scholar]
- 12.Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA. 2016;113(8):E968–E977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolonics F, Szeifert V, Timar CI, Ligeti E, Lorincz AM. The functional heterogeneity of neutrophil-derived extracellular vesicles reflects the status of the parent cell. Cell. 2020;9(12):2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathieu M, Nevo N, Jouve M, et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat Commun. 2021;12(1):4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Headland SE, Jones HR, D’Sa AS, Perretti M, Norling LV. Cutting-edge analysis of extracellular microparticles using ImageStream(X) imaging flow cytometry. Sci Rep. 2014;4:5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marki A, Buscher K, Lorenzini C, et al. Elongated neutrophil-derived structures are blood-borne microparticles formed by rolling neutrophils during sepsis. J Exp Med. 2021;218(3):e20200551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosch S, de Beaurepaire L, Allard M, et al. Trehalose prevents aggregation of exosomes and cryodamage. Sci Rep. 2016;6:36162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linares R, Tan S, Gounou C, Arraud N, Brisson AR. High-speed centrifugation induces aggregation of extracellular vesicles. J Extracell Vesicles. 2015;4:29509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorgens A, Bremer M, Ferrer-Tur R, et al. Optimisation of imaging flow cytometry for the analysis of single extracellular vesicles by using fluorescence-tagged vesicles as biological reference material. J Extracell Vesicles. 2019;8(1):1587567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Botha J, Pugsley HR, Handberg A. Conventional, high-resolution and imaging flow cytometry: benchmarking performance in characterisation of extracellular vesicles. Biomedicine. 2021;9(2):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welsh JA, Holloway JA, Wilkinson JS, Englyst NA. Extracellular vesicle flow cytometry analysis and standardization. Front Cell Dev Biol. 2017;5:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szatanek R, Baj-Krzyworzeka M, Zimoch J, Lekka M, Siedlar M, Baran J. The methods of choice for extracellular vesicles (EVs) characterization. Int J Mol Sci. 2017;18(6):1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serrano-Pertierra E, Oliveira-Rodriguez M, Matos M, et al. Extracellular vesicles: current analytical techniques for detection and quantification. Biomolecules. 2020;10(6):824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Z, Wijerathne H, Godwin AK, Soper SA. Isolation and analysis methods of extracellular vesicles (EVs). Extracell Vesicles Circ Nucl Acids. 2021;2:80–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Droste M, Tertel T, Jeruschke S, et al. Single extracellular vesicle analysis performed by imaging flow cytometry and nanoparticle tracking analysis evaluate the accuracy of urinary extracellular vesicle preparation techniques differently. Int J Mol Sci. 2021;22(22):12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong CW. Extracellular vesicles of neutrophils. Immune Netw. 2018;18(6):e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson BL III, Kuethe JW, Caldwell CC. Neutrophil derived microvesicles: emerging role of a key mediator to the immune response. Endocr Metab Immune Disord Drug Targets. 2014;14(3):210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prakash PS, Caldwell CC, Lentsch AB, Pritts TA, Robinson BR. Human microparticles generated during sepsis in patients with critical illness are neutrophil-derived and modulate the immune response. J Trauma Acute Care Surg. 2012;73(2):401–406; discussion 406-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Genschmer KR, Russell DW, Lal C, et al. Activated PMN exosomes: pathogenic entities causing matrix destruction and disease in the lung. Cell. 2019;176(1–2):113–126. e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amjadi MF, Avner BS, Greenlee-Wacker MC, Horswill AR, Nauseef WM. Neutrophil-derived extracellular vesicles modulate the phenotype of naive human neutrophils. J Leukoc Biol. 2021;110:917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Timar CI, Lorincz AM, Csepanyi-Komi R, et al. Antibacterial effect of microvesicles released from human neutrophilic granulocytes. Blood. 2013;121(3):510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossaint J, Kuhne K, Skupski J, et al. Directed transport of neutrophil-derived extracellular vesicles enables platelet-mediated innate immune response. Nat Commun. 2016;7:13464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Majumdar R, Tavakoli Tameh A, Parent CA. Exosomes mediate LTB4 release during neutrophil chemotaxis. PLoS Biol. 2016;14(1):e1002336. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Gomez I, Ward B, Souilhol C, et al. Neutrophil microvesicles drive atherosclerosis by delivering miR-155 to atheroprone endothelium. Nat Commun. 2020;11(1):214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butin-Israeli V, Bui TM, Wiesolek HL, et al. Neutrophil-induced genomic instability impedes resolution of inflammation and wound healing. J Clin Invest. 2019;129(2):712–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doran AC, Yurdagul A Jr, Tabas I. Efferocytosis in health and disease. Nat Rev Immunol. 2020;20(4):254–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li M, Liao L, Tian W. Extracellular vesicles derived from apoptotic cells: an essential link between death and regeneration. Front Cell Dev Biol. 2020;8:573511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santavanond JP, Rutter SF, Atkin-Smith GK, Poon IKH. Apoptotic bodies: mechanism of formation, isolation and functional relevance. Subcell Biochem. 2021;97:61–88. [DOI] [PubMed] [Google Scholar]
- 39.Park SJ, Kim JM, Kim J, et al. Molecular mechanisms of biogenesis of apoptotic exosome-like vesicles and their roles as damage-associated molecular patterns. Proc Natl Acad Sci USA. 2018;115(50):E11721–E11730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poon IK, Chiu YH, Armstrong AJ, et al. Unexpected link between an antibiotic, pannexin channels and apoptosis. Nature. 2014;507(7492):329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atkin-Smith GK, Tixeira R, Paone S, et al. A novel mechanism of generating extracellular vesicles during apoptosis via a beads-on-a-string membrane structure. Nat Commun. 2015;6:7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Atkin-Smith GK, Miles MA, Tixeira R, et al. Plexin B2 is a regulator of monocyte apoptotic cell disassembly. Cell Rep. 2019;29(7):1821–1831. e1823. [DOI] [PubMed] [Google Scholar]
- 43.Jiang L, Paone S, Caruso S, et al. Determining the contents and cell origins of apoptotic bodies by flow cytometry. Sci Rep. 2017;7(1):14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atkin-Smith GK, Paone S, Zanker DJ, et al. Isolation of cell type-specific apoptotic bodies by fluorescence-activated cell sorting. Sci Rep. 2017;7:39846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phan TK, Poon IK, Atkin-Smith GK. Detection and isolation of apoptotic bodies to high purity. J Vis Exp. 2018;138:58317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poon IKH, Parkes MAF, Jiang L, et al. Moving beyond size and phosphatidylserine exposure: evidence for a diversity of apoptotic cell-derived extracellular vesicles in vitro. J Extracell Vesicles. 2019;8(1):1608786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skotland T, Sandvig K, Llorente A. Lipids in exosomes: Current knowledge and the way forward. Prog Lipid Res. 2017;66:30–41. [DOI] [PubMed] [Google Scholar]
- 48.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and inter-cellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. [DOI] [PubMed] [Google Scholar]
- 49.Shi J, Fujieda H, Kokubo Y, Wake K. Apoptosis of neutrophils and their elimination by Kupffer cells in rat liver. Hepatology. 1996;24(5):1256–1263. [DOI] [PubMed] [Google Scholar]
- 50.Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120(15):e60–e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsao FH, Xiang Z, Abbasi A, Meyer KC. Neutrophil necrosis and annexin 1 degradation associated with airway inflammation in lung transplant recipients with cystic fibrosis. BMC Pulm Med. 2012;12:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dieker J, Tel J, Pieterse E, et al. Circulating apoptotic microparticles in systemic lupus erythematosus patients drive the activation of dendritic cell subsets and prime neutrophils for NETosis. Arthritis Rheumatol. 2016;68(2):462–472. [DOI] [PubMed] [Google Scholar]
- 53.Greenlee-Wacker MC. Clearance of apoptotic neutrophils and resolution of inflammation. Immunol Rev. 2016;273(1):357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22(3):285–294. [DOI] [PubMed] [Google Scholar]
- 55.Paunel-Gorgulu A, Kirichevska T, Logters T, Windolf J, Flohe S. Molecular mechanisms underlying delayed apoptosis in neutrophils from multiple trauma patients with and without sepsis. Mol Med. 2012;18:325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kohno T, Ninomiya T, Kikuchi S, Konno T, Kojima T. Staurosporine induces formation of two types of extra-long cell protrusions: actin-based filaments and microtubule-based shafts. Mol Pharmacol. 2015;87(5):815–824. [DOI] [PubMed] [Google Scholar]
- 57.Galkina SI, Stadnichuk VI, Molotkovsky JG, Romanova JM, Sud’ina GF, Klein T. Microbial alkaloid staurosporine induces formation of nanometer-wide membrane tubular extensions (cytonemes, membrane tethers) in human neutrophils. Cell Adh Migr. 2010;4(1):32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carter SB. Effects of cytochalasins on mammalian cells. Nature. 1967;213(5073):261–264. [DOI] [PubMed] [Google Scholar]
- 59.Wigler MH, Weinstein IB. A preparative method for obtaining enucleated mammalian cells. Biochem Biophys Res Commun. 1975;63(3):669–674. [DOI] [PubMed] [Google Scholar]
- 60.Roos D, Voetman AA, Meerhof LJ. Functional activity of enucleated human polymorphonuclear leukocytes. J Cell Biol. 1983;97(2):368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keller HU, Bessis M. Migration and chemotaxis of anucleate cytoplasmic leukocyte fragments. Nature. 1975;258(5537):723–724. [DOI] [PubMed] [Google Scholar]
- 62.Malawista SE, De Boisfleury CA. The cytokineplast: purified, stable, and functional motile machinery from human blood polymorphonuclear leukocytes. J Cell Biol. 1982;95(3):960–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Malawista SE, Van Blaricom G. Cytoplasts made from human blood polymorphonuclear leukocytes with or without heat: preservation of both motile function and respiratory burst oxidase activity. Proc Natl Acad Sci USA. 1987;84(2):454–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Korchak HM, Roos D, Giedd KN, et al. Granulocytes without degranulation: neutrophil function in granule-depleted cytoplasts. Proc Natl Acad Sci USA. 1983;80(16):4968–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gallin JI, Metcalf JA, Roos D, Seligmann B, Friedman MM. Organelle-depleted human neutrophil cytoplasts used to study fmet-leu-phe receptor modulation and cell function. J Immunol. 1984;133(1):415–421. [PubMed] [Google Scholar]
- 66.Haines KA, Giedd KN, Weissmann G. Leukotriene B4 synthesis and metabolism by neutrophils and granule-free cytoplasts. Biochem J. 1986;233(2):583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malawista SE, Van Blaricom G, Breitenstein MG. Cryopreservable neutrophil surrogates. Stored cytoplasts from human polymorphonuclear leukocytes retain chemotactic, phagocytic, and microbicidal function. J Clin Invest. 1989;83(2):728–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fleit HB, Lane BP. FC gamma receptor mediated phagocytosis by human neutrophil cytoplasts. Inflammation. 1999;23(3):253–262. [DOI] [PubMed] [Google Scholar]
- 69.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. [DOI] [PubMed] [Google Scholar]
- 70.Yipp BG, Petri B, Salina D, et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med. 2012;18(9):1386–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.von Bruhl ML, Stark K, Steinhart A, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209(4):819–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 2015;349(6245):316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Silvestre-Roig C, Braster Q, Wichapong K, et al. Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature. 2019;569(7755):236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiménez-Alcázar M, Rangaswamy C, Panda R, et al. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science. 2017;358(6367):1202–1206. [DOI] [PubMed] [Google Scholar]
- 75.Honda M, Kubes P. Neutrophils and neutrophil extracellular traps in the liver and gastrointestinal system. Nat Rev Gastroenterol Hepatol. 2018;15(4):206–221. [DOI] [PubMed] [Google Scholar]
- 76.Krishnamoorthy N, Douda DN, BRÜGGEMANN TR, et al. Neutrophil cytoplasts induce T(H)17 differentiation and skew inflammation toward neutrophilia in severe asthma. Sci Immunol. 2018;3(26):eaao4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hyun YM, Sumagin R, Sarangi PP, et al. Uropod elongation is a common final step in leukocyte extravasation through inflamed vessels. J Exp Med. 2012;209(7):1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lim K, Hyun YM, Lambert-Emo K, et al. Neutrophil trails guide influenza-specific CD8(+) T cells in the airways. Science. 2015;349(6252):aaa4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thanabalasuriar A, Neupane AS, Wang J, Krummel MF, Kubes P. iNKT cell emigration out of the lung vasculature requires neutrophils and monocyte-derived dendritic cells in inflammation. Cell Rep. 2016;16(12):3260–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu J, Lu Z, Jiang D, et al. Iterative tomography with digital adaptive optics permits hour-long intravital observation of 3D subcellular dynamics at millisecond scale. Cell. 2021;184:3318–3332. e17. [DOI] [PubMed] [Google Scholar]
- 81.Jiao H, Jiang D, Hu X, et al. Mitocytosis, a migrasome-mediated mitochondrial quality-control process. Cell. 2021;184(11):2896–2910. e2813. [DOI] [PubMed] [Google Scholar]
- 82.Ma L, Li Y, Peng J, et al. Discovery of the migrasome, an organelle mediating release of cytoplasmic contents during cell migration. Cell Res. 2015;25(1):24–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen Y, Li Y, Ma L, Yu L. Detection of migrasomes. Methods Mol Biol. 2018;1749:43–49. [DOI] [PubMed] [Google Scholar]
- 84.Wu D, Xu Y, Ding T, Zu Y, Yang C, Yu L. Pairing of integrins with ECM proteins determines migrasome formation. Cell Res. 2017;27(11):1397–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang Y, Zucker B, Zhang S, et al. Migrasome formation is mediated by assembly of micron-scale tetraspanin macrodomains. Nat Cell Biol. 2019;21(8):991–1002. [DOI] [PubMed] [Google Scholar]
- 86.Zhao X, Lei Y, Zheng J, et al. Identification of markers for migrasome detection. Cell Discov. 2019;5:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McDonald B, Pittman K, Menezes GB, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330(6002):362–366. [DOI] [PubMed] [Google Scholar]
- 88.Lammermann T, Afonso PV, Angermann BR, et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498(7454):371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zabeo D, Cvjetkovic A, Lasser C, Schorb M, Lotvall J, Hoog JL. Exosomes purified from a single cell type have diverse morphology. J Extracell Vesicles. 2017;6(1):1329476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yuana Y, Koning RI, Kuil ME, et al. Cryo-electron microscopy of extracellular vesicles in fresh plasma. J Extracell Vesicles. 2013;2(1):21494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Božič D, Hočevar M, Kononenko V, et al. Pursuing mechanisms of extracellular vesicle formation. Effects of sample processing. Advances in Biomembranes and Lipid Self-Assembly. Elsevier; 2020:113–155. [Google Scholar]
- 92.Arraud N, Linares R, Tan S, et al. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. J Thromb Haemost. 2014;12(5):614–627. [DOI] [PubMed] [Google Scholar]
- 93.Tersteeg C, Heijnen HF, Eckly A, et al. FLow-induced PRotrusions (FLIPRs): a platelet-derived platform for the retrieval of microparticles by monocytes and neutrophils. Circ Res. 2014;114(5):780–791. [DOI] [PubMed] [Google Scholar]
- 94.Pruenster M, Vogl T, Roth J, Sperandio M. S100A8/A9: From basic science to clinical application. Pharmacol Ther. 2016;167:120–131. [DOI] [PubMed] [Google Scholar]
- 95.Vogl T, Stratis A, Wixler V, et al. Autoinhibitory regulation of S100A8/S100A9 alarmin activity locally restricts sterile inflammation. J Clin Invest. 2018;128(5):1852–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Adrover JM, Del Fresno C, Crainiciuc G, et al. A neutrophil timer coordinates immune defense and vascular protection. Immunity. 2019;50(2):390–402 e310. [DOI] [PubMed] [Google Scholar]
- 97.Margraf A, Ley K, Zarbock A. Neutrophil recruitment: from model systems to tissue-specific patterns. Trends Immunol. 2019;40(7):613–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study


